Seasonal variation of gastro-intestinal helminths of three bat species in the dry forest of western Mexico
Valeria B. Salinas-Ramos⁎ ✉ , L. Gerardo Herrera, David I. Hernández-Mena, David Osorio-Sarabia, Virginia León-Règagnon
Studies on helminths of chiropterans are relatively uncommon compared to those of other animals, and seasonal changes in helminth load have been rarely examined. We characterized the gastro-intestinal helminth load of 3 bats species to test for the existence of seasonal changes in response to known seasonal environmental and bat prey fluctuations. We did not find seasonal variation in most of the cases. However, the prevalence of 4 endoparasite species was significantly higher during one of the seasons. The highest richness was registered in Pteronotus parnellii during the wet season. The effective number of species was higher during the dry season in the 3 species of Pteronotus . Diet seems to be an important driver of helminth infracommunity structure, but we found heterogeneous patterns in the relationship between diversity and load of helminths and seasonal patterns of bat’s diets and abundance of potential intermediate hosts.
© 2017 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:
Variación estacional de helmintos gastrointestinales en tres especies de murciélagos en el bosque tropical caducifolio del occidente de México
Resumen
Las investigaciones sobre los helmintos de quirópteros son relativamente escasas en comparación con las de otros vertebrados y los cambios estacionales en su carga han sido poco estudiados. En este estudio se caracterizó la carga de helmintos gastrointestinales de 3 especies de murciélagos para probar la existencia de cambios estacionales en respuesta a las fluctuaciones ambientales y de presas. No se encontró variación estacional en la mayoría de los casos. Sin embargo, la prevalencia de 4 especies de endoparásitos fue significativamente mayor durante una de las épocas. La mayor riqueza de especies se registró en P. parnellii durante la época de lluvias. El número efectivo de especies fue mayor durante estaciones secas en las 3 especies de Pteronotus . La dieta parece dirigir la estructura de las infracomunidades de helmintos, aunque se encontraron patrones heterogéneos en la relación entre la diversidad y la carga de helmintos y los patrones estacionales de la dieta y la abundancia de los posibles huéspedes intermediarios.
Palabras clave
Murciélago; Endoparásitos; Interacciones; Pteronotus; Estacionalidad
Introduction
All organisms, including parasites, are influenced directly or indirectly by environmental variations ( Marcogliese, 2001; Pilosof, Dick, Korine, Patterson, & Krasnov, 2012 ). The transmission, development and distribution of parasites can be regulated by abiotic factors ( Brooks & Hoberg, 2007; Gotz, Harf, Sommer, & Matthee, 2010 ). In particular, dynamics of helminths are regulated by environmental conditions such as ambient temperature, humidity and precipitation ( Appleton & Gouws, 1996; Doi & Yurlova, 2011 ; Hudson, Cattadori, Boag, & Dobson, 2006 ; Mouritsen & Poulin, 2002; M oyer, Drown, & Clayton, 2002 ; Tinsley et al., 2011 ). A few studies have also suggested that seasonal variation in parasite communities is influenced by biotic factors such as abundance, diet, reproductive behavior, and immunocompetence of hosts ( Carvalho & Luque, 2011; Esch & Fernández, 1993; Felis & Esch, 2004 ; Šimková, Jarkovsky, Koubková, Barus, & Prokes, 2005 ).
Bats are one of the most diverse and widespread of mammal orders ( Altringham, 1996; Wilson & Reeder, 2005 ). Studies of helminths in chiropteran populations are relatively uncommon compared to those of other animals ( Kirschbaum, Perkins, & Gannon, 2009 ; Lord, Parker, Parker, & Brooks, 2012 ). Chiropterans harbor a great variety of helminths, including trematodes, cestodes, and nematodes ( Cuartas-Calles & Muñoz-Arango, 1999; Lord et al., 2012 ) and most studies consist of checklists of species and new descriptions of host or localities ( Guzmán-Cornejo, García-Prieto, Pérez-Ponce de León, & Morales-Malacara, 2003 ; McAllister, Bursey, & Dowler, 2007 ; Muñoz et al., 2011; Nahhas, Yang, & Uch, 2005 ; Nogueira, de Fabio, & Peracchi, 2004 ; Shimalov, Demyanchik, & Demyanchik, 2002 ). Nevertheless, a few studies have explored the effect of seasonal variation in intensity and prevalence of bat endoparasites ( Blankespoor & Ulmer, 1970; Coggins, Tedesco, & Rupprecht, 1982 ; Lord et al., 2012; Nickel & Hansen, 1967 ). For example, studies with insectivorous bats have reported that prevalence and intensity of helminths are low during the spring, increase in the summer and reach a peak in the autumn ( Blankespoor & Ulmer, 1970; Nickel & Hansen, 1967 ). Among other factors intrinsic to host biology, seasonal changes in endoparasite abundance might be related to increased abundance of arthropods that act as intermediate hosts and that are then ingested by bats ( Lord et al., 2012).
Trematodes are the most diverse group of helminths found in bats ( Coggins, 1988; Ubelaker, 1970 ). They are found mainly within the gastrointestinal tract and in other body cavities ( Coggins, 1988; Ricci, 1995; Shimalov et al., 2002 ), and their incidence and prevalence are affected by the host’s feeding habits ( Coggins, 1988; Marshall & Miller, 1979 ). For example, most digenean species (trematodes) have been collected in insectivorous bats since they are more prone to ingest infected insects (intermediate hosts) than nectar or fruit feeding bats ( Coggins, 1988; García-Vargas, Osorio, & Pérez-Ponce de León, 1996 ; Lord et al., 2012; Ubelaker, 1970 ). Studies with other vertebrates (e.g., fishes) have reported that the diet of the host determines the abundance and richness of helminths ( Bell & Burt, 1991; Poulin & Morand, 2004; Šimková et al., 2005 ).
In this study, we investigated the seasonal variation of the endoparasitic load in 3 insectivorous [ Pteronotus davyi (Gray), P. parnellii (Gray), and P. personatus (Wagner)] bat species. Previous studies have reported the helminthological record of these bats species in Mexico ( Caballero-Caballero & Zerecero, 1942; Espericueta-Viera, 2012; García-Vargas et al., 1996; Guzmán-Cornejo et al., 2003 ; Peralta-Rodríguez, Caspeta-Mandujano, & Guerrero, 2012 ; Pérez-Ponce de León, León-Régagnon, & García-Vargas, 1996 ), but few have examined seasonal variations of infection patterns. For example, Clarke (2008) found no seasonal variation in endoparasite species composition of P. davyi and P. personatus and no seasonal difference in prevalence and abundance in a related species ( Mormoops megalophylla ) in a tropical deciduous forest in southern Mexico.
The study was conducted in a highly seasonal dry forest. Tropical dry forests have extreme changes in the physiognomy and availability of food resources during the wet and dry seasons, affecting the composition and diversity of fauna ( Castaño-Meneses, 2014 ). For instance, the abundance of arthropods in tropical dry forests experiences considerable seasonal fluctuations, reaching its highest level during the wet season ( Andresen, 2005; Castaño-Meneses, 2014; Güizado & Casas-Andreu, 2011; Leavings & Windsor, 1984 ), a pattern that has been previously reported for the study region ( Pescador-Rubio, Rodríguez-Palafox, & Noguera, 2002 ).
A previous study using DNA barcodes showed that the diet of the 3 species of Pteronotus considered in our study is more diverse during the dry season ( Salinas-Ramos, Montalvo, León-Regagnon, Arrizabalaga-Escudero, & Clare, 2015 ). For completing their transmission, some species of helminths use arthropods as intermediate hosts ( Bush, Fernández, Esch, & Seed, 2001 ; Clarke, 2008 ) and insectivorous bats as definite host ( Chitwood, 1938; García-Vargas, 1995 ). Accordingly, we predicted that the endoparasite load in insectivorous bats would exhibit seasonal changes, having the highest richness during the dry season (spring), when their diet is more diverse ( Salinas-Ramos et al., 2015 ). In contrast, we expected that the prevalence, abundance and intensity of helminths would be higher in the rainy season, when the abundance of intermediate hosts peaks ( Lord et al., 2012).
Material and methods
The 3 focal bat species roost in a cave in San Panchito Island, off the Pacific coast in Jalisco, Mexico (19°32′6″ N, 105°5′17.9″ W). The adjacent continental region is composed of tropical deciduous and tropical semideciduous forest ( Rzedowski, 1981 ), with most of the rainfall occurring from July to November ( Bullock, 1995; Méndez-Alonzo, Pineda-García, Paz, Rosell, & Olson, 2013 ; Pringle, Dirzo, & Gordon, 2012 ). We carried out 3 collecting trips during the dry season (spring: June 2012, April 2013, May 2014) and 4 in the wet season (summer: July 2013; autumn: November 2012, November 2013 and September 2014). Bats were collected with mist nests at sunset and with sweep nets inside the cave during the morning. All the specimens captured were adults and we held each individual in a cotton bag. Bats were transported to the Estación de Biología Chamela.
Bats were sacrificed with chloroform and deposited as voucher specimens in the National Mammal Collection (CNMA) of the Instituto de Biología and in the Mammal Collection of the Zoology Museum, Universidad Nacional Autónoma de México (MZFC, UNAM). The gastrointestinal tract was dissected from each bat and immersed in phosphate-buffered saline solution in a Petri dish. The stomach and intestine were examined carefully using a stereo microscope (Leica Microsystems, ES2, Wetzlar, Germany). All the helminths were fixed by sudden immersion in hot 4% formalin and preserved in 70% ethanol. Specimens were stained with Mayer’s paracarmine, dehydrated, cleared in methyl salicylate and mounted in Canada balsam ( Lamothe-Argumedo, 1997 ). Morphological data were cross-referenced with the available literature on species known to be present in bats ( García-Vargas, 1995; Justine, 1989; Moravec, 1982; Vaucher, 1992; Vaucher & Durette-Desset, 1986 ).
In order to characterize the seasonal variation in endoparasite load, we calculated the following descriptors of parasite populations: prevalence, abundance and intensity in the dry and wet seasons. We performed a chi-squared test with the prevalence data and a bootstrap test with the mean abundance and mean intensity values of each endoparasite species to evaluate significant differences between the seasons. We also calculated for each bat species the proportion of hosts infected considering the total endoparasite records, the average number of total endoparasites per host individual examined, and the average number of total endoparasites per infected host individual. Statistical analyses were conducted in Quantitative Parasitology (Version, 3.0, Reiczigel & Rózsa, 2005 ), and the significance level was set at p ≤ 0.05.
Richness, Shannon–Wiener and Simpson–Gini indexes were calculated to assess the diversity of endoparasite species during both seasons for each bat species. We transformed index values to the effective number of species ( Hill, 1973; MacArthur, 1965 ) in order to unify an intuitive interpretation of diversity ( Jost, 2006 ). Descriptive analyses and graphics were conducted in GraphPad Prism (Version 6.0 for Windows, GraphPad Software, La Jolla, CA, USA).
Results
One hundred and twenty three bats were collected during 7 fieldtrips, 40% of which had at least 1 helminth species. We found 10 species of parasites, totaling 955 individual helminths, of which 50% belonged to 5 nematode species, followed by 4 trematode species and 1 cestode species (40% and 10% of individuals, respectively). Only 6 of these species were collected in both seasons. Pteronotus personatus had the highest number of helminth individuals from the total of helminths. Two individual helminths collected in P. davyi were in too poor conditions to be identified to species and were not included in measurements of richness and diversity ( Table 1). Limatulum gastroides was the only species recorded in the 3 insectivorous bat species and it was particularly abundant in P. personatus (Table 1).
Table 1
Frequencies of helminths in 3 species of bats during the dry and wet seasons.
| Host | P. davyi | P. parnellii | P. personatus | |||
| Season | Dry | Wet | Dry | Wet | Dry | Wet |
| Bats captured/no. of infested | 24/4 | 24/8 | 20/5 | 27/12 | 11/11 | 17/9 |
| Cestoda | ||||||
| Vampirolepis elongatus | 1 | 5 | ||||
| Nematoda | ||||||
| Anoplostrongylinae gen. sp. | 1 | |||||
| Capillarida sp. | 14 | 2 | 2 | |||
| Linustrongylus pteronoti | 4 | 2 | ||||
| Physalopteridae gen. sp. | 4 | |||||
| Websternema parnellii | 6 | 3 | 2 | |||
| Trematoda | ||||||
| Parametadelphis sp. | 127 | |||||
| Lecithodendriidae gen. sp. | 96 | |||||
| Limatulum gastroides | 3 | 2 | 40 | 384 | 252 | |
| Urotrema sp. | 2 | 1 | ||||
| Unidentified specimen | 2 | |||||
| Total | 7 | 20 | 13 | 183 | 480 | 252 |
Prevalence was similar for both seasons in most endoparasite species except for Capillaria sp. and Websternema parnellii , which had a higher prevalence in the wet season in P. davyi , and Anoplostrongylinae gen. sp. and L. gastroides , in which prevalence was higher in the dry season in P. parnellii and P. personatus, respectively (Table 2 ). Mean abundance and intensity did not differ significantly between seasons for any endoparasite species ( Table 3).
Table 2
Prevalence registered in helminth parasites of Pteronotus species during the dry and wet seasons. P = values of chi-squared test with prevalence data and bootstrap test with the mean abundance and intensity data. Confidence intervals (CI) were set to 95% probability.
| Prevalence (%; 95% CI) | |||
| Dry | Wet | P | |
| P. davyi | |||
| Urotrema sp. | 4 (0.10–21.10) | 0 | 0.31 |
| L. gastroides | 8 (1.00–27.00) | 0 | 0.14 |
| Capillaria sp. | 0 | 25 (9.80–46.7) | 0.00 |
| W. parnellii | 0 | 17 (4.70–37.4) | 0.03 |
| P. parnellii | |||
| Capillaria sp. | 10 (1.20–31.70) | 4 (0.10–19.00) | 0.38 |
| L. pteronoti | 15 (3.20–37.90) | 7 (0.90–24.30) | 0.38 |
| W. parnellii | 10 (1.20–31.70) | 4 (0.10–19.00) | 0.38 |
| Anoplostrongylinae gen. sp. | 5 (0.10–24.90) | 0 | 0.00 |
| L. gastroides | 10 (1.20–31.70) | 11 (2.40–29.20) | 0.27 |
| V. elongatus | 5 (0.10–24.90) | 4 (0.10–19.00) | 0.82 |
| Parametadelphis sp. | 0 | 7 (0.90–24.30) | 0.21 |
| Urotrema sp. | 0 | 4 (0.10–19.00) | 0.38 |
| Physalopteridae gen sp. | 0 | 4 (0.10–19.00) | 0.38 |
| P. personatus | |||
| Lecithodendriidae gen. sp. | 9 (0.20–41.30) | 0 | 0.20 |
| L. gastroides | 91 (58.70–99.80) | 53 (27.80–77.00) | 0.03 |
Table 3
Mean abundance and intensity registered in helminth parasites of Pteronotus species during the dry and wet seasons. P = values of chi-squared test with prevalence data and bootstrap test with the mean abundance and intensity data. Confidence intervals (CI) were set to 95% probability.
| Mean abundance (95% CI) | Mean intensity (95% CI) | |||||
| Dry | Wet | P | Dry | Wet | P | |
| P. davyi | ||||||
| Urotrema sp. | 0.08 (0.00–0.25) | 0.00 | 0.42 | 2.00 (0.00) | 0.00 | 1.00 |
| L. gastroides | 0.12 (0. 00–0.37) | 0.00 | 0.28 | 1.50 (1.00–2.00) | 0.00 | 1.00 |
| Capillaria sp. | 0.00 | 0.58 (0.20–1.46) | 0.13 | 0.00 | 2.33 (1.17–4.33) | 1.00 |
| W. parnellii | 0.00 | 0.25 (0.04–0.54) | 0.08 | 0.00 | 1.50 (1.00–1.75) | 1.00 |
| P. parnellii | ||||||
| Capillaria sp. | 0.13 (0.00–0.20) | 0.07 (0.00–0.22) | 0.63 | 1.00 (0.00) | 2.00 (0.00) | 1.00 |
| L. pteronoti | 0.25 (0.05–0.45) | 0.07 (0.00–0.18) | 0.27 | 1.33 (1.00–1.67) | 1.00 (0.00) | 0.33 |
| W. parnellii | 0.15 (0.00–0.45) | 0.07 (0.00–0.22) | 0.48 | 1.50 (1.00–1.50) | 2.00 (0.00) | 1.00 |
| Anoplostrongylinae gen. sp. | 0.05 (0.00–0.15) | 0.00 | 0.43 | 1.00 (0.00) | 0.00 | 1.00 |
| L. gastroides | 0.12 (0.00–0.25) | 1.48 (0.03–7.11) | 0.10 | 1.00 (0.00) | 13.30 (1.00–25.70) | 0.05 |
| V. elongatus | 0.05 (0.00–0.15) | 0.18 (0.00–0.55) | 0.59 | 1.00 (0.00) | 5.00 (0.00) | 1.00 |
| Parametadelphis sp. | 0.00 | 4.70 (0.00–18.70) | 0.43 | 0.00 | 63.5 (2.00–63.5) | 1.00 |
| Urotrema sp. | 0.00 | 0.03 (0.00–0.11) | 0.45 | 0.00 | 1.00 (0.00) | 1.00 |
| Physalopteridae gen sp. | 0.00 | 0.14 (0.00–0.44) | 0.43 | 0.00 | 4.00 (0.00) | 1.00 |
| P. personatus | ||||||
| Lecithodendriidae gen. sp. | 8.73 (0.00–26.20) | 0.00 | 0.42 | 96.00 (0.00) | 0.00 | 1.00 |
| L. gastroides | 34.90 (20.00–61.40) | 14.82 (6.89–28.10) | 0.13 | 38.40 (23.9–69.50) | 28.00 (16.90–43.4) | 0.43 |
Percent of bats infected, average number of endoparasites per examined individual host and average number of endoparasites per infected individual host did not differ between seasons in most cases ( Table 4 ). The only exception to this pattern was P. personatus , in which there was a higher proportion of infected bats in the dry season ( Table 4).
Table 4
Comparative of endoparasite load between the dry and wet season in 3 species of bats. N = number of bats collected; PHI = proportion of hosts infected considering the total endoparasite records; THE = average number of total endoparasites per host individual examined; THI = average number of total endoparasites per infected host individuals. P = values of chi-squared test with PHI data and bootstrap test with THE and THI data. Confidence intervals (CI) were set to 95% probability.
| Specie | N | Infected | PHI (%) | P | THE | P | THI | P |
| P. davyi | ||||||||
| Dry season | 24 | 4 | 16.7 (0.04–0.37) | 0.29 (0.08–0.58) | 1.75 (1.00–2.00) | |||
| Wet season | 24 | 8 | 33.3 (0.15–0.55) | 0.18 | 0.83 (0.37–1.58) | 0.13 | 2.50 (1.62–3.88) | 0.28 |
| P. parnellii | ||||||||
| Dry season | 20 | 5 | 25 (0.0–0.49) | 0.65 (0.20–1.30) | 2.60 (2.00–3.20) | |||
| Wet season | 37 | 12 | 32.4 (0.18–0.49) | 0.55 | 6.7 (2.49–22.90) | 0.24 | 20.83 (8.75–55.5) | 0.26 |
| P. personatus | ||||||||
| Dry season | 11 | 11 | 100 (0.71–1.00) | 43.6 (26.50–69.30) | 43.64 (27.20–71.10) | |||
| Wet season | 17 | 9 | 52.9 (0.27–0.77) | 0.00 | 14.8 (7.30–28.50) | 0.05 | 28.00 (16.90–42.70) | 0.25 |
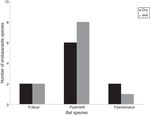
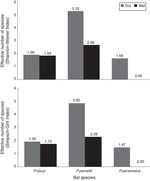
The highest helminth richness was recorded in P. parnellii in both seasons (Fig. 1 ). In terms of diversity, effective number of species calculated from Shannon–Wiener and Simpson–Gini Indexes were greater for all Pteronotus species during the dry season (Fig. 2).
Figure 1
Richness of helminth species during the dry and wet seasons in 3 species of bats.
Figure 2
Effective number of species calculated from Shannon–Wiener and Simpson–Gini indexes (A and B, respectively) during the dry and wet seasons in 3 species of insectivorous bats ( Pteronotus).
Discussion
We characterized the endoparasite load of 3 insectivorous bat species to examine the existence of seasonal changes in response to known seasonal ambient and prey fluctuations. Below, we discuss how our findings fitted to our predictions.
Our hypothesis of seasonal changes in parasite load was rejected in most cases either when comparing helminth species or the total endoparasite species. Mean abundance and intensity did not differ significantly between seasons for any endoparasite species. Our prediction of higher parasite load in the wet season was supported by the finding of higher prevalence in this season in Capillaria sp. and W. parnellii in P. davyi . In contrast, prevalence in Anoplostrongylinae gen. sp. and L. gastroides was higher in the dry season in P. parnellii and P. personatus , respectively. Percent of bats infected, average number of endoparasites per examined host individual and average number of endoparasites per infected host individual did not differ between seasons in most cases. The only exception to this pattern was P. personatus in which there was a higher proportion of infected bats in the dry season in contrast to our prediction.
Epidemiological models predict a positive relationship between host population density and abundance of macroparasite populations ( Altizer, Harvell, & Friedle, 2003 ; Anderson & May, 1978, 1991; May & Anderson, 1978 ). When the host density is high, the transmission stages (e.g., eggs, larvae) of the parasite increase their probability of finding a permanent or intermediate host ( Anderson & May, 1978; Arneberg, Skorping, Grenfell, & Read, 1998 ; Lafferty, 1997; May & Anderson, 1978 ). Our findings partly coincide with the increase in helminth abundance found in temperate insectivorous bats when abundance of intermediate hosts is presumably higher ( Blankespoor & Ulmer, 1970; Lord et al., 2012; Nickel & Hansen, 1967 ), and with a study in which no seasonal patterns in helminth abundance were found in tropical insectivorous bats ( Clarke, 2008 ). The lack of a uniform pattern in the cases when seasonal changes of parasite load were detected suggests that seasonal changes in the abundance of arthropod species that serve as intermediate hosts also might not be uniform. We based our prediction of a higher infestation rate in the wet season on the large increase in arthropod abundance during this season in the study region ( Andresen, 2005; Güizado & Casas-Andreu, 2011 ). The variation in diet of Pteronotus species in Chamela is driven by the abundance and availability of insect prey ( Salinas-Ramos et al., 2015 ), which are highly variable in time and space ( Aldridge & Rautenbach, 1987; Whitaker, 1994 ). More than 1,423 species of arthropods have been recorded in Chamela, of which 570 species are found throughout the year while 622 and 231 species are found only during the wet or dry season, respectively ( Pescador-Rubio et al., 2002 ). Seasonal changes in the abundance of some species of helminths detected in our study might reflect changes in the abundance of some arthropod species. For example, Trichoptera collected in rivers are more abundant in the dry season ( Pescador-Rubio et al., 2002 ). Further studies focused on the examination of seasonal abundance of arthropod species and their endoparasites are warranted to understand seasonal dynamics of infection rate in Pteronotus species. An alternative explanation to the finparding of higher rates of infection when the abundance of intermediate hosts is lower is the “dilution effect” ( Hamilton, 1971 ). The increase in the number of individuals of intermediate hosts leads to a decrease in an individual’s probability to be parasitized ( Krasnov, Stanko, & Morand, 2007 ; Ostfeld & Keesing, 2000 ). In other words, the probability of bats being infected by helminths decreases when the abundance of the intermediate host increases.
Our hypothesis of seasonal changes in endoparasite diversity was partly supported. Species richness was slightly higher in P. parnellii in the wet season, but it remained the same in P. davyi and P. personatus . However, following our prediction, the effective number of species was higher during the dry season for the 3 Pteronotus species. Dietary diversity of P. davyi and P. personatus increases in the dry season (Salinas-Ramos et al., 2015 ), when arthropod abundance is lower in the area ( Andresen, 2005; Güizado & Casas-Andreu, 2011 ). It has been suggested that these bat species adopt a more generalist strategy when prey are limited and they become more selective when the insect abundance increases during the wet season ( Salinas-Ramos et al., 2015). In the case of P. parnellii , it had the highest endoparasite richness and effective number of species of all insectivorous bats, which matches the more diverse diet reported in Chamela for P. parnellii compared to the diets of P. davyi and P. personatus all year long (Salinas-Ramos et al., 2015).
Host–parasite interactions could impact food webs and community structures ( Mouritsen & Poulin, 2002; Sukhdeo, 2010 ) and the studies of the factors regulating helminth communities are complex ( Lord et al., 2012 ). Our study examined the relationship between diversity and load of helminths and seasonal patterns of bat and abundance of potential intermediate hosts previously measured. We found heterogeneous patterns of this relationship that warrant further examination of seasonal abundance of intermediate hosts used as food by our focal bat species and of their helminth infection rates. These kinds of studies might be facilitated by the recent development of DNA libraries of arthropods and helminths in the study region ( Fernández-Flores, Fernández-Triana, Martínez, & Zaldívar-Riverón, 2013 ; Prosser, Velarde-Aguilar, León-Règagnon, & Hebert, 2013 ).
Acknowledgements
This work was supported by grants given by Consejo Nacional de Ciencia y Tecnología (Conacyt, Red temática del código de barras de la vida, 2013–2015) to VLR; by Dirección General de Asuntos del Personal Académico (IN202113 ) to LGHM. VBSR thanks Programa de Doctorado en Ciencias Biológicas, Universidad Nacional Autónoma de México and Conacyt for the scholarship received. We also thank Carlos A. González-Castro, Andrea Rebollo-Hernández and Alejandro Zaldívar-Riverón for their assistance in the field and the staff at the Estación de Biología Chamela, UNAM for hosting this work.
References
Aldridge and Rautenbach, 1987
H.D.J.N. Aldridge
I.L. Rautenbach
Morphology, echolocation and resource partitioning in insectivorous bats
Journal of Animal Ecology
56
1987
763-78
Altizer et al., 2003
S. Altizer
D. Harvell
E. Friedle
Rapid evolutionary dynamics and disease threats to biodiversity
Trends in Ecology and Evolution
18
2003
589-96
Altringham, 1996
J.D. Altringham
Bats biology and behavior
1996
Andresen, 2005
E. Andresen
Effects of season and vegetation type on community organization of dung beetles in a tropical dry forest
Biotropica
37
2005
291-300
Anderson and May, 1978
R.M. Anderson
R.M. May
Regulation and stability of host–parasite population interactions: I. Regulatory processes
Journal of Animal Ecology
47
1978
219-47
Anderson and May, 1991
R.M. Anderson
R.M. May
Infectious diseases of humans: dynamics and control
1991
Appleton and Gouws, 1996
C.C. Appleton
E. Gouws
The distribution of common intestinal nematodes along an altitudinal transect in KwaZulu-Natal, South Africa
Annals of Tropical Medicine Parasitology
90
1996
181-8
Arneberg et al., 1998
P. Arneberg
A. Skorping
B. Grenfell
A.F. Read
Host densities as determinants of abundance in parasite communities
Proceedings of the Royal Society of London B: Biological Sciences
265
1998
1283-9
Bell and Burt, 1991
G. Bell
A. Burt
The comparative biology of parasite species diversity: intestinal helminths of freshwater fishes
Journal of Animal Ecology
60
1991
1046-63
Blankespoor and Ulmer, 1970
H.D. Blankespoor
M.J. Ulmer
Helminths from six species of Iowa bats
Proceedings of the Iowa Academy of Sciences
77
1970
200-6
Brooks and Hoberg, 2007
D.R. Brooks
E.P. Hoberg
How will global climate change affect parasite–host assemblages?
Trends in Parasitology
23
2007
571-4
Bullock, 1995
S.H. Bullock
Plant reproduction in neotropical dry forest
Seasonally dry tropical forests
Cambridge University Press
Cambridge, UK
1995
277-303
Bush et al., 2001
A.O. Bush
J.C. Fernández
G.W. Esch
J.R. Seed
Parasitism. The diversity and ecology of animal parasites
2001
Caballero-Caballero and Zerecero, 1942
E. Caballero-Caballero
C. Zerecero
Trematodos de los murciélagos de México II. Redescripción y posición sistemática de Distommum tubiporum Braun, 1900
Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología
13
1942
97-104
Carvalho and Luque, 2011
A.R. Carvalho
J.L. Luque
Seasonal variation in metazoan parasites of Trichiurus lepturus (Perciformes: Trichiuridae) of Rio de Janeiro, Brazil
Brazilian Journal of Biology
71
2011
771-82
Castaño-Meneses, 2014
G. Castaño-Meneses
Trophic guild structure of a canopy ants community in a Mexican tropical deciduous forest
Sociobiology
61
2014
35-42
Chitwood, 1938
B.G. Chitwood
Some nematodes from the caves of Yucatán
Publications of the Carnegie Institution of Washington
491
1938
51-66
Clarke, 2008
E. Clarke
Descripción de la helmintofauna asociada a tres especies de murciélagos (Chiroptera: Mormoopidae) en el municipio de Apazapan, Veracruz (M.Sc. Thesis)
2008
Coggins, 1988
J.R. Coggins
Methods for the ecological study of bat endoparasites
Ecological and behavioral methods for the study of bats
Smithsonian Institute
Washington, D.C.
1988
475-89
Coggins et al., 1982
J.R. Coggins
J.L. Tedesco
C.E. Rupprecht
Seasonal changes and overwintering of parasites in the bat, Myotis lucifugus (Le Conte), in Wisconsin Hibernaculum
The American Midland Naturalist
107
1982
305-15
Cuartas-Calles and Muñoz-Arango, 1999
C. Cuartas-Calles
J. Muñoz-Arango
Nemátodos en la cavidad abdominal y el tracto digestivo de algunos murciélagos Colombianos
Caldasia
21
1999
10-25
Doi and Yurlova, 2011
H. Doi
N.I. Yurlova
Host–parasite interactions and global climate oscillations
Parasitology
2011
1022-8
Esch and Fernández, 1993
G.W. Esch
J.C. Fernández
A functional biology of parasitism: ecological and evolutionary implications
1993
Espericueta-Viera, 2012
J.C. Espericueta-Viera
Diversidad de murciélagos y sus nemátodos parásitos en el area de proteccion de flora y fauna Meseta de Cacaxtla Sinaloa, México (M.Sc. Thesis)
2012
Felis and Esch, 2004
K.J. Felis
G.W. Esch
Community structure and seasonal dynamics of the helminth parasites in Lepomis cyanellus e L. macrochirus from Charles pond, North Carolina: host size and species as determinants of community structure
Journal of Parasitology
90
2004
41-9
Fernández-Flores et al., 2013
S.J.L. Fernández-Flores
J. Fernández-Triana
J.J. Martínez
A. Zaldívar-Riverón
DNA barcoding species inventory of Micrograstrinae wasps (Hymenoptera, Braconidae) from a Mexican tropical dry forest
Molecular Ecology Resources
13
2013
1146-50
García-Vargas, 1995
F. García-Vargas
Helmintos parásitos de murciélagos en la Estación de Biología Chamela, Jalisco (Bachelor Thesis)
1995
García-Vargas et al., 1996
F. García-Vargas
S.D. Osorio
G. Pérez-Ponce de León
Helminths parasites of bats (Mormooopidae y Phyllostomidae) from the Estación de Biología Chamela, Jalisco State, Mexico
Bat Research News
37
1996
7-8
Gotz et al., 2010
F. Gotz
R. Harf
S. Sommer
S. Matthee
Effects of precipitation on parasite burden along a natural climatic gradient in southern Africa – implications for possible shifts in infestation patterns due to global changes
Oikos
119
2010
1029-39
Güizado and Casas-Andreu, 2011
R.M.A. Güizado
G. Casas-Andreu
Facultative specialization in the diet of the twelve-lined Whiptail, Aspidoscelis lineatissima
Journal of Herpetology
3
2011
287-90
Guzmán-Cornejo et al., 2003
C. Guzmán-Cornejo
L. García-Prieto
G. Pérez-Ponce de León
J.B. Morales-Malacara
Parasites of Tadarida brasiliensis mexicana (Chiroptera: Molossidae) from arid regions of México
Comparative Parasitology
70
2003
11-25
Jost, 2006
L. Jost
Entropy and diversity
Oikos
113
2006
363-75
Hamilton, 1971
W.D. Hamilton
Geometry for the selfish herd
Journal of Theoretical Biology
31
1971
295-311
Hill, 1973
M. Hill
Diversity and evenness: a unifying notation and its consequences
Ecology
54
1973
427-32
Hudson et al., 2006
P.J. Hudson
I.M. Cattadori
B. Boag
A.P. Dobson
Climate disruption and parasite–host dynamics: patterns and processes associated with warming and the frequency of extreme climatic events
Journal of Helminthology
80
2006
175-82
Justine, 1989
J.L. Justine
Quatre nouvelles espèces de Capillaria (Nematoda, Capillariinae) parasites de Chiroptères du Gabon
Bulletin du Muséum national d’Histoire naturelle, Paris, 4° Série
11
1989
535-61
Kirschbaum et al., 2009
K. Kirschbaum
S. Perkins
M.R. Gannon
Host–parasite interactions of tropical bats in Puerto Rico
Acta Chiropterologica
11
2009
157-62
Krasnov et al., 2007
B.R. Krasnov
M. Stanko
S. Morand
Host community structure and infestation by ixodid ticks: repeatability, dilution effect and ecological specialization
Oecologia
154
2007
185-94
Lafferty, 1997
K.D. Lafferty
Environmental parasitology: what can parasites tell us about human impacts on the environment?
Parasitology Today
13
1997
251-5
Lamothe-Argumedo, 1997
R. Lamothe-Argumedo
Manual de técnicas para preparar y estudiar los parásitos de animales silvestres
1997
Leavings and Windsor, 1984
S.C. Leavings
D.M. Windsor
Litter moisture content as a determinant of litter arthropod distribution and abundance during the dry season on Barro Colorado Island, Panama
Biotropica
16
1984
125-31
Lord et al., 2012
J.S. Lord
S. Parker
F. Parker
D.R. Brooks
Gastrointestinal helminths of pipistrelle bats ( Pipistrellus pipistrellus/Pipistrellus pygmaeus ) (Chiroptera: Vespertilionidae) of England
Parasitology
139
2012
366-74
MacArthur, 1965
R.H. MacArthur
Patterns of species diversity
Biological Reviews
40
1965
510-33
Marcogliese, 2001
D. Marcogliese
Implications of climate change for parasitism of animals in the aquatic environment
Canadian Journal of Zoology
79
2001
1331-52
Marshall and Miller, 1979
M.E. Marshall
G.C. Miller
Some digenetic trematodes from Ecuadorian bats including five new species and one new genus
Journal of Parasitololgy
65
1979
909-17
May and Anderson, 1978
R.M. May
R.M. Anderson
Regulation and stability of host–parasite population interactions: II. Destabilizing processes
Journal of Animal Ecology
47
1978
249-67
McAllister et al., 2007
C.T. McAllister
C.R. Bursey
R.C. Dowler
Acanthatrium alicatai (Trematoda: Lecithodendriidae) from two species of bats (Chiroptera: Vespertilionidae) in southwestern Texas
Southwestern Association of Naturalists
52
2007
597-600
Méndez-Alonzo et al., 2013
R. Méndez-Alonzo
F. Pineda-García
H. Paz
J.A. Rosell
M.E. Olson
Leaf phenology is associated with soil water availability and xylem traits in tropical dry forest
Trees
27
2013
745-54
Moravec, 1982
F. Moravec
Proposal of a new systematic arrangement of nematodes of the family Capillariidae
Folia Parasitologica
28
1982
119-32
Mouritsen and Poulin, 2002
K.N. Mouritsen
R. Poulin
Parasitism, community structure and biodiversity in intertidal ecosystems
Parasitology
124
2002
101-17
Moyer et al., 2002
B.R. Moyer
D.M. Drown
D.H. Clayton
Low humidity reduces ectoparasite pressure: implications for host life history evolution
Oikos
97
2002
223-8
Muñoz et al., 2011
P. Muñoz
F. Fredes
E. Raffo
D. González-Acuña
L. Muñoz
C. Cid
New report of parasite-fauna of the free-tailed bat ( Tadarida brasiliensis, Geoffroy, 1824) in Chile
Veterinary Research Communications
35
2011
61-6
Nahhas et al., 2005
F. Nahhas
M. Yang
R.S. Uch
Digenetic trematodes of Tadarida brasiliensis mexicana (Chiroptera: Molossidae) and Myotis californicus (Chiroptera:Vespertilionidae) from Northern California, U.S.A.
Comparative Parasitology
72
2005
196-9
Nickel and Hansen, 1967
P.A. Nickel
M.F. Hansen
Helminths of bats collected in Kansas, Nebraska and Oklahoma
The American Midland Naturalist
78
1967
481-6
Nogueira et al., 2004
M.R. Nogueira
S.P. de Fabio
A.L. Peracchi
Gastrointertinal helminth parasitism in fruit-eating bats (Chiroptera: Stenodermatinae) from western Amazonian Brazil
Revista de Biología Tropical
52
2004
1-5
Ostfeld and Keesing, 2000
R.S. Ostfeld
F. Keesing
Biodiversity and disease risk: the case of Lyme disease
Conservation Biology
14
2000
722-8
Peralta-Rodríguez et al., 2012
J.L. Peralta-Rodríguez
J.M. Caspeta-Mandujano
J.A. Guerrero
A new spirurid (Nematoda) parasite from mormooopid bats in Mexico
Journal of Parasitology
98
2012
1006-9
Pérez-Ponce de León et al., 1996
G. Pérez-Ponce de León
V. León-Régagnon
F. García-Vargas
Helminth parasites of bats from Neotropical regions of Mexico
Bat Research News
37
1996
3-6
Pescador-Rubio et al., 2002
A. Pescador-Rubio
A. Rodríguez-Palafox
F.A. Noguera
Diversidad y estacionalidad de Arthropoda
Historia Natural de Chamela
Instituto de Biología, Universidad Nacional Autónoma de México
México, D.F.
2002
183-201
Pilosof et al., 2012
S. Pilosof
C.W. Dick
C. Korine
B.D. Patterson
B.R. Krasnov
Effects of anthropogenic disturbance and climate on patterns of bat fly parasitism
PLoS ONE
7
2012
e41487
Poulin and Morand, 2004
R. Poulin
S. Morand
Parasite biodiversity
2004
Pringle et al., 2012
E.G. Pringle
R. Dirzo
D.M. Gordon
Plant defence, herbivory, and the growth of Cordia alliodora trees and their symbiotic Azteca ant colonies
Oecologia
170
2012
677-85
Prosser et al., 2013
S.J. Prosser
M.G. Velarde-Aguilar
V. León-Règagnon
P.D.N. Hebert
Advancing nematodes barcoding: a primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes
Molecular Ecology Resources
13
2013
1108-15
Reiczigel and Rózsa, 2005
J. Reiczigel
L. Rózsa
Quantitative Parasitology 3.0
2005
Ricci, 1995
M. Ricci
Trematode parasites of Italian bats
Parasitologia
37
1995
199-214
Rzedowski, 1981
J. Rzedowski
Vegetación de México
1981
Salinas-Ramos et al., 2015
V.B. Salinas-Ramos
L.G.H. Montalvo
V. León-Regagnon
A. Arrizabalaga-Escudero
E.L. Clare
Dietary overlap and seasonality in three species of mormoopid bats from a tropical dry forest
Molecular Ecology
24
2015
5296-307
Shimalov et al., 2002
V.V. Shimalov
M.G. Demyanchik
V.T. Demyanchik
A study of the helminth fauna of the bats (Mammalia, Chiroptera: Vespertillionidae) in Belarus
Parasitology Research
88
2002
1011
Šimková et al., 2005
A. Šimková
J. Jarkovsky
B. Koubková
V. Barus
M. Prokes
Associations between fish reproductive cycle and the dynamics of metazoan parasite infections
Parasitology Research
95
2005
65-72
Sukhdeo, 2010
M.V.K. Sukhdeo
Food webs for parasitologists: a review
Journal of Parasitology
96
2010
273-84
Tinsley et al., 2011
R.C. Tinsley
J.E. York
A.L.E. Everard
L.C. Stott
S.J. Chapple
M.C. Tinsley
Environmental constraints influencing survival of an African parasite in a north temperate habitat: effects of temperature on egg development
Parasitology
138
2011
1029-38
Ubelaker, 1970
J.E. Ubelaker
Some observations on ecto- and endoparasites of Chiroptera
About bats
Southern Methodist University
Dallas
1970
247-61
Vaucher, 1992
C. Vaucher
Revision of the genus Vampirolepis Spasskij, 1954 (Cestoda: Hymenolepididae)
Memórias do Instituto Oswaldo Cruz
87
1992
299-304
Vaucher and Durette-Desset, 1986
C. Vaucher
M.C. Durette-Desset
Trichostrongyloidea (Nematoda) parasites de chiroptères néotropicaux. I. Websternema parnellii (Webster, 1971) n. gen. n. comb. et Linustrongylus pteronoti n. gen. n. sp., parasites de Pteronotus au Nicaragua
Revue Suisse de Zoologie
93
1986
237-46
Whitaker, 1994
J.O. Whitaker
Food availability and opportunistic versus selective feeding in insectivorous bats
Bat Research News
35
1994
75-7
Wilson and Reeder, 2005
D.E. Wilson
D.M. Reeder
Mammal species of the World. A taxonomic and geographic reference
2005
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
*
Corresponding author.
Copyright © 2017 Universidad Nacional Autónoma de México, Instituto de Biología.