First report of Chaetogaster limnaei (Annelida: Naididae) in Chile based on samples retrieved from an invasive freshwater snail
Gonzalo A. Collado a, b, *, Francisco J. Cabrera c, Gabriel I. Ballesteros d, Nicolás I. Villalobos a, Karina P. Aguayo a
a Departamento de Ciencias Básicas, Facultad de Ciencias, Universidad del Bío-Bío, Avenida Andrés Bello 720, Casilla 447, Chillán, Chile
b Grupo de Investigación Biodiversidad y Cambio Global, Universidad del Bío-Bío, Avenida Andrés Bello 720, Casilla 447, Chillán, Chile
c Universidad Tecnológica de Chile INACAP, Longitudinal Sur 441, Chillán, Chile
d Centro de Ecología Molecular y Funcional, Instituto de Ciencias Biológicas, Universidad de Talca, Avenida Lircay s/n, Talca, Chile
*Corresponding author: gcollado@ubiobio.cl (G.A. Collado)
Abstract
Some naidid oligochaetes establish commensal relationships with species of molluscs worldwide. In the present study, we report the finding of Chaetogaster limnaei in Illapel River, northern Chile. This worm was found inhabiting the mantle cavity of the freshwater gastropod Physa acuta, an invasive species in this country. The taxonomic status of C. limnaei was confirmed by molecular analysis based on mitochondrial 16S ribosomal RNA gene.
Keywords:
Aquatic molluscs; Invasive species; Naidids; Oligochaetes; Physa acuta
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Primer registro de Chaetogaster limnaei (Annelida: Naididae) en Chile con base en muestras obtenidas de un caracol de agua dulce invasor
Resumen
Los oligoquetos Naididae pueden establecer relaciones comensales con especies de moluscos alrededor del mundo. En el presente estudio se informa el hallazgo del oligoqueto Chaetogaster limnaei en el río Illapel, norte de Chile. Los animales se encontraron habitando la cavidad del manto del gasterópodo de agua dulce Physa acuta, una especie invasora en este país. El estado taxonómico de C. limnaei se confirmó mediante el análisis molecular basado en secuencias de ADN del gen mitocondrial del ARN ribosomal 16S.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Molusco acuático; Especie invasora; Naididae; Oligoquetos; Physa acuta
Introduction
Naididae Ehrenberg, 1828, is a family of small aquatic oligochaetes that play an important role in many marine and freshwater ecosystems worldwide (Brinkhurst & Jamieson, 1971). The group includes detritivorous, grazers and carnivorous species (Pinder & Ohtaka, 2014). One member of the family is the genus Chaetogaster von Baer, 1827 which, in addition to free-living worms, includes Chaetogaster limnaei von Baer, 1927, an ectosymbiont species for which a close commensal association with several mollusc taxa, including gastropods and bivalves, has been broadly reported (Barbour, 1977; Buse, 1974; Conn et al., 1995; Gelder, 1980; Gruffydd, 1965a, b; Ibrahim, 2007; Streit, 1974).
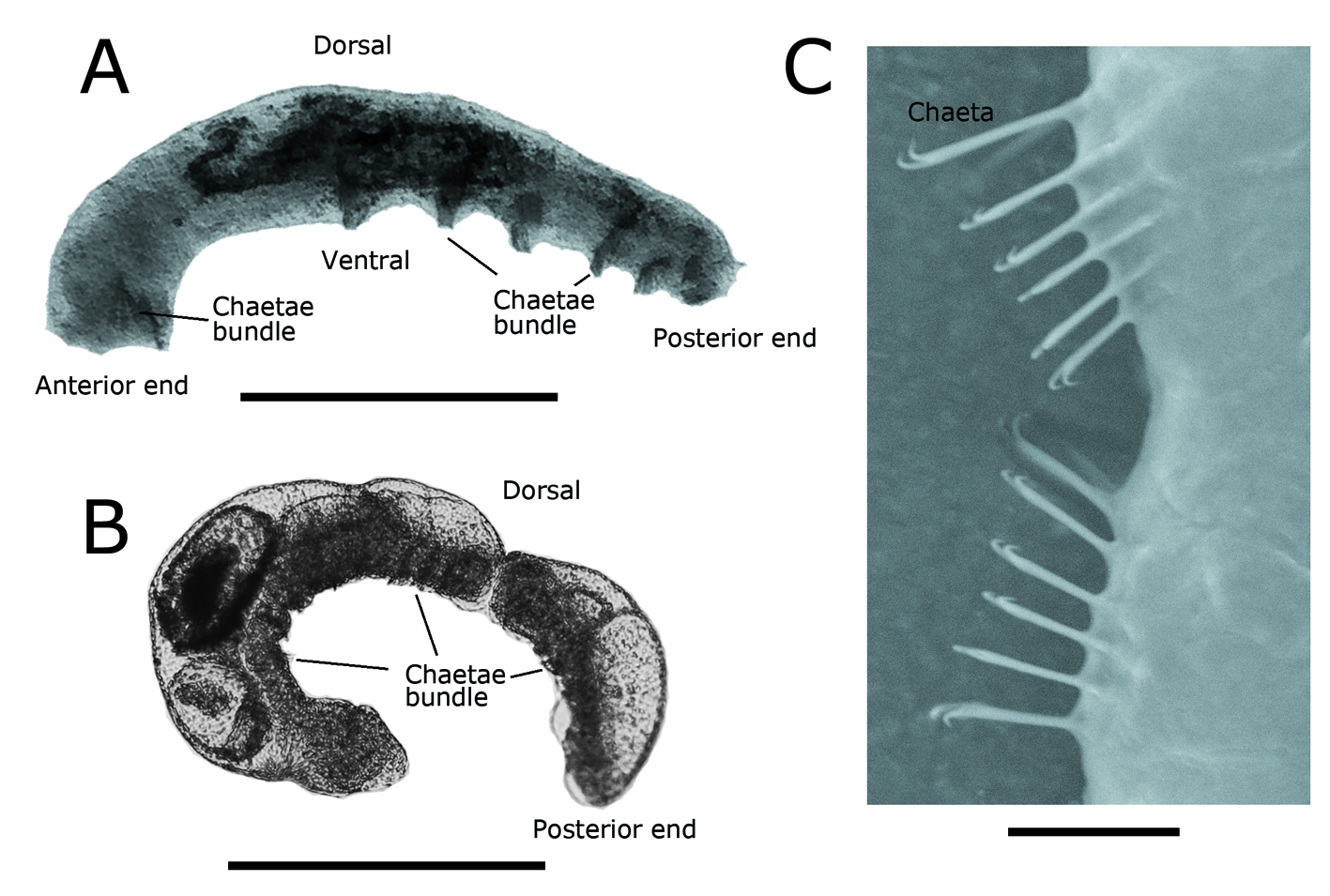
Chaetogaster limnaei has no dorsal chaetae and the ventral chaetae, up to 14-20 per bundle, are absent in segments 3 to 5 (Gruffydd, 1965a; Brinkhurst, 1986). The species is also characterized by having a vestigial prostomium and chaetae with strongly curved teeth (Gruffydd, 1965a; Gelder, 1980; Brinkhurst, 1971, 1986). These features, as well as its habitat, separate C. limnaei from the other species of the genus. Two subspecies have been proposed for C. limnaei, based on habitat and feeding differentiation, C. limnaei limnaei Von Baer, 1927 and C. limnaei vaghini Gruffydd, 1965. Chaetogaster limnaei limnaei inhabits the mantle cavity of snails, where it reduces parasite infection by feeding on trematode larvae in addition to other organisms, so it has been considered a commensal (Gruffydd, 1965a, b; Khalil, 1961; Michelson, 1964; Rodgers et al., 2005; Zimmermann et al., 2011). However, Gamble and Fried (1976) reported C. limnaei limnaei consuming host snail epithelial tissue, indicating a parasitic relationship. Chaetogaster limnaei vaghini, on the other hand, lives in the renal system of the host, where it feeds on kidney cells, so it has been considered as endoparasitic (Gruffydd, 1965a, b). This subspecies separation is not supported by recent molecular phylogenetic analysis based on COI sequences, which provides evidence that these forms belong to the same taxonomic entity (Smythe et al., 2015). In South America, C. limnaei has been found associated with snails Aplexa rivalis (Maton & Rackett, 1807) (Physidae), Biomphalaria straminea (Dunker, 1848) (Planorbidae) and Pseudosuccinea columella (Say, 1817) (Lymnaeidae) in Brazil (Callisto et al., 2005; Martins & Alves, 2008, 2010). Di Persia (1980) found C. limnaei in Argentina. In Chile, Gluzman (1990) and Valdovinos (2008) included Chaetogaster sp. in their checklists without specifying habitat or host, while Fuentealba-Jara (2011) recorded this worm associated with the freshwater snails of the genus Uncancylus Pilsbry, 1913. Only Chaetogaster diastrophus (Gruithuisen, 1828) has been recorded in freshwater ecosystems at the Chilean Altiplano (SINAB, 2017).
In the present study, we report the first finding of C. limnaei in Chile based on samples retrieved from the gastropod Physa acuta Draparnaud, 1805, an invasive snail recently reported in the country (Bousset et al., 2014; Collado, 2017).
Materials and methods
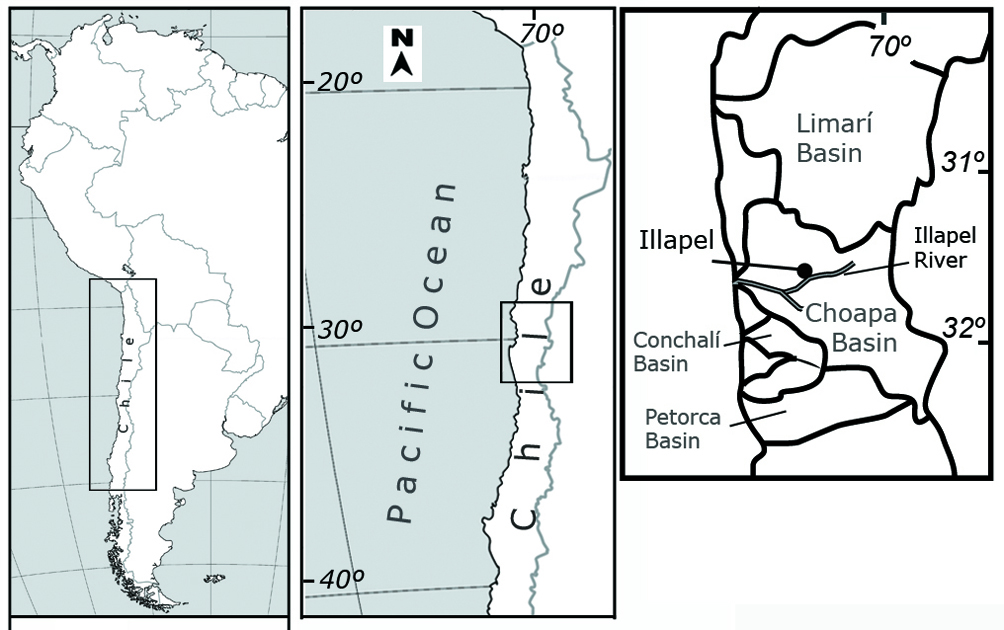
Eleven adult physid snails were sampled from the Illapel River (Fig. 1), Coquimbo Region, Northern Chile (31°37’55.30” S, 71°09’20.30” W) on March 2016 and preserved in ethanol. The snails were assigned to Physa acuta following Collado (2017). The worms were isolated by dissection of the mantle cavity of snails and observed using stereo and light microscopes (Motic). Photographs of the chaetae were obtained using a scanning electron microscope (SEM) Hitachi SU3500. The specimens were identified as C. limnaei according to taxonomic keys (Brinkhurst, 1971, 1986; Kathman & Brinkhurst, 1998) and additional literature regarding the morphology of the species (Cichy et al., 2016; Gelder, 1989; Gruffydd, 1965a, b; Khalil, 1961). Genomic DNA was extracted using the CTAB method (cetyltrimethyl ammonium bromide) (Winnepennickx et al., 1993) of one specimen assigned to this species. The mitochondrial 16S rRNA gene was amplified by polymerase chain reaction (PCR) using the primers 16Sar-L and 16Sbr-H (Palumbi, 1996). PCR profile was described by Collado and Méndez (2012). Nucleotide sequences were obtained by automatic sequencing in forward and reverse directions at Macrogen Company (South Korea). DNA sequences were edited in the software BioEdit (Hall, 1999) and aligned with Clustal X (Thompson et al., 1997). Phylogenetic analyses were performed using maximum parsimony (MP) and Bayesian inference (BI) methods. The MP analysis was based on heuristic search, the TBR algorithm and the random addition of sequences using the program PAUP* 4.0 (Swofford, 2003). The statistical confidence of the nodes was evaluated performing 100 bootstrap pseudoreplicates (Felsenstein, 1985). The BI analysis was performed in the software MrBayes v. 3.1.2 (Ronquist & Huelsenbeck, 2003), previously selecting the best model of sequence evolution with jModelTest (Posada, 2008). The analysis was run for 3 million generations sampling every 1,000 generations; posterior probabilities were obtained using a burn-in period of 10%. The analyses were performed together with 16S rRNA sequences from other congeners and naidid taxa (Bely & Sikes, 2010; Envall et al., 2006, 2012; Sjölin et al., 2005).
Genomic DNA of C. limnaei is housed in the Laboratorio de Malacología y Sistemática Molecular, Universidad del Bío-Bío, Chillán, Chile. The 16S sequence obtained in the present study was deposited in GenBank (accession number: KY436156).
Results
Specimens of C. limnaei (Fig. 2A-C) were found living in the mantle cavity of the invasive freshwater snail P. acuta sampled in Illapel River. The worms were characterized by the absence of dorsal chaetae, the absence of ventral chaetae in anterior segments and the presence of an inconspicuous prostomium. Other worm species were not present in the host snails. The incidence of C. limnaei on P. acuta was estimated to be 27.3%.
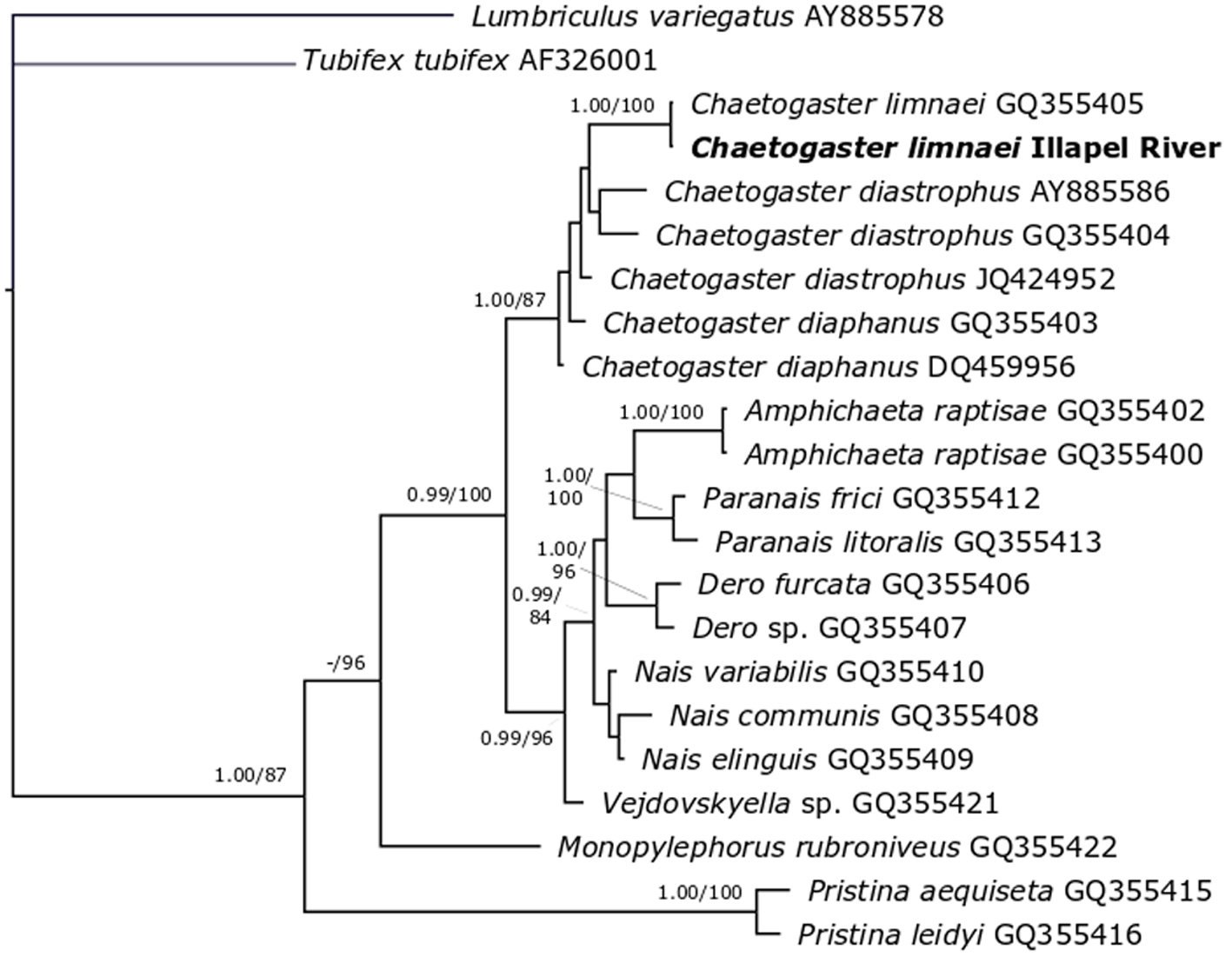
Amplification of the 16S gene in the specimen of C. limnaei from Illapel River produced a 503 base pair fragment. BLASTn analysis indicated 100% identity with a sequence of C. limnaei from Sacramento, USA (accession number: GQ355405) (Bely & Sikes, 2010). The MP analysis recovered a single tree (not shown) similar to the topology recovered by the BI analysis (Fig. 3). In both trees our sequence of C. limnaei formed a monophyletic group (1.00 posterior probability under BI; 100% bootstrap under MP) together with C. limnaei from Sacramento, USA, within a well-supported clade (1.00 posterior probability under BI; 87% bootstrap under MP) composed by other species of the genus Chaetogaster for which relationships are poorly supported.
Discussion
The present study allowed the identification of C. limnaei in the invasive P. acuta in northern Chile. The molecular approach was useful to confirm the accuracy of the species identification based on morphological characters. Although the 16S rRNA sequence obtained in the present study is identical to that of C. limnaei from Sacramento, USA (Bely & Sikes, 2010), further molecular investigation is required to establish the route of introduction of C. limnaei to Chile. Besides, it is important to note that the route of introduction of P. acuta to this country is also unknown, although more than one introduction event of the species has been suggested (Collado, 2017).
As a commensal, C. limnaei feeds in a variety of prey including diatoms, filamentous algae, ciliates, flagellates, foraminiferans, rotifers and trematode cercariae, although its omnivory is limited by particle size (Buse, 1974; Conn et al., 1996; Gelder, 1989; Gruffydd, 1965a; Khalil, 1961; Michelson, 1964; Stoll et al., 2013; Streit, 1974). In the present study, we speculate the existence of a commensal relationship between C. limnaei and P. acuta in Illapel River since the examination of the snails showed no damage to the paleal tissues, a hypothesis that needs to be further investigated. Stoll et al. (2013), on the other hand, hypothesized an epizoic antibiosis relationship between C. limnaei and P. acuta at high infestation rates as suggested by the lower growth rates of the infected snail hosts. A strong positive correlation between the size of P. acuta and the infection intensity of C. limnaei has also been observed (Mitchell & Leung, 2016).
Chaetogaster limnaei has been considered a cosmopolitan species (Brinkhurst & Jamieson, 1971; Callisto et al., 2005). It has been found associated to more than 40 species of freshwater snails from at least 10 families, mainly members of Lymnaeidae, Physidae, and Planorbidae (Smythe et al., 2015). In the present study we found C. limnaei within a population of the invasive P. acuta in Chile. Potential snail hosts in this country include native species of the genus Biomphalaria Preston, 1910, Uncancylus, Chilina Gray, 1828, Lymnaea Lamarck, 1799, Heleobia Stimpson, 1865, Physa Draparnaud, 1801 and Potamolithus Pilsbry, 1896. A more extensive sampling is necessary to test the occurrence of C. limnaei in species of these genera, as well as other freshwater ecosystems in Chile.
Acknowledgements
We thank the Subsecretaría de Pesca y Acuicultura, Ministerio de Economía, Fomento y Turismo, República de Chile for authorization to collect samples. We also thank CONICYT-FONDEQUIP Program (No EQM-140088) for the acquisition of Hitachi Scanning Electron Microscope (SEM). This work was partially funded by the projects DIUBB 153309 2/R and CONICYT-FONDECYT No. 11130697.
References
Barbour, M. T. (1977). Chaetogaster limnaei limnaei (Oligochaeta: Naididae) inhabiting the mantle cavity of the pill clam Sphaerium. Transactions of the American Microscopical Society, 96, 141–142.
Bely, A. E., & Sikes, J. M. (2010). Latent regeneration abilities persist following recent evolutionary loss in asexual annelids. Proceedings of the National Academy of Sciences, 107, 1464–1469.
Bousset, L., Pointier J. P., David, P., & Jarne, P. (2014). Neither variation loss, nor change in selfing rate is associated with the worldwide invasion of Physa acuta from its native North America. Biological Invasions, 16, 1769–1783.
Brinkhurst, R. O. (1971). A Guide for the identification of British aquatic oligochaeta. Freshwater Biological Association Scientific Publication, 22, 1–55.
Brinkhurst, R. O. (1986). Guide to the freshwater aquatic microdrile oligochaetes of North America. Canadian Special Publication of Fisheries and Aquatic Sciences, 84, 1–259.
Brinkhurst, R. O., & Jamieson, B. G. M. (1971). Aquatic oligochaeta of the world. Toronto: University of Toronto Press.
Buse, A. (1974). The relationship of Chaetogaster limnaei (Oligochaeta: Naididae) with a variety of gastropod species. Journal of Animal Ecology, 43, 821–837.
Callisto, M., Moreno, P., Gonçalves, J. F. Jr., Ferreira, W. R., & Gomes, C. L. Z. (2005). Malacological assessment and natural infestation of Biomphalaria straminea (Dunker, 1848) by Schistosoma mansoni (Sambon, 1907) and Chaetogaster limnaei (K. Von Baer, 827) in an urban eutrophic watershed. Brazilian Journal of Biology, 65, 1–13
Cichy, A., Urbańska, M., Marszewska, A., Andrzejewski, W., & Żbikowska, E. (2016). The invasive Chinese pond mussel Sinanodonta woodiana (Lea, 1834) as a host for native symbionts in European waters. Journal of Limnology, 75, 288–296.
Collado, G. A. (2017). Unraveling cryptic invasion of a freshwater snail in Chile based on molecular and morphological data. Biodiversity and Conservation, 26, 567–578.
Collado, G. A., & Méndez, M. A. (2012). Phylogenetic relationships and taxonomy of Altiplano populations of Biomphalaria (Gastropoda: Planorbidae): inference from a multilocus approach. Zoological Journal of the Linnean Society, 165, 795–808.
Conn, D. B., Ricciardi, A., Babapulle, M. N., Klein, K. A., & Rosen, D. A. (1996). Chaetogaster limnaei (Annelida: Oligochaeta) as a parasite of the zebra mussel Dreissena polymorpha, and the quagga mussel Dreissena bugensis (Mollusca: Bivalvia). Parasitology Research, 82, 1–7.
Di Persia, D. H. (1980). The aquatic oligochaeta of Argentina: current status of knowledge. In R. O. Brinkhurst, & D. G. Cook (Eds.), Aquatic oligochaete biology (pp. 225–240). New York: Plenum Press.
Envall, I., Gustavsson, L., M., & Erseus, C. (2012). Genetic and chaetal variation in Nais worms (Annelida, Clitellata, Naididae). Zoological Journal of the Linnean Society, 165, 495–520.
Envall, I., Kallersjo, M., & Erseus, C. (2006). Molecular evidence for the non-monophyletic status of Naidinae (Annelida, Clitellata, Tubificidae). Molecular Phylogenetics and Evolution, 40, 570–584.
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution, 39, 783–791.
Fuentealba-Jara, C. (2011). Uncancylus concentricus (d´Orbigny, 1835): antecedentes de la especie. Amici Molluscarum, 19, 41–43.
Gamble, H., & Fried, B. (1976). Experimental evidence for parasitism in the relationship between Chaetogaster limnaei limnaei (Oligochaeta) and Physa acuta (Gastropoda). Veliger, 18, 393–395.
Gelder, S. R. (1980). A review of the symbiotic Oligochaeta (Annelida). Zoologischer Anzeiger, 204, 69–81.
Gelder, S. R. (1989). Histophysiology of digestion and observations on the structure of the alimentary canal in the ectosymbiont Chaetogaster limnaei limnaei Von Baer, 1827 (Annelida: Oligochaeta). Hydrobiologia, 180, 115–125.
Gluzman, C. (1990). Nuevos aportes al conocimiento de los oligoquetos acuáticos de Chile. Studies on Neotropical Fauna and Environment, 25, 89–92.
Gruffydd, L. D. (1965a). Evidence for the existence of a new subspecies of Chaetogaster limnaei (Oligochaeta) in Britain. Journal of Zoology, 146, 175–196.
Gruffydd, L. D. (1965b). The population biology of Chaetogaster limnaei limnaei and Chaetogaster limnaei vaghini (Oligochaeta). Journal of Animal Ecology, 34, 667–90
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analyses. Nucleic Acids Symposium Series, 41, 95–98.
Ibrahim, M. M. (2007). Population dynamics of Chaetogaster limnaei (Oligochaeta: Naididae) in the field populations of freshwater snails and its implications as a potential regulator of trematode larvae community. Parasitology Resource, 101, 25–33.
Kathman, R. D., & Brinkhurst, R. O. (1998). Guide to the freshwater oligochaetes of North America. College Grove, TN: Aquatic Resources Center.
Khalil, J. F. (1961). On the capture and destruction of miracidia by Chaetogaster limnaei (Oligochaeta). Journal of Helminthology, 35, 269–274.
Martins, R. T., & Alves, R. G. (2008). Occurrence of Naididae (Annelida: Oligochaeta) from three gastropod species in irrigation fields in southeastern Brazil. Biota Neotropica, 8, 255–257.
Martins, R. T., & Alves, R. G. (2010). Occurrence of Chaetogaster limnaei K. von Baer, 1927 (Oligochaeta, Naididae) associated with Gastropoda mollusks in horticultural channels in Southeastern Brazil. Brazilian Journal of Biology, 70, 1055–1057
Michelson, E. H. (1964). The protective action of Chaetogaster limnaei on snails exposed to Schistosoma mansoni. Journal of Parasitology, 50, 441–444.
Mitchell, D. R., & Leung, T. L. F. (2016). Sharing the load: a survey of parasitism in the invasive freshwater pulmonate, Physa acuta (Hygrophila: Physidae) and sympatric native snail populations. Hydrobiologia, 766, 165–172.
Palumbi, S. R. (1996). Nucleic acids II: the polymerase chain reaction. In D. M. Hillis, C. Moritz, & B. K. Mable (Eds.), Molecular systematics (pp. 205–247). Sunderland, Massachusetts: Sinauer Associates, Inc.
Pinder, A. M., & Ohtaka, A. (2014). Annelida: Clitellata, Oligochaeta. In C. M. Yule, & H. S. Yong, (Eds.), Freshwater invertebrates of the Malaysian Region (pp. 162–174). Kuala Lumpur. Akademi Sains Malaysia.
Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25, 1253–1256.
Rodgers, J. K., Sandland, G. J., Joyce, S. R., & Minchella, D. J. (2005). Multi-species interactions among a commensal (Chaetogaster limnaei limnaei), a parasite (Schistosoma mansoni), and an aquatic snail host (Biomphalaria glabrata). Journal of Parasitology, 91, 709–712.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
SINAB (Sistema Nacional de Información sobre Biodiversidad). (2017). Museo Nacional de Historia Natural, Gobierno de Chile. Retrieved from: http://www.graficanimada.cl/sitios/sinab/listas-a-oligo.html
Sjölin, E., Erséus, C., & Källersjö, M. (2005). Phylogeny of Tubificidae (Annelida, Clitellata) based on mitochondrial and nuclear sequence data. Molecular Phylogenetics and Evolution, 35, 431–441.
Smythe, A. B., Forgrave, K., Patti, A., Hochberg, R., & Litvaitis, M. K. (2015). Untangling the ecology, taxonomy, and evolution of Chaetogaster limnaei (Oligochaeta: Naididae) species complex. The Journal of Parasitology, 101, 320–326.
Stoll, S., Früh, D., Westerwald, B., Hormel, N., & Haase, P. (2013). Density-dependent relationship between Chaetogaster limnaei limnaei (Oligochaeta) and the freshwater snail Physa acuta (Pulmonata). Freshwater Science, 32, 642–649.
Streit, B. (1974). Populations dynamik von Chaetogaster limnaei limnaei in einer population von Ancylus fluviatilis. Archiv für Hydrobiologie, 47, 106–118.
Swofford, D. L. (2003). PAUP*: phylogenetic analysis using parsimony (*and other methods). Ver. 4. Sunderland, Massachusetts: Sinauer Associates.
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F., & Higgins, D. G. (1997). The ClustalX windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876–4882.
Valdovinos, C. (2008). Invertebrados dulceacuícolas. In CONAMA (Ed.), Biodiversidad de Chile, patrimonio y desafíos (pp. 201–225). Santiago, Chile: Ocho Libros Editores.
Winnepennickx, B., Backeljau, T., & De Wachter, R. (1993). Extraction of high molecular weight DNA from molluscs. Trends in Genetics, 9, 407.
Zimmermann, M. R., Luth, K. E., & Esch, G. W. (2011). Complex interactions among a nematode parasite (Daubaylia potomaca), a commensalistic annelid (Chaetogaster limnaei limnaei), and trematode parasites in a snail host (Helisoma anceps). Journal of Parasitology, 97, 788–791.



