Eduardo Suárez-Morales a, *, Lourdes Vásquez-Yeomans a, Leomir Santoya b
a El Colegio de la Frontera Sur, Unidad Chetumal, Av. Centenario Km 5.5, 77014 Chetumal, Quintana Roo, Mexico
b Sarteneja Alliance for Conservation and Development (SACD), Lagunita Street, Sarteneja Village, Corozal District, Belize
*Corresponding author: esuarez@ecosur.mx (E. Suárez-Morales)
Received: 14 August 2019; accepted: 24 February 2020
http://zoobank.org/ urn:lsid:zoobank.org:pub:E432BEE7-EA79-46AE-A96D-9A62978FBF65
Abstract
Cymbasoma belizense sp. n. is described from an adult female collected during a zooplankton survey of the Corozal Bay Wildlife Sanctuary (CBWS), adjacent to Chetumal Bay, a large binational embayment on the northwestern Caribbean Basin. The new species is a member of the Cymbasoma longispinosum species-group; it resembles C. chelemense Suárez-Morales & Escamilla, 1997 from the Gulf of Mexico and C. jinigudira Suárez-Morales & McKinnon, 2014 from Australia. This is the eighth nominal species known in this species-group, the second one recorded from the Caribbean Sea Basin, and the first one in the Belizean coast. The new species is distinguished by a combination of characters, including the body proportions, the structure and armature of the fifth legs, the cephalic ornamentation, with a fringe of faint cuticular striae, the relative length and bifurcation point of the ovigerous spines, and details of the antennule armature. Comparative comments and data including the distribution and taxonomical characters of members of this species-group are presented. Records of this nominal species from different geographic areas should be revised carefully because they could represent undescribed species. A key to the females of the currently known species of this group also provided.
Keywords: Copepods; Zooplankton; Cymbasoma cf. longispinosum; Crustacean taxonomy; Belize; Western Caribbean Sea
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Nueva especie del grupo Cymbasoma longispinosum (Copepoda: Monstrilloida) de Belice, Caribe occidental
Resumen
Se describe a Cymbasoma belizense sp. n. a partir de una hembra adulta recolectada durante un estudio del zooplancton del Santuario de Vida Silvestre de Corozal (CBWS), adyacente a la bahía de Chetumal, un extenso sistema costero binacional localizado en la parte noroccidental de la cuenca del Caribe. La nueva especie es miembro del grupo de especies asociado a Cymbasoma longispinosum y es similar a C. chelemense Suárez-Morales y Escamilla, 1997, del golfo de México y a C. jinigudira Suárez-Morales y McKinnon, 2014, de Australia. Esta es la octava especie descrita como parte de este grupo de especies, la segunda de ellas registrada en la cuenca del Caribe y es la primera en aguas costeras de Belice. La nueva especie se distingue por una combinación de caracteres que incluyen las proporciones corporales, la estructura y la armadura de la quinta pata, la ornamentación cefálica, con una franja de tenues estrías, la longitud relativa y el punto de bifurcación de las espinas ovígeras, y detalles de la estructura y armamento de la anténula. Se presentan comentarios y datos comparativos que incluyen la distribución y los principales caracteres taxonómicos utilizados para distinguir a los miembros del grupo de especies de C. longispinosum. Los registros de esta especie nominal en diferentes áreas geográficas deben revisarse cuidadosamente porque podrían representar especies indescritas. Se proporciona también una clave para la identificación de las hembras de las especies actualmente reconocidas como parte de este grupo.
Palabras clave: Copépodos; Zooplancton; Cymbasoma cf. longispinosum; Taxonomía de crustáceos; Belice; Mar Caribe occidental
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Members of the copepod order Monstrilloida Sars, 1901, are rarely observed in regular plankton surveys as only 2 stages of their semi-parasitic life cycle are free-living forms: the inconspicuous early nauplius and the adult (Suárez-Morales, 2011, 2017). Their intermediate postnaupliar and juvenile stages are endoparasites of benthic invertebrates like polychaetes (including members of the families Syllidae, Capitellidae, Serpulidae, and Spionidae), gastropod and bivalve mollusks, and sponges (Huys et al., 2007; Jeon et al., 2018; Suárez-Morales, 2017; Suárez-Morales et al., 2010, 2014). Typically, adult monstrilloids lack appendages between the antennules and swimming legs, they are non-feeding reproductive stages (Grygier & Ohtsuka, 2008; Suárez-Morales, 2011). They can be caught occasionally during plankton samplings in coastal lagoons and embayments (Suárez-Morales, 2011; Suárez-Morales & Dias, 2001), particularly in reef habitats where they can be abundant and diverse (Suárez-Morales, 2001). The order is currently represented by a single family (Monstrillidae) that contains 7 valid genera: Monstrilla Dana, 1849; Cymbasoma Thompson, 1888; Monstrillopsis Sars, 1921; Maemonstrilla Grygier & Ohtsuka, 2008; Australomonstrillopsis Suárez-Morales & McKinnon, 2014; Caromiobenella Jeon, Lee & Soh, 2018; and Spinomonstrilla Suárez-Morales, 2019 (Suárez-Morales, 2011, 2019; Suárez-Morales & McKinnon, 2014;). Cymbasoma and Monstrilla are currently deemed as the most speciose genera (Suárez-Morales, 2011). There is only one previous record of a monstrilloid copepod from Chetumal Bay, a species of the genus Monstrilla (Suárez-Morales & Castellanos, 2019)
There are several nominal species whose taxonomic status is doubtful; some of these have been recorded from different geographic regions but were recognized as species groups containing distinct species (Suárez-Morales, 2011; Üstün et al., 2014). One of these groups is the Cymbasoma longispinosum species-group, which is currently known to contain at least 7 species worldwide (Üstün et al., 2014). The purpose of this study is to describe a new species of this group from the Caribbean Sea Basin and compare it with the other members of the species-group. A general overview of the distribution of the species assignable to the Cymbasoma longispinosum species-group and a key to these species are provided.
Material and methods
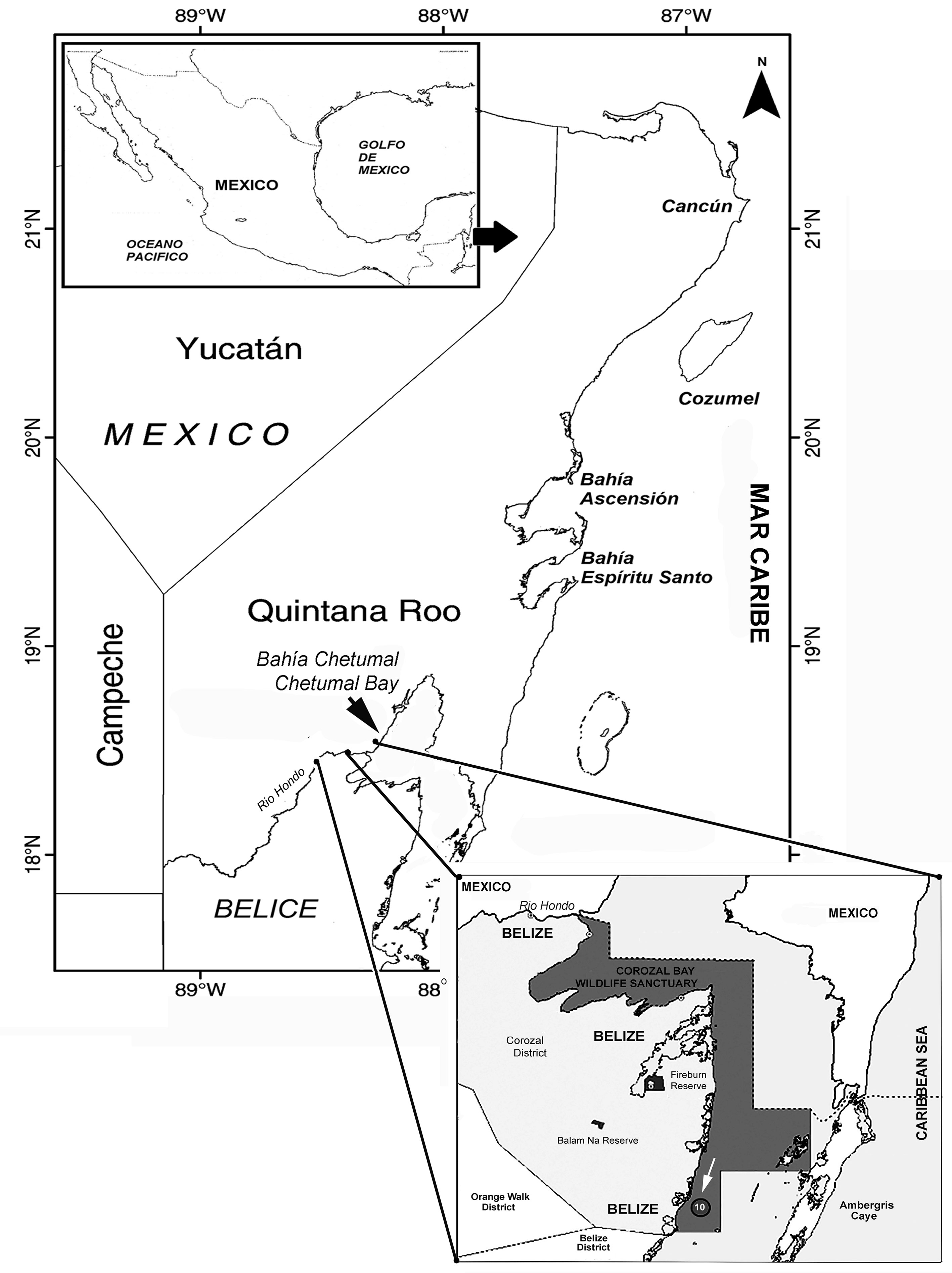
Zooplankton samples were obtained on May 9, 2019 by performing nighttime surface hauls off Chetumal Bay, a binational embayment shared by Mexico and Belize, on the southernmost part of the Mexican Caribbean coast (Fig. 1). A standard plankton net of 0.5 m mouth diameter, and 0.333 mm filtering mesh was used. During a zooplankton trawl performed at station 10 (arrowed in Fig. 1), located off the District of Corozal, Belize, Central America an adult female specimen of a monstrilloid copepod was observed and sorted for further taxonomic examination. The specimen is assignable to the genus Cymbasoma. It was found to represent a new species which is herein described in full following the current upgraded descriptive standards in monstrilloid taxonomy (Grygier & Ohtsuka, 1995, 2008) and compared with its closest congeners. The morphologic terminology follows Huys and Boxshall (1991). The holotype was deposited in the collection of zooplankton held at El Colegio de la Frontera Sur (ECOSUR) at Chetumal, Mexico (ECO-CHZ), where it is available for consultation.
Description
Order Monstrilloida Sars, 1903
Family Monstrillidae Dana, 1849
Genus Cymbasoma Thompson, 1888
Cymbasoma belizense sp. nov.
(Figs. 2, 3)
http://zoobank.org/ urn:lsid:zoobank.org:pub:E432BEE7-
EA79-46AE-A96D-9A62978FBF65
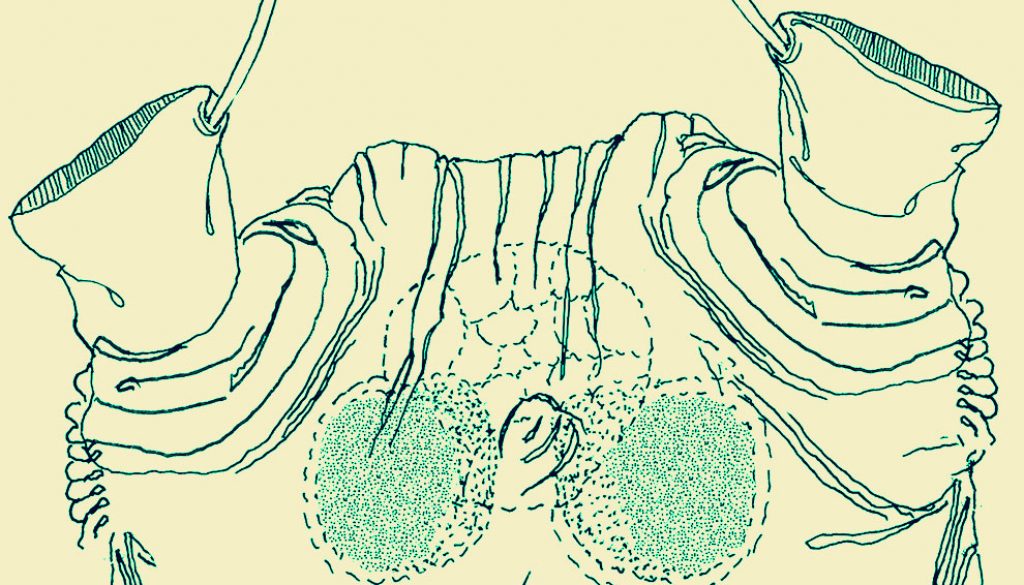
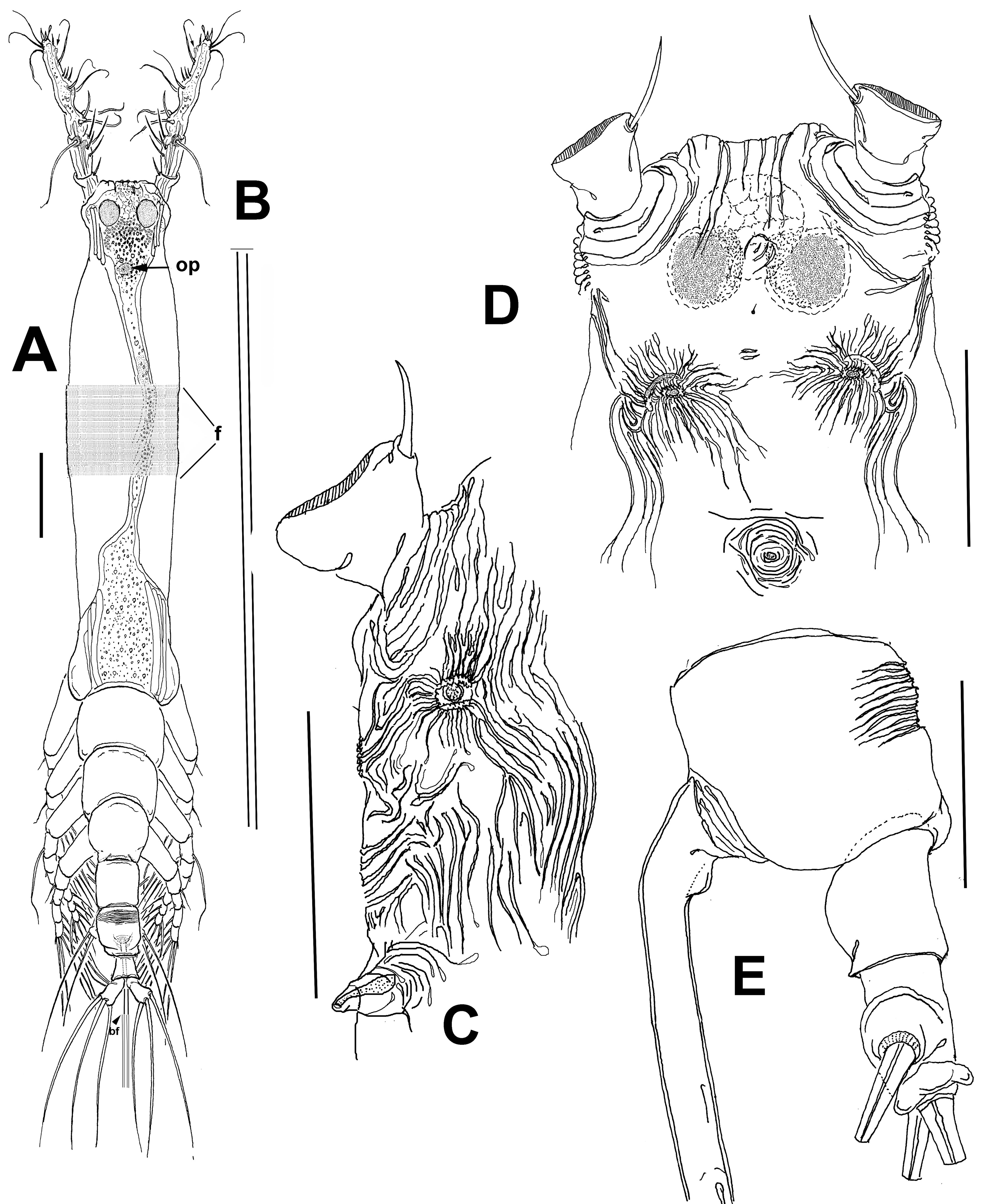
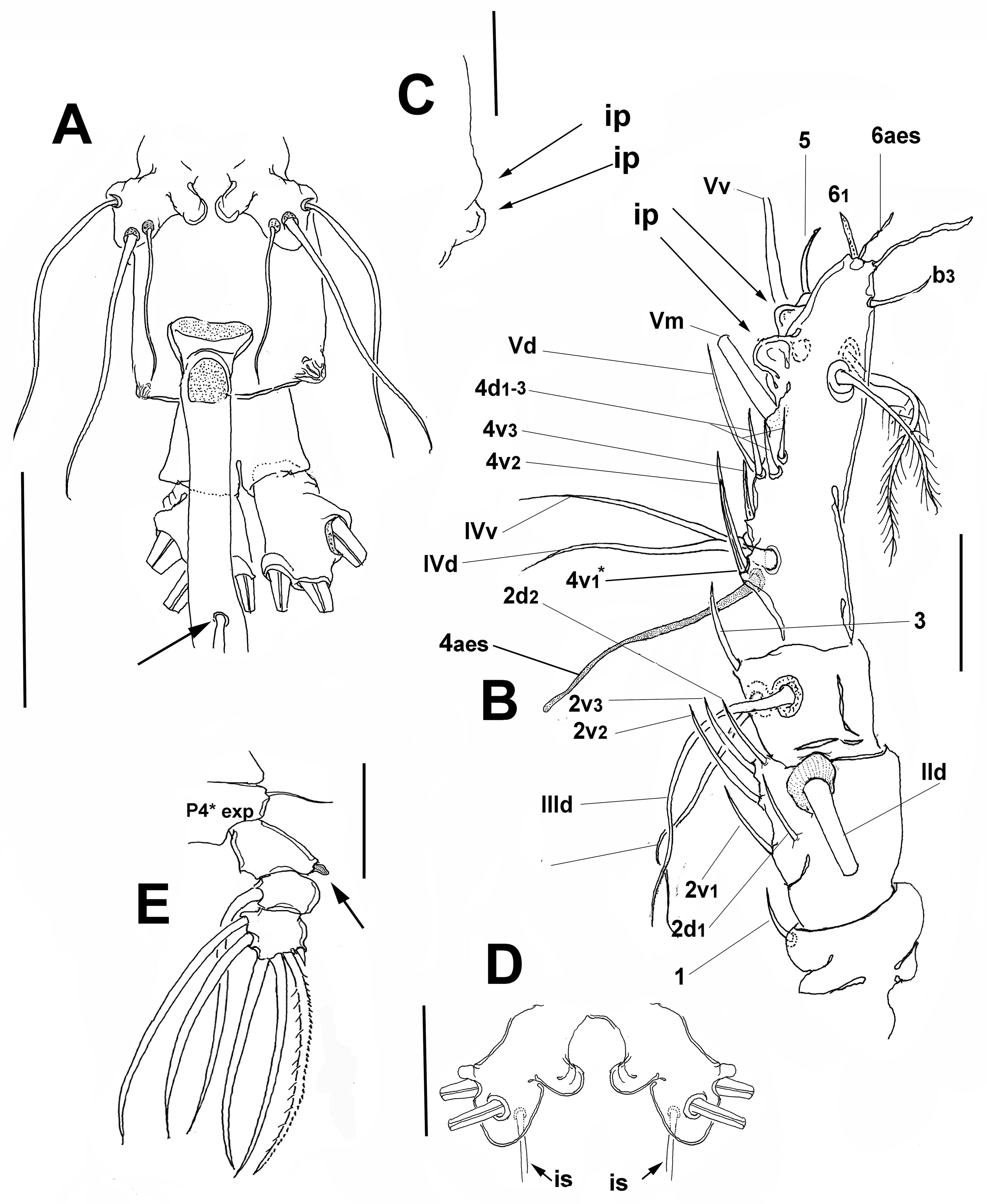
Female. Body cylindrical, cephalothorax typically elongate, slender; body length of holotype female measured from anterior end of cephalosome to posterior margin of anal somite = 2.33 mm. Cephalothorax (incorporating first pedigerous somite) approximately 1.72 mm long, representing 64% of total body length. Oral papilla conical, located ventrally at anterior 1/5 of cephalothorax. Pair of relatively large ocelli present, pigment cups moderately developed, medially conjoined, weakly pigmented; ventral cup slightly larger than lateral cups (Fig. 2A). Frontal cephalic margin with conspicuous, longitudinally arranged cuticular ridges extending onto anterior ventral surface (Fig. 2D) between the antennule bases; sensilla not observed on “forehead” area. Additional cephalic cuticular ornamentation including small, semicircular group of medial cuticular scars overlying area between ocelli on ventral surface. Ventral surface with: 1) 2 large nipple-like processes surrounded by field of wrinkles on preoral area, 2) a small medial sensillum, and 3) perioral field of wrinkles (Fig. 2C, D). Other cuticular ornamentation represented by wide transverse fringe of faint, shallow striation on cephalothorax (“f” in Fig. 2A).
Urosome consisting of fifth pedigerous somite, genital double somite, anal somite, and caudal ramus, together representing 16% of total body length (Fig. 2A, E). Genital double- somite subquadrate, dorsal surface ornamented with field of deep transverse cuticular wrinkles on proximal half and field of wrinkles on posterior corners of somite (Fig. 2E). Anal somite smooth, with ventral surface protuberant in lateral view (Fig. 2E). Caudal ramus subrectangular, 1.3 times longer than wide, armed with 3 subequally long lightly setulated caudal setae (Figs. 2D, 3C). Ovigerous spines paired, long, about 1.2 times as long as body (Fig. 2A). Spines basally conjoined, individual spines bifurcate and arise slightly beyond posterior margin of caudal ramus (arrowed in Fig. 3A). Spines slender, straight at their base and along shaft, distal part of spines broken off, thus preventing us to determine its length (Fig. 3E).
Antennule 4-segmented (Fig. 3B); antennule representing about 22% of total body length and 32% of cephalothorax length. Relative length of segments, from base to top as: 13.7; 19.7; 15.3; 51.5 = 100. Armature formula (Arabic numerals = setae, Roman numerals = spines) as: 0-I; 1-V; 2-I; 8-VIII (Fig. 2C). In terms of pattern described by Grygier and Ohtsuka (1995) for female monstrilloid antennular armature, setae (Roman numerals) and spines (Arabic numerals), element 1 present on first segment; elements on second segment: 2d1, 2d2, 2v1-3, and IId. Third segment with setal elements 3, IIId, and IIIv. Fourth segment long, representing 51.5% of antennule length; segment bearing elements 4d1-3; elements 4v1-3 well developed, thick, with remarkably long, spiniform element 4v1 reaching beyond halflength of segment; setae IVd, IVv, Vd, Vv, Vm, and aesthetasc 4aes also present on same segment; element 5 spiniform, not reaching distal end of segment. Subterminal element b3 unbranched, aesthetasc 6aes short, slender; element 61 present, element 62 absent, probably broken off during collection or handling (Fig. 2C). Fourth segment with pair of subdistal rounded processes on inner margin, adjacent to insertion of elements Vv and 5 (arrowed, “ip” in Fig. 3 B); processes observed in same position on left antennule as well (Fig. 3C).
Incorporated first pedigerous somite and succeeding 3 free pedigerous somites each bearing a pair of biramous swimming legs. Pedigerous somites 2-4 together accounting for 23% of total length in dorsal view. Structure, segmentation and armature of swimming legs 1-4 as in C. chelemense (Suárez-Morales & Escamilla-Sánchez, 1997). Legs slightly increasing in size posteriorly. Endopodites and exopodites triarticulated. Intercoxal sclerites subrectangular, widest at base, tapering distally, surface and posterior margin smooth. Basis of legs articulating with large, rectangular coxa along diagonal line. Basis with hair-like lateral seta (Fig. 4A-D); on leg 3, this seta about 2.5 times longer, lightly setulated from proximal half, slightly thicker than those on other legs (arrowed in Fig. 4C). Ramus setae all biserially plumose except short spiniform outer seta on exopod segments 1 and 3 of legs 1-4 (Fig. 4A-D). Spine on first exopodal segment of leg 4 digitiform (arrowed in Fig. 3E). Outermost apical exopodal setae of swimming legs 1-4 with inner margin naked, lightly spinulose, outer margin spinulose. Leg 4 exopod with distinctive digitiform outer spine on first segment (arrowed in Fig. 3E). Overall, the armature and segmentation of legs 1-4 is conservative in species of the longispinosum group (Mageed, 2010; Üstün et al., 2014; present data; Table 1).
Table 1
Armature formula of swimming legs.
|
Basis |
Endopodite |
Exopodite |
|
|
leg 1 |
1-0 |
0-1;0-1;1,2,2 |
I-1;0-1;I,2,2 |
|
legs 2-4 |
1-0 |
0-1;0-1;1,2,2 |
I-1;0-1;I,1,2,2 |
Fifth legs medially conjoined, bilobed, inner (endopodal) lobe thumb-like, rounded distally. Outer (exopodal) lobe with rounded protuberance on distal inner margin, at insertion of innermost seta (“is”; Fig. 3D); exopodal lobe armed with 3 setae, 1 distal, 2 inserted subdistally. Outermost and medial setae long, biserially and lightly setulated; innermost seta shortest, slender, not reaching posterior margin of genital double-somite (Fig. 3A).
Male. Unknown.
Taxonomic summary
Type material. Adult female holotype, dissected, mounted in 2 slides (ECO-CHZ-10341). Specimen collected from coastal waters off the Corozal Bay Wildlife Sanctuary, Corozal District, Belize (18°22’35.25” N, 88°21’03.62” W), NW Caribbean Sea; specimen partially dissected. Selected appendages, body and antennules on slides mounted on glycerine, sealed with Entellan®. Date of collection: 9 May, 2019 by L. Santoya (SACD- Belize) and Lourdes Vásquez-Yeomans (ECOSUR-Chetumal). Plankton sample. Slides deposited in the collection of Zooplankton at El Colegio de la Frontera Sur (ECOSUR), in Chetumal, Mexico.
Type locality. Corozal Bay (18°22’35.25” N, 88°21’03.62” W), off the Corozal District, Belize, Central America, on the western Caribbean Sea.
Etymology. The specific epiteth makes reference to the country in which the type locality is located, Belize, Central America. The name is proposed in genitive form with a neuter ending suffix to match the genus gender.
Remarks
This copepod from Belizean waters was assigned to the monstrilloid genus Cymbasoma Thompson, 1888 by virtue of the presence of a single free somite between the caudal rami and the genital double-somite, and its caudal rami being armed with only 3 caudal setae, both distinctive characters of Cymbasoma (Boxshall & Halsey, 2004; Isaac, 1975; Suárez-Morales, 2011; Suárez-Morales & McKinnon, 2014). It is assignable to the Cymbasoma longispinosum species-group by its size, body proportions with an elongate cephalothorax (+ 63% of total body length), relatively short urosome, proximally fused ovigerous spines, bilobed fifth legs with unarmed inner lobe and outer lobe bearing 3 setal elements, and the presence of conspicuous cuticular ornamentation on the genital double-somite (Grygier, 1994; Suárez-Morales, 2011; Üstün et al., 2014).
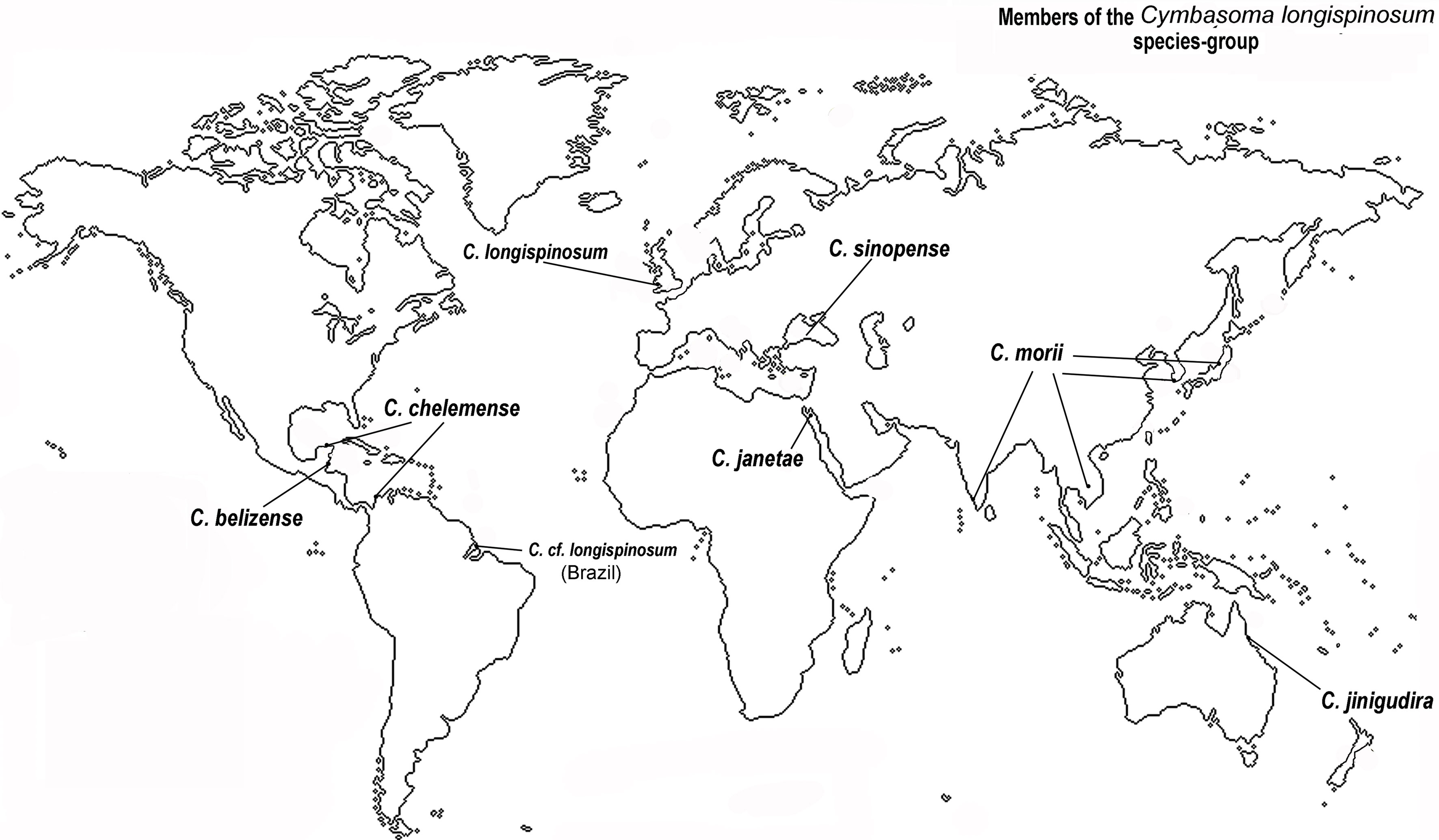
After the publication of Bourne’s (1890) original description of this species from the English Channel, the nominal C. longispinosum has been recorded from many different geographic areas and latitudes including the Mediterranean (Giesbrecht, 1893; Rose, 1933), Norway (Sars, 1921), the Australian coast and Philippines in the southern Pacific (Dakin & Colefax, 1940; Wilson, 1950), the eastern tropical Atlantic, the Red Sea (Gurney, 1927), India (Martin-Thompson, 1973), and Brazil (Dias, 1996; Dias & Bonecker, 2007; Duarte, 1999; Leite et al., 2010; Suárez-Morales et al., 2020, in press). Aside the improbability of a single monstrilloid species being so widely distributed some of these authors noted differences between their specimens and the original description of C. longispinosum. It was later realized that this nominal species contains a group of morphologically close cryptic species, each with a limited geographic distribution (Fig. 4). This notion supported the proposal of the longispinosum species-group and the subsequent description of new species linked to it (Grygier, 1994; Mageed, 2010; Suárez-Morales & Escamilla-Sánchez, 1997; Suárez-Morales & McKinnon, 2014; Suárez-Morales & Palomares-García, 1999). Only a few of the numerous records of C. longispinosum worldwide have been advanced as independent species and members of this group (Grygier, 1995). It is currently known to contain at least 7 nominal species showing subtle but consistent differences (Üstün et al., 2014) and are distributed in distinct geographical areas, including Europe (C. longispinosum s.str.), the Gulf of Mexico (C. chelemense Suárez-Morales & Escamilla-Sánchez, 1997), the Gulf of California (C. californiense Suárez-Morales & Palomares-García, 1999), Japan, Korea, Vietnam, India (C. morii Sekiguchi, 1982) (Chang, 2014; Grygier, 1994;), the Red Sea, Egypt (C. janetae Mageed, 2010), Turkey (C. sinopense Üstün, Suárez-Morales & Terbiyik, 2014), and western Australia (C. jinigudira Suárez-Morales & McKinnon, 2014) (Fig. 4). A Brazilian species of the group is currently being described (Suárez-Morales et al., in press).
Records of C. longispinosum from India (Martin-Thompson, 1973) were tentatively assigned to C. morii (Grygier, 1994), and the Brazilian specimens reported as C. longispinosum by Leite et al. (2010) and by Dias and Bonecker (2007) probably represent an undescribed species (Suárez-Morales, 2011; Suárez-Morales et al., in press; Üstün et al., 2014).
The new species from Belize most closely resembles C. morii, C. jinigudira, and C. californiense, mainly by its body size (female longer than 2 mm), relatively long cephalothorax (more than 65% of body length), the relatively long ovigerous spines bifurcating at distal end of caudal rami, and the position of oral papilla, among other characters (Suárez-Morales & McKinnon, 2014; Üstün et al., 2014). Distinctive characters of 6 known species of the C. longispinosum species-group are provided by Üstün et al. (2014).
Particularly, the new species C. belizense differs from its closest congeners, C. chelemense, C. morii, C. jinigudira, and C. californiense in several characters: 1) the peculiar arrangement of the forehead ridges, which are absent in C. morii, swirl-like in C. chelemense (Suárez-Morales & Escamilla-Sánchez, 1997), and transverse in C. californiense (Suárez-Morales & Palomares-García, 1999), thus diverging from the longitudinal ridges observed in the new species. This is a character that is probably more informative than previously thought when comparing species in this group (Suárez-Morales & Palomares-García, 1999); 2) the presence of a shell-like ventral cuticular process in the preoral surface is a relevant, unique character of C. sinopense from Turkey (Üstün et al., 2014), but it shares with most other species of the group including C. belizense, the pair of large ventral nipple-like processes (Fig. 2D); details of the cephalic ornamentation were not provided for C. janetae (Mageed, 2010) or C. longispinosum from Brazil (Leite et al., 2010); 3) in C. belizense, the last antennulary segment (51.2% of antennulary length) is among the longest of the longispinosum group (ranging between 46 and 51%), thus resembling both C. chelemense (51%) and C. californiense (51%) in this respect (Table I in Üstün et al., 2014); 4) a relatively short fifth leg innermost seta, which is longer than the fifth leg segment but not reaching the posterior margin of the genital double-somite (Fig. 3A). In other species of Cymbasoma the fifth leg innermost seta is absent or is smaller than the bearing segment; the longest fifth leg innermost seta among species of the longispinosum group was observed in C. sinopense, it is almost as long as the other 2 fifth leg setae (Üstün et al., 2014; fig. 3D); 5) in the new species the point of bifurcation of the ovigerous spines is located slightly beyond or at the distal end of the caudal ramus. This is a character shared only with the Australian C. jinigudira (Suárez-Morales & McKinnon, 2014; fig. 49B) and C. morii from Japan (Grygier, 1994; fig 2b, c); 6) the inner distal rounded processes of the fourth antennulary segment observed in the new species is a character that has not been reported in other species of the group (Fig. 3B); 7) the produced, wrinkled corners of the genital somite is also advanced as a character unique to C. belizense (Fig. 3A). In addition, with a total length of 2.62 mm, the female of Cymbasoma belizense n. sp. is arguably the longest species known in the C. longispinosum species group. Its body length is comparable with that of C. sinopense from Turkey (2.5 mm) and C. morii from Japan (1.9-3.2 mm) (Grygier, 1994; Martin-Thompson, 1973; Üstün et al., 2014). It is smaller than specimens assigned to C. longispinosum by Giesbrecht (1893) and Rose (1933) from the Mediterranean and by Sars (1921) from Norway (2.3-3.16 mm). Leite et al. (2010) reported specimens from northern Brazil with a size range between 1.6 and 2.8 mm.
The evidence presented in this paper seems to be enough to recognize a new species, C. belizense, which represents the eighth nominal species assigned to the C. longispinosum species-group. It is also the second confirmed species record of the group in waters of the Caribbean Sea (Üstün et al., 2014). Overall, it is confirmed that the diversity of monstrilloid copepods is still underestimated in this region (Suárez-Morales, 2011).
The original description and most of the subsequently published records of this species lack the details that have been used recently in distinguishing the species assigned to the longispinosum group (Üstün et al., 2014). This is the second record of a species of the C. longispinosum species-group in the Caribbean Sea after the finding of C. chelemense by Dorado-Roncancio and Dorado-Roncancio (2018) off the Caribbean coast of Colombia. Monstrilloids have been reported as parasites of different polychaete families, i.e.: Spionidae, Syllidae, Serpulidae, Capitellidae. Members of the Capitellidae and Spionidae are abundantly distributed along the coastal area of Chetumal Bay (Kuk-Dzul, 2007); these polychaetes are potential hosts for local monstrilloid copepods and their populations should be examined more closely to determine their host-parasite associations.
Key to the species currently assignable to the Cymbasoma longispinosum species-group
(modified from Üstün et al., 2014).
1. Strong cuticular ridges present on forehead between antennule bases 2
–– Cuticular ridges absent, forehead surface between antennule bases smooth 4
2. Urosome cuticular striae on genital double somite only. Relative length of cephalothorax with respect to total body length more than 68% 3
–– Urosome cuticular striae on genital double, anal, and fifth pedigerous somites. Relative length of cephalothorax with respect to total body length about 65%; fifth leg innermost seta as long as segment C. californiense Suárez-Morales & Palomares-García, 1999 (Gulf of California, Mexico)
3. Forehead ridges arranged in longitudinal parallel pattern, medial ventral process between antennule bases absent; if present, shell-like. Ovigerous spines 1.2-2.0 times the length of body 7
–– Forehead ridges arranged in complex, swirl-like pattern, medial ventral process between antennule bases absent, ovigerous spines relatively short, about as long as body C. chelemense Suárez-Morales & Escamilla-Sánchez, 1997 (Gulf of Mexico, Colombia)
4. Posterior margin of genital double-somite straight in dorsal view 5
–– Posterior margin of genital double-somite clearly curved, convex in dorsal view C. morii Sekiguchi, 1982 (Japan, India)
5. Cephalothorax representing less than 66% of total body length; point of bifurcation of ovigerous spines not reaching distal end of caudal rami C. cf. longispinosum from Brazil (Leite et al., 2010) (off Curuçá River, Brazil)
–– Cephalothorax representing more than 66% (usually 68-72%) of total body length; point of bifurcation of ovigerous spines beyond distal end of caudal rami 6
6. Point of bifurcation of ovigerous spines slightly beyond distal end of caudal rami; medial ventral process between antennule bases absent; fifth leg outer/inner lobes length ratio=1.9 C. longispinosum s.str. (Bourne, 1890) (Plymouth, United Kingdom)
–– Point of bifurcation of ovigerous spines well beyond distal end of caudal rami; medial ventral process between antennule bases present, rounded; fifth leg outer/inner lobes length ratio = 3.3 C. janetae Mageed, 2010 (Red Sea, Egypt)
7. Point of bifurcation of ovigerous spines slightly beyond posterior margin of caudal rami; medial ventral cuticular process between antennule bases distinctively shell-like; fifth legs innermost seta long, about 0.9 times as long as other setae … C. sinopense Üstün, Kurt & Suárez-Morales, 2014 (Turkey, Black Sea)
–– Large shell-like medial ventral cuticular process between antennule bases absent; fifth legs with thumb-like or globose inner lobe; cephalothorax smooth; fifth leg innermost seta 0.4–0.7 times as long as other fifth leg setae; lateral ocelli pigmentation weak or strong 8
8. Fifth legs with innermost seta 0.4-0.7 times as long as other fifth leg setae, not reaching posterior margin of genital double- somite; cephalothorax with fringe of faint cuticular striae; fifth leg inner lobe of moderate size, thumb-like; lateral ocelli weakly pigmented; posterolateral corners of genital double-somite moderately produced, with field of deep wrinkles C. belizense sp. n. (Belize, Central America)
–– Fifth legs with innermost seta 0.5–0.7 times as long as other fifth leg setae, reaching beyond posterior margin of genital double-somite; fifth leg inner lobe relatively small, globular; lateral ocelli strongly pigmented on inner margin; posterolateral corners of genital double-somite not produced, smooth C. jinigudira Suárez-Morales & McKinnon, 2014 (Western Australia)
Acknowledgements
The financial support to develop this work was provided by the project “Conservation of Coastal Marine Resources in Central America (Phase II)”, administered by MAR Fund and financed by the Government of Germany through the German Development Bank (KfW). Iván Castellanos sorted the copepods from the original samples and called our attention to the monstrilloid examined herein. Rosa María Hernández ((ECOSUR-Chetumal) deposited the specimen in the collection of zooplankton of ECOSUR, Chetumal, Mexico. The first (ESM) and second (LVY) authors recognize the remarkable efforts of the Mexican government to progressively smother all aspects of science as an additional motivation to produce and conclude this work.
References
Bourne, G. C. (1890). Notes on the genus Monstrilla, Dana. Quarterly Journal of Microscopical Science, 30, 565–578.
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. London: The Ray Society.
Chang, C. Y. (2014). Two new records of monstrilloid copepods (Crustacea) from Korea. Animal Systematics, Evolution and Diversity, 30, 206–214. https:doi.org/10.5635/ASED.2014.30.3.206
Dakin, W. J., & Colefax, A. N. (1940). The plankton of the Australian coastal waters off New South Wales, Pt.1. Sydney: Publications of the University of Sydney.
Dias, C. O. (1996). Monstrilloida (Copepoda) off the Brazilian coast. Hydrobiologia, 324, 253–256. https://doi.org/10.1007/BF00016397
Dias, C. O., & Bonecker, S. L. (2007). New records of Monstrilloida Sars, 1901 (Crustacea, Copepoda) on the Brazilian northeastern coast. Biota Neotropica, 7, 1–5.http://dx.doi.org/10.1590/S1676-06032007000200030
Dorado-Roncancio, E. F., & Dorado-Roncancio, J. (2018). First record of the copepod Cymbasoma chelemense (Copepoda: Monstrilloida) in the Colombian Caribbean Sea. Boletín del Instituto de Investigaciones Marinas y Costeras de Colombia, 47, 157–163.
Duarte, A. K. (1999). Ocorrência de Monstrilloida (Copepoda) em águas costeiras do Sul do Brasil. Nauplius, 7, 201–202.
Giesbrecht, W. (1893). Systematik und Faunistik der pelagischen Copepoden desGolfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel und der angrenzenden Meeres-Abschnitte herausgegeben von der Zoologischen Station zu Neapel. XIX [1892]. Berlin: R. Friedländer & Sohn.
Grygier, M. J. (1994). Nomenclature, redescription and new record of Cymbasoma morii Sekiguchi, 1982 (Monstrilloida). Hydrobiologia, 292/293, 23–29. https://doi.org/10.1007/978-94-017-1347-4_4
Grygier, M. J. (1995). Annotated chronological bibliography of Monstrilloida (Crustacea: Copepoda). Galaxea, 12, 1–82.
Grygier, M. J., & Ohtsuka, S. (1995). SEM observation of the nauplius of Monstrilla hamatapex, new species, from Japan and an example of upgraded descriptive standards for monstrilloid copepods. Journal of Crustacean Biology, 15, 703–719. https://doi.org/10.1163/193724095X00118
Grygier, M. J., & Ohtsuka, S. (2008). A new genus of monstrilloid copepods (Crustacea) with anteriorly pointing ovigerous spines and related adaptations for subthoracic brooding. Zoological Journal of the Linnean Society. London, 152, 459–506. https://doi.org/10.1111/j.1096-3642.2007.00381.x
Gurney, R. (1927). Report on the Crustacea-Copepoda (littoral and semi-parasitic). In Cambridge Expedition to the Suez Canal, 1924. Transactions of the Zoological Society of London, 22, 451–577.
Huys, R., & Boxshall, G. A. (1991). Copepod evolution. London: The Ray Society.
Huys, R., Llewellyn-Hughes, J., Conroy-Dalton, S., Olson, P. D., Spinks, J. N., & Johnston, D. A. (2007). Extraordinary host switching in siphonostomatoid copepods and the demise of the Monstrilloida: Integrating molecular data, ontogeny and antennulary morphology. Molecular Phylogenetics and Evolution, 43, 368–378. https://doi.org/10.1016/j.ympev.2007.02.004
Isaac, M. J. (1975). Copepoda, sub-order: Monstrilloida. Fiches pour l’. Identification du Zooplancton, 144/145, 1–10.
Jeon, D., Lee, W., & Soh, H. Y. (2018). New genus and two new species of monstrilloid copepods (Copepoda: Monstrillidae): integrating morphological, molecular phylogenetic, and ecological evidence. Journal of Crustacean Biology, 38, 45–65. https://doi.org/10.1093/jcbiol/rux095
Kuk-Dzul, J. G. (2007) Poliquetos de sustrato arenoso como bioindicadores de contaminación por materia orgánica en la zona urbana de la Bahía de Chetumal, Quintana Roo (Tesis). Universidad de Quintana Roo, Chetumal, México.
Leite, N. R., Pereira, L. C., Abrunhosa, C. F., Pires, M. A. B., & Costa, R. M. D. (2010). Occurrence of Cymbasoma longispinosum Bourne, 1890 (Copepoda: Monstrilloida) in the Curuçá River estuary (Amazon Littoral). Annals of the Brazilian Academy of Science, 82, 577–583.
Mageed, A. A. A. (2010). Cymbasoma janetae n. sp., a new monstrilloid (Copepoda, Monstrilloida) from the Gulf of Aqaba (Red Sea, Egypt). Crustaceana, 83, 513–523. https://doi.org/10.1163/156854008X390245
Martin-Thompson, P. K. (1973). Occurrence of Cymbasoma longispinosum (Copepoda: Monstrilloida) from the Indian Seas. Journal of the Marine Biological Association of India, 15, 616–620.
Rose, M. (1933). Copépodes pélagiques. Faune de France, 26, 1–374.
Sars, G. O. (1921). An account of the Crustacea of Norway with short descriptions and figures of all the species. Vol. VIII. Copepoda Monstrilloida and Notodelphyoida. Bergen: The Bergen Museum.
Suárez-Morales, E. (2001). An aggregation of monstrilloid copepods in a western Caribbean reef area: ecological and conceptual implications. Crustaceana, 74, 689–696. https://doi.org/10.1163/156854001750377966
Suárez-Morales, E. (2011). Diversity of the Monstrilloida (Crustacea: Copepoda). Plos One, 6, e22915. https://doi.org/10.1371/journal.pone.0022915
Suárez-Morales, E. (2017). Monstrilloid copepods: the best of three worlds. Bulletin of the Southern California Academy of Sciences, 107, 92–103.
Suárez-Morales, E., & Castellanos-Osorio, I. (2019). A new species of Monstrilla (Copepoda, Monstrilloida) from the plankton of a bay system of the northwestern Caribbean, with a key to species. Zookeys, 876, 111–123. https://doi.org/10.3897/zookeys.876.38400
Suárez-Morales, E., & Dias, C. (2001) Taxonomic report of some monstrilloids (Copepoda: Monstrilloida) from Brazil with description of four new species. Bulletin de l’Institut Royal des Sciences Naturelles d’Belgique, Biologie, 71, 65–81.
Suárez-Morales, E., Dias, C. O., & Bonecker, S. L. (2020). Discovery of the female of Cymbasoma rochai Suárez-Morales & Dias, 2001 (Copepoda, Monstrilloida, Monstrillidae), the first Brazilian member of the C. longispinosum species-group. Crustaceana, 93 (in press).
Suárez-Morales, E., & Escamilla-Sánchez, J. B. (1997). An undescribed monstrilloid copepod (Copepoda: Monstrilloida) from the northern Yucatán Peninsula, Mexico. Bulletin of Marine Science, 61, 539–547.
Suárez-Morales, E., Harris, L. Ferrari, F. D., & Gasca, R. (2014). Late postnaupliar development of Monstrilla sp. (Copepoda: Monstrilloida), a protelean endoparasite of benthic polychaetes. Invertebrate Reproduction and Development, 58, 60–73. https://doi.org/10.1080/07924259.2013.816787
Suárez-Morales, E., & McKinnon, A. D. (2014). The Australian Monstrilloida (Crustacea: Copepoda) I. Monstrillopsis Sars, Maemonstrilla Grygier & Ohtsuka, and Australomonstrillopsis gen. nov. Zootaxa, 3779, 301–340. https://doi.org/10.11646/zootaxa.3779.3.1
Suárez-Morales, E., Paiva-Scardua, M., & Da Silva, P. M. (2010). Occurrence and histo-pathological effects of Monstrilla sp. (Copepoda: Monstrilloida) and other parasites in the brown mussel Perna perna from Brazil. Journal of the Marine Biological Association of the United Kingdom, 90, 953–958. https://doi.org/10.1017/S0025315409991391
Suárez-Morales, E., & Palomares-García, R. (1999). Cymbasoma californiense, a new monstrilloid (Crustacea: Copepoda: Monstrilloida) from Baja California, Mexico. Proceedings of the Biological Society of Washington, 112, 189–198.
Üstün, F., Terbiyik, K. T., & Suárez-Morales, E. (2014). A new species of Cymbasoma (Copepoda, Monstrilloida) from the northern coast of Turkey (Black Sea) with comments on the C. longispinosum species-group. Crustaceana, 87, 1393–1410. https://doi.org/10.1163/15685403-00003363
Wilson, C. B. (1950). Contributions to the biology of the Philippine Archipelago and adjacent regions. Copepods gathered by the United States Fisheries Steamer “Albatross” from 1887 to 1909, chiefly in the Pacific Ocean. U.S. National Museum Bulletin, 100, 141– 441.