Threatened status of neglected and underutilised Jatropha (Euphorbiaceae) species endemic to Mexico
Cecilie S. L. Christensen a, b, Charlotte E. Seal a, Lourdes Rico-Arce c, d, *
a Seed Conservation Department, Royal Botanic Gardens, Kew, Wellcome Trust Millennium Building, Wakehurst Place, Ardingly, West Sussex RH17 6TN, England
b Department of Plant and Environmental Sciences, Faculty of Science, University of Copenhagen, Thorvaldsensvej 40, 1871 Frederiksberg C, Denmark
c Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AE, England
d Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, Liga Periférico-Insurgentes Sur, Núm. 4903, Col. Parques del Pedregal, Tlalpan, 14010 Ciudad de México, Mexico
*Corresponding author: L.Rico@kew.org (L. Rico-Arce)
Abstract
Jatropha curcas is the best known species from the genus, an oilseed crop used for biodiesel production. It deserves attention as more species show similar oil content, phytochemical properties, or as a genetic resource in J. curcas breeding programs. The closest species to J. curcas show restricted distributions and are included in the IUCN Red List. In this study, the geographical distribution and conservation status was determined for 24 species in of the subgenus Curcas endemic to the Neotropic, except J. curcas, 20 are endemic to Mexico. Nine hundred and ninety-four herbarium specimens were used to assess their status by applying the IUCN Red List Categories and Criteria. The analysis shows that 11 species are threatened: 2 are critically endangered, 6 endangered and 3 vulnerable. Additionally, 5 species were assessed as nearly threatened, 4 as least concern and 2 as data deficient. Except for J. contrerasii and J. websteri, the threatened species have a narrow altitude range (< 500 m). With a growing emphasis on the exploitation of neglected and under-utilised species such as Jatropha, it is important to establish systematic collections for the assessment of threat status and to develop management strategies for their conservation.
Keywords: Conservation; Distribution patterns; Herbarium specimens; IUCN Red List
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Estado de amenaza para las especies menos conocidas o poco utilizadas de Jatropha (Euphorbiaceae) endémicas de México
Resumen
Jatropha curcas es la especie mejor conocida del género por ser un cultivo oleaginoso utilizado para la producción de biodiesel, merece atención porque varias especies muestran un contenido similar de aceite, propiedades fotoquímicas o como un recurso genético en los programas de reproducción de J. curcas. Especies cercanas a J. curcas tienen una distribución restringida y están incluidas en la Lista Roja de la UICN. En este estudio, se determinaron la distribución geográfica y el estado de conservación de 24 especies del subgénero Curcas, todas endémicas del neotrópico; excepto J. curcas, 20 son endémicas de México. Se revisaron 994 especímenes de herbario para evaluar su estado de conservación aplicando las categorías y criterios de la Lista Roja de la UICN. El análisis muestra que 11 especies están amenazadas: 2 en peligro crítico, 6 en peligro y 3 vulnerables. Otras 5 resultaron como casi amenazadas, 4 con preocupación mínima y 2 como deficientes en datos. Las especies amenazadas tienen un rango de altitud estrecho (< 500 m), excepto J. contrerasii y J. websteri. Con el énfasis creciente en la explotación de especies poco conocidas y subutilizadas, como Jatropha, es importante establecer colecciones sistemáticas para evaluar el estado de amenaza y desarrollar estrategias de manejo para su conservación.
Palabras clave: Conservación; Patrones de distribución; Especímenes de herbario; Lista roja de la UICN
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
The value of plant biofuels as alternatives to diesel fuel continues to be researched and hotly debated (Koh et al., 2009). Whilst biofuels can partially replace diminishing fossil fuel reserves, with fewer net greenhouse gas emissions environmental concerns persist, including risks to biodiversity. Due to its ability to grow on poor quality soils and little requirement for crop management, Jatropha curcas has been promoted as a sustainable source of oilseeds for biodiesel (Bahadur et al., 2013; Carels et al., 2012; Dagar et al., 2006; Divakara et al., 2010). The seed oil content is 28 – 42% of dry weight (Heller 1996; Kaushik et al., 2007) and seed yield ranges from 0.4 – 12 t- ha-1 y-1 after 5 years of growth, depending on planting density, genetic and agronomical practise (Openshaw, 2000). Furthermore, the environmental impact of growing J. curcas is lower than that of palm oil as long as no natural ecosystems are removed for the establishment of plantations (Stone, 2007). As the main form of J. curcas in cultivation across the world produces non-edible seeds, contaminated by a phorbol ester, agricultural production of the species ought not to compete with edible oils in the food market. However, there are traditional non-toxic varieties grown in Mexico which are now at significant risk of displacement by the available commercial seed lots (Vera-Castillo et al., 2014).
Jatropha curcas is one of approximately 187 species which comprise the genus Jatropha (Euphorbiaceae) in the tribe Jatropheae of subfamily Crotonoideae (WCSP, 2013). J. curcas (section Curcas) is thought to be the ancestor to the genus and shares several anatomical features with members of both the Jatropha and Curcas subgenera (Dehgan & Schutzman, 1994). The closest related species to J. curcas are in the subgenus Curcas, section Curcas and section Platyphyllae, which was recently divided into 3 subsections Platyphyllae, Fremontioides, and Gaumeri (Dehgan, 2012). Several species in the subsection Fremontioides are from a more recent origin and some species share characteristics with those in subgenus Jatropha.
The genus is distributed worldwide throughout the sub-tropical regions, primarily in the Neotropics. Mexico is believed to be the centre of origin, with 25% of species in the genus found in the country, of which 80% are endemic (Dehgan, 2012; WCSP, 2013). A few species extend their geographical range into Guatemala, Nicaragua and Costa Rica in Central America. Jatropha species in Mexico are mainly distributed in seasonally dry tropically forest (Fresnedo-Ramírez & Orozco-Ramírez, 2012), a biome that is highly threatened by deforestation (Trejo & Dirzo, 2000). Consequently, there is a very real threat that several species in the genus, mainly the endemics with narrow distributions, might be under threat of genetic erosion and, ultimately, extinction. This is further exacerbated by the unregulated exploitation of many species due to their medicinal properties and nutritional qualities (Devappa et al., 2010a, b; Dutra et al., 1996).
The socio-economic potential of several neglected and under-utilised species related to Jatropha curcas is well documented. Several species show high seed oil content: 20 – 27% for J. glandulifera Roxb., c. 28% for J. gossypiifolia L., and 32 – 48% for J. multifida L. (Banerji et al., 1985); 39% for J. excise Griseb., 36% for J. macrocarpa Griseb. and 36% for J. hieronymi Kuntze (Aranda-Rickert et al., 2011). Closely related species to J. curcas in Mexico show other socio-economic potential: J. platyphylla is cultivated for medicinal purposes in Michoacán, is grown as an ornamental and the seeds are consumed by descendants of the Lacapaxa tribe in Sinaloa (Makkar et al., 2011); J. moranii is cultivated as an ornamental (Dehgan, 2012); J. gaumeri was used by the Mayan’s of Tixpeual and Tixcacaltuyub, Yucatán, in traditional medicine, handicraft, soap production and for its toxitity (Rico-Gray et al., 1991), and the root and latex also have medicinal value properties (Ankli et al., 2002; Dehgan, 2012). However, despite the large economic importance and management of J. curcas, little is known about the conservation status of closely related species in the genus Jatropha subgenus Curcas. This makes an assessment of their conservation status particularly important.
A recognised method to evaluate the extinction risk of a species is the IUCN Red List Categories and Criteria (IUCN, 2012), in combination with environmental information (Rodrigues et al., 2006). Where little information is available from field studies, herbarium specimens can be used as essential sources of information to clarify the distribution and conservation status of a plant species (Guerra-García et al., 2008; Willis et al., 2003). The IUCN Red List requires application of “best available evidence” to perform assessments and often herbarium specimens are the only source of information available. However, in some cases, species are only known from the type specimens in herbaria or from very few herbarium collections and conservation assessments are carried out when only limited herbarium specimens are available (Randrianasolo et al., 2002; Versieux & Wendt, 2007; Zizka et al., 2009).
The aims of this study were to apply the IUCN Red List Categories and Criteria to: 1) assess the geographical extent of the 22 species of the genus Jatropha subgenus Curcas section Curcas and section Platyphyllae; both sections are all Mexican species and 2 more species that are distributed in adjoining regions of Central America; 2) produce a Red List conservation assessment for each of the species, and 3) recommend conservation strategies for the protection of the threatened species, in order to ensure that these neglected and underutilised species are available for the future.
Table 1
Studied species of Jatropha subgenus Curcas with respective taxonomic sections. The species are arranged following the taxonomy of Dehgan (2012).
|
1) Section Curcas |
J. pseudocurcas Müll. Arg. |
|
J. curcas L.** |
J. rufescens Brandegee |
|
J. malacophylla Standl. |
J. contrerasii J. Jiménez Ram., & Mart. Gord. |
|
J. mcvaughii Dehgan & G. L. Webster |
J. andrieuxii Müll. Arg. |
|
2) Section Platyphyllae subsection Platyphyllae |
3) Section Platyphyllae subsection Fremontioides |
|
J. bartlettii Wilbur |
J. fremontioides Standl. |
|
J. websteri J. Jiménez Ram. |
J. stevensii G. L. Webster* |
|
J. chamelensis Pérez-Jim. |
J. krusei J. Jiménez Ram., & Mart. Gord. |
|
J. pereziae J. Jiménez Ram. |
J. bullockii E. J. Lott |
|
J. stephani J. Jiménez Ram., & Mart. Gord. |
J. tlalcozotitlanensis J. Jiménez Ram. |
|
J. tehuantepecana J. Jiménez Ram., & A. Campos Vilb. |
J. jaimejimenezii V. W. Steinm. |
|
J. platyphylla Müll. Arg. |
J. moranii Dehgan & G. L. Webster |
|
J. alamanii Müll. Arg. |
4) Section Platyphyllae subsection Gaumeri |
|
J. costaricensis G. L. Webster & Poveda* |
J. gaumeri Greenm.* |
The recognised taxa are based on the monograph of Dehgan (2012). However, it is worth to note that there is a nomenclature issue with between Jatropha platyphylla and J. peltata, which is discussed by Dehgan (2012: 173). *Present or **planted in some of the neighboring countries to Mexico; Belize, Guatemala, Nicaragua, El Salvador, Honduras and/or Costa Rica.
Materials and methods
The geographical extent and conservation status were assessed for 22 species of Jatropha distributed in Mexico and 2 species found in adjacent countries (Table 1).
Herbarium specimens were the primary sources of information used in this study. Specimens deposited in the herbaria of the Natural History Museum Copenhagen (C), the Natural History British Museum (BM), the Royal Botanic Gardens, Kew (K) and Izta herbarium (IZTA) in Facultad de Estudios Superiores-Iztacala (FESI) at the Universidad Nacional Autónoma de México (UNAM) [acronyms based on Index Herbarium (Thiers, 2013)] were studied and databased. Additional on-line data sources were used, including the TROPICOS database (http://www.tropicos.org/) and the National Biodiversity Information System (SNIB) from the National Commission of Biodiversity in Mexico (Conabio) (http://www.conabio.gob.mx/remib/doctos/remib_esp.html). As well as existing published data by Dehgan (2012), were used to complete our datasets where applicable. Duplicate collections and records with incomplete information, e.g., historical collections with scant information, or with little geographical information not possible to georeferenced were excluded. This resulted in the identification of 994 specimens, across 24 species of the subgenus Curcas, for analysis. Exsiccatae data was put together into a master database with the following fields: family, genus, species, collector, and collector number, year of collection, institution code, determinator, country, county, locality, latitude, longitude, altitude and remarks on occurrence. The locality position for records without latitude and longitude were geo-referenced based on collector descriptions using Google-Earth (http://earth.google.co.uk), Global Gazetteer Version 2.2 (http://www.fallingrain.com) or the digital map of Instituto Nacional de Estadística, Geografía e Informática (INEGI) http://www3.inegi.org.mx/sistemas/mapa/visualizador/Default.aspx. All data points were uploaded into Google Earth and altitude was estimated for records without this information and species distribution maps based on locality positions were completed using the online tool SimpleMappr (http://simplemappr.net).
The habitats were defined according to the Conabio eco-regions classification system (Challenger & Soberón, 2008). These data were obtained from the “Ecoregiones terrestres de México” vector data set at a scale of 1:250000 (INEGI et al., 2016). To show the habitat use, a layer of “land use and vegetation” at a scale of 1:250000 was obtained from Conabio (Conabio, 1999). Two comparator species growing outside of Mexico were analysed according to the terrestrial eco-region classified by WWF (WWF, 2013) and a layer was obtained from WorldMap (Lewis, 2011).
Species habitats were under numerous vegetation subtypes, herein, these were considered as: evergreen forest (including low, medium and high dry evergreen forest and evergreen thorny forest), semi-deciduous forest (including low, medium or high dry semi-deciduous forest) or dry deciduous forest (including low, medium and high dry deciduous forest and dry deciduous thorny forest); and species present in subtypes of vegetation within temperate mountain forest were assigned to temperate forest (including coniferous, Quercus or mixed forests) (Rzedowski, 1978).
Conservation assessments for 24 species in section Curcas and section Platyphylla in subgenus Curcas in the genus Jatropha are presented according to the IUCN Red List Categories and Criteria v 3.1 (IUCN, 2012). The species were assigned as critically endangered (CR), endangered (EN), vulnerable (VU), near threatened (NT), least concern (LC) and data deficient (DD) based on the type of data and the knowledge of the species (Table 3, Figs. 1, 2), including delimiting the risk status based on the area of geographical range (criteria B; Table 2) or the very small size of a restricted population (criteria D; Table 2).
Table 2
IUCN Red List Criteria B and D used for delimiting threat status (IUCN, 2012).
|
Criteria B. Geographic range in the form of either B1 extent of occurrence (EOO) and/or area of occupancy (AOO) |
||||
|
(Threatened categories) |
Critically Endangered |
Endangered |
Vulnerable |
|
|
B1. EOO |
< 100 km2 |
< 5,000 km2 |
< 20,000 km2 |
|
|
B2. AOO |
< 10 km2 |
< 500 km2 |
< 2,000 km2 |
|
|
And at least 2 of the following 3 conditions: |
||||
|
(a) |
Severely fragmented or number of locations |
= 1 |
≤ 5 |
≤ 10 |
|
(b) |
Continuing decline observed, estimated, inferred or projected in any of: (i) EOO; (ii) AOO; (iii) area, extent and/or quality of habitat; (iv) number of locations or subpopulations; (v) number of mature individuals |
|||
|
(c) |
Extreme fluctuations in any of: (i) EOO; (ii) AOO; (iii) number of locations or subpopulations; (iv) number of mature individuals |
|||
|
Criteria D. Very small or restricted population |
||||
|
(Threatened categories) |
Critically Endangered |
Endangered |
Vulnerable |
|
|
Number of mature individuals |
< 50 |
< 250 |
D1. < 1,000 |
|
|
D2. |
Only applies to the VU category Restricted area of occupancy or number of locations with a plausible future threat that could drive the taxon to CR or EX in very short time |
D2. typically: AOO < 20 km2 or number of locations ≤ 5 |
The extent of occurrence (EOO) and the area of occupancy (AOO) were automatically generated by uploading the dataset with latitudes and longitudes into the online tool GeoCAT (http://geocat.kew.org/). The EOO was calculated as the area within a minimum convex polygon (MCP) derived from the species occurrence points, and the AOO is sum of occupied cells (based on point occurrence data) multiplied by the cell size at the reference scale of 2 × 2 km (4 km2), as recommended by the IUCN (IUCN, 2012).
Additional criteria used to evaluate the threat category of the IUCN Red List Criteria included: a) protected areas: it was assumed that if 50% or more of the AOO is in a national protected area (NPA), the risk status was reduced to a less threatened category, though we are aware that some NPA are not properly managed. When 30% or less is in a NPA, status was left unmodified (Rodríguez et al., 2011). National protected areas include 176 biosphere reserves, national parks, natural monuments, areas for the protection of natural resources, flora and fauna, or sanctuaries, identified by Mexico´s Commission for Protected Natural Areas (Conanp, 2012) in Mexico and the World Data Base of Protected Areas (IUCN & UNEP, 2013); b) priority areas for conservation: if 50% or more of the AOO is within a priority area for conservation, the risk status was changed to a more threatened category. When 30% or less is within a priority area for conservation, status was left unmodified. Priority areas for conservation were taken into account because these are areas of high biodiversity potentially at risk from anthropogenic pressures as formally delimited by Mexico´s National Commission for the Knowledge and Use of Biodiversity, as (Conabio, 2004); c) elevation range: broad elevation range is considered an indicator for high plasticity in a species and it will therefore be found in more habitats than a species with a narrow elevation range (Holdridge, 1947). An elevation of 1,000 m or more is considered ‘broad’ and 500 m or less, is assumed to be ‘narrow’ (Rodríguez et al., 2011). Broad elevation distributions were considered to reassess the risk status to a less threatened category, this allows species to settle on a broader range, however, this does not exclude environmental threats such as over-collecting, change of land use, etc. As per narrow elevation distributions these were considered not to modify the threat status.
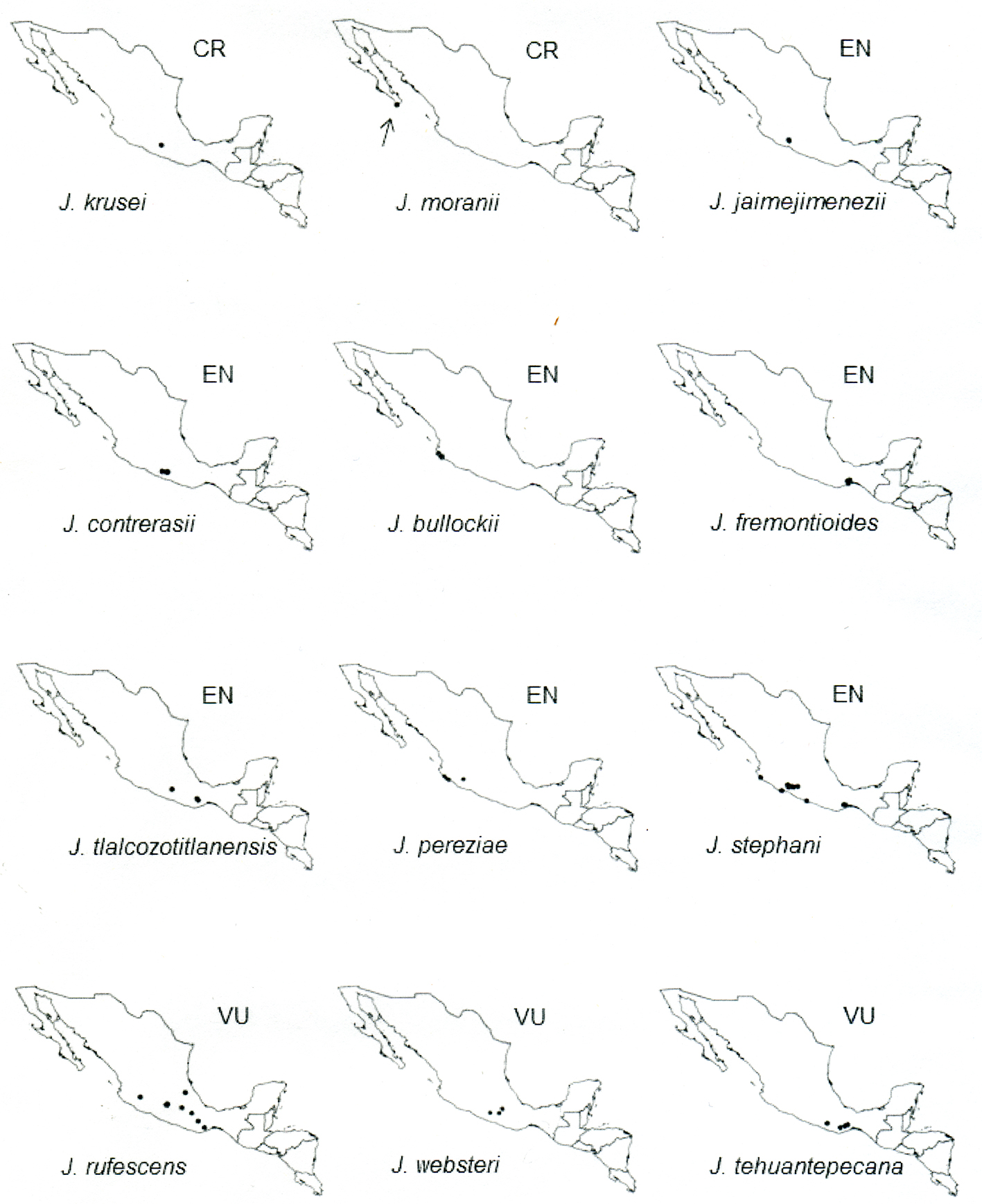
Results
The total dataset included 994 specimens representing 24 Jatropha species; however, 459 specimens alone represent J. curcas. Thirteen of the species were evaluated from more than 15 specimens, giving relative confidence level for the correct assessment based on only AOO and EOO (Rivers et al., 2011).
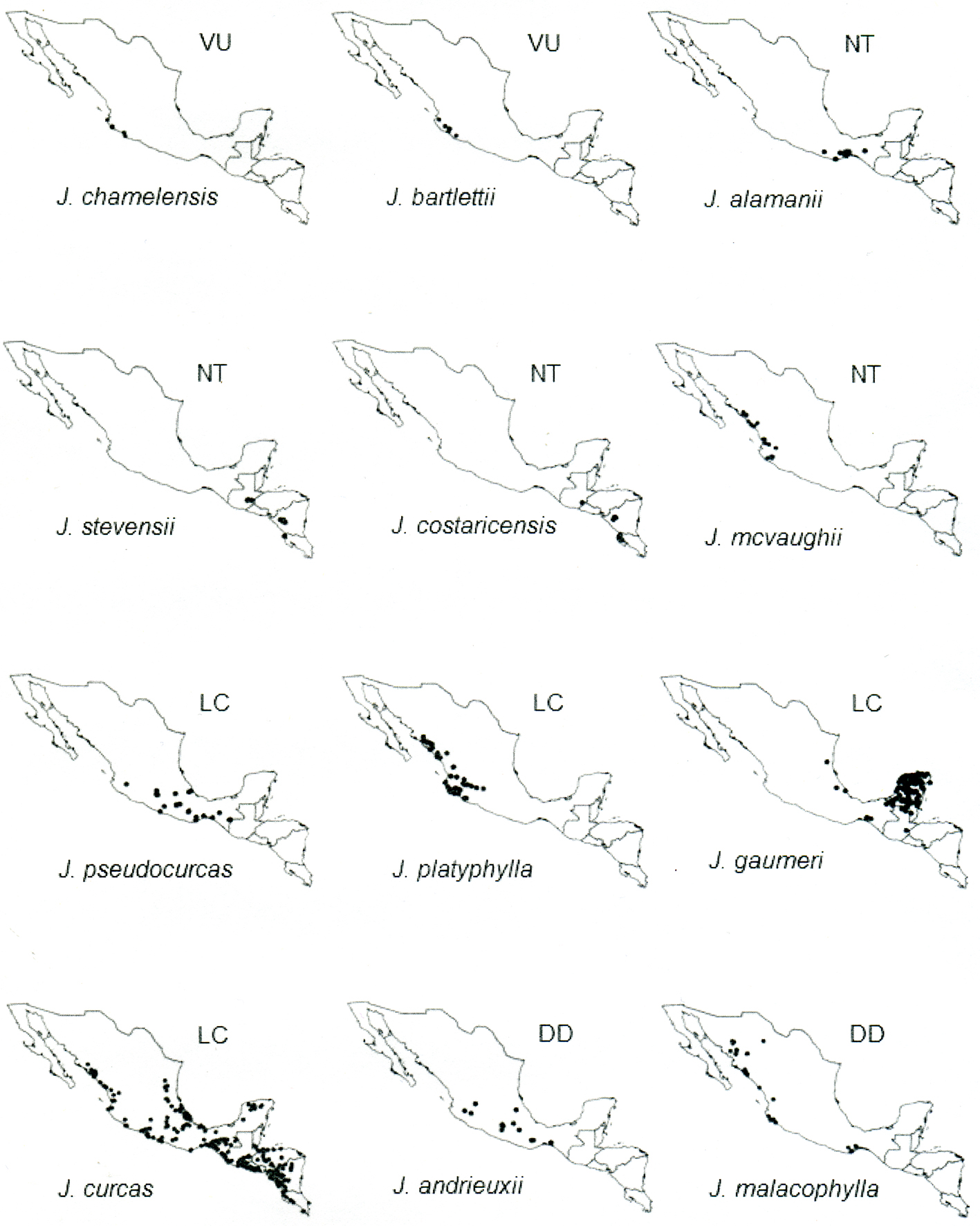
The geographic distribution of Jatropha species in section Curcas subsection Curcas and subsection Platyphyllae shows very diverse patterns, from wide to narrow ranges (Figs. 1, 2). Some species are restricted to an area of less than 20 km2 while others are distributed over > 20,000 km2. With the exception of J. curcas, all of the 24 species studied in sections Curcas and Platyphyllae are endemic to the Neotropics. However, 20 of those are endemic to Mexico and 6 species, each occurs in only 1 state (Baja California Sur, Guerrero, Jalisco, Michoacán and 2 in Oaxaca) (Table 3). For the Mexican endemics, their distributions extended south of 28°30’ N in West Mexico and south of 23°45’ N in East Mexico, and north of 15°40’ N, 91°55’ W. The majority of the species are distributed on coastal plains and hills along the Pacific Slope of Mexico, with a high concentration of species (8) in the southern part of the Isthmus of Tehuantepec. Some species are distributed in central parts of Mexico in the Balsas Basin and in the Trans- Volcanic Belt system, and few species occur in the coastal plains of the Gulf of Mexico. Only 2 species, J. gaumeri and J. curcas are distributed in the Yucatán Peninsula, and 1 species, J. moranii is present in the coastal plains of Los Cabos in Baja California Sur. Two of the studied species (J. costaricensis and J. stevensii) do not occur in Mexico; these have similar distribution patterns in the central highlands of Guatemala, Nicaragua and Costa Rica.
The majority of the species (22 of 24) studied occur in dry deciduous forest and half of the species also grow in temperate forest (Table 3). The species assessed as threatened are restricted to dry deciduous forest, except Jatropha tlalcozotitlanensis which also grows in temperate forest; and J. contrerasii is restricted to temperate forest. The species have altitudinal ranges extending from sea level to 2,650 m, with approximately half of the species found at sea level and the others adapted to higher altitudes above 600 m. The majority of the threatened species have a narrow altitude range (< 500 m), except J. contrerasii and J. websteri.
Based on the IUCN Red List Categories and Criteria ver. 3.1 (IUCN, 2012), 13 out of the 24 species are assessed as threatened: Jatropha krusei and J. moranii as CR; J. jaimejimenezii, J. contrerasii, J. bullockii, J. fremontioides, J. tlalcozotitlanensis, J. pereziae, and J. stephani as EN and J. bartlettii, J. websteri, J. tehuantepecana, and J. chamelensis as VU. Four species J. mcvaughii, J. alamanii, J. costaricensis, and J. stevensii are assessed as NT, 4 species as LC and 2 species as DD (Table 3). Six of 7 species in section Platyphyllae subsection Fremontioides are assessed as threatened for which 2 species are CR and 4 are EN. Section Platyphyllae subsection Platyphyllae comprises the most species in section Platyphyllae and they are assessed as EN (3 species), VU (5), NT (2), LC (2) and DD (1).
The species in section Curcas are assessed as NT, LC and DD, and the 1 species in section Platyphyllae subsection Gaumeri is assessed as LC (Tables 1, 3). A review of the degree of protection for the 11 threatened species reveals that 7 have no subpopulations within protected areas. In addition, 5 species occur in priority areas for conservation which currently have no management or implemented legislation (see Materials and methods additional criteria for the protected and priority areas for conservation).
Table 3
List of conservation status assessed Jatropha species with location in protected areas (letters) and priority areas for conservation (numbers) in parentheses. The distribution in Mexico and Central America (level 4 codes of TDWG), number of records and subpopulations, the extent of occurrence and area of occupation with a grid of 2 x 2 km, estimated altitude range and types of habitat.
|
Table 3 Continued |
|||||||
|
Species |
Distribution |
Records (subpopulations) |
Extent of occurrence (EOO) km2 |
Area of occupancy (AOO) km2 |
Altitude (m) |
Habitat |
Conservation status |
|
Species |
Distribution |
Records (subpopula–tions) |
Extent of occurrence (EOO) km2 |
Area of occupancy (AOO) km2 |
Altitude (m) |
Habitat |
Conservation status |
|
J. krusei |
MXS-GR |
1 |
NA |
4 |
560 |
Depression with dry deciduous forest. |
CR B1ab(i,ii,iii,iv) + B2ab(i,ii,iii,iv) |
|
J. moranii |
MXN-BS |
3 (2) |
NA |
8 |
100 – 130 |
Coastal plains and low hills with dry deciduous forest. |
CR B1ab(i,ii,iii,iv) + B2ab(i,ii,iii,iv) |
|
J. jaimejimenezii (j) |
MXS-MI |
4 (2) |
8 |
12 |
240 – 360 |
Depression with dry deciduous forest. |
EN B1ab(iii) + B2ab(iii) |
|
J. contrerasii |
MXS-GR-MI |
3 (3) |
409 |
12 |
1,100 – 1,900 |
Mountains and hills with temperate forest. |
EN B1ab(iii) + B2ab(iii) |
|
J. bullockii (c, q & 7) |
MXS-JA |
27 (6) |
417 |
48 |
0 – 275 |
Coastal plains and hills with dry deciduous forest. |
EN B1ab(i, ii,iii,iv) + B2ab(i,ii,iii,iv) |
|
J. fremontioides (23, 24) |
MXS-OA |
6 (5) |
482 |
20 |
0 – 300 |
Coastal plains, canyon and hills with dry deciduous forest. |
EN B1ab(i,iii) + B2ab(i,iii) |
|
J. tlalcozotitlanensis |
MXS-GR-OA |
5 (3) |
2,959 |
12 |
700 – 975 |
Canyon, hills and depressions with dry deciduous forest and mountains with temperate forest. |
EN B1ab(i,ii,iii,iv) + B2ab(i,ii,iii,iv) |
|
J. pereziae (c) |
MXS-CL-JA-MI |
8 (5) |
4,448 |
20 |
0 – 60 (320) |
Coastal plains, hills and depression with dry deciduous forest. |
EN B1ab(iii)+2ab(iii) |
|
J. rufescens (f & 15) |
MXC-ME-PU, MXS-JA-OA |
10 (9) |
185,810 |
36 |
1,100 – 2,000 |
Mountains and hills with temperate forests. Depressions and valleys with dry deciduous forest. |
VU B1ab(i,ii,iii,iv) + B2ab(i,ii,iii,iv) |
|
J. websteri (20) |
MXC-PU, MXS-GR |
5 (5) |
5,845 |
20 |
600 – 1,400 |
Depression with dry deciduous forest. |
VU B ab(i,ii,iii,iv) + B2 ab(i,ii,iii,iv); D2 |
|
J. tehuantepecana (23, 24) |
MXS-OA |
4 (4) |
6,975 |
16 |
1,650 – 1,900 |
Mountains with dry deciduous forest. |
VU B1ab(i,ii,iii,iv) + B2ab(i,ii,iii,iv); D2 |
|
J. chamelensis (c, r & 7, 17) |
MXS-CL-JA-MI |
24 (8) |
7,142 |
48 |
0 – 180 (440) |
Coastal plains and hills with dry deciduous forest. |
VU B1ab(i,iii) +B2ab(i,iii) |
|
J. bartlettii (d & 17) |
MXS-CL-JA-MI |
10 (10) |
4,792 |
36 |
500 – 2,200 |
Mountains with temperate forests. Few in hills and coastal plains in dry deciduous forest. |
VU B2ab(iii) |
|
J. alamanii (R & 23, 24) |
MXT-CI-OA |
26 (19) |
23,271 |
88 |
0 – 870 |
Coastal plains, hills and canyons with dry deciduous forest or evergreen forest. |
NT |
|
J. stevensii (x) |
COS, GUA, NIC |
23 (10) |
50,141 |
56 |
75 – 440 |
Central highland in dry deciduous forest and Xeric shrubland in Motagua valley. |
NT |
|
J. costaricensis (x, z) |
COS, GUA, NIC |
17 (11) |
52,461 |
52 |
0 – 700 |
Mountains and central highland in dry deciduous forest and temperate forest. |
NT |
|
J. mcvaughii (d, R & 4, 7, 8) |
MXN-SI, MXS-JA-NA |
23 (20) |
65,070 |
84 |
0 – 1,750 |
Hills and coastal plains in dry deciduous forest. Mountains or foothills with temperate forests. |
NT |
|
J. pseudocurcas (s & 23) |
MXC-ME-PU, MXG-VC, MXS-GR-JA-MI-OA, MXT-CI |
24 (>20) |
279,565 |
92 |
0 – 2,100 |
Depressions,valleys, coastal plains and hills with dry deciduous forest. Mountains and hills with temperate forests. Coastal plains and low hills with humid evergreen forest. |
LC |
|
J. platyphylla (c & 4-8, 16, 17) |
MXE-DU, MXN-SI, MXS-CL-JA-MI-NA |
65 (>20) |
187,272 |
232 |
0 – 2,650 |
Coastal plains and hills with dry deciduous forest and desert shrubs, mountains or hills with temperate forests and arid to semiarid hills and plains with desert scrubs and grassland. |
LC |
|
J. gaumeri (a, b, g, I, p, t, u, v, D, E, F & 13, 31-35) |
MXC-PU, MXE-TA, MXG-VU, MXT-CA-CI-QU-YU, BLZ, GUA |
149 (>20) |
645,072 |
480 |
0 – 300 (750, 1600) |
Plains and hills with humid semi-deciduous forest, humid evergreen forest and dry deciduous forest. Mountains and hills with temperate forests |
LC |
|
J. curcas (b, e, f, h, k, l, o, x, y, A-C, G-Q, S & 5,6 9-14, 17, 19, 21, 22, 25-30) |
*MXC-MO-PU, MXE-TA, MXG-VC, MXN-SI, MXS-CL-GR-JA-MI-NA-OA, MXT-CA-CI-YU, BLZ, COS, ELS, GUA, HON, NIC |
459 (>20) |
2,112,735 |
1,244 |
0 – 2,300 |
Hills and plains with dry to humid deciduous forest and in mountains with temperate forest. |
LC |
|
J. stephani (j & 7, 18, 24) |
MXS-GR-JA-MI-OX |
19 (14) |
89,727 |
52 |
30 – 400 |
Depression, coastal plains, hills, canyons and mountains with dry deciduous forest. |
EN B2ab(iii) |
|
J. andrieuxii (b, n & 20, 21) |
MXC-PU, MXS-JA-GR-OA |
20 (14) |
204,592 |
60 |
500 – 2,000 |
Depression, canyons, mountains, coastal plains and hills with dry deciduous forest. Mountains and hills with temperate forests. Arid to semiarid hills and plains with desert scrubs and grassland. Coastal plains and hills with humid evergreen forest. |
DD |
|
J. malacophylla(c, m & 1-3, 7, 23, 24) |
MXE-CU, MXN-SI-SO, MXS-CL-JA-NA-OA |
57 (>20) |
773,875 |
156 |
0 – 2000 |
Coastal plains, canyons and hills with dry deciduous forest. Few in mountains or foothills and plains with temperate forests. |
DD |
*Regional distribution: Mexico Federal protected – biosphere reserves: Calakmul (a), La Sepultura (b), Chamela-Cuixmala (c), Sierra de Manantlán (d), Sierra de Huautla (e), Tehuacán-Cuicatlán (f), Sian Ka’an (g), Los Tuxtlas (h), Río Lagartos (i), Zicuiran-Infiernillo (j); – national parks: El Veladero (k); – national monument: Yaxchilán (l); – areas of natural resources: Cuenca Alimentadora del Distrito de Riego 043 (m), Cuenca Hidrográfica del Río Necaxa (n); – areas of flora and fauna: Cobio Chichinautzin (o), Yum Balam (p); – sanctuary: Islas de la Bahía de Chamela (q) (CONANP, 2012). Mexican states protected areas: Zona de Uso Comun en Cerro Bandera de la Sierra Tolistoque (r), Sierra de Nanchititla (s), Kabah (t), Balam-Ku (u). Belize national protected forest reserve: Mountain Pine Ridges (v). Costa Rica national protected – national park: Santa Rosa (x); – forest reserve: Golfo Dulce (y); – wetland: Río Cañas (z). El Salvador national protected – national park: El Imposible y Balsamero (A); – natural area: Parque Walter Tilo Deininger (B); – protected area: Santa Rita (C). Guatemala national protected – national park: Mirador – Río Azul (D), Tikal (E); – biotope: Naachtún – Dos Lagunas (F). Honduras national protected wildlife reserve: Laguna de Caratasca (G). Nicaragua national protected – biosphere reserve: Zona de Amortiguamiento, Bosawas (H); – national park: Volcán Masaya (I), Archipiélago de Zapatera (J); – nature reserve: Cerro Kilambe (K), Zona de Amortiguamiento, Tisey Estanzuela (L), Zona de Amortiguamiento, C. Apante (M), Complejo Volcánico San Cristobal Casitas (N); – natural resource: Miraflor/Moropotente (O). International protected areas: El Cielo (P), Xiriualtique-Jiquilisco (Q), Marismas Nacionales (R), Zone de conservation de Guanacaste (S) (IUCN & UNEP, 2013).
Priority areas for conservation: Sierra Alta Tarahumara-Barrancas (1), Sierra Alamos-El Cuchujaqui (2), San Javier-Tepoca (3), Río Presidio (4), Pueblo Nuevo (5), Cuenca del río Jesús María (6), Chamela-Cabo Corrientes (7), Manantlán-Volcán de Colima (8), El Cielo (9), Sierra Gorda-río Moctezuma (10), Bosques Mesófilos de la Sierra Madre Oriental (11), Encinares tropicales de la planicie costera Veracruzana (12), Cuetzalan (13), Ajusco-Chichinautzin (14), Nevado de Toluca (15), Cerro Viejo-Sierras de Chapala (16), Sierra de Coalcomán (17), Infiernillo (18), Sierra Madre del Sur de Guerrero (19), Cañón del Zopilote (20), Sierras de Taxco-Huautla (21), Valle de Tehuacán-Cuicatlán (22), Sierra sur y costa de Oaxaca (23), Sierras del norte de Oaxaca-Mixe (24), Sierra de los Tuxtlas-Laguna del Ostión (25), Selva Zoque-La Sepultura (26), El Triunfo-La Encrucijada-Palo Blanco (27), El Momón-Montebello (28), Lacandona (29), Bosques mesófilos de los Altos de Chiapas (30), Dzilam-Río Lagartos-Yum Balam (31), Río Hondo (32), Zonas forestales de Quintana Roo (33), Sur del Punto Put (34), Silvituc-Calakmul (35) (Arriaga et al., 2000).
Abbreviations for geographical distribution according to Brummitt (2001): MXC, Mexico Central -DF, México Distrito Federal; -ME, México State; -PU, Puebla; MXE, Mexico Northeast -CU, Chihuahua; -DU, Durango; -GU, Guanajuato; -TA, Tamaulipas; MXB, Mexico Gulf -VC, Veracruz; MXN, Mexico Northwest -BC, Baja California; -BC Baja CaliforniaSur; -SI, Sinaloa; -SO, Sonora; MXS, Mexico Southwest -CL, Colima; -GR, Guerrero; -JA, Jalisco; -MI, Michoacán; -NA, Nayarit; -OA, Oaxaca; MXT, Mexico Southeast -CA, Campeche; -CI, Chiapas; -QR, Quintana Roo; -TB, Tabasco; -YU, Yucatán; BLZ, Belize; COS, Costa Rica; ELS, El Salvador; GUA, Guatemala; HON, Honduras; NIC, Nicaragua.
Discussion
Herbarium specimens are an essential source of information to demonstrate the distribution and conservation status of a plant species when field data is limited (Brummitt et al., 2008; Guerra-García et al., 2008). However, diversity studies based on herbarium specimens can be biased due to uneven collection intensity, particularly oversampling in areas of high species richness and in easily accessible areas (MacDougall et al., 1998). In this study, this was the case of the high density of collections from within and in close proximity to the well managed biosphere reserve of Chamela-Cuixmala [managed by the National Autonomous University of Mexico (UNAM)] in the Chamela Bay region of Jalisco. Bias was also present in the distribution of Jatropha curcas, with collection gaps in inaccessible areas along the coast. Gaps in the distribution contribute to the assessment of 2 species as DD (Fig. 2). About half of the threatened species assessed in this study are represented by less than 10 records could undermine confidence. However, a study by Rivers et al. (2011) showed that species with as few as 3 specimens can be used to classify the threat status correctly, and the IUCN Red List guidelines state assessments can be made for species only known from 1 locality (IUCN Standards and Petition Subcommittee, 2013). There are other studies assessing species threat status according to the IUCN Red List that also used few herbarium specimens, such as Randrianasolo (2002) comparing older and recent collections for 16 species of Anacardiaceae genera in Madagascar where 19 evaluations of the threat status were based on less than 10 specimens; Versieux & Wendt (2007) studying the diversity and distribution of 283 Bromeliaceae species in Brazil and the threat status of more than 200 species were based on less than 10 specimens and 50 of those species were only represented by 1 specimen; and Zizka (2009) evaluating 27 Bromeliaceae species from Chile and 10 of the species were represented by less than 10 specimens. There is a risk of extrapolating conclusion to small datasets, nonetheless, these evaluations are better than nothing, and waiting until more herbarium collection are available, to avoid species disappearing without not knowing where are present. Currently for new species descriptions a conservation assessment is suggested or requested, sometimes using 4-5 collections, evaluations are suggested such as for Opuntia delafuentiana Martínez-González, Luna-Vega, Gallegos-Vázquez & García-Sandoval (2015), Dahlia rupicola P.D. Sørensen (2018), Cestrum chiangi Mont.-Castro (2018). It is not only the geography data, it is also the nature and characteristics of the species, all features are potential data to be used, for example as is discussed below.
Bias can be reduced when considered additional criteria such as habitat and level of protected areas (see Materials and methods), considering species existence outside common habitats and combining field surveys with herbarium materials in regional herbaria. By collating information from herbarium specimens with such additional criteria (Table 3), the conservation assessment of 24 species of Jatropha subgenus Curcas section Curcas and section Platyphyllae from Mexico and Central America are presented.
The Pacific coast of in Mexico is dominated by dry deciduous forest. These forests are considered as an important biogeographical area harbouring many endemic species and a high species richness (Gentry, 1995; Lott & Atkinson, 2006). This corresponds well in the case of Jatropha species, except J. contrerasii which grows in temperate pine forest. However, the distribution of the species varies from coastal plains, depressions, hills and mountains.
The present analysis of the geographic distribution of Jatropha species showed extended distribution into areas not previously reported by Dehgan (2012); for example, J. websteri present in Guerrero; J. contrerasii, J. pereziae, and J. chamelensis in Michoacán; and J. bartlettii recorded in Colima and Michoacán. Jatropha alamanii was recorded (Cabrera 9263, MO, MEXU) in Quintana Roo and included in the distribution reported by Dehgan (2012). However, the collection point was potted far away, as outlier in the EOO polygon and therefore we studied this collection in detail. The investigation revealed it was not J. alamanii as earlier reported (verified by Jimenez, per.com., 2013) and it is now determined as J. gaumeri (verified by Casas, per.com., 2014). New records were also identified for J. stevensii in Costa Rica and Guatemala; J. costaricensis in Guatemala and Nicaragua; and J. gaumeri in Puebla, Veracruz and Chiapas.
All of 11 Jatropha species, assessed as threatened, according to the IUCN Red List Categories and Criteria v 3.1 (IUCN, 2012) (Table 3), are endemic to Mexico. The major threats are declining habitat quality due to changes of land use for livestock, plantations, expansion of infrastructure to accommodate human population growth, logging and tourism (Arriaga et al., 2000; Trejo & Dirzo, 2000; Wilson, 2008). Eight of the 11 threatened species also have a narrow altitude range, indicating specialised growth habitats (Holdridge, 1947). All species evaluated as threatened, except J. contrerasii and J. tlalcozotitlanensis, are restricted to dry deciduous forest to which a major threat is decline in area and quality (Trejo & Dirzo, 2000).
Two species were evaluated as CR: Jatropha krusei and J. moranii. The first is known from only 1 herbarium specimen collected in 1969 and has never been recorded since, even though the area it occurs in, the hills of Tepehuaje in Guerrero is a well surveyed area with many endemic species. The species grows in dry deciduous forest which is under threat from deforestation (Trejo & Dirzo, 2000) and this area is a newly defined priority area for conservation of Mexican dry forest (Ceballos et al., 2010). Furthermore, the hills of Tepehuaje are in the Papagayo River hydrologic region priority area for conservation, which is threatened by urban expansion, increase in crop and livestock production, and the over-exploitation and pollution of water resources (Arriaga et al., 2002). J. moranii is known from 3 herbarium specimens and is endemic to Los Cabos in Baja California Sur. This area is one of Mexico’s most popular touristic areas with large and growing transnational touristic companies (López-López et al., 2006), suggesting a major threat and the possible extinction of the wild populations of the species. Furthermore, this species is being cultivated as a natural bonsai tree (Dehgan, 2012) and the wild population is considered to be critically endangered.
Five species were evaluated as EN: Jatropha jaimejimenezii, J. contrerasii, J. bullockii, J. fremontioides, and J. tlalcozotitlanensis. J. jaimejimenezii is known from 4 herbarium collections and is endemic to the biosphere region Zicuiran-Infiernillo in Michoacán. Although J. jaimejimenezii is distributed in a protected area, it grows next to water resources and close to agricultural farmland which represents a potential threat to habitat quality loss (Conanp, 2012). Furthermore, the species is specialized to grow in a narrow altitude range which is considered to limit its survival to environmental changes. J. contrerasii, is known from less than 5 herbarium specimens, the habitat is severely fragmented and the species is dioecious. For insect pollinated species such as Jatropha (Neves & Viana, 2011; Reddi & Reddi, 1983; Vaknin, 2012), a combination of dioecy and severe habitat fragmentation might affect plant-pollinator interactions (Aguilar et al., 2006; Bawa, 1980; Kremen et al., 2007). J. bullockii is endemic to the coast of Jalisco and 70% of its AOO are in the protected biosphere reserve of Chamela – Cuixmala and the Islas de la Bahía de Chamela sanctuary. However, subpopulations outside of the protected areas are threatened by habitat loss (30% of the coastal forest at Jalisco has been lost in the last 2 decades) and several species in this area are considered endangered (Arriaga et al., 2000). Previous IUCN Red List assessments reported this species as EN in 1997 and VU D2 in 1998 (World Conservation Monitoring Centre, 1998a). This evaluation reports J. bullockii with a higher threat status than reported here and therefore the assessment needs to be published at the IUCN Red List (IUCN) website. J. fremontioides is endemic to the south of the Isthmus of Tehuantepec in Oaxaca. The species is only known from 6 herbarium specimens, half of which were collected close to villages and roads. Two out of 5 subpopulations grow in priority areas for conservation which have currently no management legislation and these areas are threatened by increasing population and tourism. Furthermore, a change in land use to livestock, coffee plantations and logging is also occurring, resulting in a fragmented landscape (Arriaga et al., 2000). The population of J. tlalcozotitlanensis had an AOO of only 12 km2, it is severely fragmented and the habitat is at risk of decline (Trejo & Dirzo, 2000).
Three species were assessed as VU: Jatropha websteri, J. tehuantepecana, and J. chamelensis. J. chamelensis is distributed in Jalisco, Colima and Michoacán close to the Pacific coast and are threatened by increasing tourism (Wilson, 2008). These species are dioecious and grow in fragmented habitats, as the forests of Jalisco have declined in extent due to agricultural activities and human population expansion (Arriaga et al., 2000). J. chamelensis has almost half of the subpopulations in the protected areas of the Chamela-Cuixmala biosphere reserve and the sanctuary Islas de la Bahía de Chamela. However, the remaining subpopulations are in priority areas for conservation and these areas are threatened by deforestation (Arriaga et al., 2000). Previous IUCN Red List assessments of J. chamelensis reported the species as EN in 1997 and VU D2 in 1998 (World Conservation Monitoring Centre, 1998b). These assessments were carried out more than 15 years ago and must be re-evaluated and published at the Red List (IUCN) website. Most herbarium specimens of J. websteri are collected in agricultural landscapes close to settlements and water resources, and 1 subpopulation is in the priority area for conservation Cañón del Zopilote, an area threatened by grazing, extraction of firewood and seasonal agriculture (Arriaga et al., 2000). J. tehuantepecana is known from less than 5 herbarium specimens, has severely fragmented habitats and is dioecious. It has 2 subpopulations in priority areas for conservation, Sierra Sur and Costa de Oaxaca and Sierras del Norte de Oaxaca-Mixe, which are threatened by a change in land use to livestock, coffee plantations and logging, producing a severely fragmented landscape (Arriaga et al., 2000).
Moreover, climate change plays a fundamental effect not just on presence or absence of pollinators, other physical environment interactions, such as producing CO2 carbon emissions caused by burning fossils; if biofuels, like that derived from Jatropha, are specifically conceived of as a way to reduce emissions of the anthropogenic greenhouse gases that cause climate change, this will help reduction on the carbon footprint.
The conservation assessments for Jatropha species in Mexico indicate an urgent need to implement conservation actions, at both the “ex situ” and “in situ levels”. Our current work, linking botanical institutions in Mexico, Denmark and the UK, has enabled the collection of seeds of 10 species from the Mexican states of Oaxaca and Puebla and their storage at the gene bank of Chapingo Autonomous University (CAU). In addition, this work contributed to 23 seed accessions of wild Jatropha ssp., corresponding to 14 of 48 wild Mexican species, now in storage at CAU. All species belong to the subgenus Curcas and are endemic to Mexico, except J. curcas. Seeds of 4 of the species have been added and conserved in the FESI UNAM seed bank; duplicates were sent to Kew’s Millennium Seed Bank. In total, 5 of the Kew collections are from subgenus Curcas (J. curcas, J. cinerea, J. dioica, J. rzedowskii, and J. neopauciflora). Furthermore, neglected and underutilised species, including Jatropha species, are being propagated in local villages with local communities, as part of Kew’s Project MGU-the Useful Plants Project, which is independent of this study. For at least 2 reasons, further field studies are essential. Firstly, it is important to conduct ground-truthing to confirm the existence of older specimens and for species with low specimen numbers, such as J. moranii and J. krusei which were last collected in 1969. The establishment of field surveys in areas with similar vegetation and soil to the current occupancy is a fundamental component of this. Secondly, it is difficult to make large seed collections as Jatropha species often only appear as single trees or as a few individuals at each locality. Consequently, a large scale conservation programme is required to adequately collect and conserve the seeds of this genus.
In conclusion, Jatropha species most closely related to J. curcas have both narrow and wide geographical ranges with the majority of species distributed in the dry deciduous forest of the Pacific Coast of Mexico, which is declining in extension and habitat quality. Several of the species are restricted to small areas and as a consequence, 10 of the 24 species assessed are herein evaluated as threatened according to the IUCN Red List Categories and Criteria (IUCN, 2012). With a growing emphasis on the exploitation of neglected and under-utilized species such as Jatropha, it is important to establish systematic collections for assessment of threat status and to develop management strategies for their conservation. Summing up, it is well known that plants are able to reduce the amount of carbon dioxide in the atmosphere and realized O2, hence decreasing global warming. Moreover, biofuels, when grown from plants, can thus offset their CO2 emissions because they take up the gas during growth that is produced when the fuel is burned, therefore Jatropha species are worthwhile to conserve because they are a source for biofuels. As stated “To encourage research, development and national integration of advanced technologies for generating renewable, clean energy such as tidal, solar, hydrogen, and biofuel energy sources, among others” by Semarnat-INECC (2016).
Acknowledgements
CSLC MSc degree was supported by the University of Copenhagen; mainly by M. Christian Andreasen, and the research took place at the Royal Botanic Gardens, Kew, supported by grant-in-aid from Defra, UK Government. The authors acknowledge financial support for the field work from a philanthropist based in Spain through Kew’s Useful Plants Project – Project MGU, Fællesfonden, Mindelegatet efter Hedvig og Johannes Sørensens Fond and Landbrugets Studiefond. Gill Challen, Euphorbiaceae curator at K, facilitated materials and supported this work in many ways with her friendly and professional manner, R. Laidlay for helping to enter some records of Jatropha into HerbCat, Kew; T. Ulian, J. Axayacatl Cuevas S., Y.V. Castillo, and H. Cervantes for their support on the logistics during a short field work in Mexico and Jatropha seeds incorporated into 2 seed banks in Mexico; Hugh W. Pritchard who encouraged and found financial support for the first author MSc thesis. We gratefully thank Jaime Jiménez and Javier Fernandez Casas, as Jatropha specialists in Mexico and Madrid respectively, Steve Bachman for IUCN advice, Elizabeth Moreno in Conabio, and the curators of the herbaria C, MEXU and BM. Special thanks to the reviews and editor who provided feedback; their comments and suggestion improved this contribution.
References
Aguilar, R., Ashworth, L., Galetto, L., & Aizen, M. A. (2006). Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecology Letters, 9, 968–980. https://doi.org/10.1111/j.1461-0248.2006.00927.x
Ankli, A., Heinrich, M., Bork P., Wolfram, L., Bauerfeind, P., Brun, R. et al. (2002). Yucatec Mayan medicinal plants: evaluation based on indigenous uses. Journal of Ethnopharmacology, 79, 43–52. https://doi.org/10.1016/s0378-8741(01)00355-5
Aranda-Rickert, A., Morzán, L., & Fracchia, S. (2011). Seed oil content and fatty acid profiles of five Euphorbiaceae species from arid regions in Argentina with potential as biodiesel source. Seed Science Research, 1, 63–68. https://doi.org/10.1017/s0960258510000383
Arriaga, L., Aguilar, V., & Alcocer, J. (2002). Aguas continentales y diversidad biológica de México. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. https://doi.org/10.32800/abc.2019.42.0187
Arriaga, L., Espinoza, J. M., Aguilar, C., Martínez, E., Gómez, L., & Loa, E. (2000). Regiones terrestres prioritarias de México. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. https://doi.org/10.5962/bhl.title.118644
Bahadur, B., Carels, N., & Sujatha, M. (2013). Jatropha, challenges for a new energy crop, Vol 2: genetic improvement and biotechnology. New York: Springer Science+Business Media. https://doi.org/10.1007/978-1-4614-4915-7
Banerji, R., Chowdhury, A. R., Misra, G., Sudarsanam, G., Verma, S. C., & Srivastava, G. S. (1985). Jatropha seed oils for energy. Biomass, 8, 277–282. https://doi.org/10.1016/0144-4565(85)90060-5
Bawa, K. S. (1980). Evolution of dioecy in flowering plants. Annual Review of Ecology, Evolution and Systematics, 11, 15–39. https://doi.org/10.1146/annurev.es.11.110180.000311
Brummitt, R. K. (2001). World geographical scheme for recording plant distributions. Plant taxonomic database standards No 2. http://grassworld.myspecies.info/sites/grassworld.myspecies.info/files/tdwg_geo2.pdf [accessed 14 November 2018].
Brummitt, N., Bachman, S. P., & Moat, J. (2008). Applications of the IUCN Red List: towards a global barometer for plant diversity. Endangered Species Research, 6, 127–135. https://doi.org/10.3354/esr006127
Carels, N., Sujatha, M., & Bahadur, B. (2012). Jatropha, challenges for a new energy crop, vol 1: farming, economics and biofuel. New York: Springer Science+Business Media. https://doi.org/10.1007/978-1-4614-4806-8
Ceballos, G., Martínez, L., García, A., Espinoza, E., & Bezaury, J. (2010). Áreas prioritarias para la conservación de las selvas secas del Pacífico México. In G. Ceballos, L. Martínez, A. García, E. Espinoza, J. Bezaury, & R. Dirzo (Eds.), Diversidad, amenazas y regiones prioritarias para la conservación de las selvas secas del Pacífico de México (pp. 387–392). México City: Conabio/ UNAM. https://doi.org/10.21829/abm99.2012.18
Challenger, A., & Soberón, J. (2008). Los ecosistemas terrestres. In P. Koleff, & J. Sarukhán (Eds.), Capital natural de México,Vol. 1: conocimiento actual de la biodiversidad (pp. 87–108). México City: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. https://doi.org/10.32800/abc.2019.42.0187
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (1999). Uso de suelo y vegetación modificado por Conabio. Portal de Geoinformación, Sistema Nacional de Información sobre Biodiversidad. https://doi.org/10.32800/abc.2019.42.0187. http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/usv/otras/usv731mgw [accessed 22 December 2018].
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2004). Regiones Terrestres Prioritarias. Portal de Geoinformacion, Sistema Nacional de Información sobre Biodiversidad. https://doi.org/10.32800/abc.2019.42.0187. http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/region/biotic/rtp1mgw [accessed 24 December 2018].
Conanp (Comisión Nacional de Áreas Naturales Protegidas). (2012). Áreas Protegidas Decretadas. México City: Comisión Nacional de Áreas Naturales Protegidas. https://doi.org/10.18242/anpscripta.2018.04.04.01.0001 http://sig.conanp.gob.mx/website/pagsig/felist/ [22 December 2018].
Dagar, J. C., Tomar, O. S., Kumar, Y., Bhagwan, H., Yadav, R. K., & Tyagi, N. K. (2006). Performance of some under-explored crops under saline irrigation in a semiarid climate in Northwest India. Land Degradation Development, 17, 285–299. https://doi.org/10.1002/ldr.712
Dehgan, B. (2012). Jatropha (Euphorbiaceae), Flora Neotropical Monograph 110. New York: The New York Botanical Garden Press.
Dehgan, B., & Schutzman, B. (1994). Contribution toward a monograph of neotropical Jatropha: phenetic and phylogenetic analyses. Annals of the Missouri Botanical Garden, 81, 349–367. https://doi.org/10.2307/2992102
Devappa, R. K., Makkar, H. P. S., & Becker, K. (2010a). Jatropha toxicity – a review. Journal of Toxicology and Environmental Health Part B: Critical Reviews, 13, 476–507. https://doi.org/10.1080/10937404.2010.499736
Devappa, R. K., Makkar, H. P. S., & Becker, K. (2010b). Nutritional, biochemical, and pharmaceutical potential of proteins and peptides from Jatropha: review. Journal of Agricultural and Food Chemistry, 58, 6543–6555. https://doi.org/10.1021/jf100003z
Divakara, B. N., Upadhyaya, H. D., Wani, S. P., & Gowda, C. L. L. (2010). Biology and genetic improment of Jatropha curcas L.: a review. Applied Energy, 87, 732–742. https://doi.org/10.1016/j.apenergy.2009.07.013
Dutra, F. V., Calixto, J. B., & Medeiros, Y. S. (1996). Jatrophone inhibits platelet rich plasma aggregation from man, rat and guinea-pig induced by different agents. Phytotherapy Research, 10, 271–273. https://doi.org/10.1002/(SICI)1099-1573(199605)10:3<271::AID-PTR821>3.0.CO;2-6
Fresnedo-Ramírez, J., & Orozco-Ramírez, Q. (2012). Diversity and distribution of genus Jatropha in Mexico. Genetic Resources and Crop Evolution, 60, 1087–1104. https://doi.org/10.1007/s10722-012-9906-7
Gentry, A. H. (1995). Diversity and floristic composition on neotropical dry forest. In S. H. Bullock, H. A. Mooney, & E. Medina (Eds.), Seasonally dry forest (pp. 146–194). Cambridge, England: Cambridge University Press. https://doi.org/10.1017/cbo9780511753398.007
Guerra-García, J. M., Espinosa, F., & García-Gómez, J. C. (2008). Trends in taxonomy today: an overview about the main topics in taxonomy. Zoologica Baetica, 19, 15–49.
Heller, J. (1996). Physic nut. Jatropha curcas L. Promoting the conservation and use of underutilized and neglected crops. 1. International Plant Genetic Resource Institute, Rome, Italy.
Holdridge, L. R. (1947). Determination of world plant formations from simple climatic data. Science, 105, 367–368. https://doi.org/10.1126/science.105.2727.367
INEGI Instituto Nacional de Estadística, Geografía e Informática, CONABIO Comisión Nacional para el Conocimiento y Uso de la Biodiversidad, INE Instituto Nacional de Ecología (2016). Ecorregiones Terrestres de México. Portal de Geoinformación, Sistema Nacional de Información sobre Biodiversidad. https://doi.org/10.32800/abc.2019.42.0187. http://www.conabio.gob.mx/informacion/gis/?vns=gis_root/usv/inegi/usv250s6gw [accessed 22 December 2018].
IUCN (2012) IUCN Red List Categories and Criteria: Version 3.1. Second Edition. Gland, Switzerland and Cambridge, UK: IUCN iv + 32pp. http://cmsdocs.s3.amazonaws.com/keydocuments/Categories_and_Criteria_en_web%2Bcover%2Bbckcover.pdf [accessed 15 December 2018]
IUCN & UNEP (2013). The world Database on Protected Areas (WDPA). UNEP-WCMC. Cambridge, UK. http://protectedplanet.net/ [accessed 15 December 2018].
IUCN Standards and Petitions Subcommittee (2013). Guidelines for using the IUCN Red List Categories and Criteria: Version 10. http://www.iucnredlist.org/documents/RedListGuidelines.pdf [accessed 1 August 2013].
Kaushik, N., Kumar, K., Kumar, S., Kaushik, N., & Roy, S. (2007). Genetic variability and divergence studies in seed traits and oil content of Jatropha (Jatropha curcas L.) accessions. Biomass and Bioenergy, 31, 497–502. https://doi.org/10.1016/j.biombioe.2007.01.021
Koh, L. P., Levang, P., & Ghozoul, J. (2009). Designer landscapes for sustainable biofuels. Trends in Ecology & Evolution, 24, 431–438. https://doi.org/10.1016/j.tree.2009.03.012
Kremen, C., Williams, N. M., Aizen, M. A., Gemmill-Herren, B., LeBuhn, G., Minckley, R. et al. (2007). Pollination and other ecosystem services produced by mobile organisms: a conceptual framework for the effects of land-use change. Ecology Letters, 10, 299–314.
Lewis, B. (2011). Terrestrial Ecoregions. WorldMap. https://doi.org/10.1111/j.1461-0248.2007.01018.x
http://worldmap.harvard.edu/data/geonode:wwf_terr_ecos_oRn [accessed 15 December 2018].
López-López, Á., Cukier, J., & Sánchez-Crispín, Á. (2006). Segregation of tourist space in Los Cabos, Mexico. Tourism Geographies, 8, 359–379. https://doi.org/10.1080/14616680600922054
Lott, E. J., & Atkinson, T. H. (2006). Mexican and Central American seasonally dry tropical forests. In R. T. Pennington, G. P. Lewis, & J. A. Ratter (Eds.), Neotropical savannas and seasonally dry forests (pp. 315–342). Boca Raton: CRS Press. https://doi.org/10.1201/9781420004496-13
MacDougall, A. S., Loo, J. A., Clayden, S. R., Goltz, J. G., & Hinds, H. R. (1998). Defining conservation priorities for plant taxa in southeastern New Brunswick, Canada using herbarium records. Biological Conservation, 86, 325–338. https://doi.org/10.1016/s0006-3207(98)00031-7
Makkar, H. P. S., Kumar, V., Oyeleye, O. O., Akinleye, A. O., Angulo-Escalante, M. A., & Becker, K. (2011). Jatropha platyphylla, a new non-toxoc Jatropha species: physical properties and chemical constituents including toxic and antinutritional factors of seeds. Food Chemistry, 125, 63–71. https://doi.org/10.1016/j.foodchem.2010.08.037
Neves, E. L., & Viana, B. F. (2011). Pollination efficiency of Apis mellifera Linnaeus, 1758 (Hymenoptera, Apidae) on the monoecious plants Jatropha mollissima (Pohl) Baill. and Jatropha mutabilis (Pohl) Baill. (Euphorbiaceae) in a semi-arid Caatinga area, northeastern Brazil. Brazilian Journal of Biology, 71, 107–113. http://dx.doi.org/10.1590/S1519-69842011000100016
Openshaw, K. (2000). A review of Jatropha curcas: an oil plant of unfulfilled promise. Biomass and Bioenergy, 19, 1–15. https://doi.org/10.1016/s0961-9534(00)00019-2
Randrianasolo, A., Miller, J., & Consiglio, T. (2002). Application of IUCN criteria and red list categories to species of five Anacardiaceae genera in Madagascar. Biodiversity and Conservation, 11, 1289-1300. http://dx.doi.org/10.1023/A:1016070703803
Reddi, E. U. B., & Reddi, C. S. (1983). Pollination ecology of Jatropha gossypiifolia (Euphorbiaceae). Proceedings of the Indian Academy of Sciences (Plant Sciences), 92, 215–231.
Rico-Gray, V., Chemás, A., & Mandujano, S. (1991). Uses of tropical deciduous forest species by the Yucatecan Maya. Agroforestry Systems, 14, 149–161. https://doi.org/10.1007/bf00045730
Rivers, M. C., Taylor, L., Brummitt, N. A., Meagher, T. R., Roberts, D. L., & Nic Lughadha, E. (2011). How many herbarium specimens are needed to detect threatened species? Biological Conservation, 144, 2541–2547. https://doi.org/10.1016/j.biocon.2011.07.014
Rodrigues, A. S. L., Pilgrim, J. D., Lamoreux, J. F., Hoffmann, M., & Brooks, T. M. (2006). The value of the IUCN Red List for conservation. Trends in Ecology and Evolution, 21, 71–76. https://doi.org/10.1016/j.tree.2005.10.010
Rodríguez, A., Monro, A. K., Chacón, O., Solano, D., Santamaría, D., Zamora, N., González, F., & Correa, M. (2011). Regional and global conservation assessments for 200 vascular plant species from Costa Rica and Panama. Phytotaxa, 21, 1–216. http://dx.doi.org/10.11646/phytotaxa.21.1.1
Rzedowski, J. (1978). Vegetación de México. Mexico D.F.: Editorial Limusa.
SEMARNAT-INECC (2016) Mexico’s Climate Change Mid-Century Strategy. Ministry of Environment and Natural Resources (SEMARNAT) and National Institute of Ecology and Climate Change (INECC), Mexico City, Mexico. https://unfccc.int/files/focus/long-term_strategies/application/pdf/mexico_mcs_final_cop22nov16_red.pdf [accessed 16 June 2019]
Stone, R. (2007). Can palm oil plantations come clean? Science, 317, 1491. http://dx.doi.org/10.1126/science.317.5844.1491
Thiers, B. (2013). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/ [accessed 15 December 2018].
Trejo, I., & Dirzo, R. (2000). Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biological Conservation, 94, 133–142. https://doi.org/10.1016/s0006-3207(99)00188-3
Vaknin, Y. (2012). The significance of pollination services for biodiesel feedstocks, with special reference to Jatropha curcas L.: a review. BioEnergy Research, 5, 32–40. https://doi.org/10.1007/s12155-011-9142-6
Vera-Castillo, Y. B., Pritchard, H. W., Frija, A., Chellattan-Veettil, P., Sánchez-Cuevas, J. A., van Damme, P., & van Huylenbroeck, G. (2014). Production viability and farmers’ willingness to adopt Jatropha curcas L. as a biofuel source in traditional agroecosystems in Totonacapan, Mexico. Agricultural Systems, 125, 42–49. https://doi.org/10.1016/j.agsy.2013.12.003
Versieux, L. M., & Wendt, T. (2007). Bromeliaceae diversity and conservation in Minas Gerais state, Brazil. Biodiversity and Conservation, 16, 2989–3009. http://dx.doi.org/10.1007/s10531-007-9157-7
WCSP (World Checklist of Selected Plant Families). (2013). Facilitated by the Royal Botanic Gardens, Kew. http://apps.kew.org/wcsp/prepareChecklist.do?checklist=selected_families%40%40249060920131338038 [accessed 15 December 2018].
Willis, F., Moat, J., & Paton, A. (2003). Defining a role for herbarium data in Red List assessments: a case study of Plectranthus from eastern and southern tropical Africa. Biodiversity and Conservation, 12, 1537–1552. https://doi.org/10.1023/A:1023679329093
Wilson, T. D. (2008). Economic and social impacts of tourism in Mexico. Latin American Perspectives, 35, 37-52. https://doi.org/10.1177/0094582×08315758
World Conservation Monitoring Centre (1998a). Jatropha bullockii. In IUCN 2013. IUCN red list of threatened species: version 2013.2. https://doi.org/10.2305/iucn.uk.1998.rlts.t35579a9936013.en http://www.iucnredlist.org/details/35579/0 [accessed 15 December 2018].
World Conservation Monitoring Centre (1998b). Jatropha chamelensis. In IUCN 2013. IUCN red list of threatened species: version 2013.2. https://doi.org/10.2305/iucn.uk.1998.rlts.t32635a9720143.en http://www.iucnredlist.org/details/32635/0 [accessed 15 December 2018].
WWF (2013). Central America: Patches Scateres through Mexico, Guatemala, Honduras, El Salvador, Nicaragua, and Costa Rica.World Wildlife Fund. https://doi.org/10.1515/9780691184159-078 http://worldwildlife.org/ecoregions/nt0209 [accessed 15 December 2018].
Zizka, G., Schmidt, M., Schulte, K., Novoa, P., Pinto, R., & König, K. (2009). Chilean Bromeliaceae: diversity, distribution and evaluation of conservation status. Biodiversity and Conservation, 18, 2449–2471. http://dx.doi.org/10.1007/s10531-009-9601