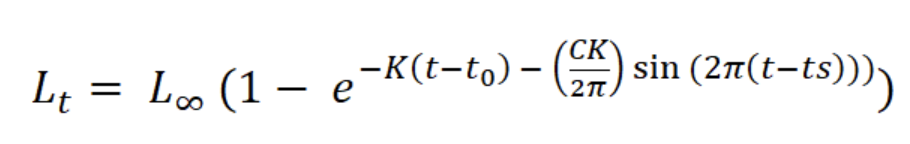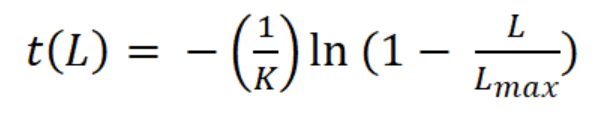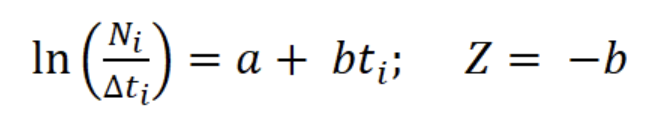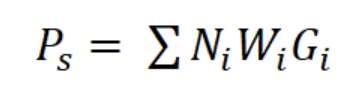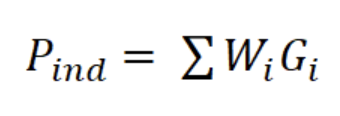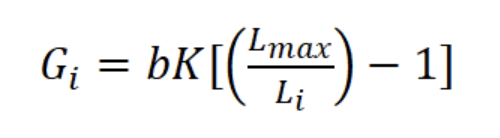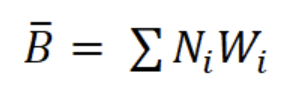Ignacio Cáceres, Esmeralda Citlali Ibarra-García, Fabián Alejandro Rodríguez-Zaragoza, Manuel Ayón-Parente *
Universidad de Guadalajara, Centro Universitario de Ciencias Biológicas y Agropecuarias, Departamento de Ecología, Laboratorio de Ecología Molecular, Microbiología y Taxonomía, Camino Ramón Padilla Sánchez Núm. 2100 Nextipac, 45110 Zapopan, Jalisco, Mexico
*Corresponding author: manuel_aparente@hotmail.com (M. Ayón-Parente)
Received: 3 November 2021; accepted: 15 June 2022
Abstract
Cambarellus chapalanus is an endemic species of the area around Lake Chapala in Mexico and knowledge about its biology and ecology is scarce. Its population dynamics were evaluated based on individual growth, mortality, production, and productivity. For this, individuals were collected between November 2011 and October 2012, in ponds located in the Sierra San Juan Cosalá, Jalisco, Mexico. The abiotic variables of ponds (depth, temperature, pH, precipitation, and evaporation) presented significant variations during the study. Abundance was similar throughout the year but was related to an increase in temperature and evaporation of ponds. Furthermore, males were more abundant than females. Growth was fast and oscillatory with slow phases in rainy periods. Mortality was high and started at medium sizes; however, high somatic production also generated high population productivity. In general, the parameters of C. chapalanus showed normal patterns for a natural population; however, were strongly influenced by temperature and pond volume, probably because it is an isolated population in Sierra San Juan Cosalá.
Keywords: Cambaridae; Isolated ponds; Growth; Mortality; Production; Productivity
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Dinámica poblacional del acocil Cambarellus chapalanus de la sierra San Juan Cosalá, Jalisco, México
Resumen
Cambarellus chapalanus es una especie endémica de la zona del Lago de Chapala en México y el conocimiento sobre su biología y ecología es escaso. Se evaluó su dinámica poblacional con base en el crecimiento individual, mortalidad, producción y productividad. Para ello, se colectaron individuos entre noviembre 2011 y octubre 2012, en charcas localizadas en la Sierra San Juan Cosalá, Jalisco, México. Las variables abióticas de las charcas (profundidad, temperatura, pH, precipitación y evaporación) presentaron variaciones significativas durante el estudio. La abundancia fue similar a lo largo del año, pero se relacionó con el incremento de la temperatura y evaporación de las charcas. Además, los machos fueron más abundantes que las hembras. El crecimiento fue rápido y oscilatorio con fases lentas en periodos de lluvias. La mortalidad fue alta e inició en tallas medianas; sin embargo, su alta producción somática también generó una alta productividad poblacional. En general, los parámetros de C. chapalanus mostraron patrones normales para una poblacional natural; sin embargo, estuvieron fuertemente influenciados por la temperatura y el volumen de las charcas, probablemente debido a que es una población aislada en la Sierra San Juan Cosalá.
Palabras clave: Cambaridae; Charcas; Crecimiento; Mortalidad; Producción; Productividad
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The family Cambaridae is found strictly in freshwater habitats and has an ample distribution in North America (Álvarez et al., 2012; Armendáriz et al., 2016; Crandall & Buhay, 2008). In Mexico, there are 3 genera (Cambarellus, Orconectes, and Procambarus) and 58 species of Cambaridae (Álvarez et al., 2012; Pedraza-Lara & Doadrio, 2015; Pedraza-Lara et al., 2021). The genus Cambarellus has 18 recognized species, of which 11 are endemic to Mexico and 2 were considered extinct by the International Union for Conservation of Nature (Hobbs, 1976; IUCN, 2022; Pedraza-Lara et al., 2012, 2021). However, Carson et al. (2015) recently rediscovered Cambarellus chihuahuae in the Ojo Solo spring, Chihuahua. In general, Cambarellus is composed of small-sized species, with low abundances, and is rarely extracted for commerce (Álvarez & Villalobos, 2016). However, in Mexico, some species, such as Cambarellus montezumae, are regularly consumed by human populations and have been proposed as a species with high potential for aquaculture (Arredondo-Figueroa et al., 2011). Also, in Jalisco, several species of the genus are used as fishing bait for largemouth bass (Micropterus salmoides), catfish (Ictalurus ochoterenai), and tilapia (Oreochromis aureus) (personal observations). Wild populations of Cambaridae can be found in a wide range of freshwater habitats. However, they preferentially live in shallow waters, with muddy bottoms and vegetation where they can also build complex burrows that provide hiding places against predators and refuge during periods of drought (Álvarez & Villalobos, 2016; Hobbs, 2001; Penn, 1950; Peterson et al., 1996; Villalobos, 1983).
Evaluation of population dynamics has become an effective tool for the description and management of aquatic populations (Butler & Rowland, 2008); age can be used to calculate individual growth, mortality, and productivity of a population (Capana, 2001). Even though the study of crustacean growth has been complicated by the processes of ecdysis, current use of indirect methods, such as size frequency analysis, tagging-recapture analysis, and quantification of lipofuscin accumulation, have been efficient to estimate the age of crustaceans (Kilada et al., 2012; Leland et al., 2011; Scalici et al., 2008a; Vogt, 2007). These methods are extensively used in ecological studies of crayfishes (e.g., Dörr & Scalici, 2013; Scalici et al., 2008b, 2010), and this has enabled the evaluation of aspects such as the capacity for invasion and adaptation, as well as to determine the conservation status of crayfish populations (Brusconi et al., 2008; Chiesa et al., 2006; Scalici & Gherardi, 2007). However, few studies have evaluated the population dynamics of Cambarellus species (e.g., Álvarez & Rangel, 2007).
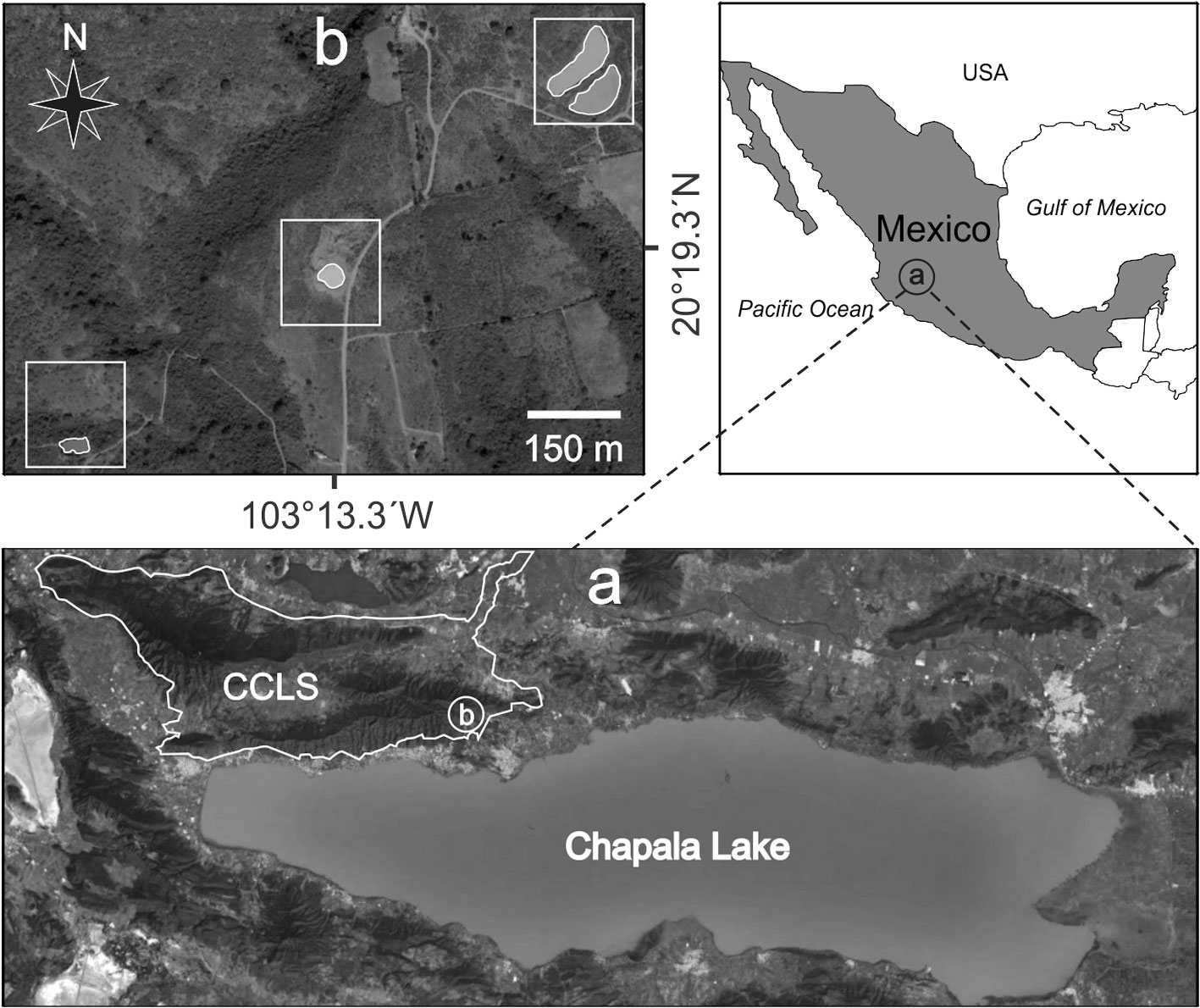
The Cerro Viejo-Chupinaya-Los Sabinos (CCLS) was declared a Natural Protected Area (NPA) in May 2013 by the State of Jalisco, Mexico (Decree 3, 2013; Sandoval-Moreno & Hernández-García, 2017). This NPA is located in a confluence zone of the Nearctic and Neotropical biogeographic regions in the center of the Lerma, Chapala, and Santiago hydrological basin. So far, the research conducted in this NPA has only evaluated the connectivity of CCLS habitats with other NPAs for the white-tailed deer Odocoileus virginianus, bird taxonomy on the shore of Lake Chapala, and the assessment of management instruments for land use of Lake Chapala, including the CCLS (Cuevas & Íñigues-Davalos, 2017; Sandoval-Moreno & Hernández-García, 2017; Villavicencio-García et al., 2017). It is important to note that the latter study mentioned that the CCLS is considered fragile due to the growth of urban development, affecting forests, biodiversity, and hydrological resources.
One of the Mexican endemic Cambarellus species is C. chapalanus (Faxon, 1898). According to Álvarez et al. (2012), the Cambarellus species recorded in the Lake Chapala zone (next to CCLS) are C. chapalanus, C. montezumae, and C. prolixus. In the present work, C. chapalanus was studied and captured in a wetland system of four ponds located inside the limits of the CCLS. These ponds have great ecological importance because they are used as drinking troughs for livestock and wildlife in the area. This study aimed to describe and evaluate the population dynamics of C. chapalanus from the San Juan Cosalá Mountain Range, specifically at the CCLS. For this, we used size frequency analysis of pooled data of 4 ponds to estimate: 1) individual growth, 2) mortality, 3) somatic and individual production, and 4) population productivity.
Materials and methods
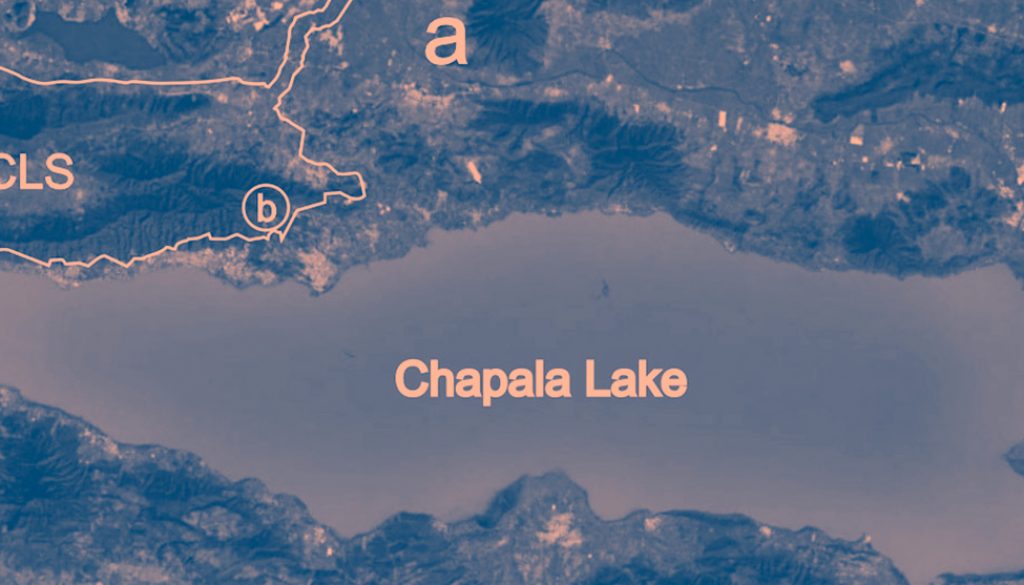
The NPA of CCLS consists of 32,129 hectares and includes 2 mountain ranges named Cerro Viejo and San Juan Cosalá. The aquatic system studied with 4 ponds is located 4 km NW of the city of Chapala, in the southeastern region of the San Juan Cosalá Mountain Range and CCLS NPA (Fig. 1). The ponds are recharged by the seasonal rains from June to August; their waters are stagnant and turbid with muddy bottoms. Vegetation is present around the ponds but not inside them, it is mainly composed of herbaceous plants and shrubs, including Aster moranensis, Barkleyanthus salicifolius, Solanum ferrugineum, and Verbesina sphaerocephala. The individual characteristics of the ponds were as follows: Pond 1, altitude 1,790 m, surface area 3,980 m2, and depth 1.47 m; Pond 2, altitude 1,787 m, surface area 4,590 m2, and depth 1.46 m; Pond 3, altitude 1,780 m, surface area 906.4 m2, and depth 0.96 m; and, Pond 4, altitude 1,770 m, surface area 900 m2, and depth 1.5 m.
Individuals of Cambarellus chapalanus were collected every month from the 4 ponds from November 2011 to October 2012, except in April of 2012. They were identified following the taxonomic criteria of Hobbs (1989) and Villalobos (1983), and compared with material deposited in the Colección Nacional de Crustáceos, Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. The material was deposited in the Reference Collection of the Laboratory of Marine Ecosystems and Aquaculture (LEMA-CR), University Center for Biological and Agricultural Sciences (CUCBA-UDG), Universidad de Guadalajara in Zapopan, Jalisco, Mexico. The study assumes that individuals from the 4 ponds belong to the same population because the distance between them is relatively short, less than 500 m. Besides, during the rainy season, small streams appear that allow the connection between them (Thomas et al., 2019). For this reason, subsequent analyses were performed with the sum of the abundances obtained from the 4 ponds.
Five bottom-level tows with 3 replicates per pond were conducted. The tows of 1 m of length were made perpendicular to the water edge every 10 m using a circular hand net (d = 20 cm, mesh size = 0.5 cm). For each individual collected, sex and cephalothorax length (CL) were recorded (from the anterior tip of rostrum to the posterior medial dorsal margin of the carapace) with a stereoscopic microscope (± 0.1 mm). Of the total samples, a subsample of 100 individuals representative of the total distribution of CL (4-14.3 mm) was collected. Individuals of the subsample were incinerated at 550 °C over 4 h and weighed on an analytical balance (± 0.0001 g) to estimate the relationship between CL and ash free dry weight (AFDW).
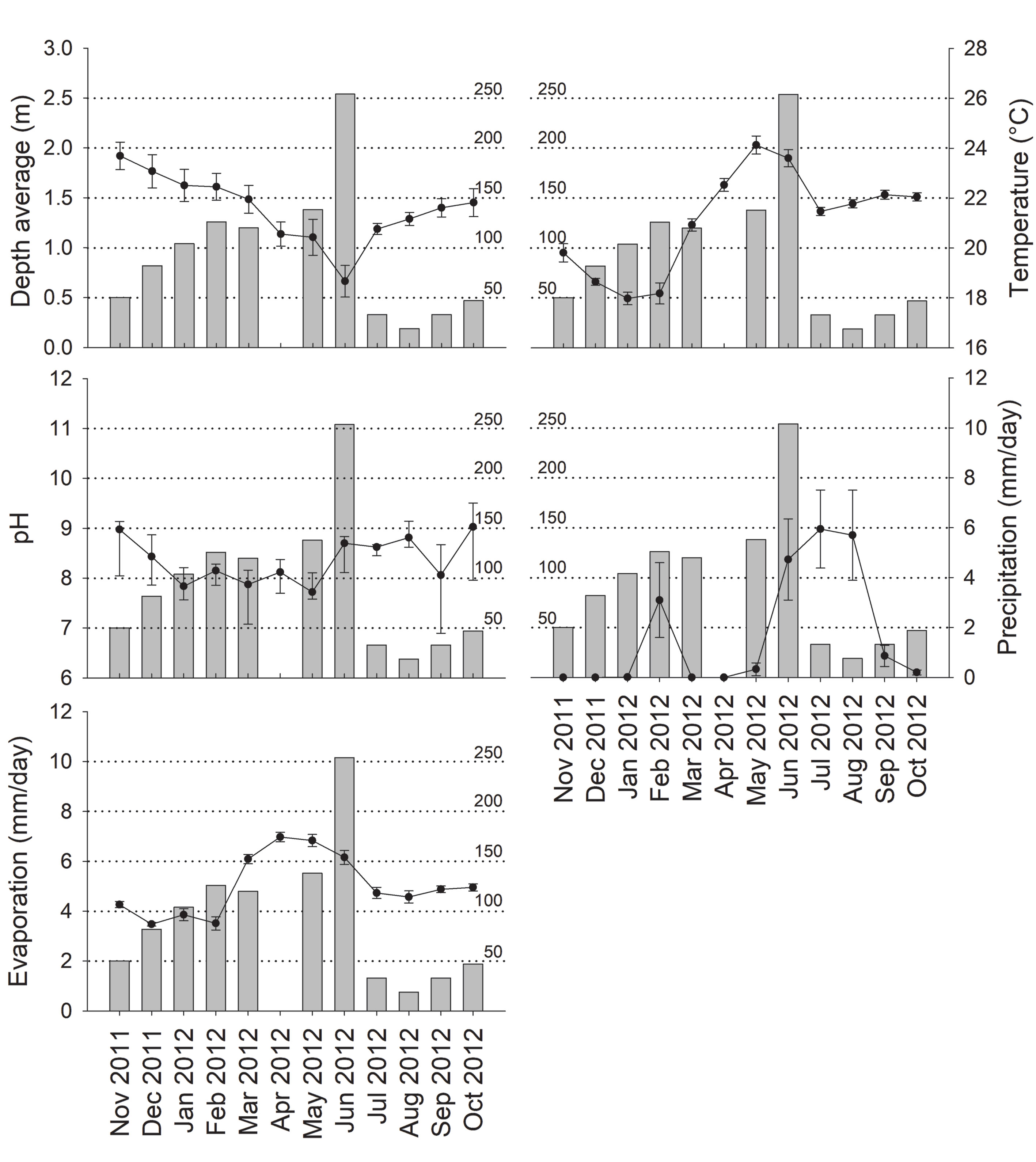
Water characteristics such as depth, temperature, and pH of each pond were recorded with 3 replicates, while the precipitation and evaporation were obtained from the meteorological station of the National Meteorological Service of Mexico, located 2 km from the study area. For the analysis of depth, temperature, and pH, the average values obtained from the 4 ponds were used, while for precipitation and evaporation, the monthly average values obtained from the daily records were used. Variation in abiotic conditions was analyzed by one-way analysis of variance (ANOVA) based on permutations, constructed with a matrix of Euclidean distance, 10,000 permutations, and a type III sum of squares in PRIMER v6.1.11 + PERMANOVA v1.01, following the criteria of Anderson et al. (2008) and Clarke and Gorley (2006). A non-restricted analysis strategy was used because the assumptions of parametric statistics were not satisfied. A multiple linear regression, constructed with a canonical redundancy analysis (RDA), was used to evaluate the linear relationship between abundance of C. chapalanus and the previously described abiotic variables. Multi-collinearity among predictive variables was reduced using Pearson correlations (r) and elimination of those with r > 0.90. Likewise, a variance inflation factor (VIF) of ≤ 10 was used. Statistical significance was tested with 10,000 permutations in CANOCO v4.5 (ter Braak & Šmilauer, 2002).
Variation in sex proportion over time was compared with a Chi-square analysis. Individual growth was estimated with a von Bertalanffy seasonal growth model:
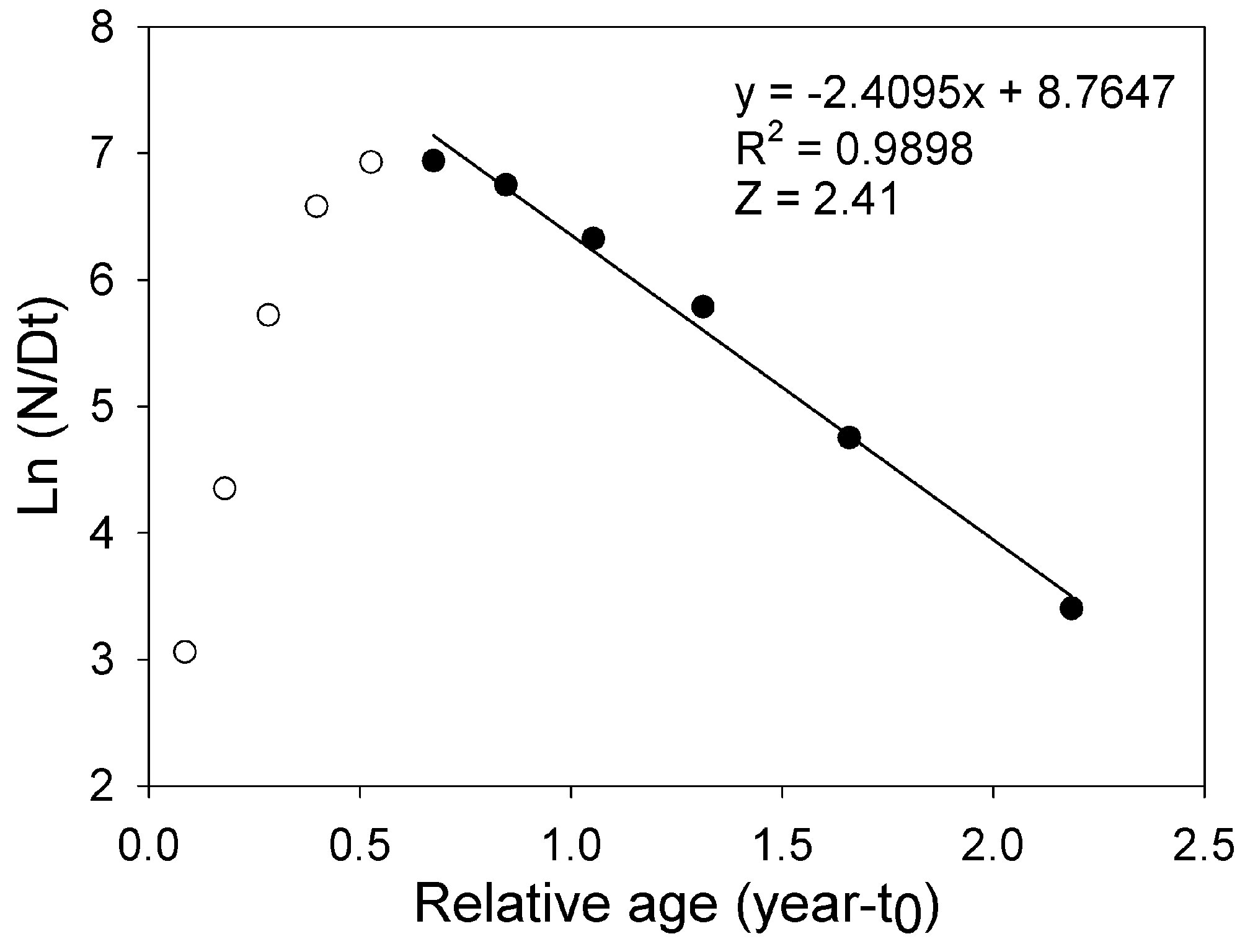
where Lt is the length L at age t (years), L∞ is the asymptotic length (mm), K is the parameter of curvature (year-1), C is the magnitude of seasonal variation in growth, ts (summer point) is the time of the year when the growth rate is highest, and t0 is the age when L is zero (Pitcher & Macdonald, 1973). The parameters K, L∞, C, and ts were estimated with the modal progression method, using the function ELEFAN of the library TropFishR for R-Project (Mildenberger et al., 2017). The parameters K and L∞ have an inverse statistical relationship that can cause underestimation of L∞ and overestimation of K (Pauly, 1979). This problem was avoided by adjusting the value of L∞ to the maximum recorded size class of CL (Lmax). For estimation of mortality Z (year-1), the sizes were converted into relative ages using the von Bertalanffy inverse function:
With the relative ages, a linearized catch curve was constructed (Pauly, 1983). Finally, Z was calculated using the following regression:
where a and b are the constants of the line, Ni is the number of individuals in length interval i; Δti is the time required to grow from one length interval to another, and ti is the age in length interval i. Annual somatic (Ps) and individual (Pind) production were calculated based on the method of Crisp (1984) as a rate of increase in specific weight, from the monthly mean density of the net tows, size frequency data, length-weight relationship, and growth parameters K and Lmax:
where Ni is the mean number of individuals (N m-3) of length interval i, Wi is the mean body weight of interval i (g AFDW), and Gi is the rate of increase in weight (years-1):
where b is the exponent of the length-weight regression equation (g AFDW = a CLb) and Li is the mean length in length interval i. Finally, productivity (Ps/B) was calculated from Ps and mean annual biomass calculated as:
Results
All the abiotic variables considered in this study presented significant variations (depth, Pseudo-F = 6.165, p = 0.0001; temperature, Pseudo-F = 55.969, p = 0.0001; pH, Pseudo-F = 3.4891, p = 0.0001, precipitation, Pseudo-F = 14.531, p = 0.0001; evaporation, Pseudo-F = 23.788, p = 0.0001). Figure 2 shows a drastic reduction in mean depth of ponds between January and June, related to a temperature and evaporation increase. The pH remained in mild alkaline ranges (between 7.62 and 8.8) throughout the year. The rainy season, in June to August of 2012, had a maximum mean precipitation of 5.95 mm; in February this was 3.1 mm.
Cambarellus chapalanus was the only crayfish present in the ponds. A total of 1,006 individuals were captured, where 549 (54.57%) were males and 457 (45.43%) females, with a proportion of 1.2:1 in favor of the males (X2 = 8.41, p < 0.05). Only 9 berried females were found during March (3), June (1), July (3), November (1), and December (1). The abundance of C. chapalanus was highest in June, with 254 individuals, and lowest in August, with 19. Between December 2011 and May 2012, the abundances presented similar values with 82 and 126 individuals, while between July 2012 and October 2012 the abundances ranged between 19 and 47 individuals (Fig. 2). On the other hand, only temperature had a significant linear relationship with abundance (Table 1).
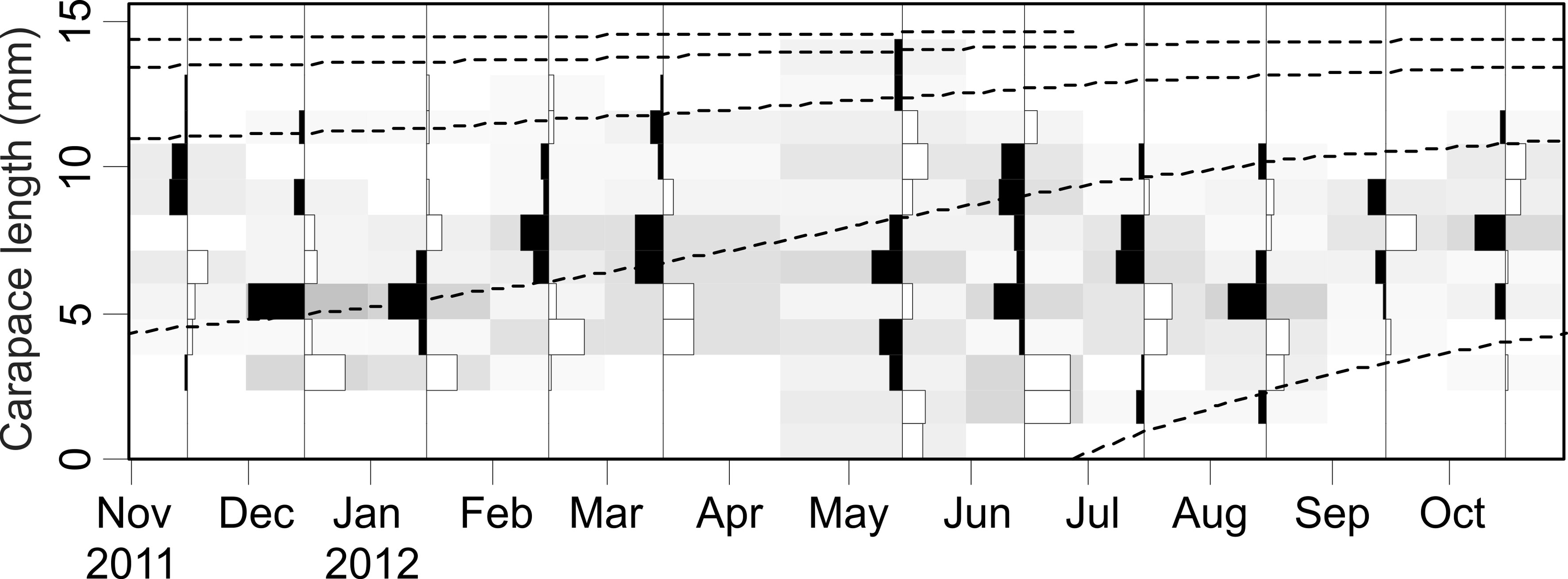
The asymptotic length L∞ estimated with the modal progression method was higher than the maximum CL recorded in the population. The Lmax used to calculate the parameters was 15 mm, producing a K of 0.97 year-1, C = 0.45, ts = 0.417 (May) and a maximum age (tmax) of 4 years. The model estimated the presence of 5 cohorts, where the first (0-4.2 mm) was present between July and October 2012, and the second (4.2-10.2 mm), third (11.4-13.8), and the fourth (13.8 mm) were present throughout the study year; the fifth (Lmax) was detected between November 2011 and June 2012. Figure 3 shows the von Bertalanffy seasonal growth curve built with the definite values of K and Lmax. Figure 4 shows the linearized catch curve with the Z value (2.41 year-1) estimated by regression, where mortality occurs from individuals with CL greater than 6 mm. The annual somatic production of the population was 6.61 g AFDW m-3, mostly sustained by individuals of sizes between 5.4 and 10.2 mm in CL, while annual individual production was 5.79 g AFDW m-3, with maximum values in individuals with CL of between 9.0 and 10.2 mm (Fig. 5). Mean annual biomass was 3.60 g AFDW m-3 and productivity (Ps/B) was 1.84. Finally, Table 2 shows all the population dynamics parameters of C. chapalanus estimated in this study.
Table 1
Results of multiple linear regression by a canonical redundancy analysis (RDA) to evaluate the linear relationship between the abundance of Cambarellus chapalanus and the abiotic variables, depth, temperature, precipitation, evaporation, and pH. Bold values show significant differences.
| Conditional effects | |||
| Variable | λ | F | p |
| Depth | 0.30 | 4.36 | 0.068 |
| Temperature | 0.27 | 7.34 | 0.028 |
| Precipitation | 0.13 | 1.95 | 0.199 |
| Evaporation | 0.06 | 1.71 | 0.239 |
| pH | 0.04 | 1.25 | 0.312 |
| Model | R2 ajust = 0.64 | F = 4.844 | p = 0.038 |
Table 2
Population parameters of the Cambarellus chapalanus population in the studied ponds. K is the growth constant, Lmax the maximum CL recorded, C the magnitude of seasonal variation, ts (summer point) the time of the year when the growth rate is highest, tmax the maximum age, Z the mortality, Ps the total annual production, Pind the individual production, and B the mean annual biomass.
| Population parameters | |
| K (year-1) | 0.97 |
| Lmax (mm) | 15.00 |
| C | 0.45 |
| ts | 0.48 |
| tmax (year) | 4.00 |
| Z (year-1) | 2.41 |
| Ps (g AFDW m-3) | 6.61 |
| Pind (g AFDW m-3) | 5.79 |
| B (g AFDW m-3) | 3.60 |
| Ps/B | 1.84 |
Discussion
Many studies have described the structure and population dynamics of crayfishes worldwide (e.g., Ghia et al., 2015; Leland et al., 2015; Wendler et al., 2015), but few evaluated the genus Cambarellus. Arana-Magallón et al. (1998) and Álvarez and Rangel (2007) are the only authors to have evaluated abundance, growth, and mortality of C. montezumae. In our study, C. chapalanus presented similar abundances throughout most of the year, similar to C. montezumae in Tlaxcala and Xochimilco (Álvarez & Rangel, 2007; Arana-Magallón et al., 1998). However, the abundance values of C. chapalanus were higher, with a maximum in June. This can be explained by the fact that individuals of C. montezumae were captured in open water bodies (reservoirs and channels), unlike our study, which was carried out in isolated ponds that increase the capture probability. Additionally, the low depth presented by the ponds in June produced an increase in the density of individuals in the ponds. Unfortunately, the densities of C. chapalanus are difficult to compare with other species of Cambarellus, because of the lack of studies on the subject. Despite this, the abundances reported herein are probably insufficient to generate density-dependence problems. Several authors have evaluated the optimal point of abundance in the growth performance and feeding of crayfishes for aquaculture purposes, where optimum densities vary between 50 and 400 individuals/m2 depending on the species (Carmona-Osalde et al., 2004; Savolainen et al., 2004). In the case of Cambarellus, Arredondo-Figueroa et al. (2010) found that the optimum density of C. montezumae was 77 individuals/m2, which is much higher than those found in this study. On the other hand, Peterson et al. (1996) mentioned that the absence of vegetation and the alkaline pH can be limiting factors for the presence and abundance of crayfishes; in our study, however, the ponds have no vegetation and the pH presented values over 8 throughout the year. This result is not unusual; C. montezumae was also found in habitats with alkaline pH (Álvarez & Rangel, 2007; Arana-Magallón et al., 1998).
In general, the reproduction of cambarid crayfishes in tropical-subtropical latitudes occurs throughout the year, where the different forms of males (Form I, reproductively active and larger carapace to chelae size ratio than the non-reproductive Form II; Stein, 1976) and berried females are always present (Álvarez & Villalobos, 2016; Arredondo-Figueroa et al., 2011). In our study, males were more abundant, differing from the 1:1 relationship expected in natural populations. This result has been observed in other cambarids, such as Orconectes immunis, O. limosus, O. williamsi and Procambarus clarkii (Chiesa et al., 2006; Chucholl, 2012; DiStefano et al., 2013); however, the opposite has been reported for the genus Cambarellus (Álvarez & Rangel, 2007). Arana-Magallón et al. (1998) found that C. montezumae males were more abundant only when there was a greater number of berried females (April and July), suggesting that females move to sheltered areas with less predator access. In our study, however, shelters for females are restricted in the ponds (e.g., no vegetation) which makes it difficult to hide. Because of this, the berried females likely move to deeper areas, use rocks as shelters, or build burrows. If C. chapalanus only uses burrows as hiding places, we can qualify it as a tertiary crayfish according to Hobbs (1981) classification because it is a free-living crayfish (field observation) and can be found in open water bodies (Gutiérrez-Yurrita et al., 2002; Villalobos, 1955). On the other hand, most of the size classes were present throughout the year, except the smaller individuals that recruited in May 2012, similar to C. montezumae, which also presented a single recruitment in December (Álvarez & Rangel, 2007). Carmona-Osalde et al. (2004) reported that spawning is induced when several factors occur simultaneously. For example, Gutiérrez-Yurrita and Montes (1999) observed that the main factors that induced spawning of P. clarkii in Doñana National Park (Spain) were temperature and flooding. Therefore, although the berried females are present throughout the year, spawning will not occur until all environmental conditions are favorable. It has even been observed that females can conserve a male spermatophore for several months (Holdich & Lowery, 1988; Reynolds, 2002). The development before hatching for Cambarellus has been reported to vary between 15 and 20 days (Arana-Magallón et al., 1998); if this is the case for C. chapalanus, the spawning likely occurred between April and May, when pond temperature begin to rise, and the first rains begin to fall. This reproductive behavior has been also reported for several North American crayfishes (Jegla, 1966).
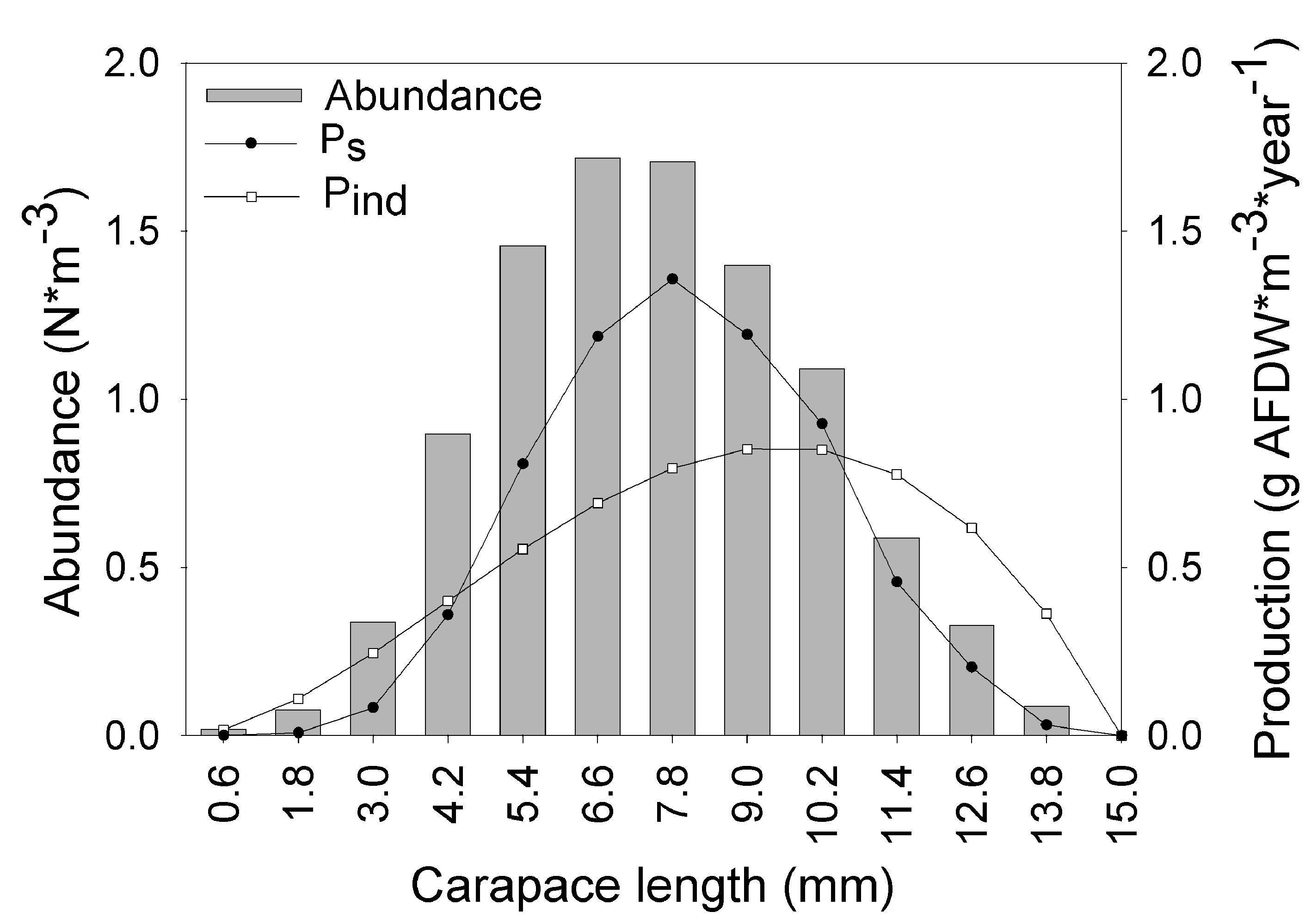
Cambarellus chapalanus has a small size, similar to other Cambarellus species, and presents an accelerated growth, similar to other cambarids such as O. limosus, P. clarkii (Chiesa et al., 2006; Scalici & Gherardi, 2007), and O. immunis (Chucholl, 2012). In our study, the growth rates of C. chapalanus increased between November 2011 and February 2012 when temperature was below 20 °C, and began to decrease in May (high-temperature period). Temperature is one of the most critical factors influencing crayfish biological processes because it regulates several physiological functions, including oxygen consumption, enzyme reaction rates, and feeding (Gutiérrez-Yurrita & Montes, 1999; Hewitt & Duncan, 2001; Newell & Branch, 1980; Seals et al., 1997). For example, Rodríguez-Serna and Carmona-Osalde (2002) reported that the ingestion rate and food efficiency assimilation of C. montezumae increases when temperatures are below 20 ºC. Similarly, Paglianti and Gherardi (2004) found that growth rates (size and weight) of Austropotamobius pallipes (Astacidae) and P. clarkii decreased when the temperature increased above 20 °C. This temperature effect on growth occurs because high temperatures (above 20 °C) increase the metabolic consumption and, if these values are approaching the ingestion rate, less energy is available for growth (Paglianti & Gherardi, 2004). High rates of growth represent a significant drain on energy reserves, and this is likely also the case for C. chapalanus because temperature was the main factor influencing growth (Rombough, 1994).
In our study, the mortality analysis has the advantage emigration and immigration are reduced due to the isolated characteristics of ponds. Additionally, the population of C. chapalanus did not present mortality due to extraction as a resource. Therefore, we assumed that mortality Z is equal to the natural mortality (M). In this context, mortality could occur due to senescence, predation, or intraspecific aggression. The life period reported for Cambarellus species is approximately 15 months (C. montezumae), and under controlled conditions, individuals have been maintained for 2 years (Álvarez & Rangel, 2007). However, the seasonal growth model estimates that C. chapalanus can live for 4 years, where the last cohort dies between June and July. Similar lifetimes were reported for other cambarid species, such as O. limosus and P. clarkii (Chiesa et al., 2006, 2008; Dörr et al., 2006; Scalici & Gherardi, 2007; Scalici et al., 2010). Our results show that mortality started from the medium sizes, which constitute the highest proportion of the population. This can be explained by the fact that this portion has a visible size and great abundance, which increases the capture probability by a predator. For this reason, we believe that predation was the main factor influencing mortality, because the study area is inhabited by several species that feed on crayfishes: birds like Eudocimus albus and Limnodromus scolopaceus, and the water snake Thamnophis melanogaster (Davis & Smith, 2001; Dorn et al., 2011; Manjarrez et al., 2013). On the other hand, crayfishes present intraspecific aggressive behaviors, including maternal aggression, territorialism, and cannibalism (Bovbjerg, 1956; Edwards & Kravitz, 1997; Edwards et al., 2003; Fero & Moore, 2008; Figler et al., 1995; Garvey & Stein, 1993; Hazlett et al., 1992; Lowe, 1956; Statzner et al., 2000). These behaviors are subject to the habitat complexity and food resources available (Jensen et al., 2005). The studied ponds can be considered relatively simple because there is no vegetation inside to provide sufficient hiding places, which increases the probability of aggressive encounters that can injure or kill the participants. The high mortality of C. chapalanus reported in this study, compared with other crayfishes (e.g., A. pallipes, Scalici et al., 2008b; O. immunis, Chucholl, 2012), might be the result of a combination of these 3 factors.
The somatic production and productivity were estimated based on the abundance, individual growth, and mortality. Therefore, the factors discussed in this study, such as habitat characteristics, temperature, predation, and intraspecific competition, also affect the production of C. chapalanus. However, the Ps and Pind per class showed an expected distribution for a natural population. For example, Ps was mainly sustained by medium-sized individuals, because this was a more abundant portion of the population. On the other hand, Pind increases gradually towards larger sizes, due to the individuals with small sizes having a lower weight and being less abundant. In addition, Arredondo-Figueroa et al. (2010) mention that in the juvenile stages, the energy costs are higher than in mature organisms, especially during the ecdysis and intermolt phases; because in this early stage, the energy allocation can vary in different processes (e.g., osmoregulation and growth; Rombough, 1994) and they have high energy cost behaviors (e.g., predator escape). In terms of energy flow, the Ps/B ratio has been documented for several cambarid crayfishes, but not for the genus Cambarellus. Also, few reports refer to populations that live in ponds; most studies have been conducted in streams, rivers, and lakes. For example, Vannote (1963) estimated a Ps/B between 1.0 and 1.4 for Orconectes propinquus, found in streams eutrophicated by agriculture; Momot and Gowing (1977) found that the Ps/B for Orconectes virilis, captured in lakes, varied between 1.0-1.4, similar to values found in rivers (1.2) by Roell and Orth (1992), who also reported the Ps/B of Orconectes sanbornii (1.9) and Cambarus sciotensis (1.5) in the same habitat; Momot and Romaire (1981) found that the values of Ps/B for P. clarkii, captured in ponds, varied between 0.87 and 1.5; whereas Griffith et al. (1996) also showed that the Ps/B in Cambarus bartonii, belonging to streams and rivers, varied between 0.58 and 1.3. Thus, our results for C. chapalanus (Ps/B = 1.84) are within the ranges observed for other crayfish species, despite that size, production, and biomass vary depending on the species and habitats.
This study represents the first quantitative evaluation of a C. chapalanus population present in 4 ponds located in the CCLS NPA of Mexico. The main difficulty of this study was contrasting our information with other populations of Cambarellus, due to the absence of studies and a standard methodology. We suggest that future studies that evaluate crayfish populations, especially Cambarellus, replicate the approach and methodologies applied here, in order to make robust comparisons and improve the understanding of the key variables that affect the dynamics of the crayfish. Our findings agree with other crayfish studies around the world, but the status of the Mexican species remains unknown.
Acknowledgements
The authors Ignacio Cáceres and Esmeralda C. Ibarra García thank Conacyt (Mexico) graduate scholarship (I.C. 584808; E.C.I.G. 277241) granted to study the Doctorado en Ciencias en Biosistemática, Ecología y Manejo de Recursos Naturales y Agrícolas (BEMARENA) of the Universidad de Guadalajara, Mexico. This work was performed with the necessary permits for field study and was done by the academic body “Ecología y Biodiversidad (UDG-CA-888)” of Universidad de Guadalajara. Finally, we are grateful for comments and observations by anonymous referees who considerably improved the quality of this work.
References
Álvarez, F., & Rangel, R. (2007). Population study of the crayfish Cambarellus montezumae (Crustacea: Decapoda: Cambaridae) in Xochimilco, Mexico. Revista Mexicana de Biodiversidad, 78, 431–437. https://doi.org/10.22201/ib.20078706e.2007.002.404
Álvarez, F., & Villalobos, J. L. (2016). The Crayfish of Middle America (pp. 448–463). In: Kawai, T. Faulkes, Z., & Scholtz, G. (Eds.) Freshwater Crayfish: A Global Overview. CRC Press.
Álvarez, F., Villalobos, J. L., Armendáriz, G., & Hernández, C. (2012). Biogeographic relationship of freshwater crabs and crayfish along the Mexican transition zone: reevaluating Rodríguez (1986) hypothesis. Revista Mexicana de Biodiversidad, 83, 1073–1083. https://doi.org/10.7550/rmb.28230
Anderson, M. J., Gorely, R. N., & Clarke, K. R. (2008). PERMANOVA+ Primer: Guide to Software and Statistical Methods. PRIMER-E Ltd., Plymouth, UK, 214.
Arana-Magallón, F., Pérez-Rodríguez, R., & Malpica-Sánchez, A. (1998). Cambáridos de tres embalses del estado de Tlaxcala, México (Crustacea: Decapoda). Revista de la Sociedad Mexicana de Historia Natural, 48, 23–35.
Armendáriz, G., Quiroz-Martínez, B., & Álvarez, F. (2016). Risk assessment for the Mexican freshwater crayfish: the roles of diversity, endemism and conservation status. Aquatic Conservation: Marine and Freshwater Ecosystems, 27, 78–89. https://doi.org/10.1002/aqc.2671
Arredondo-Figueroa, J. L., Vásquez-González, A., Barriga-Sosa, I. A., Carmona-Osalde, C., & Rodríguez-Serna, M. (2010). Effect of density on growth and feeding of the crayfish Cambarellus montezumae (Saussure, 1857). Journal of Applied Aquaculture, 22, 66–73. https://doi.org/10.1080/10454431003597579
Arredondo-Figueroa, J. L., Vásquez-González, A., Núñez-García, L. G., Barriga-Sosa, I. A., & Ponce-Palafox, J. T. (2011). Aspectos reproductivos del acocil Cambarellus (Cambarellus) montezumae (Crustacea: Decapoda: Cambaridae) en condiciones controladas. Revista Mexicana de Biodiversidad, 82, 169–178. https://doi.org/10.1080/10454431003597579
Bovbjerg, R. V. (1956). Some factors affecting aggressive behavior in crayfish. Physiological Zoology, 29, 127–136. https://doi.org/10.1086/physzool.29.2.30152201
Brusconi, S., Bertocchi, S., Renai, B., Scalici, M., Souty-Grosset, C., & Gherardi, F. (2008). Conserving indigenous crayfish: stock assessment and habitat requirements in the threatened Austropotamobius italicus. Aquatic Conservation, 18, 1227–1239. https://doi.org/10.1002/aqc.935
Butler, G. L., & Rowland, S. J. (2008). Using traditional age and growth techniques in endangered species management: eastern freshwater cod, Maccullo chellaikei. Marine & Freshwater Research, 59, 684–693. https://doi.org/10.1071/MF07188
Capana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology, 59, 197–242. https://doi.org/10.1111/j.1095-8649.2001.tb00127.x
Carmona-Osalde, C., Rodríguez-Serna, M., Olvera-Novoa, M. A., & Gutiérrez-Yurrita, P. J. (2004). Gonadal development, spawning, growth and survival of the crayfish Procambarus llamasi at three different water temperatures. Aquaculture, 232, 305–316. https://doi.org/10.1016/S0044-8486(03)00527-1
Carson, E. W., Pedraza-Lara, C., Lozano-Vilano, M. L., Rodríguez-Almaráz, G., Banda-Villanueva, I., Sepúlveda-Hernández, L. A., Vela-Valladares, L., Cantú-Garza, A., & De la Maza-Benignos, M. (2015). The rediscovery and precarious status of Chihuahuan Dwarf crayfish Cambarellus chihuahuae. Occasional Papers of the Museum of Southwestern Biology, 12, 1–7.
Chiesa, S., Celauro, D., Scalici, M., Monaco, A., Scalisi, M., & Gibertini, G. (2008). II gambero rosso della Louisiana Procambarus clarkii nella Riserva Naturale Regionale “Lago di Posta Fibreno”: problematiche di gestione e priorità di azione per il futuro. Atti della prima giornata di studio “Tutela e conservazione dell’ecosistema acquatico Lago di Posta Fibreno area SIC/ZPS IT6050015”, Università degli Studi “La Sapienza”. Orto Botanico, 100–118.
Chiesa, S., Scalici, M., & Gibertini, G. (2006). Occurrence of allochthonous freshwater crayfishes in Latium (Central Italy). Knowledge & Management of Aquatic Ecosystems, 380–381, 883–902. https://doi.org/10.1051/kmae:2006029
Chucholl, C. (2012). Understanding invasion success: life-history traits and feeding habits of the alien crayfish Orconectes immunis (Decapoda, Astacida, Cambaridae). Knowledge & Management of Aquatic Ecosystems, 404, 04. https://doi.org/10.1051/kmae/2011082
Clarke, K. R., & Gorley, R. N. (2006). PRIMER v6: User Manual/Tutorial. PRIMER E, Plymouth 866.
Crandall, K. A., & Buhay, J. E. (2008). Global diversity of crayfish (Astacidae, Cambaridae, and Parastacidae-Decapoda) in freshwater. Hydrobiologia, 595, 295–301. https://doi.org/10.1007/s10750-007-9120-3
Crisp, D. J. (1984). Energy flow measurements. In N. A. Holme, & A. D. McIntyre (Eds.), Methods for the study of marine benthos (pp. 284–372). Londres: Blackwell.
Cuevas, C., & Íñiguez-Dávalos, L. I. (2017). Birds of Puerto Interior Turístico Jocotepec in Lake Chapala, Jalisco, México. Huitzil Revista Mexicana de Ornitología, 18, 261–271. https://doi.org/10.28947/hrmo.2017.18.2.295
Davis, C. A., & Smith, L. M. (2001). Foraging strategies and niche dynamics of coexisting shore birds at stopover sites in the southern Great Plains. The Auk, 118, 484–495. https://doi.org/10.1093/auk/118.2.484
Decree 3. 2013. Diario Oficial del Estado de Jalisco. Sábado 18 de Mayo de 2013. Vol. CCCLXXVI Section IV, 6, 3–147.
DiStefano, R. J., Black, T. R., Herleth-King, S. S., Kanno, Y., & Mattingly, H. T. (2013). Life histories of two populations of the imperiled crayfish Orconectes (Procericambarus) williamsi (Decapoda: Cambaridae) in southwestern Missouri, USA. Journal of Crustacean Biology, 33, 15–24. https://doi.org/10.1163/1937240X-00002109
Dorn, N. J., Cook, M. I., Herring, G., Boyle, R. A., Nelson, J., & Gawlik D. E. (2011). Aquatic prey switching and urban foraging by the White Ibis Eudocimus albus are determined by wetland hydrological conditions. Ibis, 153, 323–335. https://doi.org/10.1111/j.1474-919X.2011.01101.x
Dörr, A. J. M., La Porta, G., Pedicillo, G., & Lorenzoni, M. (2006). Biology of Procambarus clarkii (Girard, 1852) in Lake Trasimeno. Bulletin français de la pêche et de la pisciculture, 380–381, 1155–1168. https://doi.org/10.1051/kmae:2006018
Dörr, A. J. M., & Scalici, M. (2013). Revisiting reproduction and population structure and dynamics of Procambarus clarkii eight years after its introduction into Lake Trasimeno (Central Italy). Knowledge & Management of Aquatic Ecosystems, 408, 10, 1–16. https://doi.org/10.1051/kmae/2013045
Edwards, D. H., Issa, F. A., & Herberholz, J. (2003). The neural basis of dominance hierarchy formation in crayfish. Microscopy Research and Technique, 60, 369–376. https://doi.org/10.1002/jemt.10275
Edwards, D. H., & Kravitz, E. A. (1997). Serotonin, social status and aggression. Current Opinion in Neurobiology, 7, 812–819. https://doi.org/10.1016/S0959-4388(97)80140-7
Fero, K., & Moore, P. A. (2008). Social spacing of crayfish in natural habitats: what role does dominance play? Behavioral Ecology and Sociobiology, 62, 1119–1125. https://doi.org/10.1007/s00265-007-0540-x
Figler, M. H., Finkelstein, J. E., Twum, M., & Peeke, H. V. (1995). Intruding male red swamp crayfish, Procambarus clarkii, immediately dominate members of established communities of smaller, mixed sex conspecifics. Aggressive Behavior, 21, 225–236. https://doi.org/10.1002/1098-2337(1995)21:3<225::AID-AB2480210305>3.0.CO;2-%23
Garvey, J. E., & Stein, R. A. (1993). Evaluating how chela size influences the invasion potential of an introduced crayfish (Orconectes rusticus). The American Midland Naturalist, 129, 172–181.
Ghia, D., Fea, G., Conti, A., Sacchi, R., & Nardi, P. A. (2015). Estimating age composition in alpine native populations of Austropotamobius pallipes complex. Journal of Limnology, 74, 501–511. https://doi.org/10.4081/jlimnol.2015.1139
Griffith, M. B., Wolcott, L. T., & Perry, S. A. (1996). Production of the crayfish Cambarus bartonii (Fabricius, 1798) (Decapoda, Cambaridae) in an acidic Appalachian stream (USA). Crustaceana, 69, 974–984. https://doi.org/10.1163/156854096X00411
Gutiérrez-Yurrita, P. J., & Montes, C. (1999). Bioenergetics and phenology of reproduction of the introduced red swamp crayfish, Procambarus clarkii, in Doñana National Park, Spain, and implications for species management. Freshwater Biology, 42, 561–574. http://dx.doi.org/10.1046/j.1365-2427.1999.00484.x
Gutiérrez-Yurrita, P. J., Morales-Ortíz, A., Oviedo, A., & Ramírez-Pérez, C. (2002). Distribution, spread, habitat characterization and conservation of the crayfish species (Cambaridae) in Querétaro (Central México). Freshwater Crayfish, 13, 288–297.
Hazlett, B. A., Anderson, F. E., Esman, L. A., Stafford, C., & Munro, E. (1992). Interspecific behavioral ecology of the crayfish Orconectes rusticus. Journal of Freshwater Ecology, 7, 69–76. https://doi.org/10.1080/02705060.1992.9664671
Hewitt, D. R., & Duncan, P. F. (2001). Effect of high-water temperature on the survival, moulting and food consumption of Penaeus (Marsupenaeus) japonicus (Bate, 1888). Aquaculture Research, 32, 305–313. https://doi.org/10.1046/j.1365-2109.2001.00560.x
Hobbs, H. H., Jr. (1976). Crawfishes (Astacidae) of North and Middle America. Water Pollution Research Series, Control U.S. Environmental Protection Agency, 1–73.
Hobbs, H. H., Jr. (1981). The crayfishes of Georgia. Smithsonian Contributions to Zoology, 318, 1–549. https://doi.org/10.5479/si.00810282.318
Hobbs, H. H., Jr. (1989). An illustrated checklist of the American crayfishes (Decapoda: Astacidae, Cambaridae and Parastacidae). Smithsonian Contributions to Zoology, 480, 1–236. https://dpi.org/10.5479/si.00810282.480
Hobbs, H. H. (2001). A new cave crayfish of the genus Orconectes, subgenus Orconectes, from southcentral Missouri, USA, with a key to the stygobitic species of the genus (Decapoda, Cambaridae). Crustaceana, 74, 635–646.
Holdich, D. M., & Lowery, R. S. (1988). Freshwater crayfish: biology, management and exploitation. London: Croom Helm.
IUCN, International Union for Conservation of Nature, Red List of Threatened Species. (2022). Version 2021.3. Consulted May 2022. http://www.iucnredlist.org/
Jegla, T. C. (1966). Reproductive and molting cycles in cave crayfish. The Biological Bulletin, 130, 345–358. https://doi.org/10.2307/1539741
Jensen, S. P., Gray, S. J., & Hurst, J. L. (2005). Excluding neighbours from territories: effects of habitat structure and resource distribution. Animal Behaviour, 69, 785–795. https://doi.org/10.1016/j.anbehav.2004.07.008
Kilada, R., Sainte-Marie, B., Rochette, R., Davis, N., Vanier, C., & Campana, S. (2012). Direct determination of age in shrimps, crabs and lobsters. Canadian Journal of Fisheries and Aquatic Sciences, 69, 1728–1733. https://doi.org/10.1139/cjfas-2012-0254
Leland, J. C., Bucher, D. J., & Coughran, J. (2015). Direct age determination of a subtropical freshwater crayfish (Redclaw, Cherax quadricarinatus) using ossicular growth marks. Plos One, 10, e0134966. https://doi.org/10.1371/journal.pone.0134966
Leland, J. C., Coughran, J., & Bucher, D. (2011). A preliminary investigation into the potential value of gastric mills for ageing crustaceans. In A. Asakura & C. Fransen (Eds.), New Frontiers in Crustacean Biology (I–X): Proceedings of the TCS Summer Meeting. (Crustaceana Monographs). Tokyo, Japan: Brill Academic Publishers.
Lowe, M. E. (1956). Dominance-subordinance relationships in the crawfish Cambarellus shufeldtii (Ph. D. Dissertation). Tulane University of Louisiana.
Manjarrez, J., Macías-García, C., & Drummond, H. (2013). Variation in the diet of the Mexican Black-bellied Gartersnake Thamnophis melanogaster: importance of prey availability and snake body size. Journal of Herpetology, 47, 413–420. https://doi.org/10.1670/12-174
Mildenberger, T. K., Taylor, M. H., & Wolff, M. (2017). TropFishR: an R package for fisheries analysis with length-frequency data. Methods in Ecology and Evolution, 8, 1520–1527. https://doi.org/10.1111/2041-210X.12791
Momot, W. T., & Gowing, H. (1977). Results of an experimental fishery on the crayfish Orconectes virilis. Journal of the Fisheries Board Canada, 34, 2041–2055. https://doi.org/10.1139/f77-273
Momot, W. T., & Romaire, R. P. (1981). Use of a seine to detect stunted crawfish populations in ponds, a preliminary report 1. Journal of the World Aquaculture Society, 12, 384–390. https://doi.org/10.1111/j.1749-7345.1981.tb00310.x
Newell, R. C., & Branch, G. M. (1980). The influence of temperature on the maintenance of metabolic energy balance in marine invertebrates. Advances in Marine Biology, 17, 329–396. https://doi.org/10.1016/S0065-2881(08)60304-1
Paglianti, A., & Gherardi, F. (2004). Combined effects of temperature and diet on growth and survival of young-of-year crayfish: a comparison between indigenous and invasive species. Journal Crustacean of Biology, 24, 140–148. https://doi.org/10.1651/C-2374
Pauly, D. (1979). Gill size and temperature as governing factors in fish growth: a generalization of von Bertalanffy’s growth formula. Institut für Meereskunde, Kiel, Germany, 6, 1–156. https://doi.org/10.3289/ifm_ber_63
Pauly, D. (1983). Length-converted catch curves. A powerful tool for fisheries research in the tropics (part 1). Fishbyte, The World Fish Center, 1, 9–13.
Pedraza-Lara, C., & Doadrio, I. (2015). A new species of dwarf crayfish (Decapoda: Cambaridae) from central Mexico, as supported by morphological and genetic evidence. Zootaxa, 3963, 583–594. http://dx.doi.org/10.11646/zootaxa.3963.4.5
Pedraza-Lara, C., Doadrio, I., Breinholt, J. W., & Crandall, K. A. (2012). Phylogeny and evolutionary patterns in the dwarf crayfish subfamily (Decapoda: Cambarellinae). Plos One, 7, e48233. https://doi.org/10.1371/journal.pone.0048233
Pedraza-Lara, C., Ortiz-Herrera, H. S., & Jones, R. W. (2021). A new species of crayfish of the genus Cambarellus (Decapoda: Cambaridae) from central Mexico. Revista Mexicana de Biodiversidad, 92, e923150. https://doi.org/10.22201/ib.20078706e.2021.92.3150
Penn, G. H. (1950). The genus Cambarellus in Louisiana (Decapoda, Astacidae). The American Midlland Naturalist, 44, 421–426. https://doi.org/10.2307/2421964
Peterson, M. S., Fitzpatrick, J. F., & VanderKooy, S. J. (1996). Distribution and habitat use by dwarf crayfishes (Decapoda: Cambaridae: Cambarellus). Wetlands, 16, 594–598. https://doi.org/10.1007/BF03161351
Pitcher, T. J., & MacDonald, P. D. M. (1973). Two models for seasonal growth in fishes. Journal of Applied Ecology, 10, 599–606. https://doi.org/10.2307/2402304
Reynolds, J. D. (2002). Growth and reproduction. In D.M. Holdich, (Ed.), Biology of Freshwater Crayfish (pp. 152–191), UK: Blackwell Science.
Rodríguez-Serna, M., & Carmona-Osalde, C. (2002). Balance energético del acocil Cambarellus montezumae (Saussure) (Crustacea: Astacidea: Cambaridae): pérdida de energía en la tasa metabólica. Universidad y Ciencia, 18, 128–134.
Roell, M. J., & Orth, D. J. (1992). Production of three crayfish populations in the New River of West Virginia, USA. Hydrobiologia, 228, 185–194. https://doi.org/10.1007/BF00006585
Rombough, P. J. (1994). Energy partitioning during fish development: additive or compensatory allocation of energy to support growth? Functional Ecology, 8, 178–186. https://doi.org/10.2307/2389901
Sandoval-Moreno, A., & Hernández-García, A. (2017). Transformación del territorio ribereño y la defensa del Lago de Chapala. El Cotidiano, 201, 45–58.
Savolainen, R., Ruohonen, K., & Railo, E. (2004). Effect of stocking density on growth, survival and cheliped injuries of stage 2 juvenile signal crayfish Pacifastacus leniusculus Dana. Aquaculture, 231, 237–248. https://doi.org/10.1016/j.aquaculture.2003.09.045
Scalici, M., Belluscio, A., & Gibertini, G. (2008b). Understanding population structure and dynamics in threatened crayfish. Journal of Zoology, 275, 160–171. https://doi.org/10.1111/j.1469-7998.2008.00422.x
Scalici, M., Chiesa, S., Scuderi, S., Celauro, D., & Gibertini, G. (2010). Population structure and dynamics of Procambarus clarkii (Girard, 1852) in a Mediterranean brackish wetland (Central Italy). Biological Invasions, 12, 1415–1425. https://doi.org/10.1007/s10530-009-9557-6
Scalici, M., & Gherardi, F. (2007). Structure and dynamics of an invasive population of the Red Swamp Crayfish (Procambarus clarkii) in a Mediterranean wetland. Hydrobiologia, 583, 309–319. https://doi.org/10.1007/s10750-007-0615-8
Scalici, M., Macale, D., Schiavone, F., Gherardi, F., & Gibertini, G. (2008a). Effect of urban isolation on the dynamics of river crabs. Fundamental and Applied Limnology, 172, 167–174. https://doi.org/10.1127/1863-9135/2008/0172-0167
Seals, C., Eversole, A. G., Tomasso, J. R., & Petrosky, B. R. (1997). Effects of temperature on feeding activity of the White River Crayfish Procambarus acutus acutus 1. Journal of the World Aquaculture Society, 28, 133–141. https://doi.org/10.1111/j.1749-7345.1997.tb00848.x
Statzner, B., Fievet, E., Champagne, J. Y., Morel, R., & Herouin, E. (2000). Crayfish as geomorphic agents and ecosystem engineers: biological behavior affects sand and gravel erosion in experimental streams. Limnology and Oceanography, 45, 1030–1040. https://doi.org/10.4319/lo.2000.45.5.1030
Stein, R. A. (1976). Sexual dimorphism in crayfish chelae: functional significance linked to reproductive activities. Canadian Journal of Zoology, 54, 220–227. https://doi.org/10.1139/z76-024
ter Braak, C. J. F., & Šmilauer, P. (2002). CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination. Version 4.5. USA, New York.
Thomas, J. R., Masefield, S., Hunt, R., Wood, M. J., Hart, A. G., Hallam, J., Griffiths, S., & Cable, J. (2019). Terrestrial emigration behaviour of two invasive crayfish species. Behavioural Processes, 167, 103917. https://doi.org/10.1016/j.beproc.2019.103917
Vannote, R. L. (1963). Community productivity and energy
flow in an enriched warm water stream (Ph. D. Thesis). Michigan State University, East Lansing.
Villalobos, F. A. (1955). Cambarinos de la fauna mexicana: Crustacea Decapoda (Ph. D. Thesis). Facultad de Ciencias, Universidad Nacional Autónoma de México, México, D.F.
Villalobos, F. A. (1983). Crayfishes of Mexico (Crustacea: Decapoda): [Cambarinos de la fauna mexicana: Crustacea Decapoda]. Smithsonian Institution Libraries, and the National Science Foundation. New Delhi: Amerind Publishing.
Villavicencio-García, R., Ávila-Coria, R., Guerrero-Vázquez, S., Santiago-Pérez, A. L., & Treviño-Garza, E. (2017). Conectividad del hábitat forestal de las áreas protegidas
para el venado cola blanca (Odocoileus virginianus) en el estado de Jalisco, México. Áreas Naturales Protegidas Scripta, 3, 9–31. https://doi.org/10.18242/anpscripta.2017.03.03.02.
0001
Vogt, G. (2007). Ageing and longevity in the Decapoda (Crustacea): a review. Zoologischer Anzeiger, 251, 1–25. https://doi.org/10.1016/j.jcz.2011.05.003
Wendler, F., Biss, R., & Chucholl, C. (2015). Population ecology of endangered white-clawed crayfish (Austropotamo biuspallipes s. str.) in a small rhithral river in Germany. Knowledge Management of Aquatic Ecosystems, 416, 1–24. https://doi.org/10.1051/kmae/2015020