Characterization of epibionts associated with gastropod shells inhabited by Isocheles sawayai (Crustacea: Decapoda: Anomura) on the north coast of Santa Catarina
Gilson Stanski *, Alexandre Ribeiro-da Silva, Antonio Leão-Castilho
Núcleo de Estudos em Biología, Ecología e Cultivo de Crustáceos- Grupo de Estudos sobre Biologia, Ecologia e Cultivo de Crustáceos, Departamento de Zoologia, Instituto de Biociências de Botucatu, Universidade Estadual Paulista, 18618-689, Botucatu, São Paulo, Brazil
*Corresponding author: bio.gilson@hotmail.com (G. Stanski)
Abstract
This study analyzed the taxa associated as epibionts with mollusc shells inhabited by the hermit crab Isocheles sawayai, in order to understand how they affect host crabs. A total of 575 individuals were collected, including 156 females, 103 ovigerous females, and 316 males, which occupied shells of 10 species of gastropods. The epibionts recorded in the shells of I. sawayai belonged to Cirripedia, Polychaeta, Bivalvia, Anthozoa, Demosponge, and Bryozoa. A larger percentage of infestation occurred inside the shell, which may be harmful to the hermit crab because it reduces the internal volume. Ovigerous females used shells with a smaller percentage of infestation (40%), which may be related to the need for more space for the position and development of fertilized eggs. However, a higher proportion of anemones was found in shells used by ovigerous females. The advantage of this association for the anemones and crabs is discussed. The results suggest that shells occupied by epibionts can be a favorable factor for hermit crab survival, mainly for ovigerous females.
Keywords:
Epibiosis; Habitat sharing; Ecology
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Floristic, composition and structure of vegetation communities of eastern portion of the State Reserve Ciénegas y Manglares de la
Costa Norte de Yucatán
Resumen
Los taxones asociados como epibiontes con conchas de molusco usadas por el cangrejo ermitaño Isocheles sawayai fueron analizados para comprender como afectan al cangrejo hospedero. Se recolectó un total de 575 individuos, 156 hembras, 103 hembras ovígeras y 316 machos que ocuparon conchas de 10 especies de gasterópodos. Los epibiontes registrados en las conchas de I. sawayai pertenecieron a: Cirripedia, Polychaeta, Bivalvia, Antozoa, Demospongia y Bryozoa. Dentro de la concha, el porcentaje de infestación fue mayor, lo que puede ser perjudicial para el ermitaño, ya que reduce el volumen interno. Las hembras ovígeras utilizaron conchas con menor porcentaje de infestación (40%), lo que puede estar relacionado con la necesidad de más espacio disponible para la ovoposición y el desarrollo de los huevos fertilizados. Sin embargo, hubo una mayor proporción de anémonas en conchas ocupadas por hembras ovígeras. Se discute la ventaja de esta asociación para las anémonas y los cangrejos. Los resultados muestran que las conchas ocupadas por epibiontes pueden ser un factor favorable para la supervivencia del ermitaño, especialmente para hembras ovígeras.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Epibiosis; Compartir hábitat; Ecología
Introduction
Due to the physical characteristics of the marine environment, such as tidal regimes and currents, a sessile lifestyle is possible and favorable (Wahl, 1997). Organisms like bacteria, protists, sponges, cnidarians, molluscs, rotifers, bryozoans, polychaetes, echinoderms, crustaceans, and ascidians can be sessile at some point in their life cycles (Wahl & Mark, 1999). With limited escape capabilities and dispersal mechanisms, they need to have adaptive strategies to improve their survival (Wahl & Mark, 1999). One of these strategies is to associate with other organisms of different taxa. These interspecific interactions are important and necessary in the biological cycle of many species living in terrestrial and aquatic environments (Bronstein et al., 2006), because they provide benefits for at least one of the organisms involved and increase its chances of survival (Hoeksema & Bruna, 2000). Epibiosis is an example of an ecological interspecific interaction between organisms, with epibionts and basibionts more frequently found among marine invertebrates (Wahl, 2010).
Epibionts are organisms that grow and colonize living substrates; consequently, the basibiont is the host (live substrate) that is the basis for colonization of epibionts (Wahl, 1989; Wahl & Mark, 1999). Thus, epibiosis is defined as an interspecific interaction involving at least 2 organisms of different species (Wahl, 1989). However, epibiosis provides direct and indirect relationships, resulting in benefits for both associated individuals and often disadvantages for one of them. Therefore, studying this interaction is important to explore the structure, dynamics, and evolution of a community (Wahl, 1989).
The use of gastropod shells by hermit crabs is an evolutionary adaptation to protect their fragile abdomen, and it enables them to explore different environments (Batista-Leite et al., 2005; Hazlett, 1981, 1989; Worcester & Gaines, 1997) while also benefiting from associations with epibionts.
In the southern region of Brazil, there is still a gap in studies concerning the occurrence and characterization of epibionts associated with gastropod shells occupied by hermit crabs. Ayres-Peres and Mantelatto (2010) analyzed the occurrence of epibionts in shells used by the hermit Loxopagurus loxochelis Moreira, 1901 in the coast northeast of the state of São Paulo; Turra (2003) and Turra et al. (2005) investigated the inlaying of epibionts and the adequacy of shells used by sympatric hermit crabs of the genus Clibanarius Dana, 1852 on the island of Pernambuco, Brazil; and Sant’Anna et al. (2004) studied a symbiosis in Clibanarius vittatus (Bosc, 1802) in São Vicente, São Paulo, Brazil. In this study, we used the hermit crab Isocheles sawayai Forest & Saint Laurent, 1967, on the northern coast of Santa Catarina, Brazil, to characterize the epibionts found in the shells and to evaluate different internal and external infestation types among males, females, and ovigerous females and among different sizes of animals.
Materials and methods
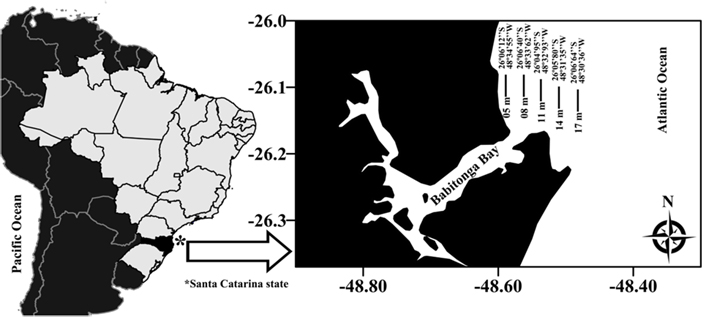
Hermit crabs were collected monthly from July 2010 to June 2011 at 5 sampling sites parallel to the shoreline at 5 depths: 5, 8, 11, 14, and 17 m. The trawls lasted 30 min in each sampling site, using a shrimp boat outfitted with double-rig nets, in adjacent areas in Babitonga Bay (Fig. 1). After collection, all specimens were sorted, frozen, and transported to the laboratory, where they were removed from their shells, counted, and identified according to Melo (1999). The sexes were recognized by verifying the position of the gonopores, and the reproductive condition was determined according to the presence of ovigerous females (Biagi & Mantelatto, 2006). These observations were performed using a stereomicroscope.
The cephalothoracic shield length (CSL) of the crabs was measured with a digital caliper (0.01 mm). The shells were weighed (0.01 g precision) under the same conditions (Gofas, 2014; Rios, 1994), and were inspected for the presence of internal and external epibionts. The species of macroinvertebrates comprising this community of epibionts were identified to the lowest possible level. Every shell used by a hermit crab that presented internal or external epibionts was considered infested, and the presence of bryozoan colonies was considered as 1 record. The hermit crabs were grouped into 9 size classes, from 3 to 11mm (3 = 3.00 – 3.99 mm; 4 = 4.00 – 4.9 mm; 5 = 5.00 – 5.99 mm; 6 = 6.00 – 6.99 mm; 7 = 7.00 – 7.90 mm; 8 = 8.00 – 8.99 mm; 9 = 9.00 – 9.99 mm; 10 = 10.00 – 10.99 mm; 11 = 11.00 – 11.99 mm), in order to compare the presence and absence of epibionts with the hermit crabs’ sizes.
The Student’s t-test (shell of the same species of gastropod) was used to detect differences in the infestation of internal and external epibionts in gastropod shells and also to compare differences in weight between shells with epibionts and shells without epibionts. Analysis of variance (Anova) and the Tukey post hoc test were used to compare differences in the infestation among demographic groups (males, females, and ovigerous females). Tests for homoscedasticity (Levene tests) and normality (Shapiro-Wilk tests) were first performed as pre-requisites for the statistical test. The variables without normal distribution were log-transformed prior to analysis (Zar, 1999).
Results
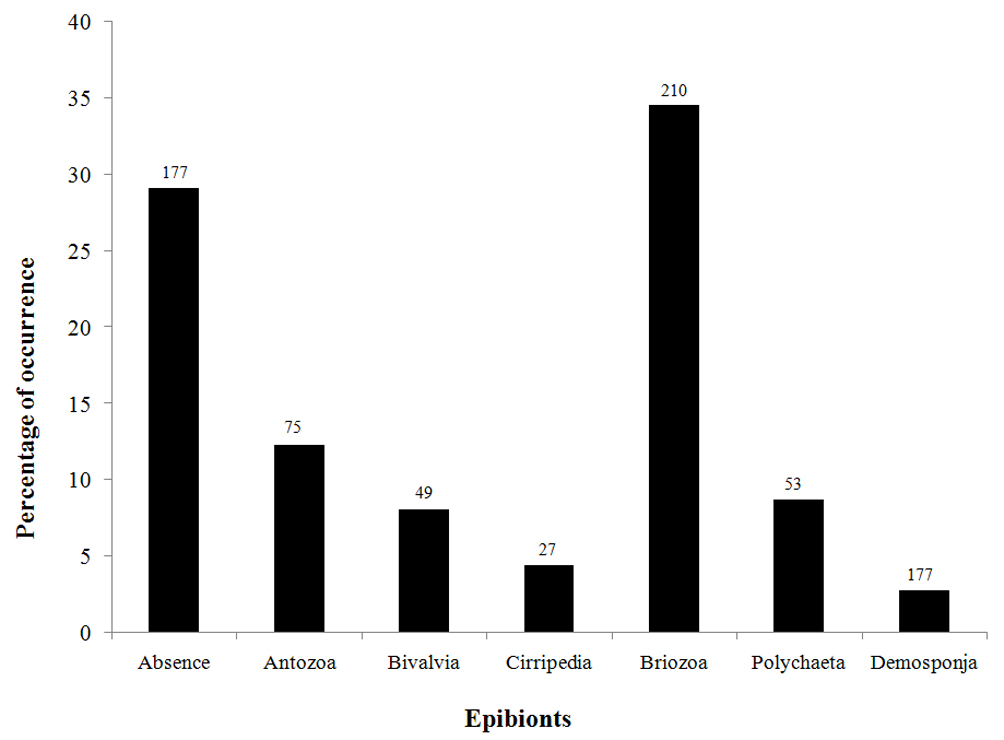
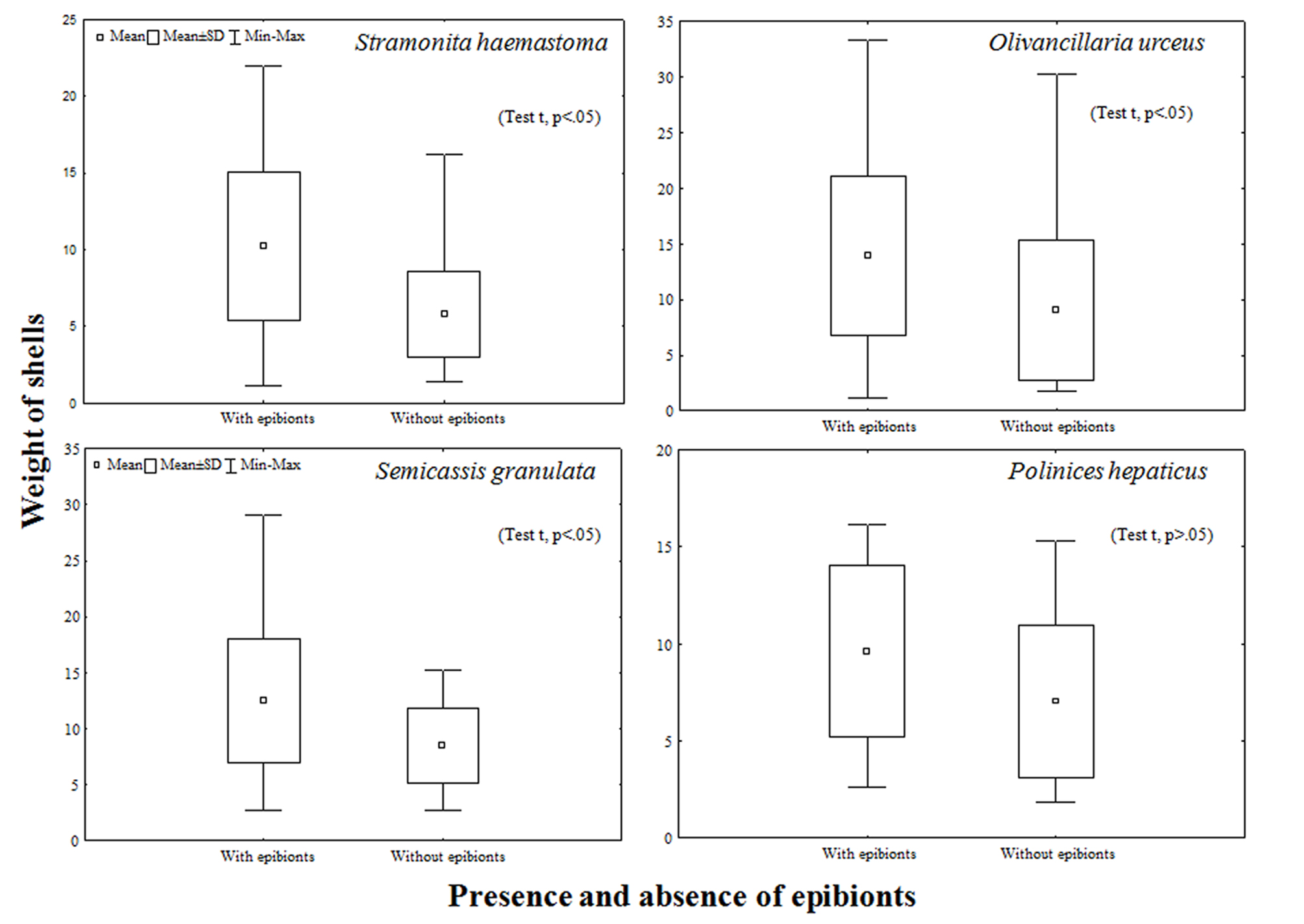
In total, 575 individuals of I. sawayai were collected, including 156 females (27%), 103 ovigerous females (18%), and 316 males (55%), which occupied shells of 10 species of gastropods: Buccinanops gradates (Deshayes, 1844), Dorsanum moniliferum (Valenciennes, 1834), Leucozonia nassa (Gmelin, 1791), Olivancillaria urceus (Roding, 1798), Olivancillaria vesica (Gmelin, 1791), Phalium granulatum (Born, 1778), Pisania auritula (Link, 1807), Polinices hepaticus (Roding, 1798), Siratus tenuivaricosus (Dautzenberg, 1927), and Stramonita haemastoma (Linnaeus, 1767). Stramonita haemastoma and O. urceus had the most used shells (202 and 148, respectively). Therefore, the statistical analyses were carried out only with the shells of these 2 species of gastropods. The epibionts found in the shells used by I. sawayai were barnacles, polychaete worm tubes, bivalves, anemones, sponges, and bryozoans (Fig. 2).
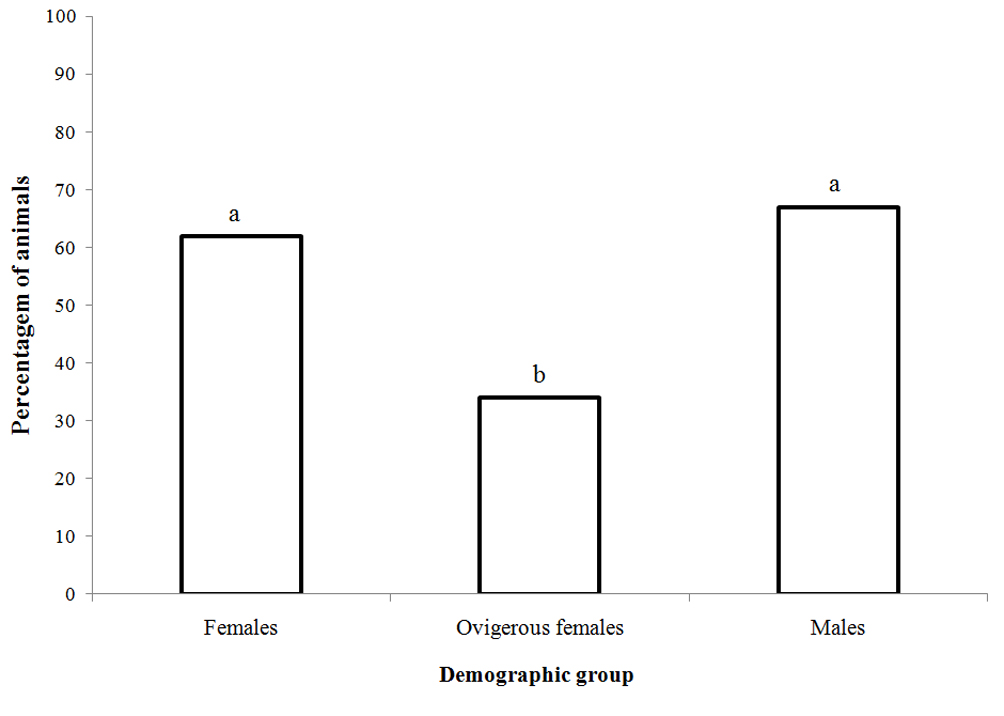
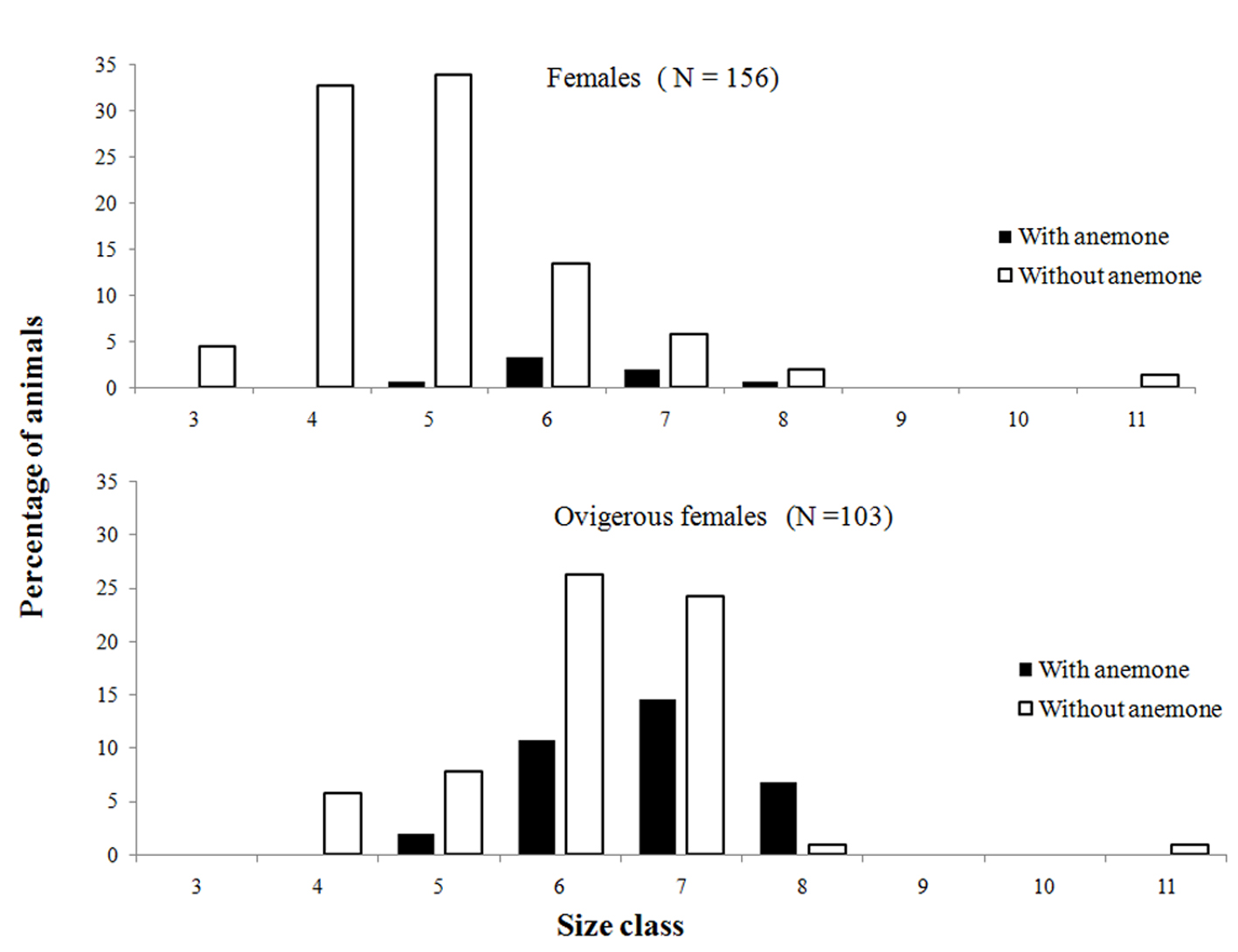
We observed differences in the prevalence of external and internal epibiont infestations (t test, p < 0.05), with a higher infestation rate inside the shell. Regarding the demographic groups, ovigerous females used shells with a lower percentage of infestation (40%), followed by males (70%) and females (80%) (Anova, Tukey post hoc, p < 0.05) (Fig. 3). However, ovigerous females had a higher proportion of anemones in comparison to non-ovigerous females (Fig. 4).
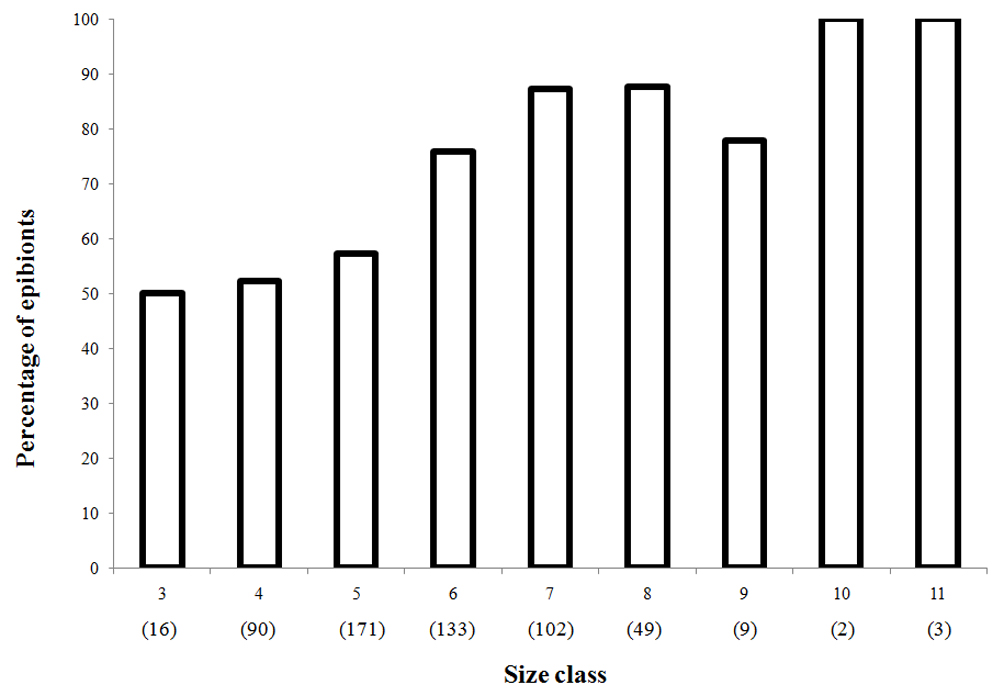
The presence of epibionts affects the average weight of shells of 3 (Student’s t-test, p < 0.05) of the 4 busiest shells, with the exception of Polinices hepaticus, which had mostly bryozoan epibionts (Student’s t-test, p > 0.05) (Fig. 5). However, regarding the presence of epibionts in the busiest shells (S. haemastoma, O. urceus), increased with increasing of CSL (Fig. 6).
Discussion
In unconsolidated benthic environments, hermit crab shells and decapod crustacean carapaces are among the few solid surfaces available, which makes them very important for attachment, survival, and dispersion of various sessile animals (Acuña et al., 2003; Conover, 1978). Bryozoans were the most abundant epibionts in our study. A similar pattern of occupation of shells by 2 species of hermit crabs was found on the Brazilian (Ayres-Peres & Mantelatto, 2010; Pereira et al., 2009) and Japanese coasts (Oba et al., 2008). As this taxon does not affect shell weight (Ayres-Peres & Mantelatto, 2010), it may be advantageous for the crab to inhabit a shell with bryozoans. In addition, bryozoans belonged to the category of “improvers” of the shell structure, because they produce calcareous colonies that can modify the morphology of the shells greatly, granting greater protection to the hermit against predators through the increased resistance of the shells or even providing camouflage (Ayres-Peres & Mantelatto, 2010).Therefore, although no species is an obligate symbiont, the high incidence of bryozoans on hermit crabs suggests a beneficial relationship between them in non-consolidated environments and, consequently, this association favors a cooperative relationship, which is an act performed by an individual that increases the fitness of another (Bergmüller et al., 2007)
Another pattern found was the constant presence of epibionts in larger animals, which could be age and molting frequency-related (Davis & White, 1994). This hypothesis has been explored by Costa et al. (2010) in Arenaeus cribrarius where they found a low epibiont presence and suggested that this condition is due to the frequent molting of the host, because the juvenile stage has a characteristic rapid growth with short inter-molt periods. Consequently, after ecdysis hermit crabs seek larger shells; otherwise their growth may be limited. As discussed in the literature, when hermit crabs use a very small shell, their growth is affected or even restrained (Fantucci et al., 2008; Ziegler & Forward, 2006). Thus, the larger the hermit crab is, the longer the inter-molt period; in other words, the hard substrate habitable by epibionts remains in movement for more time (Nogueira-Junior et al., 2006). The low frequency of epibionts found in smaller animals supports this hypothesis. In general, mature crustaceans, hermit crabs, or other taxa that undergo molting less frequently shelter a greater diversity and density of epibiont species (Dick et al., 1998; Fernández et al., 1998; Mori & Manconi, 1990).
On the other hand, ovigerous females occupied shells with fewer epibionts. According to Wait and Shoeman (2012), shell selection is a process that involves individual and sexual preferences in different dimensions, seeking the best protection for the hermit and also sufficient space for the development of embryos. Therefore the presence of epibionts causes a possible competition for space, especially when epibionts are found internally and in large numbers (conditions found in the present study), further altering shell weight (Sant’Anna et al., 2004). In addition, heavy shells can negatively affect reproduction and growth (Bertness, 1981; Osorno et al., 1998). Thus, the hypothesis that best adjusts to the use of shells with less internal infestation by ovigerous females is related to the physiological need of the species to maximize reproductive success.
Mantelatto et al. (2002), studying Paguristes tortugae Schmitt, 1933, found a positive correlation between fecundity and the internal volume of the shell, suggesting that the larger the volume, the greater the fecundity of the female. In the present study, this might explain the low prevalence of internal epibionts in the shells occupied by ovigerous females, because internal epibionts limit the space available for oviposition and development of fertilized eggs, and some polychaete epibionts of the genus Polydora use hermit crab eggs as food (Williams, 2002). However, although ovigerous females occupied shells with lower infestation of epibionts, anemones should be highlighted, as they were found mostly on the outside of the shells used by ovigerous females. A similar pattern was found in the spider crabs Libinia ferreirae Brito Capello, 1871 and Libinia spinosa Milne-Edwards, 1834 (Acuña et al., 2003; Cordeiro & Costa, 2002; Nogueira-Junior et al., 2006; Winter & Masunari, 2006) and the mud crab Scylla serrata (Forskal, 1775) (Jeffries et al., 1992), where ovigerous females had a higher occurrence of epibionts in their carapaces (anemones and cirripeds) than males and non-ovigerous females. According to the authors, ovigerous females lose the habit of burying themselves when carrying their embryos, in order to oxygenate the eggs. This fact would help to establish larger infestations in this category, due to the greater probability of contact with the epibionts, a situation that we believe also occurs in I. sawayai. This species has the habit of remaining half-buried, with only its antennae protruding from the substrate to obtain food particles in suspension and to avoid predators (Fantucci et al., 2009; Stanski et al., 2016).
Another hypothesis that should be considered for hermit crabs concerns the longer use of the shell by ovigerous females, because in this condition they do not molt, facilitating the acquisition of epibionts. Furthermore, Bach and Herrnkind (1980) inferred that the higher relative frequencies of the association between ovigerous females of Pagurus pollicaris Say, 1817 and anemones are related to higher predation pressure, because it is believed that females reduce their mobility when they are ovigerous, and thus the use of anemones for their defense can reduce the likelihood of being preyed upon.
The advantage of this association for anemones is not the use of the food remains of the host, because I. sawayai is a filter feeder (Stanski & Castilho, 2016), but the availability of a biological substrate that provides greater mobility with ability to migrate to various habitats and thus explore new resources, increasing their food availability and providing protection (Winter & Masunari, 2006). On the other hand, the main advantage that anemones provide to hermit crabs is a chemical defense mechanism against predators (Winter & Masunari, 2006).
Therefore, our results suggest that the presence of epibionts in shells can be advantageous to the survival of the hermit crab, mainly as a defense and camouflage strategy. On the other hand, epibionts can also have a negative impact if they become too numerous as to increase significantly the shell weight. Although many studies are still needed to elucidate all the fine details of the interaction between epibionts and hermit crabs, and many questions remain, it is clear that this interaction is a rich example of how evolutionary processes can interact together in the variation of life histories of the species.
Acknowledgements
The authors are indebted to foundations that provided financial support during field collections, visiting activities, and scholarships: Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP (Temático Biota 2010/50188-8), Coordenação de Aperfeiçoamento de Nível Superior – CAPES – Ciências do Mar II (23038.004310/2014-85 and 2005/2014 – 23038.004308/2014-14), Conselho Nacional de Desenvolvimento Científico e Tecnológico – CNPq (Research Scholarships PQ 304968/2014-5 and PQ 308653/2014-9), Fundação para o Desenvolvimento da Unesp – FUNDUNESP (1214/2010 – DFP), and Pró Reitoria de Pesquisa da Unesp (PROPE). We thank many colleagues from the NEBECC group who helped with sampling and laboratory analyses; and the “Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis” (IBAMA) for granting permission to collect the animals.
References
Acuña, F. H., Excoffon, A. C., & Scelzo, M. A. (2003). Mutualism between the sea anemone Antholoba achates (Drayton, 1846) (Cnidaria: Actiniaria: Actinostolidae) and the spider crab Libinia spinosa Milne-Edwards, 1834 (Crustacea: Decapoda, Majidae). Belgian Journal of Zoology, 133, 45–48.
Ayres-Peres, L., & Mantelatto, F. L. (2010). Epibiont occurrence on gastropod shells used by the hermit crab Loxopagurus loxochelis (Anomura: Diogenidae) on the northern coast of São Paulo, Brazil. Zoologia, 27, 222–227.
Bach, C. E., & Herrnkind, W. F. (1980). Effects of predation pressure on the mutualistic interaction between the hermit crab, Pagurus pollicaris Say, 1817, and the sea anemone, Calliactis tricolor (Lesueur, 1817). Crustaceana, 38, 104–107.
Batista-Leite, L. M. A., Coelho, P. A., & Calado, T. C. S. (2005). Estrutura populacional e utilização de conchas pelo caranguejo ermitão Calcinus tibicen (Herbst, 1791) (Crustacea, Decapoda, Diogenidae). Tropical Oceanography, Recife, 33, 99–118.
Bergmüller, R., Bshary, R., Johnstone, R. A., & Russell, A. F. (2007). Integrating coopeative breeding and cooperation theory. Behavioural Processes, 76, 61–72.
Bertness, M. D. (1981). The influence of shell type on hermit crab growth rate and clutch size (Decapoda, Anomura). Crustaceana, 40, 197–205.
Biagi, R., & Mantelatto, F. L. (2006). Relative growth and sexual maturity of the hermit crab Paguristes erythrops (Anomura, Diogenidae) from South Atlantic. Hydrobiologia, 559, 247–254.
Bronstein, J. L., Alarcón, R., & Geber, M. (2006). The evolution of plant-insect mutualisms. New Phytologist, 172, 412–428.
Conover, M. R. (1978). The importance of various shell characteristics to the shell-selections behavior of hermit crabs. Journal of Experimental Marine Biology and Ecology, 32, 131–142.
Cordeiro, C. A. M. M., & Costa, T. M. (2002). Relação entre categoria demográfica, tamanho do hospedeiro Libinia spinosa (Brachyura: Majoidea) e infestação por Octolasmis lowei (Cirripedia: Poecilasmatidae). In VII Congresso de Ecologia do Brasil, Caxambu – MG. Available in: https://www.researchgate.net/publication/228521796_Relacao_entre_categoria_demografica_tamanho_do_hospedeiro_Libinia_spinosa_Brachyura_Majoidea_e_infestacao_por_Octolasmis_lowei_Cirripedia
Costa, T. M., Christofoletti, R. A., & Pinheiro, M. A. A. (2010). Epibionts on Arenaeus cribrarius (Brachyura: Portunidae) from Brazil. Zoologia, 27, 387–394.
Davis, A. R., & White, G. A. (1994). Epibiosis in a guild of sessile subtidal invertebrates in southeastern Australia: a quantitative survey. Journal of Experimental Marine Biology and Ecology, 177, 1–14.
Dick, M. H., Donaldson, W. E., & Vining, I. W. (1998). Epibionts of the tanner crab Chionoecetes bairdi in the region of Kodiak Island, Alaska. Journal of Crustacean Biology, 18, 519–528.
Fantucci, M. Z., Biagi, R., & Mantelatto, F. L. (2008). Shell occupation by the endemic Western Atlantic hermit crab Isocheles sawayai (Diogenidae) from Caraguatatuba, Brazil. Brazilian Journal of Biology, 68, 859–867.
Fantucci, M. Z., Biagi, R., Meireles, A. L., & Mantelatto, F. L. (2009). Influence of biological and environmental factors on the spatial and temporal distribution of the hermit crab Isocheles sawayai Forest e Saint-Laurent, 1968 (Anomura, Diogenidae). Nauplius, 17, 37–47.
Fernández, L., Parapar, J., González-Gurriará N. E., & Muíño, R. (1998). Epibiosis and ornamental cover patterns of the spider crab Maja squinado on the glacian coast, Northwestern Spain: influence of behavioral and ecological characteristics of the host. Journal of Crustacean Biology, 18, 728–737.
Gofas, S. (2014). Gastropoda. Accessed through: World Register of Marine Species. Retrieved on March 2nd, 2016 from: http://www.marinespecies.org/aphiaphp?p=taxdetails&id=101
Grabowski, R. C., Simoes, S. M., & Castilho, A. L. (2014). Population structure, sex ratio and growth of the seabob shrimp Xiphopenaeus kroyeri (Decapoda, Penaeidae) from coastal waters of southern Brazil. Zookeys, 457, 253–269.
Hazlett, B. A. (1981). The behavioral ecology of hermit crabs. Annual Review of Ecology and Systematics, 12, 1–22.
Hazlett, B. A. (1989). Mating success of male hermit crabs in shell generalist and shell specialist species. Behavioral Ecology and Sociobiology, l25, 119–128.
Hoeksema, J. D., & Bruna, E. M. (2000). Pursuing the big questions about interspecific mutualism: a review of theoretical approaches. Oecologia, 125, 321–330.
Jeffries, W. B., Voris, H. K., & Poovachiranon, S. (1992). Age of the mangrove crab Scylla serrata at colonization by stalked barnacles of the genus Octolasmis. Biological Bulletin, 182, 188–194.
Melo, G. A. S. (1999). Manual de identificação dos crustacea Decapoda do litoral brasileiro: Anomura, Thalassinidea, Palinuridea e Astacidea. São Paulo: Editora Plêiade.
Mori, M., & Manconi, R. (1990). Macroepizoites associated with Paromola cuvieri (Risso, 1816) (Decapoda, Homolidae) of the Ligurian Sea. Crustaceana, 58, 124–129.
Mantelatto, F. L. M., Alarcon, V. F., & Garcia, R. B. (2002). Egg production strategies of the tropical hermit crab Paguristes tortugae from Brazil. Journal of Crustacean Biology, 22, 390–397.
Nogueira-Junior, M., Robert, M. C., & Haddad, M. A. (2006). Calliactis tricolor (Anthozoa, Acontiaria) epibionte em Brachyura (Crustacea, Decapoda) no litoral sul do Paraná e Norte de Santa Catarina. Acta Biológica Paranaense, 35, 233–248.
Oba, T., Wada, S., & Goshima, S. (2008). Shell partitioning of two sympatric hermit crabs, Pagurus middendorfi and P. brachiomastus, in north-eastern Hokkaido, Japan. Journal of the Marine Biological Association of the United Kingdom, 88, 103–109.
Osorno, J. L., Fernandez-Casillas, L., & Rodriguez-Juares, C. (1998). Are hermit crabs looking for light and large Shell? Evidence from natural and field induced shell exchanges. Journal of Marine Biology and Ecology, 222, 163–173.
Pereira, P. H. C., Zancaner, Jr., J., & Jacobucci, G. B. (2009). Ocupação de conchas e utilização de microambientes por caranguejos ermitões (Decapoda, Anomura) na Praia da Fortaleza, Ubatuba, São Paulo. Biotemas, 22, 65–75.
Rios, E. C. (1994). Seashells of Brasil. Rio Grande do Sul. Fundação cidade do Rio Grande/ Instituto Acqua/ Museu Oceanografico de Rio Grande, Universidade de Rio Grande.
Sant’Anna, B. S., Zangrande, C. M., Costa, T. M., & Reigada, A. L. D. (2004). Simbiose em conchas do ermitão Clibanarius vittatus (Bosc, 1802) (Crustacea, Decapoda, Anomura). In 4° Congresso Brasileiro de Pesquisas Ambientais e da e da Saúde, Santos, 1, 231–234. CD-ROM.
Stanski, G., & Castilho, A. C. (2016). Reproductive biology of the South American endemic hermit crab Isocheles sawayai (Crustacea, Anomura) from the Southern coast of Brazil. Invertebrate Reproduction & Development, 60, 103–111.
Stanski, G., Mantelatto, F. M., & Castilho, A. L. (2016). Habitat heterogeneity in the assemblages and shell use by the most abundant hermit crabs (Anomura: Diogenidae and Paguridae): does the occupied shell species differ according to gender and species? Nauplius, 2, 1–10.
Turra, A. (2003). Shell condition and adequacy of three sympatric intertidal hermit crab populations. Journal of Natural History, 37, 1781–1795.
Turra, A., Denadai, M. R., & Leite, F. P. P. (2005). Predation on gastropods by shellbreaking crabs: effects on shell availability to hermit crabs. Marine Ecology Progress Series, 286, 279–291.
Wahl, M. (1989). Marine epibiosis. I. Fouling and antifouling: some basic aspects. Marine Ecology Progress Series, 58, 175–189.
Wahl, M. (1997). Living attached: aufwuchs, fouling, epibiosis. In R. Nagabhushanam, & M. F. Thompson (Eds.), Fouling organisms of the Indian Ocean: biology and control technology (pp. 31–83). Oxford, New Delhi: Oxford & IBH Publishing Ltd.
Wahl, M. (2010). Epibiosis. In S. Dürr, & J. C. Thomason (Eds.), Biofouling (pp.100–120). Oxford: Wiley Blackwell.
Wahl, M., & Mark, O. (1999). The predominantly facultative nature of epibiosis: experimental and observational evidence. Marine Ecology Progress Series, 187, 59–66.
Wait, M., & Shoeman. D. S. (2012). Shell use, population structure, and reproduction of the hermit crab, Clibanarius virescens (Kraus, 1843) at Cape Recife, South Africa. Journal of Crustacean Biology, 32, 203–214.
Williams, J. D. (2002). The ecology and feeding biology of two Polydora species (Polychaeta: Spionidae) found to ingest the embryos of host hermit crabs (Anomura: Decapoda) from the Philippines. Journal of Zoology of London, 257, 339–351.
Winter, V., & Masunari, S. (2006). Macroepizoísmo em Libinia ferreirae (Crustacea, Brachyura, Majidae). Iheringia, Série Zoologia, 96, 135–140.
Worcester, S. E., & Gaines, S. D. (1997). Quantifying hermit crab recruitment rates and megalopal shell selection on wave swept shores. Marine Ecology Progress Series, 157, 307–310.
Zar, J. H. (1999). Biostatistical analysis. 4° Ed. New Jersey: Prentice-Hall.
Ziegler, T. A., & Forward, R. B. (2006). Larval release behaviors of the striped hermit crab, Clibanarius vittatus (Bosc): temporal pattern in hatching. Journal of Experimental Marine Biology and Ecology, 335, 245–255.



