Shelter, ecophysiology and conservation status of Plectostylus araucanus (Pulmonata: Bothriembryontidae) in the fragmented Maulino Forest, central Chile
Rodrigo M. Barahona-Segovia a, b, *, Andrea L. Riveros-Díaz c, Sebastián Zaror d, Ricardo Catalán e, Juan Francisco Araya f, g
a Laboratorio de Ecología de Ambientes Fragmentados, Facultad de Ciencias Veterinarias y Pecuarias, Universidad de Chile, Av. Sta. Rosa #11735, La Pintana, Santiago de Chile, Chile
b Centro de Estudios en Ecología Espacial y Medio Ambiente – Ecogeografía, Av. José Miguel Claro #2550, Providencia, Santiago de Chile, Chile
c Zoológico Nacional de Chile, Pío Nono #450, Recoleta, Santiago, Chile
d Facultad de Medicina y Ciencias, Universidad San Sebastián, Av. Los Leones #745, Providencia, Santiago de Chile, Chile
e Secretaría Regional Ministerial de Atacama, Ministerio del Medio Ambiente, Portales #830, Copiapó, Chile
f Centro de Investigaciones Costeras de la Universidad de Atacama, Universidad de Atacama, Copayapu 485, Copiapó, Chile
g Programa de Doctorado en Sistemática y Biodiversidad, Universidad de Concepción, Barrio Universitario s/n, Concepción, Chile
*Corresponding author: rbarahona13@gmail.com (R.M. Barahona-Segovia)
Abstract
Terrestrial mollusks are one of the least studied groups of terrestrial invertebrates, especially in the Neotropics. In Chile, there is scarce biological and ecological information about many genera, even though the group is quite diverse and occupies different habitats along the country. Plectostylus araucanus is the most recently described species and one of the few arboreal species found only in the coastal native forest of central-south of Chile. In this study, we recorded a new locality for P. araucanus in the Maule region and described ecological and physiological characteristics. The new locality is placed 204 km northwards of the type locality. Based on different records, Plectostylus araucanus is proposed as an endangered (EN) species under the distribution criterion of IUCN. Most of the specimens of P. araucanus were found living in tree cavities and away from the edge of native forest fragments. Physiological measures showed monthly differences, especially between some months of summer and fall and between months of the same season (i.e., summer). We discuss the implications of our results in the microhabitat selection, thermoregulation and habitat use by this tree snail, and the importance of this data in management and conservation for other native malacofauna.
Keywords:
Habitat fragmentation; Microhabitat; Tree cavities; Tree snail; Thermoregulation
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Refugio, ecofisiología y estado de conservación de Plectostylus araucanus (Pulmonata: Bothriembryontidae) en el bosque fragmentado Maulino, Chile central
Resumen
Los moluscos terrestres son uno de los grupos de invertebrados menos estudiados, especialmente en el neotrópico. En Chile, hay poca información biológica y ecológica sobre muchos géneros, a pesar de que el grupo es bastante diverso y ocupa diferentes hábitats a lo largo del país. Plectostylus araucanus fue la última especie descrita, con características arborícolas y restringida al bosque costero del centro-sur de Chile. En este trabajo, se describen características ecológicas y fisiológicas de P. araucanus y registramos una nueva localidad en la región del Maule, la cual se encuentra a 204 km al norte de la localidad tipo. Basados en diferentes registros, P. araucanus se propone como una especie en peligro (EN) según el criterio de distribución de la UICN. La mayoría de los especímenes de P. araucanus se encontraron viviendo en las cavidades de los árboles en fragmentos de bosque nativo y a una distancia moderada del borde de estos. Las medidas fisiológicas mostraron diferencias entre meses, especialmente entre meses de verano y otoño, y meses dentro de la misma estación (por ejemplo, verano). Se discuten las implicaciones de nuestros resultados en la selección de microhábitats, la termorregulación y el uso del hábitat de esta especie, y su uso en el manejo y conservación de otras especies de malacofauna nativa.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Fragmentación del hábitat; Microhábitat; Cavidades de los árboles; Caracol arborícola; Termorregulación
Introduction
Terrestrial mollusks are one of the least studied groups of terrestrial invertebrates (Lydeard et al., 2004). They represent a key component in the decomposition and nutrient cycle in soil (Jones et al., 1994; Meyer III et al., 2011, 2013; Valdovinos & Stuardo, 1988), provide food for other invertebrates and vertebrates (Martin, 2000; Nyffeler & Symondson, 2001), and determine plant composition (Hulme, 1996; Peters, 2007). Land snails are recognized as human perturbation indicators, due to their low dispersal capabilities and their dependence on microhabitats for survival and mating (Baur & Baur, 1988; Ström et al., 2009). Furthermore, terrestrial mollusks can be a good indicator for climate change, because of their ectothermic condition (Nicolai & Ansart, 2017).
Neotropical overview of land snails is still poor in many countries (Régnier et al., 2015), highlighting the malacological studies performed in Argentina (Cuezzo et al., 2013), Brazil (Simone, 2006), Colombia (Linares & Vera, 2012), Cuba (Espinosa & Ortea, 2009), Ecuador (Breure & Borrero, 2008) and Peru (Breure & Avila, 2016). In Chile, the study of land snails has been focused especially in the description of new species (Araya & Aliaga, 2015; Araya & Breure, 2016; Miquel & Araya, 2013, 2015; Valdovinos & Stuardo, 1988, 1989); as well as studying their distributional range and reporting new records of exotic species (Araya, 2015a, b, 2016; Araya & Catalán, 2014; Araya et al., 2016, 2017; Cádiz & Gallardo, 2007; Martínez-de los Ríos, 2017; Stuardo & Vega, 1985; Valdovinos, 1999; Valdovinos & Stuardo, 1989). Also, the study of their natural history and ecological characteristics has been poor (Cádiz & Gallardo, 2008; Jackson & Jackson, 2011; Valdovinos & Stuardo, 1988). However, in Chile the biological and ecological information about many genera is scarce, although these species are diverse and occupy many habitats along the climatic gradient of the country.
Plectostylus Beck 1837 (Pulmonata: Bothriem-bryontidae) is a recognized genus of endemic South American land snails, represented in Chile by 13 species distributed from Iquique, Tarapacá region, to del Diablo Island, Aysén region (Stuardo & Vega, 1985; Valdovinos & Stuardo, 1988). This genus is largely extant in different ecosystems, inhabiting areas from coastal deserts and coastal scrublands in northern Chile (Araya, 2016; Araya & Catalán, 2014; Jackson & Jackson, 2011), to coastal and Valdivian forest in central and southern parts of the country (Smith-Ramírez et al., 2007; Valdovinos & Stuardo, 1988). Relevant information on taxonomy and the systematic position of Plectostylus has been discussed by different authors (Breure & Romero, 2012; Valdovinos & Stuardo, 1988; Van Bruggen et al., 2016). On the other hand, several authors (Araya, 2016; Araya & Catalán, 2014; Jackson & Jackson, 2011; Valdovinos & Stuardo, 1988) highlight the deficiency in information about ecology, which is still poorly understood and represent critical gaps, especially concerning changes in land use and global warming. These threats represent some of the most important drivers in biodiversity loss of terrestrial and freshwater mollusks worldwide (Beltramino et al., 2015; Cordellier et al., 2012; Lydeard et al., 2004; Pearce & Paustian, 2013; Régnier et al., 2009, 2015).
Plectostylus araucanus Valdovinos & Stuardo, 1988 is the most recently described species of the genus, found in the forest of the Araucanía region, central-southern Chile (Valdovinos & Stuardo, 1988). This species is characterized by its large globose-ovate shell of up to a few cm in height, the presence of strong and noticeable growth lines (Fig. 1A, B), a brown periostracum, and a white columellar lip with a notorious angle in its middle part (Valdovinos & Stuardo, 1988). Currently, the known distribution for this species is from the coastal forest of the Nahuelbuta Mountains and its surrounding areas. Both P. araucanus, as well as Plectostylus vagabondiae Brooks, 1936, are true tree snails that probably feed on bryophytes living on the cortex of trees (Valdovinos & Stuardo, 1988). Due to the scarce ecological information on P. araucanus, the aim of this study is to characterize the habitat type, shelter preferences and some physiological aspects of this arboreal snail, in a locality where it was newly discovered, which belongs to the fragmented and endangered Maulino Forest (Alaniz et al., 2016; Miranda et al., 2017), with the goal to further protect this species under IUCN criteria and by national biodiversity protection laws.

Materials and methods
This study was conducted in the National Reserve Los Queules, Tregulemu (35º59’14.38” S, 72º41’21.96” W) in surrounding fragments of deciduous native forest (< 100 ha) and Monterey pine tree (Pinus radiata) plantations. Native fragments were variable in size, ranging from 29 to 242 ha. Native forest is mainly composed of endemic trees such as Gomortega keule (Molina, 1782) Baill., Aextoxicon punctatum Ruiz et Pav., Cryptocarya alba (Molina) Looser, Peumus boldus Molina, and native shrubs located in the understory (Bustamante et al., 2005; Rodríguez et al., 2018). The study site has a Mediterranean climate, with 6 months of rain (April-September) and 6 months of heat (October-March; Di Castri & Hajek, 1976). The summer is very dry and hot reaching 40 ºC in the middle of the afternoon (15:00-16:00 pm), especially in sites without canopy such as pine plantations produced by the edge effect and fragmentation (Tuff et al., 2016). However, there is a high probability of rain in summer months because the new location in which P. araucanus was found is in the coastal mountain range and has an oceanic influence that generates morning mist in the highest hills. On the other hand, falls offer more precipitations and moderate average temperatures (i.e., 12-14 ºC; Di Castri & Hajek, 1976), both in native forest as well as in pine plantations (Chen et al., 1999).
In order to build the distributional range and to, posteriorly, evaluate the conservation status of P. araucanus, localities were extracted from literature (Valdovinos & Stuardo, 1988) and personal records. The conservation status under IUCN criteria, was evaluated using the minimum convex polygon method, evaluating the B criteria by calculating the extension of occurrence (EOO) and area of occupancy (AOO) using R’s red-package (Cardoso, 2017) in R software ver.3.4.1 (R Core Team 2017).
Seven different fragments of native forest surrounded by the Monterey pine plantation were selected. These were selected because the native forest fragments were structurally very complex in contrast with the young pine plantations. These conditions were not presented by other fragments, because they were surrounded by clearcut stands or mature pine plantations. The young pines did not exceed one year of age and 152 cm of height, in average. Each fragment was sampled with perpendicular transects from the physical edge and visited every month during summer (December 2017-February 2018) and autumn (March-May 2018; N total = 42). Only one transect of 240 m was placed per fragment (120 m inside the native forest and 120 m in the pine plantations), to avoid pseudoreplication. The edge, defined as the physical limit between 2 different ecosystems (Laurance et al., 2002), was considered as the departing point in the transect. From this point, monitoring stations were placed at 2, 10, 20, 40, 80 and 120 m at both ends of the forest edge following Chen et al. (1999). In each monitoring station, we prospected a 40 m wide strip, sampling by transect a total of 4,800 m2. In addition, at each station where tree snails were found, the type of habitat that it occupied was characterized (i.e., cavities, base of the tree, morphological anomalies of the tree, etc.), the height of the forest floor to which the snail was found was measured using a measuring tape, canopy coverage (%) was calculated using discrete values (i.e., 0, 25, 50, 75, 100), and all other species present at each particular tree was noted. All fragments studied are independent from each other, or at least separated by 500 m, for an independent response. In each monitoring station, the ambient temperature (At) was measured each month using one data logger by station (KEYLOG model KTL-508 with 0.1 ºC of sensibility; accuracy from 0 °C to + 70 °C (± 0.25 °C); n = 13).
Only 10 tree snails were measured, since 2 were found damaged by the snail killing beetle (Lampyridae). To obtain thermal data of the tree snails in situ, we measured body temperature (Bt) and body moisture (Bm) each month. We took these non-invasive temperature measurements by taking the thermometer lasers to the surface of the tree snails following protocols like those used for other invertebrates (Barahona-Segovia et al., 2016; Taucare-Ríos et al., 2017). These records were always made on the shell of the animals because the soft parts of the tree snails were not always exposed. We also measured the tree cavity temperature (Ct) to compare with their habitat temperature. Differences in temperature and moisture variables were analyzed by repeated measured Anova using months as fixed factor and Bt, Bm and Ct as response variables. To meet the statistical assumptions, we used Shapiro-Wilk test and Cochran C test to probe normality and homoscedasticity, respectively. When differences were significant (P<0.05), we used a posteriori Tukey test for multiple comparisons. In addition, we used the mean value of Bt, Bm, Ct and At by month and performed Spearman rank R correlation between these variables in order to find any relation between them. All measures were taken using an infrared thermometer (EXTECH Instruments, model 42509, range: -20 to 510 °C). Statistical analyses were performed with the software STATISTICA (Statsoft, 2004).
Diagnostic characters were compared in situ following Valdovinos & Stuardo (1988) for the identification of Plectostylus araucanus (Fig. 1A, B). This species is characterized by its ovate-globose and relatively thin and slightly translucent shell, with 5 to 6 convex whorls and a last large whorl, with an oblong shape. The spire is high, conical; of about half the height of the shell. The suture line is slightly sinuous, more marked in the last whorl. The protoconch is sculptured with a weak axial striation of narrow riblets, restricted near the sutural area; while the surface of the shell is densely covered with marked and tight growth lines; with a pattern formed by a few spiral brown stripes, more noticeable on the last whorl. The aperture of P. araucanus is ovate-oblong, somewhat iridescent in the inside, of about half the height of shell; the external lip has a semi-ovate curvature, while the columellar lip has a noticeable angle in its middle part. The columella is twisted, thickened, reflected, and in some cases forming a small umbilical slit, while the columellar callus is very thin, and of white color. The periostracum of this species is of a dark brown color. Internal organs of P. araucanus were not reviewed since none of the specimens were sacrificed.
Results
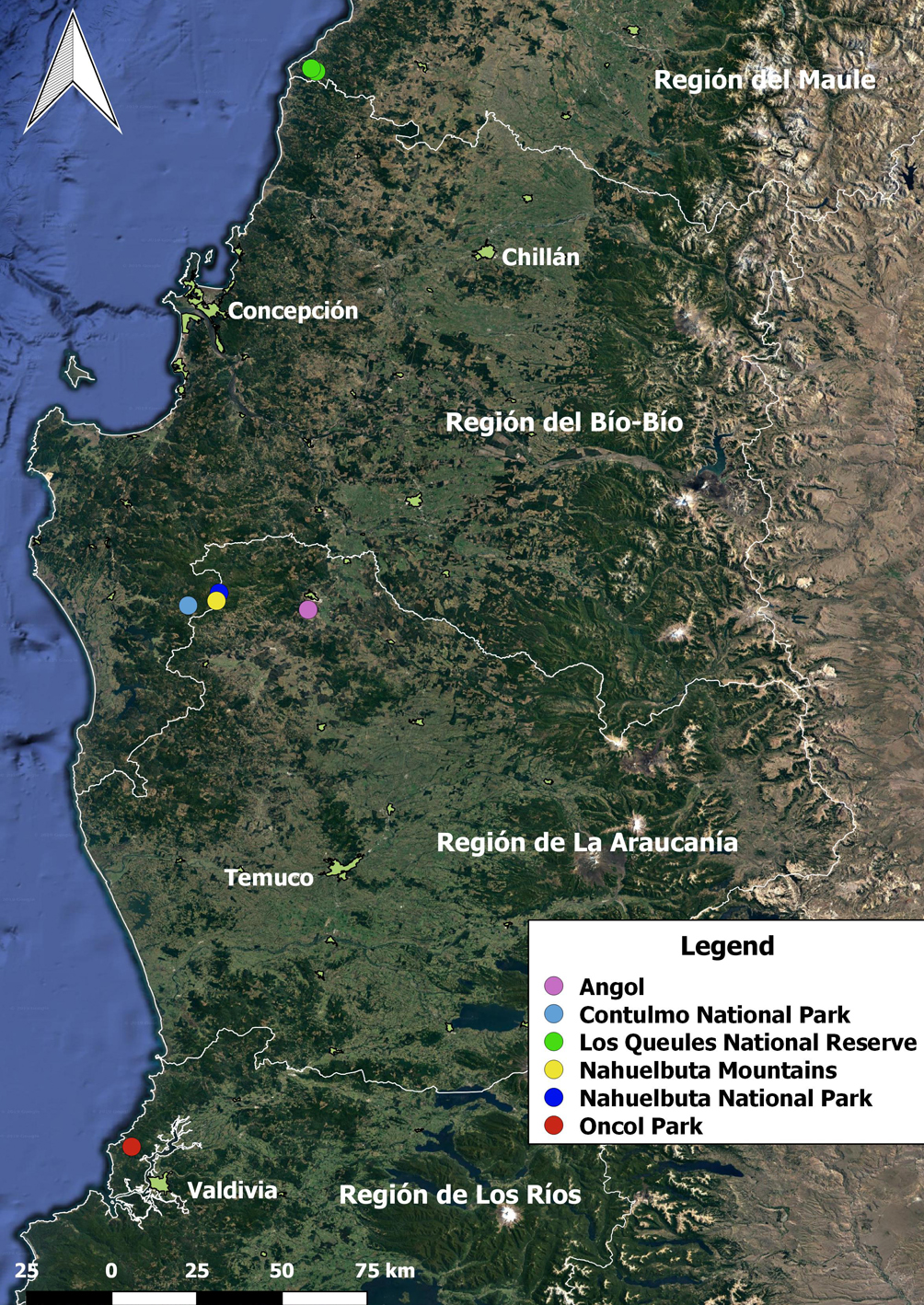
Previously, the distribution of P. araucanus was based only on 12 specimens collected from 5 localities in the mountain range Nahuelbuta Mountains and in surrounding areas of the Araucanía region. The specific localities in literature are Angol, Contulmo National Park, Nahuelbuta National Park, the west side of Nahuelbuta Mountains (Valdovinos & Stuardo, 1988), and Oncol Park, Valdivia (Valdovinos et al., 2005; Table 1, Fig. 2). The new localities recorded belong to the Los Queules National Reserve in the Maulino Forest located in the Maule region and are located 204 km northwards of the type locality (Table 1; Fig. 2).
Plectostylus araucanus is currently present in 3 localities: Nahuelbuta Mountains, Valdivian forest and the Maulino Forest ecosystem. Quantitative populations as well as habitat quality were not evaluated, and therefore, criteria A, C, D (with exception of VU D2) and E are difficult to evaluate. Nevertheless, the EOO calculated is 2880 km2 and AOO is 28 km2, with values that are within the thresholds for the endangered category using criteria B1 and criteria B2. Threats observed in the field, as wildfires or native tree exploitation could have the biggest impact. On the other hand, the increase of temperature in the edge (i.e., edge effect, Laurance et al., 2002; Tuff et al., 2016), could threaten the subpopulations in each locality, which are already highly fragmented by forestry, livestock, crops and urbanization. Therefore, we evaluated P. araucanus as “Endangered” using criterion B (EN B1ab [i, ii, iii, iv] + 2ab [i, ii, iii, iv]).
Plectostylus araucanus was only found in 2 native fragments (26.4 and 242 ha of size, respectively) in the Maulino Forest. These fragments were characterized by the presence of tree species such as A. punctatum, C. alba, P. boldus, G. keule, P. punctata, N. obliqua, and L. apiculata. Monterey pine plantation was not occupied by this tree snail species. From the 55 tree snails found between December 2017 and May 2018, 21.8% (n = 12) corresponded to P. araucanus. In the 49 studied transects, P. araucanus primarily occupied tree cavities, cracks or shadows of branches of A. punctatum (66.7%; Fig. 1C, D), C. alba (16.7%), L. apiculata (8.3%) and forest floor (8.3%) (Table 1). Canopy coverage ranged between
75-100% (Table 2). The mean height of the cavities in which the tree snails were found was of 174.27 ± 14.75 cm. The tree snails were distributed mainly between 20 and 40 m from the edge to the inside of the forest.
Table 1
Records of Plectostylus araucanus in literature and from the present study.
|
Localities |
°S |
°W |
Record |
Regional distribution |
|
Angol |
-37.846203 |
-72.716909 |
Valdovinos & Stuardo, 1988 |
Araucanía region |
|
Contulmo National Park |
-37.831643 |
-73.130988 |
Valdovinos & Stuardo, 1988 |
Araucanía region |
|
Nahuelbuta National Park |
-37.788353 |
-73.024256 |
Valdovinos & Stuardo, 1988 |
Araucanía region |
|
Nahuelbuta Mountains |
-37.815483 |
-73.033260 |
Valdovinos & Stuardo, 1988 |
Bío Bío region |
|
Oncol Park |
-39.700092 |
-73.326112 |
Valdovinos et al., 2005 |
Los Ríos region |
|
Los Queules National Reserve |
-35.987559 |
-72.689234 |
This study |
Maule region |
|
Los Queules National Reserve |
-35.977355 |
-72.706049 |
This study |
Maule region |
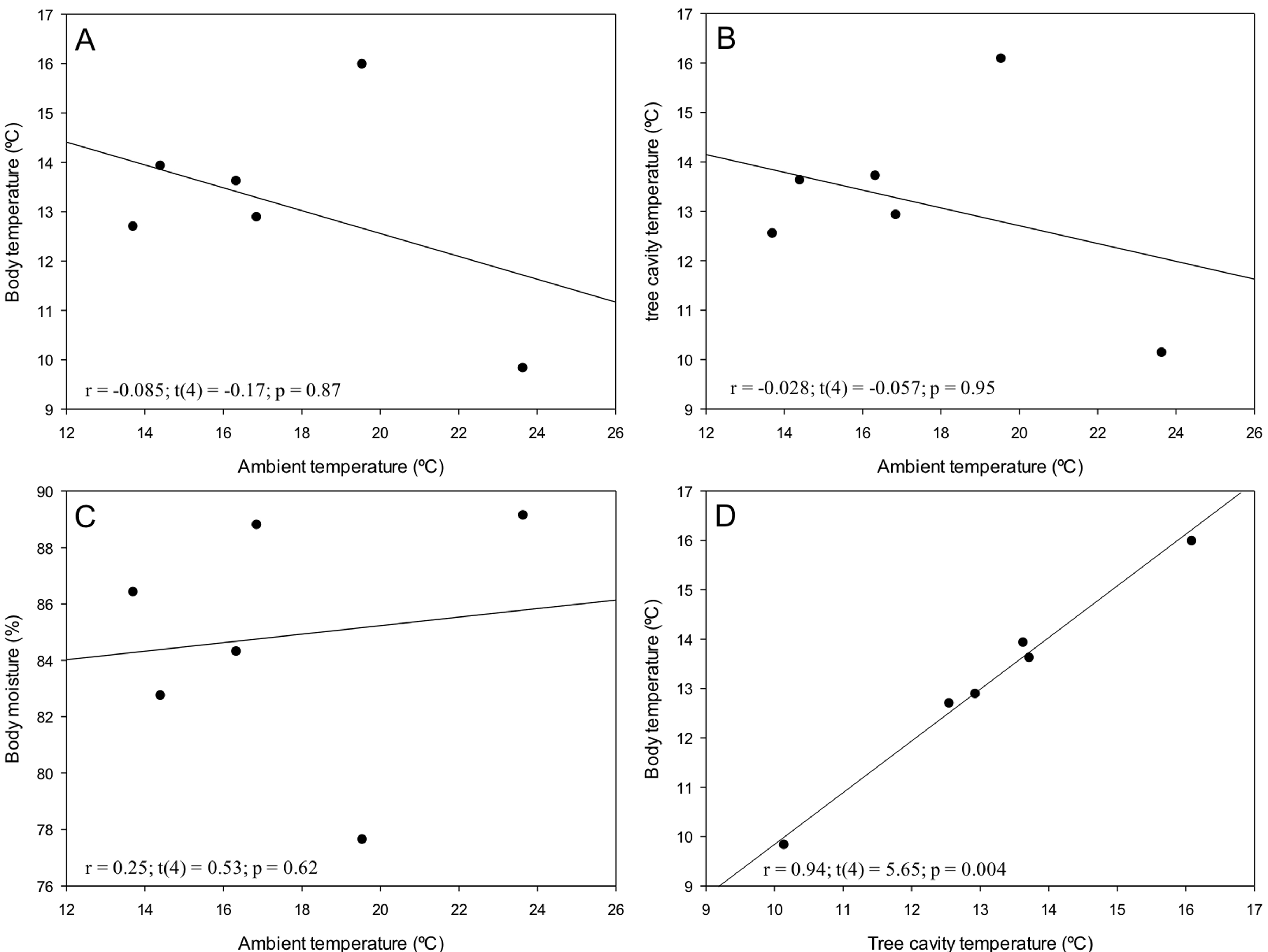
Tree snails (n = 10) had a minimum mean temperature of 9.83 ± 0.11 ºC and a maximum mean temperature of 15.99 ± 0.08 ºC, with a range of 9.1-16.5 ºC. We found statistically significant differences between months in Bt, Bm and Ct (F(4,45) = 179.31; p < 0.0001; F(4,45) = 51.10; p < 0.0001 and F(4,45) = 138.83; p < 0.0001, respectively; Fig. 3A). Bt and Ct were higher in some months (i.e. January: 15.99 ± 0.08 °C and 16.09 ± 0.10 °C respectively; Tukey p <0.05) than others (i.e., December: 9.83 ± 0.11 ºC and 10.14± 0.12 ºC respectively). However, Bm showed an opposite pattern to the other variables (Fig. 3B). Spearman correlation between At with Bt and Ct were negative and not significant (r = -0.085; p = 0.87 and r = -0.028; p = 0.95; Fig. 4A, B). On the other hand, correlation between At and Bm was positive but not significant (r = 0.25; p = 0.53; Fig. 4C). Finally, we found a positive, powerful and significant relation of Bt with Ct (r = 0.94; p = 0.0048; Fig. 4D).
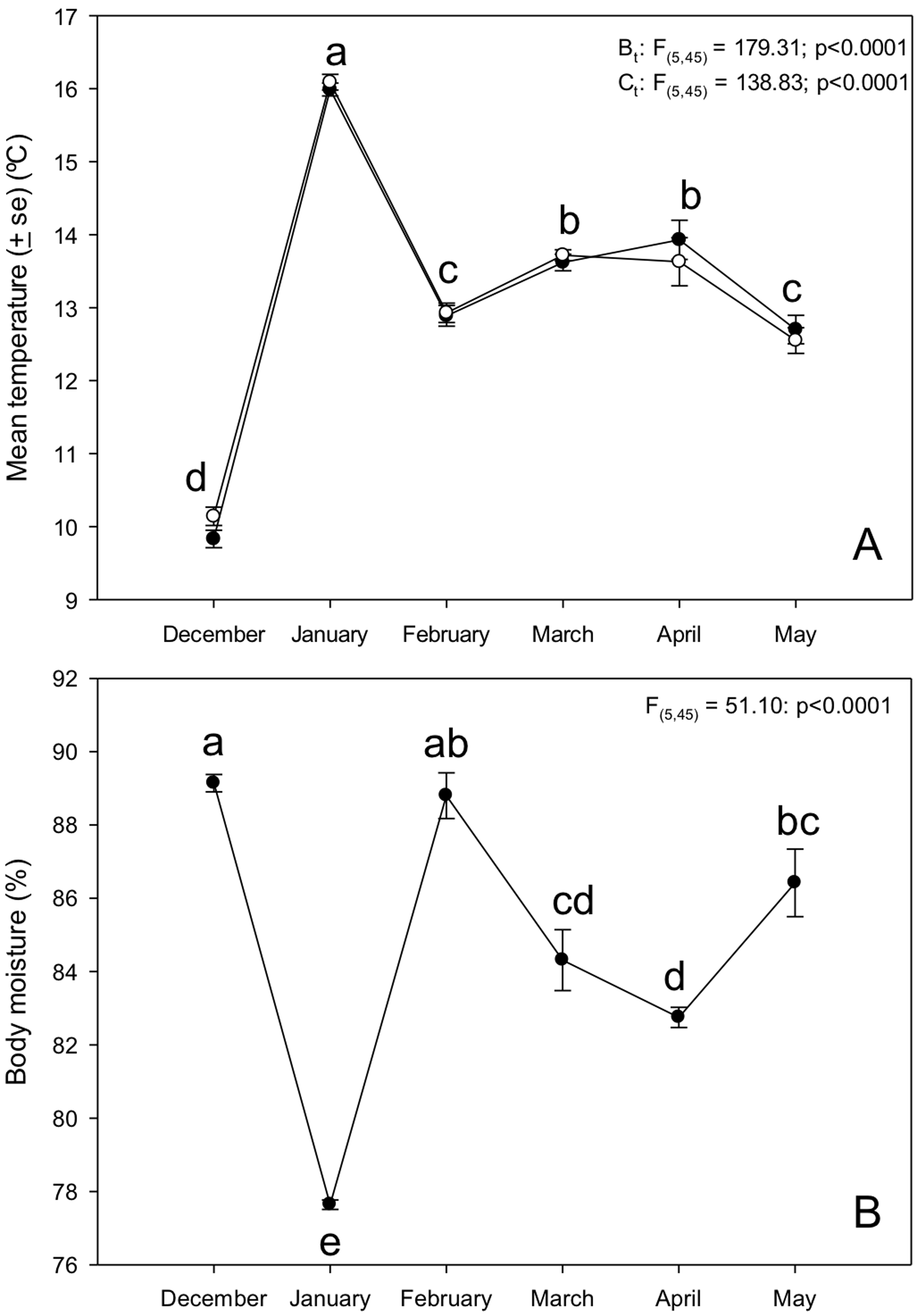
Table 2
Habitat characterization of Plectostylus araucanus Valdovinos & Stuardo 1988 in Maulino Forest fragments. Negative distances in DE represent monitoring stations in the interior of native forest. DE = Distance from tree snails to the edge; CC = canopy coverage; NA = not applicable.
|
ID |
DE (m) |
Habitat |
Tree species |
CC (%) |
Height (cm) |
|
1 |
-40 |
cavity |
Aextoxicon punctatum |
100 |
211 |
|
2 |
-40 |
cavity |
Aextoxicon punctatum |
100 |
211 |
|
3 |
-40 |
cavity |
Aextoxicon punctatum |
100 |
211 |
|
4 |
-40 |
cavity |
Aextoxicon punctatum |
100 |
211 |
|
5 |
-40 |
cavity |
Aextoxicon punctatum |
100 |
211 |
|
6 |
-40 |
cavity |
Cryptocarya alba |
75 |
186 |
|
7 |
-40 |
cavity |
Cryptocarya alba |
75 |
182 |
|
8 |
-20 |
cavity |
Aextoxicon punctatum |
75 |
155 |
|
9 |
-20 |
cavity |
Aextoxicon punctatum |
75 |
155 |
|
10 |
-20 |
cavity |
Aextoxicon punctatum |
75 |
129 |
|
11 |
-40 |
branches |
Luma apiculata |
100 |
35 |
|
12 |
-40 |
forest floor |
NA |
75 |
NA |
Discussion
The ecological and physiological knowledge, as well as the distribution of the Chilean malacofauna is still poorly understood, even when considering the large number of species cited for the country (Stuardo & Vega, 1985). Moreover, considering that many natural areas to date are severely fragmented, obtaining information on numerous aspects of these species is very useful for future work in biological conservation. Here, we report a new locality as the northernmost record for P. araucanus and provide notes about its habitat use and shelter. In addition, we evaluated physiological data of tree snails with the aim to provide a baseline of information for its conservation.
Although some information has been provided for snails of the genus Plectostylus from northern Chile, this work is the first that brings new ecological and physiological data from tree snails of the southern forest. Valdovinos & Stuardo (1988) and Valdovinos et al. (2005) mentioned that P. araucanus is an arboreal species, something that our work confirms. However, both previous studies provide few quantitative data on habitat use by this species. The few specimens documented use tree cavities, with a high mean height relative to the forest floor, and to some minimum distances away from the edges of each forest fragment (40 m inside the native forest; Barahona-Segovia et al., 2019). These cavities were almost never abandoned by snails, probably due to the fact that snails are capable of detecting and evaluating relatively small potential resource patches from a distance, enabling them to limit costly explorations (Dahirel et al., 2015). These cavities contained fecal material with different degrees of humidity, which suggests that the snails could maintain temporary homes and only go out at night.
Tree snail species use cavities as a place in which they can maintain body temperature and moisture. In our work, the tree snails sampled never left the cavities, but showed changes in Bt and Bm in different months in their microhabitats. For example, in January, tree snails increased Bt, while Bm decreased. This has been documented, for example, in other land snails, such as Theba pisana (Müller), which avoid changes in ambient temperature due to sun and wind exposure, using behavioral thermoregulation or by selecting certain microhabitats (Cowie, 1985). Another fact that reinforces the dependence of the use of microhabitats, it is the close relationship of Bt with the temperature of the cavity. Tree snails in some months (i.e., January) with thermal stress could activate metabolic down-regulation and therefore, maintain Bt by selecting microhabitats and compensating the decrease in Bm (Nicolai & Ansart, 2017). In addition, microhabitat selection is the most used behavioral alternative for decreasing body temperature (McBride et al., 1989; Chapperon & Seuront, 2011). On the other hand, P. araucanus lives at a low height level compared to the overall height of the trees in the forest (above 30 m), probably to avoid desiccation and thermal stress from the highest part of the trees, as has been documented in other land snails (Cowie, 1985). Finally, edge effect can produce a thermal gradient, with the environmental temperature increasing towards the edge of the fragment (Barahona-Segovia et al., 2019; Laurance et al., 2002; Tuff et al., 2016), which could have negative effects on the survival of land snails near the edge, especially in a global warming framework. In fact, we measured the survival of tree snails, being very low at the edge and increasing at 40 m to the interior of the native forest (Barahona-Segovia et al., 2019). Although these results suggest a potential relationship between temperature and survival, the approach of this work does not allow us to affirm that the low survival is due to physiological limitations of P. araucanus.
The Maulino Forest could represent the northern limit for many species of plants and animals, especially, for endangered animal or plant species. In recent decades, many species whose distributions were limited to the north by the Nahuelbuta Mountains range have been recorded in the reserves or native fragments associated with this type of coastal forest. Many of these species are currently classified as endangered or vulnerable by IUCN or local ministry of environment. Thus, new records in this particular ecosystem range from bryophytes (Müller & Pereira, 2006); endangered trees (Alarcón et al., 2007); endangered frogs (Cuevas & Cifuentes, 2010; Puente-Torres et al., 2017), to bats (Rodríguez-San Pedro et al., 2015). These records show that the distribution of many species was at one point continuous to the Maule region, and due to habitat loss and fragmentation produced by urbanization, crops and forest plantations (Miranda et al., 2017), these populations have been separated, with few connections between them. Our new record of P. araucanus in this area reinforces this fact, in particular, since the native forest sampled is surrounded by an anthropic matrix. It is composed principally by pine plantations, which is exposed to high solar radiation and wind fluctuations and therefore, extreme environmental conditions for tree snails (Chen et al., 1999; Laurance et al., 2002; Tuff et al., 2016), where they cannot survive. In addition, is structurally poorer than native forest and provides less or null thermal shelters to sensitive native species. In land snails, habitat fragmentation produced by any anthropogenic factor can generate metapopulations that could be genetically structured and have changes in shell morphology (Schweiger et al., 2004), homogenization of the land snail community composition (Hodges & McKinney, 2018), changes in dispersion abilities (Baur & Baur, 1990) and in diversity (Wall et al., 2018).
In Chile, the only protected terrestrial mollusks are the ones present in the Juan Fernández islands (MMA, 2018). Our new record, allows us to expand the distributional range of this species of tree snail and thus, evaluate its extinction risk using the B criteria of the IUCN (2014). Following IUCN (2014) “the term locality is defined as a distinctive geographical or ecological area in which a single threatening event can rapidly affect all individuals of the present taxon. The size of a locality depends on the area covered by the threat and may include part of one or many subpopulations of the taxon”. Therefore, our justification is based on the fact that this tree snail species only has 3 known localities that are susceptible to any anthropic event that can destroy them. For example, Chile has experienced an episode of forest fires that can be described as an extreme “fire storm” with very rapid propagations of up to 8,200 ha/hour and with exceptional heat intensities of more than 60,000 kW/m and called by expert in wildfires as sixth generation (UE, 2017). This event consumed more than 500,000 ha of plantations and forests, affecting 53 conservation areas, including parks and national reserves (CONAF, 2017). The Maulino Forest was the second most damaged native ecosystem, losing close to 1,000 ha (CONAF, 2017). Thus, our work includes the suggestion of Valdovinos et al. (2005) and allows us to place P. araucanus as an “endangered” species using our records and its previous geographical distribution in its form of EOO or AOO, confirming that this species has a low abundance as suggested by Valdovinos et al. (2005). Threats such as forest fires, habitat loss, habitat fragmentation and probably, climate change, could be the main threat for P. araucanus and, in general, for the malacofauna in Chile. Therefore, the category assigned for this species meets the thresholds necessary to evaluate it.
In conclusion, our work allowed us to obtain relevant ecological and physiological data of P. araucanus that can be used in a future for conservation work of this species, or other native snails of the same genus. Our data suggests that P. araucanus can use microhabitats for thermoregulation and maintain active its biological functions, providing a complementary overview to its natural history mentioned by Valdovinos & Stuardo (1988) and Valdovinos et al. (2005). In addition, following the proposal by Valdovinos et al. (2005), and added to the fragmentation of the habitat and its known low EOO and AOO, this species is considered as endangered. Therefore, our results can be used to develop a better protection of these species of tree snails and apply a better habitat management based on this new ecological and physiological information.
Acknowledgements
We would like to thank Carlos Reyes (CONAF), Corporación Nacional Forestal (CONAF), and MASISA for allowing us to work in the Los Queules National Reserve surrounded pine plantations, and Cecilia Smith-Ramírez for her logistic support. We thank and appreciate the photographic work of Patricia Henríquez-Piskulich on the shell of P. araucanus. Also, we thank Gretty Meza, Varbara Ramos, and Diego Ramírez for their assistance in field work; to Alejandra Fabres and M. Sc. Xochitl Vital for their comments and help to improve our work, and the LEAF to help me during my PhD. Funding and technical support from the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) by grant 1140657 to Javier Simonetti, Audrey A. Grez, Pablo Vergara y Patricio Pliscoff; postdoctoral grant 3160037 to André Rubio and CONICYT doctoral scholarship 21160404 to R.M.B.-S. supported this study.
References
Alaniz, A. J., Galleguillos, M., & Pérez-Quezada, J. F. (2016). Assessment of quality of input data used to classify ecosystems according to the IUCN Red List methodology: the case of the central Chile hotspot. Biological Conservation, 204, 378–385.
Alarcón, D., Smith-Ramírez, C., Hechenleitner, P., Ramírez de Arellano, P., Oliva, M., & Pinto, M. (2007). Nuevas poblaciones de Berberidopsis corallina Hook. F. (Berberidopsidales: Berberidopsidaceae) en la región del Bío Bío, Chile: Ubicación y conservación de su hábitat. Gayana Botánica, 64, 217–231.
Araya, J. F. (2015a). Current status of the non-indigenous molluscs in Chile, with the first record of Otala punctata (Müller, 1774) (Gastropoda, Helicidae) in the country and new records for Cornu aspersum (Müller, 1774) and Deroceras laeve (Müller, 1774). Journal of Natural History, 49, 1731–1761.
Araya, J. F. (2015b). The Bulimulidae (Mollusca: Pulmonata) from the Región de Atacama, northern Chile. PeerJ3, e1383.
Araya, J. F. (2016). On some land snails (Mollusca: Gastropoda) of Los Molles, central Chile. Revista Mexicana de Biodiversidad, 87, 1365–1368.
Araya, J. F., & Aliaga, J. A. (2015). A new species of Lilloiconcha Weyrauch, 1965 (Pulmonata: Charopidae) from central Chile. Zootaxa, 4007, 295–297.
Araya, J. F., & Breure, A. S. H. (2016). A new terrestrial snail species (Gastropoda: Bulimulidae) from the Región de Antofagasta, northern Chile. PeerJ, 5, e3538.
Araya, J. F., & Catalán, R. (2014). A review of the non-bulimulid terrestrial Mollusca from the Region of Atacama, northern Chile. Zookeys, 398, 33–51.
Araya, J. F., Madrid, M., & Breure, A. S. H. (2016). Bostryx hennahi (Gray, 1828) the largest Chilean bulimulid (Mollusca: Pulmonata), rediscovered among Tillandsia communities in northern Chile. Journal of Conchology, 42, 1–5.
Araya, J. F., Miquel, S. E., & Ríos, E. (2017). New records of terrestrial mollusks (Gastropoda: Stylommatophora) from Antofagasta, northern Chile. Revista Mexicana de Biodiversidad, 88, 769–772.
Barahona-Segovia, R. M., Crespin, S. J., Grez, A. A., & Veloso, C. (2019). Anthropogenic thermal gradient in managed landscapes determines physiological performance and explains the edge-biased distribution of ectothermic arthropods. Forest Ecology and Management, 440, 147-157.
Barahona-Segovia, R. M., Grez, A. A., & Bozinovic, F. (2016). Testing the hypothesis of greater eurythermality in invasive than in native ladybird species: from physiological performance to life-history strategies. Ecological Entomology, 41, 182–191.
Baur, A., & Baur, B. (1988). Individual movement patterns of the minute land snail Punctum pygmaeum (Draparnaud) (Pulmonata: Endodontidae). Veliger, 30, 372–376.
Baur, A., & Baur, B. (1990). Are roads barriers to dispersal in the land snail Arianta arbustorum? Canadian Journal of Zoology, 68, 613–617.
Beltramino, A. A., Vogler, R. E., Gutiérrez-Gregoric, D. E., & Rumi, A. (2015). Impact of climate change on the distribution of a giant land snail from South America: predicting future trends for setting conservation priorities on native malacofauna. Climate Change, 131, 621–633.
Breure, A. S. H., & Ávila, V. M. (2016). Synopsis of Central Andean Orthalicoid land snails (Gastropoda, Stylommatophora), excluding Bulimulidae. Zookeys, 588, 1–199.
Breure, A. S. H., & Borrero, F. J. (2008). An annotated checklist of the land snail family Orthalicidae (Gastropoda: Pulmonata: Orthalicoidea) in Ecuador, with notes on the distribution of the mainland species. Zootaxa, 1768, 1–40.
Breure, A. S., & Romero, P. E. (2012). Support and surprises: molecular phylogeny of the land snail superfamily Orthalicoidea using a three-locus gene analysis with a divergence time analysis and ancestral area reconstruction (Gastropoda: Stylommatophora). Archiv für Molluskenkunde: International Journal of Malacology, 141, 1–20.
Bustamante, R. O., Simonetti, J. A., Grez, A. A., & San-Martin, J. (2005). Fragmentación y dinámica de regeneración del bosque maulino: diagnóstico actual y perspectivas futuras. In C. Smith-Ramírez, J. J. Armesto & C. Valdovinos (Eds.), Historia, biodiversidad y ecología de los bosques de la Cordillera de la Costa. Santiago, Chile: Editorial Universitaria.
Cádiz, F. J., & Gallardo, C. S. (2007). Arion intermedius (Gastropoda: Stylommatophora); first record of this introduced slug in Chile, with notes on its anatomy and natural history. Revista Chilena de Historia Natural, 80, 99–108.
Cádiz, F. J. & Gallardo, C. S. (2008). Morphological and anatomical features of Flammulina festiva Scott, 1970 (Stylommatophora; Charopidae) from Southern Chile, with notes on its natural history. Gayana, 72, 1–8.
Cardoso, P. (2017). red – an R package to facilitate species red list assessments according to the IUCN criteria. Biodiversity Data Journal, 5, e20530.
Chapperon, C., & Seuront, L. (2011). Behavioral thermoregulation in a tropical gastropod: links to climate change scenarios. Global Change Biology, 17, 1740–1749.
Chen, J., Saunders, S. C., Crow, T. R., Naiman, R. J., Brosofske, K. D., Mroz, G. D. et al. (1999). Microclimate in forest ecosystem and landscape ecology – variations in local climate can be used to monitor and compare the effects of different management regimes. Bioscience, 49, 288–297.
CONAF (Corporación Nacional Forestal). (2017). Análisis de la afectación y severidad de los incendios forestales ocurridos en enero y febrero de 2017 sobre los usos de suelo y los ecosistemas naturales presentes entre las regiones de Coquimbo y Los Ríos de Chile. Informe Técnico, Santiago, Chile. Accessed August 20, 2018 from: http://www.conaf.cl/tormenta_de_fuego-2017/INFORME-AFECTACION-Y_SEVERIDAD-DE-INCENDIOS-FORESTALES-VERANO-2017-SOBRE-ECOSISTEMAS-VEGETACIONALES-CONAF.pdf
Cordellier, M., Pfenninger, A., Streit, B., & Pfenninger, M. (2012). Assessing the effects of climate change on the distribution of pulmonate freshwater snail biodiversity. Marine Biology, 159, 2519–2531.
Cowie, R. H. (1985). Microhabitat choice and high temperature tolerance in the land snail Theba pisana (Mollusca: Gastropoda). Journal of Zoology, 207, 201–211.
Cuevas, C. C., & Cifuentes, P. (2010). Amphibia, Anura, Ceratophryidae, Batrachyla leptopus Bell, 1843: new record. Checklist, 6, 633–636.
Cuezzo, M. G., Miranda, M. J., & Ovando, X. M. C. (2013). Species catalogue of Orthalicoidea in Argentina (Gastropoda: Stylommatophora). Malacologia, 56, 135–191.
Dahirel, M., Cholé, H., Séguret, A., Madec, L., & Ansart, A. (2015). Context dependence of the olfactory perceptual range in the generalist land snail Cornu aspersum. Canadian Journal of Zoology, 93, 665–669.
Di-Castri, F., & Hajek, E. (1976). Bioclimatología de Chile. Santiago: Ediciones Universidad Católica de Chile.
Espinosa, J., & Ortea, J. (2009). Moluscos terrestres de Cuba. Helsinki: Spartacus Säätiö.
Hodges, M. N., & McKinney, M. L. (2018). Urbanization impacts on land snail community composition. Urban Ecosystems, 21, 721–735.
Hulme, P. E. (1996). Herbivores and the performance of grassland plants: a comparison of arthropod, mollusc and rodent herbivory. Journal of Ecology, 84, 43–51.
IUCN (Red List of Threatened Species). (2014). IUCN Red List of Threatened Species. Version 2014.3. Retrieved April 23, 2018 from: http://www.iucnredlist.org
Jackson, D., & Jackson, D. (2011). El género Plectostylus Beck, 1837 (Pulmonata: Bulimulidae) en la costa de la provincia de Choapa (Coquimbo, Chile). Boletín de la Sociedad de Biología, Concepción, Chile, 80, 41–50.
Jones, C. G., Lawton, J. H., & Shachak, M. (1994). Organisms as ecosystem engineers. Oikos, 69, 373–386.
Laurance, W. F., Lovejoy, T. E., Vasconcelos, H. L., Bruna, E. M., Didham, R. K., Stouffer, P. C. et al. (2002). Ecosystem decay of Amazonian forest fragments: a 22-year investigation. Conservation Biology, 16, 605–618.
Linares, E. L., & Vera, M. L. (2012). Catálogo de los moluscos continentales de Colombia. Bogota: Instituto de Ciencias Naturales, Universidad Nacional de Colombia.
Lydeard, C., Cowie, R. H., Ponder, W. F., Bogan, A. E., Bouchet, P., Clark, S. A. et al. (2004). The Global decline of nonmarine mollusks. Bioscience, 54, 321–330.
Martin, S. M. (2000). Terrestrial snails and slugs (Mollusca: Gastropoda) of Maine. Northeast Naturalist, 7, 33–88.
Martínez-de los Ríos, E. (2017). Rediscovery of Bostryx voithianus (Pfeiffer, 1847) (Gastropoda, Pulmonata) in northern Chile, with notes on the type locality. Checklist, 13, 1125–1129.
McBride, C. J., Williams, A. H., & Henry, R. P. (1989). Effects of temperature on climbing behavior of Littorina irrorata: on avoiding a hot foot. Marine Behaviour and Physiology, 14, 93–100.
Meyer III, W.M., Ostertag, R., & Cowie, R. H. (2011). Macro-invertebrates accelerate litter decomposition and nutrient release in a Hawaiian rainforest. Soil Biology & Biochemistry, 43, 206–211.
Meyer III, W. M., Ostertag, R., & Cowie, R. H. (2013). Influence of terrestrial molluscs on litter decomposition and nutrient release in a Hawaiian rain forest. Biotropica, 45, 719–727.
Miquel, S. E., & Araya, J. F. (2013). A new Charopidae from Chile and Argentina, Stephacharopa calderaensis n. gen. and n. sp., with remarks on the taxonomy of the genus Stephadiscus Hylton Scott 1981 (Mollusca: Gastropoda Pulmonata). Archiv für Molluskenkunde, 142, 227–235.
Miquel, S. E., & Araya, J. F. (2015). New records of terrestrial mollusks of the Juan Fernández Archipelago (Chile), with the description of a new genus and species of Charopidae (Gastropoda: Stylommatophora). Archiv für Molluskenkunde, 144, 155–167.
Miranda, A., Altamirano, A., Cayuela, L., Lara, A., & González, M. (2017). Native forest loss in the Chilean biodiversity hotspot: revealing the evidence. Regional Environmental Change, 17, 285–297.
MMA (Ministerio del Medio Ambiente). (2018). Clasificación según estado de conservación. Ministerio del Medio Ambiente de Chile. Retrieved October 11, 2018 from: http://www.mma.gob.cl/clasificacionespecies/index.htm
Müller, F., & Pereira, I. (2006). The bryophyte flora of nature reserves in central Chile. 1. The moss flora of Los Ruiles Nature Reserve, near Talca. Tropical Bryology, 7, 55–66.
Nicolai, A., & Ansart, A. (2017). Conservation at a slow pace: terrestrial gastropods facing fast-changing climate. Conservation Physiology, 5, cox007.
Nyffeler, M., & Symondson, W. O. P. (2001). Spiders and harvestmen as gastropod predators. Ecological Entomology, 26, 617–628.
Pearce, T. A., & Paustian, M. E. (2013). Are temperate land snails susceptible to climate change through reduced altitudinal ranges? A Pennsylvania example. American Malacological Bulletin, 31, 213–224.
Peters, H. A. (2007). The significance of small herbivores in structuring annual grassland. Journal of Vegetation Science, 18, 175–182.
Puente-Torres, S., Barceló, M., & Simonetti, J. A. (2017). Alsodes vanzolinii (Donoso-Barros, 1974): a new locality in a disturbed habitat for a critically endangered species. Checklist, 13, 813–816.
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Austria, Vienna.
Régnier, C., Achaz, G., Lambert, A., Cowie, R. H., Bouchet, P., & Fontaine, B. (2015). Mass extinction in poorly known taxa. Proceeding of Natural Academy of Sciences, 112, 7761–7766.
Régnier, C., Fontaine, B., & Bouchet, P. (2009). Not knowing, not recording, not listing: numerous unnoticed mollusk extinctions. Conservation Biology, 23, 1214–1221.
Rodríguez, R., Marticorena, C., Alarcón, D., Baeza, C., Cavieres, L., Finot, V. L. et al. (2018). Catálogo de las plantas vasculares de Chile. Gayana Botánica, 75, 1–430.
Rodríguez-San Pedro, A., Barquez, R. A., & Simonetti, J. A. (2015). Histiotus magellanicus (Chiroptera: Vespertilionidae) is not restricted to Subantarctic forests: first record for the Coastal Maulino Forest in central Chile. Checklist, 11, 1–3.
Schweiger, O., Frenzel, M., & Durka, W. (2004). Spatial genetic structure in a metapopulation of the land snail Cepaea nemoralis (Gastropoda: Helicidae). Molecular Ecology, 13, 3645–3655.
Simone, L. R. L. (2006). Land and freshwater molluscs of Brazil. São Paulo: EGB/Fapesp.
Smith-Ramírez, C., Díaz, I., Pliscoff, P., Valdovinos, C., Méndez, M.A., Larraín, J. et al. (2007). Distribution patterns of flora and fauna in southern Chilean Coastal rain forests: integrating natural history and GIS. Biodiversity and Conservation, 16, 2627–2648.
Statsoft . (2004). STATISTICA [data analysis software system], version 7. Stasoft, Inc. www.statsoft.com
Ström, L., Hylander, K., & Dynesius, M. (2009). Different long-term and short-term responses of land snails to clear-cutting of boreal stream-side forests. Biological Conservation, 142, 1580–1587.
Stuardo, J. R., & Vega, R. (1985). Synopsis of the land Mollusca of Chile. With remarks on distribution. Studies on Neotropical Fauna and Environment, 20, 125–146.
Taucare-Ríos, A., Veloso, C., & Bustamante, R. O. (2017). Microhabitat selection in the sand recluse spider (Sicarius thomisoides): the effect of rock size and temperature. Journal of Natural History, 51, 2199–2210.
Tuff, K. T., Tuff, T., & Davies, K. F. (2016). A framework for integrating thermal biology into fragmentation research. Ecology Letters, 19, 361–374.
UE (Unión Europea). (2017). Informe técnico de la misión en Chile 2017. Análisis de los incendios forestales en regiones del Maule y Bio bio. Madrid: Lead Emergency Management Authority (LEMA).
Valdovinos, C. (1999). Biodiversidad de moluscos chilenos: base de datos taxonómica y distribucional. Gayana, 63, 111–164.
Valdovinos, C., Olmos, V., & Moya, C. (2005). Moluscos terrestres y dulceacuícolas de la cordillera de la costa. In C. Smith-Ramirez, J. J. Armesto, & C. Valdovinos (Eds.), Historia, biodiversidad y ecología de los bosques de la Cordillera de la Costa (pp. 269–285). Santiago: Editorial Universitaria.
Valdovinos, C., & Stuardo, J. R. (1988). Morfología, sistemática y distribución del género Plectostylus Beck 1837 (Pulmonata: Bulimulidae). Gayana, 52, 115–195.
Valdovinos, C., & Stuardo, J. R. (1989). Austrodiscus (Zilchogyra) solemi spec. nov. Nuevo gastrópodo humícola de Chile. Boletín de la Sociedad de Biología de Concepción, Chile, 60, 239–245.
Van Bruggen, A. C., Herbert, D. G., & Breure, A. S. H. (2016). Prestonellinae-validation of the name as a new subfamily of Bothriembryontidae (Mollusca, Gastropoda, Orthalicoidea). Zootaxa, 4084, 590–592.
Wall, A. F., Yanes, Y., Miller, J. H., & Miller, A. I. (2018). Bellwether of the Canaries: anthropogenic effects on the land snail fauna of the Canary Islands. Biodiversity and Conservation, 27, 395–415.