Eduardo Furrazolaa, Jorge Alberto Sánchez-Rendóna, Patricia Guadarramab, *, Mayté Pernúsa, Yamir Torres-Ariasa
a Instituto de Ecología y Sistemática, Ministerio de Ciencia, Tecnología y Medio Ambiente, Carretera de Varona 11835 e/ Oriente y Lindero, Calabazar, Boyeros, La Habana 19, 11900 La Habana, Cuba
b Unidad Multidisciplinaria de Docencia e Investigación, Facultad de Ciencias, Universidad Nacional Autónoma de México, Puerto de Abrigo s/n, 97356 Sisal, Yucatán, Mexico
*Corresponding author: pguadarrama@ciencias.unam.mx (P. Guadarrama)
Received: 7 May 2019; accepted: 11 June 2020
Abstract
This study describes the arbuscular mycorrhizal status of Coccothrinax crinita, an endemic endangered palm species native to western Cuba. Habitat destruction and local unregulated exploitation have caused population decline in its natural habitat and, consequently, C. crinita is on the verge of extinction. The survival of this species in stressed environments assumes its association with arbuscular mycorrhizal fungi. The present study was conducted near Las Pozas, a rural community located in Artemisa province, western Cuba. In order to determine the presence of arbuscular mycorrhizal associations in C. crinita, adult plants were selected in their natural habitat, and the roots and rhizosphere soil of the individuals collected. Root colonization percentage values were determined, as well as soil spore density and richness. An Arum–Paris mycorrhizal type was predominant in this species, with abundant coils and dark septate endophytes. Root colonization percentage averaged 60 ± 4.9% and spore density averaged 756 ± 223.33 spores 100 g soil-1. Sixteen AMF species were found, with a predominance of Glomus. Coccothrinax crinita is associated with AMF, and knowledge of the presence of an arbuscular mycorrhizal interaction in the rhizosphere of this palm is a first step towards its use as a biological tool to optimize cultivation and preservation of the species.
Keywords: Arecaceae; Threatened species; Arbuscular mycorrhizal fungi; Mycorrhizal colonization; Spore density; Richness
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Estatus micorrícico de Coccothrinax crinita (Arecaceae), una especie endémica en peligro de extinción del oeste de Cuba
Resumen
Este estudio reporta el estatus micorrícico arbuscular de Coccothrinax crinita, palma endémica en peligro de extinción del oeste de Cuba. La destrucción del hábitat y la explotación local no regulada están causando reducciones de sus poblaciones en su hábitat natural y, en consecuencia, C. crinita está al borde de la extinción. Su supervivencia en ambientes estresados puede asociarse a la presencia de hongos micorrícicos arbusculares. El presente estudio se realizó cerca de Las Pozas, una comunidad rural ubicada en la provincia de Artemisa, al oeste de Cuba. Con el fin de determinar la presencia de asociaciones micorrícicas arbusculares en C. crinita, se seleccionaron plantas adultas en su hábitat natural y se recolectaron las raíces y el suelo rizosférico de los individuos. Se determinó el porcentaje de colonización micorrícica, así como la densidad y riqueza de esporas. La micorriza tipo Arum-Paris fue predominante con abundantes ovillos y endófitos septados oscuros. El porcentaje de colonización micorrícica fue de 60 ± 4.9% y la densidad de esporas fue 756 ± 223.33 esporas 100 g suelo-1. Se registraron 16 especies con predominio del género Glomus. Ya que C. crinita se asocia a hongos micorrízico arbusculares, el conocimiento de esta interacción micorrícica en la rizósfera de la palma es el primer paso para plantear su uso como herramienta biológica para optimizar su cultivo y conservación.
Palabras clave: Arecaceae; Especies amenazadas; Hongos micorrícicos arbusculares; Colonización micorrícica; Densidad de esporas; Riqueza
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
With a total area of 109,886 km2, Cuba is the largest and most biologically rich archipelago in the Caribbean basin. It harbors more than 7,000 vascular plant species, approximately 6000 of which have flowers and 50% are endemic (Berazaín et al., 2005). There are about 30 vegetation types, including semi desert, dry forest and tropical rainforest (Vales et al., 1998). However, over 500 years, the area of forest cover has been reduced from 94% to 15%, with a consequent loss of known species and fragmentation of habitat (Berazaín et al., 2005).
With 30 threatened species, 14 categorized as Critically Endangered and 16 as Endangered, sensu IUCN, Coccothrinax (c. 54 species) is the flagship palm genus for conservation in the Caribbean Island Biodiversity Hotspot (Jestrow et al., 2017). According to the Cuban Flora Red List, which follows IUCN criteria, a total of 33 taxa of Coccothrinax (32 of them endemics) are threatened (González-Torres et al., 2016): 8 Vulnerable (VU), 12 Endangered (EN), and 13 Critically Endangered (CR), with C. crinita included in the latter (Johnson, 1996).
Coccothrinax crinita (Griseb., & H.Wendl. ex C.H.Wright) Becc. is strictly endemic to Bahía Honda municipality and is considered one of the most endangered palms in the insular territories of America (Johnson, 1996). The species, known as “old man palm” or “palma petate”, has been used to provide vegetal fiber for rope, brooms, mattress stuffing, hats, purses and for fence poles (Martínez & Miranda, 2009). Its sustainable use can contribute to the conservation of its population and ecosystem.
It is well known that preservation of tropical endangered species is the main goal of numerous conservation projects, as well as an obligation for a number of countries bound by international agreements (González-Torres et al., 2016). Therefore, there is an urgent need for applied interdisciplinary studies on plants of special concern in order to develop effective methods for the maintenance and propagation of these species.
Mycorrhizal symbiosis is distributed widely in the natural world, forming mutualistic associations with most terrestrial plants and helping them to obtain and utilize nutrients (Koide & Mosse, 2004). This symbiosis also helps the plants to cope with adverse conditions, such as drought and salinity, as well as protecting plants from rhizophagous nematodes and soil pathogens (de la Pena et al., 2006; Roldán et al., 2008; Sheng et al., 2008). In tropical forests where competition for light, nutrients and space is high, association with arbuscular mycorrhizal fungi (AMF) increases the ability of the plant to quickly absorb nutrients and thus enhances growth and adaptability (Allen et al., 2003; Zubek et al., 2009).
Mycorrhizal associations have been reported in several palm species through measurement mycorrhizal colonization levels (Carrillo et al., 2002; Janos, 1977; St. John, 1988; Wang & Qiu, 2006), but few studies have addressed the effects of the mycorrhizal fungi on their host species. Palms that have been studied to date include Cocos nucifera L. (Ambili et al., 2012), Desmoncus orthacanthos Martius (Ramos-Zapata et al., 2006a, b), Elaeis guineensis Jacq. (Corley & Tinker, 2003; Nadarajah, 1980), Roystonea spp. O.F. Cook (Zona, 1996) and Coccothrinax readii H.J.Quero (Polanco et al., 2013). In the rhizosphere of Desmoncus orthacanthos, around 400 to 3,200 spores were found per 100 g of soil (Ramos-Zapata et al., 2006b); in a coconut plantation in Kerala, India, this value ranged from 428 to 598 spores (Rajeshkumar et al., 2015), while in Bactris gasipaes Kunth in the Amazon, 27 to 48 spores were found in 50 cm3 of soil (da Silva-Júnior & Cardoso, 2006). However, as highlighted by Fisher and Jayachandran (1999), mycorrhizal associations in tropical palms have received relatively little attention.
Two general AMF anatomical types have been described: Arum and Paris (Dickson et al., 2007; Gallaud, 1905; Smith & Smith, 1997). However, the factors that determine the formation of these types are not well understood and there is contradictory information that indicates host control, fungal control, or even control by both partners (Cavagnaro et al., 2001; Dickson, 2004; Dreyer et al., 2010; van Aarle et al., 2005). These AMF anatomy types have been described for some palm species as follows: a) Paris-type: E. guineensis (Nadarajah, 1980) and D. orthacanthos (Ramos-Zapata et al., 2006a, b), and b) Arum type: Acoelorraphe wrightii (Griseb. et Wendl.) Wendl. ex Becc., Coccothrinax argentata León, Phoenix dactylifera L., Pseudophoenix sargentii Wendl., Sabal palmetto (Walt.) Lodd. ex Schult. et Schult. F., Serenoa repens (Bartram) Small, and Thrinax morrisii Wendl. (Bouamri et al., 2006; Fisher & Jayachandran, 1999, 2005).
The first step in implementing strategies of conservation and restoration is to acquire the knowledge of mutualistic interactions that can increase success in plant establishment and survival. Quantifying the presence of fungi within the roots and spores in the rhizosphere contributes to this goal. An understanding of the AMF relationships of C. crinita can contribute to conservation of the species and facilitate its cultivation for economic purposes. The objective of the present work was, therefore, to determine the mycorrhizal status of C. crinite, as well as the density and richness of AMF spores present in its rhizosphere.
Materials and methods
The study was conducted near Las Pozas (Fig. 1), a rural community located at 22°50’ N, 83°17’ W, Artemisa province, western Cuba, 17 km from the municipality of Bahía Honda. This locality is in the Pan de Guajaibón foothills, the highest elevation of Sierra del Rosario. The site presents an altitudinal range of between 36-156 m, decreasing from south to north. The carbonated soil is derived from igneous serpentinite rocks (brown tropical soil) and, in some cases, with serpentinite outcrops. Soil type is lithic Calcic Cambisol according to FAO-Unesco (1975). There are several hills at the base of Pan de Guajaibón to the north (Pinares, 2004). The habitat of C. crinita is a thorny xerophyte scrub on serpentine with secondary shrub vegetation in the most humid portion nearest to the gallery forest (Verdecia & Barrios, 2015). Annual mean temperature and precipitation values are 24.9 oC and 1,307 mm, respectively, which is characteristic of the Cajalbana district according to Borhidi (1996).

Coccothrinax crinita is a palm 8 to 10 m height, with a trunk of up to 20 cm in diameter. Its leaves are large, soft and almost orbicular, with a pod that dissolves into very long and fine cream-colored fibers, which completely cover the trunk of the specimens in the first 15 to 20 years. The rhizodermis is hard, suberified and with several layers of epidermis. The fruits are globose, pink to purple when ripe and the seeds have cracks in the surface, giving them a brain-like appearance (Leiva, 1999; Fig. 1). Adult C. crinita specimens are scarce and unevenly distributed. In an area of 500 m2, we randomly chose 10 palms and sampled the soil and roots of each tree at a distance of 15-50 cm from the main stem by extracting 3 soil sample monoliths of 10 cm × 10 cm, which were mixed in order to form a compound sample, and removing roots from a depth of 20 cm. The soil and roots were placed into plastic bags for immediate transportation to the laboratory, where the roots were thoroughly rinsed with tap water and stored in 70% ethanol at 4 °C until subsequent analysis (Brundrett et al., 1996).
The rhizodermis was introduced into lactoglycerol to be cleared and stained for assessment of mycorrhizal colonization using the Phillips and Hayman (1970) technique, and the percentage of AMF colonization was quantitatively estimated following McGonigle et al. (1990). The process of clearing and staining caused deterioration of the rhizodermis, and it was therefore difficult to observe the fungal structures (arbuscules, vesicles, hyphal tangles, etc.) in detail for quantification. It was possible, however, to observe the presence of the fungus within the roots. For each sample, roots were cut into 1 cm pieces, and 30 randomly selected segments were mounted on slides with polyvinyl-lacto-glycerol (PVLG). Roots were scored under a microscope (CARL ZEISS model AXIOSKOP 2 Plus), at magnifications of 100-400×, for the presence of the following mycorrhizal structures: arbuscules, vesicles, intraradical and extraradical hyphae. Observations made under the microscope allowed identification of fragments of the blue-stained fungal structures. The presence of non-AMF fungal endophytes was also recorded, and these were categorized into 3 main groups: dark septate endophytes (DSE), thin external blue hyphae, and Rhizoctonia-like sclerotia.
Spores of the arbuscular mycorrhizal fungi present in the rhizosphere samples were extracted from the soil by wet sieving and decanting (Gerdemann & Nicolson, 1963). Briefly, 100 g air-dried soil samples were sieved (through 1,000, 140 and 40 μm mesh) followed by a density gradient centrifugation method (Oehl et al., 2003). The spores were mounted on slides with polyvinyl–lactic acid–glycerol (PVLG) (Koske & Tessier, 1983) or a mixture (1:1; v/v) of PLVG with Melzer’s reagent and viewed under a dissecting microscope (Brundrett et al., 1994). Subsequently, they were examined under a compound microscope. Identification was based on current species descriptions and identification manuals (International Culture Collection of Arbuscular and Vesicular-Arbuscular Endomycorrhizal Fungi at http://www.invam. caf.wvu.edu/Myc_Info/Taxonomy/species.html; Schenck & Pérez, 1990) and Blaszkowski´s web page (www.agro.ar.szczecin.pl/~jblaszkowski).
Results
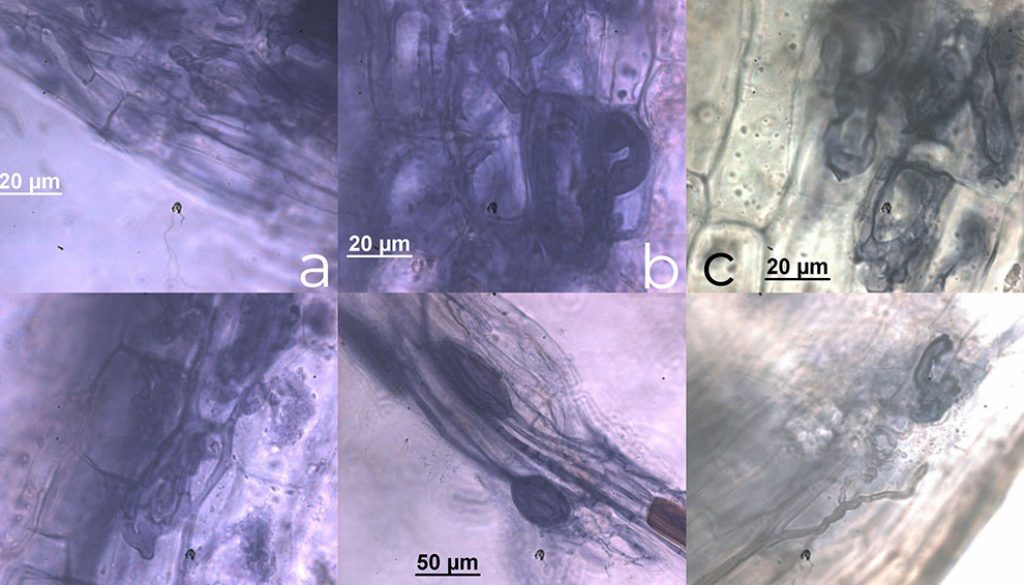
The C. crinita roots were colonized by AM fungi with a colonization average 60 ± 4.9%, being the highest root colonization 67% and the lowest 51% (Table 1). According to the presence and distribution of AMF structures in the root cortex, we propose an Arum-Paris type colonization pattern, according to Dickson (2004). Appressoria were formed by hyphae directly invading the epidermal cell with no apparent external modification. Internal arbuscules, vesicles as hyphal coils and dark septate endophytes were observed. We also observed thick extraradical hyphae running longitudinally, with some septa. The hyphae tended to form a coil within the first 3 adjacent epidermal cells and coils or loops within the adjacent exodermal and hypodermal cells (Fig. 2).
The hyphae traveled mainly in the longitudinal intercellular spaces of the inner cortical parenchyma. Coils invaded the adjacent cortical cells positioned both longitudinally and transversely to the root axis. AM fungi were never observed in the stele or endodermal cells. In old roots, hyphae (commonly septate) entered the cells of the inner cortex adjacent to the endodermis but did not penetrate the endodermal cells. Typical vesicles inter- and intracellular, elliptical or in lemon form, were observed. We also observed arbuscules, some of which had senescent aspect, and other fungi identified as dark septate endophytes (Fig. 2).
The average spore density in the rhizospheric soil was 756 ± 223.33 spores 100 g soil-1, ranging from 498 to 1,112 spores 100 g soil-1, equivalent to 5-11 spores per gram. We identified 16 morphologically distinct AMF species and/or morphospecies among the 10 samples studied (Fig. 3). More than half (7) belonged to the genus Glomus Tul and C. Tul, 2 species belonged to Funneliformis C. Walker & Schüßler, and 1 to Viscospora (T.H Nicolson) Sieverd., Oehl & G.A. Silva in the Glomerales. Three species belonged to Acaulospora Gerd. and Trappe and 1 to Kuklospora Oehl & Sieverd. in the Acaulosporales, while 1 belonged to Gigaspora Gerd. and Trappe and 1 to Scutellospora C. Walker and F.E. Sanders in the Gigasporales. Six AMF species could not be identified (Acaulospora sp. 1, Glomus sp. 1 “yellowish-brown in aggregates”, Glomus sp. 2 “brown small”, Glomus sp. 3 “reddish brown thick hyphae”, Glomus sp. 4 “big red in aggregates”, and Scutellospora sp. 1 “yellow light”). The most abundant AMF species were A. scrobiculata, G. clavisporum, G. glomerulatum and Glomus sp. 1 “yellowish-brown in aggregates”. These were found among 60-70% of all of the studied specimens. Spores of A. foveata, F. halonatum, F. mosseae and Glomus sp. 2 “brown small” were also relatively abundant, but with values of between 40 and 50% (Table 2; Fig. 3).
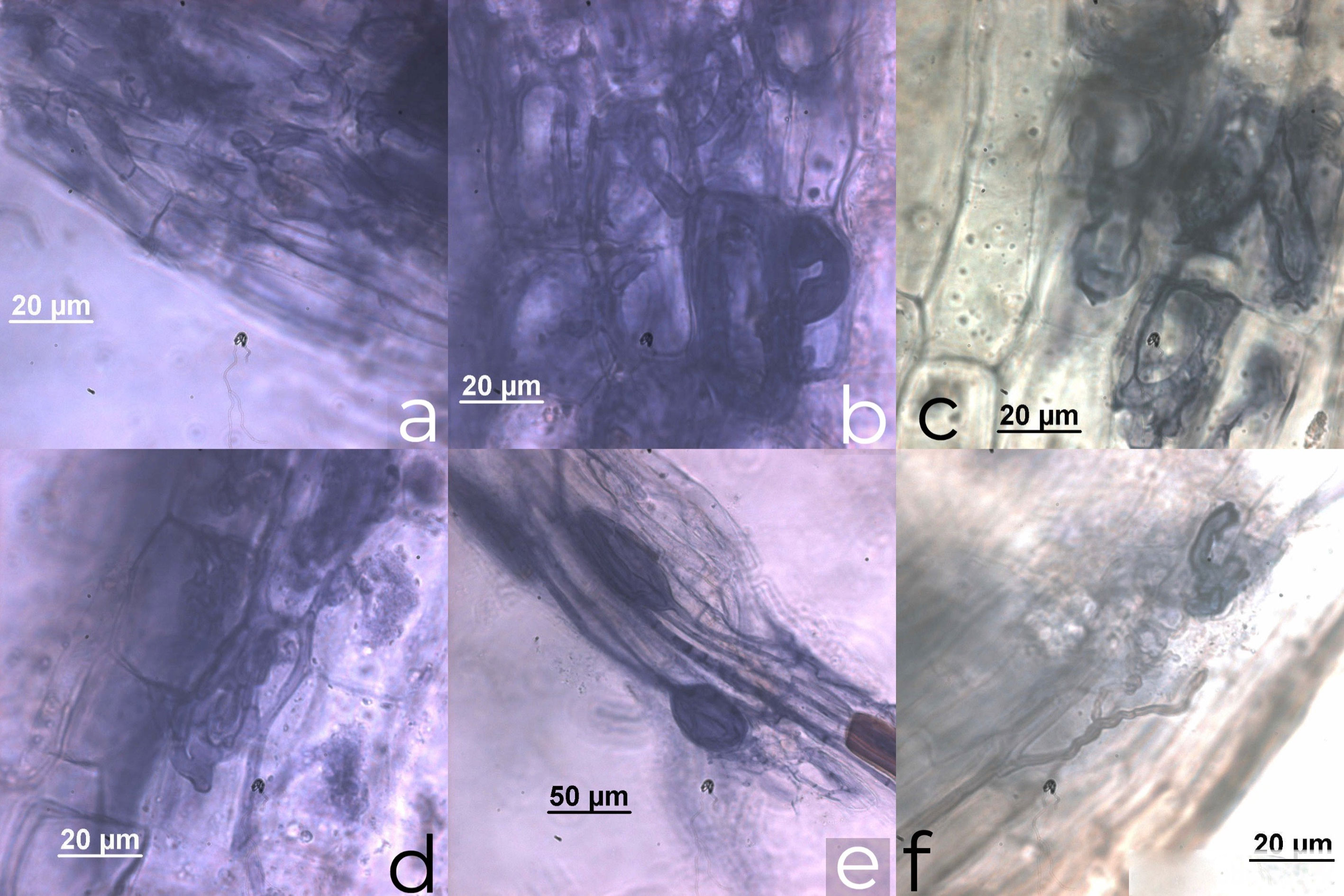
Table 1
Total root colonization in Coccothrinax crinita and fungal structures: arbuscules (A), vesicles (V), hyphal coils (HC), dark septate endophytes (DSE) and spore density in the rhizospheric soil.
| Plants | Total root colonization (%) | A | V | HC | DSE | Spore density (100 g soil) |
| 1 | 60 | x | x | x | x | 533 |
| 2 | 56 | x | x | x | 640 | |
| 3 | 59 | x | x | x | 980 | |
| 4 | 63 | x | x | x | 755 | |
| 5 | 65 | x | x | x | 1,023 | |
| 6 | 55 | x | x | x | 516 | |
| 7 | 64 | x | x | x | x | 669 |
| 8 | 67 | x | x | x | 1,112 | |
| 9 | 51 | x | x | x | 498 | |
| 10 | 60 | x | x | x | 832 | |
| Mean | 60 | 756 | ||||
| S.D. | 4.97 | 223.33 |
S.D., Mean standard deviation

Table 2
Presence-absence of arbuscular mycorrhizal fungal species in rhizospheric soil of Coccothrinax crinita.
| AMF species | Tree examined | Frequency (%) | |||||||||
| 1 | 2 | 3 | 4 | 6 | 6 | 7 | 8 | 9 | 10 | ||
| Acaulospora foveata Trappe & Janos | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 50 |
| A. scrobiculata Trappe | 1 | 1 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 60 |
| Acaulospora sp. 1 “reddish brown” | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 30 |
| Funneliformis halonatum (S.L. Rose & Trappe) Oehl, G.A. Silva & Sieverd | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 40 |
| Funneliformis mosseae (T.H. Nicolson & Gerd.) C. Walker & A. Schüssler | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 50 |
| Glomus clavisporum (Trappe) Almeida & Schenck | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 70 |
| Glomus glomerulatum Sieverd | 1 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 70 |
| Glomus tortuosum Schenck & Smith | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 20 |
| Glomus sp. 1 “yellowish-brown in aggregates” | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 70 |
| Glomus sp. 2 “brown small” | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 40 |
| Glomus sp. 3 “reddish brown thick hyphae” | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 20 |
| Glomus sp. 4 “big red in aggregates” | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 20 |
| Viscospora viscosa (T.H Nicolson) Sieverd., Oehl & G.A. Silva | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 30 |
| Kuklospora kentinensis (Wu & Liu) Oehl & Sieverd. | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 30 |
| Gigaspora decipiens Hall & Abbott | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 30 |
| Scutellospora sp. “small yellow light” | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 30 |
Discussion
The presence of AMF colonization in C. crinita roots and spores in its rhizospheric soil is important for the potential conservation of this species reported as Critically Endangered in the Flora of Cuba Red List (González-Torres et al., 2016). The species probably presents ecological specificity and its AMF could be a vital element in its survival and maintenance (McGonigle & Fitter, 1990).
Al-Yahya’ei (2008) and Fisher and Jayachandran (1999) discussed the difficulty of distinguishing AMF structures in palm roots. However, in this study, intraradical hyphae, vesicles and arbuscules were observed (Fig. 2), demonstrating the arbuscular mycorrhizal status of C. crinita. Usually the presence of arbuscules is considered the best indicator of function in the AM association (Corkidi & Rincón, 1997; Gange & Ayres, 1999); however, it has been recognized that arbuscules are not the only structures for nutrient translocation, and the vesicles and intraradical hyphae are indicators of current and past colonization (Allen, 1991). In this study, the arbuscules in the roots of C. crinita were not abundant, indicating that all fungal structures contribute to the host-fungi symbiosis (Smith & Smith, 1997). Arbuscular mycorrhizal fungal root colonization in C. crinita was higher than 60%, a value similar to that found for Astrocaryum mexicanum Liebm in tropical rainforest (Núñez-Castillo & Álvarez-Sánchez, 2003) and Thrinax radiata Lodd. ex Schult. in sand dunes (Polanco et al. 2013). On the other hand, colonization was lower in other Arecaceae, such as C. readii (27.7% during the rainy season, Polanco et al., 2013), D. orthacanthos (33% in mature tropical dry forest, Ramos-Zapata et al., 2006a) and peach palm (Bactris gasipaes Kunth) cultivated in the Amazon, from 13.54 – 43.95% (da Silva Júnior & Cardoso, 2006).
We classified the colonization pattern in C. crinita as Arum–Paris type, arbuscules with a certain degree of degradation were distinguished (Fig. 2c, d) and hyphal coils were also observed in the roots (Fig. 2a, b). As has been observed in Areca catechu L., Borassus flabellifer L. and Nypa fruticans Wurmb (Sengupta & Chaudhuri, 2002), Bactris gasipaes Kunth (da Silva Júnior & Cardoso, 2006), S. repens (Fisher & Jayachandran, 1999) and Phoenix paludosa Roxb, which is defined as a combination of “both types” according to Smith & Smith (1997).
Mycorrhizal symbiosis ranges from facultative to obligate, depending on the successional stage, seed size and nutrient requirements (Allen et al., 2003; Huante et al., 1993; Sharma et al., 2001; Siqueira & Saggin-Junior, 2001; Siqueira et al., 1998). Species such as D. orthacanthos were considered as obligate mycorrhizal in the semi-evergreen tropical forest of Quintana Roo, Mexico according to Ramos-Zapata et al. (2006a), while C. readii was considered facultative to the association with AMF fungi, as proposed by Polanco et al. (2013), since he found a low proportion of arbuscules and spore density in the soil. In our case, some experiments are necessary in order to establish the mycorrhizal dependency status of that palm. Plants with some degree of mycorrhizal dependence can increase the concentration of phosphorus in their tissues, while their seedlings have a greater survival capacity due to the hyphal network established in the soil, which is very important for a critically endangered species (Grime et al., 1987; Francis & Read, 1995).
The richness and density of spores, as well as mycorrhizal colonization, are important variables to infer the ability of the soil to produce mycorrhizae and to assess soil health (Cuenca, 2015). The spore density in rhizospheric soil of C. crinita was higher than that of the Amazon palms Attalea speciosa Mart (100 to 302 spores 100 g-1 dry soil) (Nobre et al., 2018) and B. gasipaes (54 to 96 spores 100 g-1 dry soil) (da Silva Júnior & Cardoso, 2006). On the other hand, the density was lower than that of E. guineensis (1,289-1,741 spores 100 g-1 dry soil) in coastal and Amazonian zones of Ecuador (Maldonado et al., 2008), S. repens (260-2,200 spores 100 g-1 dry soil) from the southeastern USA (Fisher & Jayachandran, 1999) and D. orthacanthos (250 and 350 spores 100 g-1 dry soil) from Quintana Roo, Mexico (Ramos-Zapata et al., 2006a).
Coccothrinax crinita has a high AMF richness similar to that reported by Nobre et al. (2018) in A. speciosa under natural conditions, but lower than that found in palm plantations in Oman (25 species) (Al-Yahya’ei et al., 2011). Glomus was the most frequently found genus in the C. crinita rhizosphere, as was observed in other palms (Fisher & Jayachandran, 1999; Guevara & López, 2007; Al-Yahya’ei et al., 2011).
Acaulospora has also been found in palms in the Brazilian Amazonia (Nobre et al., 2018; Silva et al., 2006; Trufem et al., 1994). Consequently, some Glomus and Acaulospora species could be considered as generalist AM fungi, although further studies would be necessary in order to confirm this specialist status.
The present study has shown the association of this palm with AMF in their natural habitat and, in the near future, some experiments inoculating these fungi could provide some tools to help improve the conservative management of C. crinita. An understanding of the mycorrhizal relationships of C. crinita, as well as the AMF species associated with this palm under natural conditions, could contribute to its conservation and given its multiple uses, enable cultivation for economic purposes.
References
Allen, M. F. (1991). The ecology of mycorrhizae. Cambridge: Cambridge University Press.
Allen, E. B., Allen, M. F., Egerton-Warburton, L., Corkidi, L., & Gómez-Pompa, A. (2003). Impacts of early and late seral mycorrhizae during restoration in seasonal tropical forest, Mexico. Ecological Applications, 13, 1701–1717.
Al-Yahya’ei, M. N. (2008). Arbuscular mycorrhizal fungal communities associated with date palms in a traditional and a modern experimental plantation and with desert plants in the adjacent natural habitats in Southern Arabia (Ph. D. Thesis). Università Degli Studi Di Torino. Basel.
Al-Yahya’ei, M. N., Oehl, F., Vallino, M., Lumini, E., Redecker, D., Wiemken, A. et al. (2011). Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of Southern Arabia. Mycorrhiza, 21, 195–209. https://doi.org/10.1007/s00572-010-0323-5
Ambili, K., Thomas, G. V., Indu, P., Gopal, M., & Gupta, A. (2012). Distribution of arbuscular mycorrhizae associated with coconut and arecanut based cropping systems. Agricultural Research, 1, 338–345. https://doi.org/10.1007/s40003-012-0036-4.
Berazaín, R., Areces, F., Lazcano, J. C., & González, L. R. (2005). Lista roja de la flora vascular cubana. Documento 4. Gijón: Jardín Botánico Atlántico.
Borhidi, A. (1996). Phytogeography and Vegetation Ecology of Cuba. Hungary: Akademiai Nyomda, Martonvazar.
Bouamri, R., Dalpé, Y., Serrhini, M., & Bennani, A. (2006). Arbuscular mycorrhizal fungi species associated with rhizosphere of Phoenix dactylifera L. in Morocco. African Journal of Biotechnology, 5, 510–516.
Brundrett, M., Melville, L., & Peterson, L. (1994). Practical methods in Mycorrhiza research. Mycologue Publications, University of Guelph, Guelph.
Brundrett. M., Bougher, N., Dell, B., Grove, T., & Malajczuk, N. (1996). Working with mycorrhizas in forestry and agriculture. Monograph 32. Canberra: Australian Centre for International Agricultural Research.
Carrillo, L., Orellana, R., & Varela, L. (2002). Mycorrhizal associations in three species of palms of the Yucatan Peninsula, Mexico. Palms, 46, 39–46.
Cavagnaro, T. R., Gao, L. L., Smith, F. A., & Smith, S. E. (2001). Morphology of arbuscular mycorrhizas is influenced by fungal identity. New Phytologist, 151, 469–475.
Corkidi, L., & Rincón, E. (1997). Arbuscular mycorrhizae in a tropical sand dune ecosystem on the Gulf of Mexico. I. Mycorrhizal status and inoculums potential along a successional gradient. Mycorrhiza, 7, 9–15.
Corley, R. H. B., & Tinker, P. B. H. (2003). The oil palm. 4th Edition. London: Blackwell Publishing Ltd.
Cuenca, G. (2015). Las micorrizas arbusculares: aspectos teóricos y aplicados. Caracas: Ediciones IVIC/ Instituto Venezolano de Investigaciones Científicas.
da Silva Júnior, J. P., & Cardoso, E. J. B. N. (2006). Arbuscular mycorrhiza in cupuaçu and peach palm cultivated in agroforestry and monoculture systems in Central Amazon region. Pesquisa Agropecuária Brasileira, 41, 819–825.
de la Pena, E., Rodríguez-Echeverría, S., van der Putten, W. H., Freitas, H., & Moens, M. (2006). Mechanism of control of root-feeding nematodes by mycorrhizal fungi in the dune grass Ammophila arenaria. New Phytologist, 169, 829–840.
Dickson, S. (2004). The Arum-Paris continuum of mycorrhizal symbioses. New Phytologist, 163, 187–200.
Dickson, S., Smith, F. A., & Smith, S. E. (2007). Structural differences in arbuscular mycorrhizal symbioses: more than 100 years after Gallaud, where next? Mycorrhiza, 17, 375–393.
Dreyer, B., Morte, A., López, J. A., & Honrubia, M. (2010) Comparative study of mycorrhizal susceptibility and anatomy of four palm species. Mycorrhiza, 20, 103–115. https://doi.org/10.1007/s00572-009-0266-x
FAO-Unesco (1975). Soil map of the word 1:5000000. Vol. III. México and Central American. Rome: Ed. Failli.
Fisher, J. B., & Jayachandran, K. (1999). Root structure and arbuscular mycorrhizal colonization of the palm Serenoa repens under field conditions. Plant and Soil, 217, 229–241.
Fisher, J. B., & Jayachandran, K. (2005). Presence of arbuscular mycorrhizal fungi in South Florida native plants. Mycorrhiza, 15, 580–588.
Francis, R., & Read, D. J. (1995). Mutualism and antagonism in the mycorrhizal symbiosis with special reference to impact on plant community structure. Canadian Journal of Botany, 73, 1301–1309.
Gallaud, I. (1905). Études sur les mycorrhizes endotrophs. Revue Générale de Botanique, 17, 5–48.
Gange, A. C., & Ayres, R. (1999). On the relation between arbuscular mycorrhizal colonization and plant “benefit”. Oikos, 87, 615–621.
Gerdemann, J. W., & Nicolson, T. J. (1963). Spores of mycorrhizal Endogone species extracted from soil by wet sieving and decanting. Transactions of the British Mycological Society, 46, 235–244.
González-Torres, L. R., Palmarola, A., González-Oliva, L., Bécquer, E. R., Testé, E., & Barrios, D. (Eds.) (2016). Lista Roja de la flora de Cuba. Bissea, 10 (Núm. especial), 1–352.
Grime, J. P., Mackey, J. L. M., Hillier, & Read, D. J. (1987). Floristic diversity in a model system using experimental microcosms. Nature, 328, 420–422.
Guevara, R., & López, J. C. (2007). Quality of rooting environments and patterns of root colonization by arbuscular mycorrhizal fungi in strangler figs in a Mexican palmetto woodland. Mycorrhiza, 17, 589–596. https://doi.org/10.1007/s00572-007-0136-3
Huante, P., Rincón, E., & Allen, E. B. (1993). Effect of vesicular arbuscular mycorrhizae on seedling growth of four tree species from the tropical deciduous forest in Mexico. Mycorrhiza, 2, 141–145.
Janos, D. P. (1977). Vesicular-arbuscular mycorrhizae affect the growth of Bactris gasipaes. Principes, 21, 12–18.
Jestrow, B., Peguero, B., Jiménez, F., Verdecia, R., González-Oliva, L., Moya, C. E. et al. (2017). A conservation framework for the Critically Endangered endemic species of the Caribbean palm Coccothrinax. Fauna & Flora International, 52, 452–463. https://doi.org/10.1017/S0030605317000588
Johnson, D. (Ed.) (1996). Palms: their conservation and sustained utilization. Status survey and conservation Actions plant. Gland, Switzerland: IUCN.
Koide, R. T., & Mosse, B. (2004). A history of research on arbuscular mycorrhiza. Mycorrhiza, 14, 145–163. https://doi.org/10.1007/s00572-004-0307-4
Koske, R. E., & Tessier, B. (1983). A convenient permanent slide mounting medium. Mycological Society of America Newsletter, 34, 59.
Leiva, A. T. (1999). Las palmas en Cuba. La Habana: Editorial Científico-Técnica.
Maldonado, L., Morales, R., Bernal, G., & Alcocer, I. (2008). Estudio del comportamiento de las asociaciones micorrízicas en el material germoplásmico de palma aceitera en Ecuador. Memorias del XI Congreso Ecuatoriano de la Ciencia del Suelo. Quito, 29–31 de octubre de 2008.
Martínez, B. J. I., & Miranda, J. (2009). Etnobotánica y educación para la conservación de Coccothrinax crinita subsp. crinita, palma petate (Arecaceae). Revista del Jardín Botánico Nacional de Cuba Universidad de La Habana, 30–31, 91–95.
McGonigle, T. P., & Fitter, A. H. (1990). Ecological specificity of vesicular-arbuscular mycorrhizal associations. Mycological Research, 94, 120–122.
McGonigle, T. P., Miller, M. H., Evans, D. G., Fairchild, G. L., & Swan, J. A. (1990). A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phytologist, 115, 495–501.
Nadarajah, P. (1980). Species of Endogonaceae and mycorrhizal association of Elaeis guineensis and Theobroma cacao. In P. Mikola (Ed.), Tropical mycorrhiza research (pp.233–237). Oxford: Clarendon.
Nobre, C. P., Costa, M. G. da, Goto, B. T., & Gehring, C. (2018). Arbuscular mycorrhizal fungi associated with the babassu palm (Attalea speciosa) in the eastern periphery of Amazonia, Brazil. Acta Amazonica, 48, 321–329.
Núñez-Castillo, O., & Álvarez-Sánchez, F. (2003). Arbuscular mycorrhizae of the palm Astrocaryum mexicanum in disturbed and undisturbed stands of a Mexican tropical forest. Mycorrhiza, 13, 271–276. https://doi.org/10.1007/s00572-003-0231-z
Oehl, F., Sieverding, E., Ineichen, K., Mäder, P., Boller, T., & Wiemken, A. (2003). Impact of land use intensity on the species diversity of arbuscular mycorrhizal fungi in agroecosystems of Central Europe. Applied and Environmental Microbiology, 69, 2816–2824.
Phillips, J. M., & Hayman, D. S. (1970). Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 55, 158–161.
Pinares, A. (2004). Estudios para la conservación integrada de Coccothrinax crinita Becc. (palma petate) (Master Thesis). Jardín Botánico Nacional, La Habana, Cuba.
Polanco, G., Carrillo, L., Espadas, C., Reyes-García, C., Guadarrama, P., & Orellana, R. (2013). Asociación micorrízica arbuscular en Coccothrinax readii Quero. Tropical and Subtropical Agroecosystems, 16, 223–233.
Rajeshkumar, P. P., Thomas, G. V., Gupta, A., & Gopal, M. (2015). Diversity, richness and degree of colonization of arbuscular mycorrhizal fungi in coconut cultivated along with intercrops in high productive zone of Kerala, India. Symbiosis, 65, 125–141.
Ramos-Zapata, J. A., Orellana, R., & Allen, E. B. (2006a). Mycorrhizal dynamics and dependence of Desmoncus orthacanthos Martius (Arecaceae), a native palm of the Yucatán Peninsula, Mexico. Interciencia, 31, 364–370.
Ramos-Zapata, J. A., Orellana, R., & Allen, E. B. (2006b). Establishment of Desmoncus orthacanthos Martius (Arecaceae): effect of inoculation with arbuscular mycorrhizae. Revista de Biología Tropical, 54, 65–72.
Roldán, A., Díaz-Vivancos, P., Hernández, J. A., Carrasco, L., & Caravaca, F. (2008). Superoxide dismutase and total peroxidase activities in relation to drought recovery performance of mycorrhizal shrub seedlings grown in an amended semiarid soil. Journal of Plant Physiology, 165, 715–722. https://doi.org/10.1016/j.jplph.2007.02.007
Schenck, N. C., & Pérez, Y. (1990). Manual for the identification of VA mycorrhizal fungi. 3th. Ed. Gainesville: INVAM.
Sengupta, A., & Chaudhuri, S. (2002). Arbuscular mycorrhizal relations of mangrove plant community at the Ganges river estuary in India. Mycorrhiza, 12, 169–174.
Sharma, M. P., Bhatia, N., & Adholeya, A. (2001). Mycorrhizal dependency and growth responses of Acacia nilotica and Albizzia lebbeck to inoculation by indigenous AM fungi as influenced by available soil P levels in a semi-arid Alfisol wasteland. New Forests, 21, 89–104.
Sheng, M., Tang, M., Chen, H., Yang, B. W., Zhang, F. F., & Huang, Y. H. (2008). Influence of arbuscular mycorrhizae on photosynthesis and water status of maize plants under salt stress. Mycorrhiza, 18, 287–296. https://doi.org/10.1007/s00572-008-0180-7
Silva, C. F., Pereira, M. G., Silva, E. M. R., Correia, M. E. F., & Saggin-Júnior, O. J. (2006). Fungos micorrízicos arbusculares em áreas no entorno do Parque Estadual da Serra do Mar e Ubatuba (SP). Revista Caatinga, 19, 1–10.
Siqueira, J. O., Carneiro Carbone M. A., Curi, N., Rosado da Silva, S. C., & Davide, A. C. (1998). Mycorrhizal colonization and mycotrophic growth of native woody species as related to successional groups in Southeastern Brazil. Forest Ecology and Management, 107, 241–252.
Siqueira, J. O., & Saggin-Junior, O. J. (2001). Dependency on arbuscular mycorrhizal fungi and responsiveness of some Brazilian native woody species. Mycorrhiza, 11, 245–255.
Smith, F. A., & Smith, S. E. (1997). Structural diversity in (vesicular) – arbuscular mycorrhizal symbioses. New Phytologist, 137, 373–388.
St. John, T. V. (1988). Prospects for application of vesicular-arbuscular mycorrhizae in the culture of tropical palms. Advances in Economic Botany, 6, 50–55.
Trufem, S. F. B., Malatinszky, S. M. M., & Otomo, H. S. (1994). Fungos micorrízicos arbusculares em rizosferas de plantas do litoral arenoso do Parque estadual da Ilha do Cardoso, SP, Brasil. Acta Botanica Brasilica, 8, 219–229.
Vales, M., Alvarez, A., Montes, L., & Avila, A. (1998). Estudio nacional sobre la diversidad biológica en la República de Cuba. La Habana, Madrid: PNUMA/ CenBio/ IES/ AMA/ CITMA/ CEYSTA.
van Aarle, I. M., Cavagnaro, T. R., Smith, S. E., Smith, F. A., & Dickson, S. (2005). Metabolic activity of Glomus intraradices in Arum- and Paris type arbuscular mycorrhizal colonization. New Phytologist, 166, 611–618.
Verdecia, R., & Barrios, D. (2015). Coccothrinax crinita subsp. crinita. Bissea, 9, 57.
Wang, B., & Qiu, Y-L. (2006). Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza, 16, 299–363.
Zona, S. (1996). Roystonea (Arecaceae: Acrecoideae). Flora Neotropic, 71, 1–36.
Zubek, S., Turnau, K., Tsimilli-Michael, M., & Strasser, R. J. (2009). Response of endangered plant species to inoculation with arbuscular mycorrhizal fungi and soil bacteria. Mycorrhiza, 19, 113–123.