Oscar Méndez *
Universidad Veracruzana, Facultad de Biología, Laboratorio de Calidad Ambiental, Circuito Gonzalo Aguirre Beltrán s/n, Zona Universitaria, 91090 Xalapa, Veracruz, México
*Corresponding author: omendez@uv.mx (O. Méndez)
Received: 4 April 2022; accepted: 27 March 2023
https://zoobank.org/urn:lsid:zoobank.org:pub:CFBBA921-1F5A-4823-BD5E-469EF30C4F4E
Abstract
Nine specimens of Paronatrema guillerminae n. sp. were collected from the gills of the blue shark Prionace glauca (Linnaeus) captured by artisanal fishing in the locality of Punta Belcher (Magdalena Bay), Baja California Sur, Mexico. Paronatrema guillerminae n. sp. is proposed as a new species because it presents distinctive characteristics within the genus Paronatrema (Dollfus), in the following morphological traits: body size, (larger than congeneric species), tegument with scale-like extensions, ovary shape (rounded), and testes number (more than 200). The new species is described herein.
Keywords: Trematode; Platyhelminth; Gills; Syncoeliidae
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Paronatrema guillerminae n. sp. (Trematoda: Syncoeliidae) parásito del tiburón azul Prionace glauca (Linnaeus) (Elasmobranchii: Carcharhinidae) en la costa occidental de Baja California Sur, México
Resumen
Se colectaron 9 especímenes de Paronatrema guillerminae n. sp. de las branquias del tiburón azul Prionace glauca (Linnaeus) durante la captura por pesca artesanal en la localidad de Punta Belcher (bahía Magdalena), Baja California Sur, México. Se propone a P. guillerminae n. sp. como una nueva especie dentro del género Paronatrema (Dollfus), por presentar características distintivas de otras especies cogenéricas, tales como el tamaño del cuerpo (más grande que las restantes), tegumento con extensiones en forma de escamas, forma del ovario (redondeado) y el número de testículos (más de 200 testículos). La nueva especie se describe en este trabajo.
Palabras clave: Tremátodo; Platelminto; Branquias; Syncoeliidae
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Some studies mention that the helminths fauna of Prionace glauca consists mainly of cestodes and nematodes (Curran & Caira, 1995; Henderson et al., 2002). However, in Mexico knowledge on the helminths infecting this important fishery resource is scarce (Espinal-Carrión, 2005; Méndez, 2005; Méndez & Dorantes-González, 2013). The blue shark Prionace glauca (Linnaeus) is a cartilaginous fish assigned to the family Carcharhinidae. It is considered one of the most abundant and widely distributed oceanic species, mainly in tropical and temperate areas of the Atlantic, Pacific, and Indian oceans (Nakano & Seki, 2003). It has been documented that this species makes migratory movements along the Pacific Ocean slope, from north to south during the months of October-November and from south to north during June (Mendizábal & Oriza et al., 2000). The blue shark is an epipelagic species occurring in coastal waters near the continental shelf (Compagno, 2009; Nakano & Stevens, 2008;). In the Mexican Pacific it is one of the most captured species in the oceanic shark fishery (Sosa-Nishizaki et al., 2002). On the western coast of BCS, P. glauca feeds mainly on red crab and squids, and a low proportion of teleost fish (Hernández-Aguilar, 2008; Markaida & Sosa-Nishizaki, 2010). Apparently, there are a restricted number of food items available for the blue shark in the region.
Here, the description of a new species of trematode is presented and the importance of the finding of a new species as a parasite of blue shark, Prionace glauca is discussed. Also, the reliability of some morphological traits that have been used traditionally for the classification of members of the family Syncoeliidae are reviewed.
Materials and methods
During artisanal shark fishing off the coast of Punta Belcher (Magdalena Bay) (24°15’ N, 112°05’ W), located on the western coast of Baja California Sur, from January 2003 to January 2004, 28 specimens of the blue shark, Prionace glauca were obtained. These data are part of the master’s project “Helminth infracommunities of the blue shark Prionace glauca (Linnaeus, 1758) from the western coast of Baja California Sur, Mexico”. In the gills of these elasmobranchs, 9 specimens of trematodes were collected. Trematodes were placed in 0.75% saline solution to relax them and were fixed in formalin at 10% for 48 hrs. Subsequently, they were washed in distilled water, preserved in vials with 70% ethanol, and duly labeled with their collection data for further study.
Some specimens were dehydrated with gradual series of ethanol 80º, 96º, and 100º, stained with Mayer’s Paracarmin, cleared in methyl salicylate, and mounted in Canada balsam as permanent slides (Salgado-Maldonado, 1979). Measurements were taken with a calibrated micrometric eyepiece and are presented as the range followed by the mean in parentheses. All measurements are in µm, except otherwise indicated. One specimen was dehydrated in a rotatory tissue processor and embedded in soli paraffin; with the aid a microtome, sections of 5 µm in thickness were obtained and then stained with hematoxylin and eosin. Additionally, histological sections were made in a sagittal position to describe the reproductive organs mainly. Some specimens were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México (IB-UNAM).
Description
Phylum Platyhelminthes
Clase Digenea
Family Syncoeliidae
Paronatrema guillerminae n. sp. (Méndez, 2023)
https://zoobank.org/urn:lsid:zoobank.org:act:7A5C89B3-51BC-4121-9E92-3C427627AC9C
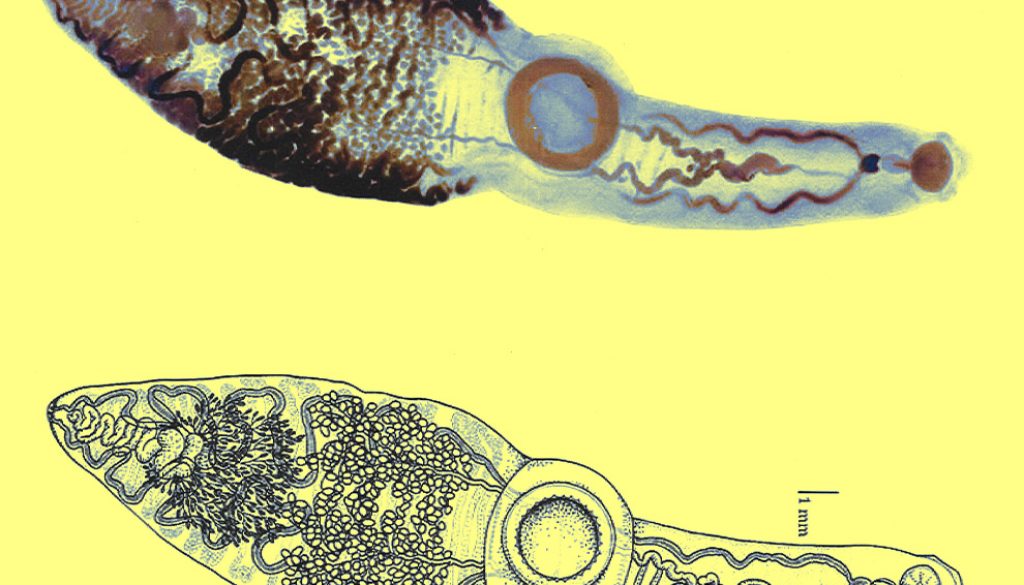
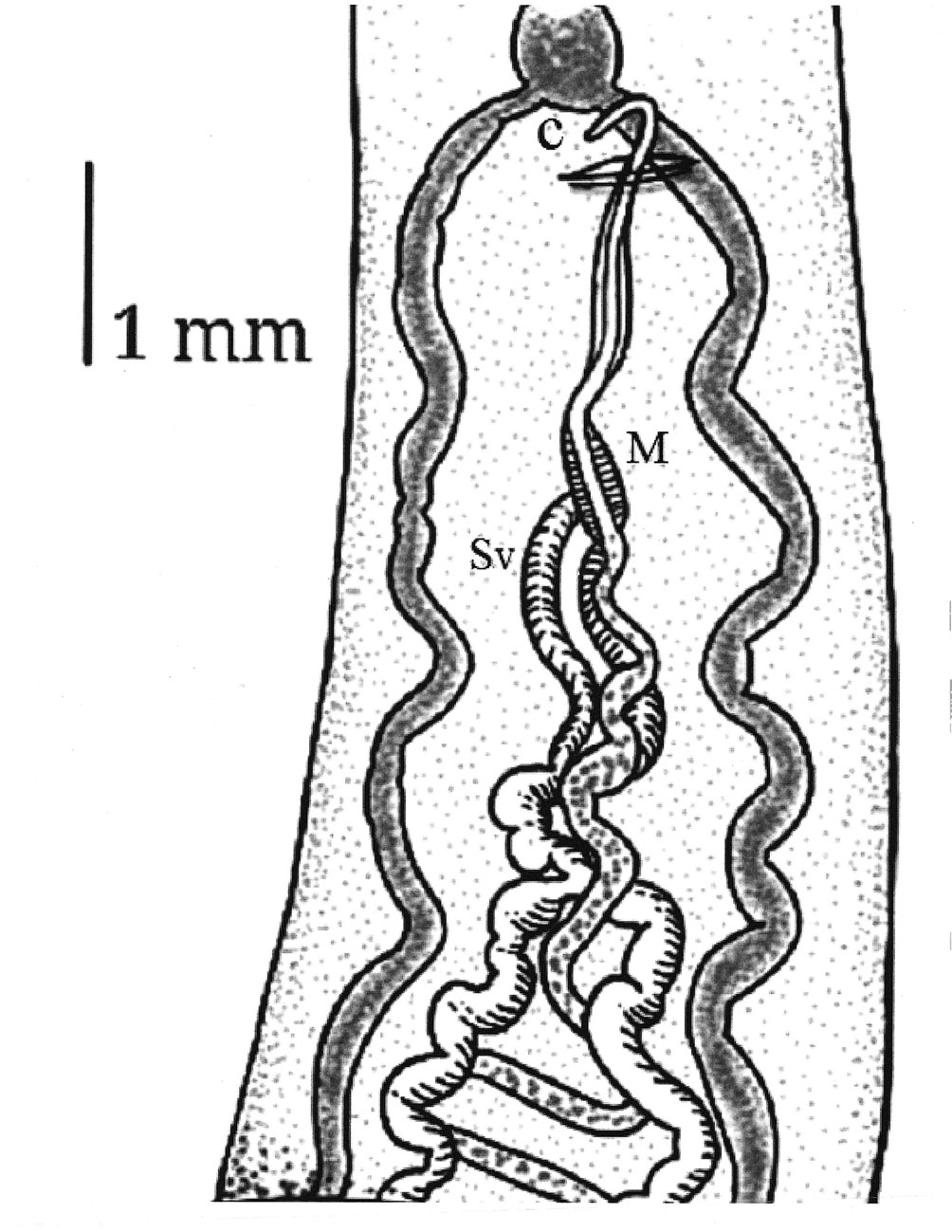
Description based on 9 specimens. Body very long, elongated, distinctively subdivided in 2, with a thin, sub-cylindrical forebody, and a wider hindbody 24.89-31.31 (28.93) long, 5.7-7.62 (6.89) wide at the level of the ventral sucker (Fig. 1).
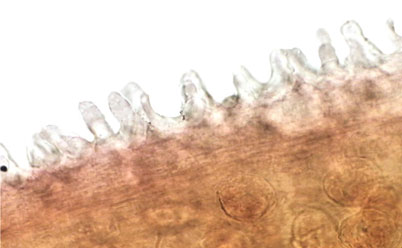
Tegument with scale-like extensions on the surface of the entire body more abundant in the region between the metraterm and the posterior end of the ventral sucker (Fig. 2).
Forebody with glandular cells in the parenchyma, more abundant laterally, decreasing toward middle part of body.
Prominent preoral lobe. Slightly transversal oral sucker, 1.4-1.7 (1.5) long by 1.7-2.1 (1.9) wide, surrounded by 2 muscular projections. Elongated pharynx, 0.9-1.2 (1.1) long by 0.6-0.8 (0.7) wide. Short, globular esophagus, sinuous intestinal caeca extending posterior end of body. Ventral sucker well developed and pre-equatorial, 3.4-4.5 (3.8) long by 3.5-4.6 (3.9) wide. Ventral sucker with a ring of small accessory suckers 36-40 (38) in the internal part (Fig. 3). Length and width ratio between oral and ventral sucker 1: 2.29-2.75 in length and 1: 1.79-2.6 in width.
Testes small, numerous (228-248), slightly oval between posterior margin of ventral sucker and posterior end of body 0.22-0.27 (0.24) long and 0.27 -0.37 (0.32) wide. Two sinuous vas deferent run into the free seminal vesicle (Fig. 4). Seminal vesicle long S-shaped surrounded by abundant prostatic cells. 0.57-0.76 (0.67) long, 0.16-0.17 (0.16) wide. Seminal vesicle joins the metraterm to form a hermaphrodictic sac containing a cirrus. Genital pore prebifurcal.
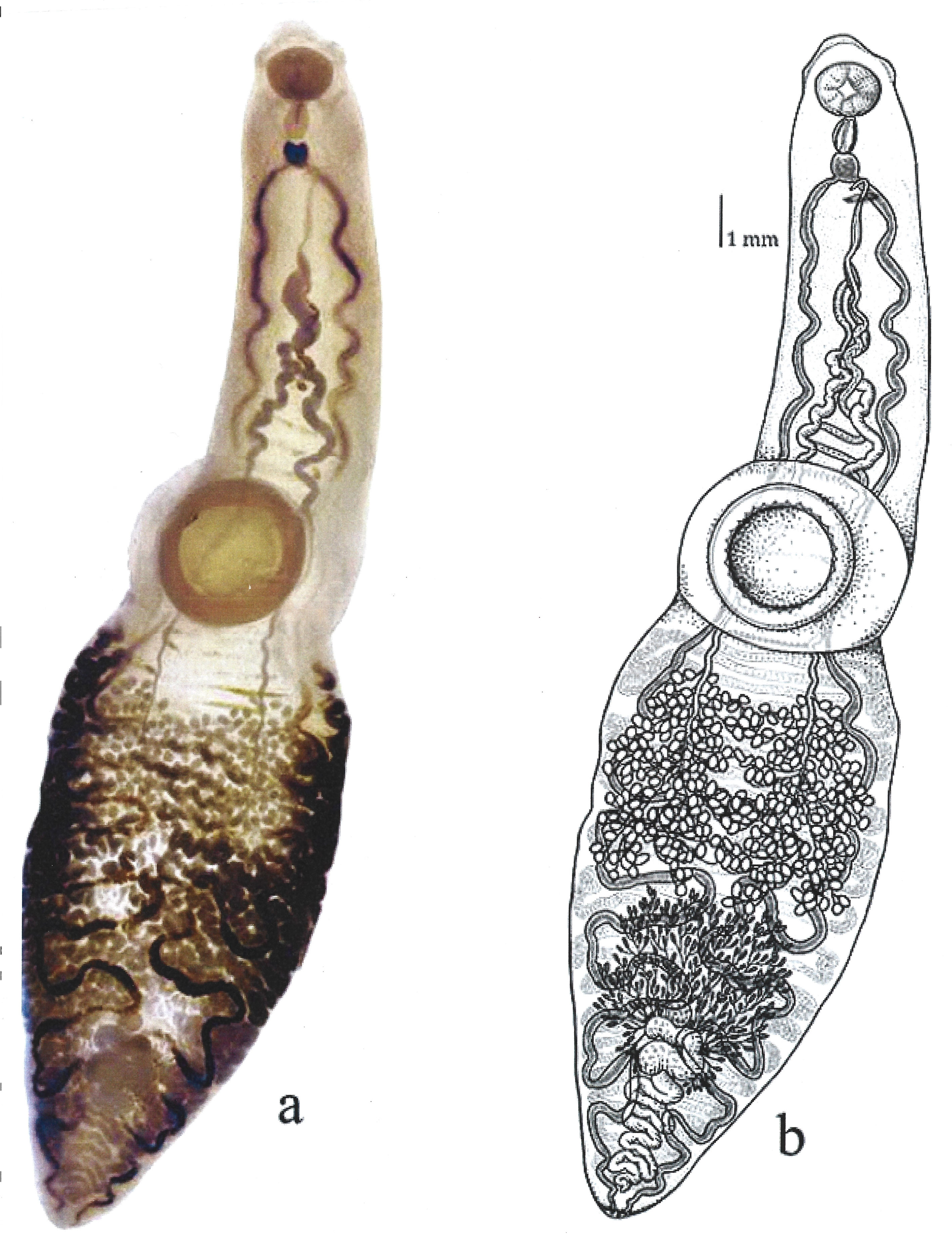
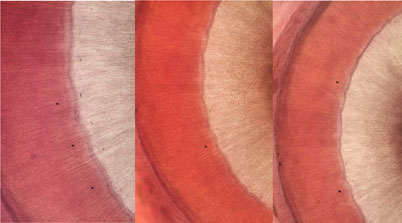
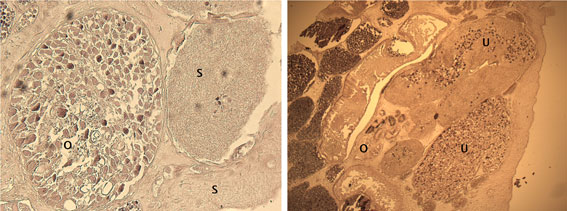
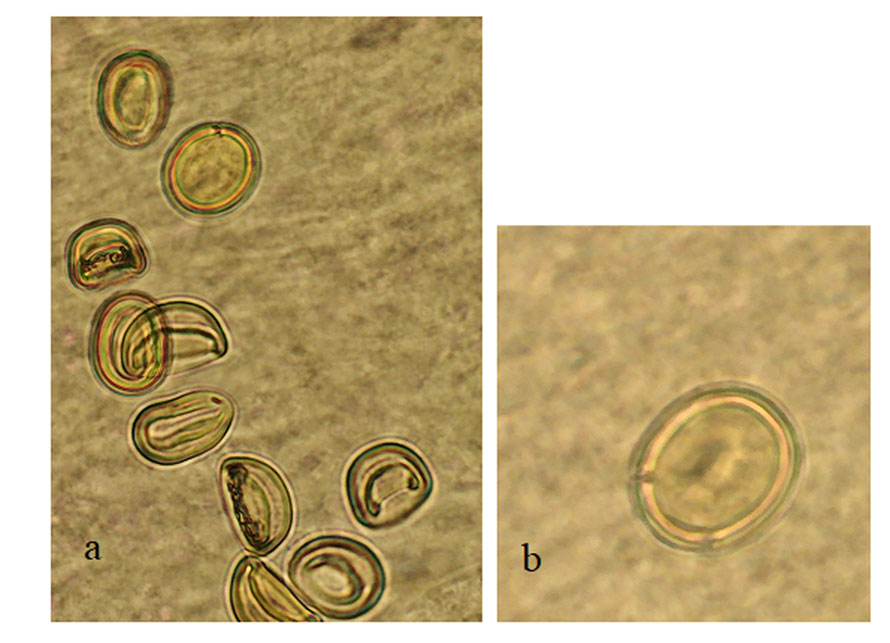
Ovary rounded, small, smooth (Figs. 5-7), post-testicular, 0.50-1.01 (0.76) long 0.58-0.92 (0.73) wide, in middle body. Mehli’s glands not observed. Seminal receptacle well-developed (Figs. 5, 6), post-ovarian filled with sperm. Tubular and sinuous uterus (Fig. 6), descending from posterior end of ovary, towards body end, turning dorsally and running anteriorly forming numerous coils to joining the metraterm at mid-level of forebody. Vitellaria follicular. Follicles 20-25 in diameter extending from the anterior part of the ovary towards the posterior region just before where the intestinal cecum ends. Y-shaped excretory vesicle, without contact with the intestinal cecum, near the end of the body and ends in a terminal excretory pore. Embryonated eggs with a small operculum and thin shell, 25-30 (27) long and 22-25 (22) wide (Fig. 7).
Taxonomic summary
Hosts: Prionace glauca (Linnaeus, 1758), blue shark (Carcharhinidae)
Site of infection: gills
Locality: Punta Belcher (Bahía Magdalena) (24°15’ N, 112°05’ W), Baja California Sur, Mexico.
Catalogue number CNHE: 11866; 11867.
Etymology: the specific name is in honor of the scientific career of M. C. Guillermina Cabañas-Carranza for her experience, dedication, and commitment to the study of parasitic helminths, but more for her overall contributions to science.
Remarks
The genus Paronatrema comprises only 3 species and is not commonly found, and there is little information regarding the diversity of the hemiouroid trematode (Gibson, 2002). Their species possess a rather complex morphology. In some descriptions, structures such as the ovary and the Mehlis´ gland have been confused and the genus might consist of species containing 1 or several ovaries (Curran & Overstreet, 2000; Manter, 1940). Three species have been described thus far (Table 1), namely Paronatrema vaginicola Dollfus 1937 in Squalus sp. from New Guinea, Paronatrema mantae Manter, 1940 in Manta birostris from Bahía Honda, Panama (Yamaguti, 1971), and P. boholana Eduardo, 2010 in Rhincodon typus from Bohol Island, Philippines (Eduardo, 2010). Additionally, P. vaginicola was recorded by Curran and Overstreet (2000) in the cloaca, gills, and oral cavity of Prionace glauca and Alopias pelagicus in the Gulf of California, Mexico, being the first record of the genus in P. glauca. This paper represents the second record of a species of the genus as parasite of the blue shark.
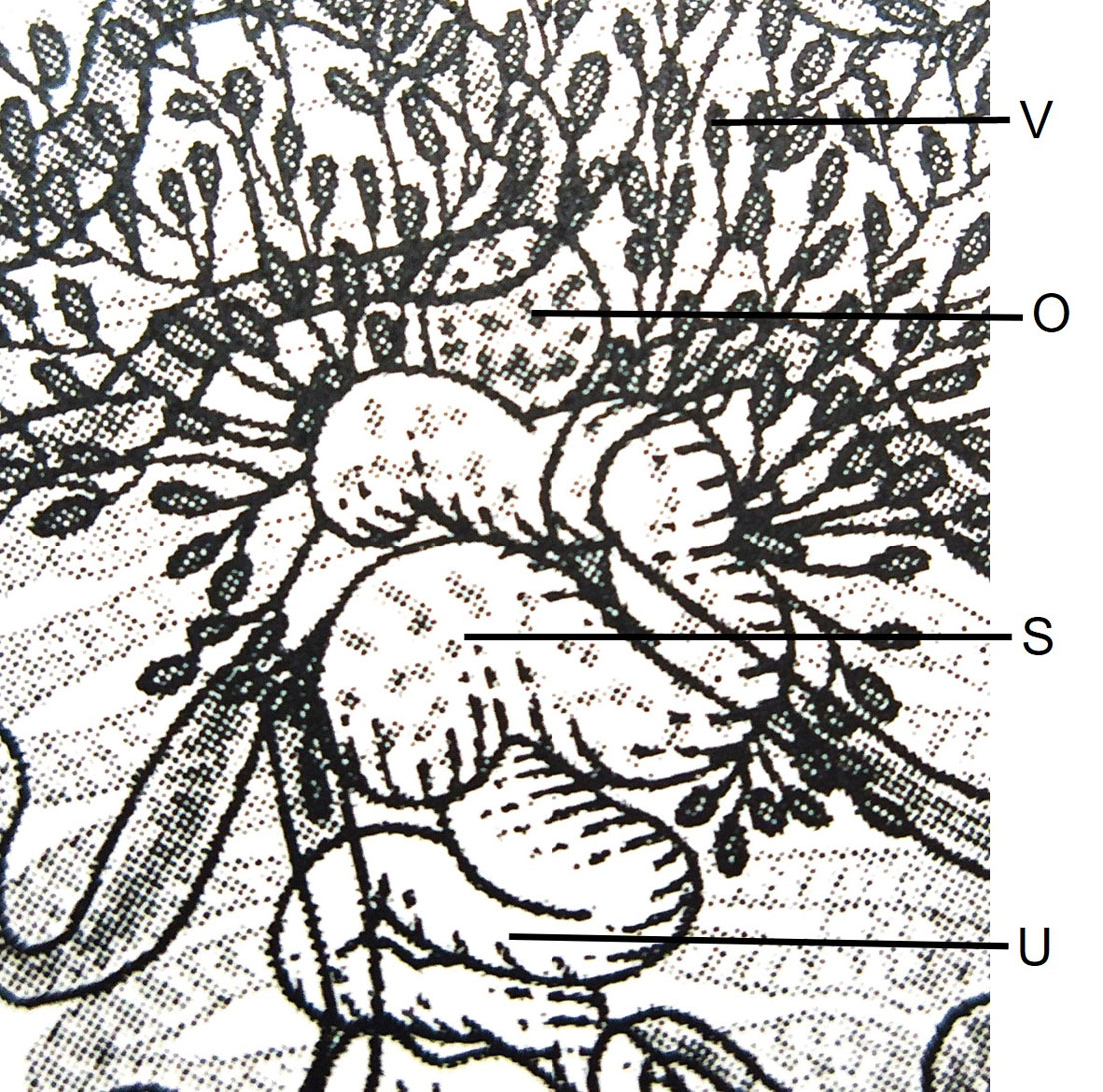
The trematode Paronatrema guillerminae n. sp. differs from the 3 species previously described by the abundant scale-like ornamentations on the body surface, the large number of testes, ovary shape, and by having embryonated eggs possessing an operculum. Additionally, the new species is the largest described for the genus (Table 1). The new species was compared with specimens of P. vaginicola deposited at the Colección Nacional de Helmintos (CNHE), Instituto de Biología (UNAM) by Curran and Overstreet (2000), from the same host species and geographical area. Paronatrema guillerminae n. sp. differs from P. vaginicola by its body size, position of the genital pore, number of testes, number of ovaries, and the abundant presence of scale-like ornamentations on the tegument of new species, extending from the metraterm towards the posterior region just before where the ventral sucker ends. Furthermore, the new species differs from P. mantae by the absence of accessory suckers on the ventral sucker and differs from P. boholana because this species possesses tubular testes and branched ovary. It is interesting to point out that in all the examined specimens of P. guillerminae n. sp., the intestinal caeca do not flow to the excretory vesicle, unlike P. vaginicola, where the caeca open into the excretory vesicle.
The distinction of species of the syncoeliid genus Paronatrema has been controversial. In some species, the ovary is tubular in shape, which can be confused with the Mehlis´ gland, and it is difficult to observe due to the uterine coil directed towards the middle part of the organism (Gibson, 2002). The ovary of P. guillerminae n. sp. is rounded as shown in figures of the detail of the female reproductive system and the cross section, unlike P. vaginicola and P. boholana where the ovary is either follicular or forms branched tubules, respectively.
Table 1. Characteristics of the species belonging to the genus Paronatrema.
| Paronatrema mantae Manter, 1940 | Paronatrema vaginicola
Dollfus, 1937 |
Paronatrema boholana
Eduardo, 2010 |
Paronatrema guillerminae n. sp. | |
| Author | Manter (1940) | Curran y Ovestreet (2000) | Eduardo (2010) | Present work |
| Host | Manta birostris | Prionace glauca and Alopias pelagicus | Rhincodon typus | Prionace glauca |
| Site of infection | Skin | Cloaca, gill arches, and bucal cavity | Skin and gills | Gills |
| Locality | Bahía Honda, Panamá | Golfo de California, México | Isla Bohol, Filipinas | Bahía Magdalena, México |
| # individuals | 4 | 5 | 20 | 9 |
| Length (L) | 17.5-19.8 | 8.0-20.5 | 4.075-12.932 (6.349) | 24.89-31.31 (28.93) |
| Wide (W) | 1.68-4.18 | 2.0-3.5 | 1.725-6.125 (3.035) | 5.70-7.62 (6.89) |
| Sucker (L) | 1.809-1.971 | 932-1,179 | 0.437-0.625 (0.497) | 1.38-1.74 (1.52) |
| Sucker (W) | Not mentioned | 1,022-1,235 | 0.537-0.812 (0.645) | 1.72-2.08 (1.93) |
| Accessory oral suckers | 41-47 | Absent | Absent | Absent |
| Pharinge (L) | 1.147-1.215 | 494-870 | 0.390-0.570 (0.475) | 0.96-1.16 (1.06) |
| Pharinge (W) | 0.634-0.815 | 371-477 | 0.200-0.400 (0.305) | 0.64-0.82 (0.74) |
| Acetabule (L) | 3.064-3.591 | 1,824-2,807 | 1.050-3.950 (1.991) | 3.40-4.49 (3.82) |
| Acetabule (W) | Not mentioned | 1,824-2,610 | 1.100-3.350 (1.878) | 3.53-4.64 (3.90) |
| Accesory suckers | 29-37 | 35-38 | 32-38 | 36-40 (38) |
| Oral sucker/Acetabule | 1:2 | 1:1.7-2.1 | 1:3.3 a 1:4.8 | 1:2.5 |
| Ovary (#) | Globular, smooth (1) | Follicles (60) | Branched tubules | Globular, smooth (1) |
| Ovary (L) | Not mentioned | 28-40 | 0.50-1.01 (0.76) | |
| Ovary (W) | Not mentioned | 20-31 | 0.58-0.92 (0.73) | |
| Testis (#) | Tubular | Pyriform (94) | Branched tubes | Oval (228-248) |
| Testis (L) | Not mentioned | 156 | – | 0.22-0.27 (0.24) |
| Testis (W) | Not mentioned | 233 | – | 0.27-0.37 (0.32) |
| Eggs (L) µm | 24-25.5 | 25-30 | 23.75-27.50 (25.86) | 25-30 (27) |
| Eggs (W) µm | 19-22 | 20-22 | 22.50-26.55 (23.59) | 22-25 (22) |
The difference in body size of P. guillerminae n. sp. in comparison with P. boholana, P. mantae, and P. vaginicola can be attributed to the different sizes and ages of the hosts; however, it should be noted that characteristics such as the presence of embryonated and operculated eggs, and the number and shape of testes are reliable characters to propose a new species. It would be worth sampling more individuals of the new species for sequencing some molecular markers and then determining more clearly the position of Syncoeliidae within the Hemiuroidea, since at the present time, only 1 species of this family has been sequenced for 2 ribosomal genes, i.e., Copiatestes filiferus (Leukart in Sars, 1885).
Acknowledgements
To Felipe Galván Magaña for his support in the collection of samples. To the scholarship No. 176166 awarded by Conacyt. I also thank Raúl Simá Álvarez from the laboratory of Aquatic Pathology of CINVESTAV-Mérida for the histological sections, and Mariana Muñoz Velasquez for making the drawings. The author is grateful to Conacyt for a postdoctoral grant from the program “Post-doctoral stays linked to the strengthening of the quality of the national postgraduate 2014”.
References
Compagno, L. J. V. (2009). Pelagic elasmobranch diversity. In M. D. Camhi, E. K. Pikitch, & E. A. Babcock (Eds.), Sharks of the open Ocean. Biology, Fisheries and Conservation. Oxford: Blackwell Publishing. https://dx.doi.org/10.1002/9781444302516.ch34
Curran, S., & Caira, J. N. (1995). Attachment site specificity and the tapeworm assemblage in the spiral intestine of the blue shark (Prionace glauca). Journal of Parasitology, 81, 149–157 https://doi.org/10.2307/3283913
Curran, S. S., & Overstreet, R. M. (2000). Syncoelium vermilionensis sp. n., (Hemiuroidea: Syncoeliidae) and new records for members of Azygiidae, Ptychogonimidae, and Syncoeliidae parasitizing elasmobranchs in the Gulf of California. Lincoln, Nebraska: Faculty Publications from the Harold W. Manter Laboratory of Parasitology. http://digitalcommons.unl.edu/parasitologyfacpubs/440
Eduardo, S. L. (2010). A new species of parasitic fluke, Paronatrema boholana Eduardo (Trematoda: Syncoeliidae) from the whale shark, Rhincodon typus Smith 1828 (Orectolobiformes: Rhincodontidae) off Bohol Island, Philippines. The Asian International Journal of Life Sciences, 19, 315–323.
Espinal-Carrión, T. (2005). Helmintos intestinales del tiburón mako Isurus oxyrinchus (Rafinesque, 1810) en Las Barrancas, Baja California Sur, México (Tesis). Área Interdisciplinaria de Ciencias del Mar. Universidad Autónoma de Baja California Sur, México.
Gibson, D. I. (2002). Family Syncoeliidae Looss 1899. In D. I., Gibson, A. Jones, & R. A. Bray (Eds.), Keys to the Trematoda, Vol. 1 (pp. 409–413). London: CABI Publishing/ The Natural History Museum.
Henderson, A. C., Flannery, K., & Dunne, J. (2002). Parasites of the blue shark (Prionace glauca L.), in the North-East Atlantic Ocean. Journal of Natural History, 36, 1995–2004. https://doi.org/10.1080/00222930110078834
Hernández-Aguilar, S. B. (2008). Espectro trófico del tiburón azul Prionace glauca (Linnaeus, 1758) en la costa occidental de Baja California Sur, México (Tesis de maestría). Instituto Politécnico Nacional. Centro Interdisciplinario de Ciencias Marinas. La Paz, Baja California Sur, Mexico. http://digitalcommons.unl.edu/parasitologyfacpubs/657
Manter, H. W. (1940). Digenetic trematodes of fishes from the Galapagos Islands and the neighboring Pacific. Lincoln, Nebraska: Faculty Publications from the Harold W. Manter Laboratory of Parasitology.
Markaida, U., & Sosa-Nishizaki, O. (2010). Food and feeding habits of the blue shark Prionace glauca caught off Ensenada, Baja California, Mexico, with a review on its feeding. Journal of the Marine Biological Association of the United Kingdom, 90, 977–994. https://doi.org/10.1017/S0025315409991597
Méndez, O., & Dorantes-González, M. A. (2013). Cestodos del tiburón toro Carcharhinus leucas en playa Chachalacas, Veracruz, México. Neotropical Helminthology, 7, 167–171. https://doi.org/10.24039/rnh201371959
Méndez, O. (2005). Infracomunidades helmínticas del tiburón azul Prionace glauca (Linnaeus. 1758) de la costa occidental de Baja California Sur, México (Tesis de maestría). Instituto Politécnico Nacional. Centro Interdisciplinario de Ciencias Marinas. La Paz, Baja California Sur, Mexico.
Mendizábal y Oriza, D., Vélez, R., Márquez, J. F. y Soriano, S. R. (2000). Tiburones oceánicos del Pacífico. In M. A. Cisneros, L. F. Beléndez, E. Zárate, M. T. Gaspar, L. C. López, C. Saucedo et al. (Eds.), Sustentabilidad y pesca responsable en México. Evaluación y manejo, 1999-2000 (pp. 179–209). México D.F.: Instituto Nacional de la Pesca-SAGARPA.
Nakano, H., & Seki, M. P. (2003). Synopsis of biological data on the blue shark (Prionace glauca Linnaeus). Bulletin of the Fisheries Research Agency of Japan, 6, 8–55.
Nakano, H., & Stevens, J. D. (2008). The biology and ecology of the blue shark, Prionace glauca. In M. D. Camhi, E. K. Pikitch, & E. A. Babcock (Eds.), Sharks of the open Ocean. Biology, fisheries and conservation (pp. 140–151). Oxford, U.K.: Blackwell Publishing. https://doi.org/10.1002/9781444302516.ch12
Sosa-Nishizaki, O., Márquez-Farías, J. F., & Villavicencio-Garayzar, C. J. (2002). Case study: pelagic shark fisheries along the west coast of Mexico. In M. D. Camhi, E. K. Pikitch, & E. A. Babcock (Eds.), Sharks of the open Ocean. Biology, fisheries and conservation (pp. 275–282). Oxford, U.K.: Blackwell Publishing.