Alejandra Ochoa-González a, b, Octavio R. Rojas-Soto c, David A. Prieto-Torres a, d, María del Coro Arizmendi e, Adolfo G. Navarro-Sigüenza a, *
a Universidad Nacional Autónoma de México, Facultad de Ciencias, Departamento de Biología Evolutiva, Museo de Zoología, Circuito Exterior s/n, Ciudad Universitaria, Apartado postal 70-399, 04510 Ciudad de México, Mexico
b Universidad Nacional Autónoma de México, Posgrado en Ciencias Biológicas, Edificio D, 1º Piso, Circuito de Posgrados, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
c Instituto de Ecología, A.C., Red de Biología Evolutiva, Laboratorio de Bioclimatología, carretera antigua a Coatepec No. 351, El Haya, 91070 Xalapa, Veracruz, Mexico
d Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Laboratorio de Biodiversidad y Cambio Global, Avenida de los Barrios Número 1, Colonia Los Reyes Ixtacala, 54090 Tlalnepantla, Estado de México, Mexico
e Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Unidad de Biología Tecnología y Prototipos, Avenida de los Barrios Número 1, Colonia Los Reyes Ixtacala, 54090 Tlalnepantla, Estado de México, Mexico
*Corresponding author: adolfon@ciencias.unam.mx (A.G. Navarro-Sigüenza)
Received: 1 March 2023; accepted: 29 September 2023
Abstract
Migratory birds move geographically by tracking specific climatic conditions through time. However, we lack information about the climatic conditions birds are tracking, especially in intratropical migrants, whose movements are contained inside the tropics. The Yellow-green Vireo Vireo flavoviridis is an intratropical migrant whose migration patterns remain only partially documented and understood. Using GBIF presence records and WorldClim monthly climatic layers, we reconstructed ecological niche for Yellow-green Vireo’ reproductive and non-reproductive seasons. Then, we used a niche overlap analysis, based on a PCA-env approach and similarity tests, to assess overlap in climatic niches between seasons. We also projected climatic niches onto their spring and fall migration to evaluate the climatic conditions tracked by the species in transitional months. Overall, models revealed significant geographic inter-prediction between seasons. Similarity analyses showed partial niche overlap between seasons; however, they failed to reject the null hypothesis of niche similarity. As expected by the hypothesis of niche conservatism in the tropics, Yellow-green Vireo is a niche follower. This information will help to clarify evolution of intratropical migration and provide ecological information for future conservation plans.
Keywords: Climate niche; Ecological niche models; Evolution of migration; Intratropical migration; Neotropics
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Hogar en los trópicos: seguimiento estacional del nicho por el vireo verdiamarillo, Vireo flavoviridis, un migrante intratropical
Resumen
Actualmente, carecemos de información sobre qué condiciones climáticas están rastreando las aves migratorias, especialmente las intratropicales, cuyos movimientos están contenidos entre los trópicos. El vireo verde amarillo Vireo flavoviridis es un migrante intratropical, cuyos patrones de migración permanecen parcialmente documentados e hipotetizamos que rastrea nichos climáticos similares entre las estaciones reproductiva y no reproductiva (tendencia de conservadurismo de nicho en los trópicos). Utilizando registros de presencia de GBIF y capas climáticas mensuales de WorldClim, reconstruimos el nicho ecológico para las temporadas reproductiva y no reproductiva. Usamos un análisis de superposición de nicho, basado en un enfoque de PCA-env y pruebas de similitud para evaluar la superposición en el nicho climático entre estaciones. Proyectamos esos nichos climáticos en su migración de primavera y otoño para evaluar las condiciones climáticas rastreadas por la especie en los meses de transición. Los modelos revelaron una significativa interpredicción geográfica entre estaciones. Los análisis de similitud mostraron una superposición parcial de nichos entre temporadas. Como era de esperar por la hipótesis del conservadurismo de nicho en los trópicos, el vireo verdiamarillo es un seguidor de nicho. Esta información ayuda a la comprensión de la migración intratropical y futuros planes de conservación.
Palabras clave: Nicho climático; Modelos de nicho ecológico; Evolución de la migración; Migración intratropical; Neotrópico
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
Intratropical migration is a complex process of behavioral strategies in which species breed and migrate within the limits of the tropics of Cancer and Capricorn (Faaborg et al., 2010a; Hayes, 1995). Migratory birds following intratropical migration could perform 3 principal movement types between their reproductive and non-reproductive areas: 1) latitudinal migration refers to movements from reproductive to non-reproductive latitudes and back. This pattern has been the most documented among species that spend the non-breeding season in the Amazon basin (Faaborg et al., 2010a), such as the Yellow-green Vireo (Vireo flavoviridis) and Piratic Flycatcher (Legatus leucophaius; Morton, 1977); 2) altitudinal migration, that is, seasonal movements along an elevational gradient, which have been documented in every mountain system in the world (Navarro-Sigüenza, 1992; Boyle, 2008, 2017); and 3) longitudinal migration, where movements are more longitudinal than latitudinal (Davenport et al., 2012) like the one performed by the Orinoco Goose (Neochen jubata; Davenport et al., 2012). Furthermore, there are also mixed and complex intratropical migrations (Faaborg et al., 2010a), in which species make a first migration from their breeding areas in temperate zones (e.g., northern North America or southern South America) to non-breeding areas in tropical zones and then make a second migration within the tropics (Callo et al., 2013; Janh et al., 2016). Ecological and behavioral roles involved in this mixed migration are not clear (e.g., Heckscher et al., 2011; Stutchbury et al., 2016), as well as intratropical migration is understudied sensu lato (Faaborg et al., 2010a; Jahn et al., 2020).
There are 2 main theories involving the evolution of bird migration: 1) the “northern home”, suggesting that some birds began their migratory behavior from temperate zones to tropical or subtropical areas (Bell, 2000; Winger et al., 2014), and 2) the “southern home”, hypothesizing that some birds began their migratory behavior from tropical or subtropical areas to temperate zones (Heckscher et al., 2015; Salewski & Bruderer, 2007; Zink, 2011). Levey and Stiles (1992) suggest these “southern home” migratory movements are predecessors of the movements towards temperate zones, in which species capable of switching environmental conditions were able to overcome climate disparities between tropical and temperate zones (Wiens & Donoghue, 2004). These 2 well-accepted theories served as a basis for a third theory, in which the concept of ecological niche defined as the environmental conditions to survive and maintain a species’ populations in a particular space has been considered (Hutchinson, 1957). Nakazawa et al. (2004) suggested that migratory species could follow 2 main patterns. They could shift between zones where environmental conditions are not similar —or niche switchers (i.e., capable of switching climatic conditions) —or between zones where environmental conditions are similar —or niche followers (i.e., not capable of switching climate conditions)–. This species’ lack of capability to move between areas with different climatic conditions has led to Wiens and Donoghue (2004) to propose a trend of niche conservatism which “forces” tropical migratory species to return to the tropics. Following/ switching migration types have been mostly studied with species moving between Neartic/Austral areas to Neotropical areas (La Sorte et al., 2017; MacPherson et al., 2018; Peña-Peniche et al., 2018; Zurell, 2018), letting the intratropical migration as an understudied theme (Faaborg et al., 2010a; Rappole, 2013; Tobón-Sampedro & Rojas-Soto, 2015; Sánchez-Barradas et al., 2017). One of the few analyses made with intratropical migration species was made under stable isotopes approach, resulting in species using similar environmental characteristics throughout the year, such as species following the same ecological niches (Guaraldo et al., 2016).
The Yellow-green Vireo (Vireo flavoviridis, Aves: Vireonidae) has been described as an intratropical migratory taxon (Morton, 1977; Styrsky et al., 2004), distributed from northern México to Panamá when breeding, and from Panamá to northern Bolivia when non-breeding (del Hoyo et al., 2010; Schulenberg, 2019), but recently in western coastal Perú (Guevara-Torres et al., 2017). Some authors have considered this species as subspecies of V. olivaceus (Battery & Klicka, 2017), but it is considered species in Clements et al. (2021) checklist. It is one of the 4 taxa within the Red-eyed Vireo group, which includes V. olivaceus, V. flavoviridis, V. altiloquus, and V. magister, and is recognized as an early divergence in such a monophyletic lineage (Battery & Klicka, 2017). It inhabits open fields with scattered trees, plantations, riparian forests and forest edges and has frugivorous/ insectivorous dietary habits (del Hoyo et al., 2010). It occurs in middle levels and canopy of forests (Skutch, 1960). Females build their 6.5 cm wide nests using a variety of plant materials, attaching them to a little branch of a tree at 1.5 to 3.5 meters from the ground (Kaufman, 2005). They commonly lay 2-3 eggs, from March to June, incubated only by the female, but the male helps in feeding (Skutch, 1960). Food availability could stimulate nesting and influence migration (Morton, 1977), going southwards by mid-October and northwards by February to March (Skutch, 1960). Probably following different routes in spring and autumn migration (Gomez et al., 2013). It is categorized as a Least Concern species by the IUCN red list (IUCN, 2022). Nevertheless, one of their breeding habitats is in danger since vegetation type from tropical dry forests has a higher rate of deforestation because it sustains activities such as agriculture and cattle raising, which has contributed to its reduction, remaining only the 35% of the original distribution, and < 10% of its distribution belongs to national protected areas (Portillo-Quintero & Sánchez-Azofeifa, 2010; Prieto-Torres et al., 2018). Additionally, the lowland tropical forests in which they live during the non-breeding season are also highly threatened by deforestation (Armenteras et al., 2017).
Here, we characterized the climatic conditions tracked by the Yellow-green Vireo, and explored whether this species migrates as a niche follower or a niche switcher. First, we build ecological niche models for reproductive and non-reproductive seasons and estimate the prediction areas between seasons. Then, we assess the similarity of niche models between seasons. Finally, we examine seasonal conditions tracked by the Yellow-green Vireo during its migration months. This study will provide new, more accurate information on the environmental needs of the species during reproductive and non-reproductive areas and during transition. Furthermore, a better understanding of species seasonal climatic niche could help us to detect which areas could be threatened, driven mainly by deforestation and accelerated climate change (Feeley & Silman, 2011; Portillo-Quintero & Sánchez-Azofeifa, 2010).
Materials and methods
Occurrence records and climate data. We obtained historical presence records for Yellow-green Vireo from the Global Biodiversity Information Facility database (GBIF; https://doi.org/10.15468/dl.rcqler; 24 July 2019). To improve the overall temporal correspondence that should exist between occurrences and environmental variables and data quality (Phillip et al., 2006), we selected occurrence records from 1950 to 2019 avoiding poor accuracy and lack of precision in the georeferencing of older data, and uncertainty in geographic coordinates that had elevational range < 1,700 m and precise coordinates (> 2 decimal places) (Marcer et al., 2022; Murphy et al., 2004). Because sampling bias in the data could affect model calibration (Anderson, 2012), we performed a spatial thinning of 20 km using the “spThin” library for R software (Aiello-Lammens et al., 2015). Occurrence records that did not match the species ranges defined by BirdLife International (https://www.birdlife.org/; 01 September 2019) and the Neotropical Birds website (https://neotropical.birds.cornell.edu; 01 September 2019) were deleted. This last step was essential to identify problematic or imprecise species occurrences with incorrect climatic values since the choice of climate baseline is also important for model performance (Boria et al., 2014; Roubiceka et al., 2010). These procedures yielded 866 historical records, spatially and temporally (January to December) unique.
We classified the records in 2 seasons, breeding and non-reproductive, based on the 3 months with the highest spatial and temporal concentration of records for V. flavoviridis in those areas defined as reproductive (northern Mexico to Panama) than those non-reproductive sites (from Panama to northern Bolivia). We obtained 373 unique occurrences for the breeding season (from May to July), across May (n = 134), June (n = 126), and July (n = 113) and 71 for the non-reproductive season (November to January) —November [29], December [26], and January [16]—. Also, to evaluate the climatic conditions tracked by the species during spring and fall migration, we obtained records from February (n = 25), March (69), April (96), August (75), September (77), and October (80). It is important to highlight that these periods were also consistent with findings in the literature (e.g., del Hoyo et al., 2010).
To build the ecological niche model, as environmental predictors, we used 5 monthly bioclimatic variables (at 30” spatial resolution [i.e., ~ 1 km2]): maximum temperature (tmax), minimum temperature (tmin), total precipitation, evapotranspiration and wind speed. To test for collinearity between variables, we performed a Pearson correlation analysis (Wei & Simko, 2017) and an exploratory Jackknife analysis assessing the contribution of them to models calculated in MaxEnt (Elith et al., 2011; Phillips et al., 2006). In this sense, we decided to: i) include both tmax and tmin variables because had higher contribution to the models; ii) discard the evapotranspiration because shows the same contribution levels that precipitation (with highest correlation values r > 0.8); iii) exclude the wind speed due to its unimportance for the overall models. From this perspective, our selection criteria were based on ecological aspects important to the species; especially considering that bird assemblages throughout the tropics may show a closer relationship with these climatic factors, such as temperature and precipitation (Prieto-Torres & Rojas-Soto, 2016; Werneck et al., 2011), compared to the availability of local resources (Santillán et al., 2018). Also, we decided not to include tmeans in the analysis since the exclusive use of averages could underestimate the true size of the species’ niche in N-dimensional space (Pérez-Navarro et al., 2020). The bioclimatic variables were downloaded directly from the WorldClim 2.1 database (available in: https://www.worldclim.org/data/monthlywth.html; Fick & Hijmans, 2017) which contains updated climate data, including weather stations installed between 1960 and 2020, for interpolation across Earth’s surface (Fick & Hijmans, 2017).
Ecological niche modelling. We decided to perform independent ENM for the reproductive and non-reproductive seasons because some migratory species can use different environmental conditions over the year (Nakazawa et al., 2004, Peña-Peniche et al., 2018). Since both seasons are composed of 3 months, we used the sum of the records for the 3 months to develop the corresponding ENM. We used 70% of the occurrence records available for each case as training data during the model calibration and the remaining 30% as testing data for internal validation of the model. In addition, we used the average precipitation over the 3 months as environmental predictors, the layer of the month with the highest maximum, and the layer of the month with the lowest minimum temperature.
Following Barve et al. (2011), we created an area for model calibration (or “M” sensu Soberón & Peterson, 2005; Supplementary material: Fig. 1) that reflects the historically accessible areas and restriction regions (e.g., including dispersal barriers) for the species. Here, we created one calibration area for all year records considering the WWF terrestrial ecoregions (Olson et al., 2001) occupied by the species and a 200 km2 buffer area around each presence record. This kind of distance constraint has been shown to reduce model overprediction (Allouche et al., 2008; Mendes et al., 2020). This consideration assumes that these regions and their boundaries are barriers that limit species distribution and represent the area that species have historically been able to explore (Barve et al., 2011; Soberón et al., 2010). The final polygon obtained was used as a GIS mask across the environmental layers used to perform the ENMs. These processes were developed using ArcGIS 10.2.1 (ESRI, 2011) and the “Terra” package (Hijmans & Etten, 2012) in R software v. 3.6.0 (R Core Team, 2017).
All models were generated by the maximum entropy algorithm in MaxEnt 3.4.1, representing the species’ ecological niche in the examined environmental dimensions based on presence-only datasets (Phillips et al., 2006). The Maxent program uses machine learning to obtain a geographic distribution of the most likely distribution of suitable conditions for the focal species as a function of localities and environmental variables (Phillips et al., 2006). We decided to use MaxEnt because it has been proven to perform better when presence-only data is available (Elith et al., 2011), as in our case. This software produces robust models if more than 15 occurrence points are available for each species, or season (Elith et al., 2011; Wisz et al., 2008).
We used the kuenm R package (Cobos et al., 2019) to perform a calibration protocol assessing the model complexity (Merow et al., 2014) and generated 5 replicate resamplings (bootstrap). The model calibration test was created considering 7 distinct regularization multipliers (0.1 to 1 at intervals of 0.3, 2 to 4 at intervals of 1), which influences how closely the obtained output distribution is fitted; values less than the default of 1.0 will produce a more localized output distribution which will fit the given presence records; and a larger regularization multiplier will produce a more extended, less localized prediction (Phillips et al., 2006). We also considered 5 feature classes: L, LP, LQ, LQP and QP (where L = linear, Q = quadratic and P = product), these are the functions to which the response curves of the species-variables are fitted (Phillips et al., 2006). We performed this step to evaluate various candidate models and select the best based on multiple model quality criteria. We allowed extrapolation by clamping, which complements by extrapolation of the response curves to each variable since we do not have comprehensive knowledge of species’ environmental limits, and it has been shown that niche extrapolation is preferable when making projections into other spatio-temporal scenarios (Owens et al., 2013).
Final models were evaluated and selected considering biological and statistical significance in the following order: partial ROC test —measures the detection efficiency of the model by comparing training data vs. testing data—, omission rates (≤ 5%), and model complexity level using the Akaike Information Criterion (AICc) (Cobos et al., 2019; Peterson et al., 2008; Warren & Seifert, 2011). When there was more than one final model selected, we used the median of the results of all replicates as the final model (Cobos et al., 2019). Then, we converted the continuous models obtained for each season into binary (presence vs. absence) maps using the tenth percentile training presence threshold, considering the error variation within presence records from different sources (e.g., Escalona et al., 2017). This reduced commission errors (i.e., areas of over-prediction) in our final binary maps (Liu et al., 2013).
Comparisons between seasonal climate niches. To define the similarity among the seasonal niches of the species, we used 2 methodological approaches to compare the climatic conditions that define the reproductive and non-reproductive periods for the species (ecological vs. geographical).
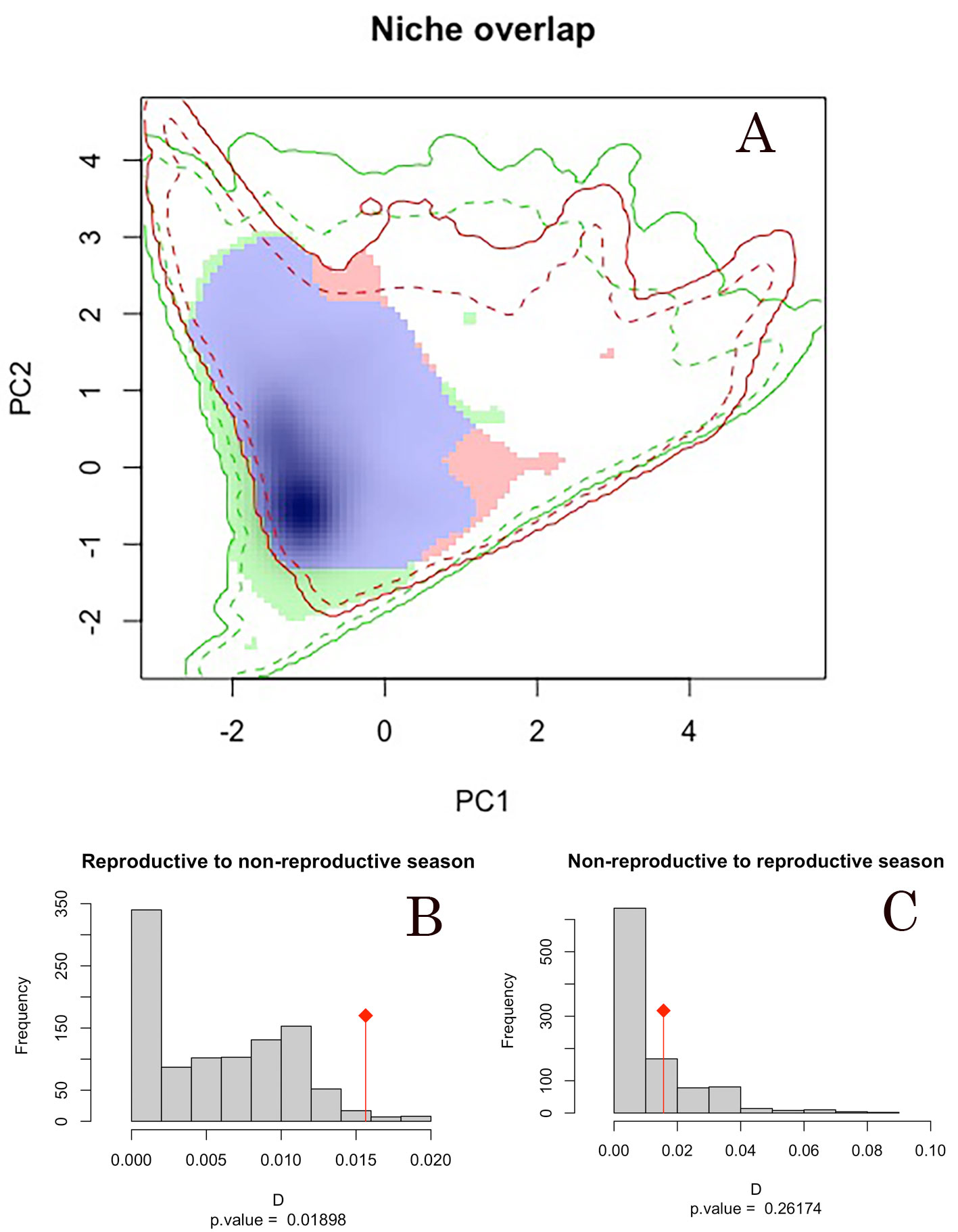
We used the ecospat R package (Di Cola et al., 2017) to understand the role of ecological conditions in the niche similarity or dissimilarity across the spatio-temporal distributional patterns of Yellow-green Vireo. We performed tests of niche similarity following the 3 steps proposed by Broennimann et al. (2012). First, we calculated the density of occurrences and environmental factors (the same 3 environmental variables described above) along the axes of a multivariate analysis (Principal Component Analysis [PCA-env]). Second, we evaluated niche overlap between the 2 selected seasons, pooling data along the gradient of the multivariate analysis by applying Schoener’s D, which generates an index from 0 [no-overlapping niches] to 1 [overlapping niches] (Schoener, 1968). Third, we performed statistical tests to compare the empirically observed distributions of Schoener’s D to 1,000 randomly generated simulated values (Broennimann et al., 2012; Warren et al., 2008). We considered that niches were more similar than random when the observed D values were significantly (p ˂ 0.05) greater than the null from the values expected for simulated overlap. In that case, the hypothesis of niche similarity (i.e., niche conservatism between seasons) was accepted (Broennimann et al., 2012; Warren et al., 2008). We developed 2 niche overlap analyses, with the summer niche as the reference and shifted only the winter niche and viceversa (rand.type = 2) (Di Cola et al., 2017).
As a second approach, the ENM generated for each season was geographically projected onto each other season’s conditions to test the inter-prediction power (i.e., the degree of geographical overlap between them). We also projected the ENM onto the transition months (February to April, and August to October) to determine which seasonal conditions birds track during their movements. To do this, we estimated the predictive ability of projected models based on the predicted total occurrence records. And finally, we create a visualization of niche in 3 environmental dimensions, with Niche Analyst 3.0 (NicheA), to explore niche overlap between seasons (Guisan et al., 2014; Qiao et al., 2016).
Results
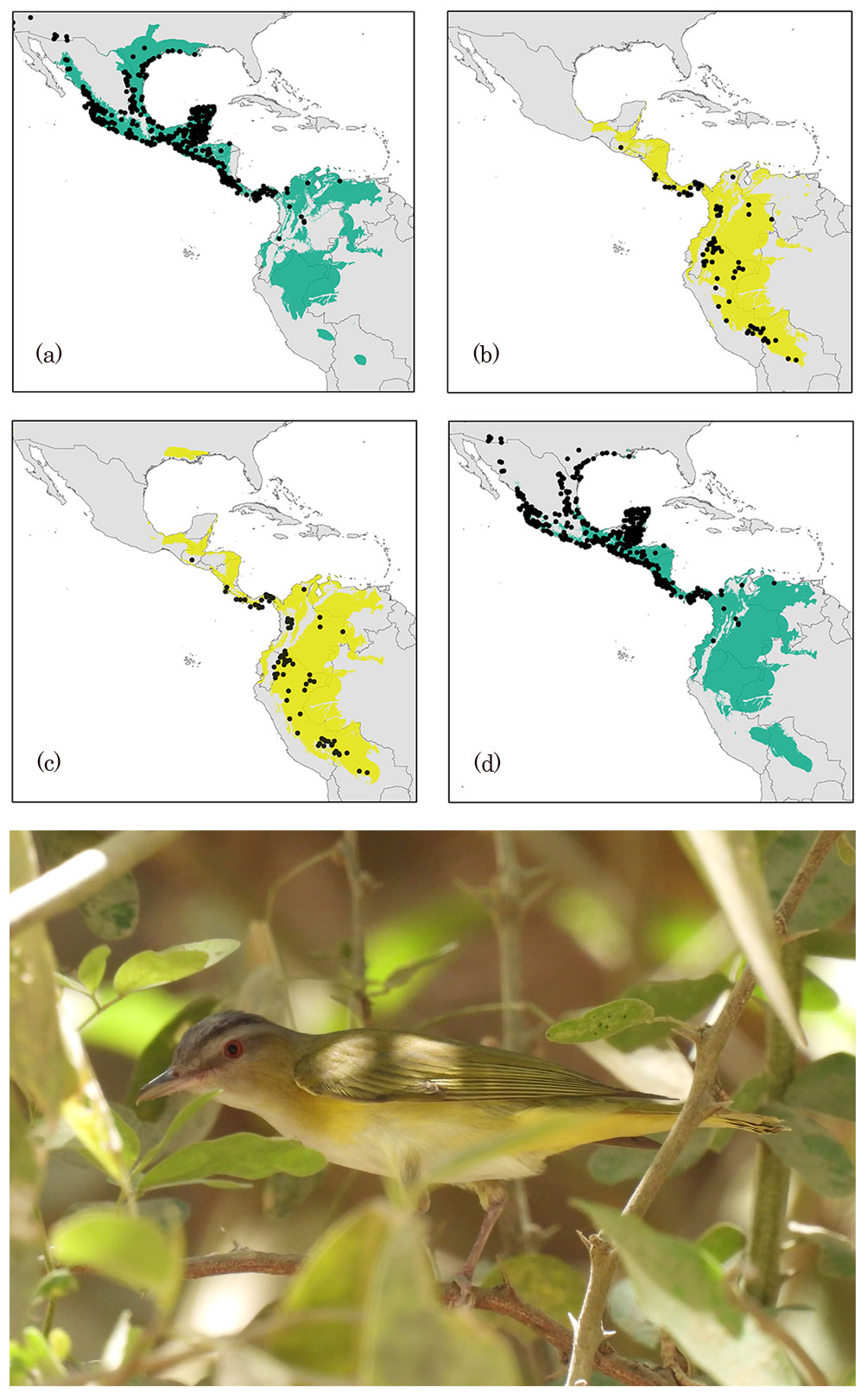
The ecological niche of the reproductive season shows a wider geographical distribution than that of the non-breeding season (Fig. 1); however, both models were significantly better than random expectations for both seasons. Performance values indicated that species’ distribution models were statistically accurate for the reproductive season with an omission rate of ~ 0.03, and the best AICc value resulted from regularization multipliers 2 with LP feature classes (Fig. 1). The model obtained showed an approximate extent of 5,965,812 km2 within the potential distributional areas, representing 64.7% of the M calibration area used. The average contribution values observed for the 3 variables were maximum temperature = 12.8%, minimum temperature = 53.9%, and precipitation = 33.3%. The non-reproductive season model with the best AICc value resulted from the combination of a regularization multiplier of 0.1 with the QP feature class (Fig. 1). The average model obtained for this season showed an area of 3,710,730 km2 for the potential distribution, representing 40.4% of M calibration area used. The average contribution values observed for the 3 variables were: maximum temperature = 29.3%, minimum temperature = 38.4%, and precipitation = 32.3%.
Comparison and similarity of seasonal niches. According to the niche overlap analyses, we observed high values of overlap index (Schoener’s D = 0.016) between the environmental conditions defining the reproductive and non-reproductive season across the Yellow-green Vireo distribution. In addition, the statistical similarity tests from reproductive to non-reproductive seasonal niches showed more similarity (p = 0. 01898) than random expectations from the 1,000 pseudo-replicated datasets. Statistical similarity tests from non-reproductive to reproductive seasonal niches showed no more similarity than expected by chance (p = 0.26174; Table 1). PCA axis 1 and 2 represent 68.79% and 27.63% of the variability, respectively. Overlap values are higher than the null distribution, which depicts niche similarity (Fig. 2); therefore, we did not reject the null hypothesis (i.e., niche similitude) between seasons.
Table 1
Significance p value by season (reproductive and non-reproductive). According to the niche overlap analyses, we observed high values of overlap index (Schoener’s D = 0.016) statistical similarity tests from reproductive to non-reproductive seasonal niches than random expectations from the 1,000 pseudo-replicated datasets.
| Reproductive ENM | Non-reproductive ENM | |
| Reproductive season | – | 0. 01898 |
| Non-reproductive season | 0.26174 | – |
Table 2
Percentage of predicted records by season (reproductive and non-reproductive) and transient months when ENMs are projected.
| Transference | Reproductive ENM | Non-reproductive ENM |
| Reproductive season | – | 38% |
| Non-reproductive season | 95% | – |
| February | 96% | 31% |
| March | 84% | 22% |
| April | 89% | 30% |
| August | 94% | 45% |
| September | 78% | 54% |
| October | 70% | 61% |
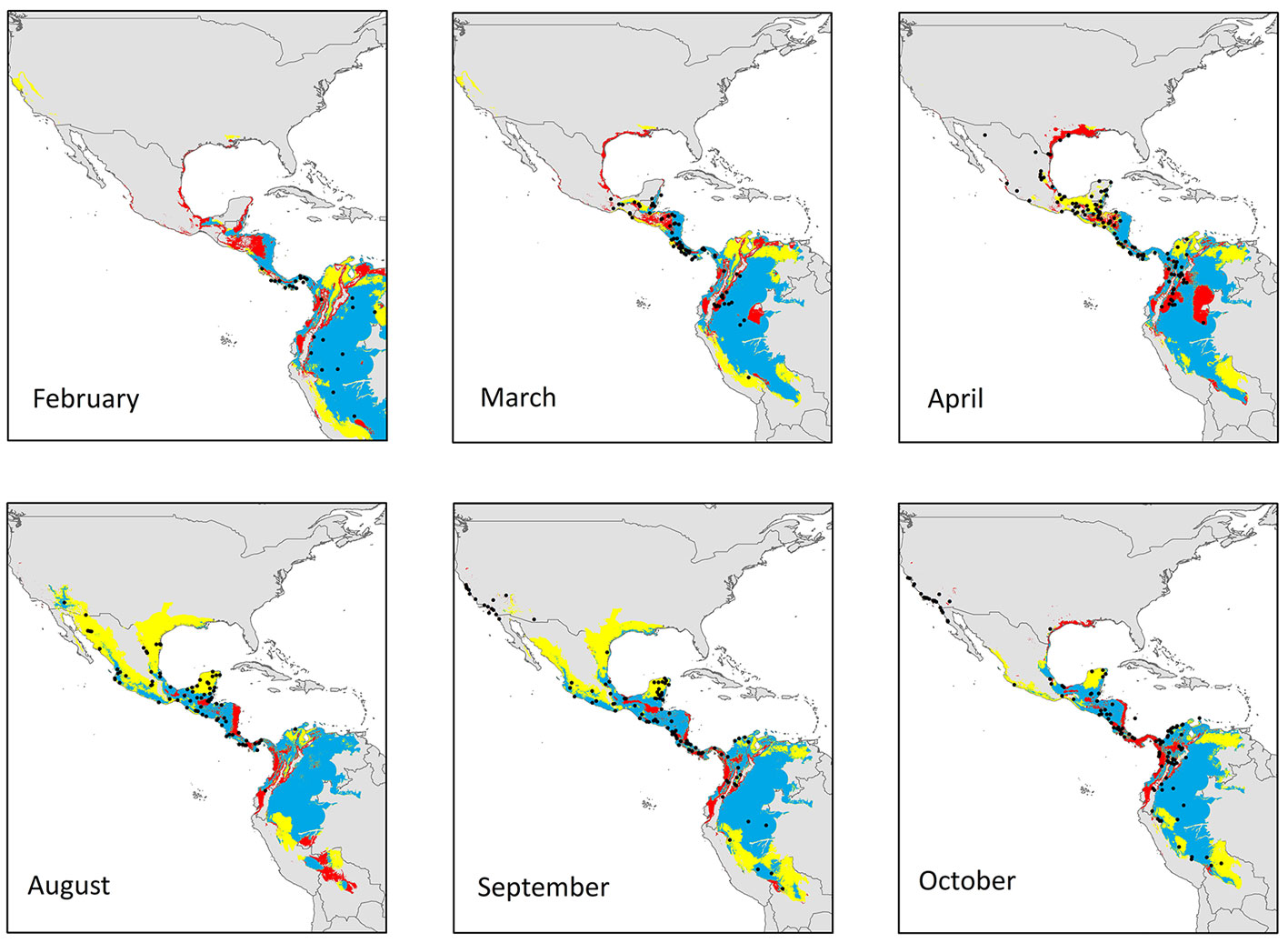
Model projections into geographic space and transferred to the opposite season are shown in Figure 1. We observed large overlapping areas in the inter-predictions made for both seasons across the M calibration areas. The ENM for reproductive season predicted 90.8% of the potential distributional areas estimated by ENM’s during the non-reproductive season, including 95% of available presence records (Table 2). Conversely, the ENM from the non-reproductive season showed an inter-prediction value of 57.0% for the potential geographical areas containing the environmental conditions defining the reproductive season. However, the seasonal model only predicted 38% of historical records associated with the breeding season.
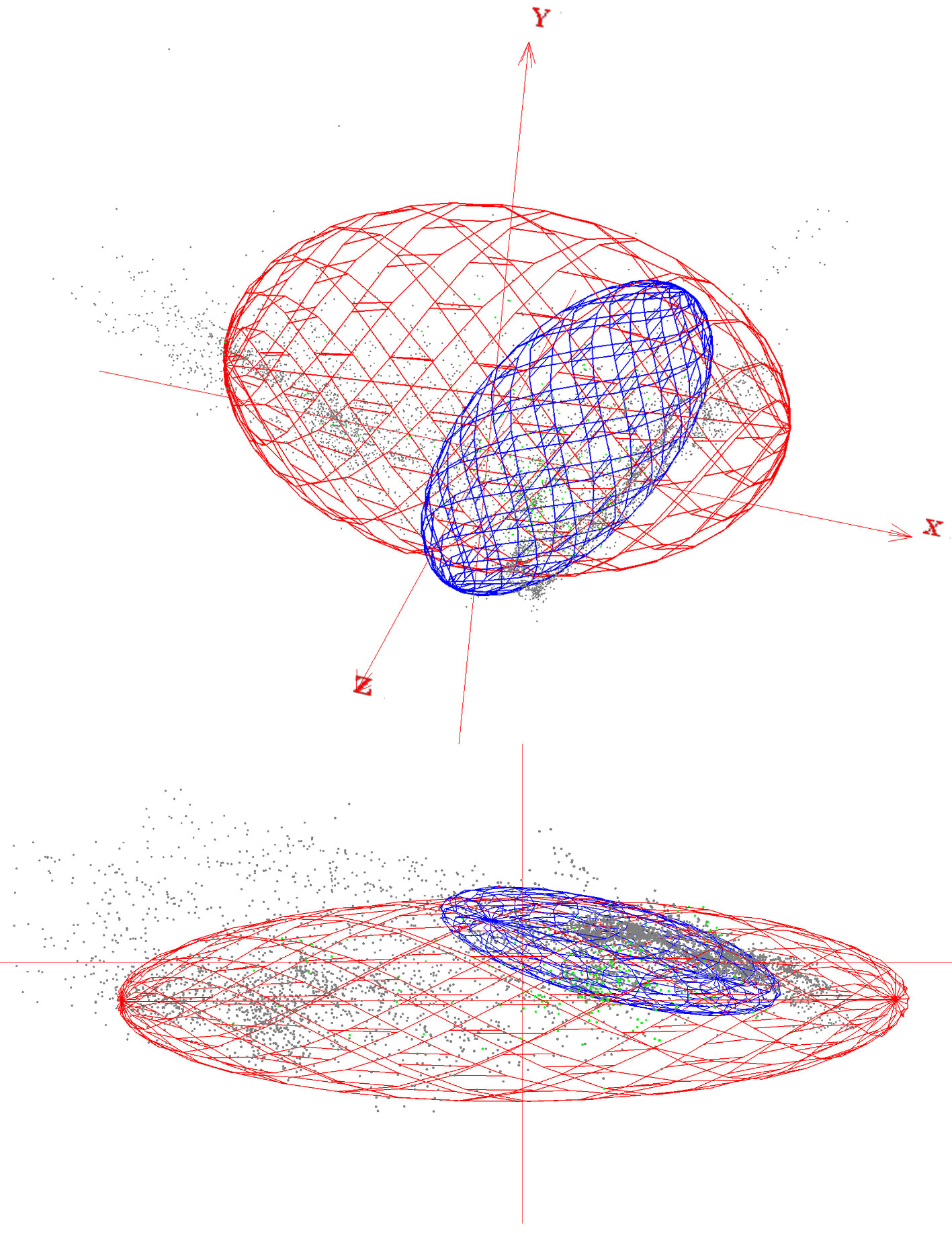
Seasonal models did not show the same predictive rate for transient months areas and records (Fig. 3). Overlapping (consensus) geographical areas predicted by both reproductive and non-reproductive models projected onto the migratory transient zones ranged from 41-63% for the reproductive and 95-100% for the non-reproductive season. Environmental conditions defining the reproductive season across Yellow-green Vireo’ potential distribution into transient months follow the records from south to north in the spring migration. Overall, we observed that projection of the ecological niche conditions from the reproductive season showed higher predictability of the presence records (ranging from 70% to 96%) into the other transition months. Contrarily, the projections from non-reproductive climate conditions to transient months showed relatively low-medium values (from 22% to 61%), especially during the spring (February to April) migratory movement (Fig. 3). Visualization of the environmental niche shows that the reproductive niche is broader than the non-reproductive niche and suggests that non-reproductive is nested inside the reproductive niche (Fig. 4).
Discussion
Intratropical migration is a complex system that has been poorly investigated (Faaborg et al., 2010a). Vireo flavoviridis is one of the few species that have been entirely recognized as an intratropical migratory bird (Morton, 1977), and presents a total migration of their populations, which makes it an excellent model to assess seasonal and transitional environmental conditions tracked by such an intratropical pattern. In this study, where we compared the climatic niches across seasons to analyze similarity or dissimilarity between the reproductive and non-reproductive grounds through a niche overlap analysis (Broennimann et al., 2012), we found that Yellow-green Vireo uses significantly similar climatic niches when comparing reproductive to non-reproductive grounds. However, when comparing non-reproductive to reproductive climatic niches, they were not significantly similar. These opposite results may be due to nested niches (i.e., one niche contains the other, Fig. 4; Guisan et al., 2014). However, these results should be taken cautiously, because the sample size difference between seasons could lead to a climatic niche not being fully captured. Conclusions will only be applicable to the climate space investigated and within analogue climates available between the 2 ranges (Guisan et al., 2014).
Based on the 3 seasonal niche patterns described by Nakazawa et al. (2004), Yellow-green Vireo corresponds to the “niche follower” pattern; in other words, it tracks similar environmental conditions between seasons. This pattern has been suggested for another intratropical migrant (Elaenia chiriquensis albivertex; Guaraldo et al., 2016). This may be because the climate in the tropics tends to be more homogeneous between seasons and therefore exerts less pressure to adapt to different climatic conditions (Levey & Stiles, 1992). Other groups of migrants follow a climatic niche seasonally; examples include the long-distance migrants of the northern hemisphere (Zurell et al., 2018), such as the New World warblers (Parulidae; Gómez et al., 2016), and Passerina buntings (Martínez-Meyer et al., 2004). However, unlike these species, some other Nearctic-Neotropical migrants switch niches over their annual cycle, like Ammodramus bairdii, (Peña-Peniche et al., 2018), Setophaga coronata, S. magnolia, S. townsendi, and Vermivora peregrina (Nakazawa et al., 2004). The “niche switcher” behavior seems to be related to a specific temperate zone in the northern USA and southern and central Canada (Nakazawa et al., 2004; Peña-Peniche et al., 2018), possibly in response to strong climatic seasonality (Nakazawa et al., 2004). Although Yellow-green Vireo seasonal niches are more similar than expected by chance from reproductive to non-reproductive season, it is not for non-reproductive to reproductive season, possibly due to differences in habitat availability within the regions they inhabit (Warren et al., 2008).
Usually, the evolution of the migration framework supports 2 main geographic origin theories: the “northern home” when resident birds from temperate zones started shifting winter areas (Jahn et al., 2020; Winger et al., 2019); and the “southern home” or “tropical home” began by colonizing high latitudes from the tropics, after which individuals began to explore increasingly far until adaptation to seasonal changes arose (Berthold, 1999; Levey & Stiles, 1992; Milá et al., 2006). This theory and recent findings gather an “evolutionary precursor theory” that suggests short-distance migration uses similar niche conditions between seasons and that the “niche follower” could be a plesiomorphic state and “niche switcher” an apomorphic state (Joseph, 1996; Joseph et al., 2003; Nakazawa et al., 2004). By suggesting that intratropical migration is a short-distance migration, our results could support the idea that short-distance migration could be a primitive stage of long-distance migration towards temperate zones (Heckscher et al., 2015; Johnson et al., 2005). This idea is consistent with the “tropical conservatism hypothesis” of Wiens and Donoghue (2004), which said that climate disparity between the tropics and temperate zones “forces” migratory species to return to the tropics, and by this mechanism, niche conservatism maintains species richness higher in the tropics. This mechanism can also be observed in the Red-eyed Vireo group since 3 of the 4 species have tropical distributions, and Yellow-green Vireo is the nearest to the common ancestor of the group (Battery & Klicka, 2017). However, the “niche follower” character is not exclusive to intratropical or short-distance migration (e.g., Gómez et al., 2016; Martínez-Meyer et al., 2004; Zurell et al., 2018). More studies about intratropical migration niche preferences are still important to consolidate a pattern of the possible evolution of migration (Levey & Stiles, 1992).
Although climatic niches between seasons are significantly similar, the ENM we used to establish which seasonal climatic conditions Yellow-green Vireo tracks in geography through transitional months (Soberón & Peterson, 2005), suggest that it also tracks climatic conditions more similar to the reproductive niche during the whole annual cycle. This is evidenced by the high predictability of the winter niche and the presence records in transitional months. This may be because migratory birds tend to have habitats programmed for the migratory route (Martin & Finch, 1995). This result indicates different climatic adaptations in reproductive niches, which could be a first clue to the directionality of the evolution of seasonal niches (Martínez-Meyer et al., 2004), from the reproductive to the non-reproductive niche. Although, the extrapolation of models can be risky and requires careful consideration, as they rely on fitted variables response curves that could be biased, especially if one species is under-sampled (Guisan et al., 2014).
The climate is not the only factor that delimits the geographic distribution of Yellow-green Vireo (Wiens & Graham, 2005), which could also be influenced by spatiotemporal variation in fruits and insects, and competition in non-reproductive grounds (Dingle & Drake, 2007; Legge et al., 2004; Morton, 1977). A more complex relationship with seasonality and resource availability may exist (MacPherson et al., 2018). On the other hand, Yellow-green Vireo has a broader winter niche in the environmental space (Fig. 3), which is consistent with other migratory species and may be related to the generalist habitat use of some migratory birds in the winter (Hutto, 1995; Peña-Peniche et al., 2018).
A notable finding is that during fall migration (August to October), Yellow-green Vireo moves further north through California before going to the wintering grounds. This would support the suggestion by Pyle (2009) that Yellow-green Vireo carries out a double fall migration to molt. Similar movements have been described in other migratory species, such as Tyrannus savanna (Jahn et al., 2016), Tyrannus verticalis (Barry et al., 2009), Piranga ludoviciana (Butler et al., 2002), and others (Rohwer et al., 2005). All these species go to eastern Arizona, New Mexico, and northwestern Mexico to use the Mexican monsoon to do a post-breeding molt, which has substantial implications for conservation (Rohwer et al., 2005).
In conclusion, our study of the Yellow-green Vireo adds information on a poorly known migration pattern, intratropical migrants, that has been less studied in the Americas as compared to the Nearctic-Neotropical migrants that have been analyzed (e.g., DeGraaf & Rappole 1995; Nakazawa et al., 2004) and monitored for several decades given that its breeding distribution is mainly in North America (e.g., Breeding Bird Survey, https://www.pwrc.usgs.gov/bbs/). Intratropical migrants, as well as other migratory bird species, have a huge impact on ecosystems functioning and balance (Faaborg et al., 2010a; Janh et al., 2020); that is relevant for several areas of biodiversity studies, for example, spread of emergent infectious diseases (Cohen et al., 2015; Peterson et al., 2004), climate change effects on biodiversity (Charmantier & Gienapp 2014), and pollination (Nava-Bolaños et al., 2023). Analyses of seasonal movement patterns of these species not only allow us to appreciate the complexity of nature, but also provides invaluable information for species protection and design of conservation areas in the region (Faaborg et al., 2010b; Heckscher et al., 2015).
Acknowledgments
This paper constitutes part of the requirements of AO-G in the PhD program in Posgrado en Ciencias Biológicas of the Universidad Nacional Autónoma de México (UNAM). AO-G was granted a Conacyt PhD scholarship, which was essential to develop this paper. We thank “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica” (PAPIIT IN214621), and “Programa de Apoyo a los Estudios de Posgrado” (PAEP-UNAM) for supporting this research. We thank the museums and data curators that provided presence records of Yellow-green Vireo in GBIF (https://doi.org/10.15468/dl.rcqler). We are grateful to Carlos Lara, Javier Fernández-López, Lynna Kiere, Susana Ochoa-González, Claudio Mota-Vargas, Ernesto Ruelas, Enrique Martínez-Meyer, Roberto Munguía-Steyer, Luis A. Sánchez-González, José de Jesús Zazueta-Algara, and two anonymous reviewers, for logistic support and comments to early versions of the manuscript.
References
Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., & Anderson, R. P. (2015). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography, 38, 541–545. https://doi.org/10.1111/ecog.01132
Allouche, O., Steinitz, O., Rotem, D., Rosenfeld, A., & Kadmon, R. (2008). Incorporating distance constraints into species distribution models. Journal of Applied Ecology, 45, 599–609. https://doi.org/10.1111/j.1365-2664.2007.01445.x
Anderson, R. P. (2012). Harnessing the world’s biodiversity data: promise and peril in ecological niche modeling of species distributions. Annals of the New York Academy of Sciences, 1260, 66–80. https://doi.org/10.1111/j.1749-6632.
2011.06440.x
Armenteras, D., Espelta, J. M., Rodríguez, N., & Retana, J. (2017). Deforestation dynamics and drivers in different forest types in Latin America: Three decades of studies (1980-2010). Global Environmental Change, 46, 139–147. https://doi.org/10.1016/j.gloenvcha.2017.09.002
Barry, J. H., Butler, L. K., Rohwer, S., & Rohwer, V. G. (2009). Documenting molt-migration in Western Kingbird (Tyrannus verticalis) using two measures of collecting effort. The Auk, 126, 260–267. https://doi.org/10.1525/auk.2009.07137
Barve, N., Barve, V., Jiménez-Valverde, A., Lira-Noriega, A., Maher, S. P., Peterson, A. T. et al. (2011). The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling, 22211, 1810–1819. https://doi.org/10.1016/j.ecolmodel.2011.02.011
Battery, C. J., & Klicka, J. (2017). Cryptic speciation and gene flow in a migratory songbird Species Complex: Insights from the Red-Eyed Vireo (Vireo olivaceus). Molecular Phylogenetics and Evolution, 113, 67–75. https://doi.org/10.1016/j.ympev.2017.05.006
Bell, C. P. (2000). Process in the evolution of bird migration and pattern in avian ecography. Journal of Avian Biology, 31, 258–265. https://doi.org/10.1034/j.1600-048X.2000.310218.x
Berthold, P. (1999). Towards a comprehensive theory for the evolution, control and adaptability of avian migration.
Ostrich, 70, 1–11. https://doi.org/10.1080/00306525.1999.96
39744
Boria, R. A., Olson, L. E., Goodman, S. M., & Anderson, R. P. (2014). Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecological Modelling, 275, 73–77. https://doi.org/10.1016/j.ecolmodel.2013.12.012
Boyle, W. A. (2008). Can variation in risk of nest predation explain altitudinal migration in tropical birds? Oecologia, 155, 397–403. https://doi.org/10.1007/s00442-007-0897-6
Boyle, W. A. (2017). Altitudinal bird migration in North America. The Auk, 134, 443–465. https://doi.org/10.1642/AUK-16-228.1
Broennimann, O., Fitzpatrick, M. C., Pearman, P. B., Petitpierre, B., Pellissier, L., Yoccoz, N. G. et al. (2012). Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography, 21, 481–497. https://doi.org/10.1111/j.1466-8238.2011.00698.x
Butler, L. K., Donahue, M. G., & Rohwer, S. (2002). Molt-migration in Western Tanagers (Piranga ludoviciana): age effects, aerodynamics, and conservation implications. The Auk, 119, 1010–1023. https://doi.org/10.1093/auk/119.4.1010
Callo, P. A., Morton, E. S., & Stutchbury, B. J. M. (2013). Prolonged spring migration in the Red-Eyed Vireo (Vireo olivaceus). The Auk, 130, 240−246. https://doi.org/10.1525/auk.2013.12213
Chapman, B., Hulthén, K., Wellenreuther, M., Hansson, L. A., Nilsson, J. A., & Brönmark, C. (2014). Patterns of animal migration. In L. A. Hansson, & S. Akesson (Eds.), Animal movement across scales (pp. 11–35). Oxford: Oxford University Press. https://doi.org/10.1093/acprof:oso/9780199677184.003.0002
Charmantier, A., & Gienapp, P. (2014). Climate change and timing of avian breeding and migration: evolutionary versus plastic changes. Evolutionary Applications, 7, 15−28. https://doi.org/10.1111/eva.12126
Clements, J. F., Schulenberg, T. S., Iliff, M. J., Billerman, S. M., Fredericks, T. A., Gerbracht, J. A. et al. (2021). The eBird/ Clements checklist of Birds of the World: v2021. Recuperado en junio de 2021 de: https://www.birds.cornell.edu/clementschecklist/download/
Cobos, M. E., Peterson, A. T., Barve, N., & Osorio-Olvera, L. (2019). kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ, 7, 1−11. https://doi.org/10.7717/peerj.6281
Cohen, E. B., Auckland, L. D., Marra, P. P., & Hamer, S. A. (2015). Avian migrants facilitate invasions of neotropical ticks and tick-borne pathogens into the United States. Applied and Environmental Microbiology, 81, 8366−8378. https://doi.org/10.1128/AEM.02656-15
Davenport, L., Nole, I., & Carlos, N. (2012). East with the Night: Longitudinal Migration of the Orinoco Goose (Neochen jubata) between Manú National Park, Peru and the Llanos de Moxos, Bolivia. Plos One, 7, e46886. https://doi.org/10.1371/journal.pone.0046886
DeGraaf, R. M., & Rappole, J. H. (1995). Neotropical migratory birds: natural history, distribution, and population change. Ithaca, NY: Cornell University Press.
del Hoyo, J., Elliott, A., & Christie, D. A. (Eds.). (2010). Handbook of the birds of the World alive. Barcelona: Lynx Edicions.
Di Cola, V., Broennimann, O., Petitpierre, B., Breiner, F. T., D’Amen, M., Randin, C. et al. (2016). ecospat: an R package to support spatial analyses and modeling of species niches and distributions. Ecography, 40, 774–787. https://doi.org/10.1111/ecog.02671
Dingle, H., & Drake, A. (2007). What is migration? Bioscience, 57, 113–121. https://doi.org/10.1641/B570206
Elith, J., Phillips, S. J., Hastie, T., Dudík, M., Chee, Y. E., & Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17, 43–57. https://doi.org/10.1111/j.1472-4642.2010.00725.x
Escalona, M., Prieto-Torres, D., & Rojas-Runjaic, F. J. (2017). Unveiling the geographic distribution of Boana pugnax (Schmidt, 1857) (Anura, Hylidae) in Venezuela: new state records, range extension, and potential distribution. Check List, 13, 671–68. https://doi.org/10.15560/13.5.671
ESRI. (2011). ArcGIS Desktop: Release 10. Redlands, CA: Environmental Systems Research Institute.
Faaborg, J., Holmes, R. T., Anders, A. D., Bildstein, K. L., Dugger, K. M., Gauthreaux, S. A. et al. (2010a). Recent advances in understanding migration systems of New World land birds. Ecological Monographs, 80, 3–48. https://doi.org/10.1890/09-0395.1
Faaborg, J., Holmes, R. T., Anders, A. D., Bildstein, K. L., Dugger, K. M., Gauthreaux, S. A. et al. (2010b). Conserving migratory land birds in the New World: Do we know enough? Ecological Applications, 20, 398–418. https://doi.org/10.1890/09-0397.1
Feeley, K. J., & Silman, M. R. (2011). The data void in modeling current and future distributions of tropical species. Global Change Biology, 17, 626–630. https://doi.org/10.1111/j.1365-2486.2010.02239.x
Fick, S. E., & Hijmans, R. J. (2017). WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology, 37, 4302–4315. https://
doi.org/10.1002/joc.5086
Gomez, C., Bayly, N. J., & Rosenberg, K. V. (2013). Seasonal variation in stopover site use: Catharus thrushes and vireos in northern Colombia. Journal of Ornithology, 154, 107–117. https://doi.org/10.1007/s10336-012-0876-5
Gómez, C., Tenorio, E. A., Montoya, P., & Cadena, C. D. (2016). Niche-tracking migrants and niche switching residents: Evolution of climatic niches in New World warblers (Parulidae). Proceedings of the Royal Society, 283, 1–9. https://doi.org/10.1098/rspb.2015.2458
Guaraldo, A. C., Kelly, J. F., & Marini, M. A. (2016). Contrasting annual cycles of an intratropical migrant and a tropical resident bird. Journal of Ornithology, 157, 695–705. https://doi.org/10.1007/s10336-016-1327-5
Guevara-Torres, D. R., Salvador, J., Antezana, M., Hernández, F., Chumpitaz, K., & Saravia, P. (2017). Registros de Vireo flavoviridis en la costa central del Perú. Boletín UNOP, 12, 15–19.
Guisan, A., Petitpierre, B., Broennimann, O., Daehler, C., & Kueffer, C. (2014). Unifying niche shift studies: insights from biological invasions. Trends in Ecology and Evolution, 29, 260–269. https://doi.org/10.1016/j.tree.2014.02.009
Hayes, F. E. (1995). Definitions for migrant birds: what is a neotropical migrant? The Auk, 112, 521–523. https://doi.org/
10.2307/4088747
Heckscher, C. M., Halley, M. R., & Stampul, P. M. (2015). Intratropical migration of a Nearctic-Neotropical migratory songbird (Catharus fuscescens) in South America with implications for migration theory. Journal of Tropical Ecology, 31, 285–289. https://doi.org/10.1017/S0266467415000024
Heckscher, C. M., Taylor, S. M., Fox, J. W., & Afanasyev, V. (2011). Veery (Catharus fuscescens) wintering locations, migratory connectivity, and a revision of its winter range using geolocator technology. The Auk, 128, 531−542. https://doi.org/10.1525/auk.2011.10280
Hijmans, R. J., Bivand, R., van Etten, J., Forner, K., Ooms, J., & Pebesma, E. (2022). Package ‘Terra’. R package version 1.5-21.
Hutchinson, G. E. (1957). Concluding remarks. Cold Spring Harbor Symposia on Quantitative Biology, 22, 415– 427. http://dx.doi.org/10.1101/SQB.1957.022.01.039
Hutto, R. L. (1995). Can patterns of vegetation change in western Mexico explain population trends in western neotropical migrants? In M. H. Wilson, & S. A. Sader (Eds.), Conservation of neotropical migratory birds in Mexico (pp. 48–58). Orono, Maine: Maine Agricultural and Forest Experiment Station, Miscellaneous Publication.
IUCN (International Union for Conservation of Nature). (2022). The IUCN Red List of Threatened Species. Version 2021-3. Recuperado el 05 de mayo de 2022 de: https://www.iucnredlist.org
Jahn, A. E., Cueto, V. R, Fontana, C. S., Guaraldo, A. C., Levey, D. J., Marra, P. P. et al. (2020). Bird migration within the Neotropics. The Auk, 137, 1–23. https://doi.org/10.1093/auk/ukaa033
Jahn, A. E., Seavy, N. E., Bejarano, V., Guzmán, M. B., Provinciato, I. C. C., Pizo, M. A. et al. (2016). Intra-tropical migration and wintering areas of Fork-tailed Flycatchers (Tyrannus savana) breeding in São Paulo, Brazil. Revista
Brasileira de Ornitologia, 24, 116–121. https://doi.org/10.
1007/BF03544339
Johnson, M., Sherry, T., Strong, A., & Medori, A. (2005). Migrants in Neotropical bird communities: An assessment of the breeding currency hypothesis. Journal of Animal Ecology, 74, 333–341. https://doi.org/10.1111/j.1365-2656.2005.00928.x
Joseph, L. (1996). Preliminary climatic overview of migration patterns in South American austral migrant passerines. Ecotropica, 2, 185–193.
Joseph, L., Wilke, T., & Alpers, D. (2003). Independent evolution of migration on the South American landscape in a long-distance temperate-tropical migratory bird, Swainson’s flycatcher (Myiarchus swainsoni). Journal of Biogeography, 30, 925–937. https://doi.org/10.1046/j.1365-2699.2003.00841.x
Kaufman Field Guide to Birds of North America. (2005). Originally published (2000) as Kaufman Focus Guide to Birds of North America. Houghton Mifflin Co., Boston.
La Sorte, F. A., Fink, D., Blancher, P. J., Rodewald, A. D., Ruiz-Gutierrez, V., Rosenberg, K. V. et al. (2017). Global change and the distributional dynamics of migratory bird populations wintering in Central America. Global Change Biology, 23, 5284–5296. https://doi.org/10.1111/gcb.13794
Legge, S., Murphy, S., Igag, P., & Mack, A. L. (2004). Territoriality and density of an Australian migrant, the Buff-breasted Paradise Kingfisher, in the New Guinean non-breeding grounds. Austral Ornithology, 104, 15–20. https://doi.org/10.1071/MU03054
Levey, D. J. (1994). Why we should adopt a broader view of neotropical migrants. The Auk, 111, 233–236.
Levey, D. J., & Stiles, F. G. (1992). Evolutionary precursors of long-distance migration: resource availability and movement patterns in Neotropical landbirds. American Naturalist, 140, 447–476. https://doi.org/10.1086/285421
Liu, C., Newell, G., & White, M. (2013). Selecting thresholds for the prediction of species occurrence with presence-only data. Journal of Biogeography, 40, 778–789. https://doi.org/
10.1111/jbi.12058
MacPherson, M. P., Jahn, A. E., Murphy, M. T., Kim, D. H., Cueto, V. R., Tuero, D. T. et al. (2018). Follow the rain? Environmental drivers of Tyrannus migration across the New World. The Auk, 135, 881–894. https://doi.org/10.1642/AUK-17-209.1
Marcer, A., Chapman, A. D., Wieczorek, J. R., Picó, F., Uribe, F., Waller, J. et al. (2022). Uncertainty matters: ascertaining
where specimens in natural history collections come from and its implications for predicting species distributions. Eco-
graphy, 2022, e06025. https://doi.org/10.1111/ecog.06025
Martin, T. E., & Finch, D. M. (1995). Ecology and management of neotropical migratory birds: a synthesis and review of critical issues. New York: Oxford University Press.
Martínez-Meyer, E., Peterson, A. T., & Navarro-Sigüenza, A. G. (2004). Evolution of seasonal ecological niches in the Passerina buntings (Aves: Cardinalidae). Proceedings of the Royal Society B, 271, 1151–1157. https://doi.org/10.1098/rspb.2003.2564
Mendes, P., Elias-Velazco, S. J., Alves-de Andrade, A. F., & De Marco, Jr. P. (2020). Dealing with overprediction in species distribution models: How adding distance constraints can improve model accuracy. Ecological Modelling, 43,109180. https://doi.org/10.1016/j.ecolmodel.2020.109180
Merow, C., Smith, M. J., Edwards, Jr. T.C., Guisan, A., Mcmahon, S. M., Normand, S. et al. (2014). What do we gain from simplicity versus complexity in species distribution models? Ecography, 37, 1267–1281. https://doi.org/10.1111/ecog.00845
Milá, B., Smith, T. B., & Wayne, R. K. (2006). Postglacial population expansion drives the evolution of long-distance migration in a songbird. Evolution, 60, 2403–2409. https://doi.org/10.1111/j.0014-3820.2006.tb01875.x
Morton, E. S. (1977). Intratropical migration in the Yellow-Green Vireo and Piratic Flycatcher. The Auk, 94, 97–106.
Murphy, P. C., Guralnick, R. P., Glaubitz, R., Neufeld, D., & Ryan, J. A. (2004). Georeferencing of museum collections: A review of problems and automated tools, and the methodology developed by the Mountain and Plains Spatio-Temporal Database-Informatics Initiative (Mapstedi). Phyloinformatics, 3, 1–29. https://doi.org/10.5281/zenodo.59792
Nakazawa, Y., Peterson, A. T., Martínez-Meyer, E., & Navarro-Sigüenza, A. G. (2004). Seasonal niches of Nearctic-Neotropical migratory birds: implications for the evolution of migration. The Auk, 121, 610–618.
Nava-Bolaños, A., Prieto-Torres, D. A., Osorio-Olvera, L., Soberón, J., Arizmendi, M. C., & Navarro-Sigüenza, A. G. (2023). Critical areas for pollinator conservation in Mexico: A cross-border priority. Biological Conservation, 283, 110119. https://doi.org/10.1016/j.biocon.2023.110119
Navarro-Sigüenza, A. G. (1992). Altitudinal distribution of birds in the Sierra Madre del Sur, Guerrero, Mexico. The Condor, 94, 29–39. https://doi.org/10.2307/1368793
Norris, D. R., & Marra, P. P. (2007). Seasonal interactions, habitat quality, and population dynamics in migratory birds. The Condor, 109, 535–547. https://doi.org/10.1093/condor/109.3.535
Olson, D. M., Dinerstein, E., Wikramanayake, E. D., Burgess, N. D., Powell, G. V. N., Underwood, E. C. et al. (2001). Terrestrial ecoregions of the world: a new map of life on Earth. Bioscience, 51, 933–938. https://doi.org/10.1641/0006-3568(2001)051[0933:TEOTWA]2.0.CO;2
Owens, H. L. Campbell, L. P., Dornak, L. L., Saupe, E. E., Barve, N., Soberón, J. et al. (2013). Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecological Modelling, 263, 10–18. https://doi.org/10.1016/j.ecolmodel.2013.04.011
Peña-Peniche, A., Ruvalcaba-Ortega, I., & Rojas-Soto, O. (2018). Climate complexity in the migratory cycle of
Ammodramus bairdii. Plos One, 13, e0202678. https://doi.org/10.1371/journal.pone.0202678
Pérez-Navarro, M. A., Broennimann, O., Esteve, M. A., Moya-Pérez, J. M., Carreño, M. F., Guisan, A. & Lloret , F. (2020) Temporal variability is key to modeling the climatic niche. Diversity and Distributions, 27, 473–484. https://doi.org/10.1111/ddi.13207
Peterson, A. T., Komar, N., Komar, O., Navarro-Sigüenza, A. G., Robbins, M. B., & Martínez-Meyer, E. (2004). Priority contribution West Nile Virus in the New World: potential impacts on bird species. Bird Conservation International, 14, 215–232. https://doi.org/10.1017/S0959270904000309
Peterson, A. T., Papeş, M., & Soberón, J. (2008). Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecological Modelling, 213, 63–72. https://doi.org/10.1016/j.ecolmodel.2007.11.008
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
Portillo-Quintero, C. A., & Sánchez-Azofeifa, G. A. (2010) Extent and conservation of tropical dry forests in the Americas. Biological Conservation, 143, 144–155. https://doi.org/10.1016/j.biocon.2009.09.020
Prieto-Torres, D. A, Nori, J., & Rojas-Soto, O. R. (2018) Identifying priority conservation areas for birds associated to endangered Neotropical dry forests. Biological Conservation, 228, 205–214. https://doi.org/10.1016/j.biocon.2018.10.025
Prieto-Torres, D. A., & Rojas-Soto, O. R. (2016). Reconstructing the Mexican Tropical Dry Forests via an autoecological niche approach: Reconsidering the ecosystem boundaries.
Plos One, 11, e0150932. https://doi.org/10.1371/journal.pone.
0150932
Pyle, P., Leitner, W. A., Lozano-Angulo, L., Avilez-Teran, F., Swanson, H., Gómez-Limón, E. et al. (2009). Temporal, spatial, and annual variation in the occurrence of molt-migrant passerines in the Mexican monsoon region. The Condor, 111, 583–590. https://doi.org/10.1525/cond.2009.090085
Qiao, H., Peterson, A. T., Campbell, L. P., Soberón, J., Ji, L., & Escobar, L. E. (2016). NicheA: creating virtual species and ecological niches in multivariate environmental scenarios. Ecography, 39, 805–813. https://doi.org/10.1111/ecog.01961
R Core Team. (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Rappole, J. (2013). The avian migrant: the biology of bird migration. New York: Columbia University Press. https://doi.org/10.7312/columbia/9780231146784.001.0001
Rohwer, S., Butler, L. K., & Froehlich, D. R. (2005). Ecology and demography of east-west differences in molt scheduling of Neotropical migrant passerines. In R. Greenberg, & P. P. Marra (Eds.), Birds of two worlds: the ecology and evolution of migration (pp. 87–105). Baltimore: Johns Hopkins University Press.
Roubiceka, A. J., Van Der Wal, J., Beaumont, L. J., Pitmanc, A. J., Wilsona, P., & Hughes, L. (2010). Does the choice of climate baseline matter in ecological niche modelling? Ecological Modelling, 221, 2280–2286. https://doi.org/10.
1016/j.ecolmodel.2010.06.021
Salewski, V., & Bruderer, B. (2007). The evolution of bird migration – a synthesis. Naturwissenschaften, 94, 268–279. https://doi.org/10.1007/s00114-006-0186-y
Sánchez-Barradas, A., Santiago-Jiménez, Q. J., & Rojas-Soto, O. (2017). Variación temporal en la distribución geográfica y ecológica de Amazona finschi, Psittaciformes: Psittacidae. Revista Biología Tropical, 65, 1194–1207. http://dx.doi.org/10.15517/rbt.v65i3.25417.
Santillán, V., Quitián, M., Tinoco, B. A., Zárate, E., Schleuning, M., Böhning-Gaese, K. et al. (2018) Spatio-temporal variation in bird assemblages is associated with fluctuations in temperature and precipitation along a tropical elevational gradient. Plos One, 13, e0196179. https://doi.org/10.1371/journal.pone.0196179
Schoener, T. W. (1968). The Anolis lizards of Bimini: resource partitioning in a complex fauna. Ecology, 49, 704–726. https://doi.org/10.2307/1935534
Schulenberg, T. S. (2019). Cornell Lab of Ornithology, Ithaca, NY, USA. Neotropical Birds Online: https://birdsoftheworld.org/bow/home [September 15, 2019].
Skutch, A. F. (1960). Life histories of Central American birds II. Pacific Coast Avifauna, Number 34. Berkeley, California: Cooper Ornithological Society.
Soberón, J. M. (2010). Niche and area of distribution modeling: a population ecology perspective. Ecography, 33, 159–167. http://dx.doi.org/10.1111/j.1600-0587.2009.06074.x
Soberón, J., & Peterson, A. T. (2005). Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodiversity Informatics, 2, 1–10.
Stutchbury, B. J. M., Siddiqui, R., Applegate, K., Hvenegaard, G. T., Mammenga, P., Mickle, N. et al. (2016). Ecological causes and consequences of intratropical migration in temperate-breeding migratory birds. American Naturalist, 188, S28–S40. http://dx.doi.org/10.1086/687531
Styrsky, S. D., Berthold, P., & Robinson, W. D. (2004). Endogenous control of migration and calendar effects in an intratropical migrant, the Yellow-Green Vireo. Animal Behaviour, 67, 1141–1499. https://doi.org/10.1016/j.anbehav.
2003.07.012
Tingley, M. W., Monahanc, W. B., Beissingera, S. R., & Moritz, C. (2009). Birds track their Grinnellian niche through a century of climate change. Proceedings of the National Academy of Sciences, 106, 19637–19643. https://doi.org/10.1073/pnas.0901562106
Tobón-Sampedro, A., & Rojas-Soto, O. (2015). The geographic and seasonal potential distribution of the little-known Fuertes’s Oriole Icterus fuertesi. Bird Conservation International, 25, 489–502. https://doi.org/10.1017/S0959270914000501
Warren, D. L., Glor, R. E., & Turelli, M. (2008). Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution, 62, 2868–2883. https://doi.org/10.1111/j.1558-5646.2008.00482.x
Warren, D. L., & Seifert, S. N. (2011). Ecological niche modeling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecological Applications, 21, 35–342. https://doi.org/10.1890/10-1171.1
Wei, T., & Simko, V. (2017). R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). https://github.com/taiyun/corrplot
Wiens J. J., & Donoghue, M. J. (2004). Historical biogeography, ecology, and species richness. Trends in Ecology and Evolution, 19, 639–644. https://doi.org/10.1016/j.tree.2004.09.011
Wiens, J. J., & Graham, C. H. (2005). Niche Conservatism: Integrating Evolution, Ecology, and Conservation Biology. Annual Review of Ecology, Evolution, and Systematics, 36, 519–39. https://doi.org/10.1146/annurev.ecolsys.36.102803.095431
Winger, B. M., Auteri, G. G., Pegan, T. M., & Weeks, B. C. (2019). A long winter for the Red Queen: rethinking the evolution of seasonal migration. Biological Reviews, 94, 737–752. https://doi.org/10.1111/brv.12476
Winger, B. M., Barker, F. K., & Ree, R. H. (2014). Temperate origins of long-distance seasonal migration in New World songbirds. Proceedings of the National Academy of Sciences, 111, 12115–12120. https://doi.org/10.1073/pnas.1405000111
Wisz, M. S., Hijmans, R. J., Li, J., Peterson, A. T., Graham, C. H., Guisan, A. et al. (2008). Effects of sample size on the performance of species distribution models. Diversity and Distributions, 14, 763–773. https://doi.org/10.1111/j.1472-4642.2008.00482.x
Zink, R. M. (2011). The evolution of avian migration. Biological Journal of the Linnean Society, 104, 237–250. https://doi.org/10.1111/j.1095-8312.2011.01752.x
Zink, R. M., & Gardner, A. S., (2017). Glaciation as a migratory switch. Science Advances, 3, e1603133. https://doi.org/10.1126/sciadv.1603133
Zurell, D., Gallien, L., Graham, C. H., & Zimmermann, N. E. (2018). Do long-distance migratory birds track their niche through seasons? Journal of Biogeography, 45, 1459–1468. https://doi.org/10.1111/jbi.13351