Valentin Mar-Silva a, b, Martina Medina-Nava b, *, Yvonne Herrerías-Diego b, c, Juan P. Ramírez-Herrejón d, Omar Domínguez-Domínguez b, c
a Universidad Michoacana de San Nicolás de Hidalgo, Programa Institucional de Doctorado en Ciencias Biológicas, Francisco J. Mujica s/n, Ciudad Universitaria, 58030 Morelia, Michoacán, Mexico
b Universidad Michoacana de San Nicolás de Hidalgo, Facultad de Biología, Francisco J. Mujica s/n, Ciudad Universitaria, 58030 Morelia, Michoacán, Mexico
c Universidad Nacional Autónoma de México, Escuela Nacional de Estudios Superiores Unidad Morelia, Laboratorio Nacional de Análisis y Síntesis Ecológica para la Conservación de Recursos Genéticos, Antigua Carretera a Pátzcuaro Núm. 8701, Ex Hacienda de San José de la Huerta, 58190 Morelia, Michoacán, Mexico
d Universidad Autónoma de Querétaro, Cátedra Conacyt-Facultad de Ciencias Naturales, Campus UAQ-Aeropuerto, Carretera a Chichimequillas s/n, Ejido Bolaños, 76140 Santiago de Querétaro, Querétaro, Mexico
*Corresponding author: mnava0424@gmail.com (M. Medina-Nava)
Received: 26 May 2020; accepted: 19 October 2020
Abstract
The Teuchitlán River in Mexico is a hotspot of fish diversity, with 3 endemic species. Pseudoxiphophorus bimaculatus has been introduced into the river, but its trophic impact on the system is unknown. We determined the importance of each food item in the diet of P. bimaculatus with a relative importance index, their feeding behavior using an omnivorous index, the trophic position with the TrophLab program, and the niche breadth using the standardized Levin index. We performed Permanova analyses to compare diet between size classes, sites, and seasons. We analyzed 631 P. bimaculatus individuals. The species consumed mainly terrestrial insects, but presented an herbivorous trend in some sites. A generalist trophic behavior was presented in the wet season and a specialist behavior in the dry season occupying different trophic levels and presenting variable trophic width. This flexible feeding strategy enables P. bimaculatus to exploit resources from different trophic levels. The high consumption (%RII > 50) of terrestrial insects could indicate that P. bimaculatus may transport allochthonous energy into the river. Furthermore, the high invasive potential of the species represents a risk for the freshwater ecosystems of central Mexico, a region that has been recognized as a hotspot for freshwater fish conservation.
Keywords: Biodiversity; Exotic ichthyofauna; Food habits; Human disturbance
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Biología trófica del guatapote manchado, Pseudoxiphophorus bimaculatus, un pez invasor en el río Teuchitlán, centro de México
Resumen
El río Teuchitlán se considera un hotspot de diversidad de peces con 3 especies endémicas. Pseudoxiphophorus bimaculatus fue introducido en el sistema fluvial, aunque se desconoce su impacto trófico en el río Teuchitlán. Se determinó la importancia de cada artículo alimenticio con el índice de importancia relativa, el comportamiento trófico usando el índice de omnívoria, la posición trófica usando el programa TrophLab y la amplitud de nicho trófico de la especie usando el índice estandarizado de Levin. Realizamos análisis de Permanova para comparar la dieta entre clases de talla, sitios y estaciones. Analizamos 631 contenidos estomacales de P. bimaculatus. La especie consume principalmente insectos terrestres, pero con tendencia a la herbivoría en algunos sitios, se presentó un comportamiento trófico generalista en la estación húmeda y especialista en la estación seca, ocupando diferentes niveles tróficos y mostrando amplitud de nicho trófico variable. Esta estrategia de alimentación flexible permite a P. bimaculatus explotar recursos de diferentes niveles tróficos. El alto consumo (%RII > 50) de insectos terrestres podría indicar que P. bimaculatus puede transportar energía alóctona al río. Además, el alto potencial invasivo de la especie es un grave riesgo para los ecosistemas de agua dulce del centro de México, una región que ha sido reconocida como un hotspot muy importante para la conservación de peces de agua dulce.
Palabras clave: Biodiversidad; Ictiofauna exótica; Hábitos alimentarios; Disturbios humanos
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Poeciliids have been widely introduced in multiple aquatic ecosystems through their popularity as ornamental species in addition to their use in biological control programs against mosquitoes (García-Vásquez et al., 2017; Pyke, 2008). For these reasons, they are considered the most abundant and widely distributed exotic freshwater fish. Additionally, Poeciliids are successful invaders, an attribute mainly credited to their high fecundity, tolerance of habitat degradation, and diet flexibility (Magurran, 2009; Pyke, 2008).
The twospot livebearer Pseudoxiphophorus bimaculatus (Heckel, 1848) is a widespread poeciliid fish species with a natural distribution ranging from the Misantla River (State of Veracruz) in Mexico to the Prianzapolka River in Nicaragua, on the Atlantic slope (Miller et al., 2009). The trophic biology of the species in its native range indicates that it is a fish with herbivorous feeding behavior (Miller et al., 2009; Vega-Cendejas et al., 1997), but with a tendency towards carnivorous-insectivorous feeding behavior outside its natural distribution exhibiting a predominance of aquatic insect larvae in its gut contents (Mercado-Silva et al., 2002; Trujillo-Jiménez & Toledo-Beto, 2007). The twospot livebearer has been translocated into several basins of Central Mexico, such as those of the Balsas River and the Lerma-Chapala River, and has recently been reported in the Teuchitlán River, a hot spot of fish diversity in the headwaters of the Ameca River (Domínguez-Domínguez et al., 2008; Mejía-Mojica et al., 2012; Ramírez-Herrejón et al., 2012; Ramírez-García et al., 2017).
The Teuchitlán River is a highly anthropized lotic system that has undergone more than 60 years of human intervention (De la Mora-Orozco et al., 2014). The anthropogenic disturbances include modifications of the riverbanks, replacing them with concrete, uncontrolled domestic wastewater discharges, changes in the river bottom substrate, water-diversion for irrigation and livestock production, and the construction of a dam for the la Vega reservoir, disrupting the continuity to the Ameca River (Webb & Miller, 1998). In the Teuchitlán River, there is an historic fish richness of 15 native and 6 exotic species (López-López & Paulo-Maya, 2001). The butterfly goodeid Ameca splendens, and 3 microendemic fishes are species documented as present in the site and the only ones that currently survive in this area: the Ameca shiner Notropis amecae and golden skiffia Skiffia francesae, both of which are now extinct in the wild, and the tequila splitfin Zoogoneticus tequila, which was reintroduced to the area and brought back to the wild in 2018 (De la Vega-Salazar et al., 2006; Domínguez-Domínguez et al., 2018; IUCN, 2020; López-López & Paulo Maya, 2001; Webb & Miller, 1998). Nowadays, only 5 native and 6 exotic species are found in the Teuchiltán River (Herrerías-Diego et al., 2019).
A key factor proposed in the local extinction of native fish in the Teuchitlán River is the competition for food resources, particularly related to a high abundance of the introduced Poeciliids (Kingston, 1978; Webb & Miller, 1998). The introduction of Poeciliids is among the most harmful threats to native freshwater fishes in the Teuchitlán River and the recently introduced P. bimaculatus may be no exception, since it has been reported to be a successful competitor against native Goodeidae fishes in other geographic regions (Magurran, 2009; Ramírez-Carrillo & Macías-García, 2014). However, there are no data regarding the trophic biology of P. bimaculatus in the site and, consequently, the impact of the species on the Teuchitlán River food web and native species remains poorly understood.
The present study therefore characterizes the trophic biology of the invasive P. bimaculatus in the Teuchitlán River, in order to: 1) determine its trophic guild, diet breadth, omnivory, trophic level, and trophic strategy, and 2) analyze its temporal, spatial, and ontogenetic variation in a highly anthropized lotic system. We also discuss the possible role of P. bimaculatus in the transport of allochthonous energy into the Teuchitlán River system as well as its invasive potential.
Materials and methods
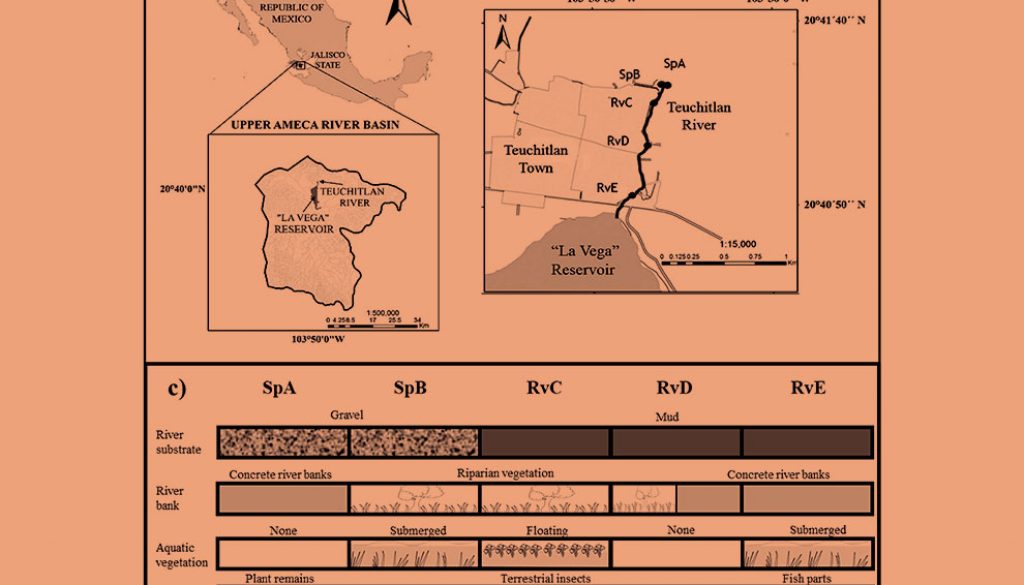
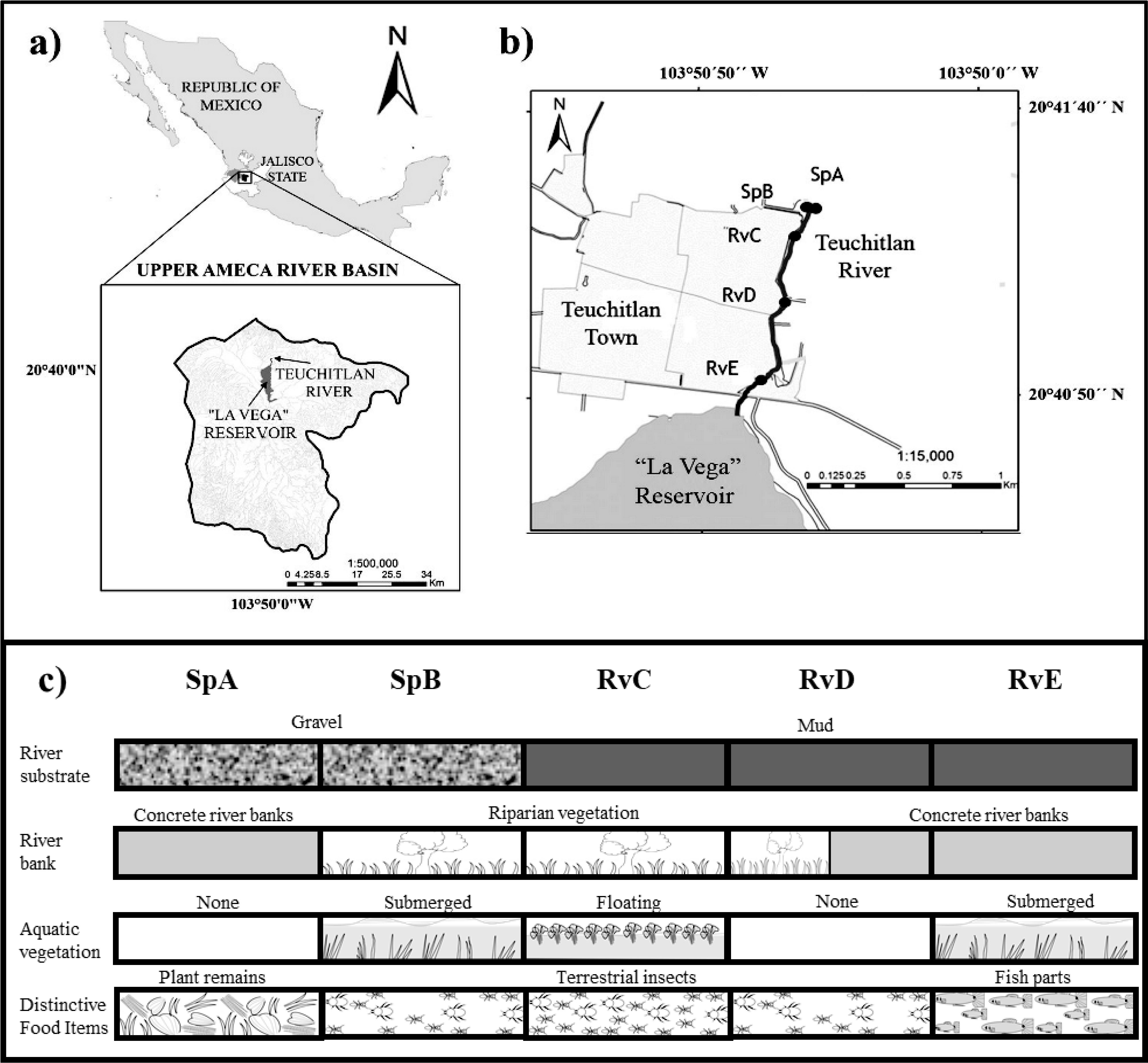
The Teuchitlán River is located in west-central Mexico, at the headwaters of the Ameca River basin (20°41’-20°40’ N, 103°51’-103°50’ W) (Fig. 1a). The lotic system has a length of 1.5 km from the origin to its termination in “La Vega” dam (López-López et al., 2004). To reflect different human impacts along the river, 5 different points were chosen as collection sites (Ramírez-García et al., 2017): “Rincón Spring” SpA, “Abrevadero Spring” SpB, “Upper part of river” RvC, “Middle part of river” RvD, and “End of the river” RvE (Fig. 1b, c).
We collected samples during the day (10:00-16:00 hrs), twice per month over 1 annual cycle (January 2016 to January 2017), using a seine net (4.5 m in length, 2.3 m in height, and mesh size 1.35 mm) and electrofishing equipment (backpack DC electrofishing model ABP-3, ETS Electrofishing Systems LLC). We determined 2 seasons according to weather variations (Jiménez-Román, 1994): dry (January to June 2016) and wet (July to November 2016).
The captured fish were sacrificed by overdosing with the anesthetic tricaine mesylate (MS-222) according to the Official Mexican Norms NOM-051-ZOO-1995 and NOM-033-SAG/ZOO-2014, then labeled and fixed in 10% formaldehyde and transferred to 70% alcohol, following the criteria of Fournie et al. (2000).
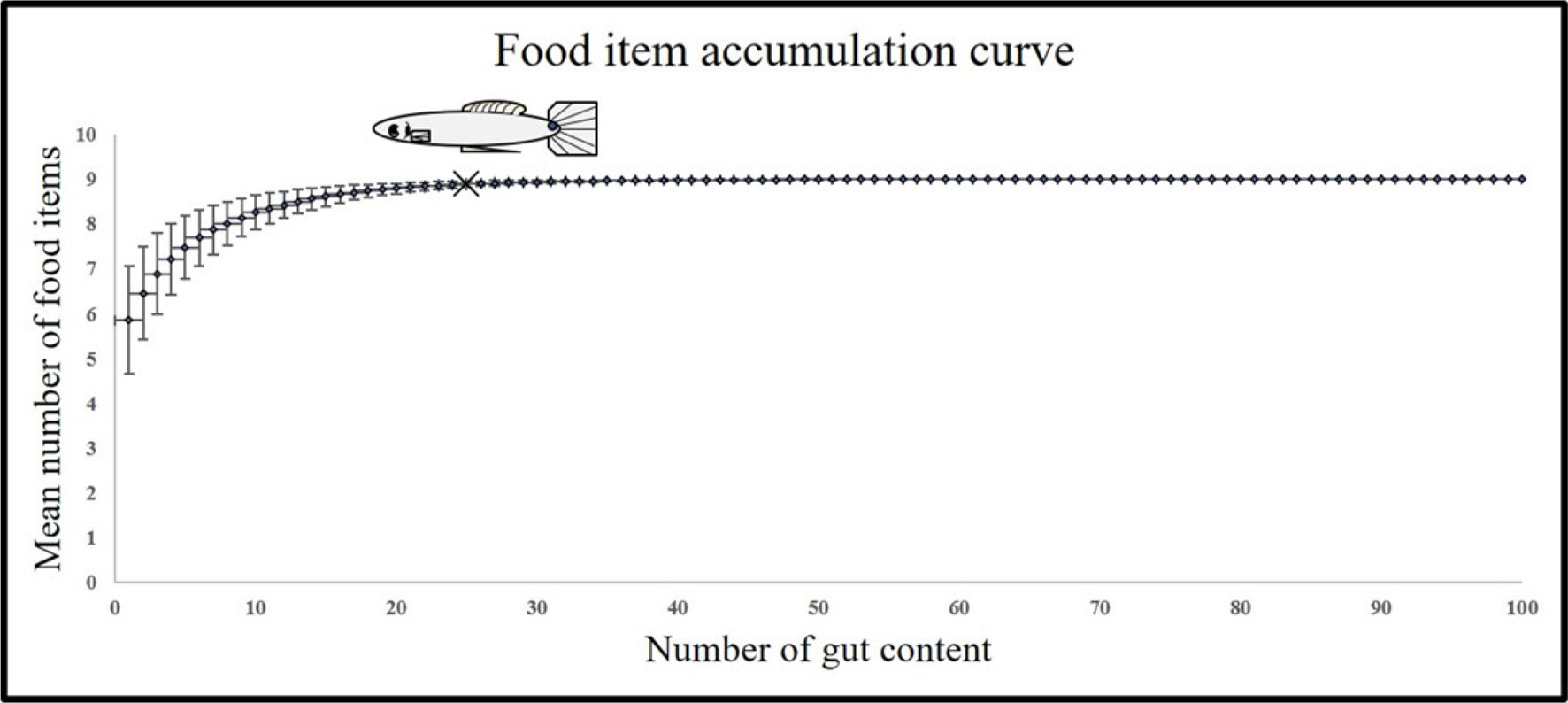
We used an exploratory analysis of prey accumulation using the Mao Tau index to determine the minimum number of individuals required to characterize the feeding habits of P. bimaculatus (Colwell et al., 2004; Appendix). We selected a minimum sample of 25 individuals and a maximum of 36 per size class, site, and season since the curve became asymptotic at 25 individual gut contents analyzed. We determined 2 size classes using a preliminary analysis of Sturges rule to obtain size class, and we used a principal component analysis on diet data and obtained the principal preys. The ANOVA test was performed on the principal preys to test differences among Sturges size classes, and we could only detect differences among 2 size classes, which correspond to size at first maturity for the species in the Teuchitlán River (Ramírez-García et al., 2017): juvenile fish (0-33.41 mm) and adult fish (> 33.42).
We obtained the weight (g) and standard length (mm) and removed the digestive tract of each fish. We measured the length of the intestine (mm) and the weight of the gut contents (mg). We performed the gut content analysis using the stomach content of P. bimaculatus because Trujillo-Jiménez and Toledo-Beto (2007) described the presence of a well-defined stomach in P. bimaculatus, as a saclike expansion of the digestive tube between the esophagus and the short intestine, which represented the first third portion of the intestine length. The gastric repletion was determined by following Borghetti et al. (1994). We used fish with a gastric repletion ≥ 50% in the trophic analysis. We evaluated the gut contents with a modification of the quadrant method (Hynes, 1950). We identified the components of the gut contents to the lowest taxonomic level possible, using the keys of Merrit and Cummins (1996) for insects, and those of Pennak (1978), and Thorp and Covich (2001) for zooplankton and other invertebrates. The insect parts for which identification was impossible due to high digestive degradation were catalogued as unidentified insect parts (UIP). We classified all unidentifiable gut content as detritus and this was excluded from the trophic analysis, along with gut samples that only had detritus in the gut content.
We evaluated the contribution of each food item to the diet of P. bimaculatus using a modified version of the relative importance index (RII) (Yáñez-Arancibia et al., 1976): RII = FO*PA/100, where FO is the frequency of occurrence and PA is the percentage of area occupied by a particular prey in the gut content. We calculated PA using a quadriculated microscope slide (1.9 mm × 1.9 mm) and the area was determined using a microscope camera (Amscope MD-35) with the software AmScope 3.7. This method has proved useful for fish with small components in their diet (e.g., microscopic algae, millimetric zooplankton, and small insect larvae) or when the gut content is difficult to separate and quantify (e.g., detritus or plant remains) (Canto-Maza & Vega-Cendejas, 2008; Ramírez-Herrejón et al., 2013; Vega-Cendejas, 1990). The RII was expressed as a percentage (Cortés, 1997).
We used the standardized Levins´ index (BI) to calculate a measure of niche breadth with the formula BA = B-1/n-1, where B = Levins´niche breadth (B = 1/Σpj2) and n = number of possible preys. The BI takes values between 0 and 1; fish are considered specialists when the BI value is lower than 0.60, and generalists when it is higher than 0.60 (Krebs, 1989). We used the omnivore index (OI) to estimate the variation in the trophic levels of the prey consumed by the species (Christensen & Pauly, 1992) with the formula OI = Σ (TLj-TL)2*DCij, where n is the number of groups in the system, TLj is the trophic level of prey j, TL is the average trophic level of the preys, and DCij is the fraction of prey (j) in the average diet of predator (i). Values equal to zero indicate that the species has only preys on 1 trophic level; large OI values indicate a variable trophic position of the species’ preys.
We estimated the trophic level (TL) with the TrophLab program (Pauly et al., 2000) using the equation TROPHi = 1+ΣDC×TROPHj, were DC represents the fraction of the prey j in the diet of species i, and TROPHj is the trophic position of species j. G is the number of species´i preys. We used Horn´s index (Krebs, 1989) to evaluate intraspecific diet overlap using the formula Ro = Σ (Pij+Pik) log (Pij+Pik) – Σpij logPij – Σpik logPik / 2 log2, where Ro represents the Horn´s overlap index among species j and species k; Pij, Pik = proportion of the resource i with respect to the total of resources shared by both species (i = 1, 2, 3…, n). The value of Horn´s index can vary from 0 when feeding resources are not shared, to 1.0 when maximum diet overlap occurs. Values higher than 0.6 are considered to represent a significant overlap due to limited resource availability (Wallace, 1981; Zaret & Rand, 1971).
We used the Costello trophic diagram to graphically determine the importance of components in the diet of the species and to identify the feeding strategy of the species (Costello, 1990). The abundance of the prey in the gut content (%PA) was placed on the axis of the ordinates and the frequency of appearance (%FO) on the axis of the abscissa. Four quadrants were determined, delimited by 50% of area and frequency of appearance. The prey items located in the upper right quadrant were considered as preferential (with a frequency of occurrence and percentage of area > 50%). Meanwhile, we considered accidental prey items those located in the lower left quadrant (with a frequency of occurrence and percentage of area < 50%)
To determine differences in the diet per site, season, and size, we conducted multivariate one-way PERMANOVAs using the prey consumption data (mm2) in PRIMER-E version 7 (Plymouth Marine Laboratory, UK) with the PERMANOVA+ package (Anderson et al., 2008). We used PERMANOVA design to detect overall differences in the diet among site, season, and size, and consequently, we performed an ANOVA analysis on the factors which presented statistically significant differences for PERMANOVA.
We conducted multi-factor analyses of variance using the R Stats Package to assess differences in prey consumption per season, site, and size class (R Core Team, 2013). We performed a posterior analysis to explore the distribution of the residuals and ensure no violation of normality and independence. A post hoc Tukey-Kramer honest significant difference test was used when the ANOVA showed significant differences (Zar, 1999).
Results
We analyzed the gut contents of 631 P. bimaculatus individuals with a standard length ranging between 11.74 and 66.31 mm. From these, 298 were sampled in the wet season and 333 in the dry season. Fifty-six percent (355) of the digestive tracts analyzed had gut repletion of between 75 and 100%. Detritus only in 18%, with a higher number (83) occurring in the wet season, compared to 29 in the dry season. Detritus-only gut content occurred at a high frequency in SpA (27), RvE (25), and RvD (16). The site with the lowest number of detritus-only gut contents was the RvC, with 1 in the dry season and 7 in the wet season.
We classified the diet composition into 10 prey categories (Table 1). Terrestrial insects showed the highest RII value (49.8-98.3) throughout the sites, size classes, and seasons. However, fish parts presented a high RII value (55.91) for adult fishes in the RvE site (Table 2). Diet differed among seasons (PERMANOVA: pseudo-F = 13.3, df = 1, p = 0.004) and sites (PERMANOVA: pseudo-F = 6.2, df = 4, p = 0.005), although not ontogenically (PERMANOVA: pseudo-F = 0.9, df = 1, p = 0.44). We found spatial and temporal differences in the consumption of plant remains (ANOVA: f = 8.5639, p < 0.001, df = 9), fish parts (ANOVA: f = 2.5409, p = 0.0388, df = 9), and terrestrial insects (ANOVA: f = 4.4135, p = 0.0016, df = 9; Fig. 2d-f), temporal differences for plant remains, fish parts, and unidentified insect parts (preferential food item by Costello: Fig. 3), and differences in the ingestion of plant remains and fish parts. In the wet season, the highest consumption of plant remains was found at site SpB, while the highest consumption of fish parts was at site RvE (ANOVA: f = 8.5, p < 0.0001, df = 1; Figs. 2a, b). Terrestrial insects were consumed to a greater extent at sites SpB, RvC, and RvD in both seasons, but the lowest ingestion of this component was found at sites SpA and RvE in the wet season (ANOVA: f = 4.4135, p = 0.0016, df = 9, Fig. 2c).
Niche breadth per size class, season, and site were variable (Bi mean = 0.22±0.18, Bi minimum = 0.007-Bi maximum = 0.66; Table 3). This indicates that the twospot livebearer has a specialist trophic niche breadth, with the lowest value presented in the dry season (Bi mean = 0.14±0.13) and a tendency towards a generalist trophic niche breadth in the wet season with the highest value (Bi mean = 0.31±0.19). The adults of the RvE site in the wet season presented the highest niche breadth value (Bi = 0.66) (Table 3).
Trophic level was variable across class size, site, and season (trophic level minimum = 2.68±0.33-trophic level maximum = 3.78±0.64). The lowest value was found for juveniles of site SpA, while the highest value was found for adults at site RvE, both in the wet season (Table 3).
The omnivorous index was also variable (OImean = 0.42 ± 0.43, OI minimum = 0.004-OI maximum = 1.31, see table 3) and mostly indicated low levels of omnivorous behavior, with the lowest value in the dry season (OImean = 0.16 ± 0.2) compared to the wet season (OImean = 0.68 ± 0.44). The highest values of the omnivorous index were for juveniles (OI = 1.31) and adults (OI = 1.2) of site SpA and juveniles (OI = 1.16) of site RvE in the wet season (Table 3).
Diet overlap between size classes was high (0.97), but the overlap presents spatial and temporal differences. The size classes of site RvE did not overlap in terms of trophic resources, with SpA, SpB, RvC, and RvD across seasons (values from 0 to 0.58). In the wet season, classes I and II of the SpA site did not present diet overlap (0.19 to 0.55). The main trophic resource in the diet overlap was unidentified insect parts (UIP).

The Costello diagrams showed a temporal difference in the feeding strategy of the twospot livebearer (Fig. 3). In the dry season, the preferred prey are terrestrial insects, while the rest of the prey items are accidental (Fig. 3b). In the wet season, no such preferential item was found (Fig. 3a).
Discussion
According to the information presented here, the invasive species P. bimaculatus presents a dynamic trophic strategy and flexible feeding behavior in the Teuchitlán River. The species is mainly a carnivorous-insectivorous fish, mostly consuming terrestrial insects (Table 2); however, we found behavior that was generalist in the wet season and specialist in the dry season, as well as an herbivorous trend in some sites, occupying different trophic levels and variable trophic width (Table 3, Figs. 2, 3). This variable feeding strategy enables P. bimaculatus to exploit resources from different trophic levels, such as plants and algae in producers and other fishes in secondary consumers.
These findings are congruent with the trophic biology data of the species throughout its native and non-native distribution; although Vega-Cendejas et al. (1997) and Miller et al. (2009) state that P. bimaculatus is herbivorous in its native range, Trujillo-Jiménez and Toledo-Beto (2007) found that the twospot livebearer is a carnivorous-insectivorous fish that feeds mainly on terrestrial insects within its non-native distribution. The morphology of structures related to trophic acquisition, such as the teeth, mouth, a well-defined stomach, and short digestive tract, indicates that the twospot livebearer is a carnivorous fish, as has been reported in invaded areas (Trujillo-Jiménez & Toledo-Beto, 2007). These data, far from being contrasting, provide evidence that the twospot livebearer can present flexible feeding habits, as we found for the Teuchitlán River according to trophic width, trophic level, and omnivory, which are similar to the flexible feeding behavior presented by other related species (Ramírez-Herrejon et al., 2013; Schaefer et al., 1994).
We did not find differences in diet among size classes (PERMANOVA pseudo-F = 0.9, df = 1, p = 0.44). Dietary shifts are expected as a function of increased body size and are common in many fish species (Davis et al., 2012; Feyrer et al., 2003; King, 2005). However, the invasive P. bimaculatus in the Teuchitlán River exhibits an ontogenetic overlap in diet, which is indicative of shared food items (Horn´s index = 0.97) (Copp, 1992; McCormick, 1998). This could be explained by the niche overlap hypothesis, which states that a high availability of prey reduces competition and allows coexistence between different species (Pianka, 1974). This theory could explain the ontogenetic diet overlap and coexistences at the same site on the river, since the ontogenetic stages of a species have been proposed as different ecological units (Davis et al., 2012; Stoner & Livingstone, 1984).
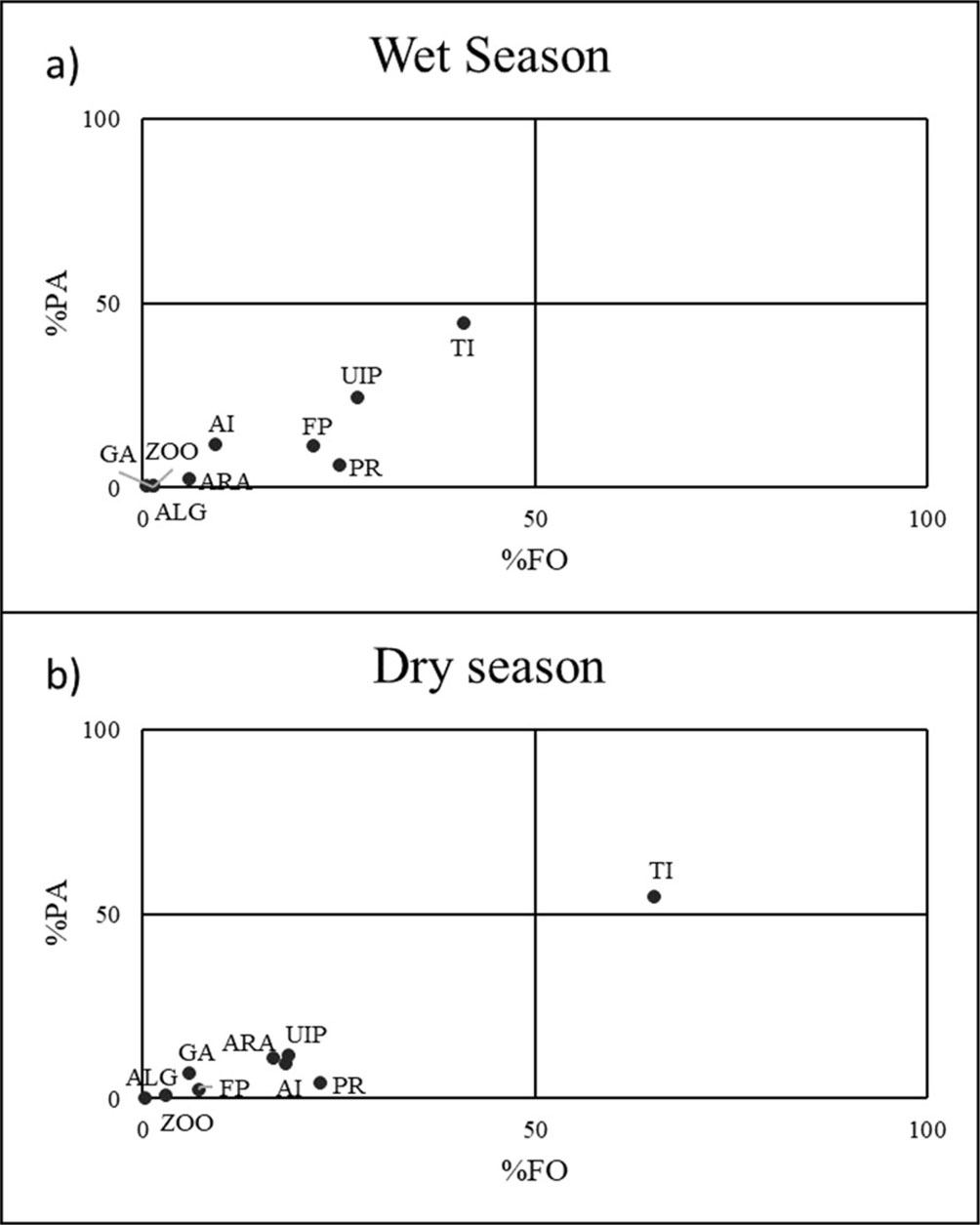
Our results from the relative importance index and the PERMANOVA of diet variation indicate the presence of spatial variation in resource consumption (Table 2). The ANOVA result indicates that consumption of terrestrial insects is statistically high in sites with the presence of riparian vegetation. This vegetation has a major role in the transfer of allochthonous energy to the river system, since it provides habitat for insects that may eventually fall into the water, increasing the availability of this food supply for the fishes (Tabacchi et al., 1998; Wipfli & Baxter, 2010). This trend of P. bimaculatus was observed in the RvC site, where we found the highest ingestion of terrestrial insects (Fig. 2c), which is congruent with the presence of well-established riparian vegetation (Fig. 1).
According to the ANOVA results, the twospot livebearer presented the highest ingestion of plant remains in the SpA. In spring-fed stream systems, the periphyton is fundamental to the web trophic dynamic (Battin et al., 2003), and the deposition of vegetal detritus from the adjacent vegetation, as well as detritus of algal origin, could represent food resources (Garman, 1992; Pound et al., 2011). The sites SpA and SpB are dominated by rocky bottom substrate, and both present concrete banks and high periphyton productivity (pers. obs), which could explain the high rate of algae consumption. In the case of the high rate of plant remains, we suggest that these 2 sites are characterized by a low abundance of macroinvertebrates and zooplankton (Escalante-Jimenez, unpublished data), and SpB is used for watering cattle, which increases the input of vegetal matter via animal defecation. The low abundance of animal prey items and increased input of vegetal material could be causes for the high consumption of primary producers in these sites (Figs. 1c, 2d). In terms of exploiting the available resources, this represents flexibility in P. bimaculatus feeding.
The results of the trophic indices (Table 3), trophic strategy (Fig. 3), and diet overlap (Horn´s index) suggest temporal differences in the diet of P. bimaculatus. The Teuchitlán micro-basin is one of the driest sites in the Ameca basin, and a severe difference in rainfall occurs between the dry and wet season (Jiménez-Román, 1994). We found that a change occurs in the feeding strategy between the wet and dry seasons. During the dry season, terrestrial insects are preferred, while in the wet season the consumption tends to be of primary producers and unidentified insects. The preference for terrestrial insects during the dry season could be the result of the temporal availability of this food. In other streams systems, a high productivity coming from the nearby riparian-terrestrial zone are reported during the summer with a consequent high availability of terrestrial insects (Nakano & Murakami, 2001). However, the consumption of plant remains in the wet season could be a result of the temporal transport of this material through the drainage system into the river via flood events (Wantzen et al., 2008). This is also supported by the increased quantity of unidentified terrestrial insects in the diet, which could also be the result of the transport of dead and partially degraded insects by rainwater (Wantzen & Junk, 2000). Rivers are known to suffer temporal changes due their variations in flood and drought (Hill & Boston, 1991). As a result, their associated biotic communities also vary seasonally and the availability of food for fishes in river systems show high spatial and temporal variation (Angermeier, 1982, 1985; Angradi. 1997; Bae et al., 2016).
Table 1
Food items of Pseudoxhiphophorus bimaculatus in the Teuchitlán River.
|
Label |
Food item |
Identified biological groups |
|
DET |
Detritus |
|
|
PR |
Plant remains |
|
|
ALG |
Algae |
Diatoms, genera: Achnanthes¸ Nitzschia, Terpsinoe |
|
ARA |
Araneae |
Spiders |
|
FP |
Fish parts |
Scales, flesh, and vertebrae |
|
GA |
Gastropoda |
Exotic snails Melanoides tuberculata and Pomacea bridgesii |
|
ZOO |
Zooplankton |
Calanoids copepods, cladocerans, ostracods |
|
UIP |
Unidentified insect parts |
|
|
AI |
Aquatic insects |
Orders: Coleoptera, Diptera, Ephemeroptera, Lepidoptera, Odonata, Trichoptera. Families: Chironomidae, Dytiscidae, Isotomidae. Stratiomyidae, Tipulidae |
|
TI |
Terrestrial insects |
Orders: Diptera, Coleoptera, Hymenoptera, Hemiptera, Thysanoptera. Families: Vespidae, Staphylinidae. Genus: the exotic crazy ant Anoplolepis sp. |
Table 2
Index of relative importance (%RII) of each prey item of Pseudoxhiphophorus bimaculatus per size class and site in the Teuchitlán River. Values in bold show the highest RII.
|
|
|
|
PR |
ALG |
ARA |
FP |
GA |
ZOO |
UIP |
AI |
TI |
|
SpA |
Wet |
C-1 |
48.79 |
0.51 |
0.52 |
4.98 |
0 |
0.13 |
35.5 |
5.87 |
3.7 |
|
C-2 |
18.51 |
0 |
0 |
17.31 |
0 |
0 |
51.97 |
0.72 |
11.49 |
||
|
Dry |
C-1 |
17.14 |
0.004 |
2.36 |
0.07 |
0 |
0.002 |
0.07 |
1.5 |
78.86 |
|
|
C-2 |
9.67 |
0 |
2.76 |
4.61 |
0 |
0 |
0 |
5.53 |
77.43 |
||
|
SpB |
Wet |
C-1 |
18.34 |
0 |
0 |
3.79 |
0 |
0.006 |
27.21 |
0 |
50.66 |
|
C-2 |
35.67 |
0 |
0 |
0.23 |
0 |
0.23 |
0 |
5.96 |
57.92 |
||
|
Dry |
C-1 |
0.79 |
0 |
0.42 |
0.87 |
0.37 |
0.25 |
0.22 |
0.91 |
96.15 |
|
|
C-2 |
0.16 |
0 |
0.14 |
2.98 |
0.46 |
0 |
0 |
1.18 |
95.08 |
||
|
RvC |
Wet |
C-1 |
0 |
0 |
0.83 |
0 |
0.03 |
0 |
13.35 |
3.77 |
82.02 |
|
C-2 |
0.01 |
0 |
0.56 |
3.86 |
0 |
0 |
7.99 |
8.46 |
79.11 |
||
|
Dry |
C-1 |
0.31 |
0 |
2.92 |
0 |
0.07 |
0 |
0 |
30.43 |
66.27 |
|
|
C-2 |
0 |
0 |
0.07 |
0 |
0 |
0 |
0 |
25.34 |
74.59 |
||
|
RvD |
Wet |
C-1 |
0.4 |
0 |
0 |
19.95 |
0.47 |
0 |
29.38 |
0 |
49.8 |
|
C-2 |
0 |
0 |
1.93 |
0.9 |
0 |
0 |
7.26 |
0 |
89.91 |
||
|
Dry |
C-1 |
3.62 |
0 |
3.71 |
0 |
1.89 |
0.02 |
5.79 |
1.98 |
82.98 |
|
|
C-2 |
6.87 |
0 |
0.15 |
1.06 |
5.87 |
0 |
16.88 |
0.009 |
69.15 |
||
|
RvE |
Wet |
C-1 |
1.18 |
0 |
0.35 |
35.81 |
0 |
0 |
61.75 |
0 |
0.9 |
|
C-2 |
12.12 |
0 |
0 |
55.91 |
0 |
0 |
31.98 |
0 |
0 |
||
|
Dry |
C-1 |
1.7 |
0 |
34.48 |
0.22 |
0.1 |
0.32 |
28.47 |
9.7 |
25 |
|
|
C-2 |
0.02 |
0 |
0.79 |
0.1 |
0 |
0 |
0.07 |
0.72 |
98.3 |
PR = Plant remains, ALG = algae, ARA = Araneae, FP = fish parts, GA = Gastropoda, ZOO = zooplankton, UIP = unidentified insect parts, AI = aquatic insects, TI = terrestrial insects.
Table 3
Niche breadth, omnivore index, and trophic level of Pseudoxhiphophorus bimaculatus by size class and sites in the Teuchitlán River. Values in bold are the highest values.
|
|
Wet |
|
Dry |
|
|
SpA |
||||
|
P. bi 1 |
P. bi 2 |
P. bi 1 |
P. bi 2 |
|
|
Diet breadth |
0.24 |
0.47 |
0.08 |
0.16 |
|
Omnivory index |
1.31 |
1.20 |
0.24 |
0.14 |
|
Trophic position |
2.68±0.33 |
3.2±0.46 |
2.98±0.36 |
3.15±0.41 |
|
SpB |
||||
|
P. bi 1 |
P. bi 2 |
P. bi 1 |
P. bi 2 |
|
|
Diet breadth |
0.43 |
0.29 |
0.01 |
0.02 |
|
Omnivory index |
0.74 |
0.49 |
0.02 |
0.007 |
|
Trophic position |
3.03±0.39 |
2.83±0.35 |
3.19±0.4 |
3.24±0.42 |
|
RvC |
||||
|
P. bi 1 |
P. bi 2 |
P. bi 1 |
P. bi 2 |
|
|
Diet breadth |
0.11 |
0.11 |
0.22 |
0.31 |
|
Omnivory index |
0.24 |
0.15 |
0.02 |
0.01 |
|
Trophic position |
3.2±0.4 |
3.25±0.42 |
3.2±0.4 |
3.2±0.4 |
|
RvD |
||||
|
P. bi 1 |
P. bi 2 |
P. bi 1 |
P. bi 2 |
|
|
Diet breadth |
0.42 |
0.08 |
0.07 |
0.16 |
|
Omnivory index |
0.55 |
0.13 |
0.16 |
0.40 |
|
Trophic position |
3.45±0.5 |
3.2±0.4 |
3.17±0.4 |
3.15±0.41 |
|
RvE |
||||
|
P. bi 1 |
P. bi 2 |
P. bi 1 |
P. bi 2 |
|
|
Diet breadth |
0.24 |
0.66 |
0.38 |
0.007 |
|
Omnivory index |
1.16 |
0.80 |
0.58 |
0.004 |
|
Trophic position |
3.64±0.57 |
3.78±0.64 |
3.18±0.4 |
3.2±0.4 |
P. bi 1 = P. bimaculatus juvenile fish (0-33.41 mm), P. bi 2 = P. bimaculatus adult fish (> 33.42).
Temporal shifts in diet have been reported for other introduced species in different freshwater systems as a response to environmental differences in the availability of food resources (Maitipe & De Silva, 1985). Our results regarding the temporal switch in feeding strategy are consistent with other fish species and for other vertebrates (Fig. 3), where the temporal shift is reported as a function of an increased abundance of food items in the wet season (Rayner et al., 2009; Wilson, 1971; Zaret & Rand, 1971). Thus, the temporal change of P. bimaculatus diet may reflect the seasonal variation of food resources; however, the abundance of food resources was not measured in the present study. Further studies should focus on the seasonal change of food resources in order to explore the effect of resource variability on the diet of P. bimaculatus.
We used a multivariate analysis (PERMANOVA) to test overall diet differences among the sites, seasons, and ontogenetic factors, but to evaluate specific differences in the use of particular resources we used multi-factor analysis (ANOVA). The PERMANOVA analysis enables us to demonstrate a spatial and temporal variation in the diet of P. bimaculatus in Teuchitlán River, but multi-factor ANOVA permits the detection of specific variation in plants remains, fish parts, and terrestrial insects among sites/seasons. The use of both ANOVA and PERMANOVA is useful to better elucidate the effects of biological invasions (Gioria & Osborne, 2009), and therefore our study shows the utility of both PERMANOVA and ANOVA in trophic studies with a community approach to understand complex biological information.
The results of the relative importance index indicate a high consumption (%RII > 50) of terrestrial insects, which was the main food item in the diet of P. bimaculatus. This could indicate that P. bimaculatus may function as a vehicle of allochthonous energy into the Teuchitlán River system. Terrestrial insects are abundant items in freshwater stream habitats with different levels of human perturbation and, as explained above, riparian vegetation seems to be key in the transfer of terrestrial insects to water, making them available for fish ingestion (Carbajal-Becerra et al., 2020; Nakano et al., 1999; Wipfli & Baxter, 2010). In other water bodies, human perturbation can affect the terrestrial-aquatic energy flux, increasing the allochthonous input by terrestrial insects (Vital-Rodríguez et al., 2017). However, study of the energetic flux is necessary to corroborate this terrestrial-aquatic trophic linkage and to evaluate the role of P. bimaculatus in the transfer of energy into the aquatic system.
A key factor proposed in the local extinction of native fish in the Teuchitlán River is the competition for food resources (Kingston, 1978; Webb & Miller, 1998). However, the main problem for evaluating the potential trophic impact of P. bimaculatus is the lack of specific information about the trophic role of the native species and how the Teuchitlán trophic web is structured. The endemic Ameca splendens is possibly an herbivorous fish based on its possession of a long and convoluted intestine and lack of stomach (Miller & Fitzimon, 1971). Because of that, trophic overlap of P. bimaculatus and A. splendens is expected to be low. However, in laboratory conditions, the interactions of poeciliids and native species have shown a disadvantage of the natives when there is low food availability (Escalera-Vázquez et al., 2016). Meanwhile, the natives Zoogoneticus tequila and Zoogoneticus purhepechus have short intestines, similar to their relative Zoogoneticus quitzoensis, which is a carnivorous fish with preference for aquatic insect preys (Acuña-Lara et al., 2006). Our results showed that P. bimaculatus are carnivorous and thus trophic competition between the native and the invasive carnivores are possible. More detailed study must be done to test this interaction. However, our study provides a baseline for further understanding of the Teuchitlán River trophic web, with the addition of the Teuchitlán River fish species trophic data to our results we could determine the trophic impact of P. bimaculatus in the site.
The poeciliid P. bimaculatus is a species with a native distribution in rivers of the Atlantic slope of Central America but has also been widely introduced by human action into several other drainages (Mejía-Mojica et al., 2012; Miller et al., 2009; Ramírez-García et al., 2017; Ramírez-Herrejón et al., 2012). The introduction of the twospot livebearer in the Teuchitlán River is relatively recent (< 15 years). It was not reported until 1996 (Dzul-Caamal et al., 2012; López-López & Paulo-Maya, 2001). And not recorded during fieldwork conducted in the area in 2008 (ODD per. obs). However, this fish species has become successfully established all along the river and is the dominant species, representing more than 50% of the fish assemblage (Herrerías-Diego et al., 2019). Studies of reproductive biology have shown that the invasive twospot livebearer in Teuchitlán is iteroparous, the dominating sex ratio being female (1.9:1, female:male), and presents early reproduction and high fecundity. This indicates the high effectiveness of the fish in terms of resource exploitation (Gómez-Márquez et al., 2016; Ramírez-García et al., 2017).
The flexible behavior in the trophic strategies of P bimaculatus presented here is indicative of a successful invasive species. Variation in the trophic biology throughout sites has been described as characteristic of an adaptive response of non-native fish species to environmental prey availability (Davis et al., 2012; Jepsen & Winemiller, 2002). Seasonally trophic flexible behavior seems to be a key factor in the abundance-dominance of the species in the Teuchitlán River (Herrerías-Diego et al., 2019), helping the twospot livebearer to tolerate anthropogenic perturbation of the water body, such as processes of habitat modification or eutrophication (Ruehl & DeWitt, 2005). The twospot livebearer is a euryphagous species, which confers an advantage in terms of avoiding seasonal food limitation and has been related to increased abundance of invasive species (Weliange & Amarasinghe, 2003). This life trait of some species of the Poeciliid fish family enables individuals to tolerate fluctuations in prey availability in their environment and has been proposed as a key factor in their invasive success (Arthington, 1991; De Carvalho et al., 2019; Pollux & Reznick, 2011). In summary, the twospot livebearer shows flexibility in its trophic biology; it can occupy different trophic levels, modify its trophic width, change its trophic guild, modify its omnivorous behavior, and utilize allochthonous and autochthonous trophic sources.
These results, as well as other biological traits such as continuous reproduction, a high proportion of female individuals (Ramírez-García et al., 2017), parental care associated with viviparity (Gross & Shine, 1981), and high tolerance to environmental degradation (Mercado-Silva et al., 2002) are consistent with successful invasive species (Sakai et al., 2001), since this success is related to their establishment, spread, and abundance (Hayes & Barry, 2008; Marchetti et al., 2004; Ricciardi, 2013; Ricciardi et al., 2013). Moreover, in the present study we found ontogenetic trophic overlap of P. bimaculatus and, considering the iteroparous reproductive biology of the species in the site (Ramírez-García et al., 2017), a clear generational overlap over time, facilitating the potential for spread and colonization and giving rise to the apparently rapid and successful establishment of P. bimaculatus in the Teuchitlán River (Bateman et al., 2015; Herrerías-Diego et al., 2019).
It is clear that P. bimaculatus should be considered a species with high invasive potential and a serious risk for the freshwater ecosystems of central Mexico, a region that has been recognized as a very important hotspot for freshwater fish conservation (Carbajal-Becerra et al., 2020; Domínguez-Domínguez et al., 2006; Miller, 1986), with endemicity of up to 70% and a dramatic decrease in native fish populations (De la Vega-Salazar, 2006; Domínguez-Domínguez et al., 2008; Lyons et al., 1998). The introduction of this species into water bodies of the area is therefore to be avoided, and more attention must be paid to the stocking process of fish species of commercial value and the release of exotic fishes for mosquito control or ornamental purposes. Educational programs to prevent the introduction of this and other species to areas of importance for the conservation of freshwater diversity must be conducted, with management plans developed and control of established populations carried out.
Acknowledgments
The first author wants to thank the Consejo Nacional de Ciencia y Tecnología (Conacyt) for doctoral fellowship no. 509825. This work was funded by the Chester Zoo, the Mohammed Bin Zayed Species Conservation Fund, the Mexican Commission for the Knowledge and Use of Biodiversity (Conabio), L´association Beauval Nature Pour la Conservation et la Recherche, Haus des Meeres Aqua Terra Zoo, Poecilia Scandinavia, European Union of Aquarium Curators, Wilhelma Zoological-Botanical Garden and Ostrava Zoo. He also thanks the División de Estudios de Posgrado of Facultad de Biología, Universidad Michoacana de San Nicolás de Hidalgo for financial support for the English editing services. Thanks to the members of the Laboratorio de Biología Acuática of the Universidad Michoacana de San Nicolás de Hidalgo (UMSNH); Rubén Hernández Morales, Luis Martín Mar Silva, Oscar Gabriel Ávila Morales, Arely Ramírez García, and Pedro de Jesús Martínez Morales for assistance with the field work.
References
Acuña-Lara, J. O., Medina-Nava, M., & Zubieta-Rojas, T. (2006). Hábitos alimentarios de dos especies de peces vivíparos de la Mintzita. Cuenca Lerma-Chapala Michoacán, México. Biológicas, 8, 47–60.
Anderson, M. J., Gorley, R. N., & Clarke, K. R. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. Plymouth, United Kingdom: PRIMER-E Ltd.
Angermeier, P. L. (1982). Resource seasonality and fish diets in an Illinois stream. Environmental Biology of Fishes, 7, 251–264. https://doi.org/10.1007/BF00002500
Angermeier, P. L. (1985). Spatio-temporal patterns of foraging success for fishes in an Illinois stream. The American Midland Naturalist, 114, 342–359. https://doi.org/10.2307/2425609
Angradi, T. R. (1997). Hydrologic context and macroinvertebrate community response to floods in an Appalachian headwater stream. The American Midland Naturalist, 138, 371–386. https://doi.org/10.2307/2426829
Arthington, A. H. (1991). Ecological and genetic impacts of introduced and translocated freshwater fishes in Australia. Canadian Journal of Fisheries and Aquatic Sciences, 48, 33–43. https://doi.org/10.1139/f91-302
Bae, M. J., Chun, J. H., Chon, T. S., & Park, Y. S. (2016). Spatio-temporal variability in benthic macroinvertebrate communities in headwater streams in South Korea. Water, 8, 99. https://doi.org/10.3390/w8030099
Bateman, A. W., Neubert, M. G., Krkošek, M., & Lewis, M. A. (2015). Generational spreading speed and the dynamics of population range expansion. The American Naturalist, 186, 362–375. https://doi.org/10.1086/682276
Battin, T. J., Kaplan, L. A., Newbold, J. D., & Hansen C. M. E. (2003). Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature, 426, 439–442. https://doi.org/10.1038/nature02152
Borghetti, J. R., Nogueira, V. S. G., Borghetti, N. R. B., & Canzi, C. (1994). The fish ladder at the Itaipu Binational hydroelectric complex on the Paraná River, Brazil. Regulated Rivers: Research & Management, 9, 127–130. https://doi.org/10.1002/rrr.3450090206
Canto-Maza, W. G., & Vega-Cendejas, M. E. (2008). Hábitos alimenticios del pez Lagodon rhomboides (Perciformes: Sparidae) en la laguna costera de Chelem, Yucatán, México. Revista de Biología Tropical, 56, 1837–1846.
Carbajal-Becerra, O., Olvera-Rodríguez, K. J., Mariscal de Souza, G., Durán-Rodríguez, O. Y., Ramírez-García, A., & Ramírez-Herrejón, J.P. (2020). Trophic strategies of the invasive Twospot livebearer (Pseudoxiphophorus bimaculatus, Teleostei: Poeciliidae) in a gradient of environmental quality in central Mexico. Neotropical Ichthyology, 18, e190080. https://doi.org/10.1590/1982-0224-2019-0080
Christensen, V., & Pauly, D. (1992). ECOPATH II—a software for balancing steady-state ecosystem models and calculating network characteristics. Ecological modelling, 61, 169–185. https://doi.org/10.1016/0304-3800(92)90016-8
Colwell, R. K., Mao, C. X., & Chang, J. (2004). Interpolating, extrapolating, and comparing incidence–based species accumulation curves. Ecology, 85, 2717–2727. https://doi.org/10.1890/03-0557
Copp, G. H. (1992). Comparative microhabitat use of cyprinid larvae and juveniles in a lotic floodplain channel. In W. Wieser, F. Schiemer, A. Goldschmidt, & K. Kotrschal (Eds.), Environmental biology of European cyprinids (pp. 181–194). Dordrecht, Netherlands: Springer Science + Business Media.
Cortés, E. (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences, 54, 726–738. https://doi.org/10.1139/f96-316
Costello, M. J. (1990). Predator feeding strategy and prey importance: a new graphical analysis. Journal of Fish Biology, 36, 261–263. https://doi.org/10.1111/j.1095-8649.1990.tb056
01.x
Davis, A. M., Blanchette, M. L., Pusey, B. J., Jardine, T. D., & Pearson, R. G. (2012). Gut content and stable isotope analyses provide complementary understanding of ontogenetic dietary shifts and trophic relationships among fishes in a tropical river. Freshwater Biology, 57, 2156–2172. https://doi.org/10.1111/j.1365-2427.2012.02858.x
De Carvalho, D. R., Flecker, A. S., Alves, C. B. M., Sparks, J. P., & Pompeu, P. S. (2019). Trophic responses to aquatic pollution of native and exotic livebearer fishes. Science of the Total Environment, 681, 503–515. https://doi.org/10.1016/j.scitotenv.2019.05.092
De La Mora-Orozco, C., Flores-López, H. E., Ruiz-Corral, A. J., Chávez-Durán, A. A., & Figueroa-Montaño, A. (2014). Impacto del cambio climático en las tendencias de la evaporación en la presa La Vega, Teuchitlán, Jalisco, México. Revista Mexicana de Ciencias Agrícolas, 10, 1993–2005.
De La Vega-Salazar. M. Y. (2006). Conservation status of Goodeidae familiy fishes (Cyprinodontiformes) from the Mexican Central Plateau. Revista de Biología Tropical, 54, 163–177.
Domínguez-Domínguez, O., Doadrio, I., & Pérez-Ponce de León, G. (2006). Historical biogeography of some river basins in central Mexico evidenced by their goodeine freshwater fishes: a preliminary hypothesis using secondary Brooks parsimony analysis. Journal of Biogeography, 33, 1437–1447. https://doi.org/10.1111/j.1365-2699.2006.01526.x
Domínguez-Domínguez, O., Zambrano, L., Escalera-Vázquez, L. H., Pérez-Rodríguez, R., & Pérez-Ponce de León, G. (2008). Cambio en la distribución de goodeidos (Osteichthyes: Cyprinodontiformes: Goodeidae) en cuencas hidrológicas del centro de México. Revista Mexicana de Biodiversidad, 79, 501–512. http://dx.doi.org/10.22201/ib.20078706e.2008.002.551
Domínguez-Domínguez, O., Morales-Hernández, R., Medina-Nava, M., Herrerías-Diego, Tafolla-Venegas, D., Escalante-Jiménez, A. L. et al. (2018). Progress in the reintroduction program of the tequila splitfin in the springs of Teuchitlán, Jalisco, Mexico. In P. S. Soorae (Ed.), Global reintroduction perspectives: 2018. Case studies from around the globe (pp. 38–42). UAE: IUCN/SSC Reintroduction Specialist Group & Environment Agency-Abu Dhabi.
Dzul-Caamal, R., Olivares-Rubio, H. F., Medina-Segura, C. G., & Vega-López, A. (2012). Endangered Mexican fish under special protection: diagnosis of habitat fragmentation, protection, and future – a review. In M. E. Lucas-Borja (Ed.), Endangered species: habitat, protection and ecological significance (pp. 109–130). New York: Nova Science Publishers.
Escalera-Vázquez, L. H., Domínguez-Domínguez, O., Hinojosa-Garro, D., & Zambrano, L. (2016). Changes in diet, growth and survivorship of the native Tequila Splitfin Zoogoneticus tequila in co-occurrence with the non-native Shortfin Molly Poecilia mexicana. Fundamental and Applied Limnology/Archiv für Hydrobiologie, 188, 341–351. https://doi.org/10.1127/fal/2016/0932
Feyrer, F., Herbold, B., Matern, S. A., & Moyle, P. B. (2003). Dietary shifts in a stressed fish assemblage: consequences of a bivalve invasion in the San Francisco Estuary. Environmental Biology of Fishes, 67, 277–288. https://doi.org/10.1023/A:1025839132274
Fournie, J. W., Krol, R. M., & Hawkins, W. E. (2000). Fixation of fish tissues. In G. K. Ostrander (Ed.), The laboratory fish (pp. 569–578). San Diego: Academic Press.
García-Vásquez, A., Razo-Mendivil, U., & Rubio-Godoy, M. (2017). Triple trouble? Invasive poeciliid fishes carry the introduced tilapia pathogen Gyrodactylus cichlidarum in the Mexican highlands. Veterinary Parasitology, 235, 37–40. https://doi.org/10.1016/j.vetpar.2017.01.014
Garman, G. C. (1992). Fate and potential significance of postspawning anadromous fish carcasses in an Atlantic coastal river. Transactions of the American Fisheries Society, 121, 390–394. https://doi.org/10.1577/1548-8659
(1992)121<0390:fapsop>2.3.co;2
Gioria, M., & Osborne, B. (2009). Assessing the impact of plant invasions on soil seed bank communities: use of univariate and multivariate statistical approaches. Journal of Vegetation Science, 20, 547–556.
Gómez-Márquez, J. L., Peña-Mendoza, B., & Guzmán-Santiago, J. (2016). Reproductive biology of Poecilia sphenops Valenciennes, 1946 (Cyprinidontiformes: Poeciliidae) at the Emiliano Zapata Reservoir in Morelos. México. Neotropical Ichthyology, 14, 1–9. https://doi.org/10.1590/
1982-0224-20140127
Gross, M. R., & Shine, R. (1981). Parental care and mode of fertilization in ectothermic vertebrates. Evolution, 35, 775–793. https://doi.org/10.2307/2408247
Hayes, K. R., & Barry, S. C. (2008). Are there any consistent predictors of invasion success? Biological Invasions, 10, 483–506. https://doi.org/10.1007/s10530-007-9146-5
Herrerías-Diego, Y., Domínguez-Domínguez, O., Medina-Nava, M., Ávila, O., & Mar-Silva, V. (2019). Comparación de la composición y abundancia de la comunidad Íctica del río Teuchitlán, Jalisco, México empleando tres artes de pesca. In C. P. Ornelas-García, F. Álvarez & A. Wegier (Eds.), Antropización: primer análisis integral (pp. 265–282). Ciudad de México: IBUNAM-Conacyt.
Hill, W. R., & Boston, H. L. (1991). Community development alters photosynthesis-irradiance relations in stream periphyton. Limnology and Oceanography, 36, 1375–1389. https://doi.org/10.4319/lo.1991.36.7.1375
Hynes, H. B. N. (1950). The food of fresh-water sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. The Journal of Animal Ecology, 19, 36–58.
IUCN. (2020). The IUCN Red List of Threatened Species. Accessed 2020 February 18. Rerieved from: https://www.iucnredlist.org/
Jepsen, D. B., & Winemiller K. O. (2002). Structure of tropical river food webs revealed by stable isotope ratios. Oikos, 96, 46–55. https://doi.org/10.1034/j.1600-0706.2002.960105.x
Jiménez-Román, A. (1994). Estudio comparativo de la precipitación y el escurrimiento en la cuenca del río Ameca, México. Revista Geográfica, 119, 59–79. http://www.jstor.org/stable/40992684
King, A. J. (2005). Ontogenetic dietary shifts of fishes in an Australian floodplain river. Marine and Freshwater Research, 56, 215–225. https://doi.org/10.1071/MF04117
Kingston, D. I. (1978). Skiffia francesae, a new species of goodeid fish from Western Mexico. Copeia, 1978, 503–508. https://doi.org/10.2307/1443618
Krebs, C. J. (1989). Ecological methodology. New York: Harper and Row.
López-López, E., & Paulo-Maya, P. (2001). Changes in the fish assemblages in the upper río Ameca, México. Journal of Freshwater Ecology, 16, 179–187. https://doi.org/10.1080/02705060.2001.9663803
López-López, E., Paulo-Maya, J., Carvajal, A. L., Ortiz-Ordóñez, E., Uría-Galicia, E., & Mendoza-Reynosa, E. (2004). Populations of the Butterfly Goodeid (Ameca splendens) in the Upper Rio Ameca Basin, Mexico. Journal of Freshwater Ecology, 19, 575–580. https://doi.org/10.1080/02705060.2004.9664737
Lyons, J., González-Hernández, G., Soto-Galera, E., & Guzmán-Arroyo, M. (1998). Decline of freshwater fishes and fisheries in selected drainages of West-Central Mexico. Fisheries, 23, 10–18. https://doi.org/10.1577/1548-8446(1998)023<0010:doffaf>2.0.co;2
Maitipe, P., & De Silva, S. S. (1985). Switches between zoophagy, phytophagy and detritivory of Sarotherodon mossambicus (Peters) populations in twelve man-made Sri Lankan lakes. Journal of Fish Biology, 26, 83–95. https://doi.org/10.1111/j.1095-8649.1985.tb04240.x
Magurran, A. E. (2009). Threats to freshwater fish. Science, 325, 1215–1216. https://doi.org/10.1126/science.1177215
Marchetti, M. P., Moyle, P. B., & Levine, R. (2004). Invasive species profiling? Exploring the characteristics of non-native fishes across invasion stages in California. Freshwater Biology, 49, 646–661. https://doi.org/10.1111/j.1365-2427.2004.01202.x
McCormick, M. I. (1998). Ontogeny of diet shifts by a microcarnivorous fish, Cheilodactylus spectabilis: relation-
ship between feeding mechanics, microhabitat selection and growth. Marine Biology, 132, 9–20. https://doi.org/10.1007/s002270050367
Mejía-Mojica, H., Rodríguez-Romero, F. D. J., & Díaz-Pardo, E. (2012). Recurrencia histórica de peces invasores en la Reserva de la Biósfera Sierra de Huautla, México. Revista de Biología Tropical, 60, 669–681. https://doi.org/10.15517/RBT.V60I2.3960
Mercado-Silva, N., Lyons, J. D., Salgado-Maldonado, G., & Medina Nava, M. (2002). Validation of a fish-based index of biotic integrity for streams and rivers of central Mexico. Reviews in Fish Biology and Fisheries, 12, 179–191. https://doi.org/10.1023/A:1025099711746
Merrit, R. W., & Cummins, K. W. (1996). An introduction to the aquatic insects of North America (3rd Ed.). Dubuque, Iowa: Kendall/Hunt.
Miller, R. R. (1986). Composition and derivation of the freshwater fish fauna of México. Anales de la Escuela Nacional de Ciencias Biológicas, 30, 121–153.
Miller, R. R., & Fitzsimons, J. M. (1971). Ameca splendens, a new genus and species of goodeid fish from western México, with Remarks on the Classification of the Goodeidae. Copeia, 1, 1–13. https://doi.org/10.2307/1441593
Miller, R. R., Minckley, W. L., & Norris, S. M. (2009). Freshwater fishes of Mexico. Chicago: University of Chicago Press.
Nakano, S., Miyasaka, H., & Kuhara, N. (1999). Terrestrial–aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology, 80, 2435–2441. https://doi.org/10.1890/0012-9658(1999)080[2435:TALRAI]2.0.CO;2
Nakano, S., & Murakami, M. (2001). Reciprocal subsidies: dynamic interdependence between terrestrial and aquatic food webs. Proceedings of the National Academy of Sciences, 98, 166–170. https://doi.org/10.1073/pnas.98.1.166
Pauly, D., Froese, R., Sala, P., Palomares, M. L., Christensen, V., & Rius, J. (2000). Trophlab Manual. Manila: ICLARM.
Pennak, W. (1978). Fresh water invertebrates of the United States. New York: John Willey and Sons.
Pianka, E. R. (1974). Niche overlap and diffuse competition. Proceedings of the National Academy of Sciences, 71, 2141–2145. https://doi.org/10.1073/pnas.71.5.2141
Pollux, B. J., & Reznick, D. N. (2011). Matrotrophy limits a female’s ability to adaptively adjust offspring size and fecundity in fluctuating environments. Functional Ecology, 25, 747–756. https://doi.org/10.1111/j.1365-2435.2011.01831.x
Pound, K. L., Nowlin, W. H., Huffman, D. G., & Bonner, T. H. (2011). Trophic ecology of a nonnative population of suckermouth catfish (Hypostomus plecostomus) in a central Texas spring-fed stream. Environmental Biology of Fishes, 90, 277–285. https://doi.org/10.1007/s10641-010-9741-7
Pyke, G. H. (2008). Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annual Review of Ecology, Evolution, and Systematics, 39, 171–191. https://doi.org/10.1146/annurev.ecolsys.39.110707.173451
R Core Team (2013). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. http://www.R-project.org/
Ramírez-Carrillo, E., & Macías-García, C. (2014). Limited options for native goodeid fish simultaneously confronted to climate change and biological invasions. Biological Invasions, 17, 245–256. https://doi.org/10.1007/s10530-014-0723-0
Ramírez-García, A., Ramírez-Herrejón, J. P., Medina-Nava, M., Hernández-Morales, R., & Domínguez-Domínguez, O. (2017). Reproductive biology of the invasive species Pseudoxiphophorus bimaculatus and Poecilia sphenops in the Teuchitlán River, México. Journal of Applied Ichthyology, 34, 81–90. https://doi.org/10.1111/jai.13543
Ramírez-Herrejón, J. P., Mercado-Silva, N., Medina-Nava, M., & Domínguez-Domínguez, O. (2012). Validación de dos índices biológicos de integridad (IBI) en la subcuenca del río Angulo en el centro de México. Revista de Biología Tropical, 60, 1669–1686. https://doi.org/10.15517/rbt.v60i4.2160
Ramírez-Herrejón, J. P., Castañeda-Sam, L. S., Moncayo-Estrada, R., Caraveo-Patiño, J., & Balart, E. F. (2013). Trophic ecology of the exotic Lerma livebearer Poeciliopsis infans (Cyprinodontiformes: Poeciliidae) in the Lago de Pátzcuaro, Central Mexico. Revista de Biología Tropical, 61, 1289–1300. http://dx.doi.org/10.15517/rbt.v61i3.11957
Rayner, T. S., Pusey, B. J., & Pearson, R. G. (2009). Spatio-temporal dynamics of fish feeding in the lower Mulgrave River, north-eastern Queensland: the influence of seasonal flooding, instream productivity and invertebrate abundance. Marine and Freshwater Research, 60, 97–111. https://doi.org/10.1071/MF08055
Ricciardi, A. (2013). Invasive species. In R. Leemans (Ed.), Ecological systems. New York: Springer.
Ricciardi, A., Hoopes, M. F., Marchetti, M. P., & Lockwood, J. L. (2013). Progress toward understanding the ecological impacts of nonnative species. Ecological Monographs, 83, 263–282. https://doi.org/10.1890/13-0183.1
Ruehl, C. B., & DeWitt, T. J. (2005). Trophic plasticity and fine-grained resource variation in populations of western mosquitofish, Gambusia affinis. Evolutionary Ecology Research, 7, 801–19.
Sakai, A. K., Allendorf, F. W., Holt, J. S., Lodge, D. M., Molofsky, J., With, K. A. et al. (2001). The population biology of invasive species. Annual Review of Ecology and Systematics, 32, 305–332. https://doi.org/10.1146/annurev.ecolsys.32.081501.114037
Schaefer, J., Heulett, S., & Farrell, T. (1994). Interactions between two poeciliid fishes (Gambusia holbrooki and Heterandria formosa) and their prey in a Florida marsh. Copeia, 2, 516–520. https://doi.org/10.2307/1447002
Stoner, A. W., & Livingston, R. J. (1984). Ontogenetic patterns in diet and feeding morphology in sympatric sparid fishes from seagrass meadows. Copeia, 1984, 174–187. https://doi.org/10.2307/1445050
Tabacchi, E., Correll, D. L., Hauer, R., Pinay, G., Planty-Tabacchi, A. M., & Wissmar, R. C. (1998). Development, maintenance and role of riparian vegetation in the river landscape. Freshwater Biology, 40, 497–516. https://doi.org/10.1046/j.1365-2427.1998.00381.x
Thorp, H. J., & Covich, P. A. (2001). Ecology and classification of North American freshwater invertebrates. San Diego: Academic Press.
Trujillo-Jiménez, P., & Toledo-Beto, H. (2007). Alimentación de los peces dulceacuícolas tropicales Heterandria bimaculata y Poecilia sphenops (Cyprinidontiformes: Poeciliidae). Revista de Biología Tropical, 55, 603–615.
Vega-Cendejas, M. E. (1990). Interacción trófica entre dos bagres Arius melanopus (Agassiz, 1829) y Arius feliz (Linnaeus, 1776) en las costas de Celestún Yucatán, México. Anuario del Instituto de Ciencias del Mar y Limnología, 15, 185–194.
Vega-Cendejas, M. E., Hernández de S. M. J., & Cruz, A. G. (1997). Los peces de la reserva de Celestún. Mérida: Pronatura Península de Yucatán, A.C./ The Nature Conservancy/ CINVESTAV-Unidad Mérida.
Vital-Rodríguez, B. E., Ramírez-Herrejón, J. P., Moncayo-Estrada, R., Caraveo-Patiño, J., & Domínguez-Domínguez, O. (2017). Feeding strategy of silverside species in eutrophic Lake Pátzcuaro, Mexico. Journal of Applied Ichthyology, 33, 93–101. https://doi.org/10.1111/jai.13248
Wallace, R. K. (1981). An assessment of diet-overlap indexes. Transactions of the American Fisheries Society, 110, 72–76. https://doi.org/10.1577/1548-8659(1981)110<72:AAODI
>2.0.CO;2
Wantzen, K. M., & Junk, W. J. (2000). The importance of stream-wetland-systems for biodiversity: a tropical perspective. In B. Gopal, W. J. Junk & J. A. Davies (Eds.), Biodiversity in wetlands: assessment, function and conservation (pp. 11–34). Leiden, The Netherlands: Backhuys Publishers.
Wantzen, K. M., Yule, C. M., Mathooko, J. M., & Pringle, C. M. (2008). Organic matter processing in tropical streams. In D. Dudgeon (Ed.), Tropical stream ecology (pp. 43–64). Londres: Academic Press.
Webb, S. A., & Miller, R. R. (1998). Zoogoneticus tequila, a new goodeid fish (Cyprinodontiformes) from the Ameca drainage of Mexico, and a rediagnosis of the genus. Occasional Papers of the Museum of Zoology the University of Michigan, 725, 1–23.
Weliange, W. S., & Amarasinghe, U. S. (2003). Seasonality in dietary shifts in size-structured freshwater fish assemblages in three reservoirs of Sri Lanka. Environmental Biology of Fishes, 68, 269–282. https://doi.org/10.1023/A:1027384
114802
Wilson, D. E. (1971). Food habits of Micronycteris hirsuta (Chiroptera: Phyllostomidae). Mammalia, 35, 107–110. https://doi.org/10.1515/mamm.1971.35.1.107
Wipfli, M. S., & Baxter, C. V. (2010). Linking ecosystems, food webs, and fish production: subsidies in salmonid watersheds. Fisheries, 35, 373–387. https://doi.org/10.1577/
1548-8446-35.8.373
Yáñez-Arancibia, A., Curriel-Gómez, J., & de Yánez, V. L. (1976). Prospección biológica y ecológica del bagre marino Galeichthys caerulescens (Gunther), en el sistema lagunar costero de Guerrero, México (Pises: Arridae). Anales del Instituto de Ciencias del Mar y Limnologia, Universidad Nacional Autonoma de Mexico, 3, 125–180.
Zar, J. H. (1999). Biostatistical analysis. New Jersey: Prentice Hall.
Zaret, T. M., & Rand, A. S. (1971). Competition in tropical stream fishes: support for the competitive exclusion principle. Ecology, 52, 336–342. https://doi.org/10.2307/1934593