Updated checklist and zoogeographic remarks of benthic amphipods (Crustacea: Peracarida: Amphipoda) of two coastal lagoons in the western Gulf of Mexico
Andrea Raz-Guzmán⁎ ✉ , Luis A. Soto
Abstract
Estuarine amphipods of the western Gulf of Mexico represent mostly eurythermal species. Sampling took place in Summer and Winter on seagrass beds, macroalgae and bare substrates, at 75 localities in Laguna Madre and 34 in Laguna de Tamiahua, Mexico, with a Renfro beam net and an otter trawl. A total of 19,398 specimens of 19 species were collected. Cymadusa compta was dominant in both lagoons with 63.4% of the total abundance. First records are 7 species for Laguna Madre and 11 species for Laguna de Tamiahua. A literature review updated the number of species from the 19 collected to 29. An analysis of the species’ distribution produced 3 zoogeographic patterns: Warm and Caribbean, Warm Temperate and Caribbean, and temperate. A hierarchical cluster analysis of the geographic distribution data of the 29 species produced 2 assemblages: one with an affinity for the warmer regions of the Gulf of Mexico, Caribbean Sea and Brazil, and another associated with the temperate conditions of the northern Gulf of Mexico, the eastern USA, Canada and Argentina, each group indicating a preference for either higher or lower water temperatures.
© 2017 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Keywords:
Lista de especies actualizada y notas zoogeográficas de anfípodos bentónicos (Crustacea: Peracarida: Amphipoda) de dos lagunas costeras en el golfo de México occidental
Resumen
Los anfípodos estuarinos del golfo de México occidental representan en su mayoría especies euritermas. El muestreo se llevó a cabo en verano e invierno en pastos marinos, macroalgas y sustratos sin vegetación, en 75 localidades de laguna Madre y 34 de laguna de Tamiahua, México, con una red Renfro y una red camaronera. Se recolectaron 19,398 ejemplares de 19 especies. Cymadusa compta fue dominante en ambas lagunas con el 63.4% de la abundancia total. Se anotaron como primeros registros 7 especies en la laguna Madre y 11 en la laguna de Tamiahua. Se actualizó el número de especies de las 19 recolectadas a 29 mediante una revisión de la literatura. El análisis de la distribución de las especies proporcionó 3 patrones zoogeográficos: cálido-caribeño, cálido-templado-caribeño y templado. El análisis de conglomerados de la distribución geográfica de las 29 especies formó 2 grupos: uno con afinidad por las regiones más cálidas del golfo de México, Caribe y Brasil, y otro asociado a las condiciones templadas del norte del golfo de México, el este de EE. UU., Canadá y Argentina, indicando cada grupo una preferencia por temperaturas más cálidas o más frescas.
Palabras clave
Gammaridea; Corophiidea; Zoogeografía; Laguna Madre; Laguna de Tamiahua; México
Introduction
Amphipods, together with polychaetes and bivalves, make up 77% of the abundance in benthic communities of continental shelves and deep seas ( Probert & Grove, 1998 ). They are a diverse, abundant and species-rich group of peracarid crustaceans. Most are free-living marine species, though some are freshwater, brackish or terrestrial. They are mostly eurythermal and euryhaline. They inhabit a wide variety of habitats and their geographic distribution is mostly cosmopolitan. They influence the structure of estuarine and coastal communities through their high abundance ( Baldo, Arias, & Drake, 2001; Saunders, Attrill, Shaw, & Rowden, 2003; Tanner, 2006 ) and varied reproductive strategies and behaviour ( Johnson, Stevens, & Watling, 2000; Thiel, 1997 ). They also affect community function by playing a part in the biogeochemical cycles of sedimentary organic matter ( Gardner, Escobar, Cruz, & Rowe, 1993; Thiel & Hinojosa, 2009 ), promoting bioturbation and sediment stabilisation in soft substrates, as parasites of some vertebrates and vectors in the transmission of diseases and microorganisms, as well as through their feeding habits ( DeBlois & Leggett, 1993; Duffy, 1990; Kamermans et al., 2002 ), nutrient recycling, linking foraging and detritivore food webs, and being prey of fish, birds and other crustaceans ( Escobar & Soto, 1997; Hill & Elmgren, 1992; Zupo & Nelson, 1999 ). They are also excellent bioindicators of environmental quality ( Montagna & Harper, 1996; Thomas, 1993b; Winfield & Ortiz, 2003 ).
Laguna Madre in Tamaulipas and Laguna de Tamiahua in Veracruz, Mexico, are large coastal lagoon systems that serve as nursery and feeding grounds for freshwater, estuarine and marine species. Their high biodiversity supports artisanal fisheries of oysters, shrimp, swimming crabs and mullet. Laguna Madre also provides feeding and refuge for migratory bird populations ( Tunnell & Judd, 2002 ). The salinity gradients, submerged aquatic vegetation and different substrate types favour a variety of habitats that are available for resident and visiting species. In particular, the part that aquatic vegetation plays in the recruitment of many estuarine invertebrate and fish species has long been recognised ( Heck & Crowder, 1991 ). High values of abundance have been recorded for species of invertebrates and fish that are temporal or permanent community components in these 2 lagoons ( Raz-Guzmán & Barba, 2000 ). However, few studies have focused on the amphipods of these lagoons, and their zoogeography has not been reported. A literature review of studies on amphipods of the Mexican Gulf of Mexico coastal lagoons provided only the papers of Corona, Soto, and Sánchez (2000) and Cházaro, Winfield, Ortiz, and Álvarez (2002) for Laguna de Términos in Campeche, Winfield, Ortiz, Franco, and Bedia (1997) , Winfield, Escobar, and Álvarez (2001) , Winfield, Cházaro-Olvera, and Álvarez (2007) , and Winfield and Ortiz (2011) for Laguna de Alvarado, Cházaro et al. (2002) and Winfield and Ortiz (2011) for Laguna Camaronera, and Winfield and Ortiz (2011) for Laguna de Sontecomapan in Veracruz, and Barba and Sánchez (2005) and Ortega (2013) for Laguna Madre in Tamaulipas. The only study found for Laguna de Tamiahua was that of Winfield and Ortiz (2011).
The zoogeographic provinces along the Western Atlantic and the Gulf of Mexico have been described by Hedgpeth (1953), Briggs (1974), and Barnwell and Thurman (1984) . Zoogeographic province boundaries are important barriers to the dispersal of tropical shallow marine species ( Thurman, 1987 ). The boundaries along the Western Atlantic include the northern tropical-temperate boundary along an imaginary line that spans from Cabo Rojo in Veracruz to Tampa Bay and Cape Canaveral in Florida and the southern tropical-temperate boundary at Cabo Frío in Brazil. Laguna de Tamiahua is zoogeographically significant as its island barrier of Cabo Rojo lies at the transition between the Caribbean-tropical and the Carolinean-Temperate provinces in the western Gulf of Mexico ( Thurman, 1987).
The recording of species along a geographical gradient provides information on the biogeographical and systematic structure of life, and the mapping of the geographic distribution of species contributes to understanding ecological and evolutionary patterns and processes. Cluster analyses assemble species with similar geographic localities, as well as geographic localities with a similar species composition, and support the establishment of zoogeographic distribution patterns ( Craw, Grehan, & Heads, 1999 ). Considering that amphipod studies in Mexican coastal lagoons are few, the purpose of this study was to define the zoogeographic patterns of 29 amphipod species recorded for Laguna Madre and Laguna de Tamiahua, based on updated data of the species’ geographic distribution that includes both published records for the Western Atlantic and the localities sampled in this study.
Materials and methods
Laguna Madre is the largest coastal lagoon in Mexico with an area of 200,000 ha. It lies at 23°45′–25°27′ N, 97°23′–97°52′ W. It is limited to the north by the delta of the Río Bravo and to the south by the Río Soto la Marina. In 1996, the sand barrier was breached by 5 inlets: Boca de Mezquital, Boca Ciega, Boca de Catán, Boca de Caballo and Boca Soto la Marina. As a result of its location in a semi-arid area, its limited communication with the sea and the reduced input of freshwater from the Río San Fernando, it is a hypersaline system with salinities of 33–62‰, water temperatures of 25–35 °C in Summer and 19–25 °C in Winter, and a maximum depth of 6 m (measured with a rope and dead weight). Further south, Laguna de Tamiahua is the third largest coastal lagoon in the Mexican Gulf of Mexico with 88,000 ha. It lies at 21°16′–22°05′ N and 97°23′–97°43′ W. It is limited to the north by the Río Pánuco and to the south by the Río Tuxpan, with the sandy barrier of Cabo Rojo to the east and 2 inlets, Tampachichi to the north and Corazones to the south. Six streams discharge into the lagoon along its western banks and contribute freshwater seasonally. For this reason, salinity varies from 22 to 38‰, water temperatures from 28 to 35 °C in Summer and 22 to 27 °C in Winter, and the maximum depth is 4 m (Fig. 1 ). Both lagoons have 2 marked climatic seasons, a dry Summer, and a rainy Winter when strong northerly winds (northers) are common ( Salcedo, Soto, Estradas-Romero, & Botello, 2017 ).
Figure 1
Laguna Madre, Tamaulipas and Laguna de Tamiahua, Veracruz, Mexico.
The submerged aquatic vegetation in both lagoons is characterised by the shoalgrass Halodule wrightii Aschers that settles along the inner margins of the sand barriers, and macroalgae along the western margins of the lagoons that include the green algae Cladophora sp. and Halimeda incrassata (Ellis) Lamouroux syn. H. tridens, the brown algae Dictyota dichotoma (Hudson) Lamouroux, Padina sp. and Rosenvingea intricata (J. Agardh) Börgensen, and the red algae Hypnea cervicornis J. Agardh, Gracilaria blodgettii Harvey, Gingicithara cylindrica Börgensen, Chondria littoralis Harvey and Digenea simplex (Wulfen) C. Agardh. Fringing mangroves in Laguna Madre are present only in the extreme south and are mostly Avicennia germinans (L.)L., while in Laguna de Tamiahua one finds small mangrove forests of Rhizophora mangle L., A. germinans, Laguncularia racemosa (L.) L. F. Gaertner and Conocarpus erectus L.
Specimens were collected in Summer (August) and Winter (December) of 1996 from 75 localities in August and 32 in December in Laguna Madre, and 34 localities in August and 23 in December in Laguna de Tamiahua ( Fig. 1 ). Regarding the sampling date, it is important to make 3 considerations after having consulted the only 3 more recent studies available: 1) that only 3 species of 13 collected by Barba and Sánchez (2005) and 3 species of 12 collected by Ortega (2013) in Laguna Madre, as well as 5 species of 6 collected by Winfield and Ortiz (2011) in Laguna de Tamiahua, were not collected in our study; 2) that in our study we recorded 9 more species than Barba and Sánchez (2005), 11 more species than Ortega (2013) and 11 more species than Winfield and Ortiz (2011) , and 3) that all the species recorded in these 3 papers plus the species collected in our study are included here, in view of which our species list may be considered to be taxonomically updated and valid.
Water temperature and salinity were recorded with a field thermometer and a refractometer respectively. Two nets were used to obtain specimens of different sizes and habitats: a Renfro beam net (1 mm mesh size, 50 m2 sampling area) and an otter trawl (1 cm mesh size, 1.5–3 min capture per unit effort). The amphipods were preserved in 10% formalin in the field, after which they were transferred to a solution of glycerin and 70% alcohol for whitening previous to dissection.
The specimens were observed in detail in order to insure a correct identification of the species. The families are listed in alphabetical order. The assignment of genera to families follows Bousfield (1973), Martín and Díaz (2003) and LeCroy (2002, 2004, 2007) . Exceptions are the Corophiidae that follow Myers and Lowry (2003) and the talitridan stat. nov. that follow Serejo (2004) . The arrangement of genera and families included in the 2 latter works were adopted since they provide more recent phylogenetic evidence of relationships within the corophiidan and talitridan amphipods. The genera and species were identified following Barnard (1969), Bousfield (1973), Myers (1982), Myers and McGrath (1984), Ledoyer (1986), Conlan (1990), Barnard and Karaman (1991), Thomas (1993a), LeCroy (2000, 2002, 2004, 2007) , Myers and Lowry (2003), Appadoo and Myers (2004), and Serejo (2004). The species list follows Martin and Davis (2001) for the Suborder Gammaridea, Myers and Lowry (2003) for the Suborder Corophiidea, with an updating following Ahyong et al. (2011) and Horton, Lowry, and De Broyer (2013) .
An Olmstead-Tükey analysis was applied to the abundance and spatial frequency data in order to identify dominant species. These and first records of species and families are reported for each lagoon, as well as the species collected in this study together with 3 species reported by Barba and Sánchez (2005) for Laguna Madre, 3 species reported by Ortega (2013) for the same lagoon (his Protohyale sp. B was not included in the analysis), and 5 species reported by Winfield and Ortiz (2011) for Laguna de Tamiahua. An updating was carried out in January 2015 for all species’ names ( http://www.itis.gov; Martin & Davis, 2001; Myers & Lowry, 2003 ) and geographic distributions. The specimens were kept in the Laboratorio de Ecología del Bentos, ICMyL, UNAM.
A map of the Western Atlantic including the important zoogeographic landmarks of Cape Cod, Cape Hatteras, Cape Canaveral and Tampa Bay in the United States, Cabo Rojo in Mexico, and Cabo Frío in Brazil, was prepared for each species considering the published data on the geographic distribution of each species (the geographic localities and the authors that reported them for each species are presented in Appendix 1 ), as well as the updated records for Laguna Madre and Laguna de Tamiahua. The Primer v.6 programme ( Clarke & Gorley, 2006 ) was used to analyse a presence-absence matrix for species-geographic localities, and group geographic points according to Pearson’s average linkage correlation to form clusters. A cluster analysis to group species provided no clear pattern.
Results
Laguna Madre and Laguna de Tamiahua experience marked seasonal changes in water temperature throughout the year. In Winter, values drop to 19 °C in Madre and to 22 °C in Tamiahua, while in Summer, there is a maximum of 35 °C in both lagoons. Salinity varied from 33 to 62‰ in Madre and from 22 to 38‰ in Tamiahua ( Table 1).
Table 1
Temperature, salinity, seasonal abundance (ind), number of species (spp.) and number of families (fam) of amphipods collected in each lagoon in this study.
| Temperature
Salinity |
Laguna Madre
19–35 °C 33–62‰ |
Laguna de Tamiahua
22–35 °C 22–38‰ |
| Abundance-species-families | 13,052 ind – 18 spp. – 14 fam | 6,346 ind – 12 spp. – 9 fam |
| Summer | 5,367 ind – 14 spp. | 4,209 ind – 10 spp. |
| Winter | 7,685 ind – 13 spp. | 2,137 ind – 11 spp. |
A total of 19,398 specimens of 19 amphipod species were collected in the 2 lagoons. Laguna Madre provided 13,052 amphipods of 18 species and 14 families, of which 5,367 specimens and 14 species were collected in August and 7,685 specimens and 13 species in December. In the case of Laguna de Tamiahua, the amphipods collected included 6,346 specimens of 12 species and 9 families, with 4,209 specimens and 10 species recorded in August, and 2,137 specimens and 11 species in December ( Table 1 ). Three species of the 13 recorded by Barba and Sánchez (2005), 3 species of the 12 recorded by Ortega (2013) , and 5 species of the 6 recorded by Winfield and Ortiz (2011) that were not collected in the present study were added to the list, providing a total of 24 species for Laguna Madre and 17 species for Laguna de Tamiahua. In total, 29 species were included in the zoogeographic analysis ( Table 2).
Table 2
Amphipod species and abundance in Laguna Madre (LM) and Laguna de Tamiahua (LT) (+ Barba & Sánchez, 2005; ++ Winfield & Ortiz, 2011; +++ Ortega, 2013).
| Family | Species | LM | LT |
| Subphylum Crustacea Brünnich, 1772 | |||
| Class Malacostraca Latreille, 1802 | |||
| Subclass Eumalacostraca Grobben, 1892 | |||
| Superorder Peracarida Calman, 1904 | |||
| Order Amphipoda Latreille, 1816 | |||
| Suborder Gammaridea Latreille, 1802 (sensu Martin & Davis, 2001) | |||
| Ampeliscidae Krøyer, 1842 | Ampelisca vadorum Mills, 1963 | 2 | 2 |
| Ampelisca venetiensis Shoemaker, 1916 | ++ | ||
| Ampelisca verrilli Mills, 1967 | ++ | ||
| Amphilochidae Boeck, 1871 | Hourstonius laguna (McKinney, 1978) | 589 | 7 |
| Bateidae Stebbing, 1906 | Batea catharinensis F. Müller, 1865 | 794 | |
| Dexaminidae Leach, 1814 | Nototropis minikoi (A.O. Walker, 1905) | 119 | 2 |
| Gammaridae Leach, 1814 | Gammarus mucronatus Say, 1818 | 42 | 847 |
| Hyalidae Bulycheva, 1957 | Apohyale prevostii (H. Milne Edwards, 1830) | 5 | 791 |
| Parhyale hawaiensis (Dana, 1853) | +++ | ||
| Melitidae Bousfield, 1983 | Elasmopus levis (S.I. Smith, 1873) | 2684 | 80 |
| Elasmopus pectenicrus (Bate, 1862) | +++ | ||
| Elasmopus rapax Costa, 1853 | +++ | ||
| Melita nitida S.I. Smith, 1873 | 1 | 54 | |
| Phoxocephalidae G.O. Sars, 1891 | Eobrolgus spinosus (Holmes, 1905) | 1 | ++ |
| Talitridae Rafinesque, 1815 | Orchestia gammarellus (Pallas, 1766) | + | |
| Orchestia grillus (Bosc, 1802) | 13 | ||
| Suborder Corophiidea Leach, 1814 (sensu Myers & Lowry, 2003 ) | |||
| Infraorder Corophiida Leach, 1814 | |||
| Superfamily Aoroidea Stebbing, 1899 | |||
| Aoridae Stebbing, 1899 | Grandidierella bonnieroides Stephensen, 1948 | 55 | |
| Lembos websteri Bate, 1857 | + | ||
| Unciolidae Myers & Lowry, 2003 | Unciola serrata Shoemaker, 1945 | ++ | |
| Superfamily Corophioidea Leach, 1814 | |||
| Ampithoidae Stebbing, 1899 | Ampithoe longimana S.I. Smith, 1873 | 10 | 1 |
| Ampithoe valida S.I. Smith, 1873 | 4 | 76 | |
| Cymadusa compta (S.I. Smith, 1873) | 8054 | 4240 | |
| Corophiidae Leach, 1814 | Monocorophium acherusicum (Costa, 1851) | 1 | |
| Monocorophium tuberculatum (Shoemaker, 1934) | 202 | ||
| Infraorder Caprellida Leach, 1814 | |||
| Superfamily Microprotopoidea Myers & Lowry, 2003 | |||
| Microprotopidae Myers & Lowry, 2003 | Microprotopus raneyi Wigley, 1966 | 1 | |
| Superfamily Photoidea Boeck, 1871 | |||
| Ischyroceridae Stebbing, 1899 | Cerapus tubularis Say, 1817 | 1 | |
| Ericthonius brasiliensis (Dana, 1853) | 674 | 245 | |
| Jassa falcata (Montagu,1808) | + | ||
| Photidae Boeck 1871 | Photis longicaudata (Bate & Westwood, 1862) | ++ | |
The amphipod species of Laguna Madre and Laguna de Tamiahua, together with the anomuran and brachyuran crabs of the 4 largest estuarine systems of the western and southwestern Gulf of Mexico, contribute to the highly diversified fauna of the Tropical Western Atlantic ( Raz-Guzmán & Sánchez, 1992a, 1992b, 1996, 1998; Guzmán, Sánchez, Soto, & Álvarez, 1986; Guzmán, Sánchez, & Soto, 1992; Raz-Guzmán, Sánchez, Peralta, & Florido, 2004 ). It is interesting to note that in spite of Cabo Rojo being the barrier island of Laguna de Tamiahua and representing a critical boundary for the northern dispersal of tropical shallow water fauna ( Thurman, 1987 ), this lagoon and Laguna Madre further north share 12 of the 29 amphipod species recorded here, a 41% affinity that may indicate that these 2 lagoons constitute habitats of positive population growth from which amphipods can successfully disperse to neighbouring areas.
Cymadusa compta (S.I. Smith, 1873) was dominant in both lagoons with 63.4% of the overall total abundance. Of the 18 species collected in this study in Laguna Madre, 2 constitute 82.3% of the total abundance: C. compta (61.7%) and Elasmopus levis (S.I. Smith, 1873) (20.6%), and are followed by Batea catharinensis F. Müller, 1865 (6.1%), Ericthonius brasiliensis (Dana, 1853) (5.2%), Hourstonius laguna (McKinney, 1978 ) (4.5%), and the other 13 species with 0.008–0.912%. Of the 12 species collected in Laguna de Tamiahua, C. compta is markedly more abundant than the others with 66.8%, and is followed by Gammarus mucronatus Say, 1818 (13.4%), Apohyale prevostii (H. Milne Edwards, 1830) (12.5%), E. brasiliensis (3.9%), and the other 8 species with 0.016–1.261% ( Table 3).
Table 3
Dominant amphipod species in Laguna Madre (LM) and Laguna de Tamiahua (LT) (% of total abundance).
| Species | Laguna Madre | Laguna de Tamiahua |
| Cymadusa compta | 61.7 | 66.8 |
| Elasmopus levis | 20.6 | |
| Gammarus mucronatus | 13.4 | |
| Apohyale prevostii | 12.5 | |
| Batea catharinensis | 6.1 | |
| Ericthonius brasiliensis | 5.2 | 3.9 |
| Hourstonius laguna | 4.5 | |
| The other 13 species in LM | 0.008–0.912 | |
| The other 8 species in LT | 0.016–1.261 | |
Seven species reported here for Laguna Madre were not recorded by Barba and Sánchez (2005) or Ortega (2013). Ampelisca vadorum Mills, 1963, H. laguna, Ampithoe longimana S.I. Smith, 1873, Ampithoe valida S.I. Smith, 1873, Melita nitida S.I. Smith, 1873, Eobrolgus spinosus (Holmes, 1905) and Orchestia grillus (Bosc, 1802) may thus be considered first records for this lagoon, as well as 2 families, the Amphilochidae and Phoxocephalidae. In the case of Laguna de Tamiahua, with the exception of G. mucronatus which was recorded previously by Winfield and Ortiz (2011), the other 11 species, A. vadorum, H. laguna, A. longimana, A. valida, C. compta, Monocorophium acherusicum (Costa, 1851), Nototropis minikoi (A.O. Walker, 1905), A. prevostii, E. brasiliensis, E. levis and M. nitida , are first records, together with the family Amphilochidae.
The mapping of the geographic distribution data of each of the 29 species recorded here made it possible to visually define 3 zoogeographic distribution patterns along the Western Atlantic from Newfoundland to Argentina: Warm and Caribbean with 5 species (17%), Warm Temperate and Caribbean with 18 species (62%) and Temperate with 6 species (21%). A hierarchical cluster analysis of the presence-absence matrix of geographic distribution data of the 29 amphipod species produced 2 groups at a correlation level of −0.05 (with other smaller groups at higher correlation levels). One group included the warmer regions of the Gulf of Mexico, Caribbean Sea and Brazil (+), and another group included the colder regions of the northern Gulf of Mexico, the eastern and northeastern United States, Canada, Greenland, and Argentina (●).
Discussion
The first records reported here for Laguna Madre ( A. vadorum, H. laguna, A. longimana, A. valida, M. nitida, E. spinosus and O. grillus) and Laguna de Tamiahua (A. vadorum, H. laguna, A. longimana, A. valida, C. compta, M. acherusicum, N. minikoi, A. prevostii, E. brasiliensis, E. levis and M. nitida ) respond to the fact that previous studies on amphipods in the state of Veracruz have focused on Laguna Camaronera ( Cházaro et al., 2002; Winfield & Ortiz, 2011 ), Laguna de Alvarado ( Winfield & Ortiz, 2011; Winfield et al., 1997, 2001; Winfield, Cházaro-Olvera, et al., 2007 ), Laguna de Sontecomapan (Winfield & Ortiz, 2011 ), the Veracruz Reef System ( Winfield & Ortiz, 2011; Winfield, Abarca-Arenas, & Cházaro-Olvera, 2007; Winfield, Cházaro-Olvera, Horta-Puga, Lozano-Aburto, & Arenas-Fuentes, 2010; Winfield, Cházaro-Olvera, Ortiz, & Palomo-Aguayo, 2011; Winfield, 2013 ) and the Veracruz continental shelf ( Winfield & Ortiz, 2011), with only that of Winfield and Ortiz (2011) for Laguna de Tamiahua, while only those of Barba and Sánchez (2005) and Ortega (2013) have dealt with Laguna Madre in the state of Tamaulipas.
The 5 Warm and Caribbean species, Ampelisca venetiensis Shoemaker, 1916, H. laguna, Parhyale hawaiensis (Dana, 1853), Photis longicaudata (Bate & Westwood, 1862) and Elasmopus pectenicrus (Bate, 1862), are mostly distributed along the eastern coast of Florida and the Gulf of Mexico (except for A. venetiensis ), throughout the Caribbean Sea, and along the coast of Brazil (except for A. venetiensis and H. laguna ). These species include a few records along the Temperate northern Gulf of Mexico which may be explained as the result of dispersal by the shallow coastal currents that flow from Louisiana and Texas (the LATEX current) to Campeche during the Winter and back during the Summer ( Fernández, Zavala, & Romero, 1998 ) (Fig. 2 ). The capacity to disperse is important in the life history of many species. Benthic invertebrates usually disperse through a swimming larval phase. However, many species with a direct development have wide geographic distributions ( Castilla & Guikez, 2000; Thiel, 2003a ). Floating and rafting have been identified as highly efficient dispersal mechanisms in these species ( Highsmith, 1985; Thiel, 2003b ), which include shallow-water isopods and amphipods ( Locke & Corey, 1989 ), many of which brood their young. This has been supported by Havermans, De Broyer, Mallefet, and Zintzen (2007) who recorded how Jassa herdmani (Walker, 1893), a tube-building amphipod, may travel long distances between hard-substrate areas, and by records of brooding benthic peracarids found in plancton nets in areas far from their native habitats ( Williams & Bynum, 1972 ). Most common floaters are small individuals that may remain suspended on the water surface for long periods of time. Small animals are also more likely to live on seagrass and macroalgae, and may become rafters when the plants break ( Norton & Mathieson, 1983 ). However, gravid females have also been observed rendering rafting a potential dispersal mechanism. It is important to note that P. hawaiensis , a species used in developmental and genetic studies, may actually occur as a species complex ( Myers, 1985).
Figure 2
Distribution of Warm and Caribbean species ( Ampelisca venetiensis, Hourstonius laguna, Parhyale hawaiensis, Photis longicaudata, Elasmopus pectenicrus ) along the Western Atlantic (■) including the Mexican lagoons (●).
The warm affinity of these species is supported by records from other warm localities that include Hawaii, the Philippines and the South China Sea for P. hawaiensis , and Hawaii, the Red Sea, Madagascar, Mauritius, India, Indonesia, the South China Sea, New Guinea and Australia for E. pectenicrus. P. longicaudata has been recorded in the Indian Ocean, however it is also present in colder localities including Sweden, Ireland, Scotland, England, Belgium, France, Spain, Greece, the Mediterranean Sea, the Black Sea and Japan (see Appendix 1).
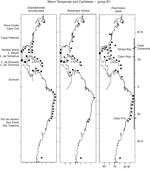
The 18 Warm Temperate and Caribbean species may be grouped into 5 sub-groups. Six species in group A1, Unciola serrata Shoemaker, 1945, Ampelisca verrilli Mills, 1967, C. compta, E. levis, E. spinosus and Microprotopus raneyi Wigley, 1966, present a mostly continuous distribution from Maine, south along the eastern coast of the United States, the Gulf of Mexico and throughout the Caribbean Sea (except for U. serrata which at present has no records in the Caribbean) ( Fig. 3 ). The species in proup A2 follow the same distribution but include temperate habitats in Nova Scotia and a northernmost point in the Gulf of St Lawrence, Canada. These are G. mucronatus, A. vadorum, A. longimana and M. nitida. It is interesting to note that G. mucronatus has 1 single record in the Caribbean, in Quintana Roo, Mexico ( Fig. 4).
Figure 3
Distribution of Warm Temperate and Caribbean species. Group A1 ( Unciola serrata, Ampelisca verrilli, Cymadusa compta, Elasmopus levis, Eobrolgus spinosus, Microprotopus raneyi ) along the Western Atlantic (■) including the Mexican lagoons (●).
Figure 4
Distribution of Warm Temperate and Caribbean species. Group A2 ( Gammarus mucronatus, Ampelisca vadorum, Ampithoe longimana, Melita nitida ) along the Western Atlantic (■) including the Mexican lagoons (●).
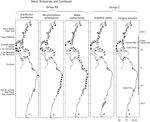
The species in group B1 are distributed along the southern half of the eastern coast of the United States, the Gulf of Mexico, the Caribbean Sea and the coast of Brazil, and include Grandidierella bonnieroides Stephensen, 1948, N. minikoi and Elasmopus rapax Costa, 1853 (Fig. 5). In particular, regarding G. bonnieroides, Myers (1970) reviewed its status and stated that specimens collected in the Gulf of Mexico, Caribbean Sea, Colombia, Venezuela, and Trinidad corresponded to this species. Asari and Myers (1982) later observed variations in specimens collected in the Western Atlantic and concluded that a complex of species was present. However, the 55 specimens from Laguna Madre were identified by A. Corona (pers comm), an expert in amphipod taxonomy, leaving no doubt as to the identity of the species. Three of the Warm Temperate and Caribbean species, C. compta, A. longimana and E. rapax , have also been recorded in Bermuda. The species in group B2, E. brasiliensis, M. acherusicum and B. catharinensis , have the same distribution but extend northwards to Nova Scotia ( Fig. 6 ). The coastline between Venezuela and Brazil with no records in the case of some species most probably indicates a lack of studies throughout the area rather than a disjunct distribution of the species.
Figure 5
Distribution of Warm Temperate and Caribbean species. Group B1 ( Grandidierella bonnieroides, Nototropis minikoi, Elasmopus rapax ) along the Western Atlantic (■) including the Mexican lagoons (●).
Figure 6
Distribution of Warm Temperate and Caribbean species. Group B2 ( Ericthonius brasiliensis, Monocorophium acherusicum, Batea catharinensis) and group C (Ampithoe valida, Cerapus tubularis ) along the Western Atlantic (■) including the Mexican lagoons (●).
The species in group C, A. valida and Cerapus tubularis Say, 1817, are distributed along the eastern coast of the United States from Maine to Florida, throughout the Gulf of Mexico, the Caribbean Sea, southern Brazil ( C. tubularis ) and include some southernmost points in Argentina ( Fig. 6).
The warm and temperate affinity of these species is reflected in the wide range of geographic locations where they have been recorded and indicates a high ecologic plasticity, as Melo (1990) stated for South American brachyurans. The geographic locations previously recorded for these species, which latitudinally include the Gulf of Mexico, validate their presence in this area. As an example of this, 12 species reported here are amphiamerican or cosmopolitan: E. spinosus (Washington to California, USA; Baja California and Gulf of California, Mexico), G. mucronatus (California, USA), A. longimana (Baja California and Gulf of California, Mexico), M. nitida (British Columbia-Canada, Washington to California, USA, Baja California, Sinaloa and Michoacán, Mexico, Costa Rica, Panama, Ecuador, United Kingdom, Netherlands, Germany), G. bonnieroides (Tanzania, Mozambique, South Africa, Madagascar, Mauritius, India, Australia), N. minikoi (East India, South China Sea, Indo-Pacific), E. rapax (United Kingdom, Italy, Mediterranean Sea, Turkey, Red Sea, Australia), E. brasiliensis (California, USA, Baja California, Mexico, Norway, United Kingdom, France, Spain, Italy, Mediterranean Sea, Mauritius, Indian Ocean, South China Sea), M. acherusicum (Alaska to California, USA, Baja California, Mexico, Hawaii, United Kingdom, Netherlands, Mediterranean Sea, France, Italy, Egypt, Tunisia, Algeria, Senegal, Cape Colony, Dar es Salaam, Mauritius, Indian Ocean, Japan, South China Sea, Australia, New Zealand), B. catharinensis (Baja California, Mexico), A. valida (British Columbia, Canada to California, USA, Japan, Korea), and C. tubularis (California, USA to Baja California, Mexico, Japan, Korea) ( Appendix 1).
Among the 6 temperate species (Fig. 7), Lembos websteri Bate, 1857, Monocorophium tuberculatum (Shoemaker, 1934) and O. grillus are distributed from Maine to eastern Florida and in the Gulf of Mexico. L. websteri also has records in an extremely northerly point in Greenland, while O. grillus extends northwards to the Newfoundland coast and southwards to Colombia (this last point is considered an isolated record). M. tuberculatum is included in this group in spite of having 1 record in Florida Bay, in consideration of its clearly northern affinity and its absence from typically tropical habitats such as Laguna de Términos, Campeche where Ledoyer (1986) and Corona et al. (2000) did not record it in spite of thorough sampling. These species present a disjunct distribution at the tip of Florida where ocean water masses are more characteristically tropical, as is the case of the Carolinian zoogeographic province ( Thurman, 1987).
Figure 7
Distribution of temperate species (Lembos websteri, Monocorophium tuberculatum, Orchestia grillus, Apohyale prevostii, Jassa falcata, Orchestia gammarellus ) along the Western Atlantic (■) including the Mexican lagoons (●).
The other 3 temperate species, A. prevostii, Jassa falcata (Montagu, 1808) and Orchestia gammarellus (Pallas, 1766), also present a northerly distribution up to Newfoundland. A. prevostii extends south to Laguna de Tamiahua, Veracruz (with 791 specimens collected in this study) at the northern tropical-temperate boundary for shallow water fauna within the western Gulf of Mexico, and includes an isolated record in eastern Brazil. J. falcata and O. gammarellus also present a southern temperate distribution with records in southern Brazil ( J. falcata ), an isolated point in eastern Brazil ( O. gammarellus ) and Argentina (both species). The presence of species in widely separated localities such as these generally responds to dispersal processes governed by ocean currents, rafting on plant debris, logs, sargassum and macroalgae, and transportation in ships’ ballast water ( Appadoo & Steele, 1998).
L. websteri, M. tuberculatum, O. grillus and J. falcata , previously recorded in the northern Gulf of Mexico, may disperse west and south to the northern tropical-Temperate boundary at Cabo Rojo, Veracruz, by floating and/or rafting on the LATEX coastal current, as is discussed above in the section on the “Warm and Caribbean species” ( Castilla & Guikez, 2000; Fernández et al., 1998; Havermans et al., 2007; Highsmith, 1985; Locke & Corey, 1989; Norton & Mathieson, 1983; Thiel, 2003a, 2003b; Williams & Bynum, 1972 ). The 6 temperate species were recorded in Laguna Madre, Tamaulipas, which lies north of this zoogeographic boundary.
As a confirmation of the temperate affinity of these species, other temperate localities have been reported for 4 of these species: for L. websteri Norway, England, France, Spain, Italy, Yugoslavia, Greece, Turkey, Israel, Egypt, the Mediterranean Sea and West Africa, for A. prevostii the Arctic Ocean, Iceland, Ireland, Norway, the United Kingdom, the Faroe Islands, the Netherlands, Belgium, France and Spain, for J. falcata Ireland, Norway, the United Kingdom, Spain, the Mediterranean Sea, British Columbia, Canada to California-United States and New Zealand, and for O. gammarellus Iceland, Norway, the United Kingdom, Spain, the Mediterranean Sea, the Black Sea, Turkey and SW Africa ( Appendix 1).
The area with no records that may be seen for some species along the Central American coasts and the northern and northeastern South American coasts may, in general, be attributed to a lack of local studies. Future studies in these areas should thus contribute to filling the information gaps, particularly in the case of species in the Warm Temperate and Caribbean distribution pattern.
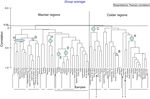
The grouping of warmer (+) and colder (●) geographic localities generated by the hierarchical cluster analysis based on the presence of gammarid and corophiid amphipods along the Western Atlantic ( Figs. 8 and 9 ) indicates that these species, despite most being eurythermal, have a physiological preference for higher or lower water temperatures ( Barba & Sánchez, 2005; d’Udekem-d’Acoz, & Verheye, 2013 ).
Figure 8
Cluster diagram of geographic points recorded for the 29 amphipod species of Laguna Madre, Tamaulipas and Laguna de Tamiahua, Veracruz, Mexico, showing one group of warmer regions and another group of colder regions. Eight smaller groups may be defined as: A, eastern and northeastern United States; B, Argentina; C, northeastern United States, Canada and Argentina (and ? Panama); D, northeastern Brazil; E, Caribbean; F, northwestern Gulf of Mexico; G, western, southern and eastern Gulf of Mexico, and H, Caribbean. Three interrogation marks (?) indicate odd localities within groups.
Figure 9
Grouping of the warmer (+) and colder (●) regions along the Western Atlantic based on the geographic points recorded for the 29 amphipod species of Laguna Madre, Tamaulipas and Laguna de Tamiahua, Veracruz, Mexico. The 2 slotted lines represent the zoogeographic boundaries that separate the warmer tropical regions from the colder Temperate regions along the Western Atlantic. The northern boundary spans along an imaginary line from Cabo Rojo in Veracruz to Tampa Bay and Cape Canaveral in Florida, and the southern boundary lies at Cabo Frío in Brazil.
This is the first comprehensive study of gammarid and corophiid amphipods of 2 major coastal lagoons in the western Gulf of Mexico that are characterised by their unique environmental conditions and are located at the northern tropical-temperate zoogeographical boundary that restricts the dispersion of tropical shallow water fauna. It may be stated that these estuarine amphipods may be further analysed, not only due to their abundance and diversity but because some species may be considered good indicators of zoogeographic provinces along the Western Atlantic.
Acknowledgements
To A. Corona for sample processing, identification of species and the note on G. bonnieroides ; N. López for identifying the macroalgae; L. Huidobro and D. Salcedo for running the cluster analyses; M. Thiel for reviewing an earlier version of the manuscript, and especially I. Winfield for reviewing the final draft. Conabio provided the funds to carry out this research (project H258).
Appendix 1
Geographic distribution and references per species (+ Barba & Sánchez, 2005; ++ Winfield & Ortiz, 2011; +++ Ortega, 2013 ). Lesser Antilles: St. Thomas, Tortola, Anegada, Jost van Dyke, Virgin Gorda, St. Croix, Anguilla, St. Martin, St. Barthélemy, Saba, St. Eustatius, Barbuda, St. Kitts, Nevis, Antigua, Montserrat, Guadeloupe, Dominica, Martinique, St. Lucia, St. Vincent, Grenadines, Grenada. References:
A = www.geocities.com/ajuarrero/amphipods.html
B = w ww.bdt.fat.org.br/workshop/costa/praias/tabela3
C = w w w & # 4 6 ; a c d & # 46;ofrj.br/mndi/Carcinologia/hp/Text/Amphipoda1.htm
| Geographic distribution | References |
| Warm and Caribbean | |
| Ampelisca venetiensis ++ Figure 2
Laguna de Tamiahua in Veracruz, Mexico; Costa Rica; Caribbean Sea; Colombia. |
Escobar, Winfield, Ortiz, Gasca, & Suárez, 2002; Escobar & Winfield, 2003; Ortiz, Martín, & Díaz, 2007; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013 |
| Hourstonius laguna Figure 2
St John’s River, Indian River Lagoon, Biscayne Bay, Florida Bay in Florida, Mississippi, and Galveston, San Antonio Bay, Corpus Christi Bay, Laguna Madre in Texas, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua, Laguna Camaronera and Laguna de Alvarado in Veracruz, Laguna de Términos in Campeche, Quintana Roo, Mexico; Bermuda; Caribbean Sea; Cuba; Colombia; Venezuela. – California, US. |
McKinney, 1978; Ledoyer, 1986; Barnard & Karaman, 1991; Ortiz & Lalana, 1993; Nelson, 1995; Camp, Lyons, & Perkins, 1998; Corona et al., 2000; Cházaro et al., 2002; LeCroy, 2002; Ortiz et al., 2007; Winfield, Cházaro-Olvera, et al., 2007; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013 |
| Parhyale hawaiensis +++ Figure 2
Florida, Louisiana, US; Gulf of Mexico; Soto la Marina in Tamaulipas, Quintana Roo, Mexico; Caribbean Sea; Cuba; Haiti; Dominican Republic; Puerto Rico; Venezuela; Aruba; Curaçao; Bonaire; Lesser Antilles; Rio de Janeiro to Paraná, Brazil. – Circumtropical; California, US; Atlantic and Pacific oceans; Hawaii; Fiji; Philippines; South China Sea; Mediterranean Sea. |
Shoemaker, 1956; Thomas, 1976; Myers, 1985; Camp et al., 1998; Wakabara & Serejo, 1998; Serejo, 1999; Lowry, 2000; Ortiz et al., 2007; Somaio & Moreira, 2008; LeCroy, Gasca, Winfield, Ortiz, & Escobar-Briones, 2009; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Ortega, 2013 |
| Photis longicaudata ++ Figure 2
Florida, US; Laguna de Tamiahua in Veracruz, Quintana Roo, Mexico; Caribbean Sea; Venezuela; Pernambuco, Alagoas, Espirito Santo, Rio de Janeiro, São Paulo, Rio Grande do Sul, Brazil. – Atlantic and Indian oceans; Sweden; Ireland; Scotland; England; Belgium; France; Spain; Greece; Mediterranean Sea; Black Sea; Japan. |
Wakabara, Tararam, Valério-Berardo, Duleba, & Pereira, 1991; Camp et al., 1998; Wakabara & Serejo, 1998; Escobar et al., 2002; Ortiz et al., 2007; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013 ; www.marinespecies.org |
| Elasmopus pectenicrus +++ Figure 2
Florida, Florida Keys, US; Gulf of Mexico; Laguna Madre in Tamaulipas, Quintana Roo, Mexico; Caribbean Sea; Cuba; Lesser Antilles; west, central and east coasts of Venezuela; Ceara to Paraná, Brazil. – Circumtropical; Atlantic, Pacific and Indian oceans; Hawaii; Mediterranean Sea; Turkey; Red Sea; western and southern Africa; Madagascar; Mauritius; India; Indonesia; South China Sea; New Guinea; Australia. |
Barnard, 1970; McKinney, 1977; Ortiz, 1978; Karaman, 1982; Wakabara, Tararam, & Takeda, 1983; Hay, Duffy, Fenical, & Gustafson, 1988; Appadoo & Steele, 1998; Camp et al., 1998; Wakabara & Serejo, 1998; Lowry, 2000; Díaz & Martín, 2001; Escobar et al., 2002; Appadoo & Myers, 2003; Martín & Díaz, 2003; Ortiz et al., 2007; LeCroy et al., 2009; Bakir, Sezgin, & Katagan, 2010; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Martín et al., 2013; Ortega, 2013; Ortega & Martín, 2013 |
| Warm Temperate and Caribbean | |
| Unciola serrata ++ Figure 3
Cape Cod in Massachusetts to Delaware Bay and Virginia, Florida, US; Laguna de Tamiahua in Veracruz, Mexico. |
Dickinson, Wigley, Brodeur, & Brown-Leger, 1980; Camp et al., 1998; Winfield & Ortiz, 2011 ; www.marinespecies.org |
| Ampelisca verrilli ++ Figure 3
Cape Cod in Massachusetts to Delaware Bay and Chesapeake Bay, Virginia to Cape Hatteras in North Carolina, Indian River Lagoon in Florida, US; Laguna de Tamiahua and Veracruz reef system in Veracruz, Campeche, Quintana Roo, Mexico; Caribbean Sea. |
Dickinson et al., 1980; Nelson, 1995; Camp et al., 1998; Eggleston, Elis, Etherington, Dahlgren, & Posey, 1999; Escobar et al., 2002; Escobar & Winfield, 2003; Winfield, Escobar, & Morrone, 2006; Ortiz et al., 2007; Winfield & Escobar, 2007; Cerrato et al., 2010; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013 ; www.marinespecies.org |
| Cymadusa compta Figure 3
Maine, New Hampshire to Cape Cod, Pleasant Bay, Nantucket, Martha’s Vineyard and Buzzards Bay in Massachusetts, Rhode Island, Connecticut, New Jersey, Delaware Bay, Chesapeake Bay, North Carolina to Indian River Lagoon, Riviera Beach, Biscayne Bay in Florida and Florida Keys to Texas, Laguna Madre in Texas, US; Laguna Madre and Soto la Marina in Tamaulipas, Laguna de Tamiahua, Laguna de Alvarado and Veracruz reef system in Veracruz, Laguna de Términos in Campeche, Quintana Roo, Mexico; Bermuda; Caribbean Sea; Cuba. |
Watling & Maurer, 1972; Bousfield, 1973; Ledoyer, 1986; Ortiz & Lalana, 1993; Thomas, 1993a; Nelson, 1995; Camp et al., 1998; Eggleston et al., 1999; Corona et al., 2000; Cházaro et al., 2002; LeCroy, 2002; Varela, Manuel, & Rogelio, 2003; Barba & Sánchez, 2005; Ortiz et al., 2007; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Martín et al., 2013; Ortega, 2013 |
| Elasmopus levis Figure 3
Maine, New Hampshire, Cape Cod, Vineyard Sound, Woods Hole, Buzzards Bay in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina to Georgia, Biscayne Bay and Florida Keys, Port Isabel, San Antonio Bay, Corpus Christi Bay, Laguna Madre in Texas, US; Gulf of Mexico; Laguna Madre and Soto la Marina in Tamaulipas, Laguna de Tamiahua, Laguna de Alvarado and Veracruz reef system in Veracruz, Laguna de Términos in Campeche, Yucatán, Quintana Roo, Mexico; Caribbean Sea; Venezuela. |
Watling & Maurer, 1972; Bousfield, 1973; McKinney, 1977; Dickinson et al., 1980; Ledoyer, 1986; Ortiz & Lalana, 1993; Camp et al., 1998; Eggleston et al., 1999; Corona et al., 2000; LeCroy, 2000; Escobar et al., 2002; Barba & Sánchez, 2005; Ortiz et al., 2007; Winfield, Abarca-Arenas, et al., 2007; Cerrato et al., 2010; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013; Ortega, 2013; Paz-Ríos & Ardisson, 2013 |
| Eobrolgus spinosus Figure 3
Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, New York to Delaware Bay, Chesapeake Bay, North Carolina, Georgia, Florida, Florida Bay, Florida Keys, Charlotte Harbor, Tampa Bay to Apalachee Bay in Florida, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua in Veracruz, southwestern Gulf of Mexico, Laguna de Términos and Campeche Bay in Campeche, Arrecife Alacranes in Yucatán, Quintana Roo, Mexico; Caribbean Sea; Cuba; Venezuela. – Washington to California, US; Bahía San Quintín, Baja California and Gulf of California, Mexico. |
Barnard, 1970; Watling & Maurer, 1972; Bousfield, 1973; Barnard & Barnard, 1981; Ledoyer, 1986; Camp et al., 1998; Eggleston et al., 1999; Corona et al., 2000; Díaz & Martín, 2001; Martín, Ortiz, & Díaz, 2002; Escobar & Winfield, 2003; Martín & Díaz, 2003; Winfield et al., 2006; Ortiz et al., 2007; Cerrato et al., 2010; Miloslavich et al., 2010; Ortiz & Lalana, 2010; LeCroy, 2011; Winfield & Ortiz, 2011; Martín et al., 2013; Paz-Ríos, Simões, & Ardisson, 2013 |
| Microprotopus raneyi Figure 3
Canada; Maine, New Hampshire, Cape Cod Bay, Martha’s Vineyard, Vineyard Sound, Buzzards Bay, Woods Hole in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, Edisto Island in South Carolina, St Simon’s Island in Georgia, north Florida, Sarasota Bay and Perdido Key in Florida, Louisiana, Texas, US; northern Gulf of Mexico; Laguna Madre in Tamaulipas, Mexico; Caribbean Sea; Colombia; Venezuela. |
Lowry, 1972; Watling & Maurer, 1972; Bousfield, 1973; Dickinson et al., 1980; Barnard & Karaman, 1991; Rakocinski, Heard, LeCroy, McLelland, & Simons, 1993; Camp et al., 1998; Eggleston et al., 1999; Díaz & Martín, 2001; Barba & Sánchez, 2005; Ortiz et al., 2007; Dubois, Gelpi, Condrey, Grippo, & Fleeger, 2009; Miloslavich et al., 2010; Martín et al., 2013; Ortega & Martín, 2013 |
| Gammarus mucronatus Figure 4
Gulf of St Lawrence and Nova Scotia, Canada; Maine, New Hampshire, Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina to Florida and Florida Keys, Louisiana, Laguna Madre in Texas, US; northern Gulf of Mexico; Laguna Madre and Soto la Marina in Tamaulipas, Laguna de Tamiahua, Laguna de Alvarado and continental shelf of Veracruz, southwestern Gulf of Mexico, Laguna de Términos in Campeche, Quintana Roo, Mexico. – California, US. |
Watling & Maurer, 1972; Bousfield, 1973; Thomas, 1976; Lincoln, 1979; Dickinson et al., 1980; Heard, 1982; Ledoyer, 1986; Ortiz & Lalana, 1993; Nelson, 1995; Winfield et al., 1997; Camp et al., 1998; Eggleston et al., 1999; Cházaro et al., 2002; Escobar & Winfield, 2003; Barba & Sánchez, 2005; Ortiz et al., 2007; Winfield, Cházaro-Olvera, et al., 2007; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013; Ortega, 2013 |
| Ampelisca vadorum Figure 4
SW Gulf of St Lawrence, Nova Scotia, Canada; Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, South Carolina, Georgia, Florida and Florida Keys, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua and continental shelf off Alvarado in Veracruz, southwestern Gulf of Mexico, Laguna de Términos and Bay of Campeche in Campeche, Yucatán, Quintana Roo, Mexico; Caribbean Sea; Cuba. |
Mills, 1963; Watling & Maurer, 1972; Bousfield, 1973; Dickinson et al., 1980; Ledoyer, 1986; Barnard & Karaman, 1991; Ortiz & Lalana, 1993; Nelson, 1995; Camp et al., 1998; Eggleston et al., 1999; Corona et al., 2000; LeCroy, 2002; Escobar & Winfield, 2003; Winfield et al., 2006; Ortiz et al., 2007; Winfield & Escobar, 2007; Cerrato et al., 2010; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Martín et al., 2013; Paz-Ríos & Ardisson, 2013 |
| Ampithoe longimana Figure 4
SW Gulf of St Lawrence, Canada; Maine, Cape Cod in Massachusetts, Chesapeake Bay to North Carolina and Indian River Lagoon, Biscayne Bay, Florida Bay, Florida Keys, Tampa Bay in Florida to South Padre Island in Texas, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua and Veracruz reef system in Veracruz, Mexico; Bermuda; Bahamas; Caribbean Sea; Venezuela – West Baja California, Gulf of California, Mexico. |
Barnard, 1963; Bousfield, 1973; Ortiz & Lalana, 1993; Nelson, 1995; Camp et al., 1998; Eggleston et al., 1999; LeCroy, 2002; Martín & Díaz, 2003; Ortiz et al., 2007; Miloslavich et al., 2010; Winfield & Ortiz, 2011; Winfield et al., 2011; Martín et al., 2013; Winfield, 2013 |
| Melita nitida Figure 4
SW Gulf of St Lawrence, Nova Scotia, Canada; Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, Georgia, Cape Romano in Florida, Louisiana, US; Gulf of Mexico; Laguna Madre in Tamaulipas, Laguna de Tamiahua and Veracruz reef system in Veracruz, Laguna de Términos in Campeche, Yucatán, Quintana Roo, Mexico; Costa Rica; Caribbean Sea; Cuba; Panama; Colombia.–British Columbia, Canada; Washington to California, US; Baja California, Mazatlán in Sinaloa, Laguna Salinas del Padre and Coahuayana River in Michoacán, Mexico; Costa Rica; Panama; Ecuador; United Kingdom; Netherlands; Germany. |
Watling & Maurer, 1972; Bousfield, 1973; Thomas, 1976; Lincoln, 1979; Dickinson et al., 1980; Heard, 1982; Ortiz, 1983; Chapman, 1988; Ortiz & Lalana, 1993; Nelson, 1995; Camp et al., 1998; Eggleston et al., 1999; Corona et al., 2000; LeCroy, 2000; Corona & Raz-Guzmán, 2003; Faasse & van Moorsel, 2003; Ortiz et al., 2007; Cerrato et al., 2010; Miloslavich et al., 2010; Winfield et al., 2011; Martín et al., 2013; Winfield, 2013 ; A |
| Grandidierella bonnieroides Figure 5
North Carolina, Georgia, east Florida, Florida Keys, west Florida, Louisiana, Texas, US; Laguna Madre and Soto la Marina in Tamaulipas, Laguna de Alvarado in Veracruz, Laguna de Términos in Campeche, Yucatán, Quintana Roo, Mexico; Caribbean Sea; Cuba; Colombia; Venezuela; Trinidad and Tobago; Aruba; Curaçao; Bonaire; Maranhao to São Paulo, Brazil. – Tanzania; Mozambique; South Africa; Madagascar; Mauritius; India; Australia. |
Fox & Bynum, 1975; Thomas, 1976; Leite, Tararam, & Wakabara, 1980; Asari & Myers, 1982; Heard, 1982; Ledoyer, 1986; Wakabara et al., 1991; Ortiz & Lalana, 1993; Nelson, 1995; Winfield et al., 1997; Camp et al., 1998; Wakabara & Serejo, 1998; Corona et al., 2000; Ortiz, 2001; Winfield et al., 2001; Cházaro et al., 2002; LeCroy, 2002; Martín & Díaz, 2003; Appadoo & Myers, 2004; Ortiz et al., 2007; Winfield, Cházaro-Olvera, et al., 2007; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Winfield & Ortiz, 2011; Martín et al., 2013; Ortega, 2013; Paz-Ríos & Ardisson, 2013 ; A, B, C |
| Nototropis minikoi Figure 5
Virginia, North Carolina, Florida, Laguna Madre in Texas, US; Laguna Madre and Soto la Marina in Tamaulipas, Laguna de Tamiahua, Laguna Camaronera and Laguna de Alvarado in Veracruz, Laguna de Términos in Campeche, Arrecife Alacranes in Yucatán, Quintana Roo, Mexico; Costa Rica; Caribbean Sea; Cuba; Colombia; Barlovento, Isla Margarita in Venezuela; Pernambuco, Rio de Janeiro and São Paulo, Brazil. – Karwar Bay, East India; South China Sea; Indo Pacific. |
Stephensen, 1948; Oliveira, 1953; Bousfield, 1973; Bynum & Fox, 1977; Naomi, 1979; Leite et al., 1980; Ledoyer, 1986; Lagarde, 1987; Barnard & Karaman, 1991; Wakabara et al., 1991; Ortiz & Lalana, 1993; Wakabara, Nicoletti, & Tararam, 1996; Wakabara & Serejo, 1998; Lowry, 2000; Cházaro et al., 2002; Lowry & Stoddart, 2003; Martín & Díaz, 2003; Barba & Sánchez, 2005; McLaughlin et al., 2005; Ortiz et al., 2007; LeCroy et al., 2009; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Winfield & Ortiz, 2011; Martín et al., 2013; Ortega & Martín, 2013; Paz-Ríos et al., 2013 ; A, B, C |
| Elasmopus rapax +++ Figure 5
Delaware Bay, South Carolina, Georgia to Florida Keys, west Florida, Louisiana, Texas, US; Gulf of Mexico; Laguna Madre in Tamaulipas, Cayo Arcas in Yucatán, Quintana Roo, Mexico; Bermuda; Caribbean Sea; Cuba; Lesser Antilles; Curaçao; east coast of Venezuela; Guyana; Paraiba to São Paulo, Brazil. – Cosmopolitan tropical and temperate; Atlantic, Pacific and Indian oceans; United Kingdom; Italy; Mediterranean Sea; Turkey; Red Sea; eastern Australia. |
Shoemaker, 1933; Barnard, 1962; Bousfield, 1973; McKinney, 1977; Ortiz, 1978; Lincoln, 1979; Ortiz, 1979; Karaman, 1982; Ruffo, 1982; Galan, 1984; González, 1991; Wakabara et al., 1991; Procaccini & Scipione, 1992; Camp et al., 1998; Wakabara & Serejo, 1998; LeCroy, 2000; Escobar et al., 2002; LeCroy, 2002; Martín & Díaz, 2003; Ortiz et al., 2007; Winfield & Escobar, 2007; LeCroy et al., 2009; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Martín et al., 2013; Ortega, 2013; Paz-Ríos & Ardisson, 2013 ; http://www.skaphandrus.com |
| Ericthonius brasiliensis Figure 6
Maine, New Hampshire, Cape Cod, Vineyard Sound in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, Georgia, Florida and Florida Keys, Perdido Key, Louisiana, Texas, Port Isabel and Laguna Madre in Texas, US; Laguna Madre and Soto la Marina in Tamaulipas, Laguna de Tamiahua, Laguna Camaronera, Laguna de Alvarado and Veracruz reef system in Veracruz, southwestern Gulf of Mexico, Laguna de Términos in Campeche, Yucatán, Quintana Roo, Mexico; Sigsbee abyssal plain; Costa Rica; Caribbean Sea; Cuba; Lesser Antilles; Colombia; Venezuela; Pernambuco, Rio de Janeiro and São Paulo, Brazil. – Circumtropical cosmopolitan; Atlantic, Pacific and Indian oceans; California, US; Bahía San Quintín, Baja California, Mexico; Norway; United Kingdom; France; Spain; Italy; Mediterranean Sea; Mauritius; South China Sea. |
Barnard, 1970; Watling & Maurer, 1972; Bousfield, 1973; McKinney, 1977; Lincoln, 1979; Dickinson et al., 1980; Leite et al., 1980; Ruffo, 1982; Myers & McGrath, 1984; Wakabara et al., 1991; Procaccini & Scipione, 1992; Ortiz & Lalana, 1993; Rakocinski et al., 1993; Thomas, 1993a; Nelson, 1995; Appadoo & Steele, 1998; Camp et al., 1998; Wakabara & Serejo, 1998; Eggleston et al., 1999; Lowry, 2000; Cházaro et al., 2002; Escobar & Winfield, 2003; Martín & Díaz, 2003; Varela et al., 2003; Appadoo & Myers, 2004; Barba & Sánchez, 2005; Winfield et al., 2006; Ortiz et al., 2007; Winfield, Abarca-Arenas, et al., 2007; Dubois et al., 2009; Cerrato et al., 2010; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Winfield et al., 2010; Winfield & Ortiz, 2011; Martín et al., 2013; Ortega, 2013; Paz-Ríos & Ardisson, 2013 ; A, B, C |
| Monocorophium acherusicum Figure 6
Bay of Fundy and Nova Scotia, Canada; Maine, New Hampshire, Massachusetts, Rhode Island, Connecticut, New Jersey, Delaware Bay, Chesapeake Bay, North Carolina, Georgia, Florida and Florida Keys, west Florida to Texas, US; Laguna de Tamiahua and Veracruz reef system in Veracruz, southwestern Gulf of Mexico, Quintana Roo, Mexico; Sigsbee abyssal plain; Cuba; Puerto Rico; Lesser Antilles; Venezuela; Pernambuco to Santa Catarina, Brazil. – Alaska to California, US; Bahía San Quintín, Baja California, Mexico; Hawaii; United Kingdom and the Netherlands south to the Mediterranean Sea including France, Italy, Egypt, Tunisia and Algeria; Senegal; Cape Colony; Dar es Salaam; Mauritius; Indian Ocean; Japan; South China Sea; Australia; New Zealand. |
Shoemaker, 1934; Barnard, 1970, 1971, 1972; Watling & Maurer, 1972; Bousfield, 1973; Lincoln, 1979; Myers, 1982; Ruffo, 1982; Barnard & Karaman, 1991; Wakabara et al., 1991; Procaccini & Scipione, 1992; Ortiz & Lalana, 1993; Nelson, 1995; Appadoo & Steele, 1998; Camp et al., 1998; Wakabara & Serejo, 1998; Eggleston et al., 1999; Lowry, 2000; Appadoo & Myers, 2004; LeCroy, 2004; Winfield et al., 2006; Ortiz et al., 2007; Somaio & Moreira, 2008; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Winfield et al., 2011; Martín et al., 2013; Ortega & Martín, 2013; Winfield, 2013 ; A, B |
| Batea catharinensis Figure 6
Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, Long Island Sound in New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, South Carolina, Georgia, Florida, Tampa Bay, Mississippi Sound, Louisiana, US; Laguna Madre in Tamaulipas, Quintana Roo, Mexico; Caribbean Sea; Cuba; Barbados to São Paulo and Santa Catarina, Brazil. – Cedros Island and Magdalena Bay in Baja California, Mexico. |
Barnard, 1972; Watling & Maurer, 1972; Bousfield, 1973; Dickinson et al., 1980; Wakabara et al., 1991; Ortiz & Lalana, 1993; Nelson, 1995; Camp et al., 1998; Wakabara & Serejo, 1998; Eggleston et al., 1999; Escobar & Winfield, 2003; LeCroy, 2004; Barba & Sánchez, 2005; Ortiz et al., 2007; Cerrato et al., 2010; Miloslavich et al., 2010; Ortiz & Lalana, 2010; Martín et al., 2013; Ortega, 2013 ; A, B, C |
| Ampithoe valida Figure 6
Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, Long Island Sound in New York, New Jersey, Delaware Bay, Chesapeake Bay, North Carolina, South Carolina, Georgia, Cape Canaveral in Florida, Apalachee Bay in Florida to Galveston Bay in Texas, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua in Veracruz, Quintana Roo, Mexico; Caribbean Sea; Venezuela; Quequén, Rio Negro, Chubut = Group A: 36°–43°S, Argentina. – British Columbia and Vancouver, Canada to Newport Bay in California, US; Japan; Korea. |
Watling & Maurer, 1972; Bousfield, 1973; Fox & Bynum, 1975; Alonso, Tablado, López-Gappa, & Magaldi, 1995; Camp et al., 1998; Eggleston et al., 1999; LeCroy, 2002; Martín & Díaz, 2003; López Gappa et al., 2006; Ortiz et al., 2007; Miloslavich et al., 2010; Martín et al., 2013 |
| Cerapus tubularis Figure 6
Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, Georgia, east Florida, Perdido Key, Louisiana, Texas, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua and Laguna de Alvarado in Veracruz, Quintana Roo, Mexico; Cuba; Colombia; São Paulo and Paraná, Brazil; Ría Deseado, Argentina. – San Diego in California, US to Ensenada in Baja California, Mexico; Japan; Korea. |
Watling & Maurer, 1972; Bousfield, 1973; Dickinson et al., 1980; Barnard & Karaman, 1991; Wakabara et al., 1991; Rakocinski et al., 1993; Nelson, 1995; Camp et al., 1998; Wakabara & Serejo, 1998; Barba & Sánchez, 2005; LeCroy, 2007; Ortiz et al., 2007; Dubois et al., 2009; Miloslavich et al., 2010 ; A, B, C |
| Temperate | |
| Lembos websteri + Figure 7
Greenland; Maine, New Hampshire, Cape Cod, Vineyard Sound in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina to Georgia and north Florida, west Florida, US; Laguna Madre in Tamaulipas, Mexico. – Norway; England; France; Spain; Italy; Yugoslavia; Greece; Turkey; Israel; Egypt; Algeria in the Mediterranean Sea; west Africa. |
Bousfield, 1973; Dickinson et al., 1980; Ruffo, 1982; Barnard & Karaman, 1991; Ortiz & Lalana, 1993; Camp et al., 1998; Eggleston et al., 1999; LeCroy, 2002; Escobar & Winfield, 2003; Barba & Sánchez, 2005; Ortiz et al., 2007 |
| Monocorophium tuberculatum Figure 7
Minas Basin, Bay of Fundy and Nova Scotia, Canada; Maine, New Hampshire, Cape Cod in Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, Georgia, Sebastian inlet in Florida, Florida Bay to Chandeleur Sound in Louisiana, US; Laguna Madre in Tamaulipas, Mexico. |
Shoemaker, 1934, 1947; Watling & Maurer, 1972; Bousfield, 1973; Dickinson et al., 1980; Bousfield & Hoover, 1997; Camp et al., 1998; LeCroy, 2004; Ortiz et al., 2007; Dubois et al., 2009; Ortega, 2013 |
| Orchestia grillus Figure 7
Newfoundland, Canada; Maine, New Hampshire, Massachusetts, Rhode Island, Connecticut, New Jersey, Delaware Bay, Chesapeake Bay, North Carolina, Georgia, Florida, Louisiana, Texas, US; Laguna Madre in Tamaulipas, Mexico; Colombia. |
Watling & Maurer, 1972; Bousfield, 1973; Thomas, 1976; Heard, 1982; Nelson, 1995; Camp et al., 1998; Ortiz et al., 2007; Miloslavich et al., 2010; Martín et al., 2013 |
| Apohyale prevostii Figure 7
Labrador, Gulf of St Lawrence, Nova Scotia, Canada; Maine, New Hampshire, Massachusetts, Rhode Island, Connecticut, US; Laguna Madre in Tamaulipas, Laguna de Tamiahua in Veracruz, Mexico; Bermuda; Pernambuco, Brazil. – Arctic Ocean; Iceland; Ireland; United Kingdom; Faroe Islands; Norway; Netherlands; Belgium; France; Spain. |
Bousfield, 1973; Lincoln, 1979; Wakabara & Serejo, 1998; Chavanich & Wilson, 2000; Barba & Sánchez, 2005; Lowry, 2014 |
| Jassa falcata + Figure 7
Newfoundland and Nova Scotia, Canada; Maine, New Hampshire, Massachusetts, Rhode Island, Connecticut, New York, New Jersey, Delaware Bay, Chesapeake Bay, Virginia, North Carolina, Georgia, Florida, Texas, US; Laguna Madre in Tamaulipas, Mexico; São Paulo, Brazil; Group A: 36°–43°S, Argentina. – Ireland; United Kingdom; Norway; Spain; Mediterranean Sea; British Columbia, Canada to California, US; South China Sea; New Zealand. |
Barnard, 1972; Watling & Maurer, 1972; Bousfield, 1973; Dickinson et al., 1980; Leite et al., 1980; Barnard & Karaman, 1991; Wakabara et al., 1991; Nelson, 1995; Camp et al., 1998; Wakabara & Serejo, 1998; Lowry, 2000; Barba & Sánchez, 2005; López Gappa et al., 2006; Ortiz et al., 2007; Cerrato et al., 2010 ; C |
| Orchestia gammarellus + Figure 7
Newfoundland to Cape Breton Island, Nova Scotia, Bay of Fundy, Canada to Maine, New Hampshire, Massachusetts, Rhode Island, Connecticut, US; Laguna Madre in Tamaulipas, Mexico; Pernambuco, Brazil; Group B: 36°–58°S, Argentina. – Iceland; Norway; United Kingdom; Spain; Mediterranean Sea; Black Sea, Turkey; southwest Africa. |
Bousfield, 1973; Soares, 1979; Wakabara & Serejo, 1998; Barba & Sánchez, 2005 ; López Gappa et al., 2006; Ortega, 2013; B, C |
References
Ahyong et al., 2011
S.T. Ahyong
J.K. Lowry
M. Alonso
R.N. Bamber
G.A. Boxshall
P. Castro
Subphylum Crustacea Brünnich, 1772
Zootaxa
Z.Q.Zhang
2011
165-91
Alonso et al., 1995
G. Alonso
A. Tablado
J. López-Gappa
N. Magaldi
Seasonal changes in an intertidal population of the amphipod Ampithoe valida Smith, 1873
Oebalia
XXI
1995
77-91
Appadoo and Myers, 2003
C. Appadoo
A.A. Myers
The genus Elasmopus (Crustacea: Amphipoda: Melitidae) from Mauritius (Indian Ocean) with description of five new species
Records of the Australian Museum
55
2003
61-84
Appadoo and Myers, 2004
C. Appadoo
A.A. Myers
Corophiidea (Crustacea: Amphipoda) from Mauritius
Records of the Australian Museum
56
2004
331-62
Appadoo and Steele, 1998
C. Appadoo
D. Steele
Shallow-water marine gammaridean amphipods of Mauritius Island
Crustaceana
71
1998
633-45
Asari and Myers, 1982
K.P. Asari
A.A. Myers
Taxonomic studies on the genus Grandidierella Coutiere (Crustacea, Amphipoda). IV. Indian species
Bulletin du Museum National d’Histoire Naturelle, Paris
4
1982
237-56
Bakir et al., 2010
K. Bakir
M. Sezgin
T. Katagan
Alien amphipods on the Turkish coasts
Zoologica Baetica
21
2010
191-6
Baldo et al., 2001
F. Baldo
A.M. Arias
P. Drake
The macrobenthic community of the Guadalquivir estuary
Boletín del Instituto Español de Oceanografía
17
2001
137-48
Barba and Sánchez, 2005
E. Barba
A.J. Sánchez
Peracarid crustaceans of central Laguna Madre, Tamaulipas in the southwestern Gulf of Mexico
Gulf of Mexico Science
23
2005
241-7
Barnard, 1962
J.L. Barnard
Benthic marine Amphipoda of Southern California: 1. Families Aoridae, Photidae, Ischyroceriae, Corophiidae, Podoceridae, 2. Families Tironidae to Gammaridae, 3. Families Amphilochidae, Leucothoidae, Stenothoidae, Argissidae, Hyalidae
Pacific Naturalist
3
1962
1-163
Barnard, 1963
J.L. Barnard
Los anfípodos bentónicos marinos de la costa occidental de Baja California
Revista de la Sociedad Mexicana de Historia Natural
XXIV
1963
205-73
Barnard, 1969
J.L. Barnard
The families and genera of marine gammaridean Amphipoda
US Natural Museum Bulletin
271
1969
1-535
Barnard, 1970
J.L. Barnard
Sublittoral gammaridean (Amphipoda) of the Hawaian Islands
Smithsonian Contributions in Zoology
34
1970
1-286
Barnard, 1971
J.L. Barnard
Keys to the Hawaiian marine Gammaridea, 0–30 meters
Smithsonian Contributions in Zoology
58
1971
1-135
Barnard, 1972
J.L. Barnard
The marine fauna of New Zealand: algae-living littoral Gammaridea (Crustacea Amphipoda)
New Zealand Department of Scientific and Industrial Research, Bulletin
210
1972
1-216
Barnard and Barnard, 1981
J.L. Barnard
C.M. Barnard
The amphipod genera Eobrolgus and Eyakia (Crustacea: Phoxocephalidae) in the Pacific Ocean
Proceedings of the Biological Society of Washington
94
1981
295-313
Barnard and Karaman, 1991
J.L. Barnard
G.S. Karaman
The families and genera of marine gammaridean Amphipoda (except marine Gammaroids)
Records of the Australian Museum (Supplement)
13
1991
1-866
Barnwell and Thurman, 1984
F.H. Barnwell
C.L. Thurman II.
Taxonomy and biogeography of the fiddler crabs (Ocypodidae: genus Uca ) of the Atlantic and Gulf coasts of eastern North America
Zoological Journal of the Linnean Society
81
1984
23-87
Bousfield, 1973
E.L. Bousfield
Shallow-water gammaridean amphipoda of New England
1973
Bousfield and Hoover, 1997
E.L. Bousfield
P.M. Hoover
The amphipod superfamily Corophioidea on the Pacific coast of North America. Part 5. Family Corophiidae: Corophiinae, new subfamily. Systematics and distributional ecology
Amphipacifica
2
1997
67-139
Briggs, 1974
J.C. Briggs
Marine zoogeography
1974
Bynum and Fox, 1977
K.H. Bynum
R.S. Fox
New and noteworthy amphipod crustaceans from North Carolina, US
Chesapeake Science
18
1977
1-33
Camp et al., 1998
D.K. Camp
W.G. Lyons
T.H. Perkins
Checklists of selected shallow-water marine invertebrates of Florida. Technical Report TR-3
1998
Castilla and Guikez, 2000
J.C. Castilla
R. Guikez
Disjoint geographical distribution of intertidal and nearshore benthic invertebrates in the Southern Hemisphere
Revista Chilena de Historia Natural
73
2000
585-603
Cerrato et al., 2010
R.M. Cerrato
R.D. Flood
L.C. Holt
Benthic mapping for habitat classification in the Peconic estuary: phase III ground truth studies
2010
Chapman, 1988
J.W. Chapman
Invasions of the northeast Pacific by Asian and Atlantic Gammaridean amphipod crustaceans, including a new species of Corophium
Journal of Crustacean Biology
8
1988
364-82
Chavanich and Wilson, 2000
S. Chavanich
K.A. Wilson
Rocky intertidal zonation of gammaridean amphipods in Long Island Sound, Connecticut
Crustaceana-International Journal of Crustacean Research
73
2000
835-46
Cházaro et al., 2002
S. Cházaro
I. Winfield
M. Ortiz
F. Álvarez
Peracarid crustaceans from three inlets in the southwestern Gulf of Mexico: new records and range extensions
Zootaxa
123
2002
1-16
Clarke and Gorley, 2006
K.R. Clarke
R.N. Gorley
PRIMER v6: user manual/tutorial
2006
Conlan, 1990
K.E. Conlan
Revision of the crustacean amphipod genus Jassa Leach (Corophioidea: Ischyroceridae)
Canadian Journal of Zoology
68
1990
2031-75
Corona and Raz-Guzmán, 2003
A. Corona
A. Raz-Guzmán
Distribución geográfica de los anfípodos e isópodos (Crustacea: Peracarida: Amphipoda e Isopoda) de los sistemas estuarinos de Michoacán, México
Contribuciones al estudio de los crustáceos del Pacífico Este 2
Instituto de Ciencias del Mar y Limnología, UNAM
Mexico, D.F.
2003
219-25
Corona et al., 2000
A. Corona
L.A. Soto
A.J. Sánchez
Epibenthic amphipod abundance and predation efficiency of the pink shrimp Farfantepenaeus duorarum (Burkenroad, 1939) in habitats with different physical complexity in a tropical estuarine system
Journal of Experimental Marine Biology and Ecology
253
2000
33-48
Craw et al., 1999
R.C. Craw
J.R. Grehan
M.J. Heads
Panbiogeography
1999
DeBlois and Leggett, 1993
E.M. DeBlois
W.C. Leggett
Impact of amphipod predation on the benthic eggs of marine fish: an analysis of Calliopius laeviusculus bioenergetic demands and predation on the eggs of a beach spawning osmeriid ( Mallotus villosus)
Marine Ecology Progress Series
93
1993
205-16
Díaz and Martín, 2001
Y.J. Díaz
A. Martín
New records of amphipods (Crustacea: Amphipoda) from shallow waters of the Caribbean coast of Venezuela
Revista de Biología Tropical
49
2001
1271-6
Dickinson et al., 1980
J.J. Dickinson
R.L. Wigley
R.D. Brodeur
S. Brown-Leger
Distribution of gammaridean Amphipoda (Crustacea) in the Middle Atlantic Bight Region
1980
Dubois et al., 2009
S. Dubois
C.G. Gelpi Jr.
R.E. Condrey
M.A. Grippo
J.W. Fleeger
Diversity and composition of macrobenthic community associated with sandy shoals of the Louisiana continental shelf
Biodiversity and Conservation
18
2009
3759-84
d’Udekem-d’Acoz and Verheye, 2013
C. d’Udekem-d’Acoz
M. Verheye
Taxocoenoses of amphipod crustaceans
J.Gutt
2013
57-67
Duffy, 1990
J.E. Duffy
Amphipods on seaweeds: partners or pests?
Oecologia
83
1990
267-76
Eggleston et al., 1999
D.B. Eggleston
W.E. Elis
L.L. Etherington
C.P. Dahlgren
M.H. Posey
Organism responses to habitat fragmentation and diversity: habitat colonization by estuarine macrofauna
Journal of Experimental Marine Biology and Ecology
236
1999
107-32
Escobar and Soto, 1997
E. Escobar
L.A. Soto
Continental shelf benthic biomass in the western Gulf of Mexico
Continental Shelf Research
17
1997
585-604
Escobar and Winfield, 2003
E. Escobar
I. Winfield
Checklist of the benthic Gammaridea and Caprellidea (Crustacea: Peracarida: Amphipoda) from the Gulf of Mexico continental shelf and slope
Belgian Journal of Zoology
133
2003
37-44
Escobar et al., 2002
E. Escobar
I. Winfield
M. Ortiz
E. Gasca
E. Suárez
Amphipoda
Conabio/UNAM
J.Llorente-BousquetsJ.J.Morrone
México, D.F.
2002
341-71
Faasse and van Moorsel, 2003
M. Faasse
G. van Moorsel
The North-American amphipods, Melita nitida Smith, 1873 and Incisocalliope aestuarius (Watling and Maurer, 1973) (Crustacea: Amphipoda: Gammaridea), introduced to the Western Scheldt estuary (The Netherlands)
Aquatic Ecology
37
2003
13-22
Fernández et al., 1998
A. Fernández
J. Zavala
R. Romero
Circulación de invierno en la plataforma de Tamaulipas y áreas adyacentes. IX Reunión Nacional SELPER-MEXICO 1998, Desarrollo Sustentable. Zacatecas, México. Memorias en CD-ROM
1998
Fox and Bynum, 1975
R.S. Fox
K.H. Bynum
The amphipod crustaceans of North Carolina estuarine waters
Chesapeake Science
16
1975
223-37
Galan, 1984
A. Galan
A systematic study of Amphipoda (Crustacea) of the Caribbean coast of Venezuela
1984
Gardner et al., 1993
W. Gardner
E. Escobar
E. Cruz
G. Rowe
Ammonium excretion by benthic invertebrates and sediment-water nitrogen flux in the Gulf of Mexico near the Mississippi River outflow
Estuaries
16
1993
799-808
González, 1991
E. González
The genus Hyale in Chile
Spixiana
14
1991
125-42
Havermans et al., 2007
C. Havermans
C. De Broyer
J. Mallefet
V. Zintzen
Dispersal mechanisms in amphipods: a case study of Jassa herdmani (Crustacea Amphipoda) in the North Sea
Marine Biology
153
2007
83-9
Hay et al., 1988
M.E. Hay
J.E. Duffy
W. Fenical
K. Gustafson
Chemical defense in the seaweed Dictyopteris delicatula: differential effects against reef fishes and amphipods
Marine Ecology Progress Series
48
1988
185-92
Heard, 1982
R.W. Heard
Guide to common tidal marsh invertebrates of the northeastern Gulf of Mexico
1982
Heck and Crowder, 1991
K.L. Heck Jr.
L.B. Crowder
Habitat structure and predator–prey interactions in vegetated aquatic systems
Habitat structure: the physical arrangement of objects in space
Chapman & Hall
London
1991
281-99
Hedgpeth, 1953
J.W. Hedgpeth
1953
107-224
Highsmith, 1985
R.C. Highsmith
Floating and algal rafting as potential dispersal mechanisms in brooding invertebrates
Marine Ecology Progress Series
25
1985
169-79
Hill and Elmgren, 1992
C. Hill
R. Elmgren
Predation by the isopod Saduria entomon on the amphipods Monoporeia affinis and Pontoporeia femorata: experiments on prey vulnerability
Oecologia
91
1992
153-6
Horton et al., 2013
T. Horton
J. Lowry
C. De Broyer
World Amphipoda database
2013
Johnson et al., 2000
W.S. Johnson
M. Stevens
L. Watling
Reproduction and development of marine peracarideans
Advances in Marine Biology
39
2000
107-220
Kamermans et al., 2002
P. Kamermans
E.J. Malta
J.M. Verschuure
L. Schrijvers
L.F. Lentz
A.T.A. Lien
Effect of grazing by isopods and amphipods on growth of Ulva spp. (Chlorophyta)
Aquatic Ecology
36
2002
425-33
Karaman, 1982
G.S. Karaman
Family Gammaridae
The Amphipoda of the Mediterranean, Part 1. Gammaridea (Acanthonotozomatidae to Gammaridae)
Memoires de l’Institut Oceanographique 13
Monaco
1982
245-364
Lagarde, 1987
G. Lagarde
Anfípodos Gammaridea del litoral de Golfo Triste y areas adyacentes
Caribbean Journal of Science
23
1987
260-77
LeCroy, 2000
S.E. LeCroy
An illustrated identification guide to the nearshore marine and estuarine gammaridean Amphipoda of Florida. Vol. 1
2000
LeCroy, 2002
S.E. LeCroy
An illustrated identification guide to the nearshore marine and estuarine gammaridean Amphipoda of Florida. Vol. 2
2002
LeCroy, 2004
S.E. LeCroy
An illustrated identification guide to the nearshore marine and estuarine gammaridean Amphipoda of Florida. Vol. 3
2004
LeCroy, 2007
S.E. LeCroy
An illustrated identification guide to the nearshore marine and estuarine gammaridean Amphipoda of Florida. Vol. 4
2007
LeCroy, 2011
S.E. LeCroy
An illustrated identification guide to the nearshore marine and estuarine gammaridean Amphipoda of Florida. Vol. 5
2011
LeCroy et al., 2009
S.E. LeCroy
R. Gasca
I. Winfield
M. Ortiz
E. Escobar-Briones
Amphipoda (Crustacea) of the Gulf of Mexico
Gulf of Mexico. Origin, waters and biota, Volume I, Biodiversity
Texas A&M University Press
College Station
2009
941-72
Ledoyer, 1986
M. Ledoyer
Faune mobile des herbiers de phanérogames marines ( Halodule et Thalassia ) de la Lagune de Términos (Mexique, Campeche). II. Les gammariens (Crustacea)
Anales del Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México
13
1986
171-200
Leite et al., 1980
F.P.P. Leite
A.S. Tararam
Y. Wakabara
Composiçao e distribuiçao da fauna de Gammaridea na regiao da Enseada da Fortaleza-Ubatuba, Estado de Sao Paulo
Boletim do Instituto Oceanografico
29
1980
297-9
Lincoln, 1979
R.J. Lincoln
British marine Amphipoda: Gammaridea
1979
Locke and Corey, 1989
A. Locke
S. Corey
Amphipods, isopods and surface currents: a case for passive dispersal in the Bay of Fundy Canada
Journal of Plankton Research
11
1989
419-30
López-Gappa et al., 2006
J. López-Gappa
G.M. Alonso
N.A. Landoni
Biodiversity of benthic Amphipoda (Crustacea: Peracarida) in the southwest Atlantic between 35°S and 56°S
Zootaxa
1342
2006
1-66
Lowry, 1972
J.K. Lowry
Taxonomy and distribution of Microprotopus along the east coast of the United States (Amphipoda: Isaeidae)
Crustaceana (Suppl.)
3
1972
277-86
Lowry, 2000
J.K. Lowry
Taxonomic status of amphipod crustaceans in the South China Sea with a checklist of known species
The Raffles Bulletin of Zoology (Suppl.)
8
2000
309-42
Lowry, 2014
Lowry, J. K. (2014). Hyale prevostii (Milne-Edwards, 1830). In T. Horton, J.K. Lowry, & C. de Broyer (2013 onwards) World Amphipoda. Retrieved from h t t p & # 5 8 ; & # 4 7 ; & # 4 7 ; w w w & # 4 6 ; m a r i n e s p e c i e s .org/amphipoda/aphia.php?p=taxdetails&id=102326 .
Lowry and Stoddart, 2003
J.K. Lowry
H.E. Stoddart
Crustacea: Malacostraca: Peracarida: Amphipoda, Cumacea Mysidacea
CSIRO Publishing
P.L.BeesleyW.W.K.Houston
Melbourne
2003
Martin and Davis, 2001
J.W. Martin
G.E. Davis
An updated classification of the recent Crustacea
Science Series
2001
39
Martín and Díaz, 2003
A. Martín
Y.J. Díaz
La fauna de anfípodos (Crustacea: Amphipoda) de las aguas costeras de la región oriental de Venezuela
Boletín del Instituto Español de Oceanografía
19
2003
327-44
Martín et al., 2002
A. Martín
M. Ortiz
Y.J. Díaz
Nuevos registros crustáceos anfípodos (Gammaridea) colectados en las costas del caribe venezolano
Boletín de Investigaciones Marinas y Costeras
31
2002
15-24
Martín et al., 2013
A. Martín
Y. Díaz
P. Miloslavich
E. Escobar-Briones
J.M. Guerra-García
M. Ortiz
Regional diversity of Amphipoda in the Caribbean Sea
Revista de Biología Tropical
61
2013
1681-720
McKinney, 1977
L.D. McKinney
The origin and distribution of shallow water gammaridean Amphipoda in the Gulf of Mexico and Caribbean Sea with notes on their ecology
1977
McKinney, 1978
L.D. McKinney
Amphilochidae (Crustacea: Amphipoda) from the western Gulf of Mexico and Caribbean Sea
Gulf Research Reports
6
1978
137-43
McLaughlin et al., 2005
P.A. McLaughlin
D.K. Camp
M.V. Angel
E.L. Bousfield
P. Brunei
R.C. Brusca
Common and scientific names of aquatic invertebrates from the United States and Canada: Crustaceans
2005
Melo, 1990
G.A.S. Melo
A presença, no litoral sudeste brasileiro, de espécies de Brachyura (Crustacea: Decapoda) originárias das regioes biogeográficas Magelânica e Argentina do Atlântico Sul
Atlântica
12
1990
71-83
Mills, 1963
E.L. Mills
A new species of Ampelisca (Crustacea: Amphipoda) from eastern North America, with notes on other species of the genus
Canadian Journal of Zoology
41
1963
971-89
Miloslavich et al., 2010
P. Miloslavich
J.M. Díaz
E. Klein
J.J. Alvarado
C. Díaz
J. Gobin
Marine biodiversity in the Caribbean: regional estimates and distribution patterns
PLoS ONE
5
2010
e11916
Montagna and Harper, 1996
P. Montagna
E. Harper
Benthic faunal long term response to offshore production platforms in the Gulf of Mexico
Canadian Journal of Fisheries and Aquatic Science
53
1996
2567-88
Myers, 1970
A.A. Myers
Taxonomic studies on the genus Grandidierella Coutiere (Crustacea: Amphipoda) with a description of G. dentimera sp. nov.
Bulletin of Marine Science
20
1970
135-47
Myers, 1982
A.A. Myers
Family Corophiidae
Memoires de l’Institut Océanographique
S.Ruffo
Monaco
1982
185-208
Myers, 1985
A.A. Myers
Shallow-water, coral reef and mangrove Amphipoda (Gammaridea) of Fiji
Records of the Australian Museum (Suppl)
5
1985
1-143
Myers and Lowry, 2003
A.A. Myers
J.K. Lowry
A phylogeny and new classification of the Corophiidea Leach 1814 (Amphipoda)
Journal of Crustacean Biology
23
2003
443-85
Myers and McGrath, 1984
A.A. Myers
D. McGrath
A revision of the north-east Atlantic species of Ericthonius (Crustacea: Amphipoda)
Journal of the Marine Biological Association of the United Kingdom
64
1984
379-400
Naomi, 1979
T.S. Naomi
On a swarm of amphipods Atylus minikoi (Walker) in the shallow waters of the Karwar Bay
Indian Journal of Fisheries
26
1979
227-8
Nelson, 1995
W.G. Nelson
Amphipod crustaceans of the Indian River Lagoon: current status and threats to biodiversity
Bulletin of Marine Science
57
1995
143-52
Norton and Mathieson, 1983
T.A. Norton
A.C. Mathieson
The biology of unattached seaweeds
Elsevier
F.E.RoundD.J.Chapman
Amsterdam/New York/Oxford
1983
333-86
Oliveira, 1953
L.P.H. Oliveira
Crustacea Amphipoda de Rio de Janeiro
Memorias del Instituto Oswaldo Cruz
51
1953
289-376
Ortega, 2013
V.M. Ortega
Taxonomía y distribución ecológica de los crustáceos peracáridos de la Laguna Madre y regiones costeras adyacentes, Tamaulipas México
2013
Ortega and Martín, 2013
I. Ortega
A. Martín
Suprabenthic amphipods from the littoral zone of Barlovento Venezuela: spatial distribution and seasonal variation
Crustaceana
86
2013
1206-23
Ortiz, 1978
M. Ortiz
Invertebrados marinos bentónicos de Cuba. I. Crustacea, Amphipoda, Gammaridea
Revista de Investigaciones Marinas, Serie 8
38
1978
3-10
Ortiz, 1979
M. Ortiz
Contribución al estudio de los anfípodos (Gammaridea) del Mediterráneo americano
Revista de Investigaciones Marinas, Serie 8
1979
1-16
Ortiz, 1983
M. Ortiz
Los anfípodos (Gammaridea) de las costas del Mar Caribe de la República de Colombia
Revista de Investigaciones Marinas
4
1983
23-31
Ortiz, 2001
M. Ortiz
Lista de invertebrados marinos, estuarinos y semiterrestres de la playa de Cojímar en la costa norte de la Provincia Ciudad de la Habana
Revista de Investigaciones Marinas
22
2001
93-102
Ortiz and Lalana, 1993
M. Ortiz
R. Lalana
Adición a la lista de especies y bibliografía de los anfípodos (Crustacea: Amphipoda) del Mediterráneo Americano
Revista de Investigaciones Marinas
14
1993
16-37
Ortiz and Lalana, 2010
M. Ortiz
R. Lalana
Distribución de los anfípodos (Crustacea, Malacostraca, Peracarida) de los subórdenes Gammaridea Caprellidea e Hyperiidea, presentes en el archipiélago cubano
Revista de Investigaciones Marinas
31
2010
75-90
Ortiz et al., 2007
M. Ortiz
A. Martín
Y.J. Díaz
Lista y referencias de los crustáceos anfípodos (Amphipoda: Gammaridea) del Atlántico occidental tropical
Revista de Biología Tropical
55
2007
479-98
Paz-Ríos and Ardisson, 2013
C.E. Paz-Ríos
P.L. Ardisson
Benthic amphipods (Amphipoda: Gammaridea and Corophiidea) from the Mexican southeast sector of the Gulf of Mexico: checklist, new records and zoogeographic comments
Zootaxa
3635
2013
137-73
Paz-Ríos et al., 2013
C.E. Paz-Ríos
N. Simões
P.L. Ardisson
Intertidal and shallow water amphipods (Amphipoda: Gammaridea and Corophiidea) from Isla Pérez Alacranes Reef, southern Gulf of Mexico
Nauplius
21
2013
179-94
Probert and Grove, 1998
P.K. Probert
S.L. Grove
Macrobenthic assemblages of the continental shelf and upper slope of the west coast of South Island, New Zealand
Journal of the Royal Society of New Zealand
28
1998
259-80
Procaccini and Scipione, 1992
G. Procaccini
M.B. Scipione
Observations on the spatio-temporal distribution of crustacean amphipods in the Fusaro coastal lagoon (Central Tyrrhenian Sea Italy) and some notes on their presence in Mediterranean lagoons
Marine Ecology
13
1992
203-24
Rakocinski et al., 1993
C.F. Rakocinski
R.W. Heard
S.E. LeCroy
J.A. McLelland
T. Simons
Seaward change and zonation of the sandy-shore macrofauna at Perdido Key, Florida, U.S.A. Estuarine
Coastal and Shelf Science
36
1993
81-104
Raz-Guzmán and Barba, 2000
A. Raz-Guzmán
E. Barba
Seagrass biomass, distribution and associated macrofauna in southwestern Gulf of Mexico coastal lagoons
Biologia Marina Mediterranea
7
2000
271-4
Raz-Guzmán and Sánchez, 1992a
A. Raz-Guzmán
A.J. Sánchez
Registros adicionales de cangrejos braquiuros (Crustacea: Brachyura) de Laguna de Términos, Campeche
Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología
63
1992
29-45
Raz-Guzmán and Sánchez, 1992b
A. Raz-Guzmán
A.J. Sánchez
Registros adicionales de cangrejos braquiuros (Crustacea: Brachyura) del sistema lagunar de Alvarado, Veracruz
Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología
63
1992
273-7
Raz-Guzmán and Sánchez, 1996
A. Raz-Guzmán
A.J. Sánchez
Catálogo ilustrado de cangrejos braquiuros (Crustacea) de la Laguna de Tamiahua, Veracruz, México. Cuadernos, 31
1996
Raz-Guzmán and Sánchez, 1998
A. Raz-Guzmán
A.J. Sánchez
Catálogo con sinonimias y notas sobre el hábitat de los cangrejos ermitaños estuarinos del suroeste del Golfo de México
Universidad y Ciencia
14
1998
17-31
Raz-Guzmán et al., 1986
A. Raz-Guzmán
A.J. Sánchez
L.A. Soto
F. Álvarez
Catálogo ilustrado de cangrejos braquiuros y anomuros de Laguna de Términos, Campeche (Crustacea: Brachyura, Anomura)
Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología
57
1986
343-83
Raz-Guzmán et al., 1992
A. Raz-Guzmán
A.J. Sánchez
L.A. Soto
Catálogo ilustrado de cangrejos braquiuros y anomuros (Crustacea) de Laguna de Alvarado, Veracruz México. Cuadernos, 14
1992
Raz-Guzmán et al., 2004
A. Raz-Guzmán
A.J. Sánchez
P. Peralta
R. Florido
Zoogeography of hermit crabs (Decapoda: Diogenidae, Paguridae) from four coastal lagoons in the Gulf of Mexico
Journal of Crustacean Biology
24
2004
625-36
Ruffo, 1982
S. Ruffo
The Amphipoda of the Mediterranean Part 1. Gammaridea (Acanthonotozomatidae to Gammaridae)
1982
13
Salcedo et al., 2017
D.L. Salcedo
L.A. Soto
A. Estradas-Romero
A. Botello
Interannual variability of soft-bottom macrobenthic communities of the NW Gulf of Mexico in relationship to the Deepwater Horizon oil spill
Marine Pollution Bulletin
114
2017
987-94
Saunders et al., 2003
J.E. Saunders
M.J. Attrill
S.M. Shaw
A.A. Rowden
Spatial variability in the epiphytic algal assemblages of Zostera marina seagrass beds
Marine Ecology Progress Series
249
2003
107-15
Serejo, 1999
C.S. Serejo
Taxonomy and distribution of the family Hyalidae (Amphipoda, Talitroidea) on the Brazilian coast
Crustaceans and the biodiversity crisis
Proceedings of the Fourth International Crustacean Congress
Amsterdam, The Netherlands, Koninklijke Brill NV, Leiden
1999
591-616
Serejo, 2004
C.S. Serejo
Cladistic revision of talitroidean amphipods (Crustacea Gammaridea), with a proposal of a new classification
Zoologica Scripta
33
2004
551-86
Shoemaker, 1933
C.R. Shoemaker
Amphipoda from Florida and the West Indies American Museum
Novitates
598
1933
1-24
Shoemaker, 1934
C.R. Shoemaker
The amphipod genus Corophium on the east coast of America
Proceedings of the Biological Society of Washington
47
1934
23-32
Shoemaker, 1947
C.R. Shoemaker
Further notes on the amphipod genus Corophium from the east coast of America
Journal of the Washington Academy of Sciences
37
1947
47-63
Shoemaker, 1956
C.R. Shoemaker
Observations on the amphipod genus Parhyale
Proceedings of the United States National Museum
106
1956
345-58
Soares, 1979
C.M.A. Soares
Estudo ecológico da região de Itamaracá, Pernambuco Brasil. III. Anfípodos das familias Talitridae e Ampithoidae. (1)
Trabalhos Oceanográficos da Universidade Federal de Pernambuco
14
1979
93-104
Somaio and Moreira, 2008
C. Somaio
R. Moreira
Introduced and cryptogenic species and their management in Paranaguá Bay Brazil
Brazilian Archives of Biology and Technology
51
2008
623-33
Stephensen, 1948
K. Stephensen
1948
1-20
Tanner, 2006
J.E. Tanner
Landscape ecology of interactions between seagrass and mobile epifauna: the matrix matters
Estuarine, Coastal and Shelf Science
68
2006
404-12
Thiel, 1997
M. Thiel
Reproductive biology of an epibenthic amphipod ( Dyopedos monacanthus) with extended parental care
Journal of the Marine Biological Association of the United Kingdom
77
1997
1059-72
Thiel, 2003a
M. Thiel
Extended parental care in crustaceans – an update
Revista Chilena de Historia Natural
76
2003
205-18
Thiel, 2003b
M. Thiel
Rafting of benthic macrofauna: important factors determining the temporal succession of the assemblage on detached macroalgae
Hydrobiologia
503
2003
49-57
Thiel and Hinojosa, 2009
M. Thiel
I.A. Hinojosa
Peracáridos – Anfípodos, Isópodos, Tanaidáceos y Cumáceos
Fauna marina bentónica de la Patagonia chilena
Nature in Focus
Santiago de Chile
2009
674-8
Thomas, 1976
J.D. Thomas
A survey of gammarid amphipods of the Barataria Bay Louisiana region
Contributions in Marine Science
20
1976
87-100
Thomas, 1993a
J.D. Thomas
Identification manual for marine Amphipoda (Gammaridea): I. Common coral reef and rocky bottom amphipods of South Florida. Tallahassee, Florida
1993
Thomas, 1993b
J.D. Thomas
Biological monitoring and tropical biodiversity in marine environments: a critique with recommendations and comments on the use of amphipods as bioindicators
Journal of Natural History
27
1993
795-806
Thurman, 1987
C.L. Thurman II
Fiddler crabs (Genus Uca ) of eastern Mexico (Decapoda, Brachyura Ocypodidae)
Crustaceana
53
1987
94-105
Tunnell and Judd, 2002
J.W. Tunnell
F.W. Judd
The Laguna Madre of Texas and Tamaulipas
2002
Varela et al., 2003
C. Varela
O. Manuel
L. Rogelio
Crustáceos (Peracarida y Decapoda) de la costa sur de la península de Guanahacabibes Cuba
Revista de Investigaciones Marinas
24
2003
73-6
Wakabara and Serejo, 1998
Y. Wakabara
C.S. Serejo
Malacostraca – Peracarida, Amphipoda, Gammaridea and Caprellidea
Catalogue of Crustacea of Brazil
Museu Nacional, Série Livros # 6
Rio de Janeiro
1998
561-94
Wakabara et al., 1983
Y. Wakabara
A.S. Tararam
A.M. Takeda
Comparative study of the amphipod fauna living on Sargassum of two Itanhaém shores
Brazilian Journal of Crustacean Biology
3
1983
602-7
Wakabara et al., 1991
Y. Wakabara
A.S. Tararam
M.T. Valério-Berardo
W. Duleba
F.P. Pereira
Gammaridean and caprellidean fauna from Brazil
Hydrobiologia
223
1991
69-77
Wakabara et al., 1996
Y. Wakabara
M. Nicoletti
A.S. Tararam
Ingestion and selection of suprabenthic crustaceans by small-sized fishes in a lower saltmarsh system
Revista Brasileira de Oceanografia
44
1996
89-103
Watling and Maurer, 1972
L. Watling
D. Maurer
Shallow water amphipods of the Delaware Bay region
Crustaceana Supplement
3
1972
251-66
Williams and Bynum, 1972
A.B. Williams
K.H. Bynum
A ten year study of meroplankton in North Carolina estuaries: amphipods
Chesapeake Science
13
1972
175-92
Winfield, 2013
I. Winfield
Catálogo de las especies de crustáceos anfípodos invasores del Parque Nacional Sistema Arrecifal Veracruzano (PNSAV) y la actualización de la base de datos (CONABIO) de los anfípodos en México
2013
Winfield and Escobar, 2007
I. Winfield
E. Escobar
Anfípodos (Crustacea: Gammaridea) del sector norte del Mar Caribe: listado faunístico, registros nuevos y distribución espacial
Revista Mexicana de Biodiversidad
78
2007
51-61
Winfield and Ortiz, 2003
I. Winfield
M. Ortiz
Anfípodos: un enfoque biológico
2003
Winfield and Ortiz, 2011
I. Winfield
M. Ortiz
Crustáceos con bolsa incubadora (Crustacea: Malacostraca: Peracarida)
Conabio, Gobierno del Estado de Veracruz, Universidad Veracruzana, Instituto de Ecología, A.C.
Comisión Nacional para el Conocimiento y Uso de la Biodiversidad
México, D.F.
2011
277-86
Winfield et al., 1997
I. Winfield
M. Ortiz
J. Franco
C. Bedia
Distribución y diversidad del superorden Peracarida asociado a pastos marinos de Alvarado Veracruz
Cuadernos Mexicanos de Zoología
3
1997
1-8
Winfield et al., 2001
I. Winfield
E. Escobar
F. Álvarez
Crustáceos peracáridos asociados a praderas de Ruppia maritima (Ruppiaceae) en la laguna de Alvarado, México
Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Zoología
72
2001
29-41
Winfield et al., 2006
I. Winfield
E. Escobar
J.J. Morrone
Updated checklist and identification of areas of endemism of benthic amphipods (Caprellidea and Gammaridea) from offshore habitats in the SW Gulf of Mexico
Scientia Marina
70
2006
99-108
Winfield et al., 2007a
I. Winfield
L.G. Abarca-Arenas
S.I. Cházaro-Olvera
Crustacean macrofoulers in the Veracruz coral reef system SW Gulf of Mexico: checklist, spatial distribution and diversity
Cahiers de Biologie Marine
48
2007
287-95
Winfield et al., 2007b
I. Winfield
S. Cházaro-Olvera
R. Álvarez
¿Controla la biomasa de pastos marinos la densidad de los peracáridos (Crustacea: Peracarida) en lagunas tropicales?
Revista de Biología Tropical
55
2007
43-53
Winfield et al., 2010
I. Winfield
S. Cházaro-Olvera
G. Horta-Puga
M.A. Lozano-Aburto
V. Arenas-Fuentes
Macrocrustáceos incrustantes en el Parque Nacional Sistema Arrecifal Veracruzano: biodiversidad, abundancia y distribución
Revista Mexicana de Biodiversidad (Suplem.)
80
2010
S165-75
Winfield et al., 2011
I. Winfield
S. Cházaro-Olvera
M. Ortiz
U. Palomo-Aguayo
Lista actualizada de las especies de anfípodos (Peracarida: Gammaridea y Corophiidea) marinos invasores en México
Revista de Biología Marina y Oceanografía
46
2011
349-61
Zupo and Nelson, 1999
V. Zupo
W.G. Nelson
Factors influencing the association patterns of Hippolyte zostericola and Palaemonetes intermedius (Decapoda: Natantia) with seagrasses of the Indian River Lagoon Florida
Marine Biology
134
1999
181-90
Peer Review under the responsibility of Universidad Nacional Autónoma de México.
*
Corresponding author.
Copyright © 2017 Universidad Nacional Autónoma de México, Instituto de Biología.