Alfonso A. González-Díaz a, *, Karem F. Ramírez-Moreno b, Wilfredo A. Matamoros b, Miriam Soria-Barreto c, Rocío Rodiles-Hernández a
a El Colegio de la Frontera Sur, Departamento Conservación de la Biodiversidad, Colección de Peces, Carretera Panamericana y Periférico Sur s/n, Barrio María Auxiliadora, 29290 San Cristóbal de Las Casas, Chiapas, Mexico
b Universidad de Ciencias y Artes de Chiapas, Instituto de Ciencias Biológicas, Laboratorio de diversidad acuática y biogeografía, Libramiento Norte Poniente 1150, Col. Lajas Maciel, 29039 Tuxtla Gutiérrez, Chiapas, Mexico
c Universidad Autónoma del Carmen, Facultad de Ciencias Naturales, Centro de Investigación de Ciencias Ambientales, Calle Laguna de Términos s/n, Col. Renovación 2ª Sección, 24115 Ciudad del Carmen, Campeche, Mexico
*Corresponding author: agonzalez@ecosur.mx (A.A. González-Díaz)
Received: 15 December 2020; accepted: 5 June 2021
Abstract
The systematics of the genus Vieja is complex because it contains many morphologically similar species that have little genetic differentiation. Examination of morphological traits can be useful for clarifying their taxonomic status. We analyzed the morphological variation of bones in the oral and lower pharyngeal jaws to determine whether these structures permit the differentiation of species and to study possible functional implications. Morphological differences were quantified using canonical variates analysis, MANOVA, and paired comparisons. Differences in the number of pharyngeal teeth in the lower pharyngeal jaw were determined using an ANCOVA. The shape of the premaxilla and lower pharyngeal jaw proved useful for the delimitation of these species. Although the morphological variation between V. breidohri and V. hartwegi is minimal, the shapes of their lower pharyngeal jaws differ in morphospace. Vieja bifasciata possesses more teeth in the lower pharyngeal jaw when compared to the other species. Variation in these bones may affect jaw biomechanics and influence feeding behavior. However, these morphological differences contradict the weak genetic differentiation observed. The geographic isolation of V. bifasciata is likely related to its morphological differentiation. The close phylogenetic relationship between V. breidohri and V. hartwegi likely explains their low morphological divergence.
Keywords: Central America; Sympatric species; Osteology; Functional morphology; Grijalva-Usumacinta Basin
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Comparación morfogeométrica de las mandíbulas orales y faríngeas inferiores de los cíclidos estrechamente relacionados Vieja bifasciata, V. breidohri y V. hartwegi (Cichliformes: Cichlidae)
Resumen
La sistemática del género Vieja es compleja, incluye especies morfológicamente similares con poca diferenciación genética. La revisión de características morfológicas puede ser útil para clarificar su taxonomía. Se analizó la variación morfológica de los huesos de las mandíbulas oral y faríngea inferior para determinar si estas estructuras ayudan a delimitar a las especies y evaluar posibles implicaciones funcionales. Las diferencias morfológicas se cuantificaron con un análisis de variables canónicas, Manova y comparaciones pareadas. Se comparó el número de dientes faríngeos en la mandíbula faríngea inferior con un Ancova. La forma de la premaxila y la mandíbula faríngea inferior mostró ser útil para delimitar estas especies. Aunque la variación morfológica entre V. breidohri y V. hartwegi fue poca, la forma de la mandíbula faríngea inferior difiere en el morfoespacio. Vieja bifasciata es la especie que posee más dientes en la mandíbula faríngea inferior. La variación en estos huesos puede afectar la biomecánica de la mandíbula e influir en la conducta alimentaria. Las diferencias morfológicas contrastan con la escasa diferenciación genética observada. El aislamiento geográfico de V. bifasciata probablemente esté relacionado con su diferenciación morfológica. Las relaciones filogenéticas entre V. breidohri y V. hartwegi podrían explicar su baja divergencia morfológica.
Palabras clave: Centroamérica; Especies simpátridas; Osteología; Morfología funcional; Cuenca Grijalva-Usumacinta
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The wide range of morphological variation exhibited by Middle American cichlids is perhaps one of their most conspicuous characteristics, which has often been correlated to the great environmental heterogeneity that characterizes this region (López-Fernández et al., 2010; Říčan et al., 2016). The Grijalva-Usumacinta River basin is shared between Guatemala and Mexico and likely one of the best examples of a watershed with such heterogeneity in habitats and environments. It has a complex and dynamic geological, volcanic, orographic, and physiographic history with wide variations in climate, vegetation, and aquatic ecosystems (Kolb & Galicia, 2012; Vaca et al., 2019). These conditions have been attributed as major contributors to the high fish diversity found in this watershed (Matamoros et al., 2015; McMahan et al., 2017). The Grijalva-Usumacinta River basin contains over 30 species of cichlids, the highest concentration of species within this family in Middle America, which exhibit great morphological and functional diversity. Additionally, this basin also has the highest number of endemic (Matamoros et al., 2015) and non-endemic species of cichlids with restricted geographic distributions (Gómez-González et al., 2018; Říčan et al., 2016; Soria-Barreto et al., 2019). The exceptional species richness and morphological variation in this region have historically led to a series of taxonomic and systematic problems reflected in several phylogenetic hypotheses and taxonomic changes in this group (López-Fernández et al., 2010; McMahan et al., 2015).
Among Central American cichlids, the genus Vieja is one of the most taxonomically complex. Its widespread distribution and high levels of morphological variability have historically prevented a clear understanding of its evolutionary history (Gómez-González et al., 2018; McMahan et al., 2010, 2015, 2019; Říčan et al., 2016). It was only recently (see McMahan et al., 2015) determined that this genus comprises 8 valid species whose distribution extends from the Papaloapan River basin in Mexico to the Chagres River in Panama on the Atlantic slope, and from the Isthmus of Tehuantepec in Mexico to Lake Coatepeque in El Salvador on the Pacific slope (Fricke et al., 2020; Gómez-González et al., 2018; McMahan et al., 2015, 2017; Říčan et al., 2016).
Although higher-level relationships of Vieja and its close relatives have largely been resolved through molecular and morphological studies, species relationships, limits and distributions remain problematic, especially among closely related species (McMahan et al., 2010, 2015; Říčan et al., 2016). While morphological characters frequently show high levels of overlap between species, there are little or no reliable diagnostic characters in the original species descriptions and the available taxonomic keys present ambiguous characters (Gómez-González et al., 2018; McMahan et al., 2019). The color patterns of live specimens have often been used to distinguish species; however, this practice also yields ambiguous results due to specimen preservation, geographic and ontogenetic variation, and ecophenotypic plasticity (Miller et al., 2005). Therefore, the incorporation and description of additional morphological characters in comparative and phylogenetic analyses are highly recommended to achieve improved taxonomic clarification and distinction among species of Vieja (Gómez-González et al., 2018; McMahan et al., 2019; Soria-Barreto et al., 2011).
Morphological characters related to feeding are useful discriminant characters in cichlids (Trewavas, 1983; Witte & van Oijen, 1990). Similar to other groups of fishes, the ecological diversification of cichlids has involved resource partitioning. In this process, the adaptive capacity of trophic morphology is crucial and includes form, function, and behavior (Burress, 2015). The trophic morphology of cichlids is closely related to the exploitation and partitioning of food resources, which are potential mechanisms for ecological divergence (Albertson et al., 2003a, b; Hulsey, 2009). Several functional morphological studies have shown that the shape of bone structures of the trophic apparatus can determine biomechanical performance in relation to the capture and processing of food (Albertson & Kocher, 2001; Albertson et al., 2003a; Meyer, 1989; Waltzek & Wainwright, 2003). The traits of these feeding structures have been used to taxonomically discriminate several genera and species of fish, including cichlids (Kassam et al., 2004; Trewavas, 1983; Witte & van Oijen, 1990; Witte & Witte-Maas, 1987). Moreover, features such as the shape and number of teeth in the lower pharyngeal jaw (LPJ) and oral jaw have traditionally been included in descriptions of Neotropical and African cichlids (Alonso et al., 2019; Oliver, 2018).
The closely related species V. bifasciata (Steindachner, 1864), V. breidohri (Werner & Stawikowski, 1987), and V. hartwegi (Taylor & Miller, 1980) possess interesting phenotypes for the study of trophic morphology. Molecular phylogenetic analyses have shown few genetic differences among these 3 species (Gómez-González et al., 2018; McMahan et al., 2010; Říčan et al., 2016). Although these species are very similar, certain differences in color pattern, body shape, and pharyngeal teeth have been observed (Gómez-González et al., 2018). Vieja breidohri is sympatric with V. hartwegi in the middle and upper reaches of the Grijalva River basin, while V. bifasciata is widespread in the entire Usumacinta River basin and does not coexist with its close congeners (Miller et al., 2005).
Since sympatric cichlids frequently exhibit trophic characteristics related to their diversification (Burress, 2015, 2016), differences in trophic anatomy are expected among these closely related species, especially in structures that are functionally important to food capture and processing. The main goal of this study was to analyze patterns of variation in the bones that form the oral jaws and LPJs of V. bifasciata, V. breidohri, and V. hartwegi to evaluate their utility as discriminating characters for species delimitation. Additionally, we explored possible functional implications of observed morphological variation, particularly among sympatric species.
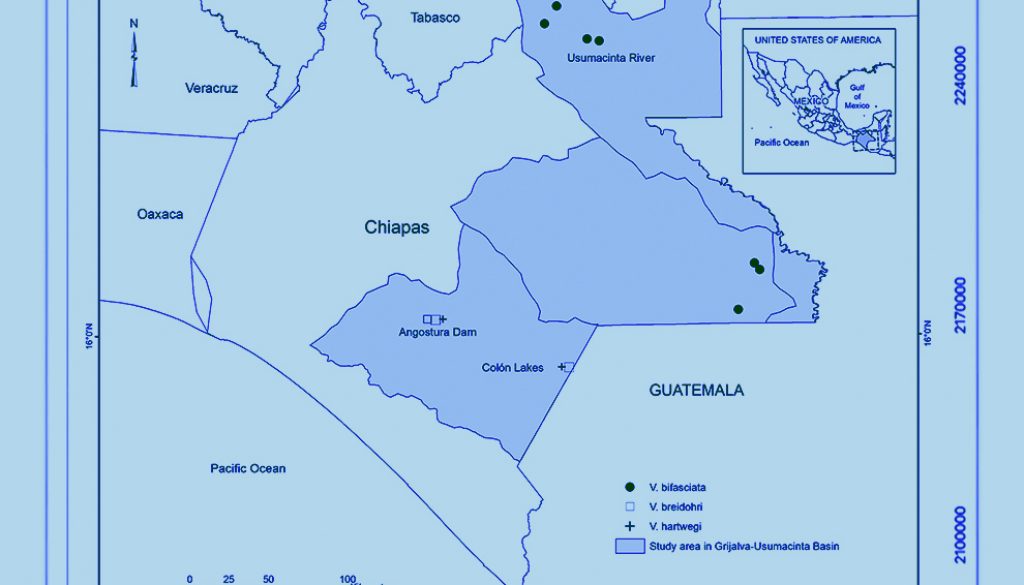
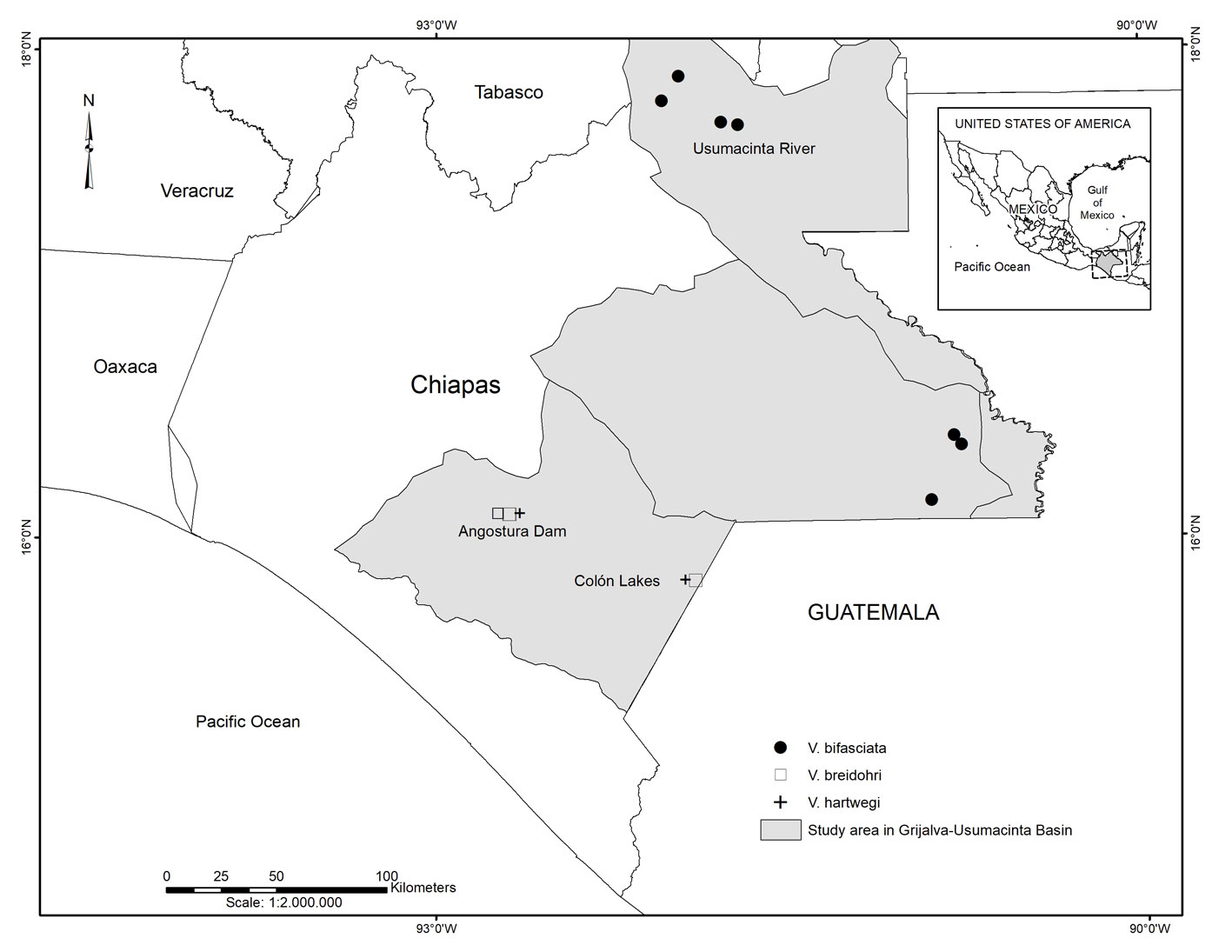
Materials and methods
For the selection of specimens for analysis, we considered their number, size, and conservation status in the fish collection. We also attempted to compare the same number of specimens and those of similar size.
A total of 55 adult specimens of the genus Vieja were obtained from the fish collection of El Colegio de la Frontera Sur (ECOSC), including V. bifasciata (n = 20, standard length, SL 62.4 – 148 mm, ECOSC 55, 731, 1621, 2381, 2387, 2675, 2746, 2760, 3353, 3362, 3878, 7509), V. breidohri (n = 21, SL 61.07 – 115.35 mm, ECOSC 4584, 6835, 6840, 6868), and V. hartwegi (n = 14, SL 59.38 – 108.29 mm, ECOSC 6838, 7455). All V. breidohri and V. hartwegi specimens were collected from the Belisario Domínguez reservoir and Lagos de Colón, located in the upper reaches of the Grijalva River in Chiapas, Mexico (Fig. 1). Moreover, all V. bifasciata specimens originated from localities in the middle reaches of the Usumacinta River. Their taxonomic determination was corroborated following Miller et al. (2005) and their original descriptions (Gómez-González et al., 2018; Steindachner, 1864; Taylor & Miller, 1980; Werner & Stawikowski, 1987). Due to high levels of overlap in morphometric and meristic characters, the key characters used to separate these species are basically body color patterns (Gómez-González et al., 2018). Preserved specimens of V. bifasciata have a curved midlateral stripe extending from the caudal fin base to the opercle, running at the mid-body level. In V. hartwegi, the longitudinal stripe is straight and has a dark blotch at the caudal fin base. Vieja breidohri has an interrupted midlateral stripe with dark blotches extending from the caudal fin base to the opercle.
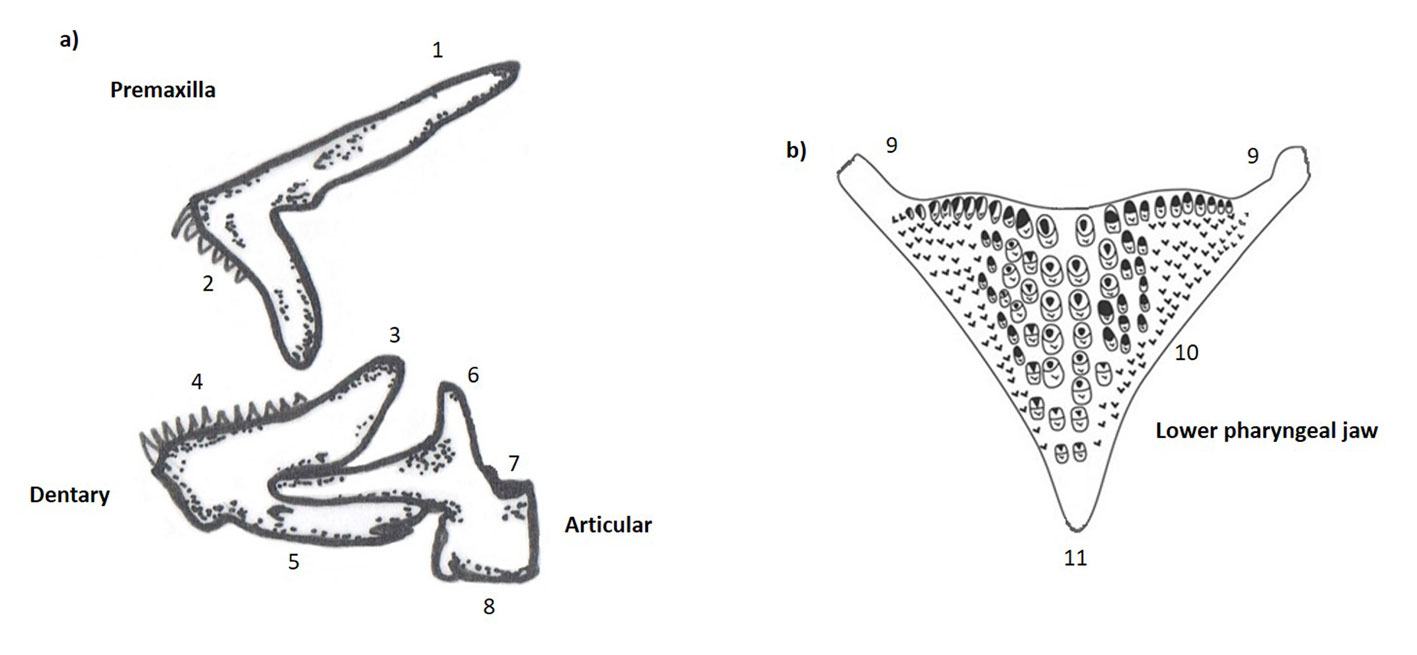
Specimens were cleared and stained following Taylor & Van Dyke (1985). The premaxilla, dentary, and articular from the oral jaw, as well as the LPJ (Fig. 2), were dissected following standardized protocols. We selected these bones because they are important in trophic morphology and have taxonomic value. The maxilla was discarded because it was difficult to recognize homologous points to define landmarks and describe their shape. Each bone was photographed with a reference scale using a Canon 70D camera mounted on an EsteREO Discovery V12 Zeiss microscope. Only LPJs were photographed dorsally. For V. breidohri, 19 premaxillas and 12 LPJs were available, while 18 premaxillas and 17 LPJs were available for V. bifasciata. Broken bones were removed from the analysis.
We defined 2D landmarks and semi-landmarks to describe the shape of each bone using MakeFan8 (Sheets, 2014) and TpsDig2 ver. 2.26 software (Rolf, 2016). The landmark and semi-landmark configurations for each bone were as follows: premaxilla (3 landmarks, 21 semi-landmarks), dentary (6, 10), articular (5, 16), and LPJ (7, 10) (Figs. 3-6). We used open curves in each bone to describe the shape of the contours between landmarks: premaxilla (3 curves), dentary (2 curves), articular (2 curves) and LPJ (3 curves). Semi-landmarks were aligned by sliding points along the curves using the minimum Procrustes distance criterion in SemiLand8 software (Sheets, 2014). To remove the effects of scale, orientation, and position, the landmark and semi-landmark configurations were superimposed using the generalized Procrustes analysis in CoordGen8 software (Sheets, 2014).
To evaluate the effect of allometry on intra- and interspecific bone shape variation, we performed linear univariate regression with the Procrustes distances of each specimen as the dependent variable and the log-transformed values of bone shape centroid size as the independent variable (Zelditch et al., 2012). This analysis tested the null hypothesis that there is no relationship between size and shape. The analysis was executed using Regress8 software with 100 permutations (Sheets, 2014). Superimposed landmarks and semi-landmarks were aligned and used to obtain partial warps with the 3 smallest specimens as references. The significance of the regression parameters was estimated through bootstrap permutations (1,000 replicates). We used the slope value (m) and explained variance (%) to determine the influence of size on bone shape. Lower values of regression slope (m) and explained variance (%) indicate no linearity between both variables and a weak effect of size on shape (Zelditch et al., 2012).
Partial warps were used in a canonical variate analysis (CVA) and multivariate analysis of variance (MANOVA) to view and quantify the intra- and interspecific bone shape differences in morphospace using CvaGen8 software (Sheets, 2014). Bartlett’s test was performed to determine the significance of CVA scores based on Wilk’s lambda (λ) values. Bone shape variation was visualized using deformation grids associated with the canonical variates. Partial Procrustes distances between the mean shapes of groups were calculated to perform paired comparisons. The interspecific differences of each bone were assessed using Goodall’s F test with Bonferroni correction and 100 permutations using Twogroup8 software (Sheets, 2014).
Finally, we tested for significant differences in the number of teeth in the LPJ to determine the effect of LPJ size on this number. The number of teeth in LPJs were counted and an analysis of covariance (ANCOVA) was performed with species as the independent variables and the number of teeth in the LPJ as the dependent variable, with centroid size as a covariate. This procedure was implemented in SPSS 15.0 (2006).
Results
The allometric analysis did not show a significant relationship between size and bone shape. At the intraspecific level, the values of the regression slope (m) were low and the percentage of shape variation explaining size (E) varied from 6.03 to 36.98%. Premaxilla: V. bifasciata (m = 0.044, E = 33.11%), V. breidohri (m = -0.019, E = 36.98%), and V. hartwegi (m = -0.003, E = 24.76%). Dentary: V. bifasciata (m = 0.03, E = 12.04%), V. breidohri (m = 0.004, E = 7.6%), and V. hartwegi (m = 0.004, E = 14.71%). Articular: V. bifasciata (m = -0.008, E = 13.48%), V. breidohri (m = -0.003, E = 17.3%), and V. hartwegi (m = -0.007, E = 8.34%). LPJ: V. bifasciata (m = 0.004, E = 6.03%), V. breidohri (m = 0.005, E = 23.91%), and V. hartwegi (m = 0.022, E = 9.44%). At the interspecific level, we detected relatively low linearity values in the regression analyses and in the variation explained for the articular (m = -0.01, E = 7.85%), dentary (m = 0.013, E = 6.09%), premaxilla (m = 0.0004, E = 21.02%), and LPJ (m = -0.009, E = 8.38%).
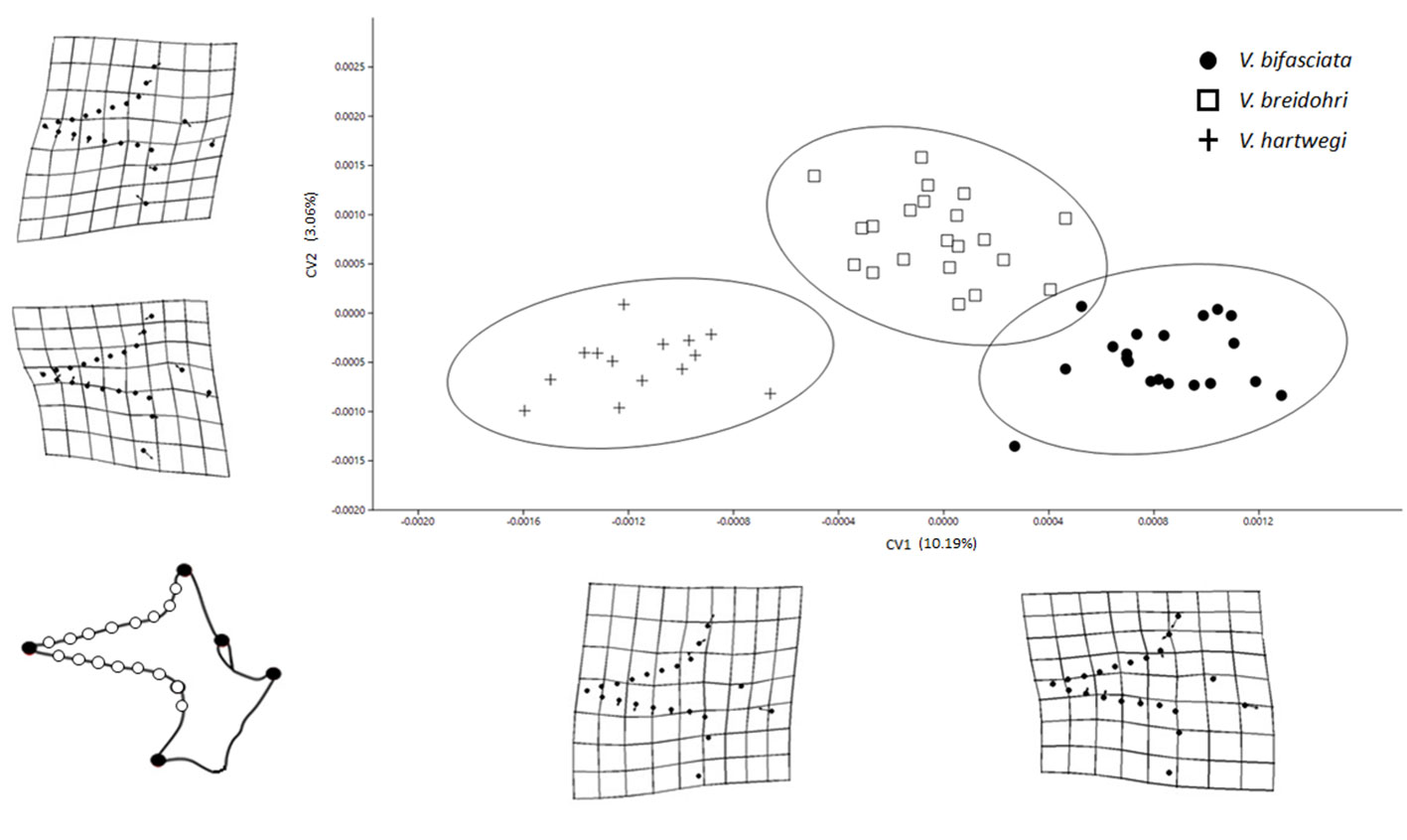
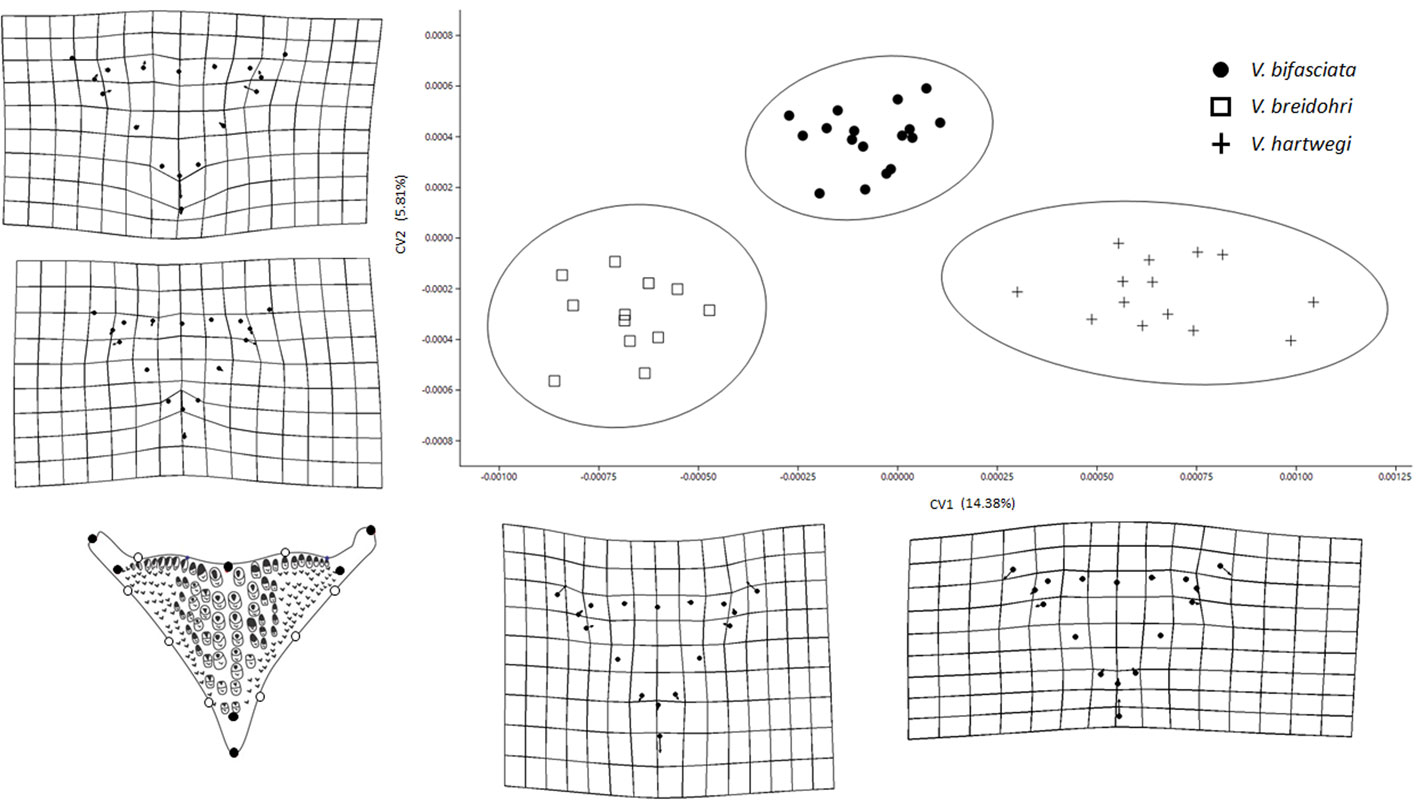
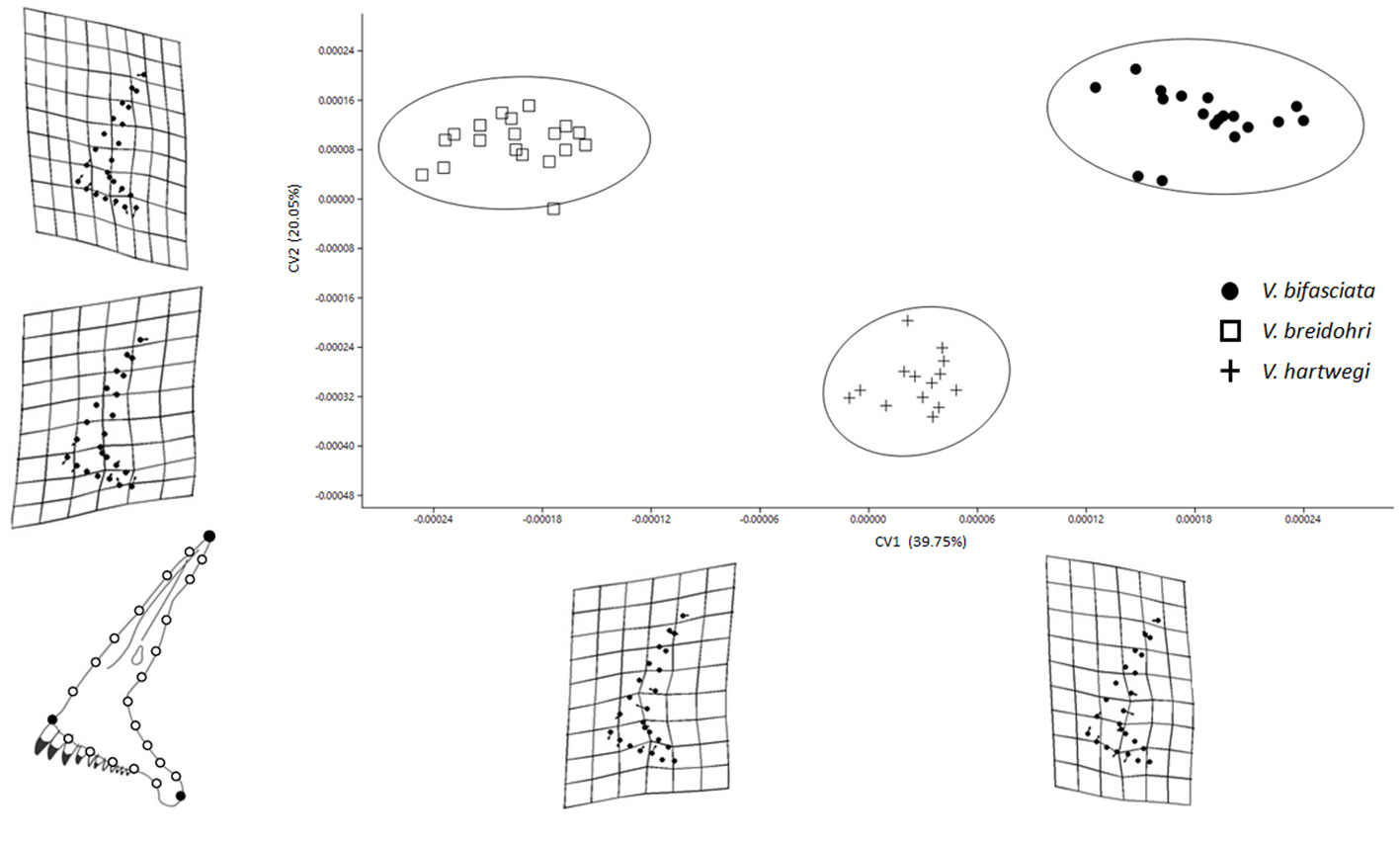
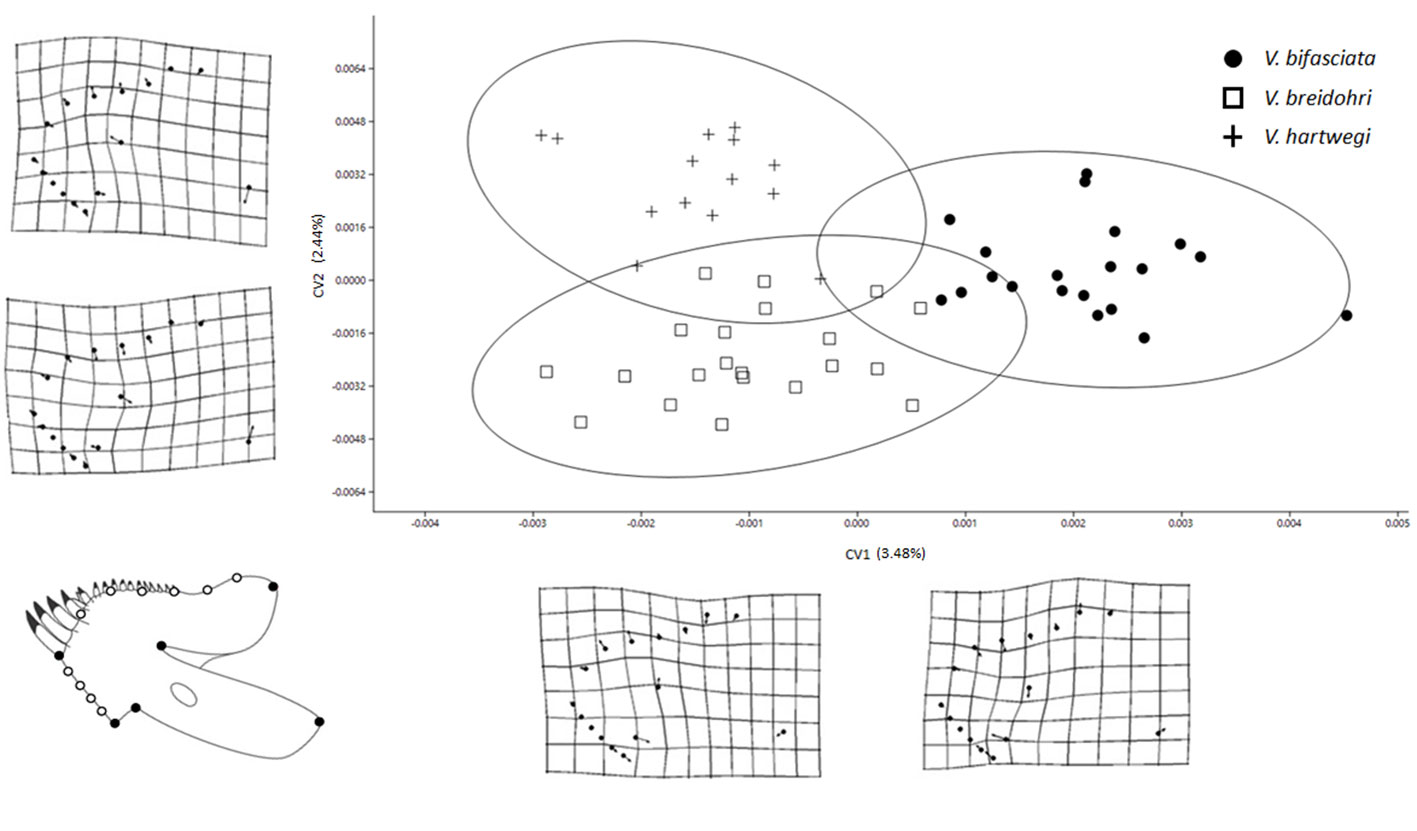
Although the CVA revealed significant differences in shape among the 4 bones in the 3 studied species (Table 1, p < 0.05), the obtained Wilks lambda (λ) values were relatively low. The premaxilla and LPJ showed the greatest differentiation among species (Figs. 3, 6) and explained variance (59.8 and 20.48%, respectively) (Table 1). The dentary exhibited significant differences in the first 2 axes (Fig. 4) with less explained variance (5.52%; Table 1). The articular only recovered a significant CV1 explaining 10.19% of the variance (Fig. 5). Paired comparisons between species also indicated significant differences in the shape of the 4 bones (Table 2).
Deformation grids showed changes in the size and orientation of the ascending process of the premaxilla as well as the height of the dentigerous process. Vieja breidohri was recovered in the negative axis of CV1 and their ascending process was elongated and thin at its base, while the dentigerous process was wider at the anterior portion. The positive axis contained V. bifasciata, in which the ascending process was short and wide at its base and the dentigerous process was narrow toward its posterior part. Vieja hartwegi possessed intermediate characteristics (Fig. 3).
In CV2, changes were associated with the shape of the ascending process and the height of the dentigerous process. Vieja hartwegi was located along the negative axis, where the ascending process was slightly longer and oriented posteriorly while the anterior section of the dentigerous process was higher with its posterior part inclined dorsally (Fig. 3).
In the dentary, changes were associated with the length of the ascending process and the heights of the dentigerous area and ventral process. Vieja breidohri and V. hartwegi were recovered along the negative axis of the CV1 and showed the shortest ascending process and the highest dentigerous area and ventral process. Vieja bifasciata was located along the positive region of CV1 with the longest ascending process and relatively short dentigerous area and ventral process. At the negative axis of CV2, V. breidohri was differentiated from V. hartwegi by a lower height of the dentigerous area, with V. bifasciata located between them (Fig. 4).
For the articular, the most notable changes were observed in the length of the coronoid process, height of the horizontal process, and size of the articular. Vieja hartwegi was located at the negative axis of CV1 with the highest coronoid process, widest horizontal process, and narrowest articular facet. Vieja bifasciata was located at the positive end of CV1 with the lowest coronoid process, narrowest horizontal process, and widest articular facet. Vieja breidohri possessed intermediate articular characteristics (Fig. 5).
The LPJ showed changes in the length of the anterior process, the position of the lateral processes, and the size of the dentigerous area. Vieja breidohri was in the negative axis of CV1 with the lateral processes displaced, anterior process and anterior part of the dentigerous area lengthened, and posterior lateral edges thinned. Vieja hartwegi was at the positive end of CV1 and showed lateral processes displaced forward, anterior process shortened, and posterior lateral edges of the dentigerous area widened. Vieja bifasciata had an intermediate form. In CV2, the most important change was in the shape of the dentigerous area. Vieja breidohri and V. hartwegi were in the negative region, showing the dentigerous area with the widest posterior lateral edges and the shortest anterior border. Vieja bifasciata was located in the positive region, with the narrowest dentigerous area at the back and an elongated front edge (Fig. 6).
Table 1
Results of CVA and MANOVA for all bones between the 3 species of Vieja
|
Bone |
CV |
λ Wilks |
χ2 |
p |
Variance % |
|
Premaxilla |
CV1 |
0.001 |
179 |
0.001 |
39.75 |
|
CV2 |
0.048 |
80.75 |
0.001 |
20.05 |
|
|
Dentary |
CV1 |
0.065 |
105.331 |
0.001 |
3.48 |
|
CV2 |
0.291 |
47.593 |
0.008 |
2.44 |
|
|
Articular |
CV1 |
0.022 |
127.487 |
0.009 |
10.19 |
|
CV2 |
——- |
——- |
——- |
3.06 |
|
|
LPJ |
CV1 |
0.008 |
122.13 |
0.001 |
14.38 |
|
CV2 |
0.128 |
52.437 |
0.004 |
6.81 |
Table 2
Goodall’s F-test for all bones between the 3 species of Vieja. Bootstrapeed F-test, significance level (p), Partial Procrustes distance between means, and degrees of freedom.
|
Bone |
V. bifasciata – V. breidohri |
V. bifasciata – V. hartwegi |
V. breidorhi – V. hartwegi |
|
|
Premaxilla |
Goodall’s F-test |
5.85 (p = 0.01) |
11.72 (p = 0.01) |
3.62 (p = 0.01) |
|
Procrustes distance |
0.034 (44, 1540) |
0.048 (44, 1320) |
0.027 (44, 1364) |
|
|
Dentary |
Goodall’s F-test |
2.65 (p = 0.01) |
10.22 ( p = 0.01) |
8.7 (p = 0.01) |
|
Procrustes distance |
0.034 (28, 1092) |
0.065 (28, 896) |
0.072 (28, 924) |
|
|
Articular |
Goodall’s F-test |
8.22 (p = 0.01) |
8.85 (p = 0.01) |
2.88 (p = 0.01) |
|
Procrustes distance |
0.056 (38, 1482) |
0.067 (38, 1216) |
0.039 (38, 1254) |
|
|
LPJ |
Goodall’s F-test |
3.82 (p = 0.01) |
23.03 (p = 0.01) |
26.19 (p = 0.01) |
|
Procrustes distance |
0.033 (30, 810) |
0.063 (30, 870) |
0.086 (30, 720) |
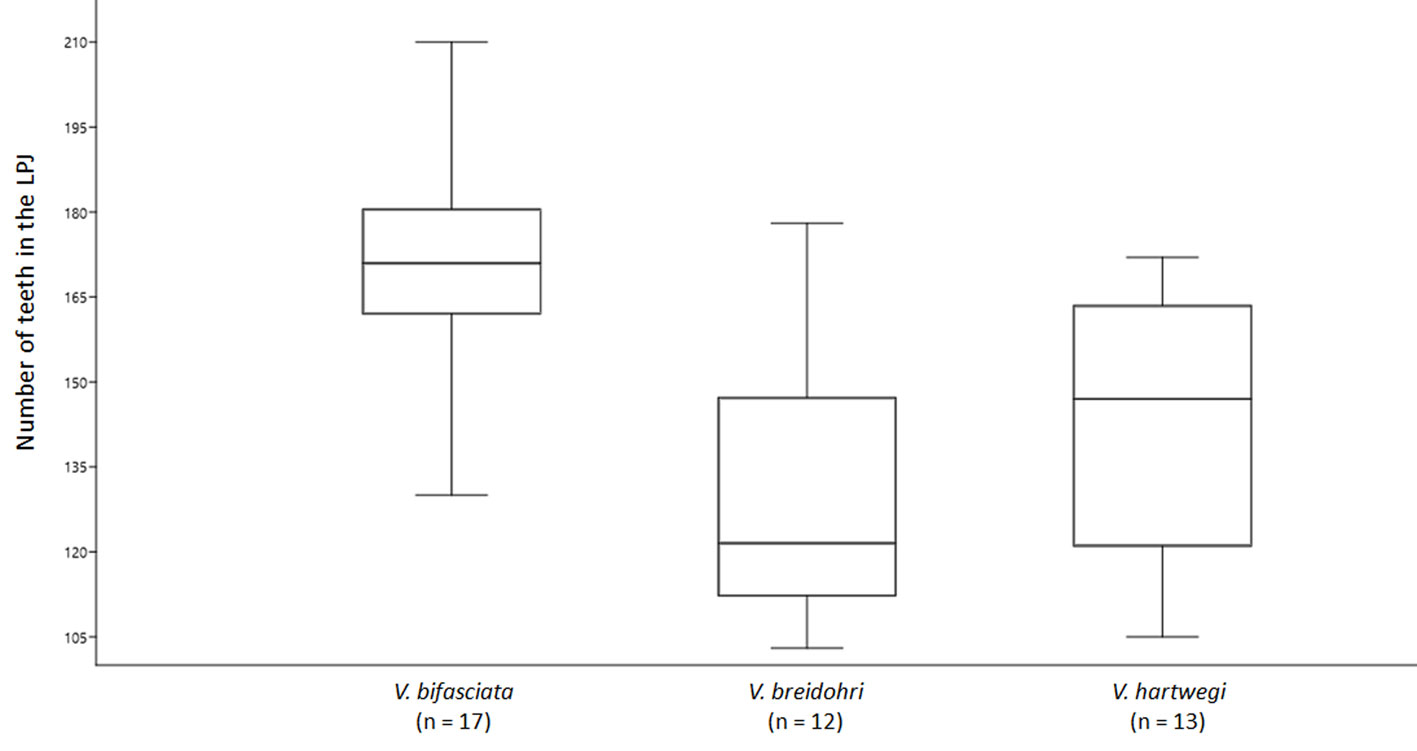
Based on the analysis of covariance (ANCOVA), no significant effect of centroid size was found (p > 0.05, F = 2.387) upon comparing the number of teeth in the LPJ. Significant differences in V. bifasciata were observed when compared to V. breidohri and V. hartwegi (p < 0.05, F = 12.96). Vieja bifasciata had 130 to 210 teeth (x = 170), V. breidohri had 103 to 178 (x =129), and V. hartwegi had 105 to 172 (x = 143) (Fig. 7).
Discussion
Our morphometric analysis showed that the shape of the bones that make up the oral jaw and LPJ differ significantly among the 3 analyzed species of the genus Vieja. These findings support the taxonomic distinction of these 3 species and can function as discriminating characters in addition to observed differentiation in color pattern, body shape, and the type of pharyngeal teeth (Gómez-González et al., 2018).
The results of the intra- and interspecific allometry tests suggest that there is no significant influence of allometry in this study. Frequently, a linear relationship between both variables can affect the morphometric comparison between species or groups. Therefore, it is very important to evaluate such relationships through this type of study and remove or account for the effect of allometry in analyses (Zelditch et al., 2012).
The shape and variation of the premaxilla explained the greatest variation among species, with the most notable differences observed in the length, width, and orientation of the ascending process. Although the length of the ascending process is functionally associated with an increase in the protrusion distance from the mouth, it is coupled with a decrease in bite force (Barel, 1983; Burress et al., 2020). These functional considerations, as well as the variation observed in the premaxilla, allow us to predict that V. bifasciata has the least amount of protrusion of the mouth and V. breidohri has the greatest. Moreover, V. hartwegi should possess a functionally intermediate form.
The dentary and articular are fused to form the lower oral jaw. The dentary is located in the anterior position, supporting the oral teeth and participating directly in the capture of food (Albertson & Kocher, 2001). In this bone, the greatest variation was observed in the height of the dentigerous area and the length of the ascending and ventral processes. All of these characters are functionally associated with bite force during food capture, with a greater bite force present when the dentigerous area is high and the length of the dorsal process is lower (Albertson et al., 2003b). Therefore, it can be inferred that bite force is likely higher in V. breidohri and V. hartwegi than in V. bifasciata.
In the articular, the majority of variation was found in the length of the coronoid process and the size of the articular facet. The coronoid process works as a lever during the closing of the mouth, and there is a direct relationship between its length and bite force (Albertson & Kocher, 2001; Otten, 1981). Under these conditions, V. hartwegi is expected to have the highest bite force. Regarding the shape of the articular facet, jaw support increases during the opening of the mouth when the ventral edge is extended (Albertson & Kocher, 2001; Otten, 1981). Based on this relationship, we expect that V. breidohri and V. bifasciata, which exhibit wider articular facets, have greater jaw support than V. hartwegi.
LPJ shape exhibited a high level of variation, with differences in the orientation of the lateral processes and the length of the anterior process. Functionally, the pharyngeal jaw processes food before it passes to the digestive tract (Barel, 1983; Burress et al., 2020; Hulsey et al., 2019; Liem, 1973). The shape of this structure is related to the bite force of the pharyngeal jaw, which increases when the distance between the lateral processes is greater and the anterior process is short (Burress et al., 2019; Muschick et al., 2011). According to our results, and from a functional perspective, V. hartwegi should have the strongest pharyngeal bite, followed by V. bifasciata, while V. breidohri should have the weakest.
Finally, the number and shape of pharyngeal teeth play a fundamental role in cichlid feeding (Burress, 2016; Liem, 1973). Vieja bifasciata was observed to have more teeth, while the differences between V. breidohri and V. hartwegi were not significant in this regard. All species of Vieja have been classified as omnivores (Říčan et al., 2016). Omnivorous species typically possess more pharyngeal teeth, which are usually used to manipulate and select food using the pharyngeal jaw (Burress, 2016). Therefore, we expect that V. bifasciata can manipulate food more efficiently than V. hartwegi and V. breidohri. Besides the number of teeth, another important aspect is the shape of the teeth and their arrangement in the LPJ. Notably, teeth can have various shapes and sizes. Those located on the back of the LPJ are often stronger and more specialized, while those on the front of the LPJ are used to grasp and transport food (Burress, 2016; Hellig et al., 2010; Liem, 1973). The shape and arrangement of these teeth were previously confirmed in a qualitative review of the LPJ (Gómez-González et al., 2018; Taylor & Miller, 1980). On the LPJ, teeth are conical in shape with the cusp facing the front and a second small cusp at the base. The anteriorly located teeth are small and increase in size toward the back, while the medial teeth are arranged in series with the posterior teeth being noticeably larger and more robust (Gómez-González et al., 2018).
Inferences regarding bite force can be tested via biomechanical analysis (Hulsey et al., 2005), but also, they can be explained by information on the diet and feeding behavior of species. The diet of V. bifasciata includes soft items such as detritus, plants, and algae (Pease et al., 2018; Soria-Barreto et al., 2019); in contrast, the diet of V. hartwegi includes plants and invertebrates (Conkel, 1993). Although the diet of V. breidohri remains unknown, the type of dentition on its LPJ suggests a diet based on harder prey such as mollusks and other invertebrates (Konings, 1989). To support this, we reviewed the stomachs contents of the fishes used in this analysis. The diet of V. breidohri in Lagos de Colón is composed mainly of mollusks, with a smaller proportion of seeds and fish scales. This information confirms that this species is omnivorous with a tendency to consume mollusks. The shape and type teeth of the LPJ are the most conspicuous morphofunctional traits related with dietary tendencies.
The differences in osteological characters observed in the present study contradict the scarce genetic differentiation reported for these species, especially between V. breidohri and V. hartwegi (Gómez-González et al., 2018; McMahan et al., 2010; Říčan et al., 2016). Under this scenario, these morphological differences may represent a case of trophic polymorphism similar to that of the widely documented case of the endemic species Herichthys minckleyi (Cohen et al., 2005; Hulsey et al., 2005, 2006; Sage & Selander, 1975) and the Amphilophus spp. complex (Barluenga & Meyer, 2010; Muschick et al., 2011), where trophic morphology is related to various ecological factors that include the distribution of food resources.
The LPJ is one of the most commonly analyzed structures in different groups of cichlids because it has diversified under sympatric conditions and its variation has been reflected in trophic adaptations that have favored the differential use of food resources and segregation of ecological niches (Burress, 2016; Takahashi & Koblmuller, 2011). In this sense, our results for V. breidohri and V. hartwegi are remarkably congruent with those reported for polymorphic species of the genus Amphilophus and Herichthys, which have no substantial differences in the structures that make up the oral jaw but exhibit differences in the form and dentition of the LPJ (Burress, 2016; Hulsey, 2006; Hulsey et al., 2005, 2016; Muschick et al., 2011; Pérez-Miranda et al., 2019).
In this study, the degree of differentiation and directions of change in the shape of the 4 bones do not have a defined pattern. A lack of integration between the components of both jaws may result from the modular organization of trophic structures (Burress et al., 2020; Cooper et al., 2011). While the oral jaw has the functional purpose of capturing food, the pharyngeal jaw processes food before it moves on to the digestive tract (Barel, 1983; Burress et al., 2020; Hulsey et al., 2019; Liem, 1973). This functional organization has also been observed in other parts of the skull for some cichlids (Parsons et al., 2018), even between the structures that control the opening and closing of the jaw (Albertson et al., 2005; Parsons et al., 2011, 2012).
Vieja bifasciata generally exhibits greater morphological differences, which are likely associated with geographic isolation and ecological interactions with other cichlids that share their area of distribution. In the case of the sympatric species V. breidohri and V. hartwegi, gradual differentiation was observed that sometimes overlaps. This morphological overlap has also been observed in the skulls of Herichthys species, where it is considered the result of selective and genetic restrictions. However, head shape differences have been observed between sympatric sister species (Pérez-Miranda et al., 2020).
In cichlids there is evidence of trophic structures from oral and mandibular jaws that have an underlying genetic basis (Fruciano et al., 2016). Making interpretations about the taxonomic value of these characters could be risky since their expression could result from phenotypic plasticity linked to ecological factors such as the availability of food resources or habitat preferences (Gómez-González et al., 2018; Říčan et al., 2016). Therefore, we support the need for further morphofunctional and ecomorphological studies to assess relationships between diet and the phenotypic expression of the trophic apparatus in cichlid fishes (Feilich & López-Fernández, 2019). This is particularly important for V. hartwegi, which shows enormous morphological variation throughout its geographic distribution and during growth (Gómez-González et al., 2018). Likewise, it is necessary to perform further genetic studies to assess the degree of differentiation between species and its effect on the phenotypic expression of oral and pharyngeal jaws.
From a biomechanical perspective, minor morphological differences can affect the capture and processing of food (Barel, 1983; Cooper et al., 2010; Kassam et al., 2004). The trophic anatomy of cichlids is one of the most complex and interesting functional systems since it can be highly variable and responds to multiple environmental and historical factors (Burress, 2016).
Acknowledgments
To the anonymous reviewers who provided helpful comments that improved the manuscript. Also to James Albert (ULL) and Caleb McMahan (FMNH) for the revision and suggestions provided that helped to improve this manuscript. To Julio Cesar Llanes for the map used in figure 1. Financial support for this study was received from the Project: “Conectividad y diversidad funcional de la cuenca del río Usumacinta” (Fondo de Investigación Científica y Desarrollo Tecnológico de El Colegio de la Frontera Sur, FID-784), coordinated by RRH. This manuscript is the result of the undergraduate thesis of KFRM.
References
Albertson, R. C., & Kocher, T. D. (2001). Assessing morphological differences in an adaptive trait: a landmark-based morphometric approach. Journal of Experimental Zoology, 289, 385–403. https://doi.org/10.1002/jez.1020
Albertson, R. C., Streelman, J. T., & Kocher, T. D. (2003a). Genetic basis of adaptive shape differences in the cichlid head. Journal of Heredity, 94, 291–301. https://doi.org/10.1093/jhered/esg071
Albertson, R. C., Streelman, J. T., & Kocher, T. D. (2003b). Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proceedings of the National Academy of Sciences, 100, 5252–5257. https://doi.org/10.1073/pnas.0930235100
Albertson, R. C., Streelman, J. T., Kocher, T. D., & Yelick, P. C. (2005). Integration and evolution of the cichlid mandible: the molecular basis of alternate feeding strategies. Proceedings of the National Academy of Sciences, 102, 16287–16292. https://doi.org/10.1073/pnas.0506649102
Alonso, F., Terán, G. E., Aguilera, G., Říčan, O., Casciotta, J., Serra, W. S. et al. (2019). Description of a new species of the Neotropical cichlid genus Gymnogeophagus Miranda Ribeiro, 1918 (Teleostei: Cichliformes) from the Middle Paraná basin, Misiones, Argentina. Plos One, 14, 1–19. https://doi.org/10.1371/journal.pone.0210166
Barel, C. D. N. (1983). Towards a constructional morphology of cichlid fishes (Teleostei, Perciformes). Netherlands Journal of Zoology, 33, 357-424. https://doi.org/10.1163/002829683X00183
Barluenga, M., & Meyer, A. (2010). Phylogeography, colonization and population history of the Midas cichlid species complex (Amphilophus spp.) in the Nicaraguan crater lakes. BMC Evolutionary Biology, 10, 326. https://doi.org/10.1186/1471-2148-10-326
Burress, E. D. (2015). Cichlid fishes as models of ecological diversification: patterns, mechanisms, and consequences. Hydrobiologia, 748, 7–27. https://doi.org/10.1007/s10750-014-1960-z
Burress, E. D. (2016). Ecological diversification associated with the pharyngeal jaw diversity of Neotropical cichlid fishes. Journal of Animal Ecology, 85, 302–313. https://doi.org/10.1111/1365-2656.12457
Burress, E. D., Martinez, C. M., & Wainwright, P. C. (2020). Decoupled jaws promote trophic diversity in cichlid fishes. Evolution, 74, 950–961. https://doi.org/10.1111/evo.13971
Burress, E. D., Tan, M., & Wainwright, P. C. (2019). Head shape modulates diversification of a classic cichlid pharyngeal jaw innovation. American Naturalist, 194, 693–706. https://doi.org/10.1086/705392
Cohen, A. E., Hendrickson, D. A., Parmesan, C., & Marks, J. C. (2005). Habitat segregation among trophic morphs of the Cuatro Cienegas cichlid (Herichthys minckleyi). Hidrobiológica, 15, 169–181.
Conkel, D. (1993). Cichlids of North and Central America. Nueva Jersey: T. F. H.
Cooper, W. J., Parsons, K. J., McIntyre, A., Kern, B., McGee-Moore, A., & Albertson, R. C. (2010). Bentho-pelagic divergence of cichlid feeding architecture was prodigious and consistent during multiple adaptive radiations within African rift-lakes. Plos One, 5, e9551. https://doi.org/10.1371/journal.pone.0009551
Cooper, W. J., Wernle, J., Mann, K., & Albertson, R. C. (2011). Functional genetic integration in the skulls of Lake Malawi cichlids. Evolutionary Biology, 38, 316–334. https://doi.org/10.1007/s11692-011-9124-9
Feilich, K. L., & López-Fernández, H. (2019). When does form reflect function? Acknowledging and supporting ecomorphological assumptions. Integrative and Comparative Biology, 52, 358–370. https://doi.org/10.1093/icb/icz070
Fricke, R., Eschmeyer, W. N., & van der Laan, R. (2020). Eschmeyer’s catalog of fishes: genera, species, references. Retrieved on January 28, 2020. http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Fruciano, C., Franchini, P., Kovacova, V., Elmer, K. R., Henning, F., & Meyer, A. (2016). Genetic linkage of distinct adaptive traits in sympatrically speciating crater lake cichlid fish. Nature communications, 7, 12736. https://doi.org/10.1038/ncomms12736
Gómez-González, A. E., Álvarez, F., Matamoros, W. A., Velázquez-Velázquez, E., Schmitter-Soto, J. J., González-Díaz, A. A. et al. (2018). Redescription of Vieja hartwegi (Taylor and Miller 1980) (Teleostei: Cichlidae) from the Grijalva River basin, Mexico and Guatemala, with description of a rheophilic morph. Zootaxa, 4375, 371–391. https://doi.org/10.11646/zootaxa.4375.3.5
Hellig, C. J., Kerschbaumer, M., Sefc, K. M., & Koblmüller, S. (2010). Allometric shape change of the lower pharyngeal jaw correlates with a dietary shift to piscivory in a cichlid fish. Naturwissenschaften, 97, 663–672. https://doi.org/10.1007/s00114-010-0682-y
Hulsey, C. D. (2006). Function of a key morphological innovation: fusion of the cichlid pharyngeal jaw. Proceedings of the Royal Society B: Biological Sciences, 273, 669–675. https://doi.org/10.1098/rspb.2005.3375
Hulsey, C. D. (2009). Cichlid genomics and phenotypic diversity in a comparative context. Integrative and Comparative Biology, 49, 618–629. https://doi.org/10.1093/icb/icp071
Hulsey, C. D., Alfaro, M. E., Zheng, J., Meyer, A., & Holzman, R. (2019). Pleiotropic jaw morphology links the evolution of mechanical modularity and functional feeding convergence in Lake Malawi cichlids. Proceedings of the Royal Society of London, B: Biological Sciences, 286, 20182358. https://doi.org/10.1098/rspb.2018.2358
Hulsey, C. D., Fraser, G. J., & Meyer, A. (2016). Biting into the genome to phenome map: developmental genetic modularity of cichlid fish dentitions. Integrative and Comparative Biology, 56, 373–388. https://doi.org/10.1093/icb/icw059
Hulsey, C. D., Hendrickson, D. A., & García de León, F. J. (2005). Trophic morphology, feeding performance and prey use in the polymorphic fish Herichthys minckleyi. Evolutionary Ecology Research, 7, 1–22.
Hulsey, C. D., Marks, J., Hendrickson, D. A., Williamson, C. A., Cohen, A. E., & Stephens M. J. (2006). Feeding specialization in Herichthys minckleyi: a trophically polymorphic fish. Journal of Fish Biology, 68, 1399–1410. https://doi.org/10.1111/j.0022-1112.2006.01021.x
Kassam, D., Adams, D. C., & Yamaoka, K. (2004). Functional significance of variation in trophic morphology within feeding microhabitat differentiated cichlid species in Lake Malawi. Animal Biology, 54, 77–90. https://doi.org/10.1163/157075604323010060
Kolb, M., & Galicia, L. (2012). Challenging the linear forestation narrative in the Neo-tropic: regional patterns and processes of deforestation and regeneration in southern Mexico. Geographical Journal, 178, 147–161. https://doi.org/10.1080/13658816.2013.770517
Konings, A. (1989). Cichlids from Central America. Nueva Jersey: T. F. H.
Liem, K. F. (1973). Evolutionary strategies and morphological innovations: cichlid pharyngeal jaws. Systematic Zoology, 22, 425–441. https://doi.org/10.2307/2412950
López-Fernández, H., Winemiller, K. O., & Honeycutt, R. L. (2010). Multilocus phylogeny and rapid radiations in Neotropical cichlid fishes (Perciformes: Cichlidae: Cichlinae). Molecular Phylogenetics and Evolution, 55, 1070-1086. https://doi.org/10.1016/j.ympev.2010.02.020
Matamoros, W. A., McMahan, C. D., Chakrabarty, P., James, S. A., & Schaefer, J. F. (2015). Derivation of the freshwater fish fauna of Central America revisited: Myers’s hypothesis in the twenty-first century. Cladistics, 31, 177–188. https://doi.org/10.1111/cla.12081
McMahan, C. D., Geheber, A. D., & Piller, K. R. (2010). Molecular systematics of the enigmatic Middle American genus Vieja (Teleostei: Cichlidae). Molecular Phylogenetics and Evolution, 57, 1293–1300. https://doi.org/10.1016/j.ympev.2010.09.005
McMahan, C. D., Ginger, L., Cage, M., David, K. T., Chakrabarty, P., Johnston, M. et al. (2017). Pleistocene to Holocene expansion of the black-belt cichlid in Central America, Vieja maculicauda (Teleostei: Cichlidae). Plos One, 12, e0178439. https://doi.org/10.1371/journal.pone.0178439
McMahan, C. D., Matamoros, W. A., Elías, D. J., & Piller, K. R. (2019). Species or population? Systematic status of Vieja coatlicue (Teleostei: Cichlidae). Neotropical Ichthyology, 17, e190004. https://doi.org/10.1590/1982-0224-20190004
McMahan, C. D., Matamoros, W. A., Piller, K. R., & Chakrabarty, P. (2015). Taxonomy and systematics of the Herichthyins (Cichlidae: Tribe Heroini), with the description of eight new Middle American Genera. Zootaxa, 3999, 211–234. https://doi.org/10.11646/zootaxa.3999.2.3
Meyer, A. (1989). Cost of morphological specialization: feeding performance of the two morphs in the trophically polymorphic cichlid fish, Cichlasoma citrinellum. Oecologia, 80, 431–436. https://doi.org/10.1007/BF00379047
Miller, R. R., Minckley, W. L., & Norris, S. M. (2005). Freshwater fishes of Mexico. Chicago: University of Chicago Press.
Muschick, M., Barluenga, M., Salzburger, W., & Meyer, A. (2011). Adaptive phenotypic plasticity in the Midas cichlid fish pharyngeal jaw and its relevance in adaptive radiation. BMC Evolutionary Biology, 11, 116. https://doi.org/10.1186/1471-2148-11-116
Oliver, M. K. (2018). Six new species of the cichlid genus Otopharynx from Lake Malaŵi (Teleostei: Cichlidae). Bulletin of the Peabody Museum of Natural History, 59, 159–197. https://doi.org/10.3374/014.059.0204
Otten, E. (1981). The development of a mouth-opening mechanism in a generalized Haplochromis species: H. elegans Trewavas, 1933 (Pisces, Cichlidae). Netherlands Journal of Zoology, 32, 31–48. https://doi.org/10.1163/002829682X00021
Parsons, K. J., Cooper, W. J., & Albertson, R. C. (2011). Modularity of the oral jaws is linked to repeated changes in the craniofacial shape of African cichlids. International Journal of Evolutionary Biology, 2011, 641501. https://doi.org/10.4061/2011/641501
Parsons, K. J., Marquez, E., & Albertson, R. C. (2012). Constraint and opportunity: the genetic basis and evolution of modularity in the cichlid mandible. American Naturalist, 179, 64–78. https://doi.org/10.1086/663200
Parsons, K. J., Son, Y. H., Crespel, A., Thambithurai, D., Killen, S., Harris, M. P. et al. (2018). Conserved but flexible modularity
in the zebrafish skull: implications for craniofacial evolvabi-
lity. Proceedings of the Royal Society B Biological Sciences, 285, 20172671. https://doi.org/10.1098/rspb.2017.2671
Pease, A. A., Mendoza-Carranza, M., & Winemiller K. O. (2018). Feeding ecology and ecomorphology of cichlid assemblages in a large Mesoamerican river delta. Environmental Biology of Fishes, 101, 867–879 https://doi.org/10.1007/s10641-018-0743-1
Pérez-Miranda, F., Mejía, O., González-Díaz, A. A., Martínez-Méndez, N., Soto-Galera, E., Zúñiga, G. et al. (2020). The role of head shape and trophic variation in the diversification of the genus Herichthys in sympatry and allopatry. Journal of Fish Biology, 96, 1370–-1378. https://doi.org/10.1111/jfb.14304
Pérez-Miranda, F., Mejía, O., Zúñiga, G., Soto-Galera, E., & Říčan, O. (2019). Feeding ecomorphologies in the fish genus Herichthys (Perciformes: Cichlidae) based on stomach content and lower pharyngeal jaw shape. Revista de Biología Tropical, 67, 643–653. https://doi.org/10.15517/RBT.V67I3.33616
Říčan, O., Piálek, L., Dragová, K., & Novák, J. (2016). Diversity and evolution of the Middle American cichlid fishes (Teleostei: Cichlidae) with revised classification, Vertebrate Zoology, 66, 1–102.
Rohlf, F. J. (2016). tpsDIG, Version 2.26. Digitize landmarks and outlines for geometric morphometrics. Department of Ecology and Evolution, State University of New York, Stony Brook, NY, USA. http://life.bio.sunysb.edu/morph/
Sage, R. D., & Selander, R. K. (1975). Trophic radiation through polymorphism in cichlid fishes. Proceedings of the National Academy of Sciences, 72, 4669–4673. https://doi.org/10.1073/pnas.72.11.4669
Sheets, H. D. (2014). IMP Series. IMP-integrated morphometrics package. Department of Physics, Canisius College, Buffalo, NY. Available from http://www.canisius.edu/~sheets/; http://www3.canisius.edu/~sheets/IMP%208.html
Soria-Barreto, M., Rodiles-Hernández, R., & González-Díaz, A. A. (2011). Morfometría de las especies de Vieja (Cichlidae) en ríos de la cuenca del Usumacinta, Chiapas, México. Revista Mexicana de Biodiversidad, 82, 569–579. https://doi.org/10.22201/ib.20078706e.2011.2.460
Soria-Barreto, M., Rodiles-Hernández, R., & Winemiller, K. O. (2019). Trophic ecomorphology of cichlid fishes of Selva Lacandona, Usumacinta, Mexico. Environmental Biology of Fishes, 102, 985–996. https://doi.org/10.1007/s10641-019-00884-5
SPSS Inc. Released. (2006). SPSS for Windows, Version 15.0. Chicago, SPSS Inc
Steindachner, F. (1864). Beiträge zur Kenntniss der Chromiden Mejico’s und Central-Amerika’s. Denkschriften der Kaiserlichen Akademie der Wissenschaften in Wien, Mathematisch-Naturwissenschaftliche Classe, 23, 57–74.
Takahashi, T., & Koblmuller, S. (2011). The adaptive radiation of cichlid fish in lake Tanganyika: a morphological perspective. International Journal of Evolutionary Biology, 2011, 1–14. https://doi.org/10.4061/2011/620754
Taylor, J. N., & Miller, R. R. (1980). Two new cichlid fishes, genus Cichlasoma, from Chiapas, Mexico. Occasional Papers of the Museum of Zoology University of Michigan, 693, 1–16.
Taylor, W. R., & van Dyke, G. C. (1985). Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium, 9, 107–119.
Trewavas, E. (1983). Tilapiine fishes of the genera Sarotherodon, Oreochromis and Danakilia. London: British Museum (Natural History). https://doi.org/10.5962/bhl.title.123198
Vaca, R., Golicher, D. J., Rodiles-Hernández, R., Castillo-Santiago, M. A., Bejarano, M., & Navarrete-Gutiérrez, D. A. (2019). Drivers of deforestation in the basin of the Usumacinta River: Inference on process from pattern analysis using generalised additive models. Plos One, 14, e0222908. https://doi.org/10.1371/journal.pone.0222908
Waltzek, T. B., & Wainwright, P. C. (2003). Functional morphology of extreme jaw protrusion in Neotropical cichlids. Journal of Morphology, 257, 96–106. https://doi.org/10.1002/jmor.10111
Werner, U., & Stawikowski, R. (1987). Ein neuer Buntbarsch aus Südmexiko: Paratheraps breidohri gen. nov., spec. nov. Die Aquarien- und Terrarienzeitschrift (DATZ), 41, 20–23.
Witte, F., & van Oijen, M. J. P. (1990). Taxonomy, ecology and fishery of haplochromine trophic groups. Zoologische Verhandelingen Leiden, 262, 1–47.
Witte, F., & Witte-Maas, E. L. M. (1987). Implications for taxonomy and functional morphology of intraspecific variation in haplochromine cichlids of Lake Victoria. In F. Witte (Ed.), From form to fishery (pp. 35–118). Leiden, The Netherlands: Leiden University,
Zelditch, M. L., Swiderski, D. L., & Sheets, H. D. (2012). Geometric morphometrics for biologists – a primer. London: Elsevier.