Your place, my place…, distribution of Agonostomus monticola and Sicydium multipunctatum in the Acahuapa Watershed
Saúl González-Murcia, Francisco S. Álvarez *
UDP Ciencias Neotropicales, Departamento de Investigación, Calle Francisco Campos 166, Colonia Escalón, CP 1101, San Salvador, El Salvador.
*Corresponding author: samuel_biologo@hotmail.com (F.S. Álvarez)
Abstract
Diadromous fish undergo habitat shifts over their life cycles, dwelling between different environments. In the Acahuapa River watershed, the distribution, abundance, and length structure of A. monticola and S. multipunctatum were assessed in its tributary rivers and main channel for 1 year. Fishes were captured using electrofishing devices and nets. Environmental variables, habitat traits, and land use around the sampling points were taken into consideration. A total of 222 A. monticola and 183 S. multipunctatum were recorded during the sampled year. Their distribution was restricted to 8 of the 17 sampling points. Both species co-occurred at 7 of 8 sites, all at elevations between 19 and 325 m asl. Both species were more abundant in lower parts of the watershed and in the tributary rivers than in the main channel. Water temperature, dissolved oxygen, river width and depth, current speed, substrata dominated by rocks and logs, and surroundings of forest and small-scale agriculture favor the presence of these fishes. The smaller individuals of A. monticola occurred at lower elevations of the watershed but fish length differences were not present at the main channel or at the tributaries. For S. multipunctatum, the larger fish were captured also at low elevations and at the main channel of the Acahuapa River watershed.
Keywords:
Size structure; Temporal and spatial patterns; Diadromous fishes; Central America; Lempa River
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Tu lugar, mi lugar…, distribución de Agonostomus monticola y Sicydium multipunctatum en la cuenca del Acahuapa
Resumen
Los peces diádromos cambian de hábitat durante su ciclo de vida ocupando ambientes diferentes. En la cuenca del río Acahuapa, la distribución, abundancia y estructura de tallas de A. monticola y S. multipunctatum fueron evaluadas en sus ríos tributarios y en el canal principal durante 1 año. Los peces fueron capturados utilizando dispositivos de electropesca y trasmallos. Las variables ambientales, características del hábitat y el uso de suelo en los puntos de muestreo fueron cuantificados. Un total de 222 ejemplares de A. monticola y 183 de S. multipunctatum fueron registrados durante el año de muestreo. Su distribución estuvo restringida a 8 de los 17 sitios de muestreo coincidiendo en 7 de ellos, en alturas entre 19 y 325 m snm. Ambas especies fueron más abundantes en las partes bajas de la cuenca y en los ríos tributarios. La temperatura del agua, oxígeno disuelto, ancho y profundidad del río, velocidad de la corriente y sustrato dominado por rocas, troncos y rodeado por bosque o agricultura a pequeña escala favorecen la presencia de estos peces. Los peces de menor tamaño de A. monticola se encontraron en la parte baja de la cuenca sin importar si este es un río tributario o el canal principal. Peces de S. multipunctatum de mayor tamaño se capturaron en la parte baja y el canal principal de la cuenca del Acahuapa.
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Estructura de talla; Patrones temporales y espaciales; Peces diádromos; Centroamérica; Río Lempa
Introduction
Fishes undertake migrations at different spatial scales that are triggered by multiple environmental or biological factors (McDowall, 2008; Smith & Kwak, 2014a, b). Diadromous fishes are the most relevant example as they regularly displace between marine, estuarine, and freshwater environments to cope with seasonal changes or to find suitable habitats, which can favor reproduction, ontogenetic development, metamorphosis, and suitable physiological conditions (McDowall, 2008). Diadromy is a geographically widespread behavior in fishes, and there are currently around 250 fish species that are considered diadromous (McDowall, 2008). Within diadromy, fishes can be categorized as anadromous, catadromous, and amphidromous due to the use of marine, brackish, or freshwater habitats in different ontogenetic stages and the migrations that they perform among these habitats, particularly for reproductive purposes (McDowall, 1988). In Central America, diadromous fish species are encompassed in the families Gobiidae, Mugilidae, and Eleotridae (McMahan, Matamoros et al., 2013). Due to their migratory behavior and complex life cycles, which include the use of a wide range of habitats, diadromous fishes are vulnerable to any kind of habitat modification, representing a challenging task for conservation that deserves special attention (Lorion et al., 2011; McDowall, 1992, 1999; Smith & Kwak, 2014a).
Two diadromous fish reported for El Salvador are the mountain mullet, Agonostomus monticola (Bancroft, 1834), and the multispotted goby, Sicydium multipunctatum Regan, 1906 (Lyons, 2005; McMahan, Matamoros et al., 2013). The mountain mullet, or tepemechin, A. monticola inhabits preferentially lotic freshwater systems with steep slopes and rapid water flow from 0 to 1500 m asl (Bussing, 2002; Cruz, 1987; Espinosa-Pérez et al., 1993; Matamoros et al., 2009; Miller et al., 2009; Phillip, 1993), although it also inhabits lentic environments (Loftus et al., 1984). Along the Atlantic slope, A. monticola occurs from North Carolina to Venezuela, including the West Indies (Smith & Kwak, 2014a). Along the Pacific slope it has been recorded from California to Ecuador (Matamoros et al., 2009; McMahan, Matamoros et al., 2013; Miller et al., 2009; Smith & Kwak, 2014a). Catadromy was suggested as the migratory behavior displayed by A. monticola (Aiken, 1998; Cruz, 1987; Ditty & Shaw, 1996; Phillip, 1993). Nevertheless, the use of telemetry and otolith microchemistry have recently determined that A. monticola behaves as an amphidromous species with eggs and larvae developing in brackish or marine waters and subsequently recruiting, growing, and living as adults mainly in freshwater systems (Smith & Kwak, 2014a, b). Their reproduction has been related with the rainy season (Aiken, 1998; Cruz, 1987; Ditty & Shaw, 1996; Phillip, 1993); however, some authors report that it occurs all year around (Ribeiro & Villalobos, 2010) and juvenile fish having been recorded in estuarine tide pools (Chicas, 2001) and fresh water systems (Matamoros et al., 2009).
Limited information is available for the biology and ecology of S. multipunctatum which occurs in Mexico, Guatemala, Honduras, and El Salvador, in rivers of relatively short distance and steep gradient (Lyons, 2005). Putatively amphidromous, scarce information exists about its migratory behavior, although it has been intensely studied for other members of the genus Sicydium (Bell & Brown, 1995; Keith, 2003; Silva-Melo, 1990). Fish of the genus Sicydium prefer areas with rocks, and the males display agonistic behavior during the parental care period (Keith, 2003); however, specific information about S. multipunctatum mainly refers to its distribution. Therefore, it is necessary to gather information about the biology and ecology of diadromous fish to generate alternatives to confront the current problems affecting freshwater habitats.
Worldwide freshwater habitats and their biodiversity are considered the most vulnerable and affected ecosystems by anthropogenic activities (Anderson et al., 2006; Liermann et al., 2012; Sala et al., 2000; Vörösmarty et al., 2010). This is particularly an issue in tropical areas where the change in land use is expected to significantly affect nutrients input, sewage disposal, sediments run off, contaminants, and the destruction of riparian areas (Sala et al., 2000). Additionally, dams represent an enormous threat for freshwater species diversity (Vörösmarty et al., 2010), leading to changes in assemblage structure (Anderson et al., 2006), modifying the dynamic of the freshwater systems from lotic to lentic, favoring settlement and competition of generalist and invasive species over specialist and endemics (Poff et al., 2007; Rahel, 2002), promoting shifts in environmental conditions like temperature (Roberts, 2001), changing the substratum and sediment movement, altering or reducing areas for refuge of nesting (Vörösmarty et al., 2003), and creating fragmentation that obstructs the movement of migratory fishes, which consequently precludes the use of spawning or feeding areas and reproduction (McDowall, 1992, 1999). Moreover they generate isolation and impede genetic exchange, decreasing genetic pools (Nielsen et al., 1997), and the exchange of biomass and nutrients (McDowall, 1992, 1999). Until now, data depicting freshwater fish distributions is limited, precluding necessary assessment of the threats to freshwater species (Liermann et al., 2012; Vörösmarty et al., 2010). However, it is clear that these ecosystems are experiencing a loss of species faster than terrestrial or marine biota (Sala et al., 2000).
In El Salvador, the loss of species follows the global trend but at an exacerbated level. Only 1% of its territory remains as a primary ecosystem and the vast majority can be categorized as collapsed, endangered, or critically endangered (Crespin & Simonetti, 2015), mainly as the result of change in land use, caused by the increase in agroproductive systems and urban areas (Crespin & Simonetti, 2016). These factors stand as the main source of freshwater ichthyofauna extinction (Sala et al., 2000). Additionally, only 12% of the rivers in El Salvador are considered to have good water quality and the remaining 88% are classified from regular to very poor based on the water quality index (WQI), limiting to some extent the natural development of aquatic organisms (MARN, 2006). The fish A. monticola is considered important in artisanal fisheries in Central America (Cruz, 1987; Matamoros et al., 2009) and its occurrence has been recorded in the south west region of El Salvador in the watersheds of the rivers Grande de Sonsonate, Mandiga Comalapa, Jiboa of the departments of Sonsonate, La Libertad, and La Paz (McMahan, Matamoros et al., 2013).
Studies of the distribution and ecology of diadromous fishes like A. monticola and S. multipunctatum in El Salvador are scarce, but considering the current environmental trends in El Salvador, it is necessary to start building up strong information about the ichthyofauna and its distribution in order to underpin a base line and understand changes in the near future that could result in local extinctions. As a consequence of the above, the objectives of this study were: 1) to describe the distribution and abundance of A. monticola and S. multipunctatum along the altitudinal gradient and among tributary rivers and the main channel in the basin of the Acahuapa River; 2) to contrast the population length structure of A. monticola and S. multipunctatum between tributary rivers and the main channel, and 3) to assess micro environmental traits of the habitat of A. monticola and S. multipunctatum. We hypothesized that A. monticola and S. multipunctatum abundance decreases with altitude; that abundance patterns differ among tributaries and the main channel and the population length structure differs between tributaries and the main channel. Therefore, we expected a decline in the abundances of fishes in tributaries and main channel at higher altitudes, higher abundances of fishes in tributary than in the main channel, and smaller size fishes in tributaries than in the main channel.
Materials and methods
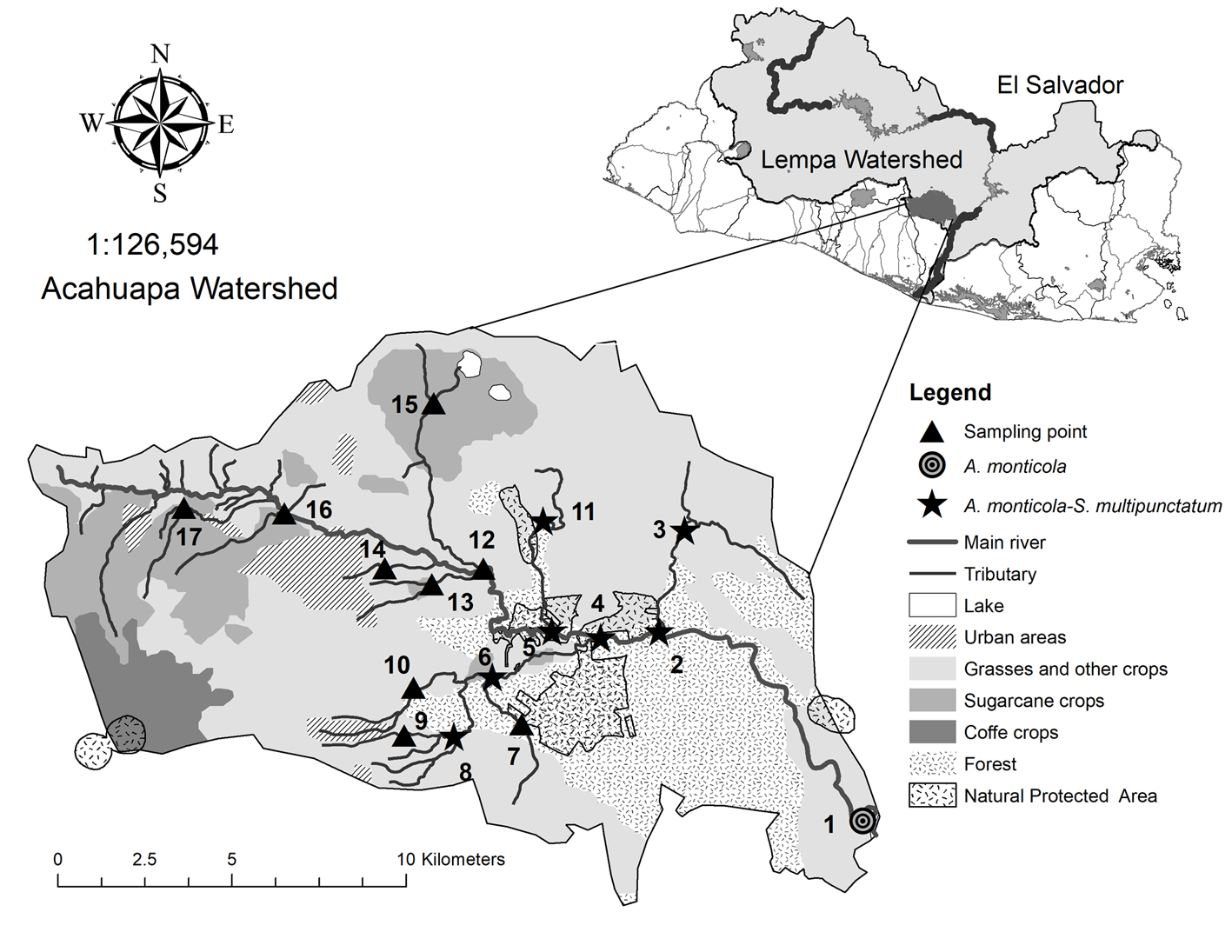
The Acahuapa River is a tributary of the Lempa River in El Salvador. Biogeographically, this area is encompassed in the Chiapas-Fonseca subregion of the Central America region (Abell et al., 2008) and was recently included in the Chiapas-El Salvador-Nacaome province within Nuclear Middle America (Matamoros et al., 2012). The watershed area of the Acahuapa River is 238.88 km2 and the river has a length of 35.33 km ranging from 13-530 m asl and 10 to 13.4 m in width, with multiple tributary rivers (Hernández et al., 2010) (Fig. 1). Tropical weather in the area has 2 marked seasons, the dry season that spans from November to April and the rainy season from May to October.
Along its course, the Acahuapa River crosses the city of San Vicente, with a population size of 198,186 habitants and areas used for stock breeding and agriculture, with crops of sugarcane, maize, and beans (Hernández et al., 2010). In the last part of the course, the Acahuapa River crosses an area of 25 ha of forest that correspond to the natural protected area La Joya to finally connect with the Lempa River (MARN, 2006). Untreated sewage from San Vicente city, pesticides, and residuals from breeding stock and agricultural activities are disposed in the Acahuapa River, and water quality has been classified as regular or poor (Hernández et al., 2010). To assess the distribution of A. monticola and S. multipunctatum, 17 sites were randomly selected and sampled in the watershed of the Acahuapa River, 6 sites were located in the main stream and 11 in the tributaries from elevations of 19 to 519 m asl. The sampling events were performed on 5 occasions during June, August, and November 2011, and February and May 2012 (Fig. 1).
An electrofishing device was used in each sampling. Stunned fish were collected with dip-nets of 1 cm mesh size. A seine-net 5 m in length and 1.5 in height, and 1 cm mesh size was deployed as supporting gear in areas of rapids to facilitate the capture of stunned fish. The mean width of the river was determined to classify the rivers in the following categories < 2 m, 2-5 m, 5-10 m, and 10-20 m and these were used to determine the length of the transect for sampling, corresponding to 50 m, 75 m, 100 m, and 200 m, respectively. A maximum sampling effort of 1 hour of electrofishing zigzag sweeps against the current was performed per site. A single sweep with the afore mentioned device was carried out in the transect in small rivers to avoid the effect of fishing gear (Angermeier & Smogor, 1995). Fish were measured with a flexible measuring tape (mm) and eventually released in the river. Voucher specimens were deposited in the Collection of the Instituto de Ciencias del Mar y Limnología, Universidad of El Salvador, under the catalogue numbers: ICMARES-UES 523-525.
At each sample point, before collecting the fish, a transversal portion of the river was subdivided into 3 sections and water physicochemical parameters such as temperature (ºC), conductivity (µS/cm), dissolved oxygen (mg/l), and pH were recorded. Additionally, water velocity was measured using a current meter (m/s) and the river width and depth recorded (cm) using a flexible measuring tape. The percentages of the substrate coverage (rocks, sand, logs, silt, and leaves) were estimated with the use of a quadrat of 100 squares of 4 cm × 4 cm each. Habitat traits like rapids, waterfalls, erosion on 1 side or both sides of the river, small and big trees, and vegetation surrounding the sampling area were assessed using the absence-presence method, represented by 0 and 1. Additionally, GIS was used in order to record the land use along the river at a radius of 1 km around each sampling point. Urban areas, forest, sugarcane crops, and agricultural use of other crops (corn, beans, sorghum, and grassland,) were estimated (m2) and used for the analysis. All the variables except the land use variables were re-measured at each sampling site.
Data analysis
A general linear model was constructed for the abundance of each species (A. monticola and S. multipunctatum) using a negative binomial distribution and Log link function. To select the best model, a step wise process was performed using the Akaike information criterion (AIC) (Agresti, 1996; Anderson et al., 1994, 2000) and the delta AIC (∆) in order to compare and discriminate among models (Anderson et al., 2000, 2001; Burnham & Anderson, 2003). The final model: abundance = altitude + river type, presented the best approximation to the observed abundance pattern and the significance of each main effect was tested with a X2 test. To assess if the length of the fish was related with altitude, a Pearson correlation test was performed using the standard length of both A. monticola and S. multipunctatum, and the altitude. In order to test if the length distribution of each A. monticola and S. multipunctatum differed between the tributaries and main channel of the Acahuapa River watershed, a 2 sample Kolmogorov-Smirnov goodness of fit test was performed. The test quantifies a distance between the empirical distributions of the 2 samples assuming that the samples are drawn from the same distribution essentially comparing the shape of those distributions. These analyses were performed in the Statistical Package R (R Core Team, 2013).
The environmental conditions of the sampled sites where A. monticola or S. multipunctatum occurred were assessed via principal components analysis (PCA) in order to detect habitat conditions preferred by the fish. The variables included in the analysis were temperature (ºC), conductivity (µS/cm), dissolved oxygen (mg/l), pH, current speed, river width and depth, the bottom structure (rocks, sand, logs, silt and leaves), habitat traits (rapids, waterfalls, erosion, vegetation, trees), and landscape variables around the river; urban areas, forest, sugar cane, crops, and agriculture (grassland, corn, beans, sorghum, etc.). All the data from these environmental variables were standardized (centering and scaling) to reduce the effect of the measurement units in the PCA. For habitat traits (rapids, waterfalls, erosion, vegetation, trees) that were described as absence and presence (0-1), a principal coordinates analysis (PCoorA) was performed to represent binary variables and transform them into quantitative variables expressed on 2 axes (PCoorA1 and PCoorA2). A logistic regression analysis was performed between the environmental variables and the values of PCoorA1 and PCoorA2 to identify the variables that best described the contribution of the 2 PCoorA axes. Only the variables with statistical significance p > 0.05 were described in the PCA. To select the number of interpretable axes in the PCA the broken stick model was computed. The PCA components that were interpreted were those whose eigenvalues were larger than the length of the corresponding piece of the broken stick model. The analyses were performed with the R and InfoStat (Di Rienzo et al., 2011) statistical packages.
Results
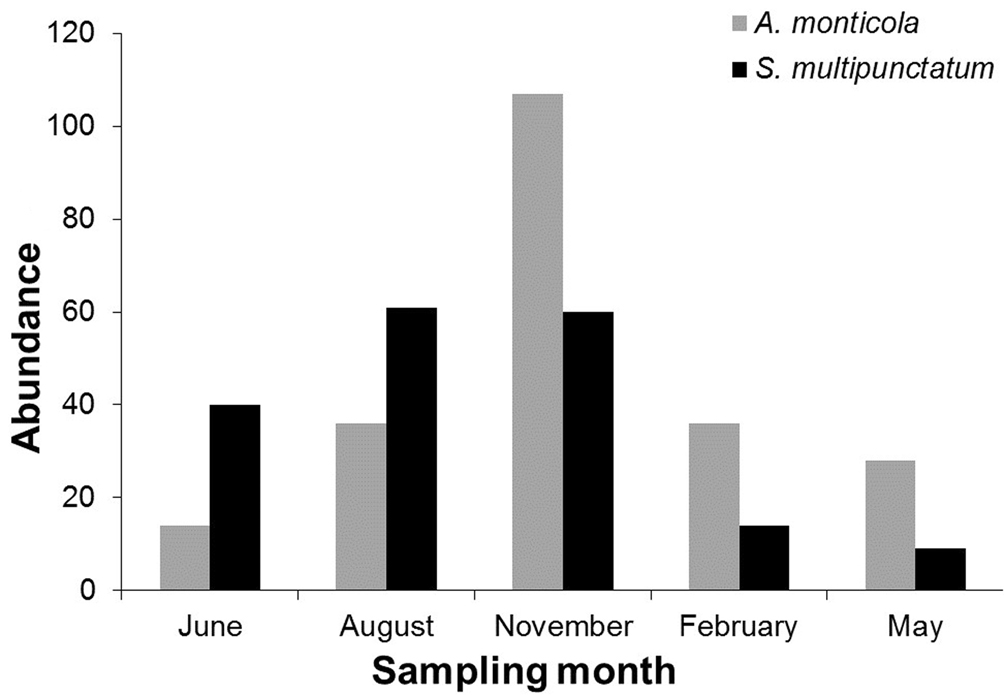
Both species occurred in almost the same sampling points but in Acahuapa-Lempa (sampling point 1), A. monticola occurred alone (Fig. 1). Agonostomus monticola occurred in 8 (47%) of the 17 sampling points, 4 in the main channel and 4 in the tributaries. In total, 222 fish were recorded during the 5 sampling events, with higher abundance in November (Fig. 2). The fish were collected from 19 to 325 m asl and fish abundance in the main channel was lower (38%) than in the tributaries (62%) decreasing with the altitude (Table 1). The average standard length for the total of individuals of A. monticola was 6.54 ± 2.97 cm. While larger fish were found at higher elevations (r = 0.20, t = 3.0649, df = 219, p = 0.0025), the population length structure of the A. monticola in the main channel and the tributaries did not differ (Kolmogorov-Smirnov GOF = 0.1376, p = 0.2334) (Fig. 3).
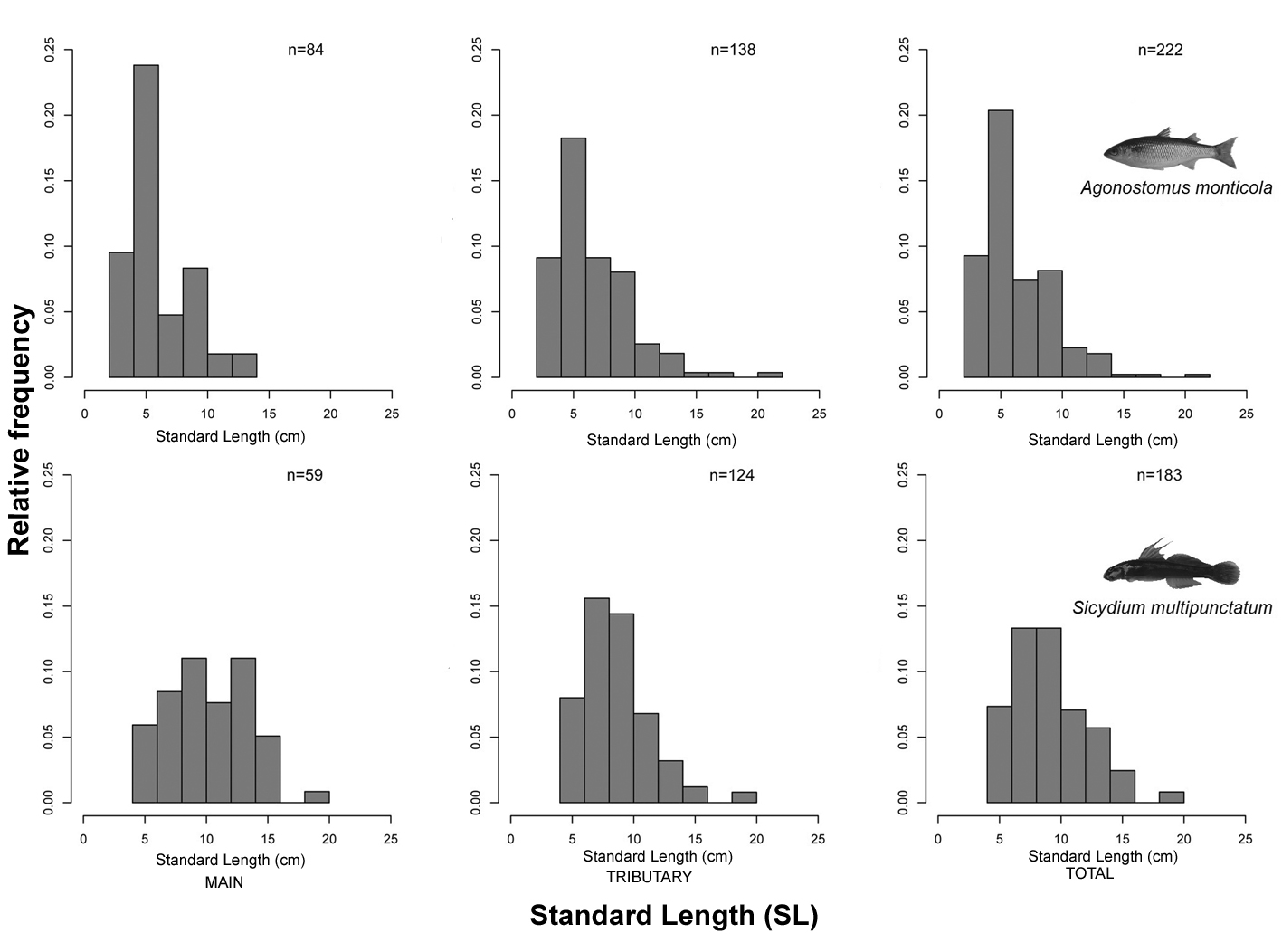
Table 1
Effect of elevation (19-530 m asl) and river type (tributary or main) on the abundance of A. monticola and S. multipunctatum.
|
Agonostomus monticola |
DF |
Residuals deviance |
DF |
Residuals deviance |
Pr (> Chi) |
|
Null |
84 |
108.378 |
|||
|
Elevation |
1 |
52.921 |
83 |
55.457 |
< 0.000 |
|
River type |
1 |
10.135 |
82 |
45.322 |
0.001 |
|
Sycidiun multipunctatum |
DF |
Residuals deviance |
DF |
Residuals deviance |
Pr (> Chi) |
|
Null |
84 |
98.103 |
|||
|
Elevation |
1 |
40.626 |
83 |
57.477 |
< 0.000 |
|
River type |
1 |
10.556 |
82 |
46.920 |
0.001 |
Sicydium multipunctatum occurred in 7 (41%) of the 17 sampling points, 3 in the main channel and 4 at the tributaries. In total, 183 fish were recorded during the 5 sampling events, with higher abundance during August and November (Fig. 2). The fish were collected from 95 to 325 m asl and the abundance in the main channel was lower (32%) than in the tributaries (68%), decreasing with altitude (Table 1). The average standard length for the total of individuals of S. multipunctatum was 9.27 ± 2.98 cm, with larger fish found at lower elevations (r = -0.14, t = -1.9801, df = 181, p = 0.0492). The population length structure of S. multipunctatum in the main channel and at the tributaries differed (Kolmogorov-Smirnov GOF = 0.2755, p = 0.000033) with larger individuals occupying the main channel (Fig. 3).
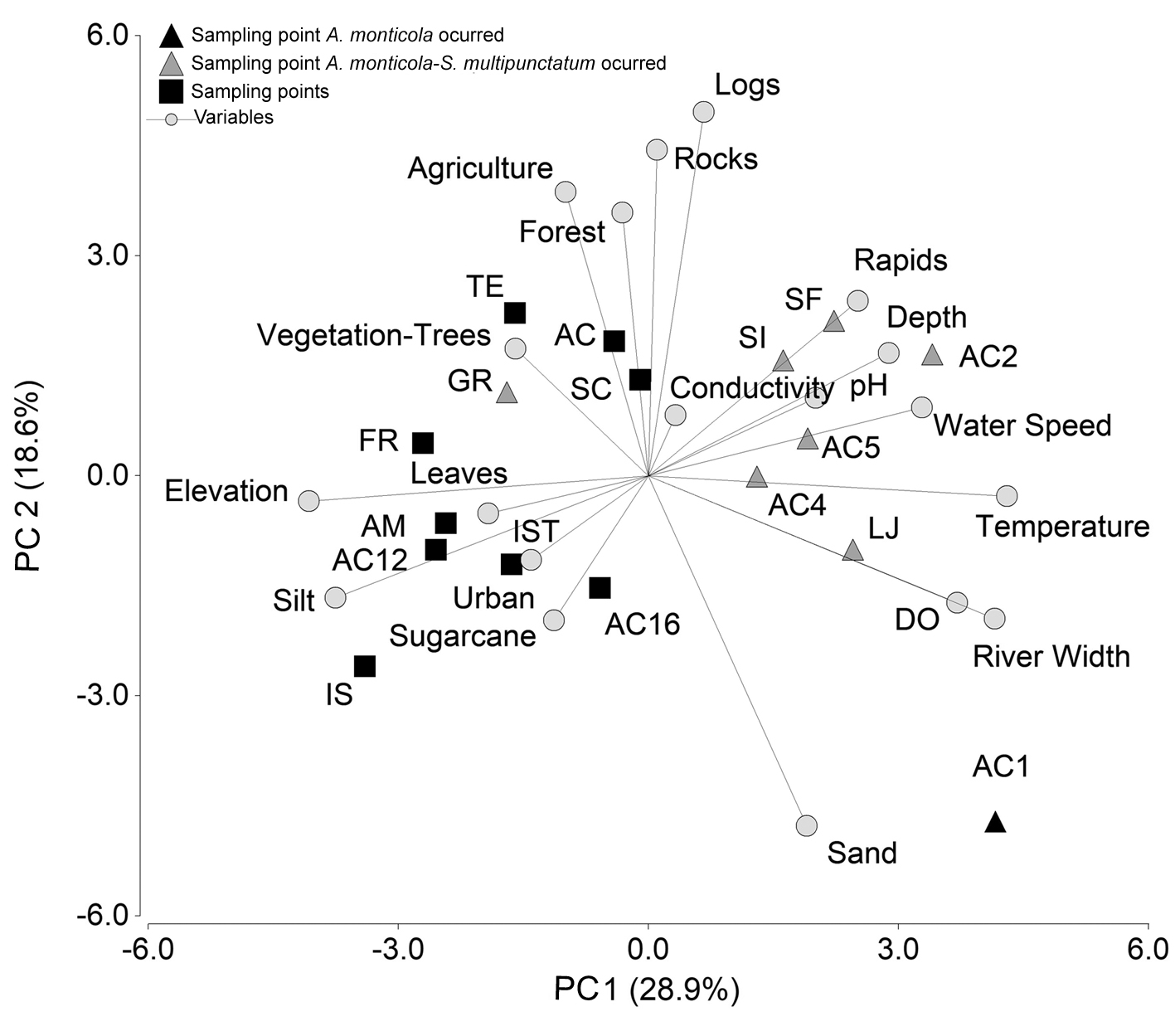
Environmental variables of the sites where the species occurred presented particular traits. The first 2 components of the PCA explained 47.50% of variance (Table 2, Fig. 4). The sites where either A. monticola or S. multipunctatum or both occurred were located in the main channel and in the tributary rivers at low elevations (19-137 m asl) and were characterized by physicochemical variables that include higher water temperatures and dissolved oxygen, and deeper and wider areas with high current speed (Table 2, Fig. 4). Complementary to these traits, the fish preferred areas that presented high cover of rock and logs and in sites where sampling points were surrounded by forest and agricultural areas. The sampling points where A. monticola and S. multipunctatum did not occur were located at elevations between 267-509 m asl and had lower temperatures, lower dissolved oxygen values, and the rivers were narrow and shallow. Usually the substratum in these areas was dominated by silt and sand and bordered by urban areas, sugarcane crops, and scattered trees. The only sampling point that had these conditions with the presence of A. monticola and S. multipunctatum was the Grande River (GR) at 325 m asl but the abundance of fish was low (3 and 8 individuals, respectively).
Discussion
The distribution and abundance of the diadromous fish A. monticola and S. multipunctatum were strongly affected by elevation. Neither species occurred in the main channel or tributaries at altitudes higher than 325 m asl. These results differ drastically with the results presented by Lorion et al. (2011) for the Sixaola River in Costa Rica where diadromous fish richness and abundance increased with elevation (including A. monticola and Sicydium spp.) in a gradient from 0 to 500 m asl. Differences in environmental conditions and habitat traits that are just available in the lower areas of the river could cause the observed altitudinal distribution pattern of both species, which has been previously shown such as a preference for areas with pebbles, rocky substratum, and strong currents (Bussing, 2002; Cruz, 1987; Eslava & Díaz, 2011; Keith, 2003; Lyons, 2005; Matamoros et al., 2009; Miller et al., 2009). Land use played an important role in the distribution of both A. monticola and S. multipunctatum. In particular, abundance was associated with areas surrounded by forest at La Joya Natural Protected Area and small-scale agriculture where water quality was depicted as regular, and did not occur in sampling points surrounded by cane crops and urban areas with poor water quality (Hernández et al., 2010). Deforestation can exert detrimental impacts in ecomorphologically unique species, which can experience sharp declines soon after habitat modification, particularly because deforestation has been coupled with reduction in habitat complexity of allochthonous origin (Brejão et al., 2017). Furthermore, geomorphological traits of the Acahuapa River watershed allowed changes in rivers flow patterns with decreasing current speed values in the low zones, differing from that expected for tropical rivers (Pouilly et al., 2006; Winemiller et al., 2008). However, the lower zone has many tributaries that could affect the current by water incorporation from tributary rivers (Hernández et al., 2010). Therefore, land use variables and their influence on habitat complexity must be carefully considered and included in detail in future studies.
|
Table 2 Results of the principal components analysis (PCA), showing the loadings of physicochemical, substratum complexity, habitat trait, and land use variables on the PC 1 and PC 2. Shaded values in bold represent values with correlation coefficients > 0.60 and statistical significance (p > 0.05). |
||
|
Variables |
PC 1 (28.9%) |
PC 2 (18.6%) |
|
Temperature |
0.39 |
-0.03 |
|
pH |
0.18 |
0.10 |
|
DO |
0.33 |
-0.16 |
|
Conductivity |
0.03 |
0.07 |
|
River Width |
0.37 |
-0.17 |
|
Depth |
0.26 |
0.15 |
|
Water Speed |
0.29 |
0.08 |
|
Sand |
0.17 |
-0.43 |
|
Rocks |
0.01 |
0.40 |
|
Leaves |
-0.17 |
-0.05 |
|
Logs |
0.06 |
0.44 |
|
Silt |
-0.34 |
-0.15 |
|
Elevation |
-0.37 |
-0.03 |
|
Urban |
-0.13 |
-0.10 |
|
Forest |
-0.03 |
0.32 |
|
Sugarcane |
-0.10 |
-0.18 |
|
Agriculture |
-0.09 |
0.35 |
|
Vegetation-Trees |
-0.14 |
0.16 |
|
Rapids |
0.23 |
0.21 |
Other factors that can potentially explain the distribution of fishes from the upper part of the watershed are natural and artificial barriers, meteorological and geological events, and sampling bias. Physical barriers like high falls or natural dams on rivers can disrupt migration of diadromous fishes, excluding them from upstream habitats (McDowall, 2008). Such physical barriers (dams or cascades) were not observed along the river, and regardless of the flow reduction during the dry season, it was considered that there was enough water to allow the movement of fish upstream. Meteorological events like the tropical rain IDA in 2009 can cause landslides and water flows dragging rocks, sediments, and riparian vegetation towards the lower part of the watershed, modifying the habitats and the natural river flow from the upper part of the basin (MARN & SNET, 2009). This natural phenomenon could have reduced the available habitats for these species or limited their distribution towards lower areas. However, diadromous fish tend to be highly resilient, recovering a few months after the unusual storms occurred (Smith & Kwak, 2014a, b). Finally, the collection of A. monticola using nets has been considered difficult in previous studies (Lyons & Navarro-Pérez, 1990) but electrofishing has been a successful technique to capture this fish (Matamoros et al., 2009). However, in this study the same set of methods were used in all the sampling points and we therefore consider that the lack of individuals at elevations higher than 325 m asl may correspond to ecological or anthropogenic factors instead of sampling bias.
High abundances of A. monticola and S. multipunctatum were recorded at lower altitudes and in tributary rivers. These rivers presented low water turbidity, fast well oxygenated waters that represent important habitat for these fish. High abundance of A. monticola in small streams with similar conditions was previously recorded by Ribeiro & Villalobos (2010). Cruz (1987) observed that terrestrial insects that are potential preys of A. monticola are abundant in these rivulets and it is easier for the fish to capture them in clear and less turbulent waters. This is a plausible explanation considering that insects are an important part of their diet (Torres-Navarro & Lyons, 1999). Probably, these habitats offer a refuge for these species, are used temporarily during their migration upstream, or currently represent their primary habitat due to the degradation of the surrounding environments in the watershed. Temporal variation in abundance of small fishes at the end of the rainy season in November for A. monticola and August-November for S. multipunctatum suggest that recruitment could be occurring during these months. Erdman (1977), Cruz (1987), Phillip (1993), Aiken (1998), and Ribeiro and Villalobos (2010) found that peaks of reproduction occur during the rainy season or after the rainy season between May and November in Puerto Rico, Honduras, Trinidad, and Jamaica, with a high abundance of juvenile fish of A. monticola returning to the river a couple of months later having a length of 20 mm (Cruz, 1987; Chicas, 2001; Ribeiro & Villalobos, 2010). This relation between rainfall and spawning in tropical fishes has been previously suggested by Payne (1975) and Lowe-McConnell (1987). To the knowledge of the authors, there is no information about the reproductive ecology of S. multipunctatum and available data only allow us to suggest some aspects of the biology of these species and highlights the caveats that exist for the study of the reproductive biology of this species. Therefore, future research should include behavioral reproduction and gonadic analysis in order to clarify this hypothesis. Disparities in abundance of both fishes are to be assessed and future research needs to be conducted in order to clarify the factors that determine these temporal and spatial discrepancies.
Spatial changes in population size and age structure in diadromous fishes are often related to elevation. Sites at lower elevations have a broad range of individuals of different ages and sizes, while at higher elevations, smaller fish tend to be less abundant or absent (McDowall 2008). This corresponds to the observed size structure pattern for A. monticola with larger fish occurring in higher altitudes and many groups of different sizes in lower areas that did not differ between the tributary rivers and the main channel.
For S. multipunctatum, the opposite occurred. Smaller fish appeared in high altitudes and population size structure differed among tributaries and the main channel. Probably small individuals of S. multipunctatum use tributary rivers as a refugee before they move into the main channel as proposed by Ribeiro & Villalobos (2010); however, these habitats could not have the same function for A. monticola populations. The size of the largest A. monticola was 22 cm, within the range reported for the area (Aiken, 1998; Cruz, 1987; Matamoros et al., 2009; Phillip, 1993). The maximum length recorded for S. multipunctatum was 19 cm, which is 72% larger than the largest individual recorded in Central America (11 cm) (Bussing, 2002). Further research on the ecology of both fish is necessary to shed light on differences in length registered for S. multipunctatum. Additionally, tagging studies could explain the level of interaction that exists among individuals in tributaries and the main channel of the river.
Global threats to the conservation of freshwater biodiversity (Abell et al., 2008; Sala et al., 2000; Vörösmarty et al., 2010) affect Salvadorian freshwater ecosystems at an exacerbated level. Cultivated land covers 82% of El Salvador´s total surface (Crespin & Simonetti, 2015), and habitat loss has probably restricted the distribution of both fishes to areas surrounded by forest cover, which maintains habitat complexity in freshwater systems (Brejão et al., 2017). The Lempa River connects to the Pacific Ocean, allowing access for diadromous fishes to move upstream until the point in which 3 hydroelectric dams create an insurmountable barrier. The Acahuapa River connects with the Lempa River prior to these barriers and it offers an alternative habitat against the detrimental effects of these structures on diadromous fish population dynamics (Anderson et al., 2006; McDowall, 1992, 1999, 2008; Smith & Kwak, 2014a). This is particularly important for the amphidromous lifestyle displayed by A. monticola and S. multipunctatum, who spending the majority of their life in fresh water, with only larvae experiencing estuarine or marine conditions after hatching in fresh water and being passively transported downstream (Keith, 2003; McDowall, 1988; Smith & Kwak, 2014a, b). However, some diadromous populations are composed of a diversity of migratory guilds that conduct full, partial, or no migration between marine and fresh waters (Kerr et al., 2009), which can confer resilience to frequent disturbance (Covich et al., 2006; Secor, 2007; Smith & Kwak, 2014b). Behavioral variation within species sets a challenge for conservation, and suggests that using watersheds as units of management will favor the effective migration of fish in all their life stages and connectivity within populations to reduce the risk of local extinctions (McDowall, 1992, 1999, 2008). This is important for A. monticola, because genetic, molecular, and morphometric analyses demonstrated that this species represents more than 1 taxon (Díaz-Murillo et al., 2017; Durand et al., 2012; McMahan, Davis et al., 2013) and for S. multipunctatum, whose ecological and biological knowledge is limited and whose taxonomy is currently under debate (Chabarria & Pezold, 2013).
Ecological aspects described for the amphidromous fish A. monticola and S. multipunctatum reveal interesting patterns for both species in the watershed of the Acahuapa River that can support insights for conservation and future research. The distribution of both fish is clearly affected by elevation and river type. Habitat traits like water temperature, dissolved oxygen, river width, depth, water speed, and substrata (rocks and logs) surrounded by forest, were identified as the preferred habitats for these fish. The abundance of A. monticola is higher in lower parts of the river and the tributaries. Smaller fish of this species occur at lower elevations but no differences exist among the length of the fish in the main channel and the tributaries of the Acahuapa River watershed. The abundance of S. multipunctatum is higher in lower parts of the river and the tributaries. Larger fish of this species occur at lower elevations and differences exist in length structure, with larger fish occurring in the main channel of the Acahuapa River watershed. Future research could test migratory, reproductive, and trophic patterns that could aid to explain the observed distribution patterns in these species. Genetic analyses would be appropriate to assess the genetic structure of the populations of A. monticola and S. multipunctatum. The information is limited at some extent, nevertheless, neglecting this information until better data are available in order to start conservation and management initiatives could be inappropriate particularly because of the environmental scenario depicted in El Salvador and its ecosystems.
Acknowledgements
To Edwin Cornejo and Arami Martínez for assistance during field sampling and data collection; SGM thanks S. González Rosales for his advice and help. We are thankful for the comments on the manuscript by the anonymous referees.
References
Abell, R., Thieme, M. L., Revenga, C., Bryer, M., Kottelat, M., Bogutskaya, N. et al. (2008). Freshwater ecoregions of the world: A new map of biogeographic units for freshwater biodiversity conservation. BioScience, 58, 403–414.
Agresti, A. (1996). Categorical data analysis. New York: John Wiley & Sons.
Aiken, K. A. (1998). Reproduction, diet and population structure of the mountain mullet, Agonostomus monticola, in Jamaica, West Indies. Environmental Biology of Fishes, 53, 347–352.
Anderson, D., Burnham, K., & White, G. (1994). Aic model selection in overdispersed capture-recapture data. Ecology, 75, 1780–1793.
Anderson, D. R., Burnham, K. P., & Thompson, W. L. (2000). Null hypothesis testing: Problems, prevalence, and an alternative. The Journal of Wildlife Management, 54, 912–923.
Anderson, D. R., Burnham, K. P., & White, G. C. (2001). Kullback-leibler information in resolving natural resource conflicts when definitive data exist. Wildlife Society Bulletin, 29, 1260–1270.
Anderson, E. P., Freeman, M. C., & Pringle, C. M. (2006). Ecological consequences of hydropower development in Central America: impacts of small dams and water diversion on neotropical stream fish assemblages. River Research and Applications, 22, 397–411.
Angermeier, P. L., & Smogor, R. A. (1995). Estimating number of species and relative abundances in stream-fish communities: effects of sampling effort and discontinuous spatial distributions. Canadian Journal of Fisheries and Aquatic Sciences, 52, 936–949.
Bell, K., & Brown, J. (1995). Active salinity choice and enhanced swimming endurance in 0 to 8-d-old larvae of diadromous gobies, including Sicydium punctatum (Pisces), in Dominica, West Indies. Marine Biology, 121, 409–417.
Brejão, G. L., Hoeinghaus, D. J., Pérez-Mayorga, M. A., Ferraz, S. F., & Casatti, L. (2017). Threshold responses of Amazonian stream fishes to timing and extent of deforestation. Conservation Biology, 32, 860–871.
Burnham, K., & Anderson, D. (2003). Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer.
Bussing, W. A. (2002). Peces de las aguas continentales de Costa Rica. San José: Universidad de Costa Rica.
Chabarria, R. E., & Pezold, F. (2013). Phylogeography and historical demography of Sicydium salvini in the eastern Pacific. Ichthyological Research, 60, 353–362.
Chicas, F. A. (2001). Peces juveniles en una poza de marea, Reserva Forestal Térraba-Sierpe, Puntarenas, Costa Rica. Revista de Biología Tropical, 49, 307–314.
Covich, A. P., Crowl, T. A., & Heartsill-Scalley, T. (2006). Effects of drought and hurricane disturbances on headwater distributions of palaemonid river shrimp (Macrobrachium spp.) in the Luquillo Mountains, Puerto Rico. Journal of the North American Benthological Society, 25, 99–107.
Crespin, S. J., & Simonetti, J. A. (2015). Predicting ecosystem collapse: Spatial factors that influence risks to tropical ecosystems. Austral Ecology, 40, 492–501.
Crespin, S. J., & Simonetti, J. A. (2016). Loss of ecosystem services and the decapitalization of nature in El Salvador. Ecosystem Services, 17, 5–13.
Cruz, G. A. (1987). Reproductive biology and feeding habits of cuyamel, Joturus pichardi and tepemechin, Agonostomus monticola (Pisces; Mugilidae) from Rio Platano, Mosquitia, Honduras. Bulletin of Marine Science, 40, 63–72.
Di Rienzo, J., Casanoves, F., Balzarini, M., González, L., Tablada, M., & Robledo, C. (2011). Infostat versión 2011. In Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. Available from http://www.infostat.com.ar.
Díaz-Murillo, B. P., Ruiz-Campos, G., Piller, K. R., McMahan, C. D., García-De León, F. J., & Camarena-Rosales, F. (2017). Assessing population-level morphometric variation of the mountain mullet Agonostomus monticola (Teleostei: Mugilidae) across its Middle American distribution. Neotropical Ichthyology, 15, e170036.
Ditty, J. G., & Shaw, R. F. (1996). Spatial and temporal distribution of larval striped mullet (Mugil cephalus) and white mullet (M. curema, Family: Mugilidae) in the Northern Gulf of Mexico, with notes on Mountain Mullet, Agonostomus monticola. Bulletin of Marine Science, 59, 271–288.
Durand, J. D., Chen, W. J., Shen, K. N., Fu, C., & Borsa, P. (2012). Genus-level taxonomic changes implied by the mitochondrial phylogeny of grey mullets (Teleostei: Mugilidae). Comptes Rendus Biologies, 335, 687–697.
Erdman, D. S. (1977). Spawning patterns of fish from the northeastern caribbean. In H. B. Stewarts (Eds.), Cooperative investigations of the Caribbean and adjacent regions (pp. 145–170). Rome: FAO Fisheries Reports.
Eslava, E. P., & Díaz, V. R. (2011). Reproducción de Joturus pichardi y Agonostomus monticola (Mugiliformes: Mugilidae) en ríos de la Sierra Nevada de Santa Marta, Colombia. Revista de Biología Tropical, 59, 1717–1728.
Espinosa-Pérez, H., Gaspar-Dillanes, M., & Fuentes-Mata, P. (1993). Listados faunísticos de México III. Los peces dulceacuícolas Mexicanos. México D.F.: Instituto de Biología, Universidad Nacional Autónoma de México.
Hernández Martínez, M., Serrano-Cervantes, L., Sermeño-Chicas, J., Paniagua, M., Pérez, D., Springer, M. et al. (2010). Atlas geográfico de los insectos acuáticos indicadores de calidad ambiental del agua de los ríos de El Salvador. In M. Springer, & J. Sermeño-Chicas (Eds.), Formulación de una guía metodológica estandarizada para determinar la calidad ambiental de las aguas de los ríos de El Salvador, utilizando insectos acuáticos (pp. 1–104). El Salvador: Proyecto Universidad de El Salvador (UES)/ Organizacion de los Estados Americanos (OEA).
Keith, P. (2003). Biology and ecology of amphidromous Gobiidae of the Indo-Pacific and the Caribbean regions. Journal of Fish Biology, 63, 831–847.
Kerr, L. A., Secor, D. H., & Piccoli, P. M. (2009). Partial migration of fishes as exemplified by the estuarine-dependent white perch. Fisheries, 34, 114–123.
Liermann, C. R., Nilsson, C., Robertson, J., & Ng, R. Y. (2012). Implications of dam obstruction for global freshwater fish diversity. BioScience, 62, 539–548.
Loftus, W. F., Kushlan, J. A., & Voorhees, S. A. (1984). Status of the mountain mullet in southern Florida. Florida Scientist, 47, 256–263.
Lorion, C. M., Kennedy, B. P., & Braatne, J. H. (2011). Altitudinal gradients in stream fish diversity and the prevalence of diadromy in the Sixaola River basin, Costa Rica. Environmental Biology of Fishes, 91, 487–499.
Lowe-McConnell, R. H. (1987). Ecological studies in tropical fish communities. London: Cambridge University Press.
Lyons, J. (2005). Distribution of Sicydium valenciennes 1837 (Pisces: Gobiidae) in Mexico and Central America. Hidrobiologica, 15, 239–243.
Lyons, J., & Navarro-Pérez, S. (1990). Fishes of the Sierra de Manantlán, west-central México. The Southwestern Naturalist, 35, 32–46.
MARN (Ministerio del Medio Ambiente y Recursos Naturales). (2006). Informe del estado actual del medio ambiente en El Salvador. El Salvador: MARN.
MARN (Ministerio de Medio Ambiente y Recursos Naturales) & SNET (Servicio Nacional de Estudios Territoriales). (2009). Informe técnico de los sistemas baja presión en el Pácífico y Huracán Ida y sus impactos ambientales. El Salvador: MARN.
Matamoros, W. A., Kreiser, B. R., & Schaefer, J. F. (2012). A delineation of nuclear middle America biogeographical provinces based on river basin faunistic similarities. Reviews in Fish Biology and Fisheries, 22, 351–365.
Matamoros, W. A., Schaefer, J., Mickle, P., Arthurs, W., Ikoma, R. J., & Ragsdale, R. (2009). First record of Agonostomus monticola (Family: Mugilidae) in Mississippi freshwaters with notes of its distribution in the southern United States. Southeastern Naturalist, 8, 175–178.
McDowall, R. (1988). Diadromy in fishes: migrations between freshwater and marine environments. BioScience, 39, 565–567.
McDowall, R. (1992). Particular problems for the conservation of diadromous fish. Aquatic Conservation: Marine and Freshwater Ecosystems, 2, 351–355.
McDowall, R. (1999). Different kinds of diadromy: different kinds of conservation problems. ICES Journal of Marine Science: Journal du Conseil, 56, 410–413.
McDowall, R. (2008). Diadromy, history and ecology: a question of scale. Hydrobiologia, 602, 5–14.
McMahan, C. D., Davis, M. P., Domínguez-Domínguez, O., García-de León, F. J., Doadrio, I., & Piller, K. R. (2013). From the mountains to the sea: phylogeography and cryptic diversity within the mountain mullet, Agonostomus monticola (Teleostei: Mugilidae). Journal of Biogeography, 40, 894–904.
McMahan, C. D., Matamoros, W. A., Álvarez, F. S., Henríquez, W. Y., Recinos, H. M., Chakrabarty, P. et al. (2013). Checklist of the inland fishes of El Salvador. Zootaxa, 3608, 440–456.
Miller, R. R., Minckley, W., & Norris, S. M. (2009). Peces dulceacuícolas de México. México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad.
Nielsen, J. L., Carpanzano, C., Fountain, M. C., & Gan, C. A. (1997). Mitochondrial DNA and nuclear microsatellite diversity in hatchery and wild Oncorhynchus mykiss from freshwater habitats in Southern California. Transactions of the American Fisheries Society, 126, 397–417.
Payne, A. (1975). The reproductive cycle, condition and feeding in Barbus liberiensis, a tropical stream-dwelling cyprinid. Journal of Zoology, 176, 247–269.
Phillip, D. A. (1993). Reproduction and feeding of the mountain mullet, Agonostomus monticola, in Trinidad, West Indies. Environmental Biology of Fishes, 37, 47–55.
Poff, N. L., Olden, J. D., Merritt, D. M., & Pepin, D. M. (2007). Homogenization of regional river dynamics by dams and global biodiversity implications. Proceedings of the National Academy of Sciences, 104, 5732–5737.
Pouilly, M., Barrera, S., & Rosales, C. (2006). Changes of taxonomic and trophic structure of fish assemblages along an environmental gradient in the Upper Beni Watershed (Bolivia). Journal of Fish Biology, 68, 137–156.
R Core Team (2013). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rahel, F. J. (2002). Homogenization of freshwater faunas. Annual Review of Ecology and Systematics, 33, 291–315.
Ribeiro, T. C. & Villalobos, G. U. (2010). Distribution of Agonostomus monticola and Brycon behreae in the Río Grande de Térraba, Costa Rica and relations with water flow. Neotropical Ichthyology, 8, 841–849.
Roberts, T. R. (2001). On the river of no returns: Thailand’s pak mun dam and its fish ladder. Natural History Bulletin of the Siam Society, 49, 189–230.
Sala, O. E., Chapin, F. S., Armesto, J. J., Berlow, E., Bloomfield, J., Dirzo, R. et al. (2000). Global biodiversity scenarios for the year 2100. Science, 287, 1770–1774.
Secor, D. H. (2007). The year-class phenomenon and the storage effect in marine fishes. Journal of Sea Research, 57, 91–103.
Silva-Melo, L. (1990). Sistemática, biología y ecología del titi Sicydium antillarum grant (Pisces: Gobiidae) en la región de Santa Marta, Colombia. Anales del Instituto de Investigaciones Marinas de Punta Betín, 19–20, 153–172.
Smith, W., & Kwak, T. J. (2014a). A capture-recapture model of amphidromous fish dispersal. Journal of Fish Biology, 84, 897–912.
Smith, W., & Kwak, T. J. (2014b). Otolith microchemistry of tropical diadromous fishes: spatial and migratory dynamics. Journal of Fish Biology, 84, 913–928.
Torres-Navarro, C. I., & Lyons, J. (1999). Diet of Agonostomus monticola (Pisces: Mugilidae) in the río Ayuquila, Sierra de Manantlán Biosphere Reserve, México. Revista de Biología Tropical, 47, 1087–1092.
Vörösmarty, C. J., McIntyre, P. B., Gessner, M. O., Dudgeon, D., Prusevich, A., Green, P. et al. (2010). Global threats to human water security and river biodiversity. Nature, 467, 555–561.
Vörösmarty, C. J., Meybeck, M., Fekete, B., Sharma, K., Green, P., & Syvitski, J. P. (2003). Anthropogenic sediment retention: major global impact from registered river impoundments. Global and Planetary Change, 39, 169–190.
Winemiller, K. O., Agostinho, A. A., & Caramaschi, É. P. (2008). Fish ecology in tropical streams. In D. Dudgeons (Eds.), Tropical stream ecology (pp. 107–146). San Diego, CA.: Academic Press.



