Fernando Álvarez *, Eduardo Torres, José Luis Villalobos
Colección Nacional de Crustáceos, Instituto de Biología, Universidad Nacional Autónoma de México, Apartado postal 70-153, 04510 Ciudad de México, México
*Corresponding author: falvarez@ib.unam.mx (F. Álvarez)
Received: 23 November 2020; accepted: 30 March 2020
http://zoobank.org/urn:lsid:zoobank.org:pub:63489F74-AF68-4CF9-A295-BBEAF38D490A
Abstract
A new species of crayfish is described from the Cave of Sótano de La Lucha, in the border between the Mexican states of Chiapas and Oaxaca. The new species is completely adapted to the cave environment with reduced eyes lacking cornea and visual pigments, an almost unpigmented body and elongated appendages. We also report on crayfish populations from the “Lagunas de Montebello” area, which fit well the description of Procambarus pilosimanus (Ortmann, 1906), a heretofore problematic species in southern Mexico.
Keywords: Stygobitic crayfish; Sótano de La Lucha; Lagunas de Montebello; Chiapas
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Especie nueva de acocil del género Procambarus y notas sobre Procambarus pilosimanus (Decapoda: Cambaridae) de Chiapas, México
Resumen
Se describe una especie nueva de acocil del Sótano de La Lucha, en la frontera entre los estados mexicanos de Chiapas y Oaxaca. La especie nueva está completamente adaptada al ambiente de cueva con ojos reducidos sin córnea ni pigmentos visuales, un cuerpo casi despigmentado y apéndices alargados. También reportamos sobre poblaciones de acociles del área de las Lagunas de Montebello, que coinciden bien con la descripción de Procambarus pilosimanus (Ortmann, 1906), que es hasta ahora una especie problemática del sur de México.
Palabras clave: Acocil estigobítico; Sótano de La Lucha; Lagunas de Montebello; Chiapas
© 2021 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
A total of 57 species of crayfish of the family Cambaridae are distributed in Mexico, 11 in the genus Cambarellus, all of them belonging to the same subgenus C. (Cambarellus), 1 species of Orconectes, O. virilis (Hagen, 1870) and 45 species of Procambarus (Pedraza-Lara & Doadrio, 2015; Álvarez & Villalobos, 2016a). Of these, 54 are endemic to Mexico, the exceptions are: P. clarkii (Girard, 1852) occurring in northern Mexico and the southeastern United States, P. pilosimanus (Ortmann, 1906) occurring in southern Mexico and Guatemala, and O. virilis which is considered an introduced species in Mexico with a native range in the central United States and southcentral Canada (Álvarez & Villalobos, 2016a).
There are 4 species of Procambarus known from Chiapas: P. llamasi Villalobos, 1954, from the northern portion of the state near Palenque; P. mirandai Villalobos, 1954, which is a common species in the Central Depression region of the state; P. pilosimanus, allegedly widely distributed in Chiapas and Guatemala, and P. sbordonii Hobbs, 1977, described from a cave in the vicinity of Bochil (Álvarez et al., 2011). Recently, P. clarkii has been introduced into Chiapas, where it has now expanded its distribution in the central portion of the state around the town of Teopisca and the city of Comitán (Torres & Álvarez, 2012).
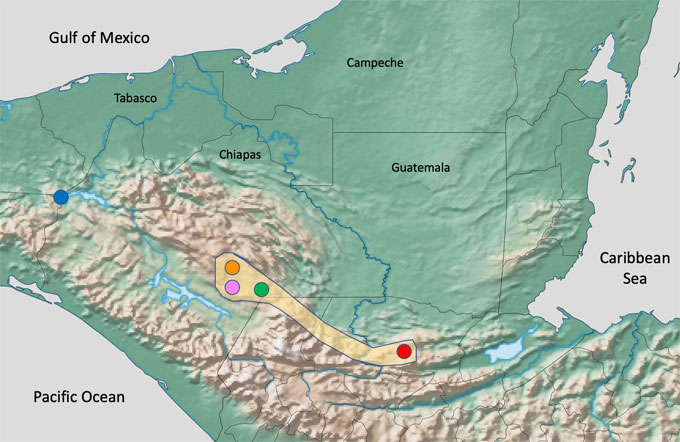
Based on a wide geographic sampling and on the revision of collection specimens, we herein describe one new species from Sótano de La Lucha Cave, a species adapted to cave life. We also comment on the identity and distribution of P. pilosimanus from Chiapas, southern Mexico, a species whose identity has been hard to define since it has a large distribution area and was described from an unknown locality in Guatemala (Ortmann, 1906; Fig. 1).
Materials and methods
Field surveys were conducted in the Central Depression, the area of “Lagunas de Montebello” and the northern portion of the Western Slopes in Chiapas (Fig. 1; Torres-Torres, 2014). From a total of 20 crayfish populations sampled, those from Sótano de La Lucha and from “Lagunas de Montebello” National Park were selected for this paper. All specimens were preserved in 96% EtOH and deposited in the National Crustacean Collection (CNCR), of the Instituto de Biología, Universidad Nacional Autónoma de México (Table 1). The obtained material was identified using Hobbs (1972, 1989), Villalobos (1954, 1955) and Torres-Torres (2014), contributions.
The external morphology (carapace, chelae, epistome, annulus ventralis, pereiopodal hooks) was observed and figured with the aid of a stereomicroscope. Scanning electron microscope (SEM) micrographs of the first pleopod of form I males were taken, covering 4 views: caudal, cephalic, lateral and mesial. The samples were critical point dried in an EMITECH K850 dryer and covered with gold in a Quorum Q150RES evaporator. Samples were photographed in a Hitachi SU1510 electron microscope.
In addition, all samples identified as P. pilosimanus deposited in the Colección Nacional de Crustáceos (CNCR), Instituto de Biología, Universidad Nacional Autónoma de México, were examined morphologically. We follow the classification proposed by Crandall and De Grave (2017) where the subgenera of Procambarus are no longer recognized.
Results
Family Cambaridae Hobbs, 1942
Genus Procambarus Ortmann, 1905
Procambarus adani n. sp.
Figs. 2-4
http://zoobank.org/urn:lsid:zoobank.org:act:B31A59B2-AC94-4843-8286-FF37B11E5EE2
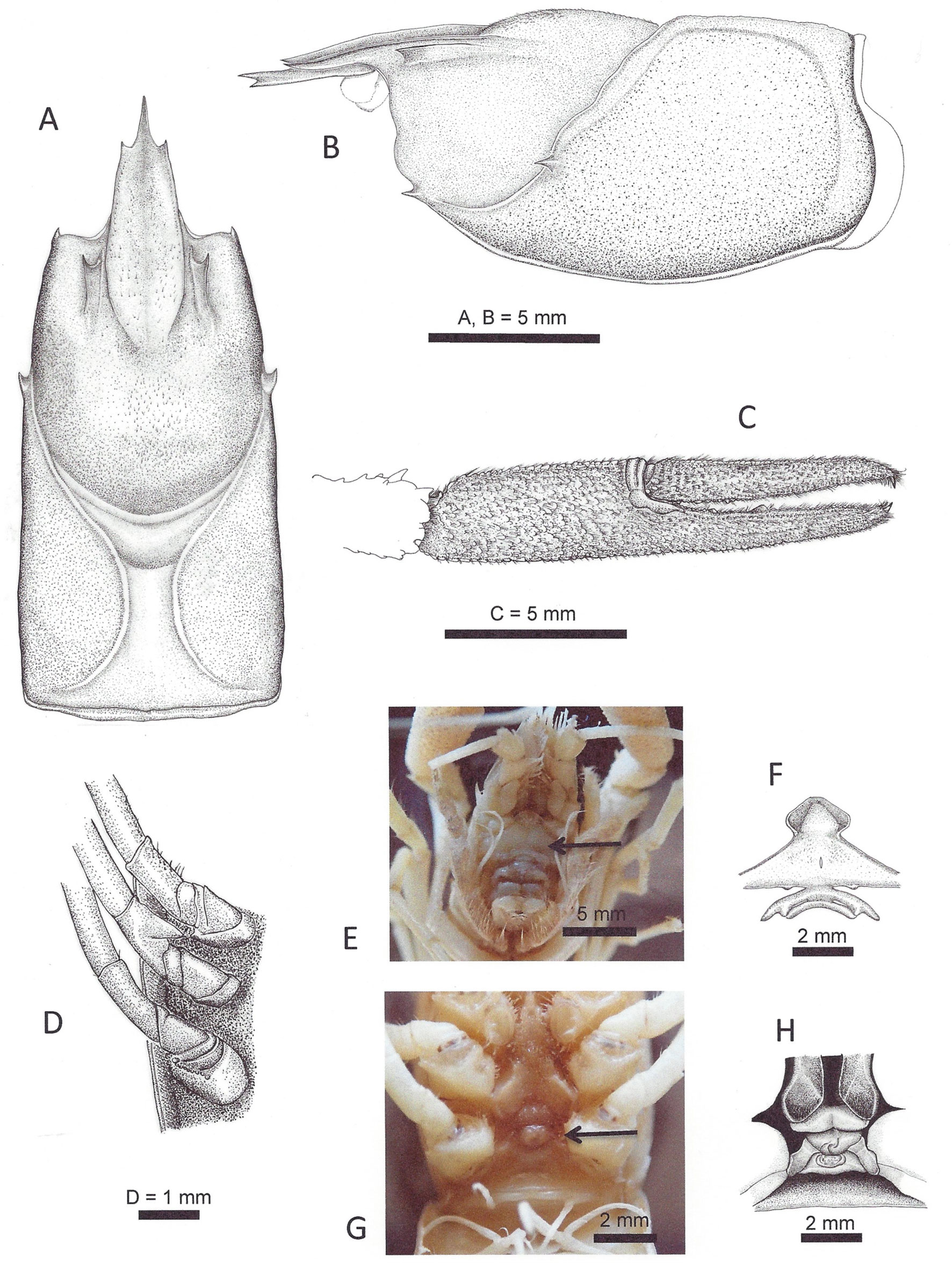
Diagnosis. Body almost unpigmented, whitish with minute orange dots dorsally (Fig. 4). Eyes reduced to a blunt peduncle, no facets or visual pigments present. Rostrum slender, acumen acute, slightly surpassing distal margin of third antennular segment, 26.4 to 31.8% (_ = 28.2%) of TCL, with marginal spines (Fig. 2A). Areola 2.3 to 3.4 times (_ = 2.8) as long as wide, 29.5 to 35.7% (_ = 32.6%) of TCL, 36.7 to 43.6% (_ = 40.6%) of postorbital length, with 4 or 5 punctuations across narrowest part. Postorbital spines prominent, cervical spine robust, single branchiostegal spine (Fig. 2A, B). Antennal scale 1.9 to 2.1 (_ = 2.0) times longer than wide, with shallow longitudinal groove. Chelipeds shorter than total body length, surface covered with minute granules with tuft of short setae; fingers longer than palm, cutting edges finely toothed (Fig. 2C).
Ischium of third pereiopod with prominent hook, ending in acute tip, reaching proximal margin of basis (Fig. 2D). Epistome with approximately pentagonal cephalic lobe, cephalomedian margin straight, lateral angles rounded, margins raised, devoid of setae; fovea slit-like, zygoma arched with 2 sublateral triangular projections on posterior margin (Fig. 2E). First pleopods (gonopods) of male form I subequal in length, reaching coxa of third pereiopod, with long plumose setae along mesial surface. In caudal view, mesial process slender, flattened, surpassing all other apical elements, inclined laterally, ending in rounded tip; lateral shoulder prominent, apex rounded, slightly acuminated; central projection as triangular plate, distally directed, acute, as long as more than 5 times its basal width (Fig. 3A). In cephalic view, apical surface with squamous granules, denser distally, central projection not visible covered by rounded boss, with reticulated surface; lateral shoulder widely rounded; mesial process slender, subacute, laterally directed (Fig. 3B). In lateral view, central projection well developed, triangular, moderately upturned, surface reticulated, small spinule on superior border; lateral shoulder surface granulated; mesial process, distal end acute, partially visible; cephalic shoulder forming straight angle, surface rough and excavated (Fig. 3C). In mesial view, central projection triangular, with slit-like groove; central projection incipient; mesial process slender, tapering distally; lateral shoulder with stout flange on apex; cephalic shoulder slightly concave, forming straight angle (90°) with respect to main axis of pleopod (Fig. 3D).
Annulus ventralis with preannular plate short with median depression, wider than annulus (Fig. 2F). Preannular plate in contact with annulus. Annulus approximately triangular, tip facing posteriorly, sinus developing anteriorly on sinistral crest, curving towards midportion, curving on dextral crest towards posterior margin (Fig 2F). Postannular plate with rounded anterior margin, extending slightly under annulus, lateral margins laterocaudally oriented, posterior margin sinuous; circular shallow depression on anterocentral portion (Fig. 2F).
Measurements of types. Provided in Table 2.
Holotypic male, form I. Body almost unpigmented, whitish with minute orange dots dorsally, eyes reduced, no visual elements, facets or pigments present. Cephalothorax 0.87 length of abdomen; posterior half wider, higher. Areola 2.9 times as long as wide, 35.7% of TCL, with 5-6 punctations across narrowest part, branchiocardiac grooves deep, well marked. Surface of carapace finely punctate, with minute setae along rostrum and cardiac region, orange dots dorsally (Fig. 4). Rostrum concave, margins raised, slightly converging anteriorly, ending in acute spines; anterior width 1.5 mm, posterior width 2.5 mm. Acumen reaching beyond distal margin of third antennular segment, length 45.7% rostrum length, ventral keel with 1 spine (Fig. 2B). Postorbital ridge as sharp keel, slightly curved in dorsal view, straight in lateral view, with acute spines anteriorly, slightly lateral directed, not reaching orbital margin (Fig. 2A, B). Suborbital margin straight, shallow indentation dividing rounded ventral portion of margin; branchiostegal spine prominent, acute. Cervical groove sinuous laterally; in lateral view cervical spine almost at same level as branchiostegal spine. Abdomen longer than carapace.
Epistome as described in Diagnosis. Chelipeds 1.6 times length of carapace, 0.73 times of total length. Chela slender, long, surface with minute punctations. Merus with irregular ventral row of spines, increasing in size distally. Carpus short, 0.4 times length of merus, strong spines on distomesial angle and distoventral margin. Palm 3.2 times longer than wide, with scattered minute setae dorsally, subequal in length to merus and dactyl. Opposable margins of fingers with minute teeth, tips ending in corneous spine. Ischium of third pereiopod with hook, surpassing basioischial articulation (Fig. 2D).
First pleopods of male form I as described in Diagnosis.
Uropods, distolateral angle of exopodite with 2 spines; endopodite wider, shorter than exopodite, with dorsal median ridge not reaching posterior margin. Telson with scattered short setae dorsally, with 2 spines on posterolateral angles of cephalic portion.
Allotypic female. Differing from holotype in following characters. Areola 3.4 times as long as wide, 34.3% of TCL. Chelipeds 1.3 times length of carapace, 0.61 times of total length. Annulus ventralis as described in Diagnosis.
Morphotypic male, form II. Differing from holotype in following characters. Mesial process of first pleopod shorter, not as inclined as in form I male; central projection triangular, apex somewhat rounded; cephalic shoulder concave, proximal angle less pronounced. Areola 2.3 times as long as wide, 29.5% of TCL. Chelipeds 1.4 times length of carapace, 0.67 times of total length. Ischium of third pereiopod with incipient acute projection.
Taxonomic summary
Disposition of types. Holotypic male form I: TL 39.0 mm, TCL 17.6 mm; cave at Sótano de La Lucha (17°03’46” N, 93°53’23” W), Ocozocuautla, Chiapas, Mexico; 25 April 2012; colls. A. Gómez, F. Álvarez, J.L. Villalobos, S. Rodríguez, L. García; CNCR 26741. Allotypic female: TL 53.2 mm, TCL 26.8 mm; same locality, date and collectors as holotype; CNCR 26741. Morphotypic male form II: TL 44.6 mm, TCL 22.0 mm; same locality, date and collectors as holotype; CNCR 26741.
Paratypes. Two females, TL 53.2, 41.4 mm, TCL 24.6. 18.9 mm; same date, locality and collectors as holotype; CNCR 35720.
Type locality. Small ponds in total darkness in the cave that leads to Sótano de La Lucha, Ocozocuautla, Chiapas, Mexico; 546 m asl.
Etymology. This species is dedicated to the memory of Adán Gómez González, outstanding biologist and friend, whose untimely passing away has been felt deeply.
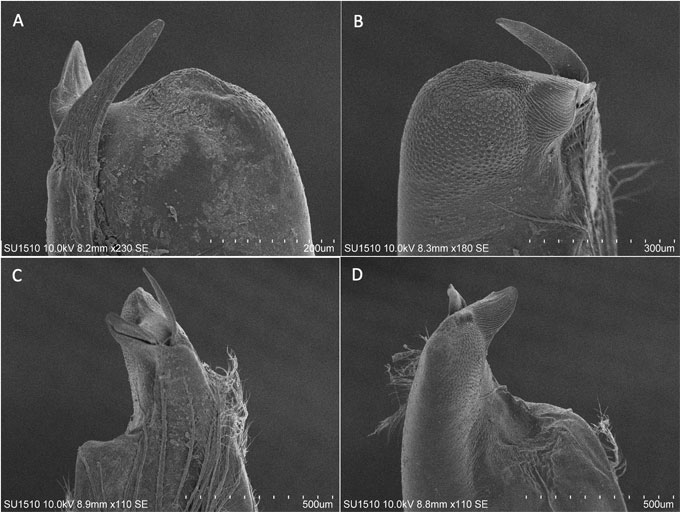
Table 1
Variation of selected characters in populations of Procambarus pilosimanus, all specimens deposited in the CNCR, Instituto de Biología, UNAM.
|
Cervical spines |
Branchiostegal spines |
Chela length/width ratio |
Pubescence on chelae |
|
|
Gruta de Zapaluta CNCR 1389 |
2 |
2 |
4.09-4.76 3.02 |
no yes |
|
Villa de las Margaritas CNCR 1390 |
2 |
2 |
3.5 |
yes |
|
Villa de las Margaritas CNCR1391 |
2 |
2 |
4.12 |
no |
|
Cueva de la Trinitaria CNCR 12319 |
2 |
2 |
3.25 |
yes |
|
Pond by El Carrizal Lake, Montebello CNCR 25397 |
0-1 |
0-1 |
3.7 |
yes |
|
Pond, km 184 Comitán-La Trinitaria Highway CNCR 25431 |
2 |
2 |
3.14 |
yes |
Remarks
Procambarus adani new species, is the 15th cave inhabiting Procambarus species from Mexico (see Mejía-Ortíz et al., 2003). This group of species show varying degrees of adaptation to cave life and are distributed from Tamaulipas to Chiapas. The occurrence of several species within this group in caves seems to be accidental, as they do not show any obvious adaptations to this environment and they also occur in epigean environments (e.g., Procambarus cuetzalanae Hobbs, 1982; P. cuevachicae Hobbs, 1941; P. ortmanni Villalobos, 1949; P. pilosimanus; P. toltecae Hobbs, 1943; P. villalobosi Hobbs, 1969; P. xochitlanae Hobbs, 1975). In other cases, there is some degree of elongation of appendages, loss or reduction of pigmentation together with epigean occurrences (e.g., Procambarus mirandai, P. sbordonii) making it difficult to define the precise habit of the species. Several species are clearly stygobitic as they occur only in caves, are unpigmented, their appendages elongated and their eyes reduced but with remnants of pigments: Procambarus rodriguezi Hobbs, 1943; P. oaxacae oaxacae Hobbs, 1973; P. oaxacae reddelli Hobbs, 1973, and P. cavernicola Mejía-Ortiz, Hartnoll & Viccon-Pale, 2003. Only 2 species, however, P. xilitlae Hobbs & Grubbs, 1982, from Hoya de las Guaguas, Aquismón, San Luis Potosí and P. adani new species, described herein, have completely reduced eyes, devoid of cornea and all other visual elements, presenting a blunt ocular peduncle (Mejía-Ortíz et al., 2003).

Table 2
Measurements in mm of type specimens of Procambarus adani, new species, from Sótano de La Lucha Cave, Chiapas, Mexico.
|
Holotypic male, form I |
Allotypic female |
Morphotypic male, form II |
|
|
Carapace |
|||
|
Total carapace length |
17.6 |
26.8 |
22.0 |
|
Height |
7.9 |
11.2 |
10.2 |
|
Width |
7.3 |
10.7 |
9.4 |
|
Postorbital carapace length |
14.5 |
21.1 |
17.2 |
|
Areola |
|||
|
Length |
6.3 |
9.2 |
6.5 |
|
Width |
2.2 |
2.7 |
2.8 |
|
Rostrum |
|||
|
Length |
5.6 |
7.5 |
6.0 |
|
Width |
2.5 |
3.6 |
3.4 |
|
Antennal scale |
|||
|
Length |
4.1 |
6.1 |
4.5 |
|
Width |
2.0 |
2.9 |
2.2 |
|
Cheliped |
|||
|
Length mesial margin palm |
8.0 |
10.2 |
8.5 |
|
Palm width |
2.5 |
3.1 |
2.4 |
|
Length lateral margin propodus |
14.8 |
20.3 |
15.4 |
|
Dactyl length |
7.3 |
10.4 |
7.5 |
|
Carpus length |
3.2 |
4.9 |
4.3 |
|
Merus length |
8.2 |
11.3 |
9.0 |
|
Abdomen |
|||
|
Length |
21.4 |
26.4 |
22.6 |
|
Width |
6.6 |
9.8 |
7.9 |
Several characters, in addition to the obvious adaptations to cave life, separate P. adani new species, from other Procambarus species from Chiapas. Regarding the external morphology, the new species has prominent spines on the anterior tip of the postorbital margin, a straight suborbital margin of the carapace, and a relatively wide areola (Fig. 2). The first pleopod bears a longer and slender mesial process than those of the other 4 species from Chiapas; and the lateral shoulder, in cephalic view, is straight towards the central portion of the pleopod, not rounded (Fig. 3) as in P. mirandai, P. llamasi (Villalobos, 1983, plates 48, 52), and P. pilosimanus (Fig. 4).
Another 2 stygobitic species from the neighboring State of Oaxaca, Procambarus oaxacae oaxacae and P. oaxacae reddelli also deserve to be compared with the new species described herein. Regarding the external morphology, P. adani is more similar to P. oaxacae reddelli due to an acute acumen, and the presence of postorbital, branchiostegal and cervical spines (Hobbs, 1973). Other external characters (e.g., epistome, shape of chelae) are similar in the 3 species; however, the morphology of the first pleopods easily differentiate all of them. Hobbs (1973) considered P. oaxacae oaxacae and P. oaxacae reddelli to be related to P. rodriguezi from Veracruz, and now with the description of P. adani the group of 4 stygobitic species align along a north-south axis in the Isthmus of Tehuantepec, a region that has been recognized as a complex mosaic where several freshwater decapod groups (e.g., crayfish, palaemonid shrimp, pseudothelphusid crabs) show a high diversity of cave adapted forms (Álvarez & Villalobos, 2016b; Álvarez et al., 2020; Hobbs, 1973).
Procambarus pilosimanus (Ortmann, 1906)
Figs. 5, 6
Cambarus (Procambarus) pilosimanus Ortmann, 1906: 6, fig. 2.
Cambarus pilosimanus Pearse, 1911: 110.
Procambarus pilosimanus Hobbs, 1942: 342 [by implication].– Villalobos, 1955: 231, pls. 53, 54; 1983: 220, pls. 53, 54.
Procambarus pelosimanus Creaser, 1962: 7 [erroneous spelling].
Procambarus (Austrocambarus) pilosimanus Hobbs, 1972: 6; 1974: 45, fig, 182; 1989: 53.
Procambarus pilosimanus Crandall & De Grave, 2017: 635.
Material examined. Two form I males, TL 47.8, 57.0 mm, TCL 24.7, 27.5 mm; 1 ovigerous female, TL 50.4 mm, TCL 25.7 mm, 60 eggs approximately, 1.5-2-6 mm in diameter, plus 16 juveniles; 29 April 2009; pond by El Carrizal Lake, Lagunas de Montebello National Park (16°06’37” N, 91°43’38” W; 1,500 m asl), La Trinitaria, Chiapas, Mexico; colls. E. Torres, J.L. Villalobos, F. Álvarez; CNCR 25397. One form I male, TL 67.1 mm, TCL 35.5 mm; 1 female, TL 64.7 mm, TCL 34.0 mm; 3 February 1986; La Trinitaria Cave (16°05’54” N, 92°02’45” W; 1,542 m asl), La Trinitaria, Chiapas, Mexico; coll. R. Medellín; CNCR 12319. Three females, TL, 30.6-39.9 mm, TCL 16.0-21 mm; 4 form II males, TL 27.4-40.0 mm, TCL 14.6-20.5 mm; 25 November 2008; pond at km 184 Comitán-La Trinitaria highway, La Trinitaria, Chiapas, Mexico; colls, E. Torres, J.L. Villalobos, F. Álvarez; CNCR 25431. Two form I males, TL 44.2-62.6 mm, TCL 18.0-31.3 mm; 3 females, TL 39.5-47.8 mm, TCL19.3-21.8 mm, plus 6 juveniles; 6 December 1949; Villa de las Margaritas, 18 km ENE from Comitán, Chiapas, Mexico; coll. A. Villalobos; CNCR 1390. One form I male, TL 51.5 mm, TCL 25.5 mm; 1 female, TL 49.5 mm, TCL 23.6 mm; 1 form II male, TL 40.8 mm, TCL 19.4 mm; 3 June 1950; Villa de Las Margaritas, 18 km ENE from Comitán, Chiapas, Mexico; coll. A. Villalobos; CNCR 1391. Four form I males, TL 57.5-71.6 mm, TCL 28.6-36.2 mm, plus 1 male and 3 females; 24 November 1950; Gruta de Zapaluta, La Trinitaria, Chiapas, Mexico; coll. A. Villalobos; CNCR 1389.
Additional material examined. Two form I males, TL 63.1, 48.0 mm, TCL 31.1, 22.7 mm; 1 form II male, TL 42.0 mm, TCL 20.1 mm; 2 females, TL 54.7, 52.2 mm, TCL 27.0, 25.5 mm; 7 April 1986; Tziscao Lake, Lagunas de Montebello National Park (16°04’59” N, 91°40’28” W; 1,485 m asl), La Trinitaria, Chiapas, Mexico; coll. J.L. Villalobos; CNCR 5580. One form I male, TL 36.0 mm, TCL 15.6 mm; 2 form II males, TL 42.4, 37.5 mm, TCL 20.1, 19.0 mm; 1 ovigerous female, TL 47.3 mm, TCL 27.0 mm, with about 40 eggs; 7 April 1986; río Paso del Soldado, rancho San Rafael del Arco, Lagunas de Montebello National Park (16°07’23” N, 91°42’47” W; 1,520 m asl), La Trinitaria, Chiapas, Mexico; coll. J.L. Villalobos; CNCR 5580. Three form I males, TL 64.5, 57.5, 43.4 mm, TCL 31.0, 25.5, 20.5 mm; 24 September 2013; river 4 km E of Tziscao, Lagunas de Montebello National Park (16°06’02” N, 91°37’18” W; 1,500 m asl), La Trinitaria, Chiapas, Mexico; colls. J.L. Villalobos, E. Moreno, J.C. Ojeda, F. Álvarez; CNCR 28382.
Remarks
Procambarus pilosimanus is, until now, an unusual widespread species occurring from central Guatemala to several localities in southern Chiapas, Mexico (Fig. 1). Due to the lack of reference material and to an apparent variable morphology of these crayfishes with pubescent chelae, the identity of several populations within this complex has been problematic. The type specimens were collected by Bocourt in “Coche, près de la rivière de Coban, Guatemala”, and deposited in the Natural History Museum of Paris. The specimens were examined and described as Cambarus (Procambarus) pilosimanus by Ortmann (1906). Regarding the exact type locality of the species Ortmann (1906) stated the following: “I have not been able to locate this place, nor a river “Coban”; but Coban is the well-known capital of the province of Alta Vera Paz. The river at Coban is called Rio Cahabon. Coban, Alta Vera Paz, is the locality for a species of Cambarus mentioned by Huxley (1878)”. Ortmann (1906) also mentioned another specimen from an unidentified locality in “Belize, British Honduras”, that is still pending for confirmation, and if found would expand the range of the species considerably. Villalobos (1955, 1983) reported several localities in Chiapas for P. pilosimanus around the city of Comitán, in Gruta de Zapaluta and Villa de Las Margaritas, based on specimens that are deposited in the CNCR. We now add samples from El Carrizal Lake, within the “Lagunas de Montebello” National Park.
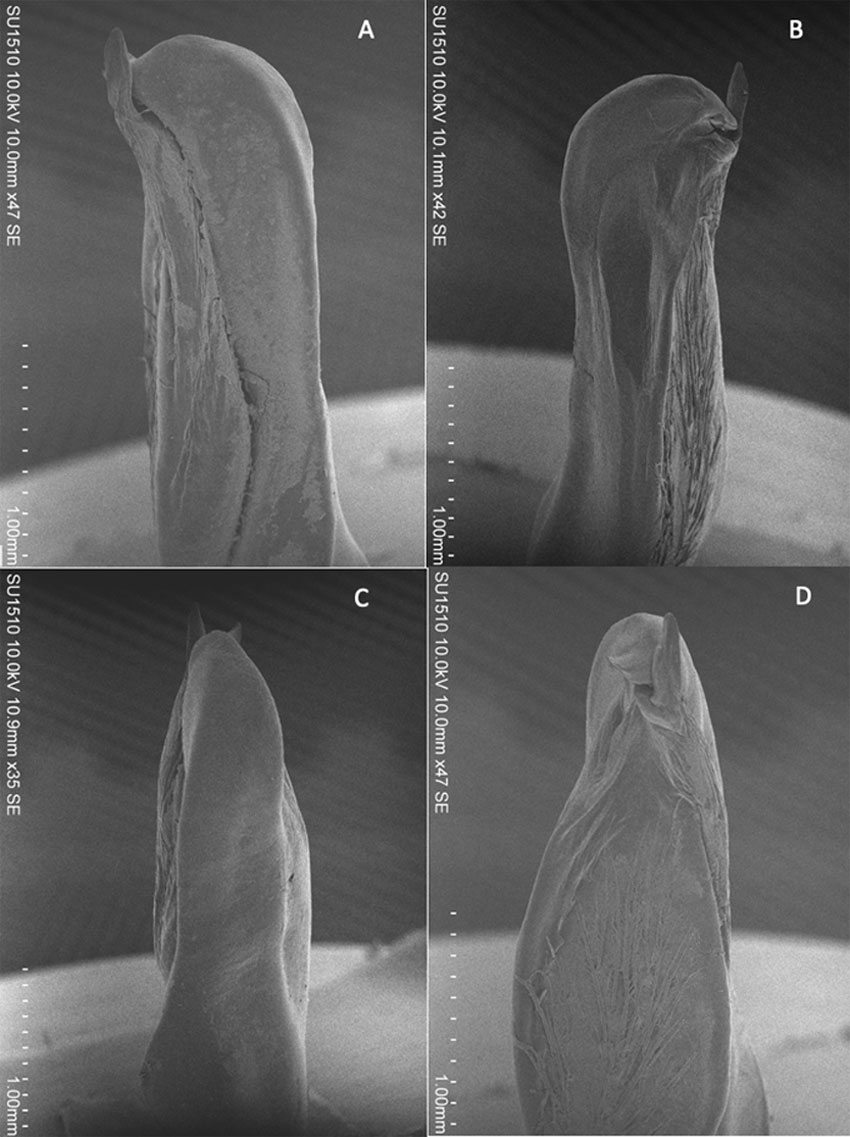
From the specimens examined we can conclude the following. The gonopod morphology of form I males is relatively constant in all the samples and corresponds to the drawings presented by Villalobos (1983; plate 54) from specimens of Gruta de Zapaluta, Municipality of La Trinitaria, Chiapas (Fig. 5). In caudal view, the size and shape of the mesial process, the broadly rounded distal margin of the lateral shoulder; in mesial view, the cephalic shoulder poorly developed, strongly inclined and the shape and size of the central projection, of the males with pubescent chelae and the figures of Villalobos (1983; pg. 253, plate 54, figs. 2, 6) are similar, but with differences in the central projection: centrocaudal process fused or not, cephalic process small or absent and with or without superior spinule. In regards to the external morphology, there is noticeable variation in the number of cervical and branchiostegal spines, and in the length/height proportion and presence of pubescence in the chelae (Table 1). It is interesting to note that in the same area between La Trinitaria and “Lagunas de Montebello” National Park, different morphologies of the chelae can be found, even within the same population. The chelae that are somewhat elongated (> 4 times as long as high) are not pubescent, while the shorter more robust ones are (Fig. 6).

In particular, the populations of Gruta de Zapaluta, Villa de Las Margaritas, Laguna de Tziscao and Río Paso del Soldado, Chiapas, the last 2 within the “Lagunas de Montebello” National Park, seem to have different morphologies of the chelae with the gonopod showing slight variations from the typical P. pilosimanus described by Ortmann (1906). Over the years, from the first samples collected in the area in 1950, until very recently, the 2 types of organisms continue to be present in the area. The more conservative conclusion is that 2 different morphs are occurring sympatrically in the area, one occupying an area from Villa de Las Margaritas to the northern section of the “Lagunas de Montebello” National Park, and with some overlap, a second one occurring towards the southern section of “Lagunas de Montebello” National Park. These observations clear out the confusion of finding in the same general area 2 types of crayfish, sometimes in the same microhabitat. It remains to be determined if there is 1 or more new species to be described from the area.
Acknowledgements
To UNAM-DGAPA PAPIIT Grant IV200319 (Área Experimental de Lagos Tropicales). E. Torres gratefully acknowledges the graduate scholarship received from Conacyt and Posgrado en Ciencias Biológicas of Universidad Nacional Autónoma de México, for their support. Berenit Mendoza, from IB-UNAM, took the SEM micrographs and Rolando Mendoza prepared the drawings.
References
Álvarez, F., & Villalobos, J. L. (2016a). The crayfish of Middle America. Chapter 18. In T. Kawai, Z. Faulkes, & G. Scholtz (Eds.), Freshwater crayfish: global overview (pp. 448–463). Boca Raton: CRC Press. https://doi.org/10.1201/b18723
Álvarez, F., & Villalobos, J. L. (2016b). Freshwater decapod diversity and conservation in Mexico. Chapter 8. In T. Kawai & N. Cumberlidge (Eds.), A global overview of the conservation of freshwater decapod crustaceans (pp. 237–266). Cham, Switzerland: Springer International Publishing AG. http://doi.org/10.1007/978-3-319-42527-6_8
Álvarez, F., Villalobos, J. L., Elías-Gutiérrez, M., & Rivera, G. (2011). Crustáceos dulceacuícolas y terrestres de Chiapas. In F. Álvarez (Ed.), Chiapas: estudios sobre su diversidad biológica (pp. 209–298). México D.F.: UNAM.
Álvarez, F., Ojeda, J. C., Souza-Carvalho, E., Villalobos, J. L., Magalhães, C., Wehrtmann, I. S. et al. (2020). Revision of the higher taxonomy of Neotropical freshwater crabs of the family Pseudothelphusidae, based on multigene and morphological analyses. Zoological Journal of the Linnean Society, 20, 1–29. https://doi.org/10.1093/zoolinnean/zlaa162
Crandall, K. A., & De Grave, S. (2017). An updated classification of the freshwater crayfishes (Decapoda: Astacidae) of the world, with a complete species list. Journal of Crustacean Biology, 37, 615–653. https://doi.org/10.1093/jcbiol/rux070
Creaser, E. P. (1962). Notes on homologies and genetic relationships in the Cambarine Crayfishes. [privately printed].
Hobbs, H. H., Jr. (1942). A generic revision of the crayfishes of the Subfamily Cambarinae (Decapoda, Astacidae) with the description of a new genus and species. American Midland Naturalist, 28, 334–357.
Hobbs, H. H., Jr. (1972). Crayfishes (Astacidae) of North and Middle America. In Biota of freshwater ecosystems. Identification Manual, 9. Chicago: Environmental Protection Agency [Water Pollution Research Control Series].
Hobbs, H. H., Jr. (1973). Three new troglobitic decapod crustaceans from Oaxaca, Mexico. Association for Mexican Cave Studies, Bulletin, 5, 25–38.
Hobbs, H. H., Jr. (1974). A checklist of the North and Middle American crayfishes (Decapoda: Astacidae and Cambaridae). Smithsonian Contributions to Zoology, 166, 1–161.
Hobbs, H. H., Jr. (1989). An illustrated checklist of the American crayfishes (Decapoda: Astacidae, Cambaridae, and Parastacidae). Smithsonian Contributions to Zoology, 480, 1–236.
Mejía-Ortíz, L. M., Hartnoll, R. G., & Viccon-Pale, J. (2003). A new stygobitic crayfish from Mexico, Procambarus cavernicola (Decapoda: Cambaridae), with a review of cave-dwelling crayfishes in Mexico. Journal of Crustacean Biology, 23, 391–401. https://doi.org/10.1163/20021975-99990349
Ortmann, A. E. (1906). Mexican, Central American and Cuban Cambari. Proceedings of The Washington Academy of Sciences, 8, 1–24.
Pearse, A. S. (1911). Report on the Crustacea collected by the University of Michigan-Walker Expedition in the State of Veracruz, Mexico. In 13th Report of the Michigan Academy of Science.
Pedraza-Lara, C., & Doadrio, I. (2015). A new species of dwarf crayfish (Decapoda: Cambaridae) from central Mexico, as supported by morphological and genetic evidence. Zootaxa, 3963, 583–594. http://dx.doi.org/10.11646/zootaxa.3963.4.5
Torres-Torres, E. (2014). Sistemática y variación genética de los acociles del complejo Procambarus (Austrocambarus) mirandai (Master Thesis). Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México, Ciudad de México.
Torres, E., & Álvarez, F. (2012). Genetic variation in native and introduced populations of the red swamp crayfish Procambarus clarkii (Crustacea, Decapoda, Cambaridae). Aquatic Invasions, 7, 235–241. http://dx.doi.org/10.3391/ai.2012.7.2.009
Villalobos, A. (1954). Estudios de los Cambarinos Mexicanos XII, Parte I. Revisión de las especies afines a Procambarus mexicanus (Erichson), con descripción de formas nuevas. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, 25, 299–379.
Villalobos, A. (1955). Cambarinos de la fauna mexicana (Crustacea Decapoda) (Ph. D. Thesis). Facultad de Ciencias, Universidad Nacional Autónoma de México, Ciudad de México.
Villalobos, A. (1983). Crayfishes of Mexico (Crustacea: Decapoda). New Delhi: Amerind Publishing.




