Mara Urdapilleta a, b, Carlos A. Galliari a, c, Tomás Navarro-Febre c, Marcela Lareschi a, c, *
a Consejo Nacional de Investigaciones Científicas y Técnicas, Godoy Cruz 2290 (C1425FQB) Ciudad Autónoma de Buenos Aires, Argentina
b Ministerio de Salud y Desarrollo Social de la Nación, Instituto Nacional de Medicina Tropical, Administración Nacional de Laboratorios e Institutos de Salud, Jujuy y Neuquén s/n, 3370 Puerto Iguazú, Misiones, Argentina
c Universidad Nacional de La Plata, Consejo Nacional de Investigaciones Científicas y Técnicas, Centro de Estudios Parasitológicos y de Vectores, Boulevard 120 s/n e/ 60 y 64, 1900 La Plata, Argentina
*Corresponding author: mlareschi@cepave.edu.ar (M. Lareschi)
Received: 8 January 2021; accepted: 23 June 2021
Abstract
Akodon montensis Thomas is the dominant rodent species in the Urugua-í Provincial Park in northeastern Argentina. Herein we analyze the effect of variables related to the hosts and the environment on the parasitic burden (PB), which includes the Ixodida, Siphonaptera and Mesostigmata (Laelapidae and Macronyssidae). In addition, we analyze host and environmental variables on specific richness (SL), and mean abundance of mites of the Laelapidae family (MAL), since these were the most abundant taxon and the only one with sufficient quantity for analysis. The variables PB, SL and MAL were used with the logarithm transformed data in the analysis. One-way ANOVA was used to test differences between rodents of different sex in an exploratory analysis, and the Levene test was then used to confirm the homogeneity of variances. Differences in these parameters were tested between the states of rodent’s sexual maturity. To test the effect of trapped site and its interaction with the sex of the rodent on MAL and SL, a two-way ANOVA was performed. The PAST software was used. The results support that the sex of the host would be the main factor that modulates PB and MAL, but not SL. The size and weight of the hosts (as a proxy for age), the reproductive stage and site of capture of the rodents, would not affect any of the variables analyzed. The results obtained in the present study contribute to the comprehension of the epidemiological role of ectoparasites, mainly laelapids.
Keywords: Laelapidae; Mite; Sigmodontine
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Efecto de los factores relacionados con el hospedador y el medio ambiente en la distribución de los ectoparásitos del ratón de pastizal pampeano Akodon montensis (Cricetidae: Sigmodontinae) en la ecorregión del Bosque Atlántico en el noreste de Argentina, con énfasis en los lelápidos (Mesostigmata)
Resumen
Akodon montensis Thomas es dominante en el Parque Provincial Urugua-í en el noreste de Argentina. Aquí analizamos el efecto de las variables relacionadas con los hospedadores y el medio ambiente sobre la carga parasitaria (PB), que incluye a los Ixodida, Siphonaptera y Mesostigmata (Laelapidae y Macronyssidae). Además, analizamos variables del hospedador y del ambiente sobre la riqueza específica (SL) y abundancia media de ácaros de la familia Laelapidae (MAL), ya que estos fueron el taxón más abundante y el único con cantidad suficiente para el análisis. Las variables PB, SL y MAL se utilizaron con los datos transformados en logaritmos en el análisis. Se usó Anova de una vía para probar las diferencias entre roedores de diferente sexo en un análisis exploratorio, y luego se usó la prueba de Levene para confirmar la homogeneidad de las varianzas. Se probaron las diferencias en estos parámetros entre los estados de madurez sexual de los roedores. Para probar el efecto del sitio de captura y su interacción con el sexo del roedor en MAL y SL, se realizó un Anova de 2 vías. Se utilizó el software PAST. Los resultados sostienen que el sexo del hospedador sería el factor principal que modula PB y MAL, pero no SL. El tamaño y peso de los hospedadores (como proxy de la edad), la etapa reproductiva y el sitio de captura de los roedores, no afectarían ninguna de las variables analizadas. Los resultados obtenidos en el presente estudio contribuyen a la comprensión del papel epidemiológico de los ectoparásitos, principalmente lelápidos.
Palabras clave: Laelapidae; Ácaro; Sigmodontino
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The Paranaense Rainforest ecoregion covers the upper basins of the Paraná and Uruguay rivers in southern Brazil, eastern Paraguay and the extreme northeast of Argentina, and is one of the fifteen ecoregions included in the Atlantic Forest ecoregional complex (Burkart et al., 1999; Plací & Di Bitetti, 2005). This ecoregion has a subtropical climate that characterizes the semi-deciduous forest with abundant rivers and streams. In Argentina, the Paranaense Rainforest occupies almost the entire territory of the Province of Misiones, contains part of the Paraná forest and Araucaria angustifolia (Bertol.) forest areas, and represents the area of greatest biodiversity and endemic species in the country (Burkart et al., 1999; Di Bitetti et al., 2003). Among the small mammal assemblage in the Paranaense Rainforest, the montane grass mouse, Akodon montensis Thomas (Rodentia, Cricetidae, Sigmodontinae), is the species with the widest distribution and dominant (D’Elía & Pardiñas, 2015; Pardiñas et al., 2003).
Usually, sigmodontine rodents are associated with a variety of ectoparasites, such as mites, ticks and fleas (Mesostigmata —Laelapidae and Macronyssidae—, Ixodida and Siphonaptera, respectively). A parasite component community represents all of the parasites of different species associated with some subset of a host species, such as a population (Bush et al., 1997). The distribution of ectoparasites among individual hosts within a component community is not random. On the contrary, host-ectoparasite associations are the result of evolutionary and ecological processes, related to factors of the parasites, the hosts and the environment (Lareschi & Krasnov, 2010; Linardi & Krasnov, 2013; Morand et al., 2006). Ectoparasites belonging to different higher taxa differ in their life histories and in the degree of their association with hosts. Individuals in a rodent population vary in ways that can affect their interactions with their parasites. For example, host specimens vary in their sex, age, reproduction condition, physiology, ecology, etc., and all of these features may influence their ectoparasite populations. In addition, since many ectoparasites spend part of their life cycle in the soil or nests of their hosts, they may be sensitive to variations in the environment (Krasnov, 2008; Marshall, 1981; Morand et al., 2006).
Most of the studies analyze the effect of host and environment features on ectoparasites of rodents on fleas (Krasnov, 2008; Morand et al., 2006). However, some have considered different taxa within the ectoparasite community (e.g., Alonso et al., 2020; Lareschi, 2007; Lareschi & Krasnov, 2010; Sponchiado et al., 2015). The aim of this study is to analyze the effect of host and environment related factors on the distribution of the ectoparasites of the montane grass mouse Akodon montensis (Cricetidae, Sigmodontinae) in the Atlantic Forest ecoregion in northeastern Argentina. In addition, we analyze the effect of the variables on the species richness and abundance exclusively for mites of the family Laelapidae.
Materials and methods
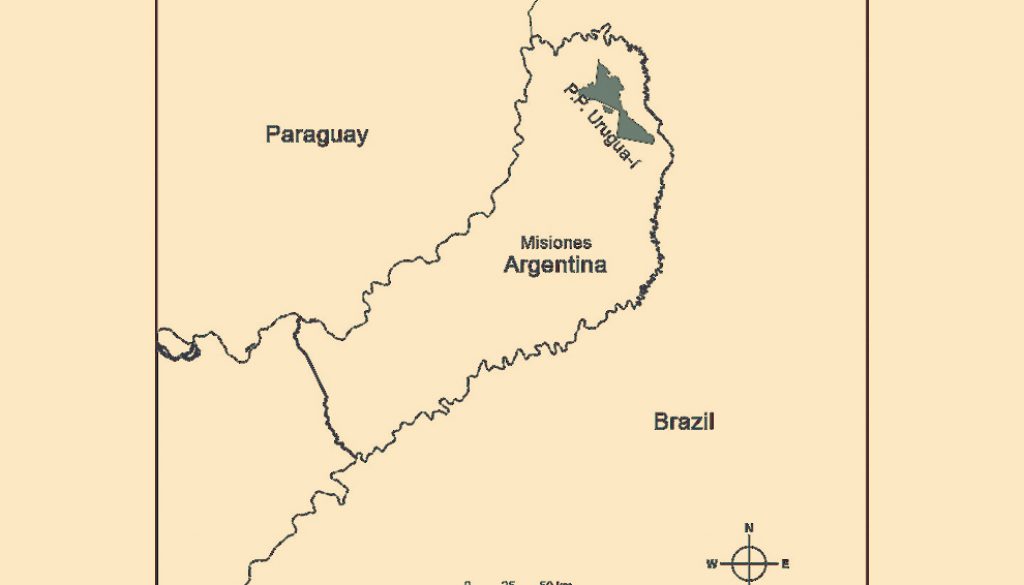
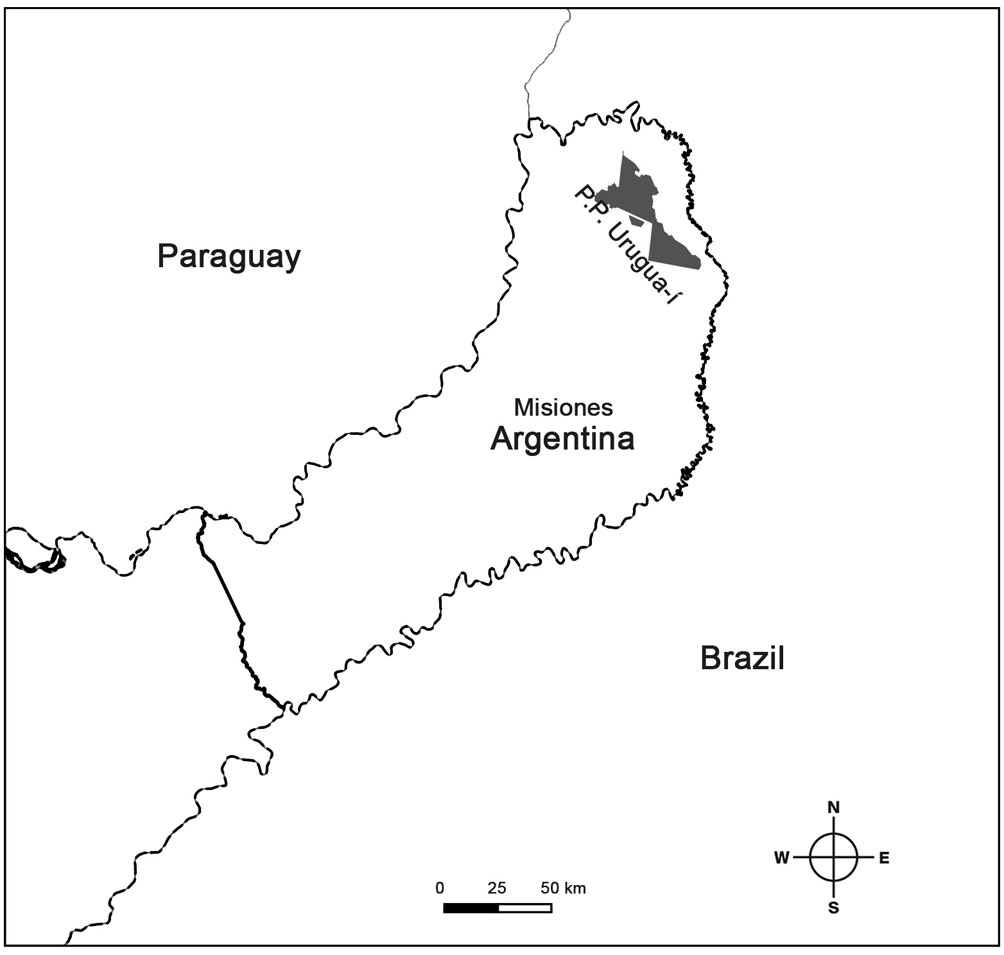
Within the Atlantic Forest ecoregion, the Paranaense Rainforest ecoregion maintains the largest amount of remnants with different degrees of forest conservation in the complex (Burkart et al., 1999; Plací & Di Bitetti, 2005). However, even the areas that are currently protected have been exploited for the selective extraction of wood (Giraudo et al., 2003). The study was carried out in the Urugua-í Provincial Park Doctor Luis Honorio Rolón, situated in the extreme northeast of the Province of Misiones (25°50’33.61” S, 54°6’7.81” W; Fig. 1). Its extension is 84,000 ha located in the municipalities of San Antonio, Bernardo de Irigoyen and Cmte. A. Guacurarí, from the Gral. Belgrano department and in the Colonia Wanda municipality, from the Iguazú department (Rolón & Chebez, 1998). Urugua-í Provincial Park integrates together with the Yacuy Provincial Park, the Urugua-í Wildlife Reserve, the Caá Porá Wildlife Refuge, the Iguazú National Park and National Reserve (all of them in Argentina) and the Iguaçú National Park (Brazil), the largest protected nucleus of Paranaense Rainforest, totaling some 255,000 ha (Cinti, 1997; Chebez & Gil, 1993; Chebez & Rolón, 1989).
Traps (N = 200) were distributed in different environments (as follow), chosen for their contrasting physiognomies (vegetation cover, tree canopy, bodies of water, etc.): 1) borders of vehicular roads (67 traps). These sites are healing vegetation, with heliophytic grasses and shrubs, which can reach heights of more than 1 meter and with almost total ground coverage. In most cases, there is a parallel vegetation cover on their outer edges (with respect to the road), of impoverished or primary forest. Internal forest trails are not included here, in which there is a continuity of the tree canopy that acts as the roof of the trail; 2) terraced forest (67 traps). These forests are associated with the low planes and foothills that surround tacuarales near river edges, on null to very slight slopes of brown soils, not at all or slightly stony and well-drained superficially. They are high mixed forest with a canopy dominated by raboitá, black laurel, and yellow laurel. The understory is dark and open with an abundance of trees, grasses, and ferns. Chusquea culeou Desvaux may be common (Srur et al., 2009); 3) streams and their edges (66 traps): almost all the streams of the Paranaense Rainforest have cyclical changes in their flow caused by regional rains, which in the dry season leaves exposed the rocky bottom which in long stretches form basalt beaches. In addition, both on the very edge of the stream and in the vicinity of its edges, in the area affected by periodic floods, there is a plant formation called sarandisal, made up of Phyllanthus sellowianus (Klotzsch) and Cephalanthus glabratus (Spreng.) shrubs, associated with the mata-ojo [Pouteria salicifolia (Spreng.)] and aguaí [(Pouteria gardneriana (A. DC.) Radlk)].
Rodents were captured alive using Sherman traps baited with oats. Two hundred traps were placed in the selected environments remaining in the field for four consecutive nights (from 23-26 August, 2013). Traps were checked daily and the bait was renewed, while those with captures were removed. Rodents were anesthetized with sulfuric ether and sacrificed by cervical dislocation. All procedures were conducted following the ethical guidelines established by the American Society of Mammalogists (Sikes, 2016).
From each rodent, capture site (1, 2 or 3), diagnostic measurements, weight, sex, and reproductive condition (males: abdominal or scrotal testicles; females: open, closed, or plugged vagina) were recorded. Taxonomy of the rodents follows D’Elía and Pardiñas (2015). Rodents will be deposited at the Colección de Mamíferos del Centro Nacional Patagónico (CNP; Puerto Madryn, Chubut Province, Argentina).
Ectoparasites were collected in the field by examining the fur of the hosts using forceps and combs and fixed in 96% alcohol in individual eppendorfs per host. Ectoparasites were identified at the higher taxa level by direct observation under magnifier binocular stereoscopic. Mites of the family Macronyssidae were identified up to genera level, and only those of the family Laelapidae were identified at a specific level. For taxonomic identification, mites were cleared in lactophenol and mounted in Hoyer´s medium. Taxonomic identification was carried out in accordance with the keys, drawings, and descriptions given by Furman (1972), Lareschi (2010a, 2018, 2020) and Radovsky (2010). Representative specimens of each ectoparasite taxa will be deposited at Colección de Entomología, Museo de La Plata, Argentina.
Independent variables were related to A) rodents: 1) sex (male/female); 2) sexual maturity: immature (IMM): females with an imperforate vagina, and males with testicles in the abdominal position; mature (MAT): females with an open vagina, with a vaginal plug, pregnant or breastfeeding, and males with testicles in the scrotum; 3) age: the weight and head-body length of the rodents were considered as substitute or proxy for calculating their age following Morris (1972); B) environment: site of capture of every rodent (1, 2 or 3, see description in materials and methods). Dependent variables related to the ectoparasites were: 1) parasitic burden (PB): total number of ectoparasites of different taxa (Siphonaptera, Ixodida and Mesostigmata —Laelapidae and Macronyssidae—) collected in a sample of a particular host species; 2) specific richness of Laelapidae mites, (SL); 3) mean abundance of Laelapidae (MAL): total number of individuals of a particular taxon in a sample of a particular host species/total number of hosts, including both infested and non-infested hosts (Begon et al., 1997; Bush et al., 1997). Analysis referring to the specific richness and the mean abundance was only calculated for Laelapidae, since these were the most abundant taxon and the only one with sufficient quantity for analysis.
The values of the parasitic burden, specific richness of laelapids, and the mean abundance of laelapids were transformed to logarithms to carry out the calculations and further analysis. One-way ANOVA was used to test differences between rodents of different sex in an exploratory analysis. The Levene test was then used to confirm the homogeneity of variances. Using the same technique, differences in these parameters were tested between the states of sexual maturity only in females, because in males the immature group contained only one case. To test the effect of habitat and its interaction with the sex of the rodent on parasite abundances and specific richness, a two-way ANOVA was performed. All analysis were carried out with the PAST software (Hammer et al., 2001).
Results
One hundred thirty-eight rodents were captured, and out of these, 56 were identified as Akodon montensis (21 females and 35 males). Sample size of the analyzed specimens according to their sex, habitat and sexual maturity is presented in Table 1. Fifty- four A. montensis were parasitized, and 946 ectoparasites were collected. From these, 228 were associated with female hosts and 721 with the males. Out of these, 806 were laelapids (Acari, Mesostigmata, Laelapidae), 122 were macronyssids (Acari, Mesostigmata, Macronyssidae), 3 were ticks (Acari, Ixodida, Ixodidae), and 15 were fleas (Hexapoda, Siphonaptera, Rhopalopsyllidae). Laelapids presented the highest mean abundance (14.4) and parasitized 95% of the rodents, followed by macronyssids (2.18; 50%), fleas (0.27; 26.80%) and ticks (0.05; 5.55%). The following 3 species of Laelapidae were identified: Androlaelaps misionalis Lareschi (N = 694), Androlaelaps fahrenholzi (Berlese) (N = 94), and Androlaelaps montensis Lareschi (N = 18). Macronyssidae mites were only identified to genera (Ornithonyssus sp.), and fleas and ticks to order level.
Table 1
Sample size of the analyzed specimens of Akodon montensis from the Atlantic Forest ecoregion, Argentina, (captured in August 23-26, 2013), according to their sex, habitat and sexual maturity. Indet. = Indeterminate; BR = borders of vehicular roads, TJ = terraced jungles, SE = streams and their edges; IMM = immature, MAT = mature.
|
|
Habitat |
Sexual maturity |
N |
|
Females N = 21 |
BR = 5 |
IMM = 1 |
1 |
|
MAT = 4 |
4 |
||
|
TJ = 11 |
IMM = 2 |
2 |
|
|
MAT = 9 |
9 |
||
|
SE = 5 |
IMM = 3 |
3 |
|
|
MAT = 2 |
2 |
||
|
Males N = 34 |
BR = 5 |
IMM = 1 |
1 |
|
MAT = 12 |
12 |
||
|
TJ = 11 |
IMM = 0 |
0 |
|
|
MAT = 17 |
17 |
||
|
SE = 5 |
IMM = 0 |
0 |
|
|
MAT = 4 |
4 |
||
|
Indet. N = 1 |
BR = 1 |
indet |
1 |
|
Total = 56 |
Specific richness of laelapids was the same in males and females of A. montensis, while the parasitic burden (721 vs. 228; p = 0.003) and mean abundance of laelapids (612 vs. 194; p = 0.003) was significantly higher in male rodents than in females (Tables 2, 3). On the contrary, parasitic burden (p = 0.65), laelapids mean abundance (p = 0.83) and laelapid specific richness (p = 0.72), may not be related with sexual maturity of females (Table 4). In addition, the two-way analysis of variance, indicated that parasitic burden, mean abundance of laelapids and specific richness of laelapids did not show significant differences related with the habitat of capture of the rodents (p = 0.10, 0.40, and 0.73, respectively), but showed significant differences between males and females for PB and MA (Table 5). Besides, there was no significant correlation between the parasitological parameters (PB, MAL, and SL) and the variables used as surrogates for age (LCC: p = 0.76; 0.82; 0.45 in females; p = 0.17; 0.23; 0.65 in males; and W: p = 0.57; 0.82; 0.45 in females; p = 0.18; 0.11; 0.95 in males) in both females and males (Tables 6, 7).
Discussion
The results support that the sex of the host would be the main factor that modulates the total parasite burden and abundance of the laelapid mites, but not the specific richness. While the size and weight of the hosts (as a proxy for age), as well as reproductive stage of female and site of capture of the rodents, would not affect any of the variables related to the ectoparasites analyzed. Laelapids preferentially feed on tissue fluids from the host. It is highly probable that if the analysis had been carried out only considering obligate hematophagous ectoparasites (such as fleas, ticks and macronyssids), the results could have been different.
Herein, laelapids are the most abundant and prevalent within the ectoparasite community (85% of the total), in agreement with other studies from northeastern and central Argentina and Brazil, where these mites were dominant over macronyssids, fleas, ticks and/or lice (Barros-Battesti et al., 1998; Lareschi & Krasnov, 2010; Lareschi et al., 2019; Sponchiado et al., 2015). Thus, the total parasite burden herein detected may be influenced by laelapids. These mites may inhabit on the fur of their hosts as well as in their nests and in the soil, but the span of time they spend on each of these microhabitats, varies within the different species (Dowling, 2006; Strandtmann & Wharton, 1958). Besides, some laelapids are notably host-specific to small mammal species (Gettinger, 1992; Lareschi & Galliari, 2014), while others, such as A. fahrenholzi, were reported from a variety of mammal and bird species worldwide (Furman, 1972; Standtmann & Wharton, 1958).
Table 2
Means ± standard error and range of parasitic burden (PB, N = 946), mean abundance of Laelapidae (N = 806) (MAL) and specific richness of Laelapidae (SL) in males (N = 35) and females (N = 21) of Akodon montensis from the Atlantic Forest ecoregion, Argentina, August 23-26, 2013.
|
PB |
MAL |
S L |
|
|
Females |
10.86 ± 2.31 |
9.24 ± 2.25 |
1.29 ± 0.16 |
|
(0-43) |
(0-41) |
(0-3) |
|
|
Males |
21.0 ± 2.66 |
17.82 ± 2.18 |
1.56 ± 0.1 |
|
(1-71) |
(0-47) |
(0-2) |
|
|
Indet. |
7 |
6 |
1 |
Table 3
ANOVA between males and females of Akodon montensis (Atlantic Forest ecoregion, Argentina, August 23-26, 2013) for parasitic burden (PB), mean abundance of laelapids (MAL) and specific richness of laelapids (SL), and Levene’s test for homogeneity of variances.
|
|
F |
p |
p test Levene |
|
PB |
9.77 |
0.003 |
0.45 |
|
MAL |
9.55 |
0.003 |
0.53 |
|
SL |
3.14 |
0.08 |
0.77 |
Table 4
ANOVA between sexually mature and immature Akodon montensis females (Atlantic Forest ecoregion, Argentina, August 23-26, 2013), and Levene’s test for homogeneity of variances.
|
|
F |
p |
p test Levene |
|
PB |
0.21 |
0.65 |
0.07 |
|
MAL |
0.05 |
0.83 |
0.17 |
|
SL |
0.13 |
0.72 |
0.48 |
Among laelapids herein identified, the most abundant was A. misionalis (86% of all laelapids). This species is specific of A. montensis, and it is a core species within the component community (Bush et al., 1997; Lareschi, 2010a, 2018). A core species is defined as a common one, with high prevalence and abundance (Bush et al., 1997). Androlaelaps misionalis is included in the Androlaelaps rotundus species group, a complex of morphological similar species, host-specific at species level within sigmodontines from the Akodontini tribe (Lareschi, 2010a, 2018; Lareschi & Galliari, 2014). The high abundance of A. misionalis detected herein may be associated with the abundance of A. montensis in the small mammal community. Within A. misionalis, like other laelapid species, females are dominant in the fur of the hosts, and the colonization of new hosts may take place mainly by contact between rodents (Lareschi & Galliari, 2014). Thus, a densodependent response to the host population may be expected.
Table 5
Two-way ANOVA between males and females of Akodon montensis (Atlantic Forest ecoregion, Argentina, August 23-26, 2013), considering habitat as the second factor of variation in parasitic parameters.
|
PB |
Sum squares |
Gl. |
Mean squares |
F |
p |
|
Sex |
1336 |
1 |
1336 |
7.72 |
0.01 |
|
Habitat |
839.1 |
2 |
419.6 |
2.43 |
0.10 |
|
Interaction |
662.9 |
2 |
331.4 |
1.92 |
0.16 |
|
Inside |
8479 |
49 |
173 |
|
|
|
Total |
1.15E+04 |
54 |
|
|
|
|
MAL |
Sum squares |
gl |
Mean squares |
F |
p |
|
Sex |
956.9 |
1 |
956.9 |
6.80 |
0.01 |
|
Habitat |
263.3 |
2 |
131.6 |
0.94 |
0.40 |
|
Interaction |
214.4 |
2 |
107.2 |
0.76 |
0.47 |
|
Inside |
6897 |
49 |
140.8 |
|
|
|
Total |
8406 |
54 |
|
|
|
|
SL |
Sum squares |
gl |
Mean squares |
F |
p |
|
Sex |
0.9683 |
1 |
0.9683 |
2.61 |
0.11 |
|
Habitat |
0.2356 |
2 |
0.1178 |
0.32 |
0.73 |
|
Interaction |
2.224 |
2 |
1.112 |
3.00 |
0.06 |
|
Inside |
18.18 |
49 |
0.371 |
|
|
|
Total |
21.64 |
54 |
|
|
|
Table 6
Regression coefficients and statistics for females of Akodon montensis (Atlantic Forest ecoregion, Argentina, August 23-26, 2013), between head-body lengths (HBL) and weight (W) with parasitological parameters (PB, MAL and SL).
|
Female rodents |
|
Coef. |
Std. Error |
p |
|
PB |
Const. |
8.54 |
27.85 |
0.76 |
|
|
HBL |
0.12 |
0.39 |
0.76 |
|
|
W |
-0.42 |
0.73 |
0.57 |
|
MAL |
Const. |
12.22 |
27.29 |
0.66 |
|
|
HBL |
0.01 |
0.38 |
0.98 |
|
|
W |
-0.17 |
0.72 |
0.82 |
|
SL |
Const. |
0.22 |
1.88 |
0.91 |
|
|
HBL |
0.02 |
0.03 |
0.48 |
|
|
W |
-0.04 |
0.05 |
0.45 |
Differences reported herein between sexes of A. montensis may be related to the month of the year when the present study took place. In Iguazú National Park, close to the study area, the pregnancy and births of A. montensis take place from September to March (Crespo, 1982). Given that the sampling took place in late August, possibly at that time the males were procuring females for copulation. Some parasites have evolved the ability to detect changes in their host populations to increase their reproductive rates and dispersion during periods in which the hosts would be more gregarious, such as during copulation (horizontal transmission) or during the birth and parental care of offspring (vertical transmission) (Clayton & Tompkins, 1994; Sponchiado et al., 2015). During the reproductive season, the possibilities of intraspecific contact may be high in males when they would be procuring females, while on the contrary, females would spend more time in the nests caring for the young. This different behaviour in rodents of different sexes would benefit the males with the possibility of being colonized more easily by laelapids. The results presented here are consistent with most of the literature that record the preference of ectoparasites for male hosts (Khokhlova et al., 2009; Krasnov et al., 2011; Patterson et al., 2015). However, there are other studies that attribute a greater abundance of ectoparasites to female hosts (e.g., Krasnov et al., 2005). Influence of host sex on ectoparasite communities associated with sigmodontines in Argentinean La Plata River marshes, situated in Pampa ecoregion, was studied. The results showed that while Oxymycterus rufus Fischer and Akodon azarae Fischer presented similar species richness and parasite burden in hosts of both sexes, in Scapteromys aquaticus Thomas and Oligoryzomys flavescens (Waterhouse) these values were higher in males (Lareschi, 2004, 2006, 2010b). However, when every laelapid species was analyzed independently, all core species in every compound community showed higher values of prevalence and mean abundance in male hosts (Lareschi, 2004, 2006, 2010b).
Table 7
Regression coefficients and statistics for males of Akodon montensis (Atlantic Forest ecoregion, Argentina, August 23-26, 2013), between head-body lengths (HBL) and weight (W) with parasitological parameters (PB, MAL and SL).
|
Male rodents |
|
Coef. |
Error Std |
p |
|
PB |
Const. |
-24.03 |
37.09 |
0.52 |
|
|
HBL |
0.62 |
0.43 |
0.17 |
|
|
W |
-0.75 |
0.55 |
0.18 |
|
MAL |
Const. |
-6.36 |
30.16 |
0.83 |
|
|
HBL |
0.43 |
0.35 |
0.23 |
|
|
W |
-0.73 |
0.45 |
0.11 |
|
SL |
Const. |
2.37 |
1.38 |
0.09 |
|
|
HBL |
-0.01 |
0.02 |
0.65 |
|
|
W |
0.00 |
0.02 |
0.95 |
Comparing mite sex and development stages, mainly females of A. misionalis and A. montensis were collected from the fur of the hosts, while most of the males and immatures supposedly remain in nests of their host (Lareschi, 2010a, 2018, 2020). On the contrary, adults of both sexes, as well as immatures of A. fahrenholzi are often found in the fur, as well as in the nest of the hosts or in the soil (Radovsky, 1985; Strandtmann & Wharton, 1958). Thus, juvenile rodents could acquire each of the 3 laelapid species from an early age not only from their mother (vertical transmission), but through the nest (horizontal transmission). This would support the fact that age (here considered by length, weight, relationship between length and weight, and reproductive status), as well as hormonal change related to age, would influence the parasitological variables directly, or indirectly, through an immunological mechanism.
Furthermore, the dominance of A. misionalis, and its preference for the fur of the host rather than the nest (Lareschi, 2010a, 2018), may explain why characteristics related to the site of capture of the rodents may not influence the parasitological parameters of ectoparasites, as detected in this study. Investigations on the effect of locality on ectoparasite parameters have mainly been carried out on large spatial scales, such as across distinct geographic regions (e.g., Krasnov et al., 2006, 2008). Considering close localities, in Argentinean Rio de La Plata marshes a significant effect of locality was observed for some species of laelapids, but not for others (Lareschi & Krasnov, 2010).
Although the results obtained show that the sex of the host is the variable that determines the distribution of the ectoparasites of A. montensis, the mechanisms of the ectoparasite-host relationships are complex and could vary under different conditions. Since the study was carried out over four consecutive days, the effects related to the different seasons of the year, both direct (e.g., hydroperiod and temperature) and indirect (related to the hormonal changes of the hosts) were not analyzed. However, there is evidence that the seasons of the year would not significantly affect the distribution of laelapids in sigmodontines, for example from the Rio de la Plata marshes in Argentina (Lareschi, 2007; Lareschi & Krasnov, 2010) and in the Cerrado in Brazil (Sponchiado et al., 2015).
Since A. montensis is frequently found in disturbed habitats (D’Elía & Pardiñas, 2015) and has been reported of epidemiological importance (Chu et al., 2009; Demoner et al., 2019), comprehensive knowledge of laelapid ecology becomes essential to understand their role in the circulation of diseases in nature. Thus, laelapid richness, distribution and abundance in relationships with individual host and environmental characteristics are relevant aspects that need to be studied to build baseline knowledge required to understand ecological and epidemiological roles of laelapids. In addition, since few studies analyze the effect of host and environment variables on a compound community, we consider that our analysis are relevant to the knowledge of ectoparasites-host-environment interaction.
Acknowledgements
To Ministerio de Ecología, Misiones Province, Argentina, for permission to collect rodents and parasites; to Ulyses Pardiñas (IDEAus, CONICET, Argentina) for his collaboration in identifying individuals of A. montensis; to Guillermo Panisse (CEPAVE), M. Cecilia Ezquiaga (CEPAVE), Jorge Barneche (CEPAVE) and Juliana Sánchez (Centro de Investigaciones y Transferencia del Noroeste de la Provincia de Buenos Aires, CITNOBA, CONICET-UNNOBA), for their help with field work; to M. Laura Morote (CEPAVE) for the figure. The study was supported by PICT2010-924 (to G. Navone), PICT2015-1564 (both Agencia Nacional de Promoción Científica y Tecnológica, Argentina) and N854 (Universidad Nacional de La Plata, Argentina) (both to M. Lareschi).
References
Alonso, R., Ruiz, M., Lovera, R., Montes De Oca, D. P., Cavia, R., & Sánchez, J. P. (2020). Norway rat (Rattus norvegicus) ectoparasites in livestock production systems from central Argentina: Influencing factors on parasitism. Acta Tropica, 203, 105299. https://doi.org/10.1016/j.actatropica.2019.105299
Barros-Battesti, D., Arzua, M., Linardi, P. M., Botelho, J. R., & Sbalqueiro, Y. J. (1998). Interrelationship between ectoparasites and wild rodents from Tijucas do Sul, State of Paraná, Brazil. Memórias do Instituto Oswaldo Cruz, 93, 719–725. https://doi.org/10.1590/S0074-02761998000600003
Begon, M. J., Harper, J. L., & Townsend, C. R. (1997). Ecología, individuos, poblaciones y comunidades. Madrid: Ed. Omega.
Burkart, R., Bárbaro, N., Sánchez, R., & Gómez, D. (1999). Eco-regiones de la Argentina. Secretaría de Recursos Naturales y Desarrollo Sustentable, Administración de Parques Nacionales, Argentina, Buenos Aires. https://sib. gob.ar/archivos/Eco-Regiones_de_la_Argentina.pdf
Bush, A. O., Lafferty, K. D., Lotz, J. M., & Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. Revisited. Journal of Parasitology, 83, 575–583. https://doi.org/10.2307/3284227
Chebez, J. C., & Gil, G. (1993). Misiones hoy. Al rescate de la selva. Nuestras Aves, 29, 5–9.
Chebez, J. C., & Rolón, L. H. (1989). Parque Provincial Urugua-í. Posadas, Misiones: Ministerio de Ecología y Recursos Naturales Renovables de la Provincia de Misiones, Subsecretaría de Ecología.
Chu, Y. K., Goodin, D., Owen, R. D., Koch, D., & Jonsson, C. B. (2009). Sympatry of 2 Hantavirus strains, Paraguay, 2003-2007. Emerging Infection Diseases, 15, 1977–1980. https://doi.org/10.3201/eid1512.090338
Cinti, R. R. (1997). Reserva de Vida Silvestre Urugua-í. La selva del oso. FVSA Vida Silvestre, 57, 4–9.
Clayton, D. H., & Tompkins, D. M. (1994). Ectoparasite virulence is linked to mode of transmission. Proceedings of Royal Society of London, 256, 211–217. https://doi.org/10.1098/rspb.1994.0072
Crespo, J. A. (1982). Ecología de la comunidad de mamíferos del Parque Nacional Iguazú, Misiones. Revista del Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Ecología, 3, 45–162.
Demoner, L. C., Silva, M. R. L., Magro, N. M., & O’Dwyer, L. H. (2019). Hepatozoon milleri sp. nov. (Adeleorina: Hepatozoidae) in Akodon montensis (Rodentia: Cricetidae: Sigmodontinae) from southeastern Brazil. Parasitology, 146, 662–669. https://doi.org/10.1017/S0031182018001956
D’Elía, G., & Pardiñas, U. F. J. (2015). Tribe Akodontini. In J. L. Patton, U. F. J. Pardiñas, & G. D’Elía (Eds.), Mammals of South America, Volume 2. Rodents (pp. 140–279). Chicago, Illinois, The University of Chicago Press. https://doi.org/10.5710/AMGH.v53i4.1
Di Bitetti, M. S., Placci, G., & Dietz, L. A. (2003). Visión de Biodiversidad para el Bosque Atlántico del Alto Paraná: Diseño de un Paisaje de Conservación de la Biodiversidad y Prioridades para las Acciones de Conservación. Washington D.C.: World Wildlife Fund.
Dowling, A. P. G. (2006). Mesostigmatid mites as parasites of small mammals: Systematics, ecology, and the evolution of parasitic associations. In S. Morand, B. R. Krasnov, & R. Poulin (Eds.). Micromammals and macroparasites: from evolutionary ecology to management (pp. 103–118), Tokyo: Springer. https://doi.org/10.1007/978-4-431-36025-4_7
Furman, D. P. (1972). Mites of the family Laelapidae in Venezuela (Acarina: Laelapidae). Brigham Young University Science Bulletin Biological Serie, 17.
Gettinger, D. (1992). Host specificity of Laelaps (Acari: Laelapidae) in central Brazil. Journal of Medical Entomology, 29, 71–77. https://doi.org/10.1093/jmedent/29.1.71
Giraudo, A. H., Belgrano, P. M., Krauczuk, E. R., Pardiñas, U., Miquelarena, A., Ligier, D. et al. (2003). Biodiversity status of the Interior Atlantic Forest of Argentina. In C. Galindo-Leal, & I. de Gusmão Cãmara (Eds.), The Atlantic Forest of South America. Biodiversity status, threats, and outlook (pp. 160–180). Buenos Aires: Island Press.
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica, 4.
Khokhlova, I., Serobyan, V., Krasnov, B., & Degen, A. (2009). Effect of host gender on blood digestion in fleas: mediating role of environment. Parasitology Research, 105, 1667–1673. https://doi.org/10.1007/s00436-009-1608-5
Krasnov, B. R. (2008). Functional and Evolutionary Ecology of fleas: a model for Ecological Parasitology. Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO
9780511542688
Krasnov, B. R., Korallo-Vinarskaya, N. P., Vinarski, M. V., Shenbrot, G. I., Mouillot, D., & Poulin, R. (2008). Searching for general patterns in parasite ecology: host identity vs. environmental influence on gamasid mite assemblages in small mammals. Parasitology, 135, 229–242. https://doi.org/10.1017/S003118200700368X
Krasnov, B. R., Morand, S., Hawlena, H., Khokhlova, I. S., & Shenbrot, G. I. (2005). Sex-biased parasitism, seasonality and sexual size dimorphism in desert rodents. Oecologia, 146, 209–217. https://doi.org/10.1007/s00442-005-0189-y
Krasnov, B. R., Stanko, M., Matthee, S., Laudisoit, A., Leirs, H., Khokhlova, I. S. et al. (2011). Male hosts drive infracommunity structure of ectoparasites. Oecologia, 166, 1099–1110. https://doi.org/10.1007/s00442-011-1950-z
Krasnov, B. R., Stanko, M., Miklisova, D., & Morand, S. (2006). Habitat variation in species composition of flea assemblages on small mammals in central Europe. Ecological Research, 21, 460–469. https://doi.org/10.1007/s11284-005-0142-x
Lareschi, M. (2004). Ectoparásitos asociados a machos y hembras de Oxymycterus rufus (Rodentia: Muridae). Estudio comparativo en la selva marginal del río de La Plata, Argentina. Revista de la Sociedad Entomológica Argentina, 63, 3–4.
Lareschi, M. (2006).The relationship of sex and ectoparasite infestation in the water rat Scapteromys aquaticus (Rodentia: Cricetidae) in La Plata, Argentina. Revista de Biología Tropical, 54, 673–679. https://doi.org/10.15517/rbt.v54i2.13970
Lareschi, M. (2007). Seasonal occurrence of ectoparasites associated with the water rat Scapteromys aquaticus (Muridae, Sigmodontinae) from Punta Lara, Argentina. In J. B. Morales-Malacara, V. Behan-Pelletier, E. Ueckermann, T. M. Pérez, E. Estrada, C. Gispert et al. (Eds.), Acarology XI: Proceedings of the International Congress. (pp. 37–44). Ciudad de México: Instituto de Biología, UNAM/ Sociedad Latinoamericana de Acarología.
Lareschi, M. (2010a). A new species of Androlaelaps Berlese, 1903 (Acari: Parasitiformes) parasitizing an akodontine rodent (Cricetidae, Sigmodontinae) in Northeastern Argentina. Systematic Parasitology, 76, 199–203. https://doi.org/10.1007/s11230-010-9244-0
Lareschi, M. (2010b). Ectoparasite occurrence associated with males and females of wild rodents Oligoryzomys flavescens (Waterhouse) and Akodon azarae (Fischer) (Rodentia: Cricetidae: Sigmodontinae) in Punta Lara’s wetlands, Argentina. Neotropical Entomology, 39, 818–822. https://doi.org/10.1590/S1519-566X2010000500022
Lareschi, M. (2018). Description of the males of Androlaelaps misionalis and Androlaelaps ulysespardinasi (Acari: Parasitiformes: Laelapidae) parasitic of sigmondontine rodents from northeastern Argentina. Journal of Parasitology, 104, 372–376. https://doi.org/10.1645/18-1
Lareschi, M. (2020). Three new species of Laelapidae mites (Mesostigmata) parasitic of species of Akodon (Rodentia, Cricetidae, Sigmodontinae) on the bases of female, male and deutonymph specimens. Veterinary Parasitology, Regional Studies and Reports, 22, 100500. https://doi.org/10.1016/j.vprsr.2020.100500
Lareschi, M., & Galliari, C. (2014). Multivariate discrimination between cryptic mites of the genus Androlaelaps (Acari: Mesostigmata: Laelapidae) parasitic of sympatric akodontine rodents (Cricetidae: Sigmodontinae) in northeastern Argentina: possible evidence of host switch followed by speciation. Experimental and Applied Acarology, 64, 479–499. https://doi.org/10.1007/s10493-014-9839-2
Lareschi, M., & Krasnov, B. R. (2010). Determinants of ectoparasite assemblage structure on rodent hosts from South American marshlands: the effect of host species, locality and season. Medical and Veterinary Entomology, 24, 284–292. http://doi.wiley.com/10.1111/j.1365-2915.2010.00880.x
Lareschi, M., Savchenko, E., & Urdapilleta, M. (2019). Ectoparasites associated with sigmodontine rodents from northeastern Argentina. Therya, 10, 103–108. https://doi.org/10.12933/therya-19-758
Linardi, P. M., & Krasnov, B. R. (2013). Patterns of diversity and abundance of fleas and mites in the Neotropics: host-related, parasite-related and environment-related factors. Medical and Veterinary Entomology, 27, 49–58. https://doi.org/10.1111/j.1365-2915.2012.01025.x
Marshall A. G. (1982). Ecology of insects ectoparasitic on bats. In T. H. Kunz (Ed.), Ecology of bats. Boston, MA: Springer. https://doi.org/10.1007/978-1-4613-3421-7_10
Morand, S., Krasnov, B. R., & Poulin, R. (Eds.). (2006). Micromammals and macroparasites: from evolutionary ecology to management. Tokyo, Japan, Springer-Verlag. https://doi.org/10.1007/978-4-431-36025-4
Morris, P. (1972). A review of mammalian age determination methods. Mammal Review, 2, 69–104. https://doi.org/10.
1111/j.1365-2907.1972.tb00160.x
Pardiñas, U. F. J., D’Elía, G., & Cirignoli, S. (2003). The genus Akodon (Muroidea: Sigmodontinae) in Misiones, Argentina. Mammalian Biology, 68, 129– 43. https://doi.org/10.1078/1616-5047-00075
Patterson, J. E. H., Neuhaus, P., Kutz, S. J., & Ruckstuhl, K. E. (2015). Patterns of ectoparasitism in North American red squirrels (Tamiasciurus hudsonicus): Sex-biases, seasonality, age, and effects on male body condition. International Journal of Parasitology. Parasites and Wildlife, 4, 301–306. https://doi.org/10.1016/j.ijppaw.2015.05.002
Plací, G., & Di Bitetti, M. (2005). Situación ambiental en la Ecorregión del Bosque Atlántico del Alto Paraná (Selva Paranaense). Una visión de biodiversidad para la Ecorregión del Bosque Atlántico del Alto Paraná: diseño de un paisaje para la conservación de la biodiversidad y prioridades para las acciones de conservación. Washington, DC: World Wildlife Fund.
Radovsky, F. J. (1985). Evolution of mammalian mesostigmatid mites. Coevolution of parasitic arthropods and mammals, New York: Wiley.
Radovsky, F. J. (2010). Revision of genera of the parasitic mite family Macronyssidae (Mesostigmata: Dermanyssoidea) of the world. West Bloomfield, MI: Indira Publishing House.
Rolón, L. H., & Chebez, J. C. (1998). Selvas naturales misioneras. Posadas, Misiones: Editorial Universitaria, Universidad Nacional de Misiones.
Sikes, R. (2016). The Animal Care and Use Committee of the American Society of Mammalogists. Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. Journal of Mammalogy, 97, 663–688. https://doi.org/10.1093/jmammal/gyw078
Sponchiado, J., Melo, G. L., Landulfo, G. A., Jacinavicius, F. C., Barros-Battesti, D. M., & Cáceres, N. C. (2015). Interaction of ectoparasites (Mesostigmata, Phthiraptera and Siphonaptera) with small mammals in Cerrado fragments, western Brazil. Experimental and Applied Acarology, 66, 369–381. https://doi.org/10.1007/s10493-015-9917-0
Srur, M., Herrera, J., & Izquierdo, A. (2009). Planificación de las Áreas Protegidas del núcleo norte de la Provincia de Misiones. Directrices Generales de manejo. Anexo II. Identificación de Ambientes. Proyecto Araucaria XXI. Administración de Parques Nacionales, Posadas, Misiones.
Strandtmann, R. W., & Wharton, G. W. (1958). Manual of mesostigmatid mites. Contribution N°4 of the Institute of Acarology. College Park: University of Maryland. https://doi.org/10.1093/aesa/52.5.566