Eduardo Gabriel Torres-Conde a, *, Jorge Gabriel Zúñiga-Delgado b,
Denise Lázara Reyes-Pérez c, Ana M. Suárez a
a Center of Marine Research, Calle 16 No. 114, Playa, 11300 La Habana, Cuba
b Universidad Nacional de San Agustín, Pabellón de Biología, Ave. Alcides Carrión, 04001 Arequipa, Perú
c Ministerio de Ciencia, Tecnología y Medio Ambiente, Instituto de Geografía Tropical, Calle F No. 302, Plaza, 10400 La Habana, Cuba
*Corresponding author: etorresconde2@gmail.com (E.G. Torres-Conde)
Received: 28 May 2020; accepted: 25 September 2020
Abstract
To date, 18 species of the algal genus Lobophora (Dictyotales, Phaeophyceae) have been reported for the Greater Caribbean Sea. Nevertheless, only 2 have been recorded for the marine flora of Cuba, Lobophora variegata and Lobophora canariensis (as Aglaozonia canariensis). To update the knowledge about this genus in Cuba, an exhaustive morphological study was performed using macroalgae collected from Western Cuba: Ecological Reserve Los Pretiles (Pinar del Río Province; May 2011), Gulf of Guanahacabibes (Pinar del Río Province; June 2014) and Calle 16 beach (La Habana Province; April and May 2019). The species found were: L. canariensis, L. declerckii, L. guadeloupensis, L. littlerorum and L. variegata. The identified species were deposited in the Cuban National Aquarium Herbarium (HANC). Morphological descriptions from Cuban material are here presented. In addition, the distribution of these species in the Greater Caribbean Sea is undertaken. We report for the first time L. guadeloupensis in the Greater Antilles. These new records increase the specific richness in Cuba and expand the distribution range of Lobophora species for the Greater Caribbean Sea.
Keywords: Greater Antilles; Habitat; Morphological taxonomy; Seaweeds; Western Cuba
Nuevos registros del género Lobophora (Dictyotales: Phaeophyceae) para la flora marina de Cuba y su distribución en el Gran Caribe
Resumen
A la fecha, 18 especies del género de alga Lobophora (Dictyotales, Phaeophyceae) han sido registradas para el Gran Caribe. Sin embargo, solo 2 especies habían sido reportadas para la flora marina de Cuba, Lobophora variegata y Lobophora canariensis (como Aglaozonia canariensis). Para actualizar el conocimiento acerca del género Lobophora (Dictyotales, Phaeophyceae) en Cuba, se realizó un estudio morfológico exhaustivo recolectando macroalgas en la Reserva Ecológica Los Pretiles (Provincia Pinar del Río; mayo 2011), en el golfo de Guanahacabibes (Provincia Pinar del Río; junio 2014) y en la playa Calle 16 (Provincia La Habana; abril y mayo 2019). Las especies encontradas fueron: L. canariensis, L. declerckii, L. guadeloupensis, L. littlerorum y L. variegata. Las especies identificadas fueron depositadas en el Herbario del Acuario Nacional de Cuba (HANC). Aquí se presentan las descripciones morfológicas del material cubano. Adicionalmente, se muestra la distribución de estas especies en el Gran Caribe y se reporta por primera vez la especie L. guadeloupensis para las Grandes Antillas. Estos nuevos reportes incrementan la riqueza específica en Cuba y el rango de distribución de las especies de Lobophora en el Gran Caribe.
Palabras claves: Antillas Mayores; Hábitat; Taxonomía morfológica; Algas; Cuba occidental
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The genus Lobophora (Dictyotales, Phaeophyceae) is one of the most common genera of marine brown algae. This genus has a pantropical distribution which includes the Atlantic, Pacific and Indian Oceans (Vieira et al., 2017). It can be found growing in a variety of habitats, from tropical to temperate, on reefs and rocky shores (Camacho et al., 2019). This genus is ecologically important in Caribbean reef systems, since it is an efficient competitor with corals for space (Camacho et al., 2019). According to Vieira et al. (2016), this is a morphologically variable genus. It can be found from crustose species tightly attached to the substratum to erect species. Historically, just 1 taxon Lobophora variegata (J.V. Lamoroux) Womersly ex E.C. Oliveira (Schultz et al., 2015) has been recognized in the Greater Caribbean Sea. The holotype of L. variegata was collected in Guadeloupe and was the only known species of the genus Lobophora during decades (Vieira, Morrow et al., 2020). This species was also recognized as displaying different morphotypes (crustose, erect and decumbent forms) by De Ruyter van Steveninck et al. (1988) and Littler and Littler (2000). However, according to Vieira et al. (2016), the genus Lobophora does not only display different morphological forms, but also can inhabit several ecological niches. Recent studies have demonstrated that the genus Lobophora is more diverse than historically stated (Camacho et al., 2019; Schultz et al., 2015; Sun et al., 2012; Vieira et al., 2016; Vieira, Henriques et al., 2020; Vieira, Morrow et al., 2020). These authors argued that different morphotypes and ecotypes can differentiate one species from another. The morphological and molecular criteria are being used to identify several new species in different regions of the Greater Caribbean Sea and the rest of the world. The external and internal characters of Lobophora are also used to distinguish between species. Currently 47 species of Lobophora are taxonomically accepted (Guiry & Guiry, 2020). For the Greater Caribbean Sea, 18 species of Lobophora have been documented and 11 are exclusive for this region (Vieira, Morrow et al., 2020). These 11 endemic species include: L. declerckii N.E. Schultz, C.W. Schneider and L. Le Gall, 2015; L. guadeloupensis N.E. Schultz, F. Rousseau and L. Le Gall, 2015; L. colombiana O. Camacho and Fredericq, 2019; L. crispata O. Camacho and Fredericq, 2019; L. tortugensis O. Camacho and Fredericq, 2019; L. aghardii Payri and C.W. Vieira, 2020; L. dickiei Payri and C.W.Vieira, 2020; L. lamourouxii Payri and C.W. Vieira, 2020; L. richardii C.W. Vieira and Payri, 2020; L. sp. 90 C.W. Vieira and Payri, 2020; and L. variegata (J.V. Lamouroux) Womersley ex E.C. Oliveira, 1977 (Ballantine et al., 2019; Camacho et al., 2019; Delnatte & Wynne, 2016; Godínez-Ortega et al., 2018; Schultz et al., 2015; Vieira et al., 2016). The Eastern and the Central Caribbean are the most diverse regions in the Greater Caribbean Sea with 16 species. Despite of all these records for the region only 2 species, L. variegata and L. canariensis (as Aglaozonia canariensis Sauvageau, 1905), have been reported for Cuba. For this reason, a taxonomic study was carried out from 3 areas of western Cuba.
Materials and methods
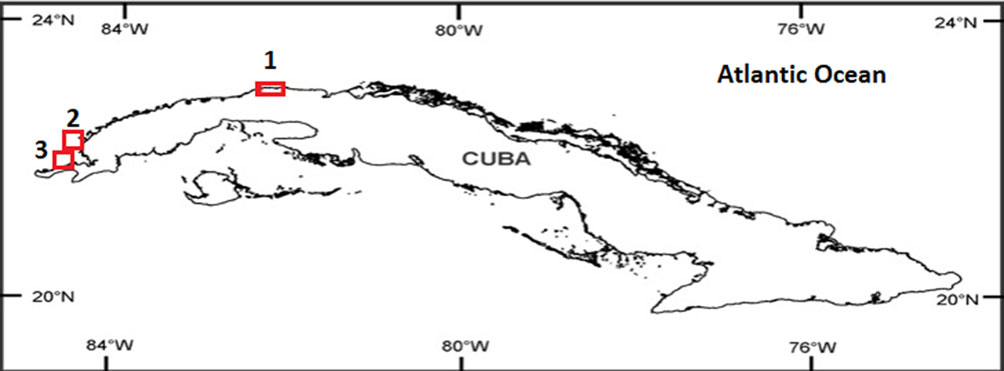
The specimens of Lobophora were collected from 3 localities in western Cuba: the first one in the Ecological Reserve Los Pretiles, Pinar del Río Province (22°22’30” N, 84°21’33.6” W) during May 2011; the second one in the Gulf of Guanahacabibes, Pinar del Río Province (22°7’60” N, 84°34’60” W) on June 2014 and the third one in the Calle 16 beach, La Habana Province (23°07’16” N, 82°25’22” W) during April and May 2019 (Fig. 1). The specimens were collected manually by scuba diving and snorkeling on a rocky-sandy substrate, mainly on rocky reefs and seagrass beds at depths ranging from 4 to 10 m (Fig. 2). The samplings were carried out during daylight hours (8:00 am: 7:00 pm). Later, the specimens collected were preserved in 70% EtOH in labeled bottles. Subsequently, the 32 specimens were analyzed in the Centre for Marine Research of Havana University (CIM-UH). For the external and internal morpho-anatomical observations a stereomicroscope (Olympus SZX7) and a photonic microscope (Olympus CX41) (maximum magnification 100×) were used. For the external observation the general appearance, habitus (crustose, decumbent, erect and fasciculate) and color of the specimens were described. For the internal observations longitudinal and transverse cross sections were made by hand using single-edged razor blades. The internal features measured were thallus height, width and thickness; medullary cell height, width and length; number of cortical layers (dorsal and ventral layers); dorsal and ventral cortex (number of cells and height) and dorsal and ventral subcortex (number of cells and height). Twenty longitudinal and transverse cross sections per species were used to obtain the range of measured features.
Photographs of the studied material were taken using a smart phone Samsung Galaxy S8 (SM-G950F). For the morphological identification specialized literature was used (Camacho et al., 2019; Godínez-Ortega et al., 2018; Schultz et al., 2015; Vieira et al., 2016; Vieira, Henriques et al., 2020; Vieira, Morrow et al., 2020). Once identified they were deposited in 95% EtOH in labeled bottles. Later, some specimens were placed in the seaweed collection of the Cuban National Aquarium Herbarium. The distribution maps of the species of Lobophora were constructed using the free software R (RStudio Team, 2021).

Descriptions
In this study, we report 5 species of the genus Lobophora for Cuba, 3 of them are new records: L. declerkii, L. guadeloupensis, and L. littlerorum. Distinct cell layers and measurements (Table 1), as well as the comparison of morphological and ecological features (Table 2) allowed us to differentiate each of the Cuban species.
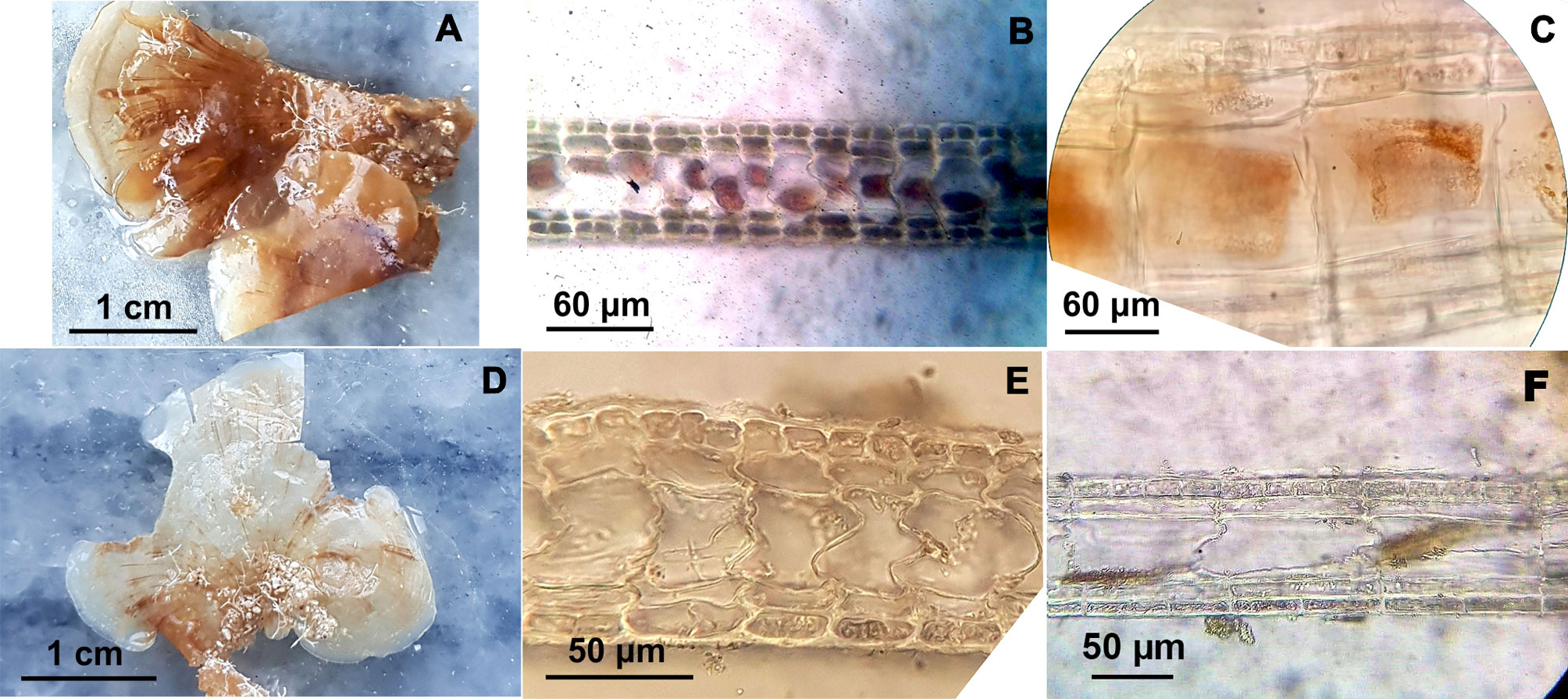
Lobophora canariensis (Sauvageau) C.W. Viera, De Clerck and Payri 2016 (Fig. 3A-C).
Basionym: Aglaozonia canariensis Sauvageau 1905.
Synonym: Lobophora payriae N.E.Schultz, C.W. Schneider and F.Rousseau 2015.
Type locality: Puerto Orotava (Puerto de la Cruz), Tenerife, Islas Canarias (Guiry & Guiry, 2020).
Specimens examined: Western Havana, Calle 16 beach, 6 m depth, rock substratum, rocky reef habitat, 18 April 2019, HANC 0954, collector: Jorge Gabriel Zúñiga Delgado; Western Havana, Calle 16 beach, 10 m depth, rock substratum, rocky reef habitat, 5 May 2019, HANC 0955, collector: Amanda Ramos; Gulf of Guanahacabibes, 6 m depth, rock substratum, rocky reef habitat, 21 June 2014, HANC 0958, collector: Roamsy Volta
Rodríguez.
The thallus is light brown and displays decumbent blades. The blade can be simple or reniform (2.3-3.5 cm long, 1.1-4.1 cm wide, 84-98 thickness). It is formed of a single-celled medulla (30-50 μm height) with 2 cortical cell layers on each side, dorsally and ventrally. The specimens of this species displayed 5 layers including the medulla. In longitudinal section a single subcortical cell layer is shown covering a medullary cell and supporting the outer cortical cell layer. The medullary cell is 72-95 μm long. The dorsal cortical cell layer showed 4 cells and the ventral cortical section displayed 2 cells. Otherwise, the dorsal subcortical cell layer displayed 1-2 cells and the ventral subcortical cell layer exhibited 1-2 cell. In transverse section a single subcortical cell layer is shown covering a medullary cell and supporting the outer cortical cell layer. The medullary cell was 30-35 μm wide. The dorsal cortical cell layer exhibited 2 cells and the ventral section displayed 1-2 cells. Additionally, the dorsal and ventral subcortical cell layer displayed only 1 cell. The dorsal cortical and subcortical sections were 14-16 μm and 14-17 μm high, respectively. Also, the ventral cortical and subcortical section showed 11-16 μm and 10-14 μm height, respectively.
Table 1
Morphological and anatomical comparison of characters among some species of Lobophora. (a) Longitudinal section, (b) transverse section. Measurements: μm.
|
Characters |
L. variegata n = 9 |
L. littlerorum n = 4 |
L. canariensis n = 7 |
L. declerckii n = 5 |
L. guadeloupensis n = 7 |
|
Thallus |
|||||
|
Color |
Yellow-green or brown |
Dark-green or brown |
Orange brown |
Brown |
Brown |
|
Blades |
Simple or lobed to lacerate |
Simple or lobed |
Simple or reniform |
Simple or lobed |
Simple |
|
Height |
2.2- 4.4 cm |
2-3.3 cm |
2.3-3.5 cm |
1.3-4.9 cm |
2-4.2 cm |
|
Width |
3.1- 5.5 cm |
3.2-4.5 cm |
1.1-4.1 cm |
1.5-6.2 cm |
1.2-3.9 cm |
|
Thickness |
128-190 |
98-135 |
8 4-98 |
65-83 |
72-98 |
|
Medullary cells |
|||||
|
Number of cell layers |
1 |
1 |
1 |
1 |
1-2 |
|
Height |
53-87 |
39-42 |
30-50 |
27-48 |
33-50 |
|
Width |
28-40 |
35-45 |
30-35 |
23-40 |
27-32 |
|
Length |
78-90 |
64-78 |
72-95 |
67-98 |
60-100 |
|
Number of cortical layers |
|||||
|
Total (including the medulla) |
5-7 |
5-6 |
5 |
3-5 |
5-7 |
|
Dorsal layers |
2-3 |
2-3 |
2 |
1-2 |
2-3 |
|
Ventral layers |
2-3 |
2 |
2 |
1-2 |
1-3 |
|
Dorsal cortex |
|||||
|
Number of cells |
2-3(4) (a), 2 (b) |
4-7 (a), 3-4 (b) |
4(a), 2 (b) |
4-2(a), 3-2 (b) |
2 |
|
Height |
10-13 |
12-13 |
14-16 |
8-12 |
10-12 |
|
Ventral cortex |
|||||
|
Number of cells |
2-3(4) (a), 2 (b) |
2 (a), 2(b) |
2(a), 1-2(b) |
1-2 |
3-2(a), 2(b) |
|
Height |
6-11 |
12-15 |
11-16 |
8-11 |
10-12 |
|
Dorsal subcortex |
|||||
|
Layers |
1-2 |
1-2 |
1 |
0-1 |
1-2 |
|
Number of cells |
1 |
2(a), 1(b) |
1-2(a), 1(b) |
1 |
1 |
|
Height |
6-12 |
13-18 |
14-17 |
6-10 |
12-14 |
|
Ventral subcortex |
|||||
|
Layers |
1-2 |
1 |
1 |
0-1 |
0-2 |
|
Number of cells |
1 |
1 |
1-2(a), 1(b) |
1 |
1 |
|
Height |
8-10 |
12-15 |
10-14 |
7-10 |
10-11 |
Table 2
Morphological and ecological features of the Cuban Lobophora species.
|
Lobophora species |
Substrate |
Morphology |
Habitat |
Depth (m) |
|
L. canariensis |
Rock |
Decumbent |
Rocky reef |
6-10 |
|
L. declerckii |
Rock |
Decumbent |
Rocky reef |
9 |
|
L. guadeloupensis |
Rock, sand |
Fasciculate |
Rocky reef/Sandy bottom |
4-5 |
|
L. littlerorum |
Rock |
Crustose |
Rocky reef |
6 |
|
L. variegata |
Rock |
Erect/Fasciculate |
Seagrass bed/Rocky reef |
6-9 |
Lobophora declerckii N.E. Schultz, C.W. Schneider and L. Le Gall 2015 (Fig. 3D-F).
Type locality: Tombant de Port-Louis, Guadeloupe, Antilles, Caribbean Sea, Western Atlantic Ocean (Schultz et al., 2015).
Specimens examined: Western Havana, Calle 16 beach, 9 m depth, rock substratum, rocky reef habitat, 5 May 2019, HANC 0956, collector: Amanda Ramos.
The thallus is brown and displays decumbent blades. The blade can be simple or lobed (1.3-4.9 cm long, 1.5-6.2 cm wide, 65-83 thickness). It is formed of a single-celled medulla (27-48 μm height) with usually 2 cortical cell layers on each side, dorsally and ventrally. The Cuban specimens displayed 3-5 cell layers including the medulla. Sometimes a subcortical cell layer may be absent. In the longitudinal section a single subcortical cell layer is usually shown covering a medullary cell and supporting the outer cortical cell layer. The medullary cell was 67-98 μm long. The dorsal cortical layer showed 4-2 cells and the ventral cortical section displayed 1-2 cells. Otherwise, the dorsal and ventral subcortical cell layer displayed only 1 cell. In transverse section a single subcortical cell layer is usually shown covering a medullary cell and supporting the outer cortical cell layer. The medullary cell was 23-40 μm width. The dorsal cortical cell layer exhibited 3-2 cells and the ventral section displayed 1-2 cells. The dorsal and subcortical cell layer showed only 1 cell. Furthermore, the dorsal cortical and subcortical section displayed 8-12 μm and 6-10 μm height, respectively and the ventral cortical and subcortical section showed 8-11 μm and 7-10 μm height, respectively.
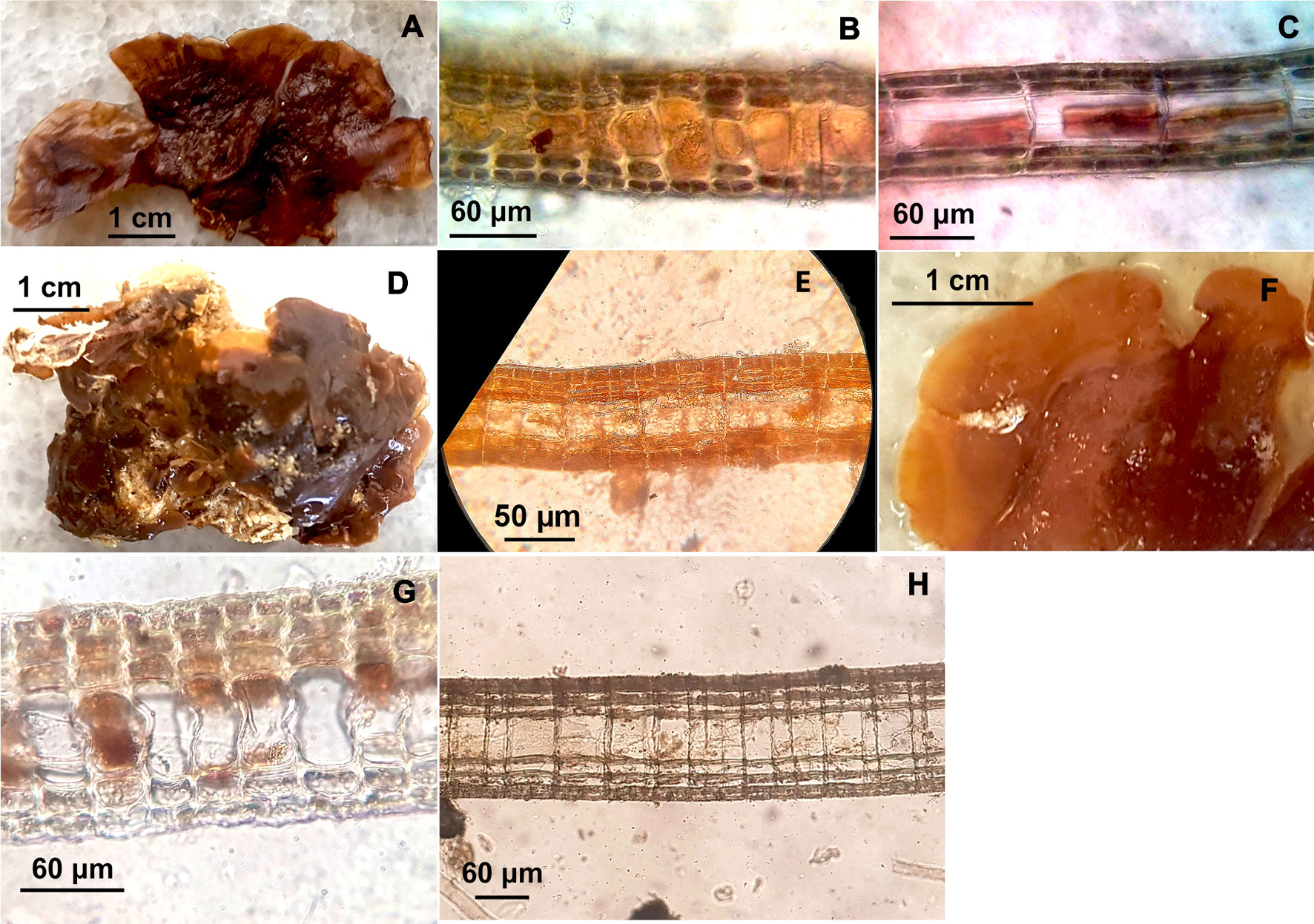
Lobophora guadeloupensis N.E. Schultz, F. Rousseau and L. Le Gall 2015 (Fig. 4A-C).
Type locality: Ilet Gosier, Guadeloupe, Lesser Antilles, Caribbean Sea, Western Atlantic Ocean, 16°11’55.428” N, 61°29’46.392” W (Schultz et al., 2015).
Specimens examined: Western Havana, Calle 16 beach, 4 m depth, rock substratum, rocky reef habitat, 18 April 2019, HANC 0953, collector: Jorge Gabriel Zúñiga Delgado; Western of Havana, Calle 16 beach, 5 m depth, sandy substratum, sand bottom, 12 May 2019, HANC 0957, collector: Eduardo Gabriel Torres Conde.
The thallus is brown. This species presented fasciculate and simple blades (2-4.2 cm long, 1.2-3.9 cm wide, 72-98 thickness). It is composed of 1-2 celled medulla (33-50 μm height) with 2-3 cortical cell layers dorsally and 1-2 cortical cell layers ventrally. The specimens from Cuban material displayed 5-7 layers including the medulla. In longitudinal section a single subcortical layer is usually shown covering a medullary cell and supporting the outer cortical layer. The medullary cell displayed 60-100 μm length. The dorsal cortical layer exhibited 2 cells and the ventral cortical section displayed 3-2 cells. Additionally, the dorsal and ventral subcortical cell layer displayed only 1 cell. In transverse section a single subcortical layer is usually displayed covering a medullary cell and supporting the outer cortical cell layer. Sometimes the dorsal or ventral subcortical cell layer may exhibit 2 layers rather 1. The medullary cell displayed 27-32 μm width. The dorsal and ventral cortical layer showed 2 cells and the dorsal and ventral subcortical layer exhibited only 1 cell. The dorsal cortical and subcortical section displayed 10-12 μm and 12-14 μm height respectively and the ventral cortical and subcortical section showed 10-12 μm and 10-11 μm height respectively.
Lobophora littlerorum C.W. Schneider, N.E. Schultz and L. Le Gall 2015 (Fig. 4D, E).
Type locality: Petit-Havre, Le Gosier, Guadalupe, West Indies, Caribbean Sea, Western Atlantic Ocean (Schultz et al., 2015).
Specimens examined: Western Havana, Calle 16 beach, 6 m depth, rock substratum, rocky reef habitat, 12 May 2019, HANC 0952, collector: Eduardo Gabriel Torres Conde.
The specimens can be dark-green or brown, displaying a crustose thallus. They present simple or lobed blades (2-3.3 cm long, 3.2-4.5 cm wide, 98-135 thickness), which are composed of a single-celled medulla (39-42 μm height) with 2-3 cortical cell layers dorsally and 2 cortical cell layers ventrally. The specimens of this species showed 5-6 layers including the medulla. In longitudinal section the specimens usually displayed 2 dorsal subcortical cell layers and a single ventral subcortical cell layer on each medullary cell supporting the outer cortical cell layer. The medullary cell displayed 64-78 μm length. The dorsal cortical cell layer showed 4-7 cells and the ventral cortical layer displayed 2 cells. Additionally, the dorsal subcortical cell layer displayed 2 cells and the ventral subcortical cell layer exhibited only 1 cell. In transverse section a single subcortical layer is usually displayed covering a medullary cell and supporting the outer cortical layer. The medullary cell displayed 35-45 μm width. The dorsal cortical layer showed 3-4 cells and the ventral section displayed 2 cells. Besides, the dorsal and ventral subcortical layer showed only 1 cell. The dorsal cortical and subcortical section displayed 12-13 μm and 13-18 μm height, respectively. Both, the ventral cortical and subcortical section showed 12-15 μm height.
Lobophora variegata (J.V.Lamouroux) Womersley ex E.C.Oliveira 1977 (Fig. 4F-H).
Basionym: Dictyota variegata J.V.Lamouroux 1809
Type locality: Antilles, West Indies (Silva et al., 1996).
Specimens examined morpho-anatomically: Ecological Reserve Los Pretiles, 9 m depth, rock substratum, seagrass bed habitat, 24 May 2011, HANC 0950, collector: Roamsy Volta Rodríguez; Guanahacabibes (Prejuicio beach), 6 m of depth, rock substratum, rocky reef habitat, 21 June 2014, HANC 0951, collector: Roamsy Volta Rodríguez; Western Havana, Calle 16 beach, 8 m depth, rock substratum, rocky reef habitat, 18 April 2019, HANC 0959, collector: Jorge Gabriel Zúñiga Delgado.
The thallus can be yellow-green or brown. It has erect-ruffled blades (2.2-4.4 cm long, 3.1-5.5 cm wide, 128-190 thickness). The specimens from Cuban material shown simple or lobed to lacerate blades, which are composed of a single-celled medulla (53-87 μm height) with 2-3 cortical cell layers on each side (ventrally and dorsally). They displayed 5-7 layers including the medulla. In longitudinal section the specimens displayed 1-2 subcortical cell layers covering a medullary cell (ventrally and dorsally) and supporting the external cortical cell layer. The medullary cell showed 78-90 μm length. The dorsal and ventral cortical cell layer displayed 2-3 (4) cells. Both, the dorsal and ventral subcortical layers displayed only 1 cell. In transverse section 2 dorsal and ventral subcortical cell layers are usually shown (each with 1 cell) covering a medullary cell and supporting the outer cortical cell layer. Sometimes the subcortical cell layers displayed 1 layer rather than 2. The medullary cell displayed 28-40 μm width. Both, the dorsal and ventral cortical cell layer exhibited 2 cells. The dorsal and ventral subcortical cell layer showed only 1 cell. Additionally, the dorsal cortical and subcortical section displayed 10-13 μm and 6-12 μm height, respectively and the ventral cortical and subcortical section showed 6-11 μm and 8-10 μm height,
respectively.
Remarks
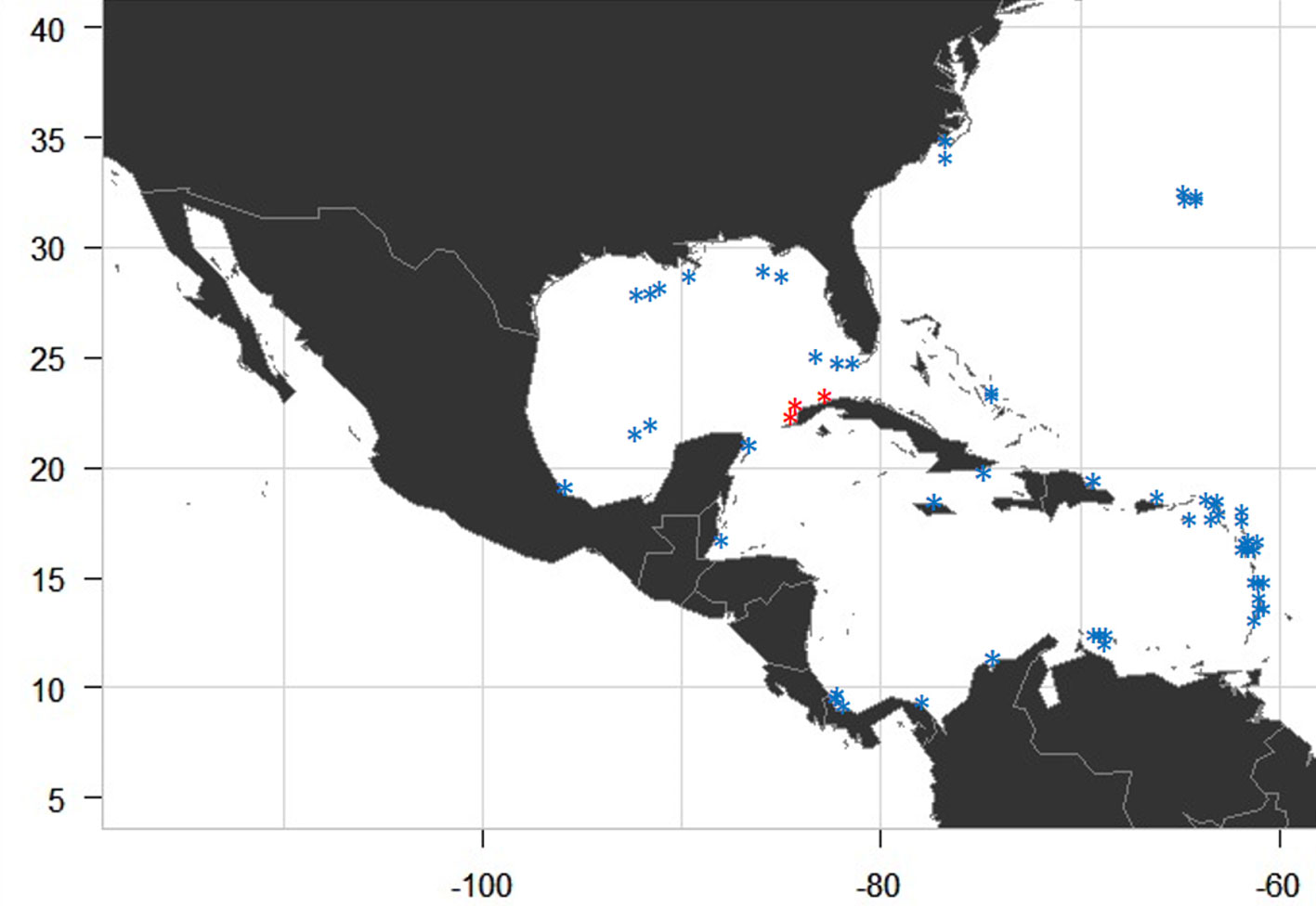
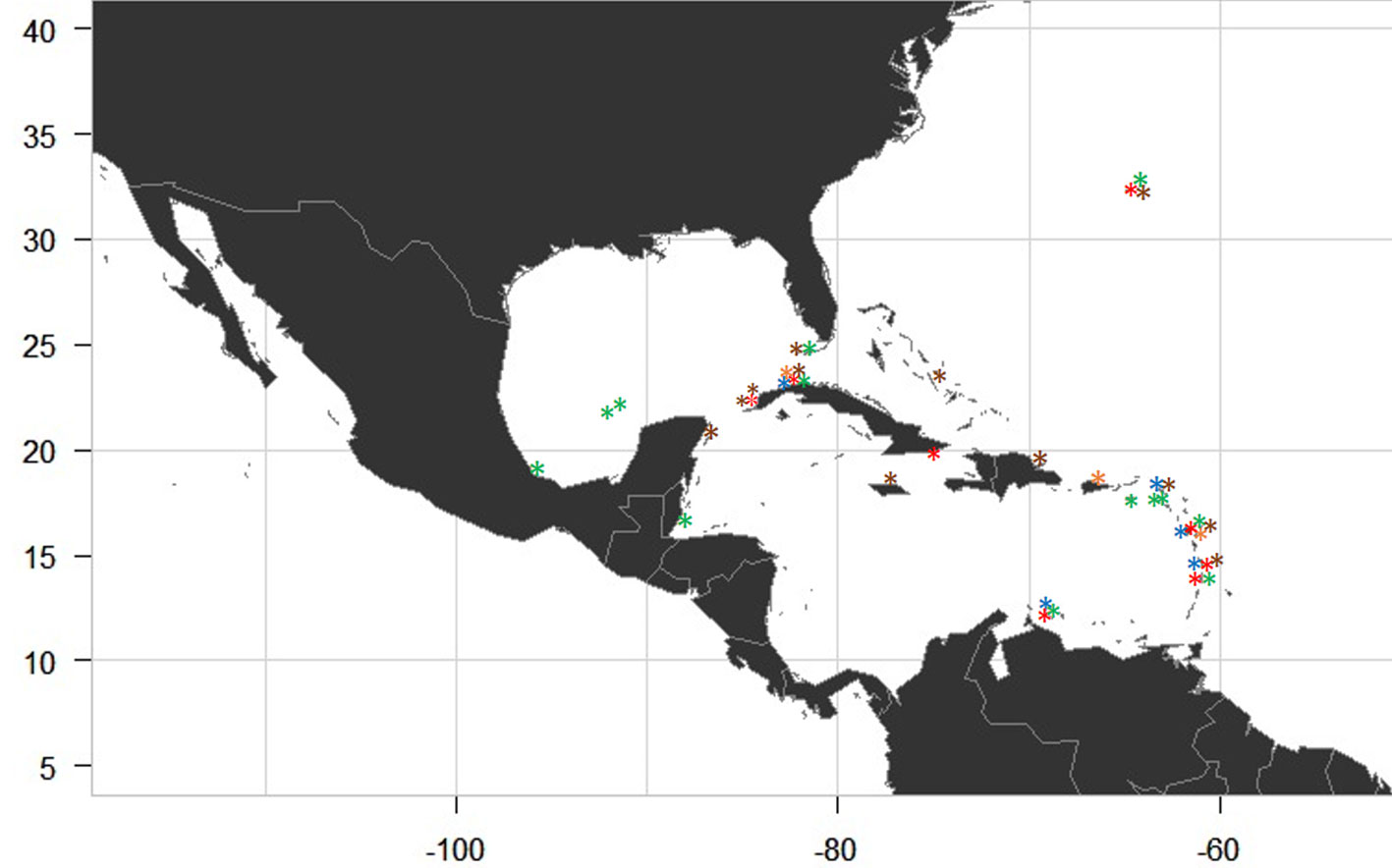
The 5 species found in Cuba have been previously reported for the Greater Caribbean Sea (Table 3). We analyzed the distribution of genus Lobophora in the Greater Caribbean Sea using the previously sampling carried out by Humm and Jackson (1955); Sun et al. (2012), Schultz et al. (2015), Vieira et al. (2016), Vieira, Morrow et al. (2020), Godínez-Ortega et al. (2018), Ballantine et al. (2019), Camacho et al. (2019) and this study (Fig. 5). Additionally, we present the distribution of the 5 Cuban Lobophora species found within the Greater Caribbean Sea (Fig. 6). We report for the first time the species L. guadeloupensis for the Greater Antilles.
Suárez et al. (2015) reported L. variegata as the only species of genus Lobophora for the Cuban island. However, Humm and Jackson (1955) had reported the presence of Aglaozonia canariensis in the Guantanamo Bay, Cuba, which is currently accepted as a synonym of L. canariensis. The morphological, ecological and cell analyses led us to report 3 new records for the Cuban macroflora (L. declerckii, L. guadeloupensis and L. littlerorum), following the species descriptions of Schultz et al. (2015), Vieira et al. (2016), Vieira, Morrow et al. (2020), and Camacho et al. (2019) for this genus. We also confirm the presence of L. variegata and L. canariensis. The Cuban material presented dorsally and ventrally cortical and subcortical cell layers surrounding medullary cell with number of cells and measurements like that proposed by Schultz et al. (2015), Vieira et al. (2016) and Vieira, Morrow et al. (2020) in longitudinal and transverse section for Lobophora variegata, L. canariensis, L. guadeloupensis, L. declerkii and L. littlerorum. The thallus form, thickness and its habitat (erect, decumbent or crustose) can also determine differences between Lobophora species according to our studies.
Lobophora canariensis is distinguished by the presence of a single subcortical cell layer with 1 cell on each side of the medulla supporting other cell layers with 1-2 cell ventrally and 2 cells dorsally in transverse section. Usually, the ventral cortex displays 2 cells rather 1 in transverse section. In the longitudinal section the dorsal cortex usually displays 4 cells on a single medullary cell. These features coincide with those proposed by Schultz et al. (2015) for the synonym L. payriae. The nomenclature of L. payriae was changed to L. canariensis by Vieira et al. (2016). Otherwise, the thallus thickness is in the range 80-112 μm presented by Vieira, Morrow et al. (2020), as well as the medullary cell is in the range of height (30-54 μm), length (60-100 μm) and width (30-40 μm). Additionally, the sum of dorsal cortical and subcortical cell layer is also in the range (26-34 μm) displayed by Vieira et al. (2016), and Vieira, Morrow et al. (2020) for this species, as well as occurred with the sum of ventral cortical and subcortical cell layer height (20-32 μm).
Table 3
Distribution of Lobophora canariensis, L. declerckii, L. guadeloupensis, L. littlerorum, and L. variegata for the Caribbean Sea. Source: Humm and Jackson (1955), Schultz et al. (2015), Delnatte and Wynne (2016), Vieira et al. (2016), Vieira, Morrow et al. (2020), Godínez-Ortega et al. (2018), Camacho et al. (2019), and this study.
|
Lobophora canariensis |
Humm and Jackson, 1955: (as A. canariensis): Guantanamo Bay, Cuba (Greater Antilles). Schultz et al., 2015: (as L. payriae): Bermuda (Central Caribbean) and Guadeloupe (Eastern Caribbean). Delnatte and Wynne, 2016: Martinique (Eastern Caribbean). Vieira, Morrow et al., 2020: Curaçao (Southern Caribbean) and Saint Lucia (Eastern Caribbean). This study (Greater Antilles): Calle 16 beach, Western Havana, Cuba and Gulf of Guanahacabibes, Pinar del Río, Cuba. |
|
Lobophora declerckii |
Schultz et al., 2015: Bermuda, Florida Keys (Northern Caribbean), Guadeloupe, Curaçao (Southern Caribbean) and Saint Croix (Eastern Caribbean). Godínez-Ortega et al., 2018: Veracruz (Southern Gulf of Mexico). Camacho et al., 2019: Ewing Bank and Northwest of Dry Tortugas (Southern Gulf of Mexico). Vieira, Morrow et al., 2020: Netherland: Saba and Sint Eustatius (Eastern Caribbean), Saint Lucia, Belize (Western Caribbean) and Curaçao. This study: Calle 16 beach, Western Havana, Cuba. Schultz et al., 2015: Bermuda. |
|
Lobophora guadeloupensis |
Schultz et al., 2015: Guadeloupe. Delnatte and Wynne, 2016: Martinique. Vieira, Morrow et al., 2020: Saint Martin (Eastern Caribbean) and Curaçao. This study: Calle 16 beach, Western Havana, Cuba. |
|
Lobophora littlerorum |
Schultz et al., 2015: Guadeloupe. Vieira et al., 2016: Guadeloupe. Ballantine et al., 2019: Puerto Rico (Greater Antilles). This study: Calle 16 beach, Western Havana, Cuba. |
|
Lobophora variegata |
Schultz et al., 2015: Bermuda, Florida Keys, Jamaica (Greater Antilles). Vieira et al., 2016: The Bahamas, Florida Keys, Guadeloupe, St. Kitts and Nevis (Eastern Caribbean). Delnatte and Wynne, 2016: Martinique. Godínez-Ortega et al., 2018: Quintana Roo, México (Western Caribbean). Camacho et al., 2019: The Bahamas and Florida Keys. Vieira, Morrow et al., 2020: Dominican Republic (Greater Antilles), Saint Martin and Martinique. This study : Ecological Reserve Los Pretiles, Pinar de Río, Cuba; Gulf of Guanahacabibes, Pinar del Río, Cuba and Calle 16 beach, Western Havana, Cuba. |
Lobophora declerckii usually looks like L. canariensis (both are decumbent) but is distinguished by the external cortical cell layer produced 1-2 cells ventrally and 2-3 cells dorsally on each medullary cell in transverse section. L. declerckii may occasionally show absence of subcortical cell layers. In longitudinal section while the dorsal external cortex of L. declerckii produced 4-2 cells on a single medullary cell, L. canariensis produced 4 cells. Additionally, L. canariensis showed thallus thickness measurements (84-98) higher than L. declerckii (65-83). These features agree with those proposed by Schultz et al. (2015) for these species. Otherwise, the thallus thickness of L. declerckii is in the range of 55-85 μm presented by Vieira, Morrow et al. (2020), as well as the medullary cell is in the range of height (30-50 μm), length (62-100 μm) and width (25-45 μm). The sum of dorsal cortical and subcortical cell layer height is also in the range (15-22 μm) displayed by Vieira et al. (2016), and Vieira, Morrow et al. (2020) for this species, as well as occurred with the sum of ventral cortical and subcortical cell layer height (14-20 μm).
Lobophora guadeloupensis has a fasciculate aspect and is usually distinguished by the presence of intermittent medulla composed of 2 cell layers, a unique aspect among the Lobophora species of the Greater Caribbean Sea (Schultz et al., 2015). The medullary cell can be single, double or beginning a cell division as is displayed in our study. This species is also distinguished from congeners by its ventral and dorsal cortices with usually 2 cells on each medullary cell in the longitudinal and transverse section. On the other hand, the specimens of these species occasionally displayed 2 subcortical cell layers rather than 1. This last feature coincides with those proposed by Vieira, Morrow et al. (2020) for this species. From the Cuban material the thallus thickness is in the range 65-95 μm presented by Vieira, Morrow et al. (2020), as well as the medullary cell is in the range of height (34-50 μm), length (55-105 μm) and width (25-32 μm). In addition, the sum of dorsal cortical and subcortical cell layer height is also in the range (18-26 μm) displayed by Vieira et al. (2016) and Vieira, Morrow et al. (2020) for this species, as well as occurred with the sum of ventral cortical and subcortical cell layer height (20-25 μm).
Lobophora littlerorum is differentiated by its crustose habitat. The thalli are strongly adhering to rocky and hard substratum. The specimens of Cuban material usually had 2 dorsal subcortical cell layers with 2 cells supporting a cortex with usually 4 cells in the longitudinal section. This agrees with the findings of Schultz et al. (2015). The thallus thickness is in the range 95-140 um reported by Vieira, Morrow et al. (2020), as well as the medullary cell is in the range of height (38-40 μm), length (60-80 μm) and width (32-46 μm). Additionally, the sum of dorsal cortical and subcortical cell layer height is also in the range (24-32 μm) displayed by Vieira et al. (2016), and Vieira, Morrow et al. (2020) for this species, as well as occurred with the sum of ventral cortical and subcortical cell layer height (24-32 μm).
Lobophora variegata is distinguished by the presence of mostly 3 cell layers below and above the medulla. However, occasionally some specimens can produce only a single subcortical cell layer dorsally or/and ventrally. Therefore, the number of cell layers can vary to 7 from 6 including the medulla. L. variegata and L. crispata display 5-7 cell layers with 2-3 cell layers dorsally and ventrally. However, in this study L. variegata showed a thallus thickness (128-190 μm) higher than the measurements proposed by Vieira, Morrow et al. (2020) for L. crispata (110-122 μm). On the other hand, while L. variegata had an erect morphology in our study, L. crispata has a decumbent morphology according to Vieira, Morrow et al. (2020). L. dispersa can also display 3 cell layers above and below the medulla (Vieira, Morrow et al., 2020) but it has smaller thallus thickness (78-164 μm) and medulla width (18-28 μm) measurements (Vieira, Morrow et al., 2020) than L. variegata (thallus thickness 128-190 μm, medulla width 28-40 μm) in our study. Additionally, L. dispersa shows a height of dorsal (40-60 μm) and ventral (38-44 μm) section (Vieira, Morrow et al., 2020) higher than the measurements of this study for L. variegata (dorsal 16-25 μm, ventral 14-21 μm height). These features coincided with those presented by Vieira et al. (2016), and Vieira, Morrow et al. (2020). Furthermore, the thallus thickness of L. variegata in our study is in the range 124-197 μm presented by Vieira et al. (2016), and Vieira, Morrow et al. (2020), as well as the medullary cell is in the range of height (50-94 μm), length (68-94 μm) and width (23-43 μm). The sum of dorsal cortical and subcortical cell layer height is also in the range (13-25 μm) displayed by Vieira et al. (2016), and Vieira, Morrow et al. (2020) for this species, as well as occurred with the sum of ventral cortical and subcortical cell layer height (13-21 μm). Vieira et al. (2016) state that a diagnostic vegetative character is the large central layer of the medulla. According to Sun et al. (2012), differences of 1 or 2 thallus layers are enough to support the genetic distinction between newly specimens of the genus Lobophora.
Ecological comments
We reported all species in shallow waters between 4-10 m depths. According to Schultz et al. (2015), the genus Lobophora has been found from the intertidal to 135 m. L. littlerorum (crustose habitat) was present only in Calle 16 beach. Vieira, Henriques et al. (2020) stated that the dominance of species with a crustose habitus versus upright species may be mediated by the pressure of herbivores. The coast of Havana has a low biomass average of fishes (12 gm-2) compared to unfished Caribbean reefs (30 gm-2) (Duran et al., 2018). According to Duran et al. (2018) the herbivore lengths were not over 20 cm, suggesting a high fishing pressure along Havana coast. This could be an influencing factor but really the presence or absence of L. littlerorum only in Calle16 beach does not mean it is due to herbivores, but by an incomplete sampling. In addition, Havana coast is an area affected by eutrophication (Duran et al., 2018), so that the benthic community is dominated by macroalgae, including Lobophora species.
The genus Lobophora has been reported at different habitats in the Greater Caribbean Sea (seagrass and macroalgae beds, coral reefs, mangroves and rocky reefs) according to Coen and Tanner (1989). From the Cuban material, we found L. canariensis, L. declerkii and L. littlerorum on rock substratum in agreement with Vieira, Morrow et al. (2020), but not on coral reef. Besides, we also found L. canariensis growing adjacent to L. declerckii on rocky reef. However, we could observe that they occupy different niches. While L. canariensis usually displayed small decumbent blades in shaded areas, L. declerckii showed large decumbent blades further exposed to the light. This ecological feature coincided with those proposed by Vieira, Morrow et al. (2020) for these species. On the other hand, we found L. guadeloupensis on rock reef and sand substratum in agreement with Vieira, Morrow et al. (2020), but not on seagrass bed. With respect to L. variegata we found this species on rock substratum and seagrass bed as it was found by Vieira, Morrow et al. (2020). The fact we did not find these species on coral reef is due to the low sampling effort. We recommend in future work to sample in other habitats such as mangroves and
coral reefs.
Distribution comments
The 5 Lobophora species reported from the Cuban material represent 27% of the Greater Caribbean Sea Lobophora species. According to Vieira, Morrow et al. (2020), the Atlantic Ocean has a lower diversity of Lobophora species (18) than the Pacific Ocean (95). However, the Greater Caribbean Sea is the area with the greatest diversity within the Atlantic Ocean, where our study focused. L. variegata historically was the unique species of this genus knowing for the Atlantic, but recently is most likely restricted to the Greater Caribbean Sea (Florida Keys, Western Caribbean, Bahamas, Greater Antilles and Eastern Caribbean (Godínez-Ortega et al., 2018; Vieira, Morrow et al., 2020). For the Greater Caribbean Sea the genus Lobophora has been reported in the Northern Caribbean (Carolinian, Florida Keys and Gulf of Mexico), Central Caribbean (Western Caribbean, Bermuda, Bahamas, Greater Antilles and Eastern Caribbean) and Southern Caribbean (Southwest Caribbean and Southern Caribbean) according to Vieira, Morrow et al. (2020). We add Western Cuba within the Greater Antilles (Central Caribbean) as a new area of distribution of Lobophora species. From the Cuban material, 4 species —L. canariensis (as A. canariensis), L. declerckii, L. variegata and L. littlerorum— have been previously reported for the Greater Antilles, but in this study, we add one new record L. guadeloupensis for this region. In addition, only 3 species found: L. declerkii, L. guadeloupensis and L. variegata, are endemic for the Greater Caribbean Sea. Lobophora canariensis and L. littlerorum share Eastern Atlantic as another area of distribution (Vieira, Morrow et al., 2020). Lobophora canariensis is distributed in the Central Caribbean (Bermuda and Eastern Caribbean) and Southern Caribbean within the Greater Caribbean Sea according to Vieira, Morrow et al. (2020). According to Vieira, Morrow et al. (2020), L. guadeloupensis is only distributed in the Eastern Caribbean and Southern Caribbean. However, we report this species for first time in the Greater Antilles. Lobophora littlerorum is now distributed in Greater Antilles (Puerto Rico and Cuba) and Eastern Caribbean (Guadeloupe) within the Central Caribbean according to Ballantine et al. (2019), Vieira, Morrow et al. (2020), and our study. From the Cuban material, L. declerckii is the one that displays the widest range of distribution within the Greater Caribbean Sea (Florida Keys, Southern Gulf of Mexico, Western Caribbean, Bermuda, Greater Antilles, Eastern Antilles and Southern Caribbean) according to Vieira, Morrow et al. (2020). Additionally, to date, only 2 sites (Guadeloupe and Havana littoral [this study]) present the 5 species L. canariensis, L. declerkii, L. guadeloupensis, L littlerorum and L. variegata within the Greater Caribbean Sea. Greater Antilles is an unknown area with only 1 sampling site in Jamaica, Dominican Republic and Puerto Rico (Ballentine et al., 2019; Vieira, Morrow et al., 2020), and 4 sampling sites in Cuba. Therefore, only 5 Lobophora species are currently documented from the 18 species reported for the Greater Caribbean Sea. For example, Eastern Caribbean is the area with most species reported (16; Vieira, Morrow et al., 2020) but it is also the area with the greatest sampling effort. We recommend in future works to strengthen the sampling effort for the Greater Antilles.
In this work, we present the diversity of this genus in Western Cuba, which is useful to extend the knowledge about the distribution of Lobophora species in the Greater Caribbean Sea. Algae diversity remains highly uncertain (De Clerck et al., 2013). In Cuba, the genus Lobophora has been highly underestimated, because it had not been taxonomically studied, and it has not been taken into account in health status studies of coral reefs. We also recommend a study of interactions between corals and Lobophora species in Cuban coral reefs as an indicator of coral health status. Moreover, a study of this genus in other areas and habitats of Cuba is required.
Acknowledgements
To all implicated people from the Centre for Marine Research of the University of Havana, who facilitated the results of this work. We also thank Beatriz Martínez-Daranas, Yelianis Orta Sánchez and Víctor Manuel Ramírez-Arrieta, for their help with the image work.
References
Ballantine, D. L., Ruiz, H., & Norris, J. N. (2019). Notes on the benthic marine algae of Puerto Rico. XII: additions to the flora. Botanica Marina, 62, 337–344. https://doi.org/10.1515/bot-2018-0117
Camacho, O., Fernández-García, C., Vieira, C., Gurgel, D., Norris, J. N., Freshwater, D. W. et al. (2019). The systematics of Lobophora (Dictyotales, Phaeophyceae) in the Western Atlantic and Eastern Pacific ocean: eight new species. Journal of Phycology, 55, 611–624. https://doi.org/10.1111/jpy.12850
Coen, L., & Tanner, C. (1989). Morphological variation and differential susceptibility to herbivory in the tropical brown alga Lobophora variegata. Marine Ecology Progress Series, 54, 287–98. https://doi.org/10.3354/MEPS054287
De Clerck, O., Guiry, M. D., Leliaert, F., Samyn, Y., & Verbruggen, H. (2013). Algal taxonomy: a road to nowhere? Journal of Phycology, 49, 215–225. https://doi.org/10.1111/jpy.12020
Delnatte, C., & Wynne, M. J. (2016). A revised checklist of marine algae and seagrasses of Martinique, French West Indies. Nova Hedwigia, 102, 415–440. https://doi.org/10.1127/nova_hedwigia/2016/0364
De Ruyter van Steveninck, E., Kamermans, P., & Breeman, A. (1988). Importance of physical and biological process in structuring tropical intertidal population of Lobophora variegata (Phaeophyceae). Marine Ecology Progress Series, 44, 77–84. https://doi.org/10.3354/meps044077
Duran, A., Shantz, A. A., Burkepile, D. E., Collado-Vides, L., Ferrer, V. M., Palma, L. et al. (2018). Fishing, pollution, climate change, and the long-term decline of coral reefs of Havana, Cuba. Bulletin of Marine Science, 94, 213–228. https://doi.org/10.5343/bms.2017.1061
Godínez-Ortega, J. L., Cabrera, L. I., García-Sandoval, R., Wynne, M. J., Olivares-Rubio, H. F., Ramírez-García, P. et al. (2018). Morphological and molecular characterization of Lobophora declerckii and L. variegata (Dictyotales, Ochrophyta) on the Atlantic coast of Mexico. Phytotaxa, 382, 57–73. https://doi.org/10.11646/phytotaxa.382.1.2. 57
Guiry, M. D., & Guiry, M. G. (2020). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. Retrieved on 14 April 2020 from: http://www.algaebase.org
Humm, H. J., & Jackson, C. R. (1955). A collection of marine algae from Guantanamo Bay, Cuba. Bulletin of Marine Science of the Gulf and the Caribbean, 5, 240–246.
Littler, D. S., & Littler, M. M. (2000). Caribbean reef plants. Washington, D.C.: OffShore Graphics.
RStudio Team (2021). RStudio. Integrated development for R. RStudio, PBC, Boston, MA URL http://www.rstudio.com/
Schultz, N. E., Lane, C. E., Gall. L. L., Bigney, D. A. R., Reviers, B. D., Rousseau, F. et al. (2015). A barcode analysis of the genus Lobophora (Dictyotales, Phaeophyceae) in the Western Atlantic Ocean with four novel species and the epitypification of L. variegata (J.V. Lamouroux) E.C. Oliveira. European Journal of Phycology, 50, 481–500. https://doi.org/10.1080/09670262.2015.1078500
Silva, P. C., Basson, P. W., & Moe, R. L. (1996). Catalogue of the benthic marine algae of the Indian Ocean. Berkeley: University of Califormia Press.
Suárez, A. M., Martínez-Daranas, B., & Alfonso, Y. (2015). Macroalgas marinas de Cuba. La Habana, Cuba: Editorial UH.
Sun, Z., Hanyuda, T., Lim, P. E., Tanaka, J., Gurgel, C. F. D., & Kawai, H. (2012). Taxonomic revision of the genus Lobophora (Dictyotales, Phaeophyceae) based on morphological evidence and analyses rbcL and cox3 gene sequences. Phycologia, 51, 500–512. https://doi.org/10.2216/11-85.1
Vieira, C., Camacho, O., Wynne, M. J., Mattio, L., Anderson, R. J., Bolton, J. J. et al. (2016). Shedding new light on old algae: Matching names and sequences in the brown algal genus Lobophora (Dictyotales, Phaeophyceae). Taxon, 65, 689–707. https://doi.org/10.12705/654.1
Vieira, C., Camacho, O., Sun, Z., Fredericq, S., Leliaert, F., Payri, C. et al. (2017). Historical biogeography of the highly diverse brown seaweed Lobophora (Dictyotales, Phaeophyceae). Molecular Phylogenetics and Evolution, 110, 81–92. https://doi.org/10.1016/j.ympev.2017.03.007
Vieira, C., Henriques, F., D’hondt, S., Neto, A. I., Almada, C. H., Kaufmann, M. et al. (2020). Lobophora (Dictyotales) species richness, ecology and biogeography across the North-Eastern Atlantic archipelagos and description of two new species. Journal of Phycology, 56, 346–357. https://doi.org/10.1111/jpy.12956
Vieira, C., Morrow, K., D´Hondt, S., Camacho, O. Engelen, A. H., Payri, C. E. et al. (2020). Diversity, ecology, biogeography, and evolution of the prevalent brown algal genus Lobophora in the Greater Caribbean Sea, including the description of five new species. Journal of Phycology, 56, 592–607. https://doi.org/10.1111/12986




