Effect of seed ingestion by birds on the germination of understorey species in cloud forest
Adelaida Pérez-Cadavid a, b, Octavio R. Rojas-Soto a, *, Martha Bonilla-Moheno b
a Red de Biología Evolutiva, Instituto de Ecología, A.C., Carretera antigua a Coatepec #351, El Haya, 91070 Xalapa, Veracruz, Mexico
b Red de Ambiente y Sustentabilidad, Instituto de Ecología, A.C., Carretera antigua a Coatepec #351, El Haya, 91070 Xalapa, Veracruz, Mexico
*Corresponding author: octavio.rojas@inecol.mx (O.R. Rojas-Soto)
Abstract
By dispersing seeds, frugivorous birds play a significant part in forest regeneration. However, there is scant information about the role of seed ingestion on the germination of cloud forest species. In this study, we evaluated whether the ingestion of the seeds of 3 woody understorey species (Conostegia xalapensis, Miconia glaberrima, M. mexicana) by frugivorous birds (Arremon brunneinucha, and Chlorospingus flavopectus) favors their percent and rate of germination. We compared the percent and rate of germination of seeds that had been ingested by birds and seeds extracted directly from their fruit. We also evaluated differences as a function of the species of bird that ingested the seed. The difference in percent seed germination was only significant for M. glaberrima ingested by A. brunneinucha; although, in general, ingested seeds germinated faster than seeds that had not been ingested, with a significant difference for all species eaten by A. brunneinucha, and for C. xalapensis eaten by C. flavopectus. Our results highlight the potential of frugivorous birds to assist the regeneration of intermediate successional species. In addition, the interspecific variation in percent germination and in germination rate suggest that certain plant-disperser interactions are very important for ecosystem maintenance.
Keywords:
Endozoochory; Forest regeneration; Frugivorous birds; Pioneer species; Seed dispersion
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Efecto de la ingestión de semillas por aves en la germinación de especies de sotobosque en el bosque mesófilo de montaña
Resumen
A través de la dispersión de semillas, las aves frugívoras contribuyen de manera importante en la regeneración de los bosques. Sin embargo, aún es escasa la información sobre el papel de la ingesta de semillas (endozoocoria) de especies del bosque mesófilo de montaña (BMM) en su germinación. En este estudio evaluamos si la ingesta de semillas de 3 especies leñosas de sotobosque (Conostegia xalapensis, Miconia glaberrima y M. mexicana) por 2 aves frugívoras (Arremon brunneinucha y Chlorospingus flavopectus), favorece el porcentaje y la tasa de germinación. Para esto, comparamos el porcentaje y velocidad de germinación de semillas que fueron ingeridas por aves, contra el de semillas extraídas directamente de los frutos. Además, evaluamos las diferencias con relación a la especie de ave que las ingirió. En general, el porcentaje de germinación fue mayor en semillas ingeridas por aves, aunque al evaluar la interacción entre especies, la diferencia solo fue significativa para semillas de M. glaberrima ingeridas por A. brunneinucha. En cuanto a la velocidad de germinación, en general, las semillas ingeridas germinaron en menos tiempo que las no ingeridas, siendo significativa esta diferencia para todas las especies consumidas por A. brunneinucha y para C. xalapensis consumida por C. flavopectus. Nuestros resultados resaltan el potencial de las aves frugívoras en la regeneración de especies de sucesión intermedia. Además, la variación interespecífica en el porcentaje y velocidad de germinación sugiere que ciertas interacciones son muy importantes para el mantenimiento del ecosistema.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Endozoocoria; Regeneración del bosque; Aves frugívoras; Especies pioneras; Dispersión de semillas
Introduction
Frugivorous birds are considered key in the maintenance of the structure and composition of forests (Wang & Smith, 2002) and their regeneration (Jacomassa & Pizo, 2010; Murray, 1988). By eating fruit and then excreting or regurgitating the seeds far from the mother tree, birds maintain genetic flow among conspecifics in different communities (Bruna, 1999). Thus, endozoochory, the passage of seeds through the digestive tract and subsequent viable dispersion (Van der Pijl, 1982) is considered one of the most efficient ways to move propagules. Through this process, frugivorous species can have an effect on germination through mechanisms such as scarification of the seed (i.e., erosion and loss of hard seed cover without affecting the embryo; Samuels & Levey, 2005; Traveset, 1998; Varela & Bucher, 2006); the removal of the pulp (which may contain germination-inhibiting chemicals); and the fertilization of the seeds (when excreted with fecal matter; Barnea et al., 1990; Samuels & Levey, 2005; Traveset, 1998).
The effectiveness of a disperser is measured by the number of seeds dispersed (the number of visits by number of seeds dispersed per visit), and the probability that a dispersed seed produces a new adult (given by the treatment in mouth and gut and the quality of seed deposition; Schupp et al., 2010). Birds, in particular can affect germination by scarification, removing the pulp and fertilizing the seed (Traveset & Willson, 1997). An increment in germination results in an increase in the potential number of individuals that can establish in the ecosystem, and thus, the endozoochory is an important process in the life cycle of some plant species (Traveset et al., 2007; Wang & Smith, 2002). A variety of studies have described the positive effect of endozoochory by birds on both germination percent and rate (LaFleur & Rubega, 2009; Lehouck et al., 2011; Lovas-Kiss et al., 2015; Paulsen & Högstedt, 2002; Silveira et al., 2012; Traveset & Verdú, 2002), though this positive contribution is not fully generalizable (Traveset, 1998), as the ingestion and passage through the gut can reduce the viability of some seeds (Domínguez-Domínguez et al., 2006; Traveset & Willson, 1997).
In degraded tropical forests, the limitation in seed dispersal is one of the main barriers for regeneration (Reid et al., 2015). Here, birds are key organisms in this process, as their vagility allows them to move among forest fragments and disturbed areas (Czarnecka & Kitowski, 2013; Schupp, 1993). Animal-dispersed pioneer tree species are fundamental for the beginning of the successional process (Martínez-Ramos & García-Orth, 2007), promoting a high density and richness of woody species beneath their canopies (Viani et al., 2015). Most of these species have small seeds and colorful fruits that are attractive for birds (Kessler-Ríos & Kattan, 2012). In fact, a large part of the woody species are pioneer dispersed by birds (Howe & Smallwood, 1982; Tabarelli & Peres, 2002; Wunderle, 1997).
The establishment and growth of pioneer species into open areas (for example, forest gaps or disturbed areas), can modify habitat conditions (such as soil organic matter, water and nitrogen), that ultimately accelerate the recovery of the nutrient cycle (Capulín-Grande et al., 2010; Dongmei & Changqun, 2008; Heer & Körner, 2002), while creating and modifying local conditions such as temperature and humidity. These changes generate a microclimate that favors the establishment of species that could not previously be established (Hooper et al., 2005). However, for these changes to occur, it is necessary the arrival of seeds to the site through dispersal mechanisms. In this context, the presence of both, pioneer species and their dispersers, indicates that important ecological interactions that catalyze the forest regeneration are in place, and brings light on the degree of conservation of an area. Even though studies have evaluated the contribution of birds to effective seeds dispersal, little is known about this process for cloud forest (hereafter CF, Baltazar, 2014; Hernández-Ladrón de Guevara et al., 2012), one of the most threatened ecosystems worldwide (Hamilton et al., 1995) and the most threaten in Mexico (González-Espinosa et al., 2012). In particular, the CF in central Veracruz, México, it is under continuous land use change pressure (Muñoz-Villers & López-Blanco, 2008), due to human population growth (González-Espinosa et al., 2012) and climate change (Challenger & Dirzo, 2009; Rojas-Soto et al., 2012). Despite being so threatened, it has the highest biological diversity per unit area compared to other types of vegetation (Conabio, 2010), and hosts between 10 and 12% of all estimated species for Mexico (Williams-Linera, 2012). Additionally, this ecosystem provides water resources (González-Espinosa et al., 2012) and contributes significantly to climate regulation and carbon capture (Conabio, 2010), which makes it a priority ecosystem for conservation (Challenger, 1998). Understanding aspects of the plant-bird interactions that contribute to the forest regeneration process, such as seed dispersal and germination, will provide important information on the resilience and maintenance of CF. In this study, we evaluate whether the consumption of seeds by frugivorous birds affects the percent and rate of germination of seeds of tree species that are important to the regeneration of CF. Understanding the potential role that seed dispersers have in secondary succession (i.e., what they consume and whether they are dispersing or preying on the seeds), will provide further knowledge on the ecology and management of the CF.
Materials and methods
The study was done in the Cloud Forest Sanctuary (CFS) located on the outskirts of Xalapa, Veracruz, Mexico (19°30’37” N, 96°56’36” W). This area is relevant for local conservation as the landscape configuration includes urban areas, fragments of degraded forest, and pasture and cropland areas (Williams-Linera et al., 2015). The area covers ~30 ha, with a mean elevation of 1400 m asl, mean annual temperature of 18 °C and mean annual precipitation of 1,500 to 2,000 mm (Williams-Linera, 2012).
In general, the cloud forest includes abundant and diverse plant species, particularly ferns and epiphytes (Rzedowski, 1996), with temperate affinity species in the canopy and species of tropical affinity in the understory (Challenger, 1998). In addition, frequent rain, cloudiness, haze, and high humidity throughout the year are common characteristics to this ecosystem (Conabio, 2010). In Mexico, the cloud forest is also recognized as the ecosystem with the highest diversity per unit area, including 10% of Mexican flora (2,500 vascular plant species) and 12% of terrestrial vertebrates (Rzedowski, 1996), with a high endemism of epiphytes.
The tree species used in the study were Conostegia xalapensis, Miconia glaberrima, and Miconia mexicana (Table 1). They were selected because of their fruit abundance during the study. These species are a good model system since they have small fruits of zoochorous dispersal, fast germination, and can establish in disturbed areas or early successional forests, generating favorable environmental conditions for the arrival and establishment of late-successional species. Other species were also offered (i.e., Palicourea padifolia, Trichilia havanensis, Poaceae spp.), but they were not sufficiently consumed by birds to be included in the study.
Field work was conducted between May and October 2015. For the experiments, we selected fruits from each plant species (approximately 30 understorey individuals) with accessible fruits. We collected 20 mature fruits from each individual, determining ripeness based on consistency and color (Almeda, 1993), and field observations. Of the 20 fruits collected per plant, 10 were used for bird-ingestion experiments, and the other 10 used as the control for the germination trials. The 10 fruits collected by each of the 3 plant species (30 in total), were offered simultaneously to each captured individual.
To evaluate the effect of bird ingestion over seeds germination we captured birds using 10 mist nets, for 2,940 net hours. We used field guides to identify the birds (Howell & Webb, 1995; Van Perlo, 2006). Captured birds were placed in cloth bags to ensure collection of all fecal material. Later, each bird was placed in a soft mesh cage (80 × 50 × 50 cm) that was hung in the forest and left for 2 to 5 h, which is the time required for ingested fruit to be defecated. Birds’ fecal samples were collected and then analyzed in the laboratory. Despite the capture of 7 frugivorous bird species (Table 2), we only present results from those 3 that had the most seeds to ensure a statistically representative number of seeds in fecal samples.
Table 1
General characteristics of the plant species used in the study.
|
Species |
Family |
Size of fruit (mm) |
Seeds per fruit |
Fructification |
|
Conostegia xalapensis1 |
Melastomataceae |
5-7 |
100-120 |
Jan-Dec |
|
Miconia glaberrima2 |
Melastomataceae |
3-5 |
~80 |
May-June |
|
Miconia mexicana |
Melastomataceae |
5-7? |
82-132? |
Sep-Dec3 |
(1Ibarra-Manriquez et al., 2015; 2Almeda, 1993; 3Ramírez-Marcial et. al., 2003; ?Pers. Obs.)
Table 2
Number of individuals of each species of bird used in the experiment and the number of seeds obtained per species of bird. Only the first 2 bird’ species, separated by a dashed line, were used for analyzes.
|
Species |
Number of birds |
Number of seeds |
|
Arremon brunneinucha |
37 |
1,719 |
|
Chlorospingus flavopectus |
11 |
746 |
|
Catharus aurantiirostris |
6 |
518 |
|
Catharus mexicanus |
7 |
80 |
|
Turdus grayi |
15 |
247 |
|
Catharus occidentalis |
3 |
65 |
|
Piranga flava |
2 |
81 |
A dissecting microscope and field guides (Almeda, 1993; Gómez-Pompa et al., 2010) were used to identify seeds. The seeds were cleaned with water, and damaged seeds were discarded. All of the seeds of the same plant species that were eaten and excreted by the same bird were considered a sample and are referred to hereafter as the ingested treatment. Seeds obtained directly from the fruit were randomly selected and used as the control group, referred to hereafter as the not ingested treatment. The number of seeds for each sample was determined according to the total of seeds of 3 plant species extracted from the excreta of each individual, an equal number of seeds to that found was extracted from the fruits to be used as a control group. The total number of samples per plant species was distributed as follows: Miconia glaberrima (51), Conostegia xalapensis (42) y Miconia mexicana (30).
For the germination experiment, samples were placed in a Petri dish with filter paper and cotton, and the dishes were placed in a growth chamber under controlled light (fluorescent lamps of 2000 lux; Ruiz et al., 2008), and temperature conditions (photoperiod: 12/12 h; 25 °C during the day and 22 °C at night). To maintain humidity, the filter paper was moistened every 3 days. Seeds were considered to have germinated once the radicle had emerged by at least 2 mm (Hartmann & Kester, 1982). The number of seeds that had germinated was counted every 2 days and the germination was evaluated for 120 days.
To determine if there were differences percent germination by treatment in: 1) total number of seeds by total number of birds, and 2) the seeds of each plant species by each of the 3 species of birds, a Mann-Whitney U test was used in R package Stats (R Core Team, 2013).
To determine if there were differences by treatment in germination rate among treatments by plant species and by bird species, we did germination curves and plotted and compared them using the Mantel-Cox test run in JMP statistical software (SAS Institute, Cary, NC, USA).
Results
From the samples used for the analysis (Table 3), a total of 2,983 seeds were obtained: 72% (2,148) M. glaberrima, 15% (445) C. xalapensis, and 13% (390) M. mexicana. The most frequently captured bird was Arremon brunneinucha, and most seeds were obtained from this species.
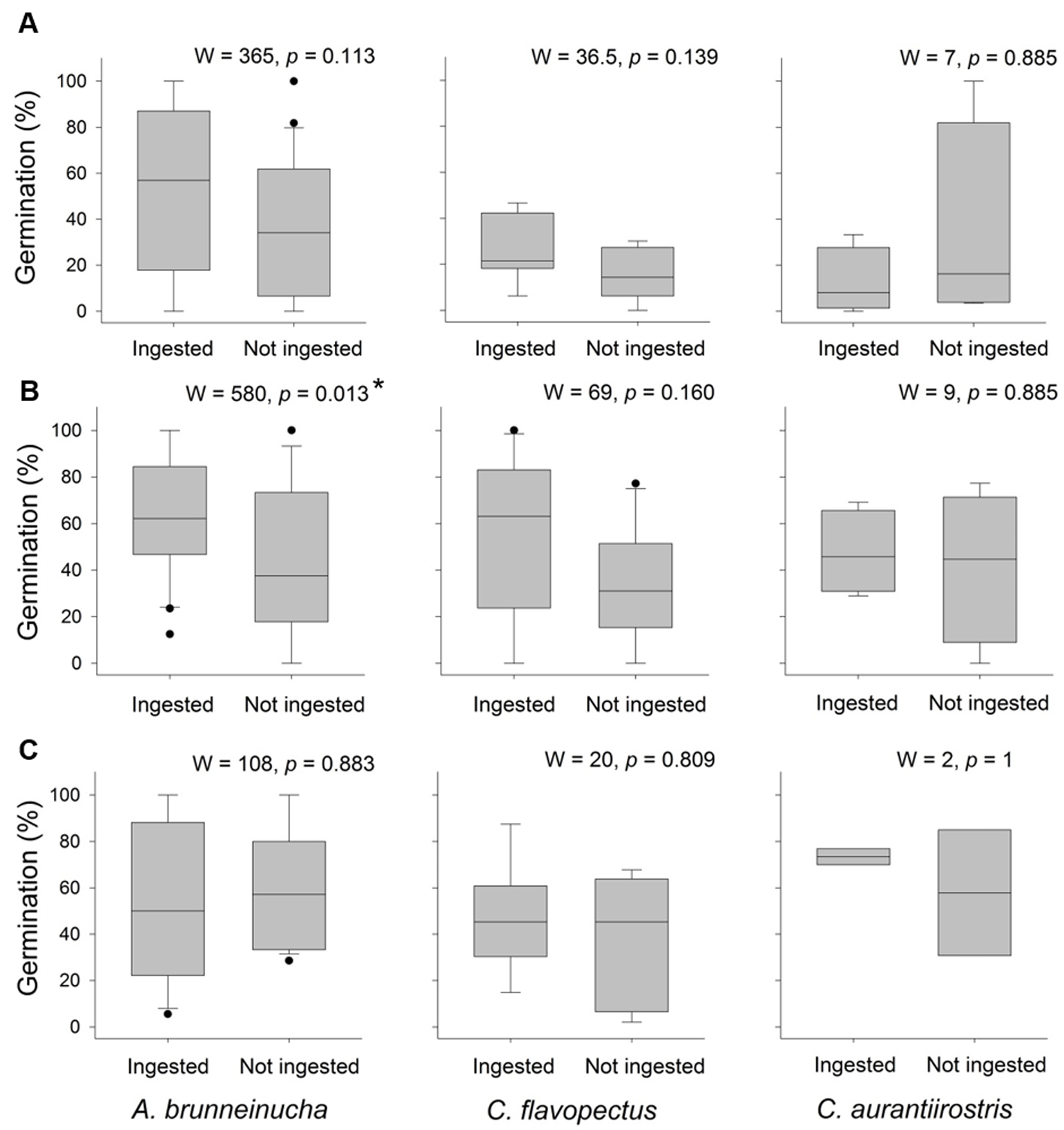
Percent germination was higher for ingested seeds than for seeds that were not ingested, and this difference was significant (W = 6230, p = 0.04); however, this result was due to the significant differences in germination of M. glaberrima seeds ingested by A. brunneinucha (Table 3; Fig. 1). In addition, germination was higher in the seeds of C. xalapensis and M. mexicana ingested by all 3 bird species.
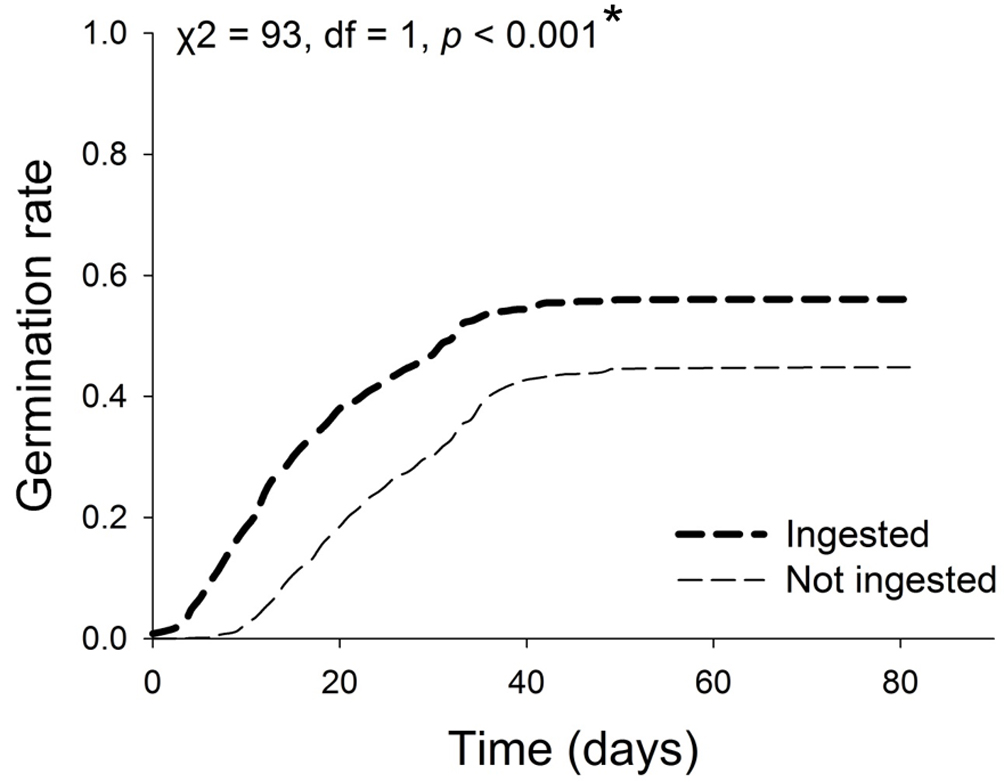
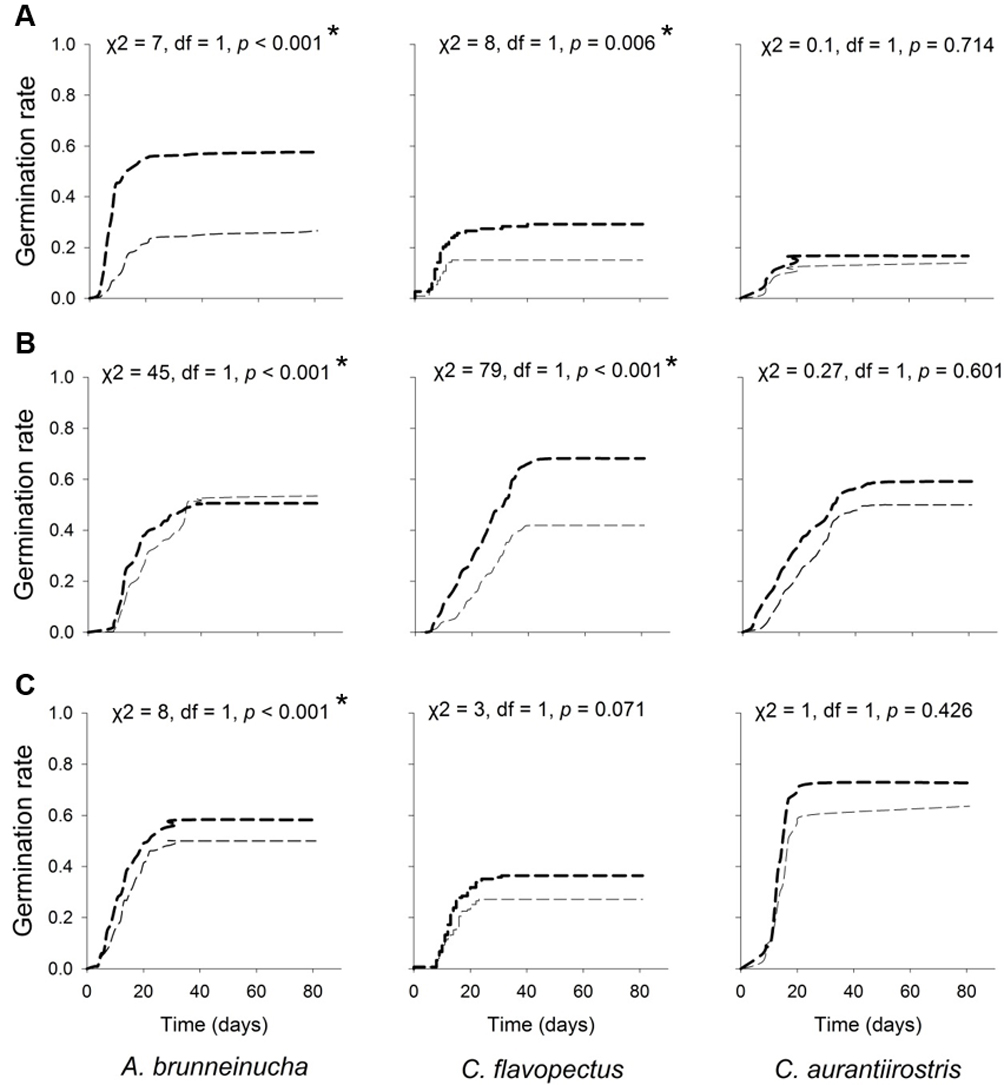
The maximum average germination time was 71 days and the minimum 24 days. The rate of germination curves for seeds ingested by all 3 species of birds differed significantly between treatments, and was greater for ingested seeds (X2 = 95 df = 1, p ˂ 0.001; Fig. 2). On comparing the rate of germination between treatments for all plant species ingested by the 3 species of bird, there were significant differences for the seeds of C. xalapensis ingested by A. brunneinucha and C. flavopectus; in the seeds of M. glaberrima ingested by A. brunneinucha and C. flavopectus, and in the seeds of M. mexicana ingested by A. brunneinucha (Fig. 3). In all cases the germination of ingested seeds was faster than that of seeds that were not ingested.
Discussion
The effect of seeds passing through the digestive tract of birds has been studied in several ecosystems (Jacomassa & Pizo, 2010; Lehouck et al., 2011; Wunderle, 1997), concluding that this is a very complex interaction that depends on the degree of specialization between the plants and their dispersers (Verdú & Traveset, 2004). Therefore, it is important to analyze this interaction in different environments, especially those that are vulnerable such as the cloud forest (Hernández-Ladrón de Guevara et al., 2012), and to determine their role in the forest regeneration processes.
Table 3
Percent germination of seeds ingested and not ingested by species of bird. Samples = number of fecal samples per bird per plant species; number seeds = total number of seeds per plant species per bird; G (%) = percent of seeds germinated, where I is ingested seeds and NI is not ingested seeds. Only the first 3 bird’ species, separated by a dashed line, were used in the analyses.
|
Plant species |
||||||||||||||
|
Conostegia xalapensis |
Miconia glaberrima |
Miconia mexicana |
||||||||||||
|
Samples |
Number of seeds |
G (%) |
Samples |
Number of seeds |
G (%) |
Samples |
Number of seeds |
G (%) |
||||||
|
I |
NI |
I |
NI |
I |
NI |
|||||||||
|
A. brunneinucha |
24 |
264 |
59 |
33 |
29 |
1,249 |
59 |
50 |
15 |
206 |
58 |
50 |
||
|
C. flavopectus |
7 |
113 |
29 |
15 |
10 |
482 |
68 |
42 |
6 |
151 |
36 |
28 |
||
|
C. aurantiirostris |
4 |
68 |
16 |
12 |
4 |
417 |
51 |
56 |
2 |
33 |
73 |
64 |
||
|
C. mexicanus |
1 |
2 |
50 |
0 |
3 |
66 |
29 |
48 |
3 |
12 |
25 |
75 |
||
|
T. grayi |
3 |
85 |
40 |
48 |
3 |
112 |
76 |
53 |
2 |
50 |
20 |
80 |
||
|
C. occidentalis |
2 |
15 |
33 |
20 |
2 |
35 |
5.7 |
29 |
2 |
15 |
73 |
– |
||
|
P. flava |
1 |
81 |
63 |
58 |
– |
– |
– |
– |
– |
– |
– |
– |
Among the factors that affect seedling survival and establishment, the number of seeds that germinate and the time they take to do so, are 2 of the most relevant ones. The percent of germination of seeds ingested by frugivorous birds was greater than for seeds that were not ingested. These results concur with those reported previously for tropical forest (Lehouck et al., 2011; Silveira et al., 2012). Higher percent germination increases the probability of survival (Baskin & Baskin, 1998), so the differences found in our study indicate that birds could play an important role in the effective dispersal, life cycle and potential recruitment of early successional plant species.
On the other hand, the curves of germination rate indicate that seed ingestion favors the number of seeds germinated and the time it takes to happen. Rapid germination can accelerate seedling establishment and may confer a competitive advantage in the acquisition of light and nutrients (Donohue et al., 2010; Dubois & Cheptou, 2012). Additionally, quick germination reduces the time seeds are exposed to predators and pathogens (Parsons, 2012; Traveset & Verdú, 2002). One of the factors that can account for increased germination rate is the time the seed is retained inside the bird, which is a function of the size and pH of the intestines (Barnea et al., 1990; Verdú & Traveset, 2004). It has been suggested that bird species that have longer and wider intestines can retain seeds for longer periods of time, intensifying the effects of abrasion during digestion (Herrera, 1984). However, several factors might be involved in this process (Traveset, 1998), such as the amount of ingested fruits and nutrients they contain (Levey & Grajal, 1991; Murray et al., 1994), and even the variability between individuals of the same species (Barnea et al., 1991).
The variation in the percent and rate of seed germination demonstrates that bird species contribute differentially to plant establishment, as observed in other studies (Lovas-Kiss et al., 2015; Reid et al., 2015; Traveset et al., 2007). In particular, the positive plant-disperser interaction between M. glaberrima and A. brunneinucha is highly relevant considering that this bird was the most captured. In addition, this species was observed in several habitats, including understory and open areas, facilitating the potential transport of propagules to distant and disturbed areas. Also, there are reports that Chlorospingus flavopectus, another species associated with the understorey, is a potential important disperser of C. xalapensis and M. mexicana based on their high consumption (Hernández-Ladrón de Guevara et al., 2012). In this study the high consumption of these species by C. flavopectus was also observed, although the difference in germination between ingested and not ingested seeds was not significant. Although there were no statistical differences in germination between treatments for any plant species ingested by C. aurantiirostris (probably due to the low sample size), the higher germination rate observed in those ingested seeds could have important biological implications as this species is also associated in the understory.
Species of the family Melastomataceae are abundant in the understorey and produce small fruit that have abundant seeds and pulp with high water and sugar contents, making them an important food for birds (Hernández-Ladrón de Guevara et al., 2012; Kessler-Ríos & Kattan, 2012; Silveira et al., 2012). In particular, the species used in this experiment, are intermediate successional plant species well represented at the understory level of the CF (Muñiz, 2008), with a high capacity for colonizing recently disturbed areas (Ramírez-Marcial et al., 2003; Williams-Linera et al., 2016). In fact, a study conducted in an adjacent abandoned pastureland, showed that after cattle were excluded, both M. glaberrima and C. xalapensis were able to germinate, highlighting their importance in the regeneration process of the forest (Williams-Linera et al., 2015, 2016).
On the other hand, it has been observed that only a few bird species of forest frugivores are willing to venture into open areas (Silva et al., 1996). In our study, those species were represented by C. flavopectus and A. brunneinucha. In addition, C. flavopectus mashes fruits extensively in the bill before swallowing, a behavior also observed in finches (such as A. brunneinucha). During this process, most of the larger seeds are separated from the pulp and dropped in situ (Murray, 1988). However, minute seeds (such as those from Melastomataceae) cannot easily be separated from the pulp, and therefore these are ingested in higher frequency (Murray, 1988). This higher frequency of mashing could increase the risk of damage to the seeds, although in our study those ingested by C. flavopectus and A. brunneinucha showed no signs of damage.
Once seeds have been ingested and deposited, they require adequate conditions (i.e, light, temperature and nutrients) to guarantee their germination and establishment (Dalling et al., 2002). While the area where this study was conducted might not be generalizable to other CF patches, and germination conditions in the laboratory might differ from natural conditions, results obtained provide evidence of the potential of frugivorous birds to assist the regeneration of intermediate successional species. At the local scale, this is valuable information given that the study site is immersed in a peri-urban landscape surrounded by a variety of land uses, making this forest a propagule reserve that could accelerate the recovery of nearby degraded forests.
Although not all the studied birds species had a significant effect on the germination percent and rate of ingested seeds, the fact that they move within and among forest fragments and degraded areas, suggest they transport seeds away from the mother plant and therefore favor their establishment and survival by reducing density-dependent mortality (Connell, 1971; Janzen, 1970), and increases the probability of seeds reaching sites with conditions favorable for their establishment (Rey & Alcántara, 2000; Samuels & Levey, 2005). The results of this study highlight the benefits that plants derive from the ingestion and potential dispersal of their seeds by birds and how this interaction is essential to ecosystem maintenance.
Acknowledgements
To the Instituto de Ecología, A.C. for the facilities provided for the study. The Consejo Nacional de Ciencia y Tecnología provided a Masters grant (number 338235) to A. Perez Cadavid and Project funding (CB 2014/01 Ref. 238831). We especially thank Javier Laborde, Miguel Martínez-Morales, Vinicio Sosa, and Jorge Galindo for suggestions to improve early versions of this manuscript and Dalia Ríos, Ana Laura Benítez, Claudio Mota-Vargas, and Rafael Vega for field assistance.
References
Almeda, F. (1993). Familia Melastomataceae. Flora del Bajío y de regiones adyacentes. Pátzcuaro. Mich.: Centro Regional del Bajío, Instituto de Ecología, A.C.
Baltazar, H. S. (2014). La importancia de la dispersión de semillas en la recuperación del bosque mesófilo de montaña del centro de Veracruz, México (M.Sc. Thesis). Instituto de Ecología, A.C. Xalapa, Veracruz.
Barnea, A., Yom-Tov, Y., & Friedman, J. (1990). Differential germination of two closely related species of Solanum in response to bird ingestion. Oikos, 57, 222–228.
Barnea, A., Yom-Tov, Y., & Friedman, J. (1991). Does ingestion by birds affect seed germination? Functional Ecology, 5, 394–402.
Baskin, C., & Baskin, J. (1998). Seeds: ecology, biogeography, and evolution of dormancy and germination. San Diego: Academic Press.
Bruna, E. M. (1999). Biodiversity: seed germination in rainforest fragments. Nature, 402, 139.
Capulín-Grande, J., Mohedano-Caballero, L., & Razo-Zárate, R. (2010). Cambios en el suelo y vegetación de un bosque de pino afectado por incendio. Terra Latinoamericana, 28, 79–87.
Challenger, A. (1998). Utilización y conservación de los ecosistemas terrestres de México: Pasado, presente y futuro. México D.F.: Conabio.
Challenger, A., & Dirzo, R. (2009). Factores de cambio y estado de la biodiversidad. In R. Dirzo, R. González e I. March (Comps.), Capital natural de México, Volumen II. Estado de conservación y tendencias de cambio (pp. 37–73). Ciudad de México: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2010). El bosque mesófilo de montaña en México: amenazas y oportunidades para su conservación y manejo sostenible. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Connell, J. H. (1971). On the role of natural enemies in preventing competitive exclusion in some marine animals and in rain forest trees. In P. J. Den Boer, & G. Gradwell (Eds.), Dynamics of populations (pp. 298–312). The Netherlands: PUDOC, Wageningen.
Czarnecka, J., & Kitowski, I. (2013). Rook spring seed dispersal in the agricultural landscape frugivory, granivory or accidental transport? Folia Geobotanica, 48, 55–73.
Dalling, J. W., Muller-Landau, H. C., Wright, S. J., & Hubbell, S. P. (2002). Role of dispersal in the recruitment limitation of neotropical pioneer species. Journal of Ecology, 90, 714–727.
Domínguez-Domínguez, L. E., Morales-Mávil, J. E., & Alba-Landa, J. (2006). Germinación de semillas de Ficus insipida (Moraceae) defecadas por tucanes (Ramphastos sulfuratus) y monos araña (Ateles geoffroyi). Revista de Biología Tropical, 54, 387–394.
Dongmei, L., & Changqun, D. (2008). Restoration potential of pioneer plants growing on lead-zinc mine tailings in Lanping, southwest China. Journal of Environmental Sciences, 20, 1202–1209.
Donohue, K., De Casas, R. R., Burghardt, L., Kovach, K., & Willis, C. G. (2010). Germination, postgermination adaptation, and species ecological ranges. Annual Review of Ecology, Evolution, and Systematics, 41, 293–319.
Dubois, J., & Cheptou, P. O. (2012). Competition/colonization syndrome mediated by early germination in non-dispersing achenes in the heteromorphic species Crepis sancta. Annals of Botany, 110, 1245–1251.
Gómez-Pompa, A. (2010). Programa Flora de Veracruz. In A. Gómez Pompa, T. Krömer, & R. Castro-Cortés (Eds.), Atlas de la flora de Veracruz: un patrimonio natural en peligro (pp. 43–56). Xalapa: Comisión del Estado de Veracruz para la Conmemoración de la Independencia Nacional y la Revolución Mexicana.
González-Espinosa, M., Meave, J. A., Ramírez-Marcial, N., Toledo-Aceves, T., Lorea-Hernández, F. G., & Ibarra-Manríquez, G. (2012). Los bosques de niebla de México: conservación y restauración de su componente arbóreo. Ecosistemas, 21, 36–52.
Hamilton, L. S, Juvik, J. O., & Scatena, F. N. (1995). Tropical montane cloud forests. Ecological Studies 110. New York: Springer Verlag.
Hartmann, H. T., & Kester D. E. (1982). Propagación de plantas; principios y prácticas. México D.F.: C.E.C.S.A.
Heer, C., & Körner, C. (2002). High elevation pioneer plants are sensitive to mineral nutrient addition. Basic and Applied Ecology, 3, 39–47.
Hernández-Ladrón de Guevara, I., Rojas-Soto, O. R., López-Barrera, F., Puebla-Olivares, F., & Díaz-Castelazo, C. (2012). Dispersión de semillas por aves en un paisaje de bosque mesófilo en el centro de Veracruz, México: su papel en la restauración pasiva. Revista Chilena de Historia Natural, 85, 89–100.
Herrera, C. M. (1984). Adaptation to frugivory of Mediterranean avian seed dispersers. Ecology, 65, 609–617.
Hooper, E., Legendre, P., & Condit, R. (2005). Barriers to forest regeneration of deforested and abandoned land in Panama. Journal of Applied Ecology, 42, 1165–1174.
Howe, H. F., & Smallwood, J. (1982). Ecology of seed dispersal. Annual Review of Ecology Evolution and Systematics, 13, 201–228.
Howell, S. N. G., & Webb, S. (1995), A guide to the birds of Mexico and northern Central America. New York: Oxford University Press.
Ibarra-Manríquez, G., Martínez-Morales, M., & Cornejo-Tenorio, G. (2015). Frutos y semillas del bosque tropical perennifolio: región de Los Tuxtlas, Veracruz. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Jacomassa, F. A. F., & Pizo, M. A. (2010). Birds and bats diverge in the qualitative and quantitative components of seed dispersal of a pioneer tree. Acta Oecologica, 36, 493–496.
Janzen, D. H. (1970). Herbivores and the number of tree species in tropical forests. American Naturalist, 104, 501–529.
Kessler-Ríos, M. M., & Kattan, H. G. (2012). Fruits of Melastomataceae: phenology in Andean forest and role as a food resource for birds. Journal of Tropical Ecology, 28, 11–21.
LaFleur, N., & Rubega, M. (2009). Does frugivory by European starlings (Sturnus vulgaris) facilitate germination in invasive plants? Journal of the Torrey Botanical Society, 136, 332–341.
Lehouck, V., Lens, L., & Spanhove, T. (2011). Avian fruit ingestion differentially facilitates seed germination of four fleshy-fruited plant species of an Afrotropical forest. Plant Ecology and Evolution, 144, 96–100.
Levey, D. J., & Grajal, A. (1991). Evolutionary implications of fruit-processing limitations in cedar waxwings. American Naturalist, 138, 171–189.
Lovas-Kiss, A., Sonkoly, J., Vincze, O., Green, A., Tackacks, A., & Molnár, A. (2015). Strong potential for endozoochory by waterfowl in a rare, ephemeral wetland plant species, Astragalus contortuplicatus (Fabaceae). Acta Societatis Botanicorum Poloniae, 84, 321–326.
Martínez-Ramos, M., & García-Orth, X. (2007). Sucesión ecológica y restauración de las selvas húmedas. Boletín de la Sociedad Botánica de México, 80, 69–84.
Muñiz, M. A. (2008). Sucesión secundaria y establecimiento de especies arbóreas nativas para restauración de bosque mesófilo de montaña en potreros abandonados del centro de Veracruz (Ph.D.Thesis). Xalapa, Veracruz, Instituto de Ecología, A.C.
Muñoz-Villers, L. E., & López-Blanco, J. (2008). Land use/cover changes using Landsat TM/ETM images in a tropical and biodiverse mountainous area of central-eastern Mexico. International Journal of Remote Sensing, 29, 1, 71–93.
Murray, K. G. (1988). Avian seed dispersal of three Neotropical gap-dependent plants. Ecological Monographs, 58, 271–298.
Murray, K. G., Russell, S., Picone, C. M., Winnett-Murray, K., Sherwood, W., & Kuhlmann, M. L. (1994). Fruit laxatives and seed passage rates in frugivores: consequences for plant reproductive success. Ecology, 75, 989–994.
Parsons, R. F. (2012). Incidence and ecology of very fast germination. Seed Science Research, 22, 161–167.
Paulsen, T. R., & Högstedt, G. (2002). Passage through bird guts increases germination rate and seedling growth in Sorbus aucuparia. Functional Ecology, 16, 608–616.
R Core Team. (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Retrieved in June 2017, from: http://www.R-project.org/
Ramírez-Marcial, N., Camacho-Cruz, A., & González-Espinosa, M. (2003). Guía para la propagación de especies leñosas nativas de los Altos y montañas del Norte de Chiapas. El Colegio de la Frontera Sur, San Cristóbal de las Casas, Chiapas.
Reid, J. L., Holl, K. D., & Zahawi, R. A. (2015). Seed dispersal limitations shift over time in tropical forest restoration. Ecological Applications, 25, 1072–1082.
Rey, P. J., & Alcántara, J. M. (2000). Recruitment dynamics of a fleshy-fruited plant (Olea europaea): connecting patterns of seed dispersal to seedling establishment. Journal of Ecology, 88, 622–633.
Rojas-Soto, O. R., Sosa, V., & Ornelas, J. F. (2012). Forecasting cloud forest in eastern and southern Mexico: conservation insights under future climate change scenarios. Biodiversity and Conservation, 21, 2671–2690.
Ruiz, B. C., Laguna, C. A., Iglesias, A. L., Damon, A., Marín, H. T., Azpíroz, R. H. et al. (2008). Germinación in vitro de semillas de Encyclia adenocaula (La llave & Lex.) Schltr (Orchidaceae). Phyton (Buenos Aires), 77, 203–215.
Rzedowski, J. (1996). Análisis preliminar de la flora vascular de los bosques mesófilos de montaña de México. Acta Botanica de México, 35, 25–44.
Samuels, I. A., & Levey, D. J. (2005). Effects of gut-passage on seed germination: do experiments answer the question they ask? Functional Ecology, 19, 365–368.
Schupp, E. W. (1993). Quantity, quality and the effectiveness of seed dispersal by animals. Vegetatio, 108, 15–29.
Schupp, E. W., Jordano, P., & Gómez, J. M. (2010). Seed dispersal effectiveness revisited: a conceptual review. New Phytologist, 188, 333–353.
Silva, J. M., Uhl, C., & Murray, G. (1996). Plant succession, landscape management, and the ecology of frugivorous birds in abandoned Amazonian pastures. Conservation Biology, 10, 491–503.
Silveira, F. A., Mafia, P. O., Lemos-Filho, J, P., & Fernandes, G. W. (2012). Species-specific outcomes of avian gut passage on germination of Melastomataceae seeds. Plant Ecology and Evolution, 145, 350–355.
Tabarelli, M., & Peres, C. A. (2002). Abiotic and vertebrate seed dispersal in the Brazilian Atlantic forest: implications for forest regeneration. Biological Conservation, 106, 165–176.
Traveset, A. (1998), Effect of seed passage through vertebrates on germination: a review. Perspectives in Plant Ecology Evolution and Systematics, 1, 151–190.
Traveset, A., Robertson, A. W., & Rodríguez-Pérez, J. (2007). Seed dispersal: theory and its application in a changing world. In A. J. Dennis, E. W. Schupp, R. J. Green, & D. A. Westcott (Eds.), A review on the role of endozoochory on seed germination (pp. 78–103). Wallingford, UK: CABI Publishing.
Traveset, A., & Verdú, M. (2002) A meta-analysis of the effect of gut treatment of seed germination. In D. J. Levey, W. R. Silva, & M. Galetti (Eds.) Seed dispersal and frugivory: ecology, evolution and conservation (pp. 339–350). Wallingford, UK/New York: CABI Publishing.
Traveset, A., & Willson, M. F. (1997). Effect of birds and bears on seed germination of fleshy-fruited plants in temperate rain forests of Southeast Alaska. Oikos, 80, 89–95.
Van Der Pijl, L. (1982). Principles of dispersal. Berlin: Springer-Verlag.
Van Perlo, B. (2006). Birds of México and Central America. Illustrated Checklist. Princeton, New Jersey: Princeton University Press.
Varela, O., & Bucher, E. H. (2006). Passage time, viability, and germination of seeds ingested by foxes. Journal of Arid Environments, 67, 566–578.
Verdú, M., & Traveset, A. (2004). Bridging meta-analysis and the comparative method: a test of seed size effect on germination after frugivores gut passage. Oecologia, 138, 414–418.
Viani, R. A., Vidas, N. B., Pardi, M. M., Castro, D. C., Gusson, e., & Brancalion, P. H. (2015). Animal-dispersed pioneer trees enhance the early regeneration in Atlantic Forest restoration plantations. Natureza & Conservação, 13, 41–46.
Wang, B. C., & Smith, T. B. (2002). Closing the seed dispersal loop. Trends in Ecology and Evolution, 7, 379–385.
Williams-Linera, G. (2012). El bosque de niebla del centro de Veracruz: ecología, historia y destino en tiempos de fragmentación y cambio climático. Xalapa: Conabio/ Instituto de Ecología, A.C.
Williams-Linera, G., Bonilla-Moheno, m., & López-Barrera, F. (2016). Tropical cloud forest recovery: the role of seed banks in pastures dominated by an exotic grass. New Forest, 47, 481–496.
Williams-Linera, G., López-Barrera, F., & Bonilla-Moheno, M. (2015). Estableciendo la línea de base para la restauración del bosque de niebla en un paisaje periurbano. Madera Bosques, 21, 89–101.
Wunderle, J. M. (1997). The role of animal seed dispersal in accelerating native forest regeneration on degraded tropical lands. Forest Ecology and Management, 99, 223–235.



