Archipelago reserves, a new option to protect montane entomofauna and beta-diverse ecosystems
Victor Moctezuma *, Gonzalo Halffter, Alfonsina Arriaga-Jiménez
Instituto de Ecología, A.C., Carretera Antigua a Coatepec 351, 91000 Xalapa, Veracruz, Mexico
* Corresponding author: abadonjvpm@hotmail.com (V. Moctezuma)
Abstract
Beta diversity is often a dominant characteristic in mountain systems and naturally fragmented ecosystems. However, natural protected areas are traditionally designed to protect ecosystems with high alpha and low beta diversity. Recent information about dung beetles of the Transmexican Volcanic Belt was used to identify the most suitable strategy for the conservation of insect biodiversity in montane and beta-diverse ecosystems. Mean alpha diversity by mountain represents 38% of regional diversity. Most of the variation in beta diversity is explained because each mountain represents a unique habitat hosting a highly differentiated community. National parks appear to be inefficient to protect the high beta diversity shown by Mexican temperate mountains, especially for insect communities adapted to fragile ecosystems. The Archipelago Reserve scheme seems to be a suitable alternative to protect montane entomofauna and beta-diverse ecosystems. Our study reveals beta diversity patterns and complementarity among sites in a montane system, representing a first step to detect a suitable region for establishing an Archipelago Reserve in the Transmexican Volcanic Belt. Nevertheless, an analysis that matches current diversity patterns and protected areas is required to establish the best configuration for future reserves.
Keywords:
Beta diversity; Biodiversity conservation; Mountain forests; National Park; Biosphere Reserve; Dung beetles; Scarabaeoidea
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Reservas archipiélago, una nueva opción para proteger a la entomofauna de montaña y ecosistemas beta-diversos
Resumen
La diversidad beta suele ser un elemento dominante en sistemas tropicales de montaña y ecosistemas fragmentados. Sin embargo, las áreas naturales protegidas fueron diseñadas para proteger ecosistemas con elevada diversidad alfa y diversidad beta reducida. Utilizamos información reciente de los escarabajos del estiércol de la Faja Volcánica Transmexicana, para determinar la estrategia de conservación más adecuada para la conservación de la entomofauna de montaña y ecosistemas beta-diversos. La diversidad alfa promedio por montaña representa 38% de la diversidad regional. La diferencia geográfica entre montañas explica la mayoría de la variación en diversidad beta (51%, p < 0.001). Aparentemente, los parques nacionales son ineficientes para proteger la elevada diversidad beta que caracteriza a las montañas templadas de México, especialmente en comunidades de insectos adaptadas a ecosistemas de montaña frágiles. El esquema de Reservas archipiélago podría ser una alternativa. Nuestro estudio representa un primer paso para detectar regiones adecuadas para establecer una Reserva archipiélago en la Faja Volcánica Transmexicana. Sin embargo, son necesarios análisis espaciales para determinar adecuadamente la configuración de nuevas reservas.
Palabras clave:
Diversidad beta; Conservación de la biodiversidad; Bosques de montaña; Parque Nacional; Reserva de la Biosfera; Escarabajos del estiércol; Scarabaeoidea
Introduction
Montane systems represent a useful model for analyzing the interaction of ecological and evolutionary factors on diversity patterns variation. Mountains differ in terms of age, size, historical stability, climatic conditions and topography, reason why they represent “natural laboratories” for examining the factors that promote the diversification and maintenance of biota (Graham et al., 2014). Montane biota distribution depends strongly on its current ecological and spatial structure, but also on its geographic history (Halffter, 1987; Mastretta-Yanes et al., 2015). In the dynamic processes that have given rise to the Mexican Transition Zone (MTZ), the mountains are occupied by lineages with different evolutionary history and origin than those occupying the lowlands. The MTZ temperate mountains play a main role as a penetration path for the Holarctic biota through the horizontal colonization process, where mountains were colonized during interglacial periods by lineages originated in higher latitudes. The Neotropical biota is scarcely represented on MTZ temperate mountains because of the vertical colonization process, where lineages from surrounding lowlands at the same latitude colonize mountains, being limited by physiological restrictions related to evolutionary history (Escobar et al., 2007; Halffter, 1987; Mastretta-Yanes et al., 2015; Moctezuma, Halffter et al., 2016). In addition, the MTZ mountains act as differentiation and speciation areas, especially in the case of the Transmexican Volcanic Belt (TMVB). The historical-geographic dynamic of the mountains can promote the appearance of endemism centers in fragile and naturally fragmented ecosystems, which are particularly susceptible to the impact of climatic change (García-López et al., 2013; García-Robledo et al., 2016; McCain & Colwell, 2011). In mountain systems, the beta component makes a prominent contribution to biological biodiversity because of processes of isolation and speciation (García-López et al., 2013; Mastretta-Yanes et al., 2015).
The National Park model appeared during the 19th century, as a response for conservation purposes to protect natural sources and scenic beauty of landscapes and for the benefit and enjoyment of the people. This model concept was expanded in the 1960’s and 1970’s with a strong influence of the classic theory of Island Biogeography (Losos & Ricklefs, 2010; MacArthur & Wilson, 1967; Whittaker & Fernández-Palacios, 2007). Since then, the application of Island Biogeography to the design of National Parks favors the protection of large size areas: the larger the surface of the conservation unit, the more species it will contain, and the more resilient against external changes (Higgs, 1981). Therefore, a debate focused on the relative value of single large or several small refuges (SLOSS) aroused. But the SLOSS debate concluded during the late 1980’s and nature reserve size became irrelevant although it achieves conservation goals such as the keystone species population viability, saving the largest possible fraction of a community and including corridors for facilitating gene flow and dispersal of individuals between reserves (Soulé & Simberloff, 1986).
Derivations of the island biogeography theory do not combine the ecological elements with productive activities (apart from tourism) in the planning of conservation (Halffter, 2002, 2005; Higgs, 1981; Whittaker & Fernández-Palacios, 2007). Given this limitation, UNESCO’s Man and Biosphere Program (MAB) developed and put into practice in the 1970’s with the concept of the Biosphere Reserve, has been modified over the years. In its current format, each Biosphere Reserve includes 1 or several core zones strictly dedicated to conservation, and buffer zones necessary to ensure the permanence of the core zones in the face of the influences of anthropic changes, as well as 1 or several areas dedicated to sustainable productive activities, thus making the conservation of the “natural capital” compatible with its use (Halffter, 1984, 2005). However, although the Biosphere Reserve (particularly the Mexican modality; Halffter, 1984) was an innovative approach in many aspects, they maintain the same approach as modern National Parks in terms of design, favoring large areas for conservation purposes. A failure of the Biosphere Reserve and the National Park models lies in the fact that both area types were not conceived considering the complementarity between the alpha, beta and gamma components of biological diversity.
Faced with the need to protect ecosystems where even an extensive area cannot comprise all of the regional diversity because of a high species turnover (e.g., mountain systems), the Archipelago Reserve model arises (Halffter, 2005, 2007). The Archipelago Reserve model is defined as a broad group of protected areas that seek to fully represent regional diversity through complementarity. In a social and political context, the Archipelago Reserve aims to be a driver to change the normativity models for land ownership and for currently protected areas, to increase interconnectivity between fragile ecosystem remnants and fragments. The Archipelago Reserve model seeks to protect traditional and rustic agroecosystems that conserve a high biodiversity, enhancing the appreciation of productive practices and achieving the sustainable development (Halffter, 2007; Peresbarbosa et al., 2007).
Due to the lack of knowledge about montane diversity patterns and its different components in the design of protected areas, it is necessary to reconsider the configuration strategy of these areas. In this article, we suggest a protection model taking as a study case some mountains of the eastern TMVB and the southern Sierra Madre Oriental. We reanalyzed the information generated in this mountain area using dung beetles as a focal group in previous biodiversity research (Arriaga-Jiménez et al., 2018; Moctezuma, Halffter et al., 2016). Therefore, a series of questions developed: How are the diversity patterns of insect communities in these mountain systems? What are the geographic factors influencing these diversity patterns? What would be the most suitable strategy for the conservation of diversity, according to the observed results? These ideas lead us to discuss the best conservation model for the mountain ranges of central Mexico and ecosystems with similar diversity patterns.
Materials and methods
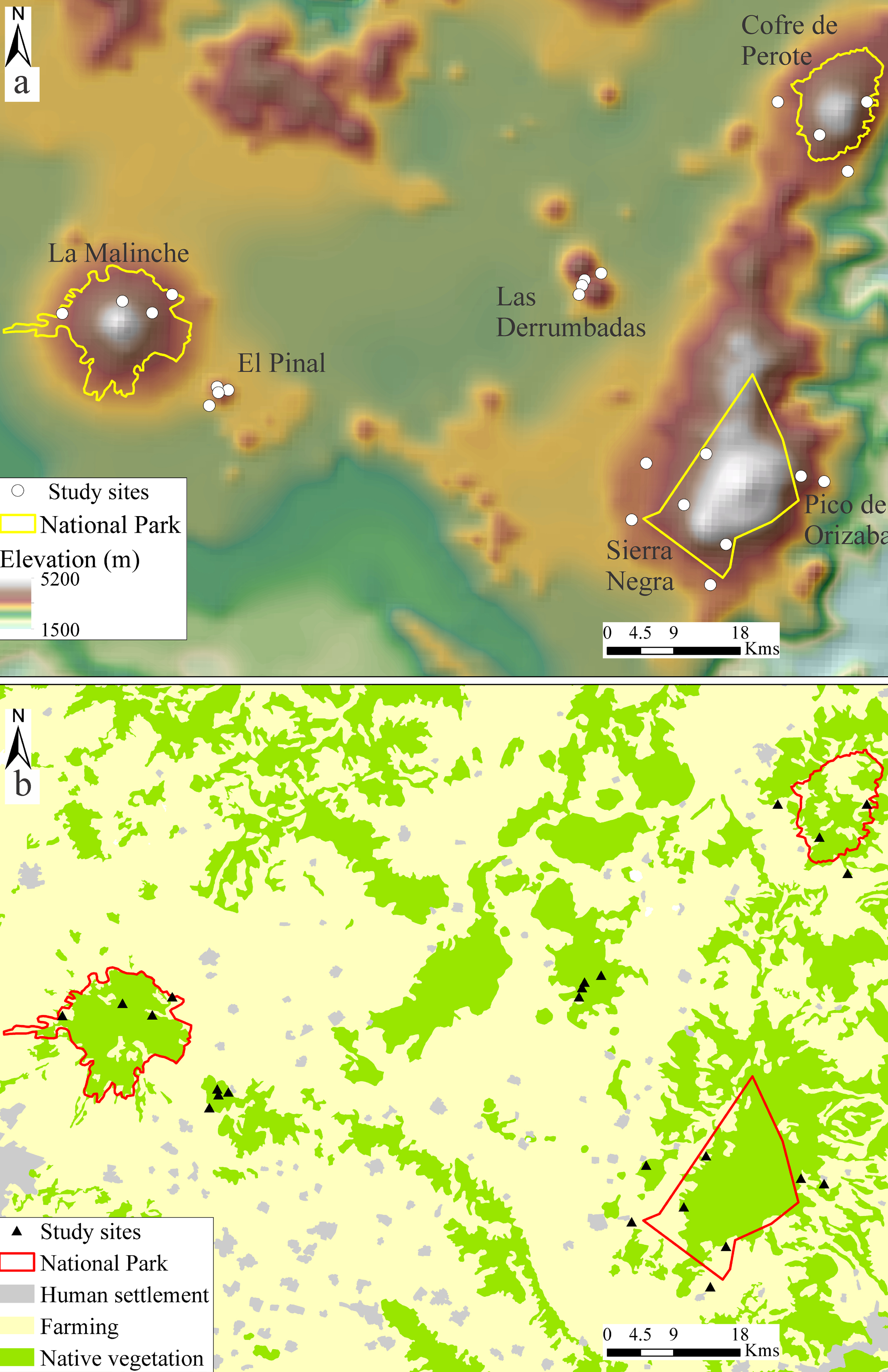
As a study area, we chose the eastern part of the TMVB (Fig. 1). This is a mountain chain in which hundreds of volcanic structures are present, with different ages (from the Miocene to the present) and ecological conditions (Mastretta-Yanes et al., 2015). The TMVB has presented periods of fragmentation and vicariance, with subsequent reconnection and colonization during interglacial events (Arriaga-Jiménez et al., 2016; Halffter, 1987; Mastretta-Yanes et al., 2015; Moctezuma, Halffter et al., 2016). Therefore, the TMVB represents for the North American montane entomofauna a colonization route and an important center of endemism and diversification, as well as presenting naturally fragmented ecosystems (Halffter, 1987; Corona et al., 2007; Escalante et al., 2009; Ruiz-Sánchez & Specht, 2013).
The eastern region of the TMVB encompasses some of the highest peaks and largest forested areas of this mountain chain, including Pico de Orizaba (5,640 m), Sierra Negra (4,580 m), La Malinche (4,440 m), Cofre de Perote (4,220 m), Las Derrumbadas (3,485 m) and El Pinal (3,280 m). Three important protected areas have been established in this region: La Malinche National Park (45,711 ha), Pico de Orizaba National Park (19,601 ha) and Cofre de Perote National Park (11,550 ha) (Inafed, 2010; Neira-Jáuregui, 2012; Semarnat & Conanp, 2016).
Dung beetles (Coleoptera: Scarabaeinae, Aphodiinae and Geotrupidae) are a highly diverse group of insects with high functional value for productive systems: they perform cleansing and fertilization of the soil, bioturbation, nutrient cycling, pest control and seed dispersion (Beynon et al., 2015; Nichols et al., 2008; Ridsdill-Smith & Edwards, 2011; Scholtz et al., 2009). In addition, dung beetles can be evaluated with standardized methods for the study of diversity (Favila & Halffter, 1997; Halffter & Favila, 1993; Nichols & Gardner, 2011; Spector, 2006). A considerable knowledge of dung beetles in the eastern region of the TMVB has been achieved, thanks to decades of intensive collection (Arriaga-Jiménez et al., 2016, 2018; Escobar et al., 2007; Halffter et al., 1995; Lobo & Halffter, 2000; Martín-Piera & Lobo, 1993; Moctezuma, Halffter et al., 2016; Moctezuma, Rossini et al., 2016; Sánchez-Huerta et al., 2015). As a result, we used information available in Moctezuma, Halffter et al. (2016) and Arriaga-Jiménez et al. (2018) that was obtained with similar methodology in 6 different mountains (Fig. 1): La Malinche, El Pinal, Las Derrumbadas, Cofre de Perote, Pico de Orizaba and Sierra Negra. On each mountain, 2 study sites were located on the eastern slope and 2 on the western slope (Table 1).
In order to evaluate the completeness of the inventories, we performed the method of sample coverage (Ĉm). This produces an estimate of the proportion of the total number of individuals of a community that belongs to the species represented in the sample, and allows direct comparison among samples of the same completeness, with a lower bias than traditional rarefaction methods. As a result, Ĉm allows robust estimates of the biodiversity of communities to be produced (Chao & Jost, 2012; Chao et al., 2014).
In order to determine diversity patterns, we estimated species richness. This parameter is considered a diversity measure (qD) of the order q = 0, forming part of the Hill Numbers, and one that is insensitive to species frequency (Hill, 1973; Jost, 2006). We contrasted the richness (0D) observed on each mountain, in protected and unprotected areas, with the regional diversity of the TMVB, through a diversity accumulation curve (Chao et al., 2014; Colwell et al., 2012). In order to estimate the magnitude of the contribution of the local diversity and of the turnover of species in the TMVB, we performed a multiplicative partition of diversity (Jost, 2007; Jost et al., 2010; Whittaker, 1972):
0Dγ = 0Dα x 0Dβ
where 0Dγ: gamma diversity, 0Dα: alpha diversity, 0Dβ: beta diversity.
We considered 0Dγ to be the regional diversity of the TMVB, 0Dα the mean diversity per mountain and 0Dβ the species turnover between mountains. We calculated the 95% confidence intervals for all of the estimates of diversity (CI95%), given that these allow statistically rigorous comparisons (Colwell et al., 2012; Wasserstein & Lazar, 2016). We used the packages INEXT and VEGETARIAN with 1000 randomizations as parameters (Charney & Record, 2014; Hsieh et al., 2013).
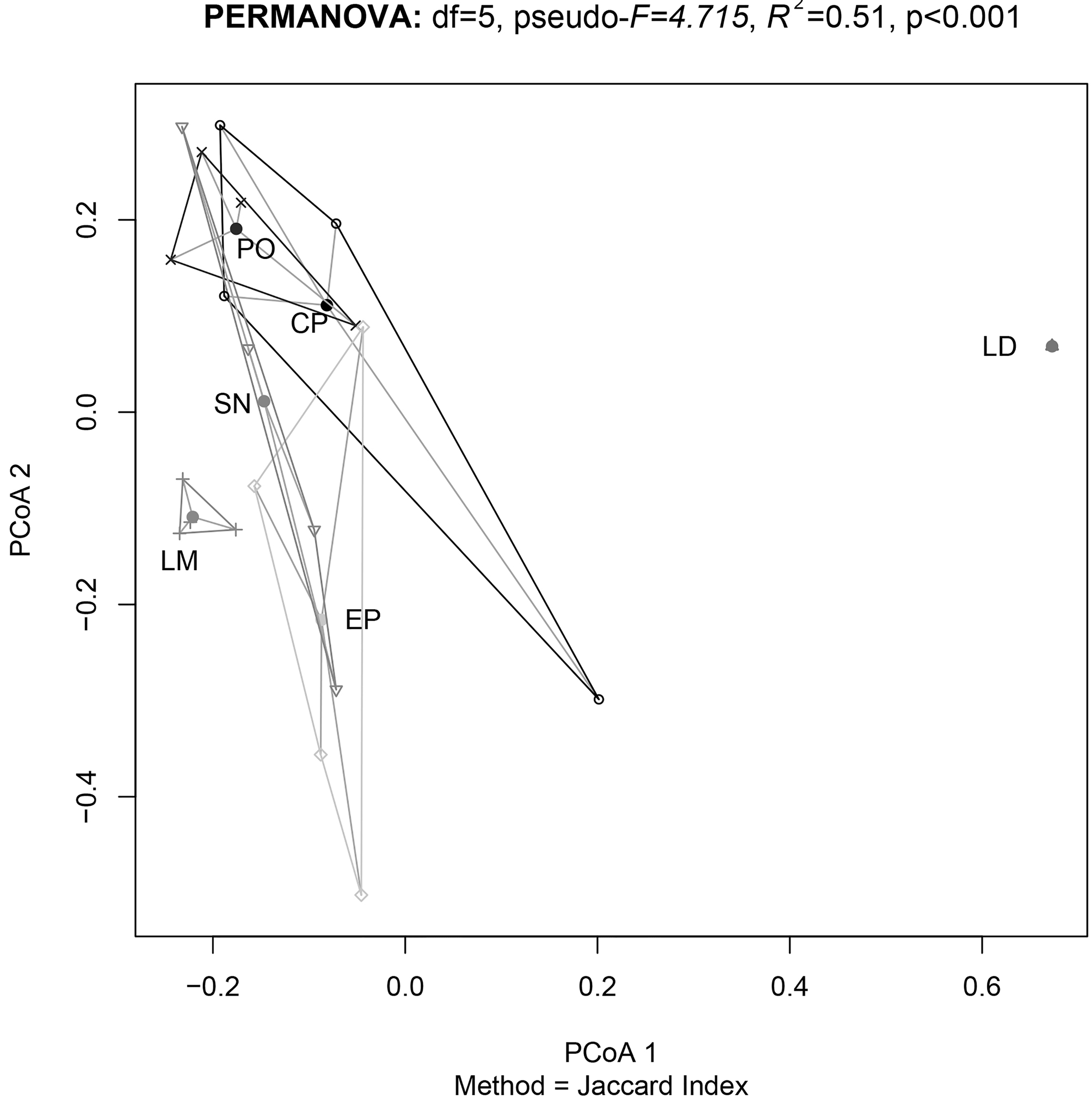
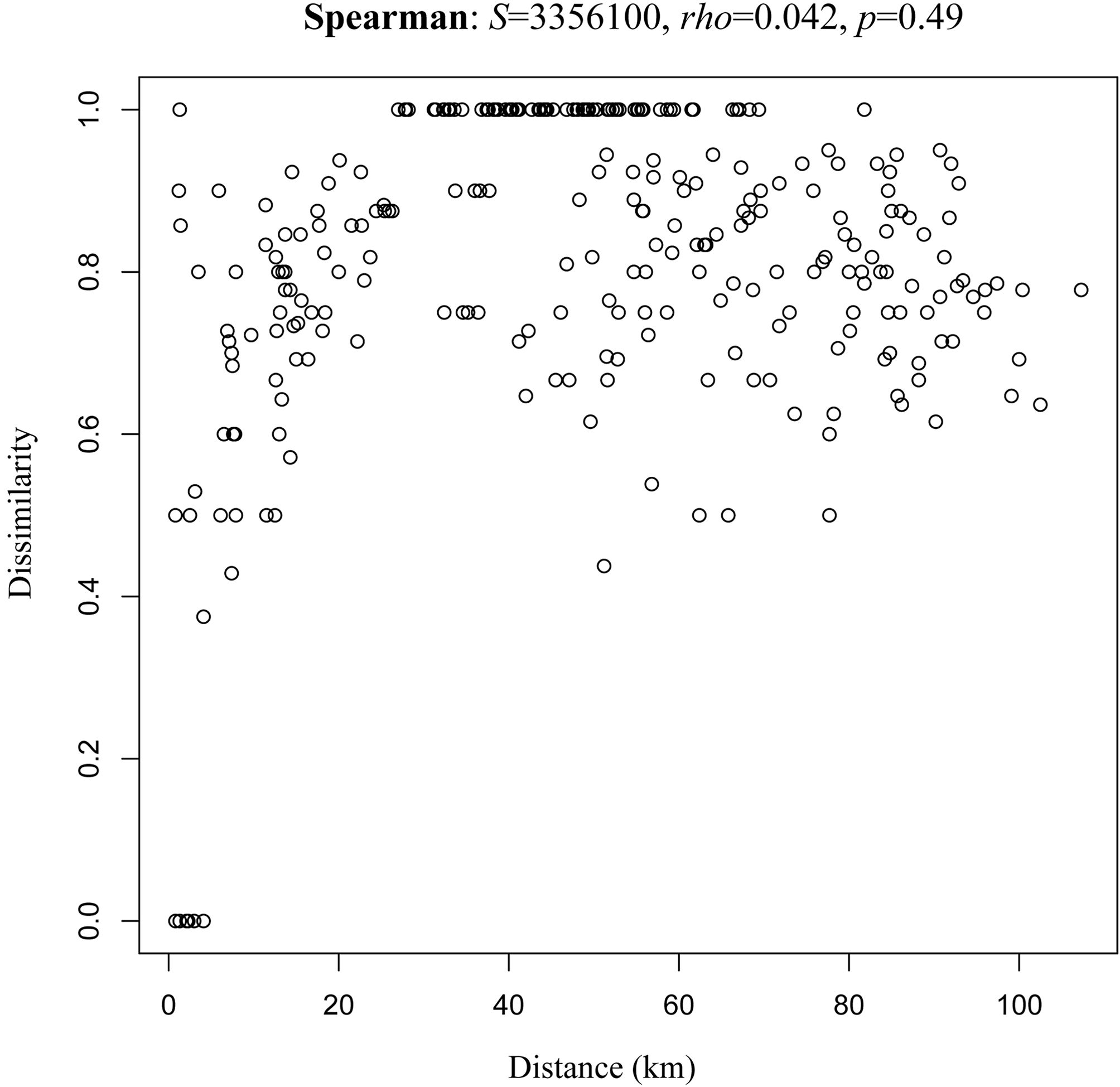
Since the study sites present variable geographic conditions (different mountains, elevations and slopes), we used a Permanova to determine which factor could best explain the variation of the beta diversity (0Dβ). The Permanova produces the partition of the dissimilarity originating from different sources of variation, and uses tests of permutation to evaluate their significance by generating pseudo-F (equivalent to the Fisher F), a value of probability and an R2 value (effect size) which shows the percentage of variation explained by the supplied sources of variation. We used the package VEGAN, the Jaccard index as a source of dissimilarity and 1,000 permutations at a level of α = 0.05 (Anderson, 2001; Anderson et al., 2006; Oksanen, 2015). We used a principle coordinates analysis (PCoA) in order to observe the differences in composition of species. Another variable that could be related to species turnover but cannot be included in the Permanova model is distance between sites. We therefore compared the matrix of dissimilarity to the distance between sites using a Pearson correlation. All analyses were performed with the program R (R Development Core Team, 2017).
Results
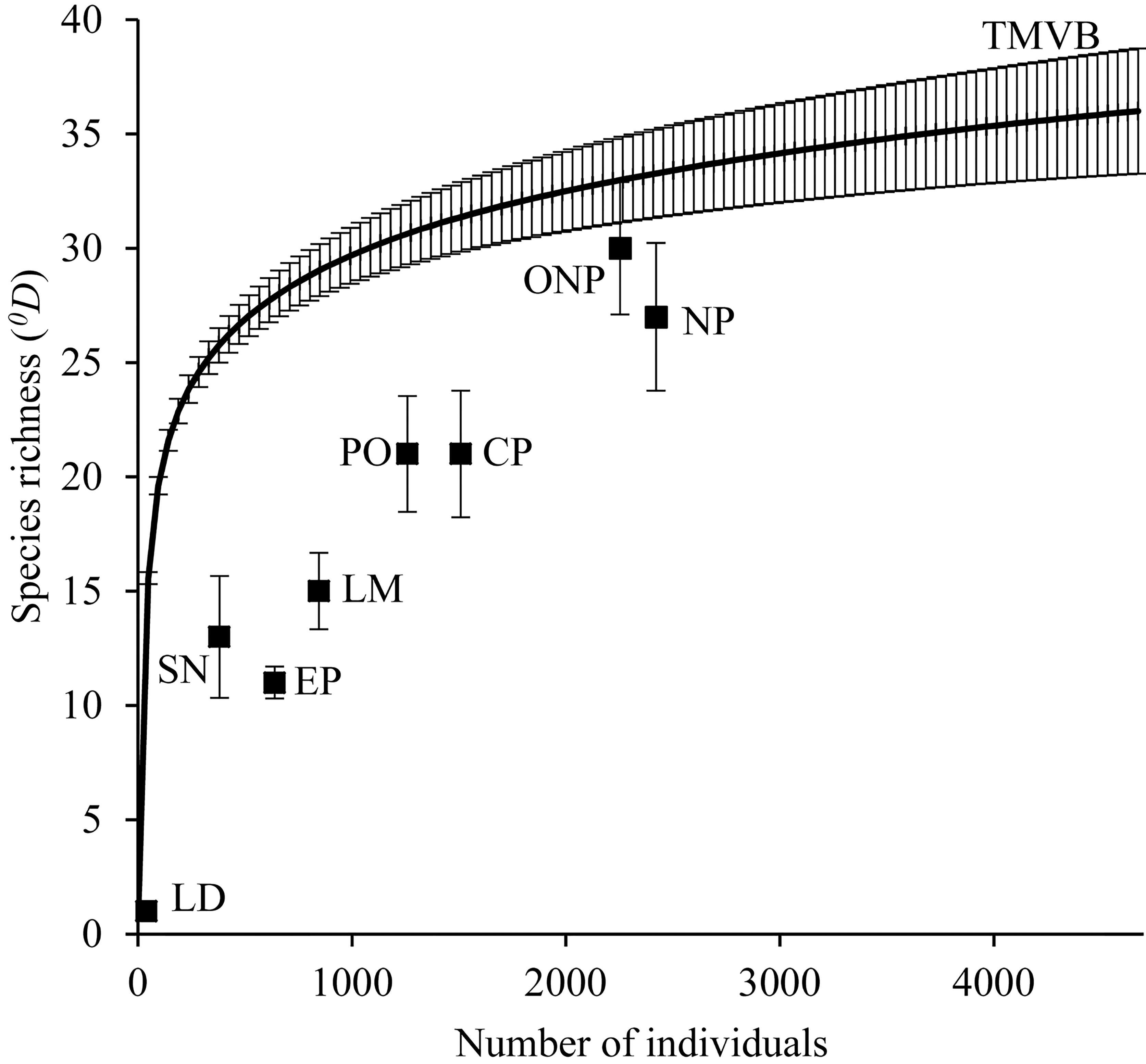
The inventories had a good sample coverage (regional = 0.999, La Malinche = 0.999, El Pinal = 0.999, Las Derrumbadas = 1, Cofre de Perote = 0.997, Pico de Orizaba = 0.998, Sierra Negra = 0.992). We estimated a regional diversity (0Dγ) of 36 ± 1.16 species, a mean alpha diversity (0Dα) per mountain equal to 13.67 ± 0.32 species and a turnover or beta diversity (0Dβ) equal to 2.63 ± 0.07. Mean alpha diversity per mountain represents only 38% of the regional diversity.
The estimates of species richness at the local level were very variable; however, all of the mountains of the TMBV examined here had a lower diversity than expected for a sample taken at random from the regional “pool” of species. The volcanoes Pico de Orizaba and Cofre de Perote presented the highest species richness of the region, while Las Derrumbadas presented the lowest. Sites included in national parks present lower species richness than those located outside these areas, and a lower richness than expected by chance (Fig. 2).
We found differences in the sources of variation for the beta diversity (Table 2). It appears that the determinant factor for species turnover are the distinct mountains, which explains a majority of the study system variation (R2 = 0.51, p < 0.001). Elevation was statistically significant (p = 0.005), but its effect size is low (R2 = 0.06). We did not find a significant effect for the factor slope or for the interactions between factors (Fig. 3). On comparison of the influence of geographic distance on the dissimilarity among sites (Fig. 4), we did not find a significant relationship between the 2 variables (rho = 0.042, p = 0.49).
Discussion
With this study, we can infer that temperate National Parks are not particularly efficient in terms of protecting the montane entomofauna in Mexico. This conclusion is based on the fact that National Parks are established in large areas overlapped in 1 or 2 mountains. In addition, they generally have their lower limit at very high elevations, leaving the zones below unprotected. We observed that national parks established on adjacent mountains (Pico de Orizaba and Cofre de Perote national parks) could protect redundant insect communities presenting a low dissimilarity in their species communities. Our study reveals that beta diversity patterns and complementarity among sites in a mountain system could represent a first step to detect a suitable region to establish an Archipelago Reserve. Nevertheless, future spatial analysis that match beta diversity patterns and current protected area coverage, are required in order establish the best spatial configuration for future Archipelago Reserve (Arriaga-Jiménez et al., 2018).
|
Table 1 Geographic characteristics of the study sites located in the mountains of the Transmexican Volcanic Belt in Central Mexico, and information of protection status is included. NP: Protected by National Park. |
||||||
|
Site |
Mountain |
Slope |
Elevation |
Latitude |
Longitude |
NP |
|
1 |
Cofre de Perote |
Eastern |
2700 |
19°25’00” N |
97°08’40” W |
no |
|
2 |
Cofre de Perote |
Eastern |
3200 |
19°30’05” N |
97°07’15” W |
yes |
|
3 |
Cofre de Perote |
Western |
2700 |
19°30’04” N |
97°13’47” W |
no |
|
4 |
Cofre de Perote |
Western |
3400 |
19°27’40” N |
97°10’43” W |
yes |
|
5 |
La Malinche |
Eastern |
2800 |
19°15’58” N |
97°58’07” W |
yes |
|
6 |
La Malinche |
Eastern |
3200 |
19°14’38” N |
97°59’35” W |
yes |
|
7 |
La Malinche |
Western |
2800 |
19°14’35” N |
98°06’10” W |
yes |
|
8 |
La Malinche |
Western |
3400 |
19°15’29” N |
98°01’46” W |
yes |
|
9 |
Pico de Orizaba |
Eastern |
2600 |
19°02’16” N |
97°10’23” W |
no |
|
10 |
Pico de Orizaba |
Eastern |
3300 |
19°02’40” N |
97°12’05” W |
no |
|
11 |
Pico de Orizaba |
Western |
2800 |
19°03’37” N |
97°23’25” W |
no |
|
12 |
Pico de Orizaba |
Western |
3400 |
19°04’18” N |
97°19’02” W |
yes |
|
13 |
Sierra Negra |
Eastern |
2800 |
18°54’42” N |
97°18’43” W |
no |
|
14 |
Sierra Negra |
Eastern |
3400 |
18°57’40” N |
97°17’34” W |
yes |
|
15 |
Sierra Negra |
Western |
2800 |
18°59’28” N |
97°24’29” W |
no |
|
16 |
Sierra Negra |
Western |
3300 |
19°00’34” N |
97°20’39” W |
yes |
|
17 |
El Pinal |
Eastern |
2700 |
19°08’59” N |
97°54’01” W |
no |
|
18 |
El Pinal |
Eastern |
2900 |
19°09’12” N |
97°54’49” W |
no |
|
19 |
El Pinal |
Western |
2600 |
19°07’50” N |
97°55’24” W |
no |
|
20 |
El Pinal |
Western |
3000 |
19°08’47” N |
97°54’44” W |
no |
|
21 |
Las Derrumbadas |
Eastern |
2500 |
19°17’32” N |
97°26’43” W |
no |
|
22 |
Las Derrumbadas |
Eastern |
2800 |
19°17’02” N |
97°27’56” W |
no |
|
23 |
Las Derrumbadas |
Western |
2800 |
19°16’38” N |
97°28’07” W |
no |
|
24 |
Las Derrumbadas |
Western |
2600 |
19°15’58” N |
97°28’20” W |
no |
Individual mountains make a significant contribution to regional diversity, because of the high species turnover among them. Indeed, it is possible that we may be severely underestimating the species turnover in the Transmexican Volcanic Belt. Our study included 6 mountains with highly complete dung beetle inventories, although the Transmexican Volcanic Belt encompasses hundreds of different volcanic structures with distinct elevations, isolation degrees, geological ages and ecological conditions that have never been sampled (Mastretta-Yanes et al., 2015). If this assumption is confirmed, the protection of regional diversity provided by the national parks could be very deficient since these constructs leave a large number of montane insect species and communities unprotected.
In our study, we found a high species turnover associated with the change among mountains: each mountain represents a unique habitat hosting a highly differentiated community. This result is explained because during interglacial periods species can be isolated in the mountains, triggering the process of vicariance and leading to unique insect communities (Arriaga-Jiménez et al., 2016; Kohlmann et al., 2018; Lobo & Halffter, 2000; Mastretta-Yanes et al., 2015; Moctezuma, Halffter et al., 2016). It has been observed that under this scenario a factor such as elevation has a limited role on our study area as a driver of species turnover (Escobar et al., 2007; Lobo & Halffter, 2000; Moctezuma, Halffter et al., 2016).
Our results refer only to species richness at a regional level, reason why the difference in elevation does not influence largely the absence/presence of species. Elevation significantly modifies the ensemble of species at local level.
The patterns of a high species turnover observed in the Transmexican Volcanic Belt are not exclusive to dung beetles. These patterns can be observed in other groups, such as other Coleoptera (Corona et al., 2007; Gutiérrez-Velázquez et al., 2012; Marshall & Liebherr, 2000; Morón, 2013), fleas (Morrone & Gutiérrez, 2005), mammals (Escalante et al., 2004; Gámez et al., 2012; Rodríguez et al., 2003), and vascular plants (Rzedowski, 2005; Suárez-Mota et al., 2013). Consequently, the low local diversity found for different taxa in the national parks of the Transmexican Volcanic Belt is a reality.
In our study, we did not find any association between distance increase and beta diversity among locations. At a small geographic scale, a complete turnover of species can be presented. In Mexico, high beta diversity patters have been recognized for different biotic groups and geographic regions, leading to the hypothesis of Mexico as a beta-diverse country (Arita, 1993, 1997; Arita & León-Paniagua, 1993; García-de Jesús et al., 2016; Moreno & Halffter, 2001; Rodríguez et al., 2003; Sarukhán et al., 1996). This is important given the fact that beta diversity must be considered a priority in the selection of conservation sites, at both large and small scales. Beta diversity is not a characteristic exclusive to Mexico or to its mountain systems, Melo et al. (2009) reported the highest beta diversity of the Americas in mountain areas (western North America, Central America and the Andes).
|
Table 2 Permanova for the beta diversity considering 3 geographic variables. We used the Jaccard index as a source of dissimilarity and 1000 permutations as a parameter. |
||||||
|
Source of variation |
df |
Sum of Sqs |
Mean Sqs |
Pseudo-F |
R2 |
p |
|
Mountain |
5 |
4.112 |
0.8224 |
4.715 |
0.51 |
< 0.001 |
|
Elevation |
1 |
0.541 |
0.541 |
3.104 |
0.067 |
0.005 |
|
Slope |
1 |
0.291 |
0.291 |
1.668 |
0.036 |
0.117 |
|
Mountain: elevation |
5 |
1.166 |
0.233 |
1.337 |
0.145 |
0.154 |
|
Mountain: slope |
5 |
0.863 |
0.173 |
0.989 |
0.107 |
0.502 |
|
Elevation: slope |
1 |
0.221 |
0.221 |
1.268 |
0.027 |
0.244 |
|
Residuals |
5 |
0.872 |
0.174 |
0.108 |
||
|
Total |
23 |
8.066 |
1 |
High beta diversity patterns have also been reported for highly endangered and fragmented ecosystems and agroecosystems, as is the case of the cloud mountain forests and shade coffee plantations (Pineda & Halffter, 2004; Pineda et al., 2005; Rös et al., 2012; Williams-Linera et al., 2007). Previous studies have proposed that cloud forest fragments, secondary forests and traditional coffee plantations can preserve complementary regional biodiversity (Williams-Linera et al., 2007). Rös et al. (2012) and suggested that Archipelago reserves could lead to the integration of heterogeneous landscapes, ensuring cloud mountain forest protection and the sustainable development. Bandeira et al. (2005) reported that a combination of factors, such as human management, original stand cover and development stage, can promote a high beta diversity in a coffee plantations system.
The Brazilian Atlantic Forest is one of the most fragmented biodiversity hotspots, but a high beta diversity among fragments allows for the permanence of regional species pool (Beca et al., 2017; Da Silva & Medina, 2016; Filgueiras et al., 2010). The Archipelago Reserve model may be able to protect the Atlantic forest fragments and to enhance connectivity through restoration measures (Beca et al., 2017). Human-disturbed and fragmented Mesoamerican tropical rain forests can be candidates to be adequately protected under the Archipelago Reserve model, since they can support fragile communities that maintain regional diversity (Arroyo-Rodríguez et al., 2012, 2013; Garmendia et al., 2013; Navarrete & Halffter, 2008; Sánchez-de Jesús et al., 2016). Archipelago reserves could also help protect the Madagascan tropical forests, which are recognized by its endemic and highly diverse biota. Studies have reported a high beta for Malagasy ant and dung beetle communities (Fisher, 1996; Viljanen et al., 2010). The Archipelago reserves could be suitable to protect transition zones between biogeographic regions (Ferro & Morrone, 2014), since they are characterized by the presentation of a high species turnover. This transition zones not only are outstanding by their beta diversity, but they are expected to preserve high phylogenetic diversity and an important number of endemisms (Ferro & Morrone, 2014; Kreft & Jetz, 2013). Therefore, transition zones should be considered a priority for world conservation strategies.
Beta-diverse ecosystems are under strong pressure from anthropic activities. However, productive and non-productive schemes can contribute to conserve insect communities within a landscape, since a diverse mosaic of agro-environmental schemes can provide a large species pool while guarantee financial benefits from production and subsides (Mader et al., 2017). Consequently, promotion of low impact activities under a scheme of conservation is vital. Biosphere Reserves allow an approach towards the sustainable use of natural resources; however, they lack the appropriate design for protecting regions with high species turnover (Halffter 1984, 2002, 2005, 2007), because they are designed to protect zones with a high local diversity (Halffter, 2007). We therefore consider that the establishment of Archipelago reserves in mountain or beta-diverse ecosystems is an urgent requirement and a priority in terms of conservation. In cases such as those discussed here, Archipelago reserves must have complementarity among their components in order to increase the total diversity under protection, with the inclusion of different local communities.
From a biological and a social point of view, the Archipelago reserves are a new and attractive proposal for biodiversity conservation. Both national parks and biosphere reserves sought to protect areas (medium or large) with exceptional richness and characteristics, while Archipelago reserves seek to protect a set of complementary areas, which together represent a unique biota. Archipelago reserves are an ecological research application facing specific problems, such as those discussed in this article. They are not intended to replace the approach of a larger area, National Park or Biosphere Reserve, but to be supplementary to them (Halffter 2007).
The creation process of Archipelago reserves is still incipient. Existing national parks could be integrated into a system of Archipelago reserves, establishing corridors of connection to protect fragments of natural vegetation, riparian zones, canyons, secondary forests and traditionally productive systems that have been shown to be important refuges of biodiversity (Arellano et al., 2004; Chazdon, 2014; Filgueiras et al., 2015; Gray et al., 2016; Pineda et al., 2005; Rös et al., 2012; Verdú et al., 2007). The first worldwide implementation of the Archipelago Reserve has been developed in the city of Xalapa (Veracruz, Mexico-2015), in which it is intended to protect the fragile remnants of the tropical cloud forest and productive ecosystems (specially shade coffee plantations) through a set of vegetation islands. We hope that this work can promote the implementation of the Archipelago Reserve on the Transmexican Volcanic Belt, particularly for ensuring insect conservation. Additionally, we aim to encourage the replication of this conservation model in landscapes with high beta diversity, where they can complement the already existing natural reserves.
Acknowledgements
We are grateful for the support of the General Direction of the Instituto de Ecología through the designation of a special project (No. 20035/30916). This project was supported by the Sectoral Research Fund for Education SEP-Conacyt Mexico (No. 257039). Victor Moctezuma thanks Conacyt-Mexico for the scholarship awarded for postgraduate studies (No. 412700). The manuscript was translated from the original Spanish version by Keith MacMillan.
References
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology, 26, 32–46.
Anderson, M. J., Ellingsen, K. E., & McArdle, B. H. (2006). Multivariate dispersion as a measure of beta diversity. Ecology Letters, 9, 683–693.
Arellano, L., Favila, M. E., & Huerta, C. (2004). Diversity of dung and carrion beetles in a disturbed Mexican tropical montane cloud forest and on shade coffee plantations. Biodiversity & Conservation, 14, 601–615.
Arita, H. T. (1993). Riqueza de especies de la mastofauna de México. In R. A. Medellín & C. Ceballos (Eds.), Avances en el estudio de los mamíferos de México (pp. 109–128). Asociación Mexicana de Mastozoología, A. C., Mexico.
Arita, H. T. (1997). The non-volant mammal fauna of Mexico: species richness in a megadiverse country. Biodiversity & Conservation, 6, 787–795.
Arita, H. T., & León-Paniagua, L. (1993). Diversidad de mamíferos terrestres. Ciencias, 7, 13–22.
Arriaga-Jiménez, A., Moctezuma, V., Rossini, M., Zunino, M., & Halffter, G. (2016). A new species of Onthophagus (Scarabaeoidea: Scarabaeinae) from the Mexican Transition Zone, with remarks on its relationships and distribution. Zootaxa, 4072, 135–143.
Arriaga-Jiménez, A., Rös, M., & Halffter, G. (2018). High variability of dung beetle diversity patterns at four mountains of the Transmexican Volcanic Belt. PeerJ, 6, e4468.
Arroyo-Rodríguez, V., Cavender-Bares, J., Escobar, F., Melo, F. P. L., Tabarelli, M., & Santos, B. A. (2012). Maintenance of tree phylogenetic diversity in a highly fragmented rain forest. Journal of Ecology, 100, 702–711.
Arroyo-Rodríguez, V., Rös, M., Escobar, F., Melo, F. P. L., Santos, B. A., Tabarelli, M. et al. (2013). Plant β-diverisy in fragmented rain forests: testing floristic homogenization and differentiation hypotesis. Journal of Ecology, 101, 1449–1458.
Bandeira, F. P., Martorell, C., Meave, J. A., & Caballero, J. (2005). The role of rustic coffee plantations in the conservation of wild tree diversity in the Chinantec region of Mexico. Biodiversity and Conservation, 14, 1225–1240.
Beca, G., Vancine, M. H., Carvalho, C. S., Pedrosa, F., Alves, R. S. C., Buscariol, D. et al. (2017). High mammal species turnover in forest patches immersed in biofuel plantations. Biological Conservation, 210, 352–359.
Beynon, S. A., Wainwright, W. A., & Christie, M. (2015). The application of an ecosystem services framework to estimate the economic value of dung beetles to the U.K. cattle industry. Ecological Entomology, 40, 124–135.
Chao, A., & Jost, L. (2012). Coverage-based rarefaction and extrapolation: standardizing samples by completeness rather than size. Ecology, 93, 2533–2547.
Chao, A., Gotelli, N. J., Hsieh, T. C., Sander, E. L., Ma, K. H., Colwell, R. K. et al. (2014). Rarefaction and extrapolation with Hill numbers: a framework for sampling and estimation in species diversity studies. Ecological Monographs, 84, 45–67.
Charney, N. & Record, S. (2014). Package ‘vegetarian’. [Accessed Jan 2016] Available at https://cran.r-project.org/web/packages/vegetarian/vegetarian.pdf
Chazdon, R. L. (2014). Second growth: the promise of Tropical Forest regeneration in an Age of deforestation. Chicago: University of Chicago Press.
Colwell, R. K., Chao, A., Gotelli, N. J., Lin, S. Y., Mao, C. X., Chazdon, R. L.et al. (2012). Models and estimators linking individual-based and sample-based rarefaction, extrapolation and comparison of assemblages. Journal of Plant Ecology, 5, 3–21.
Corona, A. M., Toledo, V. H., & Morrone, J. J. (2007). Does the Transmexican Volcanic Belt represent a natural biogeographical unit? An analysis of the distributional patterns of Coleoptera. Journal of Biogeography, 34, 1008–1015.
Da Silva, P. G., & Medina-Hernández, M. I. (2016). Spatial variation of dung beetle assemblages associated with structure remnants of southern Brazilian Atlantic Forest. Revista Brasileira de Entomologia, 60, 73–81.
Escalante, T., Rodríguez, G., & Morrone, J. J. (2004). The diversification of Nearctic mammals in the Mexican Transition Zone. Biological Journal of the Linnean Society, 83, 327–339.
Escalante, T., Szumik, C., & Morrone, J. J. (2009). Areas of endemism of Mexican mammals: reanalysis applying the optimality criterion. Biological Journal of the Linnean Society, 98, 468–478.
Escobar, F., Halffter, G., & Arellano, L. (2007). From forest to pasture: an evaluation of the influence of environment and biogeography on the structure of dung beetle (Scarabaeinae) assemblages along three altitudinal gradients in the Neotropical region. Ecography, 30, 193–208.
Favila, M. E., & Halffter, G. (1997). The use of indicator groups for measuring biodiversity as related to community structure and function. Acta Zoológica Mexicana, 72, 1–25.
Ferro, I., & Morrone, J. J. (2014). Biogeographical transition zones: a search for conceptual synthesis. Biological Journal of the Linnean Society, 113, 1–12.
Filgueiras, B. K. C., Iannuzzi, L., & Leal, I. R. (2010). Habitat fragmentation alters the structure of dung beetle communities in the Atlantic Forest. Biological Conservation, 144, 362–369.
Filgueiras, B. K. C., Tabarelli, M., Real, I. R., Vaz-de Mello, F. Z., & Iannuzzi, L. (2015). Dung beetle persistence in human-modified landscapes: Combining indicator species with anthropogenic land use and fragmentation-related effects. Ecological Indicators, 55, 65–73.
Fisher, B. L. (1996). Ant diversity patterns along an elevational gradient in the Réserve Naturelle Intégrale d’Andringitra, Madagascar. Fieldiana Zoology, 85, 93–108
Garmendia, A., Arroyo-Rodríguez, V., Estrada, A., Naranjo, E. J., & Stoner, K. E. (2013). Landscape and patch attributes in a fragmented rain forest. Journal of Tropical Ecology, 29, 331–344.
Gámez, N., Escalante, T., Rodríguez, G., Linaje, M., & Morrone, J. J. (2012). Biogeographic characterization of the Transmexican Volcanic Belt and analysis of the distributional patterns of the mammal fauna. Revista Mexicana de Biodiversidad, 83, 258–272.
García-de Jesús, S., Moreno, C. E., Morón, M. A., Castellanos, I., & Pavón, N. M. (2016). Integrando la estructura taxonómica en el análisis de la diversidad alfa y beta de los escarabajos Melolonthidae en la Faja Volcánica Transmexicana. Revista Mexicana de Biodiversidad, 87, 1033–1044.
García-López, A., Micó, E., Múrria, C., Galante, E., & Vogler, A. P. (2013). Beta diversity at multiple hierarchical levels: explaining the high diversity of scarab beetles in tropical montane forests. Journal of Biogeography, 40, 2134–2145.
García-Robledo, C., Kuprewics, E. K., Staines, C. L., Erwin, T. L. & Kress, W. J. (2016). Limited tolerance by insects to high temperatures across tropical elevational gradients and the implications of global warming for extinction. Proceedings of the National Academy of Sciences, 113, 680–685.
Graham, C. H., Carnaval, A. C., Cadena, C. D., Zamudio, K. R., Roberts, T. E., Parra, J. L. et al. (2014). The origin and maintenance of montane diversity: integrating evolutionary and ecological processes. Ecography, 37, 1–9.
Gray, C. L., Simmons, B. I., Fayle, T. M., Mann, D. J., & Slade, E. M. (2016). Are riparian forest reserves sources of invertebrate biodiversity spillover and associated ecosystem functions in oil palm landscapes? Biological Conservation, 194, 176–183.
Gutiérrez-Velázquez, A., Rojas-Soto, O., Reyes-Castillo, P., & Halffter, G. (2012). The classic theory of Mexican Transition Zone revisited: the distributional congruence patterns of Passalidae (Coleoptera). Invertebrate Systematics, 27, 282–293.
Halffter, G. (1984). Las Reservas de la Biosfera: conservación de la naturaleza para el hombre. Acta Zoológica Mexicana, 5, 48–48.
Halffter, G. (1987). Biogeography of the montane entomofauna of Mexico and Central America. Annual Review of Entomology, 32, 95–114.
Halffter, G. (2002). Conservación de la biodiversidad en el siglo XXI. Boletín de la Sociedad Entomológica Aragonesa, 31, 1–7.
Halffter, G. (2005). Towards a culture of biodiversity conservation. Acta Zoológica Mexicana, 21, 133–153.
Halffter, G. (2007). Reservas archipiélago: Un nuevo tipo de área protegida. In G. Halffter, S. Guevara, & A. Melic (Eds.), Hacia una cultura de conservación de la diversidad biológica (pp. 281–286). Zaragoza: Sociedad Entomológica Aragonesa.
Halffter, G., & Favila, M. E. (1993). The Scarabaeinae (Insecta: Coleoptera) an animal group for analyzing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biology International, 27, 15–21.
Halffter, G., Favila, M. E., & Arellano, L. (1995). Spatial distribution of three groups of coleopteran along an altitudinal transect in the Mexican Transition Zone and its biogeographical implications. Elytron, 9, 151–185.
Higgs, A. J. (1981). Island biogeography theory and nature reserve design. Journal of Biogeography, 8, 117–124.
Hill, M. O. (1973). Diversity and evenness: a unifying notation and its consequences. Ecology, 54, 427–432.
Hsieh, T. C., Ma, K. H., & Chao, A. (2013). iNEXT online: interpolation and extrapolation. [Accessed Jan 2016] Available at http://chao.stat.nthu.edu.tw/blog/software-download
Inafed (Instituto Nacional para el Federalismo y el Desarrollo Municipal). (2010). Enciclopedia de los municipios y delegaciones de México. [Accessed Jan 2016] Available at http://www.inafed.gob.mx/work/enciclopedia
Jost, L. (2006). Entropy and diversity. Oikos, 113, 363–375.
Jost, L. (2007). Partitioning diversity into independent alpha and beta components. Ecology, 88, 2427–2439.
Jost, L., DeVries, P., Walla, T., Greeney, H., Chao, A., & Ricotta, C. (2010). Partitioning diversity for conservation analyses. Diversity and Distributions, 15, 65–76.
Keft, H., & Jetz, W. (2013). Comment on ‘An update of Wallace’s zoogeographic regions of the world’. Science, 341, 343.
Kohlmann, B., Arriaga-Jiménez, A., & Rös, M. (2018) Dung beetle vicariant speciation in the mountains of Oaxaca, Mexico, with a description of a new species of Phanaeus (Coleoptera, Geotrupidae, Scarabaeidae). Zookeys, 743, 67–93.
Lobo, J. M., & Halffter, G. (2000). Variation of mountain communities of coprophagous beetles (Coleoptera: Scarabaeoidea): a comparative study. Conservation Biology and Biodiversity, 93, 115–126.
Losos, J. B., & Ricklefs, R. E. (2010). The theory of island biogeography revisited. Princeton: Princeton University Press.
MacArthur, R. H., & Wilson, E. O. (1967). The theory of Island Biogeography. Princeton: Princeton University Press.
Mader, V., Diehl, E., Fiedler, D., Thorn, S., Wolters, V., & Birkhofer, K. (2017). Trade-offs in arthropod conservation between productive and non-productive agri-environmental schemes along a landscape complexity gradient. Insect Conservation and Diversity, 10, 236–247.
Marshall, C. J., & Liebherr, J. K. (2000). Cladistic biogeography of the Mexican Transition Zone. Journal of Biogeography, 27, 203–216.
Martín-Piera, F., & Lobo, M. J. (1993). Altitudinal distribution patterns of copro-necrophage Scarabaeoidea (Coleoptera) in Veracruz, Mexico. The Coleopterists Bulletin, 47, 321–334.
Mastretta- Yanes, A., Moreno-Letelier, A., Piñero, D., Jorgensen, T. H. & Emerson, B. C. (2015). Biodiversity in the Mexican highlands and the interaction of geology, geography and climate within the Transmexican Volcanic Belt. Journal of Biogeography, 42, 1586–1600.
McCain, C., & Colwell, R. K. (2011). Assessing the threat to montane biodiversity from discordant shift in temperature and precipitation in a changing climate. Ecology Letters, 14, 1236–1245.
Melo, A. S., Rangel, T. F. L. V. B., & Diniz-Filho, J. A. F. (2009). Environmental drivers of beta-divesity patterns in New-World birds and mammals. Ecography, 32, 226–236.
Moctezuma, V., Halffter, G., & Escobar, F. (2016). Response of copronecrophagous beetle communities to habitat disturbance in two mountains of the Mexican Transition Zone: influence of historical and ecological factors. Journal of Insect Conservation, 20, 945–956.
Moctezuma, V., Rossini, M., Zunino, M., & Halffter, G. (2016). A contribution to the knowledge of the mountain entomofauna of Mexico with a description of two new species of Onthophagus Latreille, 1802 (Coleoptera, Scarabaeidae, Scarabaeinae). Zookeys, 572, 23–50.
Moreno, C. E., & Halffter, G. (2001). Spatial and temporal analysis of alpha, beta, and gamma diversities of bats in a fragmented landscape. Biodiversity & Conservation, 10, 367–382.
Morón, M. A. (2013). Three new Mexican species of Phyllophaga Harris (Coleoptera: Melolonthidae: Melolonthinae). Dugesiana, 20, 173–181.
Morrone, J. J., & Gutiérrez, A. (2005). Do fleas (Insecta: Siphonaptera) parallel their mammal host diversification in the Mexican transition zone? Journal of Biogeography, 32, 1315–1325.
Navarrete, D., & Halffter, G. (2008). Dung beetle (Coleoptera: Scarabaeidae: Scarabaeinae) diversity in continuous forest, forest fragments and cattle pastures in a landscape of Chiapas, Mexico: the effects of anthropogenic changes. Biodiversity & Conservation, 17, 2869–2898.
Neira-Jauregui, J. A. (2012). Guía de las altas montañas de México y una de Guatemala. Mexico City: Conabio.
Nichols, E. S., & Gardner, T. A. (2011). Dung beetles as a candidate study taxon in applied biodiversity conservation research. In L.W. Simmons & J. Ridsdill-Smith (Eds.), Ecology and evolution of dung beetles (pp. 267–291). West Sussex: Blackwell Publishing LTD.
Nichols, E., Spector, S., Louzada, J., Larsen, T., Amezquita, S., Favila, M. E., &The Scarabaeinae Research Network. (2008). Ecological functions and ecosystem services provided by Scarabaeinae dung beetles. Biological Conservation, 141, 1461–1474.
Oksanen, J. (2015). Multivariate analysis of ecological communities in R: vegan tutorial. [accessed Jan 2016] Available at https://www.google.com.mx/url?sa=t&rct=j&q=&esrc=s&source=web&cd=1&ved=0ahUKEwi6ranqo4jMAhWEm4MKHbtED1oQFggaMAA&url=http%3A%2F%2Fcc.oulu.fi%2F~jarioksa%2Fopetus%2Fmetodi%2Fvegantutor.pdf&usg=AFQjCNHsvyIZ380_KPgiGMqah_gA5V2jLQ&cad=rja
Peresbarbosa, R. E., Moreno-Casasola, P., Salinas, G., Ferriz, N., Castro, C., Martínez, E. et al. (2007). Reserva archipiélago: una alternativa de conservación para la costa de Veracruz. In G. Halffter, S. Guevara, & A. Melic (Eds.), Hacia una cultura de conservación de la diversidad biológica (pp. 303–310). Zaragoza: Sociedad Entomológica Aragonesa.
Pineda, E., & Halffter, G. (2004). Species diversity and habitat fragmentation: frogs in a tropical montane landscape in Mexico. Biological Conservation, 117, 499–508.
Pineda, E., Moreno, C., Escobar, F., & Halffter, G. (2005). Fog, bat and dung beetles diversity in the cloud forest and coffee agroecosystems of Veracruz, Mexico. Conservation Biology, 19, 400–410.
R development Core TEAM. (2017). R: a language and environment for statistical computing. [Accessed Feb 2018] Available at http://www.r-project.org/
Ridsdill-Smith, L. W., & Edwards, P. B. (2011). Biological control: ecosystem functions provided by dung beetles. In L.W. Simmons, & J. Ridsdill-Smith (Eds.), Ecology and evolution of dung beetles (pp. 245–266). West Sussex: Blackwell Publishing LTD.
Rodríguez, P., Soberón, J., & Arita, H. T. (2003). El componente Beta de la diversidad de mamíferos de México. Acta Zoológica Mexicana, 89, 241–259.
Rös, M., Escobar, F., & Halffter, G. (2012). How dung beetles respond to a human-modified variegated landscape in Mexican cloud forest: a study of biodiversity integrating ecological and biogeographical perspectives. Biodiversity and Distributions, 18, 377–389.
Ruiz-Sánchez, E., & Specht, C. D. (2013). Influence of the geological history of the Transmexican Volcanic Belt on the diversification of Nolina parviflora (Asparagaceae: Nolinoideae). Journal of Biogeography, 40, 1–12.
Rzedowski, J. (2005). Vegetación de México. Mexico City: Conabio.
Sánchez-de Jesús, H. A., Arroyo-Rodríguez, V., Andressen, E., & Escobar, F. (2016). Forest loss and matrix composition are the major drivers shaping dung beetle assemblages in a fragmented rainforest. Lanscape Ecology, 31, 843–854.
Sánchez-Huerta, J. L., Tonelli, M., Zunino, M., & Halffter, G. (2015). Redescription of Onthophagus halffteri Zunino (Coleoptera: Scarabaeidae: Scarabaeinae), with ecological and distributional notes. The Coleopterists Bulletin, 69, 225–230.
Sarukhán, J., Soberón, J., & Larson-Guerra, J. (1996). Biological conservation in a high beta diversity country. In E. Di Castri, & T. Younes (Eds.), Biodiversity, science and development: toward a new partnership (pp. 246–263). London: CAB International in association with the International Union of Biological Sciences.
Scholtz, C. H., Davis, A. L. V., & Kryger, U. (2009). Evolutionary biology and conservation of dung beetles. Sofia-Moscow: Pensoft.
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales) & Conanp (Comisión Nacional de Áreas Naturales Protegidas). (2016). Áreas naturales protegidas de México. [Accessed Nov 2016] Available at http://sig.conanp.gob.mx/website/pagsig/anp/nal/index.htm
Soulé, M. E., & Simberloff, D. (1986). What do genetics and ecology tell us about the design of nature reserves? Biological Conservation, 35, 19–40.
Spector, S. (2006). Scarabaeinae dung beetles (Coleoptera: Scarabaeidae: Scarabaeinae): an invertebrate focal taxon for biodiversity research and conservation. Coleopterists Society Monographs, 5, 71–83.
Suárez-Mota, M. E., Téllez-Valdés, O., Lira-Saade, R., & Villaseñor, J. L. (2013). Una regionalización de la Faja Volcánica Transmexicana con base en su riqueza florística. Botanical Sciences, 91, 93–105.
Verdú, J. R., Moreno, C. E., Sánchez-Rojas, G., Numa, C., Galante, E., & Halffter, G. (2007). Grazing promotes dung beetle diversity in the xeric landscape of a Mexican Biosphere Reserve. Biological Conservation, 140, 308–317.
Viljanen, H., Escobar, F., & Hansky, I. (2010). Low local but high beta diversity of tropical forest dung beetles in Madagascar. Global Ecology and Biogeography, 19, 886–894.
Wasserstein, R. L., & Lazar, N. A. (2016). The ASA’s statement on p-values: context, process, and purpose. The American Statistician, 70, 129–133.
Whittaker, R. H. (1972). Evolution and measurement of species diversity. Taxon, 21, 213–251.
Whittaker, R. J., & Fernández-Palacios, J. M. (2007). Island biogeography. Ecology, evolution and conservation. Oxford: Oxford University Press.
Williams-Linera, G., Guillén-Servent, A., Gómez-García, O., & Lorea-Hernández, F. (2007). Conservación en el centro de Veracruz, México. El bosque de niebla: ¿reserva archipiélago o corredor biológico? In G. Halffter, S. Guevara, & A. Melic (Eds.), Hacia una cultura de conservación de la diversidad biológica (pp. 303–310). Zaragoza: Sociedad Entomológica Aragonesa.