Differential herbivory and successional status in five tropical tree species
Néstor A. Mariano a, b, Cristina Martínez-Garza a, Raúl E. Alcalá a, *
a Departamento de Ecología Evolutiva, Centro de Investigación en Biodiversidad y Conservación, Universidad Autónoma del Estado de Morelos, Av. Universidad 1001, Chamilpa, 62209 Cuernavaca, Morelos, Mexico
b Instituto de Ambiente de Montaña y Regiones Áridas, Universidad Nacional de Chilecito, 9 de Julio 22, F5360CKB Chilecito, La Rioja, Argentina
*Corresponding author: raul.alcala@uaem.mx (R.E. Alcalá)
Abstract
Using 4 restoration plots, we performed a common garden experiment to test the hypothesis that inter-specific variation in leaf herbivory depends on the successional status of tree species of the seasonally dry tropical forest. In July of 2011, we calculated the standing levels of herbivory in 5 species at the beginning of the rainy season: Ipomoea pauciflora (early-successional), Swietenia humilis, and Pseudobombax ellipticum (intermediate-successional) and Jacaratia mexicana and Bursera linanoe (late-successional). From each individual tree, we selected 14 leaves to measure herbivory (N = 84 plants, 821 leaves). The mean leaf area lost by herbivory across the 5 tree species was 5.25%. The results evidencing differences among the categories evaluated supported our hypothesis, as herbivory increased from the late to the early-successional species. We discussed the proximate (i.e, differential leaf traits) and ultimate causes (i.e., differential selective pressures) operating on trees situated at the extreme of the successional stages that could explain the inter-specific differences in herbivory we observed. In accordance with our results, successional status should be recognized as a factor affecting herbivory in tree species in tropical dry forests.
Keywords
Common garden experiment; Early and late succession; Foliar damage; Insect herbivory; Life-history; Restoration plantings; Seasonally dry tropical forest
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Herbivoría diferencial y estatus sucesional en cinco especies de árboles tropicales
Resumen
Usando 4 parcelas de restauración, implementamos un experimento de jardín común para evaluar la hipótesis de que la variación interespecífica en la herbivoría foliar se relaciona con el estado sucesional de los árboles del bosque tropical estacionalmente seco. En julio de 2011, calculamos los niveles de herbivoría en 5 especies al inicio de la época lluviosa: Ipomoea pauciflora (sucesión temprana), Swietenia humilis y Pseudobombax ellipticum (sucesión intermedia) y Jacaratia mexicana y Bursera linanoe (sucesión tardía). De cada planta, seleccionamos aleatoriamente 14 hojas para medir la herbivoría (N = 84 plantas, 821 hojas). El promedio de área foliar perdida por herbivoría en las 5 especies de árboles fue 5.25%. Los resultados indicaron diferencias entre las categorías evaluadas que apoyaron nuestra hipótesis, mostrando que la herbivoría incrementó de las especies de sucesión tardía a las de sucesión temprana. Discutimos sobre las causas próximas (i.e., características foliares distintas) y últimas (i.e., presiones selectivas distintas) que operan sobre árboles pertenecientes a los extremos de las categorías sucesionales que podrían explicar las diferencias que observamos en los niveles de herbivoría. De acuerdo con los resultados, el estado sucesional debe reconocerse como un factor que afecta la herbivoría en especies de árboles en bosques tropicales secos.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Experimento de jardín común; Sucesión temprana y tardía; Daño foliar; Herbivoría por insectos; Historia de vida; Parcelas de restauración; Bosque tropical estacionalmente seco
Introduction
Several hypotheses have been posed to explain the concurrent observation about differences in the amount of tissue lost by insect herbivores by different groups of plants (Bryant et al., 1983; Coley et al., 1985; Herms & Mattson, 1992; Johnson, 2011; Stamp, 2003). Empirical evidence and theoretical models indicate that both, susceptibility of plants to be consumed, as well as the response of plants to herbivory (i.e., evolution of resistance or tolerance to damage), are related with resource availability (Bryant et al., 1983; Hawkes & Sullivan, 2001; Hilbert et al., 1981; Maschinski & Whitham, 1989).
Bazzaz (1979) proposed that variation in physiological and life-history traits is expressed along the successional status of species. At a one extreme, late-successional species, corresponding to those chronically subjected to limiting availability of resources display traits that enhance fitness prioritizing permanence. Late-succesional species generally express slow growth, higher allocation to defense and consequently tend to exhibit low levels of herbivory. At the other extreme, higher resource allocation to growth instead of defense is expected in species evolving in more favorable conditions; therefore, early-successional species accumulate damage at higher rates (Coley, 1983; Coley et al., 1985; Fine et al., 2006).
In specific, several studies provided abundant evidence on the relationship between variation in growth rates and successional status in seasonally dry tropical forest species (Álvarez-Aquino & Williams-Linera, 2012; Foroughbakhch et al., 2006; Huante & Rincón, 1998; Kalacska et al., 2004; Lebrija-Trejos et al., 2010; Rincón & Huante, 1992 Soriano et al., 2011). However, studies incorporating the effect of successional status as a likely factor explaining inter-specific differences in herbivory in tropical dry forests are scarce (Table 1). Thus, as far as we know, studies addressing how successional stages are related to herbivory in restoration plantings within seasonally dry tropical forests are not available. Restoration plantings could represent scenarios for the implementation of common-garden experiments, which are useful to explore the occurrence of inherent differences in the pattern of herbivory, as they provide similar environmental conditions for different tree species. Regarding herbivory, for example, it can be expected that groups of tropical tree species growing simultaneously in homogeneous conditions, should express variation in the susceptibility to herbivory related to differences in successional status (Massad et al., 2011). In this study, we predict differential herbivory in tree species growing in a seasonally dry forest located in Central Mexico. Assuming that plants within the plots are exposed to a similar environment (i.e., edaphic conditions, light regime, water availability and density of insect herbivores), herbivory is expected be greater in early-successional tree species than in late-successional species.
Materials and methods
The study site is located within the Sierra of Huautla Biosphere Reserve nearby the town El Limón de Cuauchichinola. The reserve includes a large portion of the state of Morelos, Mexico nearby the states of Guerrero and Puebla (18°20’10”-18°34’20” N, 98°51’20”-98° 08’15” W). The original vegetation in this area corresponded to seasonally dry tropical forest. After being used for maize cultivation, extensive areas of old-fields were abandoned around 30 years ago. Currently, the Reserve is a mosaic of original vegetation present in ravines and hills, which are surrounded by secondary vegetation and flatter areas heavily transformed to agricultural lands (Martínez-Garza et al., 2011).
As a part of a previous experiment, in January of 2006, 8 restoration plots (50 × 50 m) were fenced to exclude livestock and extraction of plants and the animals by the local people. At each plot, all the originally present trees [≥ 5 cm diameter at breast height (dbh = 1.3 m)] were tagged and measured, recording 33 woody species from 14 families (density of 252.5 trees/ha; basal area of 6.18 m2/ha; Martínez-Garza et al., 2011). At that moment, the most abundant species were Acacia cochliacantha (Fabaceae, 75 trees/ha), Ipomoea pauciflora (Convolvulaceae, 50 trees/ha) and Mimosa benthamii (Fabaceae, 31 trees/ha). To experimentally facilitate natural succession, 4 of these plots were enriched with seedlings (ca., 40 cm height) of 20 native tree species (Carrasco-Carballido & Martínez-Garza, 2011). In July of 2011, we used the enriched plots of that restoration experiment to test the effect of successional status on the standing levels of herbivory in 5 tree species, which included those species that had foliage starting this study. One naturally established tree species, Ipomoea pauciflora M. Martens & Galeotti (Convolvulaceae) and 4 planted native species, Bursera linanoe (La Llave) Rzed., Calderón & Molina, Jacaratia mexicana A. DC. (Caricaceae), Pseudobombax ellipticum (Kunth) Dugan (Bombacaceae) and Swietenia humilis Zucc. (Meliaceae), were used for this study.
Table 1
Herbivory measurements in tree species of the seasonally dry tropical forest. Herbivory is presented as the mean percentage of area eaten (% AE). The minimum and maximum (min-max) indicate extreme values reported per species. Values of %AE marked with * were derived from figures; those marked with ** were calculated from supplementary material. Non-marked values were directly provided by authors in the text. Information about if the studies included criterions as phenology (D, deciduous / E, evergreen) or successional stages (E, early; I, intermediate or L, late succesional) is provided.
|
Site |
N |
(% AE) |
Min-max 26.14-30.81 |
Phenology |
Successional stage |
Reference |
|
Chamela, Jalisco |
12 |
7.57 |
1.56-19.53 |
No |
No |
Filip et al., 1995 |
|
Dzibilchaltún, National Park region, Yucatán |
3 |
3.65 * |
2.45-4.65 |
D |
No |
Campo & Dirzo, 2003 |
|
Chamela, Jalisco |
3 |
14.85 * |
12.4-17.3 |
D |
No |
Boege, 2004 |
|
Chamela, Jalisco |
23 |
11.5 ** |
0.6-28.2 |
D/E |
No |
Pringle et al., 2011 |
|
Chamela, Jalisco |
2 |
21.65 |
9.9-33.4 |
D |
No |
Hernández et al., 2014 |
|
RBSH, Morelos |
2 |
28.47 |
26.14-30.81 |
D |
E |
Juan-Baeza et al., 2015 |
|
Minas Gerais, Brazil |
31 |
6.5 * |
– |
No |
No |
Neves et al., 2010 |
|
Mata Seca State Park, Minas Gerais |
1 |
8.63 * |
6.0-10.8 |
D |
E, I, L |
Silva et al., 2012 |
|
Minas Gerais, Brazil |
– |
5.36 * |
3.5-7.8 |
D |
E, I, L |
Neves et al., 2014 |
According to the literature, the 5 species were assigned to early, intermediate or late successional status (Table 2). I. pauciflora is considered an early successional tree species given its fast growth rates with high availability of resources (Huante et al., 1995) and higher density in early successional environments (Martínez-Garza et al., 2011). P. ellipticum and S. humilis were considered intermediate-successional species given their growth rates which are lower than those of early successionals (Huante et al., 2012). Bursera species (Rzedowski & Kruse, 1979) and Jacaratia mexicana (Bullock, 2002) are usually found only at late-successional stages (Table 2).
Leaf herbivory was evaluated during July 2011 (2 to 5 plants per species per plot at 4 plots; N = 84 plants). From each individual tree, we randomly selected 14 leaves to measure herbivory (N = 821 leaves). Leaves were not collected to avoid a possible impact on survivorship and growth of plants. The amount of insect damage of each individual leaf was assigned to one of 6 categories considered by Dirzo and Domínguez (1995): the category 0 represents leaves without damage (0%). The other 5 categories represent ranges of damage, as follows: 1 = 1-6%, 2 = 7-12%, 3 = 13-25%, 4 = 26-50%, 5 = 51-100%. Following Dirzo and Domínguez (1995), we calculated the index of herbivory as follows: H = Σ (Ci × ni) /N, where Ci is the category of damage, ni is the number of leaves that present damage of a given category and N is the total number of leaves.
To evaluate the effect of successional status on the level of herbivory, we used a mixed-effects model based on restricted maximum likelihood estimation of parameters. As a surrogate of the successional status (i.e., early, intermediate and late-successional), an ordered fixed factor was adopted. Species identity nested within site was specified as a random component. We tested for the presence of linear and quadratic relationships of the ordered fixed factor on damage, using a polynomial contrast. In addition, we used a different mixed-effects model to test the effect of tree species on herbivory.
Table 2
Families, seed weight (mg), successional status and references that support classification of successional status of 5 dry forest tree species in experimental restoration plots in El Limón de Cuauchichinola, Sierra de Huautla, Morelos, Mexico.
|
Species |
Family |
Seed weight |
Succesional status |
Reference |
|
Ipomoea pauciflora |
Convolvulaceae |
86.40* |
Early |
Huante et al., 1995 Martínez-Garza et. al., 2011 |
|
Swetenia humilis |
Meliaceae |
50.00* |
Intermediate |
Huante et al., 2012 |
|
Pseudobombax ellipticum |
Bombacaceae |
69.46* |
Intermediate |
Almazán-Núñez et al., 2012 |
|
Bursera linaloe |
Burseraceae |
58.74** |
Late |
Rzedowski & Kruse, 1979 Beltrán-Rodríguez et al., 2018 |
|
Jacaratia mexicana |
Caricaceae |
516.13* |
Late |
Bullock, 2002 Lebrija-Trejos et al., 2010 |
* Kattge et al. (2011); ** Mendoza-Segovia (2017)
We defined species identity as a fixed factor, whereas we specified the effect of site as a random component. We evaluated inter-specific differences between mean values of herbivory using the Tukey HSD post hoc test. For both mixed models, the index of herbivory per plant was square root-transformed to fulfill model assumptions of normality and homoscedasticity of residuals. For clarity, in all cases we reported mean and standard error values of untransformed data. All statistical analyses were carried out using R 3.2.3 (R Core Team, 2015). We fitted linear mixed effects models using the lme function in R (Pinheiro et al., 2015), and glht function in library multcomp to perform Tukey HSD test (Hothorn et al., 2008).
Results
The mixed-effects model analysis of variance showed that the effect of successional status was significant (F(2, 14) = 49.56, p < 0.001) and accounted for 69% of the total variance of observed herbivory rates. Polynomial contrasts showed a negative linear tendency between the level of herbivory and successional status (t(14) = 9.9, p < 0.001). In contrast, quadratic tendency was not statistically significant (t(14) = 0.72, p = 0.4833). Results indicate that herbivory was greater in the early-successional species, followed by intermediate-successional species and lowest in late-successional species. The early-successional species held 2.5 and 6 times more damage, than intermediate and late-successional species, respectively (Fig. 1).
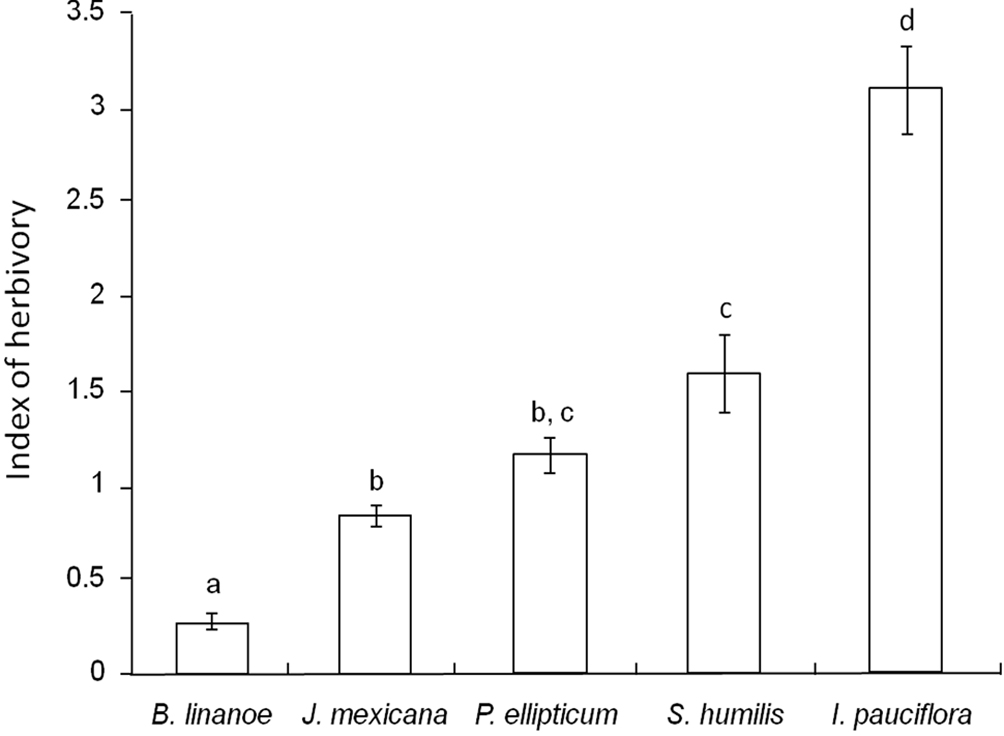
The effect of tree species identity on herbivory was also statistically significant (F(4, 76) = 70.82, p < 0.001), and accounted for 78% of total variance. Herbivory in I. pauciflora was higher than the observed for B. linanoe (Tukey HSD, p < 0.001, Fig. 2). Jacaratia mexicana had significant lower damage than S. humilis (Tukey HSD, p = 0.0042). Herbivory in P. ellipticum, was statistically similar to the observed in J. mexicana (Tukey HSD, p = 0.369) and in S. humilis (Tukey HSD, p = 0.325) (Fig. 2). Overall, the mean leaf area lost by herbivory across the 5 tree species included in our study was 5.25%, (ranging from 0.5% to 20%).
Discussion
The mean leaf area lost by herbivory across the 5 tree species included in our study was located within the range of results reported for other tree species in Mexico, in which mean percentages of herbivory varied from 3.65 to 28.47 (Table 1). Our mean value was also located within those reported for tropical dry forests in South America (Table 1), and it was very similar to the median value reported by Dirzo and Boege (2008) for tree species of tropical dry forests around the world.
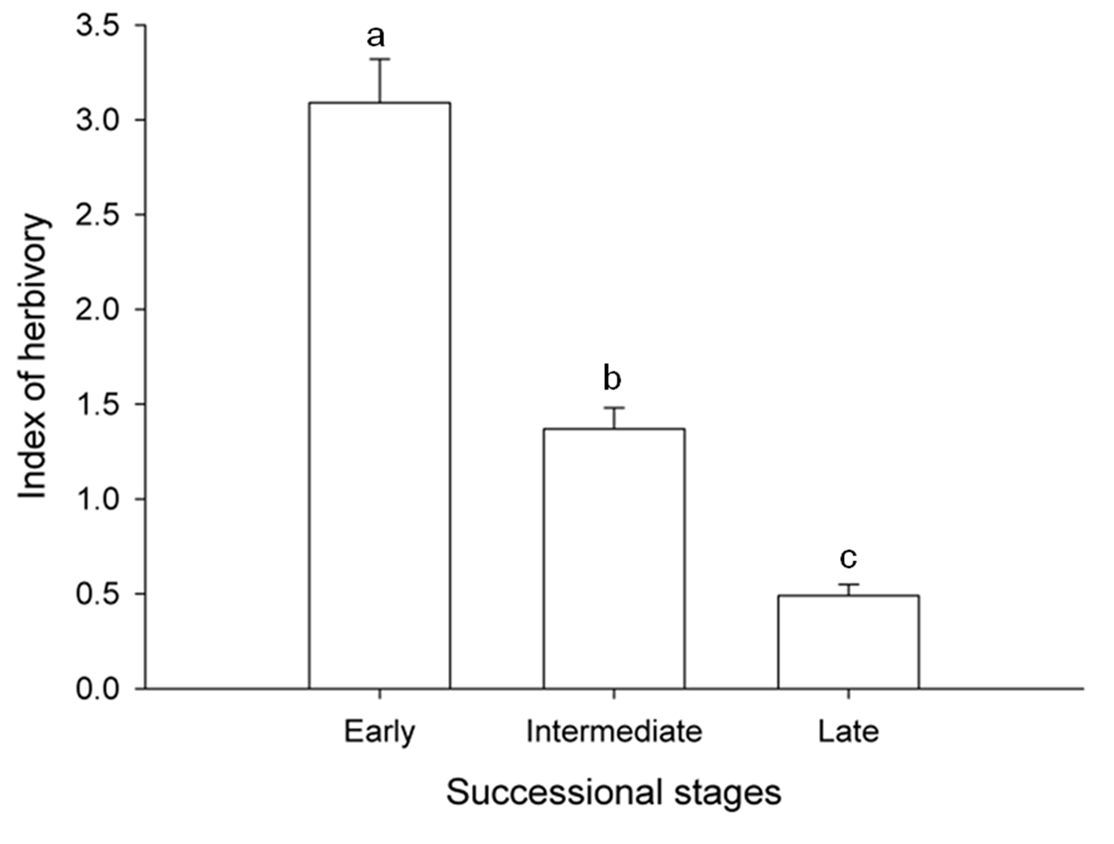
Herbivory and successional status. With regard the effect of the successional status, the evidence derived from this study supported our prediction, as we showed that herbivory increased from the late-successional to the early-successional species. These results can be explained by proximate and ultimate causes. Regarding the functional explanations that occur at the ecological level, it is known that inter-specific differences in herbivory frequently result from comparisons of correlations between defensive foliar characteristics with leaf damage (Coley, 1983, 1987). For example, low levels of herbivory are observed in species whose foliage exhibits higher toughness and pubescence and a higher concentration of fiber, cellulose and secondary metabolites (i.e., tannins, phenols). These characteristics in conjunct are hypothesized to reduce palatability and digestibility of leaf tissue. In contrast, higher herbivory is found in species with the opposite pattern for leaf traits (Coley, 1983, 1987). Interestingly, these differences are not only patent among tree species; but they are also expressed along leaf ontogeny, in which higher allocation to defense and lower herbivory occur in older than in younger leaves (Coley, 1980). Thus, inter-specific variation in traits such as secondary metabolites, nutritional quality and/or leaf toughness could be considered as proximate explanations to the variation in herbivory among tropical tree species. On the other hand, ultimate causes explaining the inter-specific variation in leaf damage are linked with the hypothesis that tropical tree species face differential selective pressures according with the availability of resources to which they are exposed. In consequence, plant species display distinct adaptive life-history traits in contrasting conditions of resource availability, such as those occurring along successional stages (Bazzaz, 1979; Bryant et al., 1985; Coley et al., 1985; Endara & Coley, 2011). For example, a higher resource allocation to growth and higher ability to compensate leaf loss should be advantageous in early-successional species. Consequently, a lower investment on defense is predicted in this group of species. Overall, the evidence available for tropical trees supports a pattern in which, on the one hand, leaves of early-successional species exhibit lower amounts of chemical compounds, foliar toughness, trichome density and higher levels of herbivory. In contrast, the rate of herbivory should be lower in late-successional species, which are exposed to limited resources. Under conditions of stress the ability to compensate the loss of foliage is limited, thus late-successional species tend to increase the allocation of resources to defensive strategies (Coley, 1980, 1983, 1987; Coley et al., 1985).
Herbivory in restoration plots. Reciprocal transplant experiments have been useful to obtain evidence supporting differential adaptive evolution of life-history traits and defensive strategies among groups of tree species. Such experimental approaches showed evidence that the expression of traits involved in growth or defense in plants has a genetic basis (Fine et al., 2004, 2006). Our results supported the expected pattern of herbivory in which level of damage is related to the successional stage of tree species. This is because the differential pattern of leaf damage exhibited among trees belonging to different successional status growing in homogeneous conditions indicates that species exhibit inherent differences in their susceptibility to herbivory (Poorter et al., 2004). Based on our experimental design and the small size of plots, it can be assumed that plant species of different successional status were exposed to similar edaphic and light conditions, as well as to similar herbivore abundance. Thus, although in this study, foliar traits were not measured, we infer that differences in intrinsic characteristics of plants explain the pattern of herbivory we observed (Coley, 1987; Coley et al., 1985; Poorter et al., 2004). In fact, as leaf characteristics correlate with growth rates, foliar traits have been utilized to predict the performance of tropical species to be selected for restoration plantings (Martínez-Garza et al., 2005, 2013).
We considered that the overall mean value of herbivory was not influenced by the occurrence of plants in restoration plots (i.e, a high density of plants). This is because other independent studies performed on the same study system, showed that herbivory levels were statistically similar between successional sites and restored plots (Hernández et al., 2014), or among different restoration treatments (with and without plantings; Juan-Baeza et al., 2015). In turn, the experimental design we used which was based on restoration plantings was successful in demonstrating inherent differences in herbivory correlated with successional status of plants, as it was shown in a large-scale restoration planting in Brazil, in which early-successional species held higher herbivory than late-successional species (Massad et al., 2011).
Species identity and herbivory. The magnitude of herbivory in I. pauciflora was 5 times higher that of the mean value obtained for the other 4 species. The index of herbivory scored in this species corresponds to a leaf area loss of about 20%, whereas damage in the other 4 species was between 1 and 6%. Ipomoea pauciflora is an early-successional tree species widely associated with disturbed conditions, being very common along roads, in agricultural lands and in secondary vegetation (Juan-Baeza et al., 2015; Martínez-Garza et al., 2011). It is known that I. pauciflora maintains a specialized interaction with Phytodectoidea quatordecimpunctata Boheman (Chrysomelidae), a beetle that produces a mean foliar damage of 25-30% of the total leaf area (Castro-Jaimez & Mariano, 2011; Juan-Baeza et al., 2015), with some individuals receiving foliar damage corresponding to 50% or higher (N. Mariano per. obs.). Thus, this specialized plant-insect interaction could explain the higher herbivory levels scored in I. pauciflora. The level of damage was much lower within the intermediate-successional plants in which damage ranged between 2 and 3% of the total leaf area for P. ellipticum and Swietenia humilis, respectively. The late-successional tree, J. mexicana received 1% of damage, which agrees with the observation of apparent very low herbivory in this dioecious tree (Bullock, 2002). However, in reproductive individuals, a higher herbivory could be expected in male than in female trees (Cepeda-Cornejo & Dirzo, 2010, and references therein). The other late-successional tree, Bursera linanoe is frequently found inhabiting pristine dry tropical forests, being a dominant element of this ecosystem (Rzedowski & Kruse, 1979). Our results show that B. linanoe received practically no damage, thus herbivory should not be a limiting factor for growth or survivorship of juveniles of this tree species.
The relationship between the levels of herbivory and successional status of plants was determined including individuals of only 5 tree species (those exhibiting foliage at the moment of the study). This could be a limitation, as studies performed at the community level have detected a marked inter-specific component on the variation in herbivory (Filip et al,. 1995), even within phenological categories (i.e., deciduous and evergreen; Pringle et al., 2011). Thus, the prediction of the relationship between successional status with herbivory should be tested including more plant species. Measurements of growth should be also carried out to directly estimate the relationship between herbivory and growth rates. For future studies, herbivory measurement in marked leaves is also recommended as index of herbivory could underestimate insect damage (Filip et al., 1995).
We have utilized a current restoration experiment of a dry tropical forest to carry out an experimental approach useful for the study of the interaction between tropical trees and their herbivores. We focused on plots previously enriched with native trees to implement a common garden experiment. Considering restoration goals, our results indicate that the pattern of herbivory shown in plantings is the expected regarding inter-specific life-history differences. Thus, plantings might restore plant-insect interactions (Juan-Baeza et al., 2015; Solis-Gabriel et al., 2017). Although some tree species in plantings could be exposed to high levels of herbivory, the effect of herbivory at the community level tends to be diminished in plots exhibiting high species richness (Massad et al., 2011). From a more ecological point of view, according to our results, the differential herbivory observed among tree species sharing a common environment, indicates that successional status should be recognized as a factor shaping the interaction between plants and their herbivores in tropical dry forests.
Acknowledgements
We are grateful to Gerardo Pacheco and Luis Castro for field assistance. The authors gratefully acknowledge financial and logistical support from the Universidad Autónoma del Estado de Morelos, the Secretary of Public Education granted to CMG (PROMEP, 103.5/05/1901), the Cuerpo Académico de Ecología Evolutiva (PROMEP) and, the National Council of Science and Technology of Mexico (Conacyt, Grant # 80027) granted to CMG to establish and maintain the experiment. Suggestions of two anonymous reviewers improved the manuscript.
References
Almazán-Núñez, R. C., Arizmendi, M. C., Eguiarte, L. E., & Corcuera, P. (2012). Changes in composition, diversity, and structure of woody plants in successional stages of tropical dry forest in southwest Mexico. Revista Mexicana de Biodiversidad, 83, 1096–1190.
Álvarez-Aquino, C., & Williams-Linera, G. (2012). Seedling survival and growth of tree species: site condition and seasonality in tropical dry forest restoration. Botanical Sciences, 90, 341–351.
Bazzaz, F. A. (1979). The physiological ecology of plant succession. Annual Review of Ecology and Systematics, 10, 351–371.
Beltrán-Rodríguez, L., Valdez-Hernández, J. I., Luna-Cavazos, M., Romero-Manzanares, A., Pineda-Herrera, A., Maldonado-Almanza, B. et al. (2018). Estructura y diversidad arbórea de bosques tropicales caducifolios secundarios en la Reserva de la Biosfera Sierra de Huautla, Morelos. Revista Mexicana de Biodiversidad, 89, 108–122.
Boege, K. (2004). Induced responses in three tropical dry forest plant species –direct and indirect effects on herbivory. Oikos, 107, 541–548.
Bryant, J. P., Chapin III, F. S., & Klein, D. R. (1983). Carbon/nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos, 40, 357–368.
Bryant, J. P., Chapin III, F. S., Reichardt, P., & Clausen, T. (1985). Adaptation to resource availability as a determinant of chemical defense strategies in woody plants. Recent Advances in Phytochemistry, 19, 219–237.
Bullock, S. J. (2002). Jacaratia mexicana. DC. (Caricaceae) Bonete. In F. A. Noguera, J. H. Vega-Rivera, A. N. García-Aldrete, & M. Quesada-Avendaño (Eds.), Historia natural de Chamela (pp. 155–158). Cd. de México: Universidad Nacional Autónoma de México.
Campo, J., & Dirzo, R. (2003). Leaf quality and herbivory responses to soil nutrient addition in secondary tropical dry forests of Yucatan Mexico. Journal of Tropical Ecology, 19, 525–530.
Carrasco-Carballido, V., & Martínez-Garza, C. (2011). Recuperación de la biodiversidad con plantaciones de especies nativas en selvas húmedas y secas de México. Tres estudios de caso. In O. Vargas-Ríos, & S. P. Reyes (Eds.), Memorias del 1er. Congreso Colombiano de Restauración Ecológica y II Simposio Nacional de Experiencias en Restauración Ecológica (pp. 297–305). Bogotá: Universidad Nacional de Colombia.
Castro-Jaimez, L. M., & Mariano, N. A. (2011). Efectos de un herbívoro especialista sobre los componentes de adecuación de un árbol pionero de la selva seca (Ipomoea pauciflora). In M. Osorio-Beristain, & C. Martínez-Garza (Eds.), Ecología de la selva seca: estudios de caso (pp. 58–61). Cuernavaca: Universidad Autónoma del Estado de Morelos.
Cepeda-Cornejo, V., & Dirzo, R. (2010). Sex-related differences in reproductive allocation, growth, defense and herbivory in three dioecious neotropical palms. Plos One, 5, e9824.
Coley, P. D. (1980). Effects of leaf age and plant life history patterns on herbivory. Nature, 284, 545–546.
Coley, P. D. (1983). Herbivory and defensive characteristics of tree species in a lowland tropical forest. Ecological Monographs, 53, 209–233.
Coley, P. D. (1987). Interspecific variation in plant antiherbivore properties: the role of habitat quality and rate of disturbance. New Phytologist (Suppl), 106, 251–263.
Coley, P. D., Bryant, J. P., & Chapin III, F. S. (1985). Resource availability and plant antiherbivore defense. Science, 230, 295–299.
Dirzo, R., & Boege, K. (2008). Patterns of herbivory and defense in tropical dry forests. In W. P. Carson, & S. A. Schnitzer (Eds.), Tropical forest community ecology (pp. 63–78). Oxford: Wiley-Blackwell.
Dirzo, R., & Domínguez, C. (1995). Plant-herbivore interactions in Mesoamerican tropical dry forests. In S. H. Bullock, H. A. Mooney & E. Medina (Eds.), Seasonally dry tropical forests (pp. 305–325). Cambridge: Cambridge University Press.
Endara, M. J., & Coley, P. D. (2011). The resource availability hypothesis revisited: a meta analysis. Functional Ecology, 25, 389–398.
Filip, V., Dirzo, R., Maass, J. M., & Sarukhán, J. (1995). Within- and among-year variation in the levels of herbivory on the foliage from a Mexican tropical deciduous forest. Biotropica, 27, 78–86.
Fine, P. V., Mesones, I., & Coley, P. D. (2004). Herbivores promote habitat specialization by trees in Amazonian forests. Science, 305, 663–665.
Fine, P. V., Miller, Z. J., Mesones, I., Irazuzta, S., Appel, H. M., Stevens, M. H. et al. (2006). The growth-defense trade-off and habitat specialization by plants in Amazonian forests. Ecology (Suppl.), 87, S150–S162.
Foroughbakhch, R., Alvarado-Vázquez, M. A., Hernández-Piñero, J. L., Rocha-Estrada, A., Guzmán-Lucio, M. A., & Treviño-Garza, E. J. (2006). Establishment, growth and biomass production of 10 tree woody species introduced for restoration and ecological restoration in northeastern Mexico. Forest Ecology and Management, 235, 194–201.
Hawkes, C. V., & Sullivan, J. J. (2001). The impact of herbivory on plants in different resource conditions: a meta-analysis. Ecology, 82, 2045–2058.
Herms, D. A., & Mattson, W. J. (1992). The dilemma of plants: to grow or defend. Quarterly Review of Biology, 67, 283–335.
Hernández, Y., Boege, K., Lindig-Cisneros, R., & del Val, E. (2014). Lepidopteran diversity in restored and successional sites in a tropical dry forest. Southwestern Naturalist, 59, 66–74.
Hilbert, D. W., Swift, D. M., Detling, J. K., & Dyer, M. I. (1981). Relative growth rates and the grazing optimization hypothesis. Oecologia, 51, 14–18.
Hothorn, T., Bretz, F., & Westfall, P. (2008). Simultaneous inference in general parametric models. Biometrical Journal, 50, 346–363.
Huante, P., Ceccon, E., Orozco-Segovia, A., Sánchez-Coronado, M. E., Acosta, I., & Rincón, E. (2012). The role of arbuscular mycorrhizal fungi on the early-stage restoration of seasonally dry tropical forest in Chamela, Mexico. Revista Árvore, 36, 279-289.
Huante, P., & Rincón, E. (1998). Responses of light changes in tropical deciduous woody seedlings with contrasting growth rates. Oecologia, 113, 53–66.
Huante, P., Rincón, E., & Acosta, I. (1995). Nutrient availability and growth rate of 34 woody species from a tropical dry deciduous forest in Mexico. Functional Ecology, 6, 849–858.
Johnson, M. T. (2011). Evolutionary ecology of plant defences against herbivores. Functional Ecology, 25, 305–311.
Juan-Baeza, I., Martínez-Garza, C., & del Val, E. (2015). Recovering more than tree cover: herbivores and herbivory in a restored tropical dry forest. Plos One, 10, 0128583.
Kalacska, M., Sánchez-Azofeifa, G. A., Calvo-Alvarado, J. C., Quesada, M., Rivard, B., & Janzen, D. (2004). Species composition, similarity and diversity in three successional stages of a seasonally dry tropical forest. Forest Ecology and Management, 200, 227–247.
Kattge, J., Díaz, S. Lavorel, S., Prentice, I. C., Leadley, P., Bönisch, G. et al. (2011). TRY-a global database of plants traits. Global Change Biology, 17, 2905-2945.
Lebrija-Trejos, E., Meave, J. A., Poorter, L., Pérez-García. E. A., & Bongers, F. (2010). Pathways, mechanisms and predictability of vegetation change during tropical dry forest succession. Perspectives in Plant Ecology, Evolution and Systematics, 12, 267–275.
Maschinski, J., & Whitham, T. G. (1989). The continuum of plant responses to herbivory: the influence of plant association, nutrient availability and timing. American Naturalist, 134, 1–19.
Martínez-Garza, C., Bongers, F., & Poorter, L. (2013). Are functional traits good predictors of species performance in restoration plantings in tropical abandoned pastures? Forest Ecology and Management, 303, 35–45.
Martínez-Garza, C., Osorio-Beristain, M., Valenzuela-Galván, D., & Nicolás-Medina, A. (2011). Intra and inter-annual variation in seed rain in a secondary dry tropical forest excluded from chronic disturbance. Forest Ecology and Management, 262, 2207–2218.
Martínez-Garza, C., Pena, V., Ricker, M., Campos, A., & Howe, H. F. (2005). Restoring tropical biodiversity: leaf traits predict growth and survival of late-successional trees in early-successional environments. Forest Ecology and Management, 217, 365–379.
Massad, T. J., Chambers, J. Q., Rolim, S. G., Jesús, R. M., & Dyer, L. A. (2011). Restoration of pasture to forest in Brazil’s Mata Atlântica: the roles of herbivory, seedling defenses, and plot design in reforestation. Restoration Ecology, 19, 257–267.
Mendoza-Segovia, Y. A. (2017). Análisis de la variación interespecífica e intraespecífica en la viabilidad de semillas del género Bursera (M. Sc.). Posgrado en Ciencias Biológicas, Facultad de Ciencias, UNAM.
Neves, F. S., Araújo, L. S., Espírito-Santo, M. M., Fagundes, M., Fernandes, G. W., Sanchez-Azofeifa, G. A. et al. (2010). Canopy herbivory and insect herbivore diversity in a dry forest-savanna transition in Brazil. Biotropica, 42, 112–118.
Neves, F. S., Silva, J. O., Espírito-Santo, M. M., & Fernandes, G. W. (2014). Insect herbivores and leaf damage along successional and vertical gradients in a tropical dry forest. Biotropica, 46, 14–24.
Pinheiro, J., Bates, D., DebRoy, S., & Sarkar, D. (2015). Nlme: linear and nonlinear mixed effects models. R package version 3.1-122, URL: http://CRAN.R-project.org/package=nlme
Poorter, L., Van de Plassche, M., Willems, S., & Boot, R. G. A. (2004). Leaf traits and herbivory rates of tropical tree species differing in successional status. Plant Biology, 6, 746–754.
Pringle, E. G., Adams, R. I., Broadbent, E., Busby, P. E., Donatti, C. I., Kurten, E. L. et al. (2011). Distinct leaf-trait syndromes of evergreen and deciduous trees in a seasonally dry tropical forest. Biotropica, 43, 299–308.
R Core Team. (2015). R: a language and environment for statistical computing [Internet]. R Foundation for Statistical Computing. Vienna, Austria. Available: URL https://www.R-project.org/
Rincón, E., & Huante, P. (1992). Growth responses of tropical deciduous tree seedlings to contrasting light conditions. Trees, 7, 202–207.
Rzedowski, J. y Kruse, J. (1979). Algunas tendencias evolutivas en Bursera. Taxon, 28, 103–106.
Silva, J. O., Espírito-Santo, M. M., & Melo, G. A. (2012). Herbivory on Handroanthus ochraceus (Bignoniaceae) along a successional gradient in a tropical dry forest. Arthropod-Plant Interactions, 6, 45–57.
Solis-Gabriel, L., Mendoza-Arroyo, W., Boege, K., & del Val, E. (2017). Restoring lepidopteran diversity in a tropical dry forest: relative importance of restoration treatment, tree identity and predator pressure. PeerJ, 5, e3344.
Soriano, D., Orozco-Segovia A, Márquez-Guzmán, J., Kitajima, K., Gamboa-de Buen, A., & Huante, P. (2011). Seed reserve composition in 19 tree species of tropical deciduous forest in Mexico and its relationship to seed germination and seedling growth. Annals of Botany, 107, 939–951.
Stamp, N. (2003). Out of the quagmire of plant defense hypotheses. Quarterly Review of Biology, 78, 23–55.



