Juana Durán-Luz a, Sergio Ibáñez-Bernal a, *, Eduardo A. Rebollar-Téllez b, Luis Arturo Ibarra-Juárez c
a Instituto de Ecología, A.C., Red Ambiente y Sustentabilidad, Carretera Antigua a Coatepec 351, Col. El Haya, 91073 Xalapa, Veracruz, Mexico
b Universidad Autónoma de Nuevo León, Facultad de Ciencias Biológicas, Departamento de Zoología de Invertebrados, Laboratorio de Entomología Médica, Av. Universidad s/n, Ciudad Universitaria, 66451 San Nicolás de los Garza, Nuevo León, Mexico
c Instituto de Ecología, A.C., Red de Estudios Moleculares Avanzados, Carretera Antigua a Coatepec 351, Col. El Haya, 91073 Xalapa, Veracruz, Mexico
*Corresponding author: sergio.ibanez@inecol.mx (S. Ibáñez-Bernal)
Received: 1 June 2022; accepted: 17 May 2023
Abstract
A sand fly fauna inventory was conducted in an ecotourism area of central Veracruz, Mexico. We recorded and analyzed the sand fly diversity at 3 different land use types (well-preserved forest, fruit-tree plantations, and human settlements) with sampling conducted 3 times in 1 year (dry, rainy, and cold seasons). A total of 891 specimens of 6 genera and 14 species was recorded. High diversity was detected in the preserved area due to a high species richness and abundance as compared with the fruit-tree plantations and human settlements, respectively. In relation to the seasons of the year, high diversity was found in the cold season as compared with the rainy and dry seasons. The variation of the Phlebotominae assemblage in space and time in the ecotourism zone is described, serving as a baseline to recommend preventive actions to settlers and travelers. Additionally, we include new species records for the state of Veracruz.
Keywords: Phlebotominae; Richness; Abundance; Spatial variation; Seasonal variation
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Diversidad y variación espacio-temporal de los flebotominos (Phlebotominae: Diptera: Psychodidae) en tres diferentes tipos de uso del suelo y temporadas en el estado de Veracruz, México
Resumen
Se realizó un inventario de la fauna de flebotominos en una zona ecoturística del centro de Veracruz, México. Se registró y analizó la diversidad de flebotominos en 3 tipos diferentes de uso del suelo (bosque, plantaciones de árboles frutales y asentamientos humanos) con muestreos realizados 3 veces en 1 año (estaciones seca, lluviosa y fría). Se registró un total de 891 ejemplares de 6 géneros y 14 especies. Se detectó una alta diversidad en el área selvática debido a una alta riqueza de especies y abundancia en comparación con las plantaciones de árboles frutales y los asentamientos humanos, respectivamente. En relación con las estaciones del año, se encontró una alta diversidad en la estación fría en comparación con las estaciones lluviosa y seca. Se describe la variación del ensamblaje de Phlebotominae en el espacio y el tiempo de una zona ecoturística, sirviendo como línea de base para recomendar acciones preventivas a colonos y viajeros. Adicionalmente, se incluyen nuevos registros de especies en el estado de Veracruz.
Palabras clave: Phlebotominae; Riqueza; Abundancia; Variación espacial; Variación estacional
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
From the nearly 1,000 species of phlebotomine sand flies known worldwide, about 500 species are found in the American continent and neighboring isles (Shimabukuru et al., 2017), and are distributed from southern Canada to northern Argentina, principally inhabiting areas with native vegetation (Munstermann, 2004). From this total, about 98 are proven or suspected vectors of Leishmania affecting humans (Killick-Kendrick, 1990, 1999; Maroli et al., 2013; WHO, 2012; Young & Duncan, 1994). Most of the American sand fly species inhabit tropical and subtropical areas, and their presence is often associated with the incidence of human leishmaniasis (Valderrama et al., 2008). In Mexico, 50 species of extant phlebotomine sand flies and 2 extinct species have been recorded (Ibáñez-Bernal & Durán-Luz, 2022), being the sylvatic southern portion the area with highest species richness and diversity (Moo-Llanes et al., 2013). Veracruz is considered one of the Mexican states with recurrent transmission of Leishmania spp. (González et al., 2011). In Veracruz, 23 species of phlebotomine sand flies have so far been recorded, corresponding to 44.2% of the total Mexican species (Ibáñez-Bernal & Durán-Luz, 2022).
In wild areas, adults of phlebotomine sand flies may be found in different ecotopes used for resting, mating, feeding, and/or undertaking oviposition. They can also be found in caves, cracks, tree-holes and rock hollows, tree-buttresses, leaf litter, rodent and other mammal burrows, and bird or insect nests (Aguiar & Vilela, 1987; Dampf, 1938; Montes de Oca-Aguilar, Moo-Llanes et al., 2013; Montes de Oca-Aguilar, Rebollar-Téllez et al., 2013; Rebollar-Téllez, Andrade-Narvaez et al., 1996; Thatcher, 1968; Vivero et al., 2015; WHO, 2012). Deforestation, and urbanization have had influence over the sand fly populations changing the pattern of Leishmania spp. transmission. Human settlements in wild areas may promote dispersal and changes in sand fly populations, with some species capable of adapting to survive in modified environments (Carvalho et al., 2011), and can be found in the peridomestic area, taking advantage of cracks, hollows, humid walls, hen houses, or getting into houses to obtain blood (endophagy) (Quate & Vockeroth, 1981; WHO, 2012). Also, it is possible to find certain species in agroecosystems, in coffee or cacao plantations (Alexander, 1987; Alexander et al., 2002; Pérez et al., 2014; Pinheiro et al., 2013; Scorza et al., 1985).
Previous studies on the diversity of phlebotomine sand flies in sites with different land use, suggest that habitat degradation affects their assemblage, diminishing species richness and population abundances as perturbation levels increase (Durán-Luz et al., 2019; Feitosa & Castellón, 2004; Jiménez et al., 2000; Machado et al., 2017; Pinheiro et al., 2013; Rebêlo et al., 2019; Travi et al., 2002; Valderrama et al., 2008).
Climate modulates the reproduction, development, activity, and abundance of phlebotomine sand flies, changing the space-temporal distribution. In general, sand fly species are sensitive to changes in temperature and humidity (Aguiar & Vilela, 1987; Moo-Llanes et al., 2013), and for that reason they seek protection in natural shelters with adequate microclimatic conditions; these sites are important as they are probably associated to the breeding places (Montes de Oca-Aguilar, Moo-Llanes et al., 2013; Parras et al., 2012). Some studies indicate that sand flies are more abundant during the hottest humid months (Carvalho et al., 2011; Córdoba-Lanús & Salomón, 2002; Pérez et al., 2014), but others report highest abundance during the hottest dry months (Durán-Luz et al., 2019; Machado et al., 2017; Pinheiro et al., 2013), and at least in the Yucatán Peninsula, their abundance is highest during the coolest months of the year (Rebollar-Téllez, Andrade-Narvaez et al., 1996; Rebollar-Téllez, Ramírez-Fraire et al., 1996; Sánchez-García et al., 2010).
In tropical countries, ecotourism is a developing industry with high economic benefits, providing more activities and revenues to the local economy. Nevertheless, these activities may represent a risk for native people and tourists, as the contact with hematophagous flies increases (Carvalho et al., 2011). Phlebotomine sand flies are the vectors of some species of Leishmania parasites, it is important to determine the species richness and how their populations change through space (land use) and time, helping to detect the probable vectors necessary for leishmaniasis prevention or control programs in endemic areas (WHO, 2012).

The main objective of this study was to document the Phlebotominae composition in an ecotourism area in central Veracruz, and to describe the composition in space and time in 3 areas with different land use in 3 seasons of the year.
Materials and methods
The municipality of Apazapan is in the lower central region of the State of Veracruz, Mexico (19°19’ N, 96°43’ W), at an altitude ranging from 100 to 520 m asl (Fig. 1). It has an area of 67.27 km2, with 18 towns and a total population of 35,347 inhabitants. It is in the Southern Gulf Coastal Plain province and the Veracruz Coastal Plain sub-province. It has an annual temperature range from 22 to 26 °C, with precipitation ranging from 900 to 1,100 mm per year (INEGI, 2010). The predominant weather is warm sub-humid with abundant rainfall during the summer and early fall. There is a marked period of drought that elapses from March to June, followed by a rainy period that comprises from July to October and subsequently a period known as “Nortes’’, which is characterized by sporadic rainfall and includes the coolest months of the year from November to February (García, 1996). The soil types are Feozem (dark, soft layer rich in organic matter and nutrients) and Rendzinas (superficial layer of organic matter resting on limestone rock); whereas the vegetation is defined as median sub-perennial tropical forest characterized by Lysiloma latisiliquum (L.) Benth, Brosimum alicastrum Swartz, Bursera simaruba (L.) Sarg, Manilkara zapota (L.) P. Royen, and Vitex gaumeri Greenm as the dominant species (Rzedowski, 1978). Most of Apazapan’s surface area (84%) is covered by agricultural land, followed by forest (12%), pastureland (3%) and urban areas (1%) (INEGI, 2010). Its main agricultural products are fruit crops such as sapodilla, banana, citrus, and mango, as well as maize, beans, and pumpkins, whereas there is also production of cattle, sheep, pig, and poultry. One of the most important activities in the area, and one that has been an important part of the region’s economy is ecotourism.
Field work consisted of 6 sampling events in 1 year, considering March-June as the dry season (DS), July-October as the rainy season (RS), and November-February as the cold season (CS). Each sampling event was conducted for 2 consecutive days, in 3 areas with different degree of disturbance: 2 areas without human settlements: a wild or sylvatic area (SA), a crop area (CA) mainly with mango, citric and sapodilla orchards, and an area with human settlements (770 inhabitants) considered as an urban area (UA).
Four CDC-UV light traps (Model 912, John W. Hock Company, Gainesville, FL, USA) were placed at each site, separated 50 m from each other. The traps were hung from tree branches, approximately 1 m above the ground, near decomposing organic matter. The traps remained active from 1 hour before sunset until 3 hours after sunset, for a total of 4 human-hours per trap per night. Additionally, 2 Malaise traps (John W. Hock Company, Gainesville, FL, USA), whose bottles contained 96% alcohol, were placed at each sampling site 100 m apart from each other. The traps were left in place for 2 days for each sampling event, and the collecting bottle was replaced twice to include the photophase (6:00-18:00 h) and the scotophase (18:00-6:00 h). The material of interest from the CDC traps was sacrificed with ethyl acetate, separated, and stored in Petri dishes (SYM Laboratorios, Mexico) prepared with glassine paper to avoid damage during transport to the laboratory.
Specimens were mounted on slides following the procedure recommended and described by Ibáñez-Bernal (2005a), using Euparal® (Bioquip Products, Inc., Rancho Dominguez, CA, USA) as a permanent mounting medium. For taxonomic species identification, we observed and analyzed the internal and external morphological characteristics of males and females under the microscopic using the dichotomous keys of Young and Duncan (1994) as well as Ibáñez-Bernal (2005a, b). The classification proposed by Galati (1995, 2003) and the abbreviations of generic names proposed by Marcondes (2007) were followed. Specimens are deposited in the Entomological Collection of the Instituto de Ecología, A. C. (INECOL), Xalapa, Veracruz, Mexico (IEXA).
To avoid biased estimates of diversity by using combined data from different types of traps, we decided to conduct calculations based on data from CDC light traps only. To evaluate the quality of the sampling based on the species richness obtained by sampling effort and repetition of collection and to determine if the samples taken in Apazapan, Veracruz were representative of the phlebotomine community, a species accumulation curve was elaborated. We used the Chao 1 estimator which is based on abundances and estimates the number of expected species considering the ratio between the number of species represented by 1 individual (singletons) and the number of species represented by 2 individuals in the samples (doubletons) (Moreno, 2001). Software EstimateS version 9.1.0 (Colwell, 2013) was used to compute these analyses.
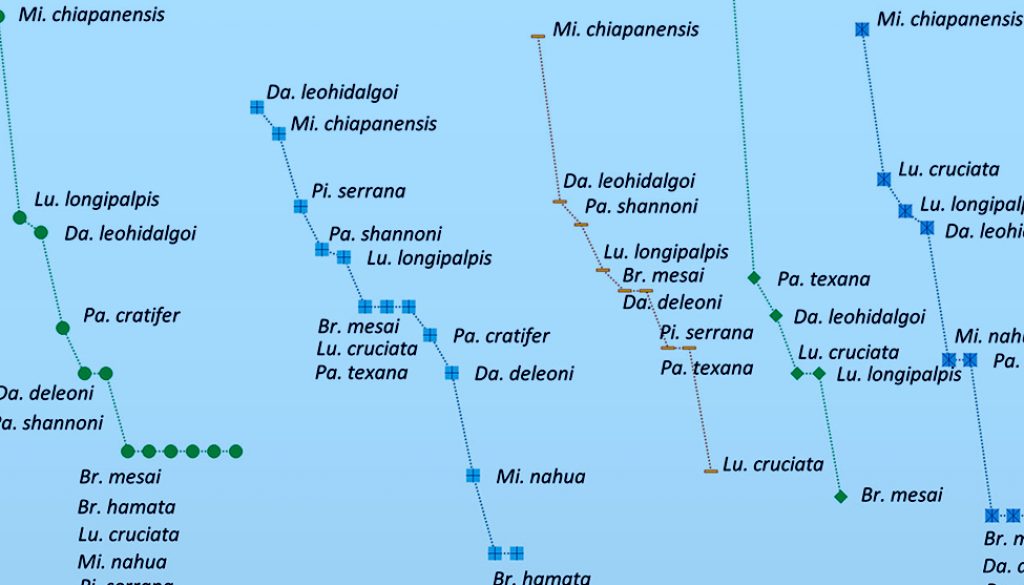
In addition, rank-abundance curves were elaborated for each area, as well as for each season, and between areas and sampling events. These curves represent a graphic comparison of species richness, relative abundances, and taxa dominance. The overall shape of the curves (equitability) display in a clear manner the sequence of each of the species that make up the community without losing their identity and were used to detect spatio-temporal changes in the structure of the phlebotomine assemblage (Feinsinger, 2001).
Alpha diversity (α) was obtained for the 3 areas and sampling seasons, which corresponds to the total number of species present in a community (Moreno, 2001). To calculate this parameter, the effective numbers of species or measures of “true diversity” were obtained, allowing an intuitive and easily comparable interpretation of species diversity (Moreno et al., 2011). Diversity of order 0 (0D) is simply equivalent to species richness (0D = S), whereas order 1 (1D) represents the exponential of Shannon’s index or true diversity, and order 2 (2D) is the inverse of Simpson’s index or equity. The iNEXT package in RStudio version 1.3.959 was used to calculate the diversity analyses. Data were plotted to visualize the effective number of species of each order of diversity, including error bars.
Finally, to graphically observe the relationship of phlebotomine sand fly species composition at the 3 sites and seasons, we generated a similarity dendrogram (Beta diversity) using the Morisita´s index multivariate cluster analysis. This index considers the abundances in species composition in relation to their similarity. The algorithm used to perform the clustering was the unweighted pairs of groups using the arithmetic mean (UPGMA). This analysis was calculated with Past version 3.18 (Hammer et al., 2001).
Table 1
Phlebotomine sand fly species collected with CDC-UV light traps in Apazapan, Veracruz, Mexico, comparing the total number of specimens, their relative abundance, and species richness, in 3 different land-use areas during 3 different seasons.
| Area | Season | Female | Male | Total | % | |||||
| Species | Sylvatic | Crop | Urban | Rainy | Cold | Dry | ||||
| Brumptomyia hamata | 2 | 0 | 0 | 1 | 1 | 0 | 0 | 2 | 2 | 0.2 |
| Brumptomyia mesai | 15 | 2 | 0 | 2 | 10 | 5 | 9 | 8 | 17 | 2.0 |
| Dampfomyia anthophora | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0.1 |
| Dampfomyia deleoni | 12 | 0 | 0 | 2 | 5 | 5 | 10 | 2 | 12 | 1.4 |
| Dampfomyia leohidalgoi | 71 | 20 | 5 | 12 | 70 | 14 | 67 | 29 | 96 | 11.3 |
| Lutzomyia cruciata | 11 | 24 | 2 | 4 | 30 | 3 | 28 | 9 | 37 | 4.3 |
| Lutzomyia longipalpis | 28 | 20 | 22 | 25 | 32 | 13 | 53 | 17 | 70 | 8.2 |
| Micropygomyia chiapanensis | 138 | 210 | 163 | 196 | 203 | 112 | 408 | 103 | 511 | 60.0 |
| Micropygomyia nahua | 3 | 4 | 2 | 1 | 6 | 2 | 7 | 2 | 9 | 1.1 |
| Pintomyia serrana | 26 | 0 | 0 | 1 | 22 | 3 | 16 | 10 | 26 | 3.1 |
| Psathyromyia cratifer | 10 | 0 | 0 | 3 | 7 | 0 | 9 | 1 | 10 | 1.2 |
| Psathyromyia dasymera | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0.1 |
| Psathyromyia shannoni | 26 | 1 | 0 | 2 | 16 | 9 | 15 | 12 | 27 | 3.2 |
| Psathyromyia texana | 14 | 14 | 4 | 10 | 14 | 8 | 21 | 11 | 32 | 3.8 |
| Total abundance | 357 | 296 | 198 | 259 | 418 | 174 | 639 | 212 | 851 | 100.0 |
| Relative abundance | 42.0 | 34.8 | 23.3 | 30.4 | 49.1 | 20.4 | 75.1 | 24.9 | 100.0 | |
| Species richness | 13 | 9 | 6 | 12 | 14 | 10 | 12 | 13 | 14 |
Results
A total of 851 specimens were captured with CDC miniature light traps comprising 6 genera and 14 species, of which 75.1% were females and 24.9% males. The highest species richness and abundance, i.e., 13 species and 357 specimens, were found in SA followed by CA and UA, respectively. Regarding the seasonality, we found that CS was the season with the highest species richness and abundance, collecting a total of 14 species and 418 specimens as compared with RS (30.4%) and DS (20.4%). Micropygomyia chiapanensis (Dampf, 1947) was the most abundant species with 511 specimens (60%), followed by Dampfomyia leohidalgoi Ibáñez-Bernal, Hernández-Xoliot & Mendoza, 2006, with 96 specimens (11.3%) (Table 1). The number of sand flies caught with Malaise traps was comparatively low, with only 40 specimens of 6 species: Brumptomyia hamata (Fairchild & Hertig, 1947) (n = 1), Brumptomyia mesai Sherlock, 1962 (n = 5), D. leohidalgoi (n = 29), Lutzomyia cruciata (Coquillett, 1907) (n = 2), M. chiapanensis (n = 2), and Pintomyia serrana (Damasceno & Arouck, 1949) (n = 1) (Table 2).
In general, for Apazapan, Veracruz, Chao 1 predicts 16 species to be present, suggesting that 2 species were not recorded. The inventory completeness was 87.5% (Fig. 2). For SA inventory completeness was 96.3%, for CA 81.9%, and 100% for UA, indicating that the sample effort in all cases was sufficient.
Of the 13 species collected in the SA, 5 were exclusive to this land use type: Brumptomyia hamata, Dampfomyia deleoni (Fairchild & Hertig, 1947), P. serrana, Psathyromyia cratifer (Fairchild & Hertig, 1961) and Psathyromyia dasymera (Fairchild & Hertig, 1961). In contrast, 9 species were recorded in CA with Dapfomyia anthophora (Addis, 1945) exclusive of this type of land use. Finally, in the UA only 6 species were recorded. Highest equitability was detected in the SA, whereas the highest dominance was found in the CA and UA, given by the large abundance of Mi. chiapanensis (Fig. 3).
About the 3 seasons, in the RS we found 12 species, 14 in CS, and 10 in DS. Dampofomyia anthophora and P. dasymera were only recorded in CS. The CS was the most equitable, whereas RS and DS had a high dominance of M. chiapanensis (Fig. 4). The results show that in SA in the CS presented highest richness with 13 species, being this area and season in which more equitability was found. Lower species richness was detected in the UA in the RS with only 3 species recorded: M. chiapanensis, Lutzomyia longipalpis (Lutz & Neiva, 1912) and Psathyromyia texana (Dampf, 1938) (Fig. 5).
Alpha diversity. For the 3 land-use areas compared, SA showed a greatest and different diversity in relation to CA and UA. Also, CA and UA were different in order 0 and 1 diversity, but they were not in order 2, as UA showed the poorest diversity (Table 3, Fig. 6). In the case of the 3 seasons, higher diversity in 0, 1, and 2 orders was found in CS as compared with the other 2 seasons. Order 0 diversity was different in CS and DS, but similar between CS and RS and RS and DS. Also, order 1 and 2 diversity were different between CS and the other 2 seasons, but similar between RS and DS (Table 3, Fig. 7).
Table 2
Phlebotomine sand fly species collected with Malaise traps in Apazapan, Veracruz, Mexico, comparing the total number of specimens, their relative abundance, and species richness, in 3 different land-use areas during 3 different seasons.
| Area | Season | Female | Male | Total | % | |||||
| Species | Sylvatic | Crop | Urban | Rainy | Cold | Dry | ||||
| Brumptomyia hamata | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 2.5 |
| Brumptomyia mesai | 5 | 0 | 0 | 0 | 3 | 2 | 1 | 4 | 5 | 12.5 |
| Dampfomyia leohidalgoi | 28 | 1 | 0 | 1 | 14 | 14 | 21 | 8 | 29 | 72.5 |
| Lutzomyia cruciata | 2 | 0 | 0 | 0 | 1 | 1 | 2 | 0 | 2 | 5.0 |
| Micropygomyia chiapanensis | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 1 | 2 | 5.0 |
| Pintomyia serrana | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 2.5 |
| Total abundance | 35 | 1 | 4 | 4 | 19 | 17 | 26 | 14 | 40 | 100.0 |
| Relative abundance | 87.5 | 2.5 | 10.0 | 10.0 | 47.5 | 42.5 | 65.0 | 35.0 | 100.0 | |
| Species richness | 3 | 1 | 3 | 3 | 4 | 3 | 5 | 4 | 6 |

Based on the Alpha diversity of land-use areas and seasons, SA in the CS presented highest diversity in the 3 orders and was different from the other areas and seasons, except SA in RS where the order 0 diversity was similar but 1 and 2 order do not (Table 4).
Beta diversity. When contrasting separately by areas, SA and CA share 8 species and exhibit 81% of similarity, whereas SA and UA showed the poorest similarity with 6 shared species (74%), and CA and UA shared 6 species but reached a 98% similarity. Comparing the seasons, a higher similarity was found between DS and RS with 10 shared species (98%), DS and CS with 10 shared species (95%) and being the least similar RS and CS with 12 shared species (89%), differences depend on the abundance of each species.
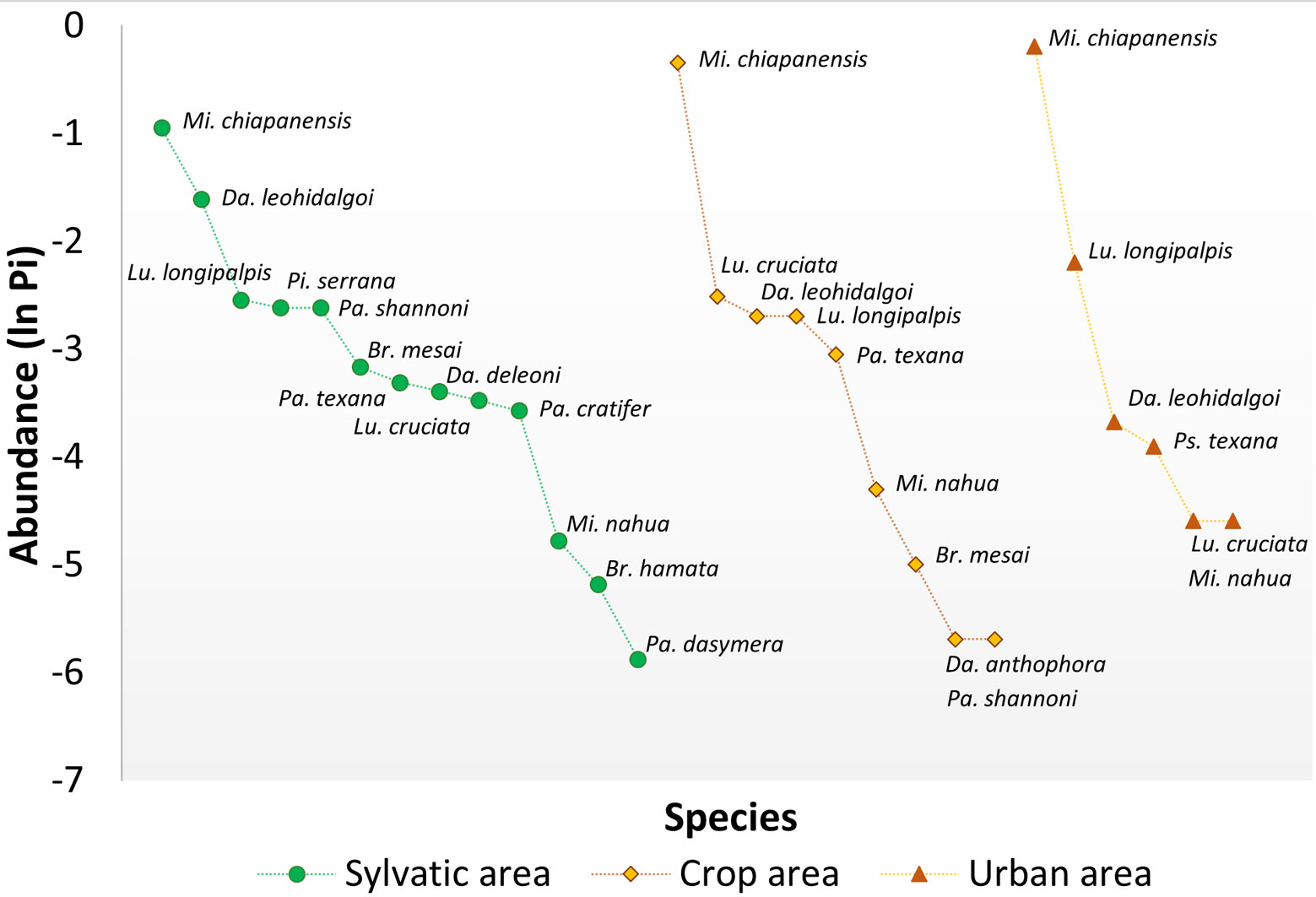
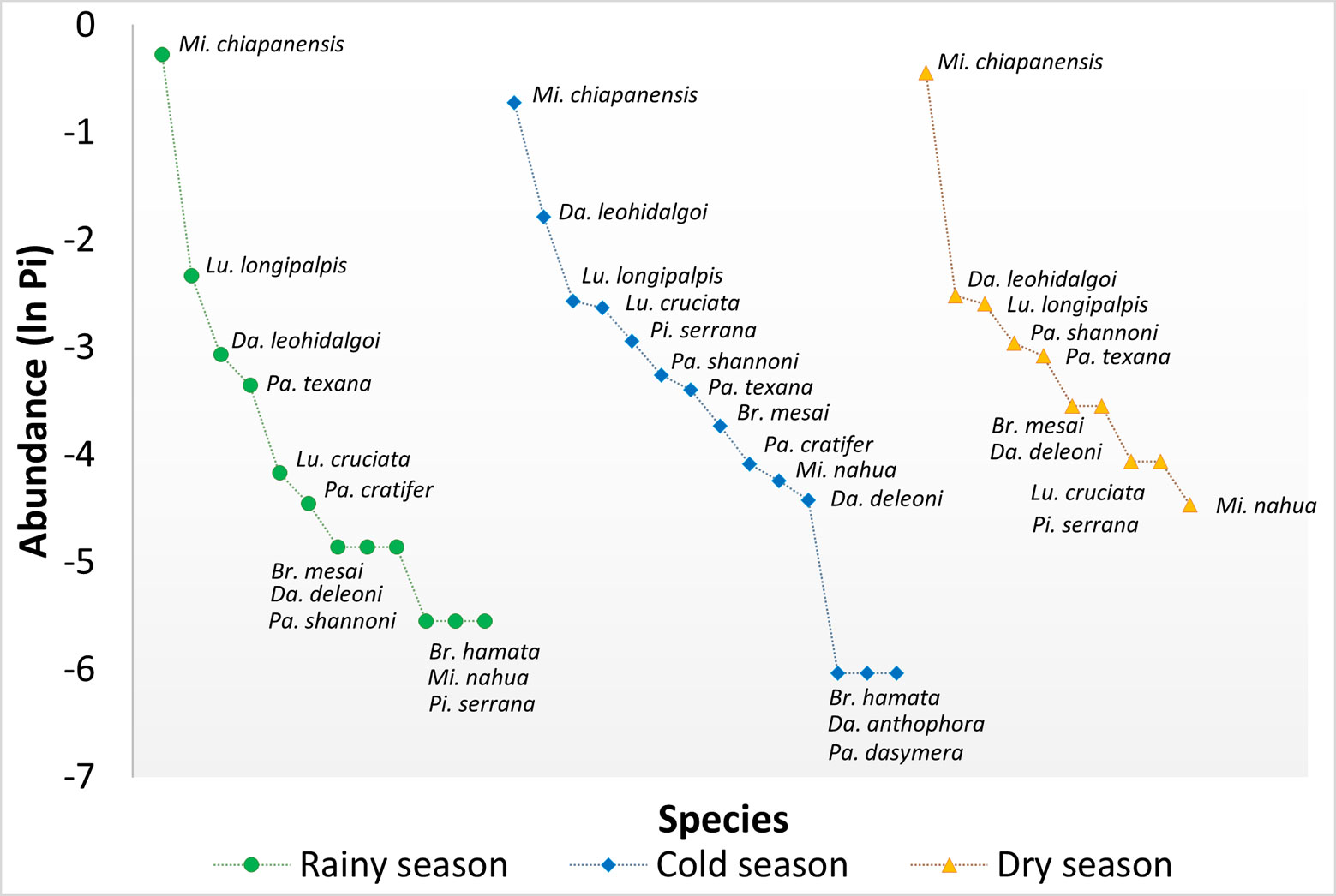

Table 3
Abundance and Alpha diversity of Phlebotominae in Apazapan, Veracruz, Mexico comparing the 3 land-use areas and seasons, by the effective number of species.
| Area | Season | |||||
| Sylvatic | Crop | Urban | Rainy | Cold | Dry | |
| Abundance | 357 | 296 | 198 | 259 | 418 | 174 |
| True diversity | ||||||
| 0D | 13 | 9 | 6 | 12 | 14 | 10 |
| 1D | 6.91 | 2.96 | 1.95 | 2.69 | 5.72 | 3.94 |
| 2D | 4.70 | 1.92 | 1.45 | 1.69 | 3.55 | 2.31 |
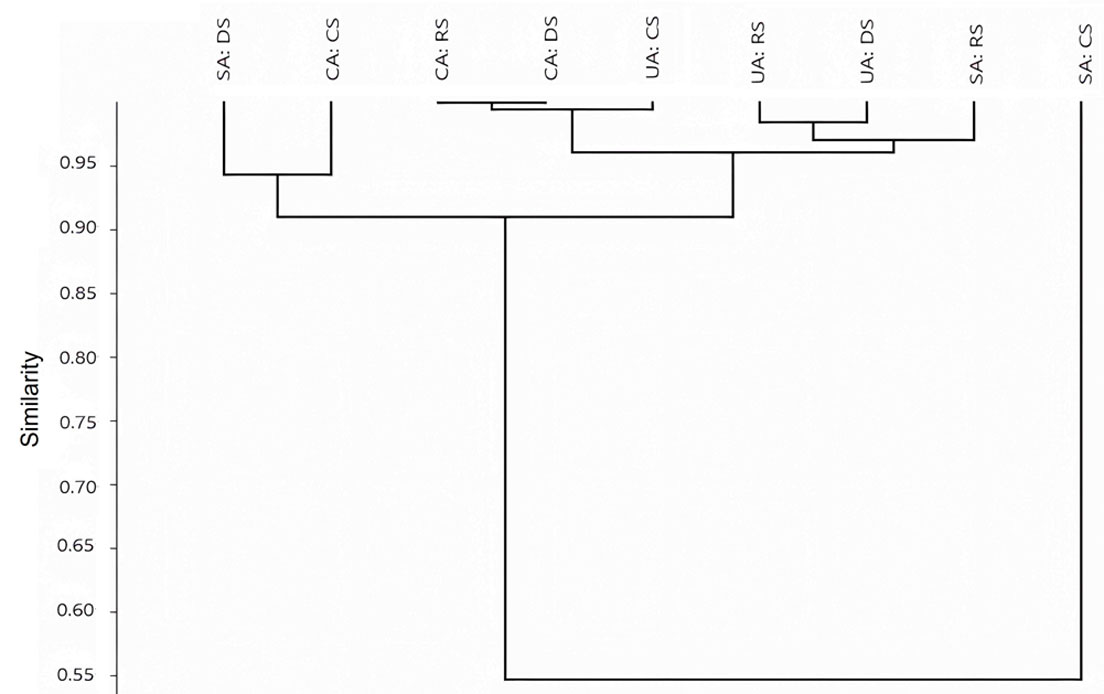
Similarity dendrograms obtained by cluster analysis using the Morisita´s index, with a correlation coefficient of 0.9 between areas and seasons, grouped all land use types except SA and CS. All combinations of CA and UA have more than 90% similarity, they shared between 3 and 6 species. Considering the sylvatic area, RS and DS display more similarity with 9 shared species (97%), followed by CS and DS with 9 shared species and 73% similarity, RS and CS were the less similar with 62% of similarity in spite that 12 species are shared. All combinations in the rainy and dry seasons have more than 88% similarity, they shared between 3 and 6 species. In the cold season the CA and UA were more similar with 5 shared species (88%), followed by SA and CA with 65% similarity and 8 shared species, the SA and UA were less similar in species composition and abundance in the cold season with 44% similarity with 5 shared species (Fig. 8).
Discussion
Except for Lutzomyia cruciata, L. longipalpis, and M. chiapanensis, all species are new records for the municipality of Apazapan (Ibáñez-Bernal et al., 2011; Montes de Oca-Aguilar, Rebollar-Téllez et al., 2017; Montes de Oca-Aguilar, Mikery-Pacheco et al., 2017). Dampfomyia anthophora and P. dasymera are new records for the state of Veracruz, increasing to 25 the known species in the state (Ibáñez-Bernal & Durán-Luz, 2022).
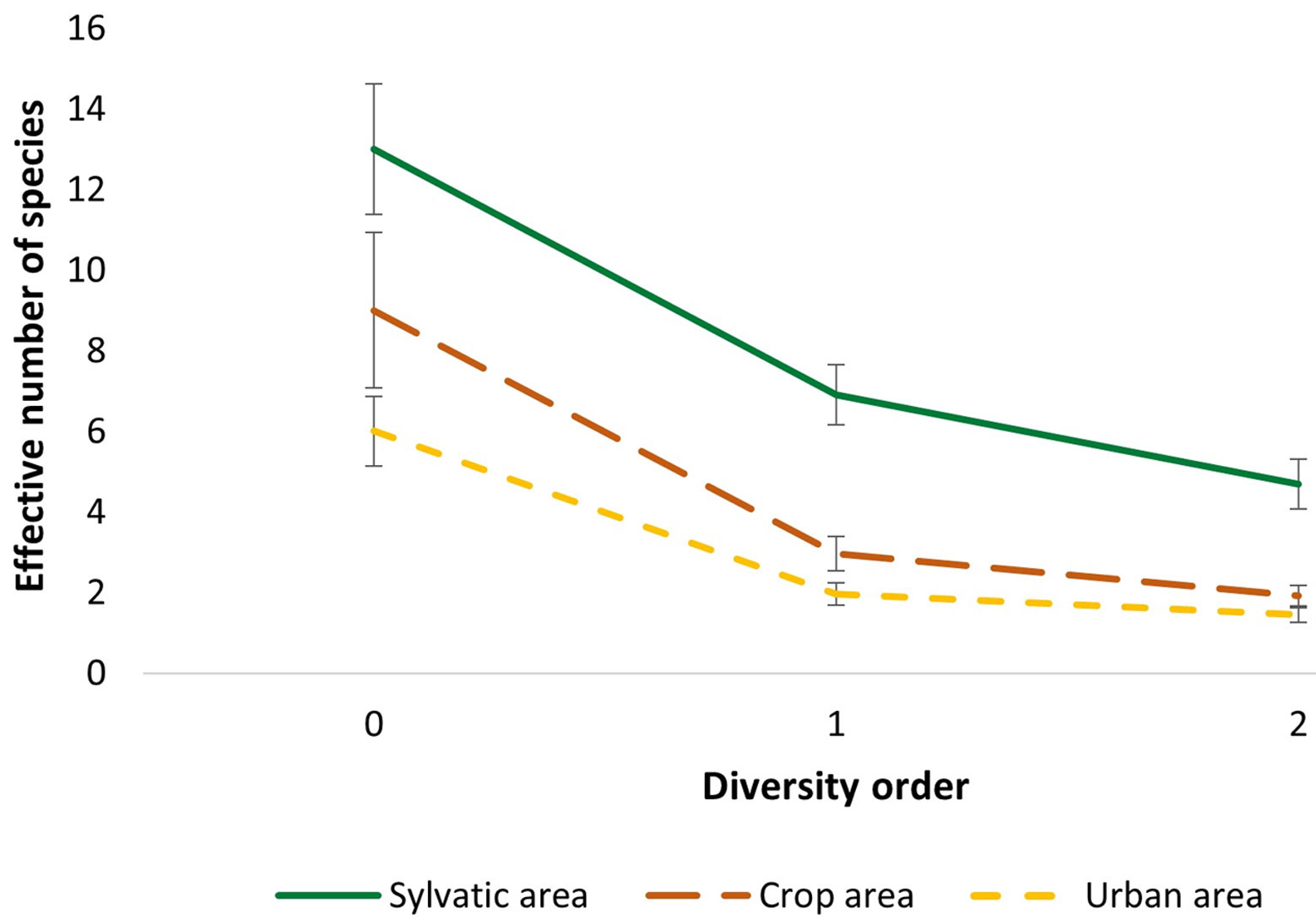

Table 4
Abundance and Alpha diversity of Phlebotominae in Apazapan, Veracruz, Mexico for each season in the 3 areas, by effective species number.
| Sylvatic area | Crop area | Urban area | |||||||
| Rainy | Cold | Dry | Rainy | Cold | Dry | Rainy | Cold | Dry | |
| Abundance | 77 | 189 | 91 | 114 | 135 | 47 | 68 | 94 | 36 |
| True diversity | |||||||||
| 0D | 12 | 13 | 9 | 6 | 9 | 5 | 3 | 5 | 6 |
| 1D | 4.09 | 8.10 | 4.93 | 2.00 | 4.03 | 1.98 | 1.79 | 1.54 | 2.85 |
| 2D | 2.37 | 6.16 | 3.17 | 1.43 | 2.76 | 1.44 | 1.54 | 1.22 | 1.96 |
In this study we found 3 species that have been considered possible vectors of Leishmania because they are anthropophilic and have been found naturally infected: Lutzomyia cruciata, L. longipalpis, and Psathyromyia shannoni (Dyar, 1929) (Biagi & Biagi, 1953; Biagi, Biagi et al., 1965; Biagi, López et al., 1965; Canto-Lara et al., 2007; Pech-May et al., 2010, 2016; Rebollar-Téllez, Andrade-Narvaez et al., 1996; Rebollar-Téllez, Ramírez-Fraire et al., 1996; Rebollar-Téllez, Reyes-Villanuaeva et al., 1996; Sánchez-García et al., 2010).
Lutzomyia cruciata and Psathyromyia shannoni have been recorded from the USA to Argentina and possess a wide distribution area in Mexico (Ibáñez-Bernal & Durán-Luz, 2022). Sánchez-García et al. (2010) and González et al. (2011) suggested that both species may be related with Leishmania transmission in areas with human cutaneous leishmaniasis in which Brumptomyia olmeca is absent. In southern Mexico (Campeche, Quintana Roo, and Yucatán) L. cruciata and P. shannoni have been found naturally infected with Leishmania (Leishmania) sp. (Canto-Lara et al., 2007) and L. mexicana (Biagi, 1953) (Pech-May et al., 2010, 2016; Sánchez-García et al., 2010). Recently, both species were also found naturally infected with Leishmania sp. in southern Veracruz (Lozano-Sardaneta et al., 2020). The other species is Lutzomyia longipalpis, which has a vast distribution range from Mexico to Uruguay and Argentina (Ibáñez-Bernal & Durán-Luz, 2022), and in this study it represents the second occasion in which it was collected in Veracruz (Ibáñez-Bernal et al., 2011). Lutzomyia longipalpis is considered the primary vector of Leishmania infantum (Nicolle, 1908) in South America (Dantas-Torres, 2006; Maroli et al., 2013), and probably in Mexico (Biagi, Biagi et al., 1965; Biagi, López et al., 1965; González et al., 2011; Ibáñez-Bernal, 1999).
Spatial variation. In this study we recorded a higher diversity of phlebotomine sand flies in a sylvatic area, than in fruit-tree plantations or human settlements, which demonstrates that habitat transformation can affect species richness and abundance, as both decrease in environments with some degree of disturbance as compared with sylvatic areas (Turrini & Knop, 2015). In Mexico, field studies describing the structure of the phlebotomine assemblages in areas with different anthropogenic disturbance are scarce. The results presented herein agree with those reported by Durán-Luz et al. (2019) from central Mexico, where higher species richness and abundance were observed in a sylvatic area in comparison to human settlements. Likewise, in a peri-urban area of Quintana Roo, Sánchez-García et al. (2010) detected low species richness in an area with human settlements, compared to a less fragmented area. Data obtained in this study agree with other studies carried out in South America in which differences were observed in phlebotomine composition, richness and abundance according to different grades of anthropogenic disturbance as compared with conserved environments, revealing that environmental quality exerts a significant influence on community structure (Feitosa & Castellón, 2004; Jiménez et al., 2000; Machado et al., 2017; Pinheiro et al., 2013; Rebêlo et al., 2019; Travi et al., 2002; Valderrama et al., 2008).
We found that sand fly abundance decreases due to habitat destruction by humans and that several species are absent from degraded sites (Travi et al., 2002). However, there are some species of medical importance that are tolerant to these changes and can survive in modified environments, sometimes even with high abundances compared to sylvatic or less disturbed sites (Mikery-Pacheco et al., 2015; Sánchez-García et al., 2010).
In this study, we observed that the abundance of Lutzomyia cruciata was higher in fruit-tree plantations than that detected in the sylvatic area and or with human settlements. This finding represents a greater risk of contact of female L. cruciata with humans entering the crop areas. This species has been recorded in urban and peri-urban areas of Campeche, Chiapas, and Quintana Roo, with high abundances, suggesting a high capacity to adapt to these modified sites (Mikery-Pacheco et al., 2015; Pech-May et al., 2016; Sánchez-García et al., 2010). Lutzomyia cruciata has also been recorded in high abundance in coffee agroecosystems in Chiapas, suggesting that Leishmania transmission is likely to occur in these sites (Pérez et al., 2014).
Psathyromyia shannoni showed a higher abundance in the sylvatic area, than in the cultivated area or the human settlements. This species has previously been found in great abundance in conserved sites with respect to degraded habitats in South America (Feitosa & Castellón, 2004; Jiménez et al., 2000; Rebêlo et al., 2019). Nonetheless, it has also been detected in high abundance in peri-urban areas of Quintana Roo and Campeche (Pech-May et al., 2016; Sánchez-García et al., 2010).
In addition, Lutzomyia longipalpis showed similar abundances in the 3 study sites, which may indicate that this species has been established in peridomiciliary areas as well as in cultivation sites, representing a risk of Leishmania spp. infection in all the sites. Our results are supported by other studies conducted in South America, which have demonstrated the ability of this species to easily adapt to human-modified environments (Jiménez et al., 2000; Quinnell & Dye, 1994; Salomón et al., 2015). There are even studies in which a greater abundance of L. longipalpis has been detected in anthropized sites compared to other more conserved ones (Machado et al., 2017; Pinheiro et al., 2013; Rebêlo et al., 2019). This could be explained by the fact that this species is commonly associated with the presence of domestic animals and their environments (Quinnell & Dye, 1994).
Seasonal variation. In the present study we recorded the highest species richness and abundance of phlebotomine sand flies during the cold season. Our results are similar to those reported by Rebollar-Téllez, Andrade-Narvaez et al. (1996), Rebollar-Téllez, Ramírez-Fraire et al. (1996) in Campeche, who found a high abundance between November and March, months corresponding to the transmission of Leishmania in that state (Andrade-Narváez et al., 2003). Our work also agrees with other studies conducted in Mexico in which it has been reported that phlebotomine sand fly captures are less numerous in periods of rainfall, for example, in coffee agroecosystems in Chiapas (Pérez et al., 2014), and in Puebla in a dry deciduous forest and in an area with human settlements (Durán-Luz et al., 2019). Other studies in South America have also shown a strong negative relationship between the species richness and abundance of phlebotomine sand flies and the amount of rainfall (Carvalho et al., 2011; Córdoba-Lanús & Salomón, 2002; Machado et al., 2017; Pinheiro et al., 2013; Quintana et al., 2010). This finding can be explained by the fact that sand flies are insects that although immature stages require high humidity conditions, are not strictly aquatic (Munstermann, 2004; Young & Duncan, 1994) and can be drown with excess water. Three species such as Lutzomyia cruciata, L. longipalpis, and Psathyromyia shannoni were found to be more abundant in the cold season, and therefore this may represent a risk of Leishmania spp. infection for the population and tourists in this season.
Acknowledgments
The first author was the recipient of a grant awarded by the Consejo Nacional de Ciencia y Tecnología (Conacyt), No. 777881. Ibáñez-Bernal was supported by the Project INECOL-10816. We thank María Teresa Suárez-Landa (INECOL, A.C.) for her valuable field support. We also appreciate the support of Arturo Báez-Hernández (Departamento de Control de Enfermedades Transmitidas por Vector, Servicios de Salud de Veracruz) and José Israel Villa-Campos (Jurisdicción Sanitaria V). For their enthusiastic help in the field, we are also grateful to the staff of Departamento de Control de Enfermedades Transmitidas por Vector, Servicios de Salud de Veracruz, especially †Carlos Roberto García-Torres and Mario Alberto Vásquez-Márquez (Unidad de Investigación Entomológica y Bioensayos). Finally, we also appreciate the support of Fabián Huerta-Zenteno in the field.
References
Aguiar, G. M., & Vilela, M. L. (1987). Aspects of the ecology of sandflies at the Serra dos Orgãos National Park, state of Rio de Janeiro. VI. Shelters and breeding places (Diptera: Psychodidae: Phlebotominae). Memórias do Instituto Oswaldo Cruz, 82, 585–586. https://doi.org/10.1590/S0074-02761987000400021
Alexander, J. B. (1987). Dispersal of Phlebotominae sandflies (Diptera: Psychodidae) in a Colombian coffee plantation. Journal of Medical Entomology, 24, 552–558. https://doi.org/10.1093/jmedent/24.5.55
Alexander, B., Oliveira, E. B., Haigh, E., & Almeida, L. L. (2002). Transmission of Leishmania in coffee plantations of Minas Gerais, Brazil. Memórias do Instituto Oswaldo Cruz, 97, 627–630. https://doi.org/10.1590/S0074-02762002000500005
Andrade-Narváez, F. J., Canto-Lara, S. B., Van Wynsberghe, N. R., Rebollar-Téllez, E. A., Vargas-Gonzalez, A., & Albertos-Alpuche, N. E. (2003). Seasonal transmission of Leishmania (Leishmania) mexicana in the state of Campeche, Yucatán Peninsula, Mexico. Memórias do Instituto Oswaldo Cruz, 98, 995–998. https://doi.org/10.1590/S0074-02762003000800002
Biagi, F. F., & Biagi, A. M. B. (1953). Datos ecológicos de algunos flebotomus mexicanos (Diptera: Psychodidae). Anales del Instituto de Biología, UNAM, 24, 445–450.
Biagi, A. M. B., Biagi, F. F., & Melinari, J. L. (1965). Kala-azar en México. Antropofilia y actividad horaria de Phlebotomus longipalpis Lutz y Neiva, 1912 (Diptera, Psychodidae). Revista del Instituto de Salubridad y Enfermedades Tropicales, 25, 13–19.
Biagi, F. F., López, R., & Biagi, A. M. B. (1965). El kala-azar en México: problema ecológico por estudiar. Revista del Instituto de Salubridad y Enfermedades Tropicales, 25, 3–12.
Canto-Lara, S. B., Bote-Sánchez, M. D., Rebollar-Téllez, E. A., & Andrade-Narváez, F. J. (2007). Detection and identification of Leishmania kDNA in Lutzomyia olmeca olmeca and Lutzomyia cruciata (Diptera: Psychodidae) by polymerase chain reaction in southern Mexico. Entomological News, 118, 217–222. https://doi.org/10.3157/0013-872X(2007)118[217:DAIOLK]2.0.CO;2
Carvalho, G. M., De Vasconcelos, F. B., Da Silva, D. G., Botelho, H. A., & Filho, J. D. (2011). Diversity of phlebotomine sand flies (Diptera: Psychodidae) in Ibitipoca State Park, Minas Gerais, Brazil. Journal of Medical Entomology, 48, 764–769. https://doi.org/10.1603/ME10258
Colwell, R. K. (2013). EstimateS: Statistical estimation of species richness and shared species from samples. Version 9.1.0. Accessed 4 April 2022 from: http://purl.oclc.org/estimates.
Córdoba-Lanús, E., & Salomón, O. D. (2002). Phlebotominae fauna in the province of Tucumán, Argentina. Revista do Instituto de Medicina Tropical de São Paulo, 44, 23–27. https://doi.org/10.1590/S0036-46652002000100005
Dampf, A. (1938). Un nuevo Phlebotomus (Insecta, Diptera, Fam. Psychodidae) procedente de Texas, E. U. A. Anales de la Escuela Nacional de Ciencias Biológicas, México, 1, 119–122.
Dantas-Torres, F. (2006). Leishmania infantum versus Leishmania chagasi: do not forget the law of priority. Memórias do Instituto Oswaldo Cruz, 10, 117–118. https://doi.org/10.1590/S0074-02762006000100024
Durán-Luz, J., Sandoval-Ruiz, C. A., & Ibáñez-Bernal, S. (2019). Phlebotominae and Trichomyiinae (Diptera: Psychodidae) diversity in a tropical dry forest of central Mexico: a comparison of conserved and anthropized habitats. Studies on Neotropical Fauna and Environment, 54, 40–47. https://doi.org/10.1080/01650521.2018.1486496
Feinsinger, P. (2001). Designing field studies for biodiversity conservation. Washington, D.C.: Island Press.
Feitosa, M. A. C., & Castellón, E. G. (2004). Fauna de flebotomíneos (Diptera: Psychodidae) em fragmentos florestais ao redor de conjuntos habitacionais na cidade de Manaus, Amazonas, Brasil. II. Estratificação horizontal. Acta Amazonica, 34, 121–127. https://doi.org/10.1590/S0044-59672004000100016
Galati, E. A. B. (1995). Phylogenetic systematics of Phlebotominae (Diptera: Psychodidae) with emphasis on American groups. Boletín de la Dirección de Malariología y Saneamiento Ambiental, 35, 133–142.
Galati, E. A. B. (2003). Morfologia e taxonomia: classificação de Phlebotominae. In E. F. Rangel, & R. Lainson (Eds.), Flebotomíneos do Brasil (pp. 23–175). Rio de Janeiro: Fiocruz.
García, E. (1996). Diversidad climático vegetal en México. In J. Llorente-Bousquets, A. N. García-Aldrete, & E. González-Soriano (Eds.), Biodiversidad, taxonomía y biogeografía de artrópodos de México: hacia una síntesis de su conocimiento, Vol. 1 (pp. 15–25). México D.F.: Conabio/ UNAM.
González, C., Rebollar-Téllez, E. A., Ibáñez-Bernal, S., Becker-Fauser, I., Martínez-Meyer, E., Peterson, A. T. et al. (2011). Current knowledge of Leishmania vectors in Mexico: how geographic distributions of species relate to transmission areas. American Journal of Tropical Medicine and Hygiene, 85, 839–846. https://doi.org/10.4269/ajtmh.2011.10-0452
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 1–9.
Ibáñez-Bernal, S. (1999). Phlebotominae (Diptera: Psychodidae) de México. I.- Brumptomyia França y Parrot; Lutzomyia França, las especies de L. (Lutzomyia) França y del grupo Verrucarum. Folia Entomologica Mexicana, 107, 61–116.
Ibáñez-Bernal, S. (2005a). Phlebotominae (Diptera: Psychodidae) de México. V. Clave ilustrada para la identificación de los machos de Lutzomyia França. Folia Entomologica Mexicana, 44, 49–66.
Ibáñez-Bernal, S. (2005b). Phlebotominae (Diptera: Psychodidae) de México. VI. Clave ilustrada para la identificación de las hembras de Lutzomyia França. Folia Entomologica Mexicana, 44, 195–212.
Ibáñez-Bernal, S., & Durán-Luz, J. (2022). An actualized catalogue of the Psychodidae (Diptera) of Mexico and their known distribution by state. Zootaxa, 5104, 347–408. https://doi.org/10.11646/zootaxa.5104.3.2
Ibáñez-Bernal, S., Suárez-Landa, M. T., & Mendoza, F. (2011). An updated checklist of the phlebotomine sandflies of Veracruz, Mexico (Diptera: Psychodidae, Phlebotominae). Zootaxa, 2928, 29–40. https://doi.org/10.11646/zootaxa.2928.1.3
INEGI (Instituto Nacional de Estadística y Geografía). (2010). Compendio de información geográfica municipal de los Estados Unidos Mexicanos. Apazapan, Veracruz de Ignacio de la Llave. Accessed 4 April 2022 from: http://www3.inegi.org.mx/contenidos/app/mexicocifras/datos_geograficos/30/30017.pdf
Jiménez, A., Rojas, J., Vargas, F., & Herrero, M. (2000). Temporal and spatial variation of Phlebotominae (Diptera, Psychodidae) community diversity in a cutaneous leishmaniasis endemic area of Costa Rica. Journal of Medical Entomology, 37, 216–221. https://doi.org/10.1603/0022-2585-37.2.216
Killick-Kendrick, R. (1990). Phlebotomine vectors of the leishmaniases: a review. Medical and Veterinary Entomology, 4, 1–24. https://doi.org/10.1111/j.1365-2915.1990.tb00255.x
Killick-Kendrick, R. (1999). The biology and control of phlebotomine sand flies. Clinics in Dermatology, 17, 279–289. https://doi.org/10.1016/S0738-081X(99)00046-2
Lozano-Sardaneta, Y. N., Sánchez-Montes, S., Sánchez-Cordero, V., Becker-Fauser, I., & Paternina, L. E. (2020). Molecular detection of Leishmania infantum in sand flies (Diptera: Psychodidae: Phlebotominae) from Veracruz, Mexico. Acta Tropica, 207, 105492. https://doi.org/10.1016/j.actatropica.2020.105492
Machado, T. D. O., Minuzzi-Souza, T. T. C., Ferreira, T. S., Freire, L. P., Timbó, R. V., Vital, T. E. et al. (2017). The role of gallery forests in maintaining Phlebotominae populations: potential Leishmania spp. vectors in the Brazilian savanna. Memórias do Instituto Oswaldo Cruz, 112, 681‒691. https://doi.org/10.1590/0074-02760170126
Marcondes, C. B. (2007). A proposal of generic and subgeneric abbreviations for Phlebotominae sandflies (Diptera: Psychodidae: Phlebotominae) of the world. Entomological News, 4, 351–356. https://doi.org/10.3157/0013-872X(2007)118[351:APOGAS]2.0.CO;2
Maroli, M., Feliciangeli, M. D., Bichaud, L., Charrel, R. N., & Gradoni, L. (2013). Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Medical and Veterinary Entomology, 27, 123–147. https://doi.org/10.1111/j.1365-2915.2012.01034.x
Mikery-Pacheco, O. F., León, J. C., Rebollar-Téllez, E. A., & Vera, A. C. (2015). Sandfly (Diptera: Psychodidae: Phlebotominae) species diversity in an urban area of the municipality of Tapachula, Chiapas, Mexico. Memórias do Instituto Oswaldo Cruz, 110, 142–144. https://doi.org/10.1590/0074-02760140351
Montes de Oca-Aguilar, A. C., Mikery-Pacheco, O., Castillo, A., Rebollar-Téllez, E. A., Piermarini, P. M., & Ibáñez-Bernal, S. (2017). Morphological variation of Lutzomyia cruciata eggs (Diptera: Psychodidae: Phlebotominae) in southern Mexico. Zootaxa, 4258, 477–489. https://doi.org/10.11646/zootaxa.4258.5.5
Montes de Oca-Aguilar, A. C., Moo-Llanes, D. A., & Rebollar-Téllez, E. A. (2013). Adult sand fly species from diurnal resting sites on the Peninsula of Yucatán, México. Southwestern Entomologist, 38, 241–250. https://doi.org/10.3958/059.038.0209
Montes de Oca-Aguilar, A. C., Rebollar-Téllez, E. A., & Moo-Llanes, D. A. (2013). Sand fly species from a karstic cave in the Peninsula of Yucatán, Mexico. Entomological News, 123, 191–200. https://doi.org/10.3157/021.123.0305
Montes de Oca-Aguilar, A. C., Rebollar-Téllez, E. A., Piermarini, P. M., & Ibáñez-Bernal, S. (2017). Descriptions of the immature stages of Lutzomyia (Tricholateralis) cruciata (Coquillett) (Diptera: Psychodidae, Phlebotominae). Neotropical Entomology, 46, 66–85. https://doi.org/10.1007/s13744-016-0439-1
Moo-Llanes, D., Ibarra-Cerdeña, C. N., Rebollar-Téllez, E. A., Ibáñez-Bernal, S., González, C., & Ramsey, J. M. (2013). Current and future niche of North and Central American sand flies (Diptera: Psychodidae) in climate change scenarios. Plos Neglected Tropical Diseases, 7, e2421. https://doi.org/10.1371/journal.pntd.0002421
Moreno, C. E. (2001). Métodos para medir la biodiversidad. M & T – Manuales y Tesis SEA. Vol. 1. Zaragoza, Mexico.
Moreno, C. E., Barragán, F., Pineda, E., & Pavón, N. P. (2011). Reanálisis de la diversidad alfa: alternativas para interpretar y comparar información sobre comunidades ecológicas. Revista Mexicana de Biodiversidad, 82, 1249–1261. https://doi.org/10.22201/ib.20078706e.2011.4.745
Munstermann, L. E. (2004). Phlebotomine sand flies, the Psychodidae. In W. C. Marquardt (Ed.), Biology of disease vectors, 2nd Ed. (pp. 141–151). San Diego, CA: Elsevier Science (USA).
Parras, M. A., Rosa, J. R., Szelag, E. A., & Salomón, O. D. (2012). Identification of the natural breeding sites of sandflies (Diptera: Psychodidae: Phlebotominae), potential vectors of leishmaniasis, in the province of Chaco, Argentina. Memórias do Instituto Oswaldo Cruz, 107, 550–552. https://doi.org/10.1590/S0074-02762012000400018
Pech-May, A., Escobedo-Ortegón, F. J., Berzunza-Cruz, M., & Rebollar-Téllez, E. A. (2010). Incrimination of four sandfly species previously unrecognized like vectors of Leishmania parasites in Mexico. Medical and Veterinary Entomology, 24, 150–161. https://doi.org/10.1111/j.1365-2915.2010.00870.x
Pech-May, A., Peraza-Herrera, G., Moo-Llanes, D. A., Escobedo-Ortegón, F. J., Berzunza-Cruz, M., Becker-Fauser, I. et al. (2016). Assessing the importance of four sandfly species (Diptera: Psychodidae) as vectors of Leishmania mexicana in Campeche, Mexico. Medical and Veterinary Entomology, 30, 310–320. https://doi.org/10.1111/mve.12169
Pérez, J., Virgen, A., Rojas, J. C., Rebollar-Téllez, E. A., Alfredo, C., Infante, F. et al. (2014). Species composition and seasonal abundance of sandflies (Diptera: Psychodidae: Phlebotominae) in coffee agroecosystems. Memórias do Instituto Oswaldo Cruz, 109, 80–86. https://doi.org/10.1590/0074-0276130224
Pinheiro, M. P., Silva, J. H., Cavalcanti, K. B., de Azevedo, P. R., & de Melo Ximenes, M. de F. (2013). Ecological interactions among phlebotomines (Diptera: Psychodidae) in an agroforestry environment of northeast Brazil. Journal of Vector Ecology, 38, 307–316. https://doi.org/10.1111/j.1948-7134.2013.12045.x
Quate, L. W., & Vockeroth, J. R. (1981). Psychodidae. In J. F. McAlpine, B. V. Peterson, G. E. Shewell, H. J. Teskey, J. R. Vockeroth et al. (Eds.), Manual of Nearctic Diptera, Vol. 1 (pp. 293–300). Monograph N°27. Hull, Quebec: Research Branch, Agriculture Canada.
Quinnell, R. J., & Dye, C. (1994). Correlates of the peridomestic abundance of Lutzomyia longipalpis (Diptera: Psychodidae) in Amazonian Brazil. Medical and Veterinary Entomology, 8, 219–224. https://doi.org/10.1111/j.1365-2915.1994.tb00502.x
Quintana, M. G., Salomón, O. D., & De Grosso, M. S. (2010). Distribution of phlebotomine sand flies (Diptera: Psychodidae) in a primary forest-crop interface, Salta, Argentina. Journal of Medical Entomology, 47, 1003–1010. https://doi.org/10.1603/ME09072
Rebêlo, J. M. M., Moraes, J. L. P., Cruz, G. B. V., Andrade-Silva, J., Bandeira, M. D. C. A., Oliveira Pereira, Y. N. et al. (2019). Influence of deforestation on the community structure of sand flies (Diptera: Psychodidae) in eastern Amazonia. Journal of Medical Entomology, 56, 1004–1012. https://doi.org/10.1093/jme/tjz014
Rebollar-Téllez, E. A., Andrade-Narvaez, F. J., Fernández-Salas, I., & Reyes-Villanueva, F. (1996). Collections of sand flies (Diptera: Psychodidae) from mammal burrows in an area of cutaneous leishmaniasis in Campeche, Mexico. Entomological News, 107, 317–321.
Rebollar-Téllez, E. A., Ramírez-Fraire, A., & Andrade-Narváez, F. J. (1996). A two-year study on vectors of cutaneous leishmaniasis. Evidence for sylvatic transmission cycle in the state of Campeche, Mexico. Memórias do Instituto Oswaldo Cruz, 91, 555–560. https://doi.org/10.1590/S0074-02761996000500004
Rebollar-Téllez, E. A., Reyes-Villanuaeva, F., Fernández-Salas, I., & Andrade-Narváez, F. J. (1996). Population dynamics and biting rhythm of the anthropophilic sand fly Lutzomyia cruciata (Diptera: Psychodidae) in southeast Mexico. Revista do Instituto de Medicina Tropical de São Paulo, 38, 29–33. https://doi.org/10.1590/S0036-46651996000100006
Rzedowski, J. (1978). Vegetación de México. México D.F.: Limusa.
Salomón, O. D., Feliciangeli, M. D., Quintana, M. G., Afonso, M. M., & Rangel, E. F. (2015). Lutzomyia longipalpis urbanisation and control. Memórias do Instituto Oswaldo Cruz, 110, 831–46. https://doi.org/10.1590/0074-02760150207
Sánchez-García, L., Berzunza-Cruz, M., Becker-Fauser, I., & Rebollar-Téllez, E. A. (2010). Sand flies naturally infected by Leishmania (Le.) mexicana in the peri-urban area of Chetumal city, Quintana Roo, México. Transactions of the Royal Society of Tropical Medicine and Hygiene, 104, 406–411. https://doi.org/10.1016/j.trstmh.2010.01.010
Scorza, J. V., Castillo, L., Rezzano, S., Márquez, M., & Márquez, J. C. (1985). El papel del cafeto en la endemicidad de la leishmaniasis cutánea en Venezuela. Boletín de la Dirección de Malariología y Saneamiento Ambiental, 25, 82–88.
Shimabukuru, P. H. F., de Andrade, A. J., & Galati, E. A. B. (2017). Checklist of American sand flies (Diptera, Psychodidae, Phlebotominae): genera, species, and their distribution. Zookeys, 660, 67–106. https://doi.org/10.3897/zookeys.660.10508
Thatcher, V. E. (1968). Arboreal breeding sites of phlebotomine sandflies in Panama. Annals of the Entomological Society of America, 61, 1141–1143. https://doi.org/10.1093/aesa/61.5.1141
Travi, B. L., Alder, G. H., Lozano, M., Cadena, H., & Montoya-Lerma, J. (2002). Impact of habitat degradation on Phlebotominae (Diptera: Psychodidae) of tropical dry forest in northern Colombia. Journal of Medical Entomology, 39, 451–456. https://doi.org/10.1603/0022-2585-39.3.451
Turrini, T., & Knop, E. (2015). A landscape ecology approach identifies important drivers of urban biodiversity. Global Change Biology, 21, 1652–1667. https://doi.org/10.1111/gcb.12825
Valderrama, A., Herrera, M., & Salazar, A. (2008). Relación entre la composición de especies del género de Lutzomyia França (Diptera: Psychodidae, Phlebotominae) y los diferentes tipos de bosques en Panamá. Acta Zoologica Mexicana, 24, 67–78. https://doi.org/10.21829/azm.2008.242704
Vivero, R. J., Torres-Gutierrez, C., Bejarano, E. E., Peña, H. C., Estrada, L. G., Florez, F. et al. (2015). Study on natural breeding sites of sand flies (Diptera: Phlebotominae) in areas of Leishmania transmission in Colombia. Parasites & Vectors, 8, 116. https://doi.org/10.1186/s13071-015-0711-y
WHO (World Health Organization). (2012). Control de la leishmaniosis. Informe de una reunión del Comité de Expertos de la OMS sobre el Control de las Leishmaniasis, Ginebra, 22 a 26 de marzo de 2010. OMS, Serie de Informes Técnicos.
Young, D. G., & Duncan, M. A. (1994). Guide to the identification and geographic distribution of Lutzomyia sand flies in Mexico, the West Indies, Central and South America (Diptera: Psychodidae). Memoirs of the American Entomological Institute, 54, 1–881.