Victoria Canova *, María del Rosario Robles, Agustín M. Abba, Graciela Minardi, Guillermo Panisse, Graciela T. Navone
Centro de Estudios Parasitológicos y de Vectores, CONICET-UNLP, Bv 120 e/ 60 y 64, 1900 La Plata, Buenos Aires, Argentina
*Corresponding author: victoriac@cepave.edu.ar (V. Canova)
Received: 14 January 2023; accepted: 27 November 2023
Abstract
Lagostomus maximus is a native rodent of South America that presents economic and biological importance. However, few studies on parasites of this rodent are available throughout its geographic distribution. The aim of this study was to explore, describe and compare the structure of helminth communities from 2 populations of L. maximus in semi-captive (SCHP) and wild (WHP) conditions in Buenos Aires Province, Argentina. The structure of helminth communities was studied considering ecological data at different levels. Seven helminth taxa were collected. Graphidioides spp. and Lagostonema ecasiense were the most prevalent species in SCHP, and Viannella viscaciae in WHP. This last species showed the highest values of mean intensity and mean abundance in both host populations. Helminths from SCHP showed higher values of abundance, mean intensity, mean abundance, diversity, and evenness, and lower values of specific richness and dominance than WHP. Specific richness, evenness and dominance allowed separating the 2 host populations. The abundances of V. viscaciae and Graphidioides spp. distinguished both host populations, suggesting possible influences of human intervention and/or environmental characteristics, as consequence of semi-captive and wild conditions.
Keywords: Argentina; Nematoda; Cestoda; Parasites; Rodents
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Descripción de la estructura de las comunidades de helmintos de dos poblaciones de vizcacha de llanura (Rodentia: Chinchillidae) en condiciones de semicautividad y silvestría
Resumen
Lagostomus maximus es un roedor autóctono de América del Sur que presenta importancia económica y biológica. Sin embargo, se dispone de pocos estudios sobre sus parásitos en toda su distribución geográfica. El objetivo de este estudio fue explorar, describir y comparar la estructura de las comunidades de helmintos de 2 poblaciones de L. maximus en la Provincia de Buenos Aires, Argentina, en condiciones de semicautividad (SCHP) y silvestría (WHP). La estructura de las comunidades de helmintos se estudió considerando datos ecológicos a diferentes niveles. Se colectaron 7 taxones de helmintos. Graphidioides spp. y Lagostonema ecasiense fueron las especies más prevalentes en SCHP y Viannella viscaciae en WHP. Esta última especie mostró los valores más altos de intensidad media y abundancia media en ambas poblaciones hospedadoras. Los helmintos de SCHP mostraron valores más altos de abundancia, intensidad media, abundancia media, diversidad y equitatividad, y valores más bajos de riqueza específica y dominancia que WHP. La riqueza específica, la equitatividad y la dominancia permitieron separar a las 2 poblaciones hospedadoras. Las abundancias de V. viscaciae y Graphidioides spp. distinguieron a ambas poblaciones hospedadoras, sugiriendo posibles influencias de la intervención humana y/o características ambientales, como consecuencia de las condiciones de semicautividad y silvestría.
Palabras clave: Argentina; Nematoda; Cestoda; Parásitos; Roedores
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
Parasite community structure and species composition can be influenced and modified by variations in biotic and abiotic factors such as human activities, translocation of host species, toxic pollution, and numerous variables related to ecological aspects (e.g., type of habitat, ecology, environmental conditions) (Ibrahim, 2012; Muñoz & Castro, 2012; Sapp & Esch, 1994; Thieltges et al., 2008). In addition, some parasites that are rarely pathogenic may become important population regulators when hosts are stressed. It is widely accepted that prolonged stress decreases immune function, leaving individuals more susceptible to infection (Sapolsky et al., 2000; Webster et al., 2002).
The Plains Viscacha, Lagostomus maximus (Desmarest, 1817) (Rodentia: Chinchillidae), is a medium size herbivorous rodent native to South America (Cirignoli & Lartigau, 2019; Spotorno & Patton, 2015). In Argentina, it is distributed in different ecoregions that include protected natural areas, agroecosystems and Wildlife Parks with different levels of human intervention (Cirignoli & Lartigau, 2019; Jackson et al., 1996; Sutton & Durette-Desset, 1987, 1995).
Some studies have been conducted on particular species of helminths found in Plains Viscacha in semi-captive (Sutton & Durette-Desset, 1987, 1995) or wild (Canova et al., 2021; Ferreyra et al., 2007; Foster et al., 2002; Martínez, 1988; Railliet & Henry, 1909; Rossanigo et al., 1986; Schuurmans-Stekhoven, 1951) host populations. However, few studies have been conducted on helminth communities and none on host populations under different environmental conditions simultaneously (Foster et al., 2002; Martínez, 1988; Rossanigo et al., 1986).
The aim of this study was to explore, describe and compare the helminth communities’ structure in semi-captive and wild populations of L. maximus from Buenos Aires Province, Argentina, considering ecological data (specific richness, abundance, prevalence, intensity, diversity, evenness, and dominance), in order to suggest possible influences of human intervention and/or environmental characteristics.
Materials and methods
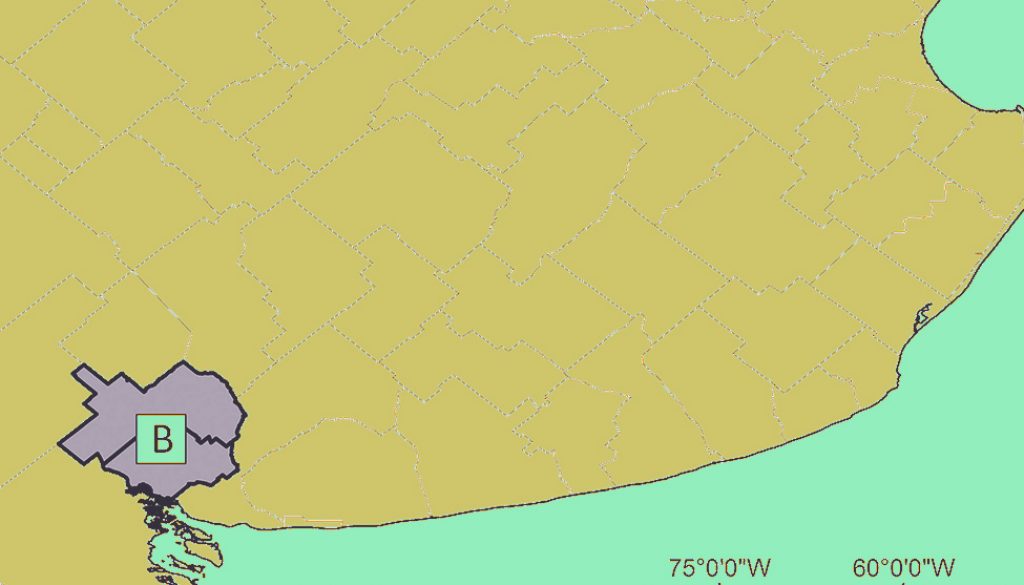
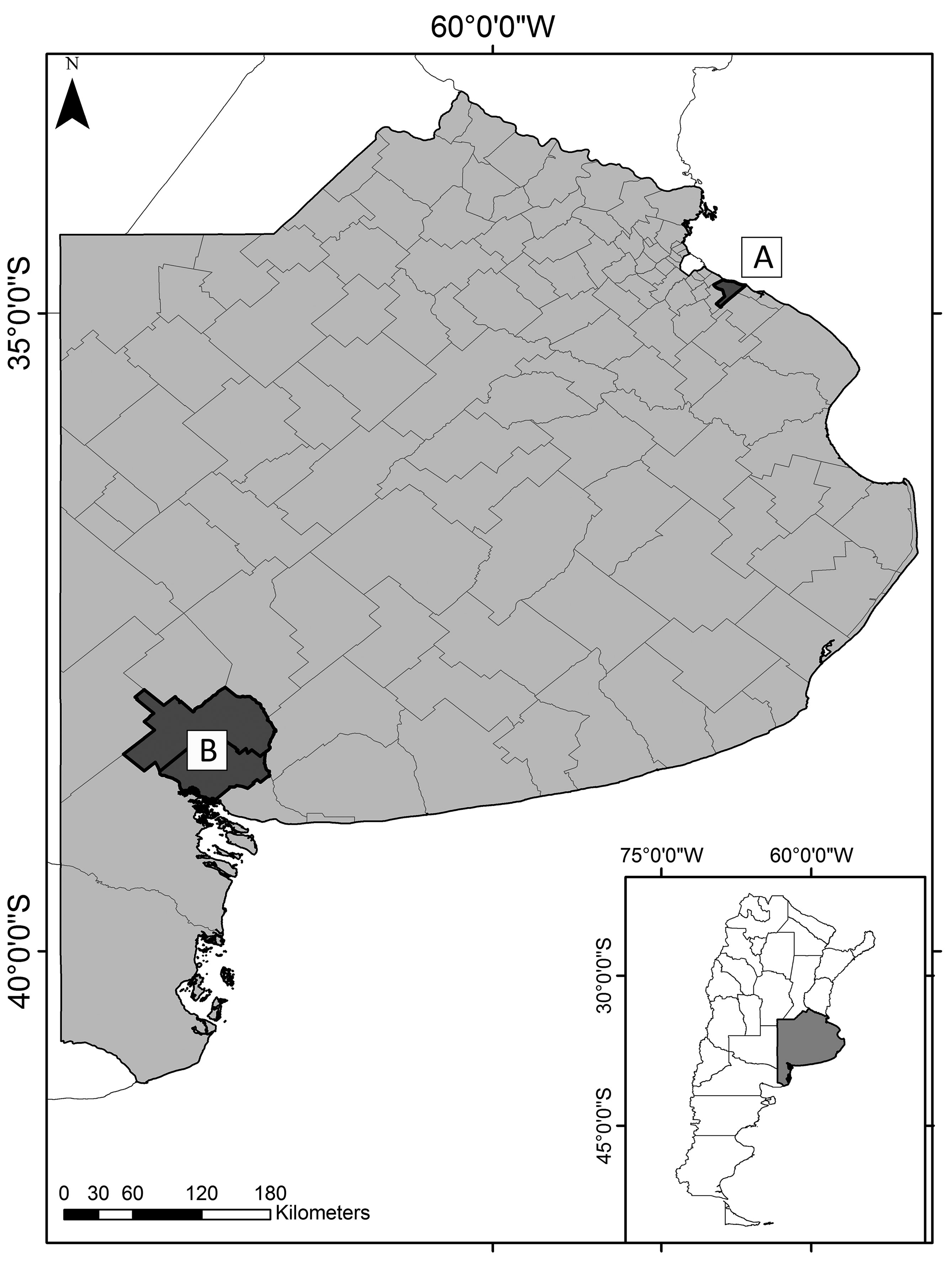
A total of 24 L. maximus specimens were collected from 2 regions of Buenos Aires Province, Argentina: 12 specimens in semi-captivity condition (SCHP: semi-captivity host population) corresponding to a northeastern region (Estación de Cría de Animales Silvestres, ECAS, Partido de Berazategui) between October 2017 and April 2018, and 12 specimens in wild condition (WHP: wild host population) corresponding to a southwest region (Estancia “La Bombilla”, Partido de Tornquist and Bahía Blanca city, Partido de Bahía Blanca) between August and November 2019.
ECAS (34°50’47.88” S, 58°7’16.48” W) has a 230 ha Wildlife Park that depends on Ministerio de Desarrollo Agrario of Buenos Aires Province, which promotes the breeding and protection of specimens in large areas fenced with wire. The Plains Viscacha specimens feed on natural food and forage that is provided to other animals on the farm, both native (e.g., Guanaco, Burrowing Owl, Black and white Tegu) and exotic (e.g., Axis Deer, Fallow Deer). Most of these species are not common in the natural habitats of Plains Viscacha, and their origin as well as the period in ECAS is unknown (www.gba.gob.ar/desarrollo_agrario/ecas). The southwest region includes Partido de Tornquist (38°06’00” S, 62°13’00” W) and Partido de Bahía Blanca (38°42’00” S, 62°16’00” W). The Plains Viscacha that inhabit this area are in a typical agroecosystem with livestock activity (Fig. 1). Currently, the distribution of most populations of Plains Viscacha coincides with peridomestic areas and agricultural-livestock activities, being the sampled environment a characteristic one of these rodents (Jackson et al., 1996).
The survey was conducted in compliance with Argentine laws. Sample collection was carried out during the fieldwork under official permits granted by the Dirección de Flora y Fauna, Buenos Aires Province and in accordance with the recommendations of the Guidelines for the capture, handling and care of mammals as approved by the American Society of Mammalogists (Sikes & The Animal Care and Use Committee of the American Society of Mammalogists, 2016). No endangered species were involved in this study.
The Plains Viscacha’s abdominal cavity, stomach, and small and large intestines were separated and preserved in 96% ethanol or 10% formalin and examined for parasites under a stereo-microscope (Olympus SZ61-TR). Helminths were collected and preserved in 70% ethanol. Nematodes were cleared with lactophenol, studied under light microscopy (Olympus BX51), and identified following specific literature. Cestodes were examined only under a stereo-microscope and most of them were immature. Representative specimens of the parasite species will be deposited in the Helminthological Collection of Museo de La Plata (MLP-He), La Plata, Buenos Aires.
The structure of helminth communities was studied at different levels following Bush et al. (1997), Esch et al. (2002) and Poulin (2004). The prevalence (P), mean intensity (MI) and mean abundance (MA) of each parasite species (component population level) were calculated for each region following Bush et al. (1997). Prevalence indices were compared with Fisher’s test (Reiczigel et al., 2019). Mean intensities and mean abundances were compared using bootstrap confidence intervals (BCα, p < 0.01) (Reiczigel et al., 2019). Moreover, the relative dominance based on the Berger-Parker dominance index (number of specimens of 1 species relative to the total number of specimens of all species) was calculated on species shared by both host populations (Magurran & McGill, 2011; Moreno, 2001).
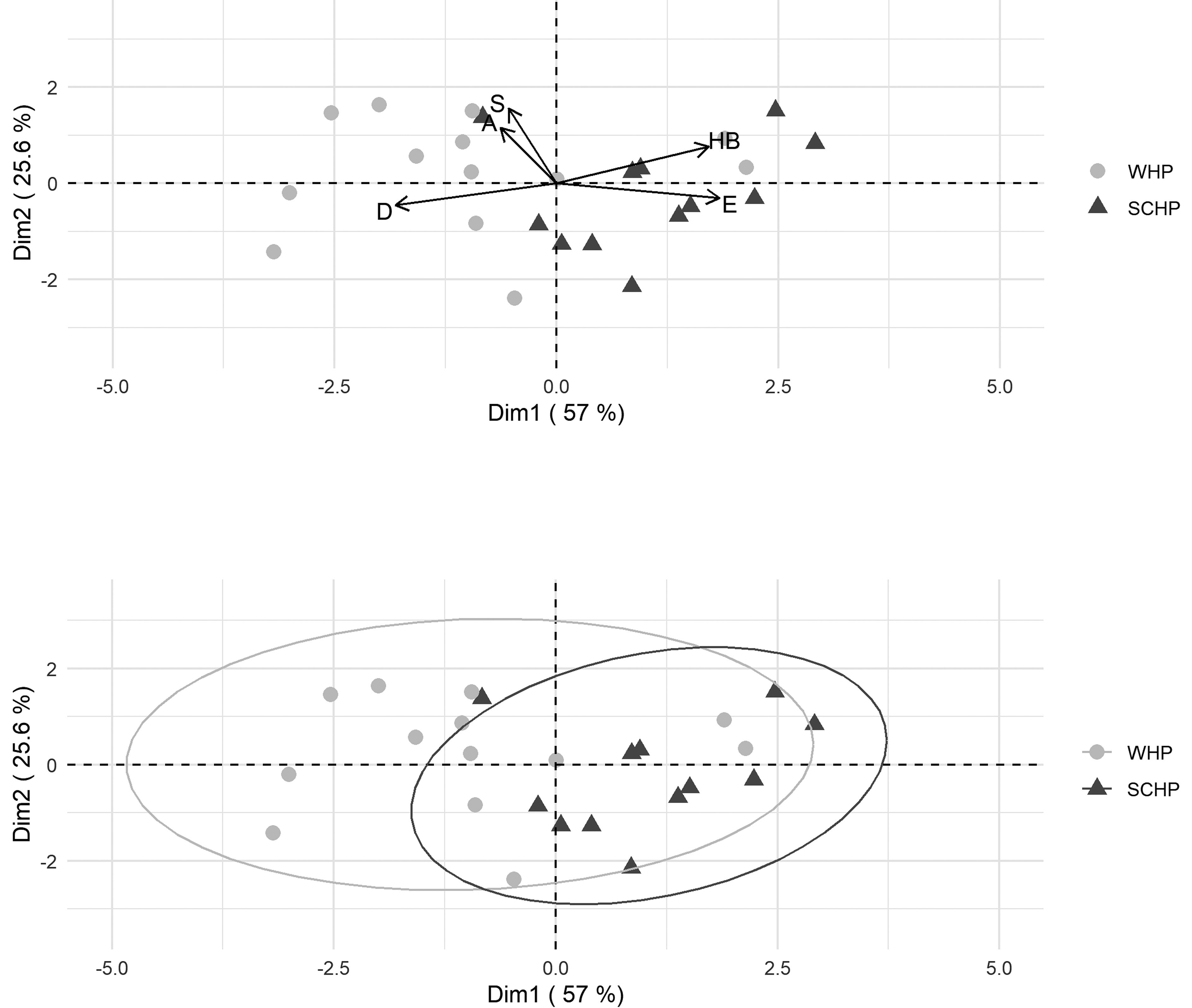
The specific richness (S), abundance (A), Brillouin diversity index (HB), evenness index (E) and simple dominance index of Berger-Parker (D) were calculated at the infracommunity level (within an individual host) of each region (Magurran & McGill, 2011; Moreno, 2001). The values are expressed as mean, standard deviation, and range between parentheses. These variables were compared with bootstrap confidence intervals in the same way that MI and MA were compared at a component population level. Also, a principal component analysis (PCA) was carried out to evaluate the contribution of each infracommunity variable and the abundance of each parasitic species.
The specific richness (S), abundance (A), prevalence (P), mean intensity (MI), mean abundance (MA), Shannon-Wiener diversity index (H’), Pielou evenness index (J’), and simple dominance index of Berger-Parker (D) were calculated to each component community (the host population) (Magurran & McGill, 2011; Moreno, 2001). Shannon-Wiener diversity index between the 2 regions was compared with the Hutcheson test (Gardener, 2017; Hutcheson, 1970). The SIMPER method was used to determine which helminth species contributed to the dissimilarity between the host populations belonging to each region. This method is based on Bray-Curtis dissimilarities, and the results are shown as the percentage of dissimilarity and the percentage contribution of each parasite species to the dissimilarities (Clarke et al., 2014). All analyses were performed with R 4.1.0 software (R Core Team, 2021).
Results
A total of 13,735 helminth specimens were collected, which belonged to 7 taxa: 6 nematodes and 1 cestode. Among these, 4 taxa from SCHP and 6 taxa from WHP were recorded. In detail, the Trichostrongylina taxa (Gibbons, 2010) were found in both host populations: Graphidioides spp., Viannella viscaciae and Lagostonema ecasiense; Heteroxynema viscaciae, Anoplocephalidae (Cestoda), and Trichuris sp. were found only in WHP and Strongyloides sp. only in SCHP (Table 1). Some taxa could not be identified at the species level due to the low number of specimens found (such as Cestoda: Anoplocephalidae) because only females were found (Trichuris sp.) or due to the difficulty of differentiating the 2 possible species under the stereo-microscope (such as Graphidioides spp.).
Table 1
Component population descriptors for helminths of Lagostomus maximus from 2 populations in semi-captive and wild conditions.
| Parasite taxa | Location | P (%) | MI | MA | Relative dominance | |||||||
| SCHP | WHP | Sig | SCHP | WHP | Sig | SCHP | WHP | Sig | SCHP | WHP | ||
| Graphidioides spp. | st | 100 | 75 | ns | 143 | 27.9 | ** | 143 | 20.9 | ** | 0.30 | 0.04 |
| L. ecasiense
Sutton and Durette-Desset, 1987 |
si | 100 | 92 | ns | 54.4 | 54.9 | ns | 54.4 | 50.3 | ns | 0.11 | 0.09 |
| V. viscaciae
Goodey, 1925 |
si | 92 | 100 | ns | 312.5 | 477.7 | ns | 286.5 | 477.7 | ns | 0.59 | 0.87 |
| Strongyloides sp. | st | 33 | – | 270.2 | – | 90.1 | – | |||||
| H. viscaciae
Hugot and Sutton, 1989 |
ca and ac | – | 83 | – | 24 | – | 20 | |||||
| Trichuris sp. | ca | – | 58 | – | 1.4 | – | 0.8 | |||||
| Cestode
Anoplocephalidae |
si | – | 58 | – | 1.4 | – | 0.8 |
P: Prevalence of each parasite taxa as percentage, MI: mean intensity, MA: mean abundance. st: Stomach, si: small intestine, ca: caecum, ac: ascending colon. SCHP: Semi-captivity host population (N = 12), WHP: wild host population (N = 12). Sig: Statistical significance ** p < 0.01, ns: no significative.
The parasites that showed the highest values of prevalence were the nematodes Graphidioides spp. and L. ecasiense in SCHP, and V. viscaciae in WHP. In both populations, V. viscaciae reached the highest values of MI and MA. The MI and MA of Graphidioides spp. showed significant statistical differences between both host populations (p < 0.01). Among the 3 taxa found in both regions, V. viscaciae was more dominant (see relative dominance), and even greater in WHP than in SCHP (Table 1).
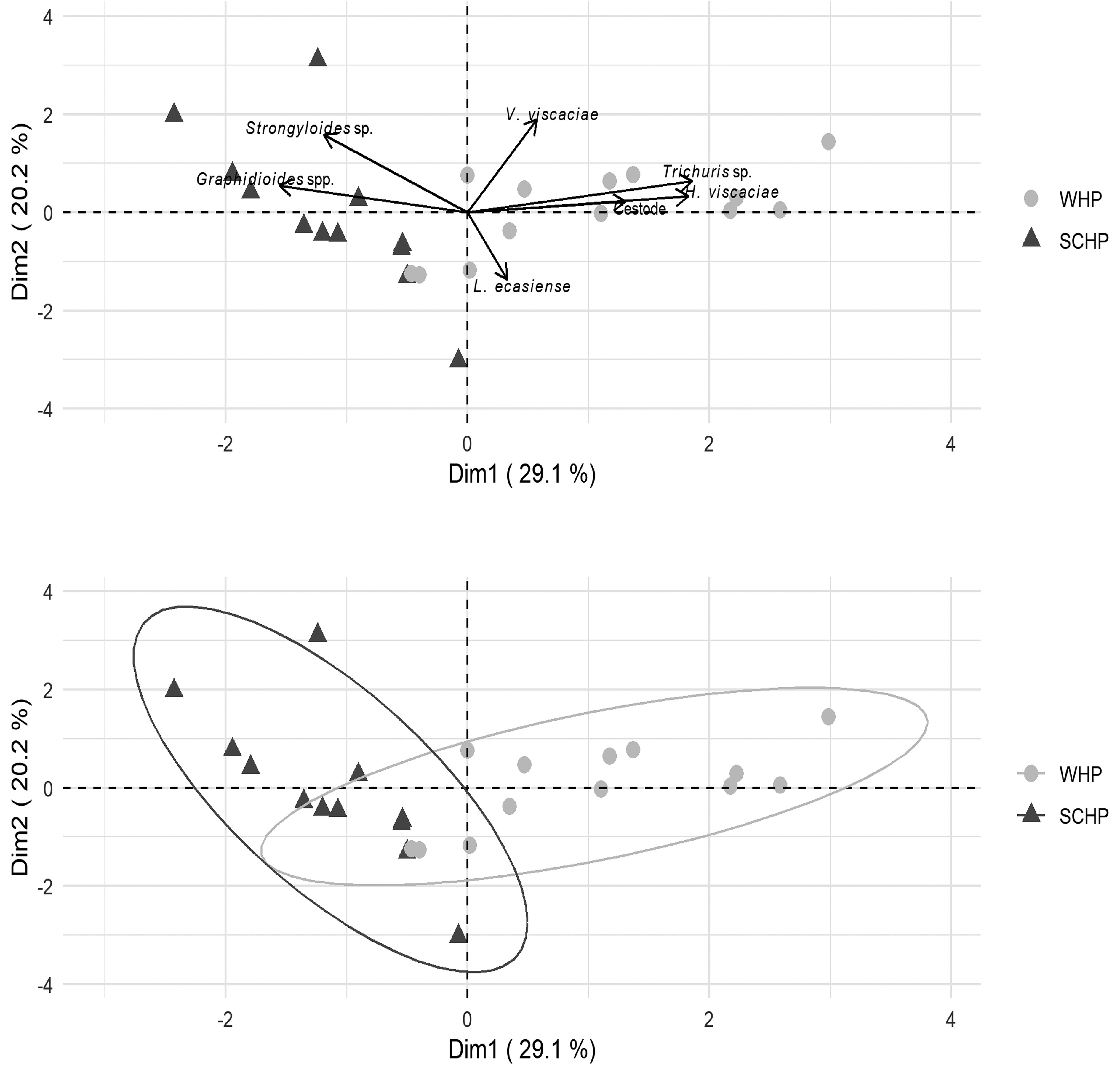
At the infracommunity level, SCHP showed higher values of abundance, diversity, and evenness, and lower values of specific richness and dominance than WHP. The bootstrap analysis showed significant statistical differences (p < 0.01) in these variables, except for the abundance (Table 2). In addition, the PCA showed that the first 2 components represented 82.6% of the variance and the infracommunities variables that most contributed to the differentiation of the host populations were specific richness, evenness and dominance (Fig. 2). Furthermore, respect to the abundance of each parasitic taxa, PCA showed that the first 2 components represented 49.3% of the variance and the helminths that most contributed to the differentiation of the host populations were H. viscaciae, Trichuris sp., Graphidioides spp. and V. viscaciae (Fig. 3).
At the component community level, helminths from SCHP showed higher values of abundance, MI, MA, Shannon-Wiener diversity and Pielou evenness index than WHP; while WHP showed higher values of specific richness and Berger-Parker dominance index than SCHP (Table 3). The Hutcheson test showed significant statistical differences in the Shannon-Wiener diversity between host populations (p = < 0.001). The SIMPER method showed a 65% dissimilarity between both populations and, V. viscaciae and Graphidioides spp. were the species that contributed the most to this dissimilarity (contribution percentage: 55% and 20%, respectively) (Table 4).
Table 2
Infracommunity descriptors for helminths of Lagostomus maximus from 2 populations in semi-captive and wild conditions.
| Infracommunity variable | SCHP | WHP | Sig |
| S | 3.25 ± 0.62 (2-4) | 4.67 ± 1.44 (2-6) | ** |
| A | 574 ± 455.55 (104-1611) | 570.6 ± 409.3 (78-1079) | ns |
| HB | 0.81 ± 0.2 (0.56-1.2) | 0.56 ± 0.25 (0.11-1.08) | ** |
| E | 0.71 ± 0.13 (0.49-0.88) | 0.39 ± 0.16 (0.16-0.69) | ** |
| D | 0.62 ± 0.14 (0.39-0.78) | 0.81 ± 0.13 (0.48-0.98) | ** |
Medium values ± standar deviation and range in parenthesis. S: Specific richness, A: abundance, HB: Brillouin diversity index, E: evenness, D: Berger-Parker dominance index. SCHP: Semi-captivity host population (N = 12), WHP: wild host population (N = 12). Sig: Statistical significance ** p < 0.01, ns: no significative.
Discussion
Previous surveys had reported a total of 15 endoparasite species in Plains Viscacha, 11 helminths (2 in SCHP and 11 in WHP), and 4 coccidians (1 in SCHP and 3 in WHP) (Canova et al., 2021; Couch et al., 2001; Cwirenbaum et al., 2021; Ferreyra et al., 2007; Foster et al., 2002; Martínez, 1988; Railliet & Henry, 1909; Rossanigo et al., 1986; Schuurmans-Stekhoven, 1951; Sutton & Durette-Desset, 1987, 1995). In the present study, Strongyloides sp. is reported for the first time in this host, and V. viscaciae, H. viscaciae, Trichuris sp., and Cestoda Anoplocephalidae are reported for the first time in Buenos Aires Province.
Furthermore, this is the first study on the helminth community structure that allows exploring possible effects of human intervention and/or environmental conditions in populations of this rodent species. In this context, the results of the abundances of helminth species, Graphidioides spp. and V. viscaciae, allow separation of both host populations (SCHP vs. WHP), so these species could be considered as tags. Moreover, the specific richness of helminths from SCHP was lower than WHP. In addition, the significant differences in specific richness, diversity, evenness and dominance, allow us to characterize both populations and use these infracommunity variables as good indicators. Additionally, the Hutcheson test at component community level shows that SCHP is characterized by a higher diversity than WHP. The effect of anthropogenic environmental changes on parasite infracommunities is known, especially in fish and can be extended to other groups of vertebrate hosts (Mascarenhas et al., 2021). The SCHP frequent environment with high human intervention, taking into account the introduction of exotic species, bare soil, and balanced feed; resulting in a possible influence on the richness and dominance of helminth species observed, shows lower values than in WHP, where the anthropogenic intervention is less. Therefore, the high parasitic diversity value obtained in SCHP is supported by the high abundance and low richness.
Table 3
Component community descriptors for helminths of Lagostomus maximus from 2 populations in semi-captive and wild conditions.
| Component community variable | SCHP | WHP |
| S | 4 | 6 |
| A | 6,888 | 6,847 |
| P | 100 | 100 |
| MI | 574 | 570.6 |
| MA | 574 | 570.6 |
| H’ | 1.21 | 0.62 |
| J’ | 0.87 | 0.35 |
| D | 0.5 | 0.84 |
S: Specific richness, A: abundance, P: prevalence as percentage, MI: mean intensity, MA: mean abundance, H’: Shannon diversity index, J’: Pielou evenness index, D: Berger-Parker dominance index. SCHP: Semi-captivity host population (N = 12),
WHP: wild host population (N = 12).
Different studies have shown that individuals fed ad libitum would have greater ingestion of parasite eggs than those with a lower consumption rate (food restricted) (Bundy & Golden, 1987). On the other hand, other surveys have shown that some plants have antiparasitic properties, and their consumption can lead to a lower parasite load (Bautista-Sopelana et al., 2022; Villalba et al., 2017). In addition, some studies of herbivorous rodents, such as capybaras, indicate that when there is little grass left (nutritional stress) they can feed close to the ground and, therefore, become more prone to ingest parasite propagules (Beldomenico & Begon, 2015). Wild animals that are maintained in captivity are forced to face situations for which they are not genetically prepared (Aguilar-Cucurachi et al., 2011). The stress caused by the new environment and the new conditions in which individuals find themselves can decrease their immune function, physiological condition and resistance to parasitic infections. In this situation, parasites will encounter less opposition to establish and reproduce in the host, producing a synergistic effect between parasitic infection and stress (Beldomenico & Begon 2009, 2015, Eberhardt et al., 2013; Khatun et al., 2014). The stress scenario described in relation to diet and the intra- and interspecific relationships between individuals is observed in the semi-captive population, supporting the high abundance of parasites observed in this study.
Parasites are considered an important source of information concerning to stability of ecosystems (Marcogliese, 2005), and numerous publications propose the role of parasites, particularly helminths, as biological tags of environmental impacts (Fuentes et al., 2010; Jankovská et al., 2009; Sures, 2001). In this paper, the study of the helminth communities at different levels permitted to distinguish both host populations. Despite the fact that in this study, both host populations share 3 helminth species, a variation in the abundance of 2 of them (V. viscaciae and Graphidioides spp.) was observed, allowing to differentiate host populations (SCHP vs. WHP), with different human intervention.
Table 4
Results of SIMPER method.
| Parasitic taxa | Average | Sd | Ratio | Ava | Avb | Cumsum |
| V. viscaciae Goodey, 1925 | 0.36 | 0.22 | 1.61 | 286.5 | 477.7 | 0.55 |
| Graphidioides spp. | 0.13 | 0.12 | 1.1 | 143 | 20.9 | 0.75 |
| L. ecasiense Sutton and Durette-Desset, 1987 | 0.07 | 0.12 | 0.64 | 54.42 | 50.3 | 0.86 |
| Strongyloides sp. | 0.06 | 0.10 | 0.63 | 90.08 | 0.00 | 0.96 |
| H. viscaciae Hugot and Sutton, 1989 | 0.02 | 0.04 | 0.53 | 0.00 | 20 | 0.997 |
| Cestode Anoplocephalidae | 0.0009 | 0.002 | 0.6 | 0.00 | 0.83 | 0.998 |
| Trichuris sp. | 0.0008 | 0.0009 | 0.9 | 0.00 | 0.83 | 1.00 |
Average: Species contribution to average between-group dissimilarity, Sd: standard deviation of contribution, Ratio: average to sd ratio, Ava/Avb: average abundances per group, Cumsum: ordered cumulative contribution.
This first study constitutes an exploration of the host and environmental distribution of different species of helminths, and the observed results are a basis for studies in other environments and with a greater number of Plains Viscacha specimens, which will allow reaching inferences in relation to the host characteristics (e.g., sex and size), and specific factors of each region.
Acknowledgments
We thank Bruno Fitte for his revision of the English version of the manuscript, Sergio Peral, Francisco Acuña, Claudio Barbeito, Alicia Flamini, Veronica Dorfman and the ECAS staff for sharing samples of Plains Viscaha’s specimens, and Mónica Rodríguez of the Dirección de Flora y Fauna from Buenos Aires Province for granting us the necessary permits to collect and transport the study material. This study was partially funded by CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Agencia Nacional de Promoción Científica y Tecnológica (PICT 2015-1348; PICT 2019- 370), and Universidad Nacional de La Plata (No. 861), Argentina.
References
Aguilar-Cucurachi, M. S., Rangel-Negrín, A., Dias, P. A. D., Chavira, R., Boeck, L., & Canales-Espinosa, D. (2011). Medición de glucocorticoides, como indicadores de estrés, durante la translocación de un grupo de monos aulladores de manto Alouatta palliata (Gray, 1849). In J. M. D. Miranda, & Z. M. B. Hirano (Eds.), A Primatologia no Brasil, Vol. 12 (pp. 282–291). Curitiba, Sociedade Brasileira de Primatologia. https://doi.org/10.13140/2.1.1210.2722
Bautista-Sopelana, L. M., Bolívar, P., Gómez-Muñoz, M. T., Martínez-Díaz, R. A., Andrés, M. F., Alonso, J. C. et al. (2022). Bioactivity of plants eaten by wild birds against laboratory models of parasites and pathogens. Frontiers in Ecology and Evolution, 10, 1027201. https://doi.org/10.3389/fevo.2022.1027201
Beldomenico, P. M., & Begon, M. (2009). Disease spread, susceptibility and infection intensity: vicious circles? Trends in Ecology & Evolution, 25, 21–27. https://doi.org/10.1016/j.tree.2009.06.015
Beldomenico, P. M., & Begon, M. (2015). Stress-host-parasite interactions: a vicious triangle? Revista FAVE Sección Ciencias Veterinarias, 14, 6–19. https://doi.org/10.14409/favecv.v14i1/2.5160
Bundy, D. A. P., & Golden, M. H. N. (1987). The impact of host nutrition on gastrointestinal helminth populations. Parasitology, 95, 623–635. https://doi.org/10.1017/S0031182000058042
Bush, A. O., Lafferty, K. D., Lotz, J. M., & Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. The Journal of Parasitology, 83, 575–583. https://doi.org/10.2307/3284227
Canova, V., Robles, M. R., & Abba, A. M. (2021). A new species of Wellcomia (Nematoda: Oxyuridae) in the Plains Viscacha (Rodentia: Chinchillidae) from Argentina, an emended diagnosis and an update of the genus Wellcomia. Parasitology Research, 120, 929–940. https://doi.org/10.1007/s00436-020-06980-1
Cirignoli, S., & Lartigau, B. (2019). Lagostomus maximus. In SAyDS-SAREM (Eds.) Categorización 2019 de los mamíferos de Argentina según su riesgo de extinción. Lista Roja de los mamíferos de Argentina. Retrieved on September 06th, 2022 from: http://cma.sarem.org.ar/es/especie-nativa/lagostomus-maximus
Clarke, K. R., Gorley, R. N., Somerfield, P. J., & Warwick, R. M. (2014). Change in marine communities: an approach to statistical analysis and interpretation, 3rd Edn. Plymouth, PRIMER-E Ltd.
Couch, L., Foster, G. W., Machicote, M., & Branch, L. C. (2001). Descriptions of two new species of Eimeria (Apicomplexa: Eimeriidae) and of Eimeria Chinchillae-like oocysts from the plains vizcacha Lagostomus maximus (Desmarest, 1817) (Rodentia: Chinchillidae) from Argentina. Journal of Parasitology, 87, 144–147. https://doi.org/10.1645/0022-3395(2001)087[0144:DOTNSO]2.0.CO;2
Cwirenbaum, R., Schmidt, A. R., Cortasa, S. A., Corso, M. C., Vitullo, A. D., Dorfman, V. B. et al. (2021). First record of an infection by tissue cyst-forming coccidia in wild vizcachas (Lagostomus maximus, Rodentia) of Argentina. International Journal for Parasitology: Parasites and Wildlife, 16, 52–58. https://doi.org/10.1016/j.ijppaw.2021.08.002
Eberhardt, A. T., Costa, S. A., Marini, M. R., Racca, A., Baldi, C. J., Robles, M. R. et al. (2013). Parasitism and physiological trade-offs in stressed capybaras. Plos One, 8, e70382.
https://doi.org/10.1371/journal.pone.0070382
Esch, G. W., Barger, M. A., & Fellis, K. J. (2002). The transmission of digenetic trematodes: style, elegance, complexity. Integrative and Comparative Biology, 42, 304–312. https://doi.org/10.1371/journal.pone.0070382
Ferreyra, H., Uhart, M. M., Romano, M. C., Beldomenico, P. M., Samartino, L., Paolicchi, F. et al. (2007). Inmovilización química y evaluación de salud de vizcachas salvajes (Lagostomus maximus) en el chaco árido argentino. Arquivos de Ciências Veterinárias e Zoologia da UNIPAR, 10, 91–99.
Foster, G. W., Branch, L. C., Machicote, M., Kinsella, J. M., Villarreal, D., & Forrester, D. J. (2002). Gastrointestinal helminths of the plains vizcacha (Lagostomus maximus) from Argentina, with observations on interspecific interactions between nematodes and cestodes. Comparative Parasitology, 69, 26–32. https://doi.org/10.
1654/1525-2647(2002)069[0026:GHOTPV]2.0.CO;2
Fuentes, M. V., Sainz-Elipe, S., Sáez-Durán, S., & Galán-Puchades, M. T. (2010). The helminth community of the wood mouse Apodemus sylvaticus in a Mediterranean ecosystem in regeneration ten years after a wildfire. Journal of Helminthology, 84, 39–48. https://doi.org/10.1017/S0022149X09990277
Gardener, M. (2017). Statistics for ecologists using R and Excel: data collection, exploration, analysis and presentation, 2nd Edn. Exeter: Pelagic Publishing.
Gibbons, L. M. (2010). Keys to the nematode parasites of vertebrates: supplementary volume. UK, CABI Publishing.
Hutcheson, K. (1970). A test for comparing diversities based on the Shannon formula. Journal of Theoretical Biology, 29, 151–154.
Ibrahim, M. M. (2012). Variation in parasite infracommunies of Tilapia zillii in relation to some biotic and abiotic factors. International Journal of Zoological Research, 8, 59–70. https://doi.org/10.3923/ijzr.2012.59.70
Jackson, J. E., Branch, L. C., & Villarreal, D. (1996). Lagostomus maximus. Mammalian Species, 543, 1–6.
Jankovská, I., Miholová, D., Langrová, I., Bejček, V., Vadlejch, J., Kolihová, D. et al. (2009). Influence of parasitism on the use of small terrestrial rodents in environmental pollution monitoring. Environmental Pollution, 157, 2584–2586. https://doi.org/10.1016/j.envpol.2009.04.008
Khatun, M. M., Begum, N., Mamun, M. A. A., Mondal, M. M. H., & Shakif-Ul-Azam, M. (2014). Coprological study of gastrointestinal parasites of captive animals at Rangpur Recreational Garden and Zoo in Bangladesh. Journal of Threatened Taxa, 6, 6142–6147. https://doi.org/10.11609/JoTT.o3093.6142-7
Magurran, A. E., & McGill, B. J. (2011). Biological diversity: frontiers in measurement and assessment. Oxford, Oxford University Press.
Marcogliese, D. J. (2005). Parasites of the superorganism: are they indicators of ecosystem health? International Journal for Parasitology, 35, 705–716. https://doi.org/10.1016/j.ijpara.2005.01.015
Martínez, F. A. (1988). Helmintofauna del Myocastor coypus y Lagostomus maximus, Nematodes. Veterinaria Argentina, 41, 33–37.
Mascarenhas, C. S., Silva, R. Z., & Müller, G. (2021). Helminth’s assemblage of Trachemys dorbigni (Testudines: Emydidae) in southern Brazil: implications of anthropogenic environments and host’s genders. Iheringia, Série Zoologia, 111, e2021011. https://doi.org/10.1590/1678-4766e2021011
Moreno, C. E. (2001). Métodos para medir la biodiversidad, Vol. 1. Zaragoza, M&T-Manuales & Tesis SEA.
Muñoz, G., & Castro, R. (2012). Comunidades de parásitos eumetazoos de peces labrisómidos de Chile central. Revista de Biología Marina y Oceanografía, 47, 565–571. http://dx.doi.org/10.4067/S0718-19572012000300018
Poulin, R. (2004). Macroecological patterns of species richness in parasite assemblages. Basic and Applied Ecology, 5, 423–434. https://doi.org/10.1016/j.baae.2004.08.003
R Core Team. (2021). R: a language and environment for statistical computing (Version 4.1. 0) [Computer software]. R Foundation for Statistical Computing.
Railliet, A., & Henry, A. (1909). Sur la classification des Strong-
ylidae: I. Metastrongylinae. Comptes Rendus Hebdomadaires des Séances et Mémoires de la Société de Biologie, 66, 85–88.
Reiczigel, J., Marozzi, M., Fábián, I., & Rózsa, L. (2019). Biostatistics for parasitologists – a primer to quantitative parasitology. Trends in Parasitology, 35, 277–281. https://doi.org/10.1016/j.pt.2019.01.003
Rossanigo, C. E., Jackson, J. E., & Lukovich, R. (1986). Endoparásitos de la vizcacha (Lagostomus maximus). Primera descripción en la República Argentina. Revista de Medicina Veterinaria, 67, 219–223.
Sapolsky, R. M., Romero, L. M., & Munck, A. U. (2000). How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocrine Reviews, 21, 55–89. https://doi.org/10.1210/edrv.21.1.0389
Sapp, K. K., & Esch, G. W. (1994). The effects of spatial and temporal heterogeneity as structuring forces for parasite communities in Helisoma anceps and Physa gyrina. The American Midland Naturalist, 132, 91–103. https://doi.org/10.2307/2426204
Schuurmans-Stekhoven, J. H. (1951). Nematodos parasitarios de anfibios, pájaros y mamíferos de la República Argentina. Acta Zoologica Lilloana, 10, 315–400.
Sikes, R. S., & The Animal Care and Use Committee of the American Society of Mammalogists. (2016). Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy, 97, 663–688. https://doi.org/10.1093/jmammal/gyw078
Spotorno, A. E., & Patton, J. L. (2015). Superfamily Chinchilloidea Bennett, 1833. In J. L. Patton, U. F. J. Pardiñas, & G. D’Elía (Eds), Mammals of South America, Vol. 2: Rodents (pp.762–786). Chicago: University of Chicago Press.
Sures, B. (2001). The use of fish parasites as bioindicators of heavy metals in aquatic ecosystems: a review. Aquatic Ecology, 35, 245–255. https://doi.org/10.1023/A:1011422310314
Sutton, C. A., & Durette-Desset, M. C. (1987). Contribution à la connaissance de la faune parasitologique argentine XVIII. Lagostonema ecasiense n. gen., n. sp. (Trichostrongyloidea, Nematoda), parasite de Lagostomus maximus (Chinchillidae, Caviomoprha). Bulletin du Muséum National d’Histoire Naturelle, Section A, Zoologie, Biologie et Ecologie Animales, 9, 127–131.
Sutton, C. A., & Durette-Desset, M. C. (1995). A description of Graphidioides kravetzi n. sp. and the revision of Graphidioides Cameron, 1923 (Nematoda Trichostrongyloidea), parasites of Neotropical rodents. Systematic Parasitology, 31, 133–145. https://doi.org/10.1007/BF02185545
Thieltges, D. W., Jensen, K. T., & Poulin, R. (2008). The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology, 135, 407–426. https://doi.org/10.1017/S0031182007000248
Villalba, J. J., Costes-Thiré, M., & Ginane, C. (2017). Phytochemicals in animal health: diet selection and trade-offs between costs and benefits. Proceedings of the Nutrition Society, 76, 113–121. https://doi.org/10.1017/S0029665116000719
Webster, J. I., Tonelli, L., & Sternberg, E. M. (2002). Neuroendocrine regulation of immunity. Annual Review of Immunology, 20, 125–163. https://doi.org/10.1146/annurev.immunol.20.082401.104914