Luis Salgado-Cruz, Casimiro Quiñonez-Velázquez *, Federico A. García-Domínguez, Carlos I. Pérez-Quiñonez
Centro Interdisciplinario de Ciencias Marinas, Instituto Politécnico Nacional, Av. IPN, Col. Playa Palo de Santa Rita, 23096 La Paz, Baja California Sur, Mexico
*Corresponding author: cquinone@ipn.mx (C. Quiñonez-Velázquez)
Received: 31 October 2019; accepted: 4 May 2020
Abstract
In a holistic approach it is important to identify potential fishing units to better understand population structure and dynamics. The objective of this work was to evaluate the potential existence of a Mugil curema population structured in stocks in La Paz Bay. For this, a total of 709 specimens were collected between 2010 and 2013. Two fish groups were identified (G1, n = 212, and G2, n = 178), and the otolith shape was compared using geometric morphometrics, L50% was estimated, age was assigned, and a multi-model approach was used to evaluate individual growth. Eight age groups were assigned for G1 (3-10) and 5 for G2 (3-7). The most adequate model to describe growth for both groups was the von Bertalanffy growth model (G1: L∞ = 421.77, k= 0.32, t0 = -0.05; G2: L∞ = 406.5, k = 0.36, t0 = -0.02). Differences in otolith shape and growth parameters between groups were significant; opposite, L50% estimates (G1 = 325 mm LT; G2 = 330 mm LT) were not significant. Results suggest the presence of a M. curema population structured in at least 2 stocks in La Paz Bay: one stock reproduces in Spring (G1) and the other in the Fall (G2).
Keywords: Population structure; Fishing stock; Multi-model approach; Individual growth; Reproductive pattern
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Detección de stocks fenotípicos de Mugil curema (Perciformes: Mugilidae) en bahía de La Paz, Baja California Sur, México, utilizando morfometría geométrica en la forma del otolito, crecimiento y parámetros reproductivos
Resumen
En un enfoque holístico es importante identificar potenciales unidades pesqueras para comprender mejor la estructura y la dinámica poblacional. El objetivo del presente estudio fue evaluar la potencial existencia de una población de Mugil curema estructurada en stocks en bahía de La Paz. Se analizaron 709 ejemplares, de 2010 a 2013. Se integraron dos grupos (G1, n = 212 y G2, n = 178) de organismos y se comparó la forma del otolito sagitta usando morfometría geométrica, se estimó la L50%, se asignó la edad y con un enfoque multimodelo se evaluó el crecimiento individual. Se identificaron 8 grupos de edad para G1 (3-10) y 5 para G2 (3-7). El modelo más adecuado para describir el crecimiento fue von Bertalanffy para ambos grupos (G1: L∞ = 421.77, k = 0.32, t0 = -0.05; G2: L∞ = 406.5, k = 0.36, t0 = -0.02). Las diferencias en la forma del otolito y en el crecimiento individual entre grupos fueron significativas, pero los estimados de L50% (G1 = 325 mm LT; G2 = 330 mm LT) no fueron significativos. Se sugiere que la población de M. curema está estructurada en al menos 2 stocks en bahía de La Paz, que se reproducen durante primavera (G1) y otoño (G2).
Palabras clave: Estructura poblacional; Stock pesquero; Enfoque multimodelo; Crecimiento individual; Patrón reproductivo
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The sustainable management of any species requires knowledge on the structure, dynamics, and demography of the population within its geographic distribution, because the condition of these parameters defines the level of exploitation and, therefore, of sustainability (Sinclair & Iles, 1988). Hence, it is important to direct research efforts towards the identification of potential fishing stocks in order to increase precision in the evaluation of populations, because most population models assume homogeneous biological parameters (e.g., growth, maturity, and mortality) and a closed life cycle, in which juveniles are produced from the same population (Cadrin et al., 2005). An approach for stock identification, generally of commercial fish, has been centered on the detection of groups based on the quantification of morphometric differences of body shape (De La Cruz-Agüero & García-Rodríguez, 2004; García-Rodríguez et al., 2011; Ibáñez-Aguirre et al., 2006; Pérez-Quiñonez et al., 2017; Silva, 2003; Tzeng, 2004; Vergara-Solana et al., 2013) and on sagitta otolith shape (Campana & Casselman, 1993; DeVries et al., 2002; Félix-Uraga et al., 2005; Stransky et al., 2008; Ramírez-Pérez et al., 2010), or on the analysis of the shape of both structures (Pérez-Quiñonez et al., 2018). The potential presence of population units is evaluated based on the assumption that individuals with high similarity (similar morphotypes) should be closely related, biologically and ecologically (Cadrin et al., 2005). Complementary demographic analyses (e.g., age, growth, reproduction, and mortality) (Begg et al., 1999; Griffiths, 1997; Ruiz-Domínguez & Quiñonez-Velázquez, 2018) evaluate parameters of biological units (morphotypes-stocks) to identify differences in the response of fish to environmental and fishing pressures (Gherard et al., 2013). For this reason, the use of the multiple approaches could provide a better understanding of population structure and dynamics, as was pointed out by Begg & Waldman (1999), who suggested a holistic effort (multiple approaches) to evaluate population structure, in such a way that this integrating approach maximizes the probability of correctly defining fishing stocks.
The white mullet, Mugil curema (Valenciennes, 1836), is an omnivorous fish that feeds mainly on the sediment surface; it is also preyed upon by a wide variety of species at upper trophic levels (Yáñez-Arancibia, 1976). This species is widely distributed on the American continent, inhabiting lagoons, estuaries, and coasts in the subtropics. In the Eastern Pacific, it occurs from California to Chile, whereas in the Western Atlantic it occurs from Cape Cod, USA, to Brazil, including the Gulf of Mexico (Castro-Aguirre, 1978; Robins et al., 1991). It spends most of its biological cycle in protected areas (estuaries, bays, lagoons, and river deltas) (González-Castro et al., 2006; Harrison, 1995; Ibáñez & Gallardo-Cabello, 2004). Adults form schools and migrate to the coastal zone to spawn. Post-larvae migrate to estuaries and coastal lagoons, where they remain until reaching the adult stage (Blaber, 1997; Marín-Espinoza et al., 2003; Polanco et al., 1987; Trape et al., 2009).
In Mexico, white mullets are captured along with the flathead grey mullet Mugil cephalus (Linnaeus, 1758) and are included in the category of “lebrancha” or “lisa” in the Mexican National Fisheries Chart (Vasconcelos et al., 1996). The group comprises an important economic resource for the artisanal fisheries of the lagoons and bays of the Mexican Pacific. Catch volumes reach over 10,000 t annually (AEAP, 2017; Ibáñez & Gallardo-Cabello, 2004). Over 75% of catches come from the states bordering the Gulf of California (Nayarit, Sinaloa, Baja California Sur, Sonora, and Baja California, in order of importance). In Baja California Sur, catches of this resource have averaged 437 annual tons during the past 18 years (AEAP, 2017). The M. curema fishery is regulated by the Official Mexican Norm NOM016-PESC-1994 (DOF, 1995) and by the National Fisheries Chart (DOF, 2006), where the minimum catch size (28 cm TL) and fisheries closure dates (April 1 to June 30) are established.
Most studies on M. curema in the Mexican Pacific have focused on estimating population parameters (Espino-Barr et al., 2005, 2013; Gallardo-Cabello et al., 2005; Ibáñez-Aguirre, 2015; Ibáñez-Aguirre & Gallardo-Cabello, 1996; Quiñonez-Velázquez & López-Olmos, 2011; Quiñonez-Velázquez & Mendoza-Guevara, 2009; Quiñonez-Velázquez et al., 2015) and reproduction parameters (Cabral-Solís et al., 2010; Lucano-Ramírez & Michel-Morfín, 1997; Yáñez-Arancibia, 1976). A recent study off the Jalisco coast found that based on the histological analysis of gonads, this species is reproductively active year-round, with a reproductive peak usually in spring and summer (Ruiz-Ramírez et al., 2017). However, the macroscopic observation of the gonads of specimens from Cuyutlán Lagoon, Colima (Cabral-Solís et al., 2010) and a study on the frequency distribution of birth dates of specimens from La Paz Bay, BCS (Quiñonez-Velázquez & Mendoza-Guevara 2009; Quiñonez-Velázquez et al., 2015) have indicated the existence of 2 reproductive peaks during the year (spring and fall). It is, therefore, reasonable to assume that the M. curema population in La Paz Bay (LPB) could comprise 2 population units, considering the 2 events of maximum reproductive activity during the year in the region. The first peak comprises organisms whose reproductive process ends with spawning in the spring (March to June), and the second peak comprises organisms spawning in the fall (October and November), as was suggested by Moore (1974) for the white mullet off the Texas coast, where the existence of 2 populations was proposed based on different spawning periods. In LPB, these events might be synchronized with the southeastern wind pattern (known as Coromuel winds), which are characteristic of the area and occur in spring-summer, and with the northwest winds in the fall-winter (Obeso-Nieblas, 2003). The surface sea temperature (SST) pattern also presents 2 well-defined seasons: winter-spring (21 to 24 ºC) and summer-fall (27 to 31 ºC) (Martínez-Flores et al., 2006; Obeso-Nieblas, 1987).
To date, no study has been done relating possible phenotypic variations with variations in population parameters. Therefore, the objective of the present study was to evaluate the potential existence of a M. curema population structured by stocks in LPB, BCS, using several approaches: the analysis of sagittal otolith shape using geometric morphometrics, the study of reproductive aspects through the macroscopic observation of gonads, and assessment of individual growth using a multi-model approach, assuming that differences between stocks are a consequence of the environmental conditions to which they are exposed.
Materials and methods
A total of 709 specimens were collected from artisanal fishery landings in LPB, BCS, Mexico, from 2010 to 2013. Up to 40 specimens per month were selected randomly, trying to represent the size structure of the catch. Samples were preserved on ice and transported to the laboratory for processing. Fish were identified to species level based on the work by Ibáñez-Aguirre and Gallardo-Cabello (2005). The total length (TL ± 0.1 cm), total weight (TW ± 0.1 g), and eviscerated weight (EW ± 0.1 g) were recorded. Gonads were extracted and weighed. The sex and maturity stage were assigned following visual observation, according to a modification of the morphochromatic criteria by Nikolsky (1963). Five gonadal developmental stages were used: 1) undifferentiated, 2) immature, 3) maturing, 4) mature, and 5) post-spawn. A pair of sagittal otoliths were extracted from each specimen and preserved dry until observation. The external face of the right otolith was photographed for growth mark readings. All images were digitized at 8x magnification including a reference for size, using a digitizing system integrated to a video camera mounted on an Olympus SZX-TR30 stereoscope connected to a computer. A subsample was selected to analyze otolith shape and the same procedure was employed for digitizing.
The reproductive pattern was described based on all collected data and the gonadal maturity changes were analyzed over an annual cycle, using 3 complementary methods. The first 2 were the monthly evaluation of the percentage of mature organisms (stages 3-5) and the gonadosomatic index (GSI) (Lucano-Ramírez et al., 2014), the third was the condition factor (CF). These indices were estimated following the equations (Rodríguez-Gutiérrez, 1992):
GSI = [EW/TW-EW] * 100 (1)
CF = [EW/TLb] * 100 (2)
where b is the value of the slope of the potential TW-TL relationship. These indices were used assuming that there was an inverse relationship between the average maximum GSI values and relatively low CF values, which usually coincides with gonadal maturity (Sánchez-Cárdenas et al., 2007). The differences between the average monthly values of each index were estimated with ANOVA using STATISTICA 7.0 program (StatSoft, 1995).
A total of 692 otolith pairs were read by 2 independent readers (Campana & Thorrold, 2001). Each reader counted the number of growth marks (composed by 1 opaque and 1 translucent band) per otolith twice, and the precision between readers and between readings was evaluated with the average percent error (APE), proposed by Beamish and Fournier (1981):
APE = 1/N∑Nj = 1[1/R∑Ri = 1 (Xij-Xj/Xj )] * 100 (3)
where N is the number of organisms for which age was determined, R is the number of readings per otolith, Xij is the ith reading of the jth otolith, and Xj is the average number of growth marks on the jth otolith. The coefficient of variation (CV) was also estimated, using the same notation and variables as in equation (3) (Chang, 1982):
CV = 1/N∑Nj=1 (√1/R∑Ri=1 ((Xij-Xj)2/Xj) (4)
In both cases, values < 10% are considered adequate (Campana et al., 1995; Morison et al., 1998). The consistency in the allocation of the first growth mark was determined by the distribution of the radio frequency to the first translucent band (R1) for all specimens (García-Contreras et al., 2009; Rocha-Olivares & Gómez-Muñoz 1993). The periodicity in the formation of growth marks was identified through the analysis of the Marginal Increment (MIA) (Lai & Liu 1979):
MIA = (R-rn)/(rn-rn-1) (5)
where R is the otolith radius, rn is the distance from the otolith nucleus to the last observed growth mark, and rn-1 is the distance from the nucleus to the penultimate observed growth mark. This analysis quantifies proportionally the formation of growth marks with respect to the last completely deposited mark, and values range from 0 to 1. Low MIA values are interpreted as the beginning of the formation of the opaque band, whereas high values are interpreted as the end of the formation of the translucent band. The differences between the monthly MIA averages were evaluated using a non-parametric Kruskal-Wallis test in the program STATISTICA 7.0 (StatSoft, 1995).
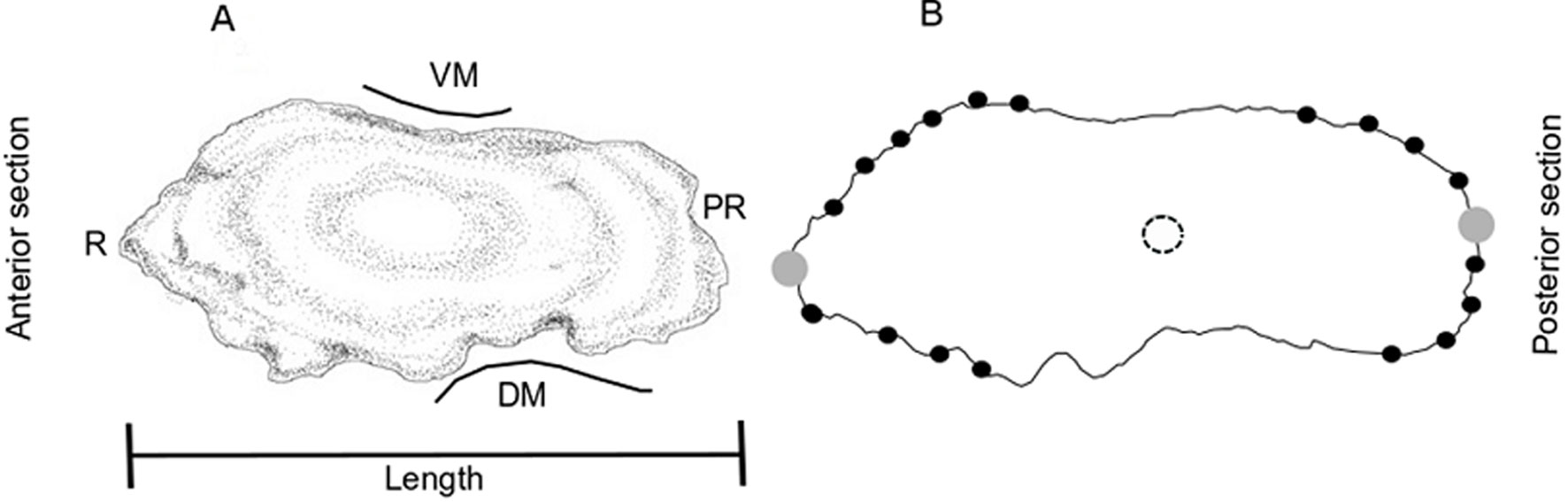
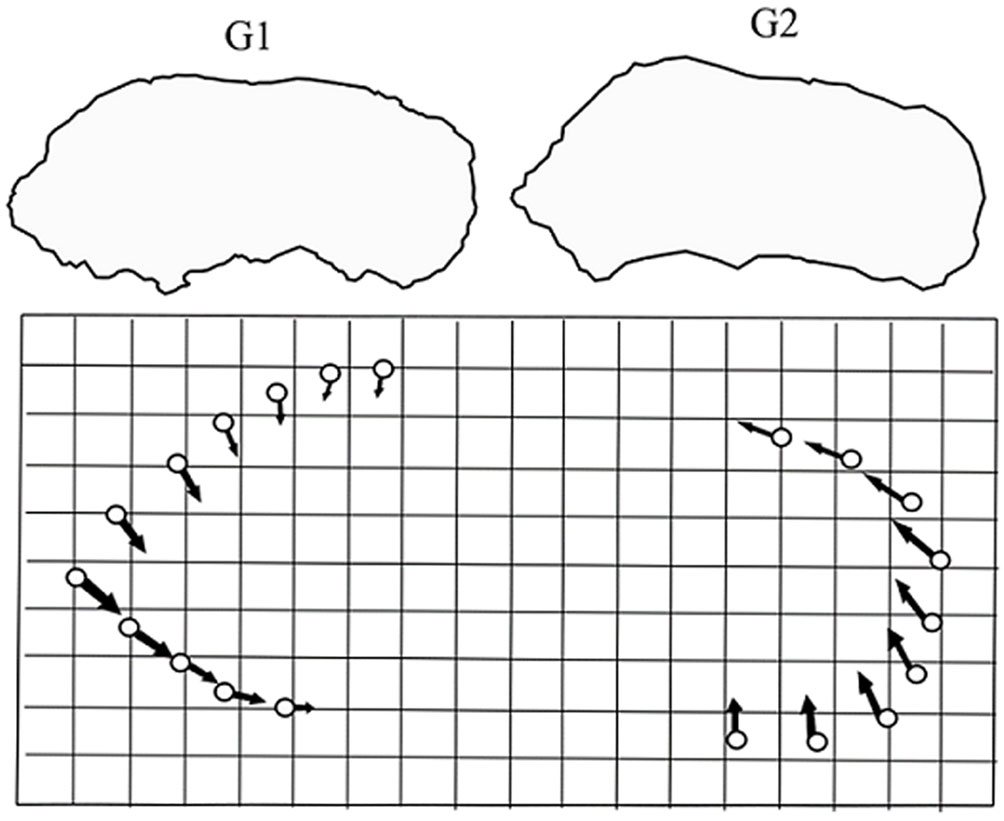
To analyze differences in sagittal otolith shape, 2 data sets were created (Spring: March-May, Fall: October-November), based on the potential existence of 2 reproductive events per year in LPB, as was suggested by Quiñonez-Velázquez and López-Olmos (2011) and by Quiñonez-Velázquez et al. (2015). The sample size for this analysis was 59 specimens for the spring (G1) and 51 specimens for the fall (G2); all specimens were ≥ 220 mm TL. The description and comparison of otolith shape were based on configurations generated by digitalization (X, Y coordinates) of natural anatomical landmarks located mainly on the outline of the structure. Because the number of landmarks was not sufficient to represent otolith shape, a reference grid with radial distances and equidistant angles was used as a reference, to assign and locate semi-landmarks along the perimeter of the otoliths using the program MakeFan (Sheets, 2004). An exploratory analysis was previously carried out by placing landmarks (n = 42) along the contour of the otolith, with the purpose of detecting those marks that presented less magnitude in the variation of the shape between analyzed groups. Selecting a total of 20 reference points to represent the shape of the otolith (Fig. 1). All digitalizations were performed using the program TpsDig (Rohlf, 2004).
For data analysis, geometric configurations were transferred, scaled, and rotated for each group using the Procrustes superposition method in the program Coordgen 6 (Sheets, 2004). To discard differences in the shape associated with allometric effects, a regression analysis was performed between partial warp scores and uniform deformation scores on the natural log (ln) of centroid size (LCS), using the Regress6k program (Sheets, 2004). To test for phenotypic differences between the organisms of the 2 groups, we compared the total procrustes distance (TPD) between each pair of average configurations. The F values (variance ratio) obtained from the observed values and the F values obtained with 100 re-samplings were compared, and the statistical significance was obtained from the percentage of times the calculated F values were equal to or greater than the F values obtained with observed data. These analyses were performed using the Two Group program (Sheets, 2004). Finally, to have a clear perspective and to be able to visualize the trend in the variation of the shape between the groups, the “thin plate spline” function was used, basically this function graphs the vectors produced from the analysis of the partial deformations and interpolates the variation occurred between homologous marks. That is, the greater the morphological differences between the 2 configurations, the deformation in the plaque will be more significant, thus defining the morphological changes. In turn, it allows to increase the degree of deformation, so that the changes are more noticeable.
A total of 390 mature specimens (stages 3, 4, and 5) were used to evaluate differences in growth and reproductive parameters between the 2 groups (G1: n = 212; G2: n = 178). Differences in length and weight between the groups were evaluated using a non-parametric Kolmogorov-Smirnov test in the program STATISTICA 7.0 (StatSoft, 1995).
Five models were evaluated to describe the individual growth of the white mullet: von Bertalanffy, Gompertz, logistical, Richards, and Shnute (Table 1). The estimation of the parameters of the models was performed by maximizing the negative of the verisimilitude logarithm using the Gauss-Newton direct search algorithm. The 95% confidence intervals of the parameters were estimated based on the verisimilitude profiles, assuming a χ2 distribution (Polacheck et al., 1993). The selection of the most adequate model and its veracity was based on Akaike´s information criterion (AIC). According to this criterion, the model with the lowest AICi (AICimin) is the most adequate model to describe growth:
AICj = 2LL + 2K (6)
where LL is the verisimilitude obtained for each adjustment and K is the number of model parameters. The AICi differences (Δi = AICi – AICmin) were estimated to assess the statistical support for each model. According to Burnham and Anderson (2002), models with Δi > 10 do not have statistical support and should be omitted from the analysis, models with Δi < 2 have high statistical support, and models with 4 < Δi < 7 have intermediate support. The credibility of each model was evaluated with the AIC weight (wi), based on the equation proposed by Burnham and Anderson (2002):
Wi = exp(-0.5 Δi)/ ∑5k=1exp(-0.5 Δk) (7)
Because the G1 and G2 groups were comprised only by organisms with a length ≥ 220 mm LT, in order to avoid overestimating the average size of the younger age groups and negatively influence the estimation of the growth coefficient, lengths back-calculated in the adjustment of the growth models to the age-length data were used. The Fraser-Lee equation was used to back-calculate previous lengths (Araya & Cubillos, 2006):
Li = a + (Lc – a) * (ri/R) (8)
where Li is the back-calculated length of growth mark i, Lc is the length of the specimen at capture, a is a model parameter, ri is the distance from the nucleus to ring i, and R is the radius of the otolith at capture. The hatch size of M. curema was used as parameter a (2.2 mm TL) (Houde et al., 1976), to reduce bias introduced from not including young specimens in the estimate of the intercept of the relationship between otolith size and fish size (Araya & Cubillos, 2006).
For reproductive aspects, the total sexual proportion and sexual proportion by group were estimated with a Chi squared (χ2 test with correction for continuity by Yates (Zar, 2010):
χ2Yates + ∑((|fi-fiexp|-0.5)2)fiexp) (9)
where fi is the frequency of males or females observed and fiexp is the expected frequency.
The length of at least 50% of individuals that had reached sexual maturity L50% was estimated, adjusting a logistic model to the percentage of mature specimens by size interval (10 mm) through the non-linear module of the Statistica 7.0 program (StatSoft, 1995). The equation defining this model is as follows:
Pi = 1/(1 + exp[-r(TLi-L50%)]) (10)
where Pi is the percentage of mature organisms, i is the size interval, TLi is the size of interval i, and r is the intercept.
Differences in parameters of growth and the sexual maturity length between groups were evaluated based on the verisimilitude test proposed by Kimura (1980):
χ2k = -Nln(SRCΩ/SRCω) (11)
where k is degrees of freedom, in this case the number of parameters, N is the total number of data, SRCΩ is the sum of squared residuals of the model adjusted to each data set, and SRCω is the total sum of squared residuals of the adjustment of the model to the data.
Table 1
Models evaluated using multi-model inference to describe the individual growth of the white mullet Mugil curema in La Paz Bay.
|
Model |
Equation |
Parameters |
|
Von Bertalanffy |
Lt = L∞ (1-e-k(t-t0)) |
Lt = length at age t L∞ = asymptotic length k = annual growth coefficient t0 = age to length 0 |
|
Gompertz |
Lt = L∞ e(-e-k1(t – t1)) |
t = age in years k1 = instantaneous rate of growth at age t1 t1 = point of inflection of the curve and age at which the absolute rate of growth begins its decline |
|
Logistic |
Lt = L∞ (1 + e-k2(t – t2))-1 |
k2 = relative parameter to the growth rate t2 = turning point of the sigmoid curve |
|
Richard |
Lt = L∞ (1 – e-k3(1 – m)(t – t3))1/(1 – m) |
k3 = growth rate at the inflection point t3 = age to the inflection point m = model adjustment parameter |
|
Shnute |
Lt = (yb1 + (yb2 – yb1) + (1-e(-a(t – t1)))/(1-e(-a(t2 – t1)))) |
b = Relative increase in the growth rate (constant over time) y1 = length at age t1 y2 = length at age t2 |
Results
The size and weight of all analyzed specimens (N = 709) ranged between 79 and 405 mm TL, and between 6 and 600 g TW. Females (F) represented 82% of the sample (TLmean = 326.6 mm, TWmean = 316.6 g), males (M) represented 12% of the sample (TLmean = 304 mm, TWmean = 263.8 g), and 6% were undifferentiated (U) (TLmean = 103.6 mm, TWmean = 13.7 g). The reproductive pattern showed modest changes during the 4 years of sampling, with ± 1 month at the beginning and end of maximum reproductive activity. Organisms with mature gonads were observed during most of the year, with 2 events of maximum reproductive activity per year. The first event occurred in March-June (G1) and the second in October-November (G2) (Fig. 2).
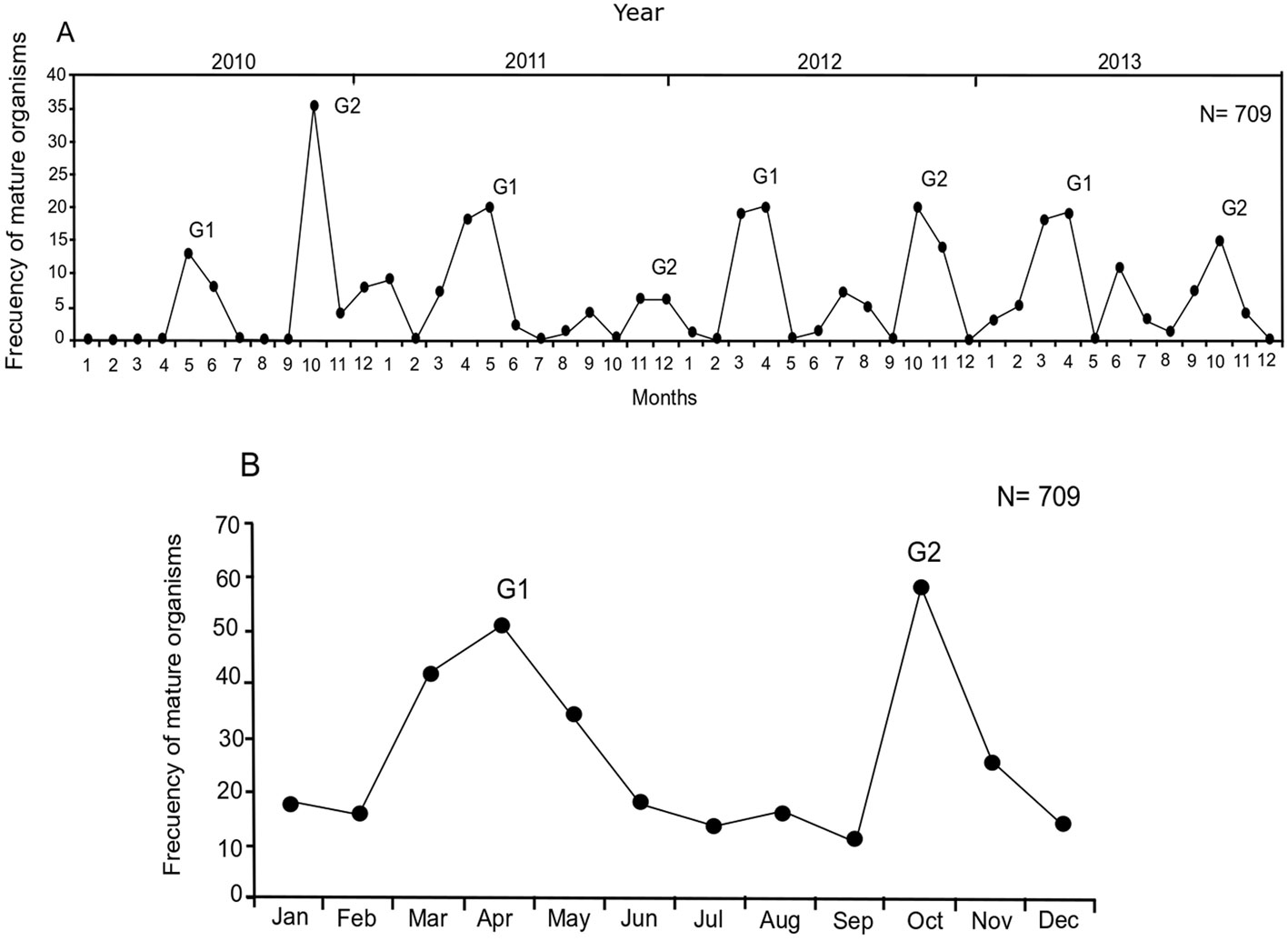
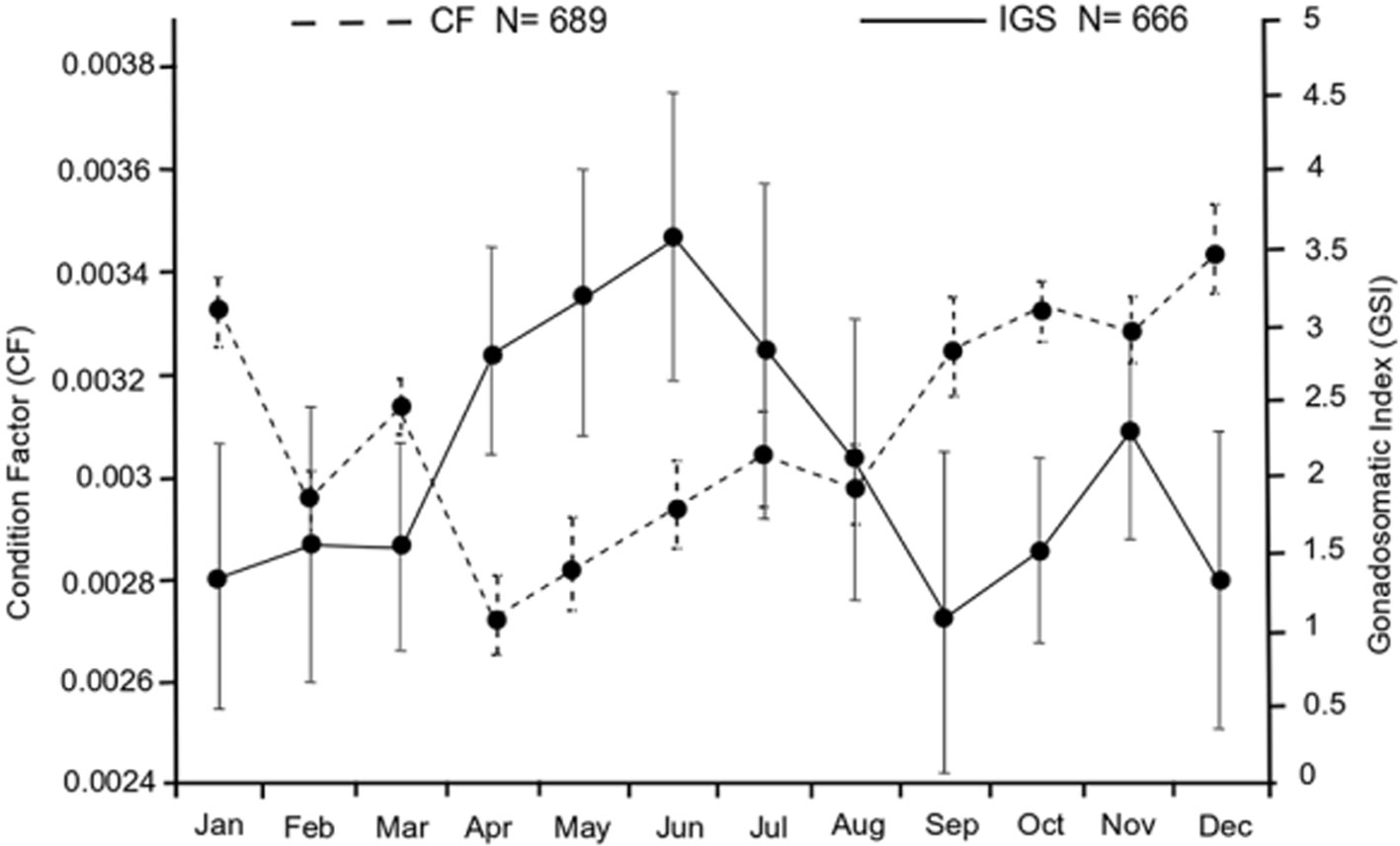
There were significant differences in monthly CF values (F = 38.74, p < 0.05). The lowest CF value was recorded in April (CF = 0.0027) and the maximum value was recorded in December (CF = 0.0034) (Fig. 3). Differences in GSI monthly values were significant (F = 3.36, p < 0.05); this pattern coincided with the 2 periods of maximum reproductive activity (G1 and G2). The greatest GSI values were recorded in April-June and November (2.7 to 3.5) (Fig. 3). There was a significant negative correlation between GSI and CF (r = -0.7, p < 0.05, N =12).
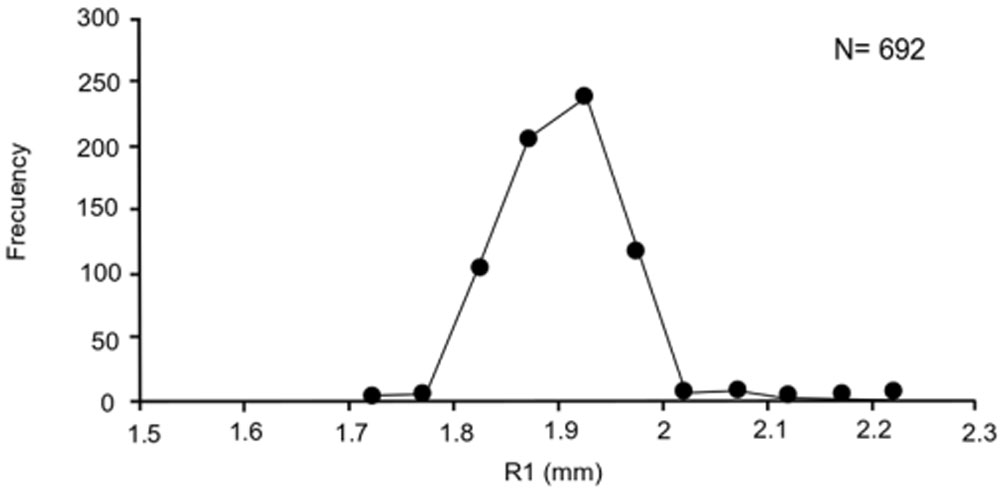
Precision in growth mark readings per reader and between readers was adequate (APE = 1.54%, CV = 2.17), and there was consistency in the assignment of the first growth mark, evidenced by a unimodal R1 pattern (Fig. 4). There were no significant differences in the monthly MIA averages (F = 1.1, p = 0.34); this index presented highest values in June and December-February, and lowest values in May and October (Fig. 5). There was a non-significant negative correlation between MIA and the monthly proportion of mature organisms (r = -0.47, p < 0.05).
No significant correlation was detected between DTP and the LCS (r = -0.0018, p > 0.05). The analysis based on the F test indicated significant differences in DTP between groups (F= 11.07, p < 0.01, DTP = 0.0239). The results in the variation of the shape between the groups (Fig. 6), occurred mainly in the dorsal area of the rostrum and in the ventral part of the postrostrum. Otoliths corresponding to G1 were considerably longer that otoliths corresponding to G2, with an average total otolith radius (RTmean-otolith) of 4.2 mm and 4.0 mm, respectively.
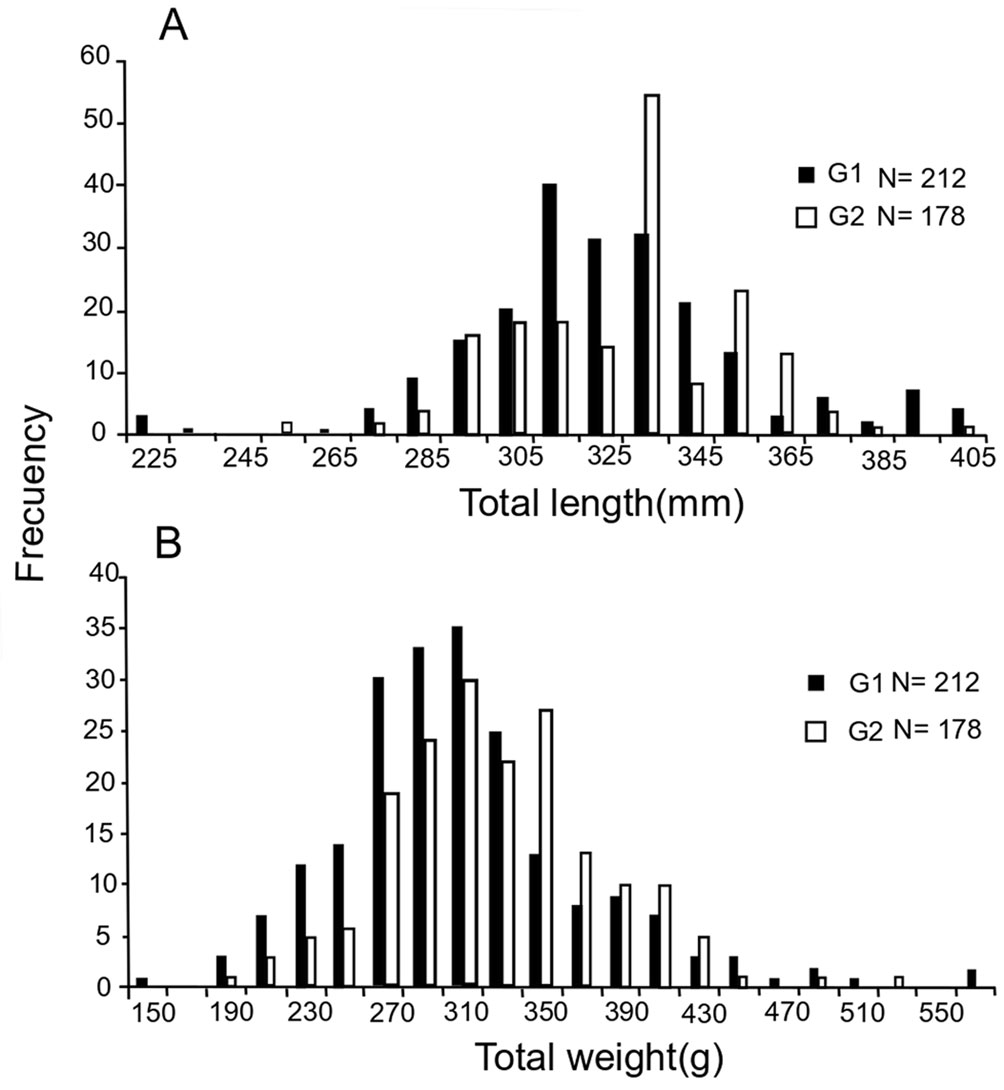
G1 fish measured 325 mm TL on average (size range: 220 to 405 mm TL) and weighed 306 g TW on average (weight range: 115 to 505 g TW); the sexual proportion was 9.8F:1M (91% F and 9% M). G2 fish measured 329 mm TL on average (size range: 254 to 401 mm TL) and weighed 324 g TW on average (weight range: 204 to 449 g TW); sexual proportion was 6.4F:1M (84% F and 16%M). Differences in size and weight between groups were significant (p < 0.01) (Fig. 7).
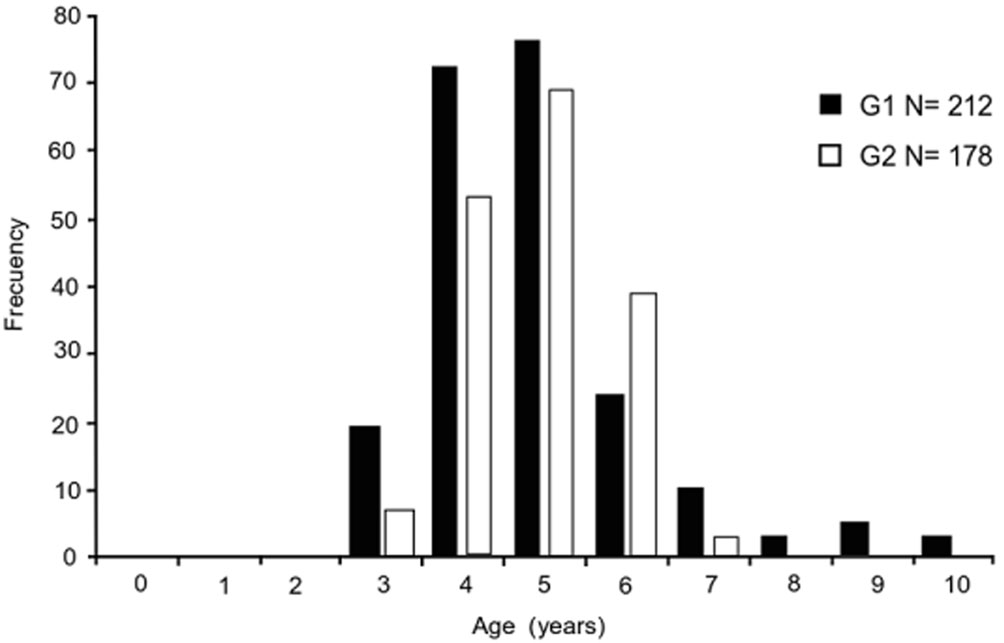
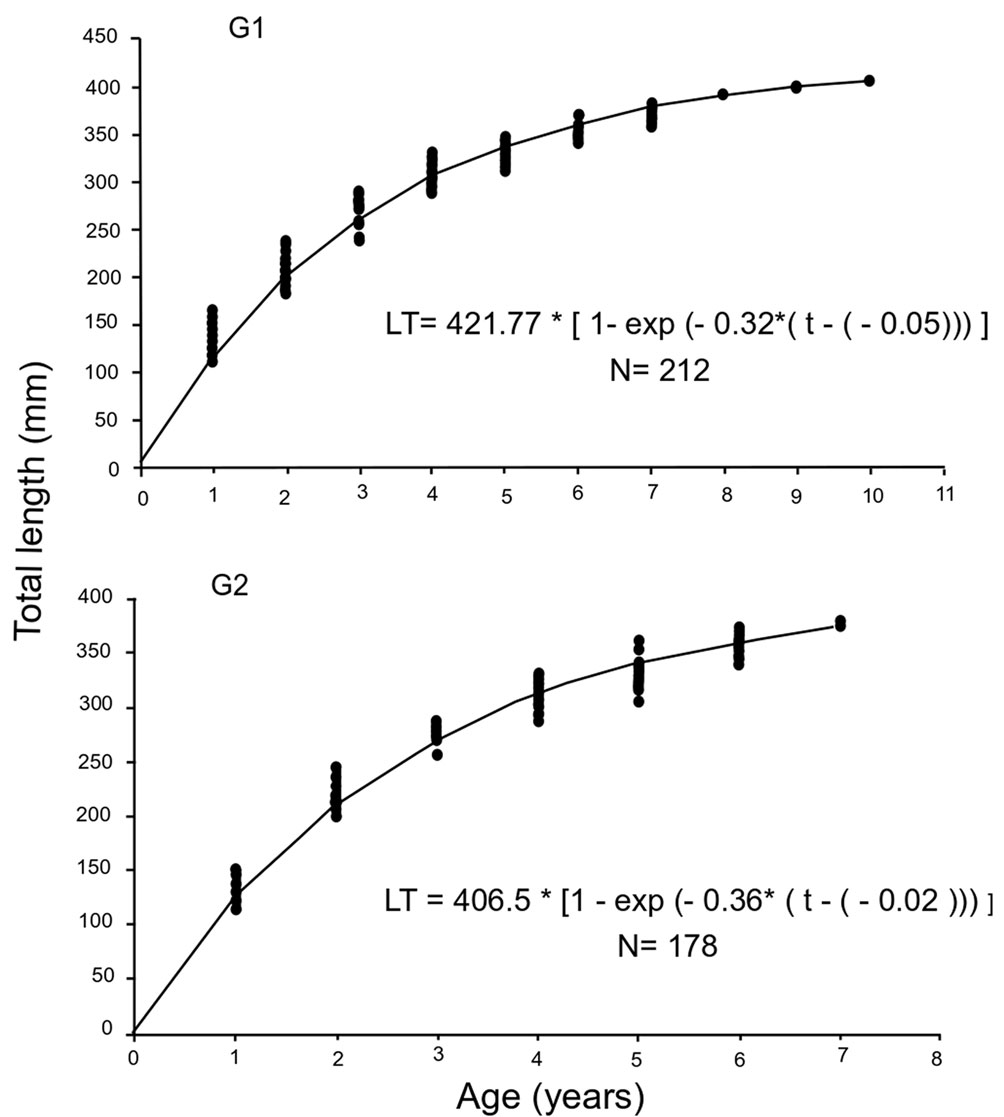
G1 comprised 8 age groups (3-10), whereas G2 comprised 5 age groups (3-7). In both cases, the best-represented age groups were year 4 (69%) and year 5 (71%) (Fig. 8). Of the models evaluated to describe the size-age relationship, the von Bertalanffy and Richard models were the most adequate for both groups. The model with the lowest AIC and greatest statistical support was the von Bertalanffy model (Table 2). Results indicated that the Gompertz, logistic, and Shnute models were not adequate to describe the growth of either white mullet group (mullet group (Δi > 10). The von Bertalanffy and Richard models had high statistical support for G1 data, whereas for G2 data the von Bertalanffy model had high statistical support (Δi < 2) and the Richard model had intermediate statistical support (4 < Δi < 7). The curve that best described growth for both groups was the von Bertalanffy model, and its parameters are presented in figure 9. Differences in growth between G1 and G2 were significant (p < 0.05). The 95% confidence intervals of estimators of the model parameters were: were: L∞ = 419.6/423.2, k = 0.314/0.324, t0 = -0.019/-0.01 for G1; and L∞ = 404/408.4, k = 0.359/0.369, t0 = -0.067/-0.001 for G2.
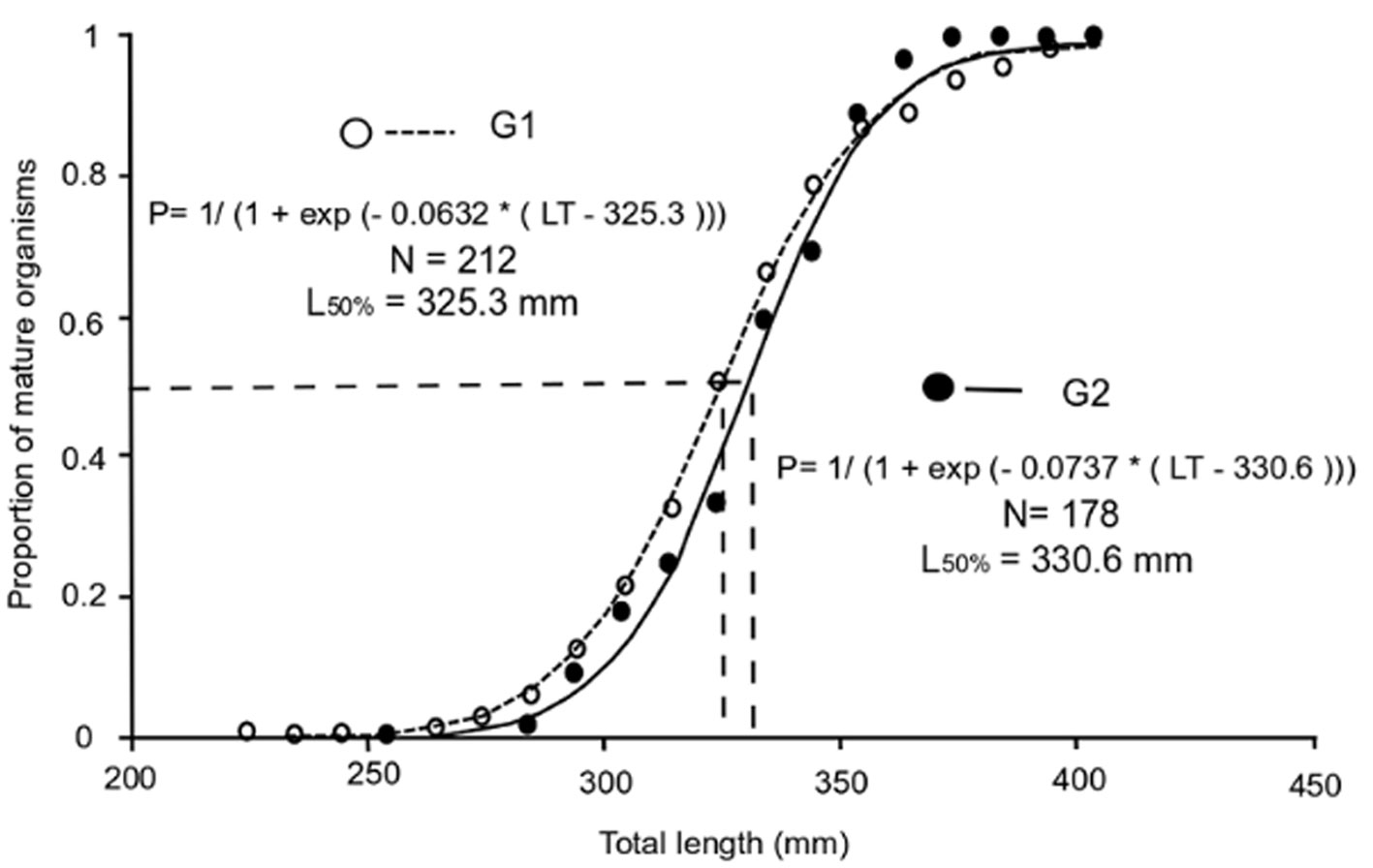
G1 had a slightly lower L50 % than G2 (G1: 325 mm TL, G2: 330 mm TL), but this difference was not significant (p > 0.05) and these sizes were reached around 5 years of age in both cases (Fig. 10).
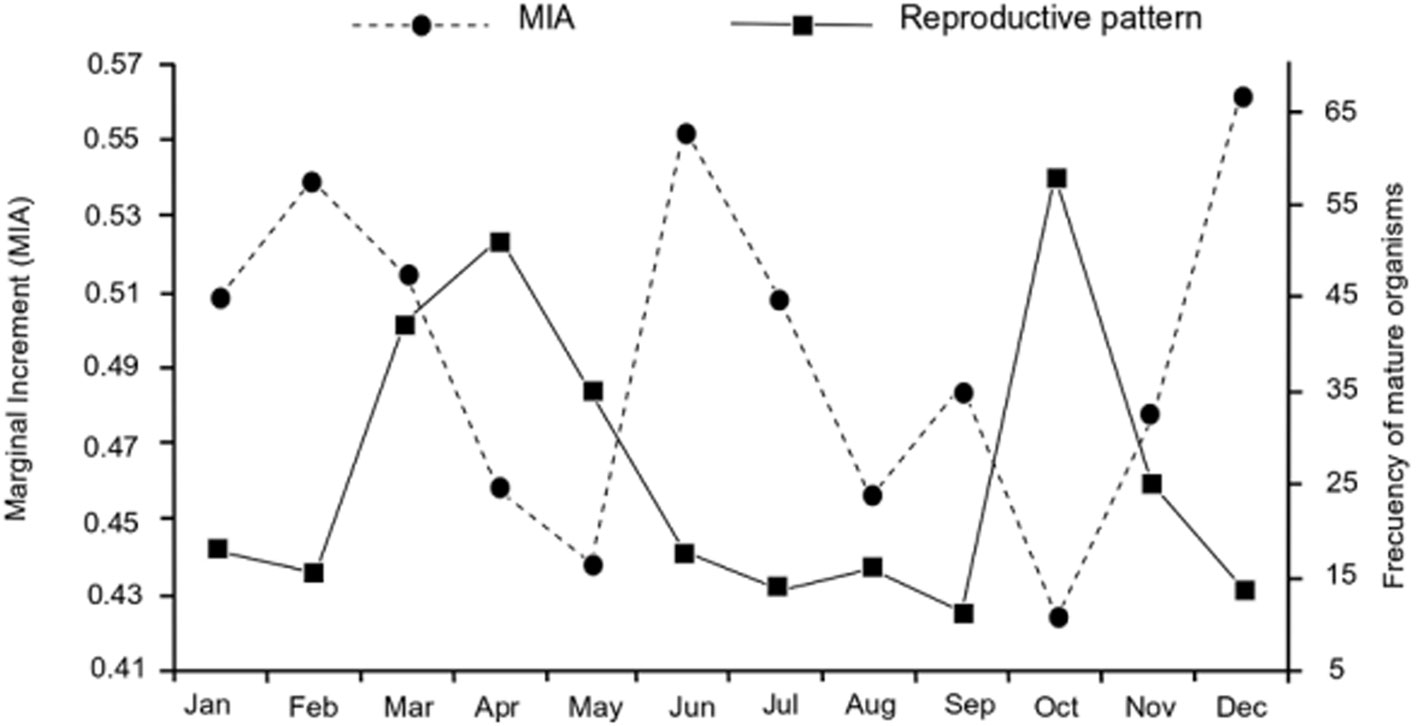
Table 2
Parameters of the models to describe the growth of white mullet Mugil curema by groups in Bahía de La Paz. t0 age at the inflection point for the Gompertz and Logistic models, and age at the zero length in von Bertalanffy, Richard and Schnute; k, coefficient of growth; L∞, asymptotic length; m, dimensionless parameter; a, relative growth rate; b, inherent constant of the growth rate; y1 and y2, length at the minimum age observed and the maximum age observed; AIC, Akaike Information Criterion and Δi Akaike difference.
|
Model |
t0 |
k |
L∞ |
m |
a |
y1 |
y2 |
b |
AIC |
Δi |
|
Group 1 (G1) |
||||||||||
|
von Bertalanffy |
-0.05 |
0.32 |
421.77 |
50.47 |
0 |
|||||
|
Gompertz |
1.32 |
0.58 |
391.38 |
57.18 |
6.71 |
|||||
|
Logistic |
2.01 |
0.82 |
384.26 |
59.82 |
9.35 |
|||||
|
Richard |
-0.47 |
0.34 |
412 |
7.4 E-5 |
52.25 |
1.78 |
||||
|
Schnute |
-0.08 |
429.23 |
0.34 |
211.28 |
415.36 |
0.99 |
57.29 |
6.82 |
||
|
Group 2 (G2) |
||||||||||
|
von Bertalanffy |
-0.02 |
0.36 |
406.5 |
38.23 |
0 |
|||||
|
Gompertz |
1.19 |
0.73 |
364.42 |
45.75 |
7.52 |
|||||
|
Logistic |
1.73 |
1.07 |
355.01 |
48.04 |
9.81 |
|||||
|
Richard |
-0.02 |
0.39 |
389.36 |
0.01 |
44.64 |
6.41 |
||||
|
Schnute |
-0.08 |
438.67 |
0.37 |
25.27 |
418.31 |
0.98 |
61.01 |
22.78 |
Discussion
The reproductive cycle of the white mullet M. curema in LPB was defined by the year-round presence of mature organisms, a similar pattern presented by other tropical fish species (Bond, 1979; Lagler et al., 1977). During the study period, the reproductive cycle of white mullet was characterized by the consistent occurrence of 2 periods of maximum reproductive activity, the first in March-May (defined as G1) and the second in October-November (defined as G2). G1 had a wider amplitude than G2, similarly to what was reported by Quiñonez-Velázquez et al. (2015) for the same study area, as well as by other authors for other regions, e.g., Marín-Espinoza (1996) for the Venezuela coast, Álvarez-Lajonchere (1976) for Cuba, Cabral-Solís et al. (2010) for Colima, Mexico, and Ditty and Shaw (1996) for the northern Gulf of Mexico. This reproductive strategy could be linked to the geographical distribution of this species and to the environmental characteristics that prevail in the region (Marín-Espinoza et al., 2003; Ruiz-Ramírez et al., 2017). Generally, fish reproduce when conditions are appropriate for the greater survival probability of larvae, avoiding periods of low food availability and, therefore, present slow growth (Chellappa et al., 2010). In areas close to the tropics, such as LPB, changes in temperature and photoperiod play an important role in the life cycle of fish, mainly in reproduction (Estrada-Godínez et al., 2014). Reyes-Salinas et al. (2003) reported a seasonal trend in primary productivity (PP) in LPB, with 2 peaks per year, the first one in March (16 mg C m-3h-1) (when the photoperiod increases and heat starts to build up), and the second in October (5 mg C m-3h-1) (when the photoperiod decreases and there is heat loss). These events coincide with the 2 reproductive periods observed in this study, suggesting a possible synchronization of gametogenic development with a sea surface temperature (SST) interval between 23 and 26 ºC (Obeso-Nieblas, 1987; Reyes-Salinas et al., 2003) and synchronization of spawning with high PP. It is also possible that white mullet stocks synchronize their reproductive resting period with the warmest months of the year (August and September), which have average SST between 28ºC and 31ºC (Martínez-Flores et al., 2006; Obeso-Nieblas, 1987) and low PP (2 mg C m-3h-1) (Reyes-Salinas et al., 2003), because the lowest proportion of mature organisms was recorded during this period during the 4 years of sampling (8%). This plasticity has also been observed in other teleost fish species off the western BCS coast (Pérez-Olivas, 2016), where the beginning of the reproductive period occurs after a seasonal increase in SST (March), and fish enter a reproductive resting period during the warmest months (August and September, > 29 ºC).
Based on the identification of significant differences in the average monthly values of the CF and the negative correlation with the GSI, an inverse relationship between the body condition of the white mullet and the reproductive activity is suggested. For this reason, CF could be used along with GSI to support the indirect identification of the reproductive period, as was done by Ibáñez and Gallardo-Cabello (2004), Kanak and Tachihara (2008), Cabral-Solís et al. (2010), and Ruiz-Ramírez et al. (2017). On the other hand, other authors (Aburto-Oropeza et al., 2008; Albieri, Araújo, & Ribeiro, 2010; Chandrasekhara & Krishnan, 2011; Estrada-Godínez et al., 2014; Volpato & Trajano, 2005) have pointed out that CF is not related to GSI in some tropical fish species because breeding fish do not stop feeding during the maturing and spawning stages. This is the opposite of what is observed in some temperate and cold water species such as salmonids, that stop feeding during the breeding period and use energy reserves from visceral fat and muscle for vital functions, which leads to a clear decrease in CF (Barnham & Baxter, 1998; Bureau et al., 2002).
The average monthly MIA values suggest that there are 2 times during the year when the white mullet population of LPB deposits a growth mark (GM), the first in summer (June-July) and the second in winter (December-February). Both seasons coincide with the end of the reproductive period of each of the detected groups. Based on this information, we identified an annual periodicity in the formation of GM. An annual GM is composed of 2 bands, an opaque band (O) usually linked to a rapid growth period and low MIA values, and a translucent band (T) linked to a slow growth period and high MIA values, both determined by food availability and energy destined towards somatic growth (Botha, 1971; Espino-Barr et al., 2005; Pannella, 1974). The reproductive process results in great energetic demands, and one can suppose that other processes such as somatic growth will tend to show a clear decrease at this time (Fernández-Palacios & Izquierdo, 2009). It should also be mentioned that after reaching gonadal maturity, white mullets migrate offshore to spawn, from protected waters towards the open ocean (Ibáñez-Aguirre & Gallardo-Cabello, 1996; Marín-Espinoza et al., 2003; Trape et al., 2009). These events could be the reason why periods of slow growth (high MIA value) were recorded in the present study, after each event of maximum reproductive activity. This white mullet strategy, which implies annual periodicity in the formation of GM, based on the relation between the formation of the T band and the reproductive period, has been previously reported for the Gulf of Mexico (Ibáñez-Aguirre & Gallardo-Cabello, 1996; Ibáñez et al., 2012) as well as for the Mexican Pacific (Cabral-Solís, 1999; Espino-Barr et al., 2005).
From the statistically significant difference in the otolith shape between groups (G1 and G2) and in the images through the thin plate spline, it was possible to observe in detail the presence of 2 white mullet morphotypes with their own phenotypic characteristics in BLP. Variations in the shape of anatomical structures such as otoliths are associated with age in the individuals of species with individual growth and tendency towards allometry (Alberch et al., 1979; Gould, 1966; Klingenberg, 1998; Pérez-Quiñonez et al., 2018). In the present study, to reduce this potential effect, the individuals analyzed were adults ≥ 220 mm LT, furthermore, we found a non-significant negative correlation between DTP and the LCS; and therefore, these detected differences in shape are not associated with allometric effects. Moreover, one of the characteristics of geometric morphometrics is that it does not consider differences between configurations that are attributable to location, scale, and orientation, leaving only differences in shape (Kendall, 1977; Zelditch et al., 2004). To date, the use of otoliths to detect phenotypic population units (stocks) through shape analysis has been very useful (Félix-Uraga et al., 2005; Pérez-Quiñonez et al., 2018; Ponton, 2006; Ramírez-Pérez et al., 2010; Vergara-Solana et al., 2013), because otoliths are structures that allow an interpretation that is relatively similar to that provided by the analysis of body shape, and it has been suggested that otoliths are adequate to discriminate stocks (Pérez-Quiñonez et al., 2018; Vergara-Solana et al., 2013). Differences in shape are a record of the life history of the individual and express ontogenetic changes, phenotypic plasticity, or evolutionary adaptations linked to environmental factors (Campana & Thorrold, 2001; Espino-Barr et al., 2005; Gallardo-Cabello et al., 2006, 2011). In the present study, the greatest variation in otolith shape occurred mainly in the dorsal rostrum-post rostrum area and allowed significant discrimination between groups. In a study by Ibáñez-Aguirre et al. (2006), which evaluated body shape of 2 white mullet populations from the Gulf of Mexico and from the Pacific Mexican coast using traditional morphometrics, only differences in eye shape were detected. In the present study, geometric morphometrics was applied to identify differences in otolith shape in the white mullet from LPB. Great intraspecific variations were found in a small geographic area, compared with that analyzed by Ibáñez-Aguirre et al. (2006). This is because geometric morphometrics allows inferences of high biological significance, based on differences between individuals and populations. Moreover, the use of otoliths has brought favorable results to delimit population units (Pérez-Quiñonez et al., 2018; Ramírez-Pérez et al., 2010; Vergara-Solana et al., 2013).
The detected variations in white mullet otolith shape could be reflecting the conditions to which individuals are exposed, such as seasonality in temperature, salinity, oxygen, photoperiod, and primary productivity in the area of residence (Ramírez-Pérez et al., 2010; Winberger, 1992), which directly influence the behavior, physiology, mortality, growth, reproduction, and demography of individuals, producing a type of phenotypic plasticity in response to variations in the environment (Miner et al., 2005). Such changes could be expressed during the individual life cycle only or during generations. However, to corroborate this information, studies are needed that include a detailed following of biological characteristics, as well as studies that focus on the discrimination of phenotypes based on geometric morphometrics (Begg & Waldman, 1999). An example of this is a case study on the Pacific thread herring off the northwest Mexican coast, in which 3 morphotypes that conserved their own growth, reproduction, and mortality parameters were detected (Pérez-Quiñonez et al., 2018; Ruiz-Domínguez & Quiñonez-Velázquez, 2018), coinciding with results from the present study on the white mullet population in LPB.
G1 fish were on average smaller and lighter (325 mm TL, 306 g TW) than G2 fish (329 mm TL, 324 g TW). Sexual proportions of G1 and G2 indicated that most of the population exploited in LPB was represented by females (> 80%), which is considerably different from what would be expected theoretically (1F:1M) (Nikolsky, 1963). This result coincides with most studies carried out to date on white mullet in the Mexican Pacific (Cabral-Solís et al., 2010; Lucano-Ramírez & Michel-Morfín, 1997; Ramos-Cruz, 1985; Ruiz-Ramírez et al., 2017), in the Gulf of Mexico (Ibáñez & Colín, 2014; Ibáñez & Gallardo-Cabello, 2004), and in Brazil (Albieri, 2009; Albieri, Araújo, & Ribeiro, 2010; Albieri, Araújo, & Uehara, 2010; Fernández & Dias, 2013). Only the studies by Oliveira, Costa et al. (2011), and Oliveira, Costa et al. (2011) off the northwest Brazil coast resulted in the expected sexual proportion (1F:1M). The greater representation of females in catches of this species could be because it is usually captured in areas near the coast. Some authors, such as Ould-Mohamed Vall (2004), argue that species from the Mugil genus tend to present segregation by age class and sex, and that males remain in areas away from the coast most of the time.
Age was assigned using the number of growth marks on otoliths. There was a greater number of age groups (3-10 years) in G1 than in G2 (3-7 years); however, the 4 and 5 age groups were the best represented in both groups. Despite the difference in the age structure between the 2 groups, the longevity estimate (A95%) was similar for both (10 years for G1 and 9 years for G2). The detected age difference could be related to the fishery closure in the region (1 April to 30 June), which occurs when G1 presents its reproductive peak; G2 is vulnerable to fishing during its maximum reproductive event in the fall.
No white mullets over 9 years old have been reported; however, A95% estimates greater than 10 years have been reported (Espino-Barr et al., 2013; Gallardo-Cabello et al., 2005; Ibáñez-Aguirre & Gallardo-Cabello, 1996; Ramos-Cruz, 1985). This could be an effect of fisheries, as was pointed out by Lagler et al. (1977), who indicated that the low abundance of organisms of advanced age in the age structure was due to a greater accumulated mortality compared with younger organisms, because the fishery was the main factor that decreased the abundance of larger organisms, as indicated by Cabral-Solís et al. (2007), who report 75% of the total mortality of organisms between 2 and 5 years of Mugil curema, as a result of fishing.
Both M. curema groups displayed fast individual growth during the first years of life (G1: 73% of L∞, G2: 75% of L∞) before reaching the average size/age at sexual maturity (G1: 325 mm TL, G2: 330 mm , 5 years in both cases). After the first reproductive event, the growth rate decreased gradually as the asymptotic length was reached, a growth pattern that is common in tropical species of the Mugil genus (Cabral-Solís, 1999; Gallardo-Cabello et al., 2005). Of the models evaluated to describe individual growth in the white mullet, the von Bertalanffy model resulted in the lowest AIC value for both groups. Moreover, it showed the highest statistical support, and was the most adequate to describe variations in age-size data. This model assumes that environmental conditions are constant and that fish growth is conditioned by metabolic physiological processes (Araya & Cubillos, 2006). The multi-model approach indicated that the Richard model was also statistically valid (G1: ∆i < 2, G2: 4 < ∆i < 7) to describe white mullet growth. This model has also been successful for the description of growth in sharks, cattle, and buffalos (Katsanevakis, 2006; Peroto et al., 1992).
The parameters obtained based on the von Bertalanffy model indicated that G1 reached greater lengths and ages than G2; however, L50% was greater for G2 than G1. The L50 % is an important parameter because it defines the moment in which the organism experiences a series of physical, metabolic, and behavioral changes. In the case of species under intense exploitation, it could function as a reference to establish minimum catch sizes (Ruiz-Ramírez et al., 2017). The difference between G1 and G2 in L50% estimates was not statistically significant, which suggests that the 2 groups matured at the same average size/age; these estimates were higher than those reported for this species in other areas of Mexico (Table 3). The large L50% estimate obtained with the analyzed specimens could point to some survival advantages because fecundity is related to the size of the female (Ibáñez & Colín, 2014). Therefore, if the size at sexual maturity is large in this area, it is probable that there is also greater fecundity and greater quality of oocytes (Lowerre-Barbieri et al., 2011). These differences could be associated mainly with environmental conditions and with the type of net used for this species’ capture (Vazzoler, 1996). Cabral-Solís et al. (2007) recommended the use of gill nets with mesh sizes between 6.98 and 7.62 cm to capture individuals measuring between 321 and 350 cm TL for the artisanal fishery of Cuyutlán Lagoon, Colima. At that size, fish would be between 4 and 5 years old and would have reproduced. McDonough et al. (2005) argued that the size at first maturity is conditioned by the particular characteristics of each species and perhaps of each population. Therefore, in the case of large detritivorouse fish with relatively long lives such as the white mullet, maturity is reached at a larger size and older age.
Previous studies on the white mullet M. curema that have focused on reproduction (Albieri, Araújo, & Ribeiro, 2010; Albieri, Araújo, & Uehara, 2010; Fernández & Dias, 2013; Ibáñez & Colín, 2014; Ibáñez & Gallardo-Cabello, 2004; Ruiz-Ramírez et al., 2017) and growth (Espino-Barr et al., 2005; Gallardo-Cabello et al., 2005; Ibáñez-Aguirre & Gallardo-Cabello, 1996; Ibáñez et al. 1999; Quiñonez-Velázquez & López-Olmos, 2011) aspects have interpreted the information obtained by supposing that the population was not structured in population units. However, results from the present study differ in this regard and indicate that the white mullet in LPB is structured by stocks. In consequence, it is necessary to consider results from the present study to perfect a management scheme that guarantees the sustainability of the white mullet resource. Cadrin et al. (2005) commented that the identification and delimitation of stocks should be considered as a prerequisite to the evaluation of populations and their fishery, especially for species that are exposed to constant pressure from fishing, such as the white mullet (Gómez-Ortiz et al., 2006). Results from this study, supported by otolith shape, reproductive aspects, and individual growth, suggest the presence of 2 stocks in LPB and therefore a potential adjustment in the fishery closure date for this species should be considered for this region.
Finally, we conclude that the unimodal pattern in the radius of the first GM, the precision in the readings between readers, and the high correspondence between Rt and TL observed in the present study, validated the use of otoliths to estimate age in the 2 white mullet groups in LPB, which presented different characteristics of average size and weight, and individual growth. Results coincided with the exploratory analysis with geometric morphometrics, the reproductive pattern, and the periodicity in the formation of growth marks. Evidence from this study supports the potential existence of 2 white mullet M. curema population units in the study area. The following step should consist of a detailed analysis gonadal development from histological methods, in addition to the variation in body shape and otoliths through ontogeny for the 2 population units in LPB.
Acknowledgements
This study was financed by the Instituto Politécnico Nacional (IPN). LSC and CIPQ received a postgraduate scholarship from Conacyt and from the Programa de Estímulo Institucional de Formación de Investigadores of the IPN. CQV and FAGD received scholarships from the Comisión de Operación y Fomento de Actividades Académicas de IPN, Estímulos al Desempeño de los Investigadores de IPN, and Sistema Nacional de Investigadores-Conacyt. We thank all who participated in collecting and processing biological material for this study. Finally, thanks to two anonymous reviewers for their comments and recommendations that improved the manuscript.
Table 3
Values of the length of first sexual maturity L50% of the white mullet Mugil curema estimated by several authors.
|
Author |
Study area |
L50% (mm) |
||
|
Both genders |
Females |
Males |
||
|
Cabral-Solís (1999) |
Colima |
271-280 |
– |
– |
|
Meléndez-Galicia & Romero-Acosta (2010) |
Michoacán |
245 |
– |
– |
|
Cabral-Solís et al. (2010) |
Colima |
– |
270 |
255 |
|
Fernández & Díaz (2013) |
Brasil |
249 |
– |
– |
|
Ruiz-Ramírez et al. (2017) |
Jalisco |
– |
245 |
217 |
|
Present study |
La Paz Bay, BCS (G1) |
325 |
– |
– |
|
Present study |
La Paz Bay, BCS (G2) |
330 |
– |
– |
References
Aburto-Oropeza, O., Erisman, B., Valdez-Ornelas, C., & Danemann, G. (2008). Serránidos de importancia comercial del Golfo de California: Ecología, pesquerías y conservación. Ciencia y Conservación, 1, 1–23.
AEAP. (2017). Anuario estadístico de acuacultura y pesca. SAGARPA, Mazatlán, Sinaloa, México.
Alberch, P., Gould, S. J., Oster, G. F., & Wake, D. B. (1979). Size and shape in ontogeny and phylogeny. Paleobiology, 5, 296–317. https://doi.org/10.1017/S0094837300006588
Albieri, R. J. (2009). Biologia reproductiva da tainha Mugil liza Valenciennes e do parti Mugil curema Valenciennes (Actinopterygii, Mugilidae) na baia de Sepetiba, RJ, Brasil (Master’s Thesis). Universidade Federal Rural Do Rio de Janeiro, Instituto de Biología. Rio de Janeiro, Brasil.
Albieri, R. J., Araújo, F. G., & Ribeiro, T. P. (2010). Gonadal development and spawning season of white mullet Mugil curema (Mugilidae) in a tropical bay. Journal of Applied Ichthyology, 26, 105–109. https://doi.org/10.1111/j.1439-0426.2009.01369.x
Albieri, R. J., Araújo, F. G., & Uehara, W. (2010). Differences in reproductive strategies between two co–occurring mullets Mugil curema Valenciennes 1836 and Mugil liza Valenciennes 1836 (Mugilidae) in a tropical bay. Tropical Zoology, 23, 51–62.
Álvarez-Lajonchère, L. S. (1976). Contribución al ciclo de vida de Mugil curema Valenciennes in Curvier et Valenciennes, 1836 (Pisces: Mugilidae). Revista Investigaciones Marinas, 28, 1–130.
Araya, M., & Cubillos, L A. (2006). Evidence of two-phase growth in elasmobranchs. Environmental Biology of Fishes, 77, 293–300. https://doi.org/10.1007/s10641-006-9110-8
Barnham, C., & Baxter, A. (1998). Condition factor, K, for salmonid fish. Fisheries Notes, 3.
Beamish, R. J., & Fournier, D. A. (1981). A method for comparing the precision of a set of age determinations. Canadian Journal of Fisheries and Aquatic Science, 38, 982–983. https://doi.org/10.1139/f81-132
Begg, G. A., Hare, J. A., & Sheehan, D. D. (1999). The role of life history parameters as indicators of stock structure. Fisheries Research, 43, 141–163. https://doi.org/10.1016/S0165-7836(99)00071-5
Begg, G. A., & Waldman, J. R. (1999). An holistic approach to fish stock identification. Fisheries Research, 43, 35–44. https://doi.org/10.1016/S0165-7836(99)00065-X
Blaber, S. J. M. (1997). Fish and fisheries of tropical estuaries. Chapman & Hall, London, United Kingdom.
Bond, C. E. (1979). Biology of fishes. Philadelphia: Saunders College Publishing.
Botha, L. (1971). Growth and otolith morphology of the Cape hakes Merluccius capensis, Cast. y M. paradoux Franca (Master’s Thesis). University of Stellenbosch, Stellenbosch, South Africa.
Bureau, D. P., Kaushik, S. J., & Young–Cho, C. (2002). Bioenergetics. In J. E. Halver, & R. W. Hardy (Eds.), Fish nutrition (pp. 1–59). Amsterdam: Academic Press.
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: a practical information-theoretic approach. Springer Science and Business Media, New York, United States of America.
Cabral-Solís, E. G. (1999). Estudio sobre el crecimiento y aspectos reproductivos de la lebrancha Mugil curema Cuvier y Valenciennes, 1836, en la laguna de Cuyutlán, Colima (Master’s Thesis). Universidad de Colima. Colima, México.
Cabral-Solís, E. G., Espino-Barr, E., Gallardo-Cabello, M., & Ibáñez-Aguirre, A. L. (2007). Fishing impact on Mugil curema stock of multi-species gill net fishery in a tropical lagoon, Colima, Mexico. Journal of Fisheries and Aquatic Science, 2, 235–242. https://doi.org/10.3923/jfas.2007.235.242
Cabral-Solís, E. G., Gallardo-Cabello, M., Espino-Barr, E., & Ibáñez, A. L. (2010). Reproduction of Mugil curema (Pisces: Mugilidae) from the Cuyutlán lagoon, in the Pacific coast of México. Avances en Investigación Agropecuaria, 14, 3–18.
Cadrin, S. X., Friedland, K. D., & Waldman, J. R. (2005). Stock identification methods an overview. In S. X. Cadrin, K. D. Friedland, & J. R. Waldman (Eds.), Amsterdam stock identification methods applications in fishery science (pp. 3–8). London: Elsevier Academic Press.
Campana, S. E., Annand, M. C., & McMillan, J. I. (1995). Graphical and statistical methods for determining the consistency of age determinations. Transactions of the American Fisheries Society, 124, 131–138. https://doi.org/10.1577/1548-8659(1995)124<0131:GASMFD>2.3.CO;2
Campana, S. E., & Casselman, J. M. (1993). Stock discrimination using otolith shape analysis. Canadian Journal of Fisheries and Aquatic Sciences, 50, 1062–1083. https://doi.org/10.
1139/f93-123
Campana, S. E., & Thorrold, S. R. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Science, 58, 30–38. https://doi.org/10.1139/f00-177
Castro-Aguirre, J. L. (1978). Catálogo sistemático de los peces marinos que penetran a las aguas continentales de México con aspectos zoogeográficos y ecológicos. Instituto Nacional de Pesca. Serie Científica. No. 19. México
Chandrasekhara, R. A., & Krishnan, L. (2011). Biochemical composition and changes in biological indices associated with maturation of the ovary in the spiny cheek grouper Epinephelus diacanthus (Valenciennes, 1828). Indian Journal of Fisheries, 58, 45–52.
Chang, W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Canadian Journal of Fisheries and Aquatic Science, 39, 1208–1210. https://doi.org/10.1139/f82-158
Chellappa, S., Lima, J. T., Araújo, A., & Chellappa, N. T. (2010). Ovarian development and spawning of Serra Spanish mackerel in coastal waters of Northeastern Brazil. Brazilian Journal of Biology, 70, 451–456.
De La Cruz-Agüero, J., & García-Rodríguez, F. J. (2004). Morphometric stock structure of the Pacific sardine Sardinops sagax (Jenyns, 1842) off Baja California, Mexico. In A. M. T. Elewa (Eds.), Morphometrics applications in biology and paleontology (pp. 115–127). New York: Springer.
DeVries, D. A., Grimes, C. B., & Prager, M. H. (2002). Using otolith shape analysis to distinguish eastern Gulf of Mexico and Atlantic Ocean stocks of king mackerel. Fisheries Research, 57, 51–62. https://doi.org/10.1016/S0165-7836(01)00332-0
Diario Oficial de la Federación (DOF). (1995). Norma Oficial Mexicana NOM-016-PESC-1994 que regula la pesca de lisa y liseta o lebrancha en aguas de jurisdicción federal del Océano Pacífico, incluyendo el Golfo de California, Golfo de México y Mar Caribe. Diario Oficial de la Federación, 24 de abril de 1995.
Diario Oficial de la Federación (DOF). (2006). Carta Nacional Pesquera. Diario Oficial de la Federación. Segunda Sección, 25 de agosto de 2006.
Ditty, J. G., & Shaw, R. F. (1996). Spatial and temporal distribution of larval Striped Mullet (Mugil cephalus) and White Mullet (M. curema, Family: Mugilidae) in the northern Gulf of Mexico, with notes on Mountain Mullet, Agnostomus monticola. Bulletin of Marine Science, 59, 271 – 288.
Espino-Barr, E., Cabral-Solís, E. G., Gallardo-Cabello, M., & Ibáñez-Aguirre, A. L. (2005). Age determination of Mugil curema Valenciennes, 1836 (Pisces: Mugilidae) in the Cuyutlán Lagoon, Colima, México. International Journal of Zoological Research, 1, 21–25. https://doi.org/10.3923/ijzr.2005.21.25
Espino-Barr, E., Gallardo-Cabello, M., Cabral-Solís, E. G., Puente-Gómez, M., & García-Boa, A. (2013). Otoliths analysis of Mugil curema (Pisces: Mugilidae) in Cuyutlan Lagoon, Mexico. Avances de Investigación Agropecuaria 17, 35–64.
Estrada-Godínez, J. A., Maldonado-García, M., Gracia-López, V., Carrillo, M., Rebollar-Prudente, R., & Spanopoulos-Zarco, M. (2014). Efecto del fotoperiodo y la temperatura sobre la composición bioquímica en reproductores silvestres de cabrilla sardinera, Mycteroperca rosacea (Streets, 1877). Latin American Journal of Aquatic Research, 42, 85–96. http://dx.doi.org/103856/vol42-issue1-fulltext-6
Félix-Uraga, R., Gómez-Muñoz, V. M., Quiñonez-Velázquez C., Melo-Barrera, F. N., Hill, K. T., & García-Franco, W. (2005). Pacific sardine (Sardinops sagax) stock discrimination off the west coast of Baja California and southern California using otolith morphometry. CalCOFI Reports, 46, 113–121.
Fernández, W. S., & Días, J. F. (2013). Aspects of the reproduction of Mugil curema Valenciennes, 1836 in two coastal systems in the southeastern Brazil. Tropical Zoology, 26, 15–32. https://doi.org/10.1080/03946975.2013.775052
Fernández-Palacios, H., & Izquierdo, M. S. (2009). Efecto de la dieta de los reproductores sobre la puesta. In M. Carrillo (Eds.), La reproducción en los peces: aspectos básicos y su aplicación en la acuicultura (pp. 337–380). Madrid: Fundación OESA.
Gallardo-Cabello, M., Cabral-Solís, E., Espino-Barr, E., & Ibáñez-Aguirre, A. L. (2005). Growth analysis of white mullet Mugil curema (Valenciennes, 1836) (Pisces: Mugilidae) in the Cuyutlán Lagoon, Colima, México. Hidrobiológica, 15, 321–325.
Gallardo-Cabello, M., Espino-Barr, E., García-Boa, A., Cabral-Solís, E. G., & Puente-Gómez, M. (2006). Morphologic and morphometric analysis and growth rings identification of otoliths: sagitta, asteriscus and lapillus of Caranx caballus (Pisces: Carangidae) in the coast of Colima, Mexico. International Journal of Zoological Research, 2, 34–47. https://doi.org/10.3923/ijzr.2006.34.47
Gallardo-Cabello, M., Espino-Barr, E., Nava-Ortega, R. A., García-Boa, A., Cabral-Solís, E. G., & Puente-Gómez, M. (2011). Analysis of the otoliths of sagitta, asteriscus and lapillus of Pacific sierra Scomberomorus sierra (Pisces: Scombridae) in the coast of Colima, Mexico. Journal of Fisheries and Aquatic Science, 6, 390–403. https://doi.org/10.3923/jfas.2011.390.403
García-Contreras, O. E., Quiñonez-Velázquez, C., Morán-Angulo, R. E., & Valdez-Pineda, M. C. (2009). Age, Growth, and Age Structure of Amarillo Snapper off the Coast of Mazatlán, Sinaloa, Mexico. North American Journal of Fisheries Management, 29, 223–230. https://doi.org/10.1577/M06-025.1
García-Rodríguez, F. J., García-Gasca, S. A., De La Cruz-Agüero, J., & Cota-Gómez, V. M. (2011). A study of the population structure of the Pacific sardine Sardinops sagax (Jenyns, 1842) in Mexico based on morphometric and genetic analyses. Fisheries Research, 107, 169–176. https://doi.org/10.1016/j.fishres.2010.11.002
Gherard, K. E., Erisman, B. E., Aburto-Oropeza, O., Rowell, K., & Allen, L. G. (2013). Growth, Development, and Reproduction in Gulf Corvina (Cynoscion othonopterus). Bulletin of the Southern California Academy of Sciences, 112, 1–18. https://doi.org/10.3160/0038-3872-112.1.1
Gómez-Ortiz, G., González-Cruz, A., & Hernández-Tabares, I. (2006). Lisa y lebrancha en el Golfo de México y Mar Caribe. In F. Arreguín-Sánchez, L. Beléndez-Moreno, I. M. Gómez-Humarán, R. Solana-Sensores, & C. Rangel-Dávalos (Eds.), Sustentabilidad y pesca responsable en México: evaluación y manejo (pp. 477–502). México D.F.: Instituto Nacional de la Pesca/Sagarpa.
González-Castro, M., Díaz de Astarloa, J. M., & Cousseau, M. B. (2006). First record of a tropical affinity mullet, Mugil curema (Mugilidae), in a temperate southwestern Atlantic coastal lagoon. Cybium 30, 90–91.
Gould, S. J. (1966). Allometry and size in ontogeny and phylogeny. Biological Reviews, 41, 587–638.
Griffiths, M. H. (1997). The life history and stock separation of silver kob, Argyrosomus inodorus, in South African waters. Fishery Bulletin, 95, 47–67.
Harrison, I. J. (1995). Mugilidae. Lisas. In W. Fischer, F. Krupp, W. Schneider, C. Sommer, K. E. Carpenter, & V. Niem (Eds.), Guía FAO para identificación de especies para los fines de la pesca. Pacífico Centro Oriental (pp. 1293–1298). Rome: FAO.
Houde, E. D., Berkeley, S. A., Klinovsky, J. J., & Schekter, R. C. (1976). Culture of larvae of the white mullet, Mugil curema Valenciennes. Aquaculture 8, 365–370. https://doi.org/10.1016/0044-8486(76)90118-6
Ibáñez-Aguirre, A. L. (2015). Geographic differences and annual stability in length-weight relationships of fish mullets (Pisces: Mugilidae). Hidrobiológica, 25, 146–150.
Ibáñez-Aguirre, A. L., Cabral-Solís, E., Gallardo-Cabello, M., & Espino-Barr, E. (2006). Comparative morphometrics of two populations of Mugil curema (Pisces: Mugilidae) on the Atlantic and Mexican Pacific coasts. Scientia Marina, 70, 139–145. http://dx.doi.org/10.3989/scimar.2006.70n1139
Ibáñez-Aguirre, A. L., & Colín, A. (2014). Reproductive biology of Mugil curema and Mugil cephalus from western Gulf of Mexico Waters. Bulletin of Marine Science, 90, 941–952. https://doi.org/10.5343/bms.2014.1004
Ibáñez-Aguirre, A. L., Chang, C. W., Hsu, C. C., Wang, C. H., Iizuka, Y., & Tzeng, W. N. (2012). Diversity of migratory environmental history of the mullets Mugil cephalus and M. curema in Mexican coastal waters as indicated by otolith Sr:Ca ratios. Ciencias Marinas, 38, 73–87. https://doi.org/10.7773/cm.v38i1A.1905
Ibáñez-Aguirre, A. L., & Gallardo-Cabello, M. (1996). Age determination of the grey mullet Mugil cephalus L. and the white mullet M. curema V. (Pisces: Mugilidae) in Tamiahua lagoon, Veracruz. Ciencias Marinas, 22, 329–345. https://doi.org/10.7773/cm.v22i3.861
Ibáñez-Aguirre, A. L., & Gallardo-Cabello, M. (2004). Reproduction of Mugil cephalus and M. curema (Pisces: Mugilidae) from coastal lagoon in the Gulf of Mexico. Bulletin of Marine Science, 75, 37–49.
Ibáñez-Aguirre, A. L., & Gallardo-Cabello, M. (2005). Identification of two Mugilidae species, Mugil cephalus and M. curema (Pisces: Mugilidae), using the ctenii of their scales. Bulletin of Marine Science, 77, 305–307.
Ibáñez-Aguirre, A. L., Gallardo-Cabello, M., & Chiappa-Carrara, X. (1999). Growth analysis of striped mullet, Mugil cephalus, and White mullet, M. curema (Pisces: Mugilidae) in the Gulf of Mexico. Fishery Bulletin, 97, 861–872.
Kanak, M. K., & Tachihara, K. (2008). Reproductive biology of common silver biddy Gerres oyena in Okinawa Island of southern Japan. Fisheries Science, 74, 265–275. https://doi.org/10.1111/j.1444-2906.2008.01521.x
Katsanevakis, S. (2006). Modelling fish growth: model selection, multi-model inference and model selection uncertainty. Fisheries Research, 81, 229–235. https://doi.org/10.1016/j.fishres.2006.07.002
Kendall, D. (1977). The diffusion of shape. Advances in Applied Probability, 9, 428–430. https://doi.org/10.2307/1426091
Kimura, M. (1980). A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. Journal of Molecular Evolution, 16, 111–120. https://doi.org/10.1007/BF01731581
Klingenberg, C. P. (1998). Heterochrony and allometry: the analysis of evolutionary change in ontogeny. Biological Reviews of the Cambridge Philosophical Society, 73, 79–123. https://doi.org/10.1111/j.1469-185X.1997.tb00026.x
Lagler, K. F., Bardach, J. E., Miller, R. R., & May-Passino, D. R. (1977). Ichthyology. México D.F.: AGT Editor. S. A.
Lai, H. L., & Liu, H. C. (1979). Age determination and growth of red snapper (Lutjanus sanguinius) in the North Australian waters. Acta Oceanographica Taiwanica, 10, 160–170.
Lowerre-Barbieri, S. K., Brown-Peterson, N. J., Murua, H., Tomkiewicz, J., Wyanski, D. M., & Saborido-Rey, F. (2011). Emerging issues and methodological advances in fisheries reproductive biology. Marine and Coastal Fisheries, 3, 32–51. https://doi.org/10.1080/19425120.2011.555725
Lucano-Ramírez, G., & Michel-Mortín, J. E. (1997). Ciclo reproductivo y aspectos poblacionales de Mugil curema Valenciennes, 1836 (Pisces: Mugilidae) en la laguna costera Agua Dulce, Jalisco, México. Oceanología, 1, 105–115.
Lucano-Ramírez, G., Ruiz-Ramírez, S., González-Sansón, G., & Ceballos-Vázquez, B. P. (2014). Biología reproductiva del pargo alazán, Lutjanus argentiventris (Pisces, Lutjanidae), en el Pacífico central mexicano. Ciencias Marinas, 40, 33–44. https://doi.org/10.7773/cm.v40i1.2325
Marín-Espinoza, B. J. (1996). Transport et recrutement du muge argenté, Mugil curema, dans une lagune côtière tropicale (PhD. Thesis). Laval University, Quebec, Canada.
Marín-Espinoza, E. B. J., Quintero, A., Bussière, D., & Dodson, J. J. (2003). Reproduction and recruitment of white mullet (Mugil curema) to a tropical lagoon (Margarita Island, Venezuela) as revealed by otolith microstructure. Fishery Bulletin, 101, 809–821.
Martínez-Flores, G., Cervantes-Duarte, R., & González-Rodríguez, E. (2006). Caracterización de la temperatura superficial del mar y climatología de la Bahía de La Paz, B.C.S., México. CICIMAR Oceánides, 21, 81–91.
McDonough, C. J., Roumillat, W. A., & Wenner, C. A. (2005). Sexual differentiation and gonad development in striped mullet (Mugil cephalus L.) from South Carolina estuaries. Fishery Bulletin, 103, 601–619.
Meléndez-Galicia, C., & Romero-Acosta, A. C. (2010). Evaluación biológico-pesquera de la lisa Mugil curema, en la costa de Michoacán. Ciencia Pesquera, 18, 67–77.
Miner, B. G., Sultan, S. E., Morgan, S. G., Padilla, D. K., & Relyea, R. A. (2005). Ecological consequences of phenotypic plasticity. Trends in Ecology & Evolution, 20, 685–692. https://doi.org/10.1016/j.tree.2005.08.002
Moore, R. H. (1974). General ecology, distribution and relative abundance of Mugil cephalus and Mugil curema on the South Texas coast. Contributions to Marine Science, 18, 241–255.
Morison, A. K, Robertson, S. G., & Smith, D. C. (1998). An integrated system for production fish aging: image analysis and quality assurance. North American Journal of Fisheries Management, 18, 587–598. https://doi.org/10.1577/1548-8675(1998)018<0587:AISFPF>2.0.CO;2
Nikolsky, G. V. (1963). The ecology of fishes. New York: Academic Press.
Obeso-Nieblas, M. (1987). Propagación de la constituyente M2 de la marea en la Bahía de La Paz, B.C.S., México, mediante un modelo hidrodinámico numérico (Master’s Thesis). Centro Interdisciplinario de Ciencias Marinas, IPN, La Paz, BCS., México.
Obeso-Nieblas, M. (2003). Variabilidad espaciotemporal de las condiciones oceanográficas de la Bahía de La Paz, BCS, México (PhD. Thesis). Centro Interdisciplinario de Ciencias Marinas, IPN, La Paz, BCS, México.
Oliveira, M. R., Costa, E. F., & Chellappa, S. (2011). Ovarian development and reproductive period of White mullet, Mugil curema in the coastal waters of Northeastern Brazil. Animal Biology Journal, 2, 199–212.
Oliveira, M. F., Costa, E. F. C., Freire, F. A. M., Oliveira, J. E. L., & Luchiari, A. C. (2011). Some aspects of the biology of white mullet, Mugil curema (Osteichthyes, Mugilidae), in the northeastern region, Brazil. Pan-American Journal of Aquatic Science, 6, 138–147.
Ould-Mohamed Vall, M. (2004). Etude de la dynamique des systèmes d’exploitation et de l’écobiologie de la reproduction de trois Mugilidae: Mugil cephalus (Linnaeus, 1758), Liza aurata (Perugia, 1892) et Mugil capurrii (Risso, 1810), analyse de leurs stratégies d’occupations des secteurs littoraux mauritaniens et de leurs possibilités d’aménagement (PhD. Thesis). University Nice-Sophia Antipolis, France.
Pannella, G. (1974). Otolith growth patterns: an aid in age determination in temperate and tropical fishes. In T. B. Bagenal (Ed.), Ageing of fish (pp. 28–39). Unwin Brothers Ltd. Surrey.
Pérez-Olivas, A. (2016). Biología reproductiva de la cabrilla sardinera (Mycteroperca rosacea, Streets 1877) en la zona costera de Santa Rosalía, BCS, México (Masters Thesis). Centro Interdisciplinario de Ciencias Marinas, IPN, La Paz, BCS, México.
Pérez-Quiñonez, C. I., Quiñonez-Velázquez, C., & Rodríguez, F. J. (2018). Detecting Opisthonema libertate (Günther 1867) phenotypic stocks in northwestern coast of Mexico using geometric morphometrics based on body and otolith shape. Latin American Journal of Aquatic Research, 46, 779–790. https://doi.org/10.3856/vol46-issue4-fulltext-15
Pérez-Quiñonez, C. I., Quiñonez-Velázquez, C., Ramírez-Pérez, J. S., Vergara-Solana, F. J., & Rodríguez, F. J. (2017). Combining geometric morphometrics and genetic analysis to identify species of Opisthonema Gill, 1861 in the eastern Mexican Pacific. Journal of Ichthyology, 33, 84–921. https://doi.org/10.1111/jai.13051
Perotto, D., Cue, R. I., & Lee, A. J. (1992). Comparison of nonlinear functions for describing the growth curve of three genotypes of dairy cattle. Canadian Journal of Animal Science, 72, 773–782. https://doi.org/10.4141/cjas92-089
Polacheck, T., Hilborn, R., & Punt, A. E. (1993). Fitting surplus production models: comparing methods and measuring uncertainty. Canadian Journal of Fisheries and Aquatic Science, 50, 2597–2607. https://doi.org/10.1139/f93-284
Polanco, J. S., Mimbela, S., & Beléndez, M. (1987). Pesquerías mexicanas: estrategias para su administración (pp. 943–1007). México D.F.: Secretaría de Pesca.
Ponton, D. (2006). Is geometric morphometric efficient for comparing otolith shape of different fish species? Journal of Morphology, 267, 750–757. https://doi.org/10.1002/jmor.10439
Quiñonez-Velázquez, C., & López-Olmos, J. R. (2011). Juvenile growth of white mullet Mugil curema (Teleostei: Mugilidae) in a coastal lagoon southwest of the Gulf of California. Latin American Journal of Aquatic. Research, 39, 25–32. https://doi.org/10.3856/vol39-issue1-fulltext-3
Quiñonez-Velázquez, C., López-Olmos, J. R., & Pérez-Quiñonez, C. I. (2015). Survival of juvenile mullet Mugil curema (Mugilidae) in a coastal lagoon. CICIMAR Oceánides, 30, 21–32.
Quiñonez-Velázquez, C., & Mendoza-Guevara, J. A. (2009). Abundancia relativa, estructura de tallas y relación longitud-peso de juveniles de lisa Mugil curema en el estero El Conchalito, La Paz, BCS, México. Ciencia Pesquera, 17, 37–46.
Ramírez-Pérez, J. S., Quiñonez-Velázquez, C., García-Rodríguez, F. J., Félix-Uraga, R., & Melo-Barrera, F. N. (2010). Using the shape of Sagitta Otoliths in the discrimination of phenotypic stocks of Scomberomorus sierra (Jordan and Starks, 1895). Journal of Fisheries and Aquatic Science, 5, 82–93. https://doi.org/10.3923/jfas.2010.82.93
Ramos-Cruz, M. S. (1985). Aspectos biológicos y determinación de algunos parámetros poblacionales de la lebrancha Mugil curema Valenciennes, en las costas de los estados de Oaxaca y Chiapas, México (Thesis). Escuela Superior de Ecología Marina. Universidad Autónoma de Guerrero, Guerrero, México.
Reyes-Salinas, A. R., Cervantes-Duarte, R., Morales-Pérez, A., & Valdez-Holguín, J. E. (2003). Variabilidad estacional de la productividad primaria y su relación con la estratificación vertical en la Bahía de la Paz, B. C. S. Hidrobiológica, 13, 103–110.
Robins, C. R., Bailey, R. M., Bond, C. E., Brooker, J. R., Lachne, E. A., Lea, R. N., & Scott W. B. (1991). Common and scientific names of fishes from the United States and Canada. American Fisheries Society Spec. Pub. 20.
Rocha-Olivares, A., & Gómez-Muñoz, V. M. (1993). Validación del uso de otolitos para determinar la edad del huachinango del Pacífico Lutjanus peru (Perciformes: Lutjanidae), en la Bahía de La Paz y aguas adyacentes, B.C.S., México. Ciencias Marinas, 19, 321–331.
Rodríguez-Gutiérrez, M. (1992). Técnicas de evaluación cuantitativas de la madurez gonádica en peces. México D.F.: AGT Editor.
Rohlf, F. J. (2004). TpsDIG Version 1.40. Department of Ecology and Evolution, State University of New York at Stony Brook, New York.
Ruiz-Domínguez, M., & Quiñonez-Velázquez, C. (2018). Age, growth, and mortality of Opisthonema libertate on the coasts of northwestern Mexico. Ciencias Marinas, 44, 235–250. https://doi.org/10.7773/cm.v44i4.2908
Ruiz-Ramírez, S., Molina-Arenas, E. G., Lucano-Ramírez, G., Aguilar-Betancourt, C., Flores-Ortega, J. R., Kosonoy-Aceves, D., & González-Sansón, G. (2017). Aspectos reproductivos de la lisa Mugil curema (Mugiliformes: Mugilidae) en la laguna costera de Barra de Navidad, Jalisco, México. Latin American Journal of Aquatic Research, 45, 443–456. http://dx.doi.org/10.3856/vol45-issue2-fulltext-19
Sánchez-Cárdenas, R., Ceballos-Vázquez, B. P., Arellano-Martínez, M., Valdez-Pineda, M. C., & Morán-Angulo, R. E. (2007). Reproductive aspects of Sphoeroides annulatus (Jenyns, 1842) (Tetraodontiformes, Tetraodontidae) inhabiting the Mazatlan coast, Sinaloa, Mexico. Revista de Biología Marina y Oceanografía, 42, 385–392. http://dx.doi.org/10.4067/S0718-19572007000300018
Sheets, H. D. (2004). IMP: Integrated Morphometrics Package. Canisius College, Buffalo. Retrieved on June 5, 2019: http://www3.canisius.edu/~sheets/morphsoft.html
Silva, A. (2003). Morphometric variation among sardine (Sardina pilchardus) populations from the northeastern Atlantic and the western Mediterranean. ICES Journal of Marine Science, 60, 1352–1360. https://doi.org/10.1016/S1054-3139(03)00141-3
Sinclair, M., & Iles, T. D. (1988). Population richness of marine fish species. Aquatic Living Resources, 1, 71–83.
StatSoft, Inc. (1995). STATISTICA (data analysis software system), version 7.0. www.statsoft.com.
Stransky, C., Murta, A. G., & Zimmermann, C. (2008). Otolith shape analysis as a tool for stock separation of horse mackerel (Trachurus trachurus) in the Northeast Atlantic and Mediterranean. Fisheries Research, 89, 159–166. https://doi.org/10.1016/j.fishres.2007.09.017
Trape, S., Durand, J. D., Guilhaumon, F., Vigliola, L., & Panfili, J. (2009). Recruitment patterns of young-of-the-year mugilid fishes in a West African estuary impacted by climate change. Estuarine Coastal and Shelf Science, 85, 357–367. https://doi.org/10.1016/j.ecss.2009.08.018
Tzeng, T. D. (2004). Morphological variation between populations of spotted mackerel (Scomber australasicus) off Taiwan. Fisheries Research, 68, 45–55. https://doi.org/10.1016/j.fishres.2004.02.011
Vasconcelos, J., Sánchez, S., & Schultz, L. (1996). La pesquería de lisa. In A. Sánchez-Palafox, D. F. Fuentes-Castellanos, & S. García-Real (Ed.), Pesquerías relevantes de México (pp. 581–594). XXX Aniversario del INP. Ciudad de México: IPN.
Vazzoler, A. E. A. M. (1996). Biología e reproducao de peixes teleósteos: teoria e practica. Sao Paulo: Ed. EDUEN.
Vergara-Solana, F. J., García-Rodríguez, F. J., & De la Cruz-Agüero, J. (2013). Comparing body and otolith shape for stock discrimination of Pacific sardine, Sardinops sagax Jenyns, 1842. Journal of Applied Ichthyology, 29, 1241–1246. https://doi.org/10.1111/jai.12300
Volpato, G. L., & Trajano E. (2005). Biological rhythms. In L. A. Val, V. M. F. A. Val, & D. J. Randal (Eds.), Fish physiology (pp. 101–153). San Diego: Elsevier.
Winberger, P. H. (1992). Plasticity of fish body shape. The effects of diet, development, family and age in two species of Geophagus (Cichlidae). Biological Journal of the Linnean Society, 45, 197–218. https://doi.org/10.1111/j.1095-8312.1992.tb00640.x
Yáñez-Arancibia, A. (1976). Observaciones sobre Mugil curema Valenciennes en áreas naturales de crianza, México. Alimentación, crecimiento, madurez y relaciones ecológicas. Anales del Centro de Ciencias del Mar y Limnología, 3, 92–124.
Zar, J. H. (2010). Biostatistical analysis. Fifth edition. Nueva Jersey: Pearson Prentice Hall.
Zelditch, M. L., Swiderski, D. L., Sheets, H. D., & Fink, W. L. (2004). Geometric morphometrics for biologists: a primer. London: Elsevier Academic Press.