Distribution and habitat of the Golden Eagle (Aquila chrysaetos) in Sonora, Mexico, 1892-2019
Aaron D. Flesch (a), *, Ricardo Rodríguez-Estrella (b), Juan Pablo Gallo-Reynoso (c), Lucila Armenta-Méndez (c), Marcelino Montiel-Herrera (d)
a University of Arizona, School of Natural Resources and the Environment, The Desert Laboratory on Tumamoc Hill, 1675 W. Anklam Road, Tucson, AZ 85745 USA
b Centro de Investigaciones Biológicas del Noroeste, S.C., Conservation and Environmental Planning, Planning Program, Av. Instituto Politécnico Nacional 195, 23096 La Paz, Baja California Sur, Mexico
c Laboratorio de Ecofisiología, Centro de Investigación en Alimentación y Desarrollo A.C., Carretera al Varadero Nacional, Km. 6.6, Colonia Las Playitas, 85480 Guaymas, Sonora, Mexico
d Departamento de Medicina y Ciencias de la Salud, Universidad de Sonora, Edificio 7K Blvd. Luis Donaldo Colosio y Reforma s/n Colonia Centro, 83000 Hermosillo, Sonora, Mexico
*Corresponding author: flesch@email.arizona.edu (A.D. Flesch)
Abstract
The Golden Eagle (Aquila chrysaetos) is listed as threatened in Mexico but there is little information on populations in the state of Sonora. We amalgamated 121 records of the Golden Eagle in Sonora between 1892 and 2019, including 49 observations by the authors between 1997 and 2016. Observations were from all months of the year, peaked during the breeding season and again in December with 53.7% representing likely breeding individuals. Most observations were from the Sky Islands region and Sonoran Desert of northern Sonora, with fewer from coastal west-central and especially southern Sonora. Most observations were from grasslands (34.3%), Madrean evergreen woodland (31.3%), and desert-scrub (30.3%), and very few were from subtropical forest and thorn-scrub (≤ 2.0%) suggesting preference for open vegetation communities that foster hunting. We found evidence of recent occupation of all general portions of Sonora that were occupied historically, except in central Sonora due possibly to changes in land use and land cover. Although our results suggest broad-scale distribution has been largely stable, more focused monitoring is needed to understand population trends. Large-scale urban and agricultural development, loss of grasslands and other open environments, electrocution, and poisoning pose major threats to Golden Eagles in Sonora.
Keywords: Birds of prey; Conservation; Deserts; Grasslands; Madrean Sky Islands; Raptors; Threats
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Distribución y hábitat del águila real (Aquila chrysaetos) en Sonora, Mexico, 1892-2019
Resumen
El águila real (Aquila chrysaetos) es una especie amenazada en México; en Sonora se conoce poco sobre su situación actual, distribución y hábitat. Aquí reportamos 121 registros de águila real obtenidos entre 1892 y 2019 en Sonora; 49 registros nuestros entre 1997 y 2016. El águila se observó durante todos los meses del año, la mayoría en temporada de apareamiento y en diciembre. El 53.7% de los registros correspondieron a probables individuos reproductivos. La mayoría de las observaciones fue en las “islas del cielo” del noreste de Sonora y en el desierto sonorense región norte; en menor número en la costa central y en el sur del estado. Frecuentemente se registró el ave en pastizales semidesérticos (34.3%), en bosques siempre verdes de la sierra Madre (31.3%), en matorral desértico (30.3%) y raramente en bosques subtropicales y matorral espinoso (≤ 2.0%), sugiriendo preferencia por vegetación abierta. Los registros del águila sugieren altos niveles de persistencia en el tiempo ya que hubo coincidencias entre avistamientos recientes e históricos, excepto en áreas del centro del estado, posiblemente debido a cambios de uso de suelo y cobertura vegetal. En conjunto, los registros indican estabilidad poblacional del águila que requiere monitoreo estratégico ya que enfrenta amenazas antropogénicas. Los desarrollos agrícolas y urbanos a gran escala, la pérdida de pastizales y de otros ambientes abiertos, la electrocución y el envenenamiento, son las amenazas más grandes para el águila real en Sonora.
Palabras claves: Aves de presa; Conservación; Desierto; Pastizales; Islas del Cielo; Rapaces; Amenazas
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
The Golden Eagle (Aquila chrysaetos) occurs in temperate regions across the northern hemisphere (Kochert et al., 2002). In North America, the subspecies A. c. canadensis is among the largest birds of prey, occurs in a variety of open and semi-open vegetation communities from deserts to tundra, and generally avoids densely forested environments (Johnsgard, 1990). Such environments provide cliffs or tall trees for nesting, and expansive open space for hunting. Golden Eagles are long-lived, have low fecundity, and with a consistent prey base and limited anthropogenic disturbance can exhibit high degrees of population stability (Kochert & Steenhof, 2002; Millsap et al., 2013; Watson, 2010).
Golden Eagles (GOEA) are indicative of undisturbed environments and depend on small to medium-sized mammals ranging in mass from 0.5-4.0 kg and sufficient visibility to detect prey (Marzluff et al., 1997; Steenhof et al., 1997; Watson, 2010; Watson et al., 1992). Although they feed primarily on leporids (cottontails and jackrabbits) and sciurids (ground squirrels and prairie dogs), GOEA also consume various birds and reptiles, coyotes, pronghorn fawns, and rarely insects (Ceballos & Pacheco, 2000; Harrison & Hallingstad, 2018; Preston et al., 2017; Stahlecker et al., 2009). Thus, large-scale changes in land use and land cover such as urbanization, agricultural development, conversion of open country into woodlands or plantations, and natural disturbance such as wildfire can influence vital and population growth rates by altering habitat, prey abundance, and prey availability (Kochert et al., 1999; Marquiss et al., 1985; Marzluff et al., 1997; Whitfield et al., 2001).
Despite intensive study in the USA and Europe (Kochert et al., 2002; Watson, 2010), information on the ecology and conservation of GOEA in Mexico is limited but increasing (Campos-Rodríguez et al., 2018; de León-Girón et al., 2016; Farías et al., 2016; Guerrero-Cárdenas et al., 2012; Nocedal et al., 2010; Nocedal & Zúñiga-Fuentes, 2012; Rodríguez-Estrella, 2002). GOEA are among the national symbols of Mexico, listed as threatened by the Mexican federal government, and of major conservation concern (Semarnat, 2010). In Mexico, GOEA inhabit open valleys and mountainous regions dominated by desert-scrub, grassland, shrubland, and open woodland, and are distributed broadly in the north and center of the country (Ceballos & Márquez-Valdelamar, 2000). Despite recent studies, major gaps of knowledge on the ecology, population trends, and threats to GOEA remain in Mexico.
Sonora is the second largest state in Mexico, supports vast areas of open country that may function as habitat for GOEA, and neighbors a broadly distributed population in adjacent Arizona, USA (Corman & Wise-Gervais, 2005). Information on the status and ecology of GOEA in Sonora, however, remains sparse. Van Rossem (1945) described GOEA as uncommon residents across northern Sonora but noted very few records. Howell and Webb (1995) classified GOEA as permanent residents in northeastern Sonora and winter visitors in the northwest. Russell and Monson (1998) described GOEA as rare permanent residents in mountainous regions of northern Sonora but reported only 21 localities and 1 nest, and noted possible declines driven by poisoning and trapping. Recently, Flesch (2008, 2018) described GOEA as a rare breeding species in 7 watershed regions in northern Sonora, and
noted actual or potential breeding populations in 13 of 26 Sky Island mountain ranges surveyed in northern and eastern Sonora between 2009 and 2012. More recently, Lafón-Terrazas et al. (2016) observed GOEA in 9 Sky Island mountain ranges, including 2 new ranges (Tigre, Oposura) not documented previously.
We assessed the status, distribution, and habitat of GOEA in Sonora based on over 3 decades of field experience (1984-2017). In addition to our own observations, we amalgamated historical and recent (1892-2019) records from published and unpublished sources, and used these data to evaluate broad-scale patterns of habitat use, potential changes in distribution, and implications for conservation and management.
Materials and methods
Sonora supports a variety of biomes and most major vegetation communities that occur in Mexico (Rzedowski, 1978). Western Sonora includes a vast arid to semi-arid coastal plain interspersed with mountain ranges that rarely reach sufficient elevation to support montane vegetation. To the east, several major north-south river valleys drain more than 30 Sky Island mountain ranges covered by temperate grassland, shrubland, woodland, and forest, which form interior valleys and foothills of the Sierra Madre Occidental (Warshall, 1995).
The Sonoran Desert of western Sonora includes the entire Plains of Sonora and portions of 3 of the 5 other subdivisions of the Sonoran Desert (Shreve, 1951). In arid northwestern Sonora, the Lower Colorado River Valley subdivision is dominated by open uplands of shrubs and subshrubs with stands of trees largely restricted to drainages (Brown, 1982). The Central Gulf Coast subdivision occupies a narrow band along the Gulf of California in west-central Sonora where cacti and stem succulents are dominant. Arizona upland desert-scrub in north-central Sonora is dominated by woodland and scrub of short trees, large columnar cacti, and many other life forms. The more mesic Plains of Sonora are dominated by open woodland and scrub with fewer cacti, and exotic grasslands. Semi-desert grasslands occur above Sonoran desert-scrub, with smaller areas of Chihuahuan desert-scrub and plains grassland at higher elevations in the northeast.
Highland temperate vegetation of Madrean affinity and lowland subtropical vegetation of Neotropical affinity replace desert-scrub and grassland in eastern and southern Sonora. Madrean evergreen woodland and forest of oaks (Quercus spp.) and pines (Pinus spp.) cover areas above ~ 1,200 m in the east (Brown, 1982). Coastal thornscrub occupies portions of the southwestern coastal plain that have not been developed for agriculture or cites, and at higher elevations, foothills thornscrub occupies uplands often below 900 m in central Sonora. Thornscrub is composed of dense, drought deciduous short trees, shrubs, and succulents often 2-8 m tall. In the southeast, thornscrub transitions to tropical deciduous forest that is often replaced by temperate vegetation above ~ 1,000 m.
We gathered observations of GOEA during surveys of diurnal birds and other wildlife, and opportunistically throughout Sonora with efforts focused between 1998 and 2018. Field effort by ADF and associates occurred during surveys of the Ferruginous Pygmy-owl (Glaucidium brasilianum) along 1,113 km of transects across lowland (< 1,200 m) Sonora in 2000 and 2001 (Flesch, 2003; Flesch & Steidl, 2007), research and monitoring of pygmy-owls in northern Sonora in 2001-2016 (Flesch, 2014, 2017; Flesch et al., 2010; Flesch, Hutto et al., 2015), and bird surveys in east-central Sonora in 2007-2013 (Flesch, Warshall et al., 2015) and northern Sonoran in 2000-2007 (Flesch, 2008; Flesch & Hahn, 2005). In highlands, ADF and associates surveyed 289 km of transects in montane vegetation in 26 Sky Islands and 4 areas in the Sierra Madre Occidental in Sonora in May-July 2009-2012 (Flesch, 2018; Flesch et al., 2016).
Effort by RRE and associates included 3 days of surveys in Sonora in 1984 (Rodríguez-Estrella, 2002) and surveys of the Pinacate Biosphere Reserve in northwestern Sonora in February and June 2015. Surveys in Pinacate were focused at 21 sampling points spaced 3-4 km apart where different observers simultaneously recorded GOEA and other raptors during 1-hr periods. This procedure allowed assessment of whether detections by different observers at nearby points were the same individuals based on timing and locations.
Effort by JPG focused on surveys of wild mammals in the Sierra el Aguaje from 1997 to 2017, and in Sierra Madre Occidental between Yécora and Basaseachi in 1998 and Sierra Huachinera in 1998-2018. To supplement field work, we also used data from automated motion-triggered cameras placed in the Sierra el Aguaje in west-central Sonora, and on the Northern Jaguar Reserve and near Mesa Tres Ríos in eastern Sonora.
We compiled data from various sources to supplement our observations and establish historical baselines from which potential changes in status and distribution could be assessed. First, we gathered records from Van Rossem (1945), Marshall (1957), Russell and Monson (1998), and literature cited therein, and used a database provided to ADF by S. M. Russell to attribute dates, locations, and other observation details. Second, we digitized a few mapped records not annotated in these sources and used VertNet (available at: http://vertnet.org/) to gather details on specimens. Third, we considered records from the Madrean Archipelago Biodiversity Assessment (MABA) database (available at: http://madrean.org/) that includes observational records from field surveys across Sonora. Fourth, we searched published and unpublished sources for additional records, used records from recent GOEA survey from northern Sonora (Lafón-Terrazas et al., 2016), and requested observations from various colleagues. Finally, we gathered records from Christmas Bird Counts (CBC, available at: http://netapp.audubon.org/CBCObservation/) in Sonora through 2017 and used the center of count circles to attribute approximate locations.
We assessed the timing, breeding status, vegetation community, watershed identity, and elevation associated with each observation across time. We presumed individuals in potential breeding habitat during the nesting and fledgling-dependency periods were breeding when observed at times migrants are typically not present (March-early September; Corman & Wise-Gervais, 2005; Kochert et al., 2002; Nocedal & Zúñiga-Fuentes, 2012). Confirmed breeding was based on presence of nests or dependent young. Individuals observed outside this period were classified as migrants or winter residents, although some were undoubtedly resident breeders. We classified the status of individuals in non-breeding habitat or areas we were unsure provided breeding habitat as unknown. We used geographic information systems to attribute observations to primary and secondary watersheds and estimate elevations. For vegetation, we used aerial imagery, vegetation maps, and field notes to classify the dominant formation around each observation in 4 categories (desert-scrub, grassland, shrubland or open woodland, temperate closed woodland or forest, and subtropical closed woodland or forest), and community in 9 categories (4 Sonoran Desert subdivisions, Chihuahuan desert-scrub, semi-desert or plains grassland, thorn-scrub, tropical deciduous forest, Madrean evergreen woodland and forest). To assess potential changes in distribution, we compared the presence and location of observations across 3 time periods, 1 historical (1892-1996) and 2 recent (1997-2008, 2009-2019) based on the timing of records. Before analyses, we censored observations that likely represented the same individuals based on timing (within 1 month but often on the same days) and location (within ~ 5-10 km), and observations of the same nest structures in years after initial detection.
Results
We obtained 121 records of GOEA between 1892 and 2019, including 51 observations by the authors between 1984 and 2016, 96% of which were from 1997-2016 (Supplementary material-Appendix A). Excluding 13 observations that were likely not independent, most observations (53.7%) were of presumed breeders including 5 that were confirmed breeding (4.7%) and 13 of pairs. Additionally, 38.0% of observations were classified as winter residents or migrants, and 8.3% as unknown. Five nests occupied between 16 Feb. and 3 June contained 1-2 young. Observations were from all months of the year, peaked between Feb. and Sept. and again in December, with fewer in fall and in January (Fig. 1). Three recent observations were photographs from camera traps from the Northern Jaguar Reserve, near Mesa Tres Ríos, and in the Sierra el Aguaje (Supplementary material-Appendix B). Across all observations, 21.3% were attributed to ADF and colleagues, 17.6% to Russell and Monson (1998) and observers cited therein, 12.0% to Lafón-Terrazas et al. (2016), 12.0% to observers noted in the MABA database, 10.2% to JPG or LAM, 9.3% to RRE and colleagues, 8.3% to the CBC, 5.6% to Van Rossem (1945) and observers cited therein, and 3.7% to other sources (Supplementary material-Appendix A).
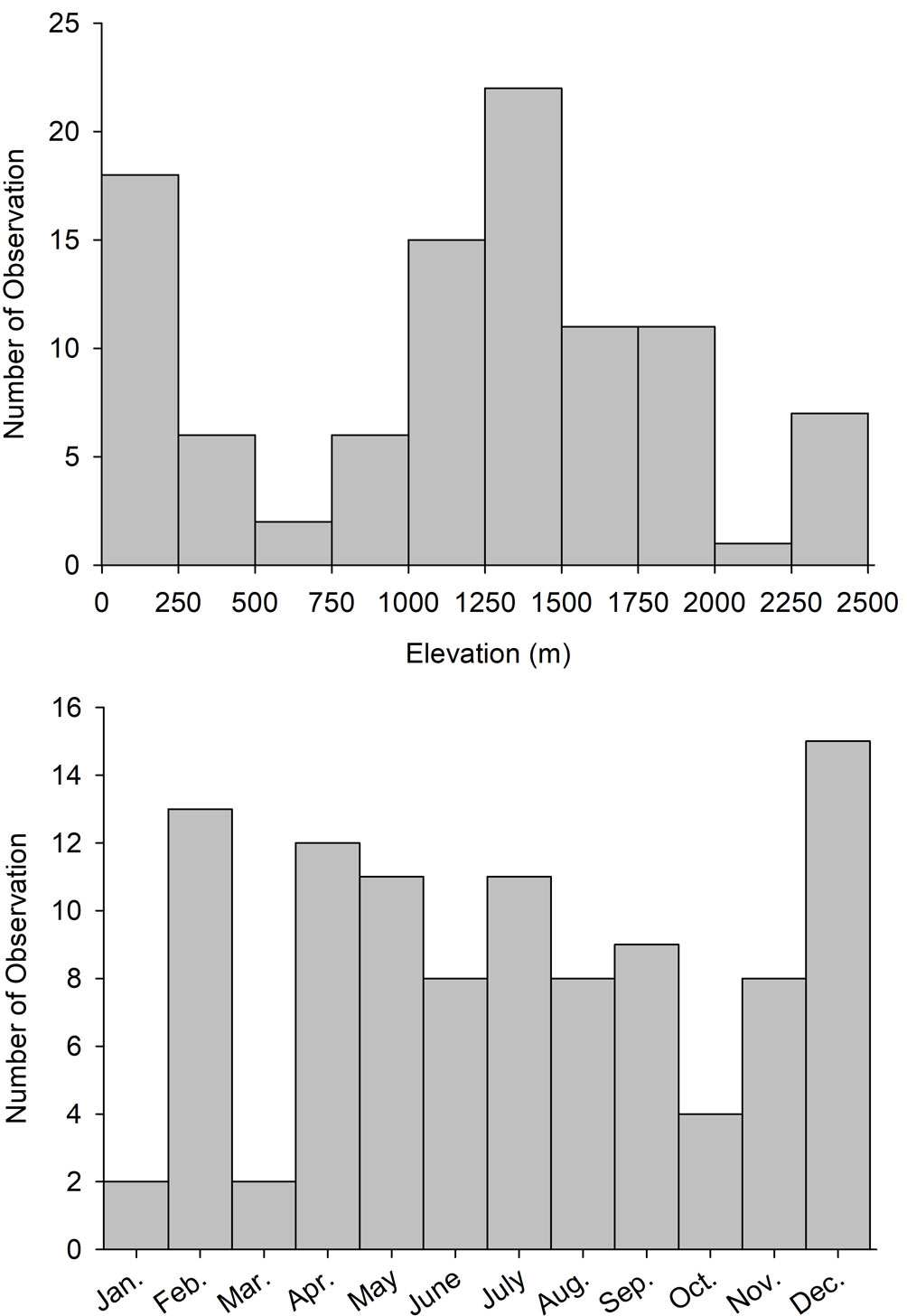
GOEA were broadly distributed across the Sky Islands region of northeast Sonora, in areas near the northern terminus of the Sierra Madre Occidental in eastern Sonora, and in portions of the borderlands of north-central and northwestern Sonora (Fig. 2). Fewer observations were from central Sonora where they were concentrated in 2 mountain ranges (Aguaje, Libre) near the central coast. Very few observations were from southern Sonora with none on the southwestern coastal plain. Most observations were from the vast Yaqui watershed (33.3%) with some in the Concepción, Sonoyta, Sonora, and Gila watersheds (12.0-13.9%), and fewer from 4 other watersheds and coastal regions (Fig. 3). In the Yaqui watershed, the majority of observations were from the largely temperate Bavispe sub-basin (65.7%) compared to the more sub-tropical Moctezuma (14.3%), lower Yaqui (11.4%), and Aros (8.6%) sub-basins. Observations ranged in elevation from 2 to 2,450 m asl, averaged 1,164 ± 68 (± SE) m overall, and peaked between 1,250 and 1,500 m. There were also numerous observations along the coast and from low deserts below 250 m, and around tall peaks > 2,250 m (Fig. 1).
Most observations were from grassland and shrubland vegetation formations (34.7%), with somewhat fewer in temperate woodland and forest (31.6%) and desert-scrub (30.6%). Among communities, most observations were from semi-desert or plains grassland (34.3%), Madrean evergreen woodland and forest (31.3%), and Sonoran desert-scrub (28.3%). Within the Sonoran Desert, most records were from in the Lower Colorado River Valley subdivision with fewer in the Central Gulf Coast (21.4%), Plains of Sonora (17.9%), and Arizona Upland (10.7%) subdivisions. Observations in Chihuahuan desert-scrub, tropical deciduous forest, and foothills thorn-scrub were rare (2.0%; Fig. 3).


Few observations were from the 1890s (3.9%), 1930s (1.9%), 50s (2.9%), and 70s (2.9%), with progressively more from the 80s (6.8%), 90s (13.6%), and 2000-09 (30.1%), and 2010-19 (37.9%). Despite being less numerous, observations from the historical period (n = 30) were distributed broadly (Fig. 4). In comparison, recent observations spanned all general areas of historical occurrence with some exceptions. These exceptions were all in central Sonora and included 2 in the central plains, 1 in the Sierra de Aconchi, and 1 on Isla Tiburón. Recent observations also included many new regions and mountain ranges such as the Sierra el Aguaje in west-central Sonora, various desert mountain ranges in north-central and northwest Sonora, numerous Sky Island mountain ranges in the northeast, near Yécora in east-central Sonora, and near Álamos in the extreme southeast.
Discussion
We documented Golden Eagles (GOEA) across much of northern Sonora and amalgamated over 5 times the number of observations reported by past accounts of the species in Sonora. Our results indicate GOEA are more broadly distributed in the Sky Islands region of north-central and northeast Sonora than previously known.

Moreover, we described the first observations of this species in and around numerous desert mountain ranges in northwest Sonora as far west as the hyper-arid Pinacate Biosphere Reserve, and more locally in portions of coastal west-central Sonora. Presence of nests and pairs in some of these areas in western Sonora combined with observations of adults throughout the breeding season, suggest GOEA breed in these arid environments. Such patterns contrast with past distributional accounts in western Sonora (Howell & Webb, 1995; Russell & Monson, 1998), but conform to observed patterns in adjacent Arizona (Corman & Wise-Gervais, 2005) and the Baja California peninsula in Mexico (de León-Girón et al., 2016). As in the northern Sonoran Desert (Millsap, 1981), however, we suspect GOEA do not nest every year in the more arid portions of their range in Sonora where nesting may depend on sufficient levels of annual primary productivity.
Assuming frequency of observation is positively associated with current densities, our results suggest GOEA are more abundant at moderate to high elevations in the Sky Islands region than in the western deserts. Such patterns may be driven by more variable and less predictable prey availability in more arid regions, and by limited nest-site availability in some areas where mountains with sheltered cliff faces are absent or rare. Although there are few estimates of home range size in arid southwestern North America, available data and known nearest-neighbor distances among nests suggest much lower densities in arid regions (Braham et al., 2015; Millsap, 1981) than in more mesic northern or high-elevation regions (Marzluff et al., 1997; Kochert et al., 2002), which is also suggested by our results and those from Arizona (Corman & Wise-Gervais, 2005).
In addition to observations across much of northern Sonora and more locally in central Sonora, we also found evidence of recent occupancy in the extreme southeast near Álamos. In one instance, this included an adult observed in late April, which is typically after migrants and winter residents have departed for their northern breeding grounds. Data reported here and elsewhere (Kochert et al., 2002; Rodríguez-Estrella, 2002), however, indicate GOEA typically avoid densely forested environments with structure similar to that of tropical deciduous forest, which dominate lowlands and foothills in southeastern Sonora. Increasing coverage of man-made vegetation clearings in tropical deciduous forest (Calderón-Aguilera et al., 2012) combined with extreme freezing events that disturbed vast areas of sub-tropical vegetation in February 2011 and 2013 (Bojórquez et al., 2019), may have augmented habitat area in this region beyond that already present locally in open pre-montane woodlands and shrublands at moderate elevations. More study in southeast Sonora is needed to better understand the status of GOEA in this region.
Populations of GOEA across the western USA seem to be largely stable but there are some suggestions of small declines in southwestern regions (Millsap et al., 2013; Nielson et al., 2014). To the south in Mexico where resident populations are smaller, there is little evidence of recent population declines but data scarcity and lack of systematic monitoring limit our understanding of population trends (de León-Girón et al., 2016; Nocedal & Zúñiga-Fuentes, 2012). In Sonora, we found evidence of recent occupation of nearly all general regions of historical occurrence suggesting, at least at broad scales, that distribution has been largely stable. Although such patterns suggest high levels of persistence and population stability, more rigorous monitoring at smaller scales is needed to understand population dynamics. Areas of historical occurrence where we found no evidence of recent occupancy have been little surveyed in recent decades (e.g., northern Plains of Sonora), and GOEA may still occur in these and other regions. Presence of a large number of new localities and regions of occupation that we report is likely due to limited past survey effort combined with extremely low natural densities of this species in arid regions of southwestern North America (Braham et al., 2015; Millsap, 1981).
Throughout their range, GOEA use a wide variety of open and semi-open environments but generally avoid extensive areas of forest and highly modified environments such as agricultural and urban areas (Johnsgard, 1990; Kochert et al., 2002; Marzluff et al., 1997). Such environments provide habitat for leporids, sciurids, and other preferred prey, and sufficient open space to foster hunting (Watson, 2010). Given the natural rarity of safe nesting sites in many of these open environments, GOEA are frequently observed near cliffs and rocky outcrops even those embedded in wooded or forested landscapes (Kochert et al., 2002; Marzluff et al., 1997; Watson, 2010). Data we report for Sonora match these patterns of broad-scale habitat use. For example, we found GOEA were more frequently observed in semi-desert and Plains grasslands despite relatively low spatial coverage of these vegetation communities on the landscape (Brown, 1982). Secondarily, GOEA were also frequently observed in Madrean evergreen woodland and forest, but at these higher elevations individuals were often detected at either relatively low (e.g., 1,000-1,500 m) or high elevations around tall peaks. Such areas typically support more open oak woodland and savannah where grasses and scattered shrubs predominate, which best foster hunting, and rocky outcrops and cliffs, which foster nesting. Finally, relatively high frequency of observation in Sonoran desert-scrub was weighted heavily toward more open vegetation of the Lower Colorado River Valley subdivision despite higher aridity, and least toward denser more mesic thorn-scrub like Arizona Uplands. In general, Sonora supports vast expanses of relatively open arid and semi-arid environments that in many cases seem to function as habitat for this threatened species in Mexico.
In Sonora, GOEA are present throughout the year with clear peaks in frequency of observation during both the wintering and breeding seasons. As in neighboring Baja California, Mexico (Rodríguez-Estrella et al., 1991), observations from Sonora suggest presence of both a non-resident migratory population in fall and winter, and a resident breeding population that nests in late winter, spring, and early summer. Presence of each of these population segments during overlapping time periods complicated our evaluations of status and breeding distribution somewhat. In many cases, individuals we presumed were winter residents or migrants may have been resident breeders, especially those detected in February in the western deserts where nesting begins earlier (ADF personal observation; Corman & Wise-Gervais, 2005; Millsap, 1981). Similarly, some birds we presumed were breeders may have been non-breeders, especially immature individuals. Long-distance migration by juvenile GOEA can entail progressively greater movements from natal areas during hatch years, and broad exploratory movements on wintering grounds where individuals may remain for more than 12 months before moving north to breed (Murphy et al., 2017; McIntyre et al., 2008; Soutullo et al., 2006). These immature individuals may use large areas of Sonora that overlap the home ranges of 1 or more breeding pairs before they move north to breed.
Developing optimal conservation and management strategies for GOEA in Sonora necessitates detailed knowledge of their ecology and primary threats. With regard to habitat use, our results suggest conservation strategies for GOEA in Sonora should focus specifically on protecting and restoring native grassland, open temperate woodland, shrubland, and desert-scrub, and cliffs and other suitable nest structures within these and other nearby vegetation communities. Given high frequency of occurrence and low landscape coverage, native semi-desert and Plains grasslands seem especially important to this species in Sonora. Grassland communities and associated bird species, however, are highly threatened in North America with alarming patterns of degradation and loss in northwest Mexico (Pool et al., 2014) and in the adjacent USA and Canada (Rosenberg et al., 2019) in recent decades. To address such threats, active measures to conserve and restore grasslands, reduce shrub encroachment, and discourage overgrazing and persecution of important prey species such as prairie dogs (Cynomys spp.) are needed.
In addition to habitat loss and degradation, a broad suite of direct threats to GOAE exist in North America (Katzner, Smith et al. 2012; Kochert & Steenhof, 2002), and many of these threats are highly relevant in Sonora and elsewhere in northern Mexico. Carcasses laced with poisons targeting animals that are perceived to be predators of livestock are serious threats to wild felids, bears, and other native mammals in northwest Mexico (Delfín-Alfonso et al., 2012). Such poisons are known to kill GOEA in the western USA (Kochert & Steenhof, 2002), constitute major threats to GOEA in Sonora, and must be controlled and regulated before the benefits of habitat conservation and restoration can be fully realized. Similarly, incidental captures in leg-hold traps and snares set for mammals can be a significant source of mortality, as can lead poisoning from ingestion of ammunition in carrion in regions where hunting is common (Katzner, Smith et al., 2012; Kramer & Redig, 1997). Electrocution from power poles is a major cause of raptor mortality in the western USA, especially for large species such as GOEA, which are more likely to make contact with energized wires and components (Harness & Wilson, 2001; Kochert & Steenhof, 2002). Efforts to retrofit power poles by increasing separation among components, insulating components, and adding materials to discourage perching on poles are essential to mitigate these threats, but must be installed carefully (Dwyer et al., 2017). Industrial-scale production of wind energy also poses significant threats to GOEA in western North America (Kochert & Steenhof, 2002). Although such facilities are not yet present in Sonora (Comisión de Energía del Estado de Sonora, 2019), careful planning and design combined with detailed information on travel routes and flight behavior of migratory and resident eagles (e.g., Bedrosian et al., 2018; Katzner, Brandes et al., 2012) should help mitigate these threats in the future. Finally, educational programs that highlight the importance of raptors in controlling small mammal populations and that correct the perception that eagles pose major threats to livestock should be developed to target landowners, cowboys, youth, and other groups. Tailoring conservation, mitigation, and educational measures to local contexts, cultures, and threats are essential for conservation of these majestic birds of prey.
Acknowledgements
To the many landowners and managers that facilitated field work including M. Mackenzie, J. L. Romero for helping in the Sierra Huachinera, Consejo Nacional de Ciencia y Tecnología for granting L. Armenta’s doctoral studies, G. Carreon of Naturalia and M. Gómez Ramírez of Northern Jaguar Project for pictures from the Northern Jaguar Reserve, and Northern Jaguar Project and Asociación Conservación del Norte for access and support of monitoring on the Northern Jaguar Reserve. We also thank Victor Hugo Cabrera and José Roberto García Martínez for pictures from Mesa Tres Ríos, S. Winckler for a photo from near Álamos, and Janitzio Égido-Villarreal for support. Finally, ADF thanks D. Ellis for sharing his insights into GOEA ecology in the region.
References
Bedrosian, B., Domenech, R., Shreading, A., Hayes, M., Booms, T. L., & Barger, C. (2018). Migration corridors of adult Golden Eagles originating in northwestern North America. Plos One, 13, e0205204. https://doi.org/10.1371/journal.pone.0205204
Bojórquez, A., Álvarez-Yépiz, J. C., Búrquez, A., & Martínez-Yrízar, A. (2019). Understanding and predicting frost-induced tropical tree mortality patterns. Global Change Biology, 25, 3817‒3828. https://doi.org/10.1111/gcb.14775
Braham, M., Miller T., Duerr A., Lanzone, M., Fesnock, A., LaPre, L. et al. (2015). Home in the heat: dramatic seasonal variation in home range of desert Golden eagles informs management for renewable energy development. Biological Conservation, 186, 225‒232. https://doi.org/10.1016/j.biocon.2015.03.020
Brown, D. (1982). Biotic communities of the American Southwest-United States and Mexico. Desert Plants, 4, 1‒342. https://doi.org/10.2307/1223522
Calderon-Aguilera, L. E., Rivera-Monroy, V. H., Porter-Bolland, L., Martínez-Yrízar, A., Ladah, L. B., Martínez-Ramos, M. et al. (2012). An assessment of natural and human disturbance effects on Mexican ecosystems: current trends and research gaps. Biodiversity and Conservation, 21, 589‒617. https://doi.org/10.1007/s10531-011-0218-6
Campos-Rodríguez, J., Flores-Leyva, X., Pérez-Valera, D., & García-Martínez, D. (2018). Anidación del águila real en el sureste de Zacatecas, México. Huitzil Revista Mexicana de Ornitología, 20, 1‒13. https://doi.org/10.28947/hrmo.2019.20.1.394
Ceballos, G., & Márquez-Valdelamar, L. (2000). Las aves de México en peligro de extinción. México D.F.: Instituto de Ecología, Universidad Nacional Autónoma de México/ Comisión Nacional para el Conocimiento y Uso de la Biodiversidad/ Fondo de Cultura Económica.
Ceballos, G., & Pacheco, J. (2000). Los perros llaneros de Chihuahua: importancia biológica y conservación. Biodiversitas, 31, 1‒5. https://doi.org/10.31381/horizonte_empresarial.v0i13.481
Comisión de Energía del Estado de Sonora (2019). Renewable energy projects as the Megaregion Sonora-Arizona. Gobierno del Estado de Sonora. Available at: http://www.coees.sonora.gob.mx
Corman, T. E., & Wise-Gervais, C. (2005). Arizona breeding bird atlas. Albuquerque, NM: University of New Mexico Press.
de León-Girón, G., Rodríguez-Estrella, R., & Ruiz-Campos, G. (2016). Current distribution status of Golden Eagle (Aquila chrysaetos) in northwestern Baja California, Mexico. Revista Mexicana de Biodiversidad, 87, 1328‒1335. https://doi.org/10.1016/j.rmb.2016.10.003
Delfín-Alfonso, C. A., López-González, C. A., & Equihua, M. (2012). Potential distribution of American black bears in northwest Mexico and implications for their conservation. Ursus, 23, 65‒78. https://doi.org/10.2192/ursus-d-11-00007.1
Dwyer, J. F., Harness, R. E., & Eccleston, D. (2017). Avian electrocutions on incorrectly retrofitted power poles. Journal of Raptor Research, 51, 293‒305. https://doi.org/10.3356/jrr-16-93.1
Farías, V., Hernández, O., Arizmendi, M. C., Téllez, O., Botello, F., Olivares, S. J. et al. (2016). Registro notable de Águila Real (Aquila chrysaetos) en la Reserva de la Biosfera Tehuacán-Cuicatlán, Puebla, México. Revista Mexicana de Biodiversidad, 87, 1153–1158. https://doi.org/10.1016/j.rmb.2016.06.001
Flesch, A. D. (2003). Distribution, abundance, and habitat of Cactus Ferruginous Pygmy-owls in Sonora Mexico (M.S. Thesis). University of Arizona, Tucson, AZ. USA.
Flesch, A. D. (2008). Distribution and status of breeding landbirds in northern Sonora Mexico. Studies in Avian Biology, 37, 28‒45.
Flesch, A. D. (2014). Spatiotemporal trends and drivers of population dynamics in a declining desert predator. Biological Conservation, 175, 110‒118. https://doi.org/10.1016/j.biocon.2014.04.021
Flesch, A. D. (2017). Influence of local and landscape factors on distributional dynamics: a species-centered fitness-based approach. Proceedings of the Royal Society of London B: Biological Sciences, 284, 20171001. https://doi.org/10.1098/rspb.2017.1001
Flesch, A. D. (2018). Patterns and drivers of long-term changes in breeding bird communities in a global biodiversity hotspot in Mexico. Diversity and Distribution, 25, 499–513. https://doi.org/ 10.1111/ddi.12862
Flesch, A. D., Epps, C. W., Cain, J. W., Clark, M., Krausman, P. R., & Morgart, J. R. (2010). Potential effects of the United States-Mexico border fence on wildlife. Conservation Biology, 24,171‒181. https://doi.org/10.1111/j.1523-1739.2009.01277.x
Flesch, A. D., & Hahn, L. E. (2005). Distribution of birds and plants at the western and southern edges of the Madrean Sky Island region in Sonora, Mexico. In G. J. Gottfried, B. S. Gebow, L. G. Eskew, & C. B. Edminster (Eds), Connecting mountain islands and desert seas: biodiversity and management of the Madrean Archipelago II (pp. 80–87). Fort Collins, Colorado: Department of Agriculture, Forest Service, Rocky Mountain Research Station.
Flesch, A. D., Hutto, R. L., Van Leeuwen, W. J., Hartfield, K., & Jacobs, S. (2015). Spatial, temporal, and density-dependent components of habitat quality for a desert owl. Plos One, 10, e0141178. https://doi.org/10.1371/journal.pone.0141178
Flesch, A. D., Sánchez, C. G., & Amarillas J. V. (2016). Abundance and habitat relationships of breeding birds in the Sky Islands and adjacent Sierra Madre Occidental of northwest Mexico. Journal of Field Ornithology, 87, 176‒195. https://doi.org/10.1111/jofo.12151
Flesch, A. D., & Steidl, R. J. (2007). Detectability and response rates of ferruginous pygmy-owls. Journal of Wildlife Management, 71, 981‒990. https://doi.org/10.2193/2006-081
Flesch, A. D., Warshall, P., & Jacobs, S. (2015). Avian richness, status, and conservation in the northwestern Neotropics in Sonora, México. Natural Areas Journal, 35, 288‒296. https://doi.org/10.3375/043.035.0209
Guerrero-Cárdenas, I. G., Gallina-Tessaro, P., Álvarez-Cárdenas, S., & Mesa-Zavala, E. (2012). Avistamientos recientes de Águila Real (Aquila chrysaetos) en la sierra El Mechudo, Baja California Sur, México. Revista Mexicana de Biodiversidad, 83, 397‒401. https://doi.org/10.7550/rmb.26780
Harness, R. E., & Wilson, K. R. (2001). Electric-utility structures associated with raptor electrocutions in rural areas. Wildlife Society Bulletin, 29, 612‒623. https://www.jstor.org/stable/3784188
Harrison, J. T., & Hallingstad, E. (2018). Direct Observation of Insectivory by a Golden Eagle. Journal of Raptor Research, 52, 261‒263. https://doi.org/10.3356/jrr-17-65.1
Howell, S. N. G., & Webb, S. (1995). A guide to the birds of México and Northern Central America. New York: Oxford University Press.
Johnsgard, P. A. (1990). Hawks, eagles, and falcons of North America. Washington, D.C. Smithsonian Institution Press.
Katzner, T. E., Brandes, D., Miller, T., Lanzone, M., Maisonneuve, C., Tremblay, J. A. et al. (2012). Topography drives migratory flight altitude of Golden Eagles: implications for on-shore wind energy development. Journal of Applied Ecology, 49, 1178‒1186. https://doi.org/10.1111/j.1365-2664.2012.02185.x
Katzner, T., Smith, B. W., Miller, T. A., Brandes, D., Cooper, J., Lanzone, M. et al. (2012). Status, biology, and conservation priorities for North America’s eastern Golden Eagle (Aquila chrysaetos) population. Auk, 129,168‒176. https://doi.org/10.1525/auk.2011.11078
Kochert, M. N., & Steenhof, K. (2002). Golden Eagles in the U.S. and Canada: Status, trends, and conservation challenges. Journal of Raptor Research, 36, 32–40.
Kochert, M. N., Steenhof, K., Carpenter, L. B., & Marzluff, J. M. (1999). Effects of fire on Golden Eagle territory occupancy and reproductive success. The Journal of Wildlife Management, 63, 773‒780. https://doi.org/10.2307/3802790
Kochert, M. N., Steenhof, K., McIntyre, C. L., & Craig, E. H. (2002). Golden Eagle (Aquila chrysaetos). In A. F. Poole, & F. B. Gill (Eds.), The birds of North America. Ithaca: Cornell Lab. of Ornithology. https://birdsna.org/Species-Account/bna/species/goleag.
Kramer, J. L., & Redig, P. T. (1997). Sixteen years of lead poisoning in eagles, 1980-95: an epizootiologic view. Journal of Raptor Research, 31, 327‒332.
Lafón-Terrazas, A., Cirett-Girón, J. M., Esquer-Robles, J. A., Higuera-Martínez, F. J., & Macías-Duarte, A. (2016). Monitoreo de la situación poblacional del águila real en las islas del Cielo, Informe final. Hermosillo, Sonora: Programa de Conservación de Especies en Riesgo (PROCER), Comisión Nacional de Áreas Naturales Protegidas.
Marquiss, M., Ratcliffe, D. A., & Roxburgh, R. (1985). The numbers, breeding success and diet of Golden Eagles in southern Scotland in relation to changes in land use. Biological Conservation, 34, 121–140. https://doi.org/10.1016/0006-3207(85)90104-1
Marshall, J. T. (1957). Birds of pine-oak woodland in southern Arizona and adjacent Mexico. Pacific Coast Avifauna 32. Berkeley: Cooper Ornithological Society.
Marzluff, J. M., Knick, S. T., Vekasy, M. S., Schueck, L. S., & Zarriello, T. J. (1997). Spatial use and habitat selection of Golden Eagles in southwestern Idaho. Auk, 114, 673‒687. https://doi.org/10.2307/4089287
McIntyre, C. L., Douglas, D. C., & Collopy, M. W. (2008). Movements of Golden Eagles (Aquila chrysaetos) from interior Alaska during their first year of independence. The Auk, 125, 214‒224. https://doi.org/10.1525/auk.2008.125.1.214
Millsap, B. A. (1981). Distributional status of Falconiformes in west central Arizona with notes on ecology, reproductive success and management. Phoenix: Department of Interior, Bureau of Land Management, Phoenix District Office.
Millsap, B. A., Zimmerman, G. S., Sauer, J. R., Nielson, R. M., Otto, M., Bjerre, E. et al. (2013). Golden Eagle population trends in the western United States: 1968–2010. The Journal of Wildlife Management, 77, 1436‒1448. https://doi.org/10.1002/jwmg.588
Murphy, R. K., Dunk, J. R., Woodbridge, B., Stahlecker, D. W., LaPlante D. W., Millsap, B. et al. (2017). First-year dispersal of Golden Eagles from natal areas in the Southwestern United States and implications for second year settling. Journal of Raptor Research, 51, 216‒233. https://doi.org/10.3356/jrr-16-80.1
Nielson, R. M., McManus, L., Rintz, T., Mcdonald, L. L., Murphy, R. K., Howe, W. H. et al. (2014). Monitoring abundance of Golden Eagles in the western United States. The Journal of Wildlife Management, 78, 721‒730. https://doi.org/10.1002/jwmg.704
Nocedal, J., Zúñiga-Fuentes, A., & Arroyo, S. I. (2010). El águila real (Aquila chrysaetos) en el Estado de Durango, México: distribución e implicaciones para su protección y conservación. El Canto del Cenzontle, 1, 134‒147.
Nocedal, J., & Zúñiga-Fuentes, A. (2012). Biología de la nidificación del águila real (Aquila chrysaetos) en el sur del desierto chihuahuense, México. El Canto del Cenzontle, 3, 26‒37.
Pool, D. B., Panjabi, A. O., Macías-Duarte, A., & Solhjem, D. M. (2014). Rapid expansion of croplands in Chihuahua, Mexico threatens declining North American grassland bird species. Biological Conservation, 170, 274–281. https://doi.org/10.1016/j.biocon.2013.12.019
Preston, C. R., Jones, R. E., & Horton, N. S. (2017). Golden Eagle diet breadth and reproduction in relation to fluctuations in primary prey abundance in Wyoming’s Bighorn Basin. Journal of Raptor Research, 51, 334‒346. https://doi.org/10.3356/jrr-16-39.1
Rodríguez-Estrella, R. (2002). A survey of Golden Eagles in northern Mexico in 1984 and recent records in central and southern Baja California Peninsula. Journal of Raptor Research, 36, 3‒9.
Rodríguez-Estrella, R., Llinas, J. G., & Cancino, J. (1991). New Golden Eagle records from Baja California. Journal of Raptor Research, 25, 68‒71.
Rosenberg, K. V., Dokter, A. M., Blancher, P. J., Sauer, J. R., Smith, A. C., Smith, P. A. et al. (2019). Decline of the North American avifauna. Science, 366, 120‒124. https://doi.org/10.1126/science.aaw1313
Russell, S. M., & Monson, G. (1998). The birds of Sonora. Tucson: University of Arizona Press.
Rzedowski, J. (1978). La vegetación de Mexico. Mexico D.F.: Limusa-Wiley.
Semarnat (Secretaría de Medio Ambiente y Recursos Naturales). (2010). (2010). Norma Oficial Mexicana NOM-059 SEMARNAT-2010. Protección ambiental – Especies nativas de México de flora y fauna silvestres – Categoría de riesgo y especificaciones para su inclusión, exclusión o cambio – Lista de especies de riesgo. Diario Oficial de la Federación. 30 de diciembre de 2010, Segunda Sección, México.
Shreve, F. (1951). Vegetation of the Sonoran Desert. Washington D.C.: Carnegie Institution of Washington.
Soutullo, A., Uríos, V., Ferrer, M., & Peñarrubia, S. G. (2006). Dispersal of Golden Eagles Aquila chrysaetos during their first year of life. Bird Study, 53, 258–264. https://doi.org/10.1080/00063650609461441
Stahlecker, D. W., Mikesic, D. G., White, J. N., Shaffer, S., de Long, J. O., Blakemore, M. R. et al. (2009). Prey remains in nests of four corners Golden Eagles, 1998-2008. Western Birds, 40, 301‒306.
Steenhof, K., Kocher, M. N., & Mcdonald, T. L. (1997). Interactive effects of prey and weather on Golden Eagle reproduction. Journal of Animal Ecology, 66, 350‒362. https://doi.org/10.2307/5981
Van Rossem, A. J. (1945). A distributional survey of the birds of Sonora, Mexico (No. 21). Baton Rouge: Louisiana State University Press.
Warshall, P. (1995). The Madrean Sky Island Archipelago in biodiversity and management of the Madrean Archipelago: the Sky Islands of Southwestern United States and Northwestern Mexico. Fort Collins: USDA Forest Service General Technical Report RM-GTR-264. Rocky Mountain Research Station.
Watson, J., Rae, S. R., & Stillman, R. (1992). Nesting density and breeding success of golden eagles in relation to food supply in Scotland. Journal of Animal Ecology, 61, 543–550. https://doi.org/10.2307/5609
Watson, J. (2010). The Golden Eagle, Second Ed. Connecticut. Yale University Press.
Whitfield, D. P., McLeod, D. R., Fielding, A. H., Broad, R. A., Evans, R. J., & Haworth, P. F. (2001). The effects of forestry on Golden eagles on the island of Mull, western Scotland. Journal of Applied Ecology, 38, 1208‒1220. https://doi.org/10.1046/j.0021-8901.2001.00675.x