Latitudinal variation in structure and function of conspicuous reef fish assemblages along the western Gulf of California
Francisco Javier Fernández-Rivera Melo a, b, Héctor Reyes-Bonilla a,* Georgina Ramírez-Ortiz c, Lorenzo Alvarez-Filip d
a Departamento de Ciencias Marinas y Costeras, Universidad Autónoma de Baja California Sur, Carretera al sur km 5.5., Col. El Mezquitito, 23080 La Paz, Baja California Sur, Mexico
b Comunidad y Biodiversidad, A.C. Isla del Peruano No. 215, 85448 Guaymas, Sonora, Mexico
c Posgrado en Uso, Manejo y Preservación de Recursos Naturales, Centro de Investigaciones Biológicas del Noroeste, 02320 La Paz, Baja California Sur, Mexico
d Unidad Académica de Sistemas Arrecifales, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México, 77580 Puerto Morelos, Quintana Roo, Mexico
* Corresponding author: hreyes@uabcs.mx (H. Reyes-Bonilla)
Abstract
The Gulf of California is characterized by great biodiversity, high biological productivity and important fisheries. Studies on community structure of the reef fish fauna in the region have been conducted mainly in central and southern areas and, except for a few studies that have focused on cryptic species, there are no comparisons of the fish assemblages along this 1,600 km sea. In this study, we examine how diversity and community structure of rocky reef fishes vary with the latitude in the north, central and southern part of the west side of the Gulf of California. We conducted stationary visual censuses in observation cylinders (5 m radius) in 6 locations between Bahía de los Ángeles (29° N) and Los Cabos (22° N) and estimated the following ecological descriptors and indexes: species richness, abundance, Shannon diversity, Pielou evenness, average taxonomic distinctness, and average trophic level. In addition, we used ordination analysis to determine the degree of similarity among study areas. Our results identified 3 distinct zones: north (29° N), central (27° N to 24° N) and south (24° N to 22° N).
Keywords:
Ichthyofauna; Rocky reefs; Sea of Cortez; Ecological indices; Trophic level
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Variacion latitudinal en la estructura y función de la comunidad íctica conspicua arrecifal en el oeste del golfo de California
Resumen
El golfo de California se caracteriza por su gran biodiversidad, alta productividad biológica y pesquerías importantes. Los estudios sobre la estructura comunitaria de la fauna de peces de arrecife en la región se han realizado principalmente en las áreas centrales y meridionales, y salvo algunos trabajos enfocados en especies crípticas, no hay comparaciones de las asociaciones de peces a lo largo de este mar de 1,600 km. En este artículo examinamos cómo la diversidad y la estructura de la comunidad de los peces conspicuos de arrecife rocosos, varían con la latitud en la zona norte, centro y sur de la costa oeste del golfo de California. Se realizaron censos visuales estacionarios en cilindros de observación (5 m de radio) en 6 áreas entre bahía de los Ángeles (29° N) y Los Cabos (22° N) y se estimaron los siguientes descriptores e índices ecológicos: riqueza de especies, abundancia, diversidad de Shannon, uniformidad de Pielou, distintividad taxonómica promedio y nivel trófico promedio. Además, se utilizó el análisis de ordenación para determinar el grado de similitud entre las áreas de estudio. Nuestros resultados mostraron la existencia de 3 zonas definidas: norte (29° N), central (27° a 24° N) y sur (24° a 22° N).
© 2018 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Palabras clave:
Ictiofauna; Arrecifes rocosos; Mar de Cortés; Índices ecológicos; Nivel trófico
Introduction
The reef ichthyofauna of the Gulf of California is remarkably diverse; according to Thomson et al. (2000), the region hosts 872 fish species, of which 271 inhabit rocky or coral reefs. Considering the composition of the reef fish assemblages, the gulf exhibits a clear biogeographic zonation in 3 parts: north, central, and south. The first one ranges from the Colorado River delta (the extreme north; 31° N) to Tiburón Island (28° N) and includes less than 300 tropical reef fish because the communities are exposed to low temperatures in winter and strong fluctuations in productivity year-round (Robertson & Allen, 2015; Viesca-Lobatón et al., 2008). The central Gulf, with a southern limit between La Paz (24° N) in the west and Guaymas (27° N) at the east, is characterized by more tropical environments; temperature gradients are weaker, and there are no severe seasonal changes (Thomson et al., 2000). The southern region, that extends from La Paz to Cabo San Lucas (22° N) on the western coast, and from Guaymas to Cabo Corrientes (20° N) on the eastern side, comprises the entrance of the Gulf of California and has several coral reef patches similar in composition and structure to those of the Mexican tropical Pacific (Glynn et al., 2017). Also, this area is influenced by the presence of 2 water masses (from the tropical Pacific and the Gulf of California) as well as the Californian Current which contribute to variable conditions of salinity, temperature and nutrients throughout the year (Lavin & Marinone, 2003).
Although the fish community structure of reefs in the Gulf of California have been studied since the 1970s (Thomson & Gilligan, 2002), it was not until the 1990s that the majority of the information was produced, first for southern localities (< 25° N, Barjau-González et al., 2012; Pérez-España et al., 1996; Rodríguez-Romero et al., 2005), and then for the north zone (> 25° N, Campos-Dávila et al., 2005; Viesca-Lobatón et al., 2008). In addition, there are several systematic checklists for specific areas of the Gulf (Abitia-Cárdenas et al., 1994; Balart et al., 1995; Del Moral-Flores et al., 2013; Sánchez-Ortiz et al., 1997; Villarreal-Cavazos et al., 2000). However, there are only a few studies that have analyzed such patterns in fish communities of the Gulf of California on a regional scale. Thomson and Gilligan (2002) collected 68 cryptic and conspicuous fish species from 28 islands and 22 continental localities, and found significant differences between islands and the mainland in the number of individuals and species diversity. Sala et al. (2002) also analyzed the composition and diversity of reef fishes from 22° to 29° N in the gulf, and found clear gradients in species richness, with the lower figures in higher latitudes. The authors used this information as well as the presence of specific ecological and oceanographic processes, and socioeconomic information, and proposed the development of a network of marine reserves along the region based exclusively on conservation arguments. Viesca-Lobatón et al. (2008) compared fish composition and abundance in Bahía de los Ángeles (28° N), Loreto (26° N), and La Paz (24° N), finding that the numerically dominant species changed depending on latitude, and also that there was an evident seasonal replacement of species composition in all studied reefs, but nevertheless, these modifications did not alter the number of functional groups present. Finally, Ramírez-Ortiz et al. (2017) investigated morpho-functional (MFG) groups in invertebrates and fishes in the Eastern Tropical Pacific, and found high biomass and richness of MFGs in the Cortés and Oceanic Islands provinces, and a decreasing pattern of MFG richness towards the tropics which demonstrates that in the ETP, the relationship between habitat heterogeneity and species diversity has been translated into functional complexity.
In spite of these efforts, there is currently no available description of the ecological gradients of reef-fish communities that exist along the west coast of the Gulf of California, with data collected specifically at the same temporal interval and using uniform sampling techniques. As these kind of information is key to develop integral studies focused to understand the structure and function of fish assemblages in the Gulf of California, the present study aims to describe and compare latitudinal variation in community and trophic structure of conspicuous reef fish assemblages along 6 regions in the west of the Gulf of California, on the basis of a series of visits conducted in a short time interval of just 4 months.
Materials and methods
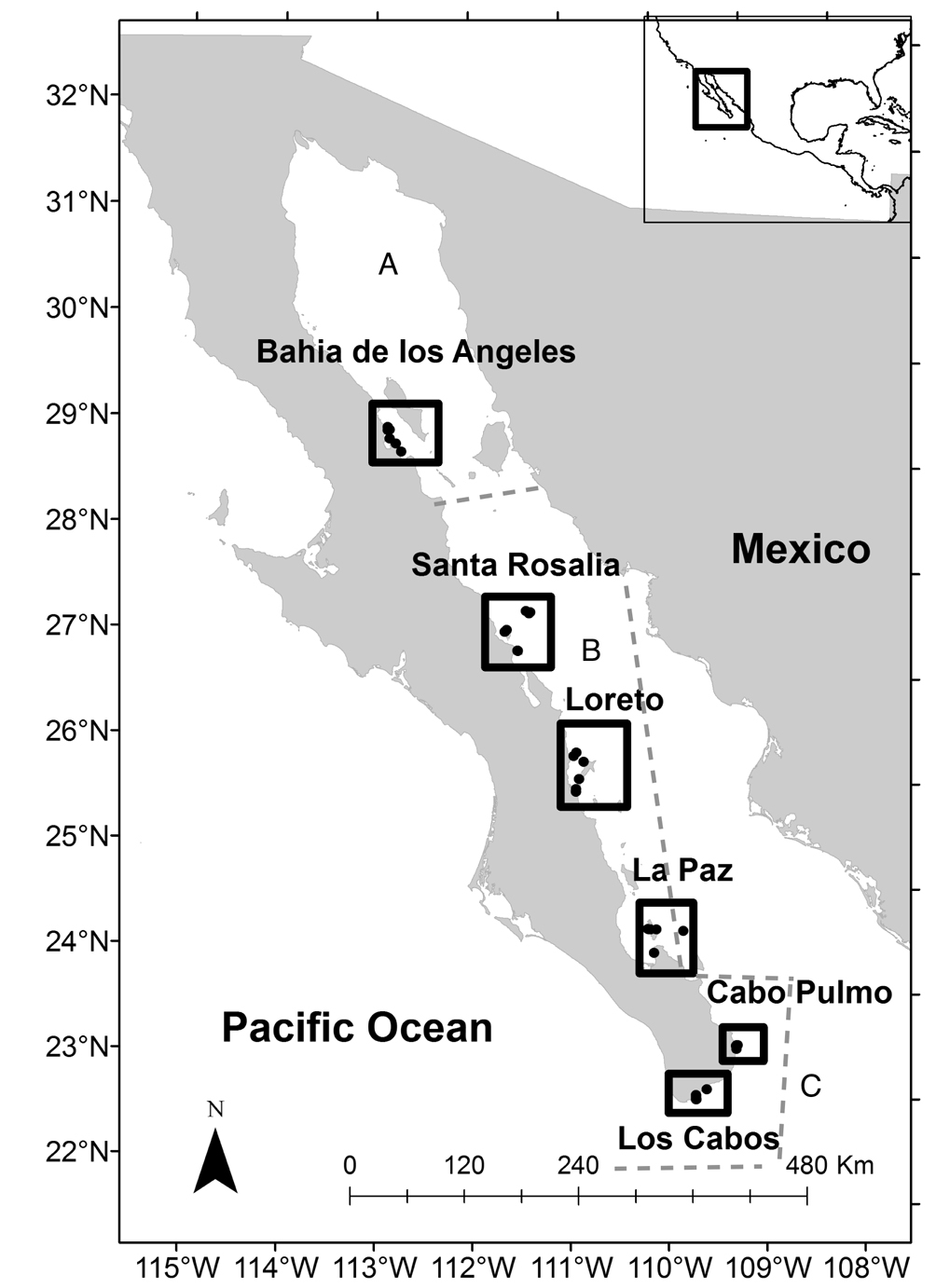
Six locations of the western coast of the Gulf of California were surveyed between August and November 2004, encompassing 850 km (Fig. 1). From north to south, the locations were as follows: Bahía de Los Ángeles (28º N; census), Santa Rosalía (27° N; 30 census), Loreto (25º N; census), La Paz (24º N; census), Cabo Pulmo (23° N; 24 census) and Los Cabos (22° N; 28 census). At each locality, fish richness and abundance were registered using the stationary census method (Bohnsack & Bannerot,1986), modified by Villarreal-Cavazos et al. (2000). This technique consists of establishing observation cylinders of 5 m radius where SCUBA diver identifies and counts all the fish that cross the census area during a period of 15 min. During this interval, 5 min involve listing the species and the other 10 min involve counting the number of individuals. The fish were counted if they displayed low abundances (up to 10 individuals), whilst the individual abundance of large schools was estimated using imaginary windows, in which the individuals in each window were counted and then the number of windows that constitute the school were calculated. The censuses were performed at depths between 5 and 8 meters.
Using the abundance per species data derived from each census, 2 frequently used indices in fish community studies were calculated: Shannon-Wiener diversity (log 10; H´) and Pielou evenness (J´). Although these indices often exhibit a lack of normality and can be biased due to sample size (Krebs, 1999), they were applied in this study to compare with previous studies in the Gulf of California. Additionally, we assessed the fish assemblages with 2 other, complementary indicators. One was the average taxonomic distinctness (Δ+) which considers the taxonomic (Linnean) distance between each pair of species as a way to incorporate qualitative aspects of the composition of taxa and is also advantageous since it is not dependent on sample size (Clarke & Warwick, 2001; Magurran, 2004; Tolimieri & Anderson, 2010). The average trophic level (NTp) was estimated per census using the trophic index of each observed species (taken from Fishbase; Froese & Pauly, 2017) and then scaled with the abundances according to the formula used by Gascuel et al. (2011).
The mean values of each of the community descriptors were compared between localities using a one-way analysis of variance (Anova, α= 0.05). Data were tested for normality and homoscedasticity with the a priori Kolmogorov-Smirnoff and Bartlett tests, respectively, (Zar, 2010). The a posteriori Tukey test was used when the index values exhibited significant differences. Only abundance was observed to be non-homogenous; therefore, the Kruskal-Wallis nonparametric test was used for this data (Zar, 2010)
To analyze the fish community composition among sites, a nonmetric multidimensional scaling (NMDS) and cluster analysis was implemented using the Bray Curtis similarity index and UPGMA coefficient (Krebs, 1999). The relative distance between points (censuses), expressed in a 2-dimension chart, and the branches in the dendrogram were used to define the groups of localities (Clarke & Warwick, 2001). The NMDS has been preferred over other ordination techniques because it adjusts well to data on species abundance and it does not require samples to be distributed normally (Clarke & Warwick, 2001).
The results of the cluster analysis and NMDS were evaluated using an analysis of similarities (Anosim), followed by a test of similarity percentages (Simper), to define the contribution of each species to the aggrupation, based on a threshold of 90% similarity. Both analysis were performed with PRIMER 6.0 software (Clarke & Warwick, 2001).
Table 1
Relative abundance of reef fishes in surveyed areas of the Gulf of California. A) Species with relative abundance higher than 5%, B) species with relative abundance from 1 to 5%); C) species with relative abundance less than 1%. Key: BLA: Bahía de los Ángeles; LOR: Loreto; LP: La Paz; CP: Cabo Pulmo; LC: Los Cabos.
|
Table 1 Continues. |
||||||||
|
Family |
Species |
BLA |
SR |
LOR |
LP |
CP |
LC |
Total |
|
Family |
Species |
BLA |
SR |
LOR |
LP |
CP |
LC |
Total |
|
Muraenidae |
Gymnothorax castaneus (Jordan & Gilbert, 1883) |
C |
C |
C |
C |
C |
||
|
Muraena lentiginosa Jenyns, 1842 |
C |
C |
C |
|||||
|
Holocentridae |
Myripristis leiognathus Valenciennes, 1846 |
C |
C |
C |
||||
|
Sargocentron suborbitalis (Gill, 1863) |
C |
C |
C |
|||||
|
Fistulariidae |
Fistularia commersonii Rüppell, 1838 |
C |
C |
C |
C |
C |
C |
|
|
Scorpaenidae |
Scorpaena plumieri mystes Bloch, 1789 |
C |
C |
C |
C |
C |
||
|
Serranidae |
Alphestes immmaculatus Breder, 1936 |
C |
C |
|||||
|
Cephalopholis panamensis (Steindachner, 1876) |
C |
C |
C |
C |
C |
C |
C |
|
|
Epinephelus analogus Gill, 1863 |
C |
C |
||||||
|
Epinephelus labriformis (Jenyns, 1840) |
C |
C |
C |
C |
C |
C |
C |
|
|
Mycteroperca rosacea (Streets, 1877) |
B |
C |
C |
C |
C |
C |
||
|
Mycteroperca xenarcha Jordan, 1888 |
C |
C |
||||||
|
Paralabrax maculatofasciatus (Steindachner, 1868) |
C |
C |
C |
|||||
|
Paranthias colonus (Valenciennes, 1846) |
C |
B |
B |
C |
C |
C |
B |
|
|
Serranus psittacinus Valenciennes, 1846 |
C |
C |
C |
C |
C |
|||
|
Carangidae |
Caranx caballus Günther, 1868 |
C |
B |
C |
||||
|
Trachinotus rhodopus Gill, 1836 |
C |
C |
||||||
|
Sciaenidae |
Pareques viola (Gilbert, 1898) |
C |
C |
C |
C |
|||
|
Lutjanidae |
Hoplopagrus guentherii Gill, 1862 |
C |
C |
C |
C |
C |
||
|
Lutjanus argentiventris (Peters, 1869) |
C |
B |
B |
C |
C |
B |
||
|
Lutjanus novemfasciatus Gill, 1862 |
C |
C |
C |
|||||
|
Lutjanus viridis (Valenciennes, 1846) |
B |
B |
C |
|||||
|
Haemulidae |
Anisotremus davidsonii (Steindachner, 1876) |
C |
C |
C |
||||
|
Anisotremus interruptus (Gill, 1862) |
C |
C |
C |
C |
C |
|||
|
Anisotremus taeniatus Gill, 1861 |
C |
C |
||||||
|
Haemulon flaviguttatum Gill, 1862 |
B |
C |
C |
|||||
|
Haemulon maculicauda (Gill, 1862) |
C |
B |
C |
B |
B |
|||
|
Haemulon sexfasciatum Gill, 1862 |
B |
C |
C |
C |
C |
C |
||
|
Haemulon steindachneri (Jordan & Gilbert, 1882) |
C |
C |
||||||
|
Mullidae |
Mulloidichthys dentatus (Gill, 1862) |
B |
C |
A |
B |
B |
||
|
Kyphosidae |
Girella simplicidens Osburn & Nicholis, 1916 |
A |
C |
C |
C |
B |
||
|
Kyphosus elegans (Peters, 1869) |
C |
C |
C |
C |
C |
C |
C |
|
|
Chaetodontidae |
Chaetodon humeralis Günther, 1860 |
C |
C |
C |
C |
C |
||
|
Forcipiger flavissimus Jordan & McGregor, 1898 |
C |
C |
||||||
|
Johnrandallia nigrirostris (Gill, 1862) |
C |
C |
C |
C |
C |
C |
C |
|
|
Pomacanthidae |
Holacanthus passer Valenciennes, 1846 |
C |
B |
B |
B |
B |
C |
B |
|
Holacanthus clarionensis Gilbert, 1890 |
C |
C |
C |
|||||
|
Pomacanthus zonipectus (Gill, 1862) |
B |
C |
C |
C |
C |
C |
||
|
Pomacentridae |
Abudefduf declivifrons (Gill, 1862) |
C |
C |
C |
C |
C |
||
|
Abudefduf troschelii (Gill, 1862) |
A |
A |
A |
A |
B |
A |
A |
|
|
Chromis atrilobata Gill, 1862 |
A |
A |
A |
A |
A |
A |
A |
|
|
Chromis limbaughi Greenfield & Woods, 1980 |
A |
C |
B |
B |
||||
|
Microspathodon bairdii (Gill, 1862) |
C |
C |
||||||
|
Microspathodon dorsalis (Gill, 1862) |
C |
C |
C |
C |
C |
C |
||
|
Stegastes acapulcoensis (Fowler, 1944) |
C |
C |
C |
C |
||||
|
Stegastes flavilatus (Gill, 1862) |
B |
B |
B |
C |
B |
B |
||
|
Stegastes rectifraenum (Gill, 1862) |
A |
A |
B |
A |
A |
B |
A |
|
|
Cirrhitidae |
Cirrhitichthys oxycephalus (Bleeker, 1855) |
B |
B |
B |
A |
B |
B |
|
|
Cirrhitus rivulatus Valenciennes, 1846 |
C |
C |
C |
C |
C |
|||
|
Mugilidae |
Mugil curema Valenciennes, 1836 |
C |
B |
C |
C |
|||
|
Labridae |
Bodianus diplotaenia (Gill, 1862) |
C |
B |
B |
B |
B |
B |
B |
|
Halichoeres chierchiae Di Caporiacco, 1948 |
C |
C |
C |
C |
C |
C |
C |
|
|
Halichoeres dispilus (Günther, 1864) |
C |
C |
C |
C |
C |
B |
C |
|
|
Halichoeres nicholsi (Jordan & Gilbert, 1882) |
B |
C |
C |
C |
C |
C |
C |
|
|
Halichoeres notospilus (Güntehr, 1864) |
C |
C |
C |
C |
||||
|
Halichoeres melanotis (Gilbert, 1890) |
C |
C |
||||||
|
Novaculichthys taeniourus (Lacepède, 1801) |
C |
C |
||||||
|
Semicossyphus pulcher (Ayres, 1854) |
C |
C |
||||||
|
Thalassoma grammaticum Gilbert, 1890 |
C |
C |
B |
B |
B |
B |
||
|
Thalassoma lucasanum (Gill, 1862) |
A |
A |
A |
A |
A |
A |
||
|
Sparidae |
Calamus brachysomus (Lockington, 1880) |
C |
C |
C |
C |
C |
||
|
Scaridae |
Nicholsina denticulata (Evermann & Radcliffe, 1917) |
C |
C |
C |
C |
C |
C |
|
|
Scarus compressus (Osburn & Nichols, 1916) |
C |
C |
C |
C |
C |
|||
|
Scarus ghobban Forsskal, 1775 |
B |
B |
B |
C |
C |
B |
||
|
Scarus perrico Jordan & Gilbert, 1882 |
C |
C |
C |
C |
C |
C |
||
|
Scarus rubroviolaceus Bleeker, 1847 |
C |
C |
C |
C |
C |
|||
|
Blenniidae |
Ophioblennius steindachneri Jordan & Evermann, 1898 |
C |
C |
C |
C |
C |
C |
|
|
Plagiotremus azaleus (Jordan & Bollman, 1890) |
C |
C |
C |
|||||
|
Acanthuridae |
Acanthurus nigricans (Linnaeus, 1758) |
C |
C |
C |
C |
|||
|
Acanthurus triostegus (Linnaeus, 1758) |
C |
C |
C |
|||||
|
Acanthurus xanthopterus Valenciennes, 1835 |
C |
C |
C |
|||||
|
Prionurus punctatus Gill, 1862 |
C |
C |
C |
A |
A |
B |
||
|
Zanclidae |
Zanclus cornutus (Linnaeus, 1758) |
C |
C |
C |
C |
|||
|
Tripterygiidae |
Crocodilichthys gracilis Allen & Robertson, 1991 |
A |
C |
B |
||||
|
Gobiidae |
Lythrypnus dalli (Gilbert, 1890) |
B |
C |
|||||
|
Balistidae |
Balistes polylepis Steindachner, 1876 |
B |
C |
C |
C |
C |
C |
|
|
Pseudobalistes naufragium (Jordan & Starks, 1895) |
C |
C |
C |
C |
||||
|
Sufflamen verres (Gilbert & Starks, 1904) |
C |
C |
C |
B |
B |
C |
||
|
Tetraodontidae |
Arothron meleagris (Anonymous, 1798) |
C |
C |
C |
||||
|
Canthigaster punctatissima (Güntehr, 1870) |
C |
B |
B |
B |
A |
B |
||
|
Sphoeroides annulatus (Jenyns, 1842) |
B |
C |
||||||
|
Sphoeroides lobatus (Steindachner, 1870) |
C |
C |
C |
C |
||||
|
Diodontidae |
Diodon holocanthus Linnaeus, 1758 |
C |
C |
C |
C |
C |
C |
C |
|
Diodon hystrix Linnaeus, 1758 |
C |
C |
C |
C |
C |
|||
|
Ostraciidae |
Ostracion meleagris Shaw, 1796 |
|
|
|
|
C |
|
C |
Results
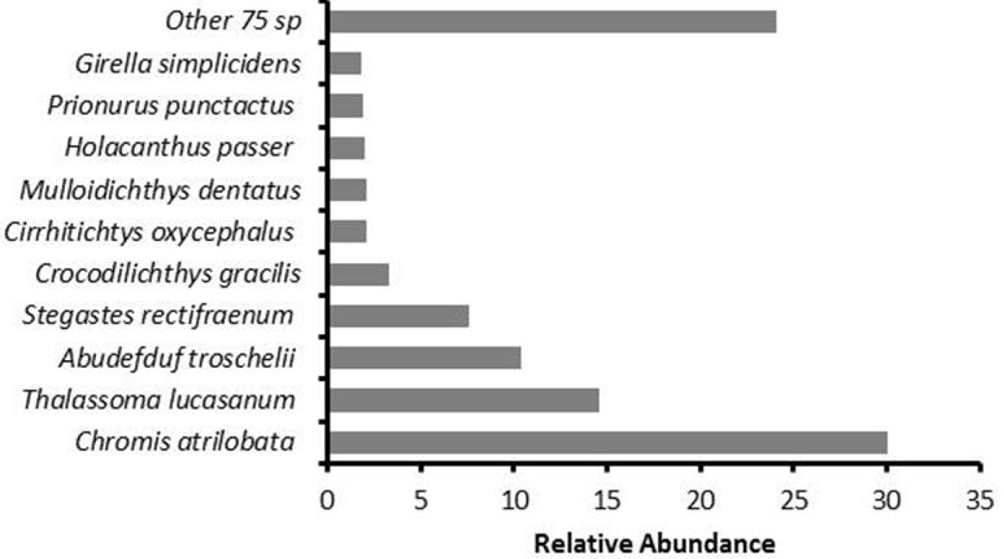
A total of 31,144 individuals were recorded in the 146 censuses; they represent 85 species, 53 genera, and 26 families (Table 1). The most abundant species were Chromis atrilobata Gill, 1862, Thalassoma lucasanum (Gill, 1862), Abudefduf troschelii (Gill, 1862), and Stegastes rectifraenum (Gill, 1862), which represented 63% of the relative abundance (Fig. 2). In addition, 15 species had moderate abundance (from 1 to 5% of the total), and 66 species exhibited abundances of less than 1%.
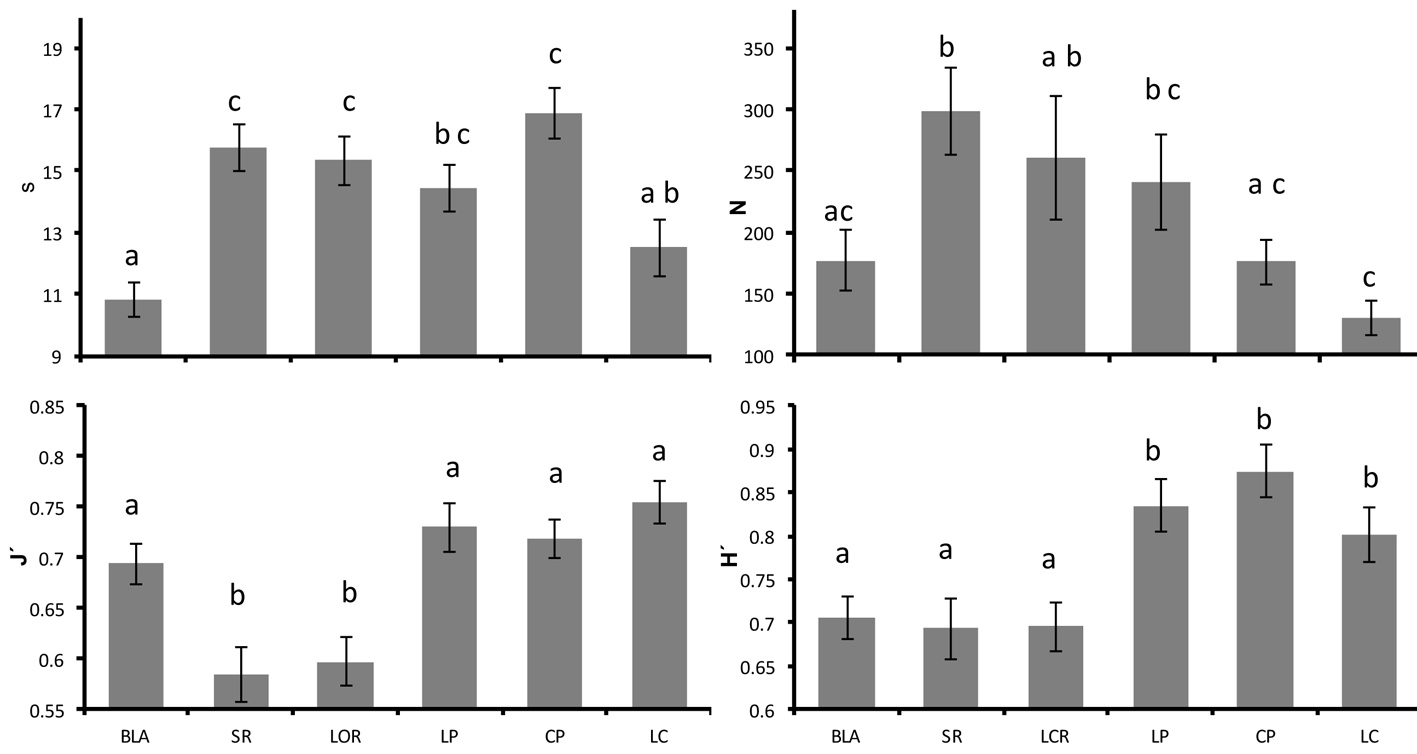
In our study, the average species richness in the western region of the Gulf of California was 14.29 ± 0.36 species per census (average ± standard error). The area with the highest richness was Cabo Pulmo (16.87 ± 0.79 spp/census), while the northernmost site (Bahía de los Ángeles) displayed the lowest (10.83 ± 0.57 spp/census). The statistical analysis showed significant differences in richness among localities (F(5,140) = 8.258, p < 0.001), and the posteriori test demonstrated that this was due to noticeable discrepancies in the number of species seen per census at Bahía de los Ángeles (lowest) and Los Cabos (highest) in comparison with the rest of the localities (Fig. 3).
On average, 213.31 ± 13.80 individuals/census were recorded in this study. However, the different localities exhibited great variability, showing a pattern of decline in abundance with respect to latitude. The differences were particularly evident between Santa Rosalía (in the central gulf) with the highest mean (297.63 ± 35.41 ind/census), and Los Cabos in the south, with the lowest mean (130 ± 13.86 ind/census; Fig. 3). The Kruskal-Wallis analysis indicated significant differences in abundance among the localities (H(5,146) = 21.887, p < 0.001).
The mean Shannon diversity values in the sampled locations in the Gulf of California was 0.76 ± 0.01. The Anova showed significant differences in H’ among localities (F(5,140) = 6.297, p = 0.003). A latitudinal trend for the Shannon diversity was also presented, with higher values in the southern Gulf (Fig. 3). The a posteriori test indicated that northern localities (Loreto, Santa Rosalía, and Bahía de los Ángeles) were similar and exhibited low diversity, while the southern localities (La Paz, Cabo Pulmo, and Los Cabos) were the most diverse sites. Complementarily, the average regional evenness was 0.67 ± 0.01, and the analysis of variance showed significant differences among localities (F(5,140) = 9.942, p < 0.001). Los Cabos, Cabo Pulmo, La Paz and Bahía de los Ángeles exhibited high evenness (J’ > 0.68), while Santa Rosalía and Loreto had the lowest (J’ < 0.60; Fig. 3).
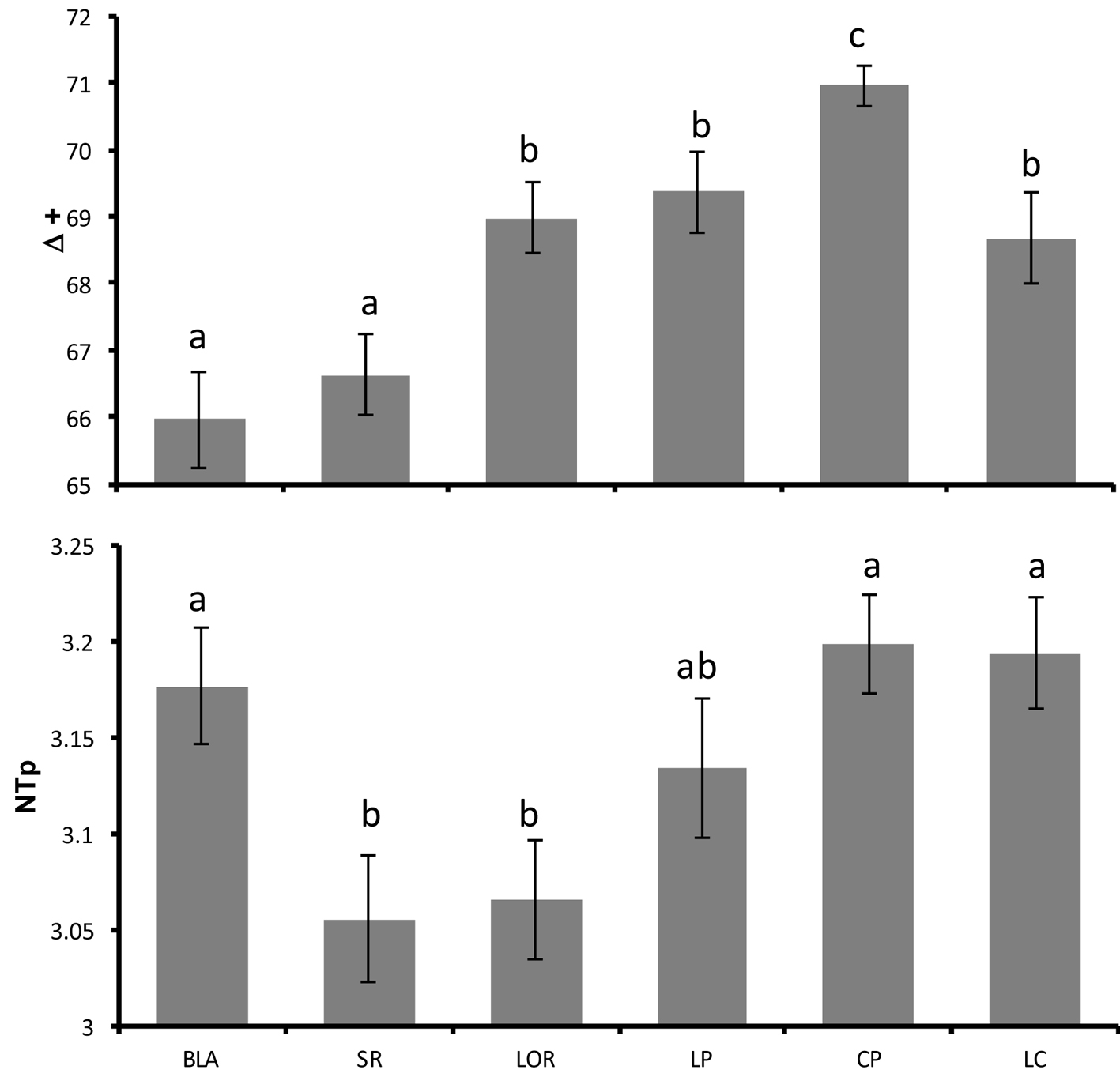
The taxonomic distinctness index displayed an average value of 68.30 ± 0.28 units in the Gulf of California, and the Anova indicated significant differences by locality (F(5,146) = 36.446, p < 0.001). Bahía de los Ángeles and Santa Rosalía exhibited low taxonomic distinctness (Δ+ < 67), while Loreto, La Paz, Cabo Pulmo and Los Cabos had the highest (Δ+ > 0.68; Fig. 4). For that reason, there was an inverted U pattern of Δ+ from north to south (Fig. 4).
The average trophic level along the study region was 3.14 ± 0.01 and the Anova showed significant differences among localities (F(5,140) = 4.579, p < 0.001), with Santa Rosalía and Loreto showing the lowest values (NTp < 3.1). In addition, an increase in the trophic level was observed as latitude decreased with the exception of Bahía de los Ángeles which presented a high average value of 3.17 (Fig. 4)
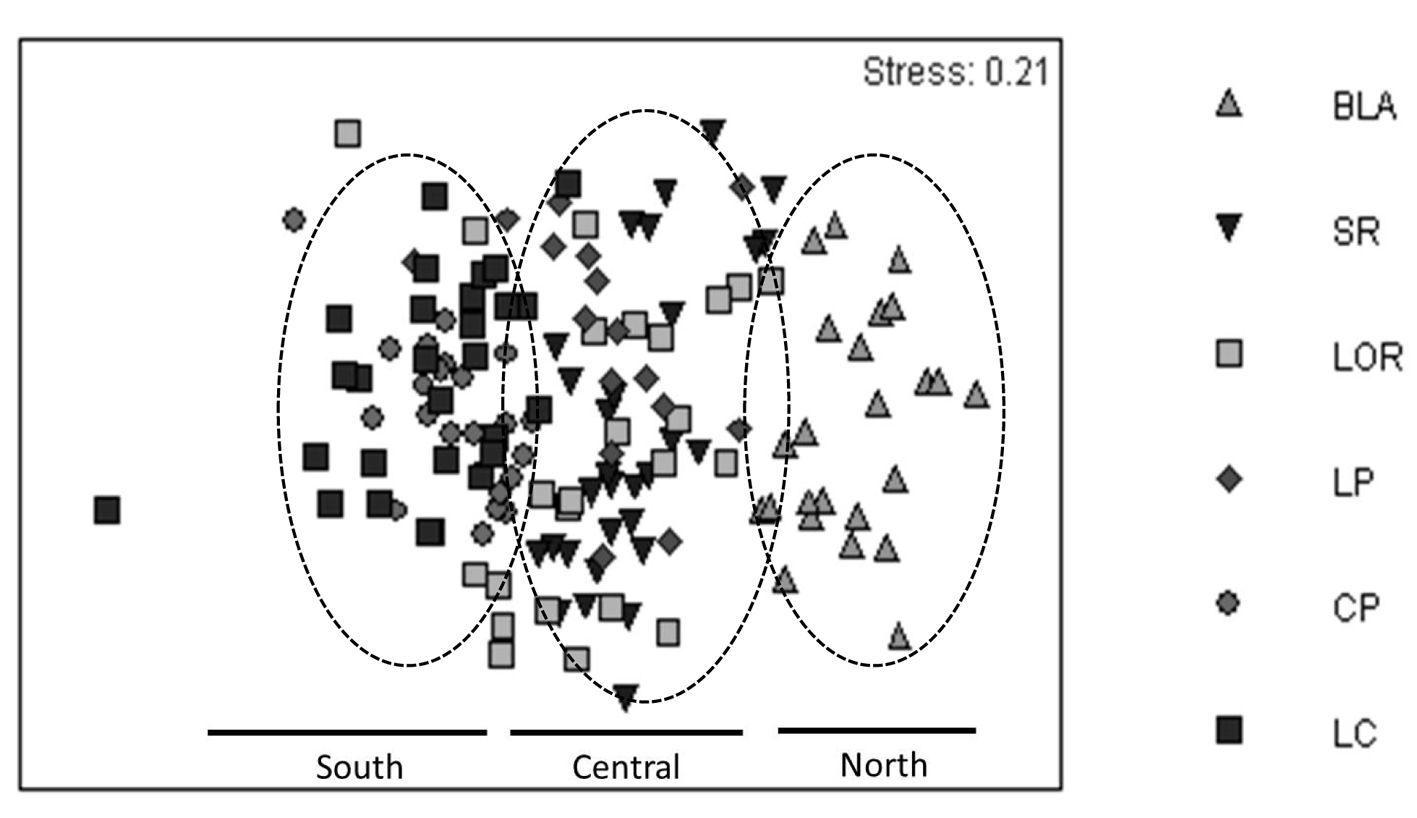
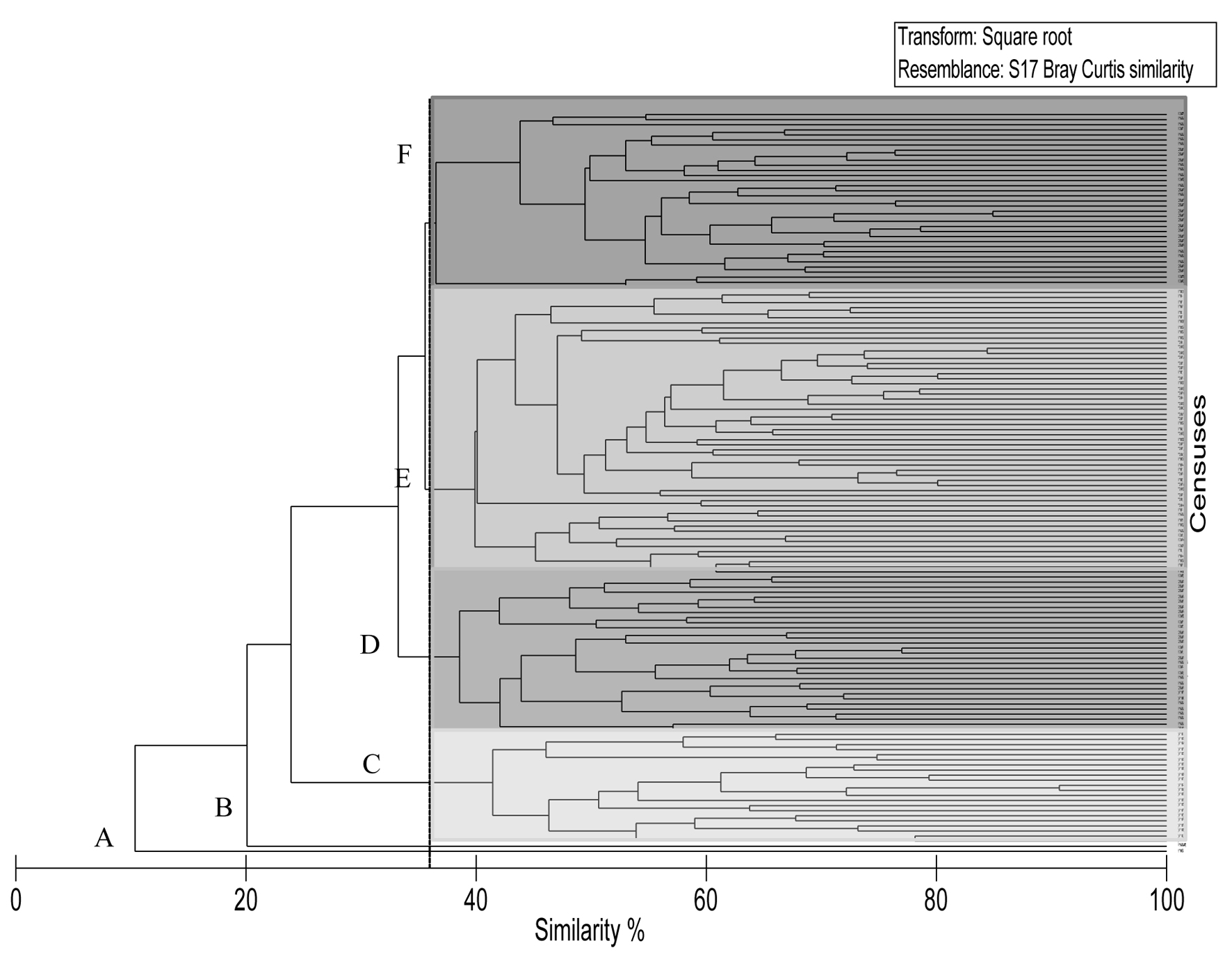
The NMDS showed a stress value of 0.21, indicating there were no well-defined groups. However, a latitudinal pattern from right to left in the horizontal axis can be observed, where the northern localities can be found to the right of the graph, and the southern localities are concentrated on the left side (Fig. 5). The cluster analysis confirmed this pattern, demonstrating the presence of 6 census groups that were 36% similar: groups A and B corresponded to anomalous censuses; group C only included Bahía de Los Ángeles (the northernmost site); groups D and F were integrated by the censuses performed in the central sites (Santa Rosalía, Loreto and La Paz); and finally the group E included surveys of the southern sites, Cabo Pulmo and Los Cabos (Fig. 6). The Anosim supported the existence of these groups, with an R value of 0.67.
The Simper analysis indicated that the 4 most abundant species (C. atrilobata, T. lucasanum, A. troschelii, and S. rectifraenum) were found in all locations surveyed; however, each group that was defined by the ordination analyses had specific species that differentiated it from the others. For group C, Crocodilichthys gracilis Allen & Roberston, 1991, Girella simplicidens Osburn & Nichols, 1916, Chromis limbaughi Greenfield & Woods, 1980 and Mycteroperca rosacea (Streets, 1877), distinguished Bahía de los Ángeles from the rest of the localities by 40%. For groups D and F (Santa Rosalía, Loreto and La Paz), the species Bodianus diplotaenia (Gill, 1862), Holacanthus passer Valenciennes, 1846 and Cirrhitichthys oxycephalus (Blecker, 1855) contributes in the arrangement, and, finally, group E (Cabo Pulmo and Los Cabos) was defined by the presence and abundance of Canthigaster punctatissima (Günther, 1870), C. oxycephalus (Blecker, 1855), Prionurus punctatus Gill, 1862 and Thalassoma grammaticum Gilbert, 1890, which together contributed 21% to the aggrupation.
Discussion
The Gulf of California is a World Heritage Site because of the great diversity and high species richness present in this relatively small body of water (UNESCO, 2005). Therefore, the conservation of the region has been a topic of great interest to government institutions and social organizations, necessitating the investigation of the organisms that inhabit the site, both locally and on large spatial scales (Lluch-Cota et al., 2007). Our study mainly focused on conspicuous reef-fish species because they are easy to observe and identify by visual census and non-extractive survey activities.
According to Thomson et al. (2000), and Robertson and Allen (2015), there are between 271 and 288 reef fish species in the entire Gulf of California, although we only found 85 in the visited locations. The difference is relevant and may be due to 3 factors: first, the observational field method used in our surveys do not take into account cryptic species; second, all the surveys were performed during the day which excludes those species with nocturnal habits; and third, our visits had a logistic limitation that restricted the availability of data spatially and temporally, as it was only focused on the western side of the Gulf and was restricted to a single season (Summer).
Considering the spatial and temporal limitation of data, we found that the fish communities of the Gulf of California are dominated by a small number of species (Fig. 2, Table 1), which have also been reported as the most abundant and frequent in other studies: A. troschelii, C. atrilobata, S. rectifraenum, and T. lucasanum, belt transects (Aburto-Oropeza & Balart, 2001; Barjau-González et al., 2012; Pérez-España et al., 1996; Sánchez-Ortiz et al., 1997; Viesca-Lobatón et al., 2008) and stationary censuses (Álvarez-Filip et al., 2006). These are non-specialist, herbivore or omnivore fishes (trophic level from 2.0 to 3.4; Froese & Pauly, 2017) that are so widely distributed that genetic studies have considered them as having a single panmictic population in the Gulf, and connectivity along the eastern Pacific (Bernardi et al., 2014; Guevara-Reyna, 2017; Meléndez & Mayor, 2014). The repetitive mention of these fishes as regionally dominant since the 1990s (Pérez-España et al., 1996), indicates that the composition of the most abundant ichthyofaunal taxa in the Gulf has not undergone great changes in the last decades, even when several species have increased their distribution ranges along this inner sea (González-Cuéllar et al., 2013).
Related to species richness, the average values in the central and southern Gulf of California were similar (between 14 to 16 spp/census), although higher when compared with the northern region (Bahía de los Ángeles) where the number of species was less (10.83 spp/census, Fig. 3). These differences in richness could be associated to the great physiographic and environmental disparity between the regions (Sala et al., 2002). The southern and central portion of the gulf are characterized by tropical water and a higher number of habitats (rocky reefs, islands, mangrove areas, rhodolith beds, coralline algae patches, submarine mountains and rocky walls), that are considered adequate habitats for reef fish over different stages of life (Aburto-Oropeza & Balart, 2001; Reyes-Bonilla et al., 2005), while in the north, the temperate water and the occurrence of reef corals and mangroves are practically absent because of the cold temperature in winter (Brusca et al., 2005). In the case of Los Cabos, the number of species observed by census was also low (12.5 spp/census), probably because the reefs are very limited in extension as the bottom reach great depths (> 100 m) at only a short distance from the coast (Brusca et al., 2005). This limited area covered by reefs due to a reduced continental shelf could explain the low local reef fish species richness (Hughes et al., 2002).
Along reefs of the western Gulf of California, fish abundance showed an inverse proportionality with latitude (Fig. 3). The atypical site was the northernmost location, Bahía de los Ángeles, that presented lower abundance values (177 ind/census). It is well documented that fish abundance is positively related to upwelling and to the presence of high biomass benthic resources, as it is very obvious in high productivity areas like those present in the Gulf of California (Campos-Dávila et al., 2005, Lluch-Cota, 2000; Schwing et al., 1996). In our case, the locations with the highest values of primary production showed the greatest abundance: Santa Rosalía (2,223 mg C/m2/d), Loreto (1,752 mg C/m2/d) and La Paz (1,236 mg C/m2/d; García et al., 2010; Fig. 3). Another explanation of this inverse pattern with the latitude, could be that currents within the Gulf are transporting larvae from south to north during the Summer-Autumn season, when most of the species are spawning (Lavin & Marinone, 2003; Sala et al., 2004), a feat that has been supported by several recent studies (Munguía-Vega et al., 2014, 2018; Soria et al., 2014). Last, it is possible that the low temperatures in Bahía de los Ángeles (average winter temperature 17 °C; Escalante et al., 2013) might be limiting the abundance of reef fish in this zone, because cold conditions negatively influence the recruitment of tropical fish species (Talbot et al., 1978). In fact, the seasonal thermal gradient present in the Midriff Island region (28° N), where conditions change abruptly from 28 °C in Summer to 17 °C in Winter (Escalante et al., 2013), represents a natural barrier between tropical and temperate fauna in the Gulf of California (Lavin & Marinone, 2003; Santamaría-del Angel et al., 1994).
The diversity and evenness of the studied reef fish assemblages of the western Gulf of California showed 2 clear groups: the northern locations (Bahía de los Ángeles, Santa Rosalía, and Loreto) dominated by few species, and the south with the highest diversity (La Paz, Cabo Pulmo and Los Cabos).
In our particular case, the high diversity in the southern localities may be associated with a variety of microenvironments (mangrove, coral reefs and different types of rocky bottoms), that are hosts to different species and allow for a more homogeneous distribution of abundance. Also, the increased availability of habitat and resources may open ecological space, as it has been shown by several authors that describe how functional diversity increases to the south of the Gulf of California and the Mexican Pacific (Aguilar-Medrano & Calderón-Aguilera, 2016; Álvarez-Filip & Reyes-Bonilla, 2006; Ramírez-Ortiz et al., 2017).
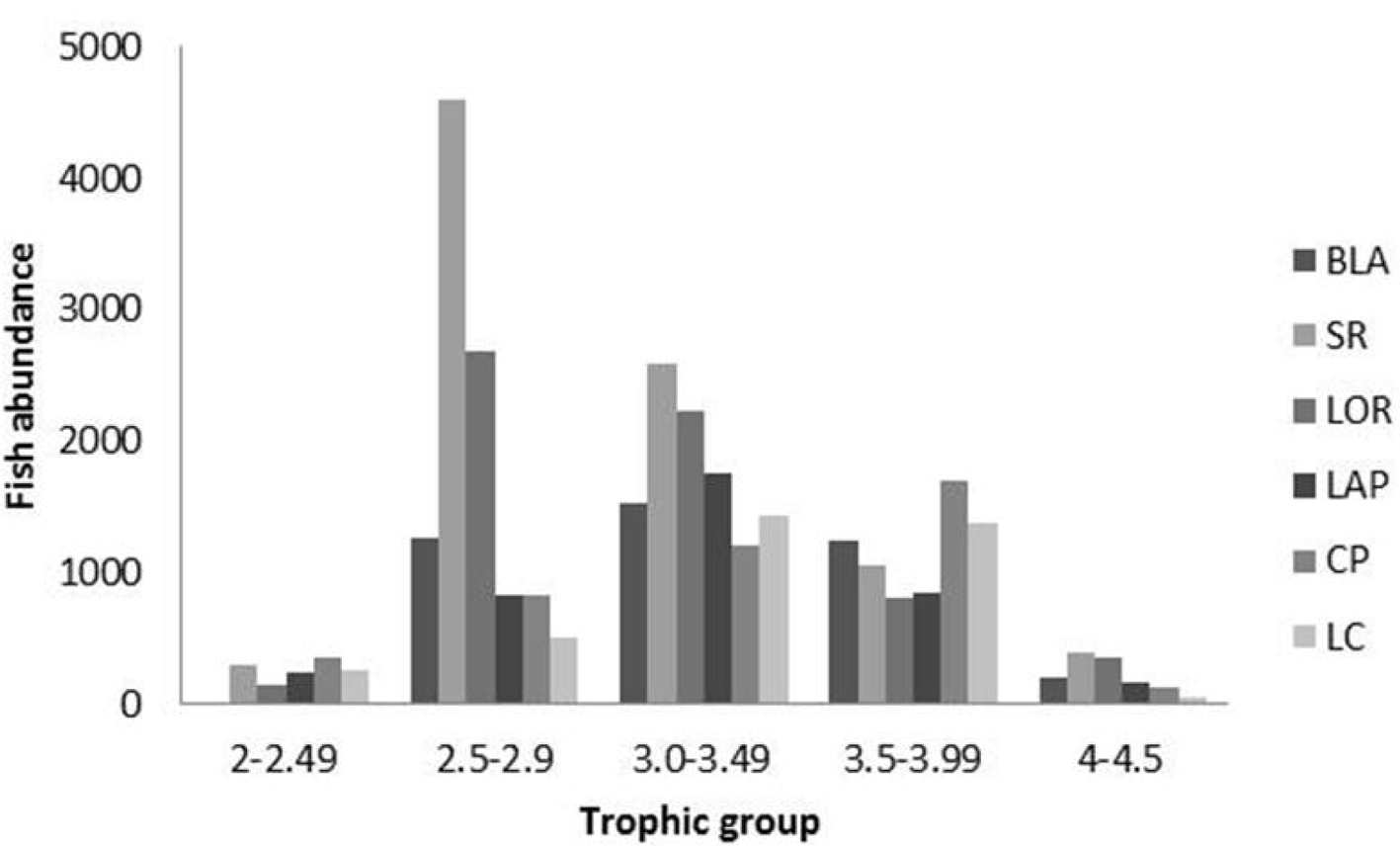
As in the case of ecological diversity, the trophic level of the fish assemblages increased towards the south (Fig. 4), which numerically is caused by the more common occurrence of carnivores such as groupers and snappers, near the entrance of the gulf. In contrast, in the central and northern parts the trophic level decreased due to the high abundance of fish with medium or low trophic levels (herbivores and omnivores; Fig. 7), probably a consequence of the high nutrient concentration and productivity of those areas (García et al., 2010).
According to the traditional representation of the trophic pyramid the first step (producers) will have high values of biomass or abundance, and as trophic value increases (consumers and predators), the number of organisms and their biomass will be lower than in each previous step (Kaiser et al., 2011). However, the ichthyofauna of the western Gulf of California did not show this typical arrangement. For instance, figure 7 showed that the abundance of omnivores (trophic level 3.00-3.49) was higher than that of herbivores. We interpret this as a consequence of a lack of a complete ecosystem assessment in our surveys because herbivory can be performed not only by fishes but also by invertebrates which have high abundance and medium and low trophic levels (Trebilco et al., 2013).
Another important point to highlight is that the average trophic level in all sites was similar, from 3.0 to 3.2 (Fig. 4), and species with a trophic level of 4 (carnivores) were present in all locations. This information indicates that the status of the reef fish communities in the Gulf of California may not be pristine, but it remains in good condition as no stage of the trophic pyramid seems to be completely depleted in abundance. Nevertheless, as there is evidence of a decrease in trophic level of the catch in specific areas of the gulf (Sala et al., 2004) and of low condition in fished reefs (Aburto-Oropeza et al., 2015), continuous monitoring of these areas is necessary to support further positive or negative changes.
The ordination analysis and NMDS indicated the presence of 3 groups (Fig. 5): the upper region (Bahía de los Ángeles; Fig. 6 group C); the central (Santa Rosalía, Loreto, and La Paz; Fig. 6 group D); and the southern Gulf formed by Cabo Pulmo and Los Cabos (Fig. 6 group E). The central Gulf comprises elements of the 2 other sections, and thus showed an intermediate ichthyofaunistic composition; this result may be influenced by the fact that during the warm season (when surveys were conducted), several fish species move temporarily to northern areas of the gulf, favored by the higher temperature and lower productivity (Aburto-Oropeza & Balart, 2001; Barjau-González et al., 2016; Campos-Dávila et al., 2005). Notwithstanding this, the Simper analysis indicated that fish assemblages of the western Gulf of California are relatively homogeneous in general because of the dominance of 4 species (Table 1), and the NMDS confirmed that the region shows gradual changes in community composition depending on latitude, probably caused by oceanographic differences, especially in temperature and productivity.
In conclusion, our results showed latitudinal variation in the community structure of reef fish along the western Gulf of California, with the highest values in richness, diversity, taxonomic distinctness and trophic level in the south, probably due to a combination of environmental conditions, a greater number of habitats, and more functional diversity of the assemblages. On the basis of the species present and their abundance, we identified 3 zones: a north zone (29° N) with the lowest values of species richness, abundance and taxonomic distinctness; a central zone (27° N to 24° N) with intermediate ecological complexity and which can be classified as a transition zone from tropical to temperate reefs; and a southern zone (24° N to 22° N), with the highest values of all diversity indicators. These results re-enforce the north, central and south zonification of the Gulf of California which has been observed in previous studies; however, those studies only considered the distribution patterns of species (Thompson et al., 2000; Walker, 1960). This study contributes to current knowledge by providing information on the latitudinal variation by using indicators of the structure and function of conspicuous reef fish.
Acknowledgements
This study was financed by grants UABCS-CIBNOR 10-2004, Conacyt FB915 and Conabio AS007. FJFRM and GRO thank the support of Consejo Nacional de Ciencia y Tecnología (Master´s scholarship 274238 and Doctoral scholarship 266599, respectively). We thank Aracely Rojas, Adriana González and Israel Sánchez, for their support and participation in the field work.
References
Abitia-Cárdenas, L. A, Rodríguez-Romero, J., Galván-Magaña, F., De La Cruz-Agüero, J., & Chávez-Ramos, H. (1994). Lista sistemática de la ictiofauna de Bahía de La Paz, Baja California Sur, México. Ciencias Marinas, 20, 159–181.
Aburto-Oropeza, O., & Balart, E. F. (2001). Community structure of reef fish in several habitats of rocky reef in the Gulf of California. Marine Ecology, 22, 283–305.
Aburto-Oropeza, O., Ezcurra, E., Moxley, J., Sánchez-Rodríguez, A., Mascarenas-Osorio, I., Sánchez-Ortiz, C. et al. (2015). A framework to assess the health of rocky reefs linking geomorphology, community assemblage, and fish biomass. Ecological Indicators, 52, 353–361.
Aguilar-Medrano, R., & Calderón-Aguilera, L. E. (2016). Redundancy and diversity of functional reef fish groups of the Mexican Eastern Pacific. Marine Ecology, 37, 119–133.
Álvarez-Filip, L., & Reyes-Bonilla, H. (2006). Comparison of community structure and functional diversity of fishes at Cabo Pulmo coral reef, B.C.S. Mexico between 1987 and 2003. Proceedings of 10th International Coral Reef Symposium, Okinawa, Japan. June 28-July 2, 2004.
Álvarez-Filip, L., Reyes-Bonilla, H., & Calderón-Aguilera, L. (2006). Community structure of fishes in Cabo Pulmo Reef, Gulf of California. Marine Ecology, 22, 253–262.
Balart, E. F., Castro-Aguirre, J. L., Aurioles-Gamboa, D., García-Rodríguez, F., & Villavicencio-Garayzar, C. (1995). Adiciones a la ictiofauna de Bahía de La Paz, Baja California Sur, México. Hidrobiológica, 5, 79–85.
Barjau-González, E., Rodríguez-Romero, J., Galván-Magaña, F., & López-Martínez J. (2012). Changes in the taxonomic diversity of the fish community of San Jose Island, Gulf of California, Mexico. Biodiversity & Conservation, 21, 3543–3554.
Barjau-González, E., Rodríguez-Romero, J., Galván-Magaña, F., & Maldonado-García, M. (2016). Seasonal shift in the taxonomic diversity of rocky reef fishes in the southwestern Gulf of California. Revista de Biología Marina y Oceanografía, 51, 11–19.
Bernardi, G., Ramón, M. L., Alva-Campbell, Y., McCosker, J. E., Bucciarelli, G., Garske, L. E., Victor, B., & Crane, N. L. (2014). Darwin’s fishes: phylogeography of Galápagos Islands reef fishes. Bulletin of Marine Science, 90, 533–549.
Bohnsack, J. A., & Bannerot, S. P. (1986). A stationary visual census technique for quantitatively assessing community structure of coral reef fishes. NOAA Technical Report NMFS, 41, 1–15.
Brusca, R. C., Findley, L. T., Hastings, P. A., Hendrickx, M. E., Torre-Cosio, J., & Van der Heiden A. M. (2005). Macrofaunal divesrsity in the Gulf of California. In J. E. Cartron, G. Ceballos, & R. S. Felger (Eds.), Biodiversity, ecosystems, and conservation in northern Mexico (pp. 179–203). New York: Oxford University Press.
Campos-Dávila, L., Cruz-Escalona, V. H., Galván-Magaña, F., Abitia-Cárdenas, A., Gutiérrez-Sánchez, F. J., & Balart, E. F. (2005). Fish assemblages in a Gulf of California Marine reserve. Bulletin of Marine Science, 77, 347–362.
Clarke, K. R., & Warwik, R. M. (2001). Change in marine communities: an approach to statistical analysis and interpretation, 2nd edition. London: PRIMER-E. Plymouth
Del Moral-Flores, L. F., González-Acosta, A. F., Espinosa-Pérez, H., Ruiz-Campos, G., & Castro-Aguirre, J. L. (2013). Lista anotada de la ictiofauna de las islas del golfo de California, con comentarios sobre sus afinidades zoogeográficas. Revista Mexicana de Biodiversidad, 84, 184–214.
Escalante, F., Valdez-Holguín, J. E., Álvarez-Borrego, S., & Lara-Lara, S. R. (2013). Temporal and spatial variation of sea surface temperature, chlorophyll a, and primary productivity in the Gulf of California. Ciencias Marinas, 39, 203–215.
Froese, R., & Pauly D. (2017). FishBase. Recovered 05 march, 2017; available: http://www.fishbase.org/search.php
García, H. E., Locarnini, R. A., Boyer, T. P., Antonov, J. I., Zweng, M. M., Baranova, O. K. et al. (2010). World ocean atlas 2009, volume 4. Nutrients (phosphate, nitrate, silicate). Washington D.C.: USA Government Printing Office.
Gascuel, D., Guénette, S., & Pauly, D. (2011). The trophic-level-based ecosystem modelling approach: theoretical overview and practical uses. ICES Journal of Marine Science, 68, 1403–1416.
Glynn, P. W., Alvarado, J. J., Banks, S., Cortés, J., Feingold, J. S., Jiménez, C. et al. (2017). Eastern Pacific coral reef provinces, coral community structure and composition: an overview. In P. W. Glynn, D. P Manzello, & I. C. Enochs (Eds.), Coral reefs of the eastern tropical Pacific (pp. 107-176). Dordrecht, Netherlands: Springer.
González-Cuéllar, O. T., Reyes-Bonilla, H., Fourriére, M., Rojo, M., Hernández-Velasco, A., Sánchez-Alcantara, I. et al. (2013). Range extensions of four species of parrotfishes (Scaridae) in the northern Gulf of California, Mexico. Cybium, 37, 223–226.
Guevara-Reyna, P. E. (2017). Variabilidad genética y filogeografía del pez arrecifal arcoiris de Cortés, Thalassoma lucasanum, en el Pacífico Oriental Tropical (M.Sc. Thesis). Centro de Investigación Científica y de Educación Superior de Ensenada, Baja California, Mexico.
Hughes, T. R., Bellwood, D. R., & Connolly, S. R. (2002). Biodiversity hotspots, centers of endemicity, and conservation of coral reefs. Ecology Letters, 5, 775–784.
Kaiser, M. J., Attrill, M. J., Jennings S., Thomas, D. N., & Barnes, K. A. (2011). Marine ecology: processes, systems, and impacts. New York: Oxford University Press.
Krebs, C. J. (1999). Ecological Methodology, 2nd Ed. San Francisco, CA: Addison-Welsey.
Lavin, M. F., & Marinone, S. G. (2003). An overview of the physical oceanography of the Gulf of California. In O. U. Velasco-Fuentes, J. Sheinbaum, & J. L. Ochoa-de la Torre (Eds.), Nonlinear processes in geophysical fluid dynamics (pp. 173–204). Dordrecht, Netherlands: Kluwer Academic Publishers.
Lluch-Cota, S. E. (2000). Coastal upwelling in the eastern Gulf of California. Oceanologica Acta, 23, 731–740.
Lluch-Cota, S. E., Aragón-Noriega, E. A., Arreguín-Sánchez, F., Aurioles-Gamboa, D., Bautista- Romero, J., Brusca, R. C. et al. (2007). The Gulf of California: review of ecosystem status and sustainability challenges. Progress in Oceanography, 73, 1–26.
Magurran, A. E. (2004). Measuring biological diversity. Oxford: Blackwell Publishing.
Meléndez, C., & Mayor, J. F. (2014). Variabilidad genética y conectividad de la jaqueta de Cortés, Stegastes rectifraenum (Gill, 1862), Perciformes: Pomacentridae, en ambas costas de la península de Baja California (Master’s degree in Marine Resources Management Thesis). Instituto Politécnico Nacional. Centro Interdisciplinario de Ciencias Marinas, La Paz, Baja California Sur, México.
Munguía-Vega, A., Jackson, A., Marinone, S. G., Erisman, B., Moreno-Baez, M., Girón-Nava, A., Pfister, T., Aburto-Oropeza, O., & Torre, J. (2014). Asymmetric connectivity of spawning aggregations of a commercially important marine fish using a multidisciplinary approach. PeerJ, 2, e511.
Munguía-Vega, A., Marinone, S. G., Paz-Díaz, D. A., Girón-Nava, A., Plomozo-Lügo, T., González-Cuellar, O. et al. (2018). Anisotoic larval connectivity and metapopulation structure driven by directional oceanic currents in a marine fish targeted by small-scale fisheries. Marine Biology, 165, 16.
Pérez-España, H., Galván-Magaña, F., & Abitia-Cárdenas, L. A. (1996). Variaciones temporales y espaciales en la estructura de la comunidad de peces arrecífales rocosos del suroeste del Golfo de California, México. Ciencias Marinas, 22, 273–294.
Ramírez-Ortiz, G., Calderón-Aguilera, L. E., Reyes-Bonilla, H., Ayala-Bocos, A., Hernández, L., Fernández-Rivera Melo, F. et al. (2017). Functional diversity of fish and invertebrates in coral and rocky reefs of the Eastern Tropical Pacific. Marine Ecology, 38, e12447.
Reyes-Bonilla, H., Calderón-Aguilera, L. E., Cruz-Piñón, G., Medina-Rosas, P., López-Pérez, R.A., Herrero-Pérezrul, M. D., Leyte-Morales, G. E. et al. (2005). Atlas de los corales pétreos (Scleractinia) del Pacífico mexicano. México D.F.: CICESE/ Conabio/ Conacyt/ UABCS/ Universidad de Guadalajara/ Universidad del Mar.
Robertson, D. R., & Allen, G. R. (2015). Fishes of the tropical eastern Pacific: online information system Smithsonian Institution. Recovered on 15 October, 2017 in: http://biogeodb.stri.si.edu/sftep/en/pages
Rodríguez-Romero, J., Muhlia-Melo, A., Galván-Magaña, F., Gutiérrez-Sánchez, F. J., & Gracia-López, V. (2005). Fish assemblages around Espíritu Santo Island and Espíritu Santo Sea mount in the lower Gulf of California, Mexico. Bulletin of Marine Science, 778, 33–50.
Sala, E., Aburto-Oropeza, O., Paredes, G., Parra, I., Barrera, J. C., & Dayton, P. K. (2002). A general model for designing networks of marine reserves. Science, 298, 1991–1993.
Sala, E., Aburto-Oropeza, O., Reza, M., Paredes, G., & López-Lemus, L. G. (2004). Fishing down coastal food webs in the Gulf of California. Fisheries, 29, 19–25.
Sánchez-Ortíz, C., Arreola-Robles, J. L., Aburto-Oropeza, O., & Cortés-Hernández, M. (1997). Peces de arrecife en la región de La Paz, B.C.S. In J. Urbán-Ramírez, & M. Ramírez-Rodríguez (Eds.), La bahía de La Paz. Investigación y Conservación (pp. 177–188). La Paz, Baja California Sur: Universidad Autónoma de Baja California Sur.
Santamaría-del Ángel, E., Álvarez-Borrego, S., & Muller-Karger, F. E. (1994). Gulf of California biogeographic regions based on coastal zone color scanner imagery. Journal of Geophysical Ressearch, 99, 7411–7421.
Schwing, F.B., O´Farrell, M. Steger, J., & Baltz, K. (1996). Coastal upwelling indices, west coast of North America, 1946-1995. NOAA Technical Memorandum NMFS-SWFSC, 231, 207.
Soria, G., Torre-Cosio, J., Munguía-Vega, A., Marinone, S. G., Lavin, M. F., Cinti, A. et al. (2014). Dynamic connectivity patterns from an insular marine protected are in the Gulf of California. Journal of Marine Systems, 129, 248–258.
Talbot, F. H., Russel, B. C., & Anderson, G. R. V. (1978). Coral reef fish communities: unstable, high-diversity systems? Ecological Monographs, 48, 425–440.
Thomson, A. D., Findley, L. T., & Kerstich, A. N. (2000). Reef fishes of the sea of Cortez. Austin: University of Texas Press.
Thomson, D. A., & Gilligan, M. (2002). The rocky shore fishes. In T. Case, M. Cody, & E. Ezcurra. A new island biogeography of the Sea of Cortes (pp. 198–229). New York: Oxford University Press.
Tolimieri, N., & Anderson, M. J. (2010). Taxonomic distinctness of demersal fishes of the California current: moving beyond simple measures of diversity for marine ecosystem-based management. Plos One, 5, e10653.
Trebilco, R., Baum, J. K., Salomon, A. K., & Dulvy, N. K. (2013) Ecosystem ecology: size-based constraints on the pyramids of life. Trends in Ecology & Evolution, 28, 423–431.
UNESCO (Organización de las Naciones Unidas para la Educación, la Ciencia y la Cultura). (2005). Decisions of the 29th Session of the World Heritage Committee (Durban, 2005). Paris: UNESCO World Heritage Center.
Viesca-Lobatón, C., Balart, E. F., González-Cabello, A., Mascareñas-Osorio, I., Reyes-Bonilla, H., & Torreblanca, E. (2008). Los peces de arrecife de bahía de los Ángeles, golfo de California. In G. Danemann, & E. Ezcurra (Eds.), Bahía de Los Ángeles: recursos naturales y comunidad (pp. 385–428). México D.F.: Instituto Nacional de Ecología.
Villareal-Cavazos, A., Reyes-Bonilla, H., Bermúdez-Almada, B., & Arizpe-Covarrubias, O. (2000). Los peces del arrecife de Cabo Pulmo, golfo de California, México: lista sistemática y aspectos de abundancia y biogeografía. Revista de Biología Tropical, 48, 413–424.
Walker, B. W. (1960). The distribution and affinities of the marine fish fauna of the Gulf of California. Systematic Zoology, 9, 123–133.
Zar, J. H. (2010). Biostatistical analysis. New Jersey: Pearson Prentice-Hall.



