Diversity and similarity of the parasites in Megalechis thoracata and Callichthys callichthys (Pisces: Callichthyidae) from Brazilian Amazon
Adriele Carolina Franco-Cardoso a, Ligia Rigôr-Neves b, Marcos Sidney Brito-Oliveira a, Marcos Tavares-Dias a, c, *
a Programa de Pós-Graduação em Biodiversidade Tropical,Universidade Federal do Amapá, Rodovia Juscelino Kubitscheck, s/n, Jardim Marco Zero, 68903-419 Macapá, Amapá, Brasil
b Programa de Pós-Graduação em Biodiversidade e Biotecnologia, Universidade Federal do Amapá, s/n, Jardim Marco Zero, 68903-419 Macapá, Amapá, Brasil
c Embrapa Amapá, Universidade, 68903-419 Macapá, Amapá, Brasil
*Corresponding author: marcos.tavares@embrapa.br (M. Tavares-Dias)
Abstract
This study compared the structure and composition of the parasite communities in Callichthys callichthys and Megalechis thoracata of a tributary of the Amazon River system, northern Brazil. A total of 447 parasites was collected from 38 specimens of each host, and 44.7% and 36.8% of fish species respectively were found to be infected with 1 or more species of parasite. The similarity in parasite assemblages between hosts was high (61%) with both infected at similar levels by Genarchella genarchella, larvae of Eustrongylides sp., Rhabdochona sp. and plerocercoids of Proteocephalidae. However, the dominant parasites found in C. callichthys were metacercariae of Posthodiplostomum sp., which did not infect M. thoracata. In M. thoracata the dominant parasite was G. genarchella, and this host species was also infected by Gorytocephalus spectabilis which did not infect C. callichthys. The parasite communities were dominated by endoparasites, and species richness of parasites, Shannon diversity index and evenness were similar for both hosts. The results indicate that these fish species are intermediate hosts of the endohelminths found. Finally, the variation in the parasite community between sympatric hosts occurred probably due to the hosts’ distribution in the environment and the diet of each host species.
Keywords:
Amazon; Diversity; Freshwater fish; Parasite ecology
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Diversidad y similitud de los parásitos de Megalechis thoracata y Callichthys callichthys (Pisces: Callichthyidae) de la Amazonia brasileña
Resumen
Este estudio comparó la estructura y composición de las comunidades de parásitos de Callichthys callichthys y Megalechis thoracata en el afluente del río Amazonas, al norte de Brasil. Un total de 447 parásitos se recolectó de 38 ejemplares de cada hospedero; 44.7% y 36.8%, respectivamente, estuvieron infectados con 1 o más especies de parásito. La similitud en los ensambles de parásitos entre estas 2 especies de hospederos fue alta (61%) debido a que ambas presentaron niveles de infección similares de Genarchella genarchella, larvas de Eustrongylides sp., Rhabdochona sp. y plerocercoides de Proteocephalidae. Sin embargo, Posthodiplostomum sp. fue encontrado solamente en C. callichthys, mientras que Gorytocephalus spectabilis fue encontrada en M. thoracata. Sin embargo, los parásitos dominantes en C. callichthys fueron metacercarias de Posthodiplostomum sp., las cuales no se encontraron en M. thoracata. En M. thoracata el parásito dominante fue G. genarchella, este hospedero también estuvo infectado por Gorytocephalus spectabilis, que no se encontró en C. callichthys. La mayoría de las infracomunidades de parásitos presentaron un patrón de dispersión agregado. Las comunidades de parásitos estuvieron dominadas por endoparásitos; la riqueza de especies, el índice de diversidad de Shannon y la equidad fueron similares para ambas especies de hospederos. El tamaño corporal de C. callichthys y M. thoracata no fueron influenciadas por los bajos índices de parasitismo. Finalmente, la variación en la comunidad de parásitos entre estas 2 especies de hospederos simpátricos se debe probablemente a la distribución de los hospederos en el cuerpo de agua y a la dieta de los hospederos.
Palabras clave:
Amazonia; Diversidad; Peces de agua dulce; Ecología de parásitos
Introduction
Callichthyidae are Siluriformes fish distributed throughout South America and in Panama, with more than 200 species grouped into 8 genera, and varying from 2 to 25 cm in size (Froese & Pauly, 2018). In Brazil, the species of this family have a wide distribution in almost all of the hydrographic basins. They inhabit different environments, such as the muddy bottoms of igarapés (small, shallow water bodies in the Amazon basin –usually a tributary or canal), lakes and rivers, including environments with low levels of dissolved oxygen (Froese & Pauly, 2018; Pinheiro et al., 2013). The phylogenetically related fish species Callichthys callichthys Linnaeus, 1758 and Megalechis thoracata Valenciennes 1840, are found in the Amazon River system in the eastern Amazon region, northern Brazil (Reis, 1998; Shimabukuro-Dias et al., 2004). Such species of Callichthyidae are omnivorous and have a diet composed of vegetation, rotifers, plant remains, algae, insects and detritus (Froese & Pauly, 2018; Hahn et al., 2004; Resende et al., 2000). Callichthys callichthys has a maximum size of 17 cm and M. thoracata of 12.4 cm (Froese & Pauly, 2018; Resende et al., 2000). These 2 species of Callichthyidae are not listed by the IUCN as endangered. Therefore, do these 2 siluriforms of the Amazon river system in Brazil have similar parasite communities structure?
Among the vertebrates, fish represent the most abundant and diverse group. Species of this group are important to humans because they are a rich source of protein and, consequently, they have economic value. Studies of the relationship between fish and their environment are more valuable when they also incorporate host-parasite interactions, as the ecology of parasites and their hosts is one of the most relevant biological factors in these relationships, owing to continuous interactions between fish and their parasites in the environment. As such, in recent years, there has been an increase in interest about the ecological interactions between different fish species, parasites and the environment (Alarcos & Timi, 2012; Alcântara & Tavares-Dias, 2015; Guidelli et al., 2006; Lima et al., 2016; Muñoz et al., 2006; Pantoja et al., 2016; Poulin & Fitzgerald, 1987; Tavares & Luque, 2008; Tavares-Dias et al., 2014).
Populations of ecologically similar and genetically or evolutionarily related fish species that inhabit the same environment (sympatry) are more likely to have similar community composition of parasites (Alarcos & Timi, 2012; Guidelli et al., 2003; Lima et al., 2016; Muñoz et al., 2006; Pantoja et al., 2016; Poulin & Fitzgerald, 1987; Tavares & Luque, 2008). However, parasite communities are highly complex and dynamic ecological systems, resulting from interactions between different biotic (age, sex, immunity, size, genetics, migration, ecology, reproduction, physiology, etc.) and abiotic factors (water quality, pH, salinity, etc.) (Alarcos & Timi, 2012; Alcântara & Tavares-Dias, 2015; Guidelli et al., 2003; Lima et al., 2016; Pantoja et al., 2016; Poulin & Fitzgerald, 1987; Tavares & Luque, 2008; Tavares-Dias et al., 2014). The influence of these factors can be magnified by the diet of fish; so, hosts with similar diets tend to have an increased probability of infection by similar endoparasites (Alarcos & Timi, 2012; Alcântara & Tavares-Dias, 2015; Lima et al., 2016; Muñoz et al., 2006; Pantoja et al., 2016). This raises the question: Does the overlap in habitat and lifestyle of C. callichthys and M. thoracata in the Amazon River system lead to the occurrence of similar parasite communities in both host species?
The aim of this study was to compare the diversity, similarity, structure, and composition of the parasite communities in C. callichthys and M. thoracata, callichthyid fish species from a tributary of the Amazon River system, northern Brazil.
Materials and methods
The Fortaleza Igarapé basin (Fig. 1), located in the municipalities of Macapá and Santana, in the state of Amapá (eastern Amazon), is a tributary of the Amazon River composed of a main channel and an extensive floodplain area. It is influenced by the high rainfall of the Amazon region and by the daily tides of the Amazon River, and as such provides shelter and food for different fish species. The waters that periodically spread out across the floodplain are rich in nutrients, because of the rapid decomposition of grasses, animal remains and the humus layer of the forest. The regional vegetation is composed of plants characteristic of floodplain forest and herbaceous fields, principally various species of macrophytes (Tavares-Dias et al., 2014; Thomaz et al., 2003). This ecosystem has been strongly influenced by eutrophication due to urbanization (Cunha et al., 2004).
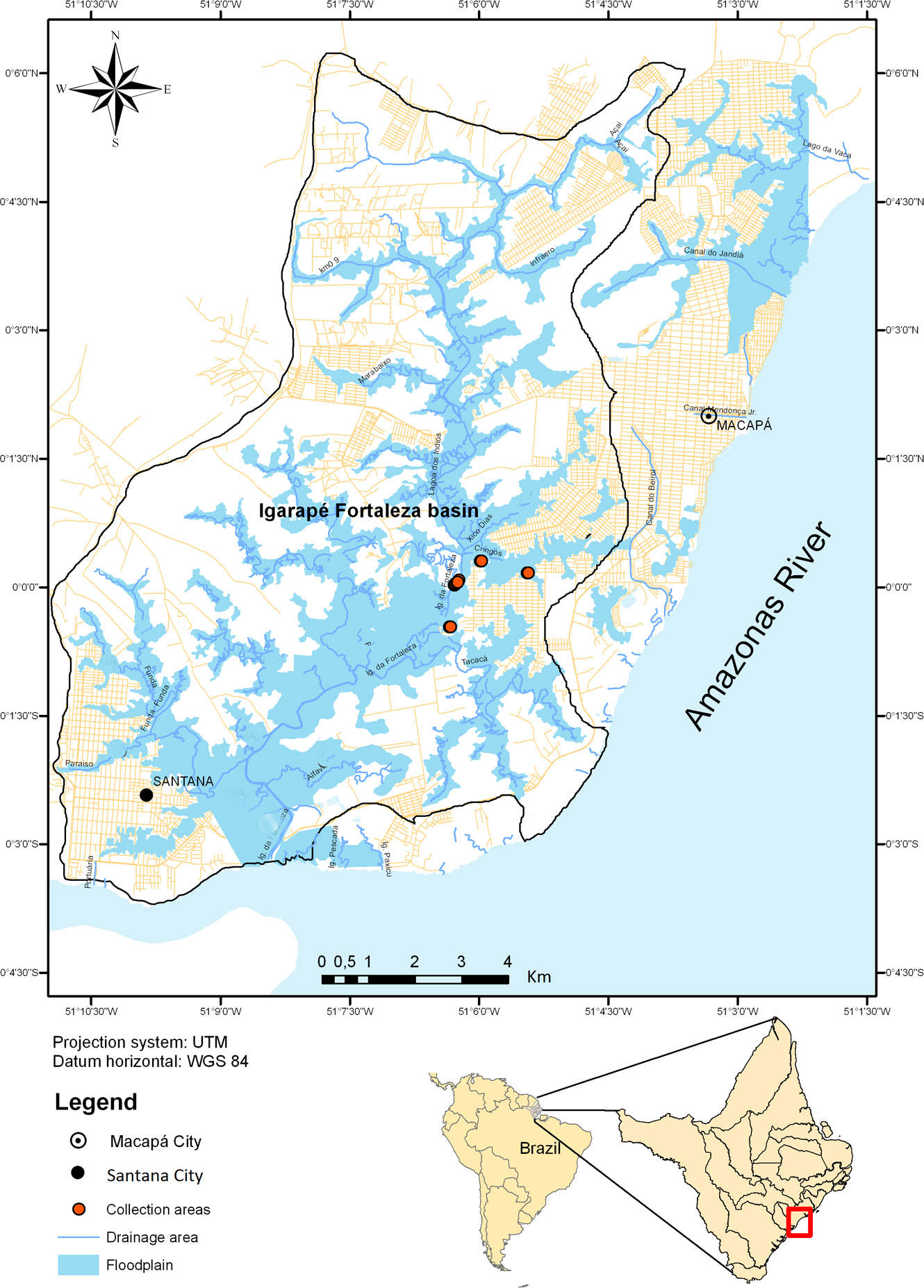
Between November and March 2013, 38 specimens of C. callichthys and 38 of M. thoracata were captured in the basin of the Fortaleza Igarapé (Fig. 1), city of Macapá, using gillnets of different sizes between 15 and 45 mm (Licence ICMBio: 23276-1). Fishes were then transported in containers of water to the Laboratory of Aquatic Animal Health of Embrapa Amapá, in Macapá (state of Amapá), for parasitological analysis. This study was developed in accordance with the principles adopted by the Brazilian College of Animal Experimentation (COBEA).
After measurement of body weight (g) and total length (cm), each fish was necropsied for parasite analysis. The mouth, opercula and gills were examined for ectoparasites and the gastro-intestinal tract was removed and examined for endo-parasites. The collection, fixation (formaldehyde 5%), preservation (70% alcohol), counting and staining of the parasites for identification followed prior recommendations (Eiras et al., 2006). The ecological terms (prevalence, mean intensity, and mean abundance) used are those recommended by Bush et al. (1997).
In order to evaluate the parasite community composition, the Shannon diversity index, evenness (E), species richness (Magurran, 2004) and dominance frequency (Rohde et al., 1995) were calculated using the software Diversity (Pisces Conservation Ltda, UK). In order to detect the pattern of distribution of the infracommunities of parasites (Rózsa et al., 2000) in species with prevalence > 10%, the dispersion index (DI) and the index of discrepancy (D) were determined using the software Quantitative Parasitology 3.0. The significance of the ID for each infracommunity was tested using the test statistic d (Ludwig & Reynolds, 1988).
The body mass weight (Wt; g) and total length (L; cm) data were used to calculate the relative condition factor (Kn) of the host fish using the length-weight relationship (Wt = aLb) after logarithmic transformation of length and weight and subsequent adjustment of 2 straight lines, thereby obtaining lny = lnA + Blnx (Le-Cren, 1951).
Principal component analysis (PCA) was carried out to compare the parasite communities of C. callichthys and M. thoracata. PCA was done using the Past-Paleontological Statistics software, version 3.0 (Hammer et al., 2001).
To measure the similarity of the parasite communities between C. callichthys and M. thoracata, 2 indices which take in to account differences in abundance of each shared parasite species were calculated –the Jaccard index (J) which is qualitative, and the Bray-Curtis index (B) which is quantitative (Ludwig & Reynolds 1988; Magurran, 2004). These indices of similarity were calculated using the software Past (Hammer et al., 2001).
The chi-squared test (χ2) was used to compare parasite prevalence between C. callichthys and M. thoracata, using a Yates correction. The mean abundance, species richness and Shannon diversity index were compared between both host species using Mann-Whiney (U) tests. Spearman’s correlation coefficient (rs) was used to determine possible correlations between length and body weight of hosts and parasite abundance, species richness and Shannon diversity index (Zar, 2010).
Results
The parasite community of C. callichthys and M. thoracata was composed by 6 species, being 4 common species to both hosts. However, Posthodiplostomum Dubois, 1936 (Diplostomidae) infected only to C. callichthys, while Gorytocephalus spectabilis Machado, 1959 infected only to M. thoracata (Table 1). Posthodiplostomum sp. was the dominant parasite in C. callichthys, but G. genarchella was the dominant parasite in M. thoracata. The majority of the infracommunities of parasites showed an aggregated pattern of distribution (Table 2).
Of the 38 specimens of C. callichthys examined, 44.7% were infected with 1 or more species of parasites; and of the 38 specimens of M. thoracata, 36.8% were infected. The prevalence (χ2 = 0.54, p = 0.464) and mean abundance (U = 622.5, p = 0.536) of Genarchella genarchella Travassos, Artigas & Pereira, 1928 (Derogenidae), did not differ between the 2 host populations. The prevalence (χ2 = 3.74, p = 0.053) of larvae of Eustrongylides Jagerskiold, 1909 (Dioctophymatidae) between both host species was different, while the mean abundance (U = 0.569, p = 0.112) was similar. The prevalence (χ2 = 4.07, p = 0.084) and mean abundance (U = 573.0, p = 0.122) of Rhabdochona Railliet, 1916 (Rhabdochonidae) were also similar between both host species. The prevalence (χ2 = 1.00, p = 0.903) and mean abundance (U = 722.0, p = 1.00) of plerocercoids of Proteocephalidae were also similar (Table 1).
Table 1. Parasites infracommunities in 2 Callichthyidae species from Brazilian Amazon. P: prevalence; MI: mean intensity; MA: mean abundance; TNP: total number of parasites, SI: site of infection.
|
Hosts |
Callichthys callichthys (n = 38) |
Megalechis thoracata (n = 38) |
|||||||
|
Parasites species |
P (%) |
MI |
MA ± SD |
TNP |
P (%) |
MI |
MA ± SD |
TNP |
SI |
|
Genarchella genarchella (metacercariae) |
20.0 |
1.7 |
0.3 ± 1.9 |
12 |
23.7 |
7.2 |
1.7 ± 4.1 |
65 |
Gills |
|
Genarchella genarchella (metacercariae and adults) |
28.9 |
3.3 |
1.0 ± 2.7 |
36 |
36.8 |
4.6 |
1.7 ± 3.3 |
65 |
Intestine |
|
Posthodiplostomum sp. (metacercariae) |
42.1 |
10.0 |
4.2 ± 9.2 |
160 |
0 |
0 |
0 |
0 |
Intestine |
|
Eustrongylides sp. (larvae) |
44.7 |
1.6 |
0.7 ± 1.0 |
27 |
23.7 |
1.8 |
0.4 ± 0.9 |
16 |
Intestine |
|
Rhabdochona sp. (larvae and adults) |
10.5 |
1.0 |
0.1 ± 0.3 |
4 |
28.9 |
3.5 |
1.0 ± 2.5 |
39 |
Intestine |
|
Proteocephalidae (plerocercoids) |
2.6 |
1 |
0.03 ± 0.2 |
1 |
2.6 |
1.0 |
0.03 ± 0.2 |
1 |
Intestine |
|
Gorytocephalus spectabilis (larvae) |
0 |
0 |
0 |
0 |
2.6 |
1.0 |
0.03 ± 0.2 |
1 |
Intestine |
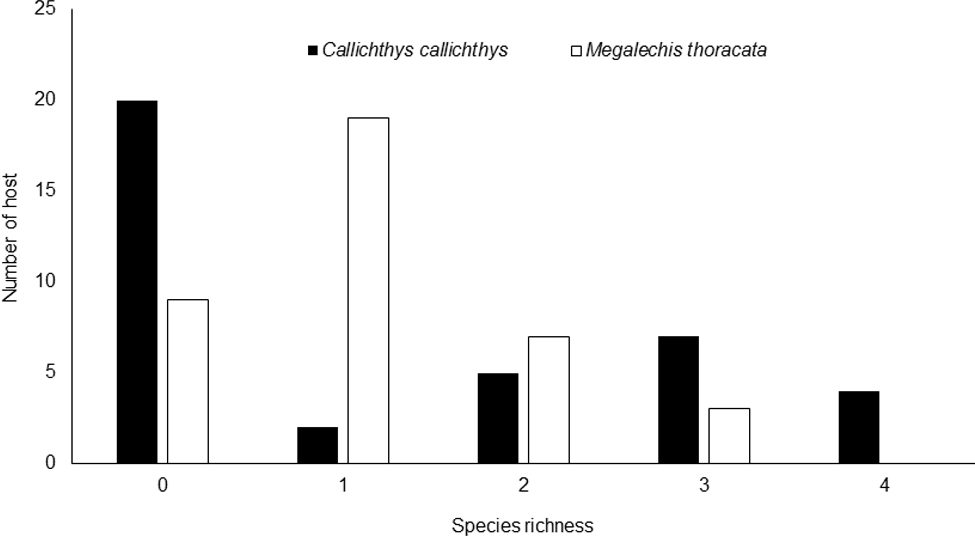
The parasite community composition of C. callichthys and M. thoracata was similar, as well as the species richness, evenness and Shannon diversity index. However, there were differences in body size of the 2 host species due to interspecific differences (Table 3). For C. callichthys, there was a predominance of non-parasitized individuals, whereas for M. thoracata there was a predominance of parasitized individuals (Fig. 2).
The size of C. callichthys was not correlated with parasite species richness (rs = 0.089, p = 0.594) or the Shannon index (rs = 0.189, p = 0.283), whereas body weight was weakly positively correlated with the Shannon index (rs = 0.328, p = 0.044), but not with parasite species richness (rs = 0.203, p = 0.222). The size of M. thoracata was not correlated with parasite species richness (rs = -0.295, p = 0.072) and the Shannon index (rs = -0.245, p = 0.138), nor was body weight correlated with the Shannon index (rs = -0.203, p = 0.221), but body weight showed a weak negative correlation with species richness of parasites (rs = -0.340, p = 0.037). Furthermore, there was no correlation (p > 0.05) between the abundance of parasites and body weight or size of C. callichthys and M. thoracata, owing to the narrow size ranges of the hosts.
The parasite community composition in C. callichthys and M. thoracata is highly similar, as shown by the Jaccard index (J = 0.67) and the Bray-Curtis similarity index (B = 0.61). However, multivariate analysis showed a difference between the parasite community composition of C. callichthys and M. thoracata, because of the presence of digeneans G. genarchella and Posthodiplostomum sp. (Fig. 3).
Table 2
Dispersion index (DI), d-statistic, discrepancy index (D) and frequency of dominance (FD) for the parasite infracommunities in 2 Callichthyidae species from Brazilian Amazon.
|
Hosts |
Callichthys callichthys |
Megalechis thoracata |
||||||||
|
Parasite species |
DI |
d |
D |
Dispersion |
FD (%) |
DI |
d |
D |
Dispersion |
FD (%) |
|
Genarchella genarchella (gills) |
2.373 |
4.51 |
0.833 |
Aggregated |
0.05 |
3.264 |
7.04 |
0.840 |
Aggregated |
0.35 |
|
Genarchella genarchella (intestine) |
2.536 |
5.15 |
0.781 |
Aggregated |
0.14 |
2.865 |
6.06 |
0.740 |
Aggregated |
0.35 |
|
Posthodiplostomum sp. |
3.410 |
7.38 |
0.711 |
Aggregated |
0.62 |
– |
– |
– |
– |
– |
|
Eustrongylides sp. |
1.114 |
0.58 |
0.625 |
Random |
0.10 |
1.466 |
1.91 |
0.795 |
Random |
0.09 |
|
Rhabdochona sp. |
0.919 |
– 0.25 |
0.872 |
Random |
0.02 |
2.544 |
5.22 |
0.773 |
Aggregated |
0.21 |
Table 3
Characteristics of the component community of parasites and body parameters of 2 Callichthyidae species from Brazilian Amazon. U = Mann-Whitney test; p = probability.
|
Parameters |
Callichthys callichthys |
Megalechis thoracata |
U |
p |
|
Length (cm) |
17.3 ± 1.3 (15.5-21.0) |
15.3 ± 1.1 (13.0-17.5) |
169.0 |
0.0001 |
|
Body mass (g) |
94.2 ± 22.3 (58.0-140.0) |
68.5 ± 16.8 (16.0-106.0) |
243.0 |
0.0001 |
|
Species richness |
1.29 ± 1.52 (0-4) |
1.11 ± 0.86 (0-3) |
692.0 |
0.755 |
|
Shannon diversity index |
0.36 ± 0.43 (0-0.99) |
0.17 ± 0.30 (0-1.08) |
551.5 |
0.076 |
|
Evenness (E) |
0.22 ± 0.27 (0-0.62) |
0.10 ± 0.19 (0-0.67) |
0.643 |
0.412 |
|
Total number of parasites |
260 |
187 |
– |
– |
|
Number of ectoparasites |
1 |
1 |
– |
– |
|
Number of endoparasites |
6 |
5 |
– |
– |
|
Endoparasites (adults) |
2 |
2 |
– |
– |
|
Endoparasites (larvae) |
7 |
6 |
– |
– |
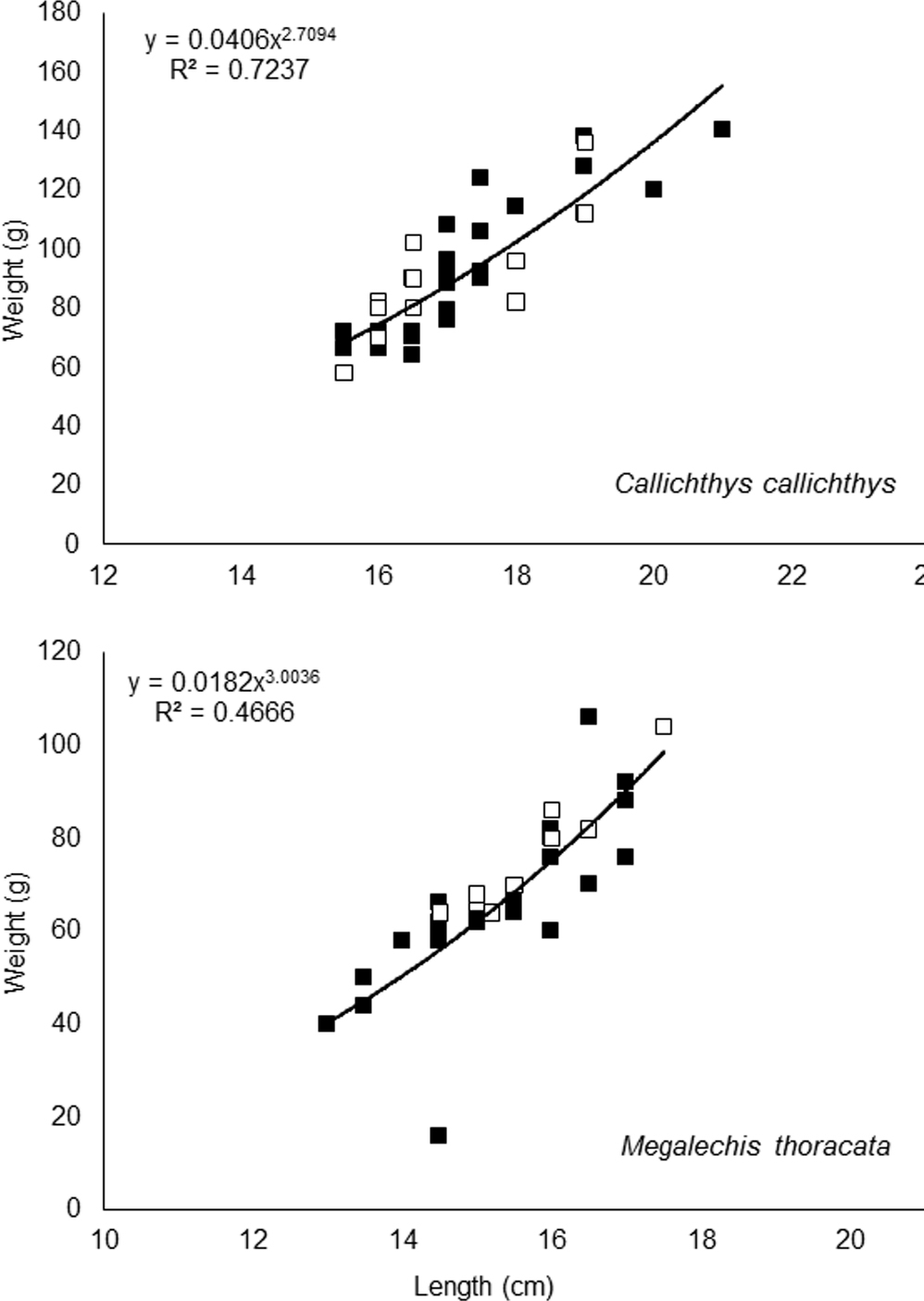
For C. callichthys, the weight-length relationship of parasitized and non-parasitized fish showed negative allometric growth (Fig. 4), which indicates a larger increase in body mass than length in these fish. The Kn of the parasitized individuals (Kn = 0.99 ± 0.02) did not differ (t = -0.23, p = 0.82) from the Kn of non-parasitized individuals (Kn = 1.00 ± 0.03). For M. thoracata the weight-length relationship of parasitized and non-parasitized fish increased isometrically (Fig. 4), indicating an increase in body mass and length in the same proportion. In this host species, the Kn of parasitized individuals (Kn = 1.01 ± 0.01) also did not differ (t = 1.85, p = 0.05) from the Kn of non-parasitized fish (Kn = 1.00 ± 0.01).
Discussion
The parasite fauna of callichthyids has been investigated for Hoplosternum littorale, a host with a rich parasite fauna made up of ectoparasites (2 Myxozoa, 2 Protozoa, 1 Crustacea, 4 Monogenea, and 2 Hirudinea) and endoparasites (6 Digenea, 2 Nematoda and 1 Cestoda) which varies according to the environment (Cohen et al., 2013; Morais & Malta, 2014; Pinheiro et al., 2013). In each host population, the structure and composition of the parasite communities are made up of a selection of the parasite species available in the environment (Alarcos & Timi, 2012; Alcântara & Tavares-Dias, 2015; Muñoz et al., 2006; Tavares-Dias et al., 2014).
The ecosystem of this study has been strongly influenced by eutrophication due to urbanization, which positively affected the ectoparasites community of some fish species (Alcântara & Tavares-Dias, 2015; Hoshino et al., 2014; Pantoja et al., 2016). However, several variables may influence the presence of endoparasites species, especially exposure to infective forms, which are directly acquired through the hosts’ feeding (Alcântara & Tavares-Dias, 2015; Pantoja et al., 2016).
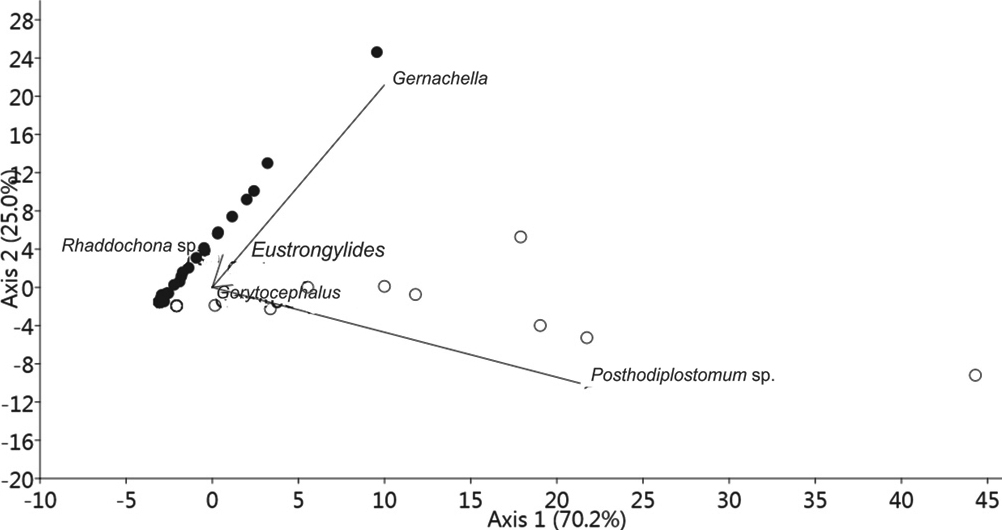
The parasite communities in C. callichthys and M. thoracata were composed of 2 species of Digenea, 2 Nematoda, 1 Acanthocephala and 1 Cestoda, with a dominance of endoparasites in the larval form, indicating that C. callichthys and M. thoracata are intermediate hosts. The similarity of the parasite communities between the 2 host species was 61%, with differences caused by digeneans G. genarchella and Posthodiplostomum sp., as shown by the analysis of variance. The similarity in the parasite communities indicates similar feeding behavior between these 2 host species, allowing relatively similar recruitment of the same species of endo-parasite from the study environment. Of the 6 species infecting C. callichthys and M. thoracata, only 4 species of parasites were common in both hosts, but the metrics of diversity, including the Shannon index, species richness and evenness were similar. The narrow range of sizes in the adult fish examined can explain the lack of correlation between the length of the 2 host species and the parasitic diversity parameters. The weight of the hosts explained only 30% of the variation in diversity of helminths found.
In C. callichthys and M. thoracata, the intestine was the most infected organ, as only 1 species of parasite was found in the gills of the hosts. Indeed, in these hosts, the infracommunities of parasites showed a highly aggregated dispersal, matching the characteristic pattern of parasites in fish populations. However, Eustrongylides sp. and Rhabdochona sp. showed a random distribution, a pattern typical of infracommunities of highly pathogenic parasites (Guidelli et al., 2003) with a different life-history strategy. Species of Eustrongylides have oligochaetes as the primary intermediate host, fish as the second intermediate host and the birds which feed on the fish (Ardeidae, Anseriformes, Gaviiformes, and Pelecaniformes) as the definitive host (Novakov et al., 2013). Species of Rhabdochona have nymphs of Ephemeroptera as intermediate hosts and fish as definitive hosts (Caspeta-Mandujano & Mejía-Mojica, 2004; Moravec, 2007). Furthermore, infection levels of Eustrongylides sp. and Rhabdochona sp. were similar in both host populations, indicating similar feeding preferences for invertebrates that are intermediate hosts in the life cycle of these nematodes.
In C. callichthys and M. thoracata the levels of infection by G. gernachella were similar, and occurred in the gills and intestine of these hosts. However, only C. callichthys was found to be infected with metacercariae of Posthodiplostomum sp., and individuals were infected at moderate levels of parasitism. This indicates contact between this fish species and mollusks containing the infectious form of this digenean, which in South America has fish as the second intermediate host and piscivorous birds as the definitive host (Ritossa et al., 2013). These parasitic helminths are frequently found in fish from the study region and at various levels of infection (Alcântara & Tavares-Dias, 2015; Bittencourt et al., 2014; Hoshino et al., 2014). Gernachella gernachella, a derogenid with a wide distribution in Brazil and Argentina (Scholz et al., 1995), has a mollusk species and some fish as intermediate hosts, but the siluriforms species are the definitive hosts (Lefebvre & Poulin, 2005; Martorelli, 1989).
Species of Diaptomus copepods or cyclopoids serve as intermediate hosts for the development of metacestodes or plerocercoids of species of Proteocephalidea, which are then ingested by a secondary intermediate fish host (Scholz, 1999; Soylu, 2013). The low infection of plerocercoids of Proteocephalidae in C. callichthys and M. thoracata indicates that these fish are intermediate hosts for these cestodes. The low level of infection by G. spectabilis occurred only in M. thoracata, and furthermore this host was infected only by larvae of this species, indicating that this acanthocephalan is using this fish as an intermediate host, completing its life-cycle in other host species in the same study region (Alcântara & Tavares-Dias, 2015; Bittencourt et al., 2014; Tavares-Dias et al., 2014).
In conclusion, the community of parasites of both hosts was characterized by low diversity, low richness and low diversity of ectoparasites. The variation in the parasite community between the 2 host species at the same life stage could be result of the distribution of the hosts in the environment, host immunology and the species composition of the parasites in the assemblage. Furthermore, there was an evident competition between the majority of the helminths species on the 2 hosts. The low levels of parasite infection did not affect body condition in C. callichthys and M. thoracata. The results suggests that the omnivorous diets of C. callichthys and M. thoracata, which include mollusks and crustaceans, favor the diversity of endohelminths in these hosts. Monogeneans, common ectoparasites in freshwater fish, were not found in C. callichthys and M. thoracata, because in general these helminths encounter hosts owing to their strict relationship with them. The co-evolutionary history of monogeneans species with their hosts is a determining factor for this relationship and, therefore, this question merits further investigation. This is the first report of G. gernachela, Rhabdochona sp., G. spectabilis, and Posthodiplostomum sp. for C. callichthys and M. thoracata.
Acknowledgements
The authors thank the “Conselho Nacional de Desenvolvimento Científico e Tecnológico” (CNPq) for the productivity scholarship awarded to Dr. M. Tavares-Dias (# 303013/2015-0).
References
Alarcos, A. J., & Timi, J. T. (2012). Parasite communities in three sympatric flounder species (Pleuronectiformes: Paralichthyidae). Parasitology Research, 110, 2155–2166.
Alcântara, N. M., & Tavares-Dias, M. (2015). Structure of the parasites communities in two Erythrinidae fish from Amazon River system (Brazil). Brazilian Journal of Veterinary Parasitology, 24, 183–190.
Bittencourt, L. S., Pinheiro, D. A., Cardenas, M. Q., Fernandes, B. M. M., & Tavares-Dias, M. (2014). Parasites of native Cichlidae populations and invasive Oreochromis niloticus (Linnaeus, 1758) in tributary of Amazonas River (Brazil). Brazilian Journal of Veterinary Parasitology, 23, 44–54.
Bush, A.O., Lafferty, K. D., Lotz, J. M., & Shostak, W. (1997). Parasitology meets ecology on its own terms: Margolis et al. Revisited. The Journal of Parasitology, 83, 575–583.
Caspeta-Mandujano, J. M., & Mejía-Mojica, H. (2004). Seasonal dynamics of the occurrence and maturation of Rhabdochona canadensis in its definitive host, Notropis boucardi, of the Chalma River, State of Morelos, Mexico. Helminthologia, 41, 121–123.
Cohen, S. C., Justo, M. C., & Kohn, A. (2013). South American Monogenoidea parasites of fishes, amphibians and reptiles. Rio de Janeiro: Oficina de Livros.
Cunha, A. C., Cunha, H. A., Brasil-Júnior, A. C. P., Daniel, L. A., & Schulz, H. E. (2004). Qualidade microbiológica da água em rios de áreas urbanas e periurbanas no baixo Rio Amazonas. O caso do Amapá. Engenharia Sanitária e Ambiente, 9, 322–328.
Eiras, J. C., Takemoto, R. M., & Pavanelli, G. C. (2006). Métodos de estudo e técnicas laboratoriais em parasitologia de peixes. Maringá: Eduem.
Froese, R., & Pauly, D. (2018). FishBase. World Wide Web electronic publication; version (02/2018). Available: www.fishbase.org
Guidelli, G. M., Isaac, A., Pavanelli, G. C., & Takemoto, R. M. (2003). Endoparasites infracommunities of Hemisorubim platyrhynchos (Valenciennes, 1840) (Pisces, Pimelodidae) of the Baia River, upper Paraná River floodplain, Brazil: specific composition and ecological aspects. Brazilian Journal of Biology, 63, 261–268.
Guidelli, G., Tavechio, W. L. G., Takemoto, R. M., & Pavanelli, G. C. (2006). Fauna parasitária de Leporinus lacustris e Leporinus friderici (Characiformes, Anostomidae) da planície de inundação do alto Rio Paraná, Brasil. Acta Scientiarum Biological Sciences, 28, 281–290.
Hahn, N. S., Fugi, R., & Andrian, J. F. (2004). Trophic ecology of the fish assemblages. In S. M. Thomaz, A. A. Agostinho, & N. S. Hahn (Eds.), The upper Paraná River and its floodplain: physical aspects, ecology and conservation (pp. 247–269). Leiden, Netherland: Backuys Publisher.
Hammer, R., Harper, D. A. T., & Ryan, P. D. (2001). PAST: paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
Hoshino, M. D. F. G., Hoshino, E. M., & Tavares-Dias, M. (2014). First study on parasites of Hemibrycon surinamensis (Characidae), a host from the eastern Amazon region. Brazilian Journal of Veterinary Parasitology, 23, 343–
347.
Le-Cren, E. D. (1951). The length-weight relationship and seasonal cycle in gonadal weight and condition in the perch (Perca fluviatilis). Journal of Animal Ecology, 20, 201–219.
Lefebvre, F., & Poulin, R. (2005). Progenesis in digenean trematodes: a taxonomic and synthetic overview of species reproducing in their second intermediate hosts. Parasitology, 130, 587–605.
Lima, L. B., Bellay, S., Giacomini, H. C., Issac, A., & Lima-Junior, D. P. (2016). Influence of host diet and phylogeny on parasite sharing by fish in a diverse tropical floodplain. Parasitology, 143, 343–349.
Ludwig, J. A., & Reynolds, J. F. (1988). Statistical ecology: a primer on methods and computing. New York: Wiley-Interscience.
Magurran, A. E. (2004). Measuring biological diversity. Oxford, UK: Blackwell Science.
Martorelli, S. R. (1989). Estudios parasitológicos en biotopos lénticos de la República Argentina. V. Dessarollo del ciclo biológico monoxeno de la metacercaria progenetica de Gernachella genarchella Travassos, 1928 (Digenea: Hemiuridae) parásita de Littoridina parchappei (Mollusca: Hidrobiidae). Revista del Museo de La Plata, Sección Zoologia, 14, 109–117.
Morais, A. M., & Malta, J. C. O. (2014). Chemical analysis trough “energy-dispersive spectroscopy (EDS)” of Digenea metacercariae found infesting specimens of Hoplosternum litoralle (Hancock, 1828) (Siluriformes: Callichthydae), captured in Manaus polluted igarapes. Neotropical Helminthology, 8, 217–225.
Moravec, F. (2007). First experimental observations on the development of Rhabdochona denudata (Nematoda: Rhabdochonidae) in the intermediate host. Folia Parasitologica, 54, 236–238.
Muñoz, G., Grutter, A. S., & Cribb, T. H. (2006). Endoparasite communities of five fish species (Labridae: Cheilininae) from Lizard Island: how important is the ecology and phylogeny of the hosts? Parasitology, 132, 363–374.
Novakov, N., Bjelic-Cabrilo, O., Cirkovic, M., Jubojevic, D., Lujic, J., Davidov, I. et al. (2013). Eustrongylidosis of European catfish (Siluris glanis). Bulgarian Journal of Agricultural Science, 19, 72–76.
Pantoja, V. M. F., Silva, L.V. F., & Tavares-Dias, M. (2016). Are similar the parasite communities structure of Trachelyopterus coriaceus and Trachelyopterus galeatus (Siluriformes: Auchenipteridae) in the Amazon basin? Brazilian Journal of Veterinary Parasitology, 25, 46–53.
Pinheiro, D. A., Tavares-Dias, M., Dias, M. K. R., Santos, E. F., & Marinho, R. G. B. (2013). Primeiro registro da ocorrência de protozoários em tamoatá Hoplosternum littorale no Brasil. Boletim do Instituto de Pesca, 39, 169–177.
Poulin, R., & Fitzgerald, G. J. (1987). The potential of parasitism in the structuring of a salt marsh stickleback community. Canadian Journal of Zoology, 65, 2793–2798.
Reis, R. E. (1998). Anatomy and phylogenetic analysis of the Neotropical callichthyid catfishes (Ostariophysi, Siluriformes). Zoological Journal of the Linnean Society, 124, 105–168.
Resende, E. K., Pereira, R. A. C., Almeida, V. L. L., & Silva, A. G. (2000). Peixes insetívoros e zooplanctófagos da planície inundável do Rio Miranda, pantanal, Mato Grosso do Sul, Brasil. Boletim de Pesquisa 17 (Embrapa Pantanal).
Ritossa, L., Flores, V., & Viozzi, G. (2013). Life-cycle stages of a Posthodiplostomum species (Digenea: Diplostomidae) from Patagonia, Argentina. The Journal of Parasitogy, l99, 777–780.
Rohde, K., Hayward, C., & Heap, M. (1995). Aspects of the ecology of metazoan ectoparasites of marine fishes. International Journal for Parasitology, 25, 945–970.
Rózsa, L., Reiczigel, J., & Majoros, G. (2000). Quantifying parasites in samples of hosts. The Journal of Parasitology, 86, 228–232.
Scholz, T. (1999). Lifes cycles of species of Proteocephalus, parasites of fishes in the Palearctich region: a review. Journal of Helminthology, 73, 1–19.
Scholz, T., Vargas-Vazquez, J., & Salgado-Maldonado, G. (1995). Revision of Genarchella species (Digenea: Derogenidae) parasitizing freshwater fishes in Mexico and Central America. Journal of Natural History, 29, 1403–1417.
Shimabukuro-Dias, C. K., Oliveira, C., Reis, R. E., & Foresti, F. (2004). Molecular phylogeny of the armored catfish family Callichthyidae (Ostariophysi, Siluriformes). Molecular Phylogenetics and Evolution, 32, 152–163.
Soylu, E. (2013). Metazoan parasites of perch Perca fluviatilis L. from Lake Sığırcı, Ipsala, Turkey. Pakistan Journal of Zoology, 45, 47–52.
Tavares, L. E. R., & Luque, J. L. (2008). Similarity between metazoan parasite communities of two sympatric brackish fish species from Brazil. The Journal of Parasitology, 94, 985–989.
Tavares-Dias, M., Oliveira, M. S. B., Gonçalves, R. A. & Silva, L. M. A. (2014). Ecology and seasonal variation of parasites in wild Aequidens tetramerus, a Cichlidae from the Amazon. Acta Parasitologica, 59, 158–164.
Thomaz, D. O., Costa-Neto, S. V., & Tostes, L. C. L. (2003). Inventario florístico das ressacas das bacias do Igarapé da Fortaleza e do Rio Curiau. In L. R. Takiyama, & A. Q. Silva (Eds.), Diagnóstico das ressacas do Estado do Amapá: bacias do Igarapé da Fortaleza e Rio Curiaú, Macapá-AP. (pp. 1–22). Macapá: CPAQ/IEPA e DGEO/ SEMA.
Zar, J. H. (2010). Biostatistical analysis. 5th Ed. New Jersey: Prentice Hall.



