Ricardo Figueroa-Huitrón a, * Leticia M. Ochoa-Ochoa b, Daniel Sánchez-Ochoa a
a Laboratorio de Ecología Evolutiva de Anfibios y Reptiles, Facultad de Estudios Superiores Iztacala, Universidad Nacional Autónoma de México, Av. de Los Barrios Núm. 1, Los Reyes Iztacala, 54090 Tlalnepantla, Estado de México, Mexico
b Museo de Zoología, Facultad de Ciencias, Universidad Nacional Autónoma de México, Circuito Exterior s/n, Ciudad Universitaria, 04510 México City, Mexico
*Corresponding author: r.figueroahuitron@ciencias.unam.mx (R. Figueroa-Huitrón)
Received: 7 February 2019; accepted: 17 March 2020
Abstract
Anuran vocalizations transmit relevant information that aids in individual recognition and sexual selection by females. When studied over time, vocalizations can help us to understand reproductive phenology. Here, we used an automated recording system to obtain calls of 4 anurans in southeastern Mexico, described the calling phenology of the species and tested its relationship with environmental variables. We performed Generalized Linear Mixed Effects Models (GLMM’s) to explore possible relationships between calling and environmental variables. The hylid species (Agalychnis callidryas, A. moreletii and Dendropsophus ebraccatus) showed a clear calling pattern; they began calling in April/May and increased in their intensity during the rainy season, from June to September. Craugastor loki showed, contrarily, no clear patterns of calling, preferring to call almost continuously throughout the year. Among the environmental factors tested, only minimum temperature and the amount of days without rain had an effect (positive and negative, respectively) on calling activity of the hylids, but we found that calling from other species had greater positive effects within the group. There was a strong phenological overlap in the calling behavior of these species, which may suggest that competition for site of calling or acoustic niche partitioning can be present in the community.
Keywords: Amphibians; Bioacoustics; Seasonal variation; Vocalizations
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Fenología de los cantos de cuatro anuros en el trópico de México
Resumen
Las vocalizaciones de los anuros transmiten información relevante que ayuda en el reconocimiento individual y la selección sexual por parte de las hembras. Cuando se estudian a través del tiempo, las vocalizaciones pueden ayudarnos a comprender la fenología reproductiva. Aquí, utilizamos un sistema de grabación automática para obtener cantos de 4 anuros en el sureste de México, describimos la fenología del canto de las especies y probamos su relación con distintas variables ambientales. Realizamos modelos lineales generalizados de efectos mixtos (GLMM) para explorar posibles relaciones entre los cantos y las variables ambientales. Los hílidos (Agalychnis callidryas, A. moreletii y Dendropsophus ebraccatus) mostraron un claro patrón de canto; comenzaron a llamar en abril/mayo y aumentaron su intensidad durante la temporada de lluvias, de junio a septiembre. Craugastor loki, por el contrario, no mostró un patrón claro de canto, prefiriendo vocalizar casi continuamente durante todo el año. Entre los factores ambientales probados, solo la temperatura mínima y la cantidad de días sin lluvia tuvieron un efecto en la actividad, pero descubrimos que las vocalizaciones de otras especies tuvieron un efecto mayor dentro de los hílidos. Hubo una fuerte superposición fenológica en el comportamiento del canto de estas especies, lo que puede sugerir que la competencia por el sitio de vocalización o la división acústica de nicho esté presente en la comunidad.
Palabras clave: Anfibios; Bioacústica; Variación estacional; Vocalizaciones
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Calling behavior plays an important role in anuran communication, especially in terms of reproduction (Bush et al., 2002; Castellano et al., 2002; Llusia et al., 2013). Anuran vocalizations contain vital information that is involved in species recognition, orientation towards breeding sites and mate choice by females (Pettitt et al., 2013; Pröhl et al., 2007; Valetti et al., 2013). This distinctive characteristic is present in most of the anurans, and therefore researchers can use this important information to search for patterns among species or groups.
By investigating calling behavior over time, the calling phenology can be analyzed. Differences in the phenology of anuran breeding and calling activity of species within a community can facilitate temporal partitioning of resources and, therefore, favor their coexistence (Canavero et al., 2008; Heard et al., 2015). Temporal partition is not the only factor relevant to the calling behavior in an anuran community; the spatial arrangement of males also plays an important role in defining the reproductive strategy of the species. In the tropical rainforests of southeastern Mexico, most hylid frogs aggregate in choruses (Briggs, 2007, 2010; Reichert, 2012), whereas direct developing frogs have a solitary calling behavior (Sarmiento-Rojas, 2014).While there are many biotic and abiotic factors that shape anuran reproductive behavior like physiological constraints, photoperiod, predator avoidance or resources partitioning, the main factors that determine it are temperature and rainfall (Both et al., 2008; Canavero et al., 2008). Both strongly influence the reproductive activity of tropical anurans, being decisive on the beginning and end of the reproductive season, the density of reproductive individuals and many other aspects of anuran’s biology. For this reason, temperature and rainfall are the main abiotic factors analyzed in phenology studies of anurans’ reproduction (Akmentins et al., 2015; Bertoluci & Rodrigues, 2002; Caldart et al., 2016; Heard et al., 2015; Schalk & Saenz, 2016).
Although studies concerning anuran bioacoustics and calling phenology have increased overall (e.g., Akmentins et al., 2015; Lowe et al., 2016; Nelson et al., 2017; Schalk & Saenz, 2016; Willacy et al., 2015) there is still a lacking of bioacoustical information of Mexican anurans. In Mexico, the research on anuran bioacoustics has focused on analyses of call characteristics of few species (Hernández-Herrera et al., 2020; Mendizával-Bevérido, 2011; Sarmiento-Rojas, 2014) and describing the anuran assembly of anuran communities (Juárez-Ortíz, 2012; Morales-Mota, 2014). Our aim was to describe the calling phenology of an anuran community in southeastern Mexico and to test whether calling behavior is related to environmental variables. We expected anuran calling activity to be positively related to temperature and precipitation.
Materials and methods
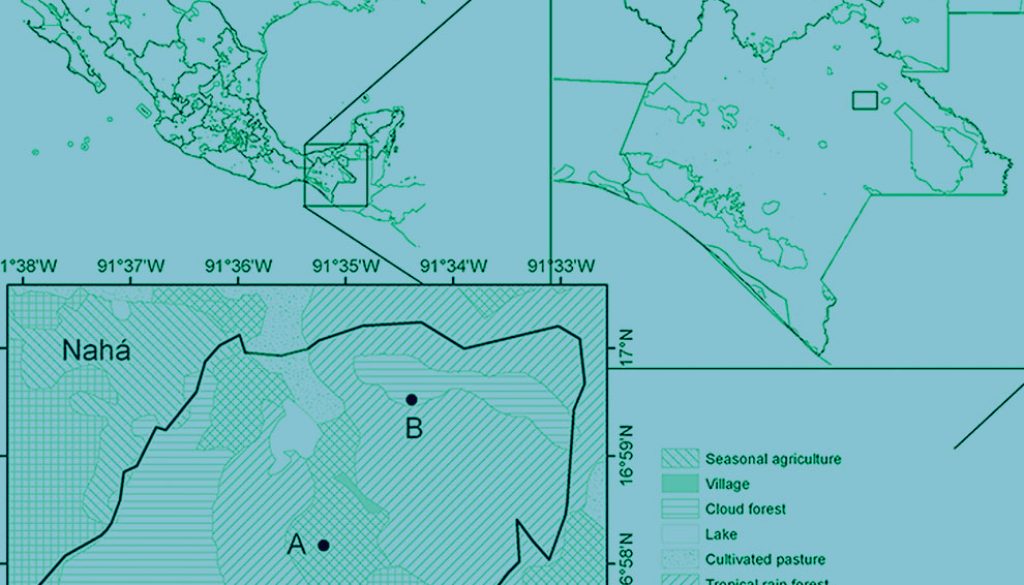
Fieldwork took place at the Nahá Natural Reserve, located in Chiapas, in south-eastern Mexico (16.98° N, 91.58° W; Fig. 1). It is an important location in the Lacandona forest due to its biological diversity (Conanp, 2006). The dominant vegetation type is tropical evergreen rainforest, followed by montane cloud forest and pine-oak forest (Conanp, 2006; Rzedowski, 2006). Previous surveys suggested that the anuran community composition between cloud montane forest and tropical rainforest may be different (Ochoa-Ochoa & Whittaker, 2014), with some species being exclusive to each vegetation type. The mean annual temperature is 23.6 °C, and ranges from 20.9 °C in January to 25.6 °C in May and June (Conanp, 2006). The climate in this area is hot sub-humid with a humid season from May to December and heavy rainfall from June to September (Conanp, 2006, Ochoa-Ochoa & Whittaker, 2014).
We obtained the recordings using an Automated Recording System (ARS) which we placed on the field in August 2013. The ARS was composed by 2 Song Meter SM2 recorders (Wildlife Acoustics, 48k Hz sampling rate, 16-bit WAV files) with 2 omnidirectional SMX-II microphones each. We secured the recorders to a tree approximately 2 m above the ground. We set up one near a temporary pond surrounded by a mixture of old and new secondary vegetation in the tropical rainforest section. The other was placed about 2 km away, at a higher elevation in the cloud montane forest, where different species can be active (personal observations). We programmed the ARS to record 5 minutes at the start of each hour from 18.00 h to 07.00 h in the next morning. We visited each location 6 times between August 2013 and November 2014 to change the batteries and download the information.
We compiled information from previous recordings of the species collected by Ochoa-Ochoa and Whittaker (2014) and personal observations in the field. With this information, we organized a catalogue of vocalizations for the purpose to facilitate the identification of the calls that would appear in the ARS recordings. We utilized Adobe Audition (ver. 3.0) to create reference spectrograms. We listened to each ARS recording and identified the vocalizations to species level (when possible) by comparing the sound and the spectrogram against the ones in the catalogue.
Data collected from the ARS was not continuous throughout the year, therefore, we analyzed the recordings obtained in 2 seasons: between August and December in 2013, and between April and June in 2014. In the ARS recordings we identified Agalychnis callidryas, A. moreletii, Dendropsophus ebraccatus and Craugastor loki with confidence; but we also found 5 potentially distinct vocalizations from Craugastor individuals. These Craugastor calls showed small variation in duration and structure, according to the spectrograms. However, vocal recognition in the field was problematic and we could not gather enough evidence to prove the vocalizations belonged to more than 1 species instead of being different calls from one species. Furthermore, many taxonomical problems still exist within the group. Therefore, we decided to treat them as calling variations of the only species of the genus that we identified with confidence, Craugastor loki.
We analyzed the recordings manually, revising each spectrogram recording and writing down the identified species on a daily basis. For each species, we assigned a daily calling intensity index (CI). This value is based on the one used by the North American Amphibian Monitoring Program (NAAMP) (Weir et al., 2005) and ranges from zero to 3 depending on the species abundance: zero when there were no individuals calling, 1 when there were only a few individuals calling without overlap, 2 when there were more individuals calling with some overlap between calls but different individuals could have been recognized from each other, and 3 when most individuals were calling in a full chorus and individual recognition would be impossible. We analyzed each recording completely before assigning the CI. When a species was active in different intensity during one night and thus presented different CI values for a date, we considered the most repeated value as the index for that specific day.
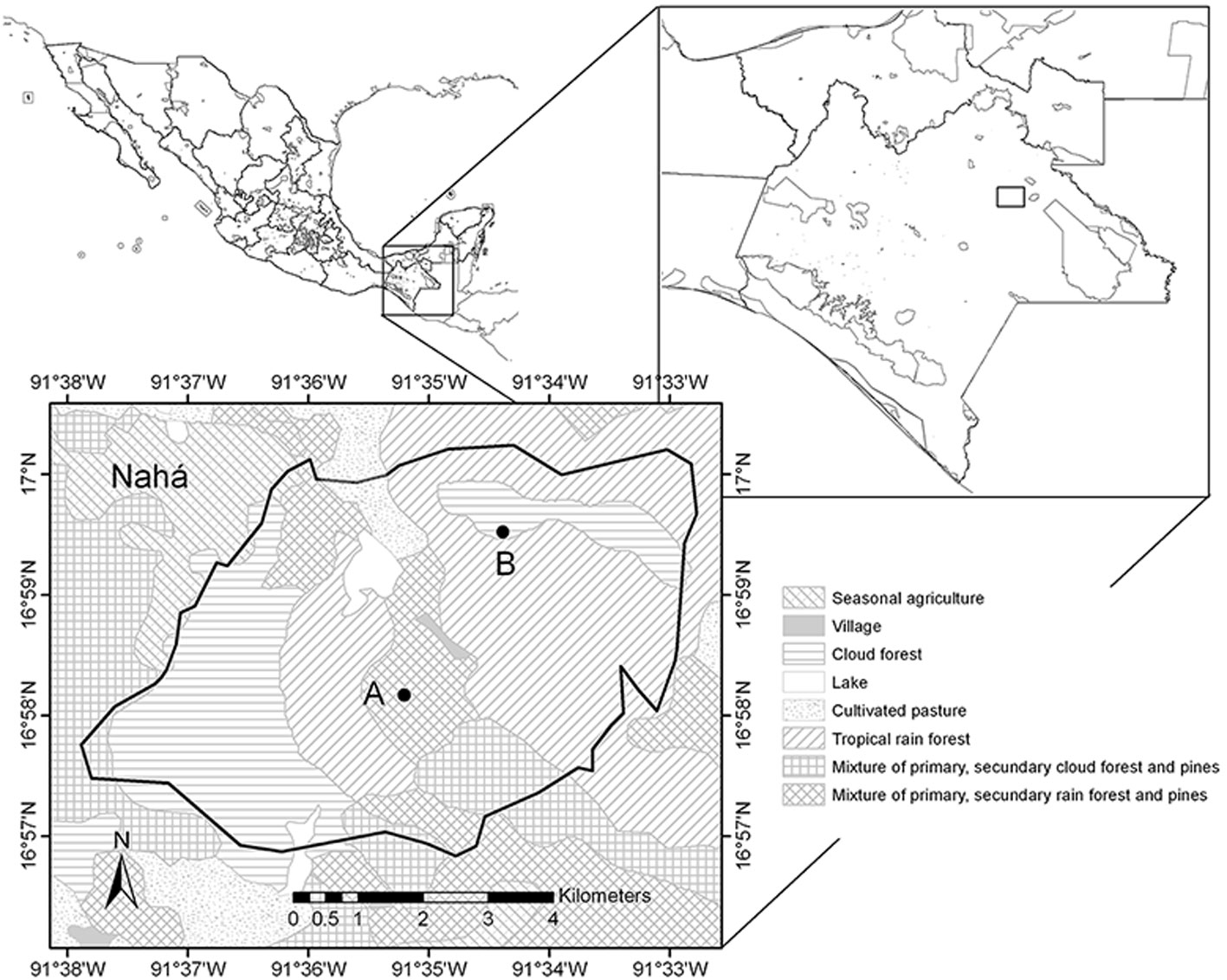
We obtained the temperature data directly from the ARS. The Song Meter recorders have a built-in temperature sensor and a data-logger, which automatically records air temperature in °C for every recording made. We selected the minimum (Tmin) and maximum temperature (Tmax) reading from each day. We did not get complete data on the exact amount of daily rain, so we assigned a daily rain intensity index (RI) using the information of the recorders. Whenever we encountered rain noise while checking the recordings, we assigned to them a RI value of 1 if it was moderate rain that allowed us to distinguish frog calls in the recording, or a value of 2 if it was heavy rain that did not allow us to distinguish frog calls. When a day had recordings with both values of RI, the value assigned was the one that was present during most of the day. The days with and without rain prior to the start of the vocalizations were also recorded. Figure 2 shows the tendency throughout the study period of the environmental variables.
We compared the distribution of the species’ CI with a Kruskal-Wallis test and a Dunn post-hoc test in R (R Core Team, 2020). To explore possible relationships between the calling intensity and the environmental factors, we performed Generalized Linear Mixed Effects Models (GLMM’s) that adjusted for temporal autocorrelation; we used the Poisson distribution and log as the link function. Our models took abiotic and biotics factors as variables that could influence the calling intensity of the species. As the fixed factors, we included daily minimum and maximum temperature, precipitation intensity, the count of the number of days with and without rain previous to each recording, and the calling intensity values of the other species within the community. As the random factor, we set the temporal structure, which refers to the temporal arrangement of our study; we organized hierarchically, first the date and then the season. We calculated all possible models and selected the best model for each species by means of the lowest Akaike’s information criterion (AIC) (Burnham & Anderson, 2002). We report the z and p values for the relevant fixed effects according to each species’ best model. For these analyses we used the R package nlme and to perform all the possible models we used the function “dredge” (Pinheiro et al., 2020).
Results
We obtained 370 hours of recordings with the 2 ARS, on which we detected 3 hylids (Agalychnis callidryas, A. moreletii, and Dendropsophus ebraccatus) and 1 craugastorid (Craugastor loki). The hylid species showed a very clear calling pattern along the sampling period, and there were statistical differences in the distribution of the species’ CI throughout the year (Kruskal-Wallis chi-squared = 33.798, p < 0.01), with the only exception of C. loki vs. A. moreletii. They called all the way from August to December, when the activity started to decline, and the beginning of the 2014 reproductive period was in April/May. D. ebraccatus showed a similar pattern but stopped calling in early November. Meanwhile, A. callidryas and A. moreletii called up until early December, but their calling intensity lowered considerably towards winter. Craugastor loki was present throughout most of the year, but there were few individuals calling in comparison to the hylid species since the CI never surpassed the value of 1 (Fig. 3).
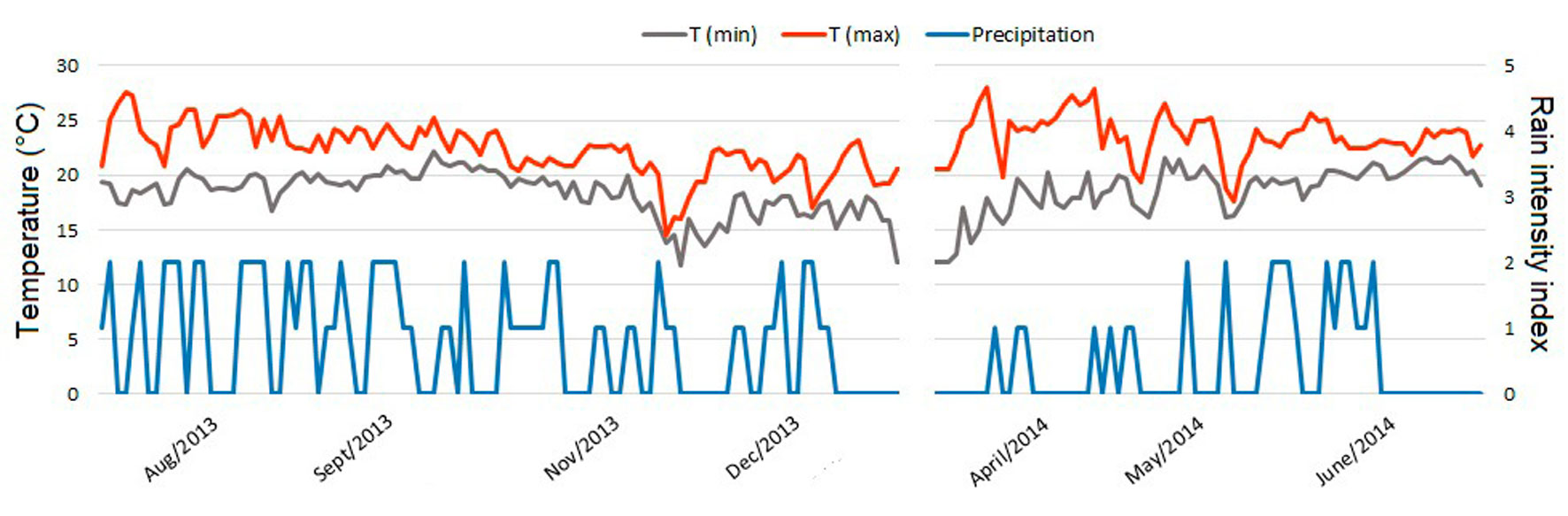
The GLMM’s showed that there were several similarly supported models for each species and, therefore, there is the possibility that other variables could be important in this phenomenon; however, the general effect of the variables was very similar within those models. Table 1 presents the results of the GLMM’s after model selection. Of the abiotic factors that we tested, Tmin had a positive effect on A. callidryas and D. ebraccatus. Days without precipitation had a negative effect on both Agalychnis species. The calling behavior of Craugastor loki and Dendropsosphus ebraccatus had negative effects with each other, which means that, in general terms, whenever one vocalized the other did not. On the other hand, the effects of the species’ calling activity within the hylids was clearly positive, which means that their calling behavior promoted calling activity of the other species within the group. The calling activity of A. callidryas triggered calling of A. moreletii and D. ebraccatus, which, on one hand, also showed positive correlation to calling activity by A. callidryas.
Table 1
Results of the GLMM. We report only the estimates for the fixed effects relevant to the best model of each species; model selection was made with AIC. *Significant values.
|
Species’ calling intensity index (CI) |
Fixed effects |
Estimate |
Std. Error |
Z value |
p value |
|
C. loki |
D. ebraccatus |
-0.269 |
0.102 |
-2.627 |
0.009 * |
|
A. callidryas |
A. moreletii |
0.294 |
0.092 |
3.190 |
0.001 * |
|
D. ebraccatus |
0.182 |
0.087 |
2.104 |
0.0354 * |
|
|
Minimum temperature |
0.138 |
0.051 |
2.699 |
0.007 * |
|
|
|
Days without precipitation |
-0.082 |
0.040 |
-2.040 |
0.041 * |
|
A. moreletii |
A. callidryas |
0.660 |
0.082 |
8.062 |
7.5e-16 * |
|
|
Days without precipitation |
-0.102 |
0.051 |
-2.019 |
0.044 * |
|
D. ebraccatus |
C. loki |
-0.410 |
0.190 |
-2.157 |
0.031 * |
|
A. callidryas |
0.973 |
0.111 |
8.758 |
2e-16 * |
|
|
|
Minimum temperature |
0.262 |
0.082 |
3.200 |
0.001 * |
Discussion
We found Craugastor loki started to call in early April, and this concurs with what Gottsberger and Gruber (2004) found; anurans that present direct development start their calling activity at the onset of the wet season in order to gain benefit throughout its duration. C. loki also called during most of the year, including November and December. This resembles the activity pattern of C. noblei, which was found emitting calls even through November and December (Salazar-Zúñiga & García-Rodríguez, 2014) in Costa Rica. In terms of abundance, the calling intensity index values were low for Craugastor loki. This means that very few individuals were calling in our study site, which is similar to that found in in Veracruz; Sarmiento-Rojas (2014) found that individuals from C. decoratus did not aggregate in choruses and that only some of those of C. rhodopis do, but never with more than 5 individuals.
On the contrary, the hylids were more abundant, as their calling intensity index presented repeatedly high values. A. callidryas and A. moreletii continued calling until December, although the intensity dropped considerably towards the end of the year. Even so, this extended period of activity is unusual. Using data from Mexico’s national water commission (Conagua , 2015) we found that November 2013 was the hottest November in the last decade and December 2013 was the fourth hottest December of that period in Chiapas. Anurans are very dependent on their habitat temperature, but mostly its humidity (Abrunhosa et al., 2006; Bevier, 1997). The data extracted from Conagua also revealed that, for the last decade, the precipitation average for November and December in Chiapas are 80 and 56.5 mm respectively, but in 2013 the rainfall heavily increased, reaching to 193.2 and 157.8 mm. This heavy rainfall, along with high temperatures, could be the reason for the lengthening of these species’ activity period. Such change in the reproductive phenology of these species, if sustained, could alter the community’s dynamics and the competition for resources (González et al., 2011; Saenz et al., 2006; Walls et al., 2013).
Regarding the results of the GLMM’s, A. callydrias and D. ebraccatus reacted positively to minimum temperature, a result opposed to that found by Segev et al. (2012), who mentioned that in high elevations of around 900 m above sea level (our study site is located between 900 and 1,000 m of altitude), low temperatures may be a limiting factor for breeding activity. However, it is in temperate regions where temperature plays a major role in amphibian reproduction, whereas in the tropics, precipitation is the most important factor (Both et al., 2008; Ficetola & Maiorano, 2016), thus, a direct effect from rainfall was expected. We found that calling activity in Agalychnis species was not related with rain intensity, but rather negatively to the number of days without rain. This means that frogs stop singing when rains are less frequent, a tendency that we observed towards the end of December 2013. It is important to note that our analysis focused on male activity, since males are the ones that call in these species. Rain intensity does influence the reproductive seasonality of females that becomes active after heavy rains, whereas males are active since the onset of the rainy season (Briggs, 2008).
As mentioned beforehand, within hylids, the calling activity of the others had a positive effect, and the estimates were higher than those of the abiotic factors. One possible explanation is that the environmental factors are in many cases important to trigger the start of the reproductive season (Oseen & Wassersug, 2002; Saenz et al., 2006), and afterwards, their activity could be influenced more by the interaction and competition with other species (Gottsberger & Gruber, 2004; Martins et al., 2006). This high association could suggest an overlap in the acoustic niches of the species, especially since they call at similar periods throughout the year. Calling through different microhabitats is a useful resource to partition the acoustic niche (Martins et al., 2006; Villanueva-Rivera, 2014). D. ebraccatus calls from low vegetation within the pond, whereas the Agalychnis species call from the higher vegetation. D. ebraccatus and C. loki presented a negative relation within themselves, which suggest a difference in the use of resources, to reduce interspecific competition and allow coexistence. The Agalychnis species, on their own, could compete for calling sites and resort to a niche partition in terms of calling frequency and call characteristics, since their vocalizations are very different.
Understanding the patterns of activity over time periods and how climate affects frogs is very important, especially when a subtle change in environmental factors can alter anuran phenology (Schalk & Saenz, 2016). The hylids in our study showed an intense activity period mostly during the rainy season, Craugastor loki showed no clear preference and showed activity throughout the year. It is commonly accepted that the activity patterns of anurans are strongly related to environmental conditions (Heard et al., 2015; Schalk & Saenz, 2016) and we found a pattern where rainfall and, in a lesser rank , temperature affect differently anurans that occupy different microhabitats. However, the calling activity of other species of the community had an even higher effect, which suggests that a complex arrangement of community structure takes place, with potential costs and benefits of interspecific interactions. The implications of these aspects on the structure of communities in the long term remain to be seen, therefore monitoring in the region must continue in order to explore if the relationship between environmental factors and anuran calling phenology changes in the future or remains constant.
Acknowledgments
To O. A. Flores-Villela, N. Mejía-Domínguez, A. Gordillo-Martínez and M. Ortiz-Ramírez for giving us logistical and theoretical support. S. Robles and H. Cayetano contributed greatly during field trips. To the Nahá community and the Comisión Nacional de Áreas Naturales Protegidas (Conanp) for hosting us during the survey. This study was partly funded by Conanp and the Consejo Nacional de Ciencias y Tecnología (Conacyt, grant No. 154093 to A. Navarro-Sigüenza).
References
Abrunhosa, P. A., Wogel, H., & Pombal, Jr. J. P. (2006). Anuran temporal occupancy in a temporary pond from the atlantic rain forest, south-eastern Brazil. Herpetological Journal, 16, 115–122.
Akmentins, M. S., Pereyra, L. C., Sanabria, E. A., & Vaira, M. (2015). Patterns of daily and seasonal calling activity of a direct-developing frog of the subtropical Andean forests of Argentina. Bioacoustics, 24, 89–99. https://doi.org/10.1080/09524622.2014.965217
Bertoluci, J., & Rodrigues, M. T. (2002). Seasonal patterns of breeding activity of Atlantic Rainforest anurans at Boracéia, Southeastern Brazil. Amphibia-Reptilia, 23, 161–167.
Bevier, C. R. (1997). Breeding activity and chorus tenure of two neotropical hylid frogs. Herpetologica, 53, 297–311. https://doi.org/10.1163/156853802760061804
Both, C., Kaefer, Í. L., Santos, T. G., & Cechin, S. T. Z. (2008). An austral anuran assemblage in the Neotropics: seasonal occurrence correlated with photoperiod. Journal of Natural History, 42, 205–222. https://doi.org/10.1080/00222930701847923
Briggs, V. S. (2007). Sexual selection and larval performance of two species of red-eyed treefrogs, Agalychnis callidryas and A. moreletii of the Chiquibul Forest Reserve, Belize. Miami: University of Miami.
Briggs, V. S. (2008). Mating patterns of red-eyed treefrogs, Agalychnis callidryas and A. moreletii. Ethology, 114, 489–498. https://doi.org/10.1111/j.1439-0310.2008.01490.x
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference. A practical information. Theoretic approach (2nd ed.). New York: Springer. https://doi.org/10.1007/978-0-387-22456-5_1
Bush, S. L., Gerhardt, H. C., & Schul, J. (2002). Pattern recognition and call preferences in treefrogs (Anura: Hylidae): a quantitative analysis using a no-choice paradigm. Animal Behaviour, 63, 7–14. https://doi.org/10.1006/anbe.2001.1880
Caldart, V. M., Iop, S., Lingnau, R., & Cechin, S. Z. (2016). Calling activity of a stream-breeding frog from the austral neotropics: Temporal patterns of activity and the role of environmental factors. Herpetologica, 72, 90–97. https://doi.org/10.1655/herpetologica-d-15-00029
Canavero, A., Arim, M., Naya, D. E., Camargo, A., da Rosa, I., & Maneyro, R. (2008). Calling activity patterns in an anuran assemblage: the role of seasonal trends and weather determinants. North-Western Journal of Zoology, 4, 29–41.
Castellano, S., Cuatto, B., Rinella, R., Rosso, A., & Giacoma, C. (2002). The advertisement call of the European treefrogs (Hyla arborea): A multilevel study of variation. Ethology, 108, 75–89. https://doi.org/10.1046/j.1439-0310.2002.00761.x
Conagua (Comisión Nacional del Agua). (2015). Resúmenes mensuales de temperaturas y lluvia. Retrieved on August 27, 2015, from: https://smn.conagua.gob.mx/es/climatologia/temperaturas-y-lluvias/resumenes-mensuales-de-temperaturas-y-lluvias
Conanp (Comisión Nacional de Áreas Naturales Protegidas). (2006). Programa de conservación y manejo Área de Protección de Flora y Fauna Nahá. México D.F.: Conanp-Semarnat.
Ficetola, G. F., & Maiorano, L. (2016). Contrasting effects of temperature and precipitation change on amphibian phenology, abundance and performance. Oecologia, 181, 683–693. https://doi.org/10.1007/s00442-016-3610-9
González, S. C., Touchon, J. C., & Vonesh, J. R. (2011). Interactions between competition and predation shape early growth and survival of two neotropical hylid tadpoles. Biotropica, 43, 633–639. https://doi.org/10.1111/j.1744-7429.2010.00748.x
Gottsberger, B., & Gruber, E. (2004). Temporal partitioning of reproductive activity in a neotropical anuran community. Journal of Tropical Ecology, 20, 271–280. https://doi.org/10.1017/s0266467403001172
Heard, G. W., Canessa, S., & Parris, K. M. (2015). Interspecific variation in the phenology of advertisement calling in a temperate Australian frog community. Ecology and Evolution, 5, 3927–3938. https://doi.org/10.1002/ece3.1666
Hernández-Herrera, C. I., & Pérez-Mendoza H. A. (2020). Acoustic and morphological variation on two populations of Dryophytes arenicolor in central México. Bioacoustics. https://doi.org/10.1080/09524622.2020.1760937
Juárez-Ortíz, J. J. (2012). Análisis de la actividad acústica en una comunidad de anuros en Tlacotepec de Porfirio Díaz, Puebla (Bachelor Thesis). Benemérita Universidad Autónoma de Puebla. Mexico.
Llusia, D., Márquez, R., Beltrán, J. F., Benítez, M., & Do Amaral, J. P. (2013). Calling behaviour under climate change: geographical and seasonal variation of calling temperatures in ectotherms. Global Change Biology, 19, 2655–2674. https://doi.org/10.1111/gcb.12267
Lowe, K., Castley, J. G., & Hero, J. -M. (2016). Calling phenology and detectability of a threatened amphibian (Litoria olongburensis) in ephemeral wetlands varies along a latitudinal cline: Implications for management. Austral Ecology, 41, 938–951. https://doi.org/10.1111/aec.12386
Martins, I. A., Almeida, S. C., & Jim, J. (2006). Calling sites and acoustic partitioning in species of the Hyla nana and rubicundula groups (Anura, Hylidae). Herpetological Journal, 16, 239–247.
Mendizával-Bevérido, N. (2011). Repertorio vocal e interacciones acústicas de machos de Ecnomiohyla miotympanum (Anura: Hylidae) (Master Thesis). Instituto de Neuroetología, Universidad Veracruzana. Xalapa, Mexico.
Morales-Mota, A. (2014). Estudio de la actividad acústica de dos especies de anuros del parque estatal Lázaro Cárdenas “Flor del Bosque” (Bachelor Thesis). Benemérita Universidad Autónoma de Puebla. Mexico.
Nelson, D. V., Garcia, T. S., & Klinck, H. (2017). Seasonal and diel vocal behavior of the northern red-legged frog, Rana aurora. Northwestern Naturalist, 98, 33–38. https://doi.org/10.1898/nwn16-06.1
Ochoa-Ochoa, L. M., & Whittaker, R. J. (2014). Spatial and temporal variation in amphibian metacommunity structure in Chiapas, Mexico. Journal of Tropical Ecology, 30, 537–549. https://doi.org/10.1017/s0266467414000388
Oseen, K. L., & Wassersug, R. J. (2002). Environmental factors influencing calling in sympatric anurans. Oecologia, 133, 616–625. https://doi.org/10.1007/s00442-002-1067-5
Pettitt, B. A., Bourne, G. R., & Bee, M. A. (2013). Advertisement Call Variation in the Golden Rocket Frog (Anomaloglossus beebei): Evidence for Individual Distinctiveness. Ethology, 119, 244–256. https://doi.org/10.1111/eth.12058
Pinheiro, J., Bates, D., DebRoy, S., Sarkar, D., & R Core Team (2020). _nlme: Linear and Nonlinear Mixed Effects Models_. R package version 3.1-144: https://CRAN.R-project.org/package=nlme
Pröhl, H., Hagemann, S., Karsch, J., & Höbel, G. (2007). Geographic Variation in Male Sexual Signals in Strawberry Poison Frogs (Dendrobates pumilio). Ethology, 113, 825–837. https://doi.org/10.1111/j.1439-0310.2007.01396.x
R Core Team (2020). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reichert, M. S. (2012). Call timing is determined by response call type, but not by stimulus properties, in the treefrog Dendropsophus ebraccatus. Behavioral Ecology and Sociobiology, 66, 433–444. https://doi.org/10.1007/s00265-011-1289-9
Rzedowski, J. (2006). Vegetación de México. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Saenz, D., Fitzgerald, L. A., Baum, K. A., & Conner, R. N. (2006). Abiotic correlates of anuran calling phenology: the importance of rain, temperature, and season. Herpetological Monographs, 20, 64–82. https://doi.org/10.1655/0733-1347(2007)20[64:acoacp]2.0.co;2
Salazar-Zúñiga, J. A., & García-Rodríguez, A. (2014). Advertisement call of Craugastor noblei: another calling species of the Craugastor gollmeri group (Anura: Craugastoridae). Phyllomedusa, 13, 67–70. https://doi.org/10.11606/issn.2316-9079.v13i1p67-70
Sarmiento-Rojas, A. (2014). Estudio bioacústico de las vocalizaciones de ocho especies de anuros en la región montañosa central de Veracruz (Master Thesis). Instituto de Ecología A.C. Xalapa, Mexico.
Schalk, C. M., & Saenz, D. (2016). Environmental drivers of anuran calling phenology in a seasonal Neotropical ecosystem. Austral Ecology, 41, 16–27. https://doi.org/10.1111/aec.12281
Segev, O., Andreone, F., Pala, R., Tessa, G., & Vences, M. (2012). Reproductive phenology of the tomato frog, Dyscophus antongili, in an urban pond of Madagascar’s east coast. Acta Herpetologica, 7, 331–340.
Valetti, J. A., Salas, N. E., & Martino, A. L. (2013). Bioacústica del canto de advertencia de Ceratophrys cranwelli (Anura: Ceratophrydae). Revista de Biologia Tropical, 61, 273–280. https://doi.org/10.15517/rbt.v61i1.11109
Villanueva-Rivera, L. J. (2014). Eleutherodactylus frogs show frequency but no temporal partitioning: implications for the acoustic niche hypothesis. PeerJ, 2, 1–14. https://doi.org/10.7717/peerj.496
Walls, S. C., Barichivich, W. J., & Brown, M. E. (2013). Drought, deluge and declines: the impact of precipitation extremes on amphibians in a changing climate. Biology, 2, 399–418. https://doi.org/10.3390/biology2010399
Weir, L. A., Royle, J. A., Nanjappa, P., & Jung, R. E. (2005). Modeling anuran detection and site occupancy on North American Amphibian Monitoring Program (NAAMP) Routes in Maryland. Journal of Herpetology, 39, 627–639. https://doi.org/10.1670/0022-1511(2005)039[0627:madaso]2.0.co;2
Willacy, R. J., Mahony, M., & Newell, D. A. (2015). If a frog calls in the forest: bioacoustic monitoring reveals the breeding phenology of the endangered Richmond Range mountain frog (Philoria richmondensis). Austral Ecology, 40, 625–633. https://doi.org/10.1111/aec.12228