Isabela R. R. Moraes a, *, Thiago M. Davanso a, b, Alexandre R. da Silva a, Valter J. Cobo c, Douglas F. R. Alves d, William Santana a, e, Fernando L. Mantelatto f, Antonio L. Castilho a
a Universidade Estadual Paulista, Instituto de Biociências, Departamento de Zoologia, Botucatu, Rua Professor Antonio Celso Wagner Zanin, 250, 18618-689 Botucatu, São Paulo, Brazil
b Universidade Paulista, Departamento de Biologia, Rua Luiz Levorato, 2-140 – Chácaras Bauruenses, Rod. Marechal Rondon – Km 335, Bauru, SP, Brazil
c Universidade de Taubaté, Instituto Básico de Biociências, Laboratório de Biologia Marinha, Avenida Tiradentes 500, Centro, 12030-180 Taubaté, SP, Brazil
d Universidade Federal de Uberlândia, Laboratório de Ecologia de Ecossistemas Aquáticos, Av. Amazonas s/n, Umuarama, Uberlândia, 38400-902 Minas Gerais, Brazil
e Universidade do Sagrado Coração, Pró-Reitoria e Pós Graduação, Laboratório de Sistemática Zoológica, Rua Irmã Arminda, 10-50m Jd Brasil, Bauru, 17011-160 SP, Brazil
f Universidade de São Paulo, Faculdade de Filosofia, Ciências e Letras de Ribeirão Preto, Departamento de Biologica, Laboratório de Bioecologia e Sistemática de Crustáceos, Av. Bandeirantes 3900, 14040-901 Ribeirão Preto, SP, Brazil
*Corresponding author: isabela.moraes@unesp.br (I.R.R. Moraes)
Received: 21 July 2020; accepted: 11 April 2021
Abstract
This study represents an unprecedented effort to assess the diversity of rocky infralittoral decapods Brachyura and Anomura from the Marine State Park of Laje de Santos, a preserved and protected area located in the southeastern Brazilian coast. Sampling was carried out quarterly for 1 year, using the combination of artificial refuge substrate (ARS) for the passive capture of specimens, and SCUBA diving for active capture. A total of 987 individuals distributed into 32 species, 22 genera, and 11 families were determined. We present a taxonomic account of the species and their distribution records are discussed. The hermit crab Pagurus brevidactylus and the crab Mithraculus forceps were the most common species of Anomura and Brachyura, respectively. As expected, the combination of the sampling methodologies showed a higher Shannon diversity index value (H = 5.7), than the individual methods of ARS
(H = 5.6) and active capture (H = 4.4). This study provides the first record of decapod crustaceans for the park, therefore it can be used as a baseline for local fauna monitoring programs and conservation planning.
Keywords: Ecology; Fauna; Marine invertebrates; Protected area
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Primer reporte de crustáceos decápodos (Anomura y Brachyura) de Laje de Santos: una reserva marina protegida en la costa sureste de Brasil
Resumen
Este estudio representa un esfuerzo sin precedentes para evaluar la diversidad de los decápodos Brachyura y Anomura del infralitoral rocoso del Parque Estatal Marino Laje de Santos, un área preservada y protegida localizada en la costa sureste de Brasil. Las muestras se tomaron trimestralmente durante 1 año con la combinación de sustrato de refugio artificial para captura pasiva y buceo autónomo para captura activa. Se determinaron un total de 987 individuos distribuidos en 32 especies, 22 géneros y 11 familias. Presentamos un reporte taxonómico de las especies y se discuten sus registros de distribución. El ermitaño Pagurus brevidactylus y el cangrejo Mithraculus forceps fueron las especies más abundantes de Anomura y Brachyura, respectivamente. Como se esperaba, la combinación de los métodos de muestreo mostró un valor más alto del índice de diversidad de Shannon (H = 5.7) que los métodos individuales de captura pasiva (H = 5.6) y captura activa (H = 4.4). Este estudio provee el primer reporte de crustáceos decápodos para el parque, por lo tanto, puede ser usado como línea base para programas de monitoreo de fauna local y en los planes de conservación.
Palabras clave: Ecología; Fauna, Invertebrados marinos; Área protegida
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
“No-take” Marine reserves (NTM) are areas completely protected from all extractive and destructive activities (Lubchenco et al., 2003). The importance of the maintenance and creation of new NTMs has been highlighted in a way to improve the preservation of the marine biodiversity and the commercial stocks (Lester et al., 2009; Rolim et al., 2019). The Marine State Park of Laje de Santos (MSPLS) is the first NTM on the southeastern Brazilian coast due to its unique peculiar island area and fauna (State Decree No. 37,537 from September 27, 1993).
The MSPLS is an area with more than 5,000 ha formed by the complex of “isolated” upright rock in the continental platform, positioned about 36 km from the São Paulo coast and the Santos city, with no close islands around it. Despite the pristine condition proportioned partially by the difficulty of access (expected by recreational SCUBA diving activities), indirect anthropogenic impacts (e.g., climate change and biological invasions through ballast water) may affect biodiversity of this region. The park is located close to the most important and biggest Brazilian harbor, which moves more than 1,000 tons of materials in daily shipments. Such region is at imminent risk of introducing non-native species through ballast water in ships traveling long distances (D’incao, 1995; Drehen-Mansur et al., 2003; Negreiros-Fransozo, 1996; Tavares & Amouroux, 2003). Despite its importance as a biodiversity conservation area, only a few biological studies have been conducted at the park area, and the record of crustacean fauna is completely non-existent (Luiz et al., 2008), expect for a recent study on the caridean shrimp fauna composition (Moraes et al., 2021).
Decapod crustaceans are one of the most representative groups in species diversity and trophic dynamics in marine environments. In different substrates, such as coral, rocky subtidal, and intertidal zones they are especially abundant (Abele, 1974; Alves et al., 2011; Coelho et al., 2008; Randall, 1967; Terossi et al., 2018). The knowledge of decapod biodiversity along the southeastern Brazilian coast has been increased in the past years (Mantelatto et al., 2018). Some shallow-water groups were targeted and studied with some degree of information, especially in the north area of São Paulo State (Almeida et al., 2018; Fransozo et al., 2002; Mantelatto & Fransozo, 2000; Terossi et al., 2018); however, the detailed information of this group inhabiting island-like regions is poorly known and limited (Almeida et al., 2013; Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Giraldes, Coelho-Filho, & Coelho, 2015; Mantelatto & Garcia, 2002; Mantelatto, Faria et al., 2004; Mantelatto, Biagi et al., 2004).
Management plans need constant monitoring and evaluation, and these processes can only be genuinely completed when there is information on local fauna. The no-take marine reserves are one of the most effective types of Marine Protected areas and deserve especial attention to their associated biota from small invertebrates to vertebrates to make an effective plan to protect all of them (Sala & Giakoumi, 2018). In this work, we provide the first records for Anomura and Brachyura crustaceans for the MSPLS. These results can be used as baseline information in the local conservation plan.
For comparative purposes, the Shannon-Wiener diversity index (H’) was also employed. This index relies on relative abundance of sampled species and works as comparative measure (Gotelli & Ellison, 2004; Stirling & Wilsey, 2001). This index will provide a diversity value which can be used to compare with other regions, island and NTMs to evaluate the state of the local diversity. Also, this index can provide comparative and baseline data for future studies on MPSLS and management plans which will be able to evaluate if the diversity decreased or increased revealing the potential efficiency of the management plan.
Materials and methods
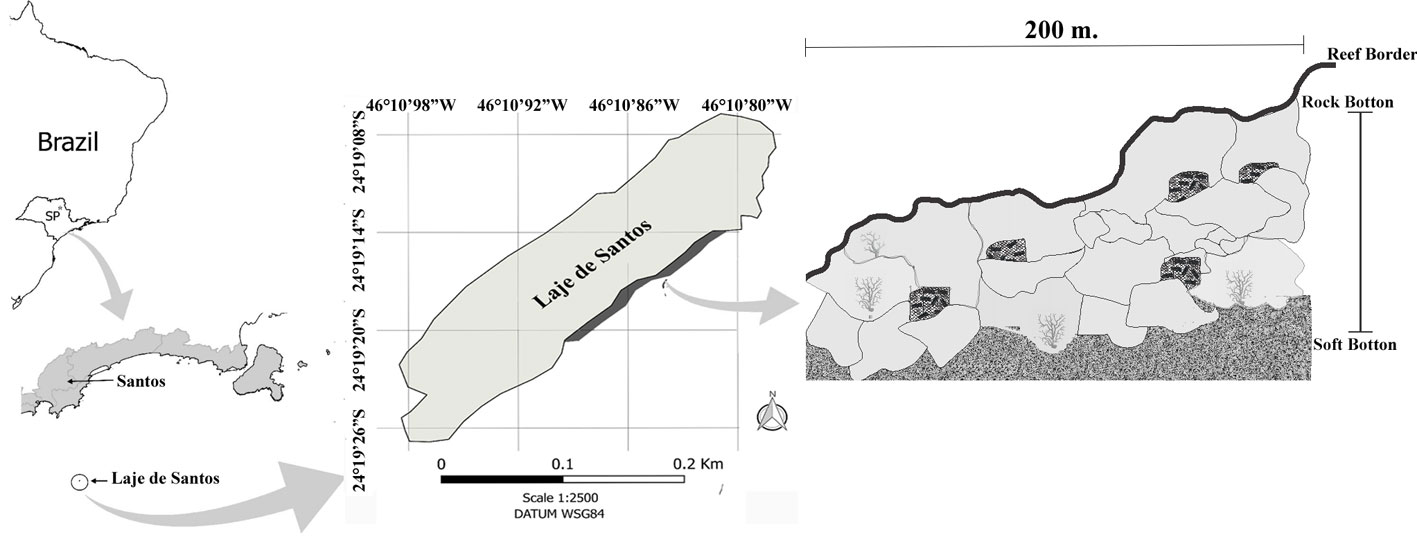
Sampling was conducted on the rocky subtidal zone of Laje de Santos (24º19’13” S, 46º10’49” W) between June/2015 and July/2016, under license permission COTEC No. 91/2015 D187/2014 BA, PROCESS SMA No. 260108-010.480/2014. We performed 4 one-day expeditions (daylight period) in intervals of 3 months. We used 2 combined different methodologies to capture the specimens: 1) the active search performed by 2 SCUBA divers during 2 hours in each campaign, and 2) the passive capture using 5 Artificial Refuge Substrates (ARS) (see Alves et al. [2019] and Moraes et al. [2021] for details about the ARS). The ARSs were left in the environment: above rocks, in crevices, and between coral and anemone groups during the intervals of 3 months to be colonized by the fauna, especially decapod crustaceans. At each sampling campaign, ARSs were bagged and thoroughly searched to find associated Brachyura and Anomura, and then, 5 new ARSs were installed for another 3-month period until the one-year sample was complete. A total of 20 ARSs were analyzed. The active search and installation of the ARSs was performed randomly along 200 m in the consolidated sublittoral of MSPLS until a 20 m depth (Figs. 1, 2).
All specimens sampled were individualized and frozen or kept alive to preserve the color and morphology, and were transported to the Laboratory of Carcinology, Institute of Biosciences of Botucatu for further analysis. Individuals were identified using specialized taxonomical keys (Ferreira & Melo, 2016; McLaughlin, 2003; Melo, 1996, 1999; Nucci & Melo, 2007, 2011, 2015). Since there was no morphological variation novelty in all specimens analyzed, all diagnosis used to identify and confirm the taxonomic status were based on specialized literature, which is properly cited in the taxonomic account. For the Anomura and taxa above infraorder level, we follow the classification proposed by Ahyong et al. (2011), and for the Brachyura the classification proposed by Ng et al. (2008). To assess the sex in the Anomura, we used the position of the gonopore, which in males is at the base of the thigh of the fifth pair of pereopods, and in females is at the base of the thigh of the third pair of pereopods (Melo, 1999). For Brachyura, the sex was determined by the shape of the pleon (a thin shape for males and an oval shape for females) and the number of pleopods (2 pairs for males and 4 pairs for females). The specimens were grouped according to sex and developmental stages as: adult male (M), adult female (F), ovigerous female (OF), and juvenile (J). The cephalothoracic shield length (SL) was measured for the Anomura (hermit crabs only), and the carapace width (CW) for porcelanid crabs and the Brachyura, always using a stereomicroscope (Zeiss Stemi SV6 with a Zeiss Stemi 2000-C image capture system) and the Axio Visio software (vers.4.8).
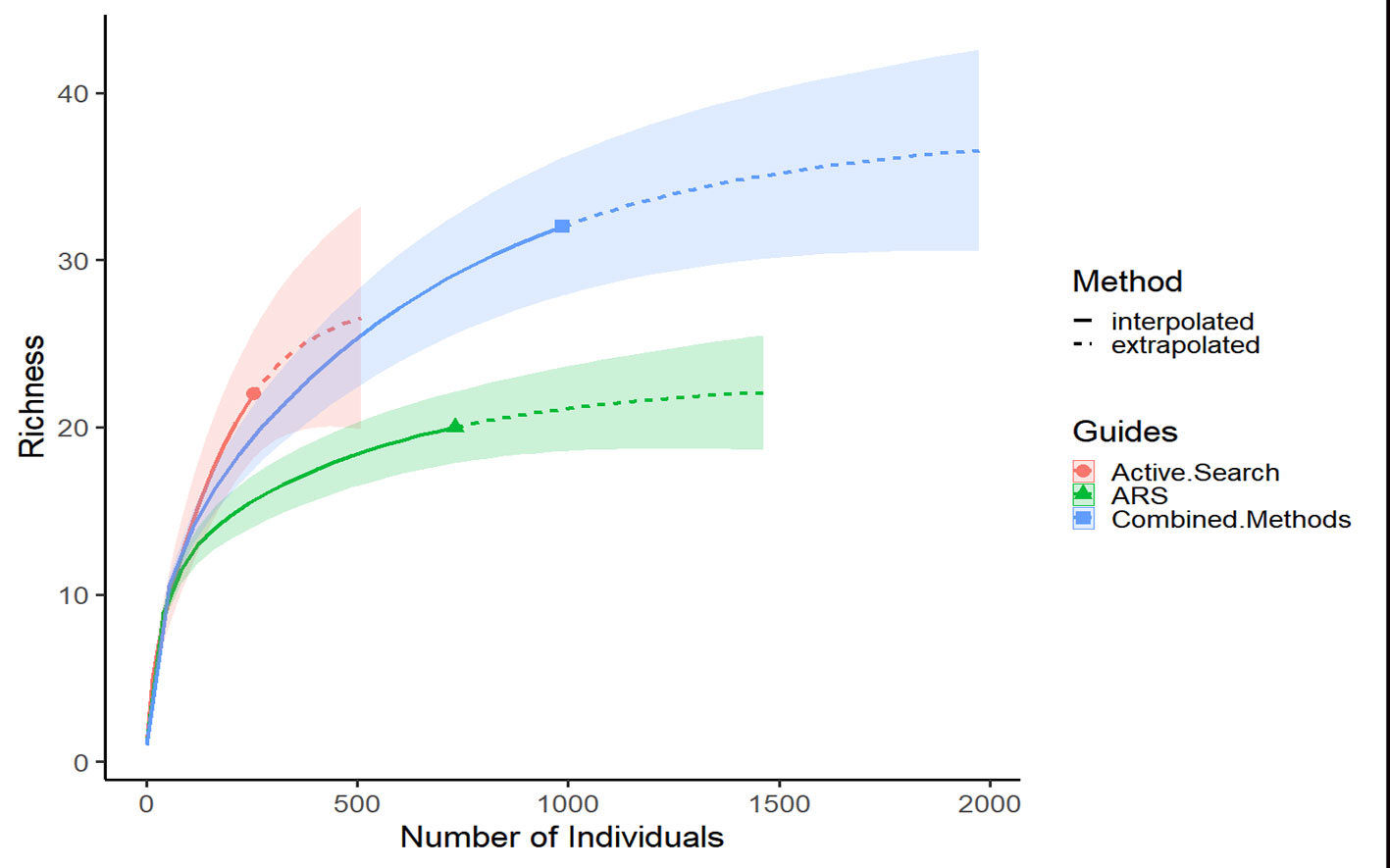
The Shannon diversity index (H) was calculated for the sampling methods: active search, ARS, and combined methods (active search + ARS). Rarefaction curves were also performed to compare the sampling methods. The analyses were carried out using the iNEXT package (Hsieh et al., 2016) in R (R-Core Team, 2020).

After analyzes, the specimens were deposited in the Crustacean Collection of the Department of Biology (CCDB) of FFCLRP, University of São Paulo, Ribeirão Preto, Brazil (CCDB/FFCLRP/USP – Authorization No. 071/2012/SECEX/CGEN).
Results
Subphylum Crustacea Brünnich, 1772
Class Malacostraca Latreille, 1802
Subclass Eumalacostraca Grobben, 1892
Superorder Eucarida Calman, 1904
Order Decapoda Latreille, 1802
Suborder Pleocyemata Burkenroad, 1963
Infraorder Anomura MacLeay, 1838
A total of 158 specimens of Anomura were sampled, representing 7 species, 6 genera, and 3 families. The Diogenidae was the most representative anomuran family, with 3 species recorded (Table 1).
Superfamily Galatheoidea Samouelle, 1819
Family Porcellanidae Haworth, 1825
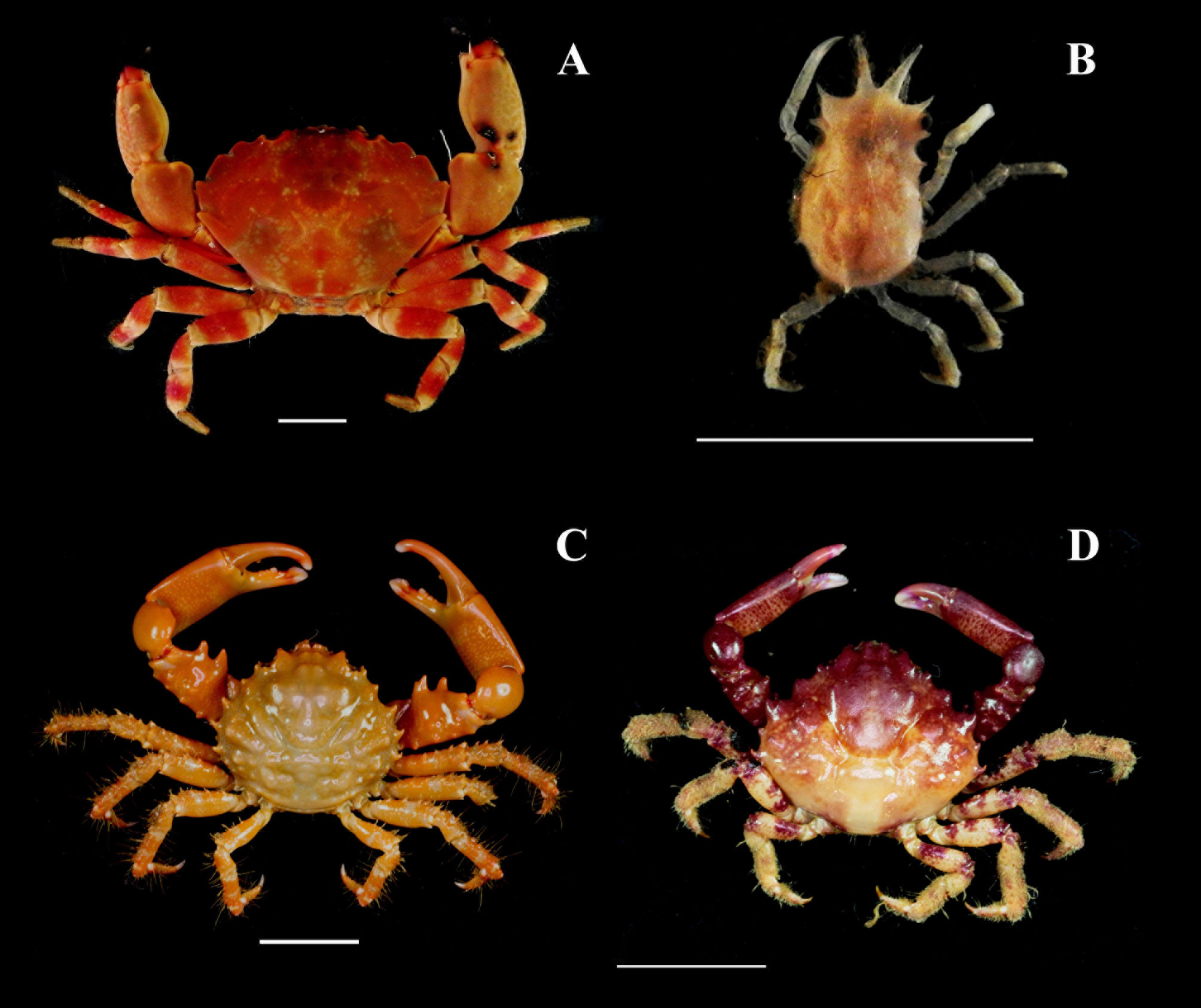
Pachycheles monilifer (Dana, 1852)
(Fig. 3A)
Diagnosis and morphological description: Melo (1999), and Ferreira & Melo (2016).
Taxonomic summary
Type locality. Rio de Janeiro, Brazil (as Porcellana monilifera) (Dana, 1852).
Geographic distribution. Western Atlantic – Gulf of Mexico, North coast of South America, and Brazil (from Pará to Santa Catarina). Eastern Pacific: Ecuador and Peru (Ferreira & Melo, 2016).
Material examined. 1 M, size: 7.75 mm CW; 1 OF, size: 7.78 mm CW. CCDB 6517.
Petrolisthes amoenus (Guérin-Méneville, 1855)
(Fig. 3B)
Diagnosis and morphological description: Melo (1999); updated morphological remarks: Ferreira & Melo (2016); morphological and coloration comments: Ferreira & Tavares (2017).
Table 1
List of studied species and number of specimens collected from the Marine State Park of Laje de Santos, Brazil organized according to taxonomical groups and sampling methodology: ARS = passive capture by the Artificial Refuge Substrate and Active search by SCUBA dive performance.
|
Infraorder |
Superfamily |
Family |
Subfamily |
Species |
ARS |
Active search |
|
Anomura |
Galatheoidea |
Porcellanidae |
Pachycheles monilifer |
0 |
2 |
|
|
Petrolisthes amoenus |
0 |
1 |
||||
|
Paguroidea |
Diogenidae |
Calcinus tibicen |
0 |
1 |
||
|
Dardanus venosus |
0 |
2 |
||||
|
Paguristes tortugae |
13 |
2 |
||||
|
Paguridae |
Pagurus brevidactylus |
106 |
14 |
|||
|
Pagurus criniticornis |
17 |
0 |
||||
|
Brachyura |
Eriphioidea |
Menippidae |
Menippe nodifrons |
2 |
1 |
|
|
Majoidea |
Epialtidae |
Stenocionops furcatus |
1 |
0 |
||
|
Inachoididae |
Stenorhynchinae |
Stenorhynchus seticornis |
42 |
2 |
||
|
Mithracidae |
Mithraculus forceps |
381 |
160 |
|||
|
Mithraculus sculptus |
0 |
1 |
||||
|
Mithrax hispidus |
0 |
1 |
||||
|
Mithrax tortugae |
1 |
2 |
||||
|
Pitho lherminieri |
0 |
2 |
||||
|
Pilumnoidea |
Pilumnidae |
Pilumninae |
Pilumnus caribaeus |
4 |
0 |
|
|
Pilumnus reticulatus |
8 |
0 |
||||
|
Portunoidea |
Portunidae |
Thalamitinae |
Cronius ruber |
0 |
1 |
|
|
Xanthoidea |
Panopeidae |
Acantholobulus bermudensis |
18 |
26 |
||
|
Acantholobulus schmitti |
0 |
1 |
||||
|
Hexapanopeus angustifrons |
1 |
1 |
||||
|
Hexapanopeus caribbaeus |
3 |
0 |
||||
|
Panopeus austrobesus |
0 |
1 |
||||
|
Panopeus harttii |
2 |
0 |
||||
|
Panopeus rugosus |
12 |
5 |
||||
|
Pseudorhombilidae |
Micropanope sculptipes |
2 |
0 |
|||
|
Scopolius nuttingi |
22 |
0 |
||||
|
Xanthidae |
Cataleptodius floridanus |
1 |
0 |
|||
|
Melybia thalamita |
77 |
24 |
||||
|
Xanthodius parvulus |
19 |
0 |
||||
|
Williamstimpsonia denticulatus |
0 |
3 |
||||
|
Zosiminae |
Platypodiella spectabilis |
0 |
2 |
Taxonomic summary
Type locality. Cuba (as Porcellana amoena) (Guérin-Méneville, 1855).
Geographic distribution. Western Atlantic – USA, Florida, Mexico: Yucatán, Cuba, Puerto Rico, Barbados, Colombia, Providence and Santa Marta Islands, Curacao and Bonaire, Venezuela, Los Roques, Cubagua and Gran Roque islands, Trinidad and Tobago, and Brazil (Fernando de Noronha and Trindade Islands, and from Maranhão to São Paulo) (Alves et al., 2006; Ferreira & Melo, 2016; Tavares et al., 2017).
Material examined. 1 OF, Size: 6.87 mm CW; CCDB 6518.
Remarks
This is the southernmost record for P. amoenus since the previous distribution was limited to the northern region of the São Paulo state (Alves et al., 2006).
Superfamily Paguroidea Latreille, 1802
Family Diogenidae Ortmann, 1892
Calcinus tibicen (Herbst, 1791)
(Fig. 3C)
Diagnosis and morphological description: Melo (1999); updated morphological remarks: Nucci and Melo (2015); coloration comments: Mandai et al. (2018).
Taxonomic summary
Type locality. Locality not given by the authors.
Geographic distribution. Western Atlantic – Bermudas Florida, Gulf of Mexico, Antilles, Panama, Colombia, Venezuela, and Brazil (Fernando de Noronha, Martim Vaz & Trindade archipelago, and from Ceará to Santa Catarina) (Lima et al., 2019; Nucci & Melo, 2015; Tavares et al., 2017).
Material examined. 1 OF; size: 3.71 mm SL;
Remarks
The genus Calcinus is fairly distributed in the Indo-Pacific region, but in the Western-Atlantic there are only 3 species recorded with only C. tibicen occurring in Brazilian waters (Nucci & Melo, 2015). Thus, being common and widely distributed in the Brazilian coast, the only specimen sampled was allocated for other purposes in our laboratory.
Dardanus venosus (H. Milne Edwards, 1848)
(Fig. 3D)
Diagnosis and morphological description: Biffar and Provenzano (1972); updated morphological remarks: Melo (1999), Nucci and Melo (2015).
Taxonomic summary
Type locality. Guadeloupe (as Pagurus venosus) (Milne Edwards, 1848).
Geographic distribution. Western Atlantic – Bermudas, Florida, Gulf of Mexico, Antilles, Colombia, and Brazil (Atol das Rocas, Fernando de Noronha, Martim Vaz & Trindade archipelago, and from Pará to São Paulo) (Lima et al., 2019; Nucci & Melo, 2007).
Material examined. 2 M, size range: 6.13 ≤ SL ≤ 7.37 mm; CCDB 6519.
Remarks
This is the southernmost record for D. venosus since the previous distribution was limited to the northern region of the São Paulo state (Mantelatto et al., 2001).
Paguristes tortugae Schmitt, 1933
(Fig. 4A)
Diagnosis and morphological description: McLaughlin and Provenzano (1974) and Melo (1999); morphological comments of Paguristes complex and key for the western Atlantic species of the complex: Lima and Santana (2017).
Taxonomic summary
Type locality. Garden Key, Dry Tortugas, Florida (Schmitt, 1933).
Geographic distribution. Western Atlantic – North Carolina, Florida, Gulf of Mexico, Antilles, Suriname, Guianas, and Brazil (from Amapá to Santa Catarina) (Nucci, 2002).
Material examined. 9 M, size range: 0.77 ≤ SL ≤ 4.87 mm; 6 F, size range: 1.72 ≤ SL ≤ 4.93 mm. CCDB 6520.
Family Paguridae Latreille, 1802
Pagurus brevidactylus (Stimpson, 1859)
(Fig. 4B)
Diagnosis and morphological description: Melo (1999); updated morphological remarks: Nucci and Melo (2007).
Taxonomic summary
Type locality. Locality not given by Stimpson (1859).
Geographic distribution. Western Atlantic – the Bermudas, Gulf of Mexico, Antilles, Central America, North of South America, and Brazil (Fernando de Noronha, and from Pernambuco to Santa Catarina) (Nucci & Melo, 2007).

Material examined. 54 M, size range: 0.86 ≤ SL ≤ 2.56 mm; 18 F, size range: 0.72 ≤ SL ≤ 2.60 mm; 34 OF, size range: 1.36 ≤ SL ≤ 2.18 mm; 14 J, size range: 0.4 ≤ SL ≤ 1.30 mm. CCDB 6521.
Pagurus criniticornis (Dana, 1852)
(Fig. 4C)
Diagnosis and morphological description: Melo (1999); updated morphological remarks: Nucci and Melo (2007).
Taxonomic summary
Type locality. Locality not given by Dana (1852).
Geographic distribution. Western Atlantic – Gulf of Mexico, Antilles, northern South America, Brazil (São Pedro Archipelago and São Paulo, and from Pernambuco to Rio Grande do Sul), Uruguay, and Argentina (Nucci & Melo, 2007).
Material examined. 11 M, size range: 0.86 ≤ SL ≤ 1.79 mm; 1 F, size: 1.38 mm SL; 5 OF, size range: 1.26 ≤ SL ≤ 1.80 mm.
Remarks
This species is very common in the Western Atlantic and in Brazilian waters (Melo, 1999; Nucci & Melo, 2007), thus the specimens sampled were directed for other purposes of the laboratory.
Infraorder Brachyura Linnaeus, 1758
A total of 829 individuals of Brachyura belonging to 25 species, 16 genera, and 8 families (Mithracidae, Epialtidae, Inachoididae, Pilumnidae, Portunidae, Xanthidae, Panopeidae, and Menippidae) were collected. The Panopeidae was the most representative family, with 7 species sampled. The most representative species was Mithraculus forceps A. Milne-Edwards, 1875, with a total of 541 specimens.
Section Eubrachyura de Saint Laurent, 1980
Subsection Heterotremata Guinot, 1977
Superfamily Eriphioidea MacLeay, 1838
Family Menippidae Ortmann, 1893
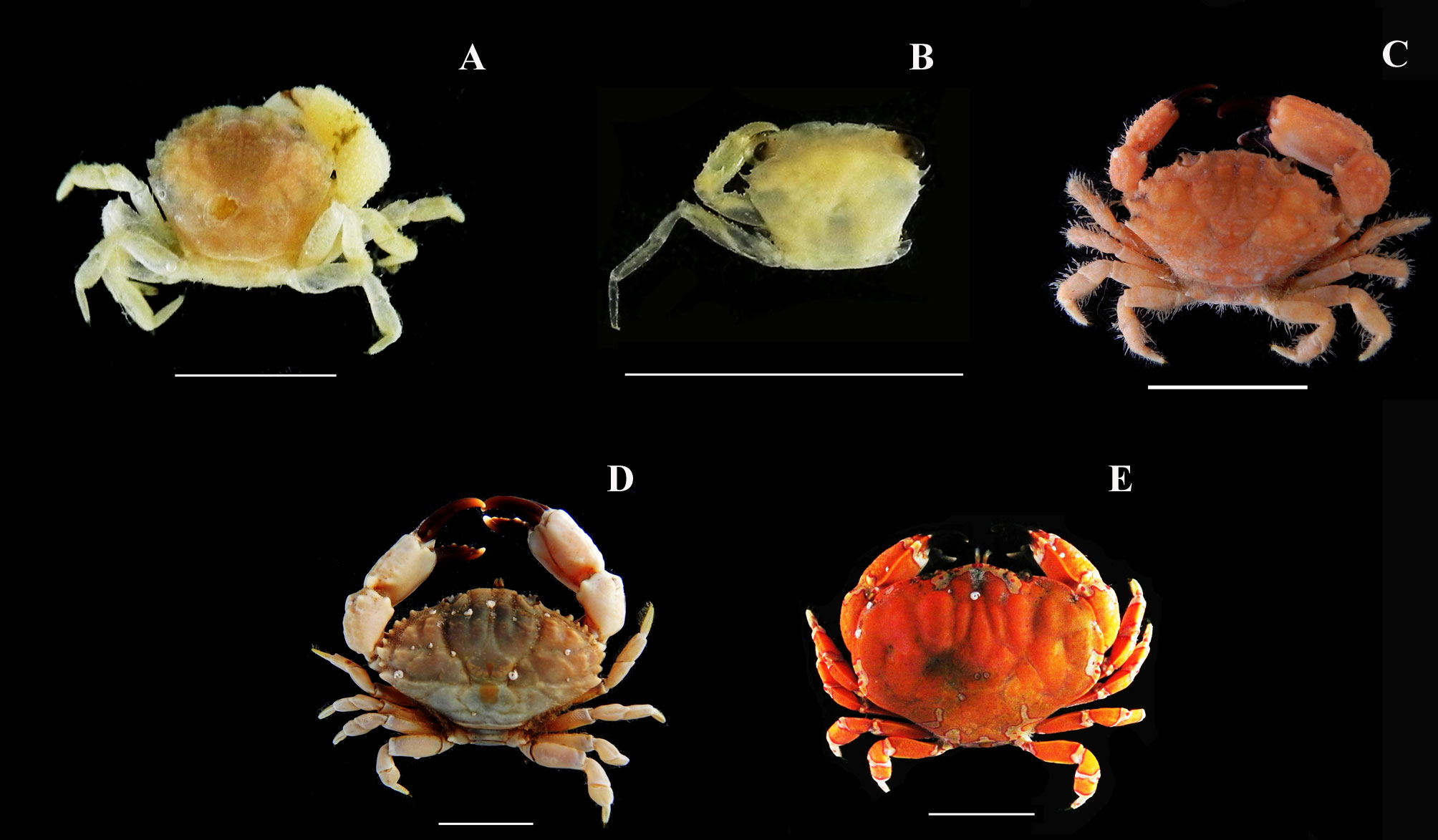
Menippe nodifrons Stimpson, 1859
(Fig. 5A)
Diagnosis and morphological description: Melo (1996).
Taxonomic summary
Type locality. Indian River, Florida (Stimpson, 1859).
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Central America, Antilles, northern South America, and Brazil (from Maranhão to Santa Catarina). Eastern Atlantic – From Cape Verde islands to Angola (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Giraldes, Coelho-Filho, & Coelho, 2015; Mantelatto et al., 2020; Melo, 1998, 2008; Pachelle et al., 2016).
Material examined. 2 M, size range: 10.19 ≤ CW ≤ 32.81 mm; 1 F, size: 7.99 mm CW. CCDB 6522.
Superfamily Majoidea Samouelle, 1819
Family Epialtidae MacLeay, 1838
Stenocionops furcatus (Oliver, 1791)
(Fig. 5B)
Diagnosis and morphological description: Melo (1996); updated morphological remarks: Colavite et al. (2016).
Taxonomic summary
Type locality. Locality not given by the authors.
Geographic distribution. Western Atlantic – Georgia, Florida, Gulf of Mexico, West Indies, Colombia, and Brazil (from Ceará to the Rio Grande do Sul) (Coelho et al., 2008; Colavite et al., 2016; Melo, 1996).
Material examined. 1 M; size: 4.20 mm CW; CCDB 6523.
Family Inachoididae Dana, 1851
Subfamily Stenorhynchinae Dana, 1851
Stenorhynchus seticornis (Herbst, 1788)
Diagnosis and morphological description: Melo (1996); details on taxonomic status of the family Inachoididae, and interpretations of S. seticornis morphology: Guinot (2012).

Taxonomic summary
Type locality. Uncertain, perhaps Guadeloupe, discussed at Rathbun (1925).
Geographic distribution. Western Atlantic – Bermuda, North Carolina, Florida, Gulf of Mexico, Antilles, Colombia, Venezuela, Guyanas, Brazil (from Amapá to Rio Grande do Sul) Uruguay, and Argentina (Alves, Barros-Alves, Cobo et al., 2012; Coelho-Filho, 2006; Guinot, 2012; Mantelatto, 2020 …en las referencias solo hay: Mantelatto et al., 2020, se refieren a esta cita??; Melo, 1998, 2008).
Material examined. 11 M, size range: 2.48 ≤ CW ≤ 12.72 mm; 23 F, size range: 3.7 ≤ CW ≤ 11.94 mm; 10 OF, size range: 6.73 ≤ CW ≤ 10.41 mm; CCDB 6524.
Family Mithracidae MacLeay, 1838
Mithraculus forceps A. Milne-Edwards, 1875 (in A. Milne-Edwards, 1873-1880)
(Fig. 5C)
Diagnosis and morphological description: Melo (1996); details on taxonomic status of the family Mithracidae, and interpretations of M. forceps morphology: Windsor and Felder (2014); integrative interpretation for taxonomic discussion of the species: Assugeni et al. (2017).
Taxonomic summary
Type locality. Guyana (Milne-Edwards, 1873-1881).
Geographic distribution. Western Atlantic – From North Carolina to the south of Florida, West Indies, Gulf of Mexico, Antilles, Venezuela, and Brazil (Fernando de Noronha and Atol das Rocas, and from Maranhão to Santa Catarina) (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Coelho-Filho, 2006; Mantelatto et al., 2020; Melo, 1998; Rieger & Giraldi 1996; Tavares et al., 2017).
Material examined. 211 M, size range: 2.86 ≤ CW ≤ 18.47mm; 143 F, size range: 3.18 ≤ CW ≤ 15.49mm; 46 OF, size range: 8.34 ≤ CW ≤ 16.01mm; 141 J, size range: 3.01 ≤ CW ≤ 8.24mm; CCDB 6525.
Mithraculus sculptus (Lamarck, 1818)
Diagnosis and morphological description: Melo (1996); details on taxonomic status of the family Mithracidae, and interpretations of M. sculptus morphology: Windsor and Felder (2014); integrative interpretation for taxonomic discussion of the species: Assugeni et al. (2017).
Taxonomic summary
Type locality. Locality not given by the authors.
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Antilles, and Brazil (Fernando de Noronha, from Rio Grande do Norte to São Paulo) (Alves, Barros-Alves, Cobo et al., 2012; Alves, Barros-Alves, Teixeira et al., 2012; Camargo et al., 2010; Mantelatto et al., 2020; Melo, 1998).
Material examined. 1 J; Size: 11.97 mm CW; CCDB 6526.
Mithrax hispidus (Herbst, 1790)
Diagnosis and morphological description: Melo (1996); details on taxonomic status of the family Mithracidae, and interpretations of M. hispidus morphology: Windsor and Felder (2014) (as Damithrax hispidus), correcting the Mithrax genera at Windsor and Felder (2017), integrative interpretation for taxonomic discussion of the species: Assugeni et al. (2017) (as M. hispidus ); morphological information about larval stages of the species: Santana et al. (2003).
Taxonomic summary
Type locality. Locality not given by the authors.
Geographic distribution. Western Atlantic – Delaware, southern Florida, Gulf of Mexico, Antilles, Colombia, and Brazil (Amapá, Pernambuco, and from São Paulo to Santa Catarina) (Almeida et al., 2008; Alves, Barros-Alves, Cobo et al., 2012; Alves, Barros-Alves, Teixeira et al., 2012; Giraldes, Coelho-Filho, & Smyth, 2015; Mantelatto et al., 2020).
Material examined. 1 F; size: 42.4 mm CW; CCDB 6527.
Mithrax tortugae Rathbun, 1920
(Fig. 5D)
Diagnosis and morphological description: Melo (1996), details on taxonomic status of the family Mithracidae, and interpretations of M. tortugae morphology: Windsor and Felder (2014) (as Damithrax tortugae), correcting for the Mithrax genera at Windsor and Felder (2017); integrative interpretation for taxonomic discussion of the species: Assugeni et al. (2017) (as M. tortugae).
Taxonomic summary
Type locality. Tortuga, Florida (Rathbun, 1920).
Geographic distribution. Western Atlantic – North Carolina to Florida, Gulf of Mexico, West Indies, Colombia, Venezuela, and Brazil (Pará, Pernambuco, Alagoas, Bahia, São Paulo, Santa Catarina) (Alves, Barros-Alves, Cobo et al., 2012; Cobo & Alves, 2009; Coelho et al., 2008; Mantelatto, Faria et al., 2004; Mantelatto et al.,2020; Melo, 1998; Rieger & Giraldi, 2001).
Material examined. 2 M size range: 14.36 ≤ CW ≤ 38.4 mm; 1 F, size: 32.2 mm CW; CCDB 6528.
Pitho lherminieri (Desbonne, in Desbonne & Schramm, 1867)
(Fig. 6A)
Diagnosis and morphological description: Melo (1996); details on taxonomic status of the family Mithracidae, and interpretations of P. lherminieri morphology: Windsor and Felder (2014).
Taxonomic summary
Type locality. Guadeloupe (Desbonne & Schram, 1867).
Geographic distribution. Western Atlantic – from North Carolina to Florida, Gulf of Mexico, Antilles, West Indies, south coast of Cuba, and Brazil (Fernando de Noronha, and from Pará to São Paulo) (Almeida & Coelho, 2008; Almeida et al., 2008; Alves, Barros-Alves, Cobo et al., 2012; Coelho-Filho, 2006; Diez-García & Capote, 2015; Giraldes, Coelho-Filho, & Smyth, 2015; Mantelatto et al., 2020; Melo, 2008).
Material examined. 1M, size: 8.56 mm CW; 1F, size: 10.97 mm CW; CCDB 6529.
Superfamily Pilumnoidea Smouelle, 1819
Family Pilumnidae Samouelle, 1819
Subfamily Pilumninae Samouelle, 1819
Pilumnus caribaeus Desbonne, in Desbonne & Schramm, 1867
(Fig. 6B)
Diagnosis and morphological description: Melo (1996); taxonomic comments for P. caribaeus species: Abele and Kim (1986).
Taxonomic summary
Type locality. Guadeloupe (Desbonne & Schram, 1867).
Geographic distribution. Western Atlantic – Florida Keys, West Indies, northern South America, and Brazil (from Pará to Santa Catarina; some occurrences in northern and northeastern Brazil, as P. brasiliensis) (Coelho & Ramos-Porto, 1980; Mantelatto et al., 2020; Melo, 1996; Miers, 1886; Powers, 1977).
Material examined. 2 M, size range: 9.9 ≤ CW ≤ 18.62 mm; 1 F, size: 15.58 mm CW; 1 J, size: 7.46 mm CW; CCDB 6544.
Pilumnus reticulatus Stimpson, 1860
(Fig. 6C)
Diagnosis and morphological description: Melo (1996); taxonomic comments for P. reticulatus species: Rathbun (1930).
Taxonomic summary
Type locality. St. Thomas, USA (Stimpson, 1860).
Geographic distribution. Western Atlantic – Central America, West Indies, and South America, and Brazil (from Pará to Rio Grande do Sul), and Argentina (Alves, Barros-Alves, Cobo et al., 2012; Bertini et al., 2010; Coelho et al., 2008; Hendrickx, 1995; Mantelatto et al., 2020; Melo, 2008).
Material examined. 5 M, size range: 3.23 ≤ CW ≤ 7.05 mm; 2 F, size range: 7.23 ≤ CW ≤ 8.09 mm; 1 OV, size: 7.01 mm CW; CCDB 6545.
Superfamily Portunoidea Rafinesque, 1815
Family Portunidae Rafinesque, 1815
Subfamily Thalhamitinae Paul’son, 1875
Cronius ruber (Lamarck, 1818)
(Fig. 6D)
Diagnosis and morphological description: Melo (1996); details on taxonomic status of the family Portunidae, and interpretations of Thalhamitinae and C. ruber morphology: Spiridonov et al. (2014).
Taxonomic summary
Type locality. Mazatlán, Mexico (as Amphitrite Edwardsii) (Lockington, 1877).
Geographic distribution. Western Atlantic – from Virginia and North Carolina to Florida, Gulf of Mexico, Central America, Antilles, Guiana, and Brazil (from Amapá to Rio Grande do Sul). Eastern Atlantic – from Mauritania to Angola, Cape Verde, Principe, São Tomé, and Annobon islands. Eastern Pacific – from Baja California to Peru and Galapagos islands (Alves, Barros-Alves, Cobo et al., 2012; Mantelatto et al., 2009, 2020; Melo, 2008).
Material examined. 1 F; Size: 38.66 mm CW; CCDB 6546.
Superfamily Xanthoidea MacLeay, 1838
Family Panopeidae Ortmann, 1893
Acantholobulus bermudensis (Benedict & Rathbun, 1891)
Diagnosis and morphological description: Melo (1996) (as Panopeus bermudensis); details on taxonomic status of the genus Acantholobulus, and interpretations of A. bermudensis morphology: Felder and Martin (2003).
Taxonomic summary
Type locality. Bermudas (as Panopeus bermudensis) (Benedict & Rathbun, 1891).
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Antilles, northern South America, West Indies, and Brazil (Ceará, São Paulo to Santa Catarina) (Barros-Alves et al., 2018; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 2008).
Material examined. 24 M, size range: 3.35 ≤ CW ≤ 13.44 mm; 14 F, size range: 5.59 ≤ CW ≤ 11.74 mm; 4 OF, size range: 7.54 ≤ CW ≤ 7.83 mm; 2 J, size range: 4.6 ≤ CW ≤ 6.27 mm; CCDB 6530.
Acantholobulus schmitti (Rathbun, 1930)
Diagnosis and morphological description: Melo (1996) (as Hexapanopeus schmitti); details on taxonomic status of the genus Acantholobulus, and interpretations of A. schmitti morphology: Felder and Martin (2003).
Taxonomic summary
Type locality. Bay of Rio de Janeiro, Brazil (as Hexapanopeus schmitti) (Rathbun, 1930).
Geographic distribution. Western Atlantic – Brazil (from Ceará to Santa Catarina), Uruguay, and Argentina (Alves, Barros-Alves, Cobo et al., 2012; Boschi et al. 1992; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 1998, 2008).
Material examined. 1 OF; size: 7.43 mm CW; CCDB 6531.
Hexapanopeus angustifrons (Benedict & Rathbun, 1891)
(Fig. 7A)
Diagnosis and morphological description: Melo (1996); taxonomic comments for H. angustifrons species: Abele and Kim (1986).

Taxonomic summary
Type locality. Long Island Sound (as Panopeus angustifrons) (Rathbun, 1930).
Geographic distribution. Western Atlantic – from Massachusetts to South Carolina, Florida, Gulf of Mexico, West Indies Antilles, and Brazil (from Pernambuco to Rio Grande do Sul) (Almeida & Coelho, 2008; Almeida et al., 2008; Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 1996; Vieira & Calazans, 2010).
Material examined. 2 M; size range: 6.33 ≤ CW ≤ 17.89 mm; CCDB 6532.
Hexapanopeus caribbaeus (Stimpson, 1871)
Diagnosis and morphological description: Melo (1996); taxonomic comments for Hexapanopeus genus and H. caribbaeus species: Sankarankutty and Ferreira (2000).
Taxonomic summary
Type locality. St. Thomas (as Micropanope caribbaeus) (Stimpson, 1871a).
Geographic distribution. Western Atlantic – the Antilles, northeastern of South America, Jamaica, Puerto Rico, Colombia, Trinidad, West Indies, and Brazil (from Piauí, Pará, Santa Catarina to Rio Grande do Sul) (Abele & Kim, 1989; Almeida & Ferraz, 2006; Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Coelho-Filho, 2006; Mantelatto et al., 2020).
Material examined. 3 M; size range: 9.33 ≤ CW ≤ 8.61 mm; CCDB 6533.
Panopeus austrobesus Williams, 1983
(Fig. 7B)
Diagnosis and morphological description: Melo (1996); morphometric and taxonomic comments of P. austrobesus species: Negreiros-Fransozo and Fransozo (2003).
Taxonomic summary
Type locality. Paranagua, Brazil (Williams, 1983).
Geographic distribution. Western Atlantic – Brazil (from Rio de Janeiro to Rio Grande do Sul), and Uruguay (Alves, Barros-Alves, Cobo et al., 2012; Mantelatto et al., 2020; Melo, 1998).
Material examined. 1 F; size: 6.75 mm CW; CCDB 6534.
Panopeus harttii Smith, 1869
Diagnosis and morphological description: Melo (1996); taxonomic comments for P. hartii species: Abele and Kim (1986).
Taxonomic summary
Type locality. Locality not given by the authors.
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Antilles, Ascension Island, and Brazil (from Maranhão to São Paulo) (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Manning & Chace, 1990; Mantelatto et al., 2020; Melo, 1998, 2008; Ng et al., 2008).
Material examined. 1 M, size: 4.98 mm CW; 1 F, size: 5.55 mm CW; CCDB 6535.
Panopeus rugosus A. Milne-Edwards, 1880
(Fig. 7C)
Diagnosis and morphological description: Melo (1996); taxonomic comments for P. rugosus species: Abele and Kim (1986).
Taxonomic summary
Type locality. Bahia, Brazil (Milne-Edwards, 1873-1881).
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Central America, Antilles, Guianas, northern South America, and Brazil (from Alagoas to Rio Grande do Sul) (Almeida & Ferraz, 2006; Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 2008; Ng et al., 2008).
Material examined. 10 M, size range: 4.27 ≤ CW ≤ 19.43 mm; 5 F, size range: 6.62 ≤ CW ≤ 10.33 mm; 1 OF, size: 8.54 mm CW; 1 J, size: 4.22 mm CW; CCDB 6536.
Family Pseudorhombilidae Alcock, 1900
Micropanope sculptipes Stimpson, 1871
(Fig. 7D)
Diagnosis and morphological description: Melo (1996); taxonomic comments for M. sculptipes species: Martin and Abele (1986).
Taxonomic summary
Type locality. Florida, USA (as Micropanope pugilator) (Milne-Edwards, 1873-1881).
Geographic distribution. Western Atlantic – North Carolina, South Carolina, Florida, Gulf of Mexico, West Indies, and Brazil (Pará, Rio de Janeiro, and São Paulo) (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 1998).
Material examined. 3 M, size range: 3.65 ≤ CW ≤ 3.86 mm; 13 F, size range: 3.33 ≤ CW ≤ 6.18 mm; 2 OF, size range: 3.67 ≤ CW ≤ 5.01 mm; 1 J size: 3.16 mm CW; CCDB 6539.
Scopolius nuttingi (Rathbun, 1898)
(Fig. 8A)
Diagnosis and morphological description: Melo (1996) (as Micropanope nuttingi); taxonomic comments for Scopolius genus and S. nuttingi species: Števčić (2011).
Taxonomic summary
Type locality. Bahamas banks (as Xanthias nuttingi) (Rathbun, 1898).
Geographic distribution. Western Atlantic – North Carolina, Florida, Gulf of Mexico, Antilles, West Indies, Surinam, and Brazil (from Amapá to São Paulo) (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 1998, 2008).
Material examined. 14 M, size range: 2.63 ≤ CW ≤ 9.96 mm; 4 F, size range: 3.81 ≤ CW ≤ 4.77 mm; 2 OF, size range: 4.06 ≤ CW ≤ 4.26 mm; 2 J, size range: 2.92 ≤ CW ≤ 3.72 mm; CCDB 6540.
Family Xanthidae MacLeay, 1838
Subfamily Xanthinae MacLeay, 1838
Cataleptodius floridanus (Gibbes, 1850)
Diagnosis and morphological description: Melo (1996); taxonomic comments for C. floridanus species: Abele and Kim (1986).

Taxonomic summary
Type locality. Key West, Florida, USA (as Chlorodius floridanus) (Gibbes, 1850).
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Bermuda, Antilles, Central America, northeastern of South America, and Brazil (from Ceará to São Paulo). Eastern Atlantic – Guinea to Gabon (Almeida & Coelho, 2008; Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Mantelatto et al., 2020; Melo, 1996).
Material examined. 1 M, size: 10.50 mm CW; CCDB 6537.
Melybia thalamita Stimpson, 1871
(Fig. 8B)
Diagnosis and morphological description: Melo (1996); taxonomic comments for M. thalamita species: Thoma and Felder (2020).
Taxonomic summary
Type locality. Tortuga islands (Stimpson, 1871b).
Geographic distribution. Western Atlantic – North Carolina, Florida, Gulf of Mexico, West Indies, northern South America, and Brazil (Amapá, Pará, Ceará to São Paulo (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Coelho-Filho, 2006; Mantelatto et al., 2020; Melo, 1998, 2008).
Material examined. 2 J, size range: 1.59 ≤ CW ≤ 2.68 mm; CCDB 6538.
Xanthodius parvulus (Fabricius, 1793)
(Fig. 8C)
Diagnosis and morphological description: Melo (1996).
Taxonomic summary
Type locality. Haiti (as Chlorodius americanos) (De Saussure 1857).
Geographic distribution. Western Atlantic – Florida, Gulf of Mexico, Cuba, Antilles, Venezuela, Bermuda, and Brazil (Atol das Rocas, Fernando de Noronha, Espírito Santo, and São Paulo) (Alves, Barros-Alves, Cobo et al., 2012; Diez-García & Capote, 2015; Mantelatto et al., 2020; Melo, 1998; Tavares et al., 2017).
Material examined. 33 M, size range: 4.45 ≤ CW ≤ 16.07 mm; 35 F, size range: 4.55 ≤ CW ≤ 12.5 mm; 9 OF, size range: 8.08 ≤ CW ≤ 15.53 mm; 24 J, size range: 2.22 ≤ CW ≤ 4.78 mm; CCDB 6541.
Williamstimpsonia denticulatus (White, 1848)
(Fig. 8D)
Diagnosis and morphological description: Melo (1996) (as Xanthodius denticulatus); description of the new genus and combination with this species: Števčić (2011).
Taxonomic summary
Type locality. West Indies (as Xantho denticulatus) (White, 1848).
Geographic distribution. Western Atlantic – Bermudas, Bahamas, Gulf of Mexico Florida, north and south coast of Cuba, Antilles, Venezuela, Panama, and Brazil (reefs of São Pedro and São Paulo, Ceará, and from Bahia to São Paulo). Central Atlantic – Ascension Island. Eastern Atlantic – Ghana, and the offshore island of the Gulf of Guinea (Alves et al., 2006; Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Diez-García & Capote, 2015; Manning & Chace, 1990; Mantelatto et al., 2020; Melo, 1998) as Xanthodius denticulatus (De Grave et al., 2014).
Material examined. 1 M, size: 18.25 mm CW; 2 OF, size range: 15.13 ≤ CW ≤ 15.77 mm; CCDB 6542.
Subfamily Zosiminae Alcock, 1989
Platypodiella spectabilis (Herbst, 1794)
(Fig. 8E)
Diagnosis and morphological description: Melo (1996); taxonomic comments for P. spectabilis species, and color variation: Martin and Zimmerman (2007) and Hartog and Türkay (1991).
Taxonomic summary
Type locality. Guadeloupe (as Cancer venustus) (Desbonne & Schramm, 1867).
Geographic distribution. Western Atlantic – Bermuda, Florida, Gulf of Mexico, Antilles, Venezuela, and Brazil (Fernando de Noronha, Trindade Island, from Rio Grande do Norte to São Paulo) (Alves, Barros-Alves, Cobo et al., 2012; Coelho et al., 2008; Giraldes, Coelho-Filho, & Coelho et al., 2015; Giraldes, Coelho-Filho, & Smyth, 2015; Mantelatto et al., 2020; Tavares et al., 2017).
Material examined. 2 M, size range: 12.35 ≤ CW ≤ 25.88 mm; CCDB 6543.
The Shannon diversity index
The combination of methodology showed higher Shannon diversity value (H = 5.71) fallowed by ARS (H = 5.6), and the active search showing the lowest diversity value (H = 4.47). The rarefaction curve showed that the combined methods are useful and increase the diversity (Fig. 9).
Discussion
The present work reveals a region with an important diversity of decapod crustaceans (crabs and anomurans). Here we register a protected by lawpreserved area, embracing the most common and especially relatively rare species on the coast of São Paulo. This demonstrates the importance of the Marine State Park of Laje de Santos (MSPLS) as an area of refuge, preservation, and maintenance of diversity, particularly as it is far from the coast and therefore has a high potential to remain a “no-take” area.
The combination of sampling methodologies was important for carrying out our survey (Fig. 9). Traps seem to be an excellent alternative for sampling cryptic habitats. There are 2 efficient types of trap for this purpose: the bait and the refuge traps – the first one is specially used to capture active individuals and sometimes can be biased to certain sizes and sexes of specimens (Calado & Narciso, 2004; Osawa et al., 2015). It is important to note that bait traps cannot remain in the environment for long periods, however, the refuge traps (like such as the present ARS), that which generally allows access to smaller individuals, like such as juveniles and small young adults, can remain in the environment for long periods (Osawa et al., 2015); and refuge traps are efficient for sampling several members of the decapod fauna.
A total of 987 individuals distributed into 32 species, 22 genera, and 11 families were determined, and 24 of them are documented with colour plates. We recorded 2 anomuran species with new meridional records. The porcellanid, Petrolisthes amoenus had its distribution extended approximately 135 km to the south; the previously southern record was from Victoria island (Alves et al., 2011). The other anomuran with a new meridional record is Dardanus venosus, which was previously recorded up to the Ubatuba region (Mantelatto et al., 2001).
For the hermit crabs, we sampled 5 species, with Pagurus brevidactylus and Paguristes tortugae being the 2 most abundant species in MSPLS. Previous studies revealed that both species were also the most abundant in the consolidated substrate of some island areas of São Paulo state (Lima et al., 2014, 2018; Mantelatto & Garcia, 2002). Pagurus criniticornis, also often found in shallow waters of the non-consolidated substrate in the region (Mantelatto & Garcia, 2002), was recorded in MSPLS in the interface from the consolidated to the non-consolidated substrate, close to the soft bottom. With the same pattern in this interface was D. venosus, which has its distribution also extended to the MSPLS with a new meridional record, approximately 135 km south from its previous record from Anchieta Island, in northern littoral of São Paulo state (Mantelatto et al., 2001). The other species sampled was C. tibicen, which is a common hermit crab found in intertidal and shallow water zones (Fransozo & Mantelatto, 1998; Garcia & Mantelatto, 2000; Hazlett, 1966), widely distributed in the Western Atlantic (Mandai et al., 2018; Nucci & Melo, 2015).
The Brachyura occur in both the rocky and the soft substrates. Many works were focused on establishing the composition of this group at the non-consolidated bottoms in São Paulo State (Bertini & Fransozo, 2004; Bertini et al., 2004, 2010; Braga et al., 2005; Fransozo et al., 1992; Mantelatto & Fransozo, 2000), and the register of this group in island and rocky subtidal zones is focused in areas of the northern coast of São Paulo (Alves, Barros-Alves, Cobo et al., 2012; Alves, Barros-Alves, Teixeira et al., 2012; Mantelatto, Faria et al., 2004; Mantelatto, Biagi et al., 2004; Mantelatto & Corrêa, 1996; Mantelatto & Souza-Carey, 1998). Alves, Barros-Alves, Cobo et al. (2012) made the richest checklist of Brachyura crabs for consolidated substrates on the Brazilian coast, with 42 species recorded. Just 3 of the species recorded in the present work were not sampled by the authors in the Victoria Archipelago (S. furcatus, P. caribbaeus, and A. bermudensis), probably because those species represent rare, or occasional species, with no more than 3 specimens of them being sampled in the present work (Barlow et al., 2010; Straatsma & Egli, 2012).
Mithraculus forceps was, by far, the most representative species in the present work. It seems to be a very common species in shallow-rocky bottoms and is very exploited as ornamental crab for the aquarium trade (Penha-Lopes et al., 2007). The biology and ecology features of this species are well-known for the São Paulo State coast (Cobo, 2002, 2006; Cobo & Okamori, 2008; Mantelatto, Biagi et al., 2004). Thus, this is a key-species that deserves attention on their conservation status, to make possible that the community remains in equilibrium with ecosystem and exploitation as ornaments.
Recognizing the importance and sensitive status of conservation of “no-take marine areas”, the MSPLS crustacean fauna is representative. Since the management plans for the area are still being implemented, this study provides the first record of decapod crustaceans for the park and therefore can be used as a baseline for local fauna monitoring programs and conservation planning.
Acknowledgments
The major support for this study was provided by Grants from the “Coordenação de Aperfeiçoamento de Pessoal de Nível Superior” (CAPES/CIMAR II 23038.004310/2014-85, 23038.004308/201414; CAPES/PROEX 23038.000802/2018-25; CAPES PRINT 88881.310767/2018-01). Additional support came from FAPESP (Process 2015/01959-5 by master’s degree scholarship program to Moraes, IRR). We thank Jéssica Colavite, Laira Lianos, Michele Mollemberg, and Nadashine Parasram for all their help with the sorting process in all material sampled. We also thank all the members of the SCUBA dive performance: Orion Dive, Ubatuba Adventure and Omnimmare, and all the people from the Laje de Santos Institute for their support during the field activities. The authors are also grateful to all members from NEBECC and LABCAM laboratory for contributing to the final version of the manuscript. ALC and FLM thank Conselho Nacional de Desenvolvimento Científico e Técnológico – CNPq for ongoing scholarships (311034/2018-7 and 304968/2014-5, respectively). Field sampling license permission COTEC No. 91/2015 D187/2014 BA, PROCESS SMA No.: 260108-010.480/2014.
References
Abele, L. G. (1974). Species diversity of Decapod Crustaceans in marine habitats. Ecology, 55, 156–161. https://doi.org/10.2307/1934629
Abele, L. G., & Kim, W. (1986). An illustrated guide to the marine decapod crustaceans of Florida, part II. State of Florida Department of Environmental Regulation. Technical Series, 8, 325–760.
Abele & Kim, W. (1989). The decapod crustaceans of the Panama Canal. Smithsonian Contributions to Zoology, 482, 1–50. https://doi.org/10.5479/si.00810282.482
Ahyong, S. T., Lowry, J. K., Alonso, M., Bamber, R. N., Boxshall, G. A., Castro, P. et al. (2011). Subphylum Crustacea Brünnich, 1772. In Z. Q. Zhang (Eds.), Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148, 165–191. https://doi.org/10.11646/zootaxa.3148.1.33
Almeida, A. O., Bezerra, L. E., Souza-Filho, J. F., Almeida, S. M., Albuquerque, D. L., & Coelho, P. A. (2008). Decapod and stomatopod crustaceans from Santo Aleixo Island, state of Pernambuco, Brazil. Nauplius, 16, 23–41.
Almeida, A. O., & Coelho, P. A. (2008). Estuarine and marine brachyuran crabs (Crustacea : Decapoda ) from Bahia , Brazil: checklist and zoogeographical considerations. Latin American Journal of Aquatic Research, 36, 183–222. https://doi.org/10.3856/vol36-issue 2-fulltext-4
Almeida, A. O., Costa-Souza, A. C., Cunha, A. M., Santos, P. S., Oliveira, M. V., & Soledade, G. O. (2013). Estuarine caridean shrimps (Crustacea: Decapoda) from Ilhéus, Bahia, Brazil: Updated checklist and a key for their identification. Check List, 9, 1396–1405. https://doi.org/10.15560/9.6.1396
Almeida, A. O., & Ferraz, N. R. (2006). Crustáceos decápodos estuarinos de Ilhéus, Bahia, Brasil. Biota Neotropica, 6, 1–24. https://doi.org/10.1590/S1676-06032006000200024
Almeida, A. O., Terossi, M., Buranelli, R. C., Castilho, A. L., Costa, R. C., Zara, F. J. et al. (2018). Checklist of decapods (Crustacea) from the coast of the São Paulo state (Brazil) supported by integrative molecular and morphological data: II. infraorder Caridea: family Alpheidae. Zootaxa, 4450, 331–358. https://doi.org/10.11646/ZOOTAXA.4450.3.2
Alves, D. F. R., Barros-Alves, S. P., & Cobo, V. J. (2011). Composition and abundance of porcellanid crabs (Crustacea: Decapoda: Anomura) from rocky bottoms off Vitória Island, southeast coast of Brazil. Zoologia (Curitiba, impresso), 28, 214–218. https://doi.org/10.1590/S1984-46702011000200009
Alves, D. F. R., Barros-Alves, S. P., Cobo, V. J., Lima, D. J. M., & Fransozo, A. (2012). Checklist of the brachyuran crabs (Crustacea: Decapoda) in the rocky subtidal of Vitória Archipelago, southeast coast of Brazil. Check List, 8, 940–950. https://doi.org/10.15560/8.5.940
Alves, D. F. R., Barros-Alves, S. P., Teixeira, G. M., & Cobo, V. J. (2012). Mithracinae (Decapoda: Brachyura) from the Brazilian coast: Review of the geographical distribution and comments on the biogeography of the group. Nauplius, 20, 51–62.
Alves, D. F. R., Cobo, V. J., & Melo, G. A. S. (2006). Extension of the geographical distribution of some brachyuran and porcellanid decapods (Crustacea) to the coast of the State of São Paulo, Brazil. Revista Brasileira de Zoologia, 23, 1280–1283. https://doi.org/10.1590/S0101-81752006000400045
Alves, D. F. R., López-Greco, L. S., Barros-Alves, S. P., & Hirose, G. L. (2019). Sexual system, reproductive cycle and embryonic development of the red-striped shrimp Lysmata vittata, an invader in the western Atlantic Ocean. Plos One, 14, 1–18. https://doi.org/10.1371/journal. pone.
0210723
Assugeni, C. O., Magalhães, T., Bolaños, J. A., Tudge, C. C., Mantelatto, F. L., & Zara, F. J. (2017). Ultrastructure of spermatozoa of spider crabs, family Mithracidae (Crustacea, Decapoda, Brachyura): Integrative analyses based on morphological and molecular data. Journal of Morphology, 278, 1628–1646. https://doi.org/10.1002/jmor.20737
Barlow, J., Gardner, T. A., Louzada, J., & Peres, C. A. (2010). Measuring the conservation value of tropical primary forests: The effect of occasional species on estimates of biodiversity uniqueness. Plos One, 5, e9609. https://doi.org/10.1371/journal.pone.0009609
Barros-Alves, S. P., Alves, D. F. R., & Cobo, V. J. (2018). Brachyuran crab (Crustacea, Decapoda) assemblage associated with Sargassum cymosum in southeastern Brazil. Marine Biodiversity, 48, 2043–2055. https://doi.org/10.1007/s12526-017-0730-3.
Benedict, J. E., & Rathbun, M. J. (1891). The genus Panopeus. Proceedings of the United States National Museum, 14, 355–385. https://doi.org/10.5479/si.00963801.14-858.355
Bertini, G., & Fransozo, A. (2004). Bathymetric distribution of brachyuran crab (Crustacea, Decapoda) communities on coastal soft bottoms off southeastern Brazil. Marine Ecology Progress Series, 279, 193–200. https://doi.org/10.3354/meps279193
Bertini, G., Fransozo, A., & Melo, G. A. S. (2004). Biodiversity of brachyuran crabs (Crustacea: Decapoda) from non-consolidated sublittoral bottom on the northern coast of São Paulo State, Brazil. Biodiverstity and Conservation, 13, 2185–2207. https://doi.org/10.1023/B:BIOC.0000047
900.96123.34
Bertini, G., Fransozo, A., & Negreiros-Fransozo, M. L. (2010). Brachyuran soft-bottom assemblage from marine shallow waters in the southeastern Brazilian littoral. Marine Biodiversity, 40, 277–291. https://doi.org/10.1007/s12526-010-0049-9
Biffar, T. A., & Provenzano, A. J. Jr. (1972). A reexamination of Dardanus venosus (H. Milne Edwards) and D. imperator (Miers), with a description of a new species of Dardanus from the western Atlantic (Crustacea, Decapoda, Diogenidae). Bulletin of Marine Science, 22, 777–805.
Boschi, E. E., Fischbach, C. E., & Iorio, M. I. (1992). Catálogo ilustrado de los Crustáceos estomatópodos y decápodos marinos de Argentina. Frente Maritimo, 10, 7–94.
Braga, A. A, Fransozo, A., Bertini, G., & Fumis, P. B. (2005). Composition and Abundance of the Crabs ( Decapoda , Brachyura ) Off Ubatuba and Caraguatatuba, Northern Coast of São Paulo, Brazil. Biota Neotropica, 5, 1–34. https://doi.org/10.1590/S1676-06032005000300004
Calado, R., & Narciso, L. (2004). An inexpensive baited trap for collecting cryptic Decapod crustaceans. Crustaceana, 77, 341–351.
Camargo, F. V., Alves, D. F. R., & Cobo, V. J. (2010). Range extensions for three majoid crabs (Crustacea, Decapoda, Brachyura) on the coast of São Paulo state, Brazil. Pan-American Journal of Aquatic Sciences, 5, 169–172.
Cobo, V. J. (2002). Breeding period of the spider crab Mithraculus forceps (A. Milne Edwards) (Crustacea, Majidae, Mithracinae) in the southeastern Brazilian coast. Revista Brasileira de Zoologia, 19, 229–234. https://doi.org/10.1590/S0101-81752002000500017
Cobo, V. J. (2006). Population Biology of the Spider Crab, Mithraculus forceps (A. Milne-Edwards, 1875) (Majidae, Mithracinae) on the Southeastern Brazilian Coast. Crustaceana, 78, 1079–1087. https://doi.org/10.1163/156854005775361016
Cobo, V. J., & Alves, D. F. R. (2009). Relative growth and sexual maturity of the spider crab, Mithrax tortugae Rathbun, 1920 (Brachyura, Mithracidae) on a continental island off the southeastern Brazilian coast. Crustaceana, 82, 1265–1273. https://doi.org/10.1163/001121609X12481627024490
Cobo, V. J., & Okamori, C. M. (2008). Fecundity of the spider crab Mithraculus forceps (Decapoda , Mithracidae) from the northeastern coast of the state of São Paulo, Brazil. Iheringia, Sér. Zool., Porto Alegre, 98, 84–87. https://doi.org/10.1590/S0073-47212008000100012
Coelho, P. A., Almeida, A. O., & Bezerra, L. E. A. (2008). Checklist of the marine and estuarine Brachyura (Crustacea: Decapoda) of northern and northeastern Brazil. Zootaxa, 58, 1–58. https://doi.org/10.11646/ZOOTAXA.1956.1.1
Coelho, P. A., & Ramos-Porto, M. (1980). Crustáceos Decápodos da costa do Maranhão, Brasil. Boletim do Instituto Oceanográfico, 29, 135–138. https://doi.org/10.1590/S0373-55241980000200028
Coelho-Filho, P. A. (2006). Checklist of the Decapods (Crustacea) from the outer continental shelf and seamounts from Northeast of Brazil – REVIZEE Program (NE III). Zootaxa, 27, 1–27. https://doi.org/10.11646/zootaxa.1184.1.1
Colavite, J., Santana, W., & Tavares, M. (2016). Morphological differences between Stenocionops furcatus (Olivier, 1791) and S. coelatus (A. Milne-Edwards, 1878) (Crustacea, Decapoda, Brachyura, Majoidea). Zootaxa, 4184, 517–528. http://doi.org/10.11646/zootaxa.4184.3.6
Dana, J. D. (1852). United States Exploring Expedition, during the years 1838, 1839, 1840, 1841, 1842. Under the command of Charles Wilkes, U.S.N.: XIII. Crustacea, part I. Philadelphia: Printed by C. Sherman. https://doi.org/10.5962/bhl.title.69333
De Grave, S., Anker, A., Dworschak, P. C., Clark, P. F., & Wirtz, P. (2014). An updated checklist of the marine Decapoda of Ascension Island, central Atlantic Ocean. Journal of the Marine Biological Association of the United Kingdom, 1990, 1–12. http://doi.org/10.1017/S0025315414001295
De Saussure, H. (1857). Diagnoses de quelques Crustacés nouveaux des Antilles et du Mexique. Revue et Magasin de Zoologie, 2, 304-308.
Desbonne, I., & Schramm, A. (1867). Brachyures. In H. Saussure, & W. Stimpson Eds, Crustacés de la Guadeloupe, d’après un manuscrit du Docteur Isis Desbonne comparé avec les échantillons de Crustacés de sa collection et les dernières publications de MM (pp. i–ii). Premiere partie. Imprimerie du Gouvernment, BasseTerre.
Diez-García, Y. L., & Capote, A. J. (2015). List of marine crabs (Decapoda: Anomura and Brachyura) of shallow littoral of Santiago de Cuba, Cuba. Check List, 112, 1–22. http://dx.doi.org/10.15560/11.2.1601
D’incao, F. (1995). Ocorrência de Metapenaeus monoceros no sul do Brasil (Decapoda: Penaeidae). Nauplius, 3, 165–167.
Drehen-Mansur, M. C. D., dos Santos, C. P., Darrigran, G., Heydrich, I., Callil, C. T., & Rossoni-Cardoso, F. (2003). Primeiros dados quali-quantitativos do mexilhão-dourado, Limnoperna fortunei (Dunker), no Delta do Jacuí, no Lago Guaíba e na Laguna dos Patos, Rio Grande do Sul, Brasil e alguns aspectos de sua invasão no novo ambiente. Revista Brasileira de Zoologia, 20, 75–84. https://doi.org/10.1590/S0101-81752003000100009
Felder, D. L., & Martin, J. W. (2003). Establishment of a new genus for Panopeus bermudensis Benedict & Rathbun, 1891 and several other xanthoid crabs from the Atlantic and Pacific oceans (Crustacea: Decapoda: Xanthoidea). Proceedings of the Biological Society of Washington, 112, 438–452.
Ferreira, L. A. A., & Melo, G. A. S. (2016). Porcelain crabs from Brazil (Crustacea: Decapoda: Anomura: Porcellanidae). Zootaxa, 4092, 175–194. http://doi.org/10.11646/zootaxa.
4092.2.2
Ferreira, L. A. A., & Tavares, M. (2017). A new species of Pachycheles (Crustacea: Anomura: Porcellanidae), with taxonomic remarks on two other porcelain crabs from the remote oceanic archipelago of Trindade and Martin Vaz, South Atlantic Ocean. Zootaxa, 4299, 546–560. https://doi.org/10.11646/zootaxa.4299.4.5
Fransozo, A., Costa, R. C., Mantelatto, F. L. M., Pinheiro, M. A. A., & Santos, S. (2002). Composition and abundance of shrimp species (Penaeidea and Caridea) in Fortaleza Bay, Ubatuba, São Paulo, Brazil. Modern Approaches to the Study of Crustacea, 1, 117–125. https://doi.org/10.1007/978-1-4615-0761-1_19
Fransozo, A., & Mantelatto, F. L. M. (1998). Population structure and reproductive period of the tropical hermit crab Calcinus tibicen (Decapoda: Diogenidae) in the region of Ubatuba, São Paulo, Brazil. Journal of Crustacean Biology, 18, 738–745. https://doi.org/10.1163/193724098X00610
Fransozo, A., Negreiros-Fransozo, M. L., Mantelatto, F. L. M., Pinheiro, M. A. A., & Santos, S. (1992). Composição e distribuição dos Brachyura (Crustacea, Decapoda) do sublitoral não consolidado na enseada da Fortaleza, Ubatuba (SP). Revista Brasileira de Biologia, 52, 667–675.
Garcia, R. B., & Mantelatto, F. L. M. (2000). Variability of shell occupation by intertidal and infralittoral Calcinus tibicen (Anomura, Diogenidae) populations. Nauplius, 8, 99–105.
Gibbes, L. R. (1850). On the carcinological collections of the United States, and an enumeration of species contained in them, with notes on the most remarkable, and descriptions of new species. Proceedings of the American Association for the Advancement of Science, 3, 165–201.
Giraldes, B. W, Coelho-Filho, P. A., & Coelho, P. A. (2015). Composition and spatial distribution of subtidal Decapoda on the “Reef Coast”, northeastern Brazil, evaluated through a low-impact visual census technique. Nauplius, 20, 187–201.
Giraldes, B. W., Coelho-Filho, P. A., & Smyth, D. M. (2015). Decapod assemblages in subtidal and intertidal zones-Importance of scuba diving as a survey technique in tropical reefs, Brazil. Global Ecology and Conservation, 3, 163–175. https://doi.org/10.1016/j.gecco.2014.11.011
Gotelli, N. J., & Ellison, A. M. (2004). A primer of ecological statistics. Sunderland: Sinauer Associates.
Guérin-Méneville, F. E. (1855). Animales articulados con piés articulados. In R. de la Sagra (Ed.), Historia física política y natural de la isla de Cuba. Segunda Parte. Historia Natural. Tomo VII (Crustaceos, Aragnides é Insectos) [1856]. Tomo VIII (Atlas de Zoologia) (pp.1–371) Calle del Sordo.
Guinot, D. (2012). Remarks on Inachoididae Dana, 1851, with the description of a new genus and the resurrection of Stenorhynchinae Dana, 1851, and recognition of the inachid subfamily Podochelinae Neumann, 1878 (Crustacea, Decapoda, Brachyura, Majoidea). Zootaxa, 3416, 22–40. https://doi.org/10.11646/zootaxa.3416.1.2
Hartog, J. C., & Türkay, M. (1991). Platypodiella georgei spec. nov. (Brachyura: Xanthidae), a new crab from the island of St. Helena, South Atlantic Ocean, with notes on the genus Platypodiella Guinot, 1967. Zoologische Mededelingen, 65, 210–220.
Hazlett, B. A. (1966). Factors affecting the aggressive behavior of the hermit crab. Zeitschrift Für Tierpsychologie, 23, 655–671. https://doi.org/10.1111/j.1439-0310.1966.tb01703.x
Hendrickx, M. E. (1995). Checklist of brachyuran crabs (Crustacea: Decapoda) from the eastern Tropical Pacific. Bulletin de l’Institut Royal Des Sciences Naturelles de Belgique, 65, 125–150.
Hsieh, T. C., Ma, K. H., & Chao, A. (2016). Interpolation and extrapolation for species diversity. Methods in Ecology and Evolution, 7, 1451–1456.
Lester, S. E., Halpern, B. S., Grorud-Colvert, K., Lubchenco, J., Ruttenberg, B. I., Gaines, S. D. et al. (2009). Biological effects within no-take marine reserves: a global synthesis. Marine Ecology Progress Series, 384, 33–46. https://doi.org/10.3354/meps08029
Lima, D. J. M., Alves, D. F. R., & Cobo, V. J. (2018). Composition, density, and shell use of hermit crabs (Crustacea: Paguroidea) from subtidal boulder fields in southeastern Brazil. Latin American Journal of Aquatic Research, 46, 72–82. http://dx.doi.org/10.3856/vol46-issue1-fulltext-9
Lima, D. J. M., Cobo, V. J., Aquino, M. A. B., & Fransozo, A. (2014). The population structure of two sympatric hermit-crab species on a subtidal rocky shore of an island in southeastern Brazil. Anais Da Academia Brasileira de Ciencias, 86, 1769–1782. http://dx.doi.org/10.1590/0001-3765201420130472
Lima, D. J. M., & Santana, W. (2017). A new hermit crab of the Paguristes tortugae complex (Crustacea: Anomura: Diogenidae), with a key to the western Atlantic species. Marine Biology Research, 13, 220–228. http://dx.doi.org/10.1080/17451000.2016.1239021
Lima, D. J. M., Tavares, M., & de Mendonça, J. B. (2019). Paguroids (Decapoda: Anomura: Diogenidae and Paguridae) of the remote oceanic Archipelago Trindade and Martin Vaz, off southeast Brazil, with new records, description of three new species and zoogeographical notes. Zootaxa, 4694, 1–063. https://doi.org/10.11646/zootaxa.4694.1.1
Lockington, W. N. (1877). Description of seventeen new species of Crustacea. Proceedings of the California Academy of Sciences, 7, 41–48. https://doi.org/10.5962/bhl.title.37841
Lubchenco, J., Palumbi, S. R., Gaines, S. D., & Andelman, S. (2003). Plugging a Hole in the Ocean: The emerging science of Marine Reserves. Ecological Applications, 13, 83–87.
Luiz, O. J., Carvalho-Filho, A., Ferreira, C. E. L., Floeter, S. R., Gasparini, J. L., & Sazima, I. (2008). The reef fish assemblage of the Laje de Santos Marine State Park, Southwestern Atlantic: annotated checklist with comments on abundance, distribution, trophic structure, symbiotic associations, and conservation. Zootaxa, 1807, 1–25. https://doi.org/10.11646/zootaxa.1807.1.1
Mandai, S. S., Buranelli, R. C., & Mantelatto, F. L. (2018). Color patterns of the hermit crab Calcinus tibicen (Herbst, 1791) fail to indicate high genetic variation within COI gene. Nauplius, 26, 1–5. https://doi.org/10.1590/2358-2936e2018008
Mandai, S. S., Buranelli, R. C., Schubart, C. D., & Mantelatto, F. L. (2018). Phylogenetic and phylogeographic inferences based on two DNA markers reveal geographic structure of the orangeclaw hermit crab Calcinus tibicen (Anomura: Diogenidae) in the western Atlantic. Marine Biology Research, 14, 565–580. https://doi.org/10.1080/17451000.2018.1497184
Manning, R. B., & Chace, F. A. (1990). Decapod and Stomatopod Crustacea from Ascension Island , South Atlantic Ocean. Smithsonian Contributions to Zoology, 503, 1–91. https://doi.org/10.5479/si.00810282.503
Mantelatto, F. L. M., Biagi, R., Faria, F. C. R., Meireles, A. L., & Melo, G. A. S. (2004). Checklist on brachyuran fauna (Decapoda) from infralittoral rocky/sandy bottom of Anchieta Island, São Paulo State, Brazil. Nauplius, 12, 135–142.
Mantelatto, F. L. M., & Corrêa, E. K. (1996). Composition and seasonal variations of the brachyuran crabs (Crustacea, Decapoda) living on Sargassum cymosum in Ubatuba region, São Paulo, Brazil. Bioikos, 9-10, 22–31.
Mantelatto, F. L. M., & Fransozo, A. (2000). Brachyuran Community in Ubatuba Bay, Northern Coast of Sao Paulo State, Brazil. Journal of Shellfish Research, 19, 701–709.
Mantelatto, F. L. M., & Garcia, R. B. (2002). Hermit crab fauna from the infralittoral zone of Anchieta Island (Ubatuba, Brazil). Modern Approaches to the Study of Crustacea, (pp. 137–143). Springer, Boston, MA. https://doi.org/10.1007/978-1-4615-0761-1_22
Mantelatto, F. L. M., Garcia, R. B., Martinelli, J. M., & Hebling, N. J. (2001). On a record of Dardanus venosus (H. Milne Edwards) (Crustacea, Anomura) from the São Paulo State, Brazil. Revista Brasileira de Zoologia, 18, 71–73. https://doi.org/10.1590/S0101-81752001000100006
Mantelatto, F. L. M., Robles, R., Schubart, C. D., & Felder, D. L. (2009). Molecular Phylogeny of the Genus Cronius Stimpson, 1860, with Reassignment of C. tumidulus and Several American Species of Portunus to the Genus Achelous De Haan, 1833 (Brachyura: Portunidae). In Crustacean Issues 18: Decapod Crustacean Phylogenetics, 1833, 567–579. https://doi.org/10.1201/9781420092592
Mantelatto, F. L. M., & Souza-Carey, M. M. (1998). Brachyura (Crustacea, Decapoda) associated to Schizoporella unicornis (Bryozoa, Gymnolaemata) in Ubatuba Bay (SP), Brazil. Brazilian Archives of Biology and Technology, 41, 212–217. https://doi.org/10.1590/S1516-89131998000200007
Mantelatto, F. L. M., Tamburus, A. F., Magalhães, T., Buranelli, R. C., Terossi, M., Negri, M. et al. (2020). Checklist of decapod crustaceans from the coast of the São Paulo state (Brazil) supported by integrative molecular and morphological data: III. Infraorder Brachyura Latreille, 1802. Zootaxa, 4872, 001–108. https://doi.org/10.11646/zootaxa.4872.1.1
Mantelatto, F. L. M., Terossi, M., Negri, M., Buranelli, R. C., Robles, R., Magalhães, T. (2018). DNA sequence database as a tool to identify decapod crustaceans on the São Paulo coastline. Mitochondrial DNA Part A: DNA Mapping, Sequencing, and Analysis, 29, 805–815. https://doi.org/10.1080/24701394.2017.1365848
Mantelatto, F. L. M., Faria, F. C. R., Biagi, R., & Melo, G. A. S. (2004). Majoid crabs community (Crustacea: Decapoda) from infralittoral rocky/sandy bottom of Anchieta Island, Ubatuba. Brazilian Archives of Biology and Technology, 47, 273–279. https://doi.org/10.1590/S1516-89132004000200015
Martin, J. W., & Abele, L. G. (1986). Notes on male pleopod morphology in the Brachyuran crab family Panopeidae Ortmann, 1893, sensu Guinot (1978) (Decapoda). Crustaceana, 50, 182–198. https://doi.org/10.1163/156854086X00205
Martin, J. W., & Zimmerman, T. L. (2007). Color Variation in the Caribbean Crab Platypodiella spectabilis (Herbst, 1794) (Decapoda, Brachyura, Xanthidae). Gulf and Caribbean Research, 19, 59–63. https://doi.org/10.18785/gcr.1901.08
McLaughlin, P. A. (2003). Illustrated keys to families and genera of the superfamily Paguroidea (Crustacea: Decapoda: Anomura), with diagnoses of genera of Paguridae. Memoirs of Museum Victoria, 60, 111–144. http://doi.org/10.24199/j.mmv.2003.60.16
McLaughlin, P. A., & Provenzano, A. J. Jr. (1974). Hermit crabs of the genus Paguristes (Crustacea: Decapoda: Diogenidae) from the Western Atlantic Part I. The Paguristes tortugae complex, with notes on variation. Bulletin of Marine Science, 2, 165–234.
Melo, G. A. S. (1996). Manual de identificação dos Brachyura (caranguejos e siris) do litoral brasileiro. São Paulo: Plêiade/FAPESP.
Melo, G. A S. (1998). Malacostraca – Eucarida, Brachyura, Oxyrhyncha and Brachyrhyncha. In Young, P.S. (Ed.), Catalogue of Crustacea of Brazil (pp. 455–515). Rio de Janeiro: Museu Nacional.
Melo, G. A S. (1999). Manual de identificação dos Crustacea Decapoda do litoral brasileiro: Anomura, Thalassinidea, Palinuridea, Astacidea. São Paulo: Plêiade/FAPESP.
Melo, G. A. S. (2008). The Brachyura (Decapoda) of Ilha Grande Bay, Rio de Janeiro, Brazil. Nauplius, 16, 1–22.
Miers, E. J. (1886). Brachyura collected by H.M.S. Challenger during the years 1873-1876. In Report on the Scientific Results of the Voyage of H.M.S. Challenger During the Years 1873–76 Under the Command of Captain George S. Nares, R.N., F.R.S. and the Late Captain Frank Tourle Thomson, R.N, 17. https://doi.org/10.5962/bhl.title.6513
Milne-Edwards, A. (1873-1881). Études sur les Xiphosures et les Crustacés de la Région Mexicaine, Mission Scientifique au Mexique et dans l’Amerique centrale. Recherches Zoologiques a l’Histoire de la Faune de l’Amerique Centrale et du Mexique (Paris), 5, 1–368. https://doi.org/10.5962/bhl.title.119681
Milne-Edwards, H. (1848). Note sur quelques nouvelles espèces du genre Pagure. Annales des Sciences Naturelles Zoologie, Paris, 3, 59–64. https://doi.org/10.5962/bhl.title.51053
Moraes, I. R. R., Almeida, A. O., Cobo, V. J., Alves, D. F. R., Davanso, T. M., & Castilho, A. L. (2021). Biodiversity of caridean shrimps on rocky bottoms of two preserved islands on the southeastern Brazilian coast. Marine Biology Research, 16, 616–631. https://doi.org/10.1080/17451000.2020.1864830
Negreiros-Fransozo, M. L. (1996). The Zoea I of Charybdis hellerii (A. Milne-Edwards, 1867) (Decapoda, Portunidae) Obtained in Laboratory. Nauplius, 4, 165–168.
Negreiros-Fransozo, M. L., & Fransozo, V. (2003). A morphometrics study of the mud crab, Panopeus austrobesus Williams, 1983 (Decapoda, Brachyura) from a subtropical mangrove in South America. Crustaceana, 76, 281–294. http://dx.doi.org/10.1163/156854003765911685
Ng, P. K. L., Davie, P. J. F., & Guinot, D. (2008). Systema Brachyurorum : Part I. an Annotated Checklist of Extant Brachyuran Crabs of the World. The Raffles Bulletin of Zoology, 17, 1–286.
Nucci, P. R. (2002). Taxonomia e biogeografia da superfamília Paguroidea Latreille (Crustacea, Decapoda, Anomura) no litoral brasileiro (Ph.D. Thesis). UNESP, Rio Claro, Brazil.
Nucci, P. R., & Melo, G. A. S. (2007). Hermit crabs from Brazil. Family Paguridae (Crustacea: Decapoda: Paguroidea): Genus Pagurus. Zootaxa, 1406, 47–59. https://doi.org/10.11646/ZOOTAXA.1406.1.6
Nucci, P. R., & Melo, G. A. S. (2011). Hermit crabs from Brazil: Family Paguridae (Crustacea: Decapoda: Paguroidea), except Pagurus. Zootaxa, 3104, 26–41. https://doi.org/10.11646/ZOOTAXA.3104.1.2
Nucci, P. R., & Melo, G. A. S. (2015). Hermit crabs from Brazil: Family Diogenidae (Crustacea: Decapoda: Paguroidea), except Paguristes. Zootaxa, 3947, 327–346. https://doi.org/10.11646/zootaxa.3947.3.2
Osawa, Y., Aoki, M. N., Bauer, R. T., & Thiel, M. (2015). Numbers and sizes of the shrimp Rhynchocinetes uritai Kubo, 1942 (Decapoda: Caridea) caught in bait and refuge traps. Journal of Crustacean Biology, 35, 768–775. https://doi.org/10.1163/1937240X-00002374
Pachelle, P. P. G., Anker, A., Mendes, C. B., & Bezerra, L. E. A. (2016). Decapod crustaceans from the state of Ceará, northeastern Brazil: an updated checklist of marine and estuarine species, with 23 new records. Zootaxa, 4131, 1–63. http://doi.org/10.11646/zootaxa.4131.1.1
Penha-Lopes, G., Figueiredo, J., & Narciso, L. (2007). Modelling survival and growth of Mithraculus forceps’ larvae and juveniles (A. Milne Edwards, 1875) (Decapoda: Brachyura: Majidae) in aquaculture. Aquaculture, 264, 285–296. http://doi.org/10.1016/j.aquaculture.2006.12.019
Powers, L. W. (1977). A catalogue & bibliography to the Crabs (Brachyura) of the Gulf of Mexico. Contributions in Marine Science, 0082–3394, 1–190.
R Core Team (2020). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Randall, J. E. (1967). Food habits of reef fishes of the West Indies. Studies in Tropical Oceanography, 5, 665–847.
Rathbun, M. J. (1898). The Brachyura of the biological expedition to the Florida Keys and the Bahamas in 1893. Bulletin of the Laboratories of Natural History of the State University of Iowa, 4, 250–294.
Rathbun, M. J. (1920). New species of spider crabs from the Straits of Florida and the Caribbean Sea. Proceedings of the Biological Society of Washington, 33, 23–24.
Rathbun, M. J. (1925). The spider crabs of America. Bulletin of the U.S. National Museum, 129, 1–613. https://doi.org/10.5479/si.03629236.129.i
Rathbun, M. J. (1930). The cancroid crabs of America of the families Euryalidae, Portunidae, Atelecyclidae, Cancridae and Xanthidae. Bulletin of the United States National Museum, 152, 1–609. https://doi.org/10.5479/si.03629236.152.i
Rieger, P. J., & Giraldi, J. L. B. (1996). Mithraculus forceps (A. M. Edwards, 1875) novo registro de Brachyura (Decapoda, Majidae) para o litoral do estado de Santa Catarina, Brasil. Trabalhos Oceanograficos Da Universidade Federal de Pernambuco, 24, 237–240. https://doi.org/10.5914/tropocean.v24i1.2712
Rieger, P. J., & Giraldi, J. L. B. (2001). Mithrax hispidus (Herbst) e Mithrax tortugae Rathbun novos registros de Brachyura (Decapoda, Majidae) para o litoral de Santa Catarina, Brasil. Revista Brasileira de Zoologia, 18, 653–654. https://doi.org/10.1590/S0101-81752001000200034
Rolim, F. A., Langlois, T., Rodrigues, P. F. C., Bond, T., Motta, F. S., Neves, L. M. et al. (2019). Network of small no-take marine reserves reveals greater abundance and body size of fisheries target species. Plos One, 14, 1–22. https://doi.org/10.1371/journal.pone.0204970
Sala, E., & Giakoumi, S. (2018). Food for Thought: No-take marine reserves are the most effective protected areas in the ocean. ICES Journal of Marine Science, 75, 1166–1168. https://doi.org/10.1093/icesjms/fsx059
Sankarankutty, C., & Ferreira, A. C. (2000). Hexapanopeus manningi, a new xanthid crab (Crustacea, Oecapoda, Xanthidae) from Brazil. Revista Brasileira de Zoologia, 17, 645–649. https://doi.org/10.1590/S0101-81752000000
300010
Santana, W., Pohle, G., & Marques, F. (2003). Zoeal stages and megalopa of Mithrax hispidus (Herbst, 1790) (Decapoda: Brachyura: Majoidea: Mithracidae): a reappraisal of larval characters from laboratory cultured material and a review of larvae of the Mithrax-Mithraculus species complex. Invertebrate Reproduction and Development, 44, 17–32. https://doi.org/10.1080/07924259.2003.9652550
Schmitt, W. L. (1933). Four new species of decapod crustaceans from Porto Rico. American Museum Novitates, 662, 1–9.
Spiridonov, V. A., Neretina, T. V., & Schepetov, D. (2014). Morphological characterization and molecular phylogeny of Portunoidea Rafinesque, 1815 (Crustacea Brachyura): implications for understanding evolution of swimming capacity and revision of the family-level classification. Zoologischer Anzeiger, 253, 404–429. https://doi.org/10.
1016/j.jcz.2014.03.003
State Decree: 37.537 (1993) “Cria o Parque Estadua Marinho da Laje de Santos e dá provivências correlatas”. Luiz Antonio Fleury. https://governo-sp.jusbrasil.com.br/legislacao/177209/decreto-37537-93
Števčić, Z. (2011). Addition to the reclassification of brachyuran crabs (crustacea: Decapoda: Brachyura) part I. new taxa. Natura Croatia, 20, 125–139.
Stimpson, W. (1859). Notes on North American Crustacea, in the Museum of the Smithsonian Institution. No. I. Annals of the Lyceum of Natural History of New York, 7, 49–93.
Stimpson, W. (1860). Notes on North American Crustacea,. No. II. Annals of the Lyceum of Natural History of New York, 7, 177–246. https://doi.org/10.1111/j.1749-6632.1862.tb00153.x
Stimpson, W. (1871a). Notes on North American Crustacea, in the museum of the Smithsonian Institution. No. III. Annals of the Lyceum of Natural History in New York, 10, 119–163.
Stimpson, W. (1871b). Preliminary report on the Crustacea dredged in the Gulf Stream in the Straits of Florida, by L.F. de Pourtales, Assist. U.S. Coast Survey. Bulletin of the Museum of Comparative Zoology at Harvard College, 2, 109–160.
Stirling, G., & Wilsey, B. (2001). Empirical Relationships between Species Richness, Evenness, and Proportional Diversity. The American Naturalist, 158, 286–299. https://doi.org/389. 10.1086/321317
Straatsma, G., & Egli, S. (2012). Rarity in large data sets: Singletons, modal values and the location of the species abundance distribution. Basic and Applied Ecology, 13, 380– 389. https://doi.org/10.1016/j.baae.2012.03.011
Tavares, M., & Amouroux, J. M. (2003). First Record of the Non-Indigenous Crab, Charybdis hellerii (A. Milne-Edwards, 1867) from French Guyana (Decapoda, Brachyura, Portunidae). Crustaceana, 76, 625–630. https://doi.org/10.1163/156854003322316254
Tavares, M., Carvalho, L., & Mendonça, J. B. Jr. (2017). Towards a review of the decapod crustacea from rhe remore oceanic archipelago of Trindade and Martin vaz, South Atlantic Ocean: new records and notes on ecology and zoogeography. Papéis Avulsos de Zoologia, 57, 157–176. https://doi.org/10.11606/0031-1049.2017.57.14
Terossi, M., Almeida, A. O., Buranelli, R. C., Castilho, A. L., Costa, R. C., Zara, F. J. et al. (2018). Checklist of decapods (Crustacea) from the coast of the São Paulo state (Brazil) supported by integrative molecular and morphological data: I. Infraorder Caridea: families Hippolytidae, Lysmatidae, Ogyrididae, Processidae and Thoridae. Zootaxa, 4370, 76–94. https://doi.org/10.11646/zootaxa.4370.1.6
Thoma, B. P., & Felder, D. L. (2020). A new genus and species of xanthoid crab (Decapoda: Brachyura) from offshore hard bank habitats in the Gulf of Mexico. Zootaxa, 4731, 403–413. https://doi.org/10.11646/ZOOTAXA.4731.3.8
Vieira, R. R. R., & de Calazans, D. K. (2010). Chave ilustrada para identificação das zoés de brachyura do estuário da lagoa dos patos (RS) e região costeira adjacente. Biota Neotropica, 10, 431–437. https://doi.org/10.1590/S1676-
06032010000300036
White, A. (1848). Short Descriptions of new or little-known Decapod Crustacea. Proceedings of the Zoological Society of London, 15, 222–228.
Williams, A. B. (1983). The mud crab, Panopeus herbstii, S.L. partition into six species (Decapoda: Xanthidae). Fishery Bulletin, 81, 863–882.
Windsor, A. M., & Felder, D. L. (2014). Molecular phylogenetics and taxonomic reanalysis of the family Mithracidae MacLeay (Decapoda : Brachyura : Majoidea). Invertebrate Systematics, 28, 145–173. https://doi.org/10.1071/IS13011
Windsor, A. M., & Felder, D. L. (2017). Corrigendum to: Molecular phylogenetics and taxonomic reanalysis of the family Mithracidae MacLeay (Decapoda : Brachyura : Majoidea). Invertebrate Systematics, 31, 232. https://doi.org/10.1071/IS13011_CO




