Climate change impact on endangered cloud forest tree species in Mexico
Daniel Jiménez-García 1, *, A. T. Peterson 2
1 Laboratorio de Biodiversidad, Centro de Agroecología y Ambiente, Instituto de Ciencias, Benemérita Universidad Autónoma de Puebla, Edificio Val. 1, Km 1.7 carretera a San Baltazar Tetela, San Pedro Zacachimalpa, 72960 Puebla, Puebla, Mexico
2 Biodiversity Institute, University of Kansas, 1450 Jayhawk Blvd, Lawrence, Kansas 66045, USA
*Corresponding author: daniel.jimenez@correo.buap.mx (D. Jiménez-García)
Abstract
Ecological niche models have seen intensive exploration as a tool in biodiversity conservation and evaluation of areas for designing protected natural areas systems, including projections of potential distributions under future conditions. Cloud forest is the most endangered ecosystem in Mexico, and yet ranks high in terms of diversity and endemism. This study focuses on 12 endangered and range-restricted tree species in Mexican cloud forests, exploring patterns of distribution and diversity under 2 future emissions scenarios (representative concentration pathways 4.5 and 8.5) as anticipated by 20 general circulation models. Our results indicate a likely strong reduction in species’ distributional areas and —consequently— species diversity manifested in different cloud forest patches across the country. The genus Quercus resulted the most sensitive to climate change. We identified cloud forest patches that are most vulnerable to climate change effects, which can and should focus priorities for protection of this ecosystem, particularly in the Sierra Madre Oriental, where cloud forest is presently lacking any protection.
Keywords: Ecological niche model; Diversity; Climatic change; Threatened species
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Impacto del cambio climático sobre las especies de árboles amenazadas del bosque mesófilo en México
Resumen
Los modelos de nicho ecológico han sido empleados como una herramienta en la Biología de la Conservación, así como en la evaluación y establecimiento de áreas naturales protegidas, incluyendo condiciones presentes y futuras. El bosque mesófilo es el ecosistema más amenazado en México y sin embargo, ocupa un lugar destacado en términos de diversidad y endemismos. Este trabajo se centró en 12 especies amenazadas y que se restringen al bosque mesófilo en México, evaluando los patrones de distribución bajo 2 escenarios de emisiones a futuro (RCP 4.5 y 8.5) bajo 20 diferentes modelos generales de circulación. Nuestros resultados muestran fuertes reducciones de las áreas de distribución de las especies; consecuentemente, ésto afecta la diversidad de los diferentes manchones de mesófilo de México. El género Quercus es el más sensible al cambio climático. Detectamos manchones de bosque mesófilo que son más vulnerables a los efectos del cambio climático que pueden y deben ser considerados como prioritarios para la protección de este ecosistema, particularmente en la sierra Madre Oriental, donde el mesófilo se encuentra, actualmente, carente de protección.
Palabras clave: Modelado de nicho ecológico; Diversidad; Cambio climático; Especies amenazadas
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
Among the most unique ecosystems in the Neotropics is cloud forest (Rzedowski, 1996; Williams-Linera, 2002). In Mexico, this forest holds more than 10% of the country’s plant species, with high levels of endemism (Rzedowski, 1996). According to González-Espinosa (2011), more than 82% of the country’s plant families are found in this ecosystem. Cloud forest is also the most endangered ecosystem in Mexico, in view of its small extent (about 1% of the total area of the country) (González-Espinosa, 2011; González-Espinosa et al., 2012). The ecological complexity of cloud forest is related to its restricted elevational range and narrow intervals of thermal and humidity conditions. These uncommon factors make cloud forest an archipelago in the highlands that may be particularly vulnerable to climate change (Foster, 2001; Gasner et al., 2010; Holder, 2004).
Cloud forest, according to Rzedowski (1996), is characterized by the persistence, frequency, and seasonality of the cloud layer at vegetation height (Aldrich et al., 1997; Foster, 2001; Gual-Díaz & Rendón-Correa, 2014). This linkage with regular cloud formation cycles provides effective fluvial precipitation, high humidity, and reduced solar radiation, as well as low rates of evapotranspiration and evaporation (Gual-Díaz & Rendón-Correa, 2014; Still et al., 1999). Cloud forests in Mexico represent a transitional ecosystem between lowland rain forest and temperate forests (Toledo-Aceves, 2010; Villaseñor, 2010), and have a complex biogeographic history (Figueroa-Rangel et al., 2009).
Cloud forests are under strong pressures from human activities, mainly in the form of conversion to agriculture and fragmentation (Moguel & Toledo, 1999; Pineda et al., 2005; Ramı́rez-Marcial et al., 2001; Williams-Linera, 2002). However, other threats like global climate change may also pose problems for this ecosystem (Correa-Ayram et al., 2017; Gasner et al., 2010; Rehm & Feeley, 2015; Rojas-Soto et al., 2012; Still et al., 1999). These changes could reduce the biological diversity, and quality of ecosystem services provided by cloud forest (Álvarez-Arteaga et al., 2013; Martínez et al., 2009; Ponce-Reyes et al., 2013; Rehm & Feeley, 2015; Toledo-Aceves et al., 2014).
Ecological niche models (ENMs) have been used frequently to estimate current distributions of species and to anticipate effects of climate change on species’ distributions in many ecosystems (Aguilera et al., 2013; Ashraf et al., 2017; Banag et al., 2015; Carroll et al., 2011; Chatterjee et al., 2012; Kearney et al., 2010), including cloud forest (Contreras-Medina et al., 2010; Cruz-Cárdenas et al., 2014; Golicher et al., 2012; Gómez-Mendoza & Arriaga, 2007; López-Mata et al., 2012; Monterroso-Rivas et al., 2013; Ponce-Reyes et al., 2012; Rojas-Soto et al., 2012; Téllez-Valdés et al., 2006; Vega et al., 2000). They also can inform about which climatic factors constrain distributions of species, and how these factors will change into the future (Luna-Vega et al., 2012). Ecological niche models, properly implemented, are presently considered to constitute the best tool with which to assess effects of climate change on distributions of species (Martínez-Meyer, 2005).
This paper assesses how climate change will likely affect tree species richness and composition in Mexican cloud forests. This work is developed under the assumption that climate is an important determinant of species diversity in each cloud forest patch, not the only factor, but one of the most important. We use ENM approaches to assess likely distributional shifts in 12 species of cloud forest tree species, under 2 greenhouse gas emissions scenarios and 20 general circulation models (GCMs; these models provide a predictive view of likely future climate conditions). The outcome is a predictive view of changes in cloud forest tree species composition that can be expected across the range of this ecosystem (with emphasis in eastern Mexico) as a consequence of global climate change.
Material and methods
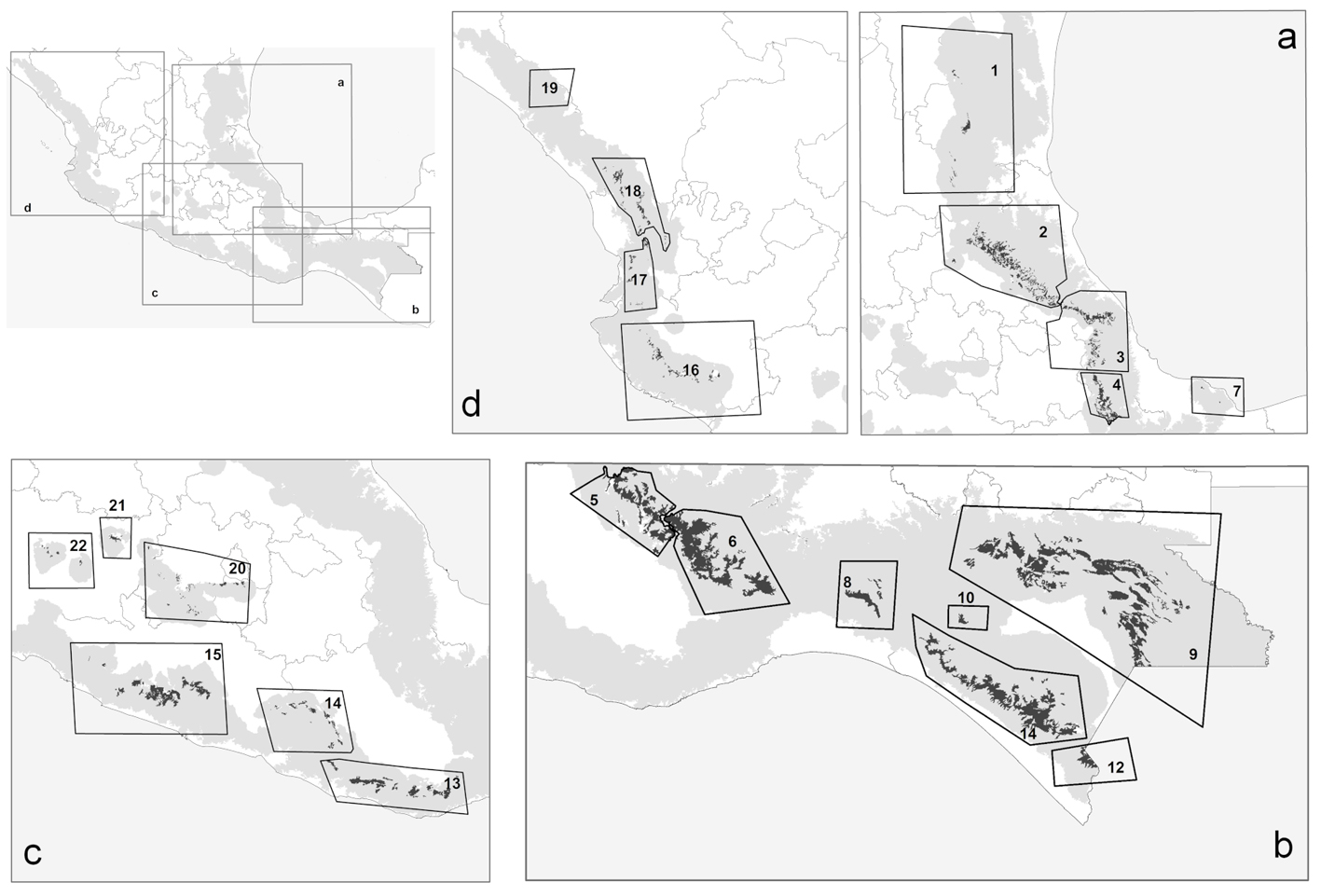
According to Rzedowski (2006), less than 1% of the surface area of Mexico is covered or was covered originally by cloud forest (INEGI, 2015). Mexican cloud forests (Fig. 1) are located mainly in the Sierra Madre Oriental (Gulf of Mexico influence: patches 1, 2, 3, 4, 5, 6, and 7), and to a lesser extent in the Sierra Madre Occidental (Pacific influence: patches 13, 14, 15, 16, 17, 18, and 19); some patches are located in the Sierra Madre del Sur (Pacific influence: patches 8, 9, 10, 11, and 12) and the Transverse Volcanic Belt (central Mexico: patches 20, 21, and 22).
Under the idea that ENMs should be calibrated across the area that has been accessible to the species in question over relevant time periods (Barve et al., 2011; Owens et al., 2013; Peterson et al., 2011), we determine our calibration area with the COSTGROW module in TerrSet (Eastman, 2016), to generate a surface that reflects effort necessary to access adjacent regions. Specifically, we used a digital elevation model (DEM) to summarize friction or resistance to colonization; this raster data layer was reclassified, such that lowlands (0 – 50 m elevation) were prohibited, and the remaining elevations guided estimates of effort necessary to increase the species’ range (i.e., low and very high elevations related to high effort to colonize). This module works with a maximum growth distance specified in cost units (buffer); we explored distances of 1 – 5 km. We chose 3.2 km because it yielded an area that approximated the historical known distribution of cloud forest (Graham, 1999; Martin & Harrell, 1957). All spatial analyses were developed using ArcGIS 10.3 and its extension SDMTools (Brown, 2014).
We focused on threatened species (Table 1, Fig. 2) in cloud forest under higher IUCN threat categories: Critical or Endangered (González-Espinosa, 2011; Rodríguez et al., 2011). Occurrence data were obtained from the Global Biodiversity Information Facility (GBIF; https://www.gbif.org/), Red Mundial de Información sobre Biodiversidad (REMIB; http://www.conabio.gob.mx/remib/doctos/remibnodosdb.html), and Herbario Nacional del Instituto de Biología, UNAM (MEXU; http://datosabiertos.unam.mx/biodiversidad/). Most of the occurrences (80%) were from the Sierra Madre Oriental (Veracruz, Puebla, Hidalgo, and Oaxaca), collected between 1972 and 2006, though we include data from across Mexico. Biasing effects of spatial autocorrelation in occurrence data were reduced with SDMTools (Brown, 2014), using a distance filter of 4.5 km, which corresponded roughly to the spatial precision of the occurrence data. We set aside a random 40% of available data for each species for model evaluation (see below).
We used 19 bioclimatic variables from WorldClim in model calibration (Hijmans et al., 2005); these variables were derived from monthly averages of temperature and precipitation over the period 1950-2000. For future conditions, we used data from 20 general circulation models obtained from Climate Change, Agriculture and Food Security (CCAFS) downscaled general circulation model (GCM) data portal (http://www.ccafs-climate.org/data_spatial_downscaling/), for 2 emissions scenarios (RCP 4.5 and 8.5) for 2050 (Table 2). All analyses were performed at a spatial resolution of 2.5´. To reduce dimensionality, we used Spearman rank correlations based on 10,000 random points inside the calibration area (Fig. 1), removing one of each pair of variables presenting correlation coefficients above 0.80, using routines in Statistica V.8.0. Variables used in final models (i.e., after variable reduction) were precipitation seasonality, isothermality, temperature seasonality, mean diurnal temperature range, and temperature annual range. When initial model evaluations were unsuccessful (see below) using the full data set, we further reduced the number of variables to only the first 3 in the list above (Table 1).
ENMs were calibrated with Maxent 3.3.3k (Phillips et al., 2006). We used a combination of model selection approaches (Warren & Seifert, 2011), significance testing, and performance testing to choose optimal parameter settings for each species. In model selection, we tested regularization multiplier values of 0.1, 0.3, 0.5, 0.7, 1, 2, 3, 5, 7, and 10, and response types including linear, quadratic, threshold, hinge, and product.
Table 1
Species, number of records, and IUCN category used in this study. We use the Pearson et al. (2006) method for species with < 25 records.
|
Family |
Species |
# records |
IUCN category |
|
Araliaceae |
Oreopanax flaccidus |
20 |
CR A4c |
|
Caprifoliaceae |
Viburnum ciliatum |
15 |
EN B1AB |
|
Fagaceae |
Quercus germana |
32 |
CR A4acd |
|
Quercus sartorii |
62 |
EN A2C |
|
|
Quercus xalapensis |
9 |
CR A2C |
|
|
Lauraceae |
Cinnamomum effusum |
54 |
EN B1ab(iii) |
|
Ocotea klotzschiana |
41 |
EN B1ab(iii) |
|
|
Ocotea psychotrioides |
53 |
EN B1ab(iii) |
|
|
Persea longipes |
14 |
EN B1AB |
|
|
Simaroubaceae |
Picramnia xalapensis |
36 |
EN A4c |
|
Symplocaceae |
Symplocos coccinea |
29 |
EN A4c; B1ab(iii) |
|
Theaceae |
Ternstroemia huasteca |
20 |
EN B1ab(iii) |
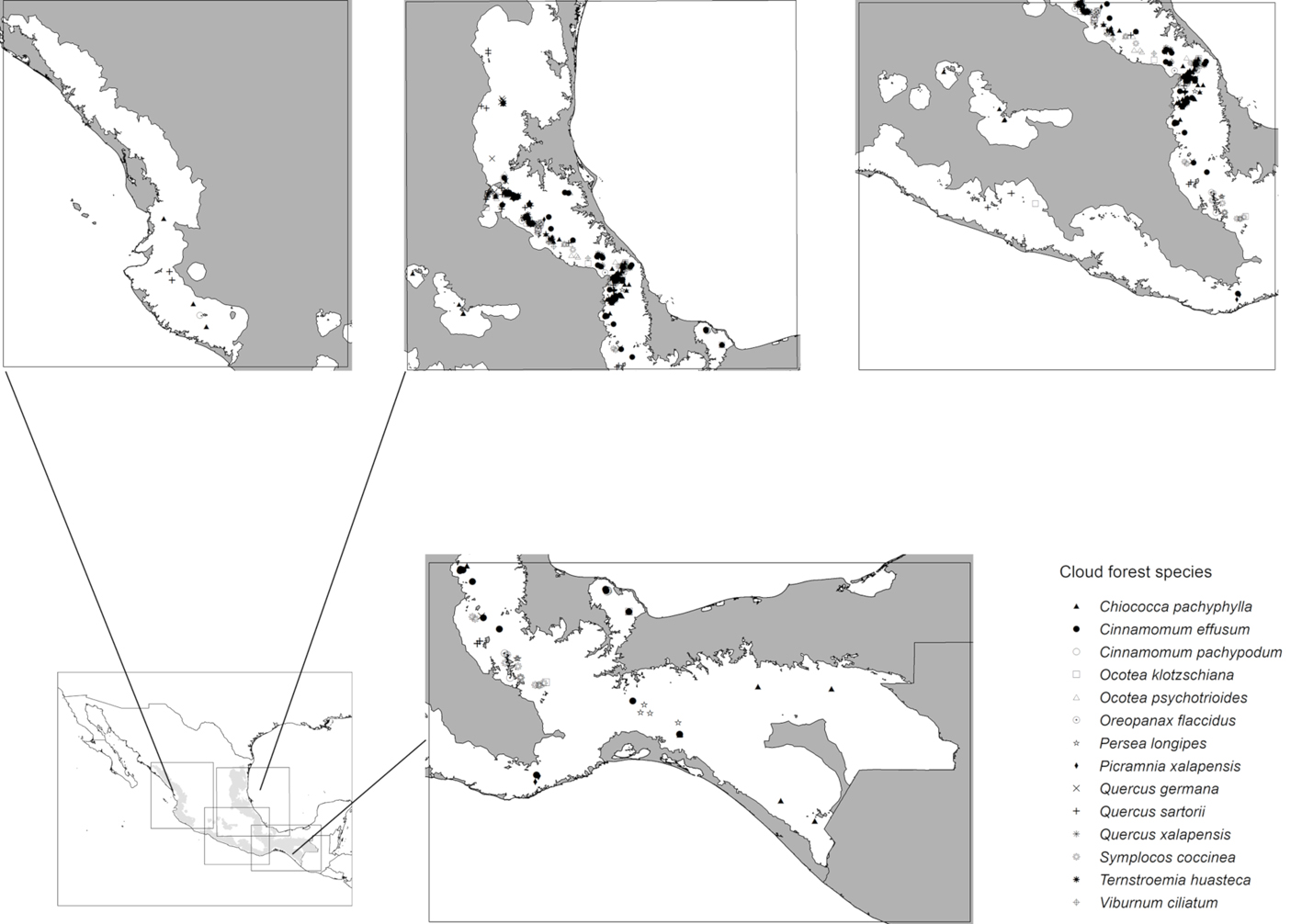
Table 2
General circulation models used in ENM projections in RCP 4.5 and RCP 8.5.
|
General circulation model acronym |
Institution |
|
bnu_esm |
Beijing Normal University Earth System Model |
|
cesm1_bgc |
NSF-DOE-NCAR |
|
cesm1_cam5 |
NSF-DOE-NCAR |
|
csiro_access1_3 |
Commonwealth Scientific and Industrial Research Organization (CSIRO) and Bureau of Meteorology (BOM), Australia |
|
csiro_access1 |
Commonwealth Scientific and Industrial Research Organization (CSIRO) and Bureau of Meteorology (BOM), Australia |
|
gfdl_cm3 |
NOAA Geophysical Fluid Dynamics Laboratory |
|
gfdl_esm2g |
NOAA Geophysical Fluid Dynamics Laboratory |
|
gfdl_esm2m |
NOAA Geophysical Fluid Dynamics Laboratory |
|
giss_e2_h_cc |
NASA Goddard Institute for Space Studies USA |
|
giss_e2_r |
NASA Goddard Institute for Space Studies USA |
|
inm_cm4 |
Russian Institute for Numerical Mathematics |
|
miroc_esm |
University of Tokyo, National Institute for Environmental Studies, and Japan Agency for Marine-Earth Science and Technology |
|
miroc_esm_chem |
University of Tokyo, National Institute for Environmental Studies, and Japan Agency for Marine-Earth Science and Technology |
|
miroc_miroc5 |
University of Tokyo, National Institute for Environmental Studies, and Japan Agency for Marine-Earth Science and Technology |
|
mohc_hadgem2_cc |
UK Met Office Hadley Centre |
|
mohc_hadgem2_es |
UK Met Office Hadley Centre |
|
mri_cgcm3 |
Meteorological Research Institute |
|
ncar_ccsm4 |
US National Centre for Atmospheric Research |
|
ncc_noresm1_m |
Norwegian Climate Centre |
|
nimr_hadgem2 |
UK Met Office Hadley Centre |
We used the Akaike information criterion with correction for small sample size (AICc) for model selection (Aho et al., 2014; Warren, & Seifert, 2011) for species with >25 occurrences; we used ENMTools version 1.4.4 (Warren et al., 2010) for AICc calculations. Partial ROC was used to evaluate model predictions, given strong concerns about the use of typical ROC approaches (Lobo et al., 2008; Peterson et al., 2008; Somodi et al., 2017). In particular, partial ROC considers only user-defined acceptable ranges of omission errors (here, up to a maximum of E = 5%). Partial ROC tests were executed in NicheToolBox (http://shiny.conabio.gob.mx:3838/nichetoolb2/), based on Peterson et al. (2008), with code developed by Osorio-Olvera et al. (2016). Probability values were determined by direct count of AUC ratios < 1.0 among 500 replicate 50% bootstrap resampling iterations. Model evaluations for species with low sample sizes (n < 25) were achieved via methods proposed and code provided by Pearson et al. (2006), using a jackknife-based approach modified from the cumulative binomial.
Models were calibrated under present-day conditions in Maxent with bootstrap subsampling and 10 random replicates. Models were then transferred to future climate scenarios (Table 3), and median outputs used to summarize outputs across the different future scenarios. For thresholding, we used a modified version of least training presence threshold, under an acceptable omission rate of E = 5% (calibration data) to create binary (presence-absence) maps from which we calculated omission rates (independent testing data). Our model selection approaches, which included dimensions of (1) statistical significance, (2) performance in terms of avoiding high omission error, and (3) model simplicity and avoidance of overfitting, should avoid or reduce many common criticisms of ENM approaches as regards underfitting or overfitting (Araújo & Peterson, 2012).
To summarize likely global climate change impacts on cloud forest tree species, we used the IUCN endangerment criteria (Rodríguez et al., 2011). Three general categories were used based on proportional change in range area as compared with the present: a) species loss, in which species lose > 80% of present-day distributional area; b) range reduction, in which species lose 20-80% of present-day area; and c) stability or increase, in which species retain > 80% of present-day distributional area. Finally, we evaluated our model transfers using a MOP analysis (Owens et al., 2013) for each combination of GCM and RCP, to identify areas of extrapolation in model transfer.
Table 3
Models selected for each of 12 cloud forest species in Mexico, providing regularization parameter values, feature, likelihood, number of parameters in the model, AICc values, AUC ratio, presence threshold, and omission rates. Entries for Cinnamomum effusum* include averages from 3 different models.
|
Family |
Species |
Regularization parameter |
Feature |
Log-likelihood |
Parameters |
AICc score |
AUC ratio |
Presence threshold |
Omission rates |
|
Araliaceae |
Oreopanax flaccidus |
0.3 |
Quadratic |
-223.5 |
4 |
457.9 |
0.2527 |
33.3 |
|
|
Caprifoliaceae |
Viburnum ciliatum |
0.1 |
Quadratic |
-136.9 |
5 |
293.8 |
0.101 |
16.7 |
|
|
Fagaceae |
Quercus germana |
3 |
Hinge |
-374.6 |
6 |
764.8 |
1.56 |
0.4922 |
0.0 |
|
Quercus sartorii |
2 |
Hinge |
-726.5 |
16 |
1497.4 |
1.13 |
0.2735 |
25.0 |
|
|
Quercus xalapensis |
2 |
Linear |
-100.3 |
1 |
203.2 |
0.4842 |
12.5 |
||
|
Lauraceae |
Cinnamomum effusum* |
1, 2, 2 |
Threshold |
-38.8 |
13, 4, 5 |
1262.1 |
1.33 |
0.2564 |
0.0 |
|
Ocotea klotzschiana |
1 |
Hinge |
-463.2 |
14 |
971.3 |
1.16 |
0.0924 |
0.1 |
|
|
Ocotea psychotrioides |
1 |
Hinge |
-581.3 |
18 |
1219.3 |
1.62 |
0.388 |
9.1 |
|
|
Persea longipes |
7 |
Quadratic |
-452.2 |
2 |
908.8 |
0.5206 |
7.7 |
||
|
Simaroubaceae |
Picramnia xalapensis |
1 |
Threshold |
-401.0 |
16 |
864.2 |
1.48 |
0.1200 |
0.0 |
|
Symplocaceae |
Symplocos coccinea |
0.7 |
Quadratic |
-338.2 |
3 |
683.4 |
1.38 |
0.2982 |
18.2 |
|
Theaceae |
Ternstroemia huasteca |
0.1 |
Quadratic |
-220.7 |
5 |
456.0 |
0.3583 |
5.3 |
Results
All models developed in this study resulted better than random expectations (all p < 0.005). For the 7 species with large sample sizes, partial ROC tests were uniformly better than null expectations (all p < 0.0002). For Quercus xalapensis, Persea longipes, Viburnum ciliatum, and Oreopanax flaccidus, for which sample sizes were lower, the Pearson small-sample test also indicated predictions better than random (p < 0.05), although in one case (Cinnamomum effusum), we had to simplify models to just 3 environmental layers.
Model selection based on AICc generally identified hinge, quadratic, and threshold response types as best. Quadratic response types were most common for small sample sizes (n < 25), except for Q. xalapensis, for which linear features were selected. AICc scores were lowest for species with small sample sizes, except for S. coccinea, probably because models with larger sample sizes generally incorporated more parameters. Regularization multiplier values were generally relatively high, which makes for relatively smooth and simple response surfaces (Table 1). The most important variables for models were related to seasonality (rather than absolute amounts) of precipitation and temperature.
Present-day predictions reflected an irregular and highly restricted distributional pattern for the 12 species within Mexican cloud forest (Fig. 3a). Southern, northwestern, and central Mexico all largely lacked habitable conditions for these species. Highest predicted species richness was in the mountains above the Mexican Gulf, in the states of Veracruz, Puebla, and Hidalgo. Lower diversity was along the Pacific Coast (of the species analyzed), where Ocotea klotzschiana, Q. sartorii, and Picramnia xalapensis had their only occurrences in our dataset. Species like O. flaccidus, Q. germana, and V. ciliatum had populations in both regions, increasing predicted species richness in the Pacific area. Another predicted hotspot for these species was in the Sierra Norte, in the northern part of the state of Oaxaca.
ENM transfers to future conditions under RCP 4.5 (Fig. 3) showed richness highest in the same regions of Puebla, Veracruz, and Hidalgo, but with lower total species richness predicted (Fig. 3a). Diversity was depressed most in patches 2, 4, and 5, (Gulf influence), and 8, 13, 15, and 16 (Pacific influence). Losses of species (Fig. 4b) were focused in patches 1, 2, 3, 5, and 6 (Gulf influence), and 20 (central Mexico). Eight patches had species losses for Cinnamomum effusum, O. flaccidus, O. klotzschiana, P. longipes, Picramnia xalapensis, and Q. sartorii (Table 4). Range reductions were predicted for O. flaccidus, O. klotzschiana, P. longipes, Q. germana, Q. sartorii, Q. xalapensis, and Symplocos coccinea. Thus, practically all the species under consideration showed losses or reductions somewhere in the region. However, stable status in patches or possible increases in distributional area were focused in the Gulf region (patches 2 and 3; Fig. 4c); in general, we observed stronger changes (both positive and negative) in the Gulf Coast-influenced patches 2, 3, 4, 5, and 6 (Fig. 4a, b).
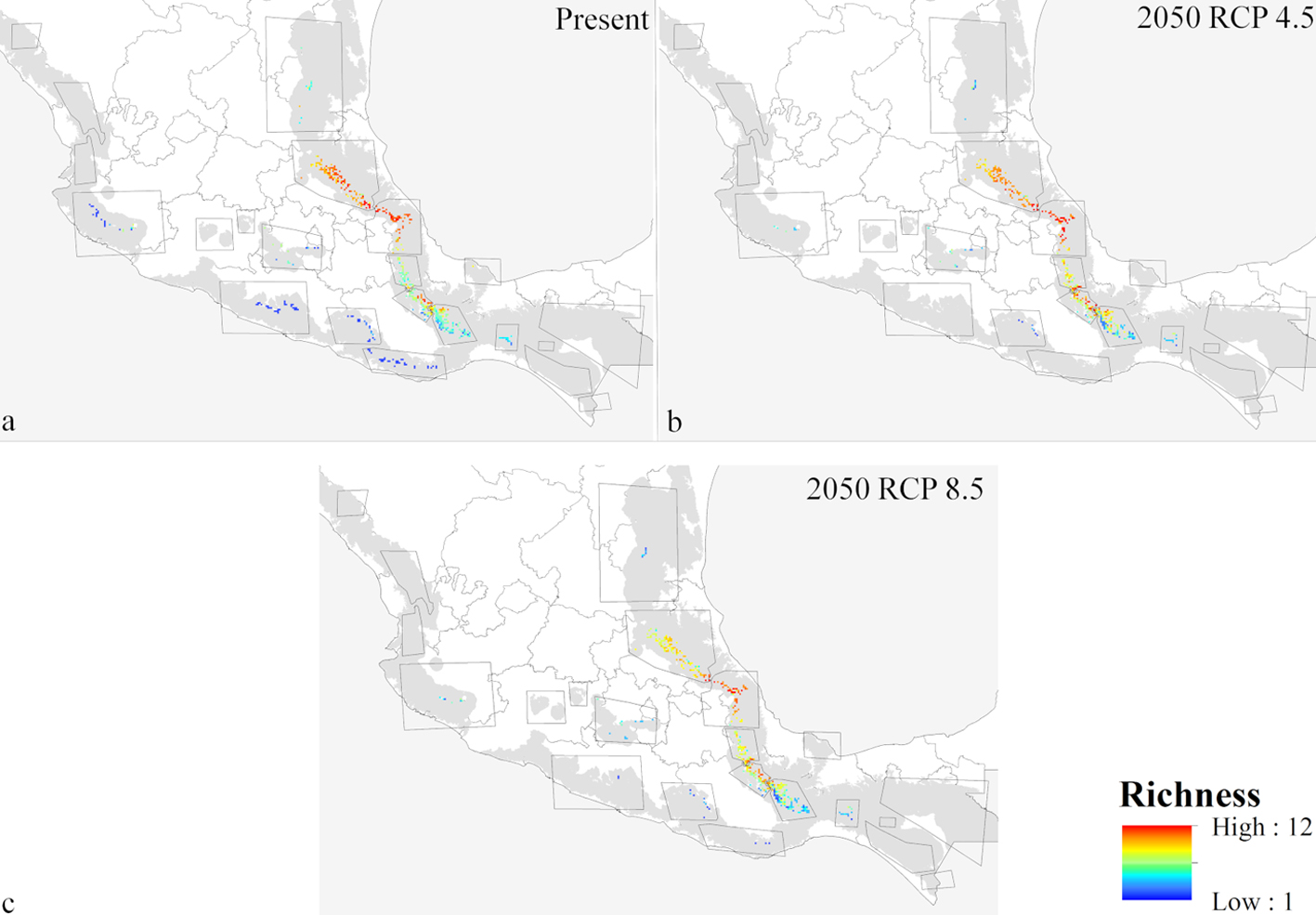
Model transfers to RCP 8.5 showed stronger differences from the present than projections to RCP 4.5 (Table 4). The biggest differences in alpha diversity were in patches 2 and 3 (Fig. 3c), although we noted species losses and range reductions in patches on both Gulf and Pacific slopes. Under this scenario, 7 species were lost from patches 2, 8, 13, and 15 (Fig. 3a), whereas range reductions included 3 species in patch 2. Species affected at the level of loss from entire patches were C. effusum, O. klotzschiana, P. longipes, P. xalapensis, Q. sartorii, Q. xalapensis, and S. coccinea. Range reductions were in patches 1, 2, 3, and 6 on the Gulf side; 16 and 20 on the Pacific side; and in central Mexico (Fig. 5b). In contrast, some species (C. effusum, O. flaccidus, and O. klotzschiana) were predicted to maintain their distributional areas without notable changes or increases (Fig. 5c). Model transfers under both scenarios were evaluated for extrapolative conditions with a MOP analysis; however, we did not observe any differences in climatic conditions between present and the 20 GCM x 2 RCP future predictions that indicated situations of model extrapolation.

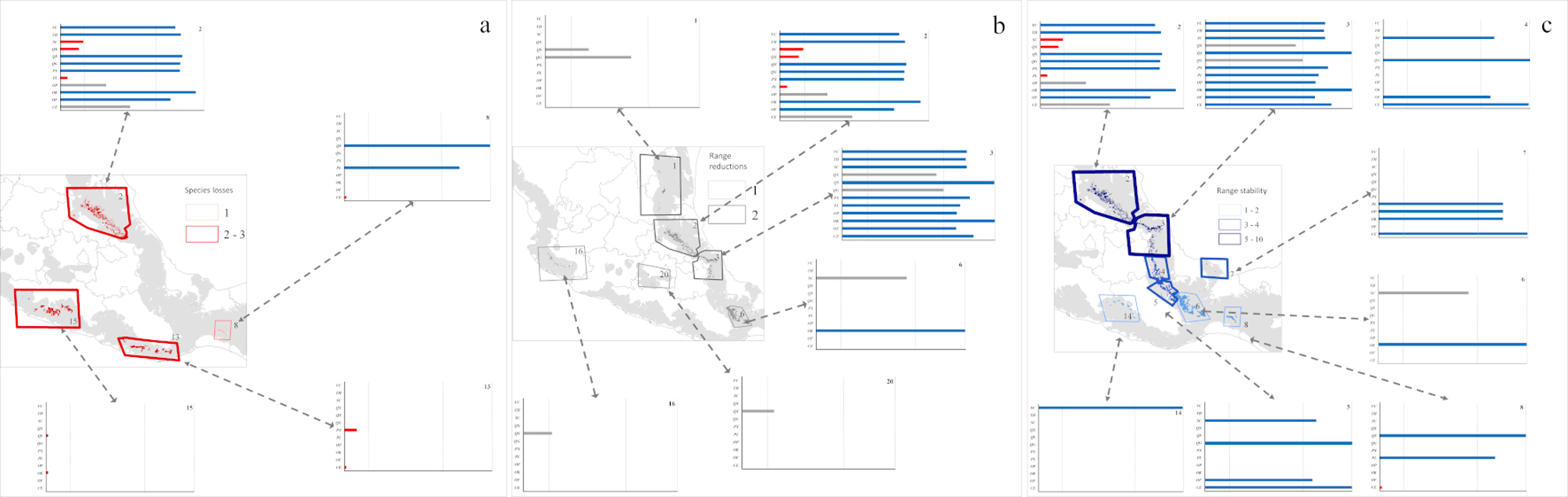
Table 4
Species sensitivity to climate change in Mexican cloud forest, including number of species losses and range reductions. Hotspots refer to cloud forest patches 2, 3, 4, and 7, whereas general refers to all patches. Species are represented with the abbreviations: C. effusum (CE), O. flaccidus (OF), O. klotzschiana (OK), O. psychotrioides (OP), P. longipes (PL), P. xalapensis (PX), Q. germana (QG), Q. sartorii (QS), Q. xalapensis (QX), S. coccinea (QC), T. huasteca (TH), and V. ciliatum (VC).
|
Transition |
RCP |
Groups |
CE |
OF |
OK |
OP |
PL |
PX |
QG |
QS |
QX |
SC |
TH |
VC |
Totals |
|
Species loss |
4.5 |
General |
1 |
2 |
1 |
0 |
1 |
1 |
0 |
2 |
0 |
0 |
0 |
0 |
8 |
|
Hotspot |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
||
|
8.5 |
General |
2 |
0 |
1 |
0 |
1 |
1 |
0 |
1 |
1 |
1 |
0 |
0 |
8 |
|
|
Hotspot |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
1 |
0 |
0 |
3 |
||
|
Species range reduction |
4.5 |
General |
0 |
1 |
1 |
0 |
1 |
0 |
1 |
2 |
1 |
1 |
0 |
0 |
8 |
|
Hotspot |
0 |
1 |
1 |
0 |
1 |
0 |
1 |
0 |
1 |
1 |
0 |
0 |
6 |
||
|
8.5 |
General |
1 |
0 |
0 |
1 |
0 |
0 |
2 |
3 |
1 |
1 |
0 |
0 |
9 |
|
|
Hotspot |
1 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
1 |
1 |
0 |
0 |
5 |
||
|
Total (loss + reduction) |
4.5 |
General |
1 |
3 |
2 |
0 |
2 |
1 |
1 |
4 |
1 |
1 |
0 |
0 |
16 |
|
Hotspot |
0 |
2 |
1 |
0 |
2 |
0 |
1 |
0 |
1 |
1 |
0 |
0 |
8 |
||
|
8.5 |
General |
3 |
0 |
1 |
1 |
1 |
1 |
2 |
4 |
2 |
2 |
0 |
0 |
17 |
|
|
Hotspot |
1 |
0 |
0 |
1 |
1 |
0 |
1 |
0 |
2 |
2 |
0 |
0 |
8 |
Discussion
Information available about cloud forest tree species is sparse, particularly regarding many of the endangered species (Rzedowski, 1996; Villaseñor, 2010). Cloud forest has an important vulnerability to different threats, particularly to land-use change (Martínez et al., 2009; Ramírez-Marcial et al., 2001) and global change (Figueroa-Rangel et al., 2009), at spatial scales ranging from local (Ledo et al., 2013; Rapp & Silman, 2012; Van Beusekom et al., 2017) to regional (Golicher et al., 2012; Rojas-Soto et al., 2012; Van Beusekom et al., 2017). Our work showed highest diversity of endangered tree species in Gulf region cloud forest (i.e., patches 2, 3, 5, and 6), where no protected areas are present (Fig. 6). Our patches 2 and 3 are the cloud forest areas considered to be the most diverse in the country (Delgadillo-Moya et al., 2017). Some protected areas are nearby, but none includes much cloud forest (Correa-Ayram et al., 2017; Ochoa-Ochoa et al., 2017). An important initiative of the Mexican government is to create a unique preserve area called the Sierra Madre Oriental Ecological Corridor (CESMO), but it is not a protected area per se, and a detailed management plan is lacking (Gillespie et al., 2012). More generally, as endangered species of cloud forest trees are not presently included inside any protected areas, mitigation strategies for climate change effects are compromised from the outset (Ponce-Reyes et al., 2012).
The Mexican government has made concerted efforts to identify endangered species and protect them via the law NOM-059-2011-Semarnat. However, this effort does not include a clear strategy by which to preserve those species (Fig. 6), especially in endangered ecosystems like cloud forest. Our results indicated that the most important hotspots for the 12 tree species were patches 2 and 3, where 12 such species are likely co-distributed (Fig. 3); these cloud forest patches, in the states of Veracruz, Puebla, and Hidalgo, hold important diversity (García-De la Cruz et al., 2013; García-Franco et al., 2008; González-Espinosa, 2011; Williams-Linera, 2002; Williams-Linera et al., 2013), mainly in a beta-diversity sense, which is most important for Mexican cloud forest biotas (Carvajal-Hernández et al., 2014; Williams-Linera et al., 2013).
Species losses have important impacts on beta diversity, often narrowing community composition and increasing floristic homogenization (Arroyo-Rodríguez et al., 2013). According to our model predictions, some patches will see strong reductions of tree diversity (Table 4), indicating high climate change sensitivity of these endangered species, as has been pointed out in previous studies (Eigenbrod et al., 2015; Figueroa-Rangel et al., 2009; Rojas-Soto et al., 2012; Vargas-Rodríguez et al., 2010).
Our models provided information about likely sensitivity of threatened tree species of cloud forest to global climate change processes, and how cloud forest will likely see significant changes in distributions of species, both increases and reductions in species diversity at particular sites (Figueroa-Rangel et al., 2009). ENMs were used by Rojas-Soto et al. (2012) to assess climate change influences on 20 Mexican cloud forest tree species, in a first exploration under a previous generation of climate change projections (IPCC, 2001, 2007). We added to this picture consideration of more cloud forest patches across Mexico (e.g., in the Sierra Madre Occidental, Transverse Volcanic Belt, and in the northeast), and by including detailed model selection and evaluation, and careful assessment of uncertainty inherent in our predictions. Both studies indicate considerable instability of Mexican cloud forest biodiversity in the face of climate change, and considerable inadequacy of the present natural protected areas system for Mexican cloud forests (Fig. 6). Téllez-Valdés et al. (2006) assessed a single, restricted-range species (Fagus grandifolia var. mexicana), and also predicted strong range reductions for that species. Our work detailed model selection exercises, and consideration of 20 GCM and 2 RCP emissions scenarios to arrive at the most detailed predictions yet for this ecosystem.
Our future-climate model projections indicated considerable potential for changes in tree species’ distributions in cloud forest (Table 4). To the extent that dispersal and colonization are feasible in a naturally fragmented and highly human affected ecosystem like cloud forest (they are probably often not feasible), alpha diversity would shift consistently southward, which is opposite of the usual expectation for the Northern Hemisphere (Zhu et al., 2012). Conservation implications of these changes center on the need for preservation of broad areas of cloud forest, yet no clear conservation strategy presently exists for this ecosystem (Aldrich et al., 1997; Foster, 2001; Ponce-Reyes et al., 2012; Van Beusekom et al., 2017; Vega et al., 2000). Our work provides a geographic summary for endangered tree species that can serve as benchmarks for future species compositions, although balanced and limited by low data availability for cloud forest, which reflects low historical and ongoing monitoring effort (González-Espinosa, 2011). We recognize that another important driver in cloud forest species loss is landscape change; this factor was not evaluated in our study for lack of detailed future scenarios for those changes.
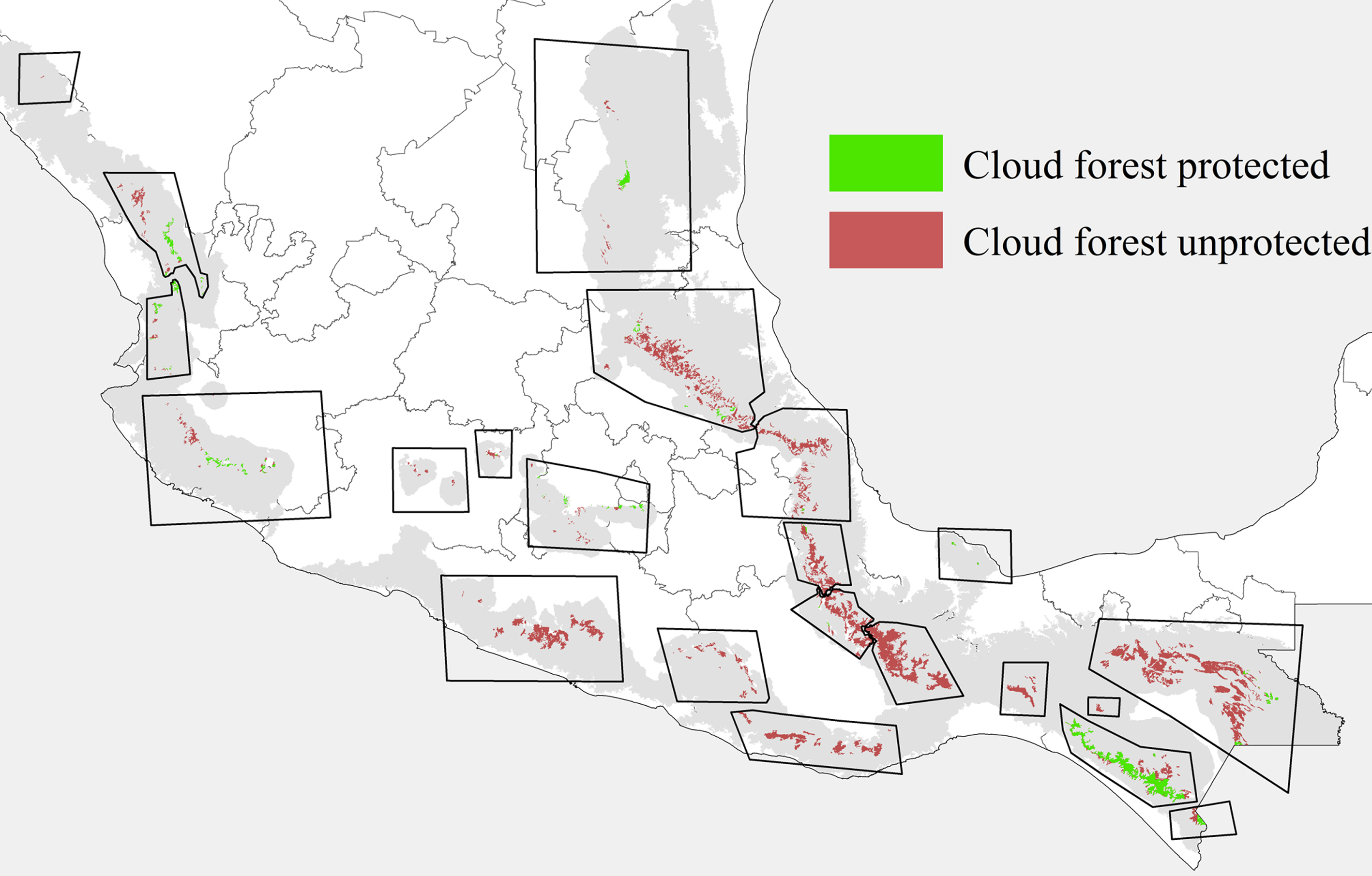
Cloud forest is projected to see significant negative effects in terms of loss of populations of threatened and endangered cloud forest trees in the face of climate change. This result is particularly concerning because the most important genus in this biome is Quercus, and this genus is precisely the one projected to see the most serious negative effects. Our results are limited to climate change implications, although land use change is important as well; however, we summarize likely conservation implications of one important factor affecting cloud forest. We explore our results as regards the conservation of this biome, focusing on the cloud forests of the eastern slopes of the Sierra Madre Oriental, which remain entirely unprotected.
Acknowledgments
We thank our colleagues Víctor Sánchez Cordero and José Luis Villaseñor for assistance with data acquisition. We are also grateful to our colleagues in the KU ENM Group for assistance in our analyses. A special acknowledgment to the Programa para el Desarrollo Profesional Docente, para el Tipo Superior (PRODEP) for supporting Daniel Jiménez-García´s research visit to University of Kansas. We thank Narayani Barve and Luis Osorio-Olvera for partial ROC scripts.
References
Aguilera, M. A., Valdivia, N., & Broitman, B. R. (2013). Spatial niche differentiation and coexistence at the edge: co-occurrence distribution patterns in Scurria limpets. Marine Ecology Progress Series, 483, 185–198. https://doi.org/10.3354/meps10293
Aho, K., Derryberry, D., & Peterson, T. (2014). Model selection for ecologists: the worldviews of AIC and BIC. Ecology, 95, 631–636.
Aldrich, M., Billington, C., Edwards, M., & Laidlaw, R. (1997). Tropical montane cloud forests: an urgent priority for conservation. WCMC Biodiversity Bulletin, 2, 1–14.
Álvarez-Arteaga, G., García-Calderón, N. E., Krasilnikov, P., & García-Oliva, F. (2013). Almacenes de carbono en bosques montanos de niebla de la Sierra Norte de Oaxaca, México. Agrociencia, 47, 171–180.
Araújo, M. B., & Peterson, A. T. (2012). Uses and misuses of bioclimatic envelope modeling. Ecology, 93, 1527–1539. https://doi.org/10.1890/11-1930.1
Arroyo-Rodríguez, V., Rös, M., Escobar, F., Melo, F. P. L., Santos, B. A., Tabarelli, M. et al. (2013). Plant β-diversity in fragmented rain forests: testing floristic homogenization and differentiation hypotheses. Journal of Ecology, 101, 1449–1458. https://doi.org/10.1111/1365-2745.12153
Ashraf, U., Peterson, A. T., Chaudhry, M. N., Ashraf, I., Saqib, Z., Rashid-Ahmad, S. et al. (2017). Ecological niche model comparison under different climate scenarios: a case study of Olea spp. in Asia. Ecosphere, 8, e01825. https://doi.org/10.1002/ecs2.1825
Banag, C., Thrippleton, T., Alejandro, G. J., Reineking, B., & Liede-Schumann, S. (2015). Bioclimatic niches of selected endemic Ixora species on the Philippines: predicting habitat suitability due to climate change. Plant Ecology, 216, 1325–1340. https://doi.org/10.1007/s11258-015-0512-6
Barve, N., Barve, V., Jiménez-Valverde, A., Lira-Noriega, A., Maher, S. P., Peterson, A. T. et al. (2011). The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling, 222, 1810–1819. https://doi.org/10.1016/j.ecolmodel.2011.02.011
Brown, J. L. (2014). SDMtoolbox: a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods in Ecology and Evolution, 5, 694–700. https://doi.org/10.1111/2041-210X.12200
Carroll, I. T., Cardinale, B. J., & Nisbet, R. M. (2011). Niche and fitness differences relate the maintenance of diversity to ecosystem function. Ecology, 92, 1157–1165.
Carvajal-Hernández, C. I., Krömer, T., & Vázquez-Torres, M. (2014). Riqueza y composición florística de pteridobiontes en bosque mesófilo de montaña y ambientes asociados en el centro de Veracruz, México. Revista Mexicana de Biodiversidad, 85, 491–501. https://doi.org/10.7550/rmb.41292
Chatterjee, H. J., Tse, J. S. Y., & Turvey, S. T. (2012). Using ecological niche modelling to predict spatial and temporal distribution patterns in Chinese gibbons: lessons from the present and the past. Folia Primatologica, 83, 85–99. https://doi.org/10.1159/000342696
Contreras-Medina, R., Luna-Vega, I., & Ríos-Muñoz, C. (2010). Distribución de Taxus globosa (Taxaceae) en México: modelos ecológicos de nicho, efectos del cambio del uso de suelo y conservación. Revista Chilena de Historia Natural, 83, 421–433.
Correa-Ayram, C. A., Mendoza, M. E., Etter, A., & Pérez-Salicrup, D. R. (2017). Potential distribution of mountain cloud forest in Michoacán, Mexico: prioritization for conservation in the context of landscape connectivity. Environmental Management, 60, 86–103. https://doi.org/10.1007/s00267-017-0871-y
Cruz-Cárdenas, G., López-Mata, L., Villaseñor, J. L., & Ortiz, E. (2014). Potential species distribution modeling and the use of principal component analysis as predictor variables. Revista Mexicana de Biodiversidad, 85, 189–199. https://doi.org/10.7550/rmb.36723
Delgadillo-Moya, C., Villaseñor, J. L., Ortiz, E., & Campos-Villanueva, Á. (2017). Floristic richness of the cloud forest moss flora of Veracruz, Mexico. Nova Hedwigia, 105, 43–63. https://doi.org/10.1127/nova_hedwigia/2017/0399
Eastman, J. R. (2016). TerrSet. Clark Labs, Clark University, Worcester. Retrieved from http://138.110.28.9/courses/tmillett/course/geog307/files/Kilimanjaro%20Manual.pdf
Eigenbrod, F., Gonzalez, P., Dash, J., & Steyl, I. (2015). Vulnerability of ecosystems to climate change moderated by habitat intactness. Global Change Biology, 21, 275–286. https://doi.org/10.1111/gcb.12669
Figueroa-Rangel, B. L., Willis, K. J., & Olvera-Vargas, M. (2009). Cloud forest dynamics in the Mexican neotropics during the last 1300 years: cloud forest dynamics in Mexico. Global Change Biology, 16, 1689–1704. https://doi.org/10.1111/j.1365-2486.2009.02024.x
Foster, P. (2001). The potential negative impacts of global climate change on tropical montane cloud forests. Earth-Science Reviews, 55, 73–106.
García-De la Cruz, Y., Olivares-López, L. A., & Ramos-Prado, J. M. (2013). Estructura y composición arbórea de un fragmento de bosque mesófilo de montaña en el estado de Veracruz. Revista Chapingo Serie Ciencias Forestales y Del Ambiente, XIX, 91–101. https://doi.org/10.5154/r.rchscfa.2012.03.025
García-Franco, J. G., Castillo-Campos, G., Mehltreter, K., Martínez, M. L., & Vázquez, G. (2008). Estructura y composición de un bosque mesófilo del centro de Veracruz, México. Boletín de La Sociedad Botánica de México, 83, 37–52.
Gasner, M. R., Jankowski, J. E., Ciecka, A. L., Kyle, K. O., & Rabenold, K. N. (2010). Projecting the local impacts of climate change on a Central American montane avian community. Biological Conservation, 143, 1250–1258. https://doi.org/10.1016/j.biocon.2010.02.034
Gillespie, T. W., Lipkin, B., Sullivan, L., Benowitz, D. R., Pau, S., & Keppel, G. (2012). The rarest and least protected forests in biodiversity hotspots. Biodiversity and Conservation, 21, 3597–3611. https://doi.org/10.1007/s10531-012-0384-1
Golicher, D. J., Cayuela, L., & Newton, A. C. (2012). Effects of climate change on the potential species richness of Mesoamerican forests: effects of climate change on potential species richness. Biotropica, 44, 284–293. https://doi.org/10.1111/j.1744-7429.2011.00815.x
Gómez-Mendoza, L., & Arriaga, L. (2007). Modeling the effect of climate change on the distribution of oak and pine species of Mexico: Mexican temperate forests and climate change. Conservation Biology, 21, 1545–1555. https://doi.org/10.1111/j.1523-1739.2007.00814.x
González-Espinosa, M. (Ed.). (2011). The red list of Mexican cloud forest trees. Cambridge: Fauna & Flora International.
González-Espinosa, M., Meave, J. A., Ramírez-Marcial, N., Toledo-Aceves, T., Lorea-Hernández, F. G., & Ibarra-Manríquez, G. (2012). Los bosques de niebla de México: conservación y restauración de su componente arbóreo. Revista Ecosistemas, 21, 36–52. Retrieved from http://www.revistaecosistemas.net/index.php/ecosistemas/article/view/26
Graham, A. (1999). The Tertiary history of the northern temperate element in the northern Latin American biota. American Journal of Botany, 86, 32–38.
Gual-Díaz, M., & Rendón-Correa, A. (2014). Bosques mesófilos de montaña en México: diversidad, ecología y manejo. Ciudad de México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. https://doi.org/10.1002/joc.1276
Holder, C. D. (2004). Rainfall interception and fog precipitation in a tropical montane cloud forest of Guatemala. Forest Ecology and Management, 190, 373–384. https://doi.org/10.1016/j.foreco.2003.11.004
INEGI (Instituto Nacional de Estadística y Geografía). (2015). Conjunto de datos vectoriales de la Carta de Uso del Suelo y Vegetación, escala 1:250 000, Serie V (Capa Unión). Retrieved October 2, 2017, from http://www.inegi.org.mx/geo/contenidos/recnat/usosuelo/doc/guia_interusosuelov.pdf
IPCC (Intergovernmental Panel on Climate Change) (Ed.). (2001). Climate change 2001: the scientific basis. Intergovernmental Panel on Climate Change. Cambridge; New York: Cambridge University Press.
IPCC (Intergovernmental Panel on Climate Change) (Ed.). (2007). Climate change 2007: the physical science basis. Intergovernmental Panel on Climate Change. Cambridge; New York: Cambridge University Press.
Kearney, M., Simpson, S. J., Raubenheimer, D., & Helmuth, B. (2010). Modelling the ecological niche from functional traits. Philosophical Transactions of the Royal Society B, 365, 3469–3483. https://doi.org/10.1098/rstb.2010.0034
Ledo, A., Burslem, D. F. R. P., Condés, S., & Montes, F. (2013). Micro-scale habitat associations of woody plants in a Neotropical cloud forest. Journal of Vegetation Science, 24, 1086–1097. https://doi.org/10.1111/jvs.12023
Lobo, J. M., Jiménez-Valverde, A., & Real, R. (2008). AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography, 17, 145–151. https://doi.org/10.1111/j.1466-8238.2007.00358.x
López-Mata, L., Villaseñor, J. L., Cruz-Cárdenas, G., Ortiz, E., & Ortiz-Solorio, C. (2012). Predictores ambientales de la riqueza de especies de plantas del bosque húmedo de montaña de México. Botanical Sciences, 90, 27–36.
Luna-Vega, I., Alcántara-Ayala, O., Contreras-Medina, R., & Ríos-Muñoz, C. A. (2012). Ecological niche modeling on the effect of climatic change and conservation of Ternstroemia lineata DC. (Ternstroemiaceae) in Mesoamerica. Botany, 90, 637–650. https://doi.org/10.1139/b2012-019
Martin, P. S., & Harrell, B. E. (1957). The Pleistocene history of temperate biotas in Mexico and eastern United States. Ecology, 38, 468. https://doi.org/10.2307/1929892
Martínez, M. L., Pérez-Maqueo, O., Vázquez, G., Castillo-Campos, G., García-Franco, J., Mehltreter, K. et al. (2009). Effects of land use change on biodiversity and ecosystem services in tropical montane cloud forests of Mexico. Forest Ecology and Management, 258, 1856–1863. https://doi.org/10.1016/j.foreco.2009.02.023
Martínez-Meyer, E. (2005). Climate change and biodiversity: some considerations in forecasting shifts in species’ potential distributions. Biodiversity Informatics, 2, 42–55.
Moguel, P., & Toledo, V. M. (1999). Biodiversity conservation in traditional coffee systems of Mexico. Conservation Biology, 13, 11–21. https://doi.org/10.1046/j.1523-1739.1999.97153.x
Monterroso-Rivas, A., Gómez-Díaz, J., & Tinoco-Rueda, J. D. (2013). Bosque mesófilo de montaña y escenarios de cambio climático: una evaluación en Hidalgo, México. Revista Chapingo Serie Ciencias Forestales y Del Ambiente, XIX, 29–43. https://doi.org/10.5154/r.rchscfa.2012.03.029
Ochoa-Ochoa, L. M., Mejía-Domínguez, N. R., & Bezaury-Creel, J. (2017). Prioritization for cloud forest conservation in Mexico. Ecosistemas, 26, 27–37. https://doi.org/10.7818/ECOS.2017.26-2.04
Osorio-Olvera, L., Vijay, B., Narayani, B., Soberón, J., & Falconi, M. (2016). Ntbox: From getting biodiversity data to evaluating species distributions models in a friendly GUI environment. R package version 0.2. 5.4. https://luismurao.github.io/
Owens, H. L., Campbell, L. P., Dornak, L. L., Saupe, E. E., Barve, N., Soberón, J. et al. (2013). Constraints on interpretation of ecological niche models by limited environmental ranges on calibration areas. Ecological Modelling, 263, 10–18. https://doi.org/10.1016/j.ecolmodel.2013.04.011
Pearson, R. G., Raxworthy, C. J., Nakamura, M., & Peterson, A. T. (2006). Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. Journal of Biogeography, 34, 102–117. https://doi.org/10.1111/j.1365-2699.2006.01594.x
Peterson, A. T., Papeş, M., & Soberón, J. (2008). Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecological Modelling, 213, 63–72. https://doi.org/10.1016/j.ecolmodel.2007.11.008
Peterson, A. T., Soberón, J. Pearson, R., Anderson, R., Martínez-Meyer, E., Nakamura, M. et al. (Eds.). (2011). Ecological niches and geographic distributions. Princeton: Princeton University Press.
Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190, 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
Pineda, E., Moreno, C., Escobar, F., & Halffter, G. (2005). Frog, bat, and dung beetle diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conservation Biology, 19, 400–410. https://doi.org/10.1111/j.1523-1739.2005.00531.x
Ponce-Reyes, R., Nicholson, E., Baxter, P. W. J., Fuller, R. A., & Possingham, H. (2013). Extinction risk in cloud forest fragments under climate change and habitat loss. Diversity and Distributions, 19, 518–529. https://doi.org/10.1111/ddi.12064
Ponce-Reyes, R., Reynoso-Rosales, V. H., Watson, J. E. M., VanDerWal, J., Fuller, R. A., Pressey, R. L. et al. (2012). Vulnerability of cloud forest reserves in Mexico to climate change. Nature Climate Change, 2, 448–452. https://doi.org/10.1038/nclimate1453
Ramírez-Marcial, N., González-Espinosa, M., & Williams-Linera, G. (2001). Anthropogenic disturbance and tree diversity in montane rain forests in Chiapas, Mexico. Forest Ecology and Management, 154, 311–326. https://doi.org/10.1016/S0378-1127(00)00639-3
Rapp, J., & Silman, M. (2012). Diurnal, seasonal, and altitudinal trends in microclimate across a tropical montane cloud forest. Climate Research, 55, 17–32. https://doi.org/10.3354/cr01127
Rehm, E. M., & Feeley, K. J. (2015). The inability of tropical cloud forest species to invade grasslands above treeline during climate change: potential explanations and consequences. Ecography, 38, 1167–1175. https://doi.org/10.1111/ecog.01050
Rodríguez, J. P., Rodríguez-Clark, K. M., Baillie, J. E. M., Ash, N., Benson, J., Boucher, T. et al. (2011). Establishing IUCN red list criteria for threatened ecosystems: IUCN red list criteria for ecosystems. Conservation Biology, 25, 21–29. https://doi.org/10.1111/j.1523-1739.2010.01598.x
Rojas-Soto, O. R., Sosa, V., & Ornelas, J. F. (2012). Forecasting cloud forest in eastern and southern Mexico: conservation insights under future climate change scenarios. Biodiversity and Conservation, 21, 2671–2690. https://doi.org/10.1007/s10531-012-0327-x
Rzedowski, J. (1996). Análisis preliminar de la flora vascular de los bosques mesófilos de montaña de México. Acta Botanica Mexicana, 35, 25–44.
Rzedowski, J. (2006). Vegetación de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. México. Retrieved from http://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/VegetacionMx_Cont.pdf
Somodi, I., Lepesi, N., & Botta-Dukát, Z. (2017). Prevalence dependence in model goodness measures with special emphasis on true skill statistics. Ecology and Evolution, 7, 863–872. https://doi.org/10.1002/ece3.2654
Still, C. J., Foster, P. N., & Schneider, S. H. (1999). Simulating the effects of climate change on tropical montane cloud forests. Nature, 398, 608.
Téllez-Valdés, O., Dávila-Aranda, P., & Lira-Saade, R. (2006). The effects of climate change on the long-term conservation of Fagus grandifolia var. mexicana, an important species of the cloud forest in eastern Mexico. Biodiversity and Conservation, 15, 1095–1107. https://doi.org/10.1007/s10531-004-1868-4
Toledo-Aceves, T. (2010). El bosque mesófilo de montaña en México: amenazas y oportunidades para su conservación y manejo sostenible. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Toledo-Aceves, T., García-Franco, J. G., Williams-Linera, G., MacMillan, K., & Gallardo-Hernández, C. (2014). Significance of remnant cloud forest fragments as reservoirs of tree and epiphytic bromeliad diversity. Tropical Conservation Science, 7, 230–243.
Van Beusekom, A. E., González, G., & Scholl, M. A. (2017). Analyzing cloud base at local and regional scales to understand tropical montane cloud forest vulnerability to climate change. Atmospheric Chemistry and Physics, 17, 7245–7259. https://doi.org/10.5194/acp-17-7245-2017
Vargas-Rodríguez, Y. L., Platt, W. J., Vázquez-García, J. A., & Boquin, G. (2010). Selecting relict montane cloud forests for conservation priorities: the case of western Mexico. Natural Areas Journal, 30, 156–173. https://doi.org/10.3375/043.030.0204
Vega, I. L., Ayala, O. A., Morrone, J., & Organista, D. E. (2000). Track analysis and conservation priorities in the cloud forests of Hidalgo, Mexico. Diversity and Distributions, 6, 137–143.
Villaseñor, J. L. (2010). El bosque húmedo de montaña en México y sus plantas vasculares. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad y Universidad Nacional Autónoma de México. Retrieved from http://ixmati.biodiversidad.gob.mx/publicaciones/librosDig/pdf/Bosque%20humedo%20de%20montana.pdf
Warren, D. L., Glor, R. E., & Turelli, M. (2010). ENMTools: a toolbox for comparative studies of environmental niche models. Ecography, 33, 607–611. https://doi.org/10.1111/j.1600-0587.2009.06142.x
Warren, D. L., & Seifert, S. N. (2011). Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications, 21, 335–342.
Williams-Linera, G. (2002). Tree species richness complementarity, disturbance and fragmentation in a Mexican tropical montane cloud forest. Biodiversity and Conservation, 11, 1825–1843.
Williams-Linera, G., Toledo-Garibaldi, M., & Hernández, C. G. (2013). How heterogeneous are the cloud forest communities in the mountains of central Veracruz, Mexico? Plant Ecology, 214, 685–701. https://doi.org/10.1007/s11258-013-0199-5
Zhu, K., Woodall, C. W., & Clark, J. S. (2012). Failure to migrate: lack of tree range expansion in response to climate change. Global Change Biology, 18, 1042–1052. https://doi.org/10.1111/j.1365-2486.2011.02571.x