Brittle stars (Echinodermata: Ophiuroidea) of coastal lagoons from the northern Yucatán Peninsula, Mexico
José Gabriel Kuk-Dzul a, b, Francisco Alonso Solís-Marín c, María Teresa Herrera-Dorantes a, Pedro-Luis Ardisson a, *
a Departamento de Recursos del Mar, Centro de Investigación y de Estudios Avanzados, Instituto Politécnico Nacional, Carretera antigua a Progreso Km 6, Apartado postal 73, Cordemex, 97310 Mérida, Yucatán, Mexico
b Cátedras Conacyt-Facultad de Ecología Marina, Universidad Autónoma de Guerrero, Av. Gran Vía Tropical No. 20, Fraccionamiento Las Playas, 39390 Acapulco Guerrero, Mexico
c Colección Nacional de Equinodermos “Dra. Ma. E. Caso Muñoz”, Laboratorio de Sistemática y Ecología de Equinodermos, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, Apartado postal 70-305, 04510 México City, Mexico
*Corresponding author: pedro.ardisson@cinvestav.mx (P.L. Ardisson)
Abstract
We present the first records of ophiuroids found in 6 tropical coastal lagoons of the northern Yucatán Peninsula. Specimens were collected from Ría Celestún, Chelem, Dzilam, Ría Lagartos, Yalahau, and Nichupté-Bojórquez lagoons, during May and June 2010. Six species belonging to the families Amphiuridae, Ophionereididae, and Ophiodermatidae were recorded. Amphiodia pulchella was recorded in all lagoons; Ophiostigma isocanthum was recorded only in Yalahau, and Ophionereis olivacea and Ophioderma appressa were recorded only in Nichupté-Bojórquez. Amphiodia cf. planispina was recorded in all the coastal lagoons except in Yalahau; all the individuals of this species were juveniles. Finally, Ophiophragmus filograneus was recorded in Ría Celestún, Ría Lagartos, and Nichupté-Bojórquez, being a new record for the southern Gulf of Mexico. The range of distribution of these species is extended and their presence in brackish waters is recorded, an environment that has not been properly studied concerning ophiuroids.
Keywords:
Benthos; Brackish waters; New records; Gulf of Mexico; Caribbean Sea
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Ofiuroideos (Echinodermata: Ophiuroidea) de las lagunas costeras del norte de la península de Yucatán, México
Resumen
Presentamos los primeros registros de ofiuroideos encontrados en 6 lagunas costeras tropicales del norte de la península de Yucatán. Los especímenes provienen de las lagunas Ría Celestún, Chelem, Dzilam, Ría Lagartos, Yalahau y Nichupté-Bojórquez, que fueron muestreados durante mayo y junio 2010. Se recolectaron 6 especies pertenecientes a las familias: Amphiuridae, Ophionereididae y Ophiodermatidae. Amphiodia pulchella se registró en todas las lagunas; Ophiostigma isocanthum se registró solo en Yalahau, y Ophionereis olivacea y Ophioderma appressa se registraron solo en Nichupté-Bojórquez. Amphiodia cf. planispina se registró en todas las lagunas con excepción de Yalahau; todos los individuos de esta especie fueron juveniles. Finalmente, se registró Ophiophragmus filograneus en Ría Celestún, Ría Lagartos y Nichupté-Bojórquez, siendo también un nuevo registro para el sur del Golfo de México. Se amplía el rango de distribución de estas especies y se registra su presencia en aguas salobres, un ambiente que no se ha estudiado adecuadamente con respecto a los ofiuroideos.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Bentos; Aguas salobres; Nuevos registros; Golfo de México; Mar Caribe
Introduction
There is a great diversity of echinoderms in the Gulf of Mexico (Durán-González et al., 2005; Pawson et al., 2009). The number of ophiuroid species that have been recorded in the Gulf of Mexico has increased through time. For example, Durán-González et al. (2005) reported 49 species of ophiuroids in the Gulf of Mexico, while Hernández-Herrejón et al. (2008) reported 68 species, and Solís-Marín et al. (2013) in the most recent review reported 79 species. In Mexico, the class Ophiuroidea represent approximately 40% (197 species) of the total species recorded (Solís-Marín et al., 2014). New records and range extensions for species of ophiuroids are often reported in the literature (Solís-Marín et al., 2015).
Benthic fauna has been studied in Ría Celestún, Chelem, Dzilam, Ría Lagartos, and Yalahau but no ophiuroid species had been reported in these lagoons (Kuk-Dzul et al., 2012; May-Kú & Ordóñez-López, 2006; May-Kú et al., 2014; Pech et al., 2007). The aims of this study were: 1) to provide an annotated checklist of ophiuroids from 6 coastal lagoons in the same geographic area, 2) to report geographical extensions for those species in the Gulf of Mexico and Caribbean Sea, and 3) to characterize the habitat occupied by the ophiuroid fauna at the sampling sites.
Material and methods
The Yucatán Peninsula has a plain calcareous soil, and its shelf is formed from calcite sand (Ellwood et al., 2006); it does not have surface rivers but it has underground flows and the weather is tropical wet-dry (Aw). Coastal lagoons are water bodies parallel to the coast, formed by barriers that separate them from the ocean; they have permanent or intermittent inlets which have a connection with the sea (Kjerfve, 1994). These ecosystems are highly productive and function as nurseries for some species; these environments are also highly variables in temperature, salinity, and turbidity (Kennish & Paerl, 2010). In coastal lagoons only a small number of species can tolerate these environmental conditions; furthermore, species numbers often decrease from the sea mouth to the inner zone (Tagliapietra et al., 2005).
The 6 tropical coastal lagoons examined in this study are located in the northern Yucatán Peninsula, 5 of them in the Gulf of Mexico (Ría Celestún, Chelem, Dzilam, Ría Lagartos, and Yalahau) and an additional lagoon in the Caribbean Sea (Nichupté-Bojórquez). Dzilam has an area of 9.4 km2 which, compared to Yalahau (275 km2), is 30 times smaller. Ría Celestún and Ría Lagartos, in their inner zone, are oligohaline and hyperhaline, respectively (Herrera-Silveira et al., 1999). The presence of seagrasses is common in these coastal lagoons, the representative species are Halodule wrightii, Ruppia maritima, and Thalassia testudinum (Herrera-Silveira & Morales-Ojeda, 2010; Herrera-Silveira et al., 1998). Ría Celestún is used for tourism and sport fishing (Córdoba-Azcárate, 2006), Chelem is a locally important port due to its proximity to the port of Progreso, Dzilam is a natural protected area, Ría Lagartos is used for tourism, Yalahau is on the maritime route to Holbox Island with moderate impact from fishing, and Nichupté-Bojórquez is a lagoon system in one of the most important tourist attractions in Mexico (Cancun, Quintana Roo). Except for Chelem and Nichupté-Bojórquez, these coastal lagoons have an environmental protection status (Conanp, 2017).
We sampled 40 sites in the 6 coastal lagoons in May and June 2010. Based on lagoon size, 6 sites per lagoon were sampled in Ría Celestún, Chelem, Dzilam, and Ría Lagartos, and 8 sites in Yalahau and Nichupté-Bojórquez (Fig. 1). At each site, depth, salinity, dissolved oxygen (DO), and pH were measured with a handheld multiparameter instrument (YSI 556), as well as oxidation-reduction-potencial (ORP). Five sediment samples were collected per site for benthic infauna analysis using a Birge-Ekman grab (total sampled area per site = 0.112 m2). Samples were stored in one-liter plastic bottles with MgCl2 (8%) to relax the benthic organisms, and after 10 min formalin (10%) was added to the bottles to fix them. One extra sediment sample was collected and preserved at 4 °C to determine sediment texture using the sand, silt, and clay content. For submerged aquatic vegetation (SAV), 3 sediment samples were collected with a 15.6 cm diameter PVC core (total sampled area per site = 0.057 m2) and then frozen for further analysis in the laboratory.
Benthic macrofauna samples were washed with tap water to eliminate excess formalin and salts, passed through a 500 µm mesh sieve, preserved with 70% alcohol, and stored in plastic bottles. Using stereoscopic and compound microscopes, ophiuroids were identified to the lowest taxonomic category, based on regional taxonomic keys (Hendler et al., 1995; Hernández-Herrejón et al., 2008; Laguarda-Figueras et al., 2009). The collected organisms were deposited in the Colección Nacional de Equinodermos de México “Dra. Ma. Elena Caso Muñoz”, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (ICML-UNAM). Extra sediment samples were dried by lyophilization at low temperature (-45 °C) and low pressure (96 × 10-6 bar). Sand, silt and clay percentage were then calculated using the pipette method (Rump & Krist, 1992). Finally, SAV samples were thawed and seagrass species identified.
Results
Ophiuroids were found in 22 of the 40 sampled sites: Ría Celestún (2 sites), Chelem (3 sites), Dzilam (5 sites), Ría Lagartos (2 sites), Yalahau (4 sites), and Nichupté-Bojórquez (6 sites) (Fig. 1). During the study period, depth ranged from 0.2 to 2.7 m, salinity from 17.9 to 41.8 PSU, and pH from 8.3 to 9.0. The oxidation-reduction potential (ORP) was negative throughout the study (-94.9 to -25.7 mV). Ophiuroids from the coastal lagoons comprised 6 species belonging to 3 families: Amphiuridae, Ophionereididae, and Ophiodermatidae (Table 1).
Family Amphiuridae Ljungman, 1857
Genus Amphiodia Verrill, 1899
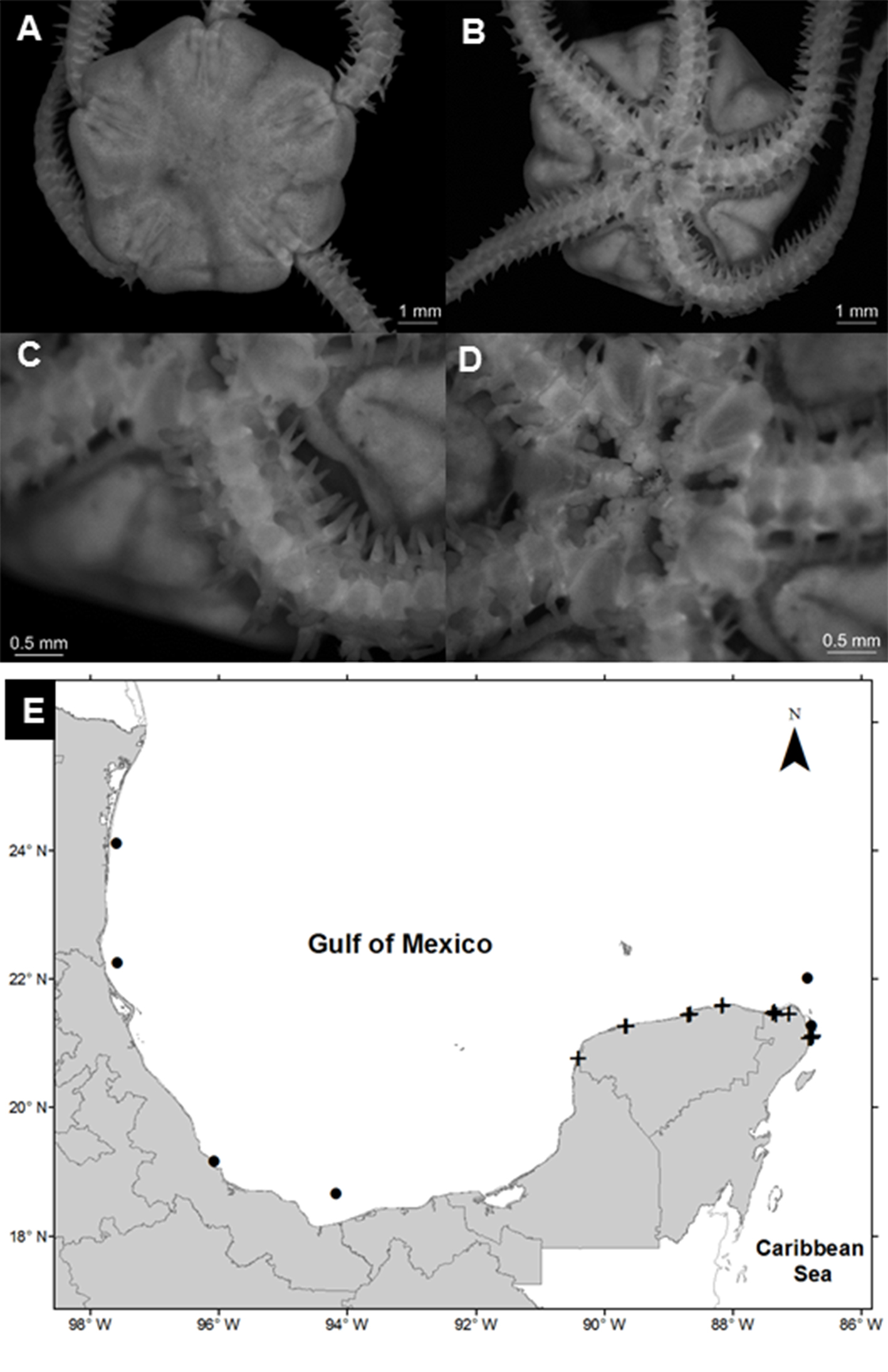
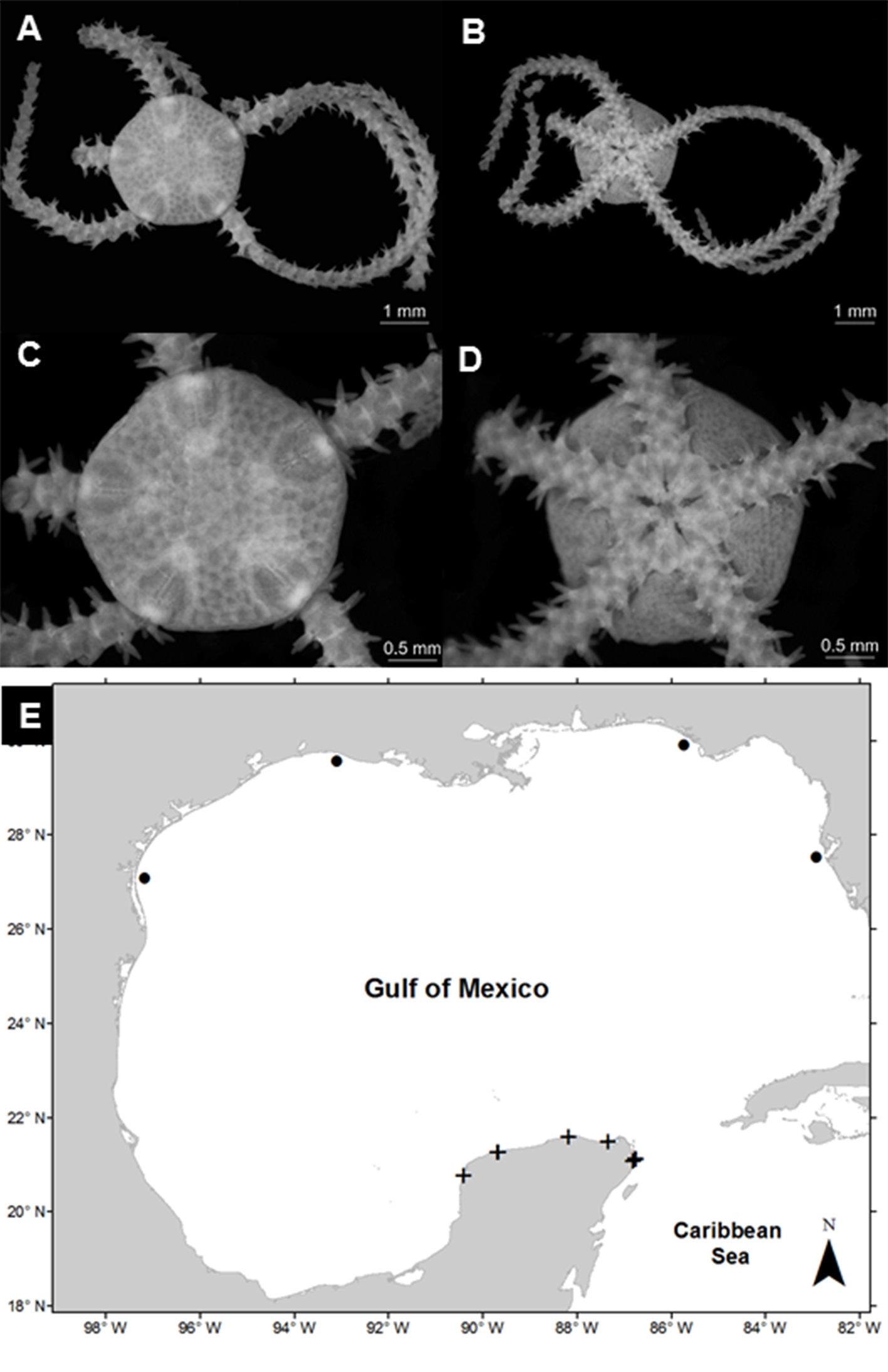
Amphiodia pulchella (Lyman, 1869)
(Fig. 2A-D)
Diagnosis (modified from Hendler et al., 1995). The disc is covered by fine scales, with radial shields in contact. The primary plates are prominent in small individuals but generally absent in large individuals. The radial shields are elongated, naked, joined in the distal part, and separated in the proximal part. The oral shields are large and subpentagonal with rounded tips. The adoral plates are large and approximately half the size of the oral shields. The oral plates are long and thin. Each jaw has 3 pairs of contiguous oval oral papillae, and the teeth are rectangular. There are 2 oval and deep genital slits in each interradial space. The dorsal arm plates are oval, and the lateral plates are thin with 3 spines of the same size; the spines are flattened, blunt in the distal part, wide in the proximal part, and with a serrated edge. The arms have an oval tentacular scale on each side of the ventral plates.
Material examined: ICML-UNAM 12497, 1 specimen, 2 December 2015, Ría Celestún (20°46’45” N, 90°24’21.2” W), depth 1.1 m; ICML-UNAM 12500, 2 specimens, 10 July 2010, Chelem Lagoon (21°16’25.9” N, 89°41’4.5” W), depth 0.8 m; ICML-UNAM 12502, 5 specimens, 2 June 2010, Chelem Lagoon (21°16’25.8” N, 89°40’14.8” W), depth 0.5 m; ICML-UNAM 12503, 1 specimen, 2 December 2015, Chelem Lagoon (21°16’27.1” N, 89°39’25.4” W), depth 0.7 m; ICML-UNAM 12505, 2 specimens, 4 June 2010, Dzilam Lagoon (21°26’59.5” N, 88°41’47.2” W), depth 0.3 m; ICML-UNAM 12507, 3 specimens, 4 June 2010, Dzilam Lagoon (21°27’10.7” N, 88°41’16.5” W), depth 0.3 m; ICML-UNAM 12509, 2 specimens; 4 June 2010, Dzilam Lagoon (21°27’52.6” N, 88°39’59.4” W), depth 0.3 m; ICML-UNAM 12510, 1 specimen, 9 July 2010, Ría Lagartos (21°35’38.4” N, 88°10’32.2” W), depth 0.8 m; ICML-UNAM 12513, 2 specimens, 9 July 2010, Ría Lagartos (21°36’6.6” N, 88°09’24.1” W), depth 0.4 m; ICML-UNAM 12515, 3 specimens, 13 July 2010, Yalahau Lagoon (21°29’59.8” N, 87°20’49.9” W), depth 0.4 m; ICML-UNAM 12516, 1 specimen, 13 July 2010, Yalahau Lagoon (21°28’16.7” N, 87°22’46.6” W), depth 0.8 m; ICML-UNAM 12518, 3 specimens, 13 July 2010, Yalahau Lagoon (21°27’05.1” N, 87°19’17.7” W), depth 0.4 m; ICML-UNAM 12519, 3 specimens, 13 July 2010, Yalahau Lagoon (21°27’55.1” N, 87°07’57.4” W), depth 0.7 m; ICML-UNAM 12522, 2 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’43” N, 86° 45’11” W), depth 1.6 m; ICML-UNAM 12523, 4 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’43” N, 86°45’11” W), depth 1.6 m; ICML-UNAM 12526, 2 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°06’54.2” N, 86°45’47.2” W), depth 1.2 m; ICML-UNAM 12529, 2 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°05’03.4” N, 86°48’52.2” W), depth 1.4 m; ICML-UNAM 12530, 3 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°05’31.3” N, 86°46’23.9” W), depth 1.1 m.
Table 1
Number of specimens of ophiuroid species in the 6 coastal lagoons of the northern Yucatán Peninsula.
|
Family/species |
Ría Celestún |
Chelem |
Dzilam |
Ría Lagartos |
Yalahau |
Nichupté-Bojórquez |
Total |
|
Amphiuridae |
|||||||
|
Amphiodia cf. planispina |
52 |
29 |
6 |
2 |
0 |
4 |
93 |
|
Amphiodia pulchella |
6 |
21 |
8 |
3 |
24 |
48 |
110 |
|
Ophiophragmus filograneus |
7 |
17 |
0 |
6 |
1 |
6 |
37 |
|
Ophiostigma isocanthum |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
|
Ophionereididae |
|||||||
|
Ophionereis olivacea |
0 |
0 |
0 |
0 |
0 |
37 |
37 |
|
Ophiodermatidae |
|||||||
|
Ophioderma appressa |
0 |
0 |
0 |
0 |
0 |
6 |
6 |
Geographic distribution: Bermuda; Bahamas; Florida, USA; Cuba; Mexico; Jamaica; Panama; Puerto Rico; Belize; Windward Islands; Leeward Antilles; Saint Lucia; Tobago; Brazil, and Argentina (Hendler et al., 1995).
Distribution in Mexico: Tampico, Tamaulipas; Sistema Arrecifal Veracruzano, near Coatzacolacos, Veracruz; Celestún, Chelem, Dzilam, Ría Lagartos, Yucatán; Cozumel, Isla Mujeres, Puerto Morelos, Yalahau Lagoon, Nichupté-Bojórquez lagoon system, and near Cabo Catoche, Quintana Roo (Fig. 2E).
Habitat: depth: 0.3-2.7 m; water salinity: 17-41 PSU; dissolved oxygen: 2-11 mg L-1; pH: 8.3-9.0; ORP: -94.9 to -35.0 mV; sediment texture: sandy silt, shells, clay, sand, silt; submerged aquatic vegetation: Halodule wrightii and Thalassia testudinum.
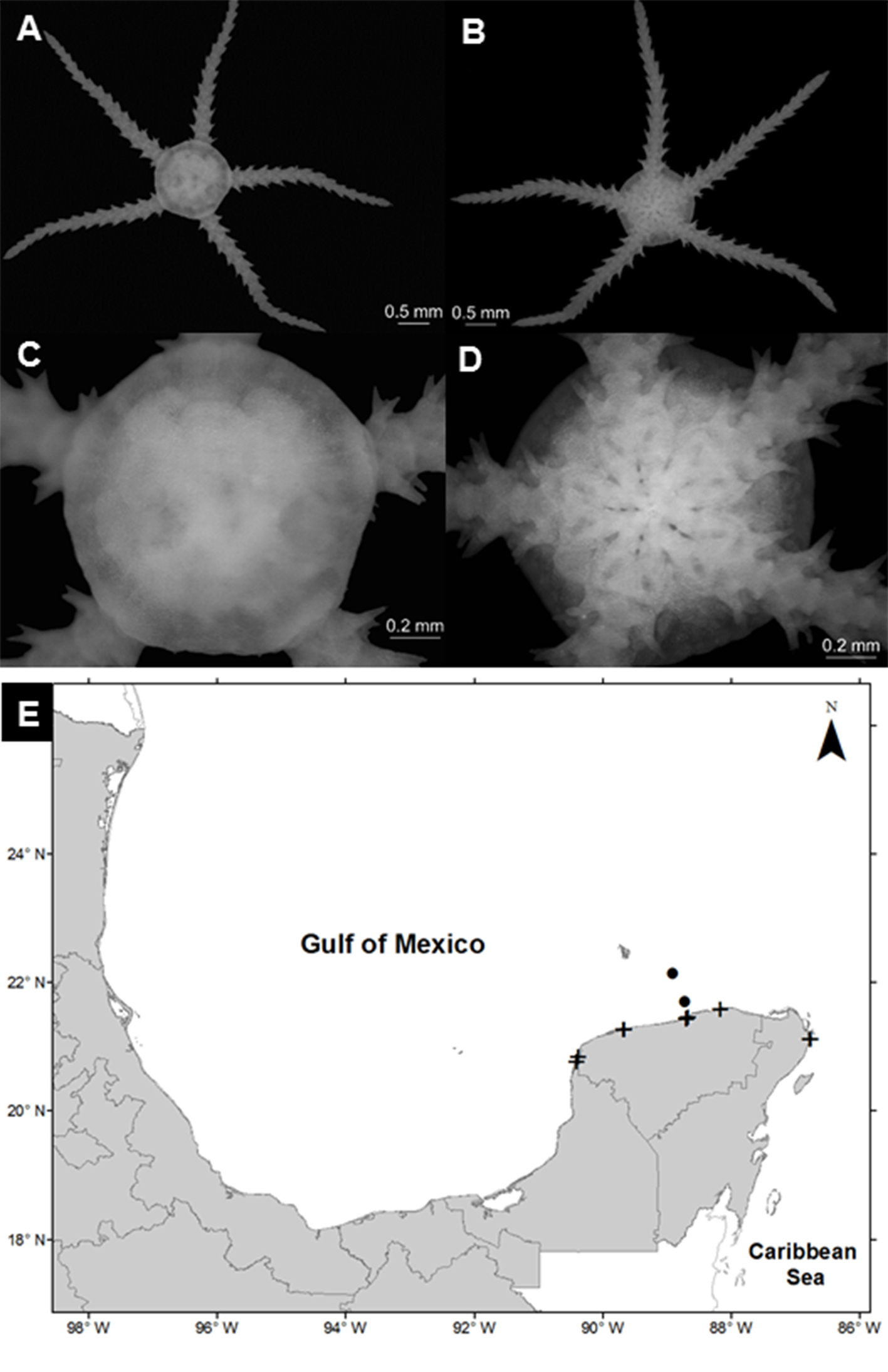
Amphiodia cf. planispina (von Martens, 1867)
(Fig. 3A-D)
Diagnosis (after von Martens, 1867; Hendler et al., 1995). Disc thick, covered with scales; such scales do not form a protruding border on the edge of the body. Two thirds of its length touches the peripheral edge of the oral disc, the lower edge approximately as long as it is wide, with adoral margin somewhat convex. Three compressed brachial spines, flattened and blunt, perpendicular to the longitudinal direction of the arms. The dorsal spine is the largest in the proximal segments of the arm, while the most ventral is the largest towards the tip of the arms.
Material examined: ICML-UNAM 12495, 1 specimen, 26 May 2010, Ría Celestún (20°50’41.5” N, 90°23’12.6” W), depth 0.5 m; ICML-UNAM 12496, 20 specimens, 26 May 2010, Ría Celestún (20°46’45” N, 90°24’21.2” W), depth 1.1 m; ICML-UNAM 12499, 5 specimens, 2 June 2010, Chelem Lagoon (21°16’25.9” N, 89°41’4.5” W), depth 0.8 m; ICML-UNAM 12501, 7 specimens, 2 June 2010, Chelem Lagoon (21°16’25.8” N, 89°40’14.8” W), depth 0.5 m; ICML-UNAM 12504, 1 specimen, 4 June 2010, Dzilam Lagoon (21°26’35.9” N, 88°41’57.4” W), depth 0.2 m; ICML-UNAM 12506, 1 specimen; 4 June 2010, Dzilam Lagoon (21°26’59.5” N, 88°41’47.2” W), depth 0.3 m; ICML-UNAM 12508, 3 specimens, 4 June 2010, Dzilam Lagoon (21°27’36.1” N, 88°40’30.4” W), depth 0.3 m; ICML-UNAM 12511, 2 specimens, 9 July 2010, Ría Lagartos (21°35’38.4” N, 88°10’32.2” W), depth 0.8 m; ICML-UNAM 12514, 1 specimen, 9 July 2010, Ría Lagartos (21°36’6.6” N, 88°09’24.1” W), depth 0.4 m; ICML-UNAM 12521, 3 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’46.3” N, 86°45’42.9” W), depth 2.7 m; ICML-UNAM 12525, 1 specimen, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’43” N, 86°46’6.3” W), depth 0.9 m.
Geographic distribution: from Florida, USA to Argentina (Pawson et al., 2009).
Distribution in Mexico: Celestún, Chelem, Dzilam, and Ría Lagartos, Yucatán; Nichupté-Bojórquez lagoon system, Quintana Roo (Fig. 3E).
Habitat: depth: 0.2-2.7 m; water salinity: 23-41 PSU; dissolved oxygen: 3.8-9 mg L-1; pH: 8.3-8.8; ORP: -53.2 to -25.7 mV; sediment texture: clayey sand, sandy silt, clayey silt, silt; submerged aquatic vegetation: Halodule wrightii.
Genus Ophiophragmus Lyman, 1865
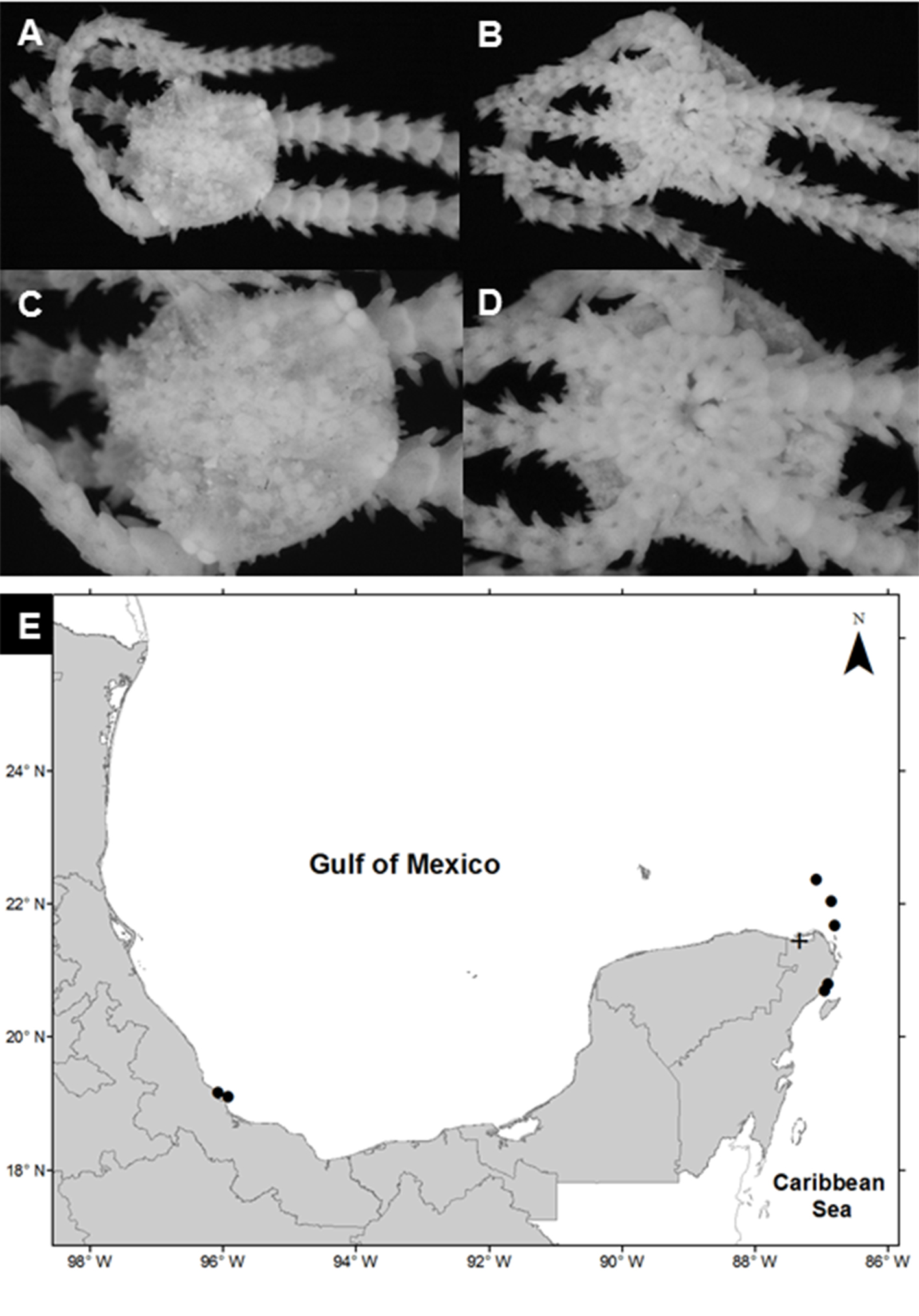
Ophiophragmus filograneus (Lyman, 1875)
(Fig. 4A-D)
Diagnosis (after Lyman, 1875). Close set granules along the margin and on part of the interbrachial spaces of disc. Radial shields short, as wide as the arms, and joined along their whole length. Three mouth-papillae on each side, the innermost one stout. Arms long and slender.
Material examined: ICML-UNAM 12498, 2 specimens, 26 May 2010, Ría Celestún (20°46’45” N, 90°24’21.2” W), depth 1.1 m; ICML-UNAM 12512, 2 specimens, 9 July 2010, Ría Celestún (21°35’38.4” N, 88°10’32.2” W), depth 0.8 m; ICML-UNAM 12520, 2 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’46.3” N, 86°45’42.9” W), depth 2.7 m.
Geographic distribution: from the southern tip of the Florida Peninsula to Pensacola Bay on the northwest coast, Mosquito Lagoon (near Cape Canaveral) in the northeast, Biscayne Bay and Whitewater Bay, USA (Hendler et al., 1995) and Mexico.
Distribution in Mexico: Celestún, Chelem and Ría Lagartos, Yucatán; Yalahau Lagoon and Nichupté-Bojórquez lagoon system, Quintana Roo (Fig. 4E).
Habitat: depth: 0.4-2.7 m; water salinity: 18-38 PSU; dissolved oxygen: 4.1-8.8 mg L-1; pH: 8.4-8.8; ORP: -74.3 to -35 mV; sediment texture: clay, sand, sandy silt, silt; submerged aquatic vegetation: Halodule wrightii and Thalassia testudinum.
Genus Ophiostigma Lüken, 1856
Ophiostigma isocanthum (Say, 1825)
(Fig. 5A-D)
Diagnosis (after Hendler et al., 1995). The disc is covered by numerous short, blunt tubercles that obscure the scales and may cover the radial shields. Usually several of the tubercles near the radial shields are markedly larger than the rest. However, the distal most oral papillae, which set it apart from the latter species, are long, opercular, and close the gaps between the jaws. The adoral shields overgrow the first ventral arm plates, both series thereby forming a nearly continuous circle of plates around the mouth. The 3 arm spines are blunt and somewhat flattened. Dorsal arm plates near the disc are subovoidal; they touch or overlap one another. The grainy texture of the lateral arm plates contrasts with the smooth dorsal and ventral arm plates. There are 2 small, slender tentacular scales.
Material examined: ICML-UNAM 12517, 1 specimen, 13 July 2010, Yalahau Lagoon (21°27’05.1” N, 87°19’17.7” W), depth 0.4 m.
Geographic distribution: Bermuda, Bahamas; from North Carolina to Florida and Florida Keys, Dry Tortugas, Texas, USA; Cuba; Jamaica; Puerto Rico; British Virgin Islands, Leeward Islands; Barbados; Tobago; Curaçao; Aruba; Costa Rica, Panama, Colombia, Venezuela and Brazil (Hendler et al., 1995).
Distribution in Mexico: Sacrificios Island, Veracruz; Puerto Morelos, Cozumel, Contoy Island, Cabo Catoche and Yalahau Lagoon, Quintana Roo (Fig. 5E).
Habitat: depth: 0.4 m; water salinity: 36.5 PSU; dissolved oxygen: 2.0 mg L-1; pH: 8.3; ORP: -94.9 mV; sediment texture: clayey sand; submerged aquatic vegetation: Halodule wrightii.
Family Ophiodermatidae Ljungman, 1867
Genus Ophioderma Müller & Troschel, 1840
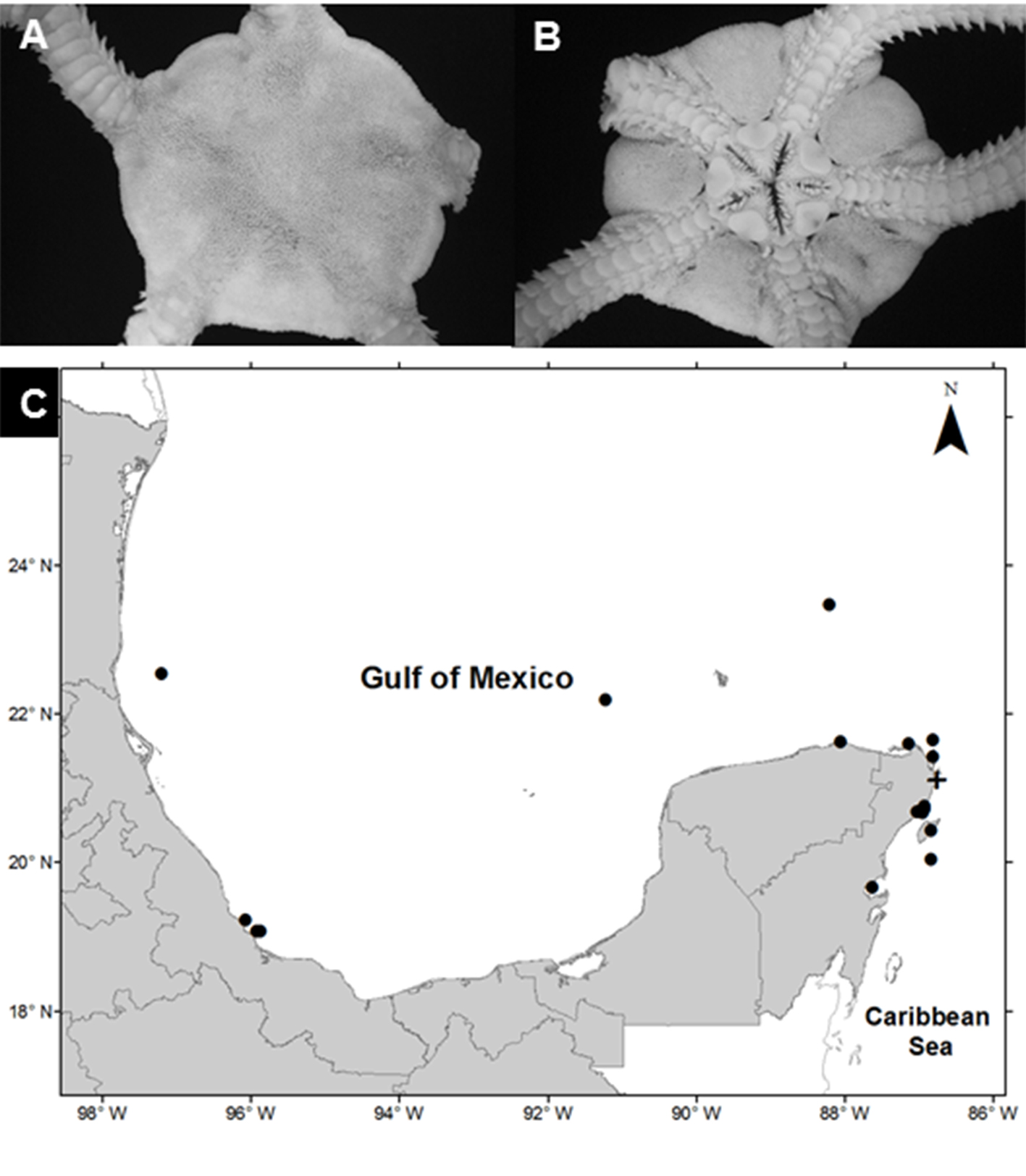
Ophioderma appressa (Say, 1825)
(Fig. 6A, B)
Diagnosis (modified from Say, 1825). Disc covered by granules with small scales below the granules. The radial shields are small, oval-shaped, and completely covered with granules. The oral shields are large in a triangular shape with rounded corners. The adoral plates are triangular, each pair is completely separated by the oral shield. The oral plates are elongated, wider proximally. Each jaw has 7 pairs of oral papillae, and the teeth are subtriangular. There are 4 genital clefts in each interradial space. The ventral arm plates are square, the dorsal plates are subrectangular, and the lateral plates have 6-9 spines, which are a conical shape, thick, roughly textured, and attached to the arms. Ventrally arms have 2 tentacular scales, the central arm is oval and slightly bigger than the lateral arm, which is approximately square.
Material examined: ICML-UNAM 12522, 2 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’43” N, 86°45’11” W), depth 1.6 m; ICML-UNAM 12528, 2 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°06’54.2” N, 86°45’47.2” W), depth 1.2 m.
Geographic distribution: Bermuda; Bahamas; South Carolina, Florida and the Florida Keys, the Dry Tortugas, Texas offshore reefs; Cuba; Jamaica; Haiti; Puerto Rico; British Virgin Islands, Leeward Islands; Barbados; Tobago, Trinidad; Curaçao; Aruba; Belize; Swan Island, Honduras; Isla de la Providencia, Colombia; Panama, Venezuela and Brazil (Hendler et al., 1995) and Mexico.
Distribution in Mexico: Tamaulipas; Sistema Arrecifal Veracruzano, Veracruz; Cayo Arenas, Campeche; Ría Lagartos, Yucatán; Cabo Catoche, Puerto Morelos, Cozumel, Bahía de la Ascensión, and Nichupté-Bojórquez lagoon system, Quintana Roo (Fig. 6C).
Habitat: depth: 1.2-1.6 m; water salinity: 32 PSU; Dissolved oxygen: 4.6-4.8 mg L-1; pH: 8.5; ORP: -74.3 to Dissolved oxygen: 4.6-4.8 mg L-1; pH: 8.5; ORP: -74.3 to -55.3 mV; Sediment texture: sandy silt; submerged aquatic vegetation: none.
Family Ophionereididae Ljungman, 1867
Genus Ophionereis Lütken, 1859
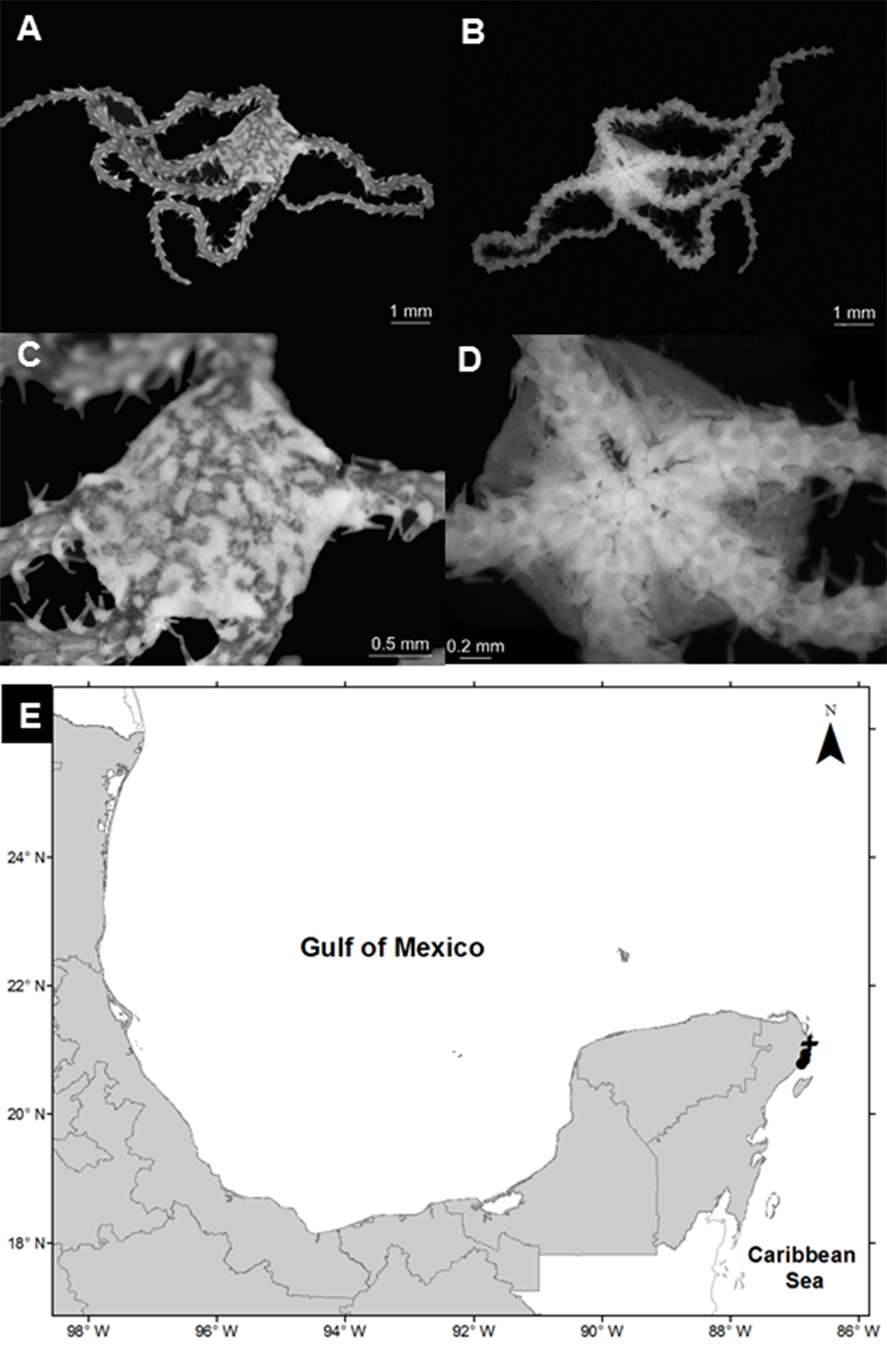
Ophionereis olivacea (H. L. Clark, 1901)
(Fig. 7A-D)
Diagnosis (modified from H. L. Clark,). The disc has a well-defined central scale and distal scales more elongated than the rest; small imbricate scales cover the whole disc. The radial shields are thin and separated. The oral shields are small and oval; the adoral plates are small, subtriangular. With 4 pairs of oral papillae in each mandible and 2 genital slits in each interradial space. The dorsal plates are small, subtriangular in the first segments, becoming wider than long towards the middle of the arms; they present an additional plate on both sides of the dorsal plate. The ventral plates of the arms are longer than they are wide; in the middle part of the arm and on each side they present a large tentacular scale. Three spines in each segment of the arms, the most ventral one is as long as the segment, the average is 2 times longer.
Material examined: ICML-UNAM 12524, 7 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°07’43” N, 86°45’11” W), depth 1.6 m; ICML-UNAM 12527, 10 specimens, 15 July 2010, Nichupté-Bojórquez Lagoon (21°06’54.2” N, 86°45’47.2” W), depth 1.2 m.
Geographic distribution: the Florida Keys, USA; Puerto Rico; Colombia; Belize (Hendler et al., 1995) and Mexico.
Distribution in Mexico: Puerto Morelos and Nichupté-Bojórquez lagoon system, Quintana Roo (Fig. 7E).
Habitat: depth: 1.1-1.6 m; water salinity: 28-32 PSU; dissolved oxygen: 4.6-4.8 mg L-1; pH: 8.5; ORP: -74.3 to -52.6 mV; sediment texture: sandy silt; submerged aquatic vegetation: Thalassia testudinum.
Discussion
In the southern Gulf of Mexico, there are no studies related to ophiuroid species in brackish environments. This study provides new records for 6 coastal lagoons from the northern Yucatán Peninsula. Amphiodia pulchella, Ophiostigma isocanthum, Ophioderma appressa, and Ophionereis olivacea have previously been recorded in
Mexican coastal waters (Laguarda-Figueras et al., 2009), whereas Amphiodia cf. planispina has been recorded only in the southeastern Gulf of Mexico (Pawson et al., 2009). Ophiophragmus filograneus has not been previously reported in Mexico. Laguarda-Figueras et al. (2009) found some individuals of Ophiostigma isocanthum in Contoy Island and Cabo Catoche in Quintana Roo, southeastern Gulf of Mexico, and now we report this species in the Yalahau coastal Lagoon. Further, Amphiodia cf. planispina and Ophiophragmus filograneus have been recorded for the first time in Quintana Roo (western Caribbean Sea).
Most studies on echinoderms in Mexico are taxonomic, and little information has been published on the ecology of echinoderms in this region (Solís-Marín et al., 2014). We found ophiuroids in a salinity range of 18 to 42, providing evidence that these organisms may live in brackish and even in hyperhaline environments (Salamon et al., 2012). Amphiodia pulchella and Ophiophragmus filograneus were recorded in this study under mesohaline and hyperhaline conditions, respectively. Ophiophragmus filograneus has been reported inhabiting brackish waters of Florida, USA (Turner & Meyer, 1980). Finally, O. filograneus has been associated with Halodule wrightii (Thomas, 1961); nevertheless, in this study, this ophiuroid species was associated with Thalassia testudinum.
In this study, ophiuroids were found in DO levels ranging from 2 to 11 ml L-1; most of the species were found in DO levels ≥ 3.8 ml L-1. However, Amphiodia pulchella and Ophiostigma isocanthum were found in sites with DO ≥2.0 ml L-1, and some ophiuroid species, such as Ophiura albida, can tolerate severe hypoxic conditions (Díaz & Rosenberg, 1995). This ecological information could be used to classify ophiuroid species as sensitive, tolerant or opportunist species with the purpose of determining the ecological quality of coastal and marine ecosystems (Borja et al., 2000; Mistri & Munari, 2008; Simboura & Zenetos, 2002).
All the ophiuroid species mentioned in this study are new records for the tropical coastal lagoons of the northern Yucatán peninsula. Besides, we present a new record of Ophiophragmus filograneus in Mexican waters. Finally, the very presence of Amphiodia pulchella and O. filograneus suggest that they are adapted to sites with high variability in water salinity and dissolved oxygen. The sampling method employed for collecting echinoderms in this study is unusual because it was intended primarily for benthic macroinfauna; in this sense, the catch may be considered incidental. These results extend the distribution range for these species and highlight their presence in brackish waters, an environment that has yet to be extensively studied worldwide concerning this Echinodermata class.
Acknowledgements
Financial support was provided by the scholarship 34691 from Conacyt to JGKD, and Cinvestav, Benthos Laboratory research fund. Thanks are due to SAGARPA and Conanp for the organism collecting permit (DGOPA.04027.080610.2754). We thank Elizabeth Real de León for her help with sediment analysis, Tania Pineda-Enríquez (University of Florida, USA) and Quetzalli Hernández Díaz (PCML, UNAM) for their help with specimen identification. Finally, we are also grateful to Alicia Durán (ICML, UNAM) for her technical support in the curation of the collection material.
References
Borja, A., Franco, J., & Pérez, V. (2000). A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin, 40, 1100–1114. https://doi.org/10.1016/s0025-326x(00)00061-8
Clark, H. L. (1901). The Echinoderms of Porto Rico. Bulletin of the United States Fish Commission, 21, 231–263.
Conanp (Comisión Nacional de Áreas Naturales Protegidas). (2019). Retrieved on June 7, 2019 from: https://www.gob.mx/conanp/
Córdoba-Azcárate, M. (2006). Between local and global, discourses and practices: rethinking ecotourism development in Celestún (Yucatán, México). Journal of Ecotourism, 5, 97–111. https://doi.org/10.1080/14724040608668449
Díaz, R., & Rosenberg, R. (1995). Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: and Annual Review, 33, 245–303.
Durán-González, A., Laguarda-Figueras, A., Solís-Marín, F. A., Buitrón-Sanchez, B. E., Ahearn, C. G., & Torres-Vega, J. (2005). Equinodermos (Echinodermata) de las aguas mexicanas del Golfo de México. Revista de Biología Tropical, 53, 53–68. https://doi.org/10.15517/rbt.v53i3.26666
Ellwood, B. B., Balsam, W. L., & Roberts, H. H. (2006). Gulf of Mexico sediment sources and sediment transport trend from magnetic susceptibility measurements of surface samples. Marine Geology, 230, 237–248. https://doi.org/10.1016/j.margeo.2006.05.008
Hendler, G., Miller, J. E., Pawson, D. L., & Kier, P. M. (1995). Sea stars, sea urchin, and allies: echinoderms of Florida and the Caribbean. Washington D.C.: Smithsonian Institution Press.
Hernández-Herrejón, L. A., Solís-Marín, F. A., & Laguarda-Figueras, A. (2008). Ofiuroideos (Echinodermata: Ophiuroidea) de las aguas mexicanas del Golfo de México. Revista de Biología Tropical, 56, 83–167. https://doi.org/10.15517/rbt.v56i3.27082
Herrera-Silveira, J. A., Martín, M., & Díaz-Arce, V. (1999). Phytoplankton variations in four coastal lagoons, Yucatán, México. Revista de Biología Tropical, 47, 47–56.
Herrera-Silveira, J. A., & Morales-Ojeda, S. M. (2010). Subtropical karstic coastal lagoon assessment, Southeast Mexico, the Yucatán peninsula case. In M. J. Kennish, & H. W. Paerl (Eds.) Coastal lagoons: critical habitats of environmental change (pp. 307–333), Boca Raton: CRC Press.
Herrera-Silveira, J. A., Ramírez, R. J., & Zaldívar, J. A. (1998). Overview and characterization of the hydrology and primary producer communities of selected coastal lagoons of Yucatán, México. Aquatic Ecosystem Health and Management, 1, 353–372. https://doi.org/10.1016/s1463-4988(98)00014-1
Kennish, M. J., & Paerl, H. W. (2010) Coastal lagoons: critical habitats of environmental change. In M. J. Kennish, & H. W. Paerl (Eds.) Coastal lagoons: critical habitats of environmental change (pp. 1–15). Boca Raton: CRC Press. https://doi.org/10.1201/ebk1420088304-c1
Kjerfve, B. (1994). Coastal lagoons. In B. Kjerfve (Ed.), Coastal lagoons processes (pp. 1–8). Amsterdam: Elsevier. https://doi.org/10.1016/s0422-9894(08)70006-0
Kuk-Dzul, J. G., Gold-Bouchot, G., & Ardisson, P. L. (2012). Benthic infauna variability in relation to environmental factors and organic pollutants in tropical coastal lagoons from the northern Yucatán Peninsula. Marine Pollution Bulletin, 64, 2725–2733. https://doi.org/10.1016/j.marpolbul.2012.09.022
Laguarda-Figueras, A., Hernández-Herrejón, L. A., Solís-Marín, F. A., & Durán-González, A. (2009). Ofiuroideos del Caribe mexicano y golfo de México. México D.F.: ICMyL-UNAM/CONABIO.
Lyman, T. (1875). II. Ophiurudae and Astrophytidae. Zoological Results of the Hassler Expedition. Illustrated Catalogue of the Museum of Comparative Zoology, at Harvard College, 8, 1–34.
May-Kú, M. A., Criales, M., Montero-Muñoz, J. L., & Ardisson, P. L. (2014). Differential use of Thalassia testudinum habitats by sympatric Penaeids in a nursery ground of the southern Gulf of Mexico. Journal of Crustacean Biology, 34, 144–156. https://doi.org/10.1163/1937240X-00002214
May-Kú, M. A., & Ordoñez-López, U. (2006). Spatial patterns of density and size structure of penaeid shrimps Farfantepenaeus brasiliensis and Farfantepenaeus notialis in a hypersaline lagoon on the Yucatán Peninsula, México. Bulletin of Marine Science, 79, 259–271.
Mistri, M., & Munari, C. (2008). BITS: A SMART indicator for soft-bottom, non-tidal lagoons. Marine Pollution Bulletin, 56, 587–599. https://doi.org/10.1016/j.marpolbul.2007.12.002
Pawson, D. L., Vance, D. J., Messing, C. G., Solis-Marin, F. A., & Mah, C. L. (2009). Echinodermata of the Gulf of Mexico. In D. L. Felder, & D. K. Camp (Eds.), Gulf of Mexico origins, waters and biota, Vol. I, Biodiversity (pp. 1177–1204). Corpus Christi: A&M University Press.
Pech, D., Ardisson, P. L., & Hernádez-Guevara, N. A. (2007). Benthic community response to habitat variation: a case of study from a natural protected area, the Celestun coastal lagoon. Continental Shelf Research, 27, 2523–2533. https://doi.org/10.1016/j.csr.2007.06.017
Rump, H., & Krist, H. (1992). Laboratory manual for the
examination of water, waste water and soil. 2nd Ed. Weinheim, Germany: VCH.
Salamon, M. A., Niedzwiedzki, R., Lach, R., Brachaniec, T., & Gorzelak, P. (2012). Ophiuroids discovered in the middle Triassic hypersaline environment. Plos One, 7, e49798. https://doi.org/10.1371/journal.pone.0049798
Say, T. (1825). On the species of the Linnaean genus Asterias inhabiting the coast of the U.S. Journal of the Academy of Natural Sciences of Philadelphia, 5, 141–154.
Simboura, N., & Zenetos, A. (2002). Benthic indicators to use in ecological quality classification of mediterranean soft bottom marine ecosystems, including a new biotic index. Mediterranean Marine Sciences, 3, 77–111. https://doi.org/10.12681/mms.249
Solís-Marín, F. A., Honey-Escandón, M. B. I., Herrero-Pérezrul, M. D., Benítez-Villalobos, F., Díaz-Martínez, J. P., Buitrón-Sánchez, B. E. et al. (2013). The echinoderms of Mexico: biodiversity, distribution and current state of knowledge. In J. J. Alvarado-Barrientos, & F. A. Solís-Marín (Eds.), Echinoderm research and diversity in Latin America (pp. 11–65). Heidelberg, Germany: Springer. https://doi.org/10.1007/978-3-642-20051-9_2
Solís-Marín, F. A., Laguarda-Figueras, A., & Honey-Escandón, M. (2014). Biodiversidad de equinodermos (Equinodermata) en México. Revista Mexicana de Biodiversidad, 85, S441–S449. https://doi.org/10.7550/rmb.31805
Solís-Marín, F. A., Pineda-Enríquez, T., Hernández-Díaz, Y. Q., Yepes-Gaurisas, D., González-Gándara, C., Granados-Barba, A. et al. (2015). First records and range extension of Ophioblenna antillensis (Echinodermata: Ophiuroidea) in the Gulf of Mexico. Revista Mexicana de Biodiversidad, 86, 306–309. https://doi.org/10.1016/j.rmb.2015.04.021
Tagliapietra, D., Rismondo, A., & Frangipane, G. (2005). Coastal lagoons: spatial patterns of benthic assemblages and bioindication. In P. Magni, G. Hyland, G. Manzella, H. Rumohr, P. Viaroli, & A. Zenetos (Eds.), Proceedings of the Workshop “Indicators of Stress in the Marine Benthos (pp. 34–35). Paris: UNESCO/ IOC, IMC.
Thomas, L. P. (1961). Distribution and salinity tolerance in the amphiurid brittlestar, Ophiophragmus filograneus (Lyman, 1875). Bulletin of Marine Science of the Gulf and Caribbean, 11, 158–160.
Turner, R. L., & Meyer, C. E. (1980). Salinity tolerance of the brackish-water echinoderm Ophiophragmus filograneus (Ophiuroidea). Marine Ecology Progress Series, 2, 249–256. https://doi.org/10.3354/meps002249
von Martens, E. (1867). Über vier neue Schlangensterne, Ophiuren, des Kgl. Zoologischen Museums vor. Sitzungsberichte der Preussischen Akademie der Wissenschaften Physikalisch-Mathematische Klasse, 1867, 345–348.