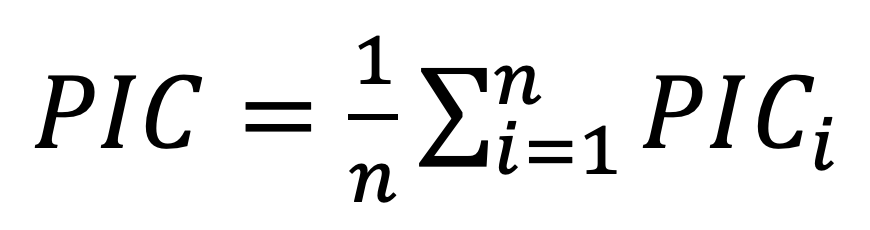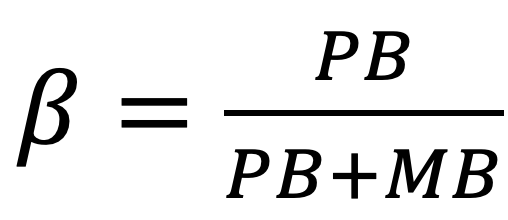Mónica Figueroa-Cabañas, Rolando T. Bárcenas *
Universidad Autónoma de Querétaro, Campus Aeropuerto, Facultad de Ciencias Naturales, Laboratorio Darwin de Sistemática Filogenética y Biogeografía Evolutiva, Campus Aeropuerto, 76140 Querétaro, Querétaro, Mexico
*Corresponding author: rtenoch@uaq.mx (R.T. Bárcenas)
Received: 13 January2023; accepted: 21 July 2023
Abstract
Three main genetic diversity hotspots were identified in a survey of the genetic variability of the populations of the candy barrel cactus Echinocactus platyacanthus, a protected and highly sought-after cacti for ornamental, culinary and livestock water source in the Chihuahuan Desert Region, Mexico. This study identified one southern, one central, and one northern population in the Chihuahuan Desert Region as first priorities for conservation based on the analysis of a matrix of 4 ISSRs for 183 individuals from 10 localities along the 900 × 300 air km polygon of the geographic distribution of the species. The genetic structure of the 183 individuals from the 10 populations studied, showed a high degree of genetic differentiation for each of the localities with low gene flow, making each of these populations important for conservation actions. The isolation by distance analysis showed that other factors apart from the geographic distance could be playing an important role in the differentiation of the genetic structure of the populations. The UPGMA tree derived from the genetic distance matrix showed that the central and the southern populations conform a mega genetic population clearly separated from the northern populations, each with its own genetic signature.
Keywords: Ex situ conservation; Seed banks; Cactaceae; Molecular markers; Arid regions
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Genética de la conservación de la biznaga de dulce protegida Echinocactus platyacanthus en la Región del Desierto Chihuahuense
Resumen
Se identificaron 3 centros de alta diversidad genética de la biznaga Echinocactus platyacanthus en el desierto Chihuahuense mexicano. Esta especie en categoría de protección especial, es apreciada por su valor ornamental, como fuente de agua para el ganado caprino y por ser fuente del dulce de acitrón. Este estudio identificó una población del sur, una del centro y una del norte del desierto Chihuahuense como las prioridades para la conservación biológica basado en 4 marcadores moleculares para 183 individuos de 10 localidades en un área de casi 900 × 300 km lineales. La estructura genética de los 183 individuos de las 10 poblaciones estudiadas exhibió un alto grado de diferenciación para cada una de las localidades, así como un bajo flujo génico, haciendo a cada una de estas poblaciones relevante para la conservación. El análisis de aislamiento por distancia mostró que pueden existir otros factores diferentes a la distancia geográfica que podrían estar jugando un papel importante en la diferenciación genética de las poblaciones. El árbol UPGMA derivado de una matriz de distancias mostró que las poblaciones del centro y las del sur conforman una megapoblación genéticamente separada de la megapoblación del norte, cada una con su propia firma genética.
Palabras clave: Conservación ex situ; Bancos de semillas; Cactaceae; Marcadores moleculares; Zonas áridas
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The candy barrel cactus Echinocactus platyacanthus is a barrel-like cactus with a high cultural, economic, and scientific value that according to the Intenational Union for Conservation of Nature (IUCN) was considered as vulnerable (as in Bárcenas, 2003). However, the information derived from an in depth conservation study of the complete family has moved the species to a near threatened (IUCN-Intenational Union for Conservation of Nature, 2022) category (Goettsch et al., 2015). The populations of E. platyacanthus are declining due to over collection of adult individuals and change in land use for livestock farming and ranching. Also, many plants are highly sought after for multipurpose uses, ranging from ornamental practises to human consumption. At the national level, E. platyacanthus is a protected native barrel cactus that can reach up to 2 metres in height. Juvenile plants are well protected from vertebrate herbivores under a layer of several thick and stiff spines making them fairly unreachable to predators. The flowers and fruits develop from an apical disk covered by thick layers of translucent trichomes and some spines and the fruit develops several black seeds from 3 to 4 mm in length that behave as orthodox seeds (Fig. 1). Its inclusion in the Mexican SEMARNAT Conservation Act (Semarnat, 2010) under the special protection category is due to the heavy exploitation as a candy source or “acitrón” (del Castillo & Trujillo, 1991), its use as an ornamental in landscaping (Bárcenas, 2003), its illegal trade as seedlings and seeds, and other anthropogenic uses (Goettsch et al., 2015). In the wild, middle sized plants are cut open by its apices and left for the cattle and goats to eat the inner tissues since this is one of the main source of food in the dry season (del Castillo & Trujillo, 1991). Some plants can regrow new stems from the damaged tissues and sprout new ramifications from the base but other cannot grow new stems and the plant eventually dies. Older plants are mainly eaten by goats, donkeys, or by other vertebrate herbivores (Bárcenas et al., 2022), from the basal region where older spines have become soft and if the apical region is damaged new smaller apical abnormal stems regrow from the disk (Fig. 1). Used as a candy, the “acitrón” is made from the parenchymatic tissues mainly of the oldest specimens, where the whole plant is cut into wide pieces. Spines, epidermal and hypodermal tissues are removed, and parenchyma is included into a crystallisation process to eventually produce small bars of candy that are sold to confectionaries, bakeries and distributed to various local markets for consumers. Although, E. platyacanthus is not the only species used for “acitrón” since other Echinocactus, Ferocactus (Fig. 1) and many tall and wide Mammillaria species are also used for this purpose, the market is mainly filled with “acitrón” from E. platyacanthus. Some candy cactus producers are registered with the national environmental authorities as legal producers of “acitrón” but the great majority of the candy comes from the unregulated trade of this species. As an ornamental, E. platyacanthus had a high availability by country but a middle availability by nursery (Bárcenas, 2003), this is, it was very common at the national level but not every nursery in the country would sell the plants. Also, it has been identified that wild collected specimens can be found in the national trade (Bárcenas, 2003). Also, E. platyacanthus played an important role in some original cultures like the Aztec people who recreated one of these barrel cacti in stone as part of a sacrificial altar
(Megged, 2010).
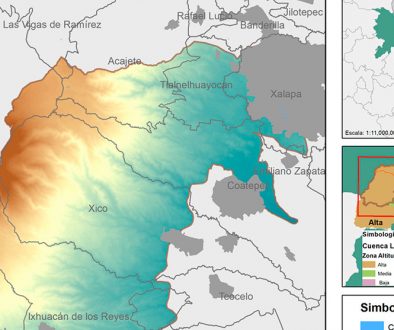
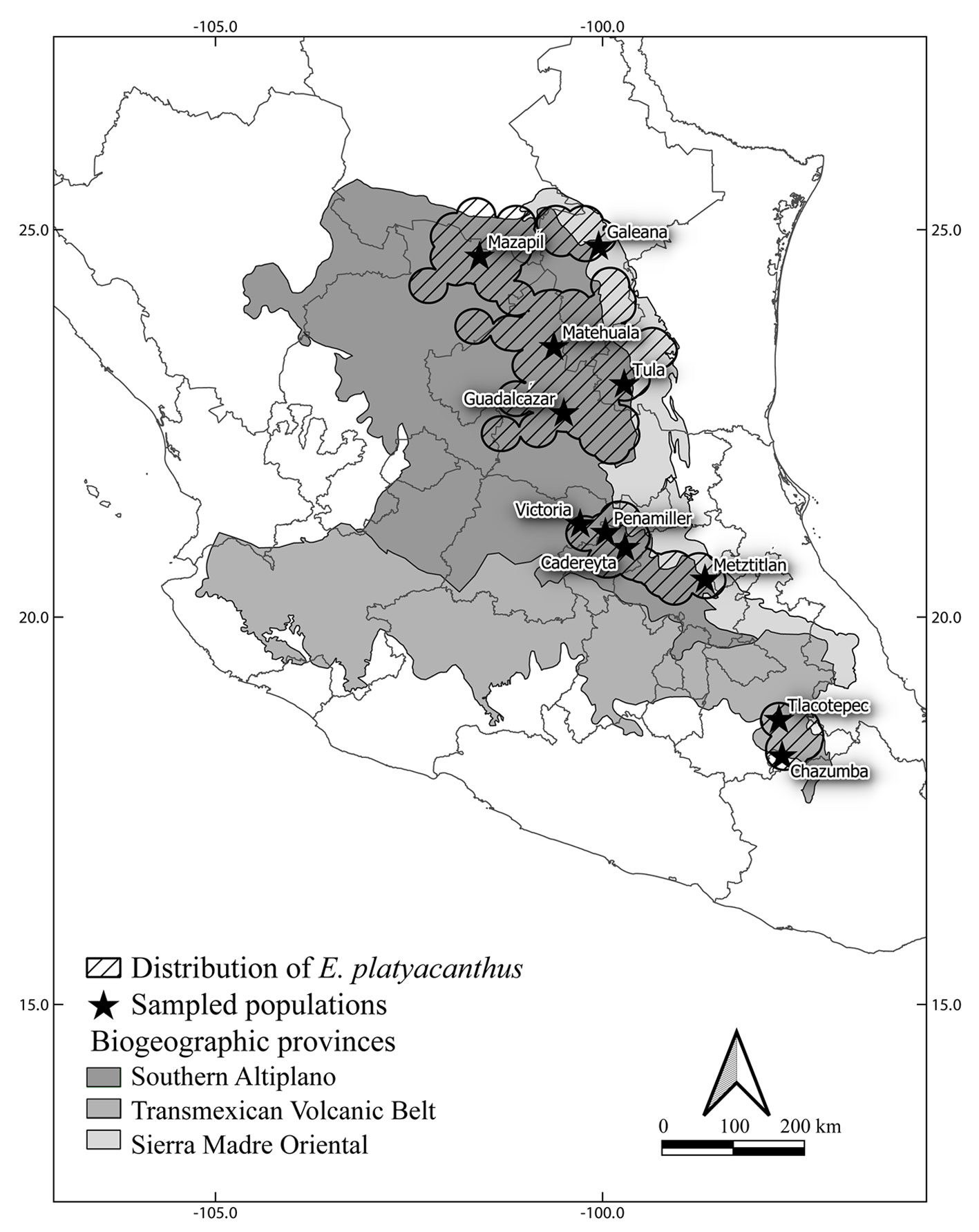
Echinocactus platyacanthus has a disjunct distribution at the north and south sides of the Transmexican Volcanic Belt and finds its eastern distribution limit at the base of the Sierra Madre Oriental in central Mexico in the eastern portions of the Chihuahuan Desert Region. Figure 2 shows the distribution of E. platyacanthus in Mexico (hatched polygons) from Hernández and Gómez-Hinostrosa (2011), the localities sampled in this study (black stars), the biogeographic provinces of the Southern Altiplano (darkest grey) in the Mexican portion of the Chihuahuan Desert Region including the Tehuacán-Cuicatlán Valley (southern limits of the disjunct Chihuahuan Desert Region), the Sierra Madre Oriental province (lightest grey polygon to the east) and the Transmexican Volcanic Belt (half grey polygon in the middle). The north to south geographic distribution of the species reaches some 900 air km and its width reaches approximately 300 air km at its widest northern limits.
Molecular variability of the populations of E. platyacanthus have not been addressed in a wide national survey and only few studies have included some samples of the species for phylogenetic reconstruction (Bárcenas et al., 2011; Hernández-Ledesma & Bárcenas, 2017; Hernández et al., 2011; Vargas-Luna et al., 2018). Also, in the Tehuacán-Cuicatlán Valley, Jiménez-Sierra et al. (2007) found that the genetic diversity of the populations is low and that only 23.5% of the loci were polymorphic with a deficit of heterozygotes. The problem with the conservation actions of widely distributed species is fundamentally different from geographically restricted species. In situ conservation of geographically restricted taxa could be achieved generating a small network of natural reserves in order to complement ex situ conservation of species in any particular region (Hernández & Bárcenas, 1996). However, the wide geographic distribution of some species, such as E. platyacanthus, raises methodological questions about the implementation of the most efficient strategy to maximise the product of conservation actions, both in situ and ex situ given the wide geographic area to be covered. The molecular diversity and variability of highly endangered species should illuminate conservation efforts in order to maximise the permanently limited resources allocated for protecting the species and their populations. The genetic diversity both at the intra and the inter population levels are crucial for the survival of the species through time. Having a clear and quantitative measure of the diversity of the populations could direct in situ and ex situ conservation actions, providing the basis for the design of conservation programs (Frankham et al., 2004). Also, international import-export trade and custom clearance could be expedited if the provenance of a plant could be known with certainty (Bárcenas, 2003); forensic investigations could demonstrate the origin of the biological samples and specific in situ conservation efforts could benefit from ex situ seed banks safeguarding the highest genetic diversity and maximising phylogenetic diversity (Prado et al., 2010; Yesson et al., 2011).

The goals of this project were to map the genetic diversity of the populations of E. platyacanthus at the national level identifying the populations with the highest genetic diversity in order to prioritise the conservation status of their populations for in situ conservation and to generate an active seed collecting plan to create ex situ conservation actions for seed and biobank reservoir.
Materials and methods
Tissue samples for DNA extractions were taken from the middle region of a healthy photosynthetic rib with a metal core borer sampler of 1.5 cm in diameter sterilising the sampler with 70% ethanol and a cotton ball before and after every sample was taken. Samples were taken under the Mexican authorisation SGPA/DGVC/01408. One voucher specimen per each of the 10 sampled populations were deposited at QMEX (Fig. 2). Tissue samples were immediately labelled and dried in 2 or 3 successive changes of silica gel indicator and stored until further use. Samples were taken from 20 randomly selected individuals per locality separated at least 25 m from each other in a ca. 1 km long × 10 m wide transect per population.
Because of the wide geographic distribution area of the species, 3 geographic hypotheses were tested in order to know if there is more than 1 genetic population. Three different analyses to test these assumptions were run. The first analysis, tested the diversity of the 10 localities to quantify their inter and their intrapopulational genetic diversity; the second analysis grouped the 10 localities into 3 different geographic populations, southern, northern and central, in partial congruence with the distribution of the 3 forms described by Bravo and Sánchez-Mejorada (1991), while the third analysis divided the 10 populations into 2 megapopulations, the northern and the southern, as a simplified version of the previous proposed distribution.
DNA extractions were done following published procedures from previous molecular studies with a 2x CTAB extraction protocol (Bárcenas, 2016; Bárcenas et al., 2011). Amplifications were carried out by duplicates in a 10 µl PCR reaction containing 1 µl of 10X DreamTaq™, 0.2 µl of dNTPs (10 µM each), 0.5 µl of primer (10 µM), 0.062 µl of DreamTaq™ and nuclease free water. Ten random ISSR primers, developed by the University of British Columbia (UBC), were screened given that they have been used in other cacti studies (Bustamante et al., 2016; García-Morales et al., 2018; Valadez-Moctezuma et al., 2015), and 4 of them were selected based on the amount of information they provided and their reproducibility. ISSR primer names and sequences with their corresponding annealing temperatures are as follow (UBC name, primer sequence and annealing temperature): UBC-879, (CTTCA)3, 43˚ C; UBC-868, (GAA)6, 43˚ C; UBC-840, (GA)8 CT, 50˚ C and UBC-814, (CT)8 A, 50˚ C. PCR conditions for the ISSRs were an initial denaturation cycle of 94° C for 1.5 minutes followed by 40 cycles of 94 °C for 40 seconds, the corresponding annealing temperatures for 40 seconds and 72 °C of extension for 1.5 minutes with a final cycle of 72 °C for 7 minutes to end reactions holding samples at 4 °C for 10 minutes.
The number of samples for the ISSRs were mostly of 20 individuals per population, except in Querétaro (n = 8) and Hidalgo (n = 15) as shown in Table 1. The ISSRs were run in duplicated agarose gels of 1.5% stained with SYBR™ Safe at 75 V for 4 hours, visualised in a Safe Imager™ 2.0 and photographed with a digital camera Canon G10. Only clearly visible bands present in both gels were recorded as presence. ISSR profiles for each individual were registered as a presence/absence character. Agarose gels produced a variable number of bands per each ISSR and these were categorised as monomorphic bands (MB), when 100% of the bands where present in every sampled individual in each ISSR. Polymorphic bands (PB) where defined as present in 1% to 99% of the sampled individuals. Rare bands (RB), where defined as those bands that were present in less than 15% of the sampled individuals. Frequent bands (FB) where defined as those bands present in more than 15% and less than 70% of the sampled individuals. Common bands (CB) were defined as those present in more than 70% of the individuals.
A reproducibility test was developed in order to test the reproducibility of the ISSR bands considering that success in the amplifications was defined as bands being present or absent in both replicated gels. Following this definition, the success index was calculated for each of the bands. This band success index was averaged for all the bands in each ISSR marker and this was considered as a measure of reproducibility of the process for each of the markers.
The polymorphic information content (PIC) for each ISSR was calculated as follows.
where “n” is the number of observed bands for each marker;
fi is the relative frequency of the i-th amplified allele (band present); and 1 ‒ fi is the frequency of null alleles. This parameter is the degree of information for each marker, it is the average of the frequencies of the detected heterozygous in each ISSR.
The effective multiplex ratio (EMR) was calculated with EMR=n*β, where “n” is the average number of amplified bands for each individual for every ISSR and β is the percent of polymorphic bands for each ISSR
The marker index (MI) was calculated as MI=PIC* EMR. This index is a measurement of the capacity of the marker to detect polymorphic loci in the system. The resolution power for each ISSR was calculated using RP= ∑Ib, where Ib=1-(2*|0.5-pi|). The value of pi is the proportion of the samples that have the i-th band.
POPGENE v. 1.32 (Yeh et al., 1999) was used to estimate the genetic variability within localities and populations with the count of observed alleles, the number of effective alleles, Nei’s genetic diversity, Shannon’s information index, the number of polymorphic loci and the percent of polymorphic loci. Nei’s genetic differentiation index (Gst) and gene flow among populations (Nm) was also calculated with POPGENE. The analysis of molecular variance (AMOVA) was developed with GenAlEx 6.5 (Peakall & Smouse, 2012) with 999 permutations to measure the partition of the genetic variation among regions and within and among populations and localities.
The deeper analysis of the population genetic structure was developed with a Bayesian approach using STRUCTURE v. 2.3.3 (Pritchard et al., 2000) in order to explore different grouping possibilities among the populations. The analysis was run with an ADMIXTURE model and the correlated allele frequencies were used with every run and a burnin of 1,000 and 100,000 Markov chains Monte Carlo replications. For every K value (genetically different number of groups) 10 replicate runs were developed and the optimal number of groups was selected using Structure Harvester v. 6.0 (Evanno et al., 2005).
Isolation by distance was tested by a regression analysis of the Nei’s genetic distance matrix (D) among every population pair versus their geographic distance in kilometres. The significance test was developed with a Mantel test based on 10,000 iterations of the matrix using VEGAN (Oksanen et al., 2022). The PCoA with ade4 and Euclidian distances allowed visualising the relationships among the different ISSR phenotypes of a distance matrix creating an UPGMA tree with TFPGA ( Dray & Dufour, 2007; Miller, 1997). The bootstrap values were derived from 10,000 resampling permutations with replacement of all the loci.
Results
Table 2 shows the reproducibility index and the number of bands amplified for each ISSR and the classification of each type of band into the previously defined categories, PB (polymorphic bands) or MB (monomorphic bands) and the descriptive statistics for each ISSR. The ISSRs had a high reproducibility index with ISSR-879 having the lowest reproducibility index (90.73) and ISSR-868 the highest reproducibility index (96.71). ISSR-840 had the highest number of bands with 31 but only had 83.87% of polymorphism while ISSR-868 had 19 bands but 94.74% of polymorphism and ISSR-814 had 92% of polymorphism. ISSR-879 only had 68.18% of polymorphic bands.
Table 1
Echinocactus platyacanthus sampled populations (state and locality), population identifier (sample), elevation (in metres), number of different individuals (n ISSR) tested for the molecular markers produced in this study and collection number of the vouchers deposited at QMEX. NB. In order to reduce the noise for a low number of individuals, samples from C3 and C5 were combined into one population and treated as the Querétaro sample due to the damage of various samples from the 2 localities.
| State | Locality | Sample | Elevation (m) | n ISSR | Voucher No. |
| Nuevo León | Galeana | C15 | 1,550 | 20 | M. Figueroa No. 15 |
| Tamaulipas | Tula | C14 | 1,200 | 20 | M. Figueroa No. 14 |
| Zacatecas | Mazapil | C11 | 2,199 | 20 | M. Figueroa No. 11 |
| San Luis Potosí 1 | Guadalcázar | C17 | 1,584 | 20 | M. Figueroa No. 17 |
| San Luis Potosí 2 | Matehuala | C16 | 1,490 | 20 | M. Figueroa No. 16 |
| Guanajuato | Victoria | C1 | 1,990 | 20 | M. Figueroa No. 1 |
| Querétaro | Cadereyta and Peñamiller | C3 and C5 | 1,942 and 1,156 | 8 | M. Figueroa No. 3 and 5 |
| Hidalgo | Metztitlán | C13 | 1,350 | 15 | M. Figueroa No. 13 |
| Puebla | Tlacotepec | C18 | 2,000 | 20 | M. Figueroa No. 18 |
| Oaxaca | Chazumba | C20 | 1,800 | 20 | M. Figueroa No. 20 |
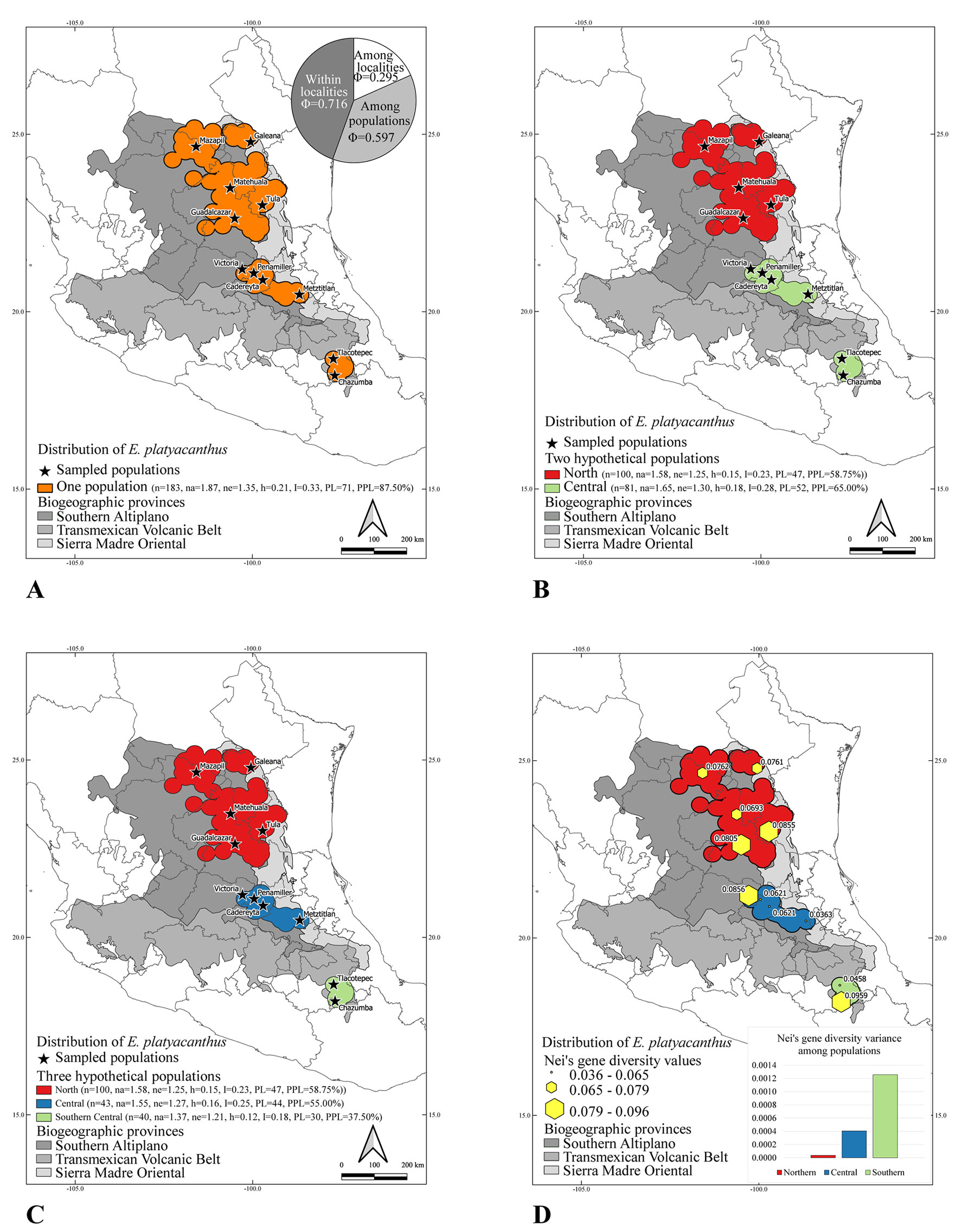
The first genetic analysis tested the ISSR intrapopulational genetic diversity for the 10 localities from which the ISSR markers were generated (Table 3). The highest Nei’s gene diversity is found in the Chazumba population (0.0959) followed by Victoria (0.0856) and Tula (0.0855). After these 3 populations, Nei’s gene diversity values range from moderate (0.0805 for Guadalcázar) to low values as the ones found in Tlacotepec (0.0458) and Metztitlán (0.0363) which are almost half of the highest diversity values found for the most diverse populations. Although the Nei’s gene diversity values closely correlate with the values of the percent of polymorphic loci, the most polymorphic population was Tula with 28.75%, followed by Chazumba (27.50%), and Victoria (26.25%). Three populations, Cadereyta, Tlacotepec, and Metztitlán, have less than 20% of polymorphic loci in their populations.
The second genetic analysis, tested the hypothesis that there were 3 populations, the northern, the southern, and the central populations. In this analysis the 10 localities were grouped as shown in Table 4 with their respective diversity indices. The central population had the highest Nei’s diversity index (h = 0.166), followed by the northern population (h = 0.154), and the southern population with the lowest Nei’s gene diversity (h = 0.125). The highest number of polymorphic loci was the northern population (47) followed by the central population with 44 and then the southern population with 30 polymorphic loci.
Table 2
ISSR statistics of the sampled individuals of Echinocactus platyacanthus in Mexico.
| ISSR marker | ISSR-879 | ISSR-868 | ISSR-840 | ISSR-814 |
| Reproducibility index | 90.73 | 96.71 | 94.48 | 95.95 |
| Total of bands | 22 | 19 | 31 | 25 |
| Polymorphic bands (PB) | 15 | 18 | 26 | 23 |
| Monomorphic bands (MB) | 7 | 1 | 5 | 2 |
| Proportion of polymorphic bands | 0.68 | 0.94 | 0.83 | 0.92 |
| Percent of polymorphic bands | 68.18 | 94.74 | 83.87 | 92.00 |
| Polymorphic information content (PIC) | 0.15 | 0.24 | 0.20 | 0.18 |
| Effective multiplex ratio (EMR) | 5.98 | 8.11 | 8.89 | 6.59 |
| Marker index (MI) | 0.88 | 1.95 | 1.78 | 1.19 |
| Resolution power (RP) | 4.26 | 6.82 | 9.34 | 6.28 |
The third analysis tested the 2 megapopulations hypothesis grouping the 10 localities into the southern and the northern megapopulations as shown in Table 5. The southern megapopulation was the most diverse (h = 0.182) and with the highest number of polymorphic loci (PPL = 65%). The northern megapopulation had slightly lower values of diversity (h = 0.154) as well as a lower number of polymorphic loci (58.75%).
The spatial distribution of the genetic diversity among and between populations is heterogeneous. The genetic diversity of the populations in the north is more evenly distributed among their populations than in either of the central or the southern populations. The northern population has Tula as the main centre of diversity and the rest of the Northern populations do not show a pronounced difference, while the difference between the most diverse population in either the southern and the central populations is more extreme (Table 3).
Table 3
ISSR intrapopulational genetic diversity of 10 geographic populations of Echinocactus platyacanthus in arid Mexico.
| Locality (code) | Population category | n | na | ne | h | I | PL | PPL |
| Chazumba (C20) | S | 20 | 1.2750 (0.4493) | 1.1707 (0.3342) | 0.0959 (0.1784) | 0.1416 (0.2547) | 22 | 27.50% |
| Victoria (C1) | C | 20 | 1.2625 (0.4428) | 1.1388 (0.2712) | 0.0856 (0.1561) | 0.1311 (0.2319) | 21 | 26.25% |
| Tula (C14) | N | 20 | 1.2875 (0.4555) | 1.1433 (0.2913) | 0.0855 (0.1600) | 0.1312 (0.2331) | 23 | 28.75% |
| Guadalcázar (C16) | N | 20 | 1.2625 (0.4428) | 1.1324 (0.2746) | 0.0805 (0.1540) | 0.1239 (0.2267) | 21 | 26.25% |
| Mazapil (C11) | N | 20 | 1.2375 (0.4282) | 1.1319 (0.2920) | 0.0762 (0.1594) | 0.1144 (0.2302) | 19 | 23.75% |
| Galeana (C15) | N | 20 | 1.2250 (0.4202) | 1.1289 (0.2803) | 0.0761 (0.1565) | 0.1146 (0.2282) | 18 | 22.50% |
| Matehuala (C17) | N | 20 | 1.2375 (0.4282) | 1.1148 (0.2623) | 0.0693 (0.1467) | 0.1066 (0.2152) | 19 | 23.75% |
| Cadereyta (C3 & C5) | C | 8 | 1.1625 (0.3712) | 1.1099 (0.2776) | 0.0621 (0.1505) | 0.0913 (0.2166) | 13 | 16.25% |
| Tlacotepec (C18) | S | 20 | 1.1375 (0.3465) | 1.0737 (0.2051) | 0.0458 (0.1221) | 0.0698 (0.1826) | 11 | 13.75% |
| Metztitlán (C13) | C | 15 | 1.1500 (0.3593) | 1.0564 (0.1754) | 0.0363 (0.1049) | 0.0580 (0.1585) | 12 | 15.00% |
| All populations | 183 | 1.875 (0.3328) | 1.3509 (0.3546) | 0.2127 (0.1843) | 0.332 (0.2516) | 71 | 87.50% |
Population categories: N = northern, C = central, S = Ssouthern; n = number of individuals analysed; na = number of observed alleles; ne = number of effective alleles (Kimura & Crow, 1964); h = Nei’s gene diversity (Nei, 1973); I = Shannon’s information index (Lewontin, 1972); PL= polymorphic loci; PPL = percent of the polymorphic loci. Values in parenthesis show standard deviation.
Figure 3 shows the graphic representation of the different population analyses with the results of treating the 10 localities as independent of each other with their genetic parameters. The analysis of molecular variance illustrates the high genetic differentiation of each of the populations (Phi = 0.716 for within localities and a Phi = 0.295 for among localities). Also, Figure 3 shows the different partitions of the genetic diversity according to the corresponding geographic scenario of assuming 2 megapopulations, the northern and the central + southern populations as well as the 3 independent megapopulations, and the evenness of the distribution of the genetic diversity (Nei’s diversity values) over the entire geographic distribution of the species. The graph shows that the northern populations (red bar of the histogram) have the smallest of the 3 genetic variances, followed by the central populations (blue bar of the histogram) and finally the southern populations (green bar of the histogram) that has a much higher genetic variance as can be also seen in the yellow hexagons of the map. The genetic differentiation value (Gst) was estimated as 0.6678 while the average gene flow (Nm) between populations was estimated as 0.2487. The genetic structure inferred with STRUCTURES showed that the optimal number of genetic populations is 8, which is consistent with the high genetic differentiation of the populations and the low gene flow among them.
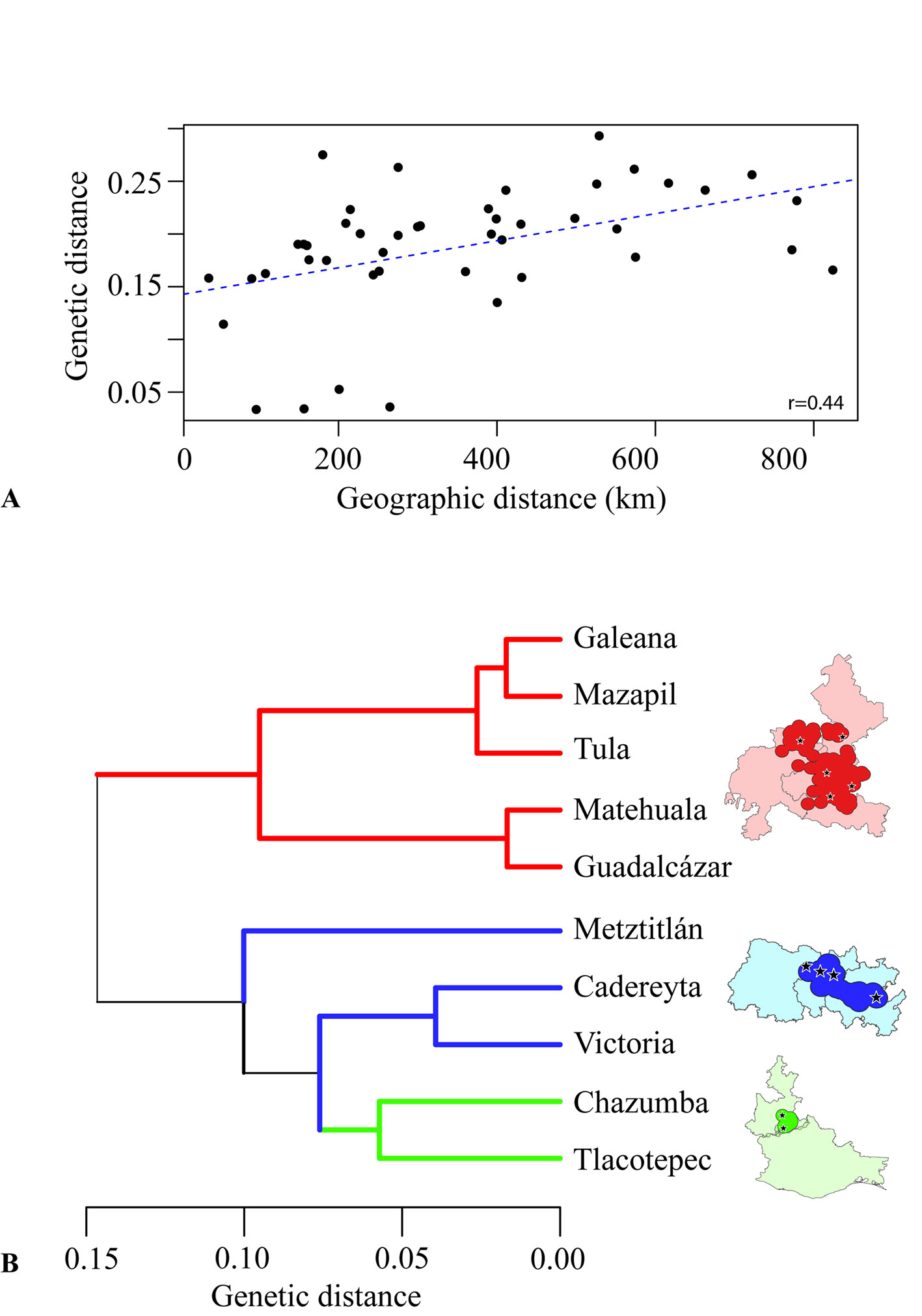
The Mantel test (Table 6) developed with the 10 populations quantified the effect of the geographic distance and the genetic isolation of the populations showing a moderate correlation (r = 0.44). This correlation suggests that different factors apart from the geographic distance are explaining the genetic diversity of the populations (Fig. 4). Although isolation by distance shows a moderate correlation, the 10 populations can be grouped according to their genetic distances into 2 main groups (Fig. 4). These 10 populations can be separated into 2 major genetic groups, the northern megapopulation and the south-central megapopulation. The northern population is also subdivided into the Matehuala-Guadalcázar group and the Galeana-Mazapil + Tula group. The second group to the northern population group is made of Metztitlán as external to a core central group (Cadereyta-Victoria) and a southern group made of Chazumba-Tlacotepec.
Table 4
Interpopulation genetic diversity of 3 geographic populations of Echinocactus platyacanthus in arid Mexico.
| Locality | n | na | ne | h | I | PL | PPL |
| Northern population
(Tula, Guadalcázar, Mazapil, Galeana, and Matehuala) |
100 | 1.5875 (0.4954) | 1.2544 (0.3412) | 0.1540 (0.1841) | 0.2395 (0.2625) | 47 | 58.75% |
| Central population
(Cadereyta, Victoria, and Metztitlán) |
43 | 1.5500 (0.5006) | 1.2763 (0.3438) | 0.1669 (0.1888) | 0.2554 (0.2723) | 44 | 55.00% |
| Southern population
(Chazumba and Tlacotepec) |
40 | 1.3750 (0.4872) | 1.2143 (0.3362) | 0.1256 (0.1874) | 0.1879 (0.2711) | 30 | 37.50% |
| Three populations | 183 | 1.8750 (0.3328) | 1.3509 (0.3546) | 0.2127 (0.1843) | 0.3320 (0.2516) | 70 | 87.50% |
n = Number of individuals analysed; na = number of observed alleles; ne = number of effective alleles (Kimura & Crow, 1964); h = Nei’s gene diversity (Nei, 1973); I = Shannon’s information index (Lewontin, 1972); PL = polymorphic loci; PPL = percent of the polymorphic loci. Values in parenthesis show standard deviation.
Discussion
Despite the wide geographic amplitude in the distribution of E. platyacanthus in the central and southern Chihuahuan Desert Region, the different populations have an internal genetic structure that is not explained exclusively by its geographic distance. Several other biotic and abiotic factors must be determining the genetic structure of these populations. It is possible that biotic factors such as foraging habits of pollinators and dispersal capacities might be affecting the Echinocactus platyacanthus populations (Kramer et al., 2011). Also, phenological differences such as flowering times could have a direct impact on the populations (Peters & Weis, 2019). Biogeographic barriers both climatic and geographic can alter gene flow among the populations of one species (O’Dwyer et al., 2021). Particularly in the central and southern populations, the stark contrast in the genetic diversity values among the richest population and the rest of the populations is extreme. Chazumba in the south had the highest diversity (0.0959) of all the localities studied, but Tlacotepec with almost half of the diversity value (0.0458) of Chazumba, had the second lowest diversity, even when the air distance between these 2 localities is only of some 50 km. Although some localities are included in national protected areas such as Metztitlán, the genetic diversity of those populations were not high, demonstrating that the creation of those reserves do not necessarily protect a high genetic diversity, at least as in the case of Echinocactus platyacanthus.
Based on the results of these analyses, 3 genetic hotspots are recognised among the northern, central and southern populations, which have their unique genetic signatures and their own conservation priorities. Between each of these 3 populations, the locality with the highest Nei’s genetic diversity has been chosen as the first priority for conservation and then prioritising the remaining populations in descending order. Chazumba has been here recognised as the first priority for conservation in the southern population since it has the highest Nei’s gene diversity index (0.095) of all the populations. Victoria is also recognised as the second priority for conservation and as a first priority in the central population since it has the second highest Nei’s gene diversity with 0.085. The Northern population has Tula as the first priority for conservation with a Nei’s gene diversity of 0.085 and is also the third highest diversity index on all the populations (Table 3). This prioritisation has an important practical ex situ conservation impact since an active seed collection plan can be developed in order to preserve the genetic diversity of these populations for current and future reintroduction programs and long-term storage of viable germplasm. Also, in terms of in situ conservation, these 3 genetic diversity hotspots can be selected for the creation of new natural reserves in their respective regions.
This genetic distribution will also establish a base line to create a hierarchal system of future collecting programs maximising the gain in diversity for ex situ seed collections. The efforts of collection of germplasm can be directed towards visiting the most genetically diverse regions and proposing the creation of a network of reserves in the Chihuahuan Desert, the Queretaroan-Hidalgoan Arid Zone and the Tehuacán-Cuicatlán Valley, high diversity arid and semi-arid regions but still poorly represented in the national protection network.
Table 5
Interpopulation genetic diversity of 2 geographic megapopulations of Echinocactus platyacanthus in arid Mexico.
| Megapopulation | n | na | ne | h | I | PL | PPL |
| Northern
(Tula, Mazapil, Galeana, Matehuala, and Guadalcázar) |
100 | 1.5875 (0.4954) | 1.2544 (0.3412) | 0.1540 (0.1841) | 0.2395 (0.2625) | 47 | 58.75% |
| Southern
(Cadereyta, Victoria, Metztitlán, Chazumba, and Tlacotepec) |
83 | 1.6500 (0.4800) | 1.3017 (0.3446) | 0.1827 (0.1900) | 0.2805 (0.2710) | 52 | 65.00% |
n = Number of individuals analysed; na = number of observed alleles; ne = number of effective alleles effective alleles (Kimura & Crow, 1964); h = Nei’s gene diversity (Nei, 1973); I = Shannon’s information index (Lewontin, 1972); PL= polymorphic loci; PPL = percent of the polymorphic loci. Values in parenthesis show standard deviation.
Table 6
Summary of the analysis of molecular variance (AMOVA) of 10 localities distributed among the 3 populations: northern, central and southern.
| Source | Degrees of freedom | Sum of squares | Mean of squares | Est. Var. | %
variation |
Phi values | p (rand > = data) |
| Among populations | 2 | 540.987 | 270.493 | 3.107 | 30% | RT = 0.295 | 0.001 |
| Among localities | 7 | 608.121 | 86.874 | 4.433 | 42% | PR = 0.597 | 0.001 |
| Within localities | 181 | 540.075 | 2.984 | 2.984 | 28% | PT = 0.716 | 0.001 |
| Total | 190 | 1,689.183 | 10.524 | 100% |
Mantel statistic r: 0.4432. Significance: 0.007992.
The genetic diversity of the populations of E. platyacanthus can clearly be subdivided into 3 geographic hotspots, the northern, the central and the southern regions. However, 2 megapopulations can be taken into account since the genetic diversity of the UPGMA tree showed that the southern region is genetically more similar to the central region than to the northern region. These 3 or 2 genetic megapopulations, also have an internal structure that allows each hotspot to have its own identity and to have a specific weight in conservation planning both ex situ and in situ. The group of Cadereyta-Victoria is genetically more similar to the group of Chazumba and Tlacotepec even when the geographic distance is close to 380 air km among these 2 hotspots. Based on geographic distance alone, the Cadereyta-Victoria group was expected to be more closely related to Metztitlán than to Chazumba-Tlacotepec based solely on geographic distance. The planning of in situ and ex situ conservation programs should take into consideration the degree of genetic differentiation and the role of geographic distance for collection of seeds and living specimens.
Further and finer studies are necessary in order to dissect ecological, geological, and biogeographical variables that are acting on the population structure of these long-lived desert cacti. The current human impacts on the natural populations of this species need immediate local and national responses from various fronts with the aim of safeguarding the majority of the genetic diversity of each of the newly identified hotspots. Echinocactus platyacanthus is an important and irreplaceable natural resource for the desert communities, both for human communities as well as for other desert native species. A source of fruits, seeds, pollen, nectar, water, and protection for local fauna, could also be intelligently managed for human uses and even exploited as a source of candy if new plants should be raised in local nurseries for human uses.
Acknowledgements
Authors would like to express their gratitude to María Teresa Reinoso Pérez and Laila M. Bárcenas for their invaluable help during field work collection, measurements, and herbarium processing. Thanks to Secretaría de Medio Ambiente y Recursos Naturales for providing the collecting permits to develop field work in Mexico, and thanks to anonymous reviewers for commenting on previous versions of this manuscript.
References
Bárcenas, R. T. (2003). Chihuahuan Desert Cacti in Mexico: an assessment of trade, management, and conservation priorities. In C. S. Robbins (Ed.), Prickly trade: trade and conservation of Chihuahuan Desert cacti (pp. 1–65). Washington D.C.: TRAFFIC North America.
Bárcenas, R. T. (2016). A molecular phylogenetic approach to the systematics of Cylindropuntieae (Opuntioideae, Cactaceae). Cladistics, 32, 335–478. https://doi.org/10.1111/cla.12135
Bárcenas, R. T., Aguilar, E. J., & Montoya, L. M. (2022). Facing feral burros and goats: conservation of cacti and succulents in the Sierra Gorda, Querétaro, México. Cactus and Succulent Journal (Los Angeles) , 94, 1–5. https://doi.org/10.2985/015.094.0202
Bárcenas, R. T., Yesson, C. J., & Hawkins, J. A. (2011). Molecular systematics of the Cactaceae. Cladistics, 27, 470–489. https://doi.org/10.1111/j.1096-0031.2011.00350.x
Bravo, H. H., & Sánchez-Mejorada, H. (1991). Las cactáceas de México. México, D.F.: Universidad Nacional Autónoma de México.
Bustamante, E., Búrquez, A., Scheinvar, E., & Eguiarte, L. E. (2016). Population genetic structure of a widespread bat-pollinated columnar cactus. Plos One, 11, e0152329. https://doi.org/10.1371/journal.pone.0152329
del Castillo, R. F., & Trujillo, S. (1991). Ethnobotany of Ferocactus histrix and Echinocactus platyacanthus (Cactaceae) in the semiarid central Mexico: past, present and future. Economic Botany, 45, 495-502.
Frankham, R., Ballou, J. D., Briscoe, D. A., & McInnes, K. H. (2004). A primer of conservation genetics. Cambridge, UK: Cambridge University Press.
García-Morales, E., Carrillo-Ángeles, I. G., Golubov, J., Piñero, D., & Mandujano, M. C. (2018). Influence of fruit dispersal on genotypic diversity and migration rates of a clonal cactus from the Chihuahuan Desert. Ecology and Evolution, 8, 12559–12575. https://doi.org/10.1002/ece3.4657
Goettsch, B., Hilton-Taylor, C., Cruz-Piñón, G., Duffy, J. P., Frances, A., Hernández, H. M. et al. (2015). High proportion of cactus species threatened with extinction. Nature Plants, 1, 1–7. http://dx.doi.org/10.1038/nplants.2015.142
Hernández, H. M., & Bárcenas, R. T. (1996). Endangered cacti in the Chihuahuan Desert: II. Biogeography and conservation. Conservation Biology, 10, 1200–1209. https://doi.org/10.1046/j.1523-1739.1996.10041200.x
Hernández, H. M., & Gómez-Hinostrosa, C. (2011). Mapping the cacti of Mexico: their geographical distribution based on referenced records. Milborne Port: dh Books.
Hernández, T., Hernández, H. M., De-Nova, J. A., Puente, R., Eguiarte, L. E., & Magallón, S. (2011). Phylogenetic relationships and evolution of growth form in Cactaceae (Caryophyllales, Eudicotyledoneae). American Journal of Botany, 98, 44–61. https://doi.org/10.3732/ajb.1000129
Hernández-Ledesma, P., & Bárcenas, R. T. (2017). Phylogenetic utility of the trnH–psbA IGR and stem-loop diversity of the 3′ UTR in Cactaceae (Caryophyllales). Plant Systematics and Evolution, 303, 299–315. https://doi.org/10.1007/s00
606-016-1372-9
IUCN-Intenational Union for Conservation of Nature. (2022). Red List 2022: Echinocactus platyacanthus. Retrieved on 16 November 2022 from: https://www.iucnredlist.org/species/152537/121477917
Jiménez-Sierra, C., Mandujano, M. C., & Eguiarte, L. E. (2007). Are populations of the candy barrel cactus (Echinocactus platyacanthus) in the desert of Tehuacán, Mexico at risk? Population projection matrix and life table response analysis. Biological Conservation, 135, 278–292.
Kimura, M., & Crow, J. F. (1964). The number of alleles that can be maintained in a finite population. Genetics, 49, 725–738. https://doi.org/10.1093/genetics/49.4.725
Kramer, A. T., Fant, J. B., & Ashley, M. V. (2011). Influences of landscape and pollinators on population genetic structure: Examples from three Penstemon (Plantaginaceae) species in the Great Basin. American Journal of Botany, 98, 109–121. https://doi.org/10.3732/ajb.1000229
Lewontin, R. C. (1972). The apportionment of human diversity. In T. Dobzhansky, M. K. Hecht, & W. C. Steere (Eds.), Evolutionary Biology (pp. 381–398). New York, NY: Springer US.
Megged, A. (2010). Social memory in ancient and colonial Mesoamerica. Cambridge; New York: Cambridge University Press.
Nei, M. (1973). Analysis of gene diversity in subdivided populations. Proceedings of the National Academy of Sciences, 70, 3321–3323. https://doi.org/10.1073/pnas.70.12.3321
O’Dwyer, J. E., Murphy, N., Tonkin, Z., Lyon, J., Koster, W., Dawson, D. et al. (2021). An investigation of genetic connectivity shines a light on the relative roles of isolation by distance and oceanic currents in three diadromous fish species. Marine and Freshwater Research, 72, 1457. https://doi.org/10.1071/MF20323
Peters, M. A. E., & Weis, A. E. (2019). Isolation by phenology synergizes isolation by distance across a continuous landscape. New Phytologist, 224, 1215–1228. https://doi.org/10.1111/nph.16041
Prado, A., Hawkins, J. A., Yesson, C., & Bárcenas, R. T. (2010). Multiple diversity measures to identify complementary conservation areas for the Baja California peninsular cacti. Biological Conservation, 143, 1510–1520. https://doi.org/10.1016/j.biocon.2010.03.033
Semarnat (Secretaría del Medio Ambiente y Recursos Naturales). (2010). Norma Oficial Mexicana NOM-059-SEMARNAT-2010, Protección ambiental – Especies nativas de México de flora y fauna silvestres – Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio – Lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010, Segunda Sección, México.
Valadez-Moctezuma, E., Samah, S., & Luna-Paez, A. (2015). Genetic diversity of Opuntia spp. varieties assessed by classical marker tools (RAPD and ISSR). Plant Systematics and Evolution, 301, 737–747. https://doi.org/10.1007/s00
606-014-1112-y
Vargas-Luna, M. D., Hernández-Ledesma, P., Majure, L. C., Puente-Martínez, R., Hernández-Macías, H. M., & Bárcenas-Luna, R. T. (2018). Splitting Echinocactus: morphological and molecular evidence support the recognition of Homalocephala as a distinct genus in the Cacteae. Phytokeys, 111, 31–59. https://doi.org/10.3897/phytokeys.111.26856
Yesson, C., Bárcenas, R. T., Hernández, H. M., Ruiz-Maqueda, M. L., Prado, A., Rodríguez, V. M. et al. (2011). DNA barcodes for Mexican Cactaceae, plants under pressure from wild collecting. Molecular Ecology Resources, 11, 775–783. https://doi.org/10.1111/j.1755-0998.2011.03009.x