Three new species of Lasioglossum (Hymenoptera: Halictidae) from Mexico, with comments on the biogeography of Mexican species of the subgenus Lasioglossum
Ricardo Ayala-Barajas a, *, Laurence Packer b
a Estación de Biología Chamela, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 21, 48980 San Patricio, Jalisco, Mexico
b Department of Biology, York University, 4700 Keele St., Toronto, M3J 1P3 2, Ontario, Canada
*Corresponding author: rayala@ib.unam.mx (R. Ayala-Barajas)
Received: 24 September 2019; accepted: 13 January 2020
http://zoobank.org/urn:lsid:zoobank.org:pub:24462BB6-052A-43D7-8712-4DD80DF9BA01
Abstract
Three new species of Lasioglossum (Lasioglossum) from Mexico, L. manantlense sp. nov., L. tacanense sp. nov. and L. colimense sp. nov., are described and figured. McGinley’s (1986) key to species south of the US-Mexican border is emended to include the new species. Comments on the biogeography of Mexican species of the subgenus are provided. An interesting feature of the femoral scopa found in 2 of the new species is described and illustrated.
Keywords: Bees; Halictinae; Chiapas Highlands; Transmexican Volcanic Belt
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Tres especies nuevas de Lasioglossum (Hymenoptera: Halictidae) de México, con comentarios sobre la biogeografía de las especies mexicanas del subgénero Lasioglossum
Resumen
Se describen e ilustran 3 especies nuevas de Lasioglossum (Lasioglossum) de México, L. manantlense sp. nov., L. tacanense sp. nov. y L. colimense sp. nov. La clave de McGinley (1986) para especies presentes al sur de la frontera de México con Estados Unidos fue modificada para dar cabida a las especies nuevas. Se comenta sobre la biogeografía de las especies mexicanas de este subgénero y se describen e ilustran los caracteres más distintivos, como la escopa femoral de 2 de las especies.
Palabras clave: Abejas; Halictinae; Tierras Altas de Chiapas; Faja Volcánica Transmexicana
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
With over 1,800 described species, Lasioglossum Curtis (Tribe Halictini) is the most speciose genus of bees in the world. With numerous additional species being described most years, it seems certain that the total number will be well over 2,000. Lasioglossum has had a complex and unstable subgeneric classification (Gibbs et al., 2013). The nominotypic subgenus has 148 described species with 33 known from Mexico and Central America (Hinojosa-Díaz, 2003; McGinley, 1986); none are known from South America.
Our purpose is to describe and illustrate 3 new species of Lasioglossum (Lasioglossum) from Mexico and to provide comments on the distribution of the Mexican species of the subgenus according to the scheme of Morrone (2010, 2019; Morrone et al., 2017).
Materials and methods
Standard morphological terminology is used (Michener, 2007) with some more specialized terms associated to the taxonomy of New World Lasioglossum from McGinley (1986). Harris’ (1979) terminology is used for surface sculpture. Changes from these terminologies are as follows: we use “metapostnotum” instead of dorsal surface of the propodeum following most recent usage (e.g., Brothers, 1976; Mir Sharifi et al., 2019); as is standard in melittology (but contra Harris, 1979), the term striate and its derivatives refer to raised rather than impressed sculpture. Body length was assessed by measuring the lengths of the head, mesosoma and metasoma separately and then summing them. Length of pubescence is given relative to the diameter of the median ocellus (MOD). IAD and AOD refer to minimum interalveolar and alveolus to inner orbit distances respectively; UOD and LOD refer to minimum upper and lower ocular distances respectively; IOC and OOC refer to minimum interocellar and ocellocular distances respectively; ITW refer to intertegular width. F, S and T refer to flagellomeres and metasomal sterna and terga, respectively. Puncture spacing is given in terms of interspace distance (i) to diameter (d) of nearby punctures, for example i ~ d means that the punctures are separated by approximately their diameters, similarly i = 1-4d indicates that punctures are separated by variable distances, from one to 4 times the diameters of adjacent punctures.
Images were taken with a Visionary Digital BK Plus imaging system (Visionary Digital Enterprises, West Hollywood, California, USA), using a Canon EOS 5D digital SLR camera. All images were processed and amalgamated into plates with Adobe Photoshop CS. Scale bars for all figures are 1 mm except the lateral habitus shots (Figs. 1, 6, 10, 12) where the scale bar indicates 2 mm. The map with the known locations for these new species was prepared using SimpleMappr (Shorthouse, 2010).
Museum acronyms are as follows: PCYU, Packer Collection at York University, Toronto, Ontario, Canada; UNAM-CNIN National Collection of Insects, Instituto de Biología, Universidad Nacional Autónoma de México, México City, Mexico.
DNA barcodes were obtained from a single right mesotibia and mesotarsus that were placed in a well-plate for processing at the Biodiversity Institute of Ontario. DNA sequences were obtained using the methods described by Ivanova et al. (2006; http//www.ccdb.ca/resources.php), with primers LepF1 and LepR1 (Hebert et al., 2003). Data were analyzed using the online BOLD platform and barcode index numbers (BINs) (Ratnasingham & Hebert, 2013) obtained from the resulting sequences. Intraspecific divergences were calculated (when more than one barcode was available for a species) as were distances to nearest neighbours (including for singletons).
Descriptions
Lasioglossum manantlense Ayala & Packer, sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:5DC07A58-
2484-4E8B-AB37-FF3F2A72AE71
(Figs. 1-5)
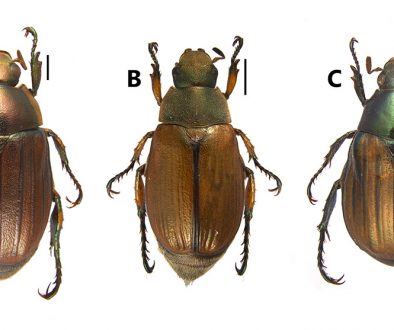
Diagnosis. Unique among species of Lasioglossum (Lasioglossum) from Mesoamerica, this species has the integument of T4-T5 orange (Figs. 1, 4), and the wings strongly marked with pale orange, with the setae making the anterior portion of the wing membrane appear smoky-orange (Fig. 1). Only a few species appear superficially similar, such as L. crocoturum (Vachal, 1904), in which T3-T5 are mostly covered in pale brown hairs rather than the integument being coloured (Fig. 4), that species also has the anterior portion of the wing coloured, but it is brown rather than pale orange.
Holotype female. Dimensions: body length 10.41mm, head width 2.9mm, wing length 9.8mm, ITW 2.4mm. Structure: head short, length/width ratio 0.9 (54:58). Labrum, apex of basal portion with a transversely oval process; distal keel moderately broad, short, approximately 1/3 total length of labrum, 11:30; distal lateral projections short not well-developed. Clypeus more than twice as wide as long, 32:14, upper and lower clypeal margins almost straight, upper margin of clypeus just above lower ocular tangent, surface without median longitudinal sulcus. Supraclypeal area evenly rounded, somewhat protuberant. UOD = LOD. IOC less than OOC, 16:20. F1 shorter than F2 on dorsal surface, 7:9. Clypeal fimbrial setae long almost reaching to apex of distal keel of labrum. Pronotal lateral a right angle; pronotal lateral ridge minutely narrowed near mid depth, somewhat rounded below. Posterior margin of scutellum not raised, continuing almost at the same level as metanotum; length of scutellum: metanotum: metapostnotum 29:17:25; metapostnotum not depressed medially, posterior margin broadly rounded; declivitous portion short, narrow. Propodeum lateral carina extending approximately three-fourths the height of posterior surface. Metatibial spur almost straight, teeth short, spur almost serrate, 13 teeth decreasing in size from base to apex and contiguous. Apical impressed areas extensive, on T2 more than 2MOD, 25:10. Sculpture: body mostly dull, largely due to dense punctation, only mandible, clypeus, pronotum, scutellum partially, legs, metasomal sterna and reflexed portions of terga shiny. Clypeal punctures large and irregularly spaced on disc, i = 0-3d; small and dense, i < d, towards upper margin; supraclypeal area weakly shiny, irregularly punctate medially, i = 1-5d; densely punctate laterally and above, i < 1.2d. Upper and lower paraocular area punctures somewhat effaced, dense i < d. Frontal area punctures contiguous, less dense, i = 0-7d, slightly larger punctures interspersed. Vertexal area punctures dense anteriorly, i < d; sparser posteriorly, i < 3d. Mesoscutum doubly punctate, small punctures crowded, larger hair-bearing punctures dense anteriorly, i ~ 2d, sparse and obscure posteriorly. Scutellum densely punctate around margins and medially, i < d; less dense, i = 0.5-3d, towards side of disc. Metanotum densely granulate. Mesopleuron sparsely rugose, more coarsely so anteriorly, more finely posteriorly; metapleuron longitudinally striate above, coarsely imbricate with minute punctures below. Metapostnotum uniformly ruguloso-striolate on imbricate background, macrosculpture weaker posteriorly (Fig. 2). Propodeum dull, finely granulate, weakly punctate, supralateral areas striate; T1-T3 minutely, densely punctate, i < d (Fig. 3); larger punctures interspersed, most dense on T3 i = 2-8d; T4 small punctures weak, larger punctures distinct, i = 3-6d; T5 tessellate, punctures continuously variable in size, i = 1-4d. S2-S6 impunctate basally, distinctly punctate apicomedially, i = 1-3d, denser towards sides. Coloration: black to black brown except as follows: mandible red brown just beyond midlength, apical 1/3 dark red brown; antenna dark brown; distitarsi brown, apically orange-brown; wing veins and stigma translucent pale orange; tegula yellow brown anteriorly; wing membranes pale straw except forewing pale orange brown as follows, radial cell anteriorly, all submarginal cells, marginal cell, and wing apex from outer posterior corner of third submarginal cell to wing apex and hind wing narrowly either side of vein R pale orange; T4-T5 orange, pygidial plate reddish brown (Fig. 4); S2-S3 red brown, S2 posterior margin orange; S4 orange except pair of transverse oval red brown marks, S5-S6 orange brown. Pubescence: pale orange brown, plumose, erect, not obscuring underlying integument < 2MOD except as follows: dark hairs on pronotal lobe, anterolaterally on scutellum and dorsally on mesepisternum; short, ~ 1/3 MOD, cream to pale orange, strongly plumose hairs obscuring underlying integument on pronotal lateral angles and basal band on each of T2-T4; longer < 2.5MOD on scutellum, metanotum and mesopleuron, on propodeum somewhat longer and with sparse long branches; mesosomal dorsum with suberect short < 0.25MOD hairs; metafemoral scopal hairs pale yellowish with long branches, strongly curved such that apices of hairs from anterior and posterior surfaces intermingle ventrally, anteroventral margin with row of more robust straighter darker hairs with short branches on posteromedial surface of rachis; metatibial scopa black brown, ventrally hairs somewhat palmate and curved apicodorsally, otherwise bearing short branches on dorsal surface of rachis on anterior tibial surface; metasoma with minute subappressed pale hairs increasing in length from < 0.2MOD on T1 to 0.4MOD on T4; longer ( < 2.3MOD) suberect hairs with branches only on anterior surface of rachis towards sides of T1-T4 and over most of T5; long
(< 3MOD) suberect hairs with anterior branches on S2-S4, similar but shorter hairs on S5.
Taxonomic summary
Holotype female: Mexico, Jalisco State, Reserva de la Biosfera Sierra de Manantlán, Estación Las Joyas, Sendero Xilosuchitlan. 19°35.215’ N, 104°16.585’ W, 1,940 m, 11/ix/2004, E.A.B. Almeida leg. On Fuchsia euclidiana (Onagraceae). UNAM-CNIN.
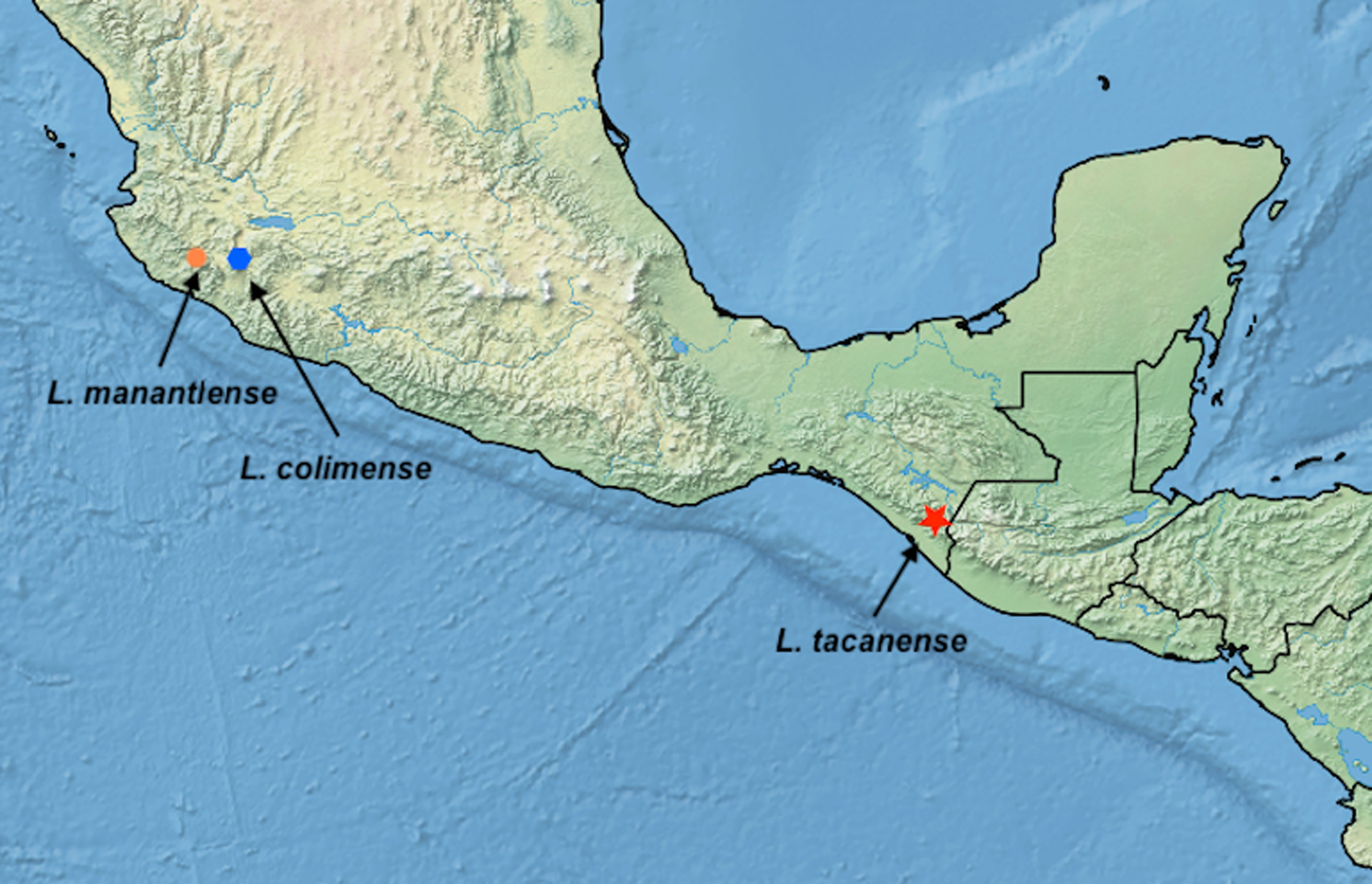
Etymology: the specific epithet refers to Manantlán a Biosphere Reserve in the state of Jalisco, Mexico, where the only known specimen was collected (Fig. 5).
Barcodes: an almost full-length sequence (613 bp) was obtained from the holotype, it has the barcode Sample ID CCDB-15262-A1 and is the sole occupant of BIN ABV5630. The closest sequence on the neighbour joining tree is the next described species which is only represented by a short sequence that is not BOLD-compliant. The closest full-length sequences belong to L. colimense sp. nov. also described below, with which it has a sequence divergence of 9.3-10.1%.
Remarks
This is arguably the most conspicuous and easily identified species in the enormous genus Lasioglossum and the only one among the new species for which a floral host is known. To identify this species based upon McGinley’s (1986) key to female Lasioglossum south of the US border with Mexico, it seems appropriate to modify couplet 13 (the species lacks the acarinarium to get to couplet 9, and has macrosculpture throughout the metapostnotum to get to couplet 13) as follows:
13(9) Integument of T4-T5 bright orange, wing membrane smoky orange anteriorly…………………………………. L. manantlense Ayala & Packer, sp. nov. – Integument of T4-T5 dark brown to black, wing membrane either hyaline or distinctly brown anteriorly…………………………………. McGinley’s original couplet 13

Lasioglossum tacanense Ayala & Packer, sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:EAB81D0B-
1360-4269-BF5F-ACDA568D6B88
(Figs. 5-9)
Diagnosis. The absence of basal hair bands on T3-T4 seems unique among New World Lasioglossum (Lasioglossum) (Fig. 6). In addition, the combination of T1 acarinarium absent [although the median portion lacks hairs there is no distinct surrounding fringe], posterior half of metapostnotum smooth (Fig. 8) and wings strongly darkened anteriorly (Fig. 6) serves to separate this species from all other Mexican and Central American species. Four other species, L. argutum McGinley, 1986, L. circinatum (Vachal, 1904), L. pharum (Vachal, 1904) and L. tropidonotum McGinley, 1986, share the first 2 characteristics, but their wings are at most indistinctly darkened anteriorly or apically. Lasioglossum manantlense sp. nov. also shares the first 2 characteristics and its forewing anterior margin is somewhat darkened, but this species has the apical terga bright orange and the wings pale orange and the 2 are readily distinguishable with the naked eye.
Holotype female. Dimensions: body length 12.3 mm, head width 2.9 mm, wing length 10.3 mm, ITW 2.2 mm. Structure: head short, length/width ratio 0.9 (55:59). Labrum, apex of basal portion with a broadly U-shaped process; distal keel moderately broad, short, less than 1/3 total length of labrum, 13:31; distal lateral projection triangular. Clypeus approximately twice as wide as long, 28:14.5, upper and lower clypeal margins almost straight, lower ocular tangent near dorsal 1/3 of clypeus, surface without median longitudinal sulcus. Supraclypeal area evenly rounded, somewhat protuberant. UOD subequal to LOD, 34:35.5. IOC less than ¾ OOC, 15:21. F1 as long as F2 on dorsal surface. Clypeal fimbrial setae ~ 2/3 as long as labrum. Pronotal lateral angle acute; pronotal lateral ridge minutely notched. Posterior margin of scutellum not raised, continuing almost at the same level as metanotum; length of scutellum: metanotum: metapostnotum 29:15:27; metapostnotum not depressed medially, posterior margin broadly rounded; declivitous portion short, triangular. Propodeum lateral carina extending approximately one half the height of posterior surface. Metatibial spur almost straight to broadly curved, teeth short, spur almost serrate, 12 teeth decreasing in size from base to apex, basal teeth U-shaped, apical teeth minute, teeth separated. Apical impressed areas weakly demarcated, extensive, on T2 more than 2.5MOD, 26:10. Sculpture: body mostly shiny, smooth to weakly imbricate. Clypeal punctures large, ill-defined, sparse on disc, i = 2-6d; small and dense, i < 1.5d, towards upper margin; supraclypeal area with few punctures on disc, punctures denser laterally and above, i = 1-3d. Lower paraocular area punctures distinct, i = 1-2d, somewhat effaced below. Upper paraocular and frontal area punctures bimodal in size, smaller ones dense, i < d, larger ones mostly i = 1-3d. Vertexal area punctures bimodal in size, minute punctures i = 2-4d, absent posteriorly; larger ones i = 2-8d. Mesoscutum doubly punctate, small punctures i = 0.5-2d, larger hair-bearing punctures, i = 1-6d anteriorly, i = 2-8d posteriorly. Scutellum punctures small around margins and medially, i < 2d; i = 1-6d towards side of disc; with scattered larger punctures. Metanotum punctures minute and dense i < 0.5d, with larger scattered punctures. Mesopleuron weakly imbricate, more strongly so posteriorly; weakly rugose-striolate, more strongly on hypoepimeral area, macrosculpture absent anteriorly; metepisternum weakly longitudinally striate above, imbricate below. Metapostnotum uniformly ruguloso-striolate basally for less than basal 1/3, middle 1/3 with short incomplete striae, apical 1/3 smooth (Fig. 8). Propodeum dull, imbricate, punctures small, i = 1-4d, weakly striolate. T1-T3 minutely punctate, i ~ d, punctures of apical impressed areas slightly larger except smaller and sparse on T3; T4 punctures i ~ 2d, apical impressed area impunctate (Fig. 9); T5 distinctly punctate, i = 1-2d. S2-S6 narrowly impunctate basally, distinctly punctate posteriorly, i = 1-4d, denser on S5-S6. Coloration: black to black brown except mandible red brown apically, all distitarsi orange brown and rest of metatarsus red brown; wing veins brown, forewing membrane dark orange brown anteriorly becoming gradually paler posteriorly and apically but only area apical to cubital and second recurrent veins hyaline, hindwing membrane pale brown anteriorly increasingly pale apically and posteriorly but only parts of membrane beyond M and cu-v hyaline. Pubescence: black to dark brown, plumose, erect, not obscuring underlying integument < 1.5MOD except as follows: longer < 2MOD on sides of mesosoma, off-white on metapleuron and propodeum; off-white and 1.5MOD on posterior surface of propodeum; dark hairs on pronotal lobe, anterolaterally on scutellum and dorsally on mesepisternum; short, ~ 1/2 MOD, white erect, strongly plumose hairs on pronotal lateral angles, around pronotal lobe and basal band on T2, these obscuring underlying integument except on pronotum; metanotum hairs short < 0.5MOD, anteriorly oriented, subapressed, pale, dense for anterior 1/4; metafemoral scopal hairs pale whitish brown with some darker hairs intermixed posteriorly, with long branches, strongly curved such that apices of hairs from anterior and posterior surfaces intermingle ventrally, anteroventral margin with row of more robust straighter darker hairs with short branches on posteromedial surface of rachis; metatibial scopa black brown, ventral hairs somewhat palmate curved apicodorsally, otherwise bearing short branches on dorsal surface of rachis, branches numerous dorsally, few anteriorly (Fig. 7); T1 hairs whitish, long, < 2MOD anterolaterally; rest of metasoma with increasingly dark and longer hairs on more posterior terga, < 3MOD on T5; sternal hairs erect, simple, mostly white, long < 2.5MOD.
Taxonomic summary
Holotype female: Mexico, Chiapas, Chiquihuite, 15°05’3979 N, 92°06’0128 W, 2,050 m, 4-6/xi/2009, L. Packer, S. Dumesh and C. Balboa; the specimen bears the GUI: MEX 09-5-033, UNAM-CNIN.
Etymology: the species is named after the Tacaná Volcano, the type locality is on its southern slopes (Fig. 5).
Barcode: a single short (271 bp) sequence was obtained. Although the sequence was too short to be barcode compliant, it does cluster with the single sequence of the previous species. Its sample id is CCDB-15262-A2.
Remarks
To identify this species through modification of McGinley’s (1986) key, see the remarks section for the following species.
Lasioglossum colimense Ayala & Packer, sp. nov.
http://zoobank.org/urn:lsid:zoobank.org:act:4C411568-
D8AB-453A-A019-A7DB06C7CDF8
(Figs. 5, 10-16)
Diagnosis. For the female, the combination of T1 acarinarium absent; basal bands of whitish pubescence on T2 and T3 (Fig. 10); anterior 1/3 of forewing dark and metapostnotum entirely granulate (Fig. 11) separates this species from all other Mexican/Central American species (Fig. 11). Other species with the first 2 characteristics: L. crocoturum (Vachal, 1904), L. eickworti McGinley, 1986, L. katyae McGinley, 1986, L. sandrae McGinley, 1986 and L. tricnicos (Vachal, 1904) all have the metapostnotum with ruguloso-striolate to striate macrosculpture, although this is weak in L. tricnicos which has the propodeal lateral angle rounded whereas that of L. colimense is briefly acute, right-angular ignoring the extreme apex; the relative lengths of scutellum and metapostnotum also differ between both, they are of similar length in L. colimense, but the metapostnotum is ~ 0.75X as long as the scutellum in L. tricnicos. For the male, the combination of anterior portion of forewing dark brown and mandible very long – attaining base of opposing mandible is sufficient to separate this species from any other Lasioglossum from Central America and Mexico except Lasioglossum eickworti. It can be differentiated from the latter species by the characters in the key below.

Holotype female. Dimensions: body length 11.1 mm, head width 2.6 mm, wing length 8.6 mm, ITW 1.9 mm (Fig. 10). Structure: head short, length/width ratio 0.9 (48:53). Labrum, apex of basal portion with a U-shaped process; distal keel moderately broad, short, less than 1/2 total length of labrum, 12:31; distal lateral projection weak. Clypeus more than twice as wide as long, 28:12.5, upper and lower clypeal margins almost straight, lower ocular tangent above midlength of clypeus, surface without median longitudinal sulcus. Supraclypeal area evenly rounded, somewhat protuberant. UOD subequal to LOD, 30.5:31. IOC more than 2/3 OOC, 13.5:19. F1 shorter than F2 on dorsal surface, 6:7. Clypeal fimbrial setae approximately as long as basal area of labrum. Pronotal lateral angle approximately right angular; pronotal lateral ridge very narrowly interrupted by oblique sulcus. Posterior margin of scutellum not raised, continuing almost at the same level as metanotum; length of scutellum: metanotum: metapostnotum 24:16:23; metapostnotum not depressed medially, posterior margin broadly rounded; declivitous portion short, triangular. Propodeum lateral carina extending approximately 3/5 the height of posterior surface. Metatibial spur almost straight to broadly curved, teeth subtriangular, longer towards apex, spur almost serrate, 11 teeth decreasing in size from base to apex, apical teeth minute weakly undulate, teeth separated. Apical impressed areas weakly demarcated, extensive, on T2 ~ 2MOD, 17:9. Sculpture: body mostly dull due to dense punctures or microgranulation unless stated otherwise. Clypeal punctures large, ill-defined, sparse on disc, i = 2-6d; small and dense, i < 1.5d, towards upper margin; supraclypeal area with few punctures on disc, punctures denser laterally and above, i = 1-3d. Lower paraocular area punctures distinct, i = 1-2d, somewhat effaced below. Upper paraocular and frontal area punctures bimodal in size, larger and denser medially on frontal area, larger punctures mostly i = 1-3d. Vertexal area punctures bimodal in size, minute punctures i = 2-4d, absent posteriorly; larger ones (small for the size of the insect) i = 2-8d. Mesoscutum doubly punctate, small punctures i = 0.5-2d, larger hair-bearing punctures, i = 1-6d anteriorly, smaller and more widely spaced, i=2-8d, posteriorly. Scutellum punctures minute around margins and medially, i < 2d. Metanotum seemingly imbricate with obscure punctures. Mesopleuron rugose, more strongly anterodorsally, weakly anteroventrally; metapleuron longitudinally striate above, becoming weakly rugulose then granulate below. Metapostnotum ruguloso-striolate basally for less than basal 1/3, rest of the surface densely granulate. Propodeum imbricate dull, punctures small, i = 1-4d. T1-T3 minutely punctate, i < d; punctures weaker on T4, stronger and sparser on T5 i = 1-4d. S1-S6 integument shiny, weakly imbricate, distinctly punctate i = 13-d, denser on S5, smaller on S6. Coloration: black to black brown, distitarsi orange brown and rest of metatarsus red brown; wing veins brown, forewing membrane dark brown a little orange anteriorly becoming gradually paler posteriorly and apically, but only area apical of cubital and second recurrent veins hyaline, hindwing membrane pale brown anteriorly increasingly pale apically and posteriorly but only parts of membrane beyond M and cu-v hyaline (Fig. 10). Pubescence: white to pale brown, with numerous small branches, erect to suberect, not obscuring underlying integument < 1.5MOD except as follows: longer < 2.5MOD on sides of mesosoma; darker brown hairs anteriorly on mesoscutum and mesepisternum, mixed with pale hairs on face; white erect, strongly plumose hairs on pronotal lateral angles and around pronotal lobe; white appressed hairs form basal band on T2 and T3 (Fig. 10), these obscuring underlying integument; metanotum hairs short < 0.5MOD, anteriorly oriented, subapressed, pale, dense for anterior 1/3; metafemoral scopal hairs pale whitish, with long branches, strongly curved such that apices of hairs from anterior and posterior surfaces intermingle ventrally, anteroventral margin without robust straighter hairs; metatibial scopa black brown, ventral hairs somewhat palmate curved apicodorsally, otherwise bearing short branches on dorsal surface of rachis; metasomal longer hairs with branches only on anterior surface of rachis, generally decreasing in length from base to apex of hair, branches sparser and more even in length on sterna; hairs darker from T1-T6, generally < 1.5MOD, longer on T5 < 3MOD; dark subappressed to appressed, short hairs, < 0.5MOD on most of rest of tergal surfaces; sternal hairs erect to suberect, < 2.5MOD.
Paratype male: body length 10.5 mm, head width 2.65 mm, wing length 7.6 mm, ITW 1.7 mm (Fig. 12). Structure: labrum with distal process moderately long, narrowly rounded. Mandible elongate, reaching opposing mandibular base, longer than the length of the eye. Clypeus more than 3X as wide as long, 62:20 (Fig. 13); upper and lower clypeal margins almost straight; clypeus produced, somewhat flattened apically, flat area a flattened D-shape in anterior view. Lower ocular tangent near midlength of clypeus. Supraclypeal area somewhat protuberant. UOD subequal to LOD, 30.5:31. IOC less than OOC 13:20. Genal area with posteroventral rounded process (Fig. 14). F1 shorter than F2 on dorsal surface 6:10. Pronotal lateral angle acute; pronotal lateral ridge strong almost lamellate, entire but weakly narrowed near mid-depth. Length of scutellum:metanotum:metapostnotum 20:12:18; metapostnotum somewhat flat, posterior margin broadly rounded. Pygidial plate broadly rounded. Terminalia: S7 with long, largely parallel-sided apicomedian process; S8 apex with three short lobes; gonostylus largely horizontally oriented, wedge-shaped, narrower basally; penis valve deep, strongly laterally compressed, bent at right angles in lateral view, lacking lateral processes (Fig. 16). Sculpture:


areas; T4-T6 weakly imbricate, t4-T5 more sparsely punctate, T6 punctures larger, i = 2-4d. Coloration: black to black brown; mandible dark, reddish toward middle; distitarsi red brown and rest of metatarsus dark red brown. Clypeus with apical flattened area and region adjacent to it yellow. Flagellum entirely dark. Wing veins brown, forewing membrane distinctly brownish for anterior ~ 1/3, rest hyaline. Pubescence: as in female except for usual sexual differences and as follows: proclinate dark hairs on clypeus < 3MOD; otherwise generally shorter < 1.5MOD throughout; longer < 2.5MOD on T6; sternal hairs whitish, branched, S1-S2 hairs erect, long < 2MOD; S3 hairs relatively even in length < 1.5MOD; S4 hairs longer towards sides < 1.5MOD; S5 hairs longer posterolaterally, ~ 2MOD than anterolaterally, < 1MOD, apical margin with pale brown subappressed posteriorly oriented hairs < 1MOD, shorter medially; S6 hairs short < 0.7MOD (Fig. 15).
Taxonomic summary
Holotype female: México, Jalisco, Volcán Nevado de Colima, 19°60.736’ N, 103°56.980’ W, 2,700 m, 7/x/2008, L. Packer, CCDB15373 A6 (Fig. 5); paratype: male; same data except: CCDB 15262 A4; paratypes: 1 female with the same data as the holotype except CCDB 15262 A5. Holotype and paratypes UNAM-CNIN, paratypes deposited in PCYU.
Etymology: the species is named after volcán Colima as all specimens were collected from the NE face of the volcano in Jalisco State. Although there is also a Mexican state called Colima, the volcano is almost entirely within Jalisco.
Barcodes: the following barcode sample identification codes refer to the holotype and paratypes: CCDB 15262 A6, A4 and A5. Its BIN is ABY5353 and there is a maximum divergence of 0.47% among the three (two sequences are identical). Other than the 5 specimens discussed below, the nearest neighbour is L. manantlense described above with a divergence of 9.36%
Remarks
We have an additional 5 specimens of what might be this species, 1 male and 4 females, which were collected in the same area, but which received different BINs in their DNA barcodes. Data for these specimens are as follows: females 09842H10 and H11 both with locality data: Mexico, Volcán Nevado de Colima, 19º.64000 N, 103º.61742 W, 2,411 m, 9/x/2010, S. Dumesh with GUIs PCYU-MEX10-0380 and 0381, and male CCDB-15262 A3 with identical locality data as for the holotype, these 3 are in BIN AAJ1725; females CCDB-00601 D08 and 09853 A01 are from the same collecting event as the holotype and the previously mentioned 2 females collected by S. Dumesh, respectively. These 2 are in BIN ACF4372. BIN AAJI1725 is separated from the type specimens of L. colimense by 1.85%, from which BIN ACF4372 is separated by 2.16%, and the 2 are separated by 1.23%. We have not been able to detect any morphological differences among the specimens in these three BINs other than minute variation in surface sculpture; the male genitalia appear identical between the paratype and CCDB 15262A4 and A3. We strongly suspect that these specimens are conspecific and indicate an example of a merge (Ratnasingham & Hebert, 2013). Nonetheless, in the absence of additional specimens and more extensive molecular data, we have chosen not to make these 5 specimens paratypes, they are labelled “L. colimense?” These specimens are shared between UNAM-CNIN and PCYU.
Lasioglossum colimense seems to be most closely related to L. eickworti McGinley, especially based upon the details of the male genitalia (McGinley, 1986: Figs. 402, 403). The very narrow retrorse lobe of the gonostylus and laterally compressed and deep penis valves being perhaps the most convincing similarities. It differs from L. eickworti in lacking the lateral processes of the penis valves and in the shape of the gonostylus (see key and associated images below).
Lacking the acarinarium and with the posterior half of the metapostnotum relatively smooth, females of both L. tacanense and L. colimense reach couplet 9 in McGinley’s (1986) key which can be modified as follows to permit their identification:
9(1) Forewing strongly darkened for at least anterior 1/3 (Figs. 1, 6, 10) 9A…………………………………. – Forewing entirely (almost entirely) hyaline…………………………………. McGinley’s original couplet 9
9A(9) Posterior half of metapostnotum smooth and shiny (Fig. 8); anterior 1/3 of forewing very dark, most of the rest of the forewing and anterior ~ 1/3 of hindwing pale brown (Fig. 6); hairs mostly dark brown to black (Fig. 6)…………………………………. L. tacanense Ayala & Packer, sp. nov. – Posterior half of metapostnotum granulate, dull (Fig. 11); wings hyaline except for anterior 1/3 of forewing brownish (Fig. 10); hairs mostly whitish to pale brown (Fig. 10)…………………………………. L. colimense Ayala & Packer, sp. nov.
Due to the lack of an anterior hair band on T1, darkened marks on wing at least on apex of marginal cell, mandibles conspicuoulsy elongate attaining opposing mandibular base and right angulate pronotal lateral angle, male L. colimense key to L. eickworti McGinley using McGinley’s (1986) key to male Lasioglossum south of the USA-Mexico border. Couplet 3 can be modified to allow separation of these species as follows:
3(2) Mandibles conspicuously elongate, reaching opposing mandibular base (Fig. 13)…………………………………. 3A
– Mandible short, just reaching opposing clypeal angle (Fig. 17)…………………………………. 4
3A(3) Gena with acute process posteroventrally (Fig. 18); mesoscutal hairs mostly dark brown to black (Fig. 19); gonostylus distinctly angulate dorsolaterally and penis valves with pair of lateral processes (Fig. 20) …………………………………. L. eickworti McGinley
– Gena with short, rounded lobe posteroventrally (Fig. 14); mesoscutal hairs mostly pale brown (Fig. 12); gonostylus not angulate dorsolaterally and lacking lateral processes (Fig. 16) ……… L. colimense Ayala & Packer, sp. nov.
Discussion
The new species have the leading edge of the forewing darkened (least so in L. manantlense). This gives them the superficial appearance of some of the vespid wasps in the region, such as Polybia occidentalis Olivier, and is shared with many other previously described species in the subgenus from Mexico and Central America (McGinley, 1986). Two species are known from single specimens, L. manantlense is from a locality that has received almost no attention from entomologists, whereas L. tacanense’s type locality has been visited several times by personnel from ECOSUR Tapachula and was also the locality where a new species of Mexalictus was discovered (Dumesh, 2013).
Within the enormous and difficult genus Lasioglossum, DNA barcodes have sped up species description rates but are not always diagnostic with several large clusters of morphologically separable species with identical barcodes (Gibbs, 2018), i.e., examples of “merges” (Ratnasingham & Hebert, 2013). Lasioglossum colimense is a putative example of the opposite difference between barcode and morphological results, with three BINs with apparently identical morphology. This is not an unknown situation among bees (Magnacca & Brown, 2010). Further investigation of this phenomenon would be of interest.
The metafemoral scopa of females in 2 of the 3 species bear a row of robust thick hairs with relatively few branches (Fig. 7). These appear to provide some structural strength to the scopa which is composed of very long hairs that bear long branches and which are curved such that those from the anterior and posterior surfaces intermingle ventrally. Inspection of other species of the subgenus available to us, including L. colimense, show that this feature may be confined to the group of relatively large species with darkened forewings, including L. eickworti, L. katyae and L. sandrae and is potentially a useful synapomorphy.
The distributions of Mexican and Central American Lasioglossum (Lasioglossum) taken from McGinley (1986) and Hinojosa-Díaz (2003) were compared with Morrone et al.’s (2017) biogeographic regionalization of the area. The results are shown in table 1. Thirteen species are restricted to the Neotropical region, 2 to the Nearctic region and 5 to the Mexican Transition Zone. Six species are found in both the Nearctic region and the Mexican Transition Zone and 3 are found in the Neotropical region and the Mexican Transition Zone. Three species are found in all 3 areas. When singleton collecting events or single locality taxa are excluded, there remain 7 taxa that may be restricted to single biogeographic provinces (Table 1): 1 each in the Baja Californian, Chihuahan Desert, Transmexican Volcanic Belt and Veracruzan provinces and 3 in the Chiapas Highlands province. These data suggest the importance of the latter province for diversification in the subgenus and suggest the importance of additional collections in that area. Further elucidation of the biogeographic history of these bees awaits molecular phylogenetic research along with node dating approaches (Packer et al., 2017), but the fact that we have described three new species suggests that there are additional species awaiting discovery that might help elucidate that history.
Table 1. Biogeographic regions and provinces for Lasioglossum (Lasioglossum) spp. found in Mexico (and L. katyae known from just outside the country). Species found in all 3 areas are stated as such, species found in 2 of the 3 have the 2 regions separated by a forward slash. Only species known from a single biogeographic province in Mexico are listed under that column heading. Occurrence records based upon McGinley (1986) and herein, regionalization based upon Morrone et al. (2017). Asterisks indicate singletons (single specimens or single localities) for which only single provinces could be assessed. Uncertainty is indicated with a “ ? ”. “ + N” and “ + S” refer to areas outside the geographic limits of Mexico to the north and south respectively.
| Species | Regions | Provinces |
| L. acarophilum McGinley | All 3 | |
| L. aequatum (Vachal) | Neotropical | |
| L. argutum McGinley | Neotropical/Mexican Transition Zone | |
| L. asaphes McGinley | Mexican Transition Zone | |
| L. bajaense McGinley | Nearctic | Baja California |
| L. bardum (Cresson) | Nearctic/Transition | |
| L. cercothrix McGinley | Nearctic/Transition | |
| L. circinatum (Vachal) | Neotropical | |
| L. colimense Ayala & Packer | Mexican Transition Zone | Sierra Madre del Sur* |
| L. costale (Vachal) | Neotropical/ Mexican Transition Zone | |
| L. crocoturum (Vachal) | Neotropical/ Mexican Transition Zone | |
| L. eickworti McGinley | Neotropical | Chiapas Highlands + S |
| L. heterorhinum (Cockerell) | All 3 | |
| L. jubatum (Vachal) | Nearctic/ Mexican Transition Zone | |
| L. katyae McGinley | Neotropical | Extralimital |
| L. manantlense Ayala & Packer | Mexican Transition Zone | Sierra Madre del Sur* |
| L. manitouellum (Cockerell) | All 3 | |
| L. morrilli (Cockerell) | Nearctic | Chihuahuan Desert + N |
| L. mystron Hinojosa-Díaz | Neotropical | |
| L. orphnaeum McGinley | Neotropical | Veracruzan |
| L. pallicorne (Vachal) | Nearctic/ Mexican Transition Zone | |
| L. parkeri McGinley | Neotropical | Balsas Basin?* |
| L. perscabrum McGinley | Neotropical | Veracruzan* |
| L. pharum (Vachal) | Neotropical | |
| L. sandrae McGinley | Neotropical | Balsas Basin?* |
| L. sisymbrii (Cockerell) | Nearctic/ Mexican Transition Zone | |
| L. tacanense Ayala & Packer | Neotropical | Chiapas Highlands* |
| L. transvorsum (Vachal) | Mexican Transition Zone | |
| L. tricnicos (Vachal) | Nearctic/ Mexican Transition Zone | |
| L. tropidonotum McGinley | Neotropical | Chiapas Highlands |
| L. uyacicola (Cokerell) | Neotropical | Chiapas Highlands + S |
| L. xyriotropis McGinley | Mexican Transition Zone | Transmexican Volcanic Belt |
Acknowledgments
The specimens described in this paper were collected under the permit FAUT.0108 from Semarnat for which we are grateful. We are grateful to Eduardo Almeida (Universidade de Sao Paulo, Brazil) for permission to describe the first species. We thank 2 anonymous referees for useful comments on the manuscript. The field trips which resulted in the specimens being collected were made possible by a Discovery Grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the junior authors salary. We thank Liam Graham (Department of Biology, York University, Canada) for taking the images and amalgamating them into plates; he is funded by a generous donation from Robert and Cecily Bradshaw. The imaging system was purchased with funds from the Canadian Foundation for Innovation and the Ontario Research Fund administered through Canadensys. We are grateful for the DNA barcoding data generated at the Canadian Centre for DNA Barcoding at the Biodiversity Institute of Ontario with funds from Genome Canada, NSERC and other sponsors listed at www.BOLNET.ca.

References
Brothers, D. J. (1976). Modifications of the metapostnotum and the origin of the “propodeal triangle” in Hymenoptera Aculeata. Systematic Entomology, 1, 177–182.
Dumesh, S. (2013). Revision of the rare Mesoamerican bee genus Mexalictus (Hymenoptera: Halictidae) with the description of 21 new species. Zootaxa, 3708, 1–117. http://dx.doi.org/10.11646/zootaxa.3708.1.1
Gibbs, J. (2018). DNA barcoding a nightmare taxon: assessing barcode index numbers and barcode gaps for sweat bees. Genome, 61, 21–31. https://doi.org/10.1139/gen-2017-0096
Gibbs, J., Packer, L., Dumesh, S., & Danforth, B. N. (2013). Revision and reclassification of Lasioglossum (Evylaeus), L. (Hemihalictus) and L. (Sphecodogastra) in eastern North America (Hymenoptera: Apoidea: Halictidae). Zootaxa, 3672, 1–117. http://dx.doi.org/10.11646/zootaxa.3672.1.1
Harris, R. A. (1979). A glossary of surface sculpturing. Occasional Papers in Entomology, 28, 1–36.
Hebert, P. D. N., Cywinska, A., Ward, S. L., & DeWaard, J. R. (2003). Biological identification through DNA barcodes. Proceedings of the Royal Society B: Biological Sciences, 270, 313–321. https://doi.org/10.1098/rspb.2002.2218
Hinojos-Díaz, I. A. (2003). A new Lasioglossum from Mexico, with the discovery of the male for L. xyriotropis (Hymenoptera: Halictidae). Journal of the Kansas Entomological Society, 76, 47–54.
Ivanova, N. V., DeWaard, J. R., & Hebert P. D. N. (2006). An inexpensive, automation–friendly protocol for recovering high-quality DNA. Molecular Biology Notes, 6, 998–1002. https://doi.org/10/1111/j/1471-8286.01428.x
Magnacca, K. N., & Brown M. F. J. (2010). Mitochondrial heteroplasmy and DNA barcoding in Hawaiian Hylaeus (Nesoprosopis) bees (Hymenoptera: Colletidae). BMC Evolutionary Biology, 10, 174. https://doi.org/10.1186/
1471-2148-10-174
Michener, C. D. (2007). The bees of the world. 2nd Edition. Baltimore, Maryland: Johns Hopkins University Press.
McGinley, R. J. (1986). Studies of Halictinae (Apoidea: Halictidae) I: revision of new world Lasioglossum Curtis. Smithsonian Contributions to Zoology, 429, 1–294.
Mir Sharifi, N., Graham, L., & Packer, L. (2019). Fifteen new species of Liphanthus Reed (Hymenoptera: Andrenidae) with two submarginal cells. Zootaxa, 4645, 1–80.
Morrone, J. J. (2010). Fundamental biogeographic patterns across the Mexican Transition Zone: an evolutionary approach. Ecography, 33, 355–361. https://doi.org/10.1111/
j.1600-0587.2010.06266.x
Morrone, J. J. (2019). Regionalización biogeográfica y evolución biótica de México: Encrucijada de la biodiversidad del Nuevo Mundo. Revista Mexicana de Biodiversidad, 90, e902980. http://dx.doi.org/10.22201/ib.20078706e.2019.90.2980
Morrone, J. J., Escalante T., & Rodríguez-Tapia, G. (2017). Mexican biogeographic provinces: Map and shapefiles. Zootaxa, 4277, 277–279. https://doi:10.11646/zootaxa.
4277.2.8
Packer, L., Litman, J., & Praz. C. J. (2017). Systematic position of a remarkable new fideliine bee from northern Chile (Hymenoptera: Apoidea: Megachilidae). Systematic Entomology, 42, 473–488. https://doi.org/10.1111/syen.
12229
Ratnasingham, S., & Hebert, P. D. N. (2013). A DNA–based registry for all animal species: the Barcode Index Number (BIN) system. Plos One, 8, e66213. https://doi.org/10.1371/journal.pone.0066213
Shorthouse, D. P. (2010). SimpleMappr, an online tool to produce publication-quality point maps. Retrieved from https://www.simplemappr.net; Accessed September 11, 2019.