Tarin Toledo-Aceves *, Martín García-Díaz
Instituto de Ecología, A.C., Red de Ecología Funcional, Carretera Antigua a Coatepec No. 351, El Haya, 91073 Xalapa, Veracruz, Mexico
*Corresponding author: tarin.toledo@inecol.mx (T. Toledo-Aceves)
Received: 13 May 2023; accepted: 7 December 2023
Abstract
Secondary cloud forests (SCF) play a valuable role in regulating the hydrological cycle, providing habitat for biodiversity and resources for local livelihoods in cloud forest landscapes. At present, there is limited information on the potential of SCF for timber production. In this study, we determined the growth rates of 4 common SCF tree species, relative to tree size, crown exposure to light, and basal area of neighbouring trees. We used dendrometric bands to measure annual diameter growth in 375 individuals for 2 years, in SCF in Veracruz, Mexico. Average diameter growth rates were: Clethra mexicana = 0.53 ± 0.04 cm y-1, Juglans pyriformis = 0.41 ± 0.03 cm y-1, Liquidambar styraciflua = 0.41 ± 0.03 cm y-1, and Trema micrantha = 0.22 ± 0.04 cm y-1. Tree size was a significant predictor of diameter growth rate; diameter growth rate increased with tree size in all 4 species. Trees with crown laterally illuminated showed higher growth than trees without direct illumination in L. styraciflua. The basal area of neighbouring trees did not affect diameter growth in any species. The results indicate that young trees have high growth potential.
Keywords: Tropical montane cloud forest; Silviculture; Diameter growth rates; Natural regeneration
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Tasa de crecimiento de Clethra mexicana, Juglans pyriformis, Liquidambar styraciflua y Trema micrantha en bosque de niebla secundario
Resumen
Los bosques de niebla secundarios (BNS) tienen un papel importante en la regulación del ciclo hidrológico, como hábitat para la biodiversidad y provisión de recursos. Actualmente, existe poca información sobre el potencial del BNS para la producción de madera. Determinamos las tasas de crecimiento de 4 especies maderables comunes en BNS y su respuesta al efecto del tamaño del árbol, la exposición de la copa a la luz (posición en el dosel) y el área basal de los árboles vecinos. Usamos bandas dendrométricas para medir el crecimiento anual diamétrico en 375 individuos durante 2 años en BNS, en Veracruz, México. Las tasas de crecimiento diamétrico fueron: Clethra mexicana = 0.53 ± 0.04 cm año-1, Juglans pyriformis = 0.41 ± 0.03 cm año-1; Liquidambar styraciflua = 0.41 ± 0.03 cm año-1 and Trema micrantha = 0.22 ± 0.04 cm año-1. En las 4 especies, la tasa de crecimiento aumentó con el tamaño del árbol. Los árboles con copas con iluminación lateral presentaron tasas de crecimiento mayores que los árboles sin iluminación directa en L. styraciflua. El área basal de los vecinos no afectó el crecimiento en ninguna de las especies. Los resultados apoyan que el arbolado joven tiene alto potencial de crecimiento.
Palabras clave: Bosque mesófilo de montaña; Silvicultura; Tasa de crecimiento diamétrico; Regeneración natural
© 2024 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
At present, secondary and degraded forests are major components in tropical landscapes, covering more area than old-growth forests (Chazdon, 2003; FAO, 2015). Following deforestation and abandonment of crop cultivation and cattle breeding (due to human migration or loss of agricultural productivity associated with erosion), new forest cover can emerge as a result of natural regeneration (Chazdon et al., 2009). This regrowth type is referred to as secondary forest, natural regeneration, fallow vegetation, succession, passive restoration, or second-growth. Increasing concerns about the destruction of tropical forests, demand for forest resources, and awareness about the significant role of forests in climate, have raised attention regarding the potential of secondary forests for the provision of ecosystem services. Comprehensive reviews of tropical moist secondary forest dynamics and management have been published for lowland forests (e.g., Finegan, 1992; Guariguata & Ostertag, 2001), but there is less information available for secondary forests growing in tropical montane cloud forest landscapes.
Tropical montane cloud forest has long been recognized as one of the most threatened ecosystems on the planet and has become a global conservation priority (Hamilton, 1995; Mata-Guel et al., 2023; Scatena et al., 2011). Even though tropical montane cloud forest has undergone important losses due to transformation into pasture and agricultural land, in recent years, these activities have been undergoing abandonment in different regions around the globe (Aide et al., 2011), opening a space for the development of secondary forests (Mulligan, 2010; Nanni et al., 2019). In Mexico, only ~55% of the original cloud forest cover remains and, of this cover, 54% corresponds to secondary vegetation (Conafor, 2017). Secondary cloud forests (SCF) are comprised of small fragments in which unplanned logging by owners and rural inhabitants for timber, firewood, and charcoal production is common (Conabio, 2010; Ramírez-Marcial et al., 2001; Williams-Linera, 2002). Unsustainable logging in these patches further contributes to forest degradation and the depletion of locally valuable tree species (Ortíz-Colín et al., 2017; Ramírez-Marcial et al., 2001). The permanence and sustainable management of SCF are of great importance because these forests play a key role in hydrological regulation and soil retention, act as refuges for biodiversity, and could contribute to the provision of both timber and ecosystem services (Del Castillo & Blanco-Macías, 2007; Holwerda et al., 2010; Muñiz-Castro et al., 2012; Toledo-Aceves et al., 2022).
Several barriers to the sustainable management of SCF have been identified in Mexico, including contradictory norms and policies and the marginalization of smallholders in timber production activities, as well as limited availability of information about the response of SCF to silvicultural interventions (Toledo-Aceves et al., 2021). Overall, there is limited information available regarding the growth rates of native tree species in SCF. Evaluating the growth rates of 3 native species in SCF in Veracruz, Mexico, Mendoza-Hernández et al. (2019) found different responses according to species and site. Since important variations can occur among species and study sites, it is important to identify the factors that control variation in tree growth for the development of sustainable SCF management schemes. Important drivers of tree growth include abiotic site characteristics such as climate, soil physicochemical properties, elevation and topography, and biotic factors such as competition (Kübler et al., 2020; Peltier & Ogle, 2020). To contribute to the knowledge about potential SCF management for timber production, in this study, we determined the diametric growth rates of 4 valuable but common tree species in SCF and analysed their response to a small subset of the variables known to cause variability in tree growth; tree size, crown exposure to light, and basal area of neighbouring trees, as a surrogate for competition.
Analysis of growth as a function of tree size is important to determine potential yields and set limits to timber harvesting. A strong relationship has been reported between tree diameter and growth rate for tropical secondary forests in Puerto Rico, with the skewed unimodal form that is typical of tree growth processes (Adame et al., 2014). The position of a tree crown relative to its neighbours is associated with access to light, and light availability is one of the most important factors regulating tree growth. Increased canopy exposure to light has a positive effect on tree growth in forest ecosystems where water availability is not limiting (Baker et al., 2003). Crown position has shown a significant relationship to tree diameter increments in tropical rainforests (Alder & Synnott, 1992). Competition with neighbouring trees can reduce tree growth in secondary forests (von Oheimb et al., 2011), and silvicultural interventions, such as thinning to reduce competition, can be effective in terms of increasing growth rates (Venturoli et al., 2015). Given that species-specific responses to silvicultural treatments have been found in tropical montane forests (Kübler et al., 2020), analysis of individual tree growth in the most valuable species is necessary. In Costa Rica, liberation thinning, which aims to enhance the degree of crown illumination and growth of immature trees, significantly increased tree growth in a young (~ 4.5 years) secondary tropical wet forest (Guariguata, 1999). With proper guidance and training, the selection of future crop trees and the decision to apply liberation thinning could be assessed and manipulated by smallholders to increase yield. Given the low volumes of timber in SCF and the low profits that can be obtained (Toledo-Aceves et al., 2022), it is essential to increase the benefits derived from harvesting to promote the permanence of these forests. Understanding the factors that influence tree growth in successional stands can contribute to the provision of basic information regarding timber yield, estimation of cutting cycles, and prescription of adequate silvicultural treatments. The main questions addressed in this study were: i) What is the diameter growth rate of 4 valuable and common tree species in a SCF? ii) What is the influence of neighbouring tree competition, crown exposure to light, and individual size on the growth rate?
Materials and methods
The common names and uses reported for the studied species are presented in Table 1. The species were selected based on the interest of the SCF owners and to contribute to filling the information gap regarding the performance of some of the most common species in SCF. A brief description of the conservation status and distribution of each of the studied species is presented below.
Clethra mexicana DC. (Clethraceae) is endemic to Mexico, distributed mainly in tropical montane cloud forests, but also found in pine forests. It is distributed between 1,800 and 3,300 m asl (González-Espinosa et al., 2011). It is a shade-tolerant species (García-Hernández et al., 2019), listed as of Least Concern according to the IUCN red list due to its abundance in disturbed habitats (González-Espinoza et al., 2011). Juglans pyriformis Liebm. (Juglandaceae) is in the category of endangered due to overexploitation because of the high value of its timber. It is endemic to Mexico and almost completely restricted to tropical montane cloud forests but has also been found growing on steep slopes and cliffs in areas of pine-oak forest, pine forest, and riparian forest. It is distributed between 1,000 and 1,900 m asl (González-Espinosa et al., 2011) and is a shade-tolerant species (García-Hernández et al., 2019). Liquidambar styraciflua L. (Altingiaceae) is listed as of Least Concern as is very abundant in the tropical montane cloud forest, oak forest, and pine-oak-liquidambar forest. It regenerates well in open areas and grows rapidly in restoration plantations. Its elevational range is 450 to 2,100 m asl (González-Espinosa et al., 2011). It is a light-demanding species and abundant in early and intermediate successional forest stands (Muñiz-Castro et al., 2012). Trema micrantha (L.) Blume (Cannabaceae) is in the category of Least Concern as it has a wide distribution in tropical montane cloud forest and sub-deciduous tropical forest, evergreen forest, low deciduous forest, oak, and pine-oak forest. Its elevational range is from 0 to 2,200 m asl (González-Espinosa et al., 2011). It is a light-demanding species, abundant in early successional forest stands (Muñiz-Castro et al., 2012; Trujillo-Miranda et al., 2018).
The study was conducted in SCF of “Las Cañadas”, in the municipality of Huatusco in central Veracruz, Mexico (19°11’23” N, 96°59’11” W; 1,353 to 1,390 m asl). The region has remnants of mature and secondary forests immersed in a matrix of crops, shade coffee plantations, and cattle pastures (Conabio, 2010). In the study area, the mean annual temperature between 2018 and 2021 (the period of the study) was 20 °C and mean annual precipitation was 1,498 mm (Geissert & Ibáñez, 2008). The original vegetation of the study area is tropical montane cloud forest (“bosque mesófilo de montaña” sensu Rzedowski (2006)). The soil is an umbric Andosol (Geissert & Ibáñez, 2008), the physicochemical characteristics of which were reported in Trujillo-Miranda et al. (2018) and are presented in Appendix 1.
In 1950, 270 ha were deforested in the study area for livestock use, which lasted for 45 years. Due to high levels of soil erosion, cattle were excluded from the grazing areas in 1996, leading to the occurrence of natural regeneration that gave rise to forest patches of different sizes that added 98 ha to the SCF where this study was conducted (Romero-González, 2018). In 2006, the cooperative “Las Cañadas” was created and the defined management objectives for the SCF were to maintain the ecosystem functions of the forest (soil protection, biodiversity reservoir, hydrological cycle), guarantee the sustainable production of timber and firewood, and generate employment for the local population. A total of 106 ha was designated for conservation (including a 30-ha fragment of old-growth cloud forest), while other areas were designated for multiple uses (agriculture, intensive livestock grazing, plantations with native and exotic species).
The studied SCF was 22 years old at the start of the project in 2018 and comprised connected stands developed in areas used for grazing in which isolated remnant trees were part of the nuclei for recolonization. Isolated trees are widespread in fragmented landscapes (Manning et al., 2006). The structure and tree composition of the SCF can be found in Trujillo-Miranda et al. (2018).
Table 1
Diameter growth rate (mean ± S.E.) of trees in a secondary cloud forest. Minimum and maximum initial diameter and individual growth rates are included in brackets. *Uses reported in González-Espinoza et al. (2011).
| Species | Common name | Uses* | Diameter (cm) | Diameter growth rate
(cm y-1) |
| Clethra mexicana | Marangola, aguacatillo, amajuastle, cuchara, jaboncillo, madroño, mamahuaxtle, mamey cimarrón, quilaguacate, tlecúhuitl, totonalcanácatl | The wood is used for handicrafts, kitchen utensils, toys, rustic constructions, and charcoal production. The trees have value as ornamentals. The branches are used for firewood | 19.3 ± 0.8
[9.6 – 35] |
0.53 ± 0.04
[0.07 – 1.46] |
| Juglans pyriformis | Nogal, cedro nogal, nogal cimarrón | High quality wood is used to make furniture and musical instruments | 19.7 ± 0.5
[10.2 – 37] |
0.41 ± 0.03
[-0.02 – 1.41] |
| Liquidambar styraciflua | Bálsamo, biito, copalillo, copalme, cozote, estoraque, icob, ien-gau-uo, ingamo, liquidámbar, maripenda, molá, nabá, nijté-pijto, nite-biito, ococote, ocozote, quirámbaro, quirámboro, somerio, so té, sots-té, suchete, toshcui, tzoté, xochiocotzocuahuitl, xochiocótzotl, yagabizigui, yaga-huille | The resin from the trunk is used to make soap, cosmetics and incense, and has some medicinal uses. The wood is used in rural constructions and to make railroad sleepers, furniture, tool handles, veneer and pulp for paper. It is also used for firewood | 21.8 ± 0.6
[9.5 – 36] |
0.41 ± 0.03
[-0.03 – 1.26] |
| Trema micrantha | Capul, capulín, capulín blanco, capulín cimarrón, chaca, chacait, equipal, guacimilla, guinda, is-pope, ixpepe, majagua colorada, mata caballo, niguo, palo de ishpepe, pellejo de vieja, pie de paloma, puam, sac-pixoy, yaco de cuero, totogapolín, wahs zak | The wood is used for construction and the bark is used to make a traditional type of paper known as “papel amate”. It also has some medicinal uses | 20. 4 ± 0.5
[10.7 – 32.2] |
0.22 ± 0.04
[-0.12 – 2.14] |
Tree growth measurements
To measure stem diameter growth, dendrometers made of stainless-steel bands and springs were installed in the SCF in 2018. This approach was adopted because the studied species do not reliably form annual rings in tropical montane cloud forests and therefore tree growth cannot be estimated from ring width measurements as it can be done in tropical forests with a distinct dry season (Briennen & Zuidema 2005). A workshop was organized with members of the cooperative “Las Cañadas” to share information about the factors that influence tree growth, define the criteria to select the trees to be measured, and install the dendrometric bands in September 2018 (Fig. 1). Bands were placed in 406 individuals in total at the start, but due to mortality, growth was measured only from 375 individuals: 55 trees of C. mexicana, 112 of J. pyriformis, 124 of L. styraciflua, and 84 of T. micrantha. The uneven number of trees per species was due to variation in their local abundance. Trees with a diameter at breast height (DBH) < 9.5 cm, mechanical damage, or bifurcation below 1.3 m in height were excluded. Tree size ranged between 9.5 and 37.0 cm DBH. Stem diameter variation was measured annually with a vernier for 2 consecutive years after placement of the dendrometers. Initial DBH, crown exposure to light, and basal area of neighbouring trees, were recorded for each individual. To categorize crown exposure to light based on direct observation of the focal tree, the 5 categories of Dawkins and Field (1978) were used: 1) crown with no significant direct illumination; 2) crown with lateral illumination only; 3) crown partially illuminated from above; 4) crown fully illuminated from above, and 5) emergent crown. Due to only 1 individual registered in category 5, this category was excluded from the analysis. The number of trees registered in each category of crown illumination can be found in Appendix 2. The basal area of the neighbouring trees was estimated with a Spiegel relascope. All trees of any species around the focal tree were counted in a circle from a central point (the focal tree) in a 360-degree rotation. The entire tree had to fall within the corresponding band, and trees with a smaller diameter were excluded from the count. The Basal Area Factor 1 was used which is recommended for irregular forest stands with maximum tree diameters of 35 cm. Using this method, site delimitation and surface area are established automatically around the observer by means of the visual angle used. Thus, in contrast to methods that use sites of fixed dimensions (Rohman, 1999), there is no need to delimit sampling sites or to determine their surface area. All data were collected by a trained forester and a local worker. The elevation of the selected trees across the SCF ranged between 1,353 and 1,374 m asl and the slope recorded ranged between 7% and 23%.

Statistical analyses
A linear model was adjusted to analyse the response of tree growth rate to the effect of initial tree size, crown exposure to light, and basal area of neighbouring trees, for each of the 4 species separately. The mean growth rate of each individual tree per year from the 2 measured years was used as the response variable. Tree initial size (DBH), basal area of neighbouring trees, and crown exposure category were included as fixed terms in the model. The quadratic term of the initial tree diameter was added, however, since it was not-significant in any of the species the term was removed from the models. Interactions between independent variables were not included. The full model included the 3 independent variables and the Akaike Information Criterion (AIC) was used to select the best model (Crawley, 2013). We used contrasts to identify which crown categories were different. All analyses were performed in R (R-Core-Team, 2022).
Results
The diameter mean growth rate of the studied species is presented in Table 1; the highest growth rate was found in C. mexicana, followed by L. styraciflua and J. pyriformis, and T. micrantha displayed the lowest growth rate. However, the maximum values found in an individual tree were in T. micrantha (Table 1). In particular, this species displayed both the lowest and highest values per individual tree (Table 1), with only 3 individuals displaying growth rates > 1 cm y-1. We recorded negative values for diameter growth, indicating that tree trunk diameters shrunk during the 2 years of monitoring.
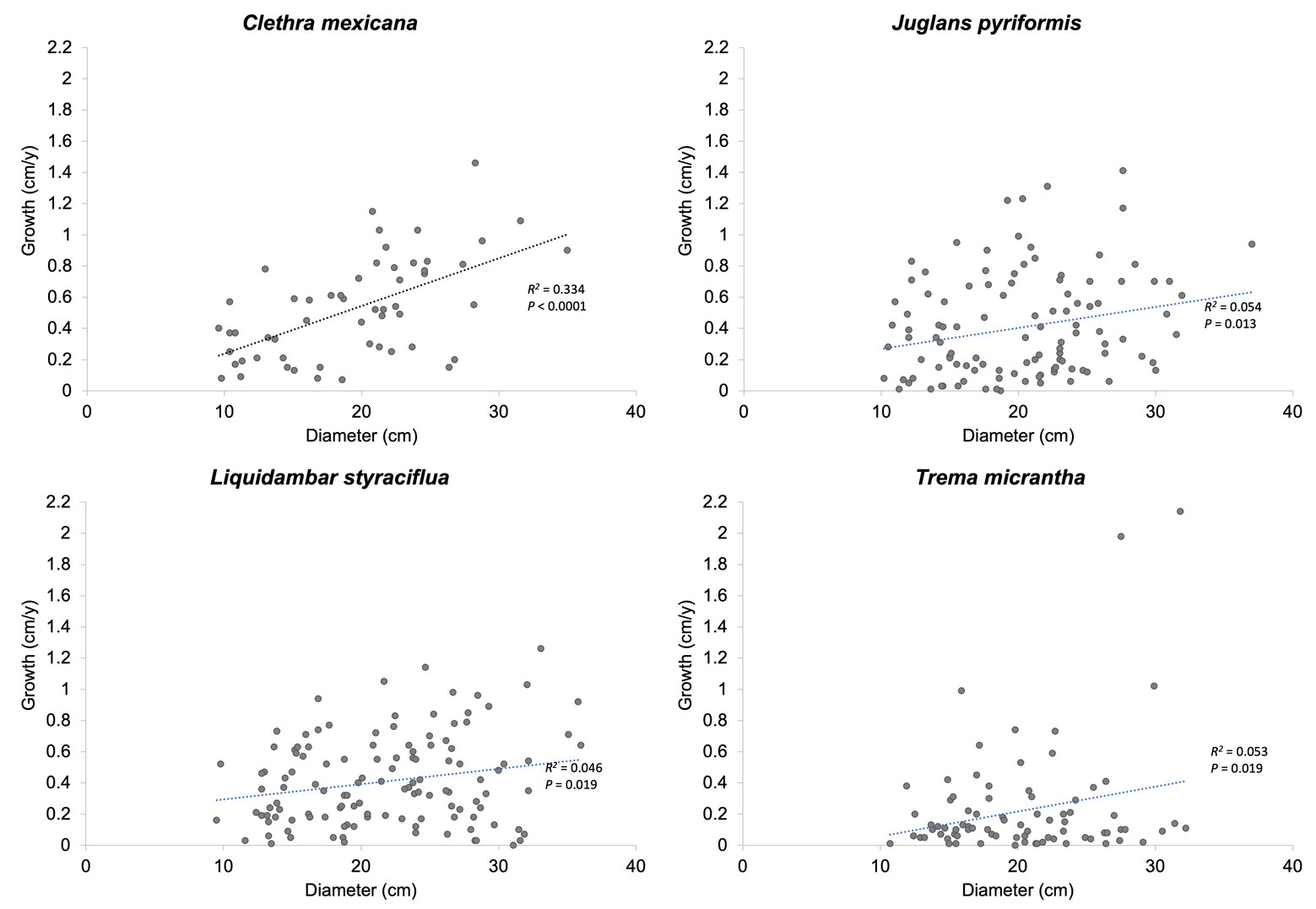
In the 4 species, the variable that explained most of the variation in tree growth was initial tree diameter (Table 2); the growth of the 4 species increased with tree DBH (Fig. 2). Crown exposure to light affected the growth of L. styraciflua but had no effect on C. mexicana, J. pyriformis, and T. micrantha; in L. styraciflua only trees in category 2 had higher growth than those in category 1 (Table 3). The basal area of the neighbouring trees had no significant effect on the growth of any of the species studied (Table 2). The percentage of variance in growth explained by the model was small in all cases, according to the values of R2 adjusted.
Discussion
The growth rates found for L. styraciflua and T. micrantha are inferior to the values reported for these species in other SCF (Mendoza-Hernández, 2015; Mendoza-Hernández et al., 2019) and for C. mexicana in periurban forests in Veracruz (Williams-Linera, 1996). Since larger trees present higher growth, the lower growth values found in the present study could be due to the lower number of trees with a DBH > 30 cm. In the present study, in comparison to the study of Mendoza-Hernández et al. (2019), 16% vs. 36% of the trees in L. styraciflua, and 7% vs. 39% in T. micrantha, were > 30 cm in DBH, respectively. In the case of J. pyriformis, to the best of our knowledge, there are no reports of its growth in natural forests with which to compare the values recorded in this study. The negative values of growth registered in some individuals indicate that tree trunks shrunk. A decrease in tree diameter has been attributed to the loss of stored water in external tissues, such as the bark, cambium and phloem, through transpiration, which dehydrate and shrink during the dry season, as opposed to the rainy season (Zweifel et al., 2000).
Table 2
Analysis of variance of linear models showing the effect of initial tree diameter, crown category, and neighbouring tree basal area (BA) on the diameter growth rates of 4 tree species in the secondary cloud forest. D.F. = Degrees of freedom; p values < 0.05 are highlighted in bold font.
| D.F. | F | p | |
| Clethra mexicana | |||
| Diameter | 1 | 26.167 | < 0.0001 |
| Crown | 3 | 1.062 | 0.373 |
| BA | 1 | 0.013 | 0.906 |
| Juglans pyriformis | |||
| Diameter | 1 | 4.945 | 0.028 |
| Crown | 3 | 0.741 | 0.529 |
| BA | 1 | 2.161 | 0.144 |
| Liquidambar styraciflua | |||
| Diameter | 1 | 6.238 | 0.013 |
| Crown | 3 | 2.745 | 0.046 |
| BA | 1 | 1.373 | 0.243 |
| Trema micrantha | |||
| Diameter | 1 | 4.457 | 0.037 |
| Crown | 3 | 0.236 | 0.870 |
| BA | 1 | 0.080 | 0.776 |
We found a positive relationship between initial tree size and diameter growth rate in all 4 species, which could be due to the size range included, which comprised mostly young trees. The behaviour of the curve denotes the early successional stage of the SCF studied and concurs with the general pattern in which diameter increase tends to peak in the early- to mid-life of a tree, then gradually declines with size and age (Bowman et al., 2013). Considering the higher growth rates found in larger trees and the higher costs per unit associated with harvesting small-diameter trees, the cost-benefit could be improved by harvesting older trees with higher volumes in more mature SCF. For example, 40 to 60-year-old secondary tropical forests with higher volumes have higher potential for timber production (Sips & van der Linden, 1998). However, small landholders often require short-term returns (Maraseni et al., 2017). Among other strategies, subsidies are therefore necessary to promote the management of SCF, as discussed in Toledo-Aceves et al. (2022).
Competition, reflected in the basal area of neighbouring trees, did not contribute to explaining tree growth in any of the species. This lack of a significant effect could be due to the low basal area of the neighbouring trees (accumulated basal area of all surrounding trees = 11.5 ± 0.17 m2 ha-1), as is common in early secondary tropical forests (Kappelle et al., 1996; Muñiz-Castro et al., 2012), as well as the reduced variation presented by this predictor variable. In contrast to the findings of this study, in other SCF, the basal area of neighbouring trees was found to range between 1 and 30 m2 ha-1 and had a significant negative effect on tree growth (Mendoza-Hernández et al., 2019). Since secondary forests are highly dynamic, the increasing basal area in the stand can be expected to have a stronger effect in more advanced successional stages. For Mexican tropical montane cloud forests (not only SCF), the basal area ranges between 13.4 and 15.4 m2 ha-1, as reported by the National Forest and Soils Inventory (Conafor, 2017). Consequently, growth rates can be expected to gradually decrease as inter-tree competition for light, water, and nutrients increases with succession (Bowman et al., 2013).
Table 3
Results of linear model showing the effect of initial tree diameter and crown category on the diameter growth rates of Liquidambar styraciflua. Contrasts among crown categories are shown. Crown 1 = crown with no significant direct illumination; crown 2 = crown with lateral illumination only; crown 3 = crown partially illuminated from above; crown 4 = crown fully illuminated from above. p Values < 0.05 are highlighted in bold font.
| Coefficient ± SE | t value | p | |
| Liquidambar styraciflua R2 adjusted = 0.078 | |||
| Intercept | 0.0372 ± 0.1105 | 0.337 | 0.736 |
| Diameter | 0.0106 ± 0.0044 | 2.373 | 0.019 |
| Crown 1 vs. 2 | 0.219 ± 0.103 | 2.129 | 0.035 |
| Crown 1 vs. 3 | 0.124 ± 0.112 | 1.109 | 0.269 |
| Crown 1 vs. 4 | 0.083 ± 0.113 | 0.734 | 0.464 |
| Crown 2 vs. 3 | 0.095 ± 0.066 | 1.433 | 0.467 |
| Crown 2 vs. 4 | 0.136 ± 0.063 | 2.158 | 0.134 |
| Crown 3 vs. 4 | 0.041 ± 0.069 | 0.590 | 0.932 |
The position of the crown influenced the growth of L. styraciflua, with higher growth found in trees with crowns laterally illuminated, compared to trees with no direct illumination. In Alnus acuminata, L. styraciflua, and Quercus xalapensis, the dominant trees also presented higher growth rates than suppressed trees in SCF (Mendoza-Hernández et al., 2019). Based on our results and those from previous studies in SCF (aus der Beek & Sáenz, 1996; Saenz & Guariguata, 2001), silvicultural treatments such as thinning have the potential to promote the growth of selected trees in montane forests. For L. styraciflua in plantations in the USA, thinning before 18 years of age is recommended, as well as elimination of poorly formed trees and those with bifurcations (Krinard, 1988). Analysis of the costs and benefits of such interventions is therefore necessary. Overall, the low R2 values of the models for 3 of the species studied indicate that only a small proportion of the tree growth variability was explained by the selected predictor variables. Other variables, such as the site characteristics of elevation, climate, and resource availability (e.g., soil fertility and water), can be main drivers of adult growth in Neotropical trees (Wagner et al., 2012), which highlights the importance of studying a wider range of sites to better represent tree growth in SCF.
Acknowledgments
The National Geographic Society, United States (#NGS-164R-18) provided financial support for the project “Secondary tropical cloud forests: assessing their potential for multiple use management”. The Instituto de Ecología, A.C., provided all the facilities to conduct the study. We are grateful to Ricardo Romero, Raúl Badín, Tania de Alba, and members of the cooperative “Las Cañadas” for all their support with the fieldwork. We thank Silvia Purata for her contribution during the workshop for installing the dendrometric bands with members of the cooperative, and Catalino López for his assistance in installing the bands and monitoring the growth. Thanks also go to Graciela Sánchez for reference formatting, Keith Macmillan for English revision, and the anonymous reviewers whose input helped us to improve this paper.
Appendix 1. Soil properties (mean ± 1 S.E.) in the secondary tropical montane cloud forest. Data reported in Trujillo-Miranda et al. (2018).
| Variable | |
| pH | 5.69 ± 0.17 |
| Bulk density | 0.53 ± 0.05 |
| C (%) | 10.46 ± 2.12 |
| N (%) | 0.96 ± 0.18 |
| P (%) | 0.08 ± 0.00 |
| C:N ratio | 10.83 ± 0.35 |
| Ca (cmol kg-1) | 9.48 ± 1.87 |
| Mg (cmol kg-1) | 4.01 ± 0.22 |
Appendix 2. Number of trees register per crown category.
| Species | Category 1 | Category 2 | Category 3 | Category 4 | Category 5 |
| Clethra mexicana | 5 | 22 | 17 | 14 | |
| Juglans pyriformis | 8 | 46 | 25 | 41 | 1 (excluded from analysis) |
| Liquidambar styraciflua | 10 | 51 | 28 | 40 | |
| Trema micrantha | 9 | 31 | 37 | 21 |
References
Adame, P., Brandeis, T. J., & Uriarte, M. (2014). Diameter growth performance of tree functional groups in Puerto Rican secondary tropical forests. Forest Systems, 23, 52–63. http://dx.doi.org/10.5424/fs/2014231-03644
Aide, T., Ruiz-Jaen, M., & Grau, H. (2011). What is the state of tropical montane cloud forest restoration. In L. S. Bruijnzeel, & F. Hamilton, L. (Ed.), Tropical montane cloud forests: Science for conservation and management (pp. 101–109). Cambridge, UK: Cambridge University Press.
Alder, D., & Synnott, T. J. (1992). Permanent sample plot techniques for mixed tropical forest. Oxford, UK: University of Oxford.
aus der Beek, R., & Sáenz, G. (1997). Impacto de las intervenciones silviculturales en los robledales de altura; estudio de caso en la Cordillera de Talamanca, Costa Rica. In Experiencias prácticas y prioridades de investigación en silvicultura de bosques naturales en América Tropical (pp. 145–159). CIFOR/CATIE, Costa Rica.
Baker, T. R., Swaine, M. D., & Burslem, D. (2003). Variation in tropical forest growth rates: combined effects of functional group composition and resource availability. Perspectives in Plant Ecology Evolution and Systematics, 6, 21–36. https://doi.org/10.1078/1433-8319-00040
Bowman, D. M., Brienen, R. J., Gloor, E., Phillips, O. L., & Prior, L. D. (2013). Detecting trends in tree growth: not so simple. Trends in Plant Science, 18, 11–17. https://doi.org/10.1016/j.tplants.2012.08.005
Brienen, R. J., & Zuidema, P. A. (2005). Relating tree growth to rainfall in Bolivian rain forests: a test for six species using tree ring analysis. Oecologia, 146, 1–12. https://doi.org/10.1007/s00442-005-0160-y
Chazdon, R. L. (2003). Tropical forest recovery: legacies of human impact and natural disturbances. Perspectives in Plant Ecology Evolution and Systematics, 6, 51–71. https://doi.org/10.1078/1433-8319-00042
Chazdon, R. L., Peres, C. A., Dent, D., Sheil, D., Lugo, A. E., Lamb, D. et al. (2009). The potential for species conservation in tropical secondary forests. Conservation Biology, 23, 1406–1417. https://doi.org/10.1111/j.1523-1739.2009.01338.x
Conabio (Comisión Nacional para el Conocimiento y Uso de la Biodiversidad). (2010). El bosque mesófilo de montaña en México: amenazas y oportunidades para su conservación y manejo sostenible. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Conafor (Comisión Nacional Forestal). (2017). Inventario Nacional Forestal y de Suelos. Informe de Resultados 2009–2014. Ciudad de México: Comisión Nacional Forestal.
Crawley, M. J. (2013). The R book. New Jersey: John Wiley & Sons.
Dawkins, H., & Field, D. (1978). A long-term surveillance system for British woodland vegetation. Oxford, UK: University of Oxford.
Del Castillo, R., & Blanco-Macías, A. (2007). Secondary succession under a slash-and-burn regime in a tropical montane cloud forest: soil and vegetation characteristics. In A. C. Newton (Ed.), Biodiversity loss and conservation in fragmented forest landscapes. The forests of montane Mexico and temperate South America. (pp. 158– 180). Oxfordshire, UK: CABI.
FAO (Food and Agriculture Organization). (2015). Global forest resource assessment. Rome.
Finegan, B. (1992). The management potential of neotropical secondary lowland rain forest. Forest Ecology and
Management, 47, 295–321. https://doi.org/10.1016/0378-1127(92)90281-d
García-Hernández, M. A., Toledo-Aceves, T., López-Barrera, F., Sosa, V. J., & Paz, H. (2019). Effects of environmental filters on early establishment of cloud forest trees along elevation gradients: Implications for assisted migration. Forest Ecology and Management, 432, 427–435. https://doi.org/10.1016/j.foreco.2018.09.042
Geissert, D., & Ibáñez, A. (2008). Calidad y ambiente físico-químico de los suelos. In R. H. Manson, V. Hernández-Ortiz, S. Gallina, & K. Mehltreter (Eds.), Agroecosistemas cafetaleros de Veracruz: biodiversidad, manejo y conservación (pp. 213–222). Xalapa: Instituto de Ecología, A.C./ INE-Semarnat.
González-Espinosa, M., Meave, J., Lorea-Hernández, F. G., Ibarra-Manríquez, G., & Newton, A. C. (2011). The Red List of Mexican cloud forest trees. Cambridge, UK: Fauna and Flora International.
Guariguata, M. R. (1999). Early response of selected tree species to liberation thinning in a young secondary forest in Northeastern Costa Rica. Forest Ecology and Management, 124, 255–261. https://doi.org/10.1016/S0378-1127(99)00072-9
Guariguata, M. R., & Ostertag, R. (2001). Neotropical secondary forest succession: changes in structural and functional characteristics. Forest Ecology and Management, 148, 185–206. https://doi.org/10.1016/S0378-1127(00)00535-1
Hamilton, L. S. (1995). Mountain cloud forest conservation and research: a synopsis Mountain Research and Development, 15, 259–266. https://doi.org/10.2307/3673933
Holwerda, F., Bruijnzeel, L. A., Muñoz-Villers, L. E., Equihua, M., & Asbjornsen, H. (2010). Rainfall and cloud water interception in mature and secondary lower montane cloud forests of central Veracruz, Mexico. Journal of Hydrology, 384, 84–96. https://doi.org/10.1016/j.jhydrol.2010.01.012
Kappelle, M., Geuze, T., Leal, M. E., & Cleef, A. M. (1996). Successional age and forest structure in a Costa Rican upper montane Quercus forest. Journal of Tropical Ecology, 12, 681–698. https://doi.org/10.1017/s0266467400009871
Krinard, R. M. (1988). Growth comparisons of planted sweetgum and sycamore. Research Note SO-RN-351. New Orleans, LA: Southern Forest Experiment Station, Forest Service, US Department of Agriculture.
Kübler, D., Hildebrandt, P., Gunter, S., Stimm, B., Weber, M., Munoz, J. et al. (2020). Effects of silvicultural treatments and topography on individual tree growth in a tropical mountain forest in Ecuador. Forest Ecology and Management, 457, 117726. https://doi.org/10.1016/j.foreco.2019.117726
Manning, A. D., Fischer, J., & Lindenmayer, D. B. (2006). Scattered trees are keystone structures – Implications for conservation. Biological Conservation, 132, 311–321. https://doi.org/10.1016/j.biocon.2006.04.023
Maraseni, T. N., Son, H. L., Cockfield, G., Duy, H. V., & Nghia, T. D. (2017). Comparing the financial returns from acacia plantations with different plantation densities and rotation ages in Vietnam. Forest Policy and Economics, 83, 80–87. https://doi.org/10.1016/j.forpol.2017.06.010
Mata-Guel, E. O., Soh, M. C. K., Butler, C. W., Morris, R. J., Razgour, O., & Peh, K. S. H. (2023). Impacts of anthropogenic climate change on tropical montane forests: an appraisal of the evidence. Biological Reviews, 98, 1200–1224. https://doi.org/10.1111/brv.12950
Mendoza-Hernández, M. (2015). Incremento diamétrico de cinco especies arbóreas con potencial maderable del bosque mesofilo de montaña en el centro de Veracruz (Tesis de maestría). Facultad de Biología, Universidad Veracruzana Xalapa, México.
Mendoza-Hernández, M., Gerez-Fernández, P., Purata-Velarde, S., & Toledo-Aceves, T. (2019). Growth rates of valuable tree species in secondary tropical montane cloud forests in Mexico: influence of tree size, crown position and competition. Madera y Bosques, 25, e2531824. https://doi.org/10.21829/myb.2019.2531824
Mulligan, M. (2010). Modeling the tropics-wide extent and distribution of cloud forest and cloud forest loss, with implications for conservation priority. In L. Bruijnzeel, & F. H. L. Scatena (Eds.), Tropical montane cloud forests: Science for conservation and management (pp. 16–38). Cambridge, UK: Cambridge University Press.
Muñiz-Castro, M. A., Williams-Linera, G., & Martínez-Ramos, M. (2012). Dispersal mode, shade tolerance, and phytogeographical affinity of tree species during secondary succession in tropical montane cloud forest. Plant Ecology, 213, 339–353. https://doi.org/10.1007/s11258-011-9980-5
Nanni, A. S., Sloan, S., Aide, T. M., Graesser, J., Edwards, D., & Grau, H. R. (2019). The neotropical reforestation hotspots: A biophysical and socioeconomic typology of contemporary forest expansion. Global Environmental Change-Human and Policy Dimensions, 54, 148–159. https://doi.org/10.1016/j.gloenvcha.2018.12.001
Ortíz-Colín, P., Toledo-Aceves, T., López-Barrera, F., & Gerez-Fernández, P. (2017). Can traditional selective logging secure tree regeneration in cloud forest? Iforest-Biogeosciences and Forestry, 10, 369–375. https://doi.org/10.3832/ifor1937-009
Peltier, D. M., & Ogle, K. (2020). Tree growth sensitivity to climate is temporally variable. Ecology Letters, 23, 1561–1572. https://doi.org/10.1111/ele.13575
R-Core-Team (2022). R: a language and environment for statistical computing, Vienna, Austria. Available at: https://www.R-project.org/
Ramírez-Marcial, N., González-Espinosa, M., & Williams-Linera, G. (2001). Anthropogenic disturbance and tree diversity in Montane Rain Forests in Chiapas, Mexico. Forest Ecology and Management, 154, 311–326. https://doi.org/10.1016/s0378-1127(00)00639-3
Rohman, C. F. (1999). Relascopia: una técnica de medición forestal. Ciudad de México: Universidad Autónoma Chapingo. División de ciencias forestales.
Romero-González, R. (2018). Ganadería agroecológica en una zona de bosque de niebla. In G. Halffter, C. Cruz, & C. Huerta (Eds.), Ganadería sustentable en el golfo de México (pp. 345–368). Xalapa: Instituto de Ecología, A.C.
Rzedowski, J. (2006). Vegetación de México. Ciudad de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Versión digital, disponible en: https://www.biodiversidad.gob.mx/
Saenz, G. P., & Guariguata, M. R. (2001). Demographic response of tree juveniles to reduced-impact logging in a Costa Rican montane forest. Forest Ecology and Management, 140, 75–84. https://doi.org/10.1016/s0378-1127(00)00278-4
Scatena, F., Bruijnzeel, L., Bubb, P., & Das, S. (2011). Setting the stage. In L. A. Bruijnzeel, F. N. Scatena, & L. S. Hamilton (Ed.), Tropical montane cloud forests science for conservation and management (pp. 38–63). Cambridge, UK: Cambridge University Press.
Sips, P. A., & van der Linden, V. A. (1998). Tropical secondary forest management: potential, constraints and recommendations. In M. R. Guariguata (Ed.), Ecology and management of tropical secondary forest: science, people and policy (pp. 1–10). San José, Costa Rica: CATIE, Turrialba.
Toledo-Aceves, T., Guariguata, M. R., Guenter, S., Porter-Bolland, L., & Merino, L. (2021). Overcoming key barriers for secondary cloud forest management in Mexico. Land, 10, 1078. https://doi.org/10.3390/land10101078
Toledo-Aceves, T., Gunter, S., Guariguata, M. R., García-Díaz, M., & Zhunusova, E. (2022). Financial revenues from timber harvesting in secondary cloud forests: a case study from Mexico. Forests, 13, 1496. https://doi.org/10.3390/f13091496
Venturoli, F., Franco, A. C., & Fagg, C. W. (2015). Tree diameter growth following silvicultural treatments in a semi-deciduous secondary forest in Central Brazil. Cerne, 21, 117–123. https://doi.org/10.1590/01047760201521011204
von Oheimb, G., Lang, A. C., Bruelheide, H., Forrester, D. I., Wasche, I., Yu, M. J. et al. (2011). Individual-tree radial growth in a subtropical broad-leaved forest: the role of local neighbourhood competition. Forest Ecology and Management, 261, 499–507. https://doi.org/10.1016/j.foreco.2010.10.035
Wagner, F., Rossi, V., Stahl, C., Bonal, D., & Herault, B. (2012). Water availability is the main climate driver of neotropical tree growth. Plos One, 7, e34074. https://doi.org/10.1371/journal.pone.0034074
Williams-Linera, G. (1996). Crecimiento diamétrico de árboles caducifolios y perennifolios del bosque mesófilo de montaña en los alrededores de Xalapa. Madera y Bosques, 2, 53–65. https://doi.org/10.21829/myb.1996.221386
Williams-Linera, G. (2002). Tree species richness complementarity, disturbance and fragmentation in a Mexican tropical
montane cloud forest. Biodiversity & Conservation, 11, 1825–1843. https://doi.org/10.1023/A:1020346519085
Zweifel, R., Item, H., & Häsler, R. (2000). Stem radius changes and their relation to stored water in stemsof young Norway spruce trees. Trees, 15, 50–57. https://doi.org/10.1007/s004680000072