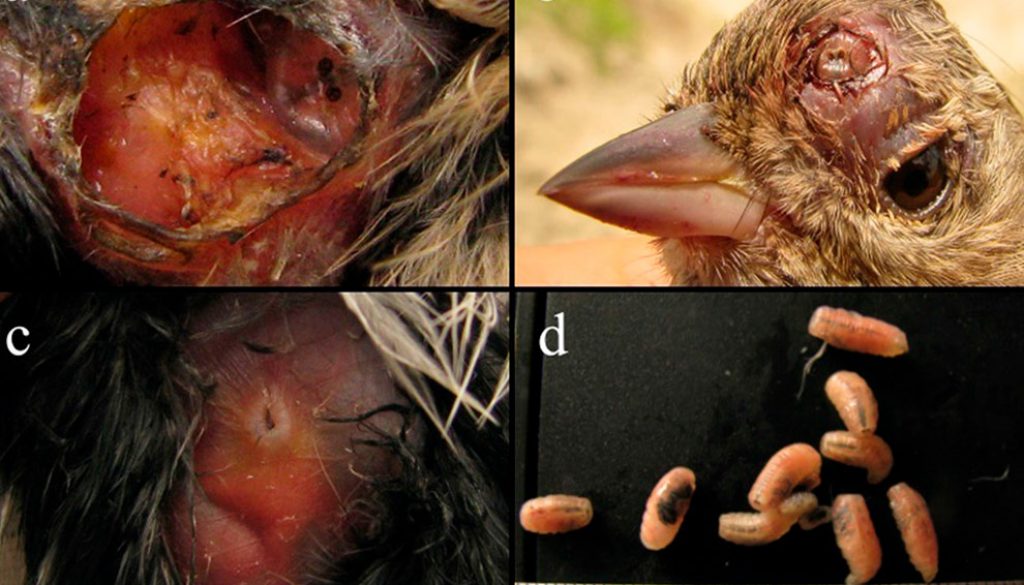
Héctor Cadena-Ortiz a, Martín Quiroga b, c, Elisa Bonaccorso d, *
a Instituto Nacional de Biodiversidad. Calle Rumipamba 341 y Av. de los Shyris, 17-07-8976, Quito, Ecuador
b Universidad Nacional del Litoral-CONICET, Instituto de Ciencias Veterinarias del Litoral, Laboratorio de Ecología de Enfermedades, Kreder 2805, S3080HOF, Esperanza, Santa Fe, Argentina
c Instituto Tecnológico de Santo Domingo, Avenida de Los Próceres #49, Los Jardines del Norte, 10602 Santo Domingo, República Dominicana
d Universidad San Francisco de Quito, Colegio de Ciencias Biológicas y Ambientales, Instituto Biósfera, Laboratorio de Biología Evolutiva, Vía Interoceánica y Diego de Robles 17-1200-841, Quito, Ecuador
*Corresponding author: elisabonaccorso@gmail.com (E. Bonaccorso)
Received: 22 June 2021; accepted: 8 March 2022
Abstract
Myiasis by Philornis is a usual phenomenon in Neotropical birds. Philornis larvae are hematophagous and are known to affect both nestlings and adults. Still, parasitism in adults seems opportunistic and has been poorly studied compared to parasitism in nestlings. Here, we inspected 1,429 adult and juvenile birds of 41 species from an Andean dry forest in northern Ecuador, searching for infestations by Philornis larvae. For the first time, we report parasitism by Philornis in adult birds of 6 species of passerine birds (Troglodytes aedon, Conirostrum cinereum, Geospizopsis plebejus, Zonotrichia capensis, Pheucticus chrysogaster, and Spinus magellanicus). We also report the first cases of infestation by the Philornis torquans complex in Ecuador and provide data on the prevalence of Philornis myiasis in this Andean dry forest. We extend both the host range and the geographic distribution of the P. torquans complex.
Keywords: Andean dry forest; Dipteran parasites; Host; Larvae; Myiasis
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Primer reporte del complejo Philornis torquans en aves adultas en Ecuador continental: ¿está siendo subestimado el parasitismo por Philornis?
Resumen
La miasis por Philornis es un fenómeno habitual en las aves neotropicales. Las larvas de Philornis son hematófagas y se sabe que afectan tanto a los polluelos como a los adultos. Sin embargo, el parasitismo en adultos parece ser oportunista y ha sido poco estudiado en comparación con el parasitismo en polluelos. En este estudio inspeccionamos 1,429 aves adultas y juveniles de 41 especies de un bosque seco andino en el norte de Ecuador, en busca de infestaciones por larvas de Philornis. Por primera vez, reportamos parasitismo por Philornis en aves adultas de 6 especies de aves paseriformes (Troglodytes aedon, Conirostrum cinereum, Geospizopsis plebejus, Zonotrichia capensis, Pheucticus chrysogaster y Spinus magellanicus). También reportamos los primeros casos de infestación por el complejo Philornis torquans en Ecuador y proveemos datos sobre la prevalencia de miasis por Philornis en este bosque seco andino. Ampliamos tanto el rango de hospedadores como la distribución geográfica del complejo P. torquans.
Palabras clave: Bosque seco andino; Parásitos dípteros; Hospedador; Larvas; Miasis
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Myiasis, or infestations by dipterous larvae that feed on vertebrate tissues (Otranto, 2001), is a usual phenomenon in birds (Sabrosky et al., 1989; Dudaniec & Kleindorfer, 2006). It is most frequently caused by hematophagous larvae that are obligate feeders on nestling birds and include members of the families Calliphoridae (Protocalliphora), Muscidae (Philornis, Passeromyia, Mydaea), and Neottiophilidae (Neottiophilum, Actinoptera), of which Philornis is present in the Neotropics (Little, 2008).
The genus Philornis encompasses near 50 species that may be host-generalist or specialists (Löwenberg-Neto, 2008). With 3 types of larval habits (coprophagous, semi hematophagous, and subcutaneous; Dudaniec & Kleindorfer, 2006), infestation by Philornis larvae can affect nestlings differently, even leading to death (McNew & Clayton, 2018; Hayes et al., 2019). Detrimental consequences detected in infested nestlings include reduced hemoglobin and hematocrit levels (Dudaniec et al., 2006; Manzoli et al., 2018), lower growth rates and body mass (Fessl et al., 2006; Norris et al., 2010; Segura & Reboreda, 2011; Segura & Palacio, 2021), reduced feather and tarsus length (Koop et al., 2011), and decreased future reproduction (McNew et al., 2020). Whereas nestling birds seem the primary hosts of Philornis, parasitism of adult birds seems to be opportunistic and is more likely to occur in adults that spend long periods in the nest (Teixeira, 1999; Dudaniec & Kleindorfer, 2006).
The impact of parasitism by Philornis on nestlings is of particular concern in most already threatened bird species (Bulgarella et al., 2019). In the Galapagos islands, the invasive Philornis downsi is known to severely affect all 14 Darwin’s finch species (Kleindorfer & Dudaniec, 2016). In continental Ecuador, Philornis falsificus and P. obscurus were found infesting nestlings in Guayas province, while P. grandis was recorded in nests from Pastaza and Napo provinces, in the Amazon basin (records compiled in Löwenberg-Neto & De Carvalho, 2013). In 2015, Bulgarella et al. found P. downsi larvae, along with pupal cases from other unidentified Philornis species in nests from Santa Elena and Guayas provinces. Finally, a Philornis larvae was found parasitizing a nestling of the Choco Screech-Owl (Megascops centralis) in Santa Elena province (Reyes & Astudillo-Sánchez, 2017). Thus, while over 145 studies have been carried on P. downsi (Quiroga et al., 2020), only 4 studies have addressed the presence of Philornis species in continental Ecuador. This situation reveals the notorious lack of information on parasitism by Philornis in continental Ecuador, where many bird species are of conservation concern (BirdLife International, 2021).
Our study is set at Bosque Protector Jerusalem (BPJ), the only track of protected Andean dry forest in the inter-Andean valleys of northern Ecuador. Between 2012 and 2014, we monitored the avian community at BPJ and surroundings, to generate a baseline for avian host-parasite dynamics in this poorly studied ecosystem. Here, we captured, inspected, and released adult birds with the next objectives: 1) detect parasitism by Philornis spp. in adult birds, 2) provide data on the prevalence of Philornis myiasis in adult birds, and 3) increase knowledge on the distribution and host range of the Philornis spp.
Materials and methods
Fieldwork was conducted at Bosque Protector Jerusalem (~10 km north of Quito; 00°00′05′′ N, 78°21′18′′ W; 2,300 m asl), a 1,110 ha protected dry forest, in the dry Andean valley of Guayllabamba, northern Ecuador. This area is located on a seasonal ecosystem, where the dry season extends from May to August (average annual rainfall of 125 mm), and the wet season extends from September to April (average annual rainfall of 360 mm) (Carvajal-Campos, 2009). Average annual temperature under shade is 19 °C (data for 2012-2013 provided by Instituto Nacional de Meteorología e Hidrología, INAMHI).
From December 2012 to June 2013, and February to August 2014, we placed 7 mist-nets (4 shelves; 12 m × 2.5 m; 36 mm mesh) for 72 h per month, for a total sampling effort of 1,008 h (or 72 h/net). We carefully inspected the body surface of captured birds for parasitism by Philornis spp. When found, subcutaneous larvae were removed from birds’ bodies and stored in 70% ethanol. Bird taxonomy used in this study follows that of Remsen et al. (2021).
In October 2020, the collected larvae were molecularly identified by sequencing the second internal transcribed spacer region (ITS2) of the rRNA gene, a barcode for insects (García-Robledo et al., 2013). Genomic DNA was extracted from the whole larvae (1 per host) using a guanidine isothiocyanate protein precipitation, followed by isopropanol DNA precipitation protocol (Peñafiel et al., 2019). We amplified a portion of ITS2 using primers ITS2-LEcEn-F and ITS2-LEcEn-R (Monje et al., 2013). Amplification products were visualized in 2% agarose gel, and unincorporated primers and dNTPs were degraded using ExoSAP-IT PCR Product Cleanup Reagent (Applied Biosystems). Purified amplicons were sequenced with big-dye chemistry and PCR primers, using capillary electrophoresis in an ABI3730xl sequencer. Chromatograms were inspected in Geneious® 11.1.5 (Biomatters Ltd., Kearse et al., 2012). For some samples, we obtained good quality sequence readings for the forward DNA chain but not for the reverse; thus, we re-sequenced the forward chain to minimize the chance of sequencing errors.
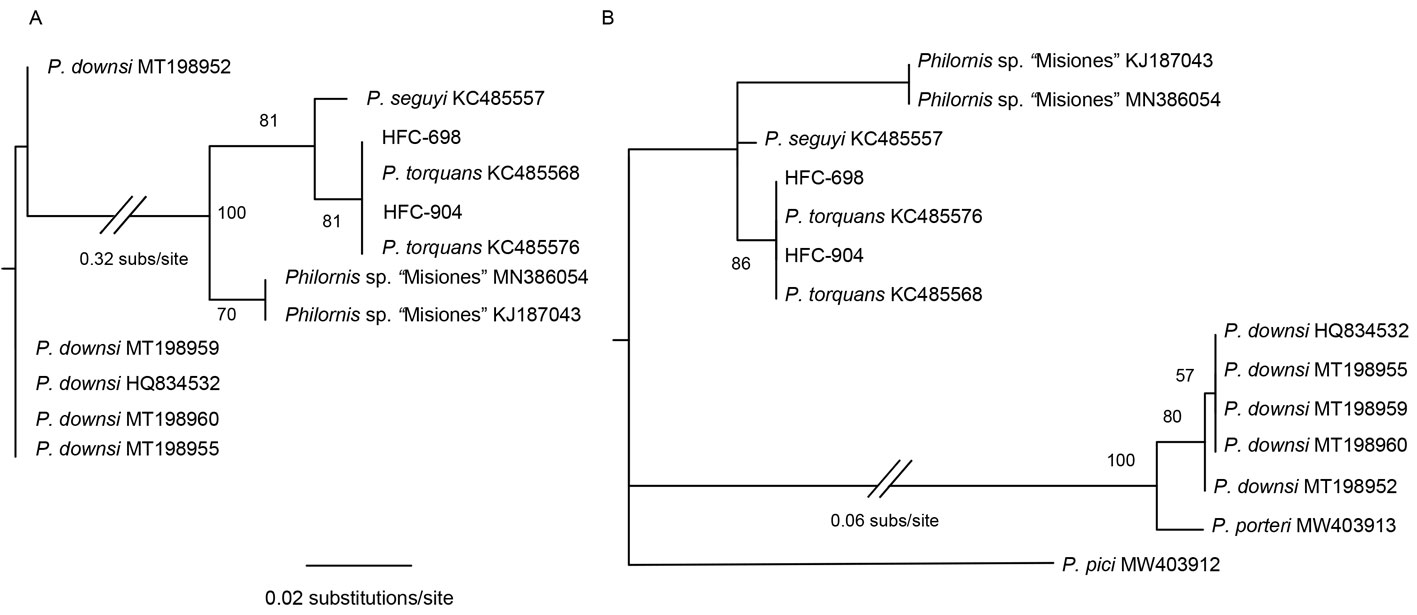
To assess the identity of our larvae, we compared the sequences obtained to those available on GenBank (https://www.ncbi.nlm.nih.gov/genbank/) using BLAST (Basic Local Alignment Search Tool; https://blast.ncbi.nlm.nih.gov/Blast.cgi). To corroborate the identity of samples from a phylogenetic perspective, we performed a maximum likelihood (ML) analysis, as follows. We assembled a matrix with our novel sequences and those of Philornis (P. torquans, P. seguyi, Philornis sp. “Misiones”, P. pici, P. porteri, and P. downsi) available on GenBank. All sequences from the same species and those obtained by us were aligned separately in Mafft (Katoh et al., 2019), using Auto alignment. Based on these preliminary alignments, we retained only the unique sequences (in terms of both nucleotide sequences and insertions/deletions). Given the presence of several ambiguous indels in ITS2 and the potential of these indels to generate very different tree topologies, we assembled 2 alignment matrices: one that contained the sequences more similar to our novel sequences (plus outgroup) and one that included sequences from all the species. Final alignments were also obtained with Mafft Auto feature. Using PartitionFinder 2 (Lanfear et al., 2016), we estimated the best-fit model of molecular evolution for both data matrices, which was the TIM + G model. For each dataset, we conducted a ML phylogenetic reconstruction as implemented in Garli 2.0 (Zwickl, 2006), using nst = 6 and gamma distribution (4 gamma categories); all other settings were left at default values. We ran 20 search replicates to obtain the ML tree, followed by 1,000 bootstrap replicates (1 search replicate each) to assess the nodal support for each clade.
Results
We sampled a total of 1,429 birds from 41 species. Among these, 15 individuals (14 adults and 1 immature) belonging to 6 species were infested by subcutaneous Philornis larvae. Parasitized species were: Troglodytes aedon (Troglodytidae), Conirostrum cinereum (Thraupidae), Geospizopsis plebejus (Thraupidae), Zonotrichia capensis (Passerellidae), Pheucticus chrysogaster (Cardinalidae), and Spinus magellanicus (Fringillidae) (Table 1; Fig. 1). The prevalence of myiasis by Philornis per bird species ranged from 0.9% (G. plebejus) to 6.9% (T. aedon), and intensity of parasitism ranged from 1 to 12 larvae (Table 1). Birds’ abdomen was the area where more larvae were found (Table 1). The complete list of species and individuals examined in this study is provided in Appendix 1. Other individuals for which we found traces (i.e., rounded wounds in the skin) of likely myiasis are summarized in Appendix 2.
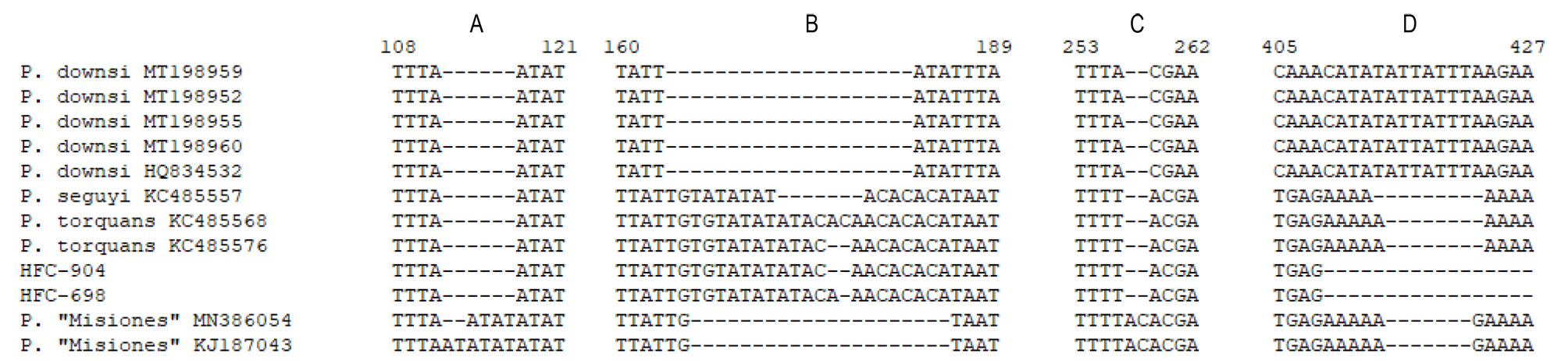
We extracted, amplified, and sequenced DNA from larvae found in 14 individuals. However, the DNA in the larvae of 8 individuals was degraded because their preserving ethanol evaporated during at least part of the storage period. Thus, we were able to obtain ITS2 sequences for larvae found on 6 individuals: 1 T. aedon, 1 G. plebejus, and 4 Z. capensis (GenBank accession numbers MW853826- MW853831). From the sequences of these 6 larvae, we obtained 2 unique haplotypes, A and B (different by 1 indel; Table 1) identified by BLAST as belonging to P. torquans. Also, phylogenetically, they were placed within the P. torquans clade (Fig. 2A, B), which was sister to the only available haplotype of P. seguyi in the reduced-dataset tree (Fig. 2A). In that same tree, Philornis sp. “Misiones” showed as sister to P. seguyi + P. torquans. We also found a few mostly unambiguous indels (Fig. 3) that may prove phylogenetically informative in the future, when more extensive haplotype sampling is available. The sequences obtained in this study allowed us to identify the sampled larvae as part of the P. torquans complex that includes both P. torquans and P. seguyi (Quiroga et al., 2016; see Discussion).
Discussion
We report, for the first time in continental Ecuador, parasitism by Philornis in adult birds of 6 species of passerine birds (T. aedon, C. cinereum, G. plebejus, Z. capensis, P. chrysogaster, and S. magellanicus). There are records Philornis myiasis in nestlings of T. aedon (Young, 1993; Quiroga & Reboreda, 2012), Z. capensis, and S. magellanicus (Salvador & Bodrati, 2013) in other latitudes (Costa Rica, Argentina), but never in adult birds. Although Philornis infecting non-nestlings is a rare event, there are at least 15 additional bird species for which adult infestation, by at least 5 species of Philornis, have been documented (Huber et al., 2010; Quiroga et al., 2020). The infestation prevalences found herein are close to the ranges summarized in Quiroga et al. (2020), when considering species with 19 or more individuals analyzed (i.e., 0.2-8.75%), except for the high prevalence shown by Margarops fuscatus (31%; Arendt, 1985).
Table 1
Birds showing Philornis myiasis at Bosque Protector Jerusalem. Specific ID is only provided for larvae identified molecularly.
| Species | Field number | Age | Date | Observations | Prevalence | ID (haplotype) |
| Troglodytes aedon | HFC-745 | Adult | 27 Apr 2013 | 1 in chest | 6.9% (2/29) | P. torquans complex (A) |
| HFC-859 | Immature | 7 Jun 2013 | 1 in abdomen | Philornis sp. | ||
| Conirostrum cinereum | HFC-121 | Adult | 16 Dec 2012 | 1 in abdomen, bird showing incubation patch | 3.6% (1/28) | Philornis sp. |
| Geospizopsis plebejus | HFC-386 | Adult | 2 Feb 2013 | 1 in forehead; bird first captured on 2 Dec 2012 with no trace of infestation | 0.9% (1/107) | Philornis sp. |
| HFC-10761 | Adult | 19 Mar 2014 | 1 in chest, 5 in abdomen | P. torquans complex (A) | ||
| Zonotrichia capensis | HFC-188 | Adult | 27 Dec 2012 | 1 in abdomen, bird showing incubation patch | 2.6% (8/303) | Philornis sp. |
| HFC-444 | Adult | 10 Feb 2013 | 2 in abdomen; bird first captured on 12 Jan 2013 with no trace of infestation, showing incubation patch | Philornis sp. | ||
| HFC-698 | Adult | 14 Apr 13 | 12 in abdomen | P. torquans complex (A) | ||
| HFC-736 | Adult | 21 Apr 2013 | 4 in abdomen | P. torquans complex (A) | ||
| HFC-904 | Adult | 19 Feb 2014 | 4 in abdomen | P. torquans complex (B) | ||
| HFC-1018 | Adult | 11 Mar 2014 | Near cloaca | Philornis sp. | ||
| No number | Adult | 4 May 2014 | Larvae above cloaca; traces of former incubation patch | Philornis sp. | ||
| HFC-1392 | Adult | 6 Jun 2014 | in abdomen | P. torquans complex (A) | ||
| Pheucticus chrysogaster | HFC-248 | Adult | 11 Jan 2013 | 1 in abdomen | 5.3% (1/19) | Philornis sp. |
| Spinus magellanicus | HFC-226 | Adult | 10 Jan 2013 | 4 in abdomen | 1.2% (1/82) | Philornis sp. |
1 Captured 3.5 km outside Bosque Protector Jerusalem. Not included in the calculation of prevalence.
Although parasitism by Philornis in adults may be more common than previously thought (Quiroga et al., 2020), nestlings seem to be more susceptible than mature birds, as they stay for a longer time in the nest and are less mobile, and then more vulnerable to infestation (Herrera & Bermúdez, 2012). In our study, at least 4 individuals showed myiasis in the abdomen with current or traces of former incubation patch and in the forehead and near the cloaca, areas that are usually less protected by contour feathers. These surfaces may be more easily reached by Philornis larvae that emerge from eggs laid in nest material (Quiroga et al., 2020).
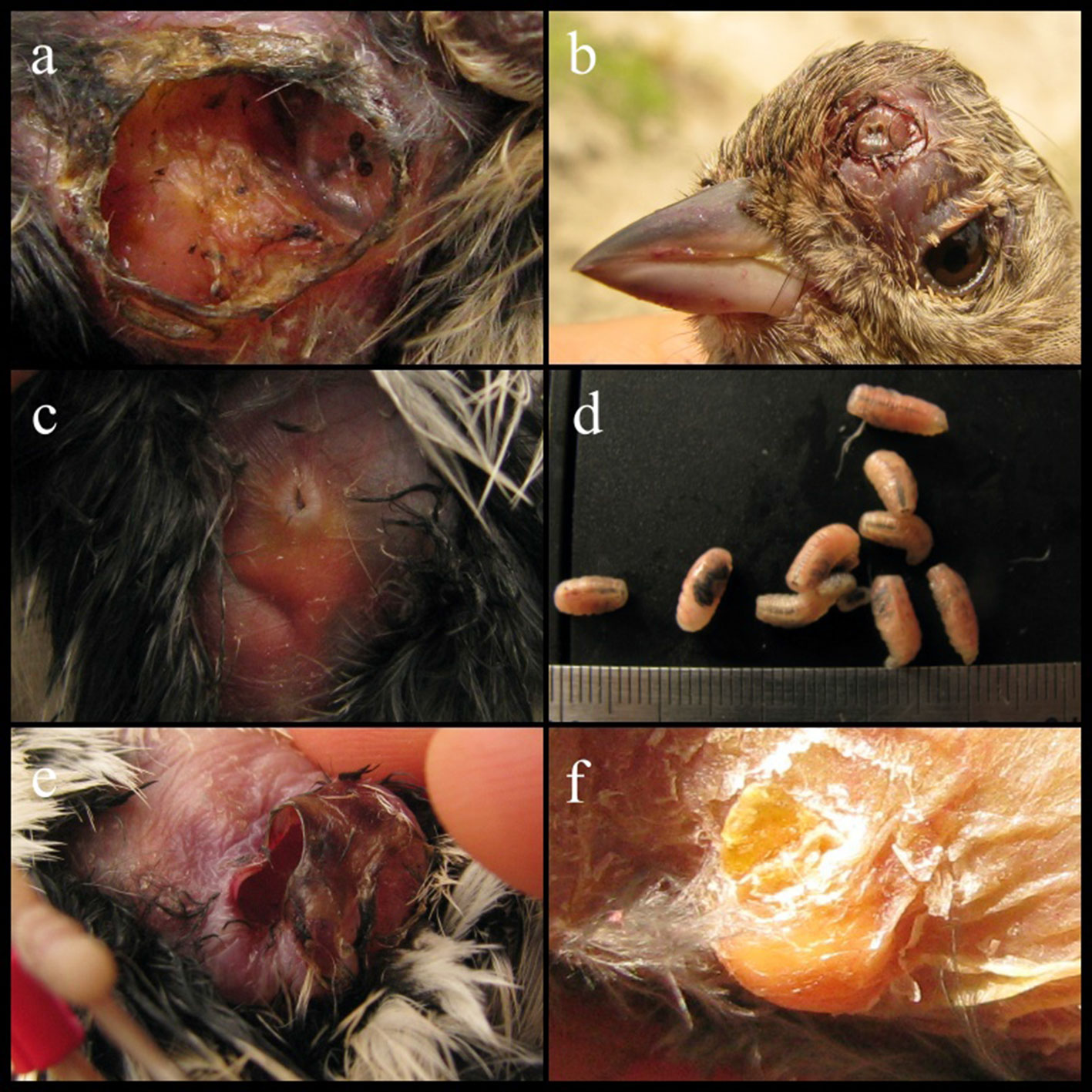
Philornis torquans was formerly reported in Argentina and Brazil (Nielsen, 1912; Löwenberg-Neto & De Carvalho, 2013), but now it is considered a complex of cryptic species from southern South America named the P. torquans complex (Quiroga et al., 2016). Here, we report the P. torquans complex in Ecuador for the first time, broadening its distribution range. This complex has been previously found affecting 34 species of birds, including T. aedon and Z. capensis (Cuervo et al., 2020). Thus, our findings expand the host range of the P. torquans complex by including a new host: G. plebejus. Furthermore, 3 other bird species —C. cinereum, P. chrysogaster, and S. magellanicus— also presented myiasis by Philornis in the same locality. The presence of the P. torquans complex along the Central and Northern Andes was predicted by the abiotic niche model of Cuervo et al. (2020). While this model was developed to predict the distribution of the P. torquans complex in southern South America, the results suggested (with some uncertainty) that this complex of cryptic species may be present in our study area. The finding of the P. torquans complex in the Andean dry forest of northern Ecuador represents the first independent confirmation of this model. This result is also consistent with those of Percara et al. (in press) who reported the P. torquans complex covering heterogeneous habitats and environmental conditions (average mean temperature 10-28 °C, mean rainfall 100-2,000 mm/year), thus encompassing the environmental conditions present at BPJ.
Few field studies have focused on the prevalence of Philornis in both adults and nestlings. To our knowledge, the most extensive research is that of Arendt (1985) on Margarops fuscatus, in Puerto Rico. In that study, adult prevalence ranged 0-92%, depending on the month of the year, whereas nestling prevalence ranged 81-100% for the same site and time period. Thus, the presence of myiasis in adults at this Andean dry forest may signal a broader Philornis infestation in the nestlings of those species (Texeira, 1999) and, likely, the nestlings of other species. Moreover, in this seasonally dry forest, infected adults may act as reservoirs for the parasites, allowing them to maintain viable parasite populations year-round. Although our sampling of the area was designed to cover the diversity of micro habitats in this dry forest, year-round sampling, including the inspection of nests and adults, is needed to support this hypothesis.
Bird populations living in this Andean dry valley are experiencing continuous landscape transformation (Aguirre et al., 2006), the presence of avian malaria parasites in high prevalence and intensity (Cadena et al., 2019), the likely expansion of the Shiny Cowbird, Molothrus bonariensis (Medrano-Vizcaíno et al., 2020; J.F. Freile pers. comm.), and probably, the non-documented effects of global climate change. Thus, they may be exposed to the synergistic effects of these factors and Philornis infestations, which are likely increasing the pressures on their reproductive effort. For these reasons, for a broader perspective on the ecology of this community and other communities along the dry Andean valleys, future studies should focus on studying how Philornis infestation, habitat degradation, malaria and cowbird parasitism, climate change, and their interactions may be affecting bird populations.
Acknowledgments
To several volunteers that assisted during fieldwork, to Nathalia Valencia for obtaining DNA sequences, and to Juan F. Freile for insightful comments on the cowbird expansion. Funding was provided by a Scopus Grant (HUBI 12434) from Universidad San Francisco de Quito. This study was authorized by Ministerio del Ambiente del Ecuador: permits 20-2012-IC-FAU-DPAP-MA and 15-2013-RIC-FAU-DPAP-MA, and Contrato de Acceso a Recursos Genéticos (MAE-DNB-CM-20 18-0 105 granted to Universidad San Francisco de Quito).
Appendix 1. Families, species, and numbers of individuals per species examined during this study.
Columbidae: Leptotila verreauxi (5), Zenaida auriculata (1), Columbina passerina (180). Caprimulgidae: Systellura longirostris (1). Trochilidae: Colibri coruscans (74), Lesbia victoriae (26), Patagona gigas (5), Myrtis fanny (64), Chaetocercus mulsant (2), Chlorostilbon melanorhynchus (34), Amazilia tzacatl (23). Picidae: Colaptes rivolii (8). Furnariidae: Synallaxis azarae (13). Tyrannidae: Camptostoma obsoletum (25), Elaenia albiceps (14), Anairetes parulus (4), Myiophobus fasciatus (2), Pyrocephalus rubinus (47), Myiotheretes striaticollis (1). Hirundinidae: Pygochelidon cyanoleuca (26). Troglodytidae: Troglodytes aedon (29). Turdidae: Turdus fuscater (14). Mimidae: Mimus gilvus (1). Fringillidae: Spinus magellanicus (82), Chlorophonia cyanocephala (16). Passerellidae: Zonotrichia capensis (303). Cardinalidae: Pheucticus chrysogaster (19). Thraupidae: Conirostrum cinereum (28), Sicalis luteola (1), Geospizopsis plebejus (107), Rhopospina alaudina (2), Catamenia analis (43), Diglossa sittoides (17), Sporophila nigricollis (13), Saltator striatipectus (55), Tiaris olivaceus (1), Asemospiza obscura (5), Pipraeidea melanonota (1), Rauenia bonariensis (25), Stilpnia vitriolina (103), Thraupis episcopus (9).
Appendix 2. Individuals that showed traces of likely current or past myiasis (by Philornis or other insects).
Pyrocephalus rubinus: HFC-604 (29/03/2013) 1 likely larval exit orifice in venter. Zonotricia capensis: HFC-1112 (4/May/2014), cavity in the pectoral area with subcutaneous infestation; HFC-1205 (6/May/2014), rugose and yellowish venter, cutaneous orifice (7 mm) above cloaca; HFC-1347 (23/May/2014), 1 likely larval exit orifice in venter; HFC-1358 (3/Jun/2014), 1 likely larval exit orifice, inflamed area around bill likely made by subcutaneous larvae. Geospizopsis plebejus: HFC-911 (25/Feb/2014), 2 likely larval exit orifices above cloaca.
References
Aguirre, Z. M., Kvist L. P., & Sánchez, O. (2006). Bosques secos en Ecuador y su diversidad. In R. Moraes, B. Øllgaard, L. P. Kvist, F. Borchsenius, & H. Balslev (Eds.), Botánica económica de los Andes Centrales (pp. 162–187). La Paz: Universidad Mayor de San Andrés.
Arendt, W. J. (1985). Philornis ectoparasitism of Pearly-eyed Thrashers. II. Effects on adults and reproduction. Auk, 102, 281–292.
BirdLife International (2021). Country profile: Ecuador. Accessed 26 May 2021 from: http://www.birdlife.org/data
zone/country/ecuador
Bulgarella, M., Quiroga, M. A., Brito-Vera, G. A., Dregni, J. S., Cunninghame, F., Mosquera, D. A. et al. (2015). Philornis downsi (Diptera: Muscidae), an avian nest parasite invasive to the Galapagos Islands, in mainland Ecuador. Annals of the Entomological Society of America, 108, 242–250. https://doi.org/10.1093/aesa/sav026
Bulgarella, M., Quiroga, M. A., & Heimpel, G. E. (2019). Additive negative effects of Philornis nest parasitism on small and declining Neotropical bird populations. Bird Conservation International, 29, 1–22.
Cadena-Ortiz, H., Mantilla, J. S., de Aguilar, J. R., Flores, D., Bahamonde, D., Matta, N. E. et al. (2019). Avian haemosporidian infections in rufous-collared sparrows in an Andean dry forest: diversity and factors related to prevalence and parasitaemia. Parasitology, 146, 765–773. https://doi.org/10.1017/S0031182018002081
Carvajal-Campos, A. (2009). Reproducción y dieta de la lagartija andina Stenocercus guentheri (Squamata: Iguania) en el Parque Protector Jerusalem (Tesis). Pontificia Universidad Católica del Ecuador. Quito.
Cuervo, P. F., Percara, A., Monje, L., Beldomenico, P. M., & Quiroga, M. A. (2020). Environmental variables determining the distribution of an avian parasite: the case of the Philornis torquans complex in South America. Medical and Veterinary Entomology, 35, 284–292. https://doi.org/10.1111/mve.124
88
Dudaniec, R. Y., & Kleindorfer, S. (2006). Effects of the parasitic flies of the genus Philornis (Diptera: Muscidae) on birds. Emu, 106, 13–20. https://doi.org/10.1071/MU04040
Dudaniec, R. Y., Kleindorfer, S., & Fessl, B. (2006). Effects of the introduced ectoparasite Philornis downsi on haemoglobin level and nestling survival in Darwin’s Small Ground Finch (Geospiza fuliginosa). Austral Ecology, 31, 88–94. https://doi.org/10.1111/j.1442-9993.2006.01553.x
Fessl, B., Kleindorfer, S., & Tebbich, S. (2006). An experimental study on the effects of an introduced parasite in Darwin’s finches. Biological Conservation, 127, 55–61. https://doi.org/10.1016/j.biocon.2005.07.013
García-Robledo, C., Erickson, D. L., Staines, C. L., Erwin, T. L., & Kress, W. J. (2013). Tropical plant-herbivore networks: reconstructing species interactions using DNA barcodes. Plos One, 8, e52967. https://doi.org/10.1371/journal.pone.0052967
Hayes, C. D., Hayes, T., McClure, C. J., Quiroga, M., Thorstrom, R. K., & Anderson D. L. (2019). Native parasitic nest fly impacts reproductive success of an Island-endemic host. Animal Conservation, 22, 157–164.
Herrera, J. M., & Bermúdez, S. E. (2012). Myiasis by Philornis spp. (Diptera: Muscidae) in Dendroica castanea (Aves: Parulidae) in Panama. Revista Mexicana de Biodiversidad, 83, 854–855. http://dx.doi.org/10.22201/ib.20078706e.2012.3.1262
Huber, S. K., Owen, J. P., Koop, J. A. H., King, M. O., Grant, P. R., Grant, B. R., & Clayton, D. (2010). Ecoimmunity in Darwin’s Finches: Invasive Parasites Trigger Acquired Immunity
in the Medium Ground Finch (Geospiza fortis). Plos One, 5, e8605. https://doi.org/10.1371/journal.pone.0008605
Katoh, K., Rozewicki, J., & Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief bioinform, 20, 1160–1166. https://doi.org/10.1093/bib/bbx108
Kearse, M., Moir, R., Wilson, A., Stone-Havas, S., Cheung, M., Sturrock, S. et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. https://doi.org/10.1093/bioinformatics/bts199
Kleindorfer, S., & Dudaniec, R. Y. (2016). Host-parasite ecology, behavior and genetics: a review of the introduced fly parasite Philornis downsi and its Darwin’s finch hosts. BMC Zoology, 1, 1. https://doi.org/10.1186/s40850-016-0003-9
Koop, J. A. H., Huber, S. K., Laverty, S. M., & Clayton, D. H. (2011). Experimental demonstration of the fitness consequen-
ces of an introduced parasite of Darwin’s finches. Plos One,
6, e19706. https://doi.org/10.1371/journal.pone.0019706
Lanfear, R., Frandsen, P. B., Wright, A. M., Senfeld, T., & Calcott, B. (2016). PartitionFinder 2: new methods for selecting partitioned models of evolution formolecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34, 772–773. https://doi.org/10.1093/molbev/msw260
Little, S. E. (2008). Myiasis in wild birds. In C. T. Atkinson, N. J. Thomas, & D. B. Hunter (Eds.), Parasitic diseases of wild birds (pp. 546–556). Ames, Iowa: Wiley-Blackwell.
Löwenberg-Neto, P. (2008). The structure of the parasite–host interactions between Philornis (Diptera: Muscidae) and neotropical birds. Journal of Tropical Ecology, 24, 575–580.
Löwenberg-Neto, P., & De Carvalho, C. J. B. (2013). Muscidae (Insecta: Diptera) of Latin America and the Caribbean: geographic distribution and check-list by country. Zootaxa, 3650, 1–147. https://doi.org/10.11646/zootaxa.3650.1.1
Manzoli, D. E., Saravia-Pietropaolo, M. J., Antoniazzi, L. R., Barengo, E., Arce, S. I., Quiroga, M. A. et al. (2018). Contrasting consequences of different defence strategies in a natural multihost-parasite system. International Journal for Parasitology, 48, 445–455. https://doi.org/10.1016/j.ijpara.2017.11.001
McNew, S., & Clayton, D. (2018). Alien invasion: Biology of Philornis flies highlighting Philornis downsi, an introduced parasite of Galapagos birds. Annual Review of Entomology, 63, 369–87.
McNew, S. M., Goodman, G. B., Yépez, R. J., & Clayton, D. H. (2020). Parasitism by an invasive nest fly reduces future reproduction in Galápagos mockingbirds. Oecologia, 192, 363–374. https://doi.org/10.1007/s00442-019-04582-y
Medrano-Vizcaíno, P., Bedoya, J., & Cadena-Ortiz, H. (2020). Dinámica de la distribución y hospederos de Molothrus bonariensis (Passeriformes: Icteridae) en Ecuador. Caldasia, 42, 38–49. https://dx.doi.org/10.15446/caldasia.v42n1.78891
Monje, L. D., Quiroga, M., Manzoli, D., Couri, M., Silvestri, L., Venzal, J. M. et al. (2013). Sequence analysis of the internal transcribed spacer 2 (ITS2) from Philornis seguyi (García, 1952) and Philornis torquans (Nielsen, 1913) (Diptera: Muscidae). Systematic Parasitology, 86, 43–51. https://doi.org/10.1007/s11230-013-9428-5
Nielsen, J. C. (1912). Mydaea anomala Jaenn., a parasite of South-American birds. Vidensk Dansk Naturh Foren, 63, 195–208.
Norris, A., Cockle, K., & Martin K. (2010). Evidence for tolerance of parasitism in a tropical cavity-nesting bird, Planalto woodcreeper (Dendrocolaptes platyrostris), in northern Argentina. Journal of Tropical Ecology, 6, 619–626.
Otranto, D. (2001). The immunology of myiasis: parasite survival and host defense strategies. Trends in Parasitology, 17, 176–182. https://doi.org/10.1016/S1471-4922(00)01943-7
Peñafiel, N., Flores, D. M., De Aguilar, J. M., Guayasamin, J. M., & Bonaccorso, E. (2019). A cost-effective protocol for total DNA isolation from animal tissue. Neotropical Biodiversity, 5, 69–74. https://doi.org/10.1080/23766808.2019.1706387
Percara, A., Quiroga, M., Beldomenico, P., & Monje, L. (in press). Genetic diversity and geographic distribution of parasitic flies of the Philornis torquans complex in southern South America. Medical and Veterinary Entomology.
Quiroga, M. A., & Reboreda, J. C. (2012). Lethal and sublethal effects of botfly (Philornis seguyi) parasitism on house wren nestlings. Condor, 114, 197–202. https://doi.org/10.1525/cond.2012.110152
Quiroga, M. A., Monje, L. D., Arrabal, J. C., & Beldomenico, P. M. (2016). Datos moleculares nuevos sobre Philornis (Diptera: Muscidae) subcutáneas del sur de Sudamérica sugieren la existencia de un complejo de especies. Revista Mexicana de Biodiversidad, 87, 1383–1386. https://doi.org/10.1016/j.rmb.2016.10.018
Quiroga, M. A., Hayes, T. I., Hayes, C. D., Garrod, H., Soares, L., Knutie, S. A. et al. (2020). More than just nestlings: incidence of subcutaneous Philornis (Diptera: Muscidae) nest flies in adult birds. Parasitology Research, 119, 2337–2342. https://doi.org/10.1007/s00436-020-06696-2
Remsen, J. V. Jr., Areta, J. I., Bonaccorso, E., Claramunt, S., Jaramillo, A., Pacheco, J. F. et al. (2021). A classification of the bird species of South America, version 1. American Ornithological Society. Accessed 30 April 2021 from: http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm
Reyes, E. M. R., & Astudillo-Sánchez, E. (2017). Notes on the nest, owlets, diet, and parasites of the Choco Screech-Owl (Megascops guatemalae centralis) in Loma Alta Communal Reserve, Western Ecuador. Wilson Journal Ornithology, 129, 377–381. https://doi.org/10.1676/16-019.1
Sabrosky, C. W., Bennett, G. F., & Whitworth, T. L. (1989). Bird blow flies (Protocalliphora) in North America (Diptera: Calliphoridae) with notes on Palearctic species. Washington DC: Smithsonian Institution Press.
Salvador, S. A., & Bodrati, A. (2013). Aves víctimas del parasitismo de moscas del género Philornis en Argentina. Nuestras Aves, 58, 16–21.
Segura, L. N., & Reboreda, J. C. 2011. Effects of botfly parasitism on nestling growth and mortality in Red-crested Cardinals. Wilson Journal of Ornithology, 123, 107–115.
Segura, L. N., & F. X. Palacio. 2021. Quantifying the relative importance of direct and indirect effects influencing bird nestling growth. Integrative Zoology, 17, 1–12. https://doi.org/10.1111/1749-4877.12544
Teixeira, D. (1999). Myiasis caused by obligate parasites, Ib. general observations on the biology of species of genus Philornis meinert. In J. Guimaraes, N. Papavero (Eds.), Myasis in man and animals in the neotropical region (pp. 71–96). Sao Paulo: Pleidae.
Young, B. E. (1993). Effects of the parasitic botfly Philornis carinatus on nestling House Wrens, Troglodytes aedon, in Costa Rica. Oecologia, 93, 256–262. https://doi.org/10.1007/BF00317679
Zwickl, D. J. (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under maximum likelihood criterion (Ph.D. Thesis). The University of Texas. Austin, USA.