Jaime Pelayo-Martínez a, Liliana Ortiz-Lozada a, Vinicio J. Sosa b, *, Claudio Mota-Vargas b, Jessica Durán-Antonio b
a SEEIA Consultores Ambientales S.A. de C.V., República de Argentina No. 35, 91070 Xalapa, Veracruz, Mexico
b Instituto de Ecología, A.C., Carretera Antigua a Coatepec No. 351, 91073 Xalapa, Veracruz, Mexico
*Corresponding author: vinicio.sosa@inecol.mx (V.J. Sosa)
Received: 20 January 2022; accepted: 18 April 2023
Abstract
Private Conservation Areas (CPAs) are a complementary resource to support mammal conservation in tropical regions of the world. However, their small surface area can exert a differential influence on the species present in terms of behavior and affect their coexistence. During the 2016 rainy season, we investigated the daily activity patterns of canopy mammals in a 100 ha CPA in a rainforest. For this, 11 camera traps were placed in the trees, 15 m high. Sciurus deppei showed diurnal activity, Potos flavus around midnight, and Caluromys derbianus and Coendou mexicanus before and after midnight. Tamandua mexicana and Didelphis marsupialis were active throughout the night. Nocturnal mammals showed great overlap in their hours of activity. Overall, there were no differences in canopy mammal activity patterns in this APC compared to those reported in larger protected natural areas. The greatest number of records occurred in the tallest trees located in the best-preserved parts of the study area, suggesting the importance of APCs being forested for the conservation of canopy mammals.
Keywords: Activity overlap; Arboreal mammals
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Actividad diaria de mamíferos del dosel amenazados en un área natural protegida privada del sureste tropical de México
Resumen
Las áreas de conservación privadas (APC) son un recurso complementario para favorecer la conservación de los mamíferos en las regiones tropicales del mundo. Sin embargo, su reducida superficie puede ejercer una influencia diferencial sobre las especies presentes en términos de comportamiento y afectar su coexistencia. Durante la temporada de lluvias de 2016 investigamos los patrones de actividad diaria de los mamíferos del dosel en una APC de 100 ha en una selva. Para ello, se colocaron 11 cámaras trampa en los árboles, a 15 m de altura. Sciurus deppei presentó actividad diurna, Potos flavus alrededor de la medianoche y Caluromys derbianus y Coendou mexicanus antes y después de la medianoche. Tamandua mexicana y Didelphis marsupialis estuvieron activos durante toda la noche. Los mamíferos nocturnos mostraron gran superposición en sus horas de actividad. En general, no hubo diferencias en los patrones de actividad de los mamíferos del dosel en esta APC en comparación con los reportados en áreas naturales protegidas más grandes. El mayor número de registros ocurrió en los árboles más altos ubicados en las partes mejor conservadas del área de estudio, lo que sugiere la importancia de que las APC estén forestadas para la conservación de los mamíferos del dosel.
Palabras clave: Traslape de actividad; Mamíferos arborícolas
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
In the Neotropics, the northern distribution limit of the tropical rain forest (also known as tropical evergreen forest) is found at the border between the States of Veracruz and San Luis Potosí, in Mexico (Leija-Loredo & Pavón, 2017). However, these tropical forests are disappearing at an alarming rate through conversion to subsistence agriculture, grazing pastures, and urban areas as a response to the needs of a growing human population and as a consequence of several complex socioeconomic factors as well as climate change (Altieri & Toledo, 2011; Laurance et al., 2014). From 1993 to 2002, the tropical rain forest of Mexico suffered a reduction of 24.8% in its covered area and, by 2016, its mature state covered only 0.6% of the national territory (Moreno-Sánchez et al., 2011; Santos-Hernández et al., 2021). Tropical forests present the most complex and developed vegetation canopies, hosting a prolific biodiversity that includes epiphytes (9% of the extant vascular plants), insects, birds, mammals, and microbiotas (bacterial and fungal assemblages) (Haysom et al., 2021; Nakamura et al., 2017).
Protected natural areas (PNAs), such as national parks or biosphere reserves, constitute one of the most common approaches for biodiversity conservation. However, since these areas are designed to protect large tracts of conserved habitat (Halffter, 2005), particularly in the tropics, there is an ever-decreasing amount of land available for decree as PNA. Small fragments of protected habitat, frequently under private ownership (Gallina & González-Romero, 2018; Stolton et al., 2014), may therefore complement or strengthen connectivity among large but isolated PNAs and contribute, given an appropriate spatial design, to regional maintenance of biodiversity and several ecosystem services (Holmes, 2013). However, private conservation areas (PCA) can present some disadvantages compared to larger protected natural areas, given the mostly small extension of the former. For example, in Latin America and the Caribbean region, the average size of PCA is 13.86 km2 (n = 1,448) compared to the 1,908.2 km2 of an average public conservation area (n = 8,373) (UNEP-WCMC, & IUCN, 2023). The disadvantages of small-sized PCA include a higher risk of impact by diseases, pests, or natural disasters, as well as a lower probability of hosting viable population sizes of those species that require extended home ranges (Hernández-Huerta, 1992; Volenec & Dobson, 2020). In addition, small PCA may differentially and detrimentally alter the behavior of some species (Wong & Candolin, 2015), and they often predominantly host common species while the rare species have been lost (Gaston et al., 2008).
A reduction in area available for the home ranges of mammals may alter their activity patterns. The daily activity pattern of mammals refers to the periods of the day during which each displays activity in order to fulfill its needs for feeding, mating, and socialization. The most common patterns are nocturnal (active during the night), diurnal (active during the day), crepuscular (activity at twilight), or cathemeral (randomly active throughout the day; Ashby, 1972). The normal activity pattern can be modified by stress factors, leading to different degrees of overlap of the activity patterns that depend on the limitation of resources (food, shelter, etc.) and/or the antagonistic interactions with sympatric species, among other factors. For example, a reduced overlap of activity patterns is often interpreted as a temporal niche partitioning that works to minimize the risk of competition (Frey et al., 2017). Thus, studies on the effect of small natural areas on the activity of the species they are supposed to conserve can be helpful to effectively manage both the protected area and the species. This is even more urgent in the case of evasive or cryptic species such as canopy mammals. The main objective of this case study was to use camera-traps to investigate the patterns of daily activity, and their degree of overlap, of arboreal mammals in a small PCA. We also assessed whether the overall activity of canopy mammals was related to the size of the trees.
Materials and methods
The study area is located in Ixhuatlán del Sureste, in the south of the State of Veracruz, Mexico, within the Ceratozamia Protection and Development Area, a private conservation area (PCA) owned by the business group Braskem Idesa. The area presents an average annual rainfall of 1,800 mm and an average annual temperature of 27 °C. It has an area of 100 ha, of which 50 ha correspond to induced pasture for livestock and 50 ha to tropical forest. The elevation of the area ranges from 15 to 65 m asl (Fig. 1). The forest area consists of a number of secondary vegetation species and remnants of evergreen tropical forest, with a tree stratum of up to 25 meters in height. The most representative species are Miconia argentea (Sw.) D.C., Guazuma ulmifolia Lam., Cupania dentata Moc. et Sessé ex D.C., Coccoloba barbadensis Jacq., Bursera simaruba (L.) Sarg., Enterolobium cyclocarpum (Jacq.) Griseb., Cecropia obtusifolia Bertol., Vochysia hondurensis Sprague, and Pithecellobium lanceolatum (Humb. & Bonpl. ex Willd.) Benth. In the lower tree stratum, the dominant species are Tabernaemontana alba Mill. and Dendropanax arboreus (L.) Decne. & Planch. (Ortiz-Lozada et al., 2017).
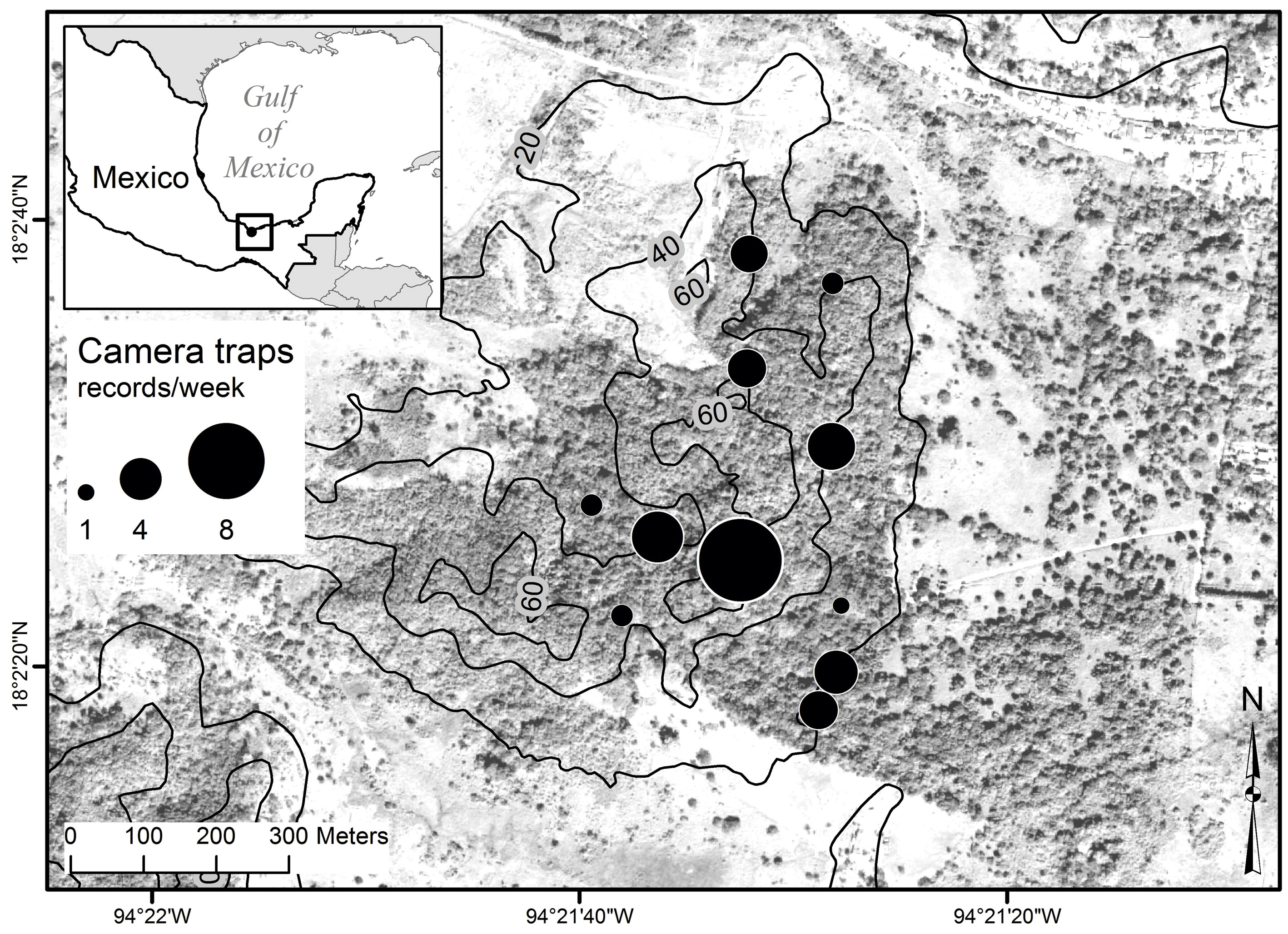
Camera traps were placed on trees with a crown in contact with the canopy of other trees and a trunk diameter at breast height (DBH) greater than 50 cm. The average distance to the nearest tree furnished with a camera was 117 m. Eleven 14-megapixel camera traps were used, each with LED light and infrared sensor (Bushnell® Trophy Cam Aggressor Brown Mod. 119774). Each camera was placed on a selected tree at an average height of 15 m and directed towards a target branch with possible crossing points. The camera traps were programmed to take 3 8-megapixel photos and a 15-second video with high-definition audio. Each camera had a 32 Gb memory card to enable storage of as much data as possible. Once programmed, the cameras were left in situ for at least 3 months during the period May-September (rainy season) in 2016. However, for some cameras their memory card got saturated fairly soon due to many windy days that caused the motion of twigs and leaves, thus activating the cameras. The average time that cameras were active was 56.5 days (range 4 -129 days). No bait was used to attract the mammal species, thus avoiding a potential artificial increase in the time spent by the animal on the tree. Mammal nomenclature followed Ramírez-Pulido et al. (2005) and Voss (2011) for Coendou only.
Table 1
Mean time of activity of 6 canopy mammals in the private “Ceratozamia Protection and Development Area” during the 2016 rainy season (May – September) and summary of results of the Rayleigh test for uniform distribution of activity. R is the test statistic, p is the probability that the null hypothesis is true.
| Mammal species | Number of records | Mean activity hour | R | p | Extinction risk** |
| Potos flavus | 90 | 00:30 | 0.412 | < 0.0001 | SP |
| Caluromys derbianus* | 31 | 00:21 | 0.726 | 0.0001 | Th |
| Coendou mexicanus* | 31 | 01:25 | 0.389 | 0.0082 | Th |
| Didelphis marsupialis? | 29 | N/A | 0.240 | 0.1886 | |
| Sciurus deppei | 25 | 08:31 | 0.607 | 0.0001 | III# |
| Tamandua mexicana? | 23 | N/A | 0.322 | 0.0914 | D |
** According to the Official Mexican Norm NOM-059 (Diario Oficial de la Federación, 2019): SP = special protection,
Th = threatened, D = in danger of extinction. * Species presented a bimodal pattern of activity; therefore, the mean activity hour should be interpreted accordingly in these cases. † Species presented a uniform distribution of activity during the night; N/A = not applicable. # CITES appendix number: trade allowed only on presentation of the appropriate permits or certificates.
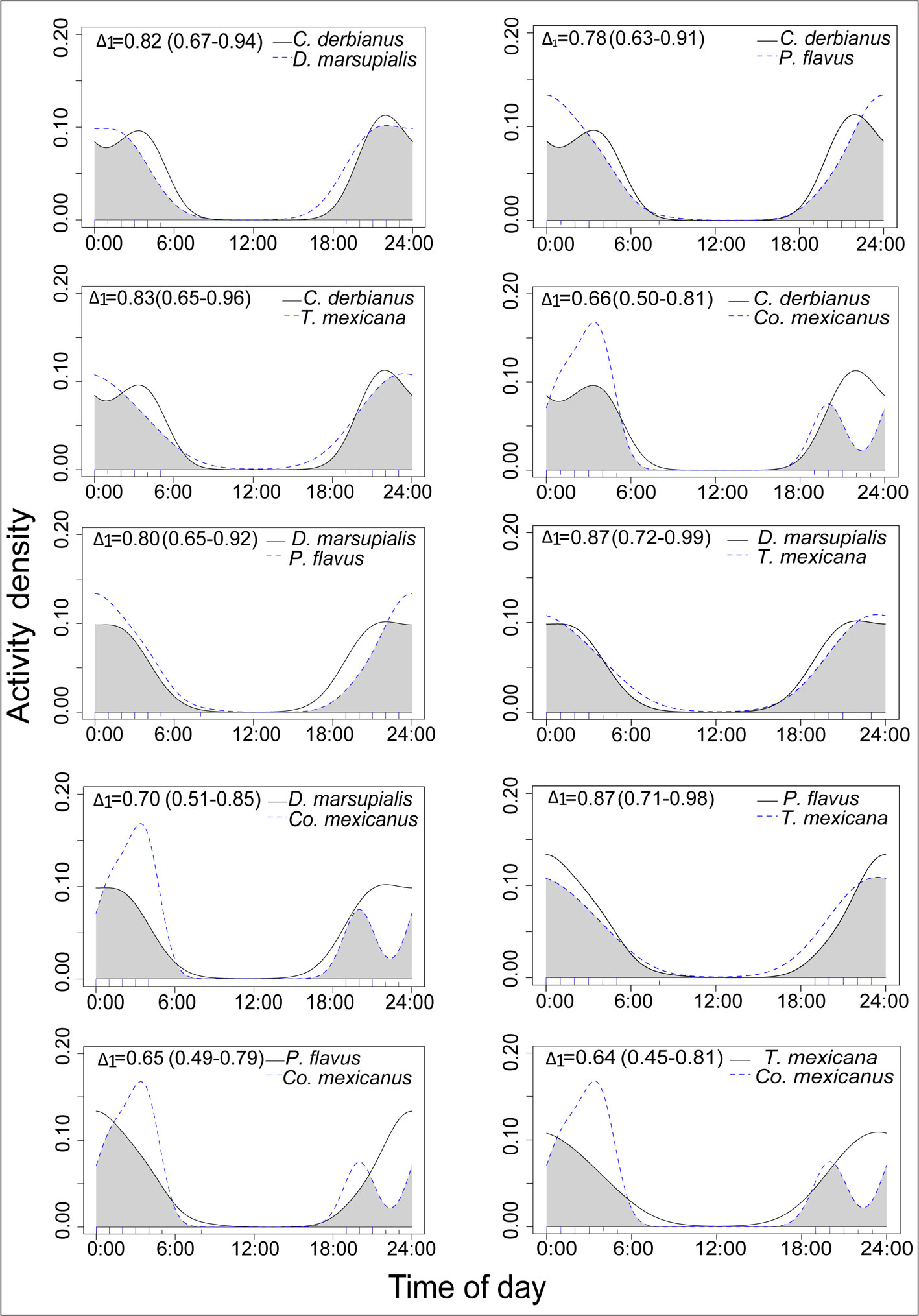
The number of records in the cameras was standardized based on the actual period each was active. To describe the daily pattern of activity, the standardized frequency of records was expressed as the number of records per hour per week, rounded to the nearest integer. To test the null hypothesis of uniform activity throughout the day or night, Rayleigh tests were run for the species, using independent records only (those separated by at least 1 hr). To determine whether there was any overlap in the activity patterns of the arboreal mammals, we used a descriptive measure of the degree of similarity between 2 Kernel density curves, estimated by the overlap coefficient ∆ (Ridout & Linkie, 2009). This measure is defined as the area in common under the 2 probability densities for the daily activity of 2 species (Schmid & Schmidt, 2006): its values range from 0, with no overlap between the activity of the 2 species, to 1, with complete activity overlap (Frey et al., 2017; Mugerwa et al., 2017). We estimated the overlap coefficient between pairs of species(which is appropriate for sample sizes smaller than 50) through the Overlap library from R (Meredith & Ridout, 2014) and its 95% confidence intervals, using bootstrap resampling with 1,000 iterations (Ridout & Linkie, 2009). During the study period, local sunrise occurred at around 07:00 and local sunset around 19:30 h. The relationship between activity (irrespective of mammal species) and tree size was investigated through a regression analysis of the values of tree diameter at breast height (DBH) —as a proxy for tree size— and the log (number of activity records). All statistical analyses were performed in R v. 3.4.3 language (R Core, 2017).
Results
With a total sampling effort of 990 camera-days, we obtained 68,287 records (51,659 photographs and 16,628 videos), of which 1,447 were considered valid. Of these records, only 233 (16%) provided sufficient elements with which to identify 9 mammal species, the most common of which are listed in Table 1. The other 3 species, the raccoon (Procyon lotor) and 2 rare marsupials, the Mexican mouse opossum (Marmosa mexicana) and the gray 4-eyed opossum (Philander opossum), had less than 3 records. Four of the recorded species are included under some category of extinction risk. In addition to mammals, we also recorded 26 species of birds (9 with video or photo records, 24 with acoustic records), and 2 species of reptiles (Iguana iguana and Anolis sp.).
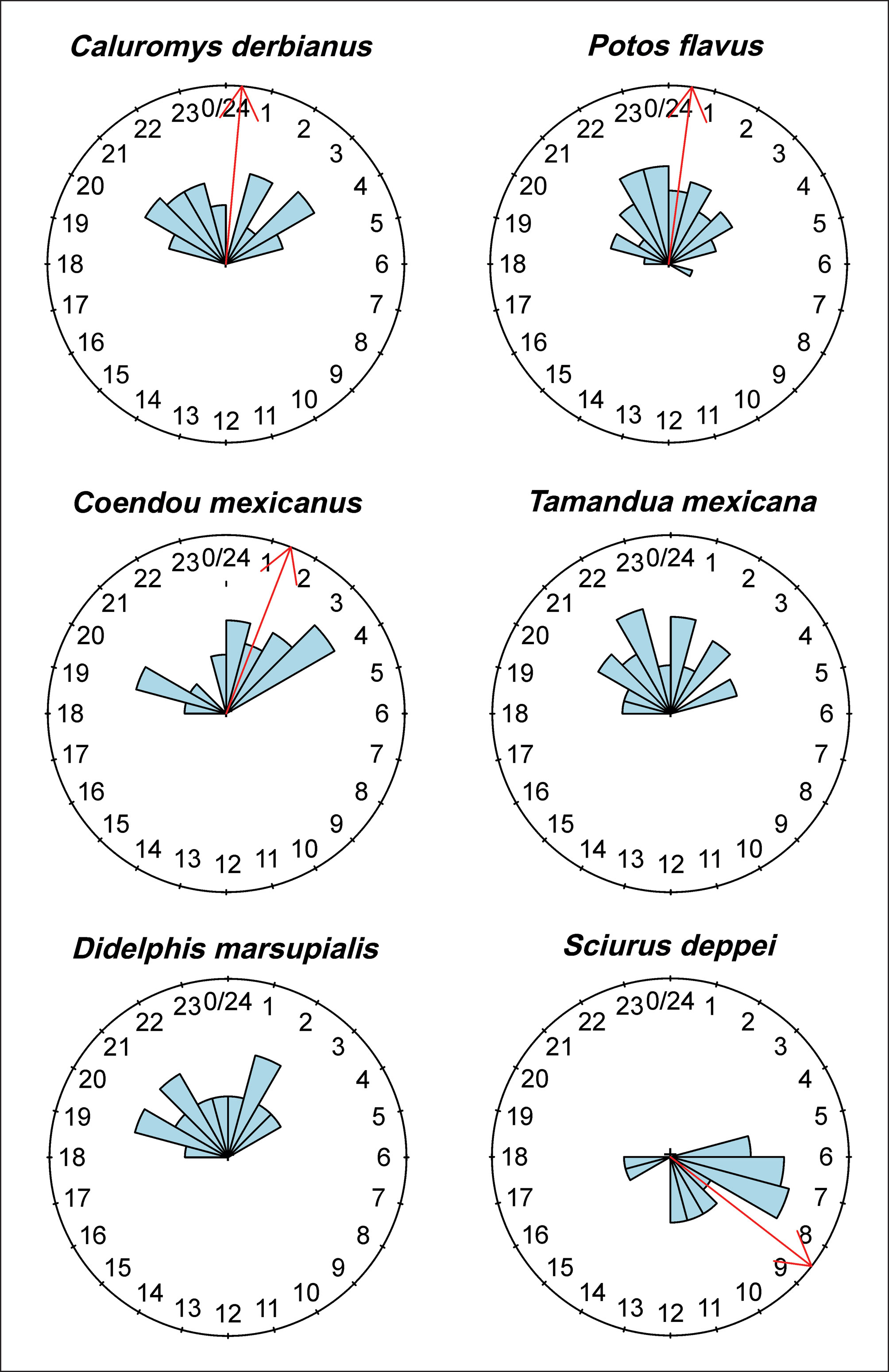
The activity patterns of the mammal species with sufficient records are presented in Figure 2. Except for Sciurus deppei (Deppe’s squirrel), these species are all nocturnal. The species that showed concentrated activity at certain times of the night were Potos flavus (kinkajou), at around 00:00 hours; Caluromys derbianus (Derby’s woolly opossum), with most activity presented before and after midnight (21:00 to 23:00 h and 1:30 – 3:30 h); and Coendou mexicanus (Mexican tree porcupine), with greatest activity recorded after midnight (01:00 to 04:00 h). The species that presented uniformly spread activity throughout the night were Tamandua mexicana (northern tamandua) and Didelphis marsupialis (common opossum; Table 1). The Deppe’s squirrel was mainly active in the early hours of the morning. In general, for the 5 nocturnal species, there was broad overlap of activity hours, with overlap coefficients ranging from 0.64 to 0.80 (Fig. 3). The number of records was positively associated with the tree DBH value (Fig. 4).
Discussion
This study presents a case in a small private conservation area (PCA) in which the patterns of activity of medium-sized canopy mammals are generally similar to those found in 2 much larger protected areas of comparable climate and vegetation: in a recent study of canopy mammals conducted in a semideciduous tropical forest of the 1,000 ha PCA Santa Gertrudis Ecological Reserve in central Veracruz, Mexico, Astiazarán-Azcárraga et al. (2020) report that the squirrel S. aureogaster was the only diurnal mammal to present an activity peak in the morning, while the kinkajou was mainly active around midnight and the common opossum (Didelphis marsupialis) presented continuous activity throughout the night. In contrast to those findings, in our study site, the northern tamandua (Tamandua mexicana) presented activity throughout the night rather than just around midnight. This difference could be explained by the reported ability of this species to adapt to different or unfavorable situations (Montgomery, 1985). In La Encrucijada Biosphere Reserve, located in Chiapas, Mexico, a 144,868 ha PNA dominated by mangrove and tropical evergreen forests, Hernández-Hernández et al. (2018) report 23:17 as the mean activity hour for D. marsupialis and 00:24 for Philander opossum, with an activity peak between 20:00 – 22:00 h. Similarly, in our studied PCA, the common opossum showed a mean activity hour near midnight, but the only 2 records of P. opossum occurred at 01:00 and 05:00. The diurnal howler monkey did not appear in our video or audio recordings, despite the fact that it is present in the studied reserve (Ortiz-Lozada et al., 2017). This is probably due to its preference for branches higher in the trees than those used to place the cameras.
The kinkajou was the mammal with most activity records concentrated around midnight. Based on characteristic markings, we estimated that at least 3 different individuals of this species were recorded throughout the study. The activity records of the Derby’s woolly opossum and the Mexican tree porcupine suggest a bimodal pattern, with marked activity before and after midnight, the time of greatest activity of the kinkajou. Further studies with the capacity to collect sufficient data throughout the year would clarify whether these species are actively avoiding encounters with this procyonid. Only 2 species, the common opossum and the northern tamandua, presented activity that was evenly distributed throughout the night. The observed activity pattern (early in the morning and before dark) of the squirrel Sciurus deppei confirms the observations reported previously (Estrada & Coates-Estrada, 1985; Goodwin, 1954). However, to the best of our knowledge, ours is the first detailed record of activity for this elusive arboreal rodent restricted to Central America.
We found a wide overlap of activity among the nocturnal canopy mammals that coincides with previous studies (Astiazarán Azcarraga et al., 2020; Mella-Mendez et al., 2019); however, because our results summarize the activity pattern of the entire protected area, we cannot dismiss the possibility that some species avoid interaction with others on particular nights, simply by foraging on different trees or feeding from different resources inside or outside the protected area (Oliveira-Santos et al., 2008). Furthermore, because of the small area of the PCA, there is a possible ongoing species spatial aggregation effect, and some of the video recordings could feature the same individual, notwithstanding the one-hour separation rule for considering independent records. At any rate, the confirmation of the presence of these threatened mammals in a relatively small PCA still supports the utility of private areas for biodiversity conservation.
We obtained the largest number of activity records from cameras placed on the trees with greater DBH, which were usually the tallest individuals and were located at the most elevated and undisturbed points within the protected area. This strongly suggests that, at least for small PCA, large trees are essential elements in the landscape for canopy mammals to find roosting sites, concealment, and/or food within them.

Despite the relatively small size of the forested area (less than 50 ha) of the PCA, we recorded the activity of canopy species, which are elusive mammals of importance given their current status as threatened or in danger of local extinction, as well as the ecosystem services they provide. Some of these species are important pollinators (Marmosa mexicana) or seed dispersers (Potos flavus, Coendou mexicanus, and Caluromys derbianus), that contribute to forest regeneration and functioning (Charles-Dominique et al., 1981). More studies with a greater sampling effort (higher number of camera-traps and a more extended sampling period), focused on specific behavior patterns (e.g. foraging and searching for a mate) of the canopy mammals in small protected natural areas will determine the extent to which the coexistence of these threatened species is assured.
Acknowledgements
To Braskem Idesa, especially to Stefan Lana Lepeki (VPE Latin America), Antonio Santos Souza Galvao (Sustainability), Ana Luisa Martínez López and Ana Paulina Demeneghi Calatayud (Environment). Special thanks go to José Alberto Toto and Sergio Trinidad VanDyck Alemán, for their support with the fieldwork, and to Teresa Hernández (Expertop México). Rosario Landgrave produced Figure 1. Graciela Sánchez helped with the reference formatting and bibliographic consultation. Keith MacMillan revised the English.
References
Altieri, M. A., & Toledo, V. M. (2011). The agroecological revolution in Latin America: rescuing nature, ensuring food sovereignty and empowering peasants. Journal of Peasant Studies, 38, 587–612. https://doi.org/10.1080/03066150.2011.582947
Astiazarán-Azcárraga, A., Gallina-Tessaro, S., & Delfin-Alfonso, C. A. (2020). Activity patterns of arboreal mammals in a tropical rain forest in México. Therya, 11, 225–231. https://doi.org/10.12933/therya-20-779
Charles-Dominique, P., Atramentowicz, M., Charles-Dominique, M., Gerard, H., Hladik, A., Hladik, C. M., & Prévost, M.-F. (1981). Les mammifères frugivores arboricoles nocturnes d’une forêt guyanaise: inter-relations plantes-animaux. Revue d’Écologie (La Terre et la Vie), 35, 341–436. https://hal.science/hal-03533285
Diario Oficial de la Federación (2019). Modificación del Anexo Normativo III, Lista de especies en riesgo de la Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental-Especies nativas de México de flora y fauna silvestres-Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo, publicada el 30 de diciembre de 2010.
Estrada, A., & Coates-Estrada R. (1985). A preliminary study of resource overlap between howling monkeys (Alouatta palliata) and other arboreal mammals in the tropical rain forest of Los Tuxtlas, Mexico. American Journal of Primatology, 9, 27–37. https://doi.org/10.1002/ajp.1350090104
Frey, S., Fisher, J. T., Burton, A. C., & Volpe, J. P. (2017). Investigating animal activity patterns and temporal niche partitioning using camera-trap data: challenges and opportunities. Remote Sensing in Ecology and Conservation, 3, 123–132. https://doi.org/10.1002/rse2.60
Gallina, S., & González-Romero, A. (2018). The conservation of medium-sized mammals in two private ecological reserves of Veracruz, Mexico. Revista Mexicana de Biodiversidad, 89, 1245–1254. https://doi.org/10.22201/ib.20078706e.2018.4.2476
Gaston, K. J., Jackson, S. E., Cantú-Salazar, L., & Cruz-Piñon, G. (2008). The ecological performance of protected areas. Annual Review of Ecology, Evolution, and Systematics, 39, 93–113. https://doi.org/10.1146/annurev.ecolsys.39.110707.173529
Goodwin, G. G. (1954). Mammals from Mexico collected by Marian Martin for the American Museum of Natural History. American Museum Novitates, 1689, 1–16. http://hdl.handle.net/2246/4908
Halffter, G. (2005). Towards a culture of biodiversity conservation. Acta Zoológica Mexicana (N.S.), 21, 133–153. https://doi.org/10.21829/azm.2005.2121991
Haysom, J. K., Deere, N. J., Wearn, O. R., Mahyudin, A., Jami, J., Reynolds, G. et al. (2021). Life in the canopy: using camera-traps to inventory arboreal rainforest mammals in Borneo. Frontiers in Forests and Global Change, 4, 673071. https://doi.org/10.3389/ffgc.2021.673071
Hernández-Huerta, A. (1992). Los carnívoros y sus perspectivas de conservación en las áreas protegidas de México.
Acta Zoológica Mexicana (N.S.), 54, 1–23. https://doi.org/10.21829/azm.1992.49541681
Hernández-Hernández J. C., Chávez, C., & List, R. (2018). Diversidad y patrones de actividad de mamíferos medianos y grandes en la Reserva de la Biosfera La Encrucijada, Chiapas, México. Revista de Biología Tropical, 66, 634–646. https://doi.org/10.15517/rbt.v66i2.33395
Holmes, G. (2013). What role do private protected areas have in conserving global biodiversity? Sustainability Research Institute (SRI) working papers.
Laurance, W. F., Sayer, J., & Cassman, K. G. (2014). Agricultural expansion and its impacts on tropical nature. Trends in Ecology & Evolution, 29, 107–116. https://doi.org/10.1016/j.tree.2013.12.001
Leija-Loredo, E. G., & Pavón, N. P. (2017). The northernmost tropical rain forest of the Americas: Endangered by agriculture expansion. Tropical Ecology, 58, 641–652.
Mella-Méndez, I., Flores-Peredo, R., Bolívar-Cimé, B., & Vázquez-Domínguez, G. (2019). Effect of free-ranging dogs and cats on medium-sized wild mammal assemblages in urban protected areas of a Mexican city. Wildlife Research, 46, 669–678. https://doi.org/10.1071/WR19074
Meredith, M., & Ridout, M. (2014). Overview of the overlap package. R Project. https://CRAN.R-project.org/package=overlap
Montgomery, G. (1985). Movements, foraging and food habits of the four extant species of neotropical vermilinguas(Mammalia; Myrmecophagidae). In M. GG (Ed.), The evolution and ecology of armadillos, sloths, and vermilinguas. (pp. 365–377). Washington D.C.: Smithsonian Institution Press.
Mugerwa, B., du Preez, B., Tallents, L. A., Loveridge, A. J., & Macdonald, D. W. (2017). Increased foraging success or competitor avoidance? Diel activity of sympatric large carnivores. Journal of Mammalogy, 98, 1443–1452. https://doi.org/10.1093/jmammal/gyx090
Nakamura, A., Kitching, R. L., Cao, M., Creedy T. J., Fayle, T. M., Freiberg, M. et al. (2017). Forests and their canopies: achievements and horizons in canopy science. Trends in Ecology & Evolution, 32, 438–451. https://doi.org/10.1016/j.tree.2017.02.020
Oliveira-Santos, L. G. R., Tortato, M. A., & Graipel, M. E. (2008). Activity pattern of Atlantic Forest small arboreal mammals as revealed by camera traps. Journal of Tropical Ecology, 24, 563–567. https://doi.org/10.1017/S0266467408005324
Ortiz-Lozada, L., Pelayo-Martinez, J., Mota-Vargas, C., Demeneghi-Calatayud, A. P., & Sosa, V. J. (2017). Absence of large and presence of medium-sized mammal species of conservation concern in a privately protected area of rain forest in southeastern Mexico. Tropical Conservation Science, 10, 1–13. https://doi.org/10.1177/1940082917738093
R Core Team. (2022). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/
Ramírez-Pulido, J., Arroyo-Cabrales, J., & Castro-Campillo, A. (2005). Estado actual y relación nomenclatural de los mamíferos terrestres de México. Acta Zoológica Mexicana (n.s.), 21, 21–82. https://doi.org/10.21829/azm.2005.2112008
Ridout, M. S., & Linkie, M. (2009). Estimating overlap of daily activity patterns from Camera trap data. Journal of Agricultural Biological and Environmental Statistics,
14, 322–337. https://link.springer.com/article/10.1198/jabes.2009.08038
Santos-Hernández, A. F., Monterroso-Rivas, A. I., Granados-Sánchez, D., Villanueva-Morales, A., & Santacruz-Carrillo, M. (2021). Projections for Mexico’s tropical rainforests considering ecological niche and climate change. Forests, 12, 119 https://doi.org/10.3390/f12020119
Schmid, F., & Schmidt, A. (2006). Nonparametric estimation of the coefficient of overlapping – theory and empirical application. Computational Statistics & Data Analysis, 50, 1583–1596. https://doi.org/10.1016/j.csda.2005.01.014
Stolton, S., Redford, K. H., & Dudley, N. (2014). The futures of privately protected areas Retrieved from https://portals.iucn.org/library/sites/library/files/documents/PATRS-001.pdf
UNEP-WCMC, & IUCN (2023), Protected planet: the World Database on Protected Areas (WDPA) and World Database on Other Effective Area-based Conservation Measures (WD-OECM) [Online], February 2023, Cambridge, UK: UNEP-WCMC and IUCN. Available at: www.protectedplanet.net
Volenec, Z. M., & Dobson, A. P. (2020). Conservation value of small reserves. Conservation Biology, 34, 66–79. https://doi.org/10.1111/cobi.13308
Voss, R. S. (2011). Revisionary notes on neotropical porcupines (Rodentia: Erethizontidae) 3. An annotated checklist of the species of Coendou Lacépède, 1799. American Museum Novitates, 3720, 1–36. http://hdl.handle.net/2246/6121
Wong, B. B. M., & Candolin, U. (2015). Behavioral responses to changing environments. Behavioral Ecology, 26, 665–673. https://doi.org/10.1093/beheco/aru183