Genetic diversity of the Common Black Hawk (Buteogallus anthracinus) population in Los Tuxtlas, Mexico, based on microsatellite markers
Héctor Hugo Barradas-García a, Jorge Éufrates Morales-Mávil a, *, María Raquel Marchán-Rivadeneira b, Liliana Cortés-Ortiz b
a Laboratorio Biología de la Conducta, Instituto de Neuroetología, Universidad Veracruzana, Av. Dr. Luis Castelazo Ayala s/n, Km. 3.5 Carretera Federal Xalapa-Veracruz, Colonia Industrial Animas, 91190 Xalapa, Veracruz, Mexico
b Department of Ecology and Evolutionary Biology, University of Michigan, 500 S State St, Ann Arbor, MI 48109-1079, USA
*Corresponding author: jormorales@uv.mx (J.E. Morales-Mávil)
Abstract
The Common Black Hawk (Buteogallus anthracinus) is a raptor associated with wetlands. Many of its populations are in decline as a consequence of habitat loss and degradation. However, there are no published studies on the genetic variation of their populations. We characterize for the first time the genetic variation of a population of B. anthracinus in southern Veracruz, Mexico. We used feathers from 19 individuals to extract DNA and amplify and genotype 9 microsatellite loci. Samples were collected from nests (n = 4), at feeding sites (n = 5), and directly from chicks (n = 2), young individuals (n = 7), and 1 adult. Six out of 9 microsatellites were polymorphic and 3 monomorphic for this population. The highest number of alleles (n = 7) was observed at the BswD107w, with an average of 4.2 alleles per polymorphic locus. Mean observed and expected heterozygosity were 0.41 and 0.53, respectively. These values are on the lower end of those found for other birds of prey, but there is no evidence of high levels of inbreeding in the population. The polymorphic microsatellite loci analyzed in this study provide a useful tool to continue characterizing the genetic variation of B. anthracinus populations and evaluate possible inter-population differences.
Keywords: DNA; Population genetics; Mangrove; Bird of prey; Veracruz
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Diversidad genética de la población de la aguililla negra menor (Buteogallus anthracinus) en Los Tuxtlas, México, basada en marcadores microsatelitales
Resumen
El Aguililla negra (Buteogallus anthracinus) es una rapaz asociada con humedales. Muchas de sus poblaciones están en declive por pérdida y transformación del hábitat. Sin embargo, no existen estudios que analicen los niveles de su variación genética. Reportamos la primera caracterización de la variabilidad genética en una población de B. anthracinus de Veracruz, México, mediante el uso de marcadores moleculares. Utilizamos plumas de 19 individuos para extraer ADN, amplificar y genotipificar 9 loci de microsatélites. Las plumas fueron recolectadas en nidos (n = 4), en sitios de alimentación (n = 5), directamente de los pollos (n = 2), de individuos inmaduros (n = 7) y de 1 individuo adulto. Seis de los 9 microsatélites resultaron polimórficos y 3 monomórficos para la población. El número más alto de alelos (n = 7) se observó en el marcador BswD107w, con un promedio de 4.2 alelos por locus polimórfico. Los promedios de heterocigosidad observada y esperada fueron 0.41 y 0.53, respectivamente. Aunque estos valores son relativamente bajos comparados con otras especies de rapaces, no se encontró evidencia de altos niveles de endogamia. Los microsatélites polimórficos analizados proveen una herramienta para continuar caracterizando genéticamente poblaciones de B. anthracinus y analizar posibles diferencias interpoblaciones.
Palabras clave: ADN; Genética de la población; Manglar; Rapaz; Veracruz.
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The development of genetic molecular tools is considered crucial for analyzing genetic diversity and to inform species management and conservation strategies. For example, genetic markers have been used to define the taxonomic boundaries of species, subspecies, and populations that require conservation attention (Haig et al., 2011), determine population structure and gene flow (Funk et al., 2007), and discern the temporal and spatial movement of migratory birds and its implications for population management (Bounas et al., 2018). Genetic data have also been used to evaluate demographic, behavioral, and ecological aspects important to design management strategies, such as the relatedness among individuals in a population (e.g., Müller et al., 2001), philopatry (Rudnick et al., 2005), extra-pair paternity and genetic mating system (Griffith et al., 2002; Kraukauer, 2008), and to measure the impact of anthropogenic disturbance and climate change on wildlife (Gebhardt et al., 2009; Martínez-Cruz et al., 2004). Particularly, microsatellite markers have been widely used in conservation genetics because they are selectively neutral, hypervariable, and possess codominant alleles (Ellegren, 1992; Nesje et al., 2000), which make them useful to evaluate levels of genetic diversity within populations and assess population structure with strong statistical power (Busch et al., 2005; Kalinowski, 2002; Sarasola et al., 2012).
Molecular markers have been used to characterize genetic diversity and structure in a number of endangered raptor species to aid in their management and conservation (e.g., Gebhardt et al., 2009; Hailer et al., 2005; Hull et al., 2007; Martínez-Cruz et al., 2002, 2004; Rudnick et al., 2005, 2008). In general, raptors are more affected by anthropogenic activities than other birds. These activities include habitat alteration and destruction, intentional killing, intentional and unintentional poisoning, electrocution, and climate change (reviewed in McClure et al., 2018). Understanding how these threats affect the genetic variation of a population is fundamental to the design of conservation and management strategies (Allendorf et al., 2013). The Common Black Hawk (Buteogallus anthracinus) is a bird of prey in the family Accipitridae that is protected under the Migratory Bird Treaty Act of 1918 in the United States of America (USFWS, 2013). Although B. anthracinus is globally considered as of “Least Concern”, many of its populations are in decline (BirdLife International, 2017, 2019). It is classified as a “Threatened” species in Arizona, New Mexico, Utah, and Texas (NatureServe 2019; Texas Parks & Wildlife, 2019), and it is subject of special protection in the Norma Oficial Mexicana (NOM-059-SEMARNAT-2010) for the protection of native wildlife in Mexico (Semarnat, 2010).
Buteogallus anthracinus inhabits mangroves and riparian vegetation, and can be found in woods on mud banks, and on beaches and swamps (Howell & Webb, 1995; Peterson & Chalif, 1989; Schennel, 1994; Sibley, 2000). It is widely distributed in the Americas, ranging from southwestern United States through Mexico and Central America, Colombia, northern Venezuela, northeastern Peru and on the Island of San Vicente in the Lesser Antilles. In Mexico, it ranges from the states of Sonora, Chihuahua, and Tamaulipas, extending southwards through the Gulf of Mexico and the Pacific coast and reaching the Yucatan peninsula (González-Salazar, 2010). Due to their obligate reliance on mangrove and riparian vegetation, B. anthracinus is strongly affected by habitat disturbance. Mexico is one of the countries that have the most mangrove vegetation in the world (5% of the world’s total), but the rate of deforestation of this ecosystem is extremely high (Aburto & Rojo, 2015), affecting the survival of all species that depend on it. In particular, the mangroves of Sontecomapan, Veracruz, Mexico have experienced high rates of destruction and fragmentation as a consequence of anthropogenic activities associated with the expansion of cattle ranching in the region (Mendoza et al., 2005).
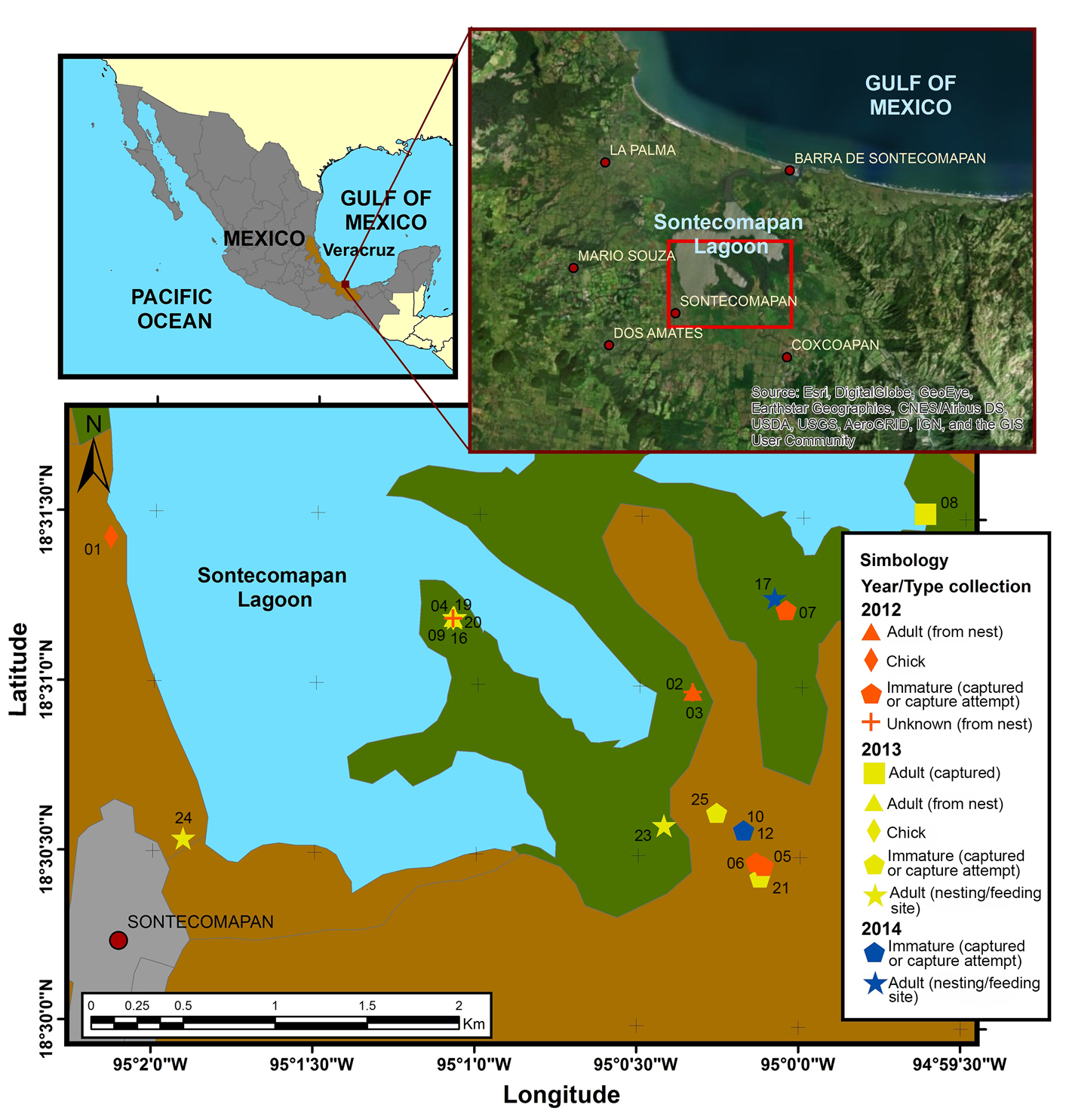
In this study, we used microsatellite markers to characterize the genetic diversity of a resident population of B. anthracinus that lives in the mangrove of Sontecomapan, Veracruz, Mexico (Fig. 1). To our knowledge, no previous studies have been published reporting genetic diversity in B. anthracinus, so this study contributes to testing heterospecific molecular markers for this species, evaluates the levels of genetic variation found in this population, and serve as an initial step for the long-term monitoring of the B. anthracinus population in Sontecomapan. This is particularly important considering the strong anthropogenic pressures on the species in the area, which not only include habitat destruction, but also poaching and disturbance by tourism (Carmona-Díaz et al., 2004).
Materials and methods
We opportunistically collected B. anthracinus feathers during the nesting (February to July) and post-nesting (August to November) periods of 2012, 2013 and 2014, in Sontecomapan, Catemaco, Veracruz, and surrounding areas (Fig. 1). Sampling occurred as part of a larger project on ecology, nesting behavior and morphology for the species (Barradas-García, 2016). We categorized sampled individuals as chicks, inmatures and adults. Chicks were individuals found in the nest and unable to fly and leave the nest (Schennel, 1994). Adults were differentiated from inmmatures based on morphological (e.g., feather color patterns) and morphometric (e.g., tail length) data (Howell & Webb, 1995; Schennel, 1994; Sibley 2000). Here we present results from 19 feathers collected from captured individuals (2 chicks, 3 immatures, and 1 adult), from nests (2 adults and 2 unknown), found in areas near nesting sites (5 adults), or those that fell from individuals during capturing attempts (4 immatures around feeding sites) (Table 1). To capture immature and adult birds we used Bal-chatri traps with the land crab Cardisoma huangumi and the blue crab Callinectes sapidus as bait. After sample collection, we hydrated the birds, placed an ID leg band, and released them at the site where they were captured. We preserved the feathers in 70% ethanol using Ziploc bags (modified from Taberlet and Bouvet [1991], and Gaur et al. [2017]). All collections were carried out under the scientific collector’s license number SGPA/DGVS/02826/12 to HHBG. Samples were transported to the University of Michigan following all legal requirements from Mexico (CITES exportation permit # 70144) and the US (USDA importation permit # 124948).
Molecular work was carried out at the Genomic Diversity Laboratory, of the Department of Ecology and Evolutionary Biology, University of Michigan. DNA was extracted from the tip of the calamus of each feather with the DNeasy Blood & Tissue Kit (Qiagen, Inc.) following the manufacturer’s protocols. DNA was stored at -20 ºC. In order to verify that the feathers belonged to B. anthracinus individuals, we amplified and sequenced a fragment of ~ 350 bp of the cytochrome b mitochondrial gen (cyt b) using primers L14996 (Sorenson et al., 1999) and CB2 (Palumbi, 1996) and compared these sequences with those publicly available on the GenBank database of the National Center for Biotechnology Information.
Amplification of the cyt b fragment was carried out through PCR in a final reaction volume of 10 μL, including 1 μL 10X Buffer, 1 μL dTNPs (2mM, of each dNTP), 0.8 μL MgCl2 (50 mM), 0.25 μL of each primer (10 mM each), 5.7 μL ddH20, 0.045 μL of Platinum Taq (Invitrogen) and 1 μL of DNA. The cycling conditions for the PCR profile were 2 min at 94 ºC, followed by 35 cycles of 45 seconds at 94 ºC, 45 seconds at 50 ºC, 1 min at 72 ºC and ending with a final extension of 2 min at 72 ºC. PCR products were visualized in 2% agarose gels using GelRed (Biotium, Inc.). Products with single bands were sent to the Sequencing Core Facilities of the University of Michigan, where they were sequenced using an ABI 3730xl DNA Analyzer.
A panel of 11 microsatellite loci previously isolated from other birds of prey phylogenetically proximate to the B. anthracinus was tested. After PCR optimization, only 9 loci successfully amplified single products (Table 2). PCR reactions were conducted using fluorescently labeled primers, following similar conditions as described above for cyt b, but with an increase in the final extension time (72 ºC for 10 minutes). Optimization of annealing temperatures (Ta) for each locus was carried out using temperature gradients before genotyping and selecting an optimal temperature at which bright single bands were observed (Table 2).
PCR products were also genotyped at the Sequencing Core Facilities of the University of Michigan using fragment analysis in an ABI 3730xl DNA Analyzer. Alleles sizes were identified using GeneMarker version 1.97 (SoftGenetics, State College, PA). Potential genotype errors such as the presence of null alleles, and errors due to stuttering and exclusion of large alleles (i.e., large allele dropout) were evaluated using a Micro-Checker version 2.3.3 (Van Oosterhout et al., 2004). GenAlEx 6.41 (Peakall & Smouse, 2006) was used to calculate the number of alleles per locus (Na), fixation index (FIS), the probability of Identity (PI), and the Queller and Goodnight’s relatedness coefficient (r) per pair of individuals. Arlequin 3.5.1.3 (Excoffier & Lischer, 2010) was used to analyze Hardy-Weinberg equilibrium (HWE), Linkage Disequilibrium (LD) and observed (Ho) and expected heterozygosity (He). Levels of statistical significance were corrected for multiple tests using the Bonferroni correction (Rice, 1989).
Table 1
List of samples used in this study with coordinates of collection site. Collection type refers to whether samples were collected directly (from chicks, immature individuals, or adults) or indirectly (from nests, or from nesting/feeding sites).
|
ID |
Collection type |
Year of collection |
Latitude |
Longitude |
|
BAST-01 |
Chick |
2012 |
-95.0356 |
18.5237 |
|
BAST-02 |
Nest |
2012 |
-95.0056 |
18.5164 |
|
BAST-03 |
Nest |
2012 |
-95.0056 |
18.5164 |
|
BAST-04 |
Nest |
2012 |
-95.0180 |
18.5199 |
|
BAST-05 |
Immature |
2012 |
-95.0018 |
18.5079 |
|
BAST-06 |
Immature |
2012 |
-95.0022 |
18.5081 |
|
BAST-07 |
Immature |
2012 |
-95.0008 |
18.5205 |
|
BAST-08 |
Adult |
2013 |
-94.9937 |
18.5253 |
|
BAST-09 |
Chick |
2013 |
-95.0180 |
18.5199 |
|
BAST-10 |
Immature |
2014 |
-95.0029 |
18.5096 |
|
BAST-12 |
Immature |
2014 |
-95.0029 |
18.5096 |
|
BAST-16 |
Adult in nest |
2013 |
-95.0180 |
18.5199 |
|
BAST-17 |
Nesting site. Adult |
2014 |
-95.0014 |
18.5210 |
|
BAST-19 |
Nesting site. Adult |
2013 |
-95.0179 |
18.5199 |
|
BAST-20 |
Nesting site. Adult |
2013 |
-95.0179 |
18.5199 |
|
BAST-21 |
Immature |
2013 |
-95.0020 |
18.5073 |
|
BAST-23 |
Nesting site. Adult |
2013 |
-95.0070 |
18.5098 |
|
BAST-24 |
Nesting site. Adult |
2013 |
-95.0317 |
18.5089 |
|
BAST-25 |
Immature |
2013 |
-95.0043 |
18.5105 |
Table 2
Microsatellite loci successfully amplified in this study for Buteogallus anthracinus. Ta °C = annealing temperature, N = number of individuals genotyped, Na = number of alleles found in our sample.
|
Locus |
Ta ºC |
N |
Na |
Allele size range (this study) |
Species from which it was originally isolated |
Original reference |
|
IEAAAG04 |
57 |
20 |
4 |
212-223 |
Aquila heliaca |
Busch et al. (2005) |
|
IEAAAG15 |
57 |
20 |
3 |
113-121 |
A. heliaca |
Busch et al. (2005) |
|
IEAAAG14 |
60 |
20 |
4 |
177-189 |
A. heliaca |
Busch et al. (2005) |
|
HaL 04 |
57 |
19 |
1 |
153 |
Haliaeetus albicilla |
Hailer et al. (2005) |
|
Bbu42 |
61 |
20 |
5 |
151-161 |
Buteo buteo |
Johnson et al. (2005) |
|
BswD107w |
61 |
17 |
7 |
160-195 |
B. swainsoni |
Hull et al. (2007) |
|
Hf-C3F2 |
57 |
19 |
1 |
169 |
Hieraaetus fasciatus |
Mira et al. (2005) |
|
Hf-C1E8 |
57 |
17 |
2 |
231-240 |
H. fasciatus |
Mira et al. (2005) |
|
Hf-C5D4 |
59 |
20 |
1 |
169 |
H. fasciatus |
Mira et al. (2005) |
Results
We recovered a small fragment of mitochondrial cyt b sequence (233-270 bp) for all sampled individuals. Sequences of all 19 feathers matched those publicly available in GeneBank for B. anthracinus specimens with 99.6-100% sequence identity. Seventeen samples had sequences that were identical to each other and to all available sequences in GeneBank, whereas 2 samples (BAST-09 and BAST-16) showed a single non-synonymous nucleotide difference in this region that changed a Tyrosine into a Histidine, representing a new cyt b haplotype for the species. The lack of variation in our samples for this mitochondrial region is not surprising, given that the amplified region is invariable across specimens from Arizona (GeneBank accession number GQ264779), Panama (AY987327, GQ264777, GQ264778), and Costa Rica (EU583331), but sequencing this fragment allowed us to verify that the analyzed feathers belonged to B. anthracinus individuals.
Of the 9 microsatellite loci analyzed, 6 were polymorphic and 3 monomorphic for the population of B. anthracinus in Sontecomapan, Veracruz (Table 2). The number of alleles per polymorphic locus varied from 2 to 7, with an average of 4.2 alleles per locus. There was no evidence of linkage disequilibrium for any of the loci. After the Bonferroni correction locus, BswD107w showed evidence of deviation from HWE (Table 2). Results from Micro-Checker detected signals of null alleles in this locus, which may explain the deviation from HWE. However, the exclusion of this locus did not qualitatively affect the results. Thus the results for all analyses we present included all 6 loci. Observed heterozygosity (Ho) per locus varied from 0.06 to 0.75, with an average of 0.41, whereas expected heterozygosity (He) per locus varied from 0.26 to 0.81, with an average of 0.53 (Table 3).
Our estimate of the probability of identity (PI) using these markers in the population was low (PI = 3.1×10-3, PIsib = 3.0×10-2), meaning that these loci have high power to differentiate individuals. Furthermore, no individuals had identical genotypes for all loci, confirming that all of them represented unique individuals. Inbreeding coefficient (FIS) was 0.19, and fourteen individuals (70%) showed high coefficients of relatedness (r > 0.5) with at least another individual, suggesting that some individuals in our sample may be related.
Table 3
Observed and expected heterozygosity (Ho and He, respectively), deviation from Hardy-Weinberg equilibrium (P) and Fixation Index (FIS; Coefficient of Inbreeding) for 6 polymorphic microsatellite loci in the population of B. anthracinus in Sontecomapan, Veracruz.
|
Locus |
Ho |
He |
p |
FIS |
|
IEAAAG04 |
0.60 |
0.58 |
ns |
-0.06 |
|
IEAAAG15 |
0.40 |
0.34 |
ns |
-0.19 |
|
IEAAAG14 |
0.50 |
0.54 |
ns |
0.05 |
|
Bbu42 |
0.75 |
0.64 |
ns |
-0.21 |
|
BswD107w |
0.18 |
0.81 |
*** |
0.77 |
|
HF-C1E8 |
0.06 |
0.26 |
ns |
0.77 |
|
Mean |
0.41 |
0.53 |
0.19 |
Discussion
The level of heterozygosity found in our study population was on the lower end of those reported using the same microsatellite loci in eagles and hawks (e.g., Aquila heliacal, A. nipalensis and Haliaeetus albicilla, Busch et al., 2005; Buteo buteo, Johnson et al., 2005; Hieraaetus fasciatus, Mira et al., 2005; Buteo swainsoni, Hull et al., 2007), and for different microsatellite loci in populations of other raptor species (e.g., Aquila adalberti, Martínez-Cruz et al., 2004; Haliaeetus albicilla, Hailer et al., 2005; Harpia harpyja, Banhos et al., 2008; Buteo ridgwayi, Woolaver et al., 2013). However, given that there is no reference data of other populations of B. anthracinus it is impossible to make appropriate interpretations on the levels of genetic diversity for the study population relative to other populations of the species.
None of the samples analyzed showed the same genotype (i.e., no sample had exactly the same alleles on each locus); therefore, we assume that they represent different individuals. However, we suggest that there is a high number of related individuals in the population, at the level of parent-offspring or siblings. Considering that our sampling method included 3 consecutive nesting seasons, and that B. anthracinus is reported to be socially monogamous (Schennel, 1994) with breeding pairs being faithful to their nesting sites in the Sontecomapan mangrove (Barradas-García & Morales-Mávil, 2007; Barradas-García et al., 2004), it is likely that our sample includes parents and offspring, or siblings, which could cause the low levels of diversity that we observed in our results. For example, in 2012 we collected a feather (BAST-04) from a nest, and in 2013 we collected a feather from an adult (BAST-16) and a feather from a chick (BAST-09) in the same nest. It is likely that all these individuals are a parent and offspring and/or siblings. Similarly, in 2012 we collected 2 feathers from the same nest, 1 from an adult (BAST-02), and another (BAST-03) presumably from its offsprings (although it could potentially be from its mate). Our inbreeding coefficient estimate shows some (although not high) level of inbreeding among our samples, which is consistent with our sampling of potentially related individuals. However, to accurately determine the actual level of relatedness among the different individuals sampled in this population, genotyping a larger number of polymorphic loci would be required to ensure reasonable statistical power.
In Mexico, B. anthracinus is a resident species that lives in different habitats, but is an obligate riparian nester with relatively low abundance and depends on the resources of the mangrove ecosystem (Arizmendi et al., 1990; Bojorges-Baños, 2011; De Labra & Escalante, 2013; Ortiz-Pulido et al., 1995; Rodríguez-Estrella & Brown, 1990; Vázquez-Pérez et al., 2009). The mangrove of Sontecomapan, Veracruz, harbors a breeding population of B. anthracinus (Barradas-García & Morales-Mávil, 2007; Barradas-García et al., 2004). However, it is unknown whether B. anthracinus individuals are phylopatric (i.e., remain in the same population where they were born) or disperse to breed in other populations in the region, so the extent at which this population is isolated from other neighboring populations remains unknown. All but one the captured birds in this study were banded with coded metal bands and the long-term monitoring and genetic characterization of individually recognized young and adult individuals in this population will allow a better understanding of the general dispersal patterns of this species. Furthermore, phylogeographic studies have found that Los Tuxtlas region harbors unique lineages for several bird species (e.g., Ornelas et al., 2013). This study sets the foundation to compare the population of B. anthracinus in Los Tuxtlas to other populations across its distribution range to understand their evolutionary history and evaluate the genetic structure among populations that could be used to ensure proper management and conservation strategies for this species.
The 6 polymorphic microsatellite loci analyzed in this study for B. anthracinus provide a useful tool that can help to characterize genetic differences among populations throughout the geographical distribution of the species. The results presented here represent an initial attempt at understanding the extent of genetic diversity in this species and could be the basis for comparative studies to determine if the genetic diversity of B. anthracinus is sub-divided into different populations that may require particular management actions.
Acknowledgements
We thank the Mexican and US authorities for granting permits to conduct this study (collector’s license No. SGPA/DGVS/02826/12, CITES No. MX 70144 and USDA 124948). This study was funded by a Conacyt grant (CVU: 170101) to HHBG. We thank Martín Quinto Charmin and family for their support in the field during the collection of biological samples. Laboratory work was conducted in the Genomic Diversity Laboratory at the University of Michigan, Ann Arbor, USA.
References
Aburto, O., & Rojo, J. (2015). “The Mangroves of Mexico – By the Numbers.” National Graphic Voices, 12 February 2015. Retrieved on July/22/2019: http://voices.nationalgeographic.com/2015/02/03/the- mangroves-of-mexico-by-numbers
Allendorf, F., Luikart, G. H., & Aitken S. N. (2013). Conservation and the genetics of populations (2nd ed.). Hoboken, NJ: Wiley & Blackwell.
Arizmendi, M. C., Berlanga, H., Márquez-Valdelamar, L. M., Navarijo, L., & Ornelas, J. F. (1990). Avifauna de la región de Chamela Jalisco. Cuadernos 4. México D.F.: Instituto de Biología, Universidad Nacional Autónoma de México.
Banhos, A., Hrbek, T., Gravena, W., Sanaiotti, T., & Farias, I. P. (2008). Genomic resources for the conservation and management of the harpy eagle (Harpia harpyja, Falconiformes, Accipitridae). Genetics and Molecular Biology, 31, 146–154. https://doi.org/10.1590/s1415-47572008000100025
Barradas-García, H. H. (2016). Caracterización de los sitios de anidación y variabilidad genética de Buteogallus anthracinus en una zona de manglar de Sontecomapan, Veracruz, México (Ph.D. Thesis), Universidad Veracruzana. Xalapa, Veracruz.
Barradas-García, H. H., Carmona-Díaz, G., & Rodríguez-Luna, E. (2004). Anidación del aguililla negra (Buteogallus anthracinus Deppe, 1830) en el manglar de Sontecomapan, Catemaco, Veracruz, México. Madera y Bosques, Número especial, 2, 37–43. https://doi.org/10.21829/myb.2004.1031265
Barradas-García, H. H., & Morales-Mávil, J. E. (2007). Parental care of Buteogallus anthracinus (Deppe 1830) (Accipitridae) in the Mangrove of Sontecomapan, Catemaco, Veracruz, México. In K. L. Bilstein, D. R. Barber, & A. Zimmerman (Eds.), Neotropical raptors (pp. 1–8). Pensilvania: Hawk Mountain Sanctuary, Raptor Conservation Science Series No. 1.
BirdLife International. (2017). Buteogallus anthracinus (amended version of 2016 assessment). The IUCN Red List of Threatened Species 2017: e.T22735514A113552417. Downloaded on 22 July 2019. http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T22735514A113552417.en
BirdLife International. (2019) Species factsheet: Buteogallus anthracinus. Downloaded on 22/07/2019 from http://www.birdlife.org
Bojorges-Baños, J. C. (2011). Riqueza y diversidad de especies de aves asociadas a manglar en tres sistemas lagunares en la región costera de Oaxaca, México. Revista Mexicana de Biodiversidad, 82, 205–215. http://dx.doi.org/10.22201/ib.20078706e.2011.1.445
Bounas, A., Tsaparis, D., Gustin, M., Mikulic, K., Sarà, M., Kotoulas, G. et al. (2018). Using genetic markers to unravel the origin of birds converging towards pre-migratory sites. Scientific Reports, 8, 8326. https://doi.org/10.1038/s41598-018-26669-x
Busch, J. D., Katzner, T. E., Bragin, E., & Keim, P. (2005). Tetranucleotide microsatellites for aquila and haliaeetus eagles. Molecular Ecology Notes, 5, 39–41. https://doi.org/10.1111/j.1471-8286.2004.00823.x
Carmona-Díaz, G., Morales-Mávil, J. E., & Rodríguez-Luna, E. (2004). Plan de manejo para el manglar de Sontecomapan, Veracruz México: una estrategia para la conservación de sus recursos naturales. Madera y Bosques, 10, 5–23. http://doi.org/10.21829/myb.2004.1031263
De Labra, M. A., & Escalante, P. (2013). Diurnal raptors in Los Tuxtlas Biosphere Reserve, Mexico: current presence and relative abundance. Journal of Raptor Research, 47, 392–399. https://doi.org/10.3356/jrr-12-18.2
Ellegren, H. (1992). Polymerase-Chain-Reaction (PCR) analysis of microsatellites a new approach to studies of genetic relationships in birds. The Auk, 109, 886–895. https://doi.org/10.2307/4088163
Excoffier, L., & Lischer, H. E. L. (2010). Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Research, 10, 564–567. https://doi.org/10.1111/j.1755-0998.2010.02847.x
Funk, W. C., Mullins, T. D., & Haig, S. M. (2007). Conservation genetics of snowy plovers (Charadrius valexandrinus) in the Western Hemisphere: population genetic structure and delineation of subspecies. Conservation Genetics, 8, 1287–1309. https://doi.org/10.1007/s10592-006-9278-7
Gaur, A., Umapathy, G., Vasudevan, K., Sontakke, S., Rao, S., Goel, S. et al. (2017). Manual for biological sample collection and preservation for genetic, reproductive and disease analyses. New Delhi: Central Zoo Authority and Laboratory for the Conservation of Endangered Species (LaCONES) CSIR- Centre for Cellular and Molecular Biology.
Gebhardt, K. J., Brightsmith, D., Powell, G., & Waits, L. P. (2009). Molted feathers from clay licks in Peru provide DNA for three large macaws (Ara ararauna, A. chloropterus, and A. macao). Journal of Field Ornithology, 80, 183–192. https://doi.org/10.1111/j.1557-9263.2009.00221.x
González-Salazar, C. (2010). Ficha técnica de Buteogallus anthracinus. In P. Escalante-Pliego. (Comp.). Fichas sobre las especies de aves incluidas en Proyecto de Norma Oficial Mexicana PROY-NOM-059-ECOL-2000. Parte 1. Instituto de Biología, UNAM. Bases de datos SNIB-Conabio. Proyecto No. W007, México D.F.
Griffith, S. C., Owens, I. P. F., & Thuman, K. A. (2002). Extra pair paternity in birds: a review of interspecific variation and adaptive function. Molecular Ecology, 11, 2195–2212. https://doi.org/10.1046/j.1365-294X.2002.01613.x
Haig, S. M., Bronaugh, W. M., Crowhurst, R. S., D’Elia, J., Eagles-Smith, C. A., Epps, C. W. et al. (2011). Genetic applications in avian conservation. The Auk, 128, 205–229. https://doi.org/10.1525/auk.2011.128.2.205
Hailer, F., Gautschi, B., & Helander, B. (2005). Development and multiplex PCR amplification of novel microsatellite markers in the White-tailed Sea Eagle, Haliaeetus albicilla (Aves: Falconiformes, Accipitridae). Molecular Ecology Notes, 5, 938–940. https://doi.org/10.1111/j.1471-8286.2005.01122.x
Howell, S. N. G., & Webb, S. (1995). A guide to the birds of México and Central America. New York: Oxford University Press.
Hull, J. M., Tufts, D., Topinka, J. R., May, B., & Ernest, H. B. (2007). Development of 19 microsatellite loci for Swainson’s hawks (Buteo swainsoni) and other buteos. Molecular Ecology Notes, 7, 346–349. https://doi.org/10.1111/j.1471-8286.2006.01604.x
Johnson, P. C. D., Fowlie, M. K., & Amos, W. (2005). Isolation of microsatellite loci from the common buzzard, Buteo buteo (Aves: Accipitridae). Molecular Ecology Notes, 5, 208–211. https://doi.org/10.1111/j.1471-8286.2005.00878.x
Kalinowski, S. T. (2002). How many alleles per locus should be used to estimate genetic distances? Heredity, 88, 62–65. https://doi.org/10.1038/sj.hdy.6800009
Krakauer, A. H. (2008). Sexual selection and genetic mating system of wild turkeys. The Condor, 110, 1–12. https://doi.org/10.1525/cond.2008.110.1.1
Martínez-Cruz, B., David, V. A., Godoy, J. A., Negro, J. J., O´Brien, S. J., & Johnson, W. E. (2002). Eighteen polymorphic microsatellite markers for the highly endangered Spanish imperial eagle (Aquila adalberti) and related species. Molecular Ecology Notes, 2, 323–326. https://doi.org/10.1046/j.1471-8286.2002.00231.x
Martínez-Cruz, B., Godoy, J. A., & Negro, J. J. (2004). Population genetics after fragmentation: the case of the endangered Spanish imperial eagle (Aquila adalberti). Molecular Ecology, 13, 2243–2255. https://doi.org/10.1111/j.1365-294X.2004.02220.x
McClure, C. J. W., Westrip, J. R. S., Johnson, J. A., Schulwitz, S. E., Virani, M. Z., Davies, R. et al. (2018). State of the world’s raptors: Distributions, threats, and conservation recommendations. Biological Conservation, 227, 390–402. https://doi.org/10.1016/j.biocon.2018.08.012
Mendoza, E., Fay, J., & Dirzo, R. (2005). A quantitative analysis of forest fragmentation in Los Tuxtlas, southeast Mexico: patterns and implications for conservation. Revista Chilena de Historia Natural, 78, 451–467. https://doi.org/10.4067/s0716-078X2005000300008
Mira, S., Wolff, K., & Cancela, L. (2005). Isolation and characterization of microsatellite markers in Bonelli’s eagle (Hieraaetus fasciatus). Molecular Ecology Notes, 5, 493–495. https://doi.org/10.1111/j.1471-8286.2005.00967.x
Müller, W., Epleen, J. T., & Lubjuhn, T. (2001). Genetic paternity analyses in little Owls (Athene noctua): does the high rate of parental care select against extra-pair young? Journal of Ornithology, 142, 195–203. https://doi.org/10.1046/j.1439-0361.2001.00069.x
NatureServe. (2019). Nature serve’s annual report championing biodiversity with science and technology. Explore our illustrated map of at-risk species. Retrieved on July/22/2019 from: http://explorer.natureserve.org/servlet/NatureServe?searchSciOrCommonName=Buteogallus%20anthracinus
Nesje, M., Roed, K. H., Bell, D. A., Lindberg, P., & Lifjeld, J. T. (2000). Microsatellite analysis of population structure and genetic variability in peregrine falcons (Falco peregrinus). Animal Conservation, 3, 267–275. https://doi.org/10.1111/j.1469-1795.2000.tb00112.x
Ornelas, J. F., Sosa, V., Soltis, D. E., Daza, J. M., González, C., Soltis, P. S. et al. (2013). Comparative phylogeographic analyses illustrate the complex evolutionary history of threatened cloud forests of Northern Mesoamerica. Plos One, 8, e56283. https://doi.org/10.1371/journal.pone.0056283
Ortiz-Pulido, R., Gómez-de Silva, H., González-García, F., & Álvarez, A. (1995). Avifauna del centro de investigaciones costeras La Mancha, Veracruz, México. Acta Zoológica Mexicana, 66, 87–118. http://azm.ojs.inecol.mx/index.php/azm/article/view/1662/1776
Palumbi, S. R. (1996). Nucleic acids II: the polymerase chain reaction. Ch. 7. In D. M. Hillis, C. Moritz, & B. K. Mable (Eds.), Molecular systematics. Sunderland, Massachusetts: Sinauer Associates.
Peakall, R., & Smouse, P. E. (2006). GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Molecular Ecology Notes, 6, 288–295. https://doi.org/10.1111/j.1471-8286.2005.01155.x
Peterson, R. T., & Chalif, E. L. (1989). Aves de México, guía de campo. México D.F.: Ed. Diana.
Rice, W. R. (1989). Analyzing tables of statistical tests. Evolution, 43, 223–225. https://www.jstor.org/stable/2409177?seq=1#page_scan_tab_contents
Rodríguez-Estrella, R., & Brown, B. T. (1990). Density and habitat use of raptors along the Rio Bavispe and Rio Yaqui, Sonora, Mexico. Journal of Raptor Research, 24, 47–55.
Rudnick, J. A., Katzner, T. E., & Bragin, E. A. (2005). Using naturally shed feathers for individual identification, genetic parentage analyses, and population monitoring in an endangered Eastern imperial eagle (Aquila heliaca) population from Kazakhstan. Molecular Ecology, 14, 2959–2967. https://doi.org/10.1111/j.1365-294X.2005.02641.x
Rudnick, J. A., Katzner, T. E., Bragin, E. A., & Dewoody, J. A. (2008). A non-invasive genetic evaluation of population size, natal philopatry, and roosting behavior of non-breeding Eastern imperial eagles (Aquila heliaca) in central Asia. Conservation Genetics, 9, 667–676. https://doi.org/10.1007%2Fs10592-007-9397-9
Sarasola, J. H., Canal, D., Solaro, C., Zanón-Martínez, J. I., Galmes, M. A., & Negro, J. J. (2012). Cross-species amplification of seventeen polymorphic microsatellite loci in the endangered Crowned Eagle (Harpyhaliaetus coronatus). Molecular Ecology Resources, 12, 779–779.
Schennel, H. (1994). Common Black-Hawk (Buteogallus anthracinus). In A. Poole, & F. Gill (Eds.), The birds of North America, No 122 (pp. 1–19). Philadelphia: The Academy of Natural Sciences, Washington D.C./ The American Ornithologists´ Union.
Semarnat (Secretaría del Medio Ambiente y Recursos Naturales). (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental – Especies nativas de México de flora y fauna silvestres – Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio – Lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010, Segunda Sección, México.
Sibley, D. A. (2000). The Sibley guide to birds. National Audubon Society. New York: Ed. Knopf.
Sorenson, M. D., Ast, J. C., Dimcheff, D. E., Yuri, T., & Mindell, D. P. (1999). Primers for a PCR-Based Approach to Mitochondrial Genome Sequencing in Birds and Other
Vertebrates. Molecular Phylogenetics and Evolution, 12, 105–114. https://doi.org/10.1006/mpev.1998.0602
Taberlet, P., & Bouvet, J. (1991) A single plucked feather as a source of DNA for bird genetic studies. The Auk, 108, 959–960. https://sora.unm.edu/sites/default/files/journals/auk/v108n04/p0959-p0960.pdf
Texas Parks, & Wildlife. (2019). Federal and State Listed Birds in Texas. Retrieved on July/22/2019 from: https://tpwd.texas.gov/huntwild/wild/wildlife_diversity/nongame/listed-species/birds.phtml
USFWS (United States Fish and Wildlife Service). (2013). Migratory bird treaty act protected species (10.13 List) (http://www.fws.gov/birds/management/managed-species/migratory-bird-treaty-act-protected-species.php). (Accessed March 24th, 2016).
Van Oosterhout, C., Hutchinson, W. F., Willis, D. P. M., & Shipley, P. (2004). MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Molecular Ecology Notes, 4, 535–538. https://doi.org/10.1111/j.1471-8286.2004.00684.x
Vázquez-Pérez, J. R., Enríquez, P. L., & Rangel-Salazar, J. L. (2009). Diversidad de aves rapaces diurnas en la Reserva de la Biosfera Selva El Ocote, Chiapas, México. Revista Mexicana de Biodiversidad, 80, 203–209. http://doi.org/10.22201/ib.20078706e2009.001.575
Woolaver, L. G., Nichols, R. K., Morton, E. S., & Stutchbury, B. J. M. (2013). Population genetics and relatedness in a critically endangered island raptor, Ridgway’s Hawk Buteo ridgwayi. Conservation Genetics, 14, 559–571. https://doi.org/10.1007%2Fs10592-013-0444-4