Rodrigo M. Barahona-Segovia a, b, *, Vicente Valdés-Guzmánb, Laura Pañinao-Monsálvez b, c
a Departamento de Ciencias Biológicas y Biodiversidad, Universidad de Los Lagos, Av. Fuschlöcher 1305, 5290000 Osorno, Chile
b Citizen Science Program Moscas Florícolas de Chile, Pje. Arizona 4067a, 8420000 Santiago, Chile
c Facultad de Ciencias Forestales, Universidad de Concepción, Victoria 500, 4030000 Concepción, Chile
*Corresponding author: rbarahona13@gmail.com (R.M. Barahona-Segovia)
Received: 4 October 2019; accepted: 26 February 2020
http://zoobank.org/urn:lsid:zoobank.org:pub:98BCC05E-4587-494C-B033-324CAB051B47
Abstract
Thick-headed flies (Conopidae) are a family of Diptera with species that are endoparasitoids of bees and aculeate wasps. Physoconops is represented by 64 species in the Neotropical and Andean regions and distributed in many countries. Only 3 species have been described for Chile, specifically from the northern area. In this work, a new species from the Valdivian evergreen forest, Physoconops tentenvilu n. sp., is described and a new key for the Chilean species is provided. In addition, P. tentevilu represents the southernmost record of this genus in Chile. Morphological aspects are discussed, as well as hosts and distribution gaps for the Chilean Physoconops species.
Keywords: Bee host; Hotspot; Mapuche myth; Megachile; Valdivian evergreen forest
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Las especies del género Physoconops (Diptera: Conopidae) de Chile, con la descripción de una especie nueva
Resumen
Las moscas de cabeza ancha (Conopidae) son una familia de Diptera cuyas especies son parasitoides de abejas y avispas. Physoconops se encuentra representado por 64 especies en las regiones Neotropical y Andina, distribuidas en varios países. Solo 3 especies han sido registradas para Chile, especialmente para la zona norte del país. En este trabajo, se describe una especie nueva del bosque valdiviano siempreverde, Physoconops tentenvilu n. sp., y se presenta una nueva clave taxonómica para los representantes chilenos de este género. Además, P. tentenvilu representa el registro más austral conocido para este género en Chile. Se discuten aspectos morfológicos, así como los huéspedes y vacíos distribucionales para las especies chilenas de Physoconops.
Palabras clave: Huésped abeja; Punto caliente; Mito mapuche; Megachile; Bosque valdiviano siempreverde
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Thick-headed flies (Diptera: Conopidae) are composed for 863 species worldwide (Stuke, 2017). This family is known due to their endoparasitoid behavior mainly on aculeate Hymenoptera, although the genus Stylogaster Macquart can also parasitize cockroaches and crickets (Freeman, 1966; Gibson & Skevington, 2013; Lopes, 1937; Marshall, 2012; Skevington et al., 2010; Stuke, 2017; Woodley & Judd, 1998). The female has a terminalia adapted for engaging bees or wasps and clasping the abdominal tergites of the host for oviposition (Smith, 1966). In order to do this, surveillance sites to detect possible hosts are used by females to assault bees or wasp with their in-flight attack system (Freeman, 1966; Marshall, 2012; Stuke, 2017). Other activities as pollination or hill toping behavior have been less studied in this family (Kendall & Solomon, 1973; Marshall, 2012; Skevington et al., 2010).
From all biogeographical regions, the Neotropical and Andean have more species described to date (221 species; Stuke, 2017). Almost all subfamilies are represented, except for Notoconopinae and Palaeomyopinae (Stuke, 2017). The most diverse subfamilies are Zodioninae, Stylogastrinae and Conopinae with 39, 73 and 96 species, respectively (Stuke, 2017). In this last subfamily, Conops Linnaeus, Physocephala Schiner and Physoconops Szilady are the most diverse genera (Stuke, 2017). The latter is composed of 64 species distributed mainly in the Nearctic and Neotropics and only 2 species are described from the Oriental region (Skevington et al., 2010; Stuke, 2017). Physoconops presents high richness with 57 species distributed from Mexico to northern Chile and central Argentina (Gibson et al., 2014; Stuke, 2017). Species of this genus have been studied in part by Kröber (1915, 1927), describing many species under the genus Conops. Camras (1955, 1957) described new species and provided taxonomic notes for other species. In addition, Stuke and Skevington (2007) provided a key for several species in Costa Rica, although many of them are present in other Neotropical countries. The hosts for many species are poorly recorded and restricted to Megachile Latreille bee species (Stuke, 2017; Stuke & Cardoso, 2013). The southernmost record for South America belongs to Physoconops rufipennis (Macquart), which has been reported for the Río Negro Province in Argentina (Gibson et al., 2014). Chile is poor in species compared to Argentina (Gibson et al., 2014; Stuke, 2017) and these have been recorded mainly in northern Chile (Kröber, 1915, 1927; Stuardo, 1946). Only 3 species have been previously mentioned: P. costatus (Fabricius), P. gracilis (Williston) and P. magnus (Williston) (Kröber, 1915, 1927; Reed, 1888; Stuardo, 1946; Stuke, 2017). The natural history or biology has never been described and represents one of the most poorly studied families of Diptera in Chile. This work is aimed to describe a new species of Physonocops from the Valdivian evergreen forest in southern Chile. In addition, we provide a checklist, new records, and a pictorial key for identification of all Physoconops species recorded for Chile.
Materials and methods
A review of the Physoconops was based on material deposited in collections from the following institutions: Instituto de Entomología, Universidad Metropolitana de Ciencias de la Educación, Santiago, Chile (UMCE); Ernesto Krahmer collection, Universidad Austral de Chile, Valdivia, Chile (UACH); Zoology Museum, Universidad de Concepción, Concepción, Chile (MZUC); and Universidad de Tarapacá, Arica, Chile (UTA). In addition, electronical records from the “Moscas Florícolas de Chile” Citizen Science Project (CSP) were obtained and deposited in a figshare repository database. From UACH, a couple of thick-headed flies were assigned to the genus studied using the keys of Camras (1955), Kröber (1915), and Stuke and Skevington (2007). The description of the new species follows the terminology proposed by Cumming and Wood (2017). Measurements were taken with a 1-mm precision ruler in the objective of a Leica S6 D microscope. Total length was measured from the head to the end of terminal tergite and wing length was measured from the base to the apex. Photographs were taken with a Nikon D7200 camera, equipped with AF-S DX Micro-NIKKOR 40mm f/2.8G and extension tubes.
Contents of each label are enclosed within double quotation marks (“ ”) and individual lines of information are separated by a single slash (/). Square brackets ([ ]) add information on specimen condition and repository collection. Original descriptions and images were used to diagnose the new species and to build the identification key. Distribution maps of the Physoconops species were created with ArcGIS v.10.4.1 (ESRI 2017).
Other acronyms of collections used were: AMNHN – American Museum of Natural History, New York, USA; SEMC – University of Kansas, Snow Entomological Museum, Lawrence, USA; UZMC – University of Copenhagen, Zoological Museum, Copenhagen, Denmark. The abbreviations used in this catalogue are: A: adult; cat.: catalog; desc.: description; distribution: geographic distribution; F: female; Fig(s).: figure(s); HT: holotype; References: references; ST: syntype; syn.: synonyms; T: type.
Results
Conopidae Latreille, 1802
Conopinae Latreille, 1802
Physoconops Szilady, 1926
Physoconops (Aconops) costatus (Fabricius, 1805), Fig. 1
Conops costata Fabricius 1805: 175.

Taxonomic summary
Type locality: America meridionali (T A UZMC).
Global distribution: Nearctic (USA) and Neotropical (Argentina, Brazil, Colombia, Paraguay, Venezuela and Chile).
References: Fabricius, 1805:175 (desc.); Kröber, 1915:141 (desc. & key); Reed, 1888:301 (cat.); Stuke, 2017:109 (cat.).
Material examined and distribution. No specimens were reviewed.
Remarks
This species only appears mentioned in the catalogs of Reed (1888) and Stuke (2017). Host(s) unknown. Most likely, this species is not distributed in Chile.
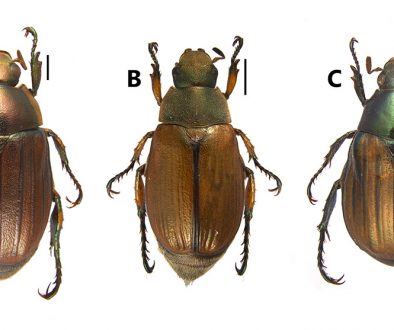
Physoconops (Pachyconops) gracilis (Willinston, 1885), Figs. 2-4
Conops gracilis Williston, 1885: 377-378.

Taxonomic summary
Type locality: Arizona, USA (HT F SEMC)
Global distribution: Nearctic (USA) and Neotropical (Brazil, Colombia, Mexico and Chile).
References: Kröber, 1915:137 (desc. & key); Kröber, 1927:140 (key); Stuardo, 1946:130 (cat.); Stuke, 2017:112 (cat.); Williston, 1885:377-378 (desc.).
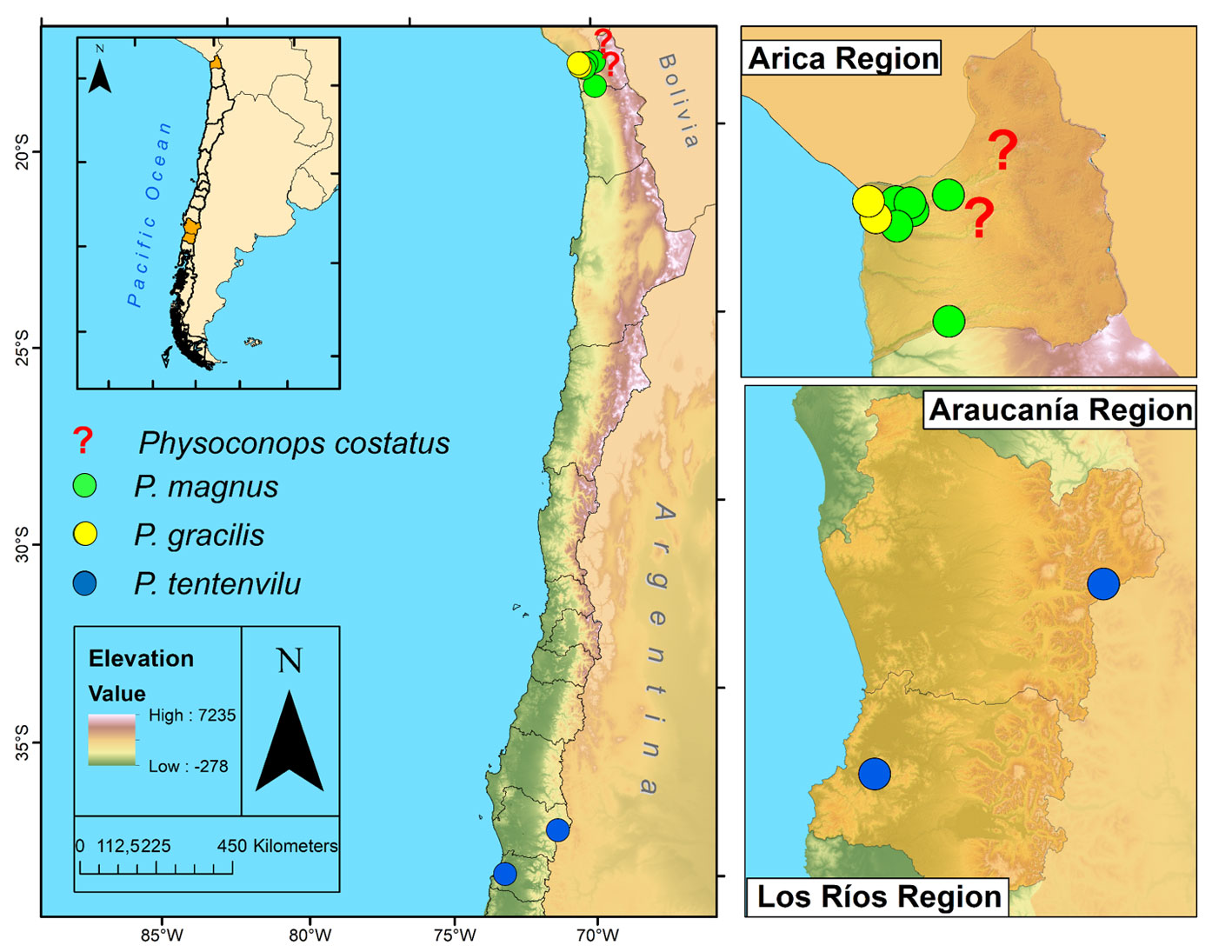
Material examined and distribution. Arica and Parinacota: mouth of the Lluta River, April-04-1924, Leg. H. Vasquez C. (UTA); Arica (Kröber, 1915) (Fig. 5).
Remarks
The natural history of this species is unknown. Possibly, Chile is the most southern limit of its distribution. Host(s) unknown.
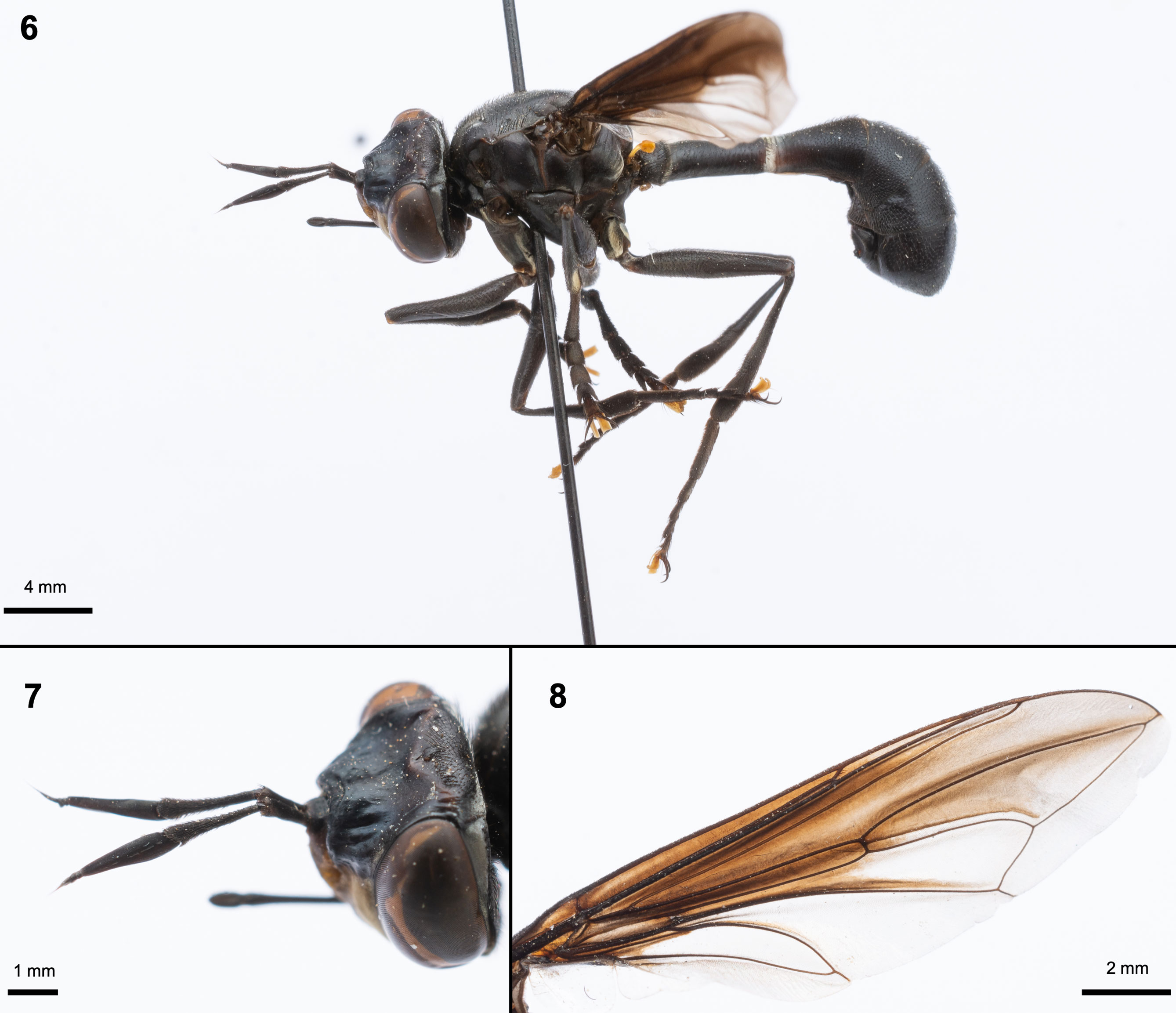
Physoconops (Pachyconops) magnus (Willinston, 1892), Figs. 6-8
Conops magnus Williston, 1892:43.
Taxonomic summary
Type locality: Chapada do Guimarães, Mato Grosso, Brazil (ST 6 F AMNHN).
Global distribution: Neotropical (Brazil, Mexico and Chile).
References: Kröber, 1915:124 (desc. & key); Kröber, 1927:139 (key); Stuardo, 1946:130 (cat.); Stuke, 2017:114 (cat.); Willinston, 1892:43 (desc.).
Material examined and distribution. Arica and Parinacota: Lluta, no date, Leg. Etcheverry (IEUMCE); valle de Lluta, km 14, Mar-1970, Leg. G. Díaz P.
(UTA); Poconchile, Jan-8-1996, Leg. J.C. Caro (MZUC); Mollepampa, Valle de Lluta, km 41-42, Jan-26-1969, Leg. J. Acuña (UTA); valle de Lluta, km 40-45, Feb-9-1969, Leg. R. Cortés (UTA); valle de Lluta, Feb-7-1986, Leg. D. Bobadilla (UTA); Azapa, Mar-2-1968, Leg. Etcheverry (IEUMCE); Valle de Camarones, Feb-4-1971, Leg. N. Hichins (UTA) (Fig. 5).
Remarks
The natural history is little known. One specimen was seen in Poconchile, Lluta Valley, attacking Xylocopa bees in agricultural lands (Mar-28-2018). This species was observed using the valleys where human activities are principally crops (i.e., alfalfa), which provided floral resources for native bees and wasps. In this habitat, we observed a single female occupying both fences as well as shrub branches to rest and prepare the fly-attack system. In 20 minutes, this female fly attacked 2 Xylocopa attaching for 3 and 6 seconds respectively. Bees fight with fly to avoid the parasitism. A third case of attack was avoided by another bee. Due to the large size of this thick-headed fly species, we suggest that Xylocopa could be its host. The female was not captured. In addition, Arica and Parinacota Region could be the southernmost limit of its distribution.
Physoconops tentenvilu n. sp. Barahona-Segovia
Figs. 9-13
http://zoobank.org/urn:lsid:zoobank.org:act:36D6E6E4-
FACE-412C-BD0A-31120BA64996
Diagnosis. Head, thorax and abdomen reddish-brown; postpronotal lobe with white pollinosity; halters yellow and legs yellow with tarsi dark brownish (Figs. 9-10).
Description: length: 12.7 mm; width: 3.5 mm (head) and 4.1 mm (thorax); wing: 13 mm.
Male. Head: reddish-brown. Eyes dark brown without ommatrichia; with a hind white fascia pollinosity in parafascia and posterior edge of the eyes. Vertex with several (10-12) black postocellar setae. Occiput dark brown with short and black bristles. Scape, pedicel and flagellum brown; pedicel 1.5 times longer than scape, and flagellum 1.5 times longer than pedicel. Pedicel with some short dark brown bristles on dorsal and anterior margins. Flagellum with 4 flagellomeres, the first enlarged and 5 times longer than others. The second article as long as the third. The final article of flagellum is slender in the posterior part (Fig. 12). Frons dark brown to rufous in the middle with lateral and upper black parts without hairs; wrinkled and concave frons in the middle before the vertex (Figs. 12, 13). Ocellar triangle black; ocelli blackish. Facial carina light brown; gena and postgena blackish with 1/3 height of the eye (Fig. 12); labrum and proboscis blackish-brown; Palp Brownish. Thorax: Postpronotal lobe reddish-brown with white pollinosity in the upper part and 5 black setae. Mesonotum completely reddish-brown with multiple and irregularly distributed setae; supra-alar area reddish-brown with 6 setae in upper part (in a same line) and 7 setae in the middle and lower parts irregularly distributed (Figs. 9, 10). Scutellum reddish-brown. Proepisternum, anepisternum, katepisternum, anepimeron, katatergite, anatergite and meron reddish-brown (Figs. 9, 10); posterior anepisternum with pale brown fringe. Katepisternum with few black setae in the middle; anterior and posterior spiracle dark red without pollinosity or setae around them (Fig. 12). Legs: trochanters and coxae of all legs are reddish-black. Trochanters of fore legs enlarged compared to mid and hind legs. Femora and tibiae orange with several short and black bristles. Anterior part of femora yellow near trochanter. The density of bristles increases in the posterior part of tibiae. Tarsi black with several black setae; claws blackish. Wings: basal C vein is dark brown with several short and blackish bristles; The R4+5, M1, CuA1, CuA2, A1, M4 veins and r-m and dm-cu cross veins are totally dark brown or at least the half; R2+3 vein is completely yellow. The bc, c, sc, br, bm and r1 cells are yellow and cover completely with blackish microtrichia; r2+3 cell yellow in ¾ parts and blackish microtrichia, and with ground black color and microtrichia of the same color in the apex forming a strong black line; r4+5 cell present ground black color and microtrichia of the same color in the anterior part; posterior part of r4+5 cell is hyaline; spurious vein lightly visible; the dm, m4 and cup cells are hyaline with black microtrichia. Knob and stem of halters are yellows and the base of this is dark brown (Fig. 11). Abdomen: Tergites 1-6 and sternites 1-7 with reddish-dark brown. Tergite 1 with several and brownish large bristles. Tergite 2 is larger than others. Posterior tergites with brownish medium size bristles (Figs. 9, 10). Genitalia: not detached. Epandrium and cerci dark brownish expanded ventrally with long brownish microtrichia distributed heterogeneously on dorsal side (Fig. 9).
Female. Length: 12.4 mm; width: 3.4 mm (head) and 4.1 mm (thorax); wing: 12.9 mm. Similar to male except for the the height of the gena, which is half of the eye height. Ventral genital plate reddish-dark brown; sternite 6 with long brownish bristles in distal portion; syntergite 8+9 with brownish medium size bristles and sternite 8 with short and brownish bristles. Cerci dark brownish densely pilosity (Fig. 10).
Taxonomic summary
Holotype: male, in excellent condition, found in Ernesto Krahmer’s collection from UACH and finally deposited in the Museo Nacional de Historia Natural de Chile (MNNC) with the following labels: “Santo Domingo, Valdivia / Oct-22-1987 / Leg. E. Krahmer”; “Holotypus / Physoconops tentenvilu / spec. nov. ♂ / det. Barahona-Segovia 2019” [red]. Paratype: female, in excellent condition, found in the Ernesto Krahmer’s collection from UACH and finally deposited in the MNNC with the following labels: “Santo Domingo, Valdivia / Oct-22-1987 / Leg. E. Krahmer”; “Holotypus / Physoconops tentenvilu / spec. nov. ♀ / det. Barahona-Segovia 2019” [yellow].
Type locality: Chile: Valdivia Province, Santo Domingo river.
Etymology: the specific epithet “tentenvilu” is related to Ten Ten-Vilú or Trentren Vilú (from the native Mapudungun language Trengtrengfilu: Trengtreng a name, and filu “snake”). This name is assigned to this species due to the enlarged form that reminds us of this Mapuche god.
History: Ten Ten-Vilú is the native god of the Mapuche people and represents the Earth (or Wallmapu in Mapudungun) and fertility of the woods and land. It is considered a protector spirit of the flora and fauna, and lord of the volcanos, according to Mapuche myths. This snake spirit is the son of Antü, a Pillan spirit of the Mapuche religion. Ten Ten-Vilú fought against Cai Cai-Vilú (currently, represented by a Chilean stonefly, Diamphipnoa caicaivilu [Vera, 2017]), another Mapuche god, since the latter flooded the earth when the human beings did not thank the sea enough for all the food he provided. Ten Ten-Vilú saved the humans and animals from drowning and ordered the earth to increase in height in order to avoid their death.
Distribution: from Icalma, La Araucanía region (-38.798108, -71.313450; CSP: https://figshare.com/s/24866642ae2689ce6833) to Valdivian evergreen forest, Valdivia, Los Ríos region, Chile (-39.903358, -73.177680; Fig. 5).

Remarks
From a biogeographic view, the Valdivian evergreen forest belongs to the Subantarctic subregion (Andean region), which extends from Valdivia to the Aysén Cordillera (Morrone, 2015). According to Smith-Ramírez et al. (2004), Valdivian evergreen forest extends for 250 km from the Toltén river (40º50’ S) to the south of the Llico river (41º30’ S) with a maximum altitude of 1,048 m in the Cordillera Pelada; rainfall varies from 4,000-5,000 mm and minimum and maximum mean temperature are 7.4 and 17.4 ºC, respectively. It is mainly characterized by the presence of Nothofagus Blume species and Aextoxicum punctatum Ruiz & Pav., and several other tree species (Smith-Ramírez, 2004). Native bee species that may represent the potential hosts of P. tentenvilu n. sp. reach 150 species (Montalva & Ruz, 2010). In addition, P. tentenvilu is the most austral species of his genus in Chile and the first record in the Valdivian evergreen forest.
Identification key for Chilean Physoconops.
1. Large size species (more than 20 mm) and body entirely black (Fig. 6) P. magnus (Willinston)
1′. Medium size species (up 20 mm) and body with other colors 2
2. Mesonotum black; pleura, anepisternum, katepisternum, anatergite, katatergite and meron with pale white color; frons reddish (Fig. 1) P. costatus (Fabricius)
2′. Mesonotum entirely or partially reddish-brown 3
3. Bright reddish-brown species with gold maculae in pleura, postpronotal lobe and scutellum, gold schiller so intense that the abdomen appears golden yellow, without a trace of black; head reddish-yellow (Fig. 2) P. gracilis (Williston)
3′. Dark reddish-brown species without golden or other color maculae or marked; head dark-brown (Figs. 9, 10) P. tentenvilu Barahona-Segovia n. sp.
Discussion
Physoconops tentenvilu represents the southernmost species record of this genus in Chile. Its morphological appearance is similar to other Neotropical Physoconops species described (Kröber, 1915, 1927; Stuke & Skevington, 2007), except for the the reddish-brown body, including the head, whitish pollinosity in parafascia, posterior margin of the eyes and postpronotal lobe, and the femora and tibiae are completely yellow. It is likely that P. tentenvilu Barahona-Segovia is one of the few species that inhabit the temperate forest in southern Chile, which has great biodiversity and is considered a hotspot (Myers et al., 2000). The natural history of this species, as well as of almost all Neotropical and Andean species of this genus, is unknown.
Physoconops is recognized for parasitizing the bee genus Megachile (Stuke, 2017; Stuke & Cardoso, 2013). In the distribution area where this thick-headed fly species has been recorded, there are only 4 Megachile species: M. euzona Pérez, 1899; M. pollinosa Spinola, 1851; M. semirufa (Sichel, 1857) and M. zaptlana (Cresson, 1878) (Montalva & Ruz, 2010). However, another 146 species of native bees are present in the same area and the potential hosts could be more (Montalva & Ruz, 2010). In addition, P. magnus showed the first field observation evidence of the potential host of this species, but for other Chilean species there is no information about the reproductive biology, habitat use or other ecological information.
Distribution for almost all thick-headed fly species present in Chile is little known and only a few studies have been developed on this subject. For example, Aubertin and Malloch (1933), in their review of the Diptera from Patagonia and southern Chile did not record any species of Physoconops in temperate forests. Other contributions in this aspect in Chile have been addressed through citizen science in other species to obtain new records and habitat information. Recently, Barahona-Segovia et al. (2017, 2018) rediscovered the rare Myopa metallica Camras in northern Chile, which provided a new distributional record and habitat description both in northern Chile as in the Mediterranean area. In this work, some new distributional records for P. magnus in Arica and Parinacota region are provided, as well as a brief habitat and behavioral description. The photographs provided here and the key for the Chilean species can be used in the future for other researchers to fill the distributional gaps and obtain new information in relation to the habitat or hosts. The morphological similarities of Physoconops with aculeate wasps may be confusing to citizen scientists and therefore, a photographic guide could possibly aid to recognize this species in the field. In conclusion, P. tentenvilu represents the southernmost Chilean species recorded to date and probably, one of the most austral records of this genus in Chile and southern South America temperate forests. Nevertheless, biological and distributional gaps for thick-headed flies are still high, therefore integral methods to capture information are needed to fill these gaps.
Acknowledgments
To Dolly Lanfranco and Cecilia Ruiz (UACH), for allowing us to work with the valuable collection of Ernesto Krahmer; to Mario Elgueta and Yasna Sepúlveda (MNNC); Danilo Cepeda (MEUC), Hugo Benítez, Héctor Vargas and Dante Bobadilla (UTA), Miriam Ramírez and José Artigas (MZUC) and Patricia Estrada and Antonio Rivera (IEUMCE) for allowing us to visit their collection, and Cecilia Smith for all the logistic support. We also thank Jeff Skevington for facilitating the photograph of Physoconops costatus; to Peter D. Lewis for valuable comments and contribution in the English review of our manuscript; to Juan Morrone for dedicating time in our manuscript and to anonymous reviewers. R.M.B.S. thanks the doctoral grant CONICYT 21160404.
References
Aubertin, D., & Malloch, J. R. (1933). Conopidae. In Diptera of Patagonia and Southern Chile. Part 6. Fascicle 3. (Natural History). London, British Museum.
Barahona-Segovia, R. M., Castillo-Tapia, R., & Pañinao-Monsálvez, L. (2017). First record of Myopa metallica Camras, 1992 (Diptera: Conopidae: Myopinae) in northern Chile after 46 years: A case study of the success of citizen science programs. Journal of Insect Biodiversity, 5, 1–8. http://doi.org/10.12976/jib/2017.5.13
Barahona-Segovia, R. M., Pañinao-Monsálvez, L., & Barceló, M. (2018). New records and updated distribution of Myopa metallica Camras 1992 (Diptera: Conopidae: Myopinae) in Chile by using integrative collection methods. Gayana, 82, 156–159. http://doi.org/10.4067/S0717-65382018000200156
Camras, S. (1955). A review of the new world flies of the genus Conops and allies (Diptera: Conopidae). Proceedings of the United State National Museum, 105, 155–187. https://doi.org/10.5479/si.00963801.3355.155
Camras, S. (1957). A review of the new world Physocephala (Diptera: Conopidae). Annals of the Entomological Society of America, 50, 213–218. https://doi.org/10.1093/aesa/50.3.213
Cumming, J. M., & Wood, D. M. (2017). Adult morphology and terminology. In A. H. Kirk-Spriggs, & B. J. Sinclair (Eds.), Manual of Afrotropical Diptera, Vol. 1. Introductory chapters and keys to Diptera families (pp. 89–133). Suricata 4. Pretoria: South African National Biodiversity Institute.
Fabricius, J. C. (1805). Systema Antliatorum, secundum ordines, genera, species: adjectis synonymis, locis, observationibus, descriptionibus. C. Reichard, Brunsvigae [=Brunswick]. https://doi.org/10.5962/bhl.title.137098
Freeman, B. A. (1966). Notes on conopid flies, including insect host, plant and phoretic relationships (Diptera: Conopidae). Journal of the Kansas Entomology Society, 39, 123–131.
Gibson, J. F., & Skevington, J. H. (2013). Phylogeny and taxonomic revision of all genera of Conopidae (Diptera) based on morphological data. Zoological Journal of the Linnean Society of London, 167, 43–81. https://doi.org/10.1111/j.1096-3642.2012.00873.x
Gibson, J. F., Skevington, J. H., & Camras, S. (2014). Conopidae (Diptera). In S. Roig-Juñent, L. E. Claps, & J. J. Morrone (Eds.), Biodiversidad de artrópodos argentinos, Vol. 4. San Miguel de Tucumán: Editorial INSUE-UNT.
Kendall, D. A., & Solomon, M. E. (1973). Quantities of pollen on the bodies of insects visiting apple blossom. Journal of Applied Ecology, 10, 627–634. http://doi.org/10.2307/2402306
Kröber, O. (1915). Die nord-und sudamerikanischen Arten der Gattung Conops. Archiv für Naturgeschichte (A), 81, 121–160.
Kröber, O. (1927). Beiträge zur Kenntnis der Conopidae. Konowia, 6, 122–143.
Latreille, P. A. (1802). Histoire naturelle, generale et particuliere, des crustaces et des insectes. Tome troisieme. Paris: Dufart.
Lopes, H. S. (1937). Contribução ao conhecimento do genero Stylogaster Macquart, 1835 (Dipt. Conopidae). Archivos de Instituto de Biologia Vegetal, 3, 257–293.
Marshall, S. A. (2012). Flies: the natural history and diversity of Diptera. New York: Firefly Book.
Montalva, J., & Ruz, L. (2010). Actualización de la lista sistemática de las abejas chilenas (Hymenoptera: Apoidea). Revista Chilena de Entomología, 35, 15–52.
Morrone, J. J. (2015). Biogeographical regionalisation of the Andean region. Zootaxa, 3936, 207–236. https://doi.org/10.11646/zootaxa.3936.2.3
Myers, N., Mittermeier, R. A., Mittermeier, C. G., da Fonseca, G. A. B., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403, 853–858. https://doi.org/10.1038/35002501
Reed, E. C. (1888). Catálogo de los Insectos Dípteros de Chile. Anales de la Universidad de Chile, Santiago, 73, 271–316.
Skevington, J., Thompson, F. C., & Camras, S. (2010). Conopidae (Thick-headed flies). In E. V. Brown, A. Borkent, J. M. Cumming, D. M. Wood, N. E. Woodley, & N. A. Zumbado (Eds.), Manual of Central American Diptera, Vol. 2 (pp. 847–856). Ottawa: NRC Research Press.
Smith, K. G. (1966). The larva of Thecophora occidensis, with comments upon the biology of Conopidae: Diptera. Journal of Zoology, 149, 263–276. https://doi.org/10.1111
/j.1469-7998.1966.tb04048.x
Smith-Ramírez, C. (2004). The Chilean coastal range: a vanishing center of biodiversity and endemism in South American temperate rainforests. Biodiversity & Conservation, 13, 373–393. https://doi.org/10.1023/B:BIOC.0000006505.67560.9f
Stuardo, C. (1946). Catálogo de los dípteros de Chile. Ministerio de Agricultura. Dirección general de Agricultura. Santiago de Chile: Imprenta Universitaria.
Stuke, J. H. (2017). Conopidae (Diptera) (World Catalogue of Insects 15). Leiden, Sweden: Brill.
Stuke, J. H., & Cardoso, C. F. (2013). Physocephala inhabilis (Walker) (Diptera: Conopidae) parasitizing Megachile (Moureapis) maculata Smith (Hymenoptera: Megachilidae). Studia dipterologica, 20, 39–43.
Stuke, J. H., & Skevington, J. (2007). The Conopidae of Costa Rica (Diptera) (Part 1: Conopinae-Conopini & Tropidomyiini). Zootaxa, 1528, 1–40. https://doi.org/10.11646/zootaxa.1528.1.1
Szilady, Z. (1926). Dipterenstudien. Annals of the Museum National of Hungary, 24, 586–611.
Vera, A. (2017). Estudio morfológico de la genitalia femenina y huevos en Diamphipnoidae (Plecoptera), con la descripción de la hembra de Diamphipnoa colberti y Diamphipnoa caicaivilu nov. sp. Revista Chilena de Entomología, 43, 25–40.
Williston, S. W. (1885). North American Conopidae: conclusion. Transactions of the Connecticut Academy of Arts and Sciences, 6, 377–394.
Williston, S. W. (1892). Diptera Brasiliana. Part II. Conops. Kansas University Quarterly, 1, 43– 46.
Woodley, N. E., & Judd, D. D. (1998). Notes on the host, egg, and puparium of Stylogaster biannulata (Say) (Diptera: Conopidae). Proceedings of the Entomological Society of Washington, 100, 658–664.