Jazmín García-Román a, b, Tonatiuh Ramírez-Reyes b y Francisco Armendáriz-Toledano b, *
a Instituto Politécnico Nacional, Escuela Nacional de Ciencias Biológicas, Prol. de Carpio y Plan de Ayala s/n, Col. Santo Tomás, Miguel Hidalgo, 11340 Ciudad de México, Mexico
b Universidad Nacional Autónoma de México, Instituto de Biología, Circuito Exterior s/n, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
*Corresponding author: farmendariztoledano@ib.unam.mx (F. Armendáriz-Toledano)
Received: 20 December 2021; accepted: 31 May 2022
Abstract
In most members of the genus Dendroctonus, the usefulness of the spermatheca for taxonomic purposes has not been evaluated in depth; therefore, the aim of this study is to describe and compare the elements that integrate the female reproductive apparatus among Dendroctonus species, clarify their nomenclature and evaluate their interspecific variation to propose useful characteristics for their identification. In addition, we evaluated whether there is a structure between the shape of the receptacle and the phylogenetic relatedness of the species. The spermatheca consists of a sperm duct, a receptacle, and a gland; of these elements, the receptacle is the most taxonomically informative structure. The most closely related species of the D. frontalis complex show wide intraspecific variation in the shape and size of this structure, which precludes recognition of diagnostic features. However, there are differences among species groups in both shape and size in less closely related species such as D. approximatus, D. mexicanus, D. micans, D. parallelocollis and D. rhizophagus. A phylogenetic signal was detected in receptacle shape, and patterns of morphological similarity and phylogenetic relatedness of species were evident in the D. frontalis complex.
Keywords: Receptacle; Receptacle gland; Geometric morphometrics
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
La espermateca en el género Dendroctonus (Curculionidae: Scolytinae): morfología, nomenclatura, caracteres potenciales para su uso taxonómico y señal filogenética
Resumen
En la mayoría de los miembros del género Dendroctonus, la utilidad de la espermateca con fines taxonómicos no ha sido evaluada en profundidad; por ello, el objetivo de este estudio es describir y comparar los elementos que integran el aparato reproductor femenino entre las especies de Dendroctonus, aclarar su nomenclatura y evaluar su variación interespecífica para proponer características útiles para su identificación. Además, evaluamos si existe una estructura entre forma del receptáculo y el parentesco filogenético de las especies. La espermateca consta de un conducto espermático, un receptáculo y una glándula; de estos elementos, el receptáculo es la estructura taxonómicamente más útil. Las especies más estrechamente relacionadas del complejo D. frontalis, presenta una amplia variación intraespecífica en la forma y el tamaño de esta estructura, lo que impide el reconocimiento de características diagnósticas. Sin embargo, hay diferencias entre los grupos de especies tanto en la forma como en el tamaño, en especies menos relacionadas, como D. approximatus, D. mexicanus, D. micans, D. parallelocollis y D. rhizophagus. Se detectó una señal filogenética en la forma del receptáculo, y los patrones de similitud morfológica y el parentesco filogenético de las especies fueron evidentes en el complejo D. frontalis.
Palabras clave: Receptáculo; Glándula del receptáculo; Morfometría geométrica
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The taxonomy of the genus Dendroctonus Erichson is traditionally based on external morphological attributes, such as the abundance, distribution, and size of cuticular ornamentation and pubescence on the different body parts (Hopkins, 1909; Wood, 1982). The use of these characteristics has shown that some of them are not useful to separate closely related species, because they present wide intraspecific geographic variation (Armendáriz-Toledano et al., 2014; Lanier et al., 1998; Valerio Mendoza et al., 2019).
Recent studies on the analysis of the morphological variation in the species of Dendroctonus have revealed new diagnostic characteristics for their identification, from the antennae, stridulatory apparatus, and genitalia (Armendáriz-Toledano et al., 2015, 2017; Armendáriz-Toledano & Zúñiga, 2017; López et al., 2014). Regarding the latter, the elements of the seminal capsule of the male genitalia (anchor and seminal rod) have proved very useful, as they present species-specific morphology for all members, allowing reliable identification (Lanier et al., 1988; Armendáriz-Toledano & Zúñiga, 2017). The morphology of the female genitalia has been used in fewer cases to differentiate species, even though it has allowed improved identification in other genera of the subfamily Scolytinae, as is the case for the genus Xileborus Eichhoff, 1864 (Pérez-Silva et al., 2015).
Of the 21 species recognized for the genus (Valerio-Mendoza et al., 2019; Víctor & Zúñiga, 2016; Wood, 1982), the anatomy of the female genitalia is only known in detail in D. ponderosae Hopkins (Cerezque, 1964) and for 7 more species (D. approximatus Dietz, D. brevicomis LeConte, D. frontalis Zimmerman, D. mesoamericanus Armendáriz-Toledano & Sullivan, D. mexicanus Hopkins, D. parallelocollis Chapuis, and D. vitei Wood) the studies have only focused on describing and comparing qualitative and quantitative attributes of the “spermatheca” (= seminal receptacle, sensu De Marzo, 2009) as well as the patterns of variation in shape, all for taxonomic purposes (Armendáriz-Toledano et al., 2014, 2017; García-Román et al., 2019; Ríos-Reyes et al., 2008; Valerio- Mendoza et al., 2019).
Morphometric analyses support that the shape of the seminal receptacle allows the recognition of distinct geographic groups in D. brevicomis, information that contributed to removing D. barberi Hopkins from synonymy (Valerio-Mendoza et al., 2019). In other congeners, this structure presents a species-specific shape, as is the case of D. approximatus-D. parallelocollis (García-Román et al., 2019) and D. mexicanus-D. vitei, whereas, in others such as D. frontalis and D. mesoamericanus, the shape of the spermatheca has only been partially differentiated (Armendáriz-Toledano et al., 2014). According to the most recent phylogenetic hypotheses for the genus (Godefroid et al., 2019; Víctor & Zúñiga, 2016), the most species in which the spermatheca has been characterized belong to the D. frontalis complex sensu Lanier et al. (1988), which is supported by genomic data (Godefroid et al., 2019) and several corresponding synapomorphies (Víctor & Zúñiga, 2016). Within this group, pairs of sister species (D. frontalis-D. mesoamericanus and D. mexicanus-D. vitei) show distinct morphological character states and shape differences in the seminal receptacle, a situation that suggests that the evolutionary pattern of the female genitalia is divergent in these taxa and, therefore, could present a phylogenetic signal or structure (Klingengberg & Guidaszewski, 2010).
However, in the rest of the members of the genus, the patterns of variation of the female genitalia have not been described, and, in general terms, their usefulness for taxonomic purposes has not been evaluated in depth. For this reason, it has not been possible to study from a phylogenetic point of view the evolution of the female genitalia and, in particular, the seminal receptacle that apparently has evolved divergently in some groups of species of Dendroctonus.
Therefore, the aim of this work is to describe and compare the elements that integrate the female reproductive apparatus, with emphasis on the spermatheca, among Dendroctonus species, clarify its nomenclature, and evaluate its interspecific variation to propose taxonomically useful characteristics for the identification of females. Furthermore, we reconstruct the evolutionary history of the shape of the seminal receptacle (spermatheca sensu Armendáriz-Toledano et al., 2014, 2017; García-Román et al., 2019; Ríos-Reyes et al., 2007; Valerio-Mendoza et al., 2019) by detecting its phylogenetic signal, thus determining whether there is a structure between the patterns of the shape variation of receptacle and the phylogenetic relatedness of species.
Materials and methods
The material examined included more than 200 female specimens corresponding to 16 species of the genus Dendroctonus (D. adjunctus Blandford, D. approximatus, D. brevicomis, D. frontalis, D. jeffreyi Hopkins, D. mesoamericanus, D. mexicanus, D. micans Kugelann, D. parallelocollis, D. ponderosae, D. pseudotsugae Hopkins, D. rhizophagus Thomas and Bright, D. rufipennis Kirby, D. simplex LeConte, D. valens LeConte, and D. vitei), collected at localities representative of their geographic distribution (Table 1). The sex of the specimens was determined by observation of the stridulatory apparatus on the seventh abdominal tergite, which in females has an entire edge and is without scrapers (present in males) (Hopkins, 1909). The species from the USA and D. micans were identified using the taxonomic key of Labonte and Valley (2013), while for those from Mexico and Central America, we used the key of Armendáriz-Toledano and Zúñiga (2017).
Females were dissected for the extraction of the female genitalia, which was clarified, mounted, and examined, following the protocol described by Armendáriz-Toledano et al. (2014). Details of the spermatheca were observed under a compound microscope at 400x, and measurements were taken with a micrometer. Moreover, to refine the initial observations, a subset of this material was processed for scanning electron microscopy (SEM). For this, the genitalia were immersed in 5% sodium hypochlorite to digest the tissues and then rinsed in distilled water. Samples were mounted on metal plates, coated with gold, and examined under a Hitachi S-2469N scanning electron microscope.
The nomenclature used to describe the general anatomy of the spermatheca was based on the study by De Marzo (2009), and the attributes used for their characterization were taken from articles corresponding to other subfamilies of Curculionidae (Anderson, 1984; Brizzola-dos Santos & Rosado-Neto, 2010; Erbey et al., 2010; Lanteri & del Río, 2008; Omar, 2012; Sanders, 1960; Velázquez-de Castro et al., 2007) and species of Scolytinae (Pérez-Silva & Equihua-Martínez, 2016; Román-Ruíz et al., 2017; Rubio et al., 2008), including those performed on Dendroctonus sp. Since, anatomically, all species of the genus studied presented the same elements of female genitalia (see results), the comparison among species was focused on the seminal receptacle, as it was the element with the greatest interspecific variation and the one that provided the greatest number of characteristics for the purposes of the study.
To analyze the morphological variation of the receptacle, 6 continuous attributes were established in the 16 species studied: diameter of the nodulus (ND), diameter of the cornu (CD), diameter of the receptacle in its median region (APM), length of the nodulus (NL), length of the cornu (CL), and distance between the cornu and the nodulus (CND) (Fig. 1). Two discrete characteristics were also analyzed: the portion of the receptacle covered by striae (PCG): (0) 1/3 of the receptacle, (1), 1/2 of the receptacle, (2), 2/3 of the receptacle; and the anatomy of the posterior region of the cornu (APR): (0), protuberance absent and (1), protuberance present (Fig. 1).
With these attributes, the receptacles of all specimens were characterized. For continuous characteristics, the mean and standard deviation per species were calculated, while for discrete ones, frequencies were calculated for each character state. The multidimensional patterns of variation of the characteristics were analyzed by ordination analyses from 2 matrices: (a) continuous and discrete attributes considering the specimens as operational taxonomic units (OTUs) and (b) discrete characteristics considering species as OTUs. Matrix (a) was analyzed by principal coordinate analysis (PCoA) using a Gower distance matrix, meanwhile, matrix (b) was analyzed by principal component analysis (PCA) from a variance-covariance matrix; since the first component (PC1) quantified almost 80% of the variation, it was used for further analysis as a variable representing receptacle morphometric variation among species.
To determine whether the body size of Dendroctonus species was associated with the morphological differences found in the seminal receptacle, a regression analysis was performed between the total body length of the species (mean value) and the principal component one (PC1) obtained from the PCA as dependent variable.
Table 1
Species, locality, and coordinates of Dendroctonus species examined in this study.
| Species | Locality | Coordinates | |
| Latitude | Longitude | ||
| D. adjunctus | Tecpan, Chimaltenango, Guatemala | 14.8 | -90.96 |
| Desierto de los Leones, CDMX, Mexico | 19.33 | -99.63 | |
| D. approximatus | Ejido El Largo, Maderas, Chihuahua, Mexico | 29.2 | -108.11 |
| El Gallo, Guerrero, Mexico | 18.27 | 100.50 | |
| Zempoala, Huitzilac, Morelos, Mexico | 19.05 | -99.31 | |
| D. brevicomis | Deer Creek Rd., 14.9 km S Nelson, British Columbia, Canada | 49.45 | -117.33 |
| Panguitch, Oregon, United States | 37.82 | -112.43 | |
| D. frontalis | Buenavista, Motozintla, Chiapas, Mexico | 15.36 | -92.25 |
| El Picacho, Cerro Tomabu, Chinandega, Nicaragua | 13.03 | -86.3 | |
| La Mesa, Charo, Michoacán, Mexico | 19.6 | -100.93 | |
| D. jeffreyi | San Pedro Mártir, Baja California, Mexico | 30.95 | -115.37 |
| D. mesoamericanus | Campestre Alta Montaña, Guatemala, Guatemala | 14.78 | -90.92 |
| Guaimaca, Francisco Morazán, Honduras | 14.53 | -86.81 | |
| D. mexicanus | Teloloapan, Guerrero, Mexico | 18.35 | -99.85 |
| Jacala, Hidalgo, Mexico | 21.01 | -99.18 | |
| D. micans | Rue des Quatre Saisons, Peyrat le Chateau, France | 45.81 | 1.77 |
| D. parallelocollis | Chinandega, Nicaragua | 19.78 | -103.49 |
| San Rafael, Tlalmanalco, Mexico State, Mexico | 19.23 | -98.75 | |
| D. ponderosae | Pinaleno Mtns. Graham, Arizona, USA | 32.65 | -109.87 |
| D. pseudotsugae | Arteaga, Coahuila, Mexico | 25.24 | -100.44 |
| D. rhizophagus | Chihuahua, Mexico | 27.97 | -107.55 |
| D. rufipennis | Aklavik, Northwest Territories, Canada | 68.22 | -135.00 |
| D. simplex | Belgrade, Kennebec, Maine, United States | 44.44 | -69.83 |
| D. valens | Álvaro Obregón, CDMX, Mexico | 14.8 | -90.96 |
| D. vitei | Ejido Aquiles Serdán, Montemorelos, Nuevo León, Mexico | 25.2 | -99.88 |
| Hidalgo, Tamaulipas, Mexico | 24.13 | -99.06 |
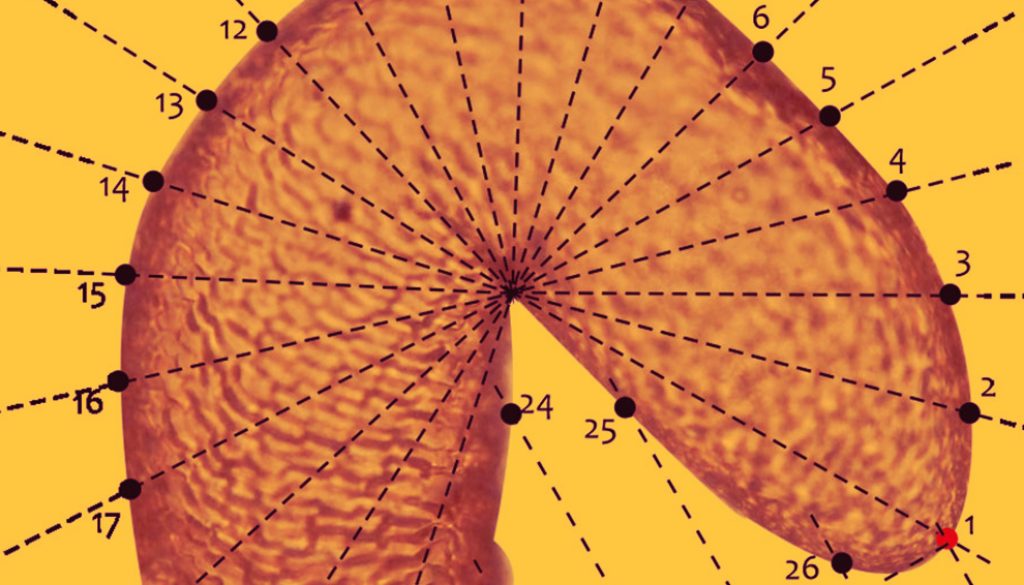
Geometric morphometrics. The variation in the shape of the seminal receptacle was studied in 16 of the 21 described species of the genus. For each species, 2 to 4 photographs were used, for a total of 48. The capture and processing of the images, as well as the morphometry protocol, were performed with some modifications, following the protocol of Armendáriz-Toledano et al. (2014), who studied the variation of the receptacle shape in D. frontalis and D. mesoamericanus through semilandmarks. To facilitate the positioning of the semilandmarks, a fan of 20 radios was used, taking as reference points the middle part of the nodulus and the cornu (Fig. 2). Semilandmarks were placed at each intersection point of each radio and the receptacle contour. In total, 26 points were digitized in the program tpsDig Version 1.40 (Rohlf, 2004), of which 3 were landmarks type I and the remaining semilandmarks sensu Bookstein (1991) (Fig. 2). Subsequently, the coordinates were adjusted to decrease the tangential variation of the semilandmarks, using the criterion of minimum procrustes distances (Pérez et al., 2006) in the program SemiLand6 of the IMP (Integrated Morphometrics Package) (Sheets, 2003), as well as to eliminate the effects of position, scale, and orientation, using a generalized procrustes analysis in the program CoordGene6f of the IPM (Sheets, 2003). From the adjusted shape coordinate matrices, the average shape configurations of each species were calculated, and a relative warps analysis (RWA) was performed in the PAST 1.95 program (Hammer et al., 2001). Patterns of variation in receptacle shape among species were evaluated by projecting the first 2 components of relative deformation.
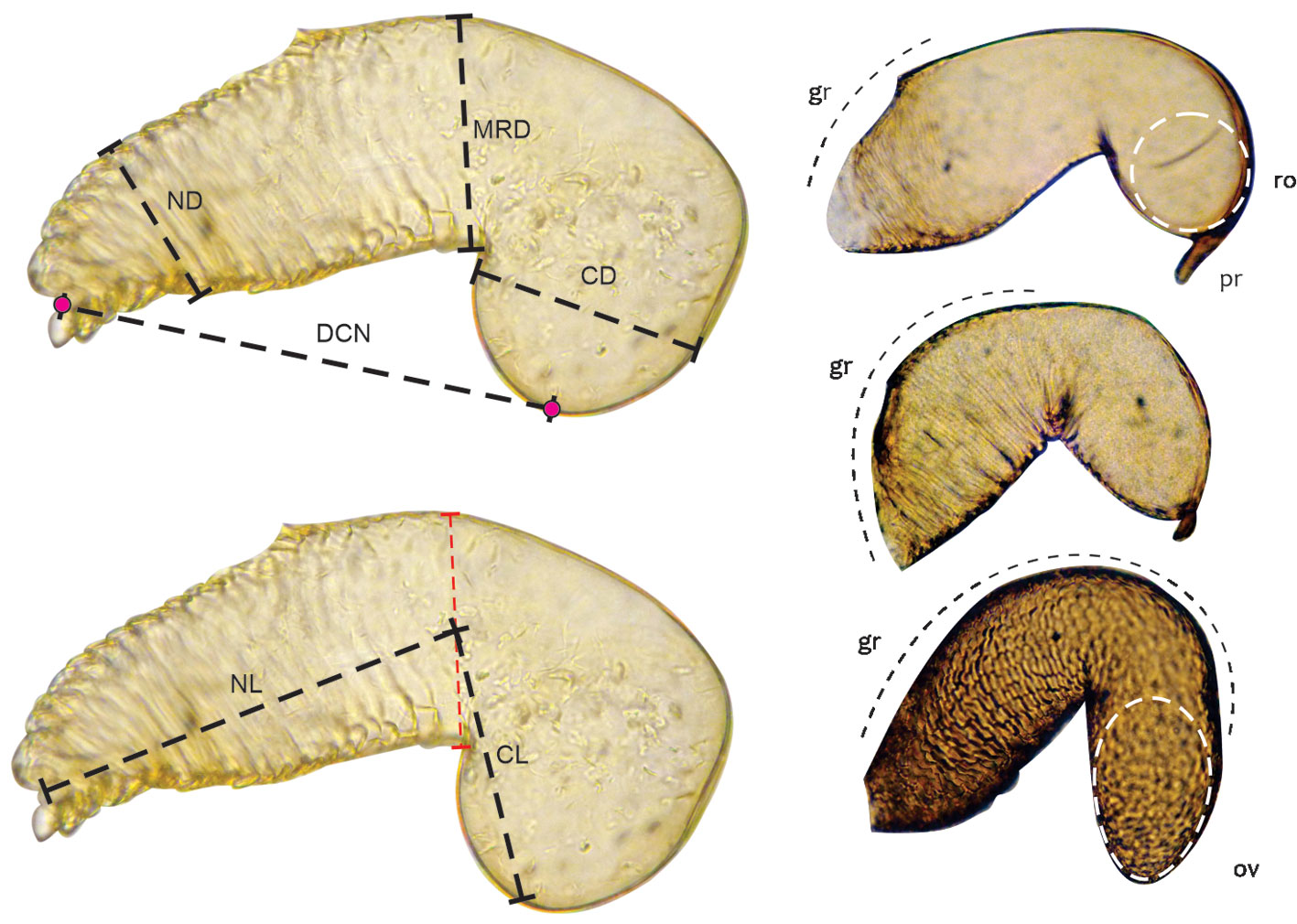
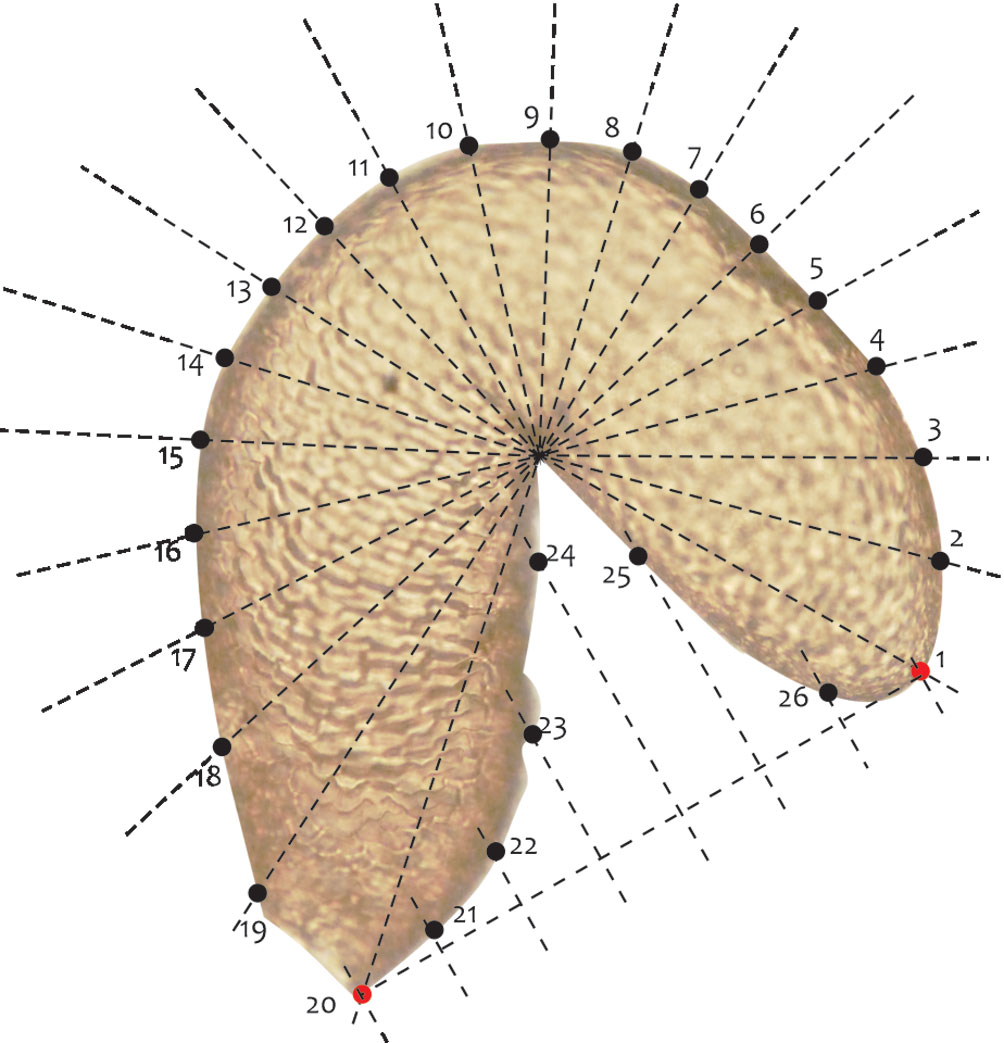
Table 2
Accession numbers and length of cytochrome oxidase gene fragment used to obtain the molecular phylogenetic hypothesis of genus Dendroctonus.
| Species | Accession number | Length (bp) |
| D. frontalis | KC855262.1, KC855261.1, KC855260.1 | 771, 771, 771 |
| D. mesoamericanus | KT364536.1 | 1,013 |
| D. mexicanus | MK734375.1, MK734374.1, MK734373.1 | 755,791,793 |
| D. vitei | KT364538.1 | 505 |
| D. approximatus | MK734350.1, MK734349.1, MK734348.1 | 797, 793, 819 |
| D. brevicomis | MK734343.1, MK734345.1 | 767, 790 |
| D. adjunctus | AF068001.1, AF067992.1 | 1,129, 1,177 |
| D. rhizophagus | MK734365.1, MK734364.1, AF067993.1 | 766, 1,233, 1,009 |
| D. valens | MK734363.1, MK734361.1 | 764, 735 |
| D. ponderosae | MK734357.1 | 770 |
| D. jeffreyi | MK734353.1, MK734352.1, MK734351.1 | 752, 749, 784 |
| D. simplex | AF067985.1 | 996 |
| D. pseudotsugae | MK840763.1 | 670 |
| D. rufipennis | MK840771.1 | 670 |
| D. parallelocollis | KT364537.1, JQ005147.1 | 977 |
| D. terebrans | AF068003.1 | 1,077 |
| D. micans | KJ964805.1 | 658 |
| Hypothenemus obscurus | KX818284.1 | 667 |
Phylogenetic signal. We obtained 2 phylogenetic hypotheses for the genus Dendroctonus, the first one based on molecular data and the second one using both molecular and morphological data. To obtain the first phylogeny, 34 sequences of the mitochondrial COI gene fragment were downloaded from GenBank, 33 for Dendroctonus and 1 for Hypotheneumus as an outgroup (Table 2). From the sequence alignment in MAFFT (online: https://www.ebi.ac.uk/Tools/msa/mafft/), a matrix of 1,233 characteristics was obtained. We then inferred a maximum likelihood tree in RaxmlGUI 2.0.5 (Edler et al., 2021). We indicated ML+ rapid bootstrap analysis (1,000 replicates), with a GTR+GAMMA model. Subsequently, the resulting maximum likelihood tree was pruned with the “drop.tip” function with the ape (Paradis & Schliep, 2019) and phytools (Revell, 2012) packages in RStudio, in order to have one representative for each species and to import it for further analysis. In the second hypothesis, we implemented a total evidence strategy (morphology and mitochondrial COI), for which we downloaded 16 COI gene sequences corresponding to Dendroctonus taxa from GenBank (Table 3). The sequences were aligned with the MAFFT program (online version: https://www.ebi.ac.uk/Tools/msa/mafft/). The aligned molecular matrix presented 1,545 characters. A morphological dataset corresponding to the 16 Dendroctonus species studied was obtained from Víctor and Zúñiga (2016); the morphological matrix contained 31 characters from adult, male genitalia, and larval morphology, in addition, 2 chromosomal characters and 3 behavior traits were added (Table S1 in Víctor & Zúñiga et al., 2016). With these matrices, we inferred a phylogenetic hypothesis through the maximum likelihood (ML) approach estimated in RaxmlGUI 2.0.5 (Edler et al., 2021). We indicated a ML+rapid bootstrap analysis (100 replicates), with a GTRCATI surrogate model.
To evaluate the phylogenetic signal in the receptacle, changes in the shape of this structure were mapped onto both phylogenetic trees, using the first 3 components of relative deformation, by means of the parsimony method of weighted squared change (Klingenberg & Gidaszewski, 2010). The hypothesis of no phylogenetic signal was tested with a permutation test with 10,000 replicates in the program Morpho J 1.01c (Klingenberg, 2011). The p-value was calculated as the percentage of permutations resulting in a tree equal to or shorter than the initial tree (Chursina & Negrobov, 2018; Gidaszewski et al., 2009; Klingenberg, 2011; Klingenberg & Gidaszewski, 2010).
Table 3
Accession numbers and length of cytochrome oxidase gene fragment used to obtain the total evidence phylogenetic hypothesis of genus Dendroctonus.
| Species | Accession number | Length (bp) |
| D. frontalis | AF067986 | 1,125 |
| D. mesoamericanus | KT364536 | 1,013 |
| D. mexicanus | AF067988 | 1,164 |
| D. vitei | KT364538 | 505 |
| D. approximatus | AF068000 | 1,178 |
| D. brevicomis | AF068002 | 1,179 |
| D. adjunctus | AF067992 | 1,177 |
| D. rhizophagus | JQ005115 | 1,545 |
| D. valens | JQ005145 | 1,545 |
| D. ponderosae | AF067987 | 1,054 |
| D. jeffreyi | AF067994 | 1,208 |
| D. simplex | AF067985 | 996 |
| D. pseudotsugae | AF067995 | 995 |
| D. rufipennis | AF067996 | 1,034 |
| D. parallelocollis | KT364537 | 977 |
| D. micans | AF067991 | 1,096 |
Results
In all species studied, the female reproductive apparatus consisted of 2 ovaries, each with a pair of ovarioles converging into a lateral oviduct, 2 oviducts connecting into a middle oviduct, a bursa copulatrix, a pair of accessory glands, and a spermatheca, the last 3 connected to the vagina (Fig. 22 in Cerezke, 1964).
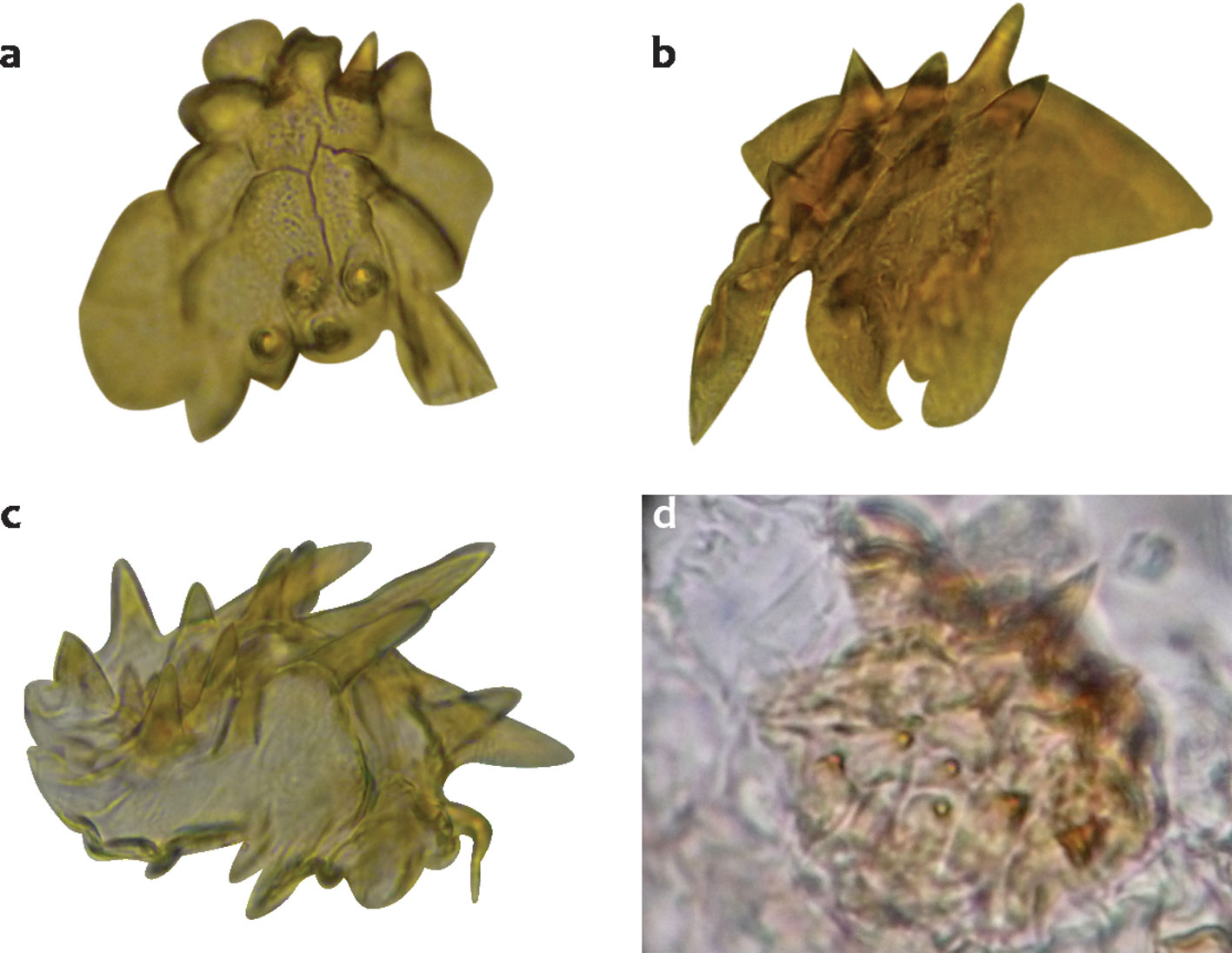
The bursa copulatrix in some species such as D. ponderosae, D. valens, D. rufipennis, and D. adjunctus present inside a pair of very small, sclerotized plates with several spines that apparently are different among species (Fig. 3). These plates were only recovered in 4 species because they are easily lost in the mounting process.
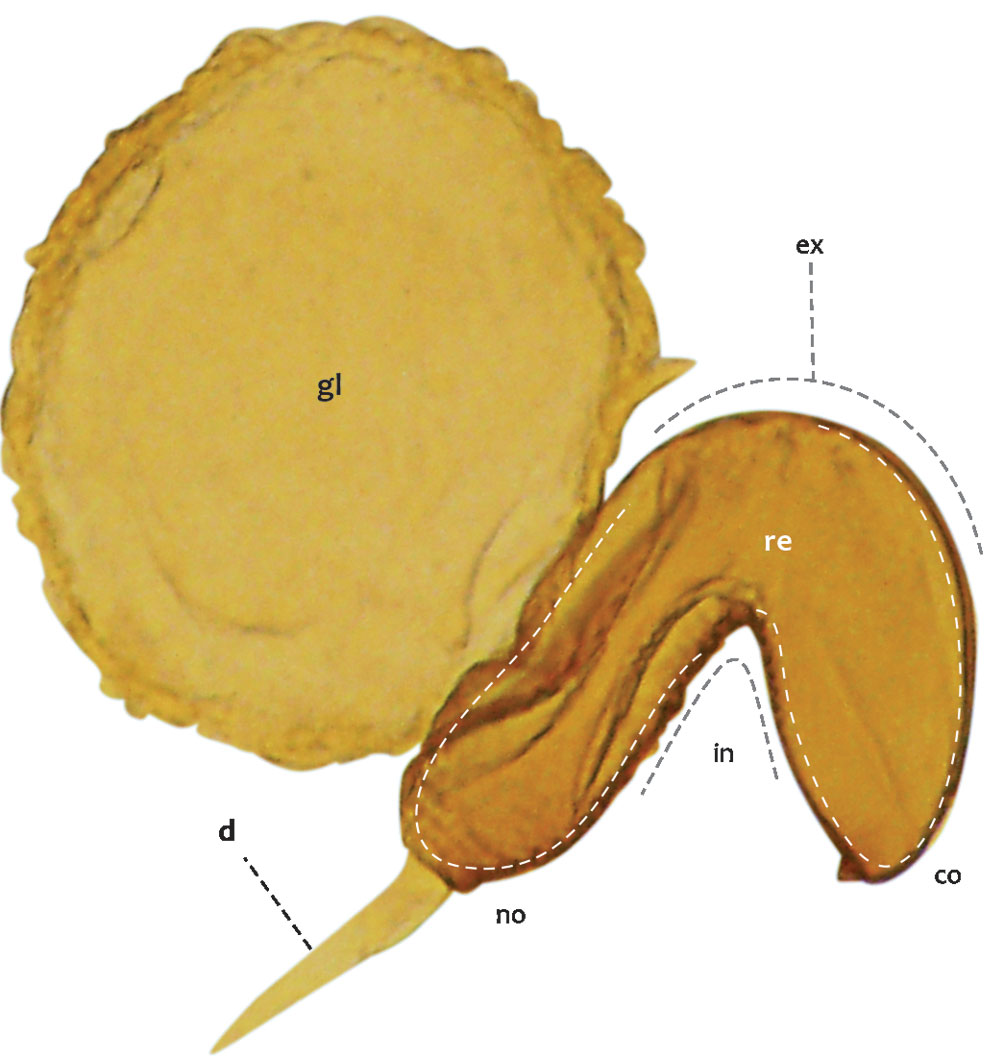
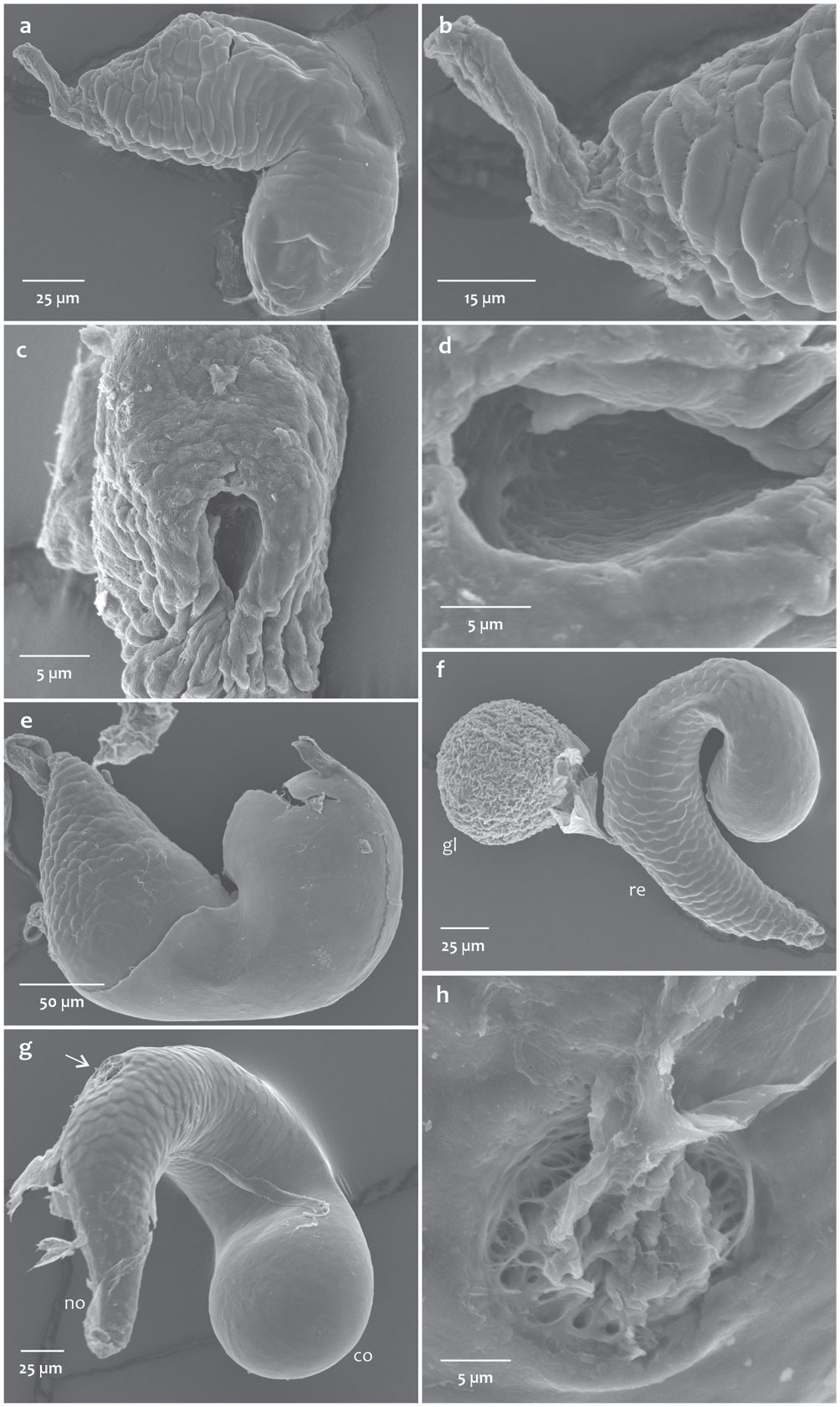
Three elements could be recognized in the spermatheca: a sperm duct, a receptacle, and an accessory gland (Figs. 4, 5). The sperm duct is a thin tube with a transparent and slightly chitinized cuticle, connecting the vagina to the receptacle (Figs. 4, 5a-d); the receptacle is a highly sclerotized kidney-shaped sac associated with muscle tissue, which stores spermatozoa; and the gland is a spherical mass of tissue that can be from 1/3 (Fig. 5f) to slightly larger than the entire receptacle (Fig. 4). The receptacle on the lateral side of the external curvature (Fig. 4) has an opening through which it connects to the accessory gland (Fig. 5g, h).
Of these 3 structures, the seminal receptacle was the one that presented differences among the species studied; the results of the evaluation of continuous and discrete characteristics of this structure in all species are presented in tables 4 and 5, respectively. Although the receptacle does not have areas delimited by sutures, it is possible to distinguish at least 2 regions, the nodulus and the cornu; which are recognized based on a pronounced curvature that gives the receptacle the appearance of a kidney (Figs. 4, 5a, b). The nodulus is the proximal portion, starting from where the sperm duct ends, up to the curvature of the receptacle, while the cornu corresponds to the distal portion, beyond the curvature (Fig. 4).
The curvature can be in the middle or in the last third of the receptacle; for example, in D. pseudotsugae, D. adjunctus, D. approximatus, D. brevicomis, D. mexicanus, D. frontalis, and D. mesoamericanus, it is located approximately in the middle of the receptacle, while in the remaining species it is in the last third (Fig. 6).
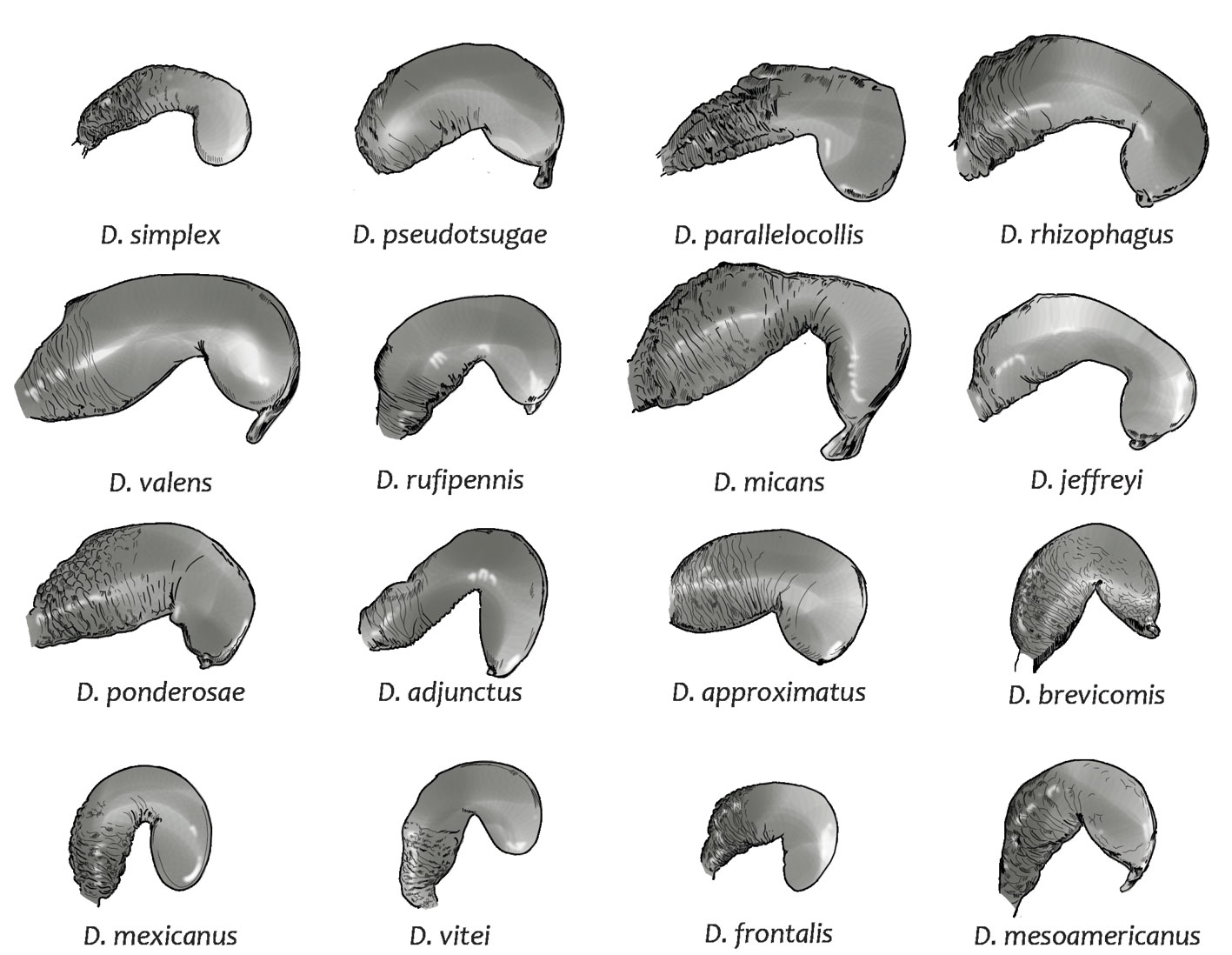
Depending on the position of the curvature of the receptacle, the relative length of the cornu and nodulus can be variable; for example, species with curvatures at the middle of the receptacle have cornu and nodulus similar in length (e.g., D. adjunctus, D. approximatus, D. mexicanus, D. frontalis, and D. pseudotsugae ) and species with curvatures in the last third have longer nodulus than cornu (e.g., D. jeffreyi, D. micans, D. parallelocollis, D. ponderosae, D. simplex, D. rhizophagus, and D. valens ) (Fig. 6; Table 4).
Table 4
The mean and standard deviation of the quantitative characteristics used to compare 16 of the 21 species of Dendroctonus.
| Characteristics (µm) | CD | ND | MRD | NL | CL | CND |
| D. adjunctus | 17.5 ± 1.99 | 15.48 ± 1.03 | 18.21 ± 0.36 | 32.98 ± 9.42 | 25.71 ± 6.8 | 39.76 ± 5.82 |
| D. brevicomis | 17.21 ± 1.79 | 18.64 ± 2.28 | 18.71 ± 2.21 | 31.21 ± 1.22 | 27.5 ± 2.7 | 33.93 ± 5.5 |
| D. frontalis | 14.29 ± 1.67 | 13.09 ± 1.6 | 15.53 ± 4.93 | 26.84 ± 5.57 | 20.83 ± 4.32 | 25.89 ± 2.46 |
| D. mesoamericanus | 15.36 ± 2.96 | 15.36 ± 2.04 | 14.38 ± 2.11 | 35.27 ± 4.33 | 25.89 ± 6.27 | 37.05 ± 3.38 |
| D. mexicanus | 15.12 ± 0.9 | 16.07 ± 1.78 | 15 ± 1.24 | 32.14 ± 3.57 | 27.38 ± 5.45 | 23.81 ± 1.03 |
| D. simplex | 15.89 ± 0.25 | 15.89 ± 5.30 | 17.86 ± 5.05 | 38.39 ± 1.26 | 24.82 ± 6.31 | 41.96 ± 6.31 |
| D. vitei | 14.76 ± 0.41 | 15.95 ± 1.97 | 13.1 ± 1.25 | 35.48 ± 2.63 | 19.17 ± 6.40 | 30.36 ± 1.79 |
| D. approximatus | 22.05 ± 2.32 | 23.93 ± 4.16 | 20.98 ± 1.07 | 43.03 ± 9.32 | 35.71 ± 11.3 | 42.96 ± 1.78 |
| D. jeffreyi | 18.21 | 20.71 | 13.21 | 46.43 | 21.43 | 44.64 |
| D. parallelocollis | 19.05 ± 1.03 | 15.48 ± 1.25 | 17.14 ± 1.24 | 47.02 ± 5.74 | 23.21 ± 3.09 | 48.81 ± 4.12 |
| D. ponderosae | 19.64 | 26.07 | 22.14 | 42.86 | 27.50 | 46.43 |
| D. pseudotsugae | 24.40 ± 3.72 | 25.48 ± 1.97 | 21.67 ± 3.32 | 41.67 ± 2.73 | 25.36 ± 4.34 | 45.83 ± 4.12 |
| D. rhizophagus | 21.43 ± 0 | 24.82 ± 2.27 | 17.68 ± 1.26 | 50.89 ± 1.26 | 25 ± 0 | 46.43 ± 0 |
| D. rufipennis | 19.7 ± 2.05 | 18.57 ± 3.74 | 17.44 ± 1.02 | 37.08 ± 4.32 | 25.54 ± 3.65 | 42.86 ± 3.91 |
| D. micans | 17.86 ± 0 | 26.78 ± 2.52 | 13.75 ± 0.76 | 59.82 ± 1.26 | 28.57 ± 5.05 | 58.03 ± 6.31 |
| D. valens | 22.5 ± 3.53 | 26.25 ± 3.28 | 22.14 ± 1.01 | 46.43 ± 5.05 | 28.93 ± 4.54 | 56.25 ± 13.89 |
Another variable characteristic among species is the degree of curvature of the receptacle. Dendroctonus parallelocollis and D. simplex presented receptacles with less pronounced curvatures, so the distance between the nodulus and cornu was relatively larger than the rest of their measurements compared to the other species (Table 4), while D. adjunctus and D. mexicanus presented the most evident receptacle curvature (Fig. 5). Therefore, the distance between the cornu and nodulus was smaller (Table 4).
The diameter of the receptacle was variable, depending on the region in which it was measured. According to this attribute, taxa may have nodulus and cornu of similar diameter along the receptacle (e.g., D. mexicanus and D. approximatus), nodulus of larger diameter than cornu (e.g., D. ponderosae, D. rhizophagus, D. valens, and D. micans) or cornu of larger diameter than nodulus (e.g., D. simplex and D. parallelocollis) (Table 4; Fig. 6). Likewise, some species show a reduction in diameter in the region of the curvature of the receptacle (constriction in the curvature), as is the case of D. jeffreyi, D. rhizophagus, D. simplex, D. valens, and D. micans (Fig. 6).
Table 5
Frequency of each discrete character state in each Dendroctonus species.
| Portion of receptacle covered by grooves | Anatomy of the posterior region of the cornu | ||||
| Species | (0) one third | (1) half | (2) two thirds | (0) with protuberance | (0) without protuberance |
| D. adjunctus | 33.33% | 66.66% | 0% | 100% | 0% |
| D. approximatus | 50% | 50% | 0% | 100% | 0% |
| D. brevicomis | 0% | 80% | 20% | 40% | 60% |
| D. frontalis | 33.33% | 66.66% | 0% | 80% | 20% |
| D. jeffreyi | 100% | 0% | 0% | 0% | 100% |
| D. mesoamericanus | 25% | 25% | 50% | 0% | 100% |
| D. mexicanus | 100% | 0% | 0% | 100% | 0% |
| D. micans | 0% | 0% | 100% | 0% | 100% |
| D. parallelocollis | 0% | 100% | 0% | 100% | 0% |
| D. ponderosae | 100% | 0% | 0% | 0% | 100% |
| D. pseudotsugae | 66.66% | 33.33% | 0% | 0% | 100% |
| D. rhizophagus | 100% | 0% | 0% | 0% | 100% |
| D. rufipennis | 66.66% | 33.33% | 0% | 33.33% | 66.66% |
| D. simplex | 100% | 0% | 0% | 100% | 0% |
| D. valens | 100% | 0% | 0% | 0% | 100% |
| D. vitei | 100% | 0% | 0% | 100% | 0% |
The surface of the receptacle is ornamented by transverse striae that completely or partially surround it; the density of these striae is much higher in the proximal region of the nodulus and decreases towards the cornu. In the taxa studied, the density of striae and the portion of the receptacle covered by them were an intraspecifically variable characteristic, since most species presented striae covering from one-third to one-half of the receptacle (Fig. 6, Table 5). Only in D. brevicomis, D. frontalis and D. pseudotsugae, were there some specimens with receptacles entirely covered by striae. In addition to the striae, some species may present a cuticular projection on the surface of the cornu (e.g., D. micans, D. valens and D. ponderosae) (Fig. 6, Table 5), which has been associated with the insertion of the contractor muscles of the receptacle (Cerezque, 1964).
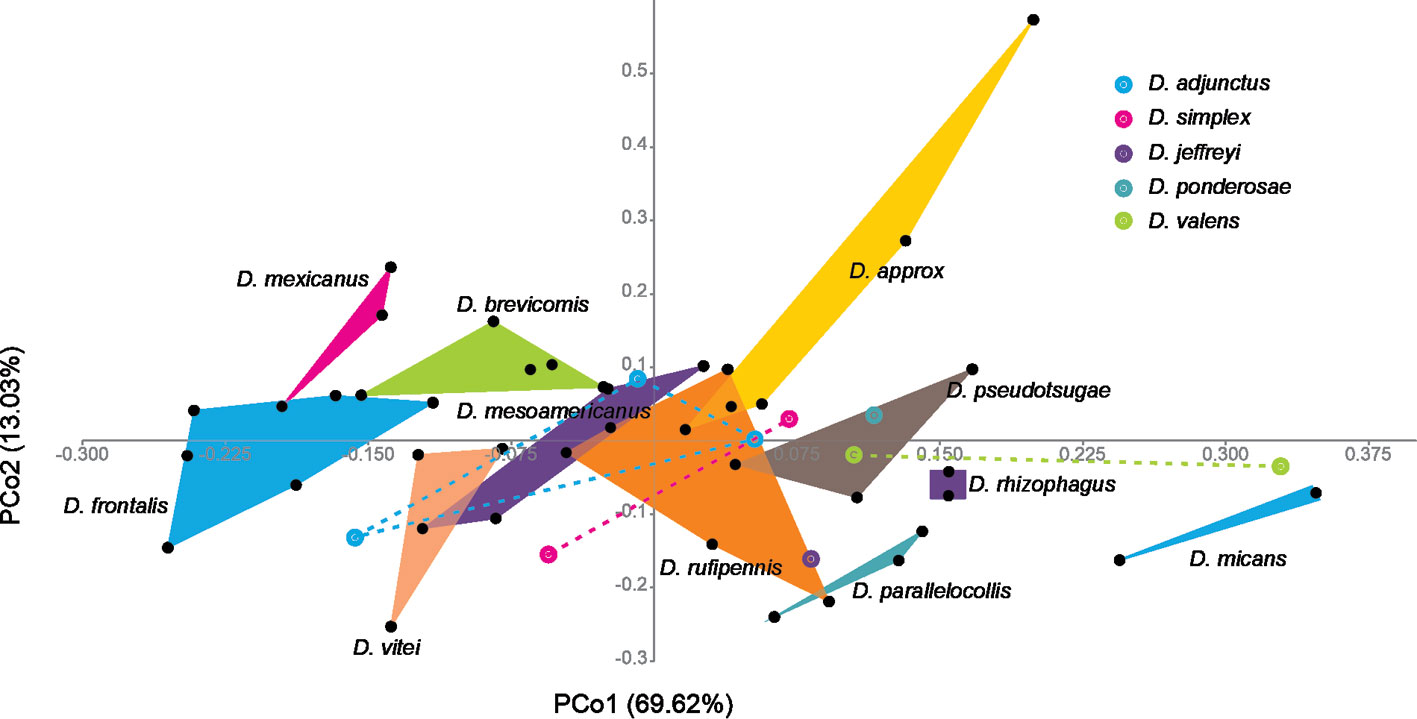
Multivariate analysis of receptacle measurements. The PCoA of the 6 continuous attributes and the 2 discrete attributes recovered 82.65% of the total variation in the first 2 coordinates (PCo1: 69.62%, PCo2: 13.03%); the scatter plot of these coordinates displayed the OTUs in multivariate space in discrete clusters corresponding to most species: D. approximatus, D. brevicomis, D. frontalis, D. micans, D. mesoamericanus, D. parallelocollis, D. pseudotsugae, D. rhizophagus, and D. vitei (Fig. 7). In the cases of D. adjunctus, D. simplex, and D. valens, the specimens showed a wide dispersal and, therefore, overlapped with other ones (Fig. 7). The intraspecific variation of D. rufipennis and D. ponderosae could not be contrasted, since only 1 receptacle per species was recovered (Fig. 7).
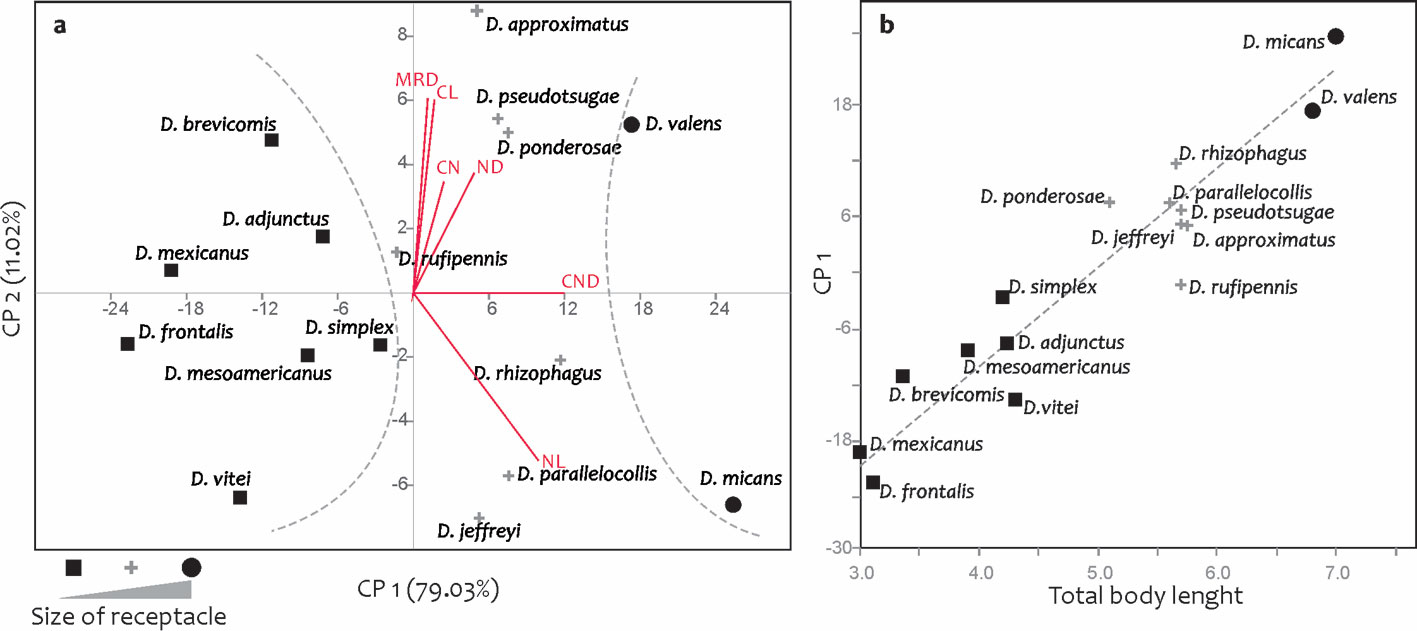
The PCA from the mean values per species of the 8 continuous attributes recovered 90.05% of the total variation in the first 2 principal components (PC1: 79.03%, PC2: 11.02%); the scatter plot between these PCs showed the segregation of the OTUs into 3 groups according to the “size” of the species’ receptacles: “small”, “medium”, and “large”. The attributes that contributed the greatest amount of variation in these components were diameter and length of nodulus (DN, LN) and distance between nodulus and cornu (DCN).
Taxa with small receptacles were D. adjunctus, D. brevicomis, D. frontalis, D. mesoamericanus, D. mexicanus, D. simplex, and D. vitei (Fig. 8a), which had nodulus diameter between 13 and 18.6 µm, nodulus length from 26.8 to 38.3 µm, and nodulus-cornu distance from 23.8 to 39.7µm (Table 4). Species with medium receptacles were D. approximatus, D. jeffreyi, D. parallelocollis, D. ponderosae, D. pseudotsugae, D. rhizophagus, and D. rufipennis (Fig. 8a), which had nodulus diameter between 18.5 and 25.4 µm, nodulus length from 37 to 50.8 µm, and nodulus-cornu distance from 42.8 to 48.8 µm (Table 4). Members with large receptacles were D. micans and D. valens (Fig. 8a), which had nodulus diameters greater than 26 µm, nodulus length greater than 46 µm, and nodulus-cornu distance greater than 56 µm (Table 4).
Differences in receptacle size among species were correlated with differences in size of the respective adults (Fig. 8b). A linear regression analysis, taking as the independent variable the body length of the adults studied and as the dependent variable the PC1 of the continuous receptacle characteristics, supported that the relationship between adult body size and receptacle dimensions was directly proportional (r = 0.94, r2 = 0.88, t = 10.44, p ≤ 0.001).
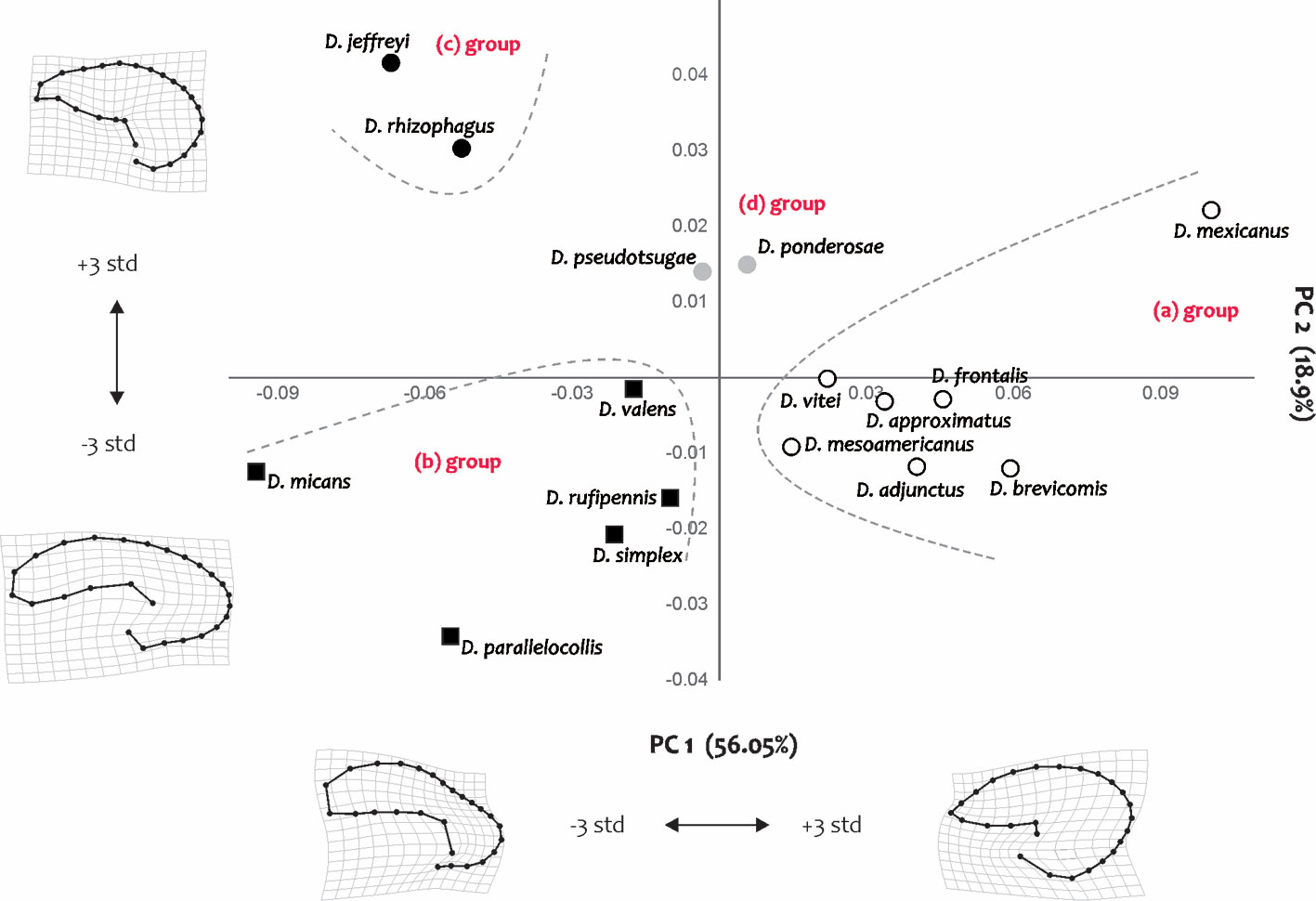
Geometric morphometrics. Superimposition of the 48 receptacle configurations of the 16 Dendroctonus species showed that the sites with the greatest variation were those describing the internal curvature of the receptacle. The analysis of relative deformations from the average configurations per species quantified 74.94% of the total variation in the first 2 components (RW1: 56.05%, RW2: 18.9%). Deformations in RW1 occurred in the degree of constriction at the height of the receptacle curvature and in the distance between the nodulus and cornu. Deformations in RW2 occurred in the internal and external curvatures of the receptacle, as well as in the diameter of the nodulus (Fig. 9).
The scatter plot of the first 2 RWs showed the segregation of the species into several sets according to similarities in receptacle shape (Fig. 9). The first of these was composed of D. adjunctus, D. approximatus, D. brevicomis, D. frontalis, D. mesoamericanus, D. mexicanus, and D. vitei, which were characterized by the absence of a constriction in the receptacle curvature, by presenting nodulus and cornu close to each other, as well as receptacles with pronounced curvature (Fig. 6). The receptacle of D. mexicanus was the most distinct of this set of species, which has the nodulus and cornu closer together and a more pronounced receptacle curvature compared with the other species (Figs. 6, 9).
Seven of the remaining species (D. jeffreyi, D. micans, D. parallelocollis, D. rhizophagus, D. rufipennis, D. simplex, and D. valens) had a constriction in the curvature of the receptacle (the diameter in this area was evidently smaller than that of the nodulus and cornu), nodulus and cornu separated from each other, as well as receptacles with less evident curvature (Figs. 6, 9). Within this set, D. jeffreyi and D. rhizophagus presented very similar shaped receptacles, with narrow nodulus in their proximal region and spherical shaped cornu in their distal portion (Figs. 6, 9). Dendroctonus ponderosae and D. pseudotsugae presented intermediate forms to the previously described groups, with receptacles absent of constriction in the curvature, as well as nodulus and cornu far from each other (Figs. 6, 9).
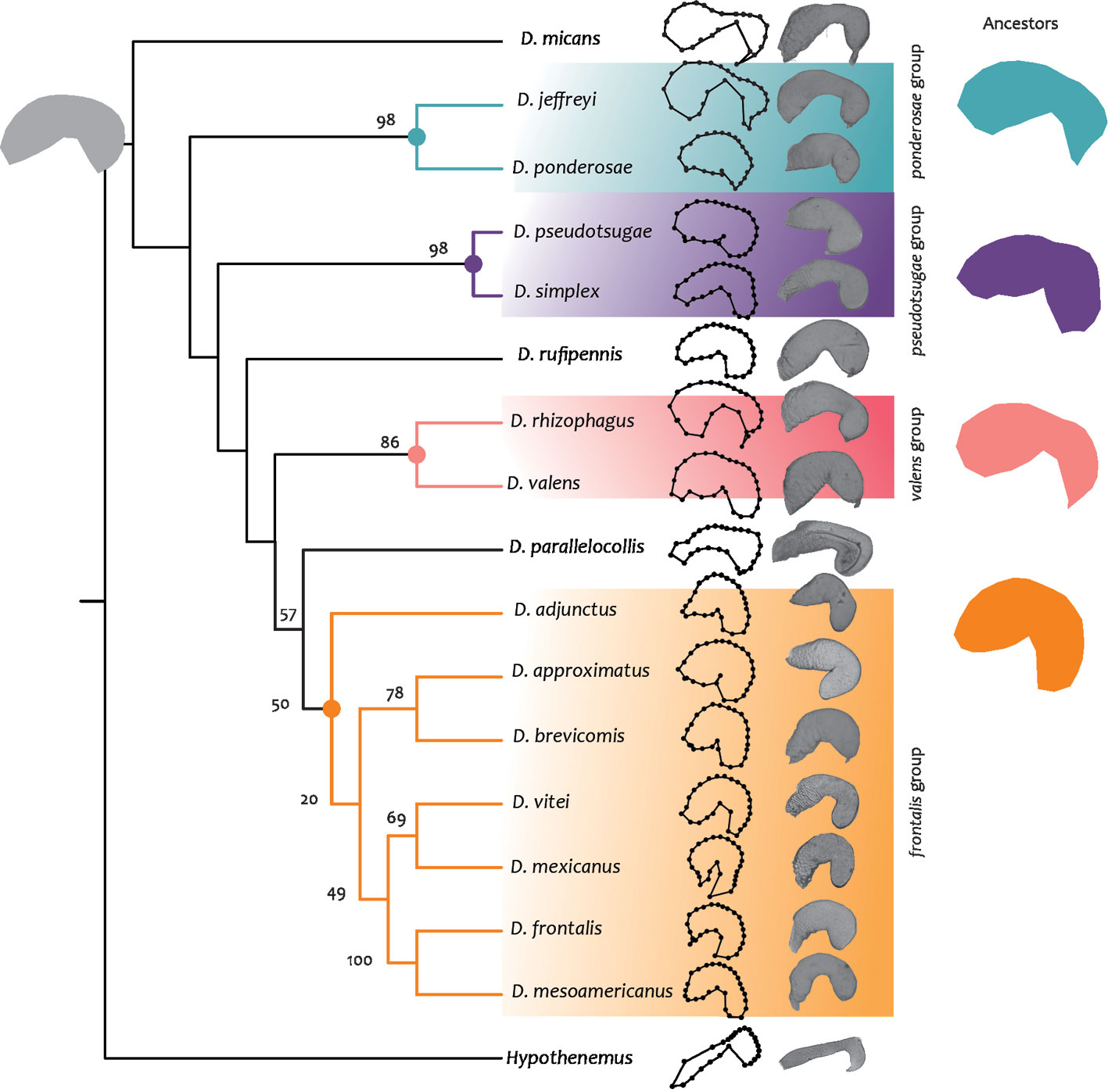
Phylogenetic signal. Both phylogenetic trees constructed from fragments of the mitochondrial COI gene and with total evidence strategy showed an arrangement like those previously reported by Godefroid et al. (2019), Reeves et al. (2012), and Víctor and Zúñiga (2016), (Figs. 10, 12). Similar to previous phylogenetic hypotesis, we recognized 4 groups, the first one corresponding to the frontalis group including: D. adjunctus, D. approximatus, D. brevicomis, D. vitei, D. mexicanus, D. frontalis and D. mesoamericanus. The second group is composed by the valens group (D. rhizophagus, D. valens); the third one corresponds to the pseudotsugae group (D. pseudotsugae, D. simplex), and the fourth one to the ponderosae group (D. jeffreyi, D. ponderosae), the remaining species (D. parallelocollis, D. rufipennis, and D. micans) were not grouped within any clade. Both phylogenies differed in the relative position of D. parallelocollis, D. rufipennis, D. adjunctus within the frontalis, ponderosae and valens groups. In the molecular hypothesis, D. adjunctus is the sister of the remainder species of the frontalis group; the frontalis group is closely related to D. parallelocollis; the valens group is the sister clade of D. parallelocollis-frontalis group; D. rufipennis is the sister of valens group, D. parallelocollis and frontalis group; D. ponderosae is a sister group of the most species of Dendroctonus less D. micans. In the total evidence hypothesis, D. adjunctus is the sister of D. brevicomis-D. approximatus; the ponderosae group is sister of the frontalis group; the pseudotsugae group is the sister of ponderosae-frontalis groups; the valens group is the sister of D. parallelocollis.
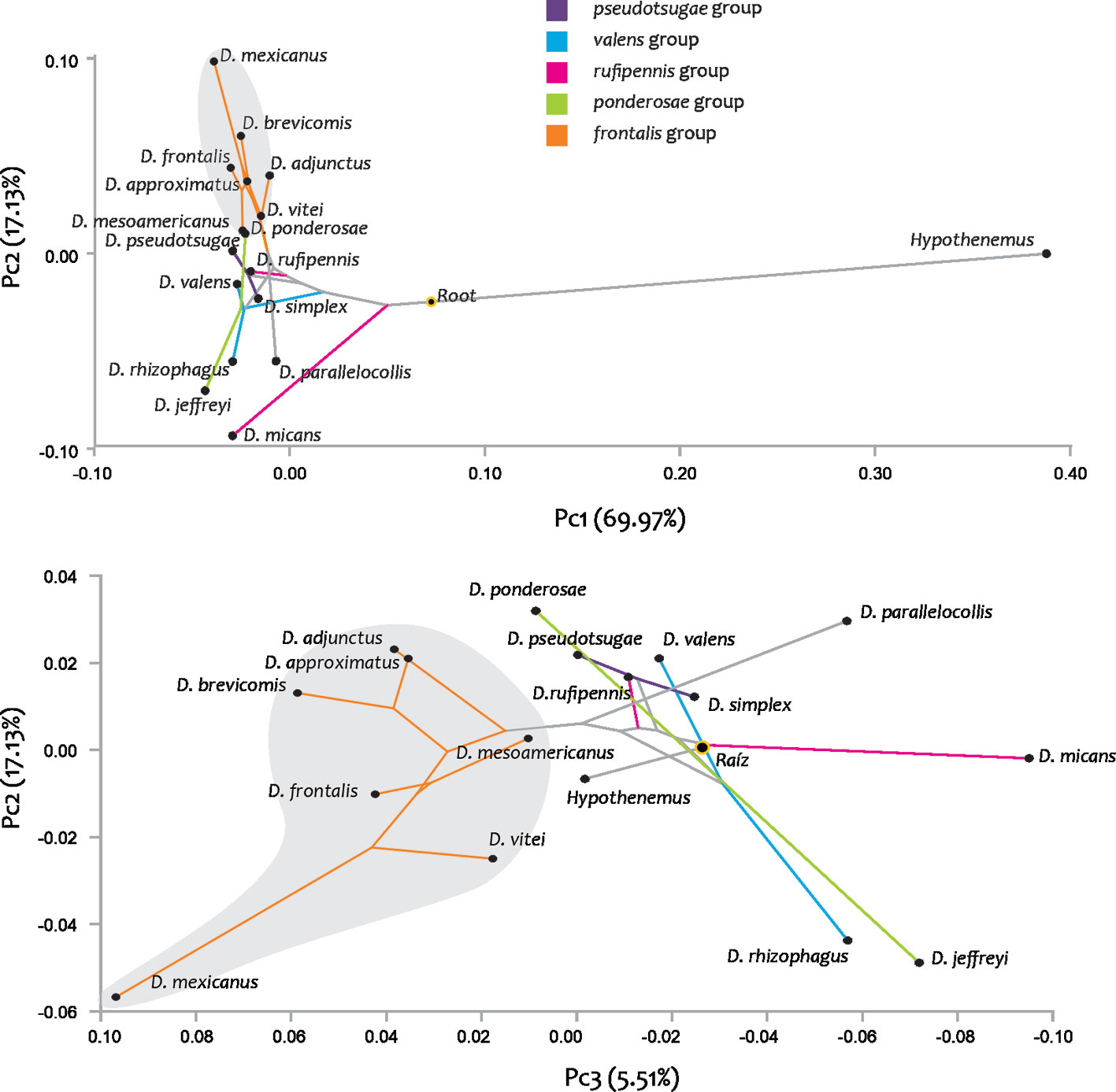
Phylogenetic signal based on molecular hypothesis. The principal component analysis to assess the shape change in phylogeny with molecular data quantified 92.61% of the variance in the first 3 PCs (PC1: 69.97%, PC2: 17.13%, and PC3: 5.51%). The mapping of the principal components of shape in the phylogenetic tree obtained provided knowledge of the changes in shape of the seminal receptacle in the hypothetical ancestors of the different nodes of the tree. The most recent common ancestor (MCRA) of Dendroctonus was characterized by a larger nodulus than the cornu, an oval-shaped cornu, and a slightly pronounced median constriction (diameter of the median constriction greater than that of the nodulus and equal to that of the cornu). The receptacle corresponding to the ancestor of the frontalis group presented a nodulus of the same size as the cornu, the latter of spherical shape, without median constriction (diameter of the median constriction equal to that of the nodulus and cornu) and the inner curvature more pronounced, so, the nodulus and cornu were close to each other. The ancestor of the valens group presented receptacles with a larger nodulus than cornu, the latter oval, with a slight median constriction (diameter of the median constriction smaller than that of the nodulus and cornu), and the internal curvature little pronounced, so, the nodulus and cornu were distant from each other. The receptacle of the ancestor of the pseudotsugae group presented a larger nodulus than cornu, the latter of spherical shape, without median constriction (diameter of the median constriction equal to that of the nodulus and cornu), and the internal curvature little pronounced, so that the nodulus and cornu were distant from each other. The ancestor of the ponderosae group had a larger nodulus than cornu, the latter being oval, with evident median constriction (diameter of the median constriction smaller than that of the nodulus and equal to that of the cornu) and the internal curvature not very pronounced, so, that the nodulus and cornu were distant from each other (Fig. 10).
The phylogenetic signal was statistically significant (p < 0.05; p = 0.049); mapping changes in shape onto phylogeny yielded a tree length of 0.15, which compared to the initial tree was shorter (37.23). In the phylomorphospace, the members of the genus were relatively dispersed and separated into 2 distinct non-overlapping groups, one composed of the members of the frontalis complex and the other with the remaining groups and species (Fig. 11). However, within each group, the pattern of species dispersal was partly consistent with the molecular phylogenetic hypothesis obtained here (Fig. 10). In the frontalis group, overlapping of the terminal branches of D. adjunctus with (D. approximatus, D. brevicomis) and of D. frontalis with the group of (D. mexicanus, D. vitei) were observed. In species not belonging to the frontalis complex, many changes in direction in the reconstructed trajectories of evolutionary change and overlapping between terminal branches were observed, indicating the presence of homoplasy in most of them.
Phylogenetic signal based on total evidence hypothesis. Principal component analysis to assess shape change in phylogeny from molecular and morphological data quantified 84.39% of the variance in the first 3 PCs (PC1: 55.99%, PC2: 18.88%, and PC3: 9.61%). The mapping of the principal components of shape in the phylogenetic tree supported similar morphological trajectories among the ancestor of all groups of the genus, which were described in the anterior section (Fig. 12).
The phylogenetic signal was statistically significant (p = 0.014); mapping changes in shape onto phylogeny yielded a tree length of 0.036, which compared to the initial tree was shorter (56). In the phylomorphospace of PC1 and PC2, members of the genus were relatively dispersed and separated into 2 distinct non-overlapping groups, one composed of the members of the frontalis group and the other with the remaining groups and species (Fig. 13). However, in the PC2 and PC3 phylomorphospace the members of the genus did not segregate, keeping the groups overlapping (Fig. 13). However, within each group, the pattern of species dispersal was only partially consistent with the phylogenetic hypothesis from molecular and morphological data obtained here (Fig. 12). In the frontalis group, overlap of the terminal branches of D. adjunctus with D. approximatus was observed. In species not belonging to the frontalis complex, many directional changes in the reconstructed trajectories of evolutionary change and overlap between terminal branches were observed, indicating the presence of homoplasy in most of them.
Discussion
In Coleoptera 5 patterns of morphological organization of the spermatheca have been recognized based on the presence and absence of 3 elements: duct, receptacle, and gland (De Marzo, 2009). The study of 16 of the 21 species of Dendroctonus showed that the type of spermatheca in the genus corresponded to the most common type in Coleoptera.
The anatomical descriptions of the male and female reproductive apparatus in Dendroctonus, Cerezque (1964) also recognized 3 parts in the spermatheca: a spermathecal duct, a driving organ (with compressor muscles), and a sac. The driving organ was described as a U-shaped sclerotized capsule, the spermathecal duct as a tube connecting in the proximal portion to the driving organ, and the sac as a mass of tissue connected at the outer curvature of the driving organ. The sac was attributed glandular function by its histologycal characteristics. According to these descriptions, the spermathecal duct would correspond to the sperm duct, the driving organ to the receptacle, and the spermathecal sac to the gland sensu De Marzo (2009).
Although the spermatheca has been studied to diagnose different groups of Curculionidae, because it presents characteristics with high taxonomic value (Aslam, 1961; Sanders, 1960), the literature presents inconsistencies in its nomenclature (Tables 4-7). A few studies recognize the spermatheca as an organ integrated by different elements (Erbey et al., 2010; Sanders, 1960), and in most of them (Anderson, 1984; Brizzola dos Santos & Rosado-Neto, 2010; Lanteri & del Río, 2008; Omar, 2012; Velázquez-de Castro et al., 2007), including those focused on species of Scolytinae (Pérez-Silva & Equihua-Martínez, 2016; Román-Ruíz et al., 2017; Rubio et al., 2008), particularly within Dendroctonus (Armendáriz-Toledano et al., 2014, 2017; García-Román et al., 2019; Ríos-Reyes et al., 2008), the term spermatheca is used to refer only to the receptacle (Table 6). Therefore, we suggest that in the future the nomenclature proposed by De Marzo (2009) be used, which can be applied to a greater number of species and larger taxonomic scales, facilitating the comparison of this organ for taxonomic and phylogenetic purposes.
Generally, most studies recognize 2 regions, the nodulus and the cornu, which are defined as the proximal and distal part of the receptacle, respectively (Anderson, 1984; Armendáriz-Toledano et al., 2014; García-Román et al., 2019; Lanteri & del Río, 2008; Pérez-Silva & Equihua-Martínez, 2016; Román-Ruíz et al., 2017; Rubio et al., 2008; Sanders, 1960; Velázquez-de Castro et al., 2007). In addition, other parts are named, such as the body or collum, which is an intermediate region between the nodulus and the cornu (Brizzola-dos Santos & Rosado-Neto 2010; Lanteri & del Río, 2008; Omar, 2012; Velázquez-de Castro et al., 2007), and the ramus, which is the junction point between the accessory gland and the receptacle (Brizzola-dos Santos & Rosado-Neto, 2010; Lanteri & del Río, 2008; Omar, 2012; Sanders, 1960). The comparison of these elements in our study allowed us to recognize that the cornu was formed only by a slight curvature of the receptacle, and the latter was not differentiated from the ramus, which has been recognized as the simplest morphological pattern compared to other families of Coleoptera (Sanders, 1960).
The characteristics associated with the striae of the receptacle were not very useful for the identification of the species of Dendroctonus; since in all evaluated members, there were individuals with different proportions of the receptacle covered by striae. Previous works, comparing the number, density, and proportion of striae covering this structure, found high intraspecific variation, which did not allow the establishment of diagnostic characteristics in species with great morphological similarity such as D. frontalis–D. mexicanus (Ríos-Reyes et al., 2008), D. approximatus–D. parallelocollis (García-Román et al., 2019), as well as between the sister species D. frontalis–D. mesoamericanus (Armendáriz-Toledano et al., 2014) and D. mexicanus–D. vitei (Armendáriz-Toledano et al., 2017). Therefore, it is suggested that characteristics associated with receptacle striae should be used with caution.
The 6 measures used to characterize the receptacle (size) (Table 4), as well as the variables to analyze its shape, showed wide intraspecific variation in the members of the genus, which prevented the recognition of discrete species-specific characteristics in most taxa. However, both continuous quantitative and discrete qualitative variables supported that receptacle morphology has been differentiated in some species and groups, as shown by PCoA in which 11 species were recovered as discrete groups.
With respect to continuous measurements, it was possible to recognize at least 3 groups based on the size of their receptacles: small (D. adjunctus, D. brevicomis, D. frontalis, D. mesoamericanus, D. mexicanus, D. simplex, and D. vitei), medium (D. approximatus, D. jeffreyi, D. parallelocollis, D. ponderosae, D. pseudotsugae, D. rhizophagus, and D. rufipennis), and large (D. micans and D. valens) (Fig. 8a). Species in these groups showed more pronounced differences in nodulus diameter, nodulus length, and the distance between the nodulus and cornu (Table 4). Although these measures do not overlap among the groups, they are recommended to be used with caution for taxonomic purposes, because receptacle size (PC1) showed a positive relationship with insect size, a measure that also exhibits wide intraspecific variation (Fig. 8b).
The shape variables also allowed us to recognize that the receptacle has been differentiated into 4 large groups within the genus: one integrated by species of the D. frontalis complex, which present receptacles with pronounced curvature and without constriction (Fig. 9, “(a) group”), a second one by the species D. micans, D. parallelocollis, D. rufipennis, D. simplex, and D. valens that present receptacles with less pronounced curvature and constriction (Fig. 9, “(b) group”), another one integrated by the species D. jeffreyi and D. rhizophagus that have narrow nodulus and cornu of spherical shape (Fig. 9, “(c) group”). Finally, another group conformed by D. ponderosae and D. pseudotsugae with receptacles absent of constriction in the curvature, as well as the nodulus and cornu far from each other (Fig. 9, “(d) group”).
Interestingly, within these groups there were cases in which the shape of the receptacle was species-specific, such as D. mexicanus, D. micans, and D. rhizophagus, allowing them to be identified from the rest of the congeners. D mexicanus showed a small receptacle with very pronounced curvature, no constriction, a cornu and nodulus of similar length and diameter, and without prolongation of the cornu; D. micans presented a larger receptacle, less evident curvature, constriction in the curvature, a nodulus of greater length and diameter than the cornu, and a long and thick prolongation on the surface of the cornu; D. rhizophagus had a medium-sized receptacle, with less obvious curvature, constriction in curvature, a nodulus of similar length and diameter as the cornu, and a short prolongation on the surface of the cornu.
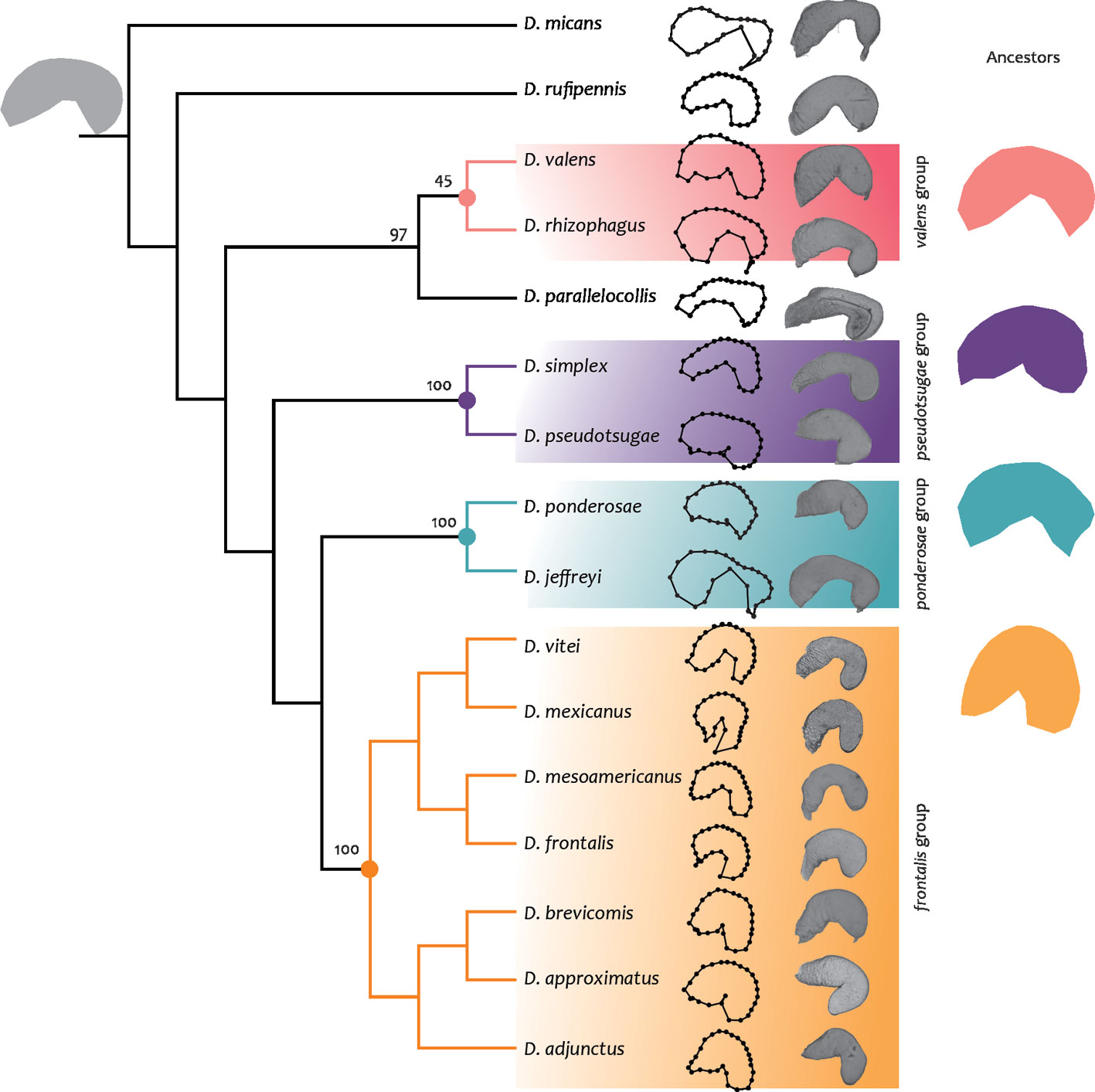
Phylogenetic hypothesis. This hypothesis based on mitochondrial data was similar to previous studies (Godefroid et al., 2019; Reeves et al., 2012; Víctor & Zúñiga, 2016), as it recovered 4 of the 5 groups proposed for the genus, frontalis (D. adjunctus ((D. approximatus, D. brevicomis) ((D. vitei, D. mexicanus) (D. frontalis, D. mesoamericanus)))), pseudotsugae (D. pseudotsugae, D. simplex), ponderosae (D. jeffreyi, D. ponderosae), and valens (D. rhizophagus, D. valens). Although the species D. micans and D. rufipennis were included, these taxa were not recovered in a monophyletic group as in previous phylogenies (Godefroid et al., 2019; Reeves et al., 2012; Víctor & Zúñiga, 2016). Similarly to the topology from Víctor and Zúñiga (2016), D. parallelocollis turned out to be a distinctive taxon that did not cluster within the major clades and was more closely related to the frontalis group in our COI hypotheses, in contrast to Víctor and Zúñiga (2016) who relate it with the valens group (Fig. 8) and with our total evidence analysis. Another difference was the position of D. adjunctus within the frontalis group, a taxon that was recovered as a sister species to (((D. frontalis, D. mesoamericanus) (D. mexicanus, D. vitei)) (D. brevicomis, D. approximatus) in our molecular tree, while in previous work and our total evidence phylogeny it constituted the sister species of the latter 2 taxa.
The relationships between the groups also showed different patterns with respect to previous phylogenies. In our tree, the valens group was sister to D. parallelocollis + frontalis group, while other authors related it to the rufipennis group (Reeves et al., 2012), to the frontalis group, (Godefroid et al., 2019) and with D. parallelocollis (Víctor & Zúñiga, 2016), the last agree with our total evidence tree. The pseudotsugae group of our molecular tree was sister to D. rufipennis + valens group + D. parallelocollis + frontalis group, and in the total evidence analysis was sister to ponderosae group + frontalis group, while Reeves et al. (2012) recovered it as sister to D. armandi, Víctor and Zúñiga (2016) as sister of the valens + rufipennis group, and Godefroid et al. (2019) related with the valens + rufipennis + frontalis group. Finally, the ponderosae group in previous studies was sister to the frontalis group (Godefroid et al., 2019; Reeves et al., 2012; Víctor & Zúñiga, 2016), while in our tree, the ponderosae group was sister to the pseudotsugae group.
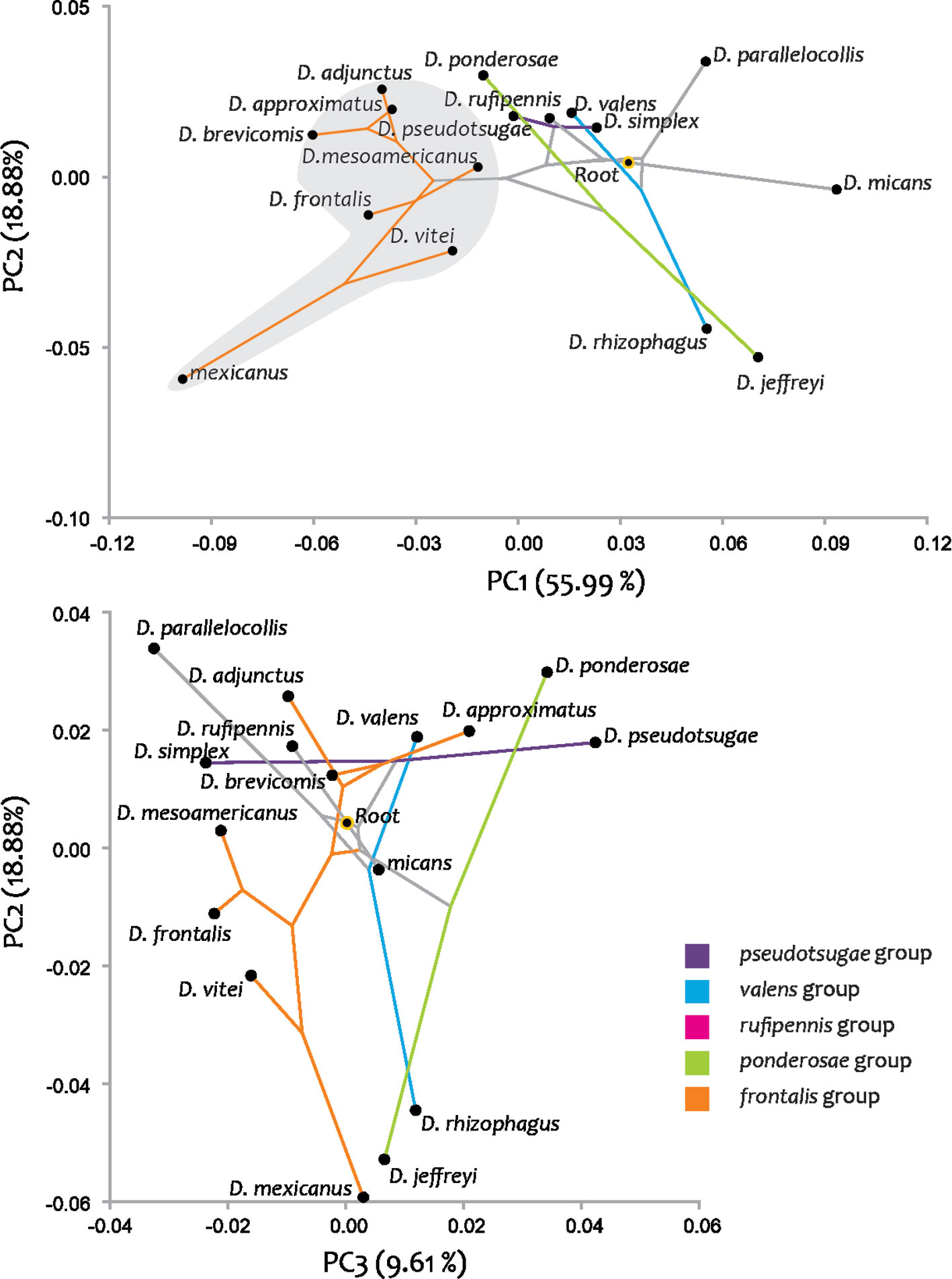
Phylogenetic signal. Hypothesis testing for the absence of a phylogenetic signal indicated that relatedness among Dendroctonus members was associated with interspecific variation in seminal receptacle shape; however, the data showed a relatively low phylogenetic signal. Using the phylogenetical tree hypothesis with molecular data, the phylogenetic signal within the frontalis group is graphically clearer than when using a total evidence hypothesis. The shape components of this structure mapped onto the phylogeny, allow us to observe that the distribution of species in the phylomorphospace, corresponded in part, to the phylogenetic pattern, since only one of the groups supported by the phylogeny was recovered, which corresponded to the frontalis group. Within the frontalis group, most of the species presented a clear relationship between relatedness and morphology, since the terminal branches were short, close, and did not overlap each other, except in 2 members of the complex. The terminal branches corresponding to the other groups and species of the genus presented different directions of evolutionary change in the reconstructed trajectories and overlapping of branches, indicating the presence of homoplasy (Figs. 11, 13).
Table 6
Nomenclature and alternative terms of the spermatheca in Curculionidae, proposed by different authors.
| Taxa | Conduct | Receptacle | Gland | Reference | |
| Dryophthorinae | Sitophilus oryzae | Spermatic duct | Spermatheca | – | Omar, 2012 |
| Entiminae | Naupactinii | Spermathecal conduct | Spermatheca | Spermathecal gland | Lanteri and del Río, 2008 |
| Entiminae | Sitonini | – | Spermatheca | – | Velázquez-de Castro de Castro et al., 2007 |
| Leptopiinae | Connatichela artemisae | Spermatic duct | Spermatheca | Spermathecal gland | Anderson, 1984 |
| Lixinae | Lixus nordmanni | Spermathecal duct | Receptaculum seminis | Accessory gland | Erbey et al., 2010 |
| Molytinae | Heilus spp. | Spermathecal duct | Spermatheca | Spermathecal gland | Brizzola dos Santos and Rosado-Neto, 2010 |
| Polyphaga | Rhynchophora | Spermathecal duct | Ductus receptaculi | Spermathecal gland | Sanders, 1960 |
| Scolitynae | Hypotenemus hampei | Spermatic duct | Spermatheca | Spermathecal gland | Román-Ruíz et al., 2017 |
| Scolitynae | Hypotenemus hampei | – | Spermatheca | Accessory gland | Rubio et al., 2008 |
| Scolitynae | Xyleborus volvus | – | Spermatheca | – | Pérez-Silva and Equihua-Martínez, 2016 |
| Scolitynae | Dendroctonus monticolae (= ponderosae) | Spermathecal duct | Driving organ | Sac | Cerezque, 1967 |
| Scolitynae | Dendroctonus mexicanus | – | Spermatheca | – | Ríos Reyes et al., 2008 |
| Scolitynae | Dendroctonus frontalis | – | Spermatheca | – | Ríos Reyes et al., 2008 |
| Scolitynae | Dendroctonus frontalis | – | Spermatheca | – | Armendáriz-Toledano et al., 2014 |
| Scolitynae | Dendroctonus mesoamericanus | – | Spermatheca | – | Armendáriz-Toledano et al., 2014 |
| Scolitynae | Dendroctonus parallelocollis | – | Spermatheca | – | García-Román et al., 2019 |
| Scolitynae | Dendroctonus approximatus | – | Spermatheca | – | García-Román et al., 2019 |
The absence of a phylogenetic signal in more than half of the species of the genus may be due to the use of an incomplete phylogenetic hypothesis, which included only 16 of the 21 species recognized for the genus. Our tree only allowed us to recover 4 of the 5 groups supported by previous studies and differed in the relationships between them. In fact, the only group in which a topology partially concordant with other works was recovered was the frontalis group, whose members have a clearer morphology relationship. Although to date there are several hypotheses of ancestry-descent in these bark beetles, all of them differ in the composition of the groups and their relative position, because they were estimated with different types and sizes of character matrices. For example, the phylogeny that included the most robust data set (Rad seq), did not include 3 members of the genus, most notably D. paralellocollis (Godefroid et al., 2019), a taxon that obscures the relationships among species, when it is included in the phylogeny (Víctor & Zúñiga, 2016). The topology of the latter authors was based on a “total evidence” approach combining morphological and molecular attributes; however, it gave weight to larval characteristics that evolved independently and, therefore, proved homoplasy considering the more recent “resolved” phylogeny of Godefroid et al. (2019).
Table 7
Nomenclature of the receptacle in members of Curculionidae, proposed by different authors.
| Taxa | Regionalization of the receptacle | Reference |
| Sitophilus oryzae | Cornu – Collum – Ramus – Apex | Omar, 2012 |
| Naupactinii | Cornu – Body – Ramus – Nodulus | Lanteri and del Río, 2008 |
| Sitonini | Cornu – Corpus- Nodulus | Velázquez-de Castro de Castro et al., 2007 |
| Connatichela artemisae | Cornu – Ramus – Nodulus | Anderson, 1984 |
| Lixus nordmanni | Apex – Basal part | Erbey et al., 2010 |
| Heilus spp. | Cornu – Collum – Ramus | Brizzola dos Santos and Rosado-Neto, 2010 |
| Rhynchophora | Cornu – Ramus – Nodulus | Sanders, 1960 |
| Hypotenemus hampei | Not mentioned | Román-Ruíz et al., 2017 |
| Hypotenemus hampei | Not mentioned | Rubio et al., 2008 |
| Xyleborus volvus | Cornu – Ramus – Nodulus | Pérez-Silva and Equihua-Martínez, 2016 |
| Dendroctonus mexicanus | Not mentioned | Ríos Reyes et al., 2008 |
| Dendroctonus frontalis | Not mentioned | Ríos Reyes et al., 2008 |
| Dendroctonus frontalis | Cornu – Nodulus | Armendáriz-Toledano et al., 2014 |
| Dendroctonus mesoamericanus | Cornu – Nodulus | Armendáriz-Toledano et al., 2014 |
| Dendroctonus parallelocollis | Cornu – Nodulus | García-Román et al., 2019 |
| Dendroctonus approximatus | Cornu – Nodulus | García-Román et al., 2019 |
Other factors obscuring the phylogenetic signal may be the plastic environmental response of species, the emergence of parallel functional adaptations (Ge et al., 2015), as well as considerable differences in the age of common ancestors in phylogeny, as older common ancestors tend not to exhibit a phylogenetic signal (Caumul & Polly, 2005). However, these factors cannot be contrasted until we have a phylogenetic hypothesis of the group that includes all members of the genus and with a greater number of genetic attributes.
Acknowledgements
To María Berenit Mendoza Gárfias and Pedro Mercado Ruaro for the scanning electron and phase contrast microphotographs and SEM micrographs, respectively (Laboratorio Nacional de Biodiversidad LaNaBio, Instituto de Biología, UNAM). J.G.-R. was a Conacyt fellow (617368), F.A.-T. was a member of Sistema Nacional de Investigadores-Conacyt. This research was funded by PAPIIT-UNAM (IA201720, IA203122) and Conacyt Fronteras de la Ciencia (139030) (F.A.-T.).
References
Anderson, R. (1984). Connatzchela artemzszae, a new genus and species of weevil from the Yukon territory (Coleoptera: Curculionidae: Leptopiinae): taxonomy, paleontology, and biogeography. The Canandian Entomologist, 116, 1571–1580. https://doi.org/10.4039/Ent1161571-11
Armendariz-Toledano, F., García, J., López, M., Sullivan, B., & Zúñiga, G. (2017). New characters and redescription of Dendroctonus vitei (Coleoptera: Curculionidae: Scolytinae). The Canadian Entomologist, 149, 413–433. https://doi.org/10.4039/tce.2017.10
Armendariz-Toledano, F., Niño, A., Sullivan, B., Kirkendall, L., & Zúñiga, G. (2015). A new species of bark beetle, Dendroctonus mesoamericanus sp. nov. (Curculionidae: Scolytinae), in southern México and Central America. Annals of the Entomological Society of America, 108, 403–414. https://doi.org/10.1093/aesa/sav020
Armendariz-Toledano, F., Niño, A., Sullivan, B., Macías, J., Víctor, J., Clarke, S. et al. (2014). Two species within Dendroctonus frontalis (Coleoptera: Curculionidae): evidence from morphological, karyological, molecular, and crossing studies. Annals of the Entomological Society of America, 107, 11–27. https://doi.org/10.1603/AN13047
Armendariz-Toledano, F., & Zúñiga, G. (2017). Illustrated key to species of the genus Dendroctonus (Coleoptera: Curculionidae) occurring in Mexico and Central America. International Journal of Insect Science, 17, 1–15. https://doi.org/10.1093/jisesa/iex009
Aslam, N. (1961). An assessment of some internal characters in the higher classification of the Curculionidae s.l. (Coleoptera). Transactions of the Royal Entomological Society of London, 113, 417–480.
Bookstein, F. (1991). Morphometrics tools for landmark data: Geometry and Biology. Cambridge University Press, United Kingdom.
Brizzola, G., & Rosado, G. (2010). Morphological aspects of the genitalia of seven species of Heilus Kuschel (Coleoptera, Curculionidae). Revista Brasileira de Entomologia, 54, 157–164. https://doi.org/10.1590/S0085-56262010000200001
Caumul, R., & Polly, P. D. (2005). Phylogenetic and environmental components of morphological variation: skull, mandible, and molar shape in marmots (Marmota, Rodentia). Evolution, 59, 2460–2472. https://doi.org/10.1111/j.0014-3820.2005.tb00955.x
Cerezke, H. (1964). The morphology and functions of the reproductive systems of Dendroctonus monticolae Hopk. (Coleoptera: Scolytidae). The Canadian Entomologist, 96, 477–500.
Chursina, M., & Negrobov, O. (2018). Phylogenetic signal in the wing shape in the subfamily Dolichopodinae (Diptera, Dolichopodidae). Entomological Review, 98, 515–527. https://doi.org/10.1134/S0013873818050019
De Marzo, L. (2009). Biodiversità della spermateca nei Coleotteri. Atti Accademia Nazionale Italiana di Entomologia, 56, 69–96.
Edler, D., Klein, J., Antonelli, A., & Silvestro, D. (2021). raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods in Ecology and Evolution, 12, 373–377. https://doi.org/10.1111/2041-210X.13512
Erbey, M., Candan, S., & Coçak, E. (2010). External morphology of the female genitalia of Lixus nordmanni Hochhuth, 1847 (Coleoptera: Curculionidae, Lixinae): a scanning electron microscope study. Gazi University Journal of Science, 23, 385–391.
García-Román, J., Armendáriz, F., Valerio, O., & Zúñiga, G. (2019). An assessment of old and new characters using traditional and geometric morphometrics for the identification of Dendroctonus approximatus and D. parallelocollis (Coleoptera: Curculionidae: Scolytinae). Journal of Insect Science, 19, 14. https://doi.org/10.1093/jisesa/iey131
Ge, Q., Wang, H., & Dai, J. (2015). Phenological response to climate change in China: a meta-analysis. Global Change Biology, 21, 265–274. https://doi.org/10.1111/gcb.12648
Gidaszewski, N., Baylac, M., & Klingenberg, C. (2009). Evolution of sexual dimorphism of wing shape in the Drosophila melanogaster subgroup. BMC Evolutionary Biology, 9, 110. https://doi.org/10.1186/1471-2148-9-110
Godefroid, M., Meseguer, A., Saune, L., Genson, G., Streito, J., Rossi, J. et al. (2019). Restriction-site associated DNA markers provide new insights into the evolutionary history of the bark beetle genus Dendroctonus. Molecular Phylogenetics and Evolution, 139, 106528. https://doi.org/10.1016/j.ympev.2019.106528
Hammer, Ø., Harper, D., & Ryan, P. (2001). PAST: Paleontological statistics software package for education and data analysis. Retrieved on 06 December, 2021 https://www.nhm.uio.no/english/research/infrastructure/past/
Hopkins, A. (1909). Contributions toward a monograph of the scolytid beetles: I the genus Dendroctonus. U.S. Department of Agriculture Bureau of Entomology Technical Series 17 (Part I). Washington D.C.: Washington Govt.
Klingenberg, C. (2011). MorphoJ: an integrated software package for geometric morphometrics. Molecular Ecology
Resources, 11, 353–357. https://doi.org/10.1111/j.1755-0998.
2010.02924.x
Klingenberg, C., & Gidaszewski, N. (2010). Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Systematic Biology, 59, 245–61. https://doi.org/10.1093/sysbio/syp106
LaBonte, J., & Valley, S. (2013). Illustrated key to the Dendroctonus of North America. Retrieved on 06 December, 2021 from: https://conference.ifas.ufl.edu/NPDN/Docs/Workshop%20Materials/Dendroctonus%20092813.pdf
Lanier, G., Hendrichs, J., & Flores, J. (1988). Biosystematics of the Dendroctonus frontalis (Coleoptera: Scolytidae) complex. Annals of Entomological Society of America, 81, 403–418.
Lantieri, A., & Del Río, M. (2008). Caracteres genitales de la hembra en la clasificación y filogenia de la tribu Naupactini (Coleoptera: Curculionidae). In J. Llorente-Bousquetes, & A. Lanteri (Eds.), Contribuciones taxonómicas de órdenes de insectos megadiversos (pp. 159–176). CDMX: Las Prensas de Ciencias, UNAM.
López, M., Armendáriz, F., Macías, J., Shibayama, M., & Zúñiga, G. (2014). Comparative study of the antenna of Dendroctonus rhizophagus and Dendroctonus valens (Curculionidae: Scolytidae): sensilla types, distribution and club shape. Annals of the Entomological Society of America, 107, 1130–1143. https://doi.org/10.1603/AN14069
Omar, Y. (2012). Morphological studies on some external and internal structures of rice weevil, Sitophilus oryzae (l.) (Coleoptera: Curculionidae), a major pest of the stored cereals in Egypt. Mansoura Journal of Plant Protection and Pathology, 3, 843–863.
Pérez, I., Bernal, V., & González, P. (2006). Differences between sliding semi-landmark methods in geometrics morphometrics, with an application to human craniofacial and dental variation. Journal of Anatomy, 208, 769–784. https://doi.org/10.1111/j.1469-7580.2006.00576.x
Pérez-Silva, M., & Equihua-Martínez, A. (2016). Distinción morfológica de dos morfotipos de Xyleborus volvulus (Fabricius) 1775 (Coleoptera: Curculionidae: Scolytinae). Revista Entomología Mexicana, 3, 955–960.
Pérez-Silva, M., Equihua-Martínez, A., Estrada-Venegas, E., Muñoz-Viveros, A., Valdez-Carrasco, J., Sánchez-Escudero, J. et al. (2015). Sinopsis de especies mexicanas del género Xyleborus Eichhoff, 1864 (Coleoptera: Curculionidae: Scolytinae). Acta Zoológica Mexicana, 31, 239–250. https://doi.org/10.21829/azm.2015.312546
Reeve, J., Anderson, F., & Kelley, S. (2012). Ancestral state reconstruction for Dendroctonus bark beetles: evolution of a tree killer. Environmental Entomology, 41, 723–730. https://doi.org/10.1603/EN11281
Revell, L. (2012). phytools: an R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution, 3, 217–223. https://doi.org/10.1111/j.2041-210X.2011.00169.x
Ríos-Reyes, A., Valdez-Carrasco, J., Equihua-Martínez, A., & Moya-Raygoza, G. (2008). Identification of Dendroctonus frontalis (Zimmermann) and D. mexicanus (Hopkins) (Coleoptera: Curculionidae: Scolytinae) through structures of the female genitalia. Coleopterist Bulletin, 62, 99–103.
Rohlf, F. (2004). tpsDig Version 1.40. Retrieved on 06 december, 2021 de: https://life2.bio.sunysb.edu/ee/rohlf/software.html
Román-Ruíz, A., Michel, B., Dufour, B., Rojas, J., Cruz-López, L., & Barrera, J. (2017). Description of the sperm and spermatheca of Hypothenemus hampei (Coleoptera: Curculionidae: Scolytinae) for the differentiation of mated and unmated females. Annals of Entomological Society of America, 110, 353–359. https://doi.org/10.1093/aesa/sax033
Rubio, J., Bustillo, A., Vallejo, L., Acuña, J., & Benavides, P. (2008). Alimentary canal and reproductive tract of Hypothenemus hampei (Ferrari) (Coleoptera: Curculionidae, Scolytinae). Neotropical Entomology, 37, 143–151. https://doi.org/10.1590/S1519-566X2008000200006
Sanders, H. (1960). The female genitalia and spermathecae of some of the Rhynchophora. The Great Basin Naturalist, 20, 1–22.
Sheets, H. (2003). IMP-integrated morphometrikage. Retrieved on 06 december, 2021 de: https://www.animal-behaviour.de/imp/
Valerio-Mendoza, O., García-Román, J., Becerril, M., Armendáriz-Toledano, F., Cuéllar, G., Negrón, J. et al. (2019). Cryptic species discrimination in western pine beetle, Dendroctonus brevicomis LeConte (Curculionidae: Scolytinae), based on morphological characters and geometric morphometrics. Insects, 10, 377. https://doi.org/10.3390/insects10110377
Velázquez-de Castro, A., Alonso, M., & Outerelo, R. (2007). Systematics of Sitonini (Coleoptera: Curculionidae: Entiminae), with a hypothesis on the evolution of feeding habits. Systematic Entomology, 32, 312–331. https://doi.org/10.1111/j.1365-3113.2006.00368.x
Víctor, J., & Zúñiga, G. (2016). Phylogeny of Dendroctonus bark beetles (Coleoptera: Curculionidae: Scolytinae) inferred from morphological and molecular data. Systematic Entomology, 41, 162–177. https://doi.org/10.1111/syen.12149
Wood, S. (1982). The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. The Great Basin Naturalist, 6, 1086.