Gabriela Felix-Nascimento a, d, Fabiano M. Vieira d, *, Ellen C. A. Gomes d, Ana Catarina L. Albinati d, Luís C. Muniz-Pereira b, c, Geraldo J. B. Moura a, Leonardo B. Ribeiro d, Jaqueline B. Oliveira a, e
a Universidade Federal Rural de Pernambuco, Programa de Pós-graduação em Biociência Animal (PPGBA), Rua Dom Manoel de Medeiros s/n, Recife 52.051-360, Brazil
b Instituto Oswaldo Cruz, FIOCRUZ, Laboratório de Helmintos Parasitos de Vertebrados, Av. Brasil 4365, Rio de Janeiro 21040-900, Brazil
c Instituto Oswaldo Cruz, FIOCRUZ, Programa de Pós-graduação em Biodiversidade e Saúde (PPGBS), Av. Brasil 4365, Rio de Janeiro 21040-900, Brazil
d Universidade Federal do Vale do São Francisco, Rodovia BR-407, Km. 12 Lote 543 s/n Projeto de Irrigação Nilo Coelho, Petrolina 56300-000, Brazil
e Universidade Federal Rural de Pernambuco, Laboratório de Parasitologia, Rua Dom Manoel de Medeiros s/n, Recife 52.051-360, Brazil
*Corresponding author: fmatosvieira@gmail.com (F.M. Vieira)
Received: 29 September 2020; accepted: 13 September 2021
Abstract
The present study aimed to describe the infective larval stage of Physaloptera sp. parasitizing Leptodactylus macrosternum and the microscopic lesions of these larvae in the stomach wall. Forty-five specimens of L. macrosternum were collected during the rainy season in May 2018, in the municipality of Petrolina, sub-middle São Francisco region, state of Pernambuco, Brazil. Twenty-seven infective larval stage (L3) specimens of Physaloptera sp. were collected attached in the stomach mucosa of 11 specimens (24.4%) of L. macrosternum. No other larval stages (L4) or adult of Physaloptera was found among the necropsied hosts. The stomach’s L3 attachment site showed macroscopic and histological lesions such as hyperaemic and ulcerated sites, mucous tunic necrotic regions, and inflammatory infiltrate eosinophilic. This is the first morphological study of Physaloptera infective larvae parasitizing L. macrosternum, and the first record of this paratenic host in the Caatinga biome. Additionally, it is also the first histopathological study of lesions caused by infective larvae of Physaloptera in this host species.
Keywords: Nematoda; Stomach; Anuran; Semiarid region
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Descripción morfológica de estadios larvarios infectantes
de Physaloptera (Spirurida: Physalopteridae), y las lesiones histológicas en el hospedador paraténico Leptodactylus macrosternum (Anura: Leptodactylidae) en el bioma de Caatinga, Brasil
Resumen
El objetivo de este estudio fue describir el estadio larvario infectante de Physaloptera sp. que parasita Leptodactylus macrosternum y los aspectos histopatológicos de esta larva en la mucosa del estómago del anuro. Se recolectaron 45 ejemplares de L. macrosternum durante la temporada de lluvias en mayo de 2018, en el municipio de Petrolina, en la región submedia de São Francisco, en Pernambuco, Brasil. Se recolectaron 27 larvas infecantes (L3) de Physaloptera sp., que se encontraban fijados en la mucosa del estómago de 11 (24.4%) individuos de L. macrosternum. No se encontraron otras larvas o adultos de Physaloptera entre los huéspedes sometidos a necropsia. El sitio de fijación de la L3 en la mucosa del estómago mostró alteraciones macroscópicas (hiperémicas y ulceradas) e histológicas (lesiones tubulares en el sitio de fijación del parásito, regiones necróticas en la túnica mucosa e infiltrado inflamatorio cerca del sitio de fijación larval). Este es el primer estudio morfológico de larvas infecciosas de Physaloptera sp. parasitando L. macrosternum, y el primer registro de Physaloptera sp. como huésped paraténico en el bioma de Caatinga. Además, es el primer estudio histopatológico de lesiones provocadas por larvas infecciosas de Physaloptera sp. en esta especie hospedera.
Palabras clave: Nematoda; Estómago; Anuro; Región semiárida
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Anurans are hosts of a wide diversity of parasites (Campião et al., 2014; Martins-Sobrinho et al., 2017). The reports of parasitism in Brazilian anurans by adult specimens of Physaloptera Rudolphi, 1819 (Nematoda: Physalopteridae) are still scarce and fragmentary. However, there are some reports of larval stages found in the anuran stomach or intestinal mucosa. The larvae are not studied morphologically in most of the records of Physaloptera in 42 anuran species in South America, so its stage of development is unknown (Aguiar et al., 2014; Alcantara et al., 2018; Campião et al., 2014; Hamann et al., 2013, 2014; Madelaire et al., 2012; Martins-Sobrinho et al., 2017; Teles, Brito et al., 2018; Toledo et al., 2015, 2018; Vrcibradic et al., 2019).
The species of Physaloptera are parasites with a heteroxenic life cycle, having arthropods as intermediate hosts. The larvae develop to the infective stage (third larval stage – L3), and occasionally paratenic hosts could be in the life cycle (Anderson, 2000). The transmission of the infective larval stage to definitive hosts occurs by ingestion of the infected arthropods or by ingestion of paratenic hosts parasitized by L3, such as anurans, lizards, snakes, and small mammals (Anderson, 1988, 2000). The cockroaches Blattella germanica (Linnaeus, 1767) (Hobmaier, 1941), some species of Orthoptera (Cawthorn & Anderson, 1976; Widmer, 1970), and Coleoptera (Anderson, 1988; 2000; Bowman, 2010; Quadros et al., 2014) are some of the insects recorded as intermediate hosts of Physaloptera with known biological cycle. The definitive hosts of Physaloptera species are terrestrial and semi-aquatic vertebrates. From 104 nominal species in this genus, only 3 are recorded as amphibian parasites: Physaloptera amphibia Linstow, 1889 from Limnonectes macrodon (Duméril & Bibron, 1841) (Anura, Dicroglossidae) in the Philippines, P. tigrinae Ali & Farooqui, 1969 from Hoplobatrachus tigrinus (Daudin, 1802) (Anura, Dicroglossidae) in India (Velarde-Aguilar et al., 2014), and P. retusa Rudolphi, 1819 from of Rhinella granulosa (Spix, 1824) (Anura, Bufonidae) and R. margaritifera (Laurenti, 1768), in Northern region of Brazil (Campião et al., 2014).
Paratenic hosts are those where the infecting parasite larva does not develop but remains infective to another host (Bowman, 2010; Gosling, 2005; Mehlhorn, 2008). Therefore, determining the larval stage is important to define the anurans’ function in the nematodes cycle (definitive host, paratenic, or second intermediate host) and better understand these interactions. In Brazil, only one study reports anurans as a possible second intermediate host of Physaloptera larvae through the analysis of cysts in the stomach of Leptodactylus leptodactyloides (Andersson, 1945), L. macrosternum Miranda-Ribeiro, 1926 (= L. ocellatus Girard, 1853), and L. petersii (Steindachner, 1864) from Cerrado biome (Goldberg et al., 2009). However, although the authors indicate the larval stage of this nematode as encysted, they did not provide morphological data or a diagnosis to confirm that this encysted larval stage is from Physaloptera or another physalopteroid (e.g., Abbreviata Travassos, 1920).
The histopathological changes caused by Physaloptera L3 attached to the stomach mucosa were described in the paratenic host Crotalus viridis snakes (Widmer, 1970). These data emphasize these vertebrates’ function as paratenic hosts since these larval stages of parasites do not secrete a cystic membrane or develop in these hosts.
Leptodactylus macrosternum (Anura, Leptodactylidae) is a medium-sized, generalist anuran with a nocturnal habit usually well-adapted to degraded environments. In Caatinga areas, this species feeds mainly on insects of Coleoptera, Hymenoptera, and Orthoptera (Teles, Rodrigues et al., 2018). As for the threat status, this anuran is classified as “Least concern” according to the IUCN red list (Heyer et al., 2010).
The morphology of Physaloptera infective larval stages in Brazil and the reports of anurans as paratenic hosts of these nematodes remains unknown. Therefore, the present study aimed to describe the infective larval stage of Physaloptera parasitizing L. macrosternum and the histopathological lesions of these larvae in the stomach wall.
Materials and methods
Forty-five specimens of L. macrosternum were collected during the rainy season in May 2018, in the municipality of Petrolina (9°20’4.68” S, 40°35’11.25” W), in the sub-middle São Francisco region, in the state of Pernambuco, Brazil. This municipality is in the semiarid of the Brazilian Northeast and presents a Caatinga vegetation sensu stricto, high environmental temperatures, irregular and scarce rainfall, and water deficit (Silva et al., 2017). The hosts were collected randomly by hand through active search and sent to the Laboratório de Morfofisiologia do Centro de Conservação e Manejo de Fauna da Caatinga (CEMAFAUNA-CAATINGA).
The anurans were euthanized through an overdose of 2% lidocaine chlorydrate, applied on the epidermis of the animals’ dorsal region, according to the CONCEA (2013) regulations. Then, the individuals were immediately necropsied under the stereomicroscope.
Live nematodes were collected, fixed in boiled 4% formalin, remaining in this fixative for 15 days at room temperature, and then stored in 70°GL ethanol. The parasites were cleared in Amann’s lactophenol and mounted on temporary slides, identified under light microscopy. The morphometry of the specimens was performed using Toup View 3.7.6701® software.
The nematodes were identified at a generic level, according to Anderson et al. (2009), and the ontogenetic stage, according to Cawthorn and Anderson (1976). The morphometric data in the description are given in micrometers, and in parentheses, the means and standard deviations were indicated. Voucher specimens of the nematodes were deposited in the Helminthological Collection of the Oswaldo Cruz Institute (CHIOC), FIOCRUZ, Rio de Janeiro, Brazil. Prevalence, mean intensity, and mean abundance of nematodes, were estimated according to Bush et al. (1997).
Six nematodes were analysed by scanning electron microscopy (SEM). The specimens were dehydrated in increasing ethanol concentrations, dried in 1,1,1,3,3,3-Hexamethyldisilazane 97% (HMDS) (Sigma-Aldrich), and coated with gold (Felix-Nascimento et al., 2020). Then, the specimens were observed in JEOL JSM 6390LV (operating 15 kV) microscope, in the Plataforma de Microscopia Eletrônica Rudolf Barth of the Oswaldo Cruz Institute, FIOCRUZ, Rio de Janeiro, Brazil.
One to 3 stomach fragments of each infected host with macroscopic lesions were collected for histopathological analysis. Through routine histology, the stomach fragments measuring about 25 and 100 mm² were fixed in 10% buffered formalin, dehydrated in increasing ethanol concentrations, clarified with xylol, and embedded in paraffin. Embedded fragments were cut to a 5 μm thickness with a microtome and 10 slides of each fragment were made, stained with hematoxylin-eosin, and analysed under the microscope light.
The study was conducted under the authorizations of the Sistema de Autorização e Informação em Biodiversidade (SISBIO), of the Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio Nº 62680-1), Brazil, and under license of the Comitê de Ética no Uso de Animais (CEUA) of the Universidade Federal do Vale do São Francisco (UNIVASF Nº 0001/221018).
Description
Physaloptera sp. – Third larval stage (L3)
(Figs. 1, 2)
Twenty-seven specimens of the infective larval stage (L3) of Physaloptera sp. (Figs. 1, 2) were collected and attached in the stomach mucosa of 11 specimens (24.4%) of L. macrosternum. No other larval (fourth larval stage – L4) or adult stages of Physaloptera was found.
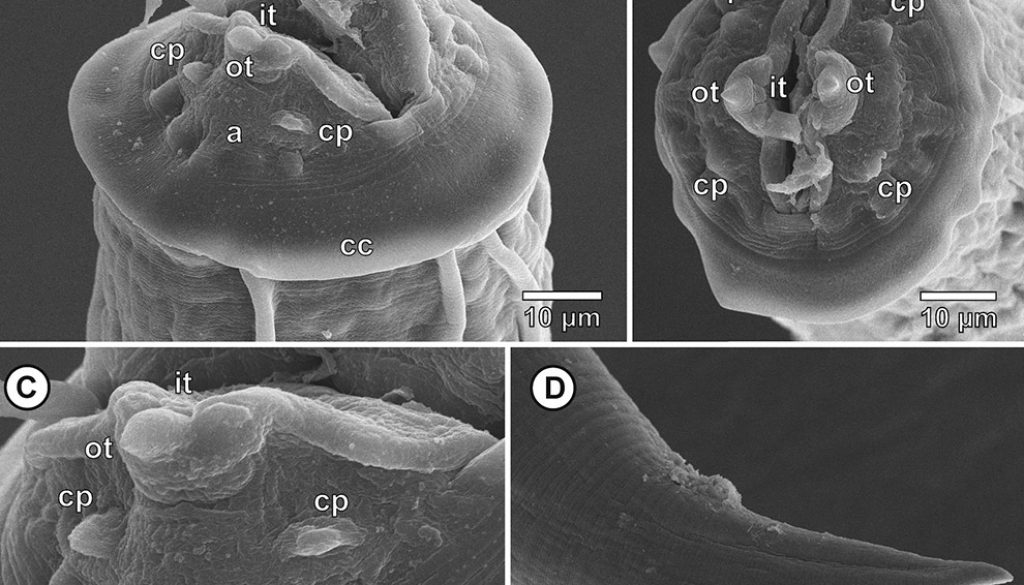
Third larval stage (n = 10 specimens analyzed): translucent nematodes before fixation. Live collected specimens were attached by the anterior region in the stomach mucosa, without cystic membrane formation or within inflammatory nodules. Thin and smooth cuticle without transverse striations (Fig. 2A), composed by the third stage larval cuticle and the cuticle sheath, forming a double cuticle typical of the infective third larval stage of Secernentea nematodes (Fig. 3E-F). Cephalic region with a circular cuticular dilatation at the base of the pseudolabia, forming a cephalic collarette (Figs. 1B, C, 2A, B). Rounded anterior end, consisting of 2 well-developed lateral pseudolabia (Figs. 1B, C, 2A-C). Each pseudolabia with 2 cephalic papillae, 1 laterodorsal and 1 lateroventral; and 1 amphid pore between these 2 cephalic papillae (Fig. 2A-C). Inner margin of pseudolabia with 1 tooth like protuberance, consisting of 1 outer tooth and 3 inner teeth, forming a trident (Fig. 2C). Buccal capsule absent. Total body length 2.7-3.7 (3.2 ± 3.8) mm, body width at the level of oesophagus intestine junction 155-213 (189 ± 21.7). Nerve ring anterior to excretory pore, 116-179 (155 ± 24) and 208-268 (248 ± 24) of the anterior region, respectively. Long muscular-glandular oesophagus (Fig. 2A), 1.2-1.4 (1.3 ± 0.1) mm long; muscular oesophagus short, 170-200 (185 ± 10.2) long; glandular oesophagus 1.0-1.2 (1.1 ± 0.2) mm long. Larvae without internal and external sexual differentiation (Fig. 1A), although with a small genital primordium located at equatorial region of body, ventral to intestine (Fig. 1A). Tail conical, without caudal filament (Figs. 1D, E, 2D), 86-138 (114 ± 16) long.
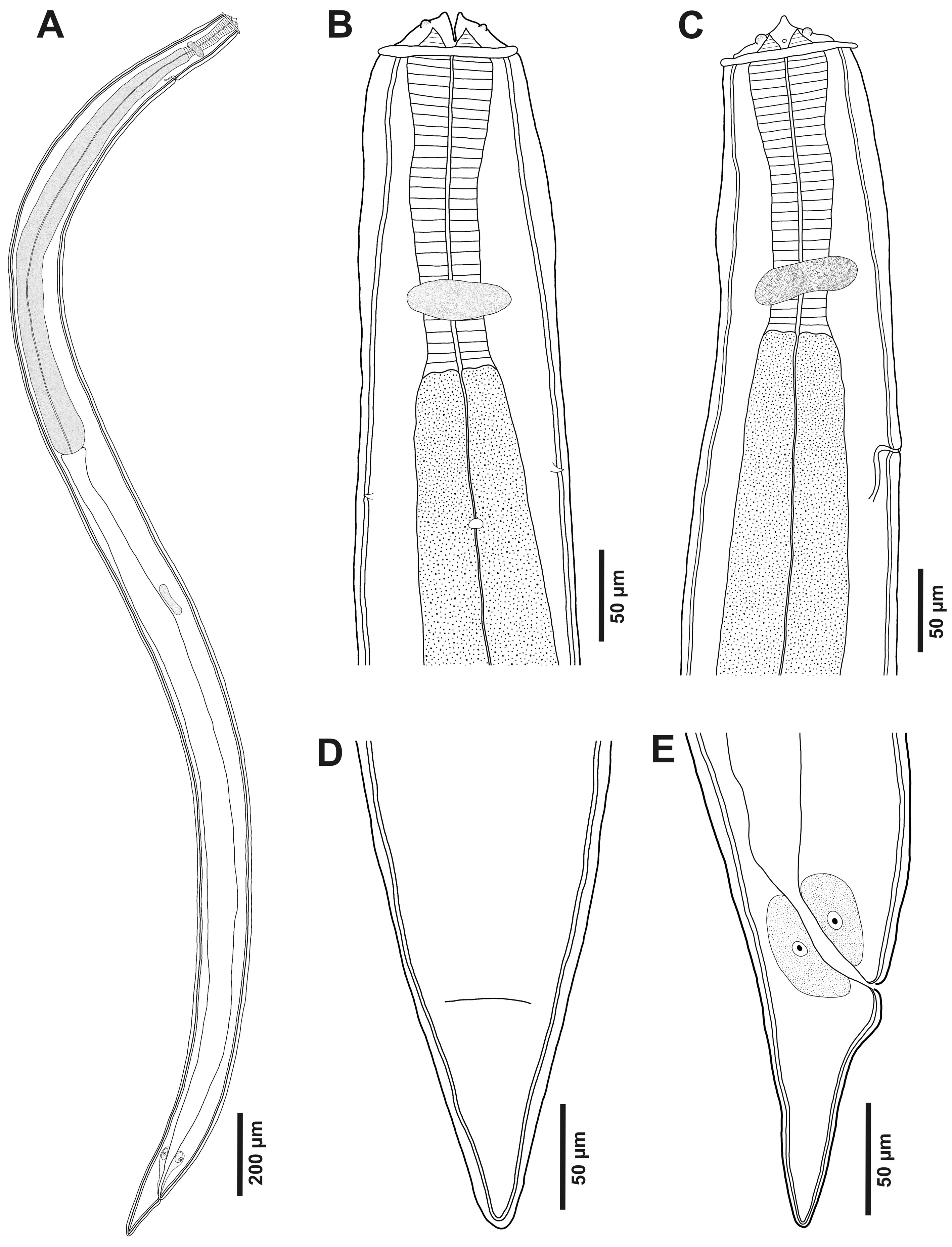
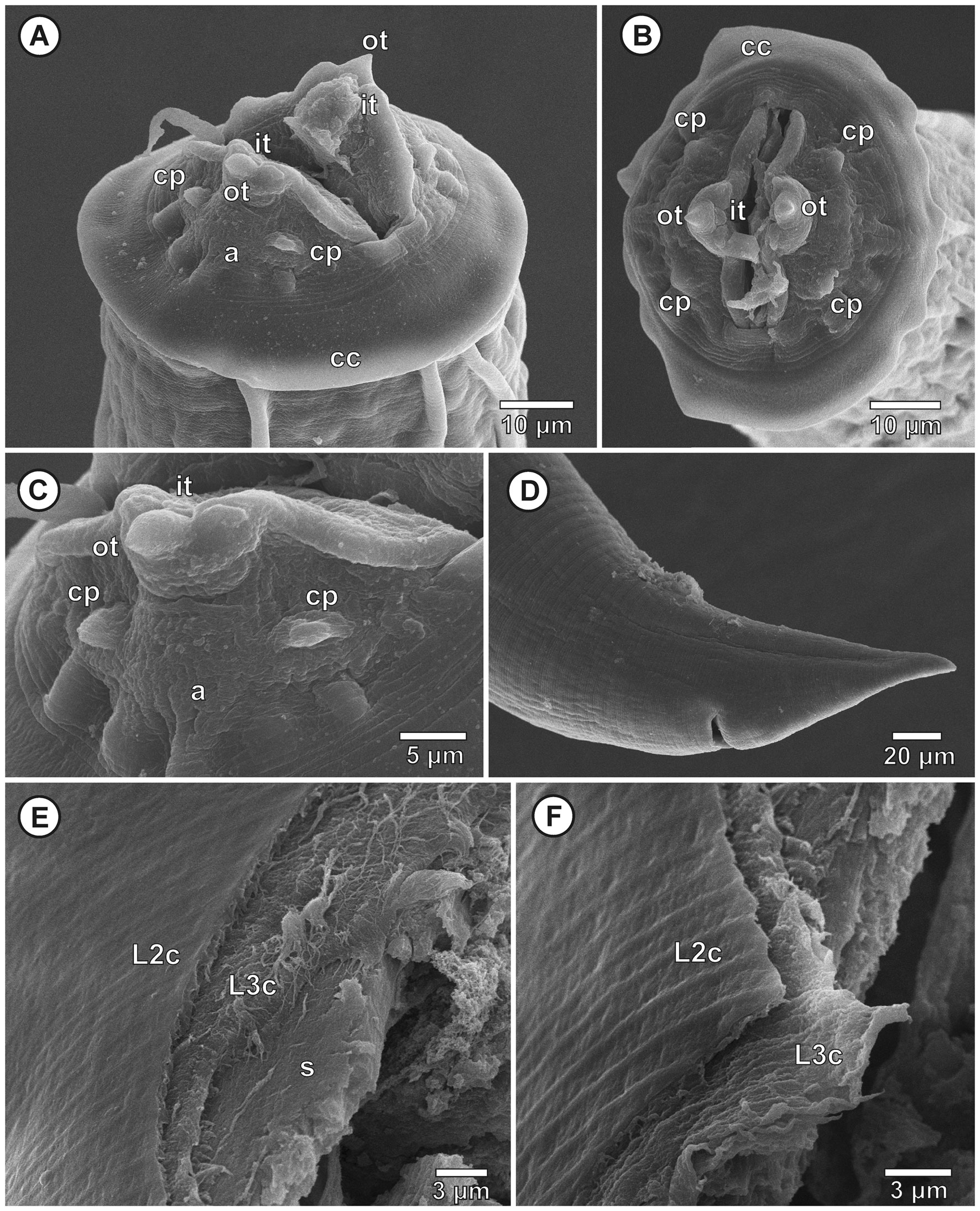
Taxonomic summary
Host: Leptodactylus macrosternum Miranda-Ribeiro 1926 (Anura, Leptodactylidae)
Collection localities: municipality of Petrolina (9°20’4.68” S, 40°35’11.25” W), state of Pernambuco, Brazil.
Site of parasitism: stomach.
Prevalence: 24.4%
Mean intensity: 2.5 ± 0.5 parasites per infected hosts.
Mean abundance: 0.6 ± 0.2 parasites per analysed hosts.
Range of infection: 1-6.
Voucher specimens: 5 specimens deposited in the CHIOC (CHIOC 38971).
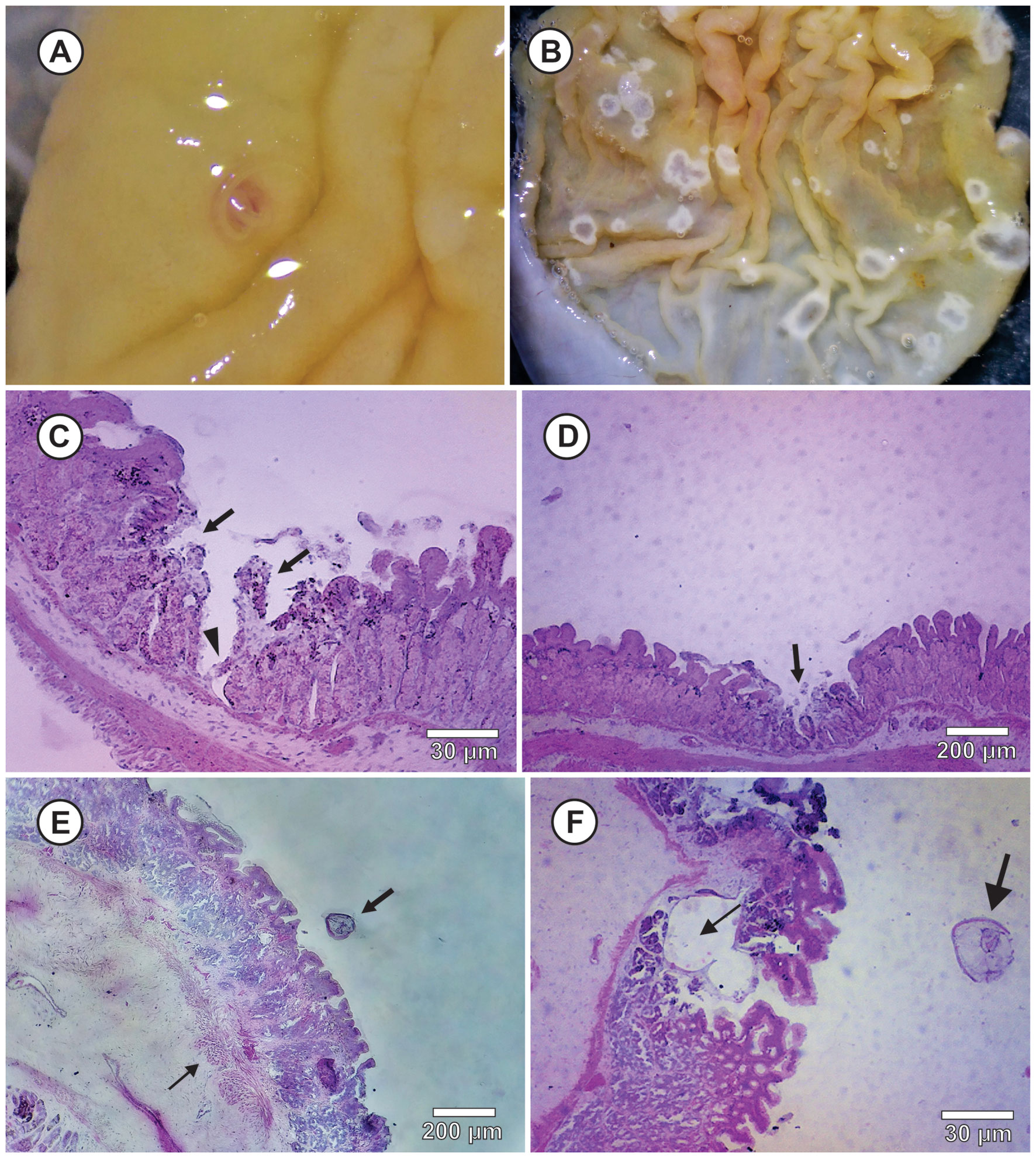
In the macroscopic analysis, it was observed that the attachment site of L3 of Physaloptera sp. at the stomach was hyperaemic and ulcerated with rounded shape and randomly distributed in the mucosa (Fig. 3A). In some parasitized animals, ulcerative lesions with whitish edges without parasite in other points of the stomach mucosa were also observed, which may correspond to a attachment site abandoned by the parasite (Fig. 3B). No exudates or edema were observed.
In histological sections, it was observed lesions at the parasite attachment site, extending to the limit of the mucosa with the submucosa (Fig. 3C), the necrotic regions in the mucous tunic (Fig. 3D), congested blood vessels that caused hyperaemia, and the areas of inflammatory infiltrate eosinophilic near the larval attachment site in the submucosa tunic (Fig. 3E). Inside the stomach mucosa or at any other parts of the digestive tract, larval stages of Physaloptera encysted by a cystic membrane or in inflammatory nodules produced by the stomach wall of hosts were not found (Fig. 3F).
Discussion
The infective larvae of Physaloptera develops in the intermediate arthropod host (Anderson, 2000), and there was no change in the nematode larval stage between the intermediate and the anuran host. Thus, the presence of free infective larval stage (L3) in the L. macrosternum stomach mucosa and the absence of other subsequent parasite developmental stages indicate that this anuran acts as a paratenic host in the biological cycle of Physaloptera. According to Anderson (1988), the L3 of Physaloptera spp. can remain attached to paratenic hosts’ stomach mucosa (e.g., frogs, snakes, and small mammals) for extended periods. In this work, the L3 was characterized by a well-developed genital primordium and a double cuticle. Besides, in this larval stage, the cephalic structures that distinguish this genus from others are the presence of a well-developed cephalic collarette, 2 pseudolabia with 3 inner and outer teeth, 1 pair of cephalic papillae in each pseudolabia, and 1 evident amphid, corroborating the morphology of the L3 of Physaloptera described in other studies (Cawthorn & Anderson, 1976; Hobmaier, 1941; Lincoln & Anderson, 1975; Petri, 1950; Schell, 1952). Physaloptera maxillaris, P. rara, and P. hispida have their ontogenetic developmental stages described in the literature. For these species, the infective larval stage (L3) presented a cephalic region morphologically similar to the adult stages (Lincoln & Anderson, 1975; Petri, 1950; Schell, 1952).
The most recent records of host-parasite associations between helminths and South American anurans highlights the occurrence of an unknown developmental stage of Physaloptera larvae in anurans of the families Leptodactylidae, Hylidae, and Bufonidae (Aguiar et al., 2014; Alcantara et al., 2018; Campião et al., 2014; Goldberg et al., 2009; Hamann, 2014; Hamann et al., 2013; Madeleire et al., 2012; Martins-Sobrinho et al., 2017; Teles, Brito et al., 2018; Toledo et al., 2015, 2018; Vrcibradic et al., 2018). In the Caatinga biome, the Physaloptera was recorded for Rhinella granulosa (Campião et al., 2014; Teles, Brito et al., 2018), Physalaemus albifrons (Spix, 1824) (Oliveira et al., 2019), and Dermatonotus muelleri (Boettger, 1885) (Alcantara et al., 2018), in the state of Ceará. The studies reporting Physaloptera larvae in South American anurans do not mention the developmental stage of these larvae, although some authors discuss the possibility of anurans acting as paratenic (Aguiar et al., 2014; Alcantara et al., 2018; Goldberg et al., 2009; Madelaire et al., 2012; Martins-Sobrinho et al., 2017) or intermediate hosts (Hamann et al., 2013; Toledo et al., 2018). Only one study reports the presence of Physaloptera larvae cysts (Goldberg et al., 2009) in the stomach of L. macrosternum (=L. ocellatus), L. petersii, and L. leptodactyloides, in Tocantins State, Brazil, however without describing its larval stage.
Anurans are among the most preyed tetrapods by vertebrates and invertebrates (Andrade et al., 2012; Pergentino & Ribeiro, 2017; Ribeiro & Freire 2009; Toledo, 2005). There are some reports of L. macrosternum predation by snakes (Oliveira et al., 2014) and birds (Andrade et al., 2013), which may act as definitive hosts of Physaloptera spp. In Mexican anurans, the parasitism by L3 of Physaloptera in Lithobates montezumae (Baird, 1854) was described by Velarde-Aguilar et al. (2014). These authors performed a detailed morphological study about the infective larvae of this nematode. Based on anurans’ role in the trophic web of definitive hosts of Physaloptera, they claim to be evident that anurans commonly act as paratenic hosts of this nematode genus, being carnivorous mammals and snakes their definitive hosts. In the parasites’ life cycle, the paratenic host presents a more ecological than physiological function since the parasites do not develop in its host (Anderson 2000). The paratenic host allows the parasite’s infective stage to be transmitted between intermediate and definitive hosts, filling an ecological gap in the definitive host trophic web (Goater et al., 2014; Loker & Hofkin, 2015; Roberts & Janovy, 2009).
The diet of L. macrosternum in the Caatinga biome is mainly composed of arthropods, mostly Hymenoptera, Coleoptera, and Orthoptera (Teles, Rodrigues et al., 2018). These last 2 insects’ orders represent the main intermediate hosts of Physaloptera infective larval stage (Anderson, 1988; Cawthorn & Anderson 1976; Quadros et al., 2014; Widmer, 1970). However, their potential predators, which are probably the definitive hosts of this species of Physalaptera, are still unknown in our sampling area.
The histopathological findings observed in the present study are similar to those described by Widmer (1970) for L3 of Physaloptera sp. in the paratenic host Crotalus viridis Rafinesque, 1818 (Squamata, Colubridae), in Weld County, Colorado, USA. The author reported tubular lesions in the stomach mucosa of the snake, with liquefaction zones in the areas of the larvae attachment. Domestic cats were experimentally infected to confirm that the studied L3 were Physaloptera rara Hall & Wigdor, 1918. In this study, necrotic lesions were observed both macroscopically and microscopically. The absence of L3 in some of these lesions suggests that the larvae may have detached from the attachment site despite maintaining the mucosa’s inflammatory reaction, perhaps due to death or migration to another site in the stomach mucosa. According to Anderson (2000), Physaloptera adheres to the host’s stomach with the help of the large dentate pseudolabia and the cephalic collarette that presses the gastric mucosa however this parasite does not feed on the host’s tissues. Thus, the lesions observed in the present study are probably the result of fixing the parasite on the mucosa, which generates an inflammatory response with subsequent necrosis. Other histopathological studies of Australian reptiles and anurans parasitized by L3 of other species of Spirurida report inflammatory processes in the stomach tissue in response to parasitism. However, these larvae were encapsulated in inflammatory nodules in these hosts’ submucosal layer (Jones, 1995; Kelehear & Jones, 2010), different from those observed in the histopathological evidence in the hosts of the current study.
Therefore, the current record is the first morphological study of the infective larvae of Physaloptera parasitizing L. macrosternum and the first record of L. macrosternum as a paratenic host of Physaloptera sp. in the Caatinga biome. It indicates that the anurans play an essential ecological role in maintaining this nematode’s life cycle, filling a gap in the food web between intermediate and definitive hosts. Additionally, it is also the first histopathological study of infective larvae of Physaloptera in this host species.
Acknowledgements
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Gabriela Felix do Nascimento was supported by a Doctoral fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) of the Programa de Pós-graduação em Biociência Animal da Universidade Federal Rural de Pernambuco (PPGBA-UFRPE), Recife, state of Pernambuco, Brazil. Fabiano M. Vieira was supported by a Research fellowship from Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE), state of Pernambuco, Brazil. We are grateful to Centro de Conservação e Manejo de Fauna da Caatinga (CEMAFAUNA CAATINGA) and to Ministério do Desenvolvimento Regional (MDR) for providing the infrastructure and equipament used in the study. Sincere thanks are given to Wasley Carlos Gonçalves de Matos by aid in the histological processing, and Valdelice Albuquerque Castelo Branco, Jhonatan Santos Nascimento and Manoel da Silva Santos Junior for the logistic support during the collections.
References
Aguiar, A., Morais, D. H., Cicchi, P. J. P., & Da Silva, R. J. (2014). Evaluation of helminths associated with 14 amphibian species from a neotropical Island near the southeast coast of Brazil. Herpetological Review, 45, 227–236.
Alcantara, E. P., Ferreira-Silva, C., Silva, L. A. F., Lins, A. G. S., Ávila, R. W., Morais, D. H., & da Silva, R. J. (2018). Helminths of Dermatonotus muelleri (Anura: Microhylidae) from Northeastern Brazil. Journal of Parasitology, 104, 550–556. https://doi.org/10.1645/16-160
Anderson, R. C. (1988). Nematode transmission patterns. Journal of Parasitology, 74, 30–45.
Anderson, R. C. (2000). Nematode parasites of vertebrates: their development and transmission, 2nd Ed. New York: CABI Publishing.
Anderson, R. C., Chabaud, A., & Willmott, S. (2009). Keys to the nematode parasites of vertebrates: archival volume. New York: CABI Publishing.
Andrade, E. B., Lima-Júnior, T. B., Leite-Júnior, J. M. A., & Leite, J. R. S. A. (2012). Predation by native fish and feeding by crab species on Leptodactylus macrosternum Miranda-Ribeiro, 1926 (Anura: Leptodactylidae) in northeastern, Brazil. Herpetology Notes, 5, 173–175.
Andrade, E. B., Melo, S. C. A., & Cunha, J. A. S. (2013). Predation on Leptodactylus macrosternum (Anura: Leptodactylidae) by Botaurus pinnatus (Pelecaniformes: Ardeidae) in Northeastern Brazil. Herpetology Notes, 6, 201–202.
Bowman, D. D. (2010). Parasitologia veterinária de Georgis, 9th Ed. Rio de Janeiro: Saunders Elsevier.
Bush, A. O., Lafferty, K. D., Lotz, J. M., & Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited*. Journal of Parasitology, 83, 575–583. https://doi.org/10.2307/3284227
Campião, K. M., Morais, D. H., Dias, O. T., Aguiar, A., Toledo, G. M., Tavares, L. E. R. et al. (2014). Checklist of helminth parasites of amphibians from South America. Zootaxa, 3843, 1–93. https://doi.org/10.11646/zootaxa.3843.1.1
Cawthorn, R. J., & Anderson, R. C. (1976). Development of Physaloptera maxillaris (Nematoda: Physalopteroidea) in skunk (Mephitis mephitis) and the role of paratenic and other hosts in its life cycle. Canadian Journal of Zoology, 54, 13–323. https://doi.org/10.1139/z76-035
Chapman, J. M., Marcogliese, D. J., Suski, C. D., & Cooke, S. J. (2015). Variation in parasite communities and health indices of juvenile Lepomis gibbosus across a gradient of watershed land-use and habitat quality. Ecological Indicators, 57, 564–572. https://doi.org/10.1016/j.ecolind.2015.05.013
Christin, M. S., Gendron, A. D., Brousseau, P., Ménard, L., Marcogliese, D. J., Cyr, D. et al. (2003). Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environmental Toxicology and Chemistry, 22, 1127–1133. https://doi.org/10.1002/etc.5620220522
CONCEA (Conselho Nacional de Controle de Experimentação Animal). (2013). Resolução Normativa nº. 13, 20 de setembro de 2013. Baixa as diretrizes da prática de eutanásia do Conselho Nacional de Controle de Experimentação Animal -CONCEA. Diário Oficial da União, 5. Brazil.
Felix-Nascimento, G., Vieira, F. M., Muniz-Pereira, L. C., Moura, G. J. B., Ribeiro, L. B. & Oliveira, J. B. (2020). Two new species of Cosmocercidae (Nematoda: Cosmocercoidea) of Leptodactylus macrosternum Miranda-Ribeiro (Anura: Leptodactylidae) from Caatinga Biome, Brazil. Zootaxa, 4877, 274–290. https://doi.org/10.11646/zootaxa.4877.2.3
Ferracini, V. L., Pessoa, M. C. Y. P., Silva, A. S., & Spadotto, C. A. (2001). Análise de Risco de Contaminação das Águas Subterrâneas e Superficiais da Região de Petrolina (PE) e Juazeiro (BA). Pesticidas: Revista de Ecotoxicologia e Meio Ambiente, 11, 1–16.
Gendron, A. D., Marcogliese, D. J., Barbeau, S., Christin, M. S., Brousseau, P., Ruby, S. et al. (2003). Exposure of leopard frogs to a pesticide mixture affects life history characteristics of the lungworm Rhabdias ranae. Oecologia, 135, 469–476. https://doi.org/10.1007/s00442-003-1210-y
Goater, T. M., Goater, C. P., & Esch, G. W. (2014). Parasitism: the diversity and ecology of animal parasites, 2nd Ed. New York: Cambridge University Press.
Goldberg, S. R., Bursey, C. R., Caldwell, J. P., & Shepard, D.B. (2009). Gastrointestinal helminths of 6 sympatric species of Leptodactylus from Tocantins State, Brazil. Comparative Parasitology, 76, 258–266. https://doi.org/10.1654/4368.1
Gosling, P. J. (2005). Dictionary of Parasitology. Boca Raton: Taylor & Francis Group.
Hamann, M. I., Kehr, A. I., & González, C. E. (2013). Helminth communities in the burrowing toad, Rhinella fernandezae, from Northeastern Argentina. Biologia, 68, 1155–1162. https://doi.org/10.2478/s11756-013-0272-5
Hamann, M. I., Kehr, A. I., & González, C. E. (2014). Helminth community structure in the Argentinean bufonid Melanophryniscus klappenbachi: importance of habitat use and season. Parasitology Research, 113, 3639–3649. https://doi.org/10.1007/s00436-014-4029-z
Hernandez, A. D., Bunnell, J. F., & Sukhdeo, M. V. K. (2007). Composition and diversity patterns in metazoan parasite communities and anthropogenic disturbance in stream ecosystems. Parasitology, 134, 91–102. https://doi.org/10.1017/S0031182006001247
Heyer, R., Langone, J., La Marca, E., Azevedo-Ramos, C., di Tada, I., Baldo, D. et al. (2010). Leptodactylus latrans. The IUCN Red List of Threatened Species 2010: e.T57151A11592655. Downloaded on 30 March 2021 in https://www.iucnredlist.org/species/57151/11592655
Hobmaier, M. (1941). Extramammalian Phase of Physaloptera maxillaris Molin, 1860 (Nematoda). Journal of Parasitology, 27, 233–235. https://doi.org/10.2307/3273016
Jones, H. I. (1995). Pathology associated with physalopterid larvae (Nematoda: Spirurida) in the gastric tissues of australian reptiles. Journal of Wildlife Diseases, 31, 299–306. https://doi.org/10.7589/0090-3558-31.3.299
Kelehear, C., & Jones, H. I. (2010). Nematode larvae (Order Spirurida) in gastric tissues of australian anurans: a comparison between the introduced cane toad and sympatric native frogs. Journal of Wildlife Diseases, 46, 1126–1140. https://doi.org/10.7589/0090-3558-46.4.1126
Koprivnikar, J. (2010). Interactions of environmental stressors impact survival and development of parasitized larval amphibians. Ecological Applications, 20, 2263–2272. https://doi.org/10.1890/09-1558.1
Koprivnikar, J., Marcogliese, D. J., Rohr, J. R., Orlofske, S. A., Raffel, T. R., & Johnson, P. T. J. (2012). Macroparasite infections of amphibians: What can they tell us? Ecohealth, 9, 342–360. https://doi.org/10.1007/s10393-012-0785-3
Lafferty, K. D., & Holt, R. D. (2003). How should environmental stress affect the population dynamics of disease? Ecology Letters, 6, 654–664. https://doi.org/10.1046/j.1461-0248.2003.00480.x
Lincoln, R. C., & Anderson, R. C. (1975). Development of Physaloptera maxillaris (Nematoda) in the common field cricket (Gryllus pennsylvanicus). Canadian Journal of Zoology, 53, 385–390. https://doi.org/10.1139/z75-051
Loker, E. S., & Hofkin, B. V. (2015). Parasitology: a conceptual approach. New York: Garland Science.
Madelaire, C. B., Gomes, F. R., & Silva, R. J. (2012). Helminth parasites of Hypsiboas prasinus (Anura: Hylidae) from two Atlantic Forest fragments, São Paulo State, Brazil. Journal of Parasitology, 98, 560–564. https://doi.org/10.1645/JP-GE-2665.1
Marcogliese, D. J., King, K. C., Salo, H. M., Fournier, M., Brousseau, P., Spear, P. et al. (2009). Combined effects of agricultural activity and parasites on biomarkers in the bullfrog, Rana catasbeiana. Aquatic Toxicology, 91, 126–134. https://doi.org/10.1016/j.aquatox.2008.10.001
Martins-Sobrinho, P. M., Silva, W. G. O., Santos, E. G., Moura, G. J. B., & Oliveira, J. B. (2017). Helminths of some tree frogs of the families Hylidae and Phyllomedusidae in an Atlantic rainforest fragment, Brazil. Journal of Natural History, 51, 1639–1648. https://doi.org/10.1080/00222933.2017.1337945
McKenzie, V. J. (2007). Human land use and patterns of parasitism in tropical amphibian hosts. Biological Conservation 137, 102–116. https://doi.org/10.1016/j.biocon.2007.01.019
Mehlhorn, H. (2008) Encyclopedia of Parasitology, 3rd Edition. Heidelberg: Springer Berlin.
Oliveira, C. R., Ávila, R. W., & Morais, D. H. (2019). Helminths associated with three Physalaemus species (Anura: Leptodactylidae) from Caatinga Biome, Brazil. Acta Parasitologica, 64, 205–212. https://doi.org/10.2478/s11686-018-00022-8
Oliveira, R. H., Silva, M. C., & Ávila R. W. (2014). Predation of Leptodactylus macrosternum Miranda-Ribeiro, 1926 (Anura: Leptodactylidae) by Lygophis dilepis Cope, 1862 (Squamata: Dipsadidae). Herpetology Notes, 7, 357–358.
Pereira, J. R., & Siqueira, F. B. (1979). Alterações nas características químicas de um oxissolo sob irrigação. Pesquisa Agropecuária Brasileira, 14, 189–195.
Pergentino, H. E. S., & Ribeiro, L. B. (2017). Anurophagy by the snake Thamnodynastes phoenix (Squamata: Dipsadidae: Tachymenini) in dry forested areas of Northeastern Brazil. Herpetology Notes, 10, 597–600.
Petri, L. H. (1950). Life cycle of Physaloptera rara Hall and Wigdor, 1918 (Nematoda: Spiruroidea) with the cockroach, Blatella germanica, serving as the intermediate host. Transactions of the Kansas Academy of Science, 53, 331–337. https://doi.org/10.2307/3626145
Quadros, R. M., Marques, S. M. T., Moura, A. B., & Antonelli, M. (2014). Primeiro registro do nematoide Physaloptera praeputialis parasitando puma. Neotropical Biology and Conservation, 9, 186–189. https://doi.org/10.4013/nbc.2014.93.07
Ribeiro, L. B., & Freire, E. M. X. (2009). Tropidurus hispidus (Ncn). Frog predation. Herpetological Review, 40, 228.
Roberts, L. S., & Janovy, J. (2009). Gerald D. Schmidt & Larry S. Roberts’ foundations of parasitology, 8th Ed. New York: McGraw-Hill.
Schell, S. C. (1952). Studies on the life cycle of Physaloptera hispida Schell (Nematoda: Spiruroidea) a parasite of the cotton rat (Sigmodon hispidus littoralis Chapman). The Journal of Parasitology, 38, 462–472. https://doi.org/10.2307/3273926
Schludermann, C., Konecny, R., Laimgruber, S., Lewis, J. W., Schiemer, F., Chovanec, A. et al. (2003). Fish macroparasites as indicators of heavy metal pollution in river sites in Austria. Parasitology, 126, 61–69. https://doi.org/10.1017/S0031182003003743
Silva, J. M. C., Barbosa, L. C. F., Leal, I. R., & Tabarelli, M. (2017). The Caatinga: understanding the challenges. In J. M. C. Silva, I. R. Leal, & M. Tabarelli (Eds.), Caatinga the largest tropical dry forest region in South America (pp. 3–19). Cham: Springer.
Sures, B., Nachev, M., Selbach, C., & Marcogliese, D. J. (2017). Parasite responses to pollution: what we know and where we go in ‘Environmental Parasitology.’ Parasites and Vectors, 10, 1–19. https://doi.org/10.1186/s13071-017-2001-3
Teles, D. A., Brito, S. V., Araujo-Filho, J. A., Ribeiro, S. C., Teixeira, A. A. M., Mesquita, D. O. et al. (2018). Nematodes of the Rhinella granulosa Spix, 1824 (Anura: Bufonidae) from the Semiarid Northeastern Caatinga Region of Brazil. Comparative Parasitology, 85, 208–211. https://doi.org/10.1654/1525-2647-85.2.208
Teles, D. A., Rodrigues, J. K., Teixeira, A. A. M., De Araujo-Filho, J. A., Sousa, J. G. G., & Ribeiro, S. C. (2018). Diet of Leptodactylus macrosternum (Miranda-Ribeiro, 1926) (Anura: Leptodactylidae) in the Caatinga domain, Northeastern Brazil, Neotropical region. Herpetology Notes, 11, 223–226.
Toledo, G. M., Morais, D. H., Silva, R. J., & Anjos, L. A. (2015). Helminth communities of Leptodactylus latrans (Anura: Leptodactylidae) from the Atlantic rainforest, south-eastern Brazil. Journal of Helminthology, 89, 250–254. https://doi.org/10.1017/S0022149X1300076X
Toledo, G. M., Schwartz, H. O., Nomura, H. A. Q., Aguiar, A., Velota, R. A. M. V., Da Silva, R. J. et al. (2018). Helminth community structure of 13 species of anurans from Atlantic rainforest remnants, Brazil. Journal of Helminthology, 92, 438–444. https://doi.org/10.1017/S0022149X17000608
Toledo, L. F. (2005). Predation of juvenile and adult anurans by invertebrates: current knowledge and perspectives. Herpetological Review, 36, 395–400.
Velarde-Aguilar, M. G., Romero-Mayén, A. R., & León-Règagnon, V. (2014). First report of the genus Physaloptera (Nemato-
da: Physalopteridae) in Lithobates montezumae (Anura: Ranidae) from Mexico. Revista Mexicana de Biodiversidad, 85, 304–307. https://doi.org/10.7550/rmb.36480
Vrcibradic, D., Fusinatto, L. A., Nascimento, B. B., & Campião, K. M. (2018). Boana secedens (Barro Branco Treefrog). Endoparasites. Herpetological Review, 49, 300.
Widmer, E. A. (1970). Development of third-stage Physaloptera larvae from Crotalus viridis Rafinesque 1818 in cats with notes on pathology of the larvae in the reptile. (Nematoda, Spiruroidea). Journal of Wildlife Diseases, 6, 89–93. https://doi.org/10.7589/0090-3558-6.2.89