Rüdiger Riesch a, *, Amber M. Makowicz b, c, Brandon Joachim c, Francisco J. García-De León d, Ingo Schlupp c
a Department of Biological Sciences, Royal Holloway University of London, Egham Hill, Egham, TW20 0EX, United Kingdom
b Department of Biological Sciences, Florida State University, 319 Stadium Dr., Tallahassee, Florida 32306, USA
c Department of Biology, University of Oklahoma, 730 Van Vleet Oval, Norman, Oklahoma 73019, USA
d Laboratorio de Genética para la Conservación, Centro de Investigaciones Biológicas del Noroeste, Instituto Politécnico Nacional, 195,
Playa Palo de Santa Rita Sur, 23096 La Paz, Baja California Sur, Mexico
*Corresponding author: rudiger.riesch@rhul.ac.uk (R. Riesch)
Received: 17 June 2019; accepted: 20 May 2020
Abstract
In the face of human-induced environmental change, basic biological data for species threatened by the impacts of human activities are sorely needed to devise and execute proper conservation strategies. Here we provide aspects of basic life-history data for 2 populations of the critically endangered Tamesí molly Poecilia latipunctata (Poeciliidae), which is native to the headwaters of the Río Tamesí in northeastern Mexico. Furthermore, we compare their life history to that of 2 syntopic species, the Atlantic molly (Poecilia mexicana) and the Amazon molly (Poecilia formosa). Life histories of P. latipunctata are largely similar to that of other mollies: male size was normally distributed in both populations, and females produced 1 clutch at a time, while relying predominantly on yolk for embryo provisioning. However, at our 2 sample sites, P. latipunctata males were smaller than P. mexicana males, and P. latipunctata females produced significantly larger offspring than either P. formosa or P. mexicana. Based on patterns of male and offspring size, we cautiously suggest that, besides anthropogenic disturbance, P. latipunctata might also be suffering from strong interspecific competition, and we call on more research into the basic biology of this species (including its competitive ability).
Keywords: Competition; Endangered species; Poecilia formosa; Poecilia mexicana; Poeciliidae
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Aspectos de la historia de vida del topote del Tamesí, Poecilia latipunctata, en dos poblaciones de la cuenca de río Tamesí del noreste de México
Resumen
Frente al cambio ambiental inducido por el hombre, se requieren datos biológicos básicos para las especies amenazadas por los impactos de las actividades humanas para diseñar y ejecutar estrategias adecuadas de conservación. Aquí reportamos datos básicos de algunos aspectos de la historia de vida de 2 poblaciones del topote del Tamesí Poecilia latipunctata (Poeciliidae), nativa de los manantiales del río Tamesí en el noreste de México y catalogada en peligro crítico. Además, comparamos su historia de vida con la de 2 especies sintópicas, el topote del Atlántico (Poecilia mexicana) y el topote del Amazonas (Poecilia formosa). Las historias de vida de P. latipunctata son, en gran parte, similares a las de otros topotes: el tamaño del macho se distribuyó normalmente en ambas poblaciones y las hembras produjeron huevos de forma discreta, predominantemente con vitelo para el aprovisionamiento de embriones. Sin embargo, en nuestros 2 sitios de muestreo, los machos de P. latipunctata fueron más pequeños que los machos de P. mexicana y las hembras de P. latipunctata produjeron crías significativamente más grandes que P. formosa o P. mexicana. Basándonos en los patrones de tamaño de los machos y de los descendientes, sugerimos que, además de la perturbación antropogénica, P. latipunctata también esté sufriendo una fuerte competencia interespecífica, por lo que recomendamos más investigaciones sobre la biología básica de esta especie (incluida su capacidad competitiva).
Palabras clave: Competencia; Especies amenazadas; Poecilia formosa; Poecilia mexicana; Poeciliidae
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
It is well-established that human activities are having a negative, sometimes catastrophic, impact on biodiversity and ecosystem functioning (Barnosky et al., 2011; Pimm et al., 2014). Aquatic ecosystems, for example, provide important ecosystem services that are closely tied to human well-being (Constanza et al., 2014; Geist, 2011), but are also exposed to a variety of human-induced changes, including the impacts of invasive species (Gozlan et al., 2010), pollution (Conkle et al., 2018) or ocean acidification (Sunday et al., 2017). This is having a drastic impact on aquatic biodiversity (Janse et al., 2015). However, in order to devise and employ proper conservation and species management plans, we first need to have a good understanding of the basic biology of the focal species, which, unfortunately, is often lacking (Stroud & Thompson, 2019).
While there are many different biological traits that can be investigated to gain a better understanding of the basic biology of a species under threat of extinction, life histories are of particular relevance in this regard (Stearns, 1992). For example, they tell us how organisms invest their acquired resources into growth, maintenance, and reproduction, but the specific life-history strategies employed by a species can also help us understand how they might interact with other syntopic species (Stearns, 1992). This makes life-history traits ideal for advancing our species-specific biological knowledge, because they help provide essential demographic data relating to fertility and population growth. This type of data can, for example, be used to gauge a species’ competitive ability
(Bashey, 2008).
The Tamesí molly, Poecilia latipunctata, is a small livebearing fish of the family Poeciliidae, is critically endangered, and native to headwaters of the Río Tamesí in northeastern Mexico (Contreras-Balderas & Almada-Villela, 1996; Tobler & Schlupp, 2009). The species has a relatively small range, which makes P. latipunctata especially vulnerable to habitat changes (Miller et al., 2005). The most important threats to the survival of this species stem from invasive species as well as fragmentation and deterioration of suitable habitat as a result of local agricultural practices (Tobler & Schlupp, 2009). Biologically, this species is of special interest for at least 3 reasons. First, despite the fact that it looks like a short-fin molly (Fig. 1A-E), it actually belongs to the long-fin mollies (Ptacek & Breden, 1998; Schartl et al, 1995). Second, it is sympatric with 2 other mollies, the Atlantic molly (Poecilia mexicana) and the Amazon molly (Poecilia formosa), and has been reported to be one of the few natural sperm donors for P. formosa (Niemeitz et al., 2002), an all-female hybrid species that reproduces via gynogenesis (i.e., sperm-dependent parthenogenesis; Schlupp & Riesch, 2011). This role is critical as P. latipunctata is a sperm donor species that was not involved in the hybridization event that led to Amazon mollies, and the mating system in the area of Ciudad Mante is one of very few described to have more than 1 sperm donor species (Joachim & Schlupp, 2012; Schlupp, 2009). Third, it is also invasive in at least 1 habitat outside its natural range. While it seems that an introduction into Florida, USA, has failed (there are no confirmed observations since 1971; Neilson, 2019), it has established a thriving population in La Media Luna, San Luis Potosí, México (Miller et al., 2005; Palacio-Núñez et al., 2015).

Nonetheless, very little is currently known about the basic biology of P. latipunctata, and how it might interact with syntopic P. formosa and P. mexicana. Here, we therefore report on basic life histories for 2 natural populations of P. latipunctata. Moreover, we compare aspects of male and female life histories of P. latipunctata to those of syntopic P. mexicana and P. formosa. With regards to the latter, we address 2 different questions: 1) What are some of the life-history differences between the 3 species? 2) Are these life-history differences similar or different between the 2 different
population?
Material and methods
We collected fishes from 2 different localities in northern México, near Ciudad Mante in May 2010, and preserved all specimens in 10% formaldehyde under the guidelines granted in the collection permit from the Mexican government (SEMARNAT=SGPA/DGVS/02221/10). Due to the endangered species status of P. latipunctata we were only able to collect a small number of fishes. Furthermore, P. latipunctata has a small geographic range with low abundance and thus, the number of sites we could use for comparison was limited in our study (García-De León et al., 2018; Tobler & Schlupp, 2009). We therefore focused on 2 sites: one was located in the canalized headwaters of the Río Mante (Mante Canal or MC hereonafter: 22°42’54.79” N, 99°1’18.83” W; Río Guayalejo/Pánuco drainage). This is a wide channel that takes water a short distance from the spring to provide water for agriculture. The second was a small ditch in between sugarcane fields within the same river drainage north of Ciudad Mante called El Limón (EL: 22°48’44.57” N, 99°0’44.55” W; Río Guayalejo/Pánuco drainage). At MC, we collected P. latipunctata and P. mexicana, while at EL, we collected all 3 species: P. latipunctata, P. mexicana, and P. formosa. Using a Hydrolab© Multisonde, we also measured several physicochemical characteristics: water temperature, pH, turbidity, oxygen content, salinity, chlorophyll and specific conductance. Our qualitative measurements included: the presence of a current, agriculture, predators, the type of substrate, contact vegetation, if the water level was managed, and the level of abundance of water plants (Table 1). All preserved specimens were then transferred to the University of Oklahoma in Norman, Oklahoma (USA) for further analyses.
To quantify selected life-history traits, fishes were dissected following well-established protocols (Riesch et al., 2015, 2016). In short, we first measured fish standard length (SL), and then removed testes for males and ovaries with (if present) developing embryos for females. Fishes and dissected reproductive tissues were then dried for 24 hrs at 55 ºC, after which they were weighed to establish dry weights. Soluble fats were extracted with a petroleum ether bath, then we weighed all fish and tissues again to establish lean weights. Thus, we quantified the following traits: male and female SL (mm), male and female dry and lean weights (g), male and female fat content (%; relative to dry weight), fecundity (number of fertilized and developing oocytes), offspring dry and lean weight (mg), and offspring fat content (%; relative to embryo dry weight). We further calculated the gonadosomatic index (GSI) for males [testis dry weight / (testis dry weight + somatic dry weight)] and reproductive allocation (RA) for females [total offspring dry weight / (total offspring dry weight + somatic dry weight)]. Finally, we evaluated the reproductive mode (i.e., if these poeciliids are capable of superfetation, which is the existence of more than a single brood at the same time; Pires et al., 2011).
Table 1
Descriptive statistics for single-point biotic and abiotic parameters collected at El Limón and Mante Canal at the same time as fish were caught for this study. The following variables were measured: quantitatively = water temperature (oC), specific conductivity (SpC), pH, dissolved oxygen (DO), chlorophyll a (Chloro), and salinity; qualitatively = the presence of a current; the presence of agriculture, the types of fish predators, the substrate type, the type of contact vegetation, if the water level is managed, and the abundance level of water plants.
| Biotic/abiotic variable | Field sites | |
| El Limón | Mante Canal | |
| Water temperature (oC) | 28.45 | 27.26 |
| SpC (mS/cm) | 1.479 | 1.008 |
| pH | 7.84 | 7.48 |
| DO (% Saturation) | 35.65 | 59.55 |
| DO (mg/l) | 2.60 | 4.77 |
| Chloro (ug/l) | 1.28 | 3.30 |
| Salinity (0/00) | 0.79 | 0.53 |
| Current present | Yes | Yes |
| Agriculture present | Yes | Yes |
| Fish predators | Mexican tetra | Cichlids |
| Substrate type | Sand | Sand, silt |
| Contact vegetation | Herbs, shrubs, trees | Herbs |
| Water level managed | Yes | Yes |
| Water plant abundance | Low | High |
For females, we further calculated the matrotrophy index [MI] to indirectly evaluate rates of post-fertilization maternal provisioning. The MI equals the estimated dry weight of the embryo at birth divided by the estimated dry weight of the oocyte at fertilization (Pollux et al., 2009). Thus, if the eggs were fully provisioned by yolk prior to fertilization (lecithotrophy), we would expect the embryos to lose 30-40% of their dry weight during development (MI ≤ 0.75). On the other hand, in the case of continuous maternal provisioning even after fertilization (matrotrophy), one would expect the embryos to lose less weight (MI between 0.75 and 1.00) or even to gain weight during development (MI ≥ 1.00; Pollux et al., 2009). Due to a low sample size, we did not calculate MI for P. mexicana females from MC nor for P. formosa from EL.
In preparation for additional subsequent analyses, we then log-transformed (SL and all lean weights), square-root transformed (fecundity) and arcsine-transformed (all fat contents, GSI and RA) all variables, and subsequently z-transformed them to meet model assumptions and to remove scaling effects.
For statistical analyses, we conducted 2 sex-specific univariate general linear models (ANOVA) on SL. For the male model, the factors were population (EL or MC) and species (P. latipunctata or P. mexicana). Since we did not have any life-history data for P. mexicana from MC, we did not include the species-by-population interaction effect. For the female model, we included the factors population (EL or MC), species (P. latipunctata, P. mexicana, and P. formosa), and the species-by-population interaction.
In a second step, we then ran 2 sex-specific multivariate general linear models (MANCOVA) on the remaining life histories. For the male model, we included the traits lean weight, fat content and GSI as dependent variables, species and population were again the factors, and SL now served as a covariate. We further included the interactions between SL and the 2 factors in the initial model but removed any non-significant interactions with p > 0.1 from the final model. For the female model, we included the traits lean weight, fat content, fecundity, offspring lean weight, offspring fat content, and RA as dependent variables, species and population were the factors, with SL and offspring stage of development now served as covariates. We further included all possible two-way interactions between the factors and covariates in the initial model, but again removed any non-significant interactions (p > 0.1) from the final model.
All tests were conducted in IBM® SPSS® Version 21 (IBM Corp. 2012). Assumptions of normality of residuals and homocedasticity were met for all models.
Results
Descriptive statistics for life histories of males and females can be found in table 2. Poecilia latipunctata and P. mexicana were characterized by a pronounced sexual size dimorphism, with males being smaller than females.
Males. Male size distribution in P. latipunctata from EL and MC and in P. mexicana from EL did not deviate from normal distributions (Shapiro-Wilk test, P. latipunctata, EL: W15 = 0.951, p = 0.536; MC: W14 = 0.892, p = 0.087; P. mexicana, EL: W10 = 0.968, p = 0.876; Fig. 2A-C).). Our ANOVA on male SL uncovered a significant species effect, because P. mexicana males were larger than P. latipunctata males (Tables 2, 3). Our multivariate analysis of male lean weight, fat content and GSI revealed a significant effect of the covariate SL, but the species —and population— factors were also significant (Table 3). Post-hoc ANCOVA revealed that SL had a significant effect on lean weight (F1,35 = 1663.629, p < 0.001) and GSI (F1,35 = 16.441, p < 0.001), which both increased with increasing SL. Significant species —and populations— factors were only uncovered for fat content (F1,35 = 13.191, p = 0.001 and F1,35 = 20.028, p < 0.001, respectively). At EL, P. mexicana had more body fat than P. latipunctata, and P. latipunctata from MC had more body fat than conspecifics from EL (Table 2). GSI further exhibited a non-significant trend between species (F1,35 = 4.061, p = 0.052), with P. latipunctata showing a trend for a lower investment into reproduction (estimated marginal means of GSI corrected for SL = 33.33 mm, P. latipunctata, EL: 0.83%; MC: 0.91%; P. mexicana, EL: 1.10%).
Table 2
Descriptive statistics (mean ± SD) for life-history traits of male and female P. formosa, P. latipunctata, and P. mexicana from El Limón (EL), and Mante Canal (MC) in northeastern Mexico. GSI: gonadosomatic index; RA: reproductive allocation.
| Species | Population | Sex | N | SL [mm] | Lean weight [g] | Fat content [%] | Fecundity | Embryo lean weight [mg] | Embryo fat content [%] | GSI/RA [%] |
| P. formosa | EL | ♀ | 5 | 46.40 ± 8.65 | 0.500 ± 0.255 | 2.26 ± 2.03 | 22.20 ± 14.31 | 3.20 ± 0.42 | 17.47 ± 2.61 | 14.37 ± 4.63 |
| P. latipunctata | EL | ♂ | 15 | 33.80 ± 5.56 | 0.187 ± 0.094 | 0.90 ± 1.34 | – | – | – | 0.85 ± 0.27 |
| ♀ | 11 | 38.09 ± 3.42 | 0.280 ± 0.087 | 1.37 ± 1.43 | 9.18 ± 2.27 | 5.00 ± 1.04 | 17.24 ± 2.00 | 16.51 ± 2.80 | ||
| MC | ♂ | 14 | 30.29 ± 5.93 | 0.145 ± 0.112 | 5.30 ± 3.23 | – | – | – | 1.10 ± 0.49 | |
| ♀ | 9 | 45.33 ± 2.45 | 0.445 ± 0.088 | 6.90 ± 6.26 | 21.89 ± 10.99 | 4.81 ± 0.93 | 17.42 ± 2.58 | 19.64 ± 6.76 | ||
| P. mexicana | EL | ♂ | 10 | 39.30 ± 5.62 | 0.299 ± 0.132 | 5.37 ± 4.62 | – | – | – | 0.92 ± 0.25 |
| ♀ | 15 | 45.07 ± 6.88 | 0.444 ± 0.213 | 3.23 ± 3.58 | 23.93 ± 15.73 | 3.10 ± 0.56 | 16.16 ± 5.87 | 15.18 ± 5.29 | ||
| MC | ♀ | 7 | 43.43 ± 8.26 | 0.401 ± 0.244 | 9.07 ± 3.99 | 30.71 ± 20.89 | 2.89 ± 0.94 | 20.99 ± 6.42 | 19.20 ± 5.60 |
Females. The ANOVA on female SL only found a significant effect of the interaction species-by-population, because P. latipunctata females were smaller than P. mexicana females in EL, but this pattern was reversed in MC (Table 4). In the MANCOVA on the remaining female life-history traits, we uncovered significant effects of the covariate SL as well as the factors species and population, while embryonic stage of development was not significant (Table 4). Post-hoc ANCOVA revealed that SL had a significant effect on female lean weight, fecundity, and embryo lean weight (all 3 traits increased with increasing SL). Species differed significantly only in embryo lean weight (F2,41 = 32.350, p < 0.001) with P. latipunctata producing the largest offspring (EMMs of embryo lean weight at SL = 42.91 mm, P. formosa: 3.11 mg, P. latipunctata: 4.86 mg, P. mexicana: 2.88 mg), while lean weight and fecundity only exhibited non-significant trends (F2,41 = 2.896, p = 0.067 and F2,41 = 3.054, p = 0.058, respectively). Populations differed significantly only in fat content (F1,41 = 13.621, p = 0.001), but there were also non-significant trends for fecundity (F1,41 = 3.781, p = 0.059), embryo fat (F1,41 = 3.188, p = 0.082), and RA (F1,41 = 3.071, p = 0.087; Table 2).
Table 3
Results of univariate analysis of variance (ANOVA) and multivariate analyses of covariance (MANCOVA) examining life-history differences between males of Poecilia latipunctata (from 2 populations: EL and MC) and P. mexicana (EL only). F-ratios in the MANCOVAs were approximated using Wilks’ values, partial variance was estimated using Wilks’ partial η2, interaction terms in brackets were removed (p > 0.1) from the final model.
| Effect | F | df | p | Partial variance |
| (a) SL (ANOVA) | ||||
| Species | 5.015 | 1, 36 | 0.031 | |
| Population | 3.273 | 1, 36 | 0.079 | |
| (b) Male life histories (MANCOVA) | ||||
| SL | 555.316 | 3, 33 | < 0.001 | 0.981 |
| Species | 4.872 | 3, 33 | 0.006 | 0.307 |
| Population | 6.466 | 3, 33 | 0.001 | 0.370 |
| (SL × species) | 0.327 | 3, 31 | 0.806 | |
| (SL × population) | 1.700 | 3, 32 | 0.187 |
Table 4
Results of univariate analysis of variance (ANOVA) and multivariate analyses of covariance (MANCOVA) examining life-history differences between pregnant females of Poecilia latipunctata (from 2 populations: EL and MC), P. formosa (EL only) and P. mexicana (EL and MC). F-ratios in the MANCOVAs were approximated using Wilks’ values, partial variance was estimated using Wilks’ partial η2, and interaction terms in brackets were removed (P > 0.1) from the final model.
| Effect | F | df | p | Partial variance |
| (a) SL (ANOVA) | ||||
| Species | 1.909 | 2, 42 | 0.161 | 0.083 |
| Population | 2.371 | 1, 42 | 0.131 | 0.053 |
| Species × population | 6.242 | 1, 42 | 0.016 | 0.129 |
| (b) Female life histories (MANCOVA) | ||||
| SL | 170.052 | 6, 36 | < 0.001 | 0.966 |
| Stage of development | 1.288 | 6, 36 | 0.287 | 0.177 |
| Species | 4.982 | 12, 72 | < 0.001 | 0.454 |
| Population | 4.051 | 6, 36 | 0.003 | 0.403 |
| (SL × species) | 1.473 | 12, 68 | 0.156 | |
| (Stage × species) | 1.632 | 12, 64 | 0.106 | |
| (SL × population) | 1.783 | 6, 31 | 0.135 | |
| (Species × population) | 1.539 | 6, 30 | 0.200 | |
| (Stage × population) | 0.524 | 6, 29 | 0.785 |
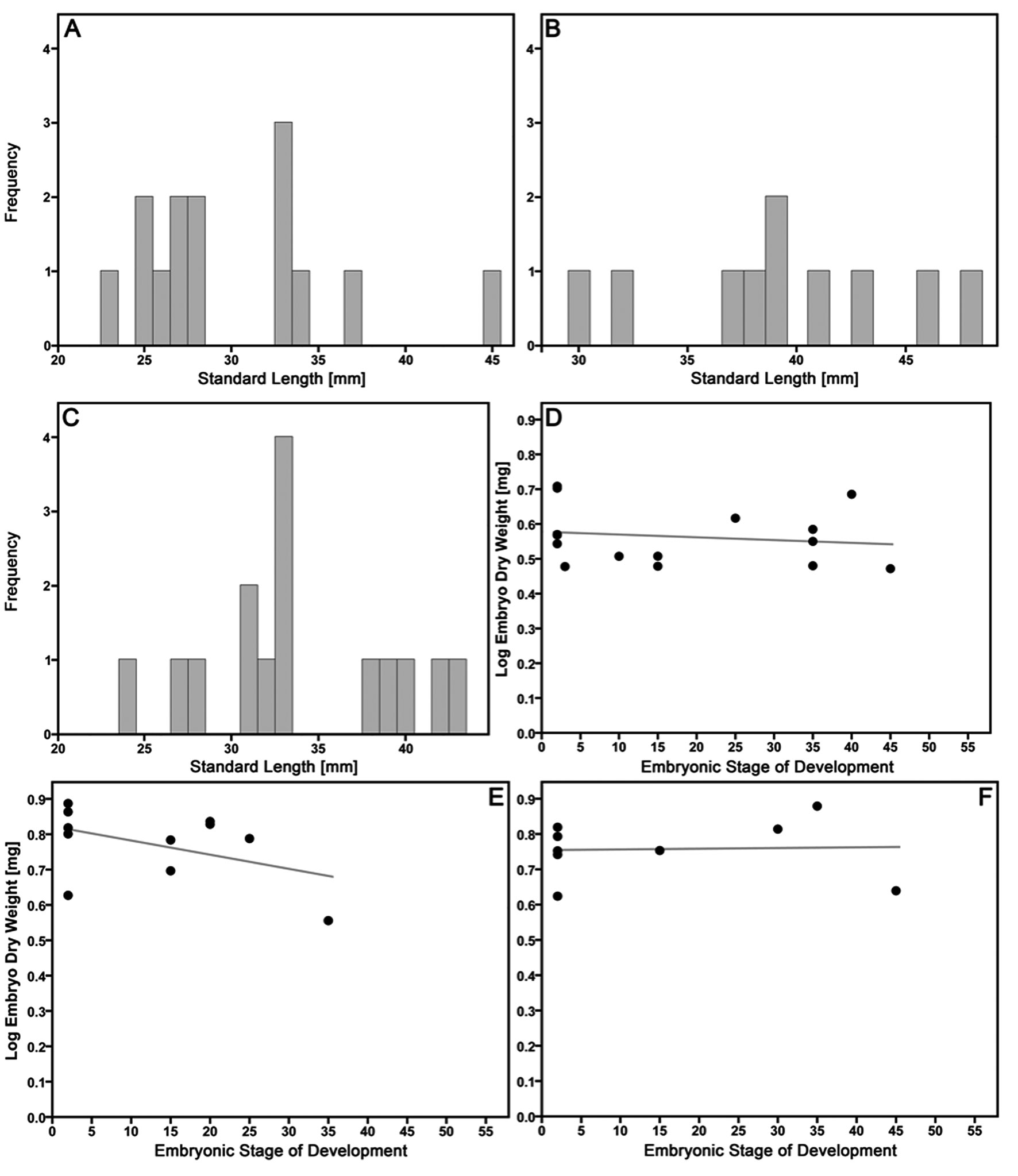
Embryo dry weight either remained more or less constant or decreased strongly with developmental stage, which is congruent with a predominantly lecithotrophic provisioning strategy (MI, P. latipunctata, EL = 0.73; MC = 1.01; P. mexicana, EL = 0.90; Fig. 2D-F). This confirmed the pattern we found for embryo lean weight (see above), because based on this, P. latipunctata produced larger neonates than P. mexicana (estimated dry weight at birth, P. latipunctata, EL = 4.00 mg, MC = 5.82 mg; P. mexicana, EL = 3.33 mg). We did not find any evidence for superfetation in P. latipunctata, P. mexicana, and P. formosa, as all developing embryos per female were always of approximately the same
developmental stage.
Discussion
As is typical for most poeciliids, P. latipunctata was characterized by strong sexual size dimorphism, with males being smaller than females (Pires et al., 2011). Moreover, size distributions for P. latipunctata did not deviate significantly from a normal distribution. At present it is too early to conclusively determine whether male size in P. latipunctata is genetically determined or not. However, bimodal size distributions in this family are usually strong evidence for size at maturity being genetically determined (Kallman, 1989), so this would suggest that size at maturity in P. latipunctata is not genetically determined. On the other hand, while males of some poeciliids exhibit a bimodal size distribution, the interpopulation variation in male size distributions is common, and environmental effects are known to sometimes supersede genetic determination (Kallman, 1989; Kolluru & Reznick, 1996; Reznick et al., 2007).
Overall, adult male and female life histories, as well as most offspring life histories were quite similar to those of other long-fin (e.g., P. latipinna: Martin et al., 2009; Riesch et al., 2012) and short-fin (e.g., P. mexicana: Riesch et al., 2010, 2011; this study) mollies (including the lack of superfetation; Pires et al., 2011). However, P. latipunctata was notably different from most other non-superfetating poeciliids from regular (i.e., non-extreme) aquatic habitats, in that they produced very large offspring. This is not fitting the general pattern within poeciliids well, whereby smaller species usually produce smaller offspring and larger species larger offspring (Pires et al., 2011). In fact, the offspring sizes we uncovered here for both populations, are much more similar to those found in non-superfetating poeciliids inhabiting toxic sulfide springs or dark caves (Riesch et al., 2010, 2011, 2016).
With respect to maternal provisioning, P. latipunctata was characterized by a largely lecithotrophic provisioning strategy (i.e., resources needed for embryo development are almost exclusively stored in the yolk prior to fertilization; Marsh-Matthews, 2011; Pires et al., 2011). However, similar to some previous studies in other poeciliids (Trexler, 1985; reviewed by Pires et al., 2011), we found inter-population variation in the extent of maternal provisioning as quantified via the MI (we have to point out, though, that sample sizes for estimating MI were quite low and late-stage embryos were largely missing). Overall, this is also congruent with the fact that some of the other male and female life histories differed to varying degree between these 2 populations. This suggests potential differences in resource availability between EL and MC but could also result from genetic differences or other selective forces that differ between these 2 populations (Johnson & Bagley, 2011). Based on the abiotic parameters we measured at the time of sampling (Table 1), some of these differences could, for example, be based on the observed differences between habitats in conductivity, oxygen content, and chlorophyll a (a proxy for productivity) content. However, we do not know if these single point measurements represent more permanent differences between habitats. Moreover, both habitats are exposed to different levels of human disturbance, which could also have a measurable impact here. EL is a small drainage ditch in the midst of surrounding agriculture, mainly sugarcane fields, while MC is a fairly large irrigation channel in the midst of agricultural fields. We have observed EL to change over time due to removal of silt from the ditch, which temporarily turned the ditch into a relatively fast flowing habitat (which is not ideal habitat for poeciliids; Meffe & Snelson, 1989), but siltation and regrowth of plants returned the ditch quickly to a state that seemed more suitable for mollies.
Our sampling scheme allowed us to directly compare aspects of life histories between 3 different species (with respect to females) of poeciliid fishes that are all syntopic in the same 2 habitats in northeastern Mexico. For males, we could unfortunately only compare P. mexicana with P. latipunctata from EL due to sampling constraints (nonetheless, it is important to note that all 3 species did occur in both habitats). Here, male P. mexicana were significantly larger and also had significantly more body fat, suggesting that P. mexicana males might, at least at EL, have a competitive advantage over P. latipunctata males.
For females, we were able to compare all 3 species for EL, and P. mexicana and P. latipunctata at MC. At EL, P. formosa and P. mexicana were of roughly similar size, and also had very similar fecundities, RA, and produced offspring of roughly similar size. Given that P. formosa is a hybrid species originating from hybridization between P. mexicana and P. latipinna, this similarity is not necessarily surprising (Schlupp & Riesch, 2011). However, it is interesting to note that P. latipunctata females at EL were considerably smaller, and produced significantly larger but fewer offspring, resulting in a somewhat similar investment into reproduction (RA). We largely found the same similarities and differences when comparing P. mexicana and P. latipunctata females at MC, but in this population, P. latipunctata females were actually larger than P. mexicana females. The significantly larger offspring of P. latipunctata compared to the syntopic P. mexicana and P. formosa, suggest strong selection on offspring’s competitive ability, as larger offspring have been demonstrated to have better competitive abilities in poeciliid fishes (Bashey, 2008). Future experiments should investigate directly the relative competitive ability of the offspring of these 3 different species in these habitats.
The present study represents the first characterization of important life-history traits of the critically endangered P. latipunctata. While it already provides us with relevant insights into their life histories and reveals some similarities and differences relative to life histories of other mollies, more research into their basic biology is needed. One interesting aspect of P. latipunctata life histories was the large offspring size. If, as we speculate above, there is indeed strong competition between the 3 species (given their relatively similar niches, this is not necessarily surprising), this could be an additional factor on top of anthropogenic disturbance that might help explain why the species seems to be doing poorly in its native range. Additional studies from the area where P. latipunctata is invasive (La Media Luna), would be important in this context. We therefore call on more research into this species; without this it will be extremely difficult to plan and implement meaningful conservation efforts to save this charismatic little fish from extinction within its native range.
Acknowledgements
FJGdL thanks CIBNOR for its support for this study. We also wish to thank the Mexican Federal Government for issuing permits for field collections. All fish were collected under standard protocols approved by IUCUC Protocol R07-004 administered by the University of Oklahoma. Financial support came from the University of Oklahoma.
References
Barnosky, A. D., Matzke, N., Tomiya, S., Wogan, G. O. U., Swartz, B., Quental, T. B. et al. (2011). Has the Earth’s sixth mass extinction already arrived? Nature, 471, 51–57. https://doi.org/10.1038/nature09678
Bashey, F. (2008). Competition as a selective mechanism for larger offspring size in guppies. Oikos, 117, 104–113. https://doi.org/10.1111/j.2007.0030-1299.16094.x
Conkle, J. L., Báez-Del Valle, C. D., & Turner, J. W. (2018). Are we underestimating microplastic contamination in aquatic environments? Environmental Management, 61, 1–8. https://doi.org/10.1016/j.envpol.2020.114721
Costanza, R., de Groot, R., Sutton, P., van der Ploeg, S., Anderson, S. J., Kubiszewski, I. et al. (2014). Changes in the global value of ecosystem services. Global Environmental Change, 26, 152–158. https://doi.org/10.1016/j.gloenvcha.2014.04.002
Contreras-Balderas, S., & Almada-Villela, P. (1996). Poecilia latipunctata. The IUCN Red List of Threatened Species 1996:e.T17832A7522399. https://dx.doi.org/10.2305/IUCN.UK.2019-2.RLTS.T17832A1531379.en
García-De León, F. J., Hernández-Sandoval, A. I., Contreras-Catala, F., Sánchez-Velasco, L., & Ruiz-Campos, G. (2018). Distribution of fishes in the Río Guayalejo-Río Tamesí system and relationships with environmental factors in northeastern Mexico. Environmental Biology of Fishes, 101, 167–180. https://doi.org/10.1007/s10641-017-0689-8
Geist, J. (2011). Integrative freshwater ecology and biodiversity conservation. Ecological Indicators, 11, 1507–1516. https://doi.org/10.1016/j.ecolind.2011.04.002
Gozlan, R. E., Britton, J. R., Cowx, I., & Copp, G. H. (2010). Current knowledge on non-native freshwater fish introductions. Journal of Fish Biology, 76, 751–786. https://doi.org/10.1111/j.1095-8649.2010.02566.x
Janse, J. H., Kuiper, J. J., Weijters, M. J., Westerbeek, E. P., Jeuken, M. H. J. L., Bakkenes, M. et al. (2015). GLOBIO-Aquatic, a global model of human impact on the biodiversity of inland aquatic ecosystems. Environmental Science & Policy, 48, 99–114. https://doi.org/10.1016/j.envsci.2014.12.007
Joachim, B. L., & Schlupp, I. (2012). Mating preferences of Amazon mollies (Poecilia formosa) in multi-host populations. Behaviour, 149, 233–249. https://doi.org/10.1163/156853912X636302
Johnson, J. B., & Bagley, J. C. (2011). Ecological drivers of life-history divergence. In J. P. Evans, A. Pilastro, & I. Schlupp (Eds.), Ecology and evolution of poeciliid fishes (pp. 38–49). Chicago: University of Chicago Press.
Kallman, K. D. (1989). Genetic control of size at maturity in Xiphophorus. In G. K. Meffe, & F. F. Snelson, Jr. (Eds.), Ecology & evolution of livebearing fishes (pp. 163–184). Englewood Cliffs: Prentice Hall.
Kolluru, G. R., & Reznick, D. N. (1996). Genetic and social control of male maturation in Phallichthys quadripunctatus (Pisces: Poeciliidae). Journal of Evolutionary Biology, 9, 695–715. https://doi.org/10.1046/j.1420-9101.1996.9060695.x
Marsh-Matthews, E. (2011). Matrotrophy. In J. P. Evans, A. Pilastro, & I. Schlupp (Eds.), Ecology and evolution of poeciliid fishes (pp. 18–27). Chicago: University of Chicago Press.
Martin, S. B., Hitch, A. T., Purcell, K. M., Klerks, P. L., & Leberg, P. L. (2009). Life history variation along a salinity gradient in coastal marshes. Aquatic Biology, 8, 15–28. https://doi.org/10.3354/ab00203
Meffe, G. K., & Snelson, Jr., F. F. (1989). An ecological overview of poeciliid fishes. In G. K. Meffe, & F. F. Snelson, Jr. (Eds.), Ecology and evolution of livebearing fishes (Poeciliidae) (pp. 13–31). Englewood Cliffs, New Jersey: Prentice Hall.
Miller, R. R., Minckley, W. L., & Norris, S. M. (2005). Freshwater fishes of Mexico. Chicago: University of Chicago Press.
Neilson, M. E. (2019). Poecilia latipunctata Meek, 1904: U.S. Geological Survey, Nonindigenous Aquatic Species Database, Gainesville, Florida. Retrieved on 7th June, 2019 from: https://nas.er.usgs.gov/queries/FactSheet.aspx?SpeciesID=860
Niemeitz, A., Kreutzfeldt, R., Schartl, M., Parzefall, J., & Schlupp, I. (2002). Male mating behaviour of a molly, Poecilia latipunctata: a third host for the sperm-dependent Amazon molly, Poecilia formosa. Acta Ethologica, 5, 45–49. https://doi.org/10.1007/s10211-002-0065-2
Palacio-Núñez, J., Martínez-Montoya, J. F., Olmos-Oropeza, G., Martínez-Calderas, J. M., Clemente-Sánchez, F., & Enríquez, J. (2015). Distribución poblacional y abundancia de los peces endémicos de la llanura de Rioverde, S. L. P., México. AGRO Productividad, 8, 17–24.
Pimm, S. L., Jenkins, C. N., Abell, R., Brooks, T. M., Gittleman, J. L. et al. (2014). The biodiversity of species and their rates of extinction, distribution, and protection. Science, 344, 1246752. https://doi.org/10.1126/science.1246752
Pires, M. N., Banet, A. I., Pollux, B. J. A., & Reznick, D. N. (2011). Variation and evolution of reproductive strategies. In J. P. Evans, A. Pilastro, & I. Schlupp (Eds.), Ecology and evolution of poeciliid fishes (pp. 28–37). Chicago: University of Chicago Press.
Pollux, B. J. A., Pires, M. N., Banet, A. I., & Reznick, D. N. (2009). Evolution of placentas in the fish family Poeciliidae: an empirical study of macroevolution. Annual Review of Ecology, Evolution and Systematics, 40, 271–289. https://doi.org/10.1146/annurev.ecolsys.110308.120209
Ptacek, M. B., & Breden, F. (1998). Phylogenetic relationships among the mollies (Poeciliidae: Poecilia: Mollienesia group) based on mitochondrial DNA sequences. Journal of Fish Biology, 53 (Supplement A), 64–81. https://doi.org/10.1111/j.1095-8649.1998.tb01018.x
Reznick, D., Hrbek, T., Caura, S., De Greef, J., & Roff, D. (2007). Life history of Xenodexia ctenolepis: implications for life history evolution in the family Poeciliidae. Biological Journal of the Linnean Society, 92, 77–85. https://doi.org/
10.1111/j.1095-8312.2007.00869.x
Riesch, R., Easter, T., Layman, C. A., & Langerhans, R. B. (2015). Rapid human-induced divergence of life-history strategies in Bahamian livebearing fishes (family Poeciliidae). Journal of Animal Ecology, 84, 1732–1743. https://doi.org/10.1111/1365-2656.12425
Riesch, R., Plath, M., Makowicz, A. M., & Schlupp, I. (2012) Behavioural and life-history regulation in a unisexual/bisexual mating system: does male mate choice affect female reproductive life histories? Biological Journal of the Linnean Society, 106, 598–606. https://doi.org/10.1111/j.1095-8312.2012.01886.x
Riesch, R., Plath, M., & Schlupp, I. (2010). Toxic hydrogen sulfide and dark caves: life-history adaptations in a livebearing fish (Poecilia mexicana, Poeciliidae). Ecology, 91, 1494–1505. https://doi.org/10.1890/09-1008.1
Riesch, R., Plath, M., & Schlupp, I. (2011). Toxic hydrogen sulphide and dark caves: pronounced male life-history divergence among locally adapted Poecilia mexicana (Poeciliidae). Journal of Evolutionary Biology, 24, 596–606. https://doi.org/10.1111/j.1420-9101.2010.02194.x
Riesch, R., Tobler, M., Lerp, H., Jourdan, J., Doumas, T., Nosil, P. et al. (2016). Extremophile Poeciliidae: multivariate insights into the complexity of speciation along replicated ecological gradients. BMC Evolutionary Biology, 16, 136. https://doi.org/10.1186/s12862-016-0705-1
Schartl, M., Wilde, B., Schlupp, I., & Parzefall, J. (1995). Evolutionary origin of a parthenoform, the Amazon molly Poecilia formosa, on the basis of a molecular genealogy. Evolution, 49, 827–835. https://doi.org/10.1111/
j.1558-5646.1995.tb02319.x
Schlupp, I. (2009). Behavior of fishes in the sexual/unisexual mating system of the Amazon molly (Poecilia formosa). In H. J. Brockmann, T. J. Roper, M. Naguib, K. E. Wynne-Edwards, J. C. Mitani, & L. W. Simmons (Eds.), Advances in the study of behavior. Vol. 39 (pp. 153–183). Amsterdam: Academic Press. https://doi.org/10.1016/S0065-3454(09)39005-1
Schlupp, I., & Riesch, R. (2011). Evolution of unisexual reproduction. In J. P. Evans, A. Pilastro, & I. Schlupp (Eds.), Ecology and evolution of poeciliid fishes (pp. 50–58). Chicago: University of Chicago Press.
Stearns, S. C. (1992). The evolution of life histories. Oxford: Oxford University Press.
Stroud, J. T., & Thompson, M. E. (2019). Looking to the past to understand the future of tropical conservation: The importance of collecting basic data. Biotropica, 51, 293–299. https://doi.org/10.1111/btp.12665
Sunday, J. M., Fabricius, K. E., Kroeker, K. J., Anderson, K. M., Brown, N. E., Barry, J. P. et al. (2017). Ocean acidification can mediate biodiversity shifts by changing biogenic habitat. Nature Climate Change, 7, 81–85. https://doi.org/10.1038/nclimate3161
Tobler, M., & Schlupp, I. (2009). Threatened fishes of the world: Poecilia latipunctata Meek, 1904 (Poeciliidae). Environmental Biology of Fishes, 85, 31–32. https://doi.org/10.1007/s10641-009-9451-1
Trexler, J. C. (1985). Variation in the degree of viviparity in the sailfin molly, Poecilia latipinna. Copeia, 1985, 999–1004. https://doi.org/10.2307/1445254