Diego Francis P. Viana a, b, Letícia Larcher b, Ângelo P. C. Rabelo b, Rafael Hoogesteijn c, Fernando Rodrigo Tortato c, Grasiela E. O. Porfírio a, *
a Universidade Católica Dom Bosco, Avenida Tamandaré, 6000, CEP 79117-900, Campo Grande, Brazil
b Instituto Homem Pantaneiro, Ladeira José Bonifácio 171, Centro, CEP 79.300-010, Corumbá, Brazil
c Panthera Brasil, Rua Comandante Costa, 1155, Sala 01, Centro-Sul, CEP 78.020-400, Cuiabá, Brazil
*Corresponding author: grasi_porfirio@hotmail.com (G.E.O. Porfirio)
Received: 21 June 2021; accepted: 18 April 2022
Abstract
We conducted a comparative study of jaguar and puma activity patterns within a mosaic of protected areas (AMR) and on a cattle ranch (CR) in Pantanal, Brazil, to better understand their activity patterns in these landscapes. We hypothesized that the activity patterns of the jaguar and puma would be biased to the nocturnal period within the cattle ranch but not in the protected areas. We used data from camera traps analyzed through a non-parametric kernel density approach to explore interspecific and intraspecific temporal relationships between these species at both sites. We obtained 71 jaguar and 29 puma independent records at AMR, and 85 jaguar and 26 puma independent records at CR. Activity patterns of jaguars and pumas differed between sites, both being cathemeral in AMR, but nocturnal at CR with moderate to high overlaps, concordant with our hypothesis. Overall, our data suggest that the cattle ranching is not incompatible with the existence of jaguars and pumas but does shape their pattern of activities.
Keywords: Activity patterns; Camera trapping; Conservation; Neotropical felids; Wetland
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
¿La ganadería determina los patrones de actividad del jaguar (Panthera onca) y el puma (Puma concolor) en el Pantanal brasileño?
Resumen
Realizamos un estudio comparativo de los patrones de actividad del jaguar y el puma dentro de un mosaico de áreas protegidas (AMR) y en un rancho ganadero (CR) en Pantanal, Brasil, para comprender mejor sus patrones de actividad en estos paisajes. Presumimos que los patrones de actividad del jaguar y el puma estarían sesgados al período nocturno en la ganadería, pero no en las áreas protegidas. Utilizamos datos de cámaras trampa analizados a través de un enfoque de densidad kernel no paramétrico para explorar las relaciones temporales interespecíficas e intraespecíficas entre estas especies en ambos sitios. Obtuvimos 71 registros independientes de jaguar y 29 de puma en AMR, y 85 registros independientes de jaguar y 26 de puma en CR. Los patrones de actividad de jaguares y pumas difirieron entre sitios, siendo ambos catemerales en AMR, pero nocturnos en CR con superposiciones de moderadas a altas, de acuerdo con nuestra hipótesis. En general, nuestros datos sugieren que la ganadería no es incompatible con la existencia de jaguares y pumas, sino que moldea su patrón de actividades.
Palabras clave: Patrones de actividad; Cámara trampa; Conservación; Felinos neotropicales; Humedal
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Jaguar (Panthera onca) and puma (Puma concolor) are large felids, sympatric throughout most of the range of jaguars in the Neotropics (de la Torre et al., 2017; Núñez et al., 2000; Scognamillo et al., 2003). As the largest terrestrial predators in this region, these species play essential roles in the functioning and dynamics of its terrestrial ecosystems (Miller et al., 2001; Moreno et al., 2006; Terborgh, 1990). Both jaguars and pumas have experienced significant contractions in their historical geographical range due to human activities (Nielsen et al., 2016; Quigley et al., 2018), primarily due to habitat loss, changes in prey abundance, and persecution derived from its predation of livestock (Farrell, 2001; Mazzolli, 2009; Novack et al., 2005; Palmeira et al., 2008; Romero-Muñoz et al., 2010). These threats have intensified in recent years (Quigley et al., 2018) and, therefore, it is important to evaluate how anthropic factors influence different aspects of jaguar and puma ecology.
The jaguar in the Pantanal has almost twice the average weight of the puma (average jaguar weight = 86 kg; average puma weight = 48.2 kg) (Almeida, 1990). Typically, the coexistence of similar species is related to differential use of trophic, spatial and/or temporal resources (Schoener, 1974), with such differences reducing exploitative and interference competition (Albrecht & Gotelli, 2001; Valeix et al., 2007). On the other hand, when resources are abundant, competition may be relaxed, and resource sharing likely does not negatively affect coexisting species (Núñez et al., 2000).
Although space and food are generally the primary resources associated with niche partitioning and coexistence (Schoener, 1974), the temporal dimension also influences similar sympatric species that can potentially exhibit interference competition (Carothers & Jaksić, 1984). Some previous studies have demonstrated that jaguar and puma may avoid each other temporally to reduce competition (Monroy-Vilchis et al., 2009; Romero-Muñoz et al., 2010). However, in areas with a higher overlap of the temporal niche, with the jaguar and puma active both day and night, coexistence can be explained by the availability of prey and/or low human interference (Ávila-Nájera et al., 2016; Hernández-Saintmartín et al., 2013; Porfírio et al., 2017). Since felids are sensitive to human disturbance to varying degrees (Zanin et al., 2014), this variable cannot be disregarded as a factor also influencing activity patterns and intra/interspecific relationships between jaguar and puma (Ávila-Nájera et al., 2016; Monroy-Vilchis et al., 2009; Paviolo et al., 2009).
The activity patterns of jaguars and pumas in the Pantanal, one of the most extensive wetlands globally, have been studied since the 1980s (Crawshaw & Quigley, 1991; Foster et al., 2013; Porfírio et al., 2017; Silveira, 2004). The Pantanal is one of the most critical areas for wildlife conservation in South America, representing an important stronghold for several threatened species, including some felids (Harris et al., 2005; Tomás et al., 2019). Although some areas of this biome retain their natural vegetation, mainly due to periodic flooding and consequently inhibited human access and agricultural production, most of the surrounding plateaus and floodplains undergo drastic landscape change due to anthropogenic activities (Alho & Sabino, 2011; Roque et al., 2016).
One of the most effective strategies for safeguarding biodiversity is to create protected areas (Alho & Sabino, 2011; Lourival et al., 2011). Nevertheless, ~ 95% of the Brazilian Pantanal is privately owned. Therefore, working in partnership with the landowners is a priority for the conservation agenda of the Pantanal (Harris et al. 2005; Quigley & Crawshaw, 1992; Tomás et al., 2019). Indeed, most of the ecological research on jaguar and puma in the Pantanal has been carried out on cattle ranches (Azevedo & Murray, 2007; Cavalcanti & Gese, 2010; Schaller & Crawshaw, 1980; Silveira, 2004; Soisalo & Cavalcanti, 2006; Tortato et al., 2015). With that, it is necessary to evaluate the ecological aspects of the jaguar and puma in protected areas of the Pantanal without human interference, thus enabling comparisons with the ecology of these cats within the cattle ranches prevailing in the Pantanal landscape. In this study, we conducted a comparative analysis of jaguar and puma activity patterns in a mosaic of protected areas and a cattle ranch in the Brazilian Pantanal using camera trapping data. We sought to: (a) describe the activity patterns of both species in each area; (b) compare the activity patterns of each species between areas; and (c) evaluate the extent of temporal overlap between jaguar and puma in both areas. Moreover, we sought to investigate differences in activity patterns between males and females. Our main hypothesis is that the activity patterns of the jaguar and puma will be biased to the nocturnal period in the cattle ranching areas, caused by the predominance of diurnal human activities associated with the historical human-felid conflict in the Pantanal (Marchini & Macdonald, 2012; Quigley & Crawshaw, 1992). In protected areas, activity patterns will be shaped by natural factors, for example, the availability of prey (Porfírio et al., 2017).
Materials and methods
Camera trapping data were collected in a mosaic of protected areas located at the Amolar Mountain Ridge (AMR) at 3 sites: (1) Private Natural Heritage Reserve (RPPN) Acurizal; (2) RPPN Engenheiro Eliezer Batista, and (3) Santa Tereza ranch; and on a cattle ranch (CR): (4) Br PEC (Fig. 1). A Private Natural Heritage Reserves (RPPN) is a category of protected area established by the Brazilian Federal Decree 98.914 of 1990, and updated by Decree 1992 of 1996, where citizens voluntarily engage the protection of representative Brazilian ecosystems (Porfirio et al., 2014). Although Santa Tereza ranch does not have a protected area status, less than 16% of its area is used for cattle ranching (Tortato et al., 2015). In this ranch, monitoring and controlling of environmental crimes (e.g., illegal hunting) is integrated with the neighboring protected areas.
The AMR study site comprised almost 970 km2, and the main activities are related to scientific research, environmental education, and controlled visits. AMR is located on the limits of the western Brazilian Pantanal, close to the border with Bolivia (Porfirio et al., 2016). The climate in Pantanal is classified as Am and Aw, warm, and markedly seasonal, with annual rainfall between 1,300 and 1,600 mm (Alvares et al., 2014). The rainy season is from October to April, and the dry season is from May to September (Junk et al., 2006). The vegetation is mainly dry and flooded savannah, open grasslands on mountain tops, seasonal deciduous and semi-deciduous forests, and gallery and riparian forests (Porfirio et al., 2016).
The CR site consists of 116,000 hectares dedicated mainly to cattle ranching and some crop production areas. A highway crosses the area, and it is about 72 km from the city of Miranda. The vegetation on the ranch is principally natural and exotic pastures, crop fields, semideciduous forest, and dry and flooded savannahs. The climate is seasonal, with a rainy season from October to March and a dry season from April to September. Hunting is not allowed on the ranch.

Camera trapping surveys were carried out in 3 areas of AMR: 1) Acurizal RPPN – January to November 2016; 2) Engenheiro Eliezer Batista RPPN – January 2016 to January 2017; and 3) Santa Tereza – June to September 2016. Five digital camera trap stations (Bushnell Trophy Cam® and Panthera V3) were installed at each of these 3 areas along dirt roads, positioned 45 cm from the ground and at an average distance of ~1.5 km from each other in different habitats. Camera traps were programmed to operate 24 hours/day and take videos of 20-second duration at 10-second intervals. We did not use bait to attract animals. The total sampling effort at AMR was 2,330 camera-days (Acurizal RPPN: 770 camera-days; Engenheiro Eliezer Batista RPPN: 1,295 camera-days; Santa Tereza: 265 camera-days).
We deployed 10 camera trap stations at CR (Bushnell HD®), installed along dirt roads at 45 cm above the ground and ~1.5 km apart, which operated as in AMR. The first CR survey was carried out from May to December 2016 (1,500 camera-days), and a second survey from February to April 2017 (430 camera-days). The total sampling effort at CR was 1,930 camera-days. In both study areas, camera stations were checked at 15-day intervals to download pictures and change batteries whenever necessary. As each camera station was composed of a single camera trap, we could not establish how many individuals
were monitored.
All data obtained from both study areas (AMR and CR) were screened to ensure sample independence, allowing one hour between records of the same species unless individuals could be identified (in which case records were considered independent) (Paviolo et al., 2009). Females and males were identified based on secondary characters whenever possible. We converted the time-stamp of each record to solar time using the software Solardials (http://www.art-spaces.com/solardials/). Then each observation was classified as diurnal (if the record occurred between 1 hour after sunrise and 1 hour before sunset), nocturnal (if the record occurred between 1 hour after sunset and 1 hour before sunrise), or crepuscular (if the record occurred up to 1 hour before and after sunrise and sunset) following Foster et al. (2013). Based on the frequency of records in each category, jaguar and puma from each study site were classified as either diurnal, nocturnal, crepuscular, or cathemeral (if record frequency was equivalent between diurnal and nocturnal categories). Independence between records of each species in each category was assessed using a Chi-squared test (Rucco et al., 2019).
To compare the activity patterns of each species between study areas, evaluate the extent of temporal overlap between jaguar and puma, and assess sex-biased activity patterns, we used a non-parametric approach widely used to investigate activity patterns and temporal ecology (Foster et al., 2013; Linkie & Ridout, 2011; Mella-Méndez et al., 2019; Monterroso et al., 2014; Porfirio et al., 2016; Ridout & Linkie, 2009; Wang et al., 2015). We only considered cases where ≥ 10 independent detections had been obtained (Monterroso et al., 2014). We generated activity curves for each sex and species in both areas by kernel density. Then we measured the extent of activity pattern overlap in each case according to a coefficient of overlap appropriate for small sample sizes (Δ1) that varied from 0 (no overlap) to 1 (complete overlap) (Ridout & Linkie, 2009). We obtained 95% confidence intervals of Δ1 through a bootstrap procedure with 500 samples (Foster et al., 2013; Linkie & Ridout, 2011). Temporal interaction was classified as low overlap (Δ1 ≤ 0.50), moderate overlap (0.51 < Δ1 ≤ 0.75), or high overlap (Δ1 > 0.75) (Monterroso et al., 2014). We employed Watson’s 2-sample homogeneity test for circular data to establish if activity patterns were significantly different (Jammalamadaka & Sengupta, 2001; Porfirio et al., 2016). All statistical analyses were performed in R software (R Development Core Team, 2019) using the package “overlap” (Ridout & Linkie, 2009).
Results
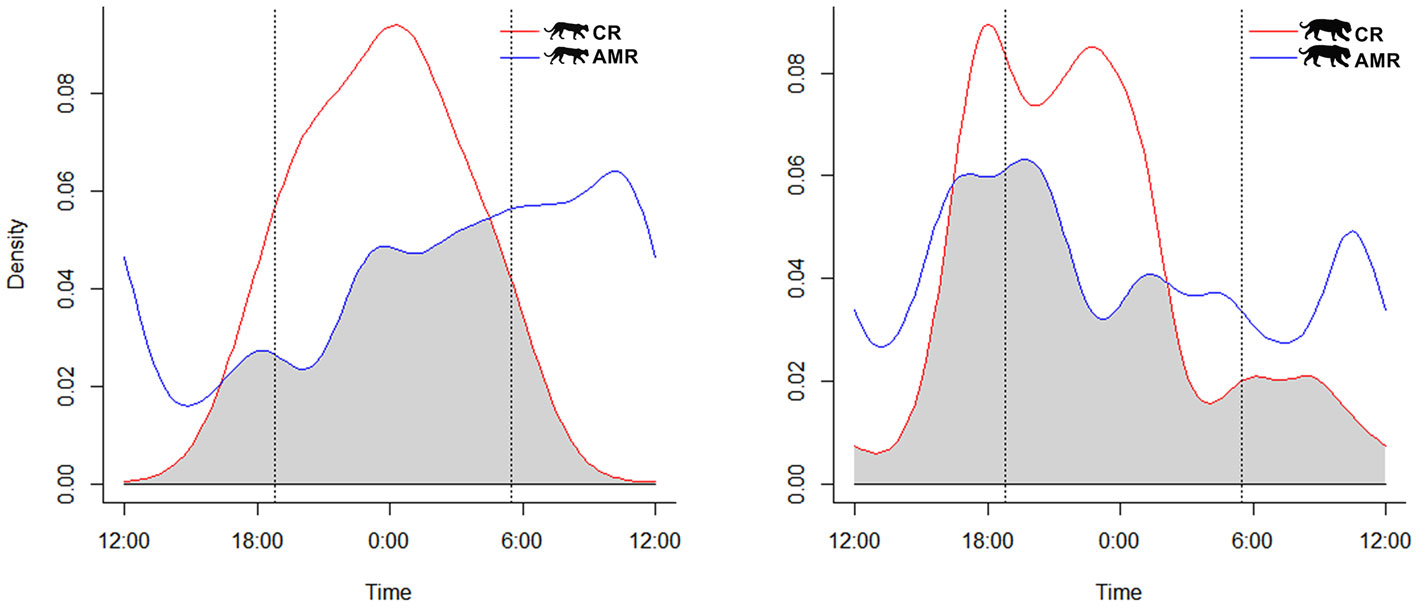
We obtained 71 independent records of jaguar and 29 of puma at AMR, and 85 of jaguar and 26 of puma at CR. Jaguars and pumas at AMR could be assigned as cathemeral (no clear daily activity pattern), since the proportion of records during the day and night were similar (X2 = 0.385, df = 2; p = 0.824). However, jaguar and puma were both nocturnal at CR (X2 = 0.800, df = 2; p = 0.012) (Table 1). As each camera station was composed by a single camera trap, we could not establish how many individuals were monitored.
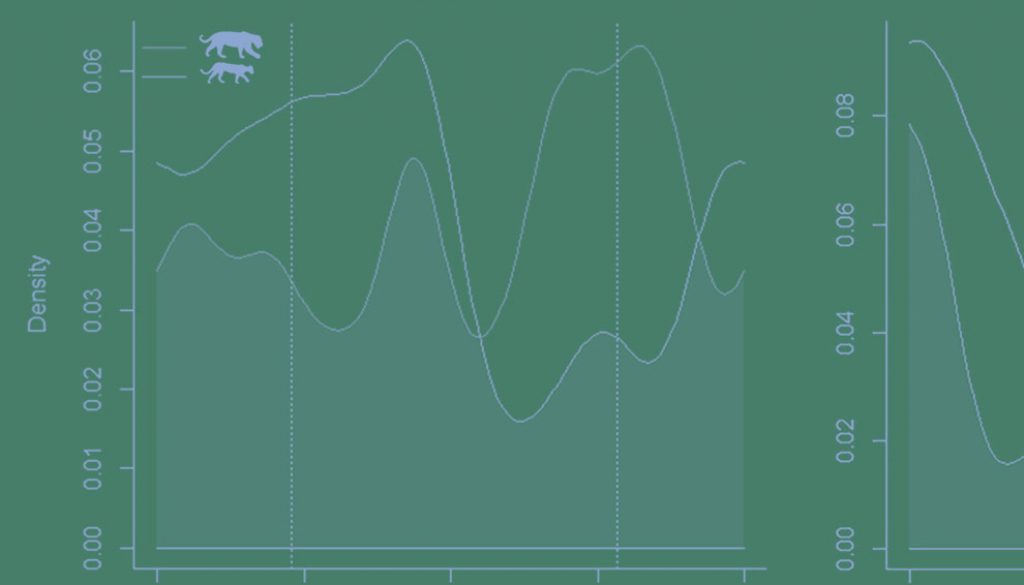
Activity patterns of each species differed significantly between study areas (jaguar: U2 = 0.3474, p < 0.05; puma: U2 = 0.3109, p < 0.05). However, we observed moderate activity overlap for jaguars at AMR and CR [Δ1 = 0.73 (0.63-0.81)]. Puma activity patterns overlapped less between study sites but still represented moderate overlap [Δ1 = 0.60 (0.42-0.65)] (Fig. 2). Pumas were never recorded during the daytime at CR (Table 1).
Examining study sites separately, the activity of jaguars moderately overlapped with that of pumas at AMR 73% of the time, perhaps unsurprisingly since their cathemeral activity patterns did not differ significantly [Δ1 = 0.73 (0.54-0.81); U2 = 0.1688, p > 0.05]. Temporal overlap between jaguars and pumas was higher at CR, likely owing to their similarly nocturnal activity patterns there [Δ1 = 0.75 (0.56-0.81); U2 = 0.1588, p > 0.05] (Fig. 3).
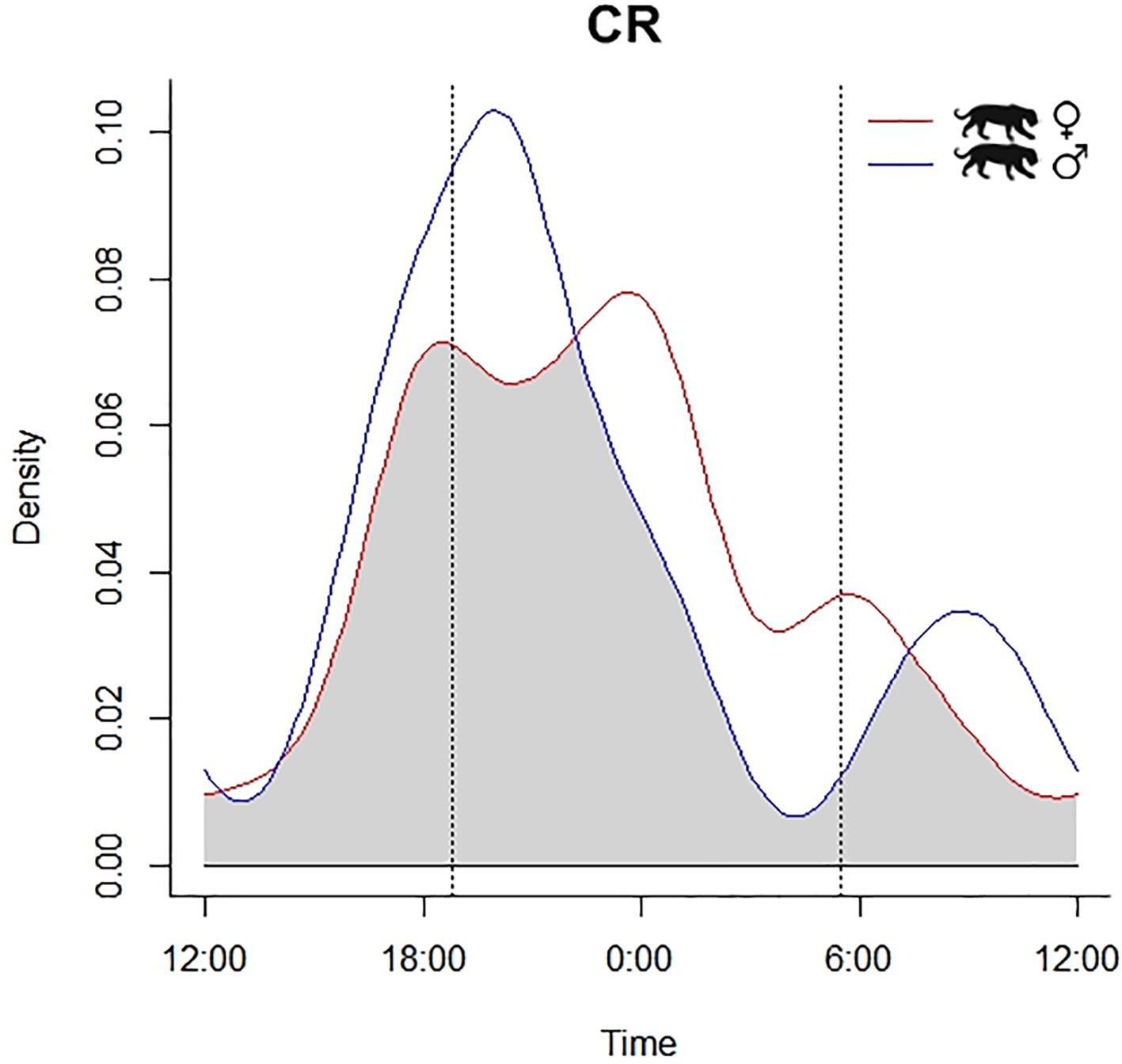
Due to image quality, we could distinguish the sex of jaguars and pumas only at CR (jaguar: 41 female records, 26 male, 18 unidentified; pumas: 7 female, 9 male, and 10 unidentified). Given the number of records, we could assess differences in activity patterns between female and male at CR only of jaguar. Our data did not exhibit any temporal segregation between female and male jaguars at CR (X2 = 1.436, df = 2; p = 0.487), with high overlap in their nocturnal activity patterns [Δ1 = 0.78 (0.56-0.84); U2 = 0.1081, p > 0.05] (Fig. 4).
Table 1
Periods of activity for jaguar (Panthera onca) and puma (Puma concolor) recorded by camera trapping. Records of camera trapping at the Amolar Mountain Ridge (AMR) and a cattle ranch (CR), both located in the Brazilian Pantanal based on surveys carried out from 2016 to 2017.
| Species | Number of records (%) | |||||
| Crepuscular | Diurnal | Nocturnal | ||||
| AMR | CR | AMR | CR | AMR | CR | |
| Jaguar | 10 (14) | 25 (29) | 27 (38) | 11 (13) | 34 (48) | 49 (58) |
| Puma | 5 (17.2) | 3 (11.6) | 12 (41.4) | 0 (0) | 12 (41.4) | 23 (88.4) |

Discussion
Our study investigated the activity patterns between jaguars and pumas in one of the most conserved areas of the Brazilian Pantanal (AMR) (Bertassoni et al., 2012; Porfírio et al., 2017), and a traditional area of cattle ranching in the Pantanal. Jaguars and pumas at AMR were cathemeral, as reported previously by Porfirio et al. (2017) in a similar study in this region. Cathemerality exhibited by both species has been mentioned as a mechanism that helped to diminish competition since jaguars and pumas may potentially share the same species of prey (Porfírio et al., 2017; Taber et al., 1997). Moreover, we observed that jaguars and pumas were less active around midday at AMR, probably to avoid the hottest period. These results were similar to that observed by Crawshaw and Quigley (1991) for the jaguar activity in the southern Pantanal. Furthermore, this tendency to avoid movement during the hottest hours of the day was also reported in the subtropical ecosystem of San Luis Potosí, México (Hernández-Saintmartín et al., 2013).
In contrast, both species were nocturnal on the cattle ranch, confirming our hypothesis, exhibiting drastically reduced activity patterns before sunrise, particularly for the jaguar (Figs. 2, 3). We did not record puma during the daylight on CR, and most of the puma activity (> 85%) occurred in the nocturnal period during the night (Table 1). Although jaguars and puma are now protected in CR, they have suffered persecution in previous years, and maybe maintaining this more prudent and nocturnal behavior. This predominance of night activity for jaguars has also been reported in other cattle ranching areas, including elsewhere in the Pantanal (Foster et al., 2013) and the Venezuelan llanos (Scognamillo et al., 2002). The same is valid for the puma, which, even being considered more tolerant to human disturbance than jaguars (De Angelo et al., 2011), was nocturnal in the cattle ranch, reinforcing the results of Paviolo et al. (2009), which observed predominantly nocturnal activities for pumas in low protected areas in the Upper Parana Atlantic Forest. This nocturnal adjustment in the activity patterns of jaguars and pumas, in response to human disturbances (Ávila-Nájera et al., 2016; Foster et al., 2013; Paviolo et al., 2009; Scognamillo et al., 2002), has been observed for leopard (Panthera pardus), Amur tigers (Panthera tigrisaltaica) ocelot (Leopardus pardalis), and southern tiger cat (Leopardus guttulus), mainly outside protected areas (Carter et al., 2015; Cruz et al., 2018; Yang et al., 2019). Different contexts can drive this change in the patterns of wildlife activity. In California, Bobcats (Lynx rufus) became more nocturnal due to human recreational activities in a California nature reserve (George & Crooks, 2006). In a global meta-analysis, Gaynor et al. (2018) showed how human disturbances make mammals more nocturnal, generating several consequences like population persistence and community interactions. Furthermore, predators shift their activity times and their prey (Shamoon et al., 2018).
A previous study performed in AMR reported that pumas and jaguars did not avoid each other, at least temporally (Porfírio et al., 2017). In that study, both species were cathemeral (Δ1 = 0.88), and tended to follow the same activity patterns of their main prey in the area, i.e., gray brocket deer (Mazama gouazoubira), collared peccary (Pecari tajacu), and capybara (Hydrochoerus hydrochaeris). However, given that jaguars and pumas were mainly nocturnal on the cattle ranch and the temporal overlap coefficient was higher than for AMR, we suggest 2 probable and potentially linked scenarios. First, felids adopt the same activity patterns as their prey, thereby increasing the likelihood of encounters (as reported by Ávila-Nájera et al. [2016] and Foster et al. [2013]). Secondly, human disturbance restricts the activity of both predator and prey, as also observed by Shamoon et al. (2018). Therefore, both scenarios respond to human activity, in this case, cattle ranching, crop production, and its effects since Gaynor et al. (2018) demonstrated an increase in nocturnality by wildlife as a response to human disturbance across several continents and different habitats worldwide. Whether cattle ranching influences the activity patterns of predators and prey, the impacts can affect other species behaviors, several ecological interactions, such as trophic interactions, community structure, and, finally, evolutionary processes, as highlighted by Gaynor et al. (2018). However, to distinguish between or prove these 2 possibilities, further investigation and comparison of prey availability, prey activity patterns, feeding habits of jaguar and puma, and the extent of human interference in both areas are needed.
Although we did not have sufficient data to fully assess sex-biased activity patterns of jaguars and pumas within and between areas, our results exhibited that activity patterns of male and female jaguars on the cattle ranch were similar. We found only one other published study comparing male and female jaguar and puma activity patterns using camera trapping data (Romero-Muñoz et al., 2010). That study, carried out in the dry forests of Bolivia, exhibited no gender-mediated differences in jaguar and puma activity patterns. However, given that human interference may influence jaguar activity in our study site, these data may not reflect what occurs in protected areas or areas with little human influence.
Therefore, our study suggests that the effects of human disturbances caused the activities of jaguars and pumas on cattle ranches to be almost exclusively nocturnal. Both species shown cathemeral activity in protected areas, driven mostly by natural factors.
Acknowledgements
This study was supported by Rede de Proteção e Conservação da Serra do Amolar (Network for Protection and Conservation of Amolar Mountain Ridge), Instituto Homem Pantaneiro (IHP) and BRPEC Company. Special thanks to Panthera Brasil that loaned camera traps to cover the study site at AMR, and camera traps and working support at CR. To Wesley Arruda Gimenes Nantes that kindly improved the figures, to Wagner Tolone da Silva Ferreira that built the map of the study sites, and to John O’Brien for the English proofread. The first author thanks Universidade Católica Dom Bosco (UCDB) for grant CAPES [88887.364520/2019-00]. This study was financed in part by Universidade Federal de Mato Grosso do Sul – UFMS, MEC, Brazil and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code [001].
References
Albrecht, M., & Gotelli, N. J. (2001). Spatial and temporal niche partitioning in grassland ants. Oecologia, 126, 134–141. https://doi.org/10.1007/s004420000494
Alho, C. J., & Sabino, J. (2011). A conservation agenda for the Pantanal’s biodiversity. Brazilian Journal of Biology, 71, 327–335. https://doi.org/10.1590/S1519-69842011000200012
Almeida, T. de. (1990). Jaguar hunting in the Mato Grosso and Bolivia. Long Beach, California: Safari Press.
Alvares, C. A., Stape, J. L., Sentelhas, P. C., Gonçalves, J. L. M., & Sparovek, G. (2014). Koppen’s climate classification map for Brazil. Meteorologische Zeitschrift, 22, 711–728. https://doi.org/10.1127/0941-2948/2013/0507
Ávila-Nájera, D. M., Chávez, C., Lazcano-Barreto, M. A., Mendoza, G. D., & Pérez-Elizalde, S. (2016). Overlap in activity patterns between big cats and their main prey in northern Quintana Roo, Mexico. Therya, 7, 439–448. https://doi.org/10.12933/therya-16-379
Azevedo, F. C. C., & Murray, D. L. (2007). Spatial organization and food habits of jaguars (Panthera onca) in a floodplain forest. Biological Conservation, 137, 391–402. https://doi.org/10.1016/j.biocon.2007.02.022
Bertassoni, A., Xavier, N. L., Rabelo, F. A., Leal, S. P., Porfírio, G. E. O., & Moreira, V. F. (2012). Paraguay River environmental monitoring by Rede de Proteção e Conservação da Serra do Amolar, Pantanal, Brazil. Pan American Journal of Aquatic Science, 72, 77–84.
Carothers, J. H., & Jaksic, F. M. (1984). Time as a niche difference: the role of interference competition. Oikos, 42, 403–406. https://doi.org/10.2307/3544413
Carter, N., Jasny, M., Gurung, B., & Liu, J. (2015). Impacts of people and tigers on leopard spatiotemporal activity patterns in a global biodiversity hotspot. Global Ecology and Conservation, 3, 149–162. https://doi.org/10.1016/j.gecco.2014.11.013
Cavalcanti, S. M., & Gese, E. M. (2010). Kill rates and predation patterns of jaguars (Panthera onca) in the southern Pantanal, Brazil. Journal of Mammalogy, 91, 722–736. https://doi.org/10.1644/09-MAMM-A-171.1
Crawshaw, P. G. Jr., & Quigley, H. B. (1991). Jaguar spacing, activity and habitat use in a seasonally flooded environment in Brazil. Journal of Zoology, 223, 357–370. Https://Doi.Org/10.1111/J.1469-7998.1991.Tb04770.X
Cruz, P., Iezzi, M. E., De Angelo, C., Varela, D., Di Bitetti, M. S., & Paviolo A. (2018). Effects of human impacts on habitat use, activity patterns and ecological relationships among medium and small felids of the Atlantic Forest. Plos One, 13, e0200806. https://doi.org/10.1371/journal.pone.0200806
de Angelo, C., Paviolo, A., & Di Bitetti, M. (2011). Differential impact of landscape transformation on pumas (Puma concolor) and jaguars (Panthera onca) in the Upper Paraná Atlantic Forest. Diversity and Distributions, 17, 422–436. https://doi.org/10.1111/j.1472-4642.2011.00746.x
de la Torre, J. A., Núñez, J. M., & Medellín, R. A. (2017). Spatial requirements of jaguars and pumas in Southern Mexico. Mammalian Biology, 84, 52–60. https://doi.org/10.1016/j.mambio.2017.01.006
Farrell, L. E. (2001). Molecular scatology as a conservation tool. Endangered Species Update, 18, 133–137.
Foster, V. C., Sarmento, P., Sollmann, R., Tôrres, N., Jácomo, A. T. A., Negrões, N. et al. (2013). Jaguar and puma activity patterns and predator-prey interactions in four Brazilian biomes. Biotropica, 45, 373–379. https://doi.org/10.1111/btp.12021
Gaynor, K. M., Hojnowski, C. E., Carter, N. H., & Brashares, J. S. (2018). The influence of human disturbance on wildlife nocturnality. Science, 360, 1232–1235. https://doi.org/ 10.1126/science.aar7121
George, S. L., & Crooks, K. R. (2006). Recreation and large mammal activity in an urban nature reserve. Biological Conservation, 133, 107–117. https://doi.org/10.1016/j.biocon.2006.05.024
Harris, M. B., Tomas, W., Mourão, G., Da Silva, C. J., Guimarães, E., Sonoda, F. et al. (2005). Safeguarding the Pantanal wetlands: threats and conservation initiatives. Conservation Biology, 19, 714–720. https://doi.org/10.1111/j.1523-1739.2005.00708.x
Hernández-Saintmartín, A. D., Rosas-Rosas, O. C., Palacio-Núñez, J., Tarango-Arámbula, L. A., Clemente-Sánchez, F., & Hoogesteinj, A. (2013). Activity patterns of jaguar, puma and their potential prey in San Luis Potosí, Mexico. Acta Zoológica Mexicana, 29, 520–533.
Jammalamadaka, S. R., & Sengupta, A. (2001). Topics in circular statistics. Singapore: World Scientific Press.
Junk, W. J., Da Cunha, C. N., Wantzen, K. M., Petermann, P., Strüssmann, C., Marques, M. I. et al. (2006). Biodiversity and its conservation in the Pantanal of Mato Grosso, Brazil. Aquatic Science, 68, 278–300. https://doi.org/10.1007/s00027-006-0851-4
Linkie, M., & Ridout, M. S. (2011). Assessing tiger-prey interactions in Sumatran rainforests. Journal of Zoology, 284, 224–229. https://doi.org/10.1111/j.1469-7998.2011.00801.x
Lourival, R., Drechsler, M., Watts, M. E., Game, E. T., & Possingham, H. P. (2011). Planning for reserve adequacy in dynamic landscapes; maximizing future representation of vegetation communities under flood disturbance in the Pantanal wetland. Diversity and Distributions, 17, 297–310. https://doi.org/10.1111/j.1472-4642.2010.00722.x
Marchini, S., & Macdonald, D. W. (2012). Predicting ranchers’ intention to kill jaguars: case studies in Amazonia and Pantanal. Biological Conservation, 147, 213–221. https://doi.org/10.1016/j.biocon.2012.01.002
Mazzolli, M. (2009). Loss of historical range of jaguars in Southern Brazil. Biodiversity and Conservation, 18, 1715–1717. https://doi.org/10.1007/s10531-008-9552-8
Mella-Méndez, I., Flores-Peredo, R., Pérez-Torres, J., Hernández-González, S., González-Uribe, D. U., & Bolívar-Cimé, B. S. (2019). Activity patterns and temporal niche partitioning of dogs and medium-sized wild mammals in urban parks of Xalapa, Mexico. Urban Ecosystems, 22, 1061–1070. https://doi.org/10.1007/s11252-019-00878-2
Miller, B., Dugelby, B., Foreman, D., Martinez-Del Rio, C., Noss, R., Phillips, M. et al. (2001). The importance of large carnivores to healthy ecosystems. Endangered Species Update, 18, 202–210.
Monroy-Vilchis, O., Urios, V., Zarco-González, M., & Rodríguez-Soto, C. (2009). Cougar and jaguar habitat use and activity patterns in central Mexico. Animal Biology, 59, 145–157. https://doi.org/10.1163/157075609X437673
Monterroso, P., Alves, P. C., & Ferreras, P. (2014). Plasticity in circadian activity patterns of mesocarnivores in Southwestern Europe: implications for species coexistence. Behavioral Ecology and Sociobiology, 68, 1403–1417. https://doi.org/10.1163/157075609X437673
Moreno, R. S., Kays, R. W., & Samudio, R. (2006). Competitive release in diets of ocelot (Leopardus pardalis) and puma (Puma concolor) after jaguar (Panthera onca) decline. Journal of Mammalogy, 87, 808–816. https://doi.org/10.1644/05-MAMM-A-360R2.1
Nielsen, C., Thompson, D., Kelly, M., & López-Gonzáalez, C. A. (2016). Puma concolor. The IUCN Red List of Threatened Species 2015. Retrieved in 27 August, 2019 from http://iucnredlist.org
Novack, A. J., Main, M. B., Sunquist, M. E., & Labisky, R. F. (2005). Foraging ecology of jaguar (Panthera onca) and puma (Puma concolor) in hunted and non-hunted sites within the Maya Biosphere Reserve, Guatemala. Journal of Zoology, 267, 167–178. https://doi.org/10.1017/S0952836905007338
Núñez, R., Miller, B., & Lindzey, F. (2000). Food habits of jaguars and pumas in Jalisco, Mexico. Journal of Zoology, 252, 373–379. https://doi.org/10.1111/j.1469-7998.2000.tb00632.x
Palmeira, F. B., Crawshaw Jr, P. G., Haddad, C. M., Ferraz, K. M. P., & Verdade, L. M. (2008). Cattle depredation by puma (Puma concolor) and jaguar (Panthera onca) in central-western Brazil. Biological Conservation, 141, 118–125. https://doi.org/10.1016/j.biocon.2007.09.015
Paviolo, A., Di Blanco, Y. E., De Angelo, C. D., & Di Bitetti, M. (2009). Protection affects the abundance and activity patterns of pumas in the Atlantic Forest. Journal of Mammalogy, 90, 926–934. https://doi.org/10.1644/08-MAMM-A-128.1
Porfirio, G., Sarmento, P., Xavier-Filho, N. L., Cruz, J., & Fonseca, C. (2014). Medium to large size mammals of southern Serra do Amolar, Mato Grosso do Sul, Brazilian Pantanal. Check List, 10, 473–482. https://doi.org/10.15560/10.3.473
Porfirio, G., Foster, V. C., Fonseca, C., & Sarmento, P. (2016). Activity patterns of ocelots and their potential prey in the Brazilian Pantanal. Mammalian Biology, 81, 511–517. https://doi.org/10.1016/j.mambio.2016.06.006
Porfirio, G., Sarmento, P., Foster, V., & Fonseca, C. (2017). Activity patterns of jaguars and pumas and their relationship to those of their potential prey in the Brazilian Pantanal. Mammalia, 81, 401–404. https://doi.org/10.1515/mammalia-2015-0175
Quigley, H. B., & Crawshaw Jr, P. G. (1992). A conservation plan for the jaguar Panthera onca in the Pantanal region of Brazil. Biological Conservation, 61, 149–157. https://doi.org/10.1016/0006-3207(92)91111-5
Quigley, H., Foster, R., Petracca, L., Payan, E., Salom, R., & Harmsen, B. (2018). Panthera onca. The IUCN Red List of Threatened Species 2017. Retrieved in 27 august, 2019 from: http://iucnredlist.org
R Core Team. (2019). R: A language and environment for statistical computing. Software. https://www.R-project.org/
Ridout, M. S., & Linkie, M. (2009). Estimating overlap of daily activity patterns from camera trap data. Journal of Agriculture Biology and Environmental Statistics, 14, 322–337. https://doi.org/10.1198/jabes.2009.08038
Romero-Muñoz, A., Maffei, L., Cuéllar, E., & Noss, A. J. (2010). Temporal separation between jaguar and puma in the dry forests of Southern Bolivia. Journal of Tropical Ecology, 26, 303–311. https://www.jstor.org/stable/40665237
Roque, F. O., Ochoa-Quintero, J., Ribeiro, D. B., Sugai, L., Costa-Pereira, R., Lourival, R. et al. (2016). Upland habitat loss as a threat to Pantanal wetlands. Conservation Biology, 30, 1131–1134. https://doi.org/10.1111/cobi.12713
Rucco, A. C., Porfirio, G. E. O., Santos, F. M., Nascimento, L. F., Foster, V. C., Fonseca, C.et al. (2019). Padrões de atividade de duas espécies de cervídeos simpátricos (Mazama americana e Mazama gouazoubira) no Maciço do Urucum, Corumbá, MS. Oecologia Australis, 23, 440–450. https://doi.org/10.4257/oeco.2019.2303.04
Schaller, G. B., & Crawshaw Jr, P. G. (1980). Movement patterns of jaguar. Biotropica, 12, 161–168. https://doi.org/10.2307/2387967
Schoener, T. W. (1974). Resource partitioning in ecological communities. Science, 185, 27–39. https://doi.org/10.1126/science.185.4145.27
Scognamillo, D., Maxit, I., Sunquist, M., & Farrell, L. (2002). Ecología del jaguar y el problema de la depredación de ganado en un hato de los Llanos Venezolanos. In R. A. Medellín, C. L. B. Cequihua, P. G. Chetkiewicz, A. Crawshaw, K. H. Rabinowitz, R. G. Redford, E. W. G. Sanderson y A. B. Taber (Eds), El jaguar en el nuevo milenio (pp. 139–150). Mexico City: Ediciones Científicas Universitarias.
Scognamillo, D., Maxit, I. E., Sunquist, M., & Polisar, J. (2003). Coexistence of jaguar (Panthera onca) and puma (Puma concolor) in a mosaic landscape in the Venezuelan llanos. Journal of Zoology, 259, 269–273. https://doi.org/10.1017/S0952836902003230
Shamoon, H., Maor, R., Saltz, D., & Dayan, T. (2018). Increased mammal nocturnality in agricultural landscapes results in fragmentation due to cascading effects. Biological Conservation, 226, 32–41. https://doi.org/10.1016/j.biocon.
2018.07.028
Silveira, L. (2004). Ecologia comparada e conservação da onça-pintada (Panthera onca) e onça-parda (Puma concolor), no Cerrado e Pantanal. Tesis Doctoral. Universidade de Brasília. Brasil.
Soisalo, M. K, & Cavalcanti, S. M. (2006). Estimating the density of a jaguar population in the Brazilian Pantanal using camera-traps and capture-recapture sampling in combination with GPS radio-telemetry. Biological Conservation, 129, 487–496. https://doi.org/10.1016/j.biocon.2005.11.023
Taber, A. B., Novaro, A. J., Neris, N., & Colman, F. H. (1997). The Food Habits of Sympatric Jaguar and Puma in the Paraguayan Chaco. Biotropica, 29, 204–213. https://doi.org/10.1111/j.1744-7429.1997.tb00025.x
Terborgh, J. (1990). The role of felid predators in the neotropical forest. Vida Silvestre Neotropical, 2, 3–5.
Tomás, W. M. et al. (2019). Sustainability agenda for the Pantanal Wetland: perspectives on a collaborative interface for science, policy, and decision-making. Tropical Conservation Science, 12, 1940082919872634. https://doi.org/10.1177/1940082919872634
Tortato, F. R., Layme, V., Crawshaw Jr, P. G., & Izzo, T. J. (2015). The impact of herd composition and foraging area on livestock predation by big cats in the Pantanal of Brazil. Animal Conservation, 18, 539–547. https://doi.org/10.1111/acv.12207
Valeix, M., Chamaillé-Jammes, S., & Fritz, H. (2007). Interference competition and temporal niche shifts: elephants and herbivore communities at waterholes. Oecologia, 153, 739–748. https://doi.org/10.1007/s00442-007-0764-5
Yang, H., Han, S., Xie, B., Mou, P., Kou, X., Wang, T. et al. (2019). Do prey availability, human disturbance and habitat structure drive the daily activity patterns of Amur tigers (Panthera tigris altaica)? Journal of Zoology, 307, 131–140. https://doi.org/10.1111/jzo.12622
Wang, Y., Allen, M. L., & Wilmers, C. C. (2015). Mesopredator spatial and temporal responses to large predators and human development in the Santa Cruz Mountains of California. Biological Conservation, 190, 23–33. https://doi.org/10.1016/j.biocon.2015.05.007
Zanin, M., Palomares, F., & Brito, D. (2014). What we (don’t) know about the effects of habitat loss and fragmentation on felids. Oryx, 49, 96–106. https://doi.org/10.1017/S0030605313001609