Large-scale human environmental intervention is related to a richness reduction in Mexican odonates
Karina Cuevas-Yáñeza , Mariana Benítezb c , Maya Rochaa , Alex Córdoba-Aguilara ⁎ ✉
It is unclear how land use change, reduction in tree cover and human footprint impact species occurrence and co-occurrence especially at a large regional scale. This is particularly prevalent for species with complex life cycles, for example odonates (dragonflies and damselflies). We evaluated richness of odonates in Mexico in terms of land use, tree cover and human footprint. We also analyzed how odonate species co-occur to interpret our richness analysis using a community perspective. We used odonate collecting records from year 2000 to 2014. Odonate geographical records were more abundant in forest and agricultural areas, and decreased in areas without vegetation. Although our results may suffer of incomplete samplings, there was a positive relationship between species richness and tree cover, and a quadratic relationship with human footprint was observed. These results indicate that some degree of forest disturbance may still sustain relatively high odonate richness levels. Finally, species tend to co-occur in particular ensembles with some species being key in their ecological communities. Further studies should detail the role these key species play in their environments to provide community stability.
© 2017 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:
La intervención humana a gran escala está relacionada con la reducción de la riqueza de las libélulas y caballitos del diablo mexicanos
Resumen
No es claro cómo el cambio de uso de suelo, la reducción en la cobertura arbórea y la huella humana impactan la ocurrencia y coocurrencia de las especies especialmente a una gran escala regional. Esto es particularmente predominante en especies con ciclos de vida complejos, por ejemplo los odonatos (libélulas y caballitos del diablo). Por lo tanto, se evalúa aquí la riqueza de los odonatos en México en términos de uso de suelo, cobertura arbórea y huella humana. También se analiza cómo las especies de odonatos coocurren para interpretar nuestros análisis de riqueza usando una perspectiva de la comunidad. Se usaron registros de recolectas de odonatos del 2000 al 2014. Los registros geográficos fueron más abundantes en áreas de bosque y de agricultura, y decrecieron en áreas sin vegetación. Aunque nuestros resultados pueden tener el problema de muestreos incompletos, existió una relación positiva entre la riqueza de especies y la cobertura arbórea, y una relación cuadrática con la huella humana. Estos resultados indican que un cierto grado de disturbio arbóreo puede mantener niveles relativamente altos de riqueza de odonatos. Finalmente, las especies de odonatos tienden a coocurrir en ensambles particulares con otras especies siendo claves en sus ambientes proveyendo estabilidad en la comunidad.
Palabras clave
Libélulas; Caballitos del diablo; Uso de suelo; Huella humana; Riqueza de especies; Bosque; Copresencia; México
Introduction
At a global and local scale, changes in land use impose enormous pressures on biodiversity ( Foley et al., 2005; Newbold, Hudson, Hill, Contu, & Lysenko, 2015 ). Three variables linked with changes in land use are agricultural conversion, deforestation and human footprint ( Meyer & Turner, 1992 ). In the case of Mexico, the country has experienced a large change in land use ( González-Abraham et al., 2015; Mas et al., 2004 ; Robson and Berkes, 2011 ). For example, Mexican agricultural areas have dramatically increased over the last 20 years ( Hansen et al., 2013 ). At the same time, per capita forest area decreased by almost a third between 1980 and 2000 ( Mas et al., 2004; Velázquez et al., 2002 ). The human footprint in Mexico has been large and heterogeneous, and is explained by the complex physical geography, historic human settlements, technology use, and recent demographic explosion ( González-Abraham et al., 2015 ). Although it is intuitive that land use change, tree cover reduction and increased human footprint are driving Mexican organic diversity to extinction, precise measurements or even a rough approximation of this is lacking.
Land use change and deforestation have been shown to affect the biodiversity of many taxa ( Barragán, Moreno, Escobar, Halffter, & Navarrete, 2011 ; Brown, 1997; Schulze et al., 2004; Vidal, López-García, & Rendón-Salinas, 2014 ). However, such relationships are not necessarily straightforward and linear. For example, agricultural areas may drive reduced species richness but such effects can be more acute with the prevalence of cultivated species, intensive land management and short cultivated cycles ( Scales & Marsdens, 2008 ). In contrast, take the case of some coffee plantations which, despite altering vegetation structure of a community, still provide protection to a number of native species ( Martínez et al., 2005; Perfecto & Vandermeer, 2015 ; Perfecto, Rice, Greenberg, & Van der Voort, 1996 ). The impacts of land use change and deforestation on biodiversity can be taxa-dependent as some taxonomic groups are more resilient than others ( Hansen et al., 2001 ). For example, birds are commonly thought to be highly sensitive to land use change. However, some particular bird taxa are more likely than others to go extinct ( Fischer et al., 2007 ). In this case, the simplicity of landscape structure may render some species to become more likely to disappear if such species do not find their vital resources ( Fischer et al., 2007).
Conservation biologists have recently come to consensus that beyond species, there is a need to assess community level interactions ( Rodewald, Rohr, Fortuna, & Bascompte, 2014 ; Tilianakis, Laliberté, Nielsen, & Bascompte, 2010 ). Ecological interactions between species can affect community composition and dynamics (e.g. Faust & Raes, 2012; Thébault & Fontaine, 2010; Verdú & Valiente-Banuet, 2008 ). A classic example is that of microbial communities in which the presence or absence of certain species will affect the interactions of all species ( Rodríguez-Martínez & Pascual, 2006 ). This implies that communities occur non-randomly and that, in conservation terms, the community structure and dynamics should be assessed. There are several methods to systematically characterize and study ecological networks ( Pascual & Dunne, 2005 ). Among these methods, co-occurrence networks provide a valuable tool to infer statistical relationships among species or other taxonomic units in relatively large datasets, allowing one to go beyond composition and abundance and examine community structure ( Deng et al., 2012; Faust & Raes, 2012 ). Two of the structural parameters that have been proposed to reflect the relevance of particular nodes in an ecological network: (1) the number of connections a focal node has with other nodes, and (2) a focal node’s betweenness centrality (the proportion of the shortest paths between all i and j pair of nodes that contains the focal node) ( Jordan, 2009; Jordán, Liu, & Davis, 2006 ). Many ecological networks exhibit modular organization, meaning that their elements tend to form semi-autonomous groups with more interactions within them than with other groups. Such a modular structure suggests that ecological interactions lead to the formation of well-defined groups of species, which could in turn be relevant with respect to conservation and ecosystem stability ( Pascual & Dunne, 2005; Thébault & Fontaine, 2010 ).
One relatively neglected functional group in conservation biology are complex life cycle (CLC) organisms. CLC animals are those that face an abrupt habitat shift as they go through different developmental stages and so they may be exposed to radically different ecological conditions and stressors ( Werner, 1988 ). For example, many animals with CLC spend their larval stage being aquatic while the adult is terrestrial. In terms of conservation strategies, CLC animals present a more complicated scenario for their protection as their different transitions (e.g. aquatic and terrestrial) need to be considered both for the stressors involved and their habitat protection. Odonate insects (dragonfly and damselflies) are an example of a CLC animal that has gained increased conservation attention (e.g. Bried, Hassall, Simaika, Corser, & Ware, 2015 ; Clausnitzer et al., 2009; Kalkman et al., 2008; Samways, McGeoch, & New, 2013 ). Calls for conservation efforts directed toward odonates focus on the transformation of the body waters they use as larvae and their aerial surroundings used by flying adults ( Bried & Samways, 2015; Corbet, 1999; New, 2009 ). The relationship between land use and odonate species richness shows mixed results. On one hand, it has been recorded that forest areas of agricultural areas may contribute to odonate richness as in the case of European farms ( Sahlén, 2006; Ruggiero, Céréghino, Figuerola, Marty, & Angélibert, 2008 ), but this is not necessarily so for tropical areas (e.g. Costa Rica; Hofhansl & Schneeweihs, 2008 ). Indeed, habitat changes affect populations of odonates very quickly and dramatically ( Harabis & Dolny, 2011). For example, Junior et al. (2015) observed that urbanization led to changes in odonate species composition and richness. Habitat quality (i.e. physical structure) affected odonate assemblages. Oliveira-Junior et al. (2015) found that moderately-degraded areas may host high species richness. Kadoya, Akasaka, Aoki, and Takamura (2011) conducted a heuristic analysis of contributing factors affecting odonate diversity and found that although water quality conditions largely explain species losses, land use change can also contribute ( Kadoya et al., 2011 ). Note, however, that many of these analyses have relied on simplified land use changes such as forest conversion to farms. Thus, further analyses are needed to examine how general is the relation between land use (at a broad scale level) and odonate diversity. Moreover, such analyses can be enriched if the component of community-level interactions is added. Some authors have begun such a community-level analyses: odonate communities have been related to habitat types (e.g. using canonical correspondence analysis; Clausnitzer, 2003; Stewart & Samways, 1998 ). Although valuable, these efforts do not provide information with regard to the role of species in maintaining community stability as the co-occurrence networks we explained above.
In this paper we examine how deforestation and land use affect odonate species richness in the Mexican territory. This was done using incidence of odonate geographical records according to the different land uses reported in Mexico from the year 2000 to 2014. As a second aim, we have analyzed the co-occurrence of species presence in the Mexican territory as a preliminary attempts at a community-level analysis.
Materials and methods
We searched for odonate species records in Mexico from 2000 to 2014 using Conabio (2015) databases, published literature and unpublished data. Records were depurated and georeferenced, if needed (note that 1 geographical species record consists of only 1 species, but 1 geographical coordinate may contain more than 1 species). We considered presence-absence species data as we did not take into account the abundance of total species records of each species in 1 geographical coordinate. This abundance data may have provided several useful resources to our study such as, for example, how human impact may have impacted species richness at the population level. Odonate species records were counted (even if there were several for the same species) per geographic coordinate and assigned to the corresponding land use entities as referred by the Mexican Land Use and Vegetation map, series V (Layer Union) ( Inegi, 2011) (Table 1 ). In each land use entity, we counted all species and geographic records. This was taken as species richness and presence of records respectively.
Table 1
Land use entities taken from land use and vegetation maps (Series V, INEGI 2011) and number of odonate species records between 2000 and 2014.
| Land use or vegetation entity | Description | Odonate species richness | Odonate species records |
| Agricultural | Rainfed and irrigation agriculture | 196 | 1,282 |
| Agricultural-grassland | Rainfed agriculture predominance, cultivated or induced grass in less proportion | 61 | 95 |
| Agricultural-rainforest | Rainfed agriculture, secondary vegetation of low, medium or high tropical forest | 96 | 181 |
| Area without vegetation | Without apparent vegetation cover | 1 | 1 |
| Urban area | Cities, towns or urban sprawls | 129 | 315 |
| Forest/Rainforest | Pine, oak-pine, oyamel, or cloud forest/low, medium or high tropical forest | 288 | 1,326 |
| Waterbody | Maritime, or internal perennial water body | 31 | 42 |
| Scrub | Microphyte desert, rosetophilous, crassicaule, subtropical, thorn scrub, | 93 | 247 |
| Other vegetation | Mangrove, mesquite, halophytic vegetation | 42 | 52 |
| Grassland | Cultivated or induced (or natural) grass | 106 | 256 |
| Grassland-Agricultural | Cultivated or induced grass, rainfed agriculture | 30 | 33 |
| Grassland-Rainforest | Cultivated or induced grass, secondary vegetation of low, medium or high tropical forest | 25 | 29 |
| Rainforest-Agricultural | Secondary vegetation of low, medium or high tropical forest, rainfed agriculture | 56 | 58 |
| Rainforest-Grassland | Secondary vegetation of low, medium or high tropical forest, induced or cultivated grass | 75 | 154 |
A grid of 15 × 15 km approximately for each pixel size was used. This grid size was used as the best logistical compromise between collection effort and the probability of finding a collected odonate. For example, a smaller grid size could have been used but given that there are few collections, richness could not have been calculated. Each pixel with at least 1 species was considered. All odonate species that fell inside each pixel were counted. The total species number observed per pixel was considered as a measure of species richness (herein, SOBS). Given that we were unaware of how complete richness was per pixel, we also calculated the Chao2 Index ( Chao, 1987 ; CHAO2). Both calculations were made with DIVA-GIS v.7.5 ( Hijmans, Guarino, Cruz, & Rojas, 2002 ).
To assess the relationship between odonate richness with tree cover presence, we obtained the tree coverage according to Global Forest Change ( Hansen et al., 2013 ). Trees were defined as vegetation taller than 5 meters, and so we used the series 2000 percent tree cover ( Hansen et al., 2013 ). Human footprint was assessed as the influence index (HII) from Socioeconomic Data and Applications Center (SEDAC). HII provides an updated view of anthropogenic environmental impact, considering human population density, human land and infrastructure, and human access (roads, railroads, navigable rivers or coastlines) (WCS, CIESIN, 2005 ). In each grid pixel, we calculated the average value of tree cover percentage (AVGTC) and the human influence index (AVGHII) with the ArcMap extension of ArcGIS v.9.3 ( ESRI, 2008 ). Then, average values of AVGTC and AVGHII were regressed between the SOBS and CHAO2 respectively. We searched for the best fit of a model regression in each case. Analyses were made using Minitab v.17 software (Minitab Inc.).
From the odonate species records, we generated a file containing a list of all species in the records and their count in each of the land-use types. This file was processed using CoNet ( http://psbweb05.psb.ugent.be/conet/ ), a Cytoscape 3.2.1 application that has been used to infer statistical relationships among species or other taxonomical units within communities ( Faust & Raes, 2012; Faust et al., 2012 ). In order to infer the significant co-occurrence links among Odonata species in the records, we set CoNet to use the count mode and the Pearson correlation index with a threshold of 0.9 . Co-occurrence links were graphed using Cytoscape 3.2.1. This rendered a network in which nodes represent odonate species and edges stand for significant co-occurrence relationships. Connectivity and betweenness centrality of the nodes were calculated using the Cytoscape 3.2.1 built-in network analyzer. Finally, the network modules (potential communities) were identified with the Cytoscape 3.2.1 application ModuLand ( Szalay-Bekö et al., 2012).
Results
We found 4,071 species records from 275 odonate species (information available upon request). Species richness and records were mainly from forest/rainforest and agricultural areas which accounted for approximately 60%, with around 30% of all species records for each area. Forest in general encompassed 23.4% of total richness, followed by agricultural areas with 15.9% of the whole species total, and urban areas with 10.5%.
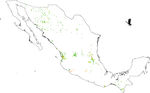
Our results suggest that odonate species and records occurring in the Mexican territory are scarce in areas without vegetation and with grassland-rainforest ( Table 1, Figs. 1 and 2 ). Odonates collected in urban areas represented 7.7% of records and 10.5% of species richness. Desert areas contributed with 7.6% of the species richness (6.1% of the records). There was a significant correlation between odonate records and species richness per land use (Pearson r = 0.987, p < 0.00001). The highest SOBS and CHAO2 values were found mostly for pixels located in Michoacán, Nayarit, San Luis Potosí and Veracruz states, while the lowest values were found in Sonora, Chihuahua, Coahuila and Zacatecas states with only 1 species per pixel ( Figs. 3 and 4).
Figure 1
Land use, vegetation entity, odonate species richness (black bars) and geographical records (gray bars) in the Mexican territory 2.
Figure 2
Odonate species records for the Mexican territory. Collection range spanned from 2000 to 2014.
Figure 3
Observed odonate species (SOBS) in a 15 × 15 km (approximately) pixel size for the Mexican territory. Green pixels indicate from 1 to 23 species, yellow pixels indicate 21–26 species, orange pixels indicate 27–40 species, while red pixels indicate 40–65 species.
Figure 4
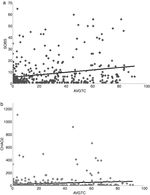
Number of odonate observed spe cies (SOBS) (a) and Chao 2 (CHAO2) (b) regressed against tree cover average (AVGTC).
A positive linear relation between SOBS and AVGTC ( n = 406, linear R 2 = 0.05, p < 0.005) was found. CHAO2 showed a similar tendency ( n = 406, R 2 = 0.0139, p = 0.017) (Fig. 4 a, b). The regression model with the best fit was quadratic for both SOBS ( n = 406, quadratic R 2 = 0.0437, p = 0.001; Fig. 5a) and CHAO2 (n = 406, quadratic R 2 = 0.0139, p < 0.05; Fig. 5b) versus AVGHII.
Figure 5
Number of observed species (SOBS) (a) and Chao 2 (CHAO2) (b) regressed against human influence index (AVGHII).
Regarding the analysis at the community level, the co-occurrence network generated from the records suggest that Odonata species indeed tended to associate in well-defined modules or communities ( Fig. 6 a). Moreover, this analysis uncovers some species as nodes with a particular high connectivity or betweenness centrality, pointing at potentially relevant species in terms of the conservation of whole communities ( Fig. 6b; Table 2).
Figure 6
Structure of the co-occurrence network inferred from the odonate species counts in the different land-use types. Nodes represent species and edges correspond to the statistically significant co-occurrence links. Colors in (a) represent the different modules that were identified and that could correspond to different communities. In (b), the size of each node is proportional to its betweenness centrality, while its color corresponds to how densely connected a node’s neighborhood is.
Table 2
Odonate species ranked (most important-less important) according to their role in centrality and betweenness as suggested by the network analysis.
| Centrality | Betweenness |
| Macrothemis ultima | Macrothemis imitans |
| Brechmorhoga tepeaca | Erpetogomphus bothrops |
| Enallagma praevarum | Argia pallens |
| Orthemis discolor | Erythrodiplax fervida |
| Remartinia luteipennis | Phyllocycla elongata |
| Ischnura capreolus | Ischnura ramburii |
| Brechmorhoga praecox | Gynacantha nervosa |
| Erythrodiplax fusca | |
| Cannaphila insularis | |
| Orthemis levis | |
| Micrathyria didyma | |
| Epigomphus crepidus | |
| Erythemis haematogastra | |
| Protoneura aurantiaca | |
| Perithemis tenera | |
| Argia barretti | |
| Palaemnema paulitoyaca | |
| Erythemis vesiculosa | |
| Macrothemis hemichlora | |
| Brechmorhoga vivax | |
| Enallagma novaehispaniae | |
| Argia pulla | |
| Dythemis nigra | |
| Hetaerina occisa | |
| Argia garrisoni | |
| Erpetogomphus constrictor | |
| Perithemis mooma | |
| Protoneura cara | |
| Argia oenea |
Discussion
The relatively low number of odonate records in this analysis may affect our results and thus our discussion below has to be framed within this situation. However, waiting to increase sampling effort in such a big country may be meaningless for Mexico given that the number of specialists has not augmented in the last 3 decades ( González-Soriano & Novelo-Gutiérrez, 2014 ). This suggests that sampling effort will not increase.
In relation to the land use, our results indicated (not surprisingly) a prominent role of forest or tropical forest areas in preserving odonate presence (i.e. over one third of records were collected in these areas). It is well-known that oligiotrophic waters (with macrophyte presence) and good riparian vegetation coverage are essential components for the odonate life cycle (e.g. Remsburg & Turner, 2009; Samways & Steytler, 1996; Stewart & Samways, 1998 ). These components can be present in forested and even agricultural water bodies. Coherent with this, a substantial portion of the records (30%) and 20% of all species were located in agricultural areas. This indicates that agricultural areas are also important reservoirs for Mexican odonate species. This finding echoes previous odonate studies in other countries. For example, in lowland Pacific Costa Rica, species’ accumulation curves showed highest species richness in forest margin habitats followed by agricultural areas ( Hofhansl & Schneeweihs, 2008 ). Not all agricultural practices impact species richness negatively (e.g. Hofmann & Mason, 2005; Lenat, 1984 ). The precise mechanism(s) that explains why cultivated areas are key for odonate species richness and communities in the Mexican scenario is unclear. We speculate that conditions that affect larval and adult survival in aquatic and terrestrial places play a key role in explaining this pattern. With such a large number of odonate species in our study, describing such conditions is an impossible task. Agricultural area sampling was more intense in our database than forested area sampling: 60% of records are from agricultural areas while only 6% of records are from forested areas. Rather than odonates being less common in these areas, an alternative explanation is that forested areas with combined land use have been less collected. A bias in collecting effort is actually one problem in our analysis. This may explain, for example, why we found a positive relationship between odonate records and species richness. As a matter of fact, odonate collection in the country of Mexico has rarely been systematically planned with the exception of research dissertations ( González-Soriano & Novelo-Gutiérrez, 2014 ). This may have resulted in biased sampling such as collecting near roads or populated areas ( Cuevas-Yáñez, Rivas, Muñoz, & Córdoba Aguilar, 2015 ), a phenomenon that has been also described for other insects such as butterflies ( New, 2009 ). Surprisingly, richness was fairly high in urban areas. Urban aquatic areas are stressed sites where odonate richness is usually impaired. At the general level, 3 sources of stress can negatively affect odonate diversity: landscape fragmentation (e.g. Chovanec et al., 2000 ), vegetation loss/or modification ( Jeanmougin, Leprieur, Loís, & Clergeau, 2014 ) and sewage discharge and/or stormwater runoff ( Henriques-Oliveira, Baptista, & Nessimian, 2007 ). Despite these stressors, a prominent number of odonate species have been found to occur in urban areas (reviewed by Villalobos-Jiménez, Dunn, & Hassall, 2016 ). These odonates include generalists, specialists and even threatened species. This indicates: (a) a prominent role for urban aquatic ecosystems, and (b) a large level of tolerance to urbanization by odonates. For our study, one case is that of Mexico City which despite being one of the largest cities in the world, it has a fairly high diversity ( González-Soriano & Novelo-Gutiérrez, 2007 ). One reason is that some areas have remained safe to the stressors above despite being within the urban boundary.
Our results also indicated some geographical areas of particular importance for odonate conservation: including regions in Michoacán, Nayarit, San Luis Potosí and Veracruz states. González-Soriano, Noguera, and Oñate (2011) suggested the Huasteca region of San Luis Potosí as an odonate hot spot which concurs with our findings. Clearly, these areas deserve immediate attention in terms of conservation. Sadly, studies that assess the odonate conservation status in the country of Mexico have been rare (e.g. Cuevas-Yáñez et al., 2015 ). This, along with current Mexican legislation which does not include any odonate as threatened (NOM-059-Semarnat-2010; Semarnat, 2010 ), implies that efforts should be directed at working with local and federal governments to outline an action plan toward odonate conservation assessment.
A weak but positive relationship between tree cover and odonate species richness was also observed. With regards to how novel these results are, scarce information exists on tree cover and dragonfly richness in tropical areas. In fact, Clausnitzer et al. (2009) highlighted that the highest odonate richness are in running waters in tropical forests partly arguing in favor of the role of the tree cover effect. Mexican neotropical forest regions have highly complex habitats. Forest complexity provides odonates habitats where they may shelter at night, or safely secure against adverse weather conditions (e.g. rain or strong wind) or predation. If farm sites are near forest, the latter can function as a refuge or buffer that allows establishment of odonate species ( Paulson, 2006 ). Although it is true that shaded conditions have a negative effect on odonate presence ( Remsburg & Turner, 2009 ), forest cover still plays an important role in almost every part of the life cycle of the average odonate. Mechanisms that mediate odonate survival in general can be manifold – thus there is not a sole role for landscape biotic integrity. For example, in Japan, more than half of the odonate species depend on its forest, although at a broad-scale, climate largely explains species distribution ( Tsubaki & Tsuji, 2006 ). Despite this, the Mexican scenario for odonate conservation should emphasize the maintenance of tree cover even after native forests are transformed. One example is the case of coffee plantations which retain canopy integrity and, in combination with native forest, may become key refuges for several local plant and animal species ( Martínez et al., 2005; Perfecto & Vandermeer, 2015; Perfecto et al., 1996 ). Actually, odonate species that are currently in danger, e.g. Paraphlebia zoe , are likely to be protected thanks to such combination of coffee plantation-original cloud forests in Veracruz and Hidalgo states in Mexico ( Cuevas-Yáñez et al., 2015 ).
Our analysis showed a quadratic relationship between human influence and odonate richness and richness completeness. Nonlinear regressions have already described for odonate community structure (e.g. Southern High Plains in the USA; Hernández, Reece, & McIntyre, 2006 ). Notice, however, that our regression coefficients were low. This suggests that other variables not considered in our study are involved in predicting odonate richness. The effects of human activity on odonate populations have been documented in lentic (standing) waters in temperate zones ( Findlay & Houlahan, 1997; Hamasaki et al., 2009; Hall, McCauley, & Fortin, 2015 ; Raebel et al., 2012 ), but rarely in tropical zones and lotic systems ( Monteiro-Junior, Juen, & Hamada, 2015 ). Anthropogenic disturbance in the form of, for example, road construction, may have significant effects on wetlands biodiversity ( Findlay & Houlahan, 1997 ). In our analysis, the highest richness was in sites with an intermediate human footprint. This tendency has been also observed in odonates of the Amazon basin ( Junior et al., 2015). Stewart and Samways (1998) also noticed that high dragonfly species richness occurs in streams with moderate disturbance, although the species that live in such places are not endangered or threatened. This phenomenon may be explained by a high metapopulation succession dynamics in sites altered by anthropogenic factors or that such high dynamics are weighted by less complex adaptations in habitat selection or may function as refuges for species survival ( Harabis & Dolny, 2011 ). Another explanation is that collection and sampling may be biased by the collectors’ preferences for places close to urban areas (e.g. aquatic places close to roads) to avoid the challenge implied by areas of difficult access.
The co-occurrence network suggested that odonate species are organized in non-random communities and that some species may reinforce each other’s presence. While this network offers a first look at the potential community organization of Odonata species, it is important to bear in mind that it represents statistical relationships and that more in-depth studies will be necessary to uncover the direct or indirect interactions underlying co-occurrences. Related to this, among the future challenges is the functional or taxonomical characterization of the modules, or proposed communities, which could help uncover some of the principles behind their assembly. Species with high connectivity or betweenness centrality seem to show very diverse biological properties and/or ecological needs: modules typically (1) include both odonate suborders: Anisoptera (e.g. Macrothemis imitans) and Zygoptera (e.g. Ischnura capreola ); (2) include small (e.g. Ischnura ramburi) and big (e.g. Remartinia luteipennis ); (3) engage in territorial (e.g. Hetaerina titia ) and non-territorial (e.g. Enallagma novahispaniae ) male mating behavior; (4) use lotic (e.g. Brechmorhoga tepeaca), and lentic (e.g. Orthemis discolor ) waters, and, (5) use open (e.g. Erythemis vesiculosa) but also closed (e.g. Brechmorhoga praecox ) habitats. Nevertheless, if anything, these species seem to share a widespread distribution. How this property (or others) may affect community stability deserves further studies.
Finally, what is to be learned in terms of the functional property of odonates being CFC organisms and our results? Given that odonates make use of aquatic and terrestrial areas, we need to pay attention to the different stressors that occur in such distinct places and the preservation of both habitats ( Stoks & Córdoba-Aguilar, 2010 ). On one hand, stressors in the aquatic environment are usually agrochemicals which in many cases increase eutrophication or decrease macrophyte presence (e.g. Kadoya et al., 2011; Raebel et al., 2012; Weisner et al., 2007 ). On the other hand, stressors such as the reduction of tree cover imply a number of negative actions for odonates such as: fewer perching refuges for resting and predation protection, less available food, increased sun exposure and less humidity. Although the aim of our paper is not to detail a conservation plan, in general the above stressors need to be reduced to preserve odonate presence. Indeed, the challenge is huge.
Acknowledgements
We thank R. Behrstock, S. Upson, and D. Danforth who kindly provided part of the literature used in this research. To C. Anderson for key comments and English editing. KC-Y acknowledges a graduate grant provided by the Consejo Nacional de Ciencia y Tecnología (226198/210553) and the support by the Posgrado en Ciencias Biológicas (Universidad Nacional Autónoma de Mexico, UNAM). Thanks also to J.C. Rivera for the logistic support in the database polishing as well as to M. Rivas for GIS suggestions. This project was financed by PAPIIT IN203115 and Conacyt 221341 grants.
Referencias
Barragán, F., Moreno, C.E., Escobar, F., Halffter, G. & Navarrete, D. (2011). Negative impacts of human land use on dung beetle functional diversity PLoS One 6:e17976-e17976.
Brown, K. S. (1997). Diversity disturbance and sustainable use of Neotropical forests: insects as indicators for conservation monitoring. Journal of Insect Conservation, 11, 25-42.
Bried, J. T., Hassall, C., Simaik a, J. P., Corser, J. D. & Ware, J. (2015). Directions in dragonfly applied ecology and conservation science. Freshwater Science, 35, 1020-1022.
Bried, J. T. & Samways, M. J. (2015). A review of odonatology in freshwater applied ecology and conservation science. Freshwater Science, 35, 1023-1031.
Chao, A. (1987). Estimating the population size for capture-recapture data with unequal catchability. Biometrics, 43, 783-791.
Clausnitzer, V. (2003). Dragonfly communities in coastal habitats of Kenya: indication of biotope quality and the need of conservation measures Biodiversity and Conservation, 12, 333-356.
Clausnitzer, V., Kalkman, V. J., Ram, M., Collen, B., Baillie, J. E., Bedjanič, M, & Wilson, K. (2009). Odonata enter the biodiversity crisis debate: the first global assessment of an insect group. Biological Conservation, 142, 1864-1869.
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad (2015) Portal de geoinformación Sistema Nacional de Información sobre Biodiversidad http://wwwconabiogobmx/informacion/gis/. Accessed on 20 August 2015
Corbet, P. S. (1999). Dragonflies Behavioral and Ecology of Odonata. Colchester Harley Books
Cuevas‐Yáñez, K., Rivas, M., Muñoz, J. & Córdoba‐Aguilar, A. (2015). Conservation status assessment of Paraphlebia damselflies in Mexico. Insect Conservation and Diversity, 8, 517-524.
Deng, Y., Jiang, Y. H., Yang, Y., He, Luo, F. & Zhou, J. (2012). Molecular ecological network analyses BMC Bioinformatics, 13, 31.
ESRI, 2008 ArcGIS released 93 Software CA ESRI (wwwesricom)
Faust, K. & Raes, J. (2012). Microbial interactions: from networks to models. Nature, 10, 538-550.
Faust, K., Sathirapongsasuti, J. F., Izard, J., Segata, N., Gevers, D., Raes, J, & Huttenhower, C. (2012). Microbial co-occurrence relationships in the human microbiome. PLoS Comput Biol 8:e100260
Findlay, C. S. & Houlahan, J. (1997). Anthropogenic correlates of species richness in southeastern Ontario wetlands. Conservation Biology, 11, 1000-1009.
Fischer, J., Lindenmayer, S. P., Blomberg, S. P., Montague-Drake, R., Felton, A, & Stein, J. A. (2007). Functional richness and relative resilience of bird communities in regions with different land use intensities. Ecosystems, 10, 964-974.
Foley, J. A., DeFries, R., Asner, G. P., Barford, C., Bonan, G., Carpenter, S. R., Chapin, F. S, Coe, M. T., Daily, G. C., Gibbs, H. K., Helkowski, J. H., Holloway, T., Howard, E. A., Kucharik, C. J., Monfreda, C., Patz, J. A., Prentice, C., Ramankutty, N, & Snyder, P. K. (2005). Global consequences of land use. Science, 309. 570-574,
González-Abraham, C., Ezcurra, E., Garcillán, P. P., Ortega-Rubio, A., Kolb, M. & Bezaury, C. J. (2015). The human footprint in Mexico: physical geography and historical legacies. PloS One 10:e0121203
González-Soriano, E., & Novelo-Gutiérrez, R. (2014). Biodiversidad de Odonata en México. Revista Mexicana de Biodiversidad, 85, 243-251.
González-Soriano, E., Noguera, F. & Oñate, L. (2011). A biodiversity hotspot for odonates in Mexico: The Huasteca Potosina San Luis Potosí. Odonatologica, 40, 179–190.
Hall, A. M., McCauley, S. J, & Fortin, M. J. (2015). Recreational boating landscape configuration and local habitat structure as drivers of odonate community composition in an island setting. Insect Conservation and Diversity, 8, 31-42.
Hamasaki, K., Yamanaka, T., Tanaka, K., Nakatani, Y., Iwasaki, N. & Sprague, D. S. (2009). Relative importance of within-habitat environment land use and spatial autocorrelations for determining odonate assemblages in rural reservoir ponds in Japan. Ecological Research, 24, 597-605.
Hansen, A. J., Neilson, R. P., Dale, V. H., Flather, C. H., Iverson, L. R., Currie, D. J., Shafer, S., Cook, R. & Bartlein, P. J. (2001). Global change in forests: responses of species communities and biomes. BioScience 51, 765-779.
Hansen, M. C., Potapov, P. V., Moore, R., Hancher, M., Turubanova, S. A., Tyukavina, A. & Townshend, J. R. G. (2013). High-resolution global maps of 21st-century forest cover change Science, 342, 850-853.
Harabis, F. & Dolny, A. (2011). The effect of ecological determinants on the dispersal abilities of central European dragonflies (Odonata). Odonatologica, 40, 17-26.
Hernandez, K. M., Reece, B. A. & McIntyre, N. E. (2006). Effects of anthropogenic land use on Odonata in playas of the Southern High Plains West N. American Naturalist, 66, 273-278.
Hijmans, R. J., Guarino, L., Cruz, M. & Rojas, E. (2002). Computer tools for spatial analysis of plant genetic resources data: 1 DIVA-GIS. Pl. Genetical Research, 127, 15-19.
Hofhansl, F. P. & Schneeweihs, S. (2008). Banderillas: Effects of deforestation on dragonflies (Insecta Odonata) in the Pacific lowland of Costa Rica. Zugleich Kataloge der oberosterreichischen Landesmuseen Neue Serie, 80, 237-247.
Hofmann, T. A, & Mason, C. F. (2005) Habitat characteristics and the distribution of Odonata in a lowland river catchment in eastern England. Hydrobiologia, 539, 137-147.
INEGI. (2011). Carta de uso de suelo y vegetación Capa Unión Serie V.
Jordán, F. (2009). Keystone species and food webs. Philosophical Transactions of the Royal Society B, 364, 1733-1741.
Jordán, F., Liu, W. C. & Davis, A. J. (2006). Topological keystone species: measures of positional importance in food webs. Oikos, 112, 535-546.
Junior, J. M. B., Shimano, Y., Gardner, T. A., Hughes, R. M., Marco, Júnior P. & Juen, L. (2015). Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral Ecology, DOI: 101111/aec12242
Kadoya, T., Akasaka, M., Aoki, T. & Takamura, N. (2011). A proposal of framework to obtain an integrated biodiversity indicator for agricultural ponds incorporating the simultaneous effects of multiple pressures. Ecological Indicators, 11, 1396-1402.
Kalkman, V. J., Clausnitzer, V., Dijkstra, K. D. B., Orr, A. G., Paulson, D. R. & van Tol, J. (2008). Global diversity of dragonflies (Odonata) in freshwater. Hydrobiologia, 595, 351-363.
Lenat, D. R. (1984). Agriculture and stream water quality: a biological evaluation of erosion control practices. Environmental Management, 8, 333-343.
Martínez, M. L., Pérez-Maqueo, O., Vázquez, G., Castillo-Campos, G., García-Franco, J., Mehltreter, K., Equihua, M. & Landgrave, R. (2005). Effects of land use change on biodiversity and ecosystem services in tropical montane cloud forest of Mexico. Forest Ecology and Management, 258, 1856-1863.
Mas, J. F., Velázquez, A., Díaz-Gallegos, J. R., Mayorga-Saucedo, R., Alcántara, C., Bocco, G. & Pérez-Vega, A. (2004). Assessing land use/cover changes: a nationwide multidate spatial database for Mexico. International Journal of Applied Earth Observation and Geoinformation, 5, 249-261.
Meyer, W. B. & Turner, B. L. (1992). Human population growth and global land-use/cover change. Annual Review of Ecology, Evolution and Systematics, 23, 39-61.
Minitab 17 Statistical Software (2013). State College PA: Minitab Inc (wwwminitabcom)
Monteiro Junior,. C. S., Juen, L. & Hamada, N. (2015). Analysis of urban impacts on aquatic habitats in the central Amazon basin: adult odonates as bioindicators of environmental quality. Ecological Indicators, 48, 303-311.
New, T. R. (2009). Insect Species Conservation. Cambridge University Press Cambridge
Newbold, T., Hudson, L. N., Hill, S. L., Contu, S., Lysenko, I., Senior, R. A. & Purvis, A. (2015). Global effects of land use on local terrestrial biodiversity. Nature, 520, 45-50.
NOM-059-SEMARNAT (2010). Protección ambiental – especies nativas de México de Flora y Fauna Silvestres Categorías de riesgo y especificaciones para su inclusión exclusión o cambio – Lista de especies en riesgo available at http://bibliotecasemarnatgobmx/janium/Documentos/Ciga/agenda/DOFsr/DO2454pdf. Last accessed Nov 13 2015.
Pascual, M., & Dunne, J. A. (Eds) (2005) Ecological Networks: Linking Structure to Dynamics in Food Webs. Oxford University Press
Paulson, D. (2006). The importance of forests to neotropical dragonflies Forests and dragonflies (ed A Cordero Rivera). 79-101 Pensoft Publishers Sofia
Perfecto, I. & Vandermeer, J. (2015). Coffee Agroecology: A New Approach to Understanding Agricultural Biodiversity Ecosystem Services and Sustainable Development. Routledge
Perfecto, I., Rice, R. A., Greenberg, R. & Van der Voort, M. E. (1996). Shade coffee: a disappearing refuge for biodiversity. BioScience, 46, 598-608.
Raebel, E. M., Merckx, T., Feber, R. E., Riordan, P., Macdonald, D. W. & Thompson, D. J. (2012). Identifying high‐quality pond habitats for Odonata in lowland England: implications for agri‐environment schemes. Insect Conservation and Diversity, 5, 422-432.
Remsburg, A. J. & Turner, M. G. (2009). Aquatic and terrestrial drivers of dragonfly (Odonata) assemblages within and among north-temperate lakes. Journal of the North American Benthological Society, 28, 44-56.
Robson, J. P. & Berkes, F. (2011). Exploring some of the myths of land use change: Can rural to urban migration drive declines in biodiversity? Global Environmental Change, 21, 844-854.
Rodewald, A. D., Rohr, R. P., Fortuna, M. A. & Bascompte, J. (2014). Community-level demographic consequences of urbanization: an ecological network approach. Journal of Animal Ecology, 83, 1409-1417.
Rodríguez-Martínez, J. M. & Pascual, A. (2006). Antimicrobial resistance in bacterial biofilms. Reviews in Medical Microbiology, 17, 65-75.
Ruggiero, A., Céréghino, R., Figuerola, J., Marty, P. & Angélibert, S. (2008). Farm ponds make a contribution to the biodiversity of aquatic insects in a French agricultural landscape. Comptes Rendus Biologies, 331, 298-308.
Sahlén, G. (2006). Specialists vs generalists in the Odonata–the importance of forest environments in the formation of diverse species pools In: Cordero A (ed) Forests and Dragonflies. Pensoft Publishers Sofia, pp 153-179
Samways, M. J. & Steytler, N. S. (1996). Dragonfly (Odonata) distribution patterns in urban and forest landscapes and recommendations for riparian management. Biological Conservation, 78, 279-288.
Samways, M. J., McGeoch, M. A. & New, T. R. (2013). Insect Conservation A Handbook of Approaches and Methods. Oxford University Press Oxford
Scales, B. R. & Marsden, S. J. (2008). Biodiversity in small-scale tropical agroforests: a review of species richness and abundance shifts and the factors influencing them. Environmental Conservation, 35, 160-172.
Schulze, C. H., Waltert, M., Kessler, P. J., Pitopang, R., Veddeler, D., Mühlenberg, M. & Tscharntke, T. (2004). Biodiversity indicator groups of tropical land-use systems: comparing plants birds and insects. Ecological Applications, 14, 1321-1333.
Stewart, D. A. & Samways, M. J. (1998). Conserving dragonfly (Odonata) assemblages relative to river dynamics in an African savanna game reserve. Conservation Biology, 12, 683-692.
Stoks, R. & Córdoba-Aguilar, A. (2010). Evolutionary ecology of odonata: a complex life cycle perspective. Annual Review of Entomology, 57, 249-265.
Szalay-Bekő, M., Palotai, R., Szappanos, B., Kovács, I.A., Papp, B. & Csermely, P. (2012). ModuLand plug-in for Cytoscape: determination of hierarchical layers of overlapping network modules and community centrality. Bioinformatics, 28, 2202-2204.
Thébault, E. & Fontaine, C. (2010). Stability of ecological communities and the architecture of mutualistic and trophic networks. Science, 329, 853-856.
Tilianakis, J. M., Laliberté, E., Nielsen, A. & Bascompte, J. (2010). Conservation of species interaction networks. Biological Conservation, 143, 2270-2279.
Tsubaki, Y. & Tsuji, N. (2006). Dragonfly distributional predictive models in Japan: relevance of land cover and climatic variables. In: Cordero, A. (ed) Forests and Dragonflies. Pensoft Publishers Sofia pp 181-208
Velázquez, A., Mas, J. F., Gallegos, J. R. D., Mayorga-Saucedo, R., Alcántara, P. C., Castro, R. & Palacio, J. L. (2002). Patrones y tasas de cambio de uso del suelo en México. Gaceta Ecológica, 62, 21-37.
Verdú, M. & Valiente-Banuet, A. (2008). The nested assembly of plant facilitation networks prevents species extinctions. American Naturalist, 172, 751-760.
Vidal, O., López‐García, J. & Rendón‐Salinas, E. (2014). Trends in deforestation and forest degradation after a decade of monitoring in the Monarch Butterfly Biosphere Reserve in Mexico. Conservation Biology, 28, 177-186.
Weisner, S. E., Strand, J. A., Sahlén, G., Thiere, G., Ehde, P. M. & Svensson, J. M. (2007). Combating eutrophication and biodiversity loss in Sweden: importance of constructed wetlands in the agricultural landscape. In: International Conference on Multi Functions of Wetland Systems: Legnaro Italy. 26-29 June 2007 PAN pp 60-61
Werner, E. E. (1988). Size scaling and the evolution of complex life cycles. In: Size-Structured Populations. Ebenman, B Person L (eds). Springer-Verlag Berlin pp 60-81
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
*
Corresponding author.
Copyright © 2017 Universidad Nacional Autónoma de México, Instituto de Biología.