Population traits and reproduction of the hippolytid shrimp Tozeuma carolinense (Decapoda: Caridea) at Laguna de Términos, Campeche, southwestern Gulf of Mexico
Mario Martínez-Mayén (a, *), Jesús Romero-Rodríguez (b), Marco A. Martínez-Muñoz (c)
a Unidad Académica de Ecología y Biodiversidad Acuática, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apartado postal 70-305, 04510 Ciudad de México, Mexico
b Colección Nacional de Crustáceos, Instituto de Biología, Universidad Nacional Autónoma de México. Apartado postal 70-153, 04510 Ciudad de México, Mexico
c Unidad Profesional Interdisciplinaria de Ingeniería y Ciencias Sociales y Administrativas, Instituto Politécnico Nacional, Calle Té 950, Granjas México, Iztacalco, 08400 Ciudad de México, Mexico
*Corresponding author: mariom@cmarl.unam.mx (M. Martínez-Mayén)
Abstract
This study explored the population biology and reproduction of Tozeuma carolinense in Laguna de Términos, Campeche, southwestern Gulf of Mexico, collected bimonthly from June 2009 to June 2010. The sample of 2,109 specimens analysed had a sex ratio of 0.39 males per female. On average, females were significantly larger (6.16 ± 2.02 mm carapace length, CL) than males (4.42 ± 0.70 mm CL). Recruitment was continuous, with the highest percentages of juveniles during June 2009 and June 2010. The estimated asymptotic length with the von Bertalanffy model was 10.13 mm CL for females and 6.62 mm CL for males. Longevity was 6 months for females and 5 months for males, with males having a higher mortality (Z = 1.08) than females (Z = 0.85). The reproduction was continuous, with highest incidence of berried specimens from August 2009 to April 2010. The estimated size at sexual maturity for females was 6.67 ± 1.30 mm CL. There was a significant correlation between female size and the number of embryos, with a mean fecundity of 268 ± 131 embryos. During the incubation period females lost 46% of the embryos.
Keywords: Hippolytidae; Growth; Sexual maturity; Fecundity; Embryo loss
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Características poblacionales y reproducción del camarón hipolítido Tozeuma carolinense (Decapoda: Caridea) en la laguna de Términos, Campeche, suroeste del golfo de México
Resumen
Se examinó la biología poblacional y reproducción de Tozeuma carolinense en la laguna de Términos, Campeche, suroeste del golfo de México, de especímenes recolectados bimestralmente de junio de 2009 a junio de 2010. Se capturaron 2,109 individuos de los que se calculó una proporción de 0.39 machos por hembra. Las hembras fueron más grandes (6.16 ± 2.02 mm de longitud de caparazón, LC) que los machos (4.42 ± 0.70 mm LC). El reclutamiento fue continuo, observándose el mayor porcentaje de juveniles durante junio de 2009 y junio de 2010. La longitud asintótica estimada con el modelo de von Bertalanffy fue 10.13 mm CL para hembras y 6.62 mm CL para machos. La longevidad se estimó en 6 meses para hembras y 5 meses para machos, los cuales tuvieron mayor mortalidad (Z = 1.08) que las hembras (Z = 0.85). La reproducción fue continua, incrementándose entre agosto de 2009 y abril de 2010. La talla estimada de madurez sexual en hembras fue 6.67 ± 1.30 mm LC. La fecundidad media fue 268 ± 131 embriones y se correlacionó significativamente con la talla de las hembras. Durante la incubación, las hembras perdieron 46% de los embriones.
Palabras clave: Hippolytidae; Crecimiento; Madurez sexual; Fecundidad; Pérdida de embriones
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Population traits and reproductive biology are important in studies of crustaceans since they provide an approximation of the ecology and life history of any population (Alon & Stancyk, 1982; Corey & Reid, 1991). Sex ratio and fecundity indicate the population structure and its reproductive potential, while embryo size and volume reflect the action of selective pressures on reproductive effort and larval development (Bauer, 1991). Therefore, it is necessary to gather information about species from diverse habitats, latitudes and depths in order to distinguish between phylogenetic and environmental sources of variation in the life history of a species (Bauer, 2004).
Tozeuma carolinense is a caridean shrimp endemic to the western Atlantic Ocean, with records from Vineyard Sound, Massachusetts to São Paulo, Brazil, and from Bermuda to Saint Lucia Island and Curaçao (Román-Contreras & Martínez-Mayén, 2009). It is highly abundant in shallow waters among beds of the seagrasses Syringodium filiforme, Thalassia testudinum, and Halodule wrightii (Hays, 2005). In coastal and estuarine systems of the Gulf of Mexico and Mexican Caribbean Sea it is the second dominant species after Hippolyte zostericola (Barba, 2012). It feeds on algae epiphytic on the seagrass or other structures, and thereby allows the seagrass beds to grow unhindered (Hays, 2005).
In spite of its wide geographical distribution, information on the population structure and some reproductive aspects of the species has been published only by Ewald (1969) and partly by Heck (1977), Dugan (1983), and Corey and Reid (1991). Other studies have focused on diverse experimental aspects (Cournoyer & Cohen, 2011; Hays, 2005; Main, 1985), regional faunal inventories and distribution (Barba, 2012; Ledoyer, 1986; Román-Contreras & Martínez-Mayén, 2009; Rouse, 1970), and prevalence of infestation by parasitic isopods (Romero-Rodríguez & Martínez-Mayén, 2018).
To address the lack of studies of the population biology and reproduction of T. carolinense along the Atlantic coast of Mexico, the present study evaluates the population structure and reproduction of this species in Laguna de Términos, Campeche, southwestern Gulf of Mexico, focusing on the size structure, sex ratio, sexual maturity, breeding season, fecundity and brood loss. Additionally, we provide data for the first time about the growth and mortality of this species; these results may provide a better understanding of the biology of this widely distributed caridean shrimp.
Materials and methods
Shrimps were collected every 2 months from June 2009 to June 2010 at the inner margin of Isla del Carmen, Laguna de Términos, Campeche, Mexico (19°10’-18°05’ N, 92°12’-91°10’ W) by 3 minute trawls at depth of 1 m with a Colman-Segrove sledge net (mouth aperture: 0.2 m height × 0.7 m width) equipped with a mesh size of 800 μm; the net was dragged from a boat over Thalassia testudinum meadows. The captured shrimps were preserved in 100% ethanol and then transferred into 70% ethanol. During the collections, water temperature fluctuated from 30 to 32.8 °C and the salinity from 33.9 to 38.8 psu. The characteristics of the Laguna de Términos were described in detail by Yáñez-Arancibia and Day (1982) and Raz-Guzmán and Sánchez (1996).
Samples were separated according to sex. Shrimps were classified as males or females by the presence or absence of the appendix masculina on the second pair of pleopods. The sex-ratio was estimated as the quotient between the number of males and the total number of individuals collected in each sampling (Grabowski et al., 2014). A chi-square test (χ2) was used to measure deviation from the expected 1:1 male:female for the total number of individuals in each size class and each date. The body size of the individuals was established as carapace length (CL), measured from the postorbital margin of the ocular orbit to the mid-posterior end of the carapace. Carapace length data were organized into 12 size-classes of 0.75 mm intervals calculated according to Sturges (1926) in order to construct length frequency distributions for each sex and deduce the population structure of this species. The individuals in the smallest size-class were considered to be recruits.
Growth of T. carolinense was calculated with the von Bertalanffy (1938) equation CLt = CL∞(1 – e-k (t – to)), where CLt is the carapace length at time t, CL∞ is the asymptotic length, k is the growth coefficient, and t0 the theoretical age when size is 0. These parameters were determined with the FiSAT II software version 1.1.3 (Gayanilo et al., 1995). Growth performances were compared in terms of the growth performance index (φ′), which is preferred to the use of CL∞ and k individually (Pauly & Munro,1984); it was computed as: φ′ = log10k + 2log10CL∞. The longevity of each sex was estimated by inverting the von Bertalanffy growth model (D’Incao & Fonseca, 2000), considering t0 = 0 and CLt/CL∞ = 0.99; thus, longevity = 0-(1/k) ln[1-(CLt/CL∞)] (Simões et al., 2013). Tozeuma carolinense is not a commercially exploited species, hence the total mortality (Z) was considered as natural mortality (Cházaro-Olvera, 2009). Since mortality is the inverse of survival (Ricker, 1975), the following formula was used to estimate total mortality (Z): loge (Nyc) = -Zyc + loge (a), where Nyc is the number of shrimps per year class, yc is the number of year classes and a is a constant.
All the females with sizes equal to or greater than the smallest ovigerous female found were considered mature (Alon & Stancyk, 1982; Bauer, 1989) and were used to estimate the size at sexual maturity (CL50) using a logistic curve fitted between the proportion of sexually mature females (991 specimens) in each size class (P) and the carapace length (CL) with the equation P = 1/(1 + exp [- (a + b × CL)]), where a and b represent the parameters obtained of the regression between P and CL. The CL50 corresponds to 50% sexually mature females as calculated from the relationship CL50 = -(a/b) (Oh et al., 2002).
The entire brood mass of each female was separated from the pleopods with fine forceps, and was assigned to 1 of 2 stages of embryonic development (Corey & Reid, 1991): stage I, embryos without eyespot, and stage II, eyespot fully defined and well developed appendages. Embryo volume (EV) was calculated on the basis of 120 ovigerous females (60 of each developmental stage) taking a random subsample of 10 embryos per individual to measure their individual length and width and estimate their volume with the formula EV = EL × π × (EW/2)2 as proposed by Corey and Reid (1991) for caridean shrimps.
In order to avoid errors related to embryo loss during incubation (Kuris, 1991), fecundity (total number of embryos attached to the pleopods of a female) was assessed by direct counting in females that carried a compact brood with stage I embryos. Data from females with embryos in stage II were used to estimate the average increase in volume and the possible brood loss.
One-way Anova was applied to evaluate the changes in embryo volume between stage I and stage II. Simple linear regression of loge transformed data was determined for the number of embryos (stage I) on carapace length, and the parameters that describe the equation between the 2 variables were obtained with the least squares method (Corey & Reid, 1991). All statistical analyses were based on Zar (2010), and the differences were considered significant at 95% (p < 0.05) confidence level.
Results
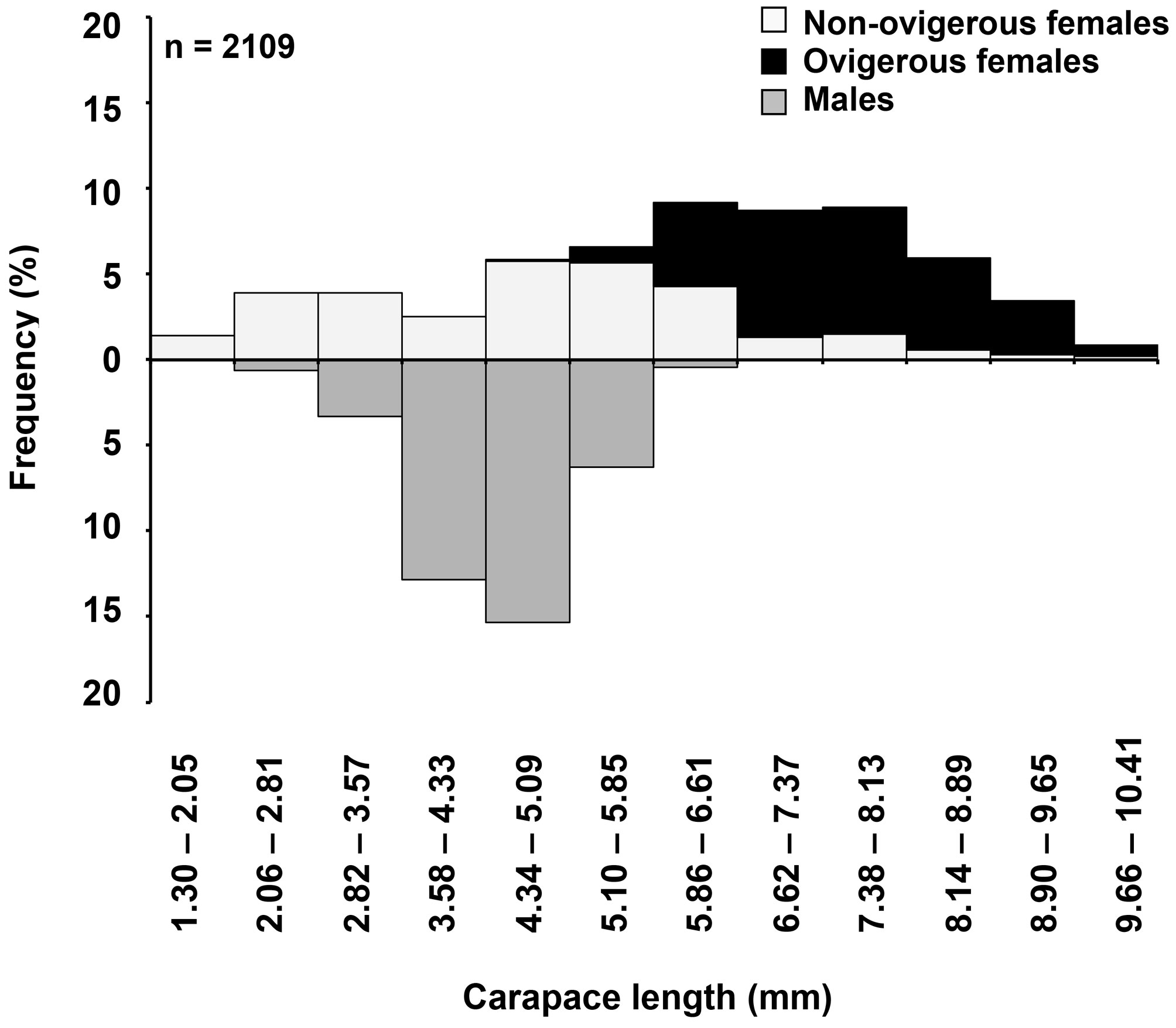
The sample consisted of 2,109 individuals of Tozeuma carolinense, of which 1,294 were females (634 of them ovigerous) and 815 were males. The highest abundance occurred in February 2010 (n = 705) and the minimum in October 2009 (n = 110). The average size ± standard deviation differed significantly between females (6.16 ± 2.02 mm) and males (4.42 ± 0.70 mm) (t– test = 89.11, p < 0.05). Females measured from 1.37 to 10.35 mm CL and males from 2.25 to 6.13 mm (Fig. 1). The overall sex-ratio was 0.39 males: 1 female, which differed significantly from the ratio of 1:1 (χ2 test = 108.33, p < 0.05). Females also significantly outnumbered males in every sampling period (χ2 test = 12.93, p < 0.05) (Fig. 2). Recruitment took place throughout the year, but the highest percentages of juveniles were observed during June 2009 and June 2010 (Fig. 3).

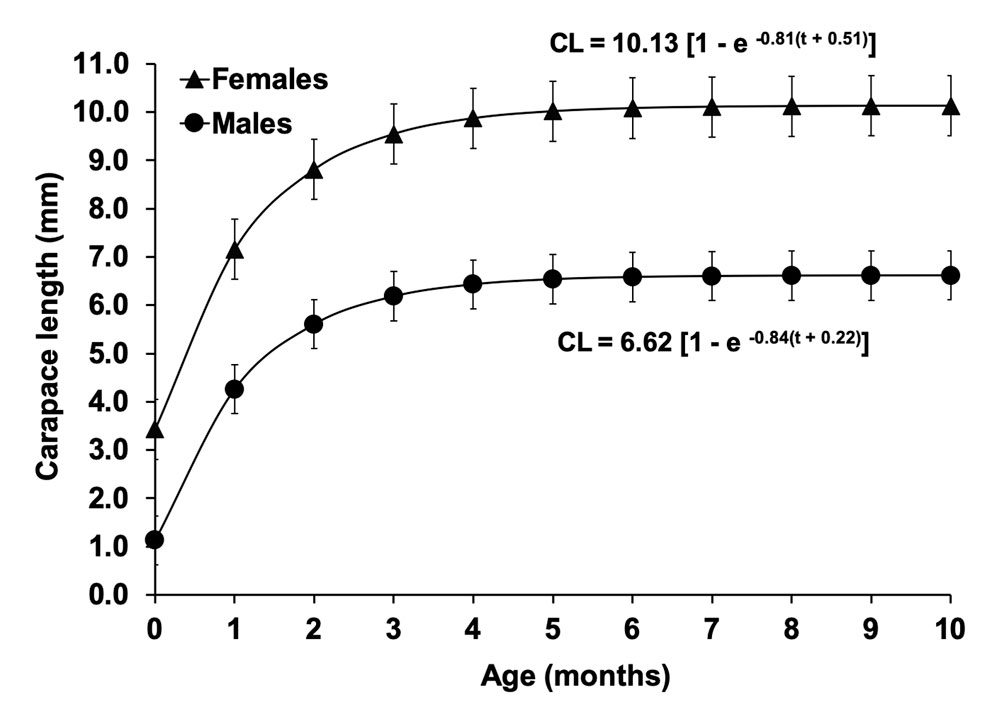
According to the estimated parameters of the von Bertalanffy equation (Fig. 4), the asymptotic carapace length for females (10.13 mm) was greater than that in males (6.62 mm), but the growth coefficient was similar in females (k = 0.81year -1) and males (k = 0.84 year -1). The growth performance index was higher in females (φ′ = 1.92) than in males (φ′ = 1.57). The estimated longevity was 6 months for females and 5 months for males. Mortality (Z) was 0.85 for females and 1.08 for males.
The size (CL ± 95% confidence interval) at which 50% of females are mature was 6.67 ± 1.30 mm CL, a value similar to the mean population size of females. The logistic function that expressed the relationship between CL and the proportion of sexually mature females was P = 1/[(1 + exp (9.1385 – 1.3701 × CL)].
Size of ovigerous females ranged from 4.90 to 10.25 mm CL (7.58 ± 1.06 mm CL); they were collected throughout the study period, but the highest incidence of berried specimens occurred from August 2009 to April 2010 (Table 1). The mean number of embryos (stage I) produced by females was 268 ± 131 and varied between 42 and 726. There was a significant relationship between the fecundity and female size (t = 27.55, p < 0.05), indicating that the larger females carry more embryos, although fecundity varied widely in females of similar body sizes (Fig. 5).
Embryos had a mean length of 0.46 ± 0.05 mm at stage I, and of 0.65 ± 0.07 mm at stage II. Their average volume varied from 0.050 ± 0.012 mm3 in stage I to 0.104 ± 0.036 mm3 in stage II, an increase of 108% (F = 1,193.31, p < 0.05). Females lost on average 46 % of their embryos during the incubation period.
Discussion
The abundance of Tozeuma carolinense in Laguna de Términos throughout the year was variable, a pattern also observed in Panamá by Heck (1977) and in Florida by Dugan (1983). The last author registered the highest abundance from July to November (490-1,798 individuals) and the minimum during February, May and June (167-310 individuals); in our study, the highest and lowest abundance occurs in February 2010 and October 2009, respectively (see Fig. 3). Heck (1977) and Dugan (1983) suggested that the variability in abundance is related to changes in water salinity following the rainy seasons. During the present study, the variation of this parameter was minimal (4.9 psu), hence the notable difference in abundances possibly are given by factors such as changes in the species composition and extent of the seagrass meadows into the surrounding areas (Ewald, 1969; Heck, 1977).
The continuous recruitment of T. carolinense is similar to other tropical caridean shrimps that inhabit seagrass meadows (Bauer, 1992; Romero-Rodríguez & Román-Contreras, 2013). According to Bauer (1989) the variations in recruitment throughout the year can be caused by factors such as differences in the breeding intensity of the population, temporal mortality of planktonic larvae or the recruits recently settled in seagrass beds, as well as changes in currents. The latter could explain, partially, the higher number of recruits of T. carolinense in both June samples since changes in circulation patterns returns the planktonic larvae to seagrass meadows increasing the abundance (Bauer, 1989).
The predominance of females throughout the year in Laguna de Términos concurs with observations of T. carolinense in Florida, USA (Ewald, 1969), and of caridean shrimps in other regions (Bae & Oh, 2014; Nye & Copley, 2014; Oya, 1987; Oya & Oka, 1985; Torres et al., 2007; Zupo, 1994). The high proportion of females could be explained, partially, by a heterogeneous distribution of the sexes on the substratum, associated with hiding or feeding (Main, 1985), or may be attributable to a higher mortality of males (see below). Berglund (1981) pointed out that the predominance of one sex also could be determined by a sexual dimorphism in size.
The growth parameters calculated indicates that females of T. carolinense grew faster and reached larger sizes than males, this pattern is equal to that reported for other caridean shrimps (Bae & Oh, 2014; Cházaro-Olvera, 2009; Manjón-Cabeza et al., 2009; Paschoal et al., 2016; Romero-Rodríguez & Román-Contreras, 2013). On the other hand, despite the longevity estimated for both sexes was similar, the larger lifespan of the females coincides with the results obtained in other crustacean studies, e.g., 12 months for females and 8 months for males of Hippolyte inermis (Manjón-Cabeza et al., 2009), 16 months for females and 13 months for males of Palaemon argentinus (as Palaemonetes argentinus) (Schuldt et al., 1988), and 10.2 months for females and 8.9 months for males of Hippolyte zostericola (Romero-Rodríguez & Román-Contreras, 2013). Thus, both observed patterns in T. carolinense could be related with the mating system known as pure searching reported in many gonochoristic carideans with females larger than males (Bauer, 2004), in which males are more vulnerable to predation because they have to move constantly in order to find and fertilize as many females as possible (Correa & Thiel, 2003; Bauer, 2004), which also explain that they presenting a higher mortality respect to the females.
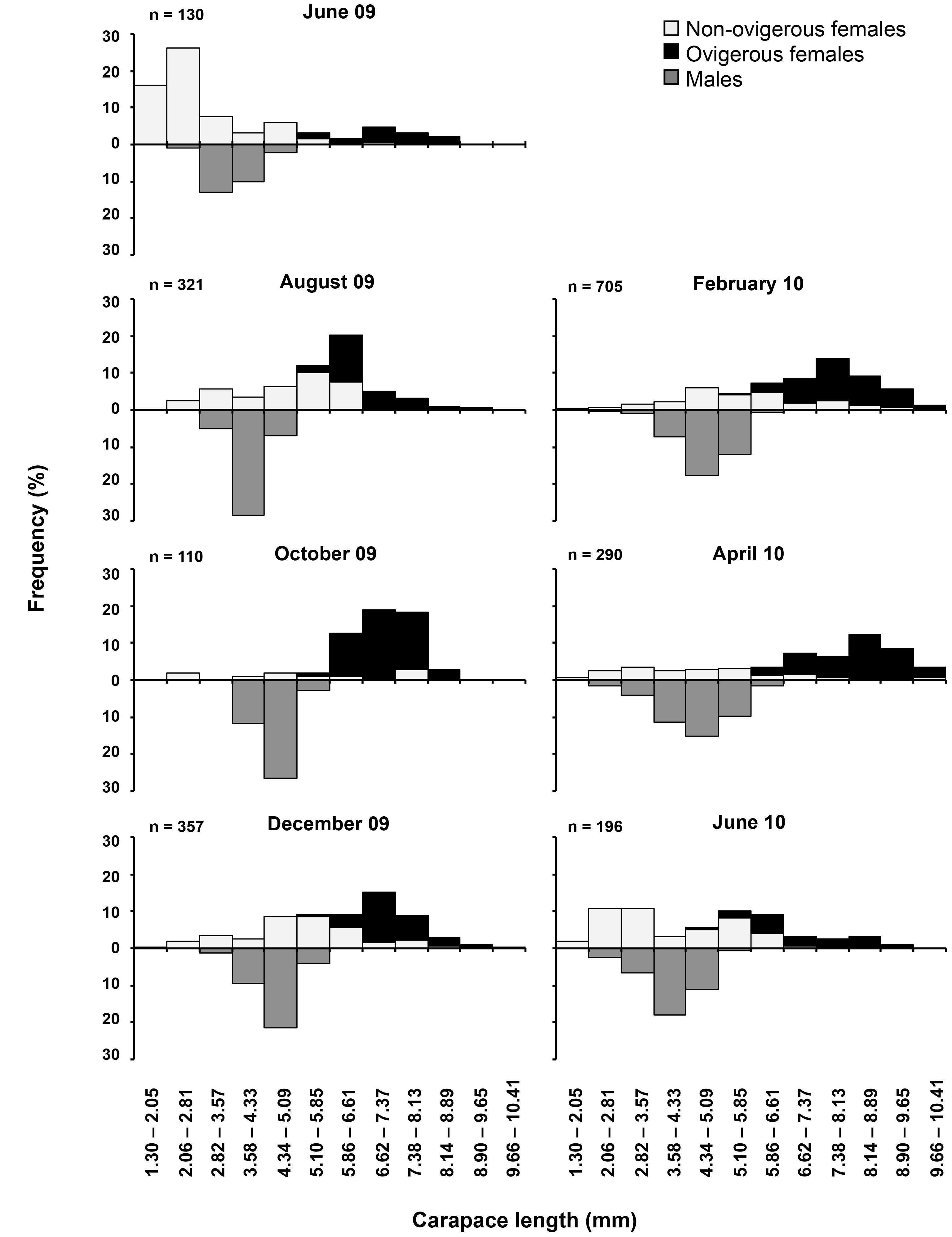
In the tropics, the majority of the species breed for a long period of time, but a seasonal increase in the number of ovigerous females may occur, as was observed in T. carolinense in the study area, this could be related to rise of food resources in the lagoon during some months (Contreras et al., 1996) creating better conditions for larval development and settlement of the individuals. Continuous reproduction in this species has also been recorded from Bear Cut, southeast waters of Florida (Ewald, 1969) and on the Caribbean coast of Panama (Heck, 1977). In contrast, in Apalachee Bay, Florida, Dugan (1983) collected berried females only from March to October when water temperatures were greater than 15°C. Continuous reproduction has also been reported for H. zostericola in the area of the present study (Romero-Rodríguez & Román-Contreras, 2013), and for other caridean shrimp species in Puerto Rico (Bauer, 1989) such as Hippolyte obliquimanus (as Hippolyte curacaoensis), Latreutes fucorum, Latreutes parvulus, and Thor manningi.
In decapod crustaceans with a continuous reproductive pattern, sexual maturity of females is reached at a relatively small size, thus the size of the smallest ovigerous female has been set as the size of sexual maturity or size at onset of sexual maturity (Bauer, 1989; Anger & Moreira, 1998) because it represents the stage where the specimens are able to generate new individuals. In our study area, T. carolinense starts to produce embryos at a minor size (4.90 mm CL) than that reported for the same species in Florida, ~ 6.9 mm CL (Ewald, 1969), 5.76 mm CL (Corey & Reid 1991), and 35 mm total length (~5.75 mm CL) (Dugan, 1983). High water temperatures (28-33 °C) would favour rapid gonadal development, sexual maturity and spawning, as well as continuous reproduction (Bauer, 1989). However, temperature cannot be the sole controlling factor, since temperatures in Laguna de Términos were similar to those reported for Florida (Mason & Zengel, 1996). Therefore, this discrepancy in size at the onset sexual maturity in other regions may indicate adaptations to local environmental conditions including quality and quantity of food sources, as well as population density and predation pressure (Bas et al., 2007; Hines, 1989).
Table 1
Number and percentage of ovigerous females of Tozeuma carolinense collected between June 2009 and June 2010.
|
Month |
Number of individuals |
||
|
Females |
Ovigerous |
(%) |
|
|
June 2009 |
96 |
16 |
16.66 |
|
August 2009 |
192 |
79 |
41.14 |
|
October 2009 |
65 |
55 |
84.61 |
|
December 2009 |
226 |
98 |
43.36 |
|
February 2010 |
432 |
246 |
56.94 |
|
April 2010 |
163 |
107 |
65.64 |
|
June 2010 |
120 |
33 |
27.5 |
|
Total |
1,294 |
634 |
48.99 |
Fecundity of caridean shrimps is variable and highly dependent upon the body size of the female. The positive relationship between carapace length and number of embryos in the present study corroborated other fecundity studies in tropical caridean groups including T. carolinense (Anger & Moreira, 1998; Bauer, 1991; Corey & Reid, 1991; Ewald, 1969). The average fecundity calculated for T. carolinense differs from the value reported by Corey and Reid (1991) in the same species. It is also higher than other caridean shrimp species, except for Exhyppolysmata oplophoroides, Lysmatta boggessi, and Trachycaris restricta (Table 2). The clutch size is highly plastic and may vary from one geographical area to another even in the same species (Bas et al., 2007). This variation has been attributed to many factors such as embryo size and genetic features of the populations (Martínez-Mayén & Romero-Rodríguez, 2018), exhaustion of females due to production of successive clutches during a season (Palacios et al., 1999), embryo mortality (Oh et al., 2002), reproductive senescence (Pandian, 2016), small sample size and the method of measurement used. However, the difference in fecundity recorded here could also reflect the smaller size of the females analysed in previous studies (Table 2).
Table 2
Average carapace length of ovigerous females, fecundity and embryo volume in stage I for 12 caridean shrimps species.
|
Species |
Carapace length (mm) |
Fecundity |
Embryo volume (mm3) |
Locality |
Reference |
|
Exhippolysmata oplophoroides |
11.51 |
2,742 |
0.015 |
Ubatuba, São Paulo, Brazil |
Chacur & Negreiros-Fransozo (1999) |
|
Hippolyte nicholsoni |
0.96 |
25 |
0.018 |
Florida Middle Ground, USA |
Corey & Reid (1991) |
|
Hippolyte obliquimanus |
2.4 |
153 |
0.005 |
Dorado, Puerto Rico |
Bauer (1991) (as Hippolyte curacaoensis) |
|
2.43 |
187 |
0.012 |
Limón, Costa Rica |
Terossi et al. (2010) |
|
|
2.09 |
141 |
0.015 |
Ubatuba, São Paulo, Brazil |
||
|
Hippolyte zostericola |
2.77 |
147 |
0.037 |
Indian River, Florida, USA |
Corey & Reid (1991) |
|
2.41 |
144 |
0.015 |
Laguna de Términos, Campeche, México |
Romero-Rodríguez & Román-Contreras (2013) |
|
|
Latreutes fucorum |
2.3 |
57 |
0.012 |
Dorado, Puerto Rico |
Bauer (1991) |
|
3.16 |
127 |
0.030 |
Florida, USA, and Virgin Islands |
Corey & Reid (1991) |
|
|
2.62 |
96 |
0.013 |
Bahía de la Ascensión, Quintana Roo, Mexico |
Martínez- Mayén & Román-Contreras (2011) |
|
|
Latreutes parvulus |
2.0 |
59 |
0.012 |
Dorado, Puerto Rico |
Bauer (1991) |
|
Lysmata boggessi |
7.5-8.24 |
554-829 |
0.0862-0.1017 |
West central coast of Florida, USA |
Baeza et al. (2014) |
|
Lysmata vittata |
6.38 |
3.93 |
0.038 |
Sergipe, Brazil |
Almeida et al. (2018) |
|
Nauticaris magellanica |
NA |
NA |
0.031-0.038 |
Guanaqueros and Metri, Chile |
Wehrtmann & Kattner (1998) |
|
Thor manningi |
1.62 |
37 |
0.036 |
Indian River, Florida, USA |
Corey & Reid (1991) |
|
Trachycaris restricta |
6.10 |
431 |
0.220 |
Virgin Gorda, Virgin Islands |
Corey & Reid (1991) |
|
Tozeuma carolinense |
6.79 |
190 |
0.057 |
Indian River, Florida, USA |
Corey & Reid (1991) |
|
7.63 |
268 |
0.050 |
Laguna de Términos, Campeche, Mexico |
Present study |
NA, data not available

Embryo size determines the reproductive output and correlates with the development pattern of organisms. Tozeuma carolinense produces small embryos (0.46 mm) that generate an extended larval development (10 larval stages, see Ewald, 1969). The average volume recorded herein agrees with the values reported for other caridean shrimp species except L. boggessi and T. restricta, which produce larger embryos (Table 2). Embryo volume in this study (0.050 mm3) is practically the same as that calculated by Corey and Reid (1991) in Florida (0.057 mm3), presumably reflecting a similar investment of energy in the production of embryos (Clarke, 1993).
The 108% increase in embryo volume during ontogeny was higher than the value of 84% calculated by Corey and Reid (1991) for this species. An increase of more than twice the volume has also been recorded for other caridean shrimp species from the Americas (Corey & Reid, 1991; Terossi et al., 2010; Wehrtmann & Kattner, 1998). The increase is a consequence of gradual water absorption by the embryo, changes in biochemical composition during embryonic development (Wehrtmann & Kattner, 1998) and embryo growth.
Brood loss during incubation diminishes reproductive potential but is common among embryo-carrying crustaceans (Oh & Hartnoll, 1999); the reduction of the clutch size may favour the survival of the remaining embryos because there is more space in the abdomen for a better accommodation of the embryos and a better oxygenation of the ovigerous mass (Nazari et al., 2003). Tozeuma carolinense here lost 46% of the embryos during the incubation period, a value higher than that reported previously (31-41%) in the same species (Corey & Reid, 1991), but that fits well with the percentages of brood loss tabulated (12-74%) for various caridean shrimps (Oh & Hartnoll, 1999). Factors that may influence embryo loss during development include increase in embryo volume during development, parental care, mechanical stress, and parasites (for a review, see Kuris, 1991). In T. carolinense, the major reason appears to be the increase in embryo volume, because this can strain and break the attachment structures.
The present study is the first regarding biological aspects of T. carolinense for the Atlantic coast of Mexico. Our results increase current understanding, but additional studies along the geographic distribution are required, in order to elucidate the possible differences among populations of the same species.
Acknowledgements
To the Instituto de Ciencias del Mar y Limnología, UNAM, for financing the field trips; to A. Reda-Deara and H. Álvarez-Guillén (Estación El Carmen, ICMyL-UNAM) for their support in the field work; to Ann Grant for her comments and improvements to the first English version, and the two anonymous reviewers for critical reading and valuable suggestions on the manuscript.
References
Almeida, A. S., Barros-Alves, S. P., Hirose, G. L., & Alves, D. F. R. (2018). Reproductive output of the ornamental shrimp Lysmata vittata (Stimpson, 1860) (Decapoda, Caridea) in wild populations and under different maturation diets. Invertebrate Reproduction and Development, 62, 257–267. https://doi.org/10.1080/07924259.2018.1509903
Alon, N. C., & Stancyk, S. E. (1982). Variation in life-history patterns of the grass shrimp Palaemonetes pugio in two South Carolina estuarine systems. Marine Biology, 68, 265–276. https://doi.org/10.1007/bf00409593
Anger, K., & Moreira, G. S. (1998). Morphometric and reproductive traits of tropical caridean shrimps. Journal of Crustacean Biology, 18, 823–838. https://doi.org/10.2307/1549156
Bae, H. J., & Oh, C. W. (2014). Reproduction and growth of the spiny lebbeid shrimp, Lebbeus groenlandicus (Fabricius, 1775) (Caridea, Hippolytidae) in the east sea of Korea. Crustaceana, 87, 1430–1446. https://doi.org/10.1163/15685403-00003357
Baeza, J. A., Behringer, D. C., Hart, R. J., Dickson, M. D., & Anderson, J. R. (2014). Reproductive biology of the marine ornamental shrimp Lysmata boggessi in the south-eastern Gulf of Mexico. Journal of the Marine Biological Association of the United Kingdom, 94, 141–149. https://doi.org/10.1017/s0025315413001185
Barba, M. E. (2012). Faunistic analysis of the caridean shrimps inhabiting seagrasses along the NW coast of the Gulf of Mexico and Caribbean Sea. Revista de Biología Tropical, 60, 1161–1175. https://doi.org/10.15517/rbt.v60i3.1765
Bas, C. C., Spivak, E. D., & Anger, K. (2007). Seasonal and interpopulational variability in fecundity, egg size, and elemental composition (CHN) of eggs and larvae in a grapsoid crab, Chasmagnathus granulatus. Helgoland Marine Research, 61, 225–237. https://doi.org/10.1007/s10152-007-0070-y
Bauer, R. T. (1989). Continuous reproduction and episodic recruitment in nine shrimp species inhabiting a tropical seagrass meadow. Journal of Experimental Marine Biology and Ecology, 127, 175–187. https://doi.org/10.1016/0022-0981(89)90183-4
Bauer, R. T. (1991). Analysis of embryo production in a caridean shrimp guild from a tropical seagrass meadow. In A. Wenner, & A. Kuris (Eds.), Crustacean issues 7. Crustacean egg production (pp. 181–191). Rotterdam: A. A. Balkema.
Bauer, R. T. (1992). Testing generalizations about latitudinal variation in reproduction and recruitment patterns with sicyoniid and caridean shrimp species. Invertebrate Reproduction and Development, 22, 193–202. https://doi.org/10.1080/07924259.1992.9672272
Bauer, R. T. (2004). Remarkable shrimps: adaptations and natural history of the carideans. Norman, OK: University of Oklahoma Press.
Berglund, A. (1981). Sex dimorphism and skewed sex ratios in the prawn species Palaemon adspersus and P. squilla. Oikos, 36, 158–162. https://doi.org/10.2307/3544440
Chacur, M. M., & Negreiros-Fransozo, M. L. (1999). Aspectos biológicos do camarão-espinho Exhippolysmata oplophoroides (Holthuis, 1948) (Crustacea, Caridea, Hippolytidae). Revista Brasileira de Biologia, 59, 173–181. https://doi.org/10.1590/s0034-71081999000100022
Cházaro-Olvera, S. (2009). Growth, mortality, and fecundity of Palaemonetes pugio from a lagoon system inlet in the southwestern Gulf of Mexico. Journal of Crustacean Biology, 29, 201–207. https://doi.org/10.1651/08-3055r.1
Clarke, A. (1993). Egg size and egg composition in polar shrimps (Caridea; Decapoda). Journal of Experimental Marine Biology and Ecology, 168, 189–203. https://doi.org/10.1016/0022-0981(93)90259-q
Contreras, E. F., Castañeda, L. O., Torres-Alvarado, R., & Gutiérrez, M. F. (1996). Nutrientes en 39 lagunas costeras mexicanas. Revista de Biología Tropical, 44, 417–425.
Corey, S., & Reid, D. M. (1991). Comparative fecundity of decapod crustaceans I. The fecundity of thirty-three species of nine families of caridean shrimp. Crustaceana, 60, 270–294. https://doi.org/10.1163/156854091×00056
Correa, C., & Thiel, M. (2003). Mating systems in caridean shrimp (Decapoda: Caridea) and their evolutionary consequences for sexual dimorphism and reproductive biology. Revista Chilena de Historia Natural, 76, 187–203. https://doi.org/10.4067/s0716-078×2003000200006
Cournoyer, B. L., & Cohen, J. H. (2011). Cryptic coloration as a predator avoidance strategy in seagrass arrow shrimp color morphs. Journal of Experimental Marine Biology and Ecology, 402, 27–34. https://doi.org/10.1016/j.jembe.2011.03.011
D’Incao, F., & Fonseca, D. B. (2000). Performance of the von Bertalanffy growth curve in penaeid shrimp: a critical approach. In J. C. von Vaupel Klein, & F. R. Schram (Eds.), The biodiversity crisis and Crustacea (pp. 733–737). Proceedings of the Fourth International Crustacean Congress; July 1998; Amsterdam, The Netherlands. Rotterdam: A. A. Balkema.
Dugan, P. J. (1983). Seasonal and geographic distribution of seven decapod crustaceans in Apalachee Bay, Florida. Contributions in Marine Science, 26, 65–79.
Ewald, J. J. (1969). Observations on the biology of Tozeuma carolinense (Decapoda, Hippolytidae) from Florida, with special reference to larval development. Bulletin of Marine Science, 19, 510–549.
Gayanilo, F. C. Jr., Sparre, P., & Pauly, D. (1995). FAO-ICLARM stock assessment tools (FiSAT) user’s guide. Rome: FAO Computerized Information Series (Fisheries) Number 8.
Grabowski, R. C., Simões, S. M., & Castilho A. L. (2014). Population structure, sex ratio and growth of the seabob shrimp Xiphopenaeus kroyeri (Decapoda, Penaeidae) from coastal waters of southern Brazil. Zookeys, 457, 253–269. https://doi.org/10.3897/zookeys.457.6682
Hays, C. G. (2005). Effect of nutrient availability, grazer assemblage and seagrass source population on the interaction between Thalassia testudinum (turtle grass) and its algal epiphytes. Journal of Experimental Marine Biology and Ecology, 314, 53–68. https://doi.org/10.1016/j.jembe.2004.08.017
Heck, K. L. Jr. (1977). Comparative species richness, composition, and abundance of invertebrates in Caribbean seagrass (Thalassia testudinum) meadows (Panamá). Marine Biology, 41, 335–348. https://doi.org/10.1007/bf00389099
Hines, A. H. (1989). Geographic variation in size at maturity in brachyuran crabs. Bulletin of Marine Science, 45, 356–368.
Kuris, A. M. (1991). A review of patterns and causes of crustacean brood mortality. In A. Wenner, & A. Kuris (Eds.), Crustacean Issues 7. Crustacean egg production (pp. 117–141). Rotterdam: A. A. Balkema. https://doi.org/10.1017/s0025315400070211
Ledoyer, M. (1986). Faune mobile des herbiers de phanérogames marines (Halodule et Thalassia) de la Laguna de Términos (Mexique, Campeche) I. Les Caridea (Crustacea Decapoda) et aperçu sur la faune globale. Anales del Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, 13, 147–170. https://doi.org/10.20937/rica.2018.34.04.12
Main, K. L. (1985). The influence of prey identity and size on selection of prey by two marine fishes. Journal of Experimental Marine Biology and Ecology, 88, 145–152. https://doi.org/10.1016/0022-0981(85)90034-6
Manjón-Cabeza, M. E., Cobos, V., García-Muñoz, J. E., & García-Raso, J. E. (2009). Structure and absolute growth of a population of Hippolyte inermis Leach 1815 (Decapoda: Caridea) from Zostera marina (L.) meadows (Malaga, southern Spain). Scientia Marina, 73, 377–386. https://doi.org/10.3989/scimar.2009.73n2377
Martínez-Mayén, M., & Román-Contreras, R. (2011). Some reproductive aspects of Latreutes fucorum (Fabricius, 1798) (Decapoda, Hippolytidae) from Bahía de la Ascensión, Quintana Roo, Mexico. Crustaceana, 84, 1353–1365. https://doi.org/10.1163/156854011×596964
Martínez-Mayén, M., & Romero-Rodríguez, J. (2018). Population structure and reproduction of the longtail grass shrimp Urocaris longicaudata (Decapoda, Caridea, Pontoniinae) in Laguna de Términos, SW Gulf of Mexico. Invertebrate Reproduction and Development, 62, 10–18. https://doi.org/10.1080/07924259.2017.1362483
Mason, W. T. Jr., & Zengel, S. A. (1996). Foods of juvenile spotted seatrout in seagrasses at Seahorse Key, Florida. Gulf of Mexico Science, 14, 89–104. https://doi.org/10.18785/goms.1402.05
Nazari, E. M., Simões-Costa, M. S., Müller, Y. M. R., Ammar, D., & Dias, M. (2003). Comparisons of fecundity, egg size, and egg mass volume of the freshwater prawns Macrobrachium potiuna and Macrobrachium olfersi (Decapoda, Palaemonidae). Journal of Crustacean Biology, 23, 862–868. https://doi.org/10.1651/c-2387
Nye, V., & Copley, J. T. (2014). Reproductive ecology of a hippolytid shrimp, Lebbeus virentova (Caridea: Hippolytidae) at the Von Damm Field, Mid-Cayman Spreading Centre. Marine Biology, 161, 2371–2380. https://doi.org/10.1007/s00227-014-2512-9
Oh, C. W., & Hartnoll, R. G. (1999). Brood loss during incubation in Philocheras trispinosus (Decapoda) in Port Erin bay, Isle of Man. Journal of Crustacean Biology, 19, 467–476. https://doi.org/10.2307/1549255
Oh, C. W., Suh, H. L., Park, K. Y., Ma, C. W., & Lim, H. S. (2002). Growth and reproductive biology of the freshwater shrimp Exopalaemon modestus (Decapoda: Palaemonidae) in a lake of Korea. Journal of Crustacean Biology, 22, 357–366. https://doi.org/10.1651/0278-0372(2002)022[0357:garbot]2.0.co;2
Oya, F. (1987). Growth, stomach contents and parasites in a tidepool population of the hippolytid shrimp Heptacarpus futilirostris, with reference to reproduction. Marine Biology, 96, 225–234. https://doi.org/10.1007/bf00427022
Oya, F., & Oka, K. (1985). Growth and breeding ecology of the hippolytid shrimp Eualus sinensis (Yu). Zoological Science, 2, 257–263.
Palacios, E., Pérez-Rostro, C. I., Ramírez, J. L., Ibarra, A. M., & Racotta, I. S. (1999). Reproductive exhaustion in shrimp (Penaeus vannamei) reflected in larval biochemical composition, survival and growth. Aquaculture, 17, 309–321. https://doi.org/10.1016/s0044-8486(98)00393-7
Pandian, T. J. (2016). Series on reproduction and development in aquatic invertebrates. Vol 1. Reproduction and development in Crustacea. Boca Raton: CRC Press.
Paschoal, L. R. P., Guimarães, F. J., & Couto, E. C. G. (2016). Growth and reproductive biology of the amphidromous shrimp Palaemon pandaliformis (Decapoda: Caridea) in a neotropical river from northeastern Brazil. Zoologia, 33, 1–14. https://doi.org/10.1590/s1984-4689zool-20160060
Pauly, D., & Munro, J. L. (1984). Once more on the comparison of growth in fish and invertebrates. ICLARM Fishbyte, 2, 21.
Raz-Guzmán A., & Sánchez, A. J. (1996). Trophic structure related to seagrass habitat complexity. In J. Kuo, R. C. Phillips, D. I. Walker, & H. Kirkman (Eds.), Seagrass biology (pp. 241–248). Proceedings of an international workshop, Rottnest Island, Western Australia; Jan 5-29; Perth, Western Australia: University of Western Australia Press. https://doi.org/10.1016/s0304-3770(00)00097-8
Ricker, W. E. (1975). Computation and interpretation of biological statistics of fish populations. Bulletin of the Fisheries Research Board of Canada, 191, 1–382.
Román-Contreras, R., & Martínez-Mayén, M. (2009). Shallow water hippolytid shrimps (Crustacea: Decapoda: Caridea) from the Mexican Caribbean coast. Hidrobiológica, 19, 119–128.
Romero-Rodríguez, J., & Martínez-Mayén, M. (2018). Rediscovery of the bopyrid isopod Parabopyrella thomasi (Nierstrasz & Brender à Brandis, 1929), parasite of the arrow shrimp Tozeuma carolinense Kingsley, 1878 (Decapoda, Caridea) in the Caribbean region. Crustaceana, 91, 1183–1194. https://doi.org/10.1163/15685403-00003811
Romero-Rodríguez, J., & Román-Contreras, R. (2013). Population structure and reproduction of the seagrass shrimp Hippolyte zostericola (Decapoda: Hippolytidae) at Laguna de Términos, Campeche, Mexico. Journal of the Marine Biological Association of the United Kingdom, 93, 675–682. https://doi.org/10.1017/s0025315411001974
Rouse, W. L. (1970). Littoral Crustacea from southwest Florida. Quarterly Journal of the Florida Academy of Sciences, 32, 127–152.
Schuldt, M., Freyre, L. R., & Damborenea, M. C. (1988). Infestación de Palaemonetes argentinus (Crustacea Palaemonidae) con Probopyrus cf. oviformis (Crustacea Bopyridae) en el canal Villa Elisa (selva marginal de Punta Lara, Provincia Buenos Aires, Argentina). II. Crecimiento de los consortes. Anales de la Sociedad Científica Argentina, 218, 37–48. https://doi.org/10.1016/0022-2011(88)90047-x
Simões, S. M., D’Incao, F., Fransozo, A., Castilho, A. L., & da Costa, R. C. (2013). Sex ratio, growth and recruitment of the pelagic shrimp Acetes americanus on the southeastern coast of Brazil. Journal of Crustacean Biology, 33, 1–9. https://doi.org/10.1163/1937240x-00002108
Sturges, H. A. (1926). The choice of a class interval. Journal of the American Statistical Association, 21, 65–66.
Terossi, M., Wehrtmann, I. S., & Mantelatto, F. L. (2010). Interpopulation comparison of reproduction of the Atlantic shrimp Hippolyte obliquimanus (Caridea: Hippolytidae). Journal of Crustacean Biology, 30, 571–579. https://doi.org/10.1651/09-3233.1
Torres, P., Penha-Lopes, G., Macia, A., & Paula, J. (2007). Population structure and egg production of the grass shrimp Hippolyte kraussiana Stimpson, 1860 (Decapoda: Hippolytidae) at Inhaca Island, Mozambique. Invertebrate Reproduction and Development, 50, 145–153. https://doi.org/10.1080/07924259.2007.9652239
von Bertalanffy, L. (1938). A quantitative theory of organic growth. (Inquiries on growth laws. II). Human Biology, 10, 181–213.
Wehrtmann, I. S., & Kattner, G. (1998). Changes in volume, biomass, and fatty acids of developing eggs in Nauticaris magellanica (Decapoda: Caridea): a latitudinal comparison. Journal of Crustacean Biology, 18, 413–422. https://doi.org/10.2307/1549406
Yáñez-Arancibia, A., & Day, J. W. Jr. (1982). Ecological characterization of Términos Lagoon, a tropical lagoon-estuarine system in the southern Gulf of Mexico. In P. Laserre, & H. Postma (Eds.), Coastal lagoons. Oceanologica Acta. Special Publication, 5, 431–440. https://doi.org/10.1016/b978-0-12-404060-1.50044-7
Zar, J. H. (2010). Biostatistical analysis. Upper Saddle, NJ: Pearson Prentice Hall.
Zupo, V. (1994). Strategies of sexual inversion in Hippolyte inermis Leach (Crustacea, Decapoda) from a Mediterranean seagrass meadow. Journal of Experimental Marine Biology and Ecology, 178, 131–145. https://doi.org/10.1016/0022-0981(94)90229-1



