Fish fauna and its environmental relationship in an endorheic basin of Zacatecas, Mexico
Fernando Solís-Carlos a, Gorgonio Ruiz-Campos a, *, María de Lourdes Lozano-Vilano b y Juana Claudia Leyva-Aguilera a
a Facultad de Ciencias, Universidad Autónoma de Baja California, Carretera Transpeninsular Ensenada-Tijuana Núm. 3917, Colonia Playitas, 22860 Ensenada, Baja California, México
b Laboratorio de Ictiología, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Ciudad Universitaria, 66450 San Nicolás de los Garza, Nuevo León, México
*Corresponding author: gruiz@uabc.edu.mx (G. Ruiz-Campos)
Abstract
We studied the fish fauna of the Río Saín Alto (Río Aguanaval endorheic basin) in Zacatecas, Mexico, during the dry (May) and wet (October) seasons of 2011 in order to evaluate changes in the distribution of the species and its relationship with environmental variables. A total of 12 fish species (6 native and 6 exotic) were registered. The longitudinal distribution of the species in the river during both seasons combined was 2 native species (Gila conspersa and Catostomus nebuliferus) with ample distribution in the river (upper, middle and lower zones), 2 native species (Campostoma ornatum and Notropis nazas) in 2 zones of the river (middle and lower), 4 species (2 native: Astyanax mexicanus and Etheostoma pottsii, and 2 exotic: Cyprinus carpio and Oreochromis sp.) in the middle zone of the river, and 4 exotic species (Gambusia affinis, Lepomis cyanellus, Lepomis macrochirus, and Micropterus salmoides) with distribution confined to the lower zone of the river, especially in reservoirs. Species richness decreased with altitude and after a flooding event. The factor analysis model determined that both altitude and flow velocity were the factors that best explained the variation in the distribution of fish species during the rainy season, especially for the exotic component.
Keywords:
Fishes; Aguanaval basin; Distribution; Composition; Environmental factors
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Ictiofauna y su relación ambiental en una cuenca endorreica de Zacatecas, México
Resumen
Se investigó la ictiofauna del río Saín Alto (cuenca endorreica río Aguanaval) en Zacatecas, México, durante las estaciones seca (mayo) y de lluvias (octubre) del 2011, con el objetivo de evaluar los cambios en la distribución longitudinal de las especies y su relación con las variables ambientales. Se registró un total de 12 especies (6 nativas y 6 exóticas). La distribución longitudinal de las especies en el río durante ambas estaciones combinadas fue de 2 especies nativas (Gila conspersa y Catostomus nebuliferus) con distribución amplia en el río (zonas alta, media y baja), 2 especies nativas (Campostoma ornatum y Notropis nazas) en 2 zonas del río (media y baja), 4 especies (2 nativas: Astyanax mexicanus y Etheostoma pottsii, y 2 exóticas: Cyprinus carpio y Oreochromis sp.) en la zona media del río, y 4 especies exóticas (Gambusia affinis, Lepomis cyanellus, Lepomis macrochirus y Micropterus salmoides) confinadas a la zona baja del río, especialmente en reservorios. La riqueza de especies disminuyó con la altitud y después de un evento de inundación. El modelo de análisis de factores determinó que la altitud y la velocidad del flujo fueron las variables ambientales que mejor explicaron la variación en la distribución de las especies durante la estación de lluvias, especialmente para el componente exótico.
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Peces; Cuenca Aguanaval; Distribución; Composición; Factores ambientales
Introduction
The spatial-temporal distribution of fish populations through rivers and streams, as well as their causal factors, has been the object of numerous studies on ecology of communities in North America during the last decade (Frimpong & Angermeier, 2010). Fishes are one of the best examples of this research topic due to their strict dependence on the aquatic environments and their relationships with specific environmental factors within each hydrological basin. These conditions make them excellent indicators of the health status and functioning of rivers (Contreras-Balderas et al., 2005; García-De León et al., 2018; Hocutt & Stauffer, 1980; Lozano-Vilano et al., 2009; Lyons et al., 1995, 2000; Mercado-Silva et al., 2006; Schmitter-Soto et al., 2011).
Several biotic and abiotic factors have been determined as causal agents of the composition and longitudinal distribution of fish species in river and stream ecosystems, such as altitude (Quist et al., 2005), water chemistry (Castillo-Rivera & Zárate-Hernández, 2001; Cech et al., 1990; Facey & Grossman,1990), temperature (Hugueny et al., 2010; Mejía-Mojica et al., 2015), habitat availability and heterogeneity (Castillo-Rivera & Zárate-Hernández, 2001; García-De León et al., 2018; Gido et al., 1997; Trujillo-Jiménez et al., 2010), river hydrodynamics (Olden & Kennard, 2010; Pearsons & Li, 1992; Tedesco & Hugueny, 2006), and the interaction with exotic fishes (Bomford et al., 2010; Olden et al., 2006; Ruiz-Campos et al., 2006). Over time, the integration and understanding of the effects of all these factors has led to the formulation of different hypothesis and terminologies on the ecological study of fish communities in streams and rivers (Frimpong & Angermeier, 2010). One of the hypotheses that explains the changes in fish composition is allusive to the idea that environmental heterogeneity along the river is the main causal factor that drives changes in the fish assemblage (Petry & Schultz, 2006).
Despite the different studies mentioned above, a high number of studies concerning the evaluation of the specific relationships of fish communities with the types of habitats, connectivity, alpha and beta biodiversity in rivers, and loss of these attributes by human activities are still needed (cf. Mercado-Silva et al., 2006). Such studies are relevant in predicting the behavior of biological communities, and in the formulation of conservation policies, restoration works, and mitigation measures (Quist et al., 2005; Sánchez, 2007).
In this study, we analyzed the longitudinal distribution of fishes in the Río Saín Alto (Central Mexican Plateau) and its relationship with environmental factors during 2 contrasting climatic conditions (dry and rainy season) of 2011, in order to identify the main causal environmental factors of the distribution of fish species in this endorheic basin.
Materials and methods
This study was carried out in the main course of the Río Saín Alto (RSA), a major tributary of the Río Aguanaval, endorheic basin in the state of Zacatecas, Mexico (Fig. 1). Their geographical coordinates are 19°34’28.36” N, -103°14’43.79” W, with an altitudinal range from 1,912 to 2,867 m asl. This river has a drainage area of 1,162.64 km2 (Inegi, 2010a, b) and an approximate length of 50 km from its beginning until the confluence with the Río Trujillo, forming the Río Aguanaval (CNA, 2009). The RSA drainage pattern is dendritic (FISRWG, 1998) with a pattern of drying in the headwaters (Lake, 2003) and a maximum level of branching (Strahler’s classification) of seventh order (Inegi, 2010b) that is reached where the main river confluences with its major tributary, the Río Frío. Thereafter, the flow of this river becomes permanent throughout the year.
The prevailing climate in the area is template semi-arid with rains in summer and warm sub-humid conditions in the mountains. The annual average rainfall and temperature is between 400-700 mm and 12-18 °C, respectively (Inegi, 2010a). It is important to note the occurrence of a severe drought in the study region during 2011, which decreased the annual average precipitation approximately 50% (Albanil-Encarnación et al., 2011), causing a very low flow event during the dry and rainy season. The riparian zone is mainly represented by Mexican pinyon (Pinus cembroides), oak (Quercus eduardii), yucca (Yucca discipiens), and mule-fat (Baccharis salicifolia) in the upper part of the basin. Other riparian species such as willow (Salix nigra), cottonwood (Populus spp.) and mule-fat are widely distributed along the river. Aquatic plants occur mainly in the middle and lower parts of the basin, represented by Chara sp., Hydrocotyle ranunculoides, Lemna gibba, Potamogeton sp., Azolla sp., Nostoc sp. and Eleocharis montevidensis.
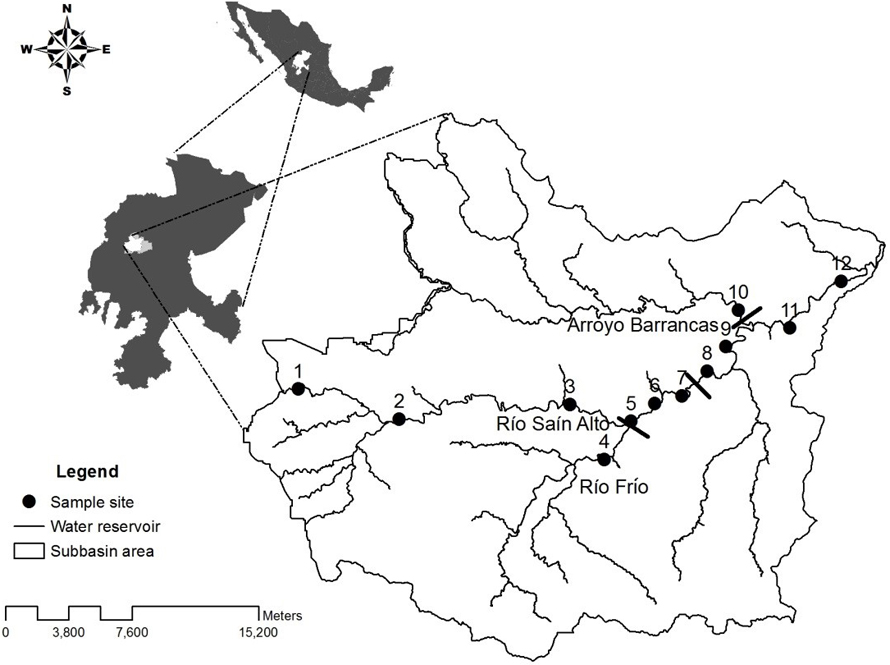
Two sampling events for fish and environmental variables were carried out at 12 sites along a segment of 40 km of the RSA during 2 contrasting seasons in 2011 (dry season in May and rainy season in October), based on the presence of water and fish (Fig. 1). The sampling sites were located in 3 zones along the river based on physiography and elevation (Solís-Carlos, 2013) as follows: upper zone (2,161 to 2,245 m asl) represented by mountain biotopes (sites of Arriba de Luis Moya [LM] and Los Sauces [LS]); middle zone (1,991 to 2,064 m asl) characterized by alluvial valleys or plateaus (sites of Emiliano Zapata [EZ], Miguel Hidalgo [MH], Puente Atotonilco [PA], Saín Alto [SA], La Boquilla [LB], and El Castro [EC]); and lower zone (1,952 to 1,997 m asl) represented by low hills (sites of El Alamillo [EA], Crucero a Barrancas [CB], Saín Bajo [SB], and La Laborcita [LL]).
Water physical-chemical variables such as conductivity (Cond; mS/cm), salinity.
(Sal, ppm), pH, total dissolved solids (TDS, g/l) and temperature (T, °C) were measured in 3 points of each site of sampling using a Hydrolab Scout 2 multi-analyzer (Hydrolab Co., Austin, TX). The physiographic characteristics of the river and riparian zone including turbidity, dominant bottom type and percentage of riparian vegetation cover were visually determined and scored on the basis of the following qualitative criteria: turbidity (clear = 1, turbid = 2), dominant substrate (sand = 1, gravel = 2, pebbles = 3, bedrock = 4), and percentage of riparian vegetation cover (0-100%). Flow velocity was measured in m/s with a Swoffer model 2100 current velocity meter and scored by ranges as follows: zero (0.0 = 1), slow (0.01-0.20 = 2), moderate (0.21-0.40 = 3) and fast (> 0.40 = 4). The riparian vegetation cover was quantified for trees and shrubs, but not for seasonal grasses or herbs. Other variables measured in situ were channel width (m) and depth (m) as well as altitude (m asl). The values of all these environmental variables for each sampling site in the study area are presented in appendix 1.
The fishes were collected by means of the combined use of a minnow seine (3 m long × 1.5 m high and 0.5 cm mesh size) for shallow habitats (riffles, runs and pools < 1.5 m deep) and minnow traps (n = 3) for sites with depths > 1.5 m. In the sampling event of October 2011 (rainy season), in most of the sampled sites for fishes, the catches were not standardized by types of fishing gear, situation that complicated the quantitative comparison among sites. Therefore, we presented the species composition for this season in terms of presence or absence. For the sampling event in May 2011 (dry season), at each site an area of 6 × 50 m was sampled with the minnow seine during an average effort time of 30 min; while 3 minnow traps were deployed during 24 h. The abundance of each species by sampled site is expressed as percent of the total capture (all the species combined).
Fish specimens collected were fixed in 10% formalin (neutralized with sodium borate), and after 7 days they were placed in water for 24 h, and finally preserved in 50% isopropanol. The taxonomic identification of the species was based on Trautman (1981) and Miller et al. (2005). The fish material was cataloged and deposited in the Fish Collection of the Universidad Autónoma de Baja California (UABC) at Ensenada, Baja California, México.
The nomenclature and systematic arrangement of species follows Page et al. (2013). The current conservation status of the species was based on the Mexican Official Policy for the protection of wild flora and fauna (Semarnat, 2010).
Based on the qualitative nature of the data obtained in the present study, as well as the type of variables considered here, an exploratory multivariate analysis was used to process this information. The species similarity among sites was calculated using the Bray-Curtis index, based on the presence-absence of species; the result matrix was used to generate clusters in mode complete linkage of PRIMER software version 5.2.9 (Clarke & Gorley, 2001). Subsequently, a factor analysis (FA) by means of the method of extracting main components was performed to identify the environmental factors that are involved in explaining the presence or absence of fish species in the study area for each contrasting season. Although this method is very similar to the principal component analysis (PCA), it differs in the variance that is taken account; in this respect, PCA assumes that all common variance is explained by the output components, while FA split up common and unique variance (variable specific variance and error variance). Additionally, FA also allows for the rotation and definition of the structure of the output data, facilitating their interpretation (Johnson, 2000; Rietveld & Van Hout, 1993).
This analysis was developed using Statistica 7.0 software (StatSoft, Inc., Tulsa, OK, 2002), and was based on width and depth of the river, river order, altitude, substrate, turbidity, percentage of riparian vegetation, water physicochemical variables (temperature, pH, conductivity, TDS, and salinity), current velocity, and presence or absence of fish species. Raw data were preferred to avoid the loss of significance of variables, since the statistical program (Statistica 7.0) works on the correlation matrix of the variables and on standardized data (Johnson, 2000); likewise, the factors were subject to rotation using the varimax method.
Results
During the fish samplings along the main course of the RSA, a total of 12 species (6 native and 6 exotic) belonging to 7 families were recorded. The native species were Campostoma ornatum, Gila conspersa, Notropis nazas, Catostomus nebuliferus, Astyanax mexicanus, and Etheostoma pottsiii; while exotic or non-native species were Cyprinus carpio, Gambusia affinis, Lepomis cyanellus, Lepomis macrochirus, Micropterus salmoides and Oreochromis sp.
The longitudinal distribution of the species in the river based on presence or absence during the May and October 2011 sampling events, was of combined manner as follows: 2 native species (G. conspersa and C. nebuliferus) occurring through the 3 zones of the river (upper, middle and lower), 2 native species (C. ornatum and N. nazas) distributed in 2 zones of the river (middle and lower), 4 species (2 native A. mexicanus and E. pottsiii, and 2 exotic C. carpio and Oreochromis sp.) occurring only in the middle zone of the river, and 4 exotic species (G. affinis, L. cyanellus, L. macrochirus and M. salmoides) with distribution confined to the lower zone of the river, especially in reservoirs (Table 1).
In the sampling event of May 2011 (dry season) in La Laborcita site (LL), 2 types of isolated pools were sampled and analyzed separately, a large and deep pool (LL-a) with dominance of exotic fishes, and a small and shallow pool (LL-b) with dominance of native fishes (Table 1).
During the sampling event of October 2011 (rainy season), the most abundant species were the native species; Gila conspersa, C. nebuliferus, and N. nazas with 38.7%, 23% and 14.4%, respectively (Table 1). Other species had abundances of 10.4% (G. affinis), 5.7% (L. cyanellus), 3.4% (C. ornatum) and 3.1% (E. pottsiii); the remaining species had abundances below 1% (Table 1).
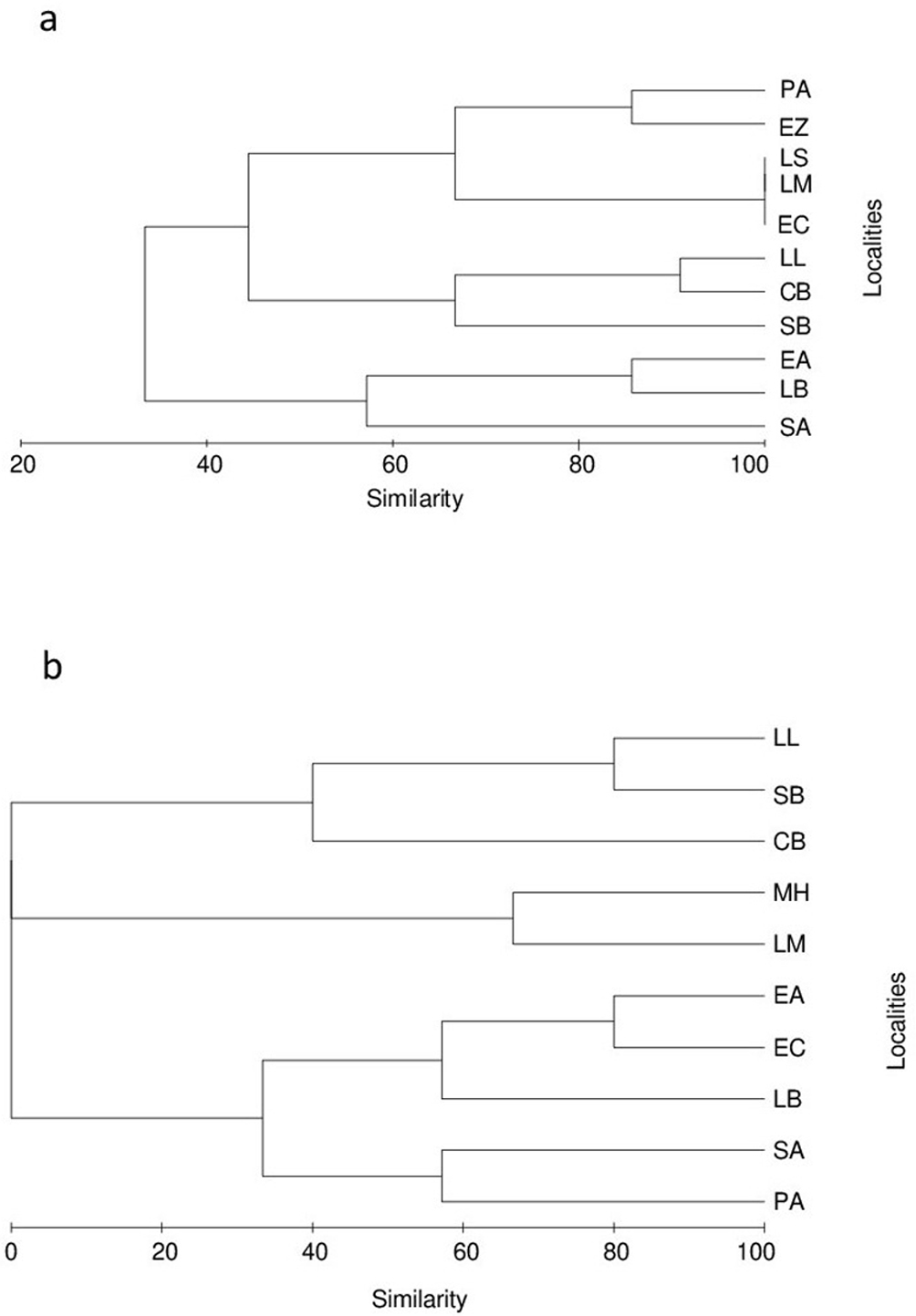
Cluster analysis of similarity among sampling sites (Fig. 2), based on presence-absence of species during May (dry season) (Fig. 2a), showed the formation of 2 different groups at a 40% similarity level. The first group composed by 2 sites of the middle zone (LB and SA) plus one site of the lower zone (EA). The second group was formed by the 2 subgroups, being the first subgroup composed by 2 sites of the upper zone (LM and LS) and 3 sites of the middle zone (PA, EZ and EC). In this first subgroup, 3 sites (LS, LM and EC) showed 100% similarity sharing native species such as G. conspersa and C. nebuliferus; in the case of the PA and EZ sites, both shared 85% of the species, including the native N. nazas. The second subgroup was represented by 3 sites of the lower zone (LL, CB and SB) sharing more than 60% of species, especially exotic species as L. cyanellus and L. macrochirus.
In October (rainy season), the species similarity among sites was very different compared with that of May (dry season). Three different major groups are distinguished in the tree diagram (Fig. 2b), being the first group formed by 2 sites of the lower zone (LL and SB, 80% of similarity), a second group composed by 1 site of the upper zone (LM) and another of the middle zone (MH) with 65% of similarity, and a third group dominated by sites of the middle zone (EC, LB, SA and PA) plus 1 site of the lower zone (EA) with similarities among them lesser than 60%. In most sites, at least 1 species was absent, and even, at ones as LS and EZ, there was absence of fish. Another important difference was the new record of the Mexican tetra, A. mexicanus, at PA and MH sites. The presence of exotics such as C. carpio and Oreochromis sp. was recorded at SA and LB, and at La Boquilla and El Castro sites, respectively.
Table 1
Composition of fish species by collecting sites along the Río Saín Alto, Zacatecas, Mexico, during the dry (May) and rainy (October) season of 2011. See description of abbreviations of sites in figure 1. Relative abundance of species per collecting site was calculated only for May, while in October includes data on presence of species. *No relative abundance was estimated for the location and species. La Laborcita site (LL) classified in 2 types of habitat: LL-a= large and
deep pool with dominance of exotic fishes, and LL-b= small and shallow pool with dominance of native fishes.
|
Upper Zone |
Middle Zone |
Lower Zone |
||||||||||||||||||||||||||
|
Site |
LM |
LS |
EZ |
MH* |
PA |
SA |
LB |
EC |
EA |
CB |
SB |
LL |
LL (a) |
LL (b) |
Abun. Rel. |
|||||||||||||
|
Date of Sampling |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
May/Oct |
Total (%) |
|||||||||||||
|
C. ornatum |
X |
X |
X |
X |
X |
X |
X |
|||||||||||||||||||||
|
Relative abundance |
3.7 |
29.4 |
1.7 |
2.9 |
5.3 |
5.8 |
3.4 |
|||||||||||||||||||||
|
G. conspersa |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
||||||||||
|
Relative abundance |
76.1 |
70.1 |
60.4 |
10.5 |
22.1 |
69.4 |
76.3 |
3.7 |
10.2 |
39.5 |
43.5 |
38.7 |
||||||||||||||||
|
N. nazas |
X |
X |
X |
X |
X |
X |
X |
X |
X |
|||||||||||||||||||
|
Relative abundance |
3.8 |
84.2 |
7.4 |
13.2 |
43.9 |
9.5 |
14.5 |
15.9 |
14.4 |
|||||||||||||||||||
|
C. nebuliferus |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
X |
||||||||||
|
Relative abundance |
23.9 |
29.9 |
35.8 |
2.6 |
7.4 |
35.3 |
30.6 |
22 |
4.9 |
19.5 |
32.9 |
28.6 |
33.3 |
23.0 |
||||||||||||||
|
A. mexicanus* |
X |
X |
||||||||||||||||||||||||||
|
E. pottsi |
X |
X |
X |
X |
||||||||||||||||||||||||
|
Relative abundance |
2.6 |
81.5 |
3.1 |
|||||||||||||||||||||||||
|
C. carpio* |
X |
X |
||||||||||||||||||||||||||
|
G. affinis |
X |
X |
X |
X |
||||||||||||||||||||||||
|
Relative abundance |
5.5 |
10.4 |
||||||||||||||||||||||||||
|
L. cyanellus |
X |
X |
X |
X |
X |
X |
X |
X |
||||||||||||||||||||
|
Relative abundance |
46.3 |
5.3 |
42.8 |
1.4 |
5.7 |
|||||||||||||||||||||||
|
L. macrochirus |
X |
X |
X |
X |
||||||||||||||||||||||||
|
Relative abundance |
1.2 |
1.5 |
2.6 |
28.6 |
0.7 |
|||||||||||||||||||||||
|
M. salmoides |
X |
|||||||||||||||||||||||||||
|
Relative abundance |
2.9 |
0.5 |
||||||||||||||||||||||||||
|
Oreochromis sp.* |
X |
X |
||||||||||||||||||||||||||
|
Total of species |
2 |
1 |
2 |
0 |
3 |
0 |
0 |
2 |
4 |
4 |
4 |
3 |
4 |
5 |
2 |
3 |
3 |
2 |
5 |
2 |
7 |
3 |
6 |
2 |
3 |
2 |
5 |
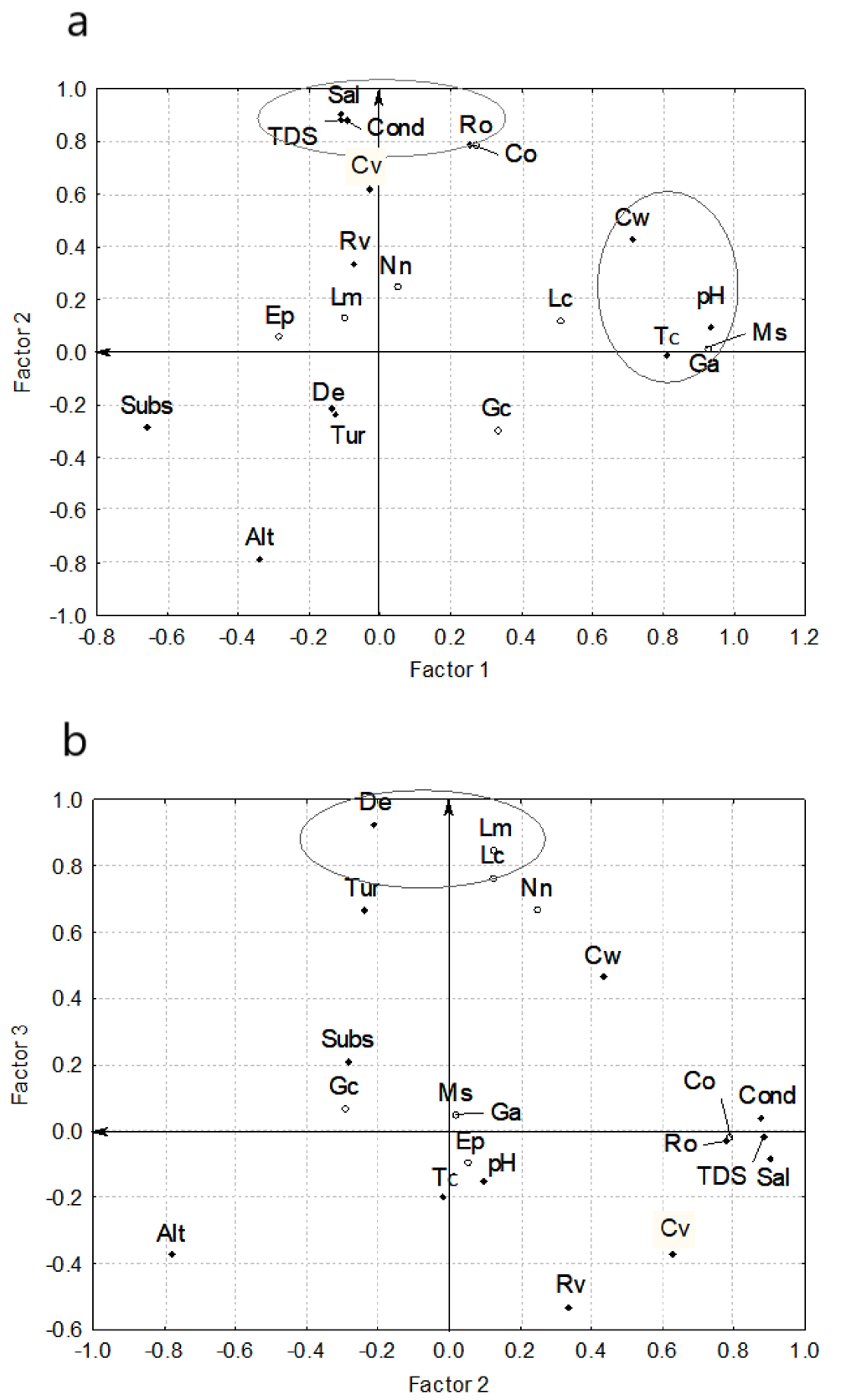
The multivariate analysis resulted in the extraction of 3 main factors to the set of variables analyzed for the fish sampling of May 2011 (Fig. 3), giving a cumulative variance of 14.22 that represents 67.7% of the total cumulative variance. These 3 factors extracted explained 24%, 25%, and 19% of the total variance. Considering a load value (correlation) ≥ 0.7 for factor 1, the set of variables significantly correlated were channel width (0.71), pH (0.94), temperature (0.81), M. salmoides (0.92) and G. affinis (0.92); while for factor 2, 6 variables were significantly correlated (altitude, -0.78; conductivity, 0.88; salinity, 0.91; total dissolved solids, 0.88; river order, 0.78; and presence of C. ornatum, 0.79) (Fig. 3a); finally for factor 3, 3 variables were significantly correlated (depth, 0.93; L. macrochirus, 0.85; and L. cyanellus, 0.77) (Fig. 3b).
Summarizing these results, we can interpret the following trends: a) as the channel width increases, pH, temperature and occurrences of G. affinis and M. salmoides also increase; b) at lower altitudes, conductivity, salinity, total dissolved solids, river order and the occurrence of C. ornatum increased, and c) as the depth of river increases, the probability of occurrence of fishes of lentic habitats such as L. macrochirus and L. cyanellus also increases.
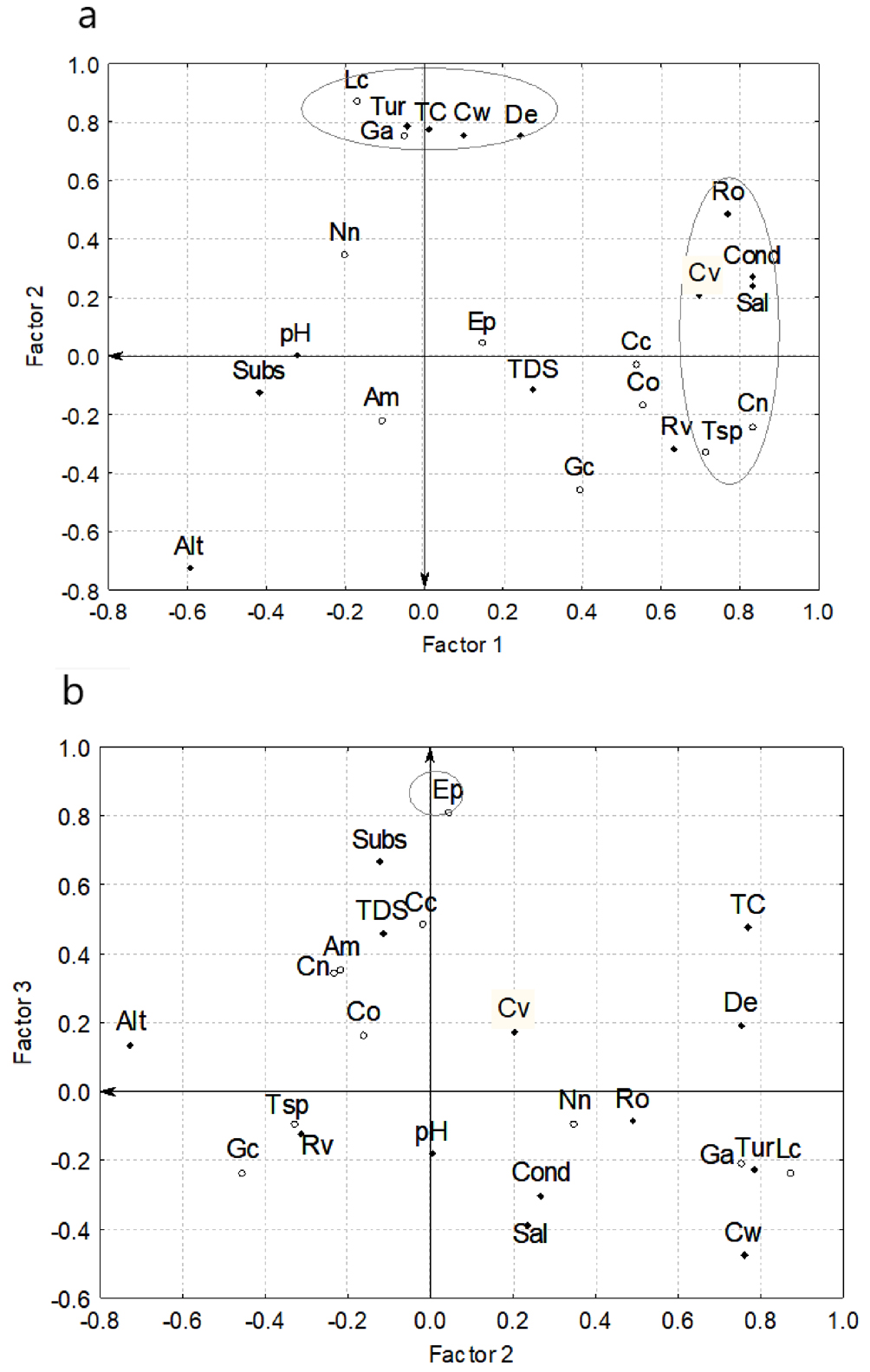
Analysis of October 2011 sampling (Fig. 4), showed a cumulative variance of 13.89, which means 60% of the total variance. The first extracted factor explained 25% (5.72), the second 23% (5.3), and the third 12% (2.86). The set of variables correlated for each factor were as follows: river flow velocity (0.7), conductivity (0.83), salinity (0.83), river order (0.77), C. nebuliferus (0.83) and Oreochromis sp. (0.71) for factor 1; turbidity (0.79), channel width (0.76), L. cyanellus (0.87) and G. affinis (0.75) for factor 2 (Fig. 4a); and E. pottsiii (0.81) as a single variable or tribal factor (Fig. 4b). Integrating all these results we can infer that: a) as the river order increases, the flow velocity, conductivity and salinity also increase of concomitant manner with the presence of C. nebuliferus and Orecochromis sp.; b) as the water turbidity and channel width increases, the presence of exotic species (L. cyanellus and G. affinis) also increase, and c) the environmental variables in the site of distribution of E. pottsii are notably different than in other sites of the river where this darter is absent.
Discussion
The native fish fauna of Río Saín Alto (RSA), Zacatecas is composed of 6 species, of which 5 (C. ornatum, G. conspersa, N. nazas, C. nebuliferus, and E. pottsiii) have been previously reported for this sub-basin, except the Mexican tetra (A. mexicanus) (cf. Miller et al., 2005; Salas-Martínez, 1971; Solís-Carlos et al., 2009). Most of species recorded in the RSA are shared with other basins from northern Mexico, such as those of the Yaqui, Mayo, Tunal and Conchos rivers among others (Contreras-Balderas, 1978; Hendrickson et al., 1980; Lozano-Vilano et al., 2009; Miller et al., 2005; Varela-Romero & Hendrickson, 2009).
Two of the species (N. nazas and G. conspersa) registered in the RSA are endemic to the Nazas-Aguanaval system (Miller et al., 2005), where G. conspersa along with E. pottsii and C. nebuliferus are considered to be categorized as threatened status by the Mexican Official Norm (Semarnat, 2010).
Based on the presence-absence of species along the 3 different zones of RSA (upper, middle and lower), the higher species richness was observed in the middle and lower zones during the rainy season (sampling event of October 2011), and a wide variation in the presence-absence of species was notable for all 3 zones after the rainy season. In the same way, the results of factor analysis (FA) indicated that the variables of altitude and flow velocity were the main factors explaining the longitudinal distribution of species in the river. This behavior has been previously described by some authors such as Quist et al. (2005), Trujillo-Jiménez et al. (2010), Gido et al. (1997) and Grossman et al. (2010), who pointed out these same variables along with others (availability and habitat heterogeneity, and temperature) as predictors of the distribution and structure of the fish community in streams and rivers.
The upper zone of RSA was dominated by 2 native species, G. conspersa and C. nebuliferus, which are typical forms in mountain streams (Bond, 1996; Miller at al., 2005) and better adapted to the wide seasonal changes in flow and temperature. Myrick and Cech (2000) showed that suckers such as Catostomus occidentalis were less affected by changes in temperature in comparison to other native species in the Sacramento River, California. In addition, both G. conspersa and C. nebuliferus have the largest and most hydrodynamic bodies of the 6 native species that inhabit the Sacramento River, features that seems to allow them a better swimming performance against the current (Cech et al., 1990; Chun et al., 2011) and thus can reach sites upstream.
In the middle zone of RSA, the habitat heterogeneity increases (Bond, 1996; García-De León et al., 2018; Sánchez, 2007), a condition that promotes the presence of all native fish species, especially from the point where the river becomes a seventh order stream and maintains a constant flow throughout the year. Similarly, Gido et al. (1997) found that the fish community in secondary channels of the San Juan River (New Mexico, USA) was more diverse in areas where habitat conditions were more stable and diverse. Likewise, García-De León et al. (2018) argued that the fish diversity increased in the Río Guayalejo system from northeastern Mexico as a function of habitat availability and heterogeneity. It is important to note that native darter, E. pottsii, known to occur in very specific habitats in northern Mexico (Miller et al., 2005) is confined to a segment of the RSA between the sites of Puente Atotonilco and Saín Alto, where the habitat conditions are suitable for this species. Therefore, it is necessary to implement measures for the conservation of this threatened fish and its habitat (Semarnat, 2010).
Another species with confined distribution in the RSA, is A. mexicanus, which was recorded for the first time in this sub-basin of the Río Aguanaval in the sites of Puente Atotonilco and Miguel Hidalgo. Its low abundance and distribution is indicative of very specific habitat requirements and of low tolerance to environmental changes. This hypothesis fits with that of Solís-Carlos et al. (2009), who reported it at 2 sites of the Río Aguanaval of Zacatecas, downstream of the junction with RSA, in areas with low environmental degradation.
On the other hand, the occurrence of exotic species in this area as common carp (Cyprinus carpio) in Saín Alto and La Boquilla sites, as well as Oreochromis sp. in La Boquilla and El Castro, during sampling of October 2011, might be explained due to the presence of artificial reservoirs and/or the dispersal of individuals via flooding during the rainy season. A similar case was observed by Ruiz-Campos et al. (2006) in the Río San Ignacio basin of Baja California (Mexico), who documented that the dispersal of exotic fishes along the main river was promoted by flooding events during periods of hurricanes.
The species C. ornatum was recorded in high number at the site of La Boquilla, which is located on a plateau area that favors the presence of riffles and runs with high flows along the year, representing the preferred habitats for these species (Burr, 1976; Miller et al., 2005; Solís-Carlos et al., 2009). The absence of this species in the upper zone of the RSA was notable during dry and rainy seasons.
On the other hand, N. nazas showed a high abundance both in the middle and lower zones of the RSA, at the sites of Puente Atotonilco and Crucero Barrancas. In this last site, where the construction of a dam promotes pool habitats, high abundances of the native minnow were detected in spite of the presence of exotic green sunfish (L. cyanellus) that is considered as a voracious carnivore (Miller et al., 2005) that might potentially predate on N. nazas. A possible explanation for the above is the segregation of habitat, while L. cyanellus were mainly confined to deepest pools, N. nazas were confined to shallowest pools, avoiding the predator-prey interaction. A different scenario was observed for the native species G. conspersa and C. nebuliferus in this same site, whose low abundances suggest a high degree of predation by exotic species. Dudley and Matter (2000) found a similar situation in populations of Gila intermedia at sites in the Sabino Creek, Arizona (USA), where L. cyanellus could be found. Their results showed that populations of G. intermedia were abundant in the streams with the absence of this predator (L. cyanellus), whereas in areas with this predator, only adult specimens of G. intermedia were present.
The presence of exotic species in the middle and lower zones of the RSA is key evidence of the important impact on the habitat and native fish community caused by the construction of hydraulic works such as levees and dams (Havel et al., 2005). These works promote unfavorable habitat conditions for native species, increasing depth, reducing stream flow, increasing temperatures and promoting sedimentation, all of which facilitates the entry and establishment of exotic species with greater tolerance to degradation (Johnson et al., 2008; Olden & Poff, 2005). This relationship was evident in the results of the FA, where M. salmoides, G. affinis, L. cyanellus and L. macrochirus were the species that showed the highest correlations with the variables of channel width, pH, temperature and depth.
In summary, the structure of fish communities in the RSA is mainly determined by variables related to the altitudinal gradient and flow velocity for native species or impound and hydraulic infrastructure for exotic species. Finally, the RSA should be a conservation priority area because of its importance as a potential gene bank ideal for future stocking of fish in the Río Aguanaval basin due to the high number of species in common.
Acknowledgements
To Consejo Zacatecano de Ciencia y Tecnología (Cozcyt), Cuerpo Académico Estudios Relativos a la Biodiversidad (Universidad Autónoma de Baja California), and the Red de Especies Exóticas de México (UANL-UABC-Umar) for the funds provided to this study. Asunción Andreu-Soler and Jorge A. Contreras-Lozano for the support during the fish sampling in May and October 2011, respectively. Evarista Arellano-García helped in the statistical analysis. Finally, to the Universidad Autónoma de Zacatecas and the Mayor’s office of the Municipality of Saín Alto, Zacatecas for the logistic support.
Appendix 1. Environmental variables measured in the Río Saín Alto, Zacatecas, Mexico, during dry (May) and rainy (October) of 2011. Unities for each variable are: temperature (Temp., °C), conductivity (Cond., mS/cm), salinity (Salin., ppt), total of dissolved solids (TDS), turbidity (Turbid., clear = 1, and turbid = 2), current velocity (Cv) as zero (0.0 = 1), slow (0.01-0.20 = 2), moderate (0.21-0.40 = 3) and fast (> 0.40 = 4); depth (m), substratum (sand= 1, gravel= 2, pebbles= 3, and bedrock= 4), percent of riparian vegetation cover (% Rip.veg.), channel width (Chann. Width, m), altitude (Altit., m asl), and stream order (Strahler’s classification). Measurements of water quality are expressed as average of 3 measurements. * = absence of water.
|
Environmental variables |
||||||||||||||
|
Sites |
Month |
Temp. |
Cond. |
Salin. |
pH |
TDS |
Turbid. |
Cv |
Depth |
Subst. |
% Rip. veg. |
Chann. width |
Altit. |
Riv. order |
|
Luis Moya |
May |
18.6 |
0.3 |
0.2 |
8.4 |
0.2 |
1.0 |
2.0 |
0.5 |
4.0 |
30.0 |
10.0 |
2245.0 |
4.0 |
|
Oct. |
11.7 |
0.2 |
0.1 |
7.6 |
0.2 |
1.0 |
2.0 |
0.3 |
||||||
|
Los Sauces |
May |
20.7 |
0.0 |
0.1 |
8.4 |
0.0 |
1.0 |
2.0 |
0.1 |
2.0 |
90.0 |
6.0 |
2161.0 |
6.0 |
|
Oct. |
17.6 |
0.2 |
0.1 |
7.3 |
0.4 |
1.0 |
1.0 |
0.3 |
||||||
|
Emiliano Zapata |
May |
23.3 |
0.4 |
0.2 |
8.1 |
0.3 |
2.0 |
1.0 |
0.6 |
3.0 |
40.0 |
16.0 |
2064.0 |
6.0 |
|
Oct. |
15.1 |
0.4 |
0.2 |
7.5 |
0.3 |
2.0 |
1.0 |
0.4 |
||||||
|
Puente Atotonilco |
May |
23.2 |
0.2 |
0.1 |
8.7 |
0.3 |
1.0 |
2.0 |
0.5 |
3.0 |
50.0 |
12.0 |
2049.0 |
6.0 |
|
Oct. |
21.8 |
0.4 |
0.2 |
7.9 |
0.8 |
1.0 |
2.0 |
0.6 |
||||||
|
Saín Alto |
May |
17.6 |
0.5 |
0.3 |
8.1 |
0.3 |
2.0 |
3.0 |
0.4 |
3.0 |
80.0 |
15.0 |
2033.0 |
7.0 |
|
Oct. |
14.2 |
0.3 |
0.2 |
7.4 |
0.3 |
1.0 |
2.0 |
0.7 |
||||||
|
La Boquilla |
May |
16.2 |
0.6 |
0.3 |
8.3 |
0.4 |
1.0 |
3.0 |
0.1 |
3.0 |
85.0 |
12.0 |
1991.0 |
7.0 |
|
Oct. |
19.0 |
0.4 |
0.2 |
7.5 |
0.3 |
1.0 |
3.0 |
0.7 |
||||||
|
El Castro |
May |
22.7 |
0.7 |
0.3 |
8.5 |
0.4 |
1.0 |
3.0 |
0.1 |
1.0 |
90.0 |
11.0 |
2035.0 |
7.0 |
|
Oct. |
18.1 |
0.5 |
0.3 |
7.2 |
0.4 |
1.0 |
2.0 |
0.5 |
||||||
|
El Alamillo |
May |
26.7 |
0.7 |
0.4 |
8.9 |
0.5 |
2.0 |
3.0 |
0.1 |
1.0 |
95.0 |
25.0 |
1983.0 |
7.0 |
|
Oct. |
13.4 |
0.6 |
0.3 |
7.3 |
0.4 |
1.0 |
2.0 |
0.5 |
||||||
|
Crucero a Barrancas |
May |
18.8 |
0.5 |
0.2 |
8.4 |
0.3 |
2.0 |
2.0 |
1.2 |
2.0 |
70.0 |
23.0 |
1997.0 |
6.0 |
|
Oct. |
13.7 |
0.6 |
0.3 |
7.8 |
0.2 |
2.0 |
1.0 |
0.6 |
||||||
|
Saín Bajo |
May |
28.6 |
0.4 |
0.2 |
9.7 |
0.2 |
2.0 |
2.0 |
0.3 |
1.0 |
50.0 |
33.0 |
1965.0 |
7.0 |
|
Oct. |
23.2 |
0.4 |
0.2 |
8.1 |
0.3 |
1.0 |
2.0 |
0.6 |
||||||
|
La Laborcita |
May |
18.8 |
0.6 |
0.3 |
8.4 |
0.4 |
2.0 |
3.0 |
0.7 |
3.0 |
30.0 |
22.0 |
1952.0 |
7.0 |
|
Oct. |
18.9 |
0.5 |
0.3 |
7.9 |
0.3 |
1.0 |
2.0 |
1.3 |
||||||
|
Miguel Hidalgo |
May |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
* |
||
|
Oct. |
23.4 |
0.4 |
0.2 |
7.7 |
0.2 |
1.0 |
1.0 |
0.7 |
2.0 |
70.0 |
14.8 |
2057.0 |
6.0 |
References
Albanil-Encarnación, A., Pascual-Ramírez, R., & Lobato-Sánchez, R. (2011). Reporte del clima en México. Servicio Meteorológico Nacional (SMN), Gerencia de Meteorología y Climatología; Subgerencia de Pronóstico a Mediano y Largo Plazo. México D.F.: Comisión Nacional del Agua, 16.
Bomford, M., Barry, S. C., & Lawrence, E. (2010). Predicting establishment success for introduced freshwater fishes: a role for climate matching. Biological Invasions, 12, 2559–2571.
Bond, C. E. (1996). The biology of fishes. 2nd Edition. Philadelphia: Saunders.
Burr, B. M. (1976). A review of the mexican stoneroller Campostoma ornatum, Girard (Pisces: Cyprinidae). Transactions of the San Diego Society of Natural History, 18, 127–143.
Castillo-Rivera, M., & Zárate-Hernández, R. (2001). Patrones espacio-temporales de la abundancia de peces en la Laguna de Pueblo Viejo, Veracruz. Hidrobiológica, 11, 75–84.
Cech, J. J., Mitchell, S. J., Castleberry, D. T., & McEnroe, M. (1990). Dsitribution of California stream fishes: influence of environmental temperature and hypoxia. Environmental Biology of Fishes, 29, 95–105.
Chun, S. N., Cocherell, S. A., Cocherell, D. E., Miranda, J. B., Jones, G. J., Graham, J. et al. (2011). Displacement, velocity preference, and substrate use of three native California stream fishes in simulated pulsed flows. Environmental Biology of Fishes, 90, 43–52.
Clarke, K. R., & Gorley, R. N. (2001). Primer v5. Roborough, Plymouth, Plymouth Marine Laboratory, UK. Available: www.primer-e.com
CNA (Comisión Nacional del Agua). (2009). Actualización de la disponibilidad media anual de agua subterranea, acuífero (3216) SaínAlto, Zacatecas. Zacatecas: Comisión Nacional del Agua, Gerencia de Aguas Subterráneas.
Contreras-Balderas, S. (1978). Speciation aspects and man made community composition in Chihuahuan Desert Fishes. In R. H. Wauer, & D. H. Riskind (Eds.), Transactions of the Symposium of the Biological Resources of the Chihuahuan Desert Region (pp. 405–431). U.S. Natl. Park. Serv. Trans. Proc. Ser 3.
Contreras-Balderas, S., Lozano-Vilano, M. L., & García-Ramírez, M. E. (2005). Historical changes in the index of biological integrity for the Lower Río Nazas, Durango, México. American Fisheries Society Symposium, 45, 225–237.
Dudley, R. K., & Matter, W. J. (2000). Effects of small green sunfish (Lepomis cyanellus) on recruitment of Gila Chub (Gila intermedia) in Sabino Creek, Arizona. The Southwestern Naturalist, 45, 24–29.
Facey, D. E., & Grossman, G. D. (1990). Metabolic cost of mantaining position for four North American stream fishes: effects of season and velocity. Physiological Zoology, 63, 757–776.
FISRWG (Federal Interagency Stream Restoration Working Group). (1998). Stream corridor restoration: principles, processes, and practices. Federal Interagency Stream Restoration Working Group, 15 Federal Agencies of the U.S. Government.
Frimpong, E. A., & Angermeier, P. L. (2010). Trait-based approaches in the analysis of stream fish communities. In K. B. Gido & D. A. Jackson (Eds.), Community ecology of stream fishes: concepts, approaches and techniques (pp. 109–135). Bethesda, Maryland: American Fisheries Society.
García-De León, F. J, Hernández-Sandoval, A. I., Contreras-Catala, F., Sánchez-Velasco, L., & Ruiz-Campos, G. (2018). Distribution of fishes in the Río Guayalejo-Río Tamesí system and relationships with environmental factors in northeastern Mexico. Environmental Biology of Fishes, 101, 167–180.
Gido, K. B., Propst, D. L., & Molles, M. C. (1997). Spatial and temporal variation of fish communities in secondary channels of the San Juan River, New Mexico and Utah. Environmental Biology of Fishes, 49, 417–434.
Grossman, G. D., Ratajczak, R. E., Farr, M. D., Wagner, M. C., & Petty, J. T. (2010). Why there are fewer fish upstream.. In K. B. Gido, & D. A. Jackson (Eds.), Community ecology of stream fishes: concepts, approaches, and techniques (pp. 63–81). Bethesda, Maryland: American Fisheries Society.
Havel, J. E., Eunmi-Lee, C., & Vander-Zanden, M. J. (2005). Do Reservoirs Facilitate Inavsions Into Landscapes? Bioscience, 55, 518–525.
Hendrickson, D. A., Minckley, W. L., Miller, R. R., Siebert, D. J., & Minckley, P. H. (1980). Fishes of the Río Yaqui Basin, Mexico and United States. Journal of the Arizona-Nevada Academy of Science, 15, 65–106.
Hocutt, C. H., & Stauffer, J. R. (1980). Biological monitoring of fish. Lexington, Massachusetts: Lexington Books/ D. C. Heath Company.
Hugueny, B., Oberdorff, T., & Tedesco, P. A. (2010). Community ecology of river fishes: a large-scale perspective. In K. B. Gido, & D. A. Jakson (Eds.), Community ecology of stream fishes: concepts, approaches and techniques (pp. 29–62). Bethesda, Maryland: American Fisheries Society.
Inegi (Instituto Nacional de Estadística y Geografía). (2010a). Prontuario de información geográfica municipal de los Estados Unidos Mexicanos, Saín Alto, Zacatecas. Página Oficial del Instituto Nacional de Estadística y Geografía. Retrieved on 12 February 2011from: http://mapserver.inegi.org.mx/mgn2k/
Inegi (Instituto Nacional de Estadística y Geografía). (2010b). Red hidrográfica. Escala 1:50,000, Edición 2.0. Documento Técnico Descriptivo de la Red Hidrográfica Escala 1:50,000. Aguascalientes: Instituto Nacional de Estadística Geografía e Informática, Dirección General de Geografía y Medio Ambiente, 105.
Johnson, D. E. (2000). Métodos multivariados aplicados al análisis de datos. México D.F.: International Thomson Editores.
Johnson, P. T. J., Olden, J. D., & Vander-Zanden, M. J. (2008). Dam invaders: hydrologic impoundments enhance ecosystem invasibility. Frontiers in Ecology and the Environment, 6, 357–363.
Lake, P. S. (2003). Ecological effects of perturbation by drought in flowing waters. Freshwater Biology, 48, 1161–1172.
Lozano-Vilano, M. L., García-Ramírez, M. E., & De la Maza-Benignos, M. (2009). Índice biológico de integridad histórico (IBIh) e índice de similaridad de sitios de Jaccard. In M. De la Maza-Benignos (Ed.), Los peces del Río Conchos (pp. 139–174). Chihuahua: Alianza WWF-FGRA/ Gobierno del Estado de Chihuahua, Mexico.
Lyons, J., Gutiérrez-Hernández, A., Díaz-Pardo, E., Soto-Galera, E., Medina-Nava, M., & Pineda-López, R. (2000). Development of a preliminary index of biotic integrity (IBI) based of fish assemblages to asses ecosystem condition in the lakes of Central Mexico. Hidrobiologia, 418, 57–72.
Lyons, J., Navarro-Pérez, S., Cochran, P. A., Santana, E., & Guzmán-Arroyo, M. (1995). Index of biotic integrity based on fish assemblages for the conservation of the streams and rivers in West Central Mexico. Conservation Biology, 9, 569–584.
Mejía-Mojica, H., Contreras-MacBeath, T., & Ruiz-Campos, G. (2015). Relationship of environmental and geographic factors and the distribution of exotic fishes in tributaries of the Rio Balsas basin, Mexico. Environmental Biology of Fishes, 98, 611–621.
Mercado-Silva, N., Lyons, J., Díaz-Pardo, E., Gutiérrez-Hernandez, A., Ornelas-García, P., Pedraza-Lara, C. et al. (2006). Long-term changes in the fish assemblage of the Laja River, Guanajuato, Central Mexico. Aquatic Conservation: Marine and Freshwater Ecosystem, 16, 533–546.
Miller, R. R., Minckley, W. L., & Norris, S. M. (2005). Freshwater fishes of Mexico. Chicago: University of Chicago Press.
Myrick, C. A., & Cech, J. J. (2000). Swiming performances of four California stream fishes: temperature effects. Environmental Biology of Fishes, 58, 289–295.
Olden, J. D., & Kennard, M. J. (2010). Intercontinental comparison of fish life history strategies along a gradient of hydrologic variability. American Fisheries Symposium, 73, 83–107.
Olden, J. D., & Poff, N. L. (2005). Long-term trends of native and non-native fish faunas in the America Soutwest. Animal Biodiversity and Conservation, 28, 75–89.
Olden, J. D., Poff, N. L., & Bestgen, K. R. (2006). Life-History strategies predict fish invasions and extirpations in the Colorado River Basin. Ecological Monographs, 76, 25–40.
Page, L. M., Espinosa-Pérez, H., Findley, L. T., Gilbert, C. R., Lea, R. N., Mandrak, N. E. et al. (2013). Common and scientific names of fishes from the United States, Canada and Mexico, 7th Ed. Special Publication 34. Bethesda, Maryland: American Fisheries Society.
Pearsons, T. N., & Li, H. W. (1992). Influence of habitat complexity on resistance to flooding and resilience to stream fish assemblages. Transactions of the American Fisheries Society, 121, 427–436.
Petry, A. C., & Schulz, U. H. (2006). Longitudinal changes and indicator species of the fishfauna in the subtropical Sinos River, Brazil. Journal of Fish Biology, 69, 272–290.
Quist, M. C., Rahel, F. J., & Hubert, W. A. (2005). Hierarchical faunal filters: an approach to assessing effects of habitat and nonnative species on native fishes. Ecology of Fresh Water Fish, 14, 24–39.
Rietveld, T., & Van-Hout, R. (1993). Statistical techniques for the study of lenguage and lenguage behavior. Berlin: Gruyter & Co.
Ruiz-Campos, G., Camarena-Rosales, F., Contreras-Balderas, S., Reyes-Valdez, C. A., De La Cruz-Agüero, J., & Torres-Balcázar, E. (2006). Distribution and abundance of the endangered killifish, Fundulus lima (Teleostei: Fundulidae), in oases of Central Baja California Peninsula, México. The Southwestern Naturalist, 51, 502–509.
Salas-Martínez, M. G. (1971). Ictiofauna del complejo de cuencas Nazas, Aguanaval, Parras y del Chorro de los Estados de Durango, Zacatecas y Coahuila, México (Bachelor thesis). Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, San Nicolás de los Garza, Nuevo León, México.
Sánchez, O. (2007). Ecosistemas acuáticos: diversidad, procesos, problemática y conservación. In O. Sánchez, M. Herzing, E. Peters, R. Márquez, & L. Zambrano (Eds.), Perspectivas sobre conservación de ecosistemas acuáticos en México (pp. 11–36). México D.F.: Instituto Nacional de Ecología (INE-Semarnat).
Schmitter-Soto, J. J., Ruiz-Cauich, L. E., Herrera, R. L., & Gonzáles-Solís, D. (2011). An index of biotic integrity of shallow streams of the Hondo River basin, Yucatán Peninsula. Science of the Total Environment, 409, 844–852.
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales) . (2010). Norma Oficial Mexicana, NOM-059-SEMARNAT-2010. Protección ambiental-Especies nativas de México de Flora y Fauna silvestres- Categorías de riesgo y especificaciones para su inclusión, exclusión o cambio-Lista de especies en riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010, Segunda Sección, México.
Solís-Carlos, F. (2013). Propuesta de plan de manejo para el Río Saín Alto, subcuenca del Río Aguanaval, Zacatecas, México (Tesis de Maestría en Manejo de Ecosistemas de Zonas Áridas), Facultad de Ciencias, Universidad Autónoma de Baja California, México.
Solís-Carlos, F., Lozano-Vilano, M. L., García-Ramírez, M. E., & Contreras-Balderas, J. A. (2009). Estudio taxonómico y distribucional de la ictiofauna de áreas selectas en el norte del estado, Zacatecas, México. 112th Annual Meeting of the Texas Academy of Sciences, San Nicolás de los Garza, Nuevo León, México. 5-7 March, 2009. 130 p. [abstract].
Tedesco, P., & Hugueny, B. (2006). Life history strategies affect climate based spatial synchrony in population dynamics of West African freshwater fishes. Oikos, 115, 117–127.
Trautman, M. B. (1981). The fishes of Ohio. Columbus, Ohio: Ohio State University Press.
Trujillo-Jiménez, P., López-López, E., Díaz-Pardo, E., & Camargo, J. A. (2010). Patterns in the distribution of fish assemblages in Río Amacuzac, Mexico: influence of abiotic factors and biotic factors. Reviews in Fish Biology and Fisheries, 20, 457–469.
Varela-Romero, A., & Hendrickson, D. A. (2009). Peces dulceacuícolas. In F. E. Molina-Freaner, & T. R. Van-Devender (Eds.), Diversidad biológica de Sonora (pp. 339–356). México D.F.: Universidad Nacional Autónoma de México.



