First record of Brachylaima (Trematoda: Brachylaimidae) in Salvator merianae (Squamata: Teiidae)
Nayely Martínez-Meléndez⁎ ✉ , Manuel Martínez-Meléndez, Roberto García-Martínez
Twenty-four gastrointestinal tracts of Salvator merianae were examined for helminths research. Immature forms of Brachylaima sp. were identified with a prevalence of 4.16%. This is the first occurrence the Brachylaimidae for this host species in Brazil and in South America since the previous records of Brachylaima sp. in lizards were known only from Europe and Eurasia.
© 2017 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:
Argentine black and white tegu; Trematoda; Parasites
Primer registro de Brachylaima (Trematoda: Brachylaimidae) en Salvator merianae (Squamata: Teiidae)
Resumen
Se revisó el tubo gastrointestinal de 24 tegus argentinos Salvator merianae en busca de helmintos. Se encontraron formas inmaduras de Brachylaima sp. con una prevalencia del 4.16%. Este es el primer registro de Brachylaimidae en esta especie de hospedero en Brasil y en América del Sur, ya que los registros previos de Brachylaima sp. en lagartos se tenían solo de Europa y Eurasia.
Palabras clave
Tegu blanquinegro argentino; Trematoda; Parásitos
The geographical distribution of Salvator merianae (Duméril and Bibron, 1839) (Squamata: Teiidae), Argentine black and white tegu comprises eastern Bolivia, Argentina, Uruguay and Brazil ( Embert, Fitzgerald, & Waldez, 2010; Loebmann & Quintela, 2009 ). The lizard is omnivorous, feeding on fruits, insects, snails, fish, amphibians, reptiles, rodents, adult birds, nestlings and eggs ( Achaval & Olmos, 2003).
Regarding the helminths of S. merianae , the diversity is composed of 6 species of Nematoda: Cruzia travassosi Kalil and Vogelsangi, 1932 (Kathlaniidae); Physaloptera retusa Rudolphi, 1819 (Physalopteridae); Diaphanocephalus galeatus (Rudolphi, 1819) (Diaphanocephalidae); Spinicauda spinicauda Olfers, 1819 (Heterakidae); Physaloptera tupinambae Pereira, Alves, Rocha, Lima, and Luque, 2012 (Physalopteridae); Physaloptera bainae Pereira, Alves, Rocha, Lima, and Luque, 2014 (Physalopteridae) and 1 species of Cestoda, Oochoristica sp. (Cyclophyllidea) ( Ávila & Silva, 2010, 2011; Pereira, Alves, Rocha, Lima, & Luque, 2012, 2014; Vieira, Bernardon, & Müller, 2016 ). In this context, the present study had the aim to report the occurrence of Brachylaimidae (Trematoda) and their parasitological indexes in S. merianae.
Twenty-four S. merianae were necropsied (collected in the period of 2007–2015), 20 collected on highways of southern Brazil (in Rio Grande do Sul State) under the autorization “Instituto Chico Mendes de Conservação da Biodiversidade – Sistema de Autorização e Informação em Biodiversidade” (ICMBIO-SISBIO 38913-1) from municipalities of Pelotas (31°46′19″ S, 52°20′33″ W) ( n = 13), Capão do Leão (31°46′3″ S, 52°26′55″ W) ( n = 10), and Morro Redondo (31°35′18″ S, 52°37′47″ W) ( n = 1), and 4 were donated by the “Núcleo de Reabilitação da Fauna Silvestre e Centro de Triagem de Animais Silvestres da Universidade Federal de Pelotas” (NURFS-CETAS/UFPel).
The gastrointestinal tracts were sectioned into esophagus, stomach, small intestine and large intestine. These were dissected and washed with a water jet through a sieve (150 μm) and the resulting contents and mucous were examined under an Olympus SZ 51 stereomicroscope. The trematodes were quantified, fixed in AFA, stored in alcohol 70% and dyed with Carmin’s Langeron and mounted on slides with Canada’s balsam. These were viewed with an Olympus CX21 microscope for morphological and morphometric identification according to Gibson, Jones, and Bray (2002) and Valente, Diaz, Salomón, and Navone (2016) .
Vouchers specimens are deposited in “Coleção de Helmintos do Laboratório de Parasitologia de Animais Silvestres do Instituto de Biologia, Universidade Federal de Pelotas (CHLAPASIL/UFPel)” (Nos. 637 and 638).
Measurements are expressed in millimeters (the mean shown in parentheses) and the photographs were taken with an Olympus BX 41 microscope with attached camera system. The parameters calculated were prevalence (P), mean abundance (MA) and mean intensity of infection (MI) according to Bush, Lafferty, Lotz, and Shostak (1997) .
Class Trematoda Rudolphi, 1808
Subclass Digenea Carus, 1863
Family Brachylaimidae Joyeux & Foley, 1930
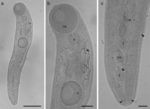
Genus Brachylaima Dujardin, 1843: (immature forms) ( Fig. 1a–c):
Figure 1
(a–c) Brachylaima Dujardin, 1843 (Trematoda: Brachylaimidae) parasite of Salvator merianae (Duméril and Bibron, 1839) from southern of Brazil. (a) Body of Brachylaima (bar = 0.42 mm); (b) OS = oral sucker; P = pharynx; A = acetabulum (bar = 0.1 mm); (c) GP = genital pore; OV = ovary; T = testes; arrow heads indicate the cecum (bar = 0.06 mm).
Characterization: based on 8 specimens (5 were measured). Length of body 1.66–2.35 (2.04 ± 0.34); width of body (level of acetabulum) 0.3–0.6 (0.43 ± 0.12); length of oral sucker 0.25–0.36 (0.31 ± 0.02); width of oral sucker 0.25–0.35 (0.31 ± 0.02); length of pharynx 0.1–0.18 (0.13 ± 0.03); width of pharynx 0.12–0.16 (0.14 ± 0.01); length of acetabulum 0.23–0.28 (0.26 ± 0.02); width of acetabulum 0.18–0.27 (0.23 ± 0.04); length of ovary 0.04–0.1 (0.07 ± 0.02); width of ovary 0.04–0.08 (0.05 ± 0.02); length of the anterior testicle 0.05–0.1 (0.08 ± 0.02) and width of anterior testicle 0.04–0.09 (0.06 ± 0.02); length of the posterior testicle 0.05–0.1 (0.08 ± 0.02) and width of the posterior testicle 0.03–0.08 (0.06 ± 0.02). The specimens do not present eggs.
Although the specimens found are immature forms, the following morphological characteristics allowed us to assign them to the genus Brachylaima : body elongated; acetabulum located in the anterior half of the body; presence of muscular pharynx; caeca long slightly sinuous, extending to the posterior extremity of the body; uterus intercaecal; vitelline fields extending between the posterior margin of the acetabulum to the anterior margin of the anterior testis; genital pore located in the anterior margin of anterior testis; gonads located in the posterior half of the body near the posterior extremity of the body.
Eight trematodes were found parasitizing the small intestine of only 1 S. merianae (P = 4.16%; MA = 0.33; MI = 8) from Pelotas. The specimens when compared to adults are smaller in size with respect to morphology, exhibit testis, ovary and vitelline glands reduced, and the absence of eggs.
The history of Brachylaima Dujardin, 1843 is confusing at first, because some authors, generating various synonyms, used different spelling. Brachylaima Dujardin, 1843 (Syns Brachylaime Dujardin, 1843; Brachylaimus Dujardin, 1845; Brachylaemus Blanchard, 1847; Harmostomum Braun, 1899; Heterolope Looss, 1899; Entosiphonus Sinitsin, 1931; Centrodes Travassos & Kohn, 1964; Mazzantia Travassos & Kohn, 1964; Rallitrema Travassos & Kohn, 1964) ( Gibson et al., 2002). According to Pojmanska (2002) , Brachylaimidae Joyeux and Foley, 1930 is composed of Brachylaiminae Joyeux and Foley, 1930 and Ityogoniminae Yamaguti, 1958. Brachylaiminae includes Brachylaima; Glaphyrostomum Braun, 1901; Parabrachylaima Lotz and Corkum, 1975; Postharmostomum Witenberg, 1923 and Ectosiphomus Sinitsin, 1931 (Gibson et al., 2002).
In Brazil, 4 species of Brachylaima, Brachylaima advena Dujardin, 1843; Brachylaima centrodes (Braun, 1901); Brachylaima marsupium (Braun, 1901) and Brachylaima mazzantii (Travassos, 1927) have been registered in mammals (Muridae, Cricetidae; Didelphidae) and birds (Columbidae, Odontophoridae, Tinamidae) ( Travassos, Teixeira-de Freitas, & Kohn, 1969 ). Although there are no reports of Brachylaima sp. parasiting S. merianae , other digeneans were found parasitizing Teiidae, in Tupinambis teguixin Linnaeus, 1758: Pulchrosomoides elegans Freitas and Lent, 1937 (Cathaemasiidae); Paradistomum parvissimum (Travassos, 1918) (Pronocephalidae); Dasymetra tupinambis Nasir and Diaz, 1971 (Plagiorchiidae) and in Tupinambis rufescens (Günther, 1871): Styphlodora condita Faria, 1911 (Plagiorchiidae) ( Ávila & Silva, 2010).
Brachylaima species are generally found in the alimentary tract of birds, mammals and rarely in amphibians ( Gibson et al., 2002 ). For lizards, (Squamata) immature forms were registered in the Spain by Roca, López-Balaguer, and Hornero (1989) in Podarcis bocagei (Seoane, 1884) (Lacertidae) (P = 0.9%). For the islands (Balearic Archipelago) in Podarcis pityusensis (Boscá, 1883) [P = 0.2% (n = 564)] and in Podarcis lilfordi (Günther, 1874) [P = 0.8%; MA = 0.01; MI = 1.3 (n = 386)] (Roca, 1996).
In Portugal, Galdón, Roca, Barbosa and Carretero (2006) recorded in P. bocagei [P = 0.4% (n = 249)] and Podarcis carbonelli Pérez Mellado, 1981 (Lacertidae [P = 1.2%; MA = 4.7; MI = 0.05) (n = 257)]. In Turkey, Yildirimhan, Bursey, and Altunel (2011) in Lacerta trilineata Bedriaga, 1886 (Lacertidae) [P = 9%; MA = 0.4; MI = 5 (n = 38)] and Incedogan, Yildirimhan, and Bursey (2014) in Chalcides ocellatus (Forskal, 1775) (Scincidae) [P = 2.2%; MA = 0.02; MI = 12 (n = 45)]. In Poland, registered in Anguis fragilis Linnaeus, 1758 (Anguidae) and Lacerta agilis Linnaeus, 1758 (Lacertidae) ( Lewin, 1990, 1992 apud Incedogan, Yildirimhan, & Bursey, 2014 ).
In this study, the prevalence, mean abundance and mean intensity values of infection found for Brachylaima sp. in S. merianae were low (P = 4.16%; MA = 0.33; MI = 8), corroborating with previous studies which indicate that this is a parasite atypical in lizards ( Galdón, Roca, Barbosa, & Carretero, 2006; Incedogan et al., 2014; Roca et al., 1989; Yildirimhan, Bursey, & Altunel, 2011 ).
The life cycle of Brachylaima spp. has 2 terrestrial gastropods as intermediate hosts and birds, mammals and less frequently amphibians and reptiles as definitive hosts ( Butcher & Grove, 2001) . It is probable that the infestation of Brachylaima sp. in S. merinae occurred through the feeding cycle ( Kiefer & Sazima, 2002).
Cases of infection Brachylaima spp. in humans were recorded in South Australia ( Butcher, 2016; Butcher & Grove, 2001 ). The patients (2 children and 1 adult) had gastrointestinal symptoms such as abdominal pain and chronic diarrhea. After treatment, adults and eggs of the trematodes were obtained and through experimental infection were identified as Brachylaima cribbi Butcher and Groove, 2001 (Butcher, 2016 ). Both patients lived in rural areas, with plenty of snails, which after analysis of these cases were confirmed as the source of infection in children and adults, the ingestion of raw snails and vegetables contaminated respectively ( Butcher, 2016 ). Although in Brazil there are no cases of infection in humans, the occurrence of Brachylaima sp. in S. merianae serves as an alert for possible infections, and in other animals that are natural predators such as the carnivorus mammals: Procyon cacrivorus (Cuvier, 1789), and Canis lupus familiaris Linnaeus, 1758 ( Quintela, Lob, & Artioli, 2014; Rangel & Neiva, 2013 ), once the digenetic has zoonotic potential, and the predators may hold their dispersion.
For the first time in Brazil and the South American continent, immature forms of Brachylaima sp. were registered in S. merianae presenting the morphological characterization, parasitological indexes and photographies.
We thank the ICMBio for authorization, the NURFS-CETAS/UFPel for donating dead lizards, and Coordenação de Aperfeiçoamento do Pessoal de Nível Superior by financial support through the edict 2010/032.
Referencias
Achaval, F. & Olmos, A. (2003). Anfíbios y Reptiles del Uruguay (2ª ed.). Montevideo: Montevideo, Graphis.
Ávila, W. R. & Silva, J. R. (2010). Checklist of helminths from lizards and amphisbaenians (Reptilia: Squamata) of South America. The Journal of Venomous Animals and Toxins including Tropical Diseases, 16, 543-572.
Ávila, W. R. & Silva, J. R. (2011). Helminths of Lizards (Reptilia: Squamata) from Mato Grosso State, Brazil. Comparative Parasitology, 78, 129-139.
Bush, A. O., Lafferty, K. D., Lotz J. M., & Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. The Journal of Parasitology, 83, 575-583.
Butcher, A. R., & Grove, D. I. (2001). Description of the life-cycle stages of Brachylaima cribbi n. sp. (Digenea: Brachylaimidae) derived from eggs recovered from human faeces in Australia. Systematic Parasitology, 49, 211-221.
Butcher, A. R. (2016). Children, snails and worms: the Brachylaima cribbi story. Microbiology Australia. 37, 30-33.
Embert, D., Fitzgerald, L. & Waldez, F. (2010). Salvator merianae. The IUCN Red List of Threatened Species.Version 2014.3. Retrieved on April 19, 2016 from: www.iucnredlist.org.
Galdón, M. A., Roca, V., Barbosa, D., & Carretero, M. A. (2006). Intestinal helminth communities of Podarcis bocagei and Podarcis carbonelli (Sauria: Lacertidae) in NW Portugal. Helminthologia, 43, 37-41.
Gibson, D. I; Jones, A., & Bray, R. A. (2002). Keys to the Trematoda. London: UK: Ed. CABI.
Incedogan, S., Yildirimhan, H. S., & Bursey, C. R. (2014). Helminth parasites of the ocellated skink, Chalcides ocellatus (Forskal, 1775) (Scincidae) from Turkey. Comparative Parasitology, 81, 260-269.
Kiefer, M. C., & Sazima, I. (2002). Diet of juvenile tegu lizard Tupinambis merianae (Teiidae) in southeastern Brazil. Amphibia-Reptilia, 23, 105-108.
Lewin, J. (1990). Parasitic worms in a slowworm (Anguis fragilis L.) population from the Bieszczady Mountains (Poland). Acta Parasitologica Polonica, 35, 207–215.
Lewin, J. (1992). Parasites of the sand lizard (Lacerta agilis L.) in Poland. Acta Parasitologica, 37, 19–24.
Loebmann, D., & Quintela, F. M. (2009) Guia Ilustrado – Os Répteis da Região Costeira do Extremo Sul do Brasil. 1ªed, Rio Grande do Sul: USEB: Pelotas.
Pereira, F. B., Alves, V. P., Rocha, B. M., Lima, S. S., & Luque, J. L. (2012). A New Physaloptera (Nematoda: Physalopteridae) Parasite of Tupinambis merianae (Squamata: Teiidae) from South eastern Brazil. Journal of Parasitology, 98, 1227-1235.
Pereira, F. B., Alves, V. P., Rocha, B. M., Lima, S. S., & Luque, J. L. (2014). Physaloptera bainae n. sp. (Nematoda: Physalopteridae) Parasitic in Salvator merianae (Squamata: Teiidae), with a Key to Physaloptera Species Parasitizing Reptiles from Brazil. Journal of Parasitology, 100, 221-227.
Quintela, F. M., Lob, G., & Artioli, L. G. S. (2014). Diet of Procyon cancrivorus (Carnivora, Procyonidae) in restinga and estuarine environments of southern Brazil. Iheringia. Série Zoologia, 104, 143-149.
Roca, V., López-Balaguer, E., & Hornero, M. J. (1989). Helmintofauna de Podarcis hispanica (Steindachner, 1870) y Podarcis bocagei (Seoane, 1884) (Reptilia: Lacertidae) em el Cuadrante Noroccidental de la Península Ibérica. Revista Ibérica de Parasitología, 49, 127-135.
Roca, V. (1996). The effect of some factors on the helminth parasite infracommunities of Podarcis lizards in the Balearic Islands (Western Mediterranean). Bolleti de la Societat d’Historia Natural de les Balears, 39, 65-76.
Rangel, C. H., & Neiva, C. H. M. B. (2014). Predação de vertebrados por cães Canis lupus F. Familiaris (Mammalia: Carnivora) no Jardim Botânico do Rio de Janeiro. Biodiversidade Brasileira, 2, 261-269.
Travassos, L., Teixeira de Feitas, J. F., &Kohn, A. (1969) Trematódeos do Brasil. Memórias do Instituto Oswaldo Cruz, 67, 1-886.
Valente, R., Diaz, J. I., Salomón, O. D., & Navone, G. T. (2016). The role of Phyllocaulis variegatus (Mollusca: Veronicellidae) on the transmission of digenean parasites. Revista Mexicana de Biodiversidad, 87, 255-257.
Vieira, T. D., Bernardon, F.F., & Müller, G. (2016). Diaphanocephalus galeatus (Nematoda: Diaphanocephalidae), parasite of Salvator merianae (Squamata: Teiidae) in southern Brazil. Revista Mexicana de Biodiversidad, 87, 512-515.
Yildirimhan, H. S., Bursey, C.R., & Altunel, F. N. (2011). Helminth parasites of the Balkan green lizard, Lacerta trilineata Bedriaga 1886, from Bursa, Turkey. Turkish Journal of Zoology. 35, 519-535.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
*
Corresponding author.
Copyright © 2017 Universidad Nacional Autónoma de México, Instituto de Biología.