Sergio Díaz-Martínez a, *, Lisandro Hernández-Anaya a, Alejandrina G. Avila-Ortiz a, Lidia I. Cabrera-Martínez b, Giuseppe C. Zuccarello c
a Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Zaragoza, Carrera de Biología, Herbario FEZA, Batalla de 5 de mayo s/n, Col. Ejército de Oriente, 09230 Ciudad de México, Mexico
b Universidad Nacional Autónoma de México, Instituto de Biología, Departamento de Botánica, Apartado postal 70-367, 04510 Ciudad de México, Mexico
c Victoria University of Wellington, School of Biological Sciences, PO Box 600, Wellington 6140, New Zealand
*Corresponding author: sergio.dm@comunidad.unam.mx (S. Díaz-Martínez)
Received: 16 January 2023; accepted: 2 May 2023
Abstract
Species of Lobophora (Dictyotales) are distributed throughout the sub-tropical and tropical seas worldwide. Recent analyses have revealed high species diversity in regions previously presumed to host only a single species, such as the Bismarck Sea, Eastern Pacific, Western Atlantic, Mediterranean Sea, and Greater Caribbean. Here, samples from Veracruz and Quintana Roo, Mexico, were collected, and 2 genetic markers (cox3 and psbA) were sequenced. The results confirmed the presence of L. dispersa and L. variegata. Lobophora dispersa is recorded for the first time on the Mexican coast. The distribution of its cox3 haplotypes shows genetic differentiation within the Greater Caribbean and Gulf of Mexico, possibly indicating limited dispersal and isolation by distance. Lobophora variegata exhibits lower genetic variability compared to L. dispersa, but its haplotypes did not show any obvious pattern. Lobophora declerckii, previously reported in the “Anegada de Afuera” reef, Veracruz, was not found, possibly due to its affinity to subtidal depths. Morphologically, L. dispersa and L. variegata align with previous descriptions, although we observed more variation in thallus cell thickness in L. dispersa. However, relying solely on morphological characters is insufficient to confidently identify the species, necessitating further sampling to determine the species diversity in Mexico.
Keywords: Brown algae; Haplotype network; Molecular systematics; Lobophora variegata
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Lobophora dispersa (Dictyotaceae: Phaeophyceae), un registro nuevo para la costa de Veracruz y perspectivas de su diferenciación genética en el golfo de México y el mar Caribe
Resumen
Las especies de Lobophora (Dictyotales) se distribuyen en mares tropicales y subtropicales del mundo. Estudios recientes revelaron una alta diversidad de especies en regiones en las que se había reportado únicamente una especie, como el mar de Bismarck, Pacífico oriental, Atlántico occidental, mar Mediterráneo y el gran Caribe. En este estudio, se recolectaron ejemplares de Veracruz y Quintana Roo, México, y se secuenciaron 2 genes (cox3 y psbA). Los resultados confirmaron la presencia de L. dispersa y L. variegata. Lobophora dispersa se reporta por primera vez en la costa mexicana. La distribución de sus haplotipos muestra diferenciación genética dentro del gran Caribe y golfo de México, posiblemente indicando dispersión limitada y aislamiento por distancia. Lobophora variegata es menos variable y sus haplotipos no muestran un patrón definido. Lobophora declerckii, reportada en el arrecife “Anegada de Afuera”, Veracruz, no fue encontrada, posiblemente debido a su afinidad por profundidades submareales. Morfológicamente, L. dispersa y L. variegata concuerdan con descripciones previas, aunque se encontró mayor variación en el grosor del talo en L. dispersa. Sin embargo, la morfología es insuficiente para determinar las especies con confianza, requiriendo un mayor muestreo para conocer la diversidad de especies en México.
Palabras clave: Algas pardas; Red de haplotipos; Sistemática molecular; Lobophora variegata
© 2023 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Lobophora J. Agardh is distributed on tropical and subtropical coasts worldwide (Vieira, Henriques et al., 2020). It is characterized by multilayered fronds growing from a continuous row of apical cells. It has a highly variable morphology, displaying crustose and erect species with fan-shaped, reniform, or dichotomously divided blades, sometimes resembling Zonaria C. Agardh (Vieira et al., 2016). Lobophora can inhabit several ecological niches such as rocky shores and reefs, from the upper intertidal zone to depths of more than 130 m (Camacho et al., 2019; Puk et al., 2020; Vieira, Henriques et al., 2020).
In recent years, this genus has been the subject of several taxonomic studies, including morphological and molecular data, leading to a re-interpretation of the species limits and distributions (Camacho et al., 2019; Vieira, Henriques et al., 2020). The number of formally described species worldwide increased from 28 (Camacho et al., 2019; Godínez-Ortega et al., 2018; Vieira, Morrow et al., 2020) to 71 (Guiry & Guiry, 2022). Currently, a total of 5 species can be found in the Gulf of Mexico and adjacent regions: L. declerckii N.E. Schultz, C.W. Schneider & L. Le Gall; L. delicata Camacho & Fredericq; L. dispersa Camacho, Freshwater & Fredericq, L. schneideri C.W. Vieira; and L. variegata (J.V. Lamouroux) Womersley ex E.C. Oliveira (Vieira, Morrow et al., 2020). Until 2018, only L. variegata was recognized from the Atlantic coast of Mexico (Dreckmann, 1998; Godínez-Ortega et al., 2018; Ortega et al., 2001). This changed with the first record of L. declerckii at the “Anegada de Afuera” coral reef near Veracruz (Godínez-Ortega et al., 2018). The occurrence of L. variegata in Mexico was confirmed with molecular data for Cancun (Quintana Roo) in the Caribbean (Godínez-Ortega et al., 2018). However, the actual species of Lobophora ocurring on Mexican coasts remain uncertain.
The high number of recently discovered species of Lobophora around the world, the morphological similarities between species making the identification difficult, and the historical morphology-based records of Lobophora in several localities on the Atlantic coast of Mexico (as L. variegata; Dreckmann, 1998; García-García et al., 2021; Ortega et al., 2001) prompted the need to re-evaluate the taxonomic identity of Lobophora species in Mexico using molecular data. In this study, DNA sequences of the mitochondrial cox3 and plastid psbA were compared from specimens collected from the states of Veracruz and Quintana Roo, Mexico, to investigate the species diversity and the occurrence of genetic differentiation in Lobophora.
Materials and methods
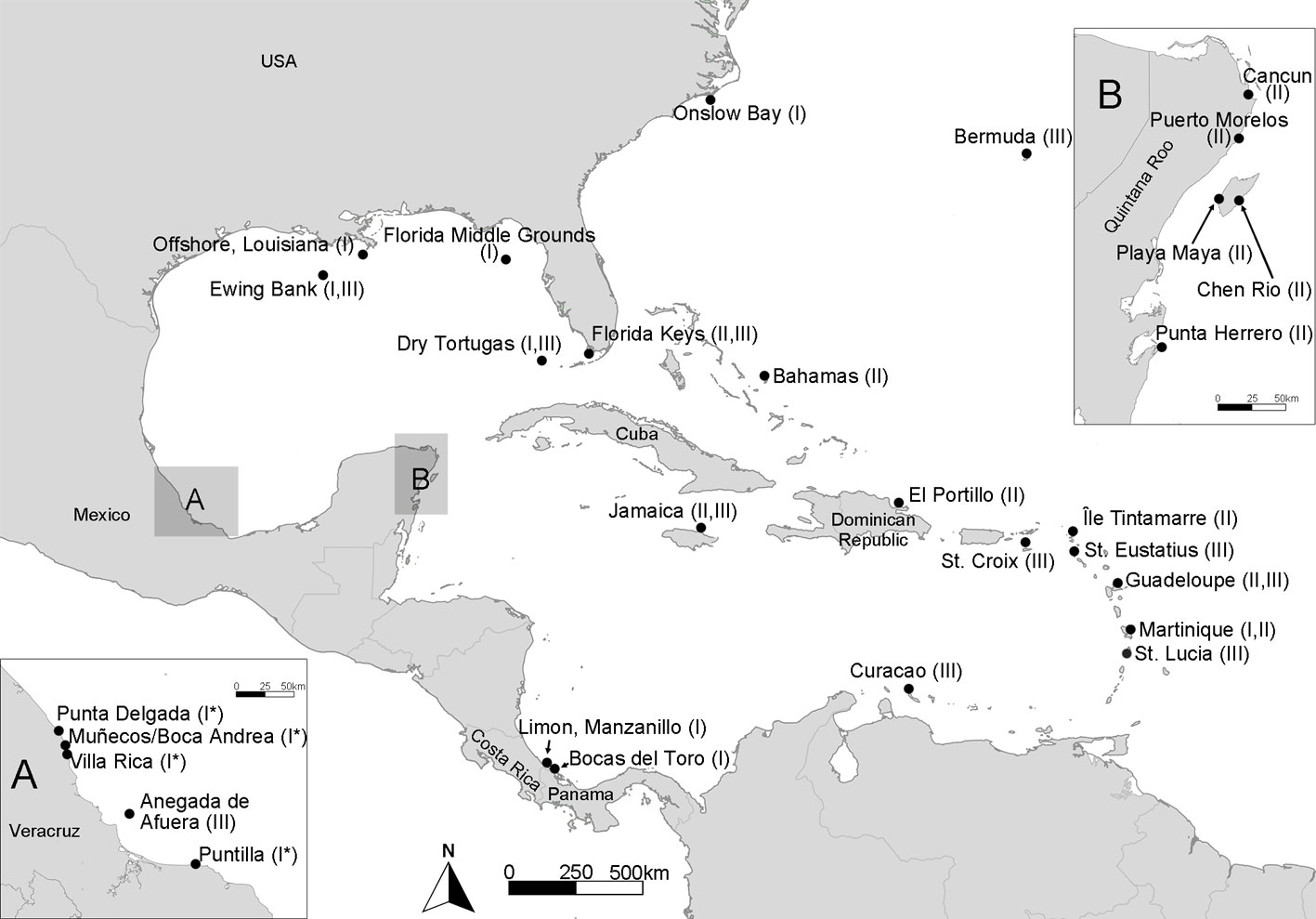
Specimens of Lobophora from Veracruz and Quintana Roo, Mexico (Fig. 1), were collected in the intertidal zone and by snorkeling up to 2 m depth. Specimens were prepared as herbarium vouchers, and a small fragment from the apex of the thallus was brushed and rinsed with distilled water to remove contaminants. The fragment was preserved in silica gel for DNA extraction. Vouchers were deposited in the FEZA herbarium (Thiers, 2022) at the Facultad de Estudios Superiores Zaragoza, UNAM. Other collected vouchers preserved in 4% formalin were morphologically examined.
The CTAB (cetyltrimethylammonium bromide; Doyle & Doyle, 1987) method was used for DNA extractions with the addition of 2% (w/v) polyvinylpyrridoline (PVP). The cytochrome c oxidase subunit 3 (cox3) and D1 protein of Photosystem II (psbA) genes were used following previous studies within Lobophora (Camacho et al., 2019; Vieira et al., 2016; Vieira, De Clerckm et al., 2019). The DNA was amplified with the MyTaq Polymerase Kit (Bioline, Meridian Bioscience Inc., USA) with the following primers: cox3-44F/cox3-739R for cox3 (Silberfeld et al., 2013) and psbA-F/psbA-R1 for psbA (Yoon et al., 2002). The amplification profile consisted of 3 minutes at 94 °C for initial denaturing, followed by 30 cycles of 1 minute at 94 °C, 46 °C for 1 minute and 72 °C for 1 minute, and 7 minutes at 72 °C for final extension. Amplification success was evaluated visually by electrophoresis on 1% agarose. Amplicons were sent to Macrogen (Seoul, Korea) for Sanger sequencing using the amplification primers. A total of 14 specimens of Lobophora were sequenced: 9 from the coast of Veracruz and 5 from Quintana Roo (Supplementary material: Table S1). The chromatograms were assembled and edited using Geneious 6 (Biomatters Ltd. available from http://www.geneious.com/).
Independent data matrices were created and aligned in Mega X ver. 10.2.4. using default settings for each gene due to the uneven sequences available (Kumar et al., 2018). Additional sequences of Lobophora were downloaded from GenBank (Supplementary material: Table S2). The selected sequences were mainly based on the study of Vieira, Morrow et al. (2020) for the Greater Caribbean region and complemented with others from previous publications (Camacho et al., 2019; Schultz et al., 2015; Vieira et al., 2014, 2016; Vieira, Rasoamanendrika et al., 2021; Vieira, Steen et al., 2021) as well as sequences of Mexican L. declerckii and L. variegata (Godínez-Ortega et al., 2018). Padina gymnospora (Kützing) Sonder was used as an outgroup. Both matrices were analyzed using maximum likelihood (ML) and Bayesian inference (BI). The best molecular model and partition scheme for each codon and data matrix were calculated with PartitionFinder v1.1.0 (Lanfear et al., 2012) using the BIC value model selection option. The selected model for both markers was GTR+I+G in a single partition. In addition, uncorrected pairwise distances (“p” distance) were calculated in Mega X.
For ML, IQ-TREE ver. 2.2.0 (Nguyen et al., 2015) was employed, and branch support was calculated by 500 nonparametric bootstrap (BS) replicates. For BI, MrBayes ver. 3.2 (Huelsenbeck & Ronquist, 2001) was executed in CIPRES Science Gateway V. 3.3. (http://www.phylo.org/sub_sections/portal/). Two parallel analyses were performed running for 10,000,000 generations, sampling every 1,000 generations, with unlinked partitions. The stationarity of the likelihood curve was examined visually, and convergence was analyzed with Tracer 1.7 (Rambaut et al., 2018). The first 2,500,000 generations were discarded as burn-in, and a summary tree including average branch lengths and posterior probability (PP) values was calculated from the remaining trees.
Haplotype networks of the cox3 sequences that grouped with our samples of L. dispersa and L. variegata were calculated to explore insights of genetic differentiation in PopART ver. 1.7 (Leigh & Bryant, 2015) using the TCS method (Clement et al., 2000), which implements the statistical parsimony algorithm. The haplotypes found were placed on a map to explore potential geographic patterns.
Morphological examination. Thirty-four specimens (23 from Veracruz and 11 from Quintana Roo; Supplementary material: Table S1) were examined morphologically using a Nikon SMZ660 stereoscope. Cross-sections were made by hand with a single-edged razor blade in longitudinal and transverse orientations. Measurements of the medullar, subcortical, and cortical cells were made following the anatomical description used by Schultz et al. (2015), using a Nikon Eclipse 50i microscope. Maximum and minimum values were obtained for each trait and compared with previous descriptions (Camacho et al., 2019; Godínez-Ortega et al., 2018; Torres-Conde et al., 2021; Vieira, Henriques et al., 2020; Vieira, Morrow et al., 2020).
Results
Molecular data from both markers allow the recognition of Lobophora dispersa from the coast of Veracruz (Gulf of Mexico) and L. variegata from Quintana Roo (Caribbean Sea).
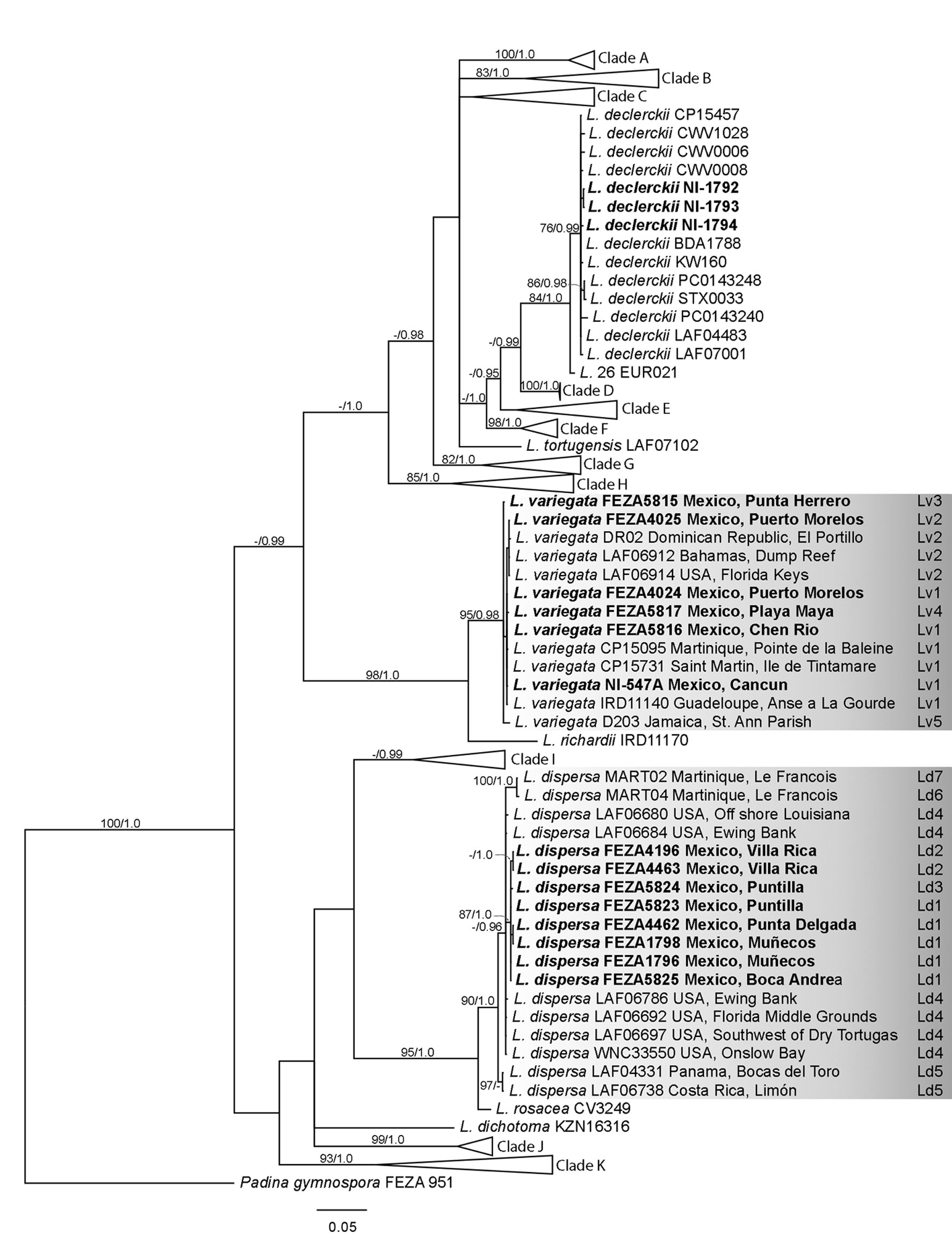
The cox3 data matrix consisted of 697 base pairs (bp) and 107 sequences (13 newly sequenced). BI (Fig. 2) and ML resulted in similar topologies differing only in poorly supported branches. Lobophora dispersa from Mexico grouped with the holotype (WNC 33550; USA: North Carolina, Onslow Bay; sequence in Supplementary material: Table S2), while the Mexican samples of L. variegata grouped with sequences of L. variegata sensu Vieira et al. (2016; see Supplementary material: Table S2). The Lobophora dispersa clade was well supported (BS = 90%, PP = 1.0) with low intraspecific genetic variation (0.9 % pairwise difference within the clade). The Mexican samples of L. dispersa were found only in Veracruz and grouped in a subclade (BS = 87%, PP = 1.0) different from samples from other localities (e.g., North Carolina, upper Gulf of Mexico, Dry Tortugas). The sister species of L. dispersa was L. rosacea C.W. Vieira, Payri & De Clerck, as shown in previous studies (BS = 95%, PP = 1.0; Vieira, Morrow et al., 2020) with a genetic distance of 2.9%. Lobophora variegata was also well supported (BS = 95%; PP = 0.98). The Mexican samples of L. variegata were collected only from Quintana Roo and are highly similar to sequences from other localities (e.g., Bahamas, Dominican Republic, Florida Keys, Guadeloupe, Jamaica; distance within the clade of 0.2%). Its sister species was L. richardii C.W. Vieira & Payri (BS = 98%, PP = 1.0; Vieira, Morrow et al., 2020) with a genetic distance of 6.7%.
The psbA data matrix consisted of 956 bp and 76 sequences (6 sequenced here). As in the cox3 dataset, the BI tree is used for discussion (Fig. 3). The psbA phylogeny was congruent with the cox3 topology. Lobophora dispersa was recovered in a well-supported clade (BS = 92%, PP = 1.0; distance within the clade of 0.4%) with L. rosacea as sister species (BS = 95%, PP = 1.0) with a genetic distance of 1.3%. Mexican samples of L. dispersa were grouped in a subclade as well (BS = 81%, PP = 0.97). Lobophora variegata was recovered in a well-supported clade with no genetic differentiation (BS = 100%, PP = 1.0; distance within the clade of 0.0%), sister to L. richardii (genetic distance of 3.4%) as in the cox3 topology.
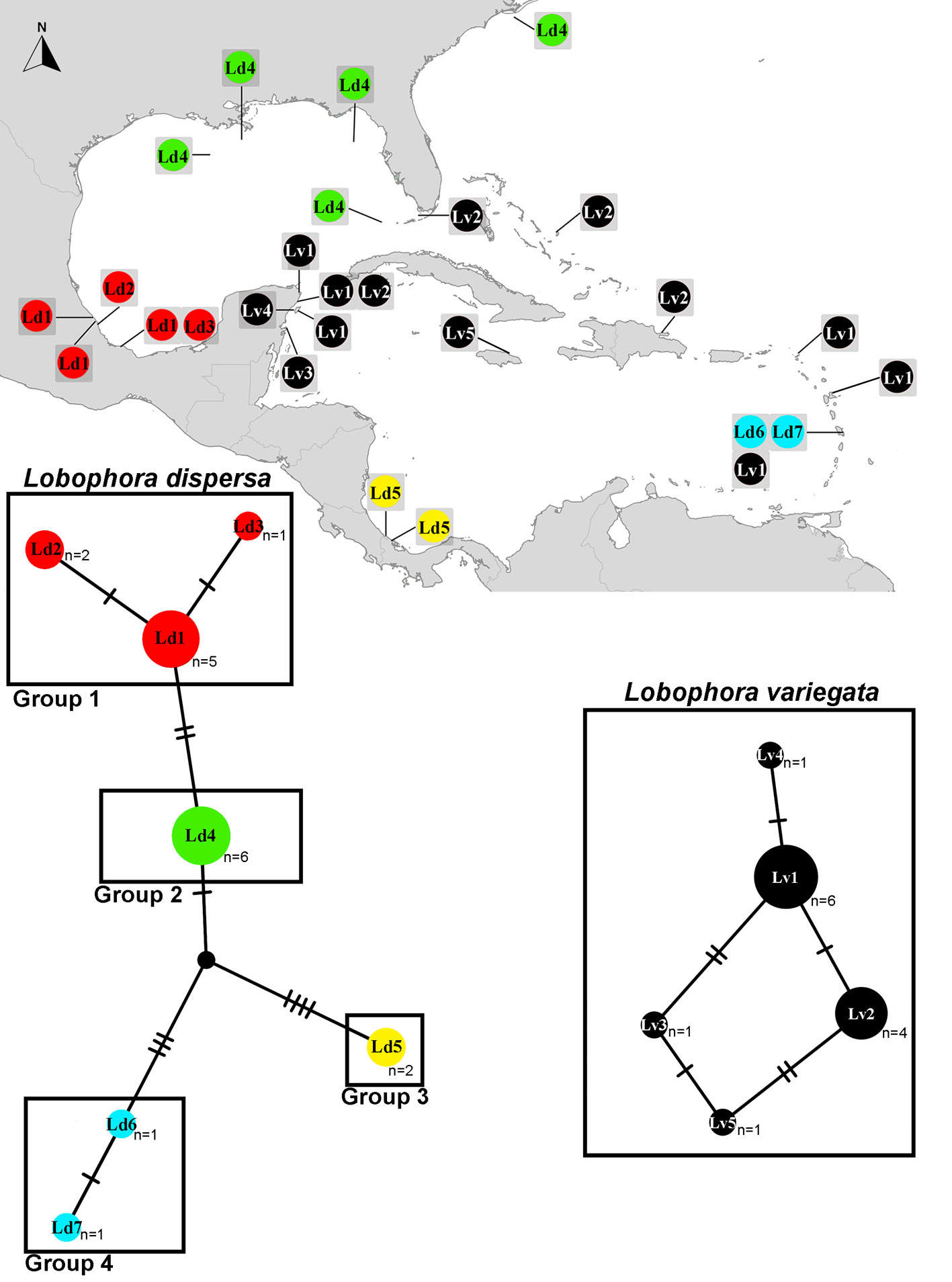
The cox3 haplotype networks built for L. dispersa (n = 18) recovered a total of 7 haplotypes (Ld1-7; Fig. 4) and 4 subgroups. Group 1 included samples from Veracruz, Mexico (Ld1-3); group 2 included samples from the northern Gulf of Mexico, Florida, and North Carolina, USA (Ld4); group 3 contained samples from Costa Rica and Panama in the southern Caribbean (Ld5); and group 4 included samples from Martinique in the eastern Caribbean region (Ld6, 7). For L. variegata (n = 13), a total of 5 haplotypes were found (Lv1-5; Fig. 4). Two haplotypes appear to be widely distributed in the Greater Caribbean: Lv1 occurred along the Mexican Caribbean coast and in Saint Martin, and Lv2 occurred in Mexico, the Dominican Republic, and Florida, USA. Haplotypes Lv3 and Lv4 were found only in Mexico, and Lv5 solely in Jamaica.
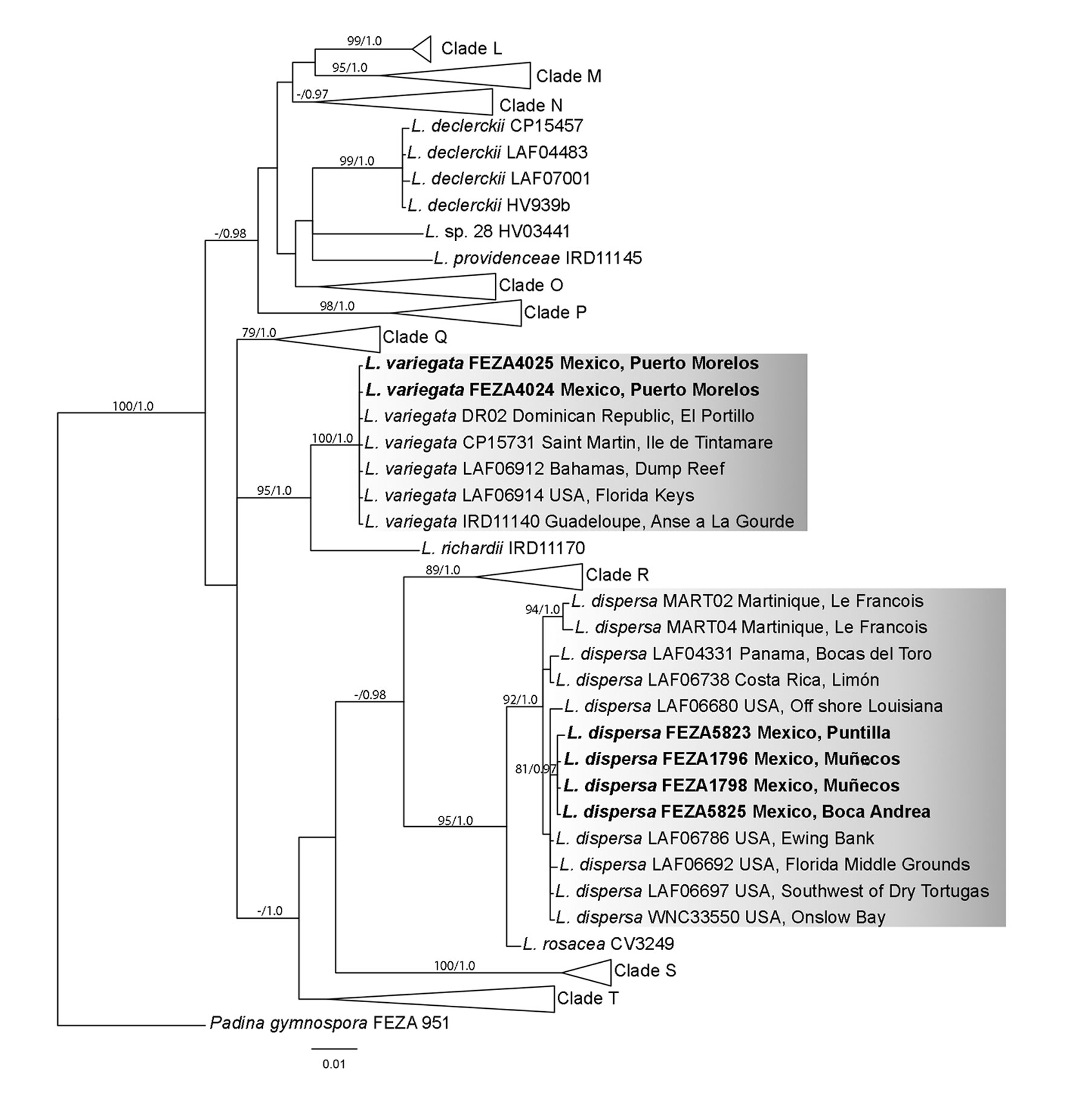
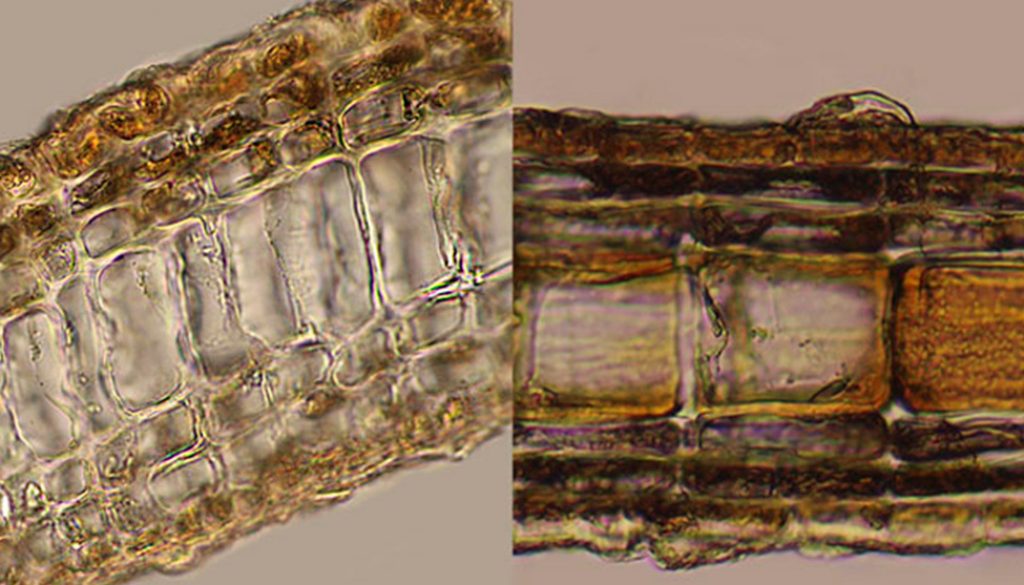
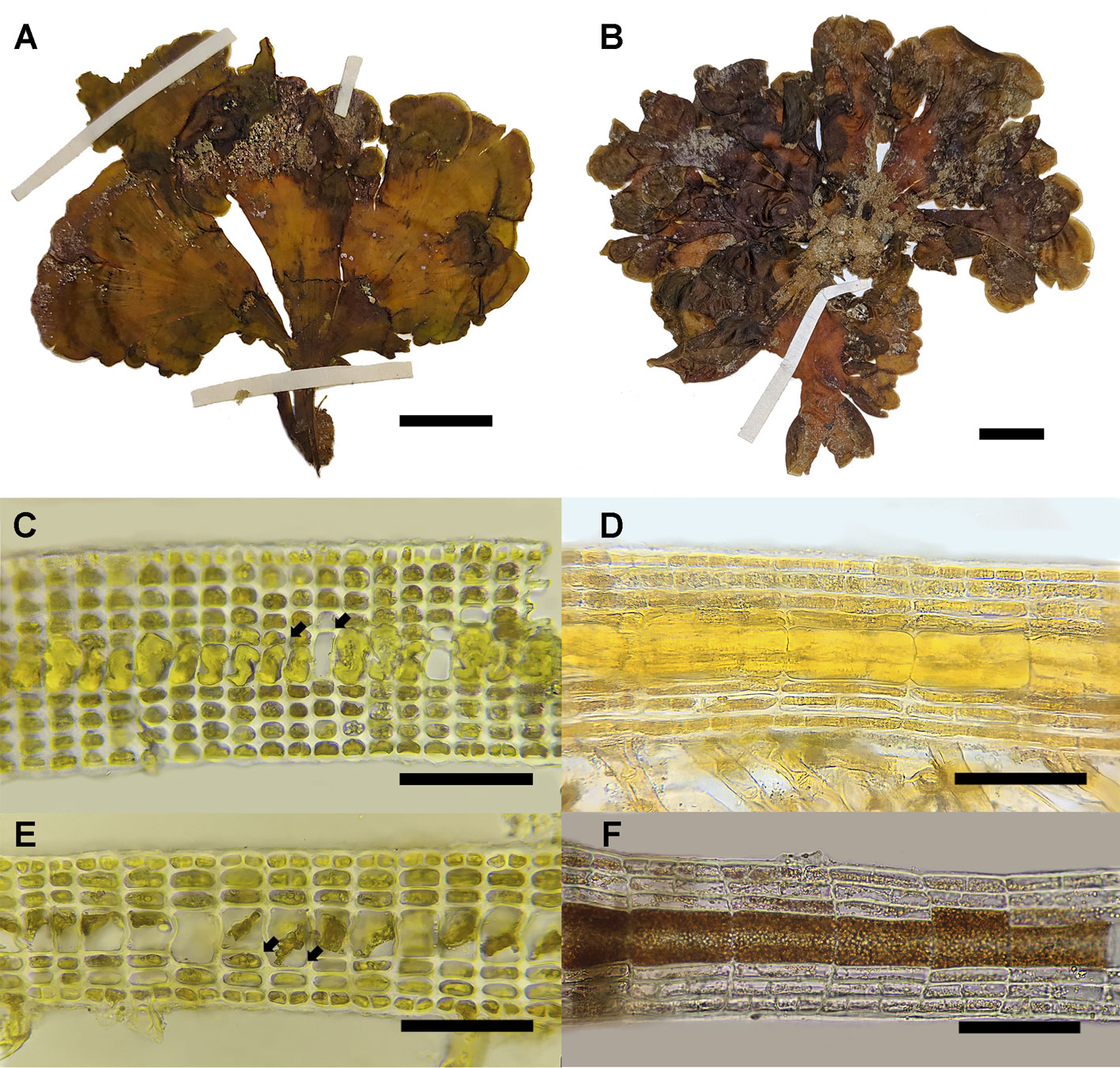
Morphological results. The individuals of Lobophora dispersa display lobed procumbent blades, either entire or slightly divided (Fig. 5A) to highly divided close to the base of the thallus (Fig. 5B). The color ranged from light brown to yellowish-green but turning darker towards the stipe. Examined thalli were up to 3.2 cm in height and 3.7 cm wide. Anatomically, the thalli consisted of 1 layer of medullary cells, with multiple layers of subcortical and cortical cells on both sides of the medulla (Fig. 5C-F). The basal section of the thallus ranged from 9-10 layers of cells (Fig. 5C, D) to 6-7 in the middle sections (Fig. 5E, F). The ventral side consists of 2-4 layers (1 cortical layer), and the dorsal side of 3-5 layers (1 cortical) depending on the part of the thallus examined and the age of the individual. Transverse sections occasionally reveal the division of the medullary cells (Fig. 5C, E). Measurements of cells can be found in Table 1.
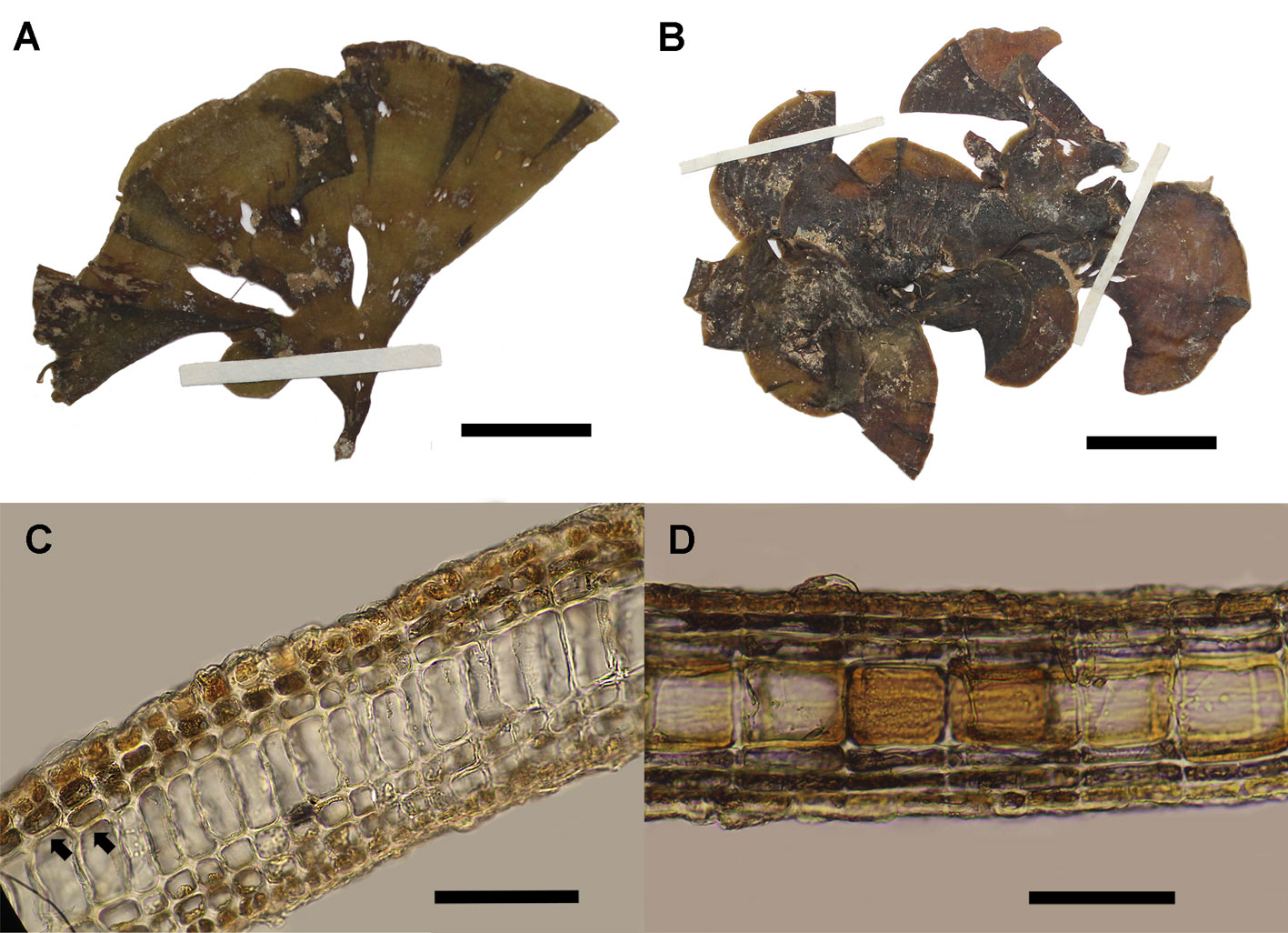
The analyzed individuals of L. variegata formed lobed erect blades (Fig. 6A), stipitate to decumbent, and are sometimes divided (Fig. 6B). The color ranged from brown to light brown and yellow-brown, turning darker toward the stipe. The analyzed thalli were up to 7.5 cm in height and 6.1 cm wide. Anatomically, the thalli consisted of 1 layer of medullary cells, plus 3 ventral cell layers (1 cortical layer) and 3 layers on the dorsal side (1 cortical) (Fig. 6C, D). Other measurements are shown in Table 1.
Discussion
The molecular data show the occurrence of L. dispersa on the coast of Veracruz because the Mexican sequences are highly similar to the type material (Camacho et al., 2019; see above). However, the Veracruz samples correspond to newly discovered haplotypes. The genetic variation and haplotype network of cox3 for this species suggests genetic differentiation based on the collection site. Such genetic differences could be attributed to reproductive strategies or isolation by distance (Couceiro et al., 2011; Krueger-Hadfield et al., 2011; Li et al., 2016). Interestingly, no reproductive structures have been reported so far for L. dispersa (Camacho et al., 2019; Vieira, Aharonov et al., 2019; Vieira, Morrow et al., 2020) raising questions regarding its dispersal capabilities, phenology, and life cycle. Some of these questions may be answered with further sampling at different times and scale, and finer population genetic analyses. Similarly, patterns of genetic differentiation through the Gulf of Mexico can be found in Padina gymnospora and P. boergesenii Allender & Kraft, where most individuals from Veracruz are genetically distinct from those in Campeche and the Yucatán Peninsula (Díaz-Martínez et al., 2016). Ocean currents in the Caribbean and the Gulf of Mexico, which originated from the rising of the Yucatán Peninsula and the closure of the Central American isthmus (Pindell & Kennan, 2009), have been invoked to explain the distribution and endemicity of red algae in the region, particularly in relation to the isolation of populations in Campeche, located in the southeastern Gulf of Mexico (Dreckmann et al., 2018; Hernández et al., 2021; Núñez-Resendiz et al., 2017, 2019), either improving or reducing the connectivity between populations. The genetic pattern observed in L. dispersa could be influenced by the heterogeneity of habitats in the region (because of the influence of currents and geological history; Dreckmann & Sentíes, 2013) and/or other intrinsic species factors such as reproductive modes (Ardehed et al., 2015; Krueger-Hadfield et al., 2013).
The occurrence of L. variegata on the Mexican Caribbean coast is also corroborated as the cox3 sequences are highly similar to the type material (Antilles, West Indies) studied by Viera et al. (2016). For this species, the genetic distances in both markers, cox3 and psbA, was low, even considering specimens from distant regions, contrasting with L. dispersa. The haplotype network analysis revealed the presence of at least 3 distinct haplotypes from the Caribbean coast of Mexico, with no obvious differentiation patterns observed. At first glance, this finding appears to be consistent with other macroalgae in the Western Atlantic such as Hypnea sp. 1 (Nauer et al., 2019) and Gracilaria usneoides (C. Agardh) J. Agardh (Núñez-Resendiz et al., 2017), where surface ocean currents could be promoting connectivity and genetic exchange between populations. However, the genetic diversity observed in Hypnea sp. and Gracilaria usneoides is higher compared to Lobophora variegata. To further test these hypotheses, additional sampling and molecular studies, such as population genetics, phylogeography, and time-calibrated analyses, will be necessary.
Table 1
Comparison of morphological and anatomical characters of Lobophora dispersa, L. variegata, and L. declerckii. Measurements are in μm, except for thallus height and width (cm). ‘-’ = Not available.
| Lobophora dispersa | Lobophora variegata | Lobophora declerckii | |||||||
| Color | Light brown to dark green | Light to dark brown | Light to dark brown, olive green | Dark orange, brown to dark green | Light brown | Yellow- green | Light green | Light brown | Brown |
| Growth form | Flabellate, fan-shaped; blade entire to highly divided;
procumbent |
Erect, procumbent; fan-shaped, stipitate | Flabellate, fan-shaped; blade entire to highly divided; decumbent | Decumbent, fasciculate, ruffled | Erect; simple or lobed | Simple or lobed to lacerate | Conk-like/ decumbent | Decumbent, simple or lobed | Simple or lobed |
| Thallus height | 1.8-3.7 | – | 2.3-6.1 | – | 1-3 | 2.2-4.4 | – | 1-5 | 1.3-4.9 |
| Thallus width | 1.9-3.2 | – | 1.2-7.6 | – | 7-13 | 3.1-5.5 | – | 2-7 | 65-83 |
| Blade thick | 110-167.5 | 78-164 | 140-180 | 124-197 | 135-145 | 128-190 | 55-85 | 70-110 | 65-83 |
| Total number of cells | 6-10 | 5-8 | 7 | 5-7 | 5-7 | 5-7 | 3-5 | 5 | 3-5 |
| Number of dorsal cells | 3-5 | 2-4 | 3 | 2-3 | 3 | 2-3 | 1-2 | 2 | 1-2 |
| Number of cortical cells | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Number of ventral cells | 2-4 | 2-3 | 3 | 2-3 | 3 | 2-3 | 1-2 | 1-2 | 1-2 |
| Dorsal side cells | |||||||||
| Cortical cells height | 4.3-14.5 | – | 8.9-13.5 | – | 5-12 | 10-13 | 15-22 | 17-16 | 8-12 |
| Cortical cells length | 10.8-50.9 | – | 20.8-37.9 | – | 24-50 | – | – | 24-42 | – |
| Cortical cells width | 7.14-14.8 | – | 11.1-11.7 | – | 12-19 | – | – | 8-29 | – |
| Subcortical cells height | 9.1 -15.2 | – | 8.8-16.5 | – | 10-24 | 6-12 | – | 7-15 | 6-10 |
| Subcortical cells length | 27.4-74.9 | – | 45.0-65.9 | – | 54-107 | – | – | 44-104 | – |
| Subcortical cells width | 14.3-25.1 | – | 15.5-21.4 | – | 20-39 | – | – | 8-41 | – |
| Medullar cells | |||||||||
| Cells height | 20.1-45.3 | 23-60 | 42.1 -54.2 | 50-94 | 35-73 | 53-87 | 30-50 | 27-75 | 27-48 |
| Cells length | 59.3-107.7 | 48-125 | 46.5-65.2 | 68-94 | 53-91 | 78-90 | 62-100 | 53-103 | 67-98 |
| Cells width | 13.9-23.6 | 18-28 | 18.8-22.4 | 23-43 | 24-40 | 28-40 | 25-45 | 18-41 | 23-40 |
| Ventral side cells | |||||||||
| Subcortical cells height | 8.7-16.4 | – | 9.7-13.1 | – | 11-27 | 8-10 | – | 7-15 | 6-10 |
| Subcortical cells length | 28.8 -68.2 | 46.3-69.5 | 60-100 | 44-104 | |||||
| Subcortical cells width | 9.82-23.3 | 18.6-21.1 | 23-37 | 8-41 | |||||
| Cortical cells height | 7.8-16.4 | 7.6-14.2 | 7-18 | 6-11 | 14-20 | 7-17 | 8-11 | ||
| Cortical cells length | 17.9-47.0 | 23.3-55.6 | 34-64 | 15-79 | |||||
| Cortical cells width | 8.7-24.0 | 7.8-19.0 | 11-17 | 9-35 | |||||
| Sporangia | |||||||||
| Diameter | Not observed | Not observed | Not observed | Not observed | Not observed | Not observed | 90-105 | Not observed | Not observed |
| Height | 125-150 | ||||||||
| References | This study | Camacho et al., 2019, Vieira, Aharonov et al., 2019; Vieira, Henriques et al., 2020; Vieira, Morrow et al., 2020 | This study | Vieira et al., 2016 | Godínez-Ortega et al., 2018 | Torres-Conde et al., 2021 | Vieira, Morrow et al., 2020 | Godínez-Ortega et al., 2018 | Torres-Conde et al., 2021 |
The morphology of Lobophora dispersa found in Mexico mostly fits with previous descriptions, although some variations in cortical and subcortical cells are newly reported (Table 1). Remarkably, we have found specimens with up to 10 layers, 2 more than previously reported. In L. variegata, the specimens examined are similar to previous reports from Mexico (Godínez-Ortega et al., 2018), Cuba (Torres-Conde et al., 2021), and other Caribbean localities (Camacho et al., 2019; Vieira et al., 2016; Vieira, Henriques et al., 2020; Vieira, Morrow et al., 2020), but the overall measurement ranges were smaller. This could be attributed to where sections were made or the developmental stage of the samples, which can make identification difficult based only on thallus thickness (Vieira et al., 2014).
With the occurrence of L. dispersa, the current number of Lobophora species reported on the Mexican coast of the Gulf of Mexico and the Caribbean Sea rises to 3. Based only on morphology, it has been proposed that some species can be differentiated using the number of cell layers, thickness, and growth pattern (Vieira et al., 2014). Lobophora species in Mexico cannot be confidently identified based only on these traits. The number of cell layers and thickness of thalli between L. dispersa (5-10 layers) and L. variegata (5-7 layers) overlap, although they can be distinguished by growth form: L. dispersa is procumbent while L. variegata is erect to decumbent. On the other hand, both can be mistaken with L. declerckii which, despite being thinner, its number of cell layers (5) is similar to the slender individuals of L. dispersa and L. variegata. Lobophora declerckii also can share a decumbent growth with L. variegata, although a “conk-like” form similar to a shelf is reported in L. declerckii as well (Vieira, 2020; Vieira, Morrow et al., 2020). Having these similar and overlapping features between species, it is clear that molecular tools are necessary to support species identification (Puk et al., 2020; Vieira et al., 2014).
Interestingly, Lobophora dispersa, L. variegata, and L. declerckii were not found at the same sites. In previous studies, Lobophora species are reported to co-exist due to microhabitat preferences in the same localities and limited intraspecific and interspecific competition (Vieira, Morrow et al., 2020). However, niche preferences could be influencing the distribution of the species in the region at small and broad scales. In this regard, local studies in Palau (Micronesia) have shown the existence of ecologically generalist and specialist species where wave exposure is an important factor determining the Lobophora species assemblages at large scales, while wave exposure, depth, and herbivory are relevant at small scales (Puk et al., 2020). In addition, other studies in red algae such as Bostrychia intricata (Bory) Montagne (Muangmai et al., 2015) and Caloglossa ogasawaraensis Okamura (Kamiya & West, 2014) have shown a correlation between distinct genetic lineages adapted to specific growth conditions and, therefore, different microhabitats. For L. declerckii, it is possible that the only record from “Anegada de Adentro” coral reef is related to an affinity for deeper zones, while L. dispersa could be more adapted to the intertidal and shallow shores of Veracruz. Therefore, exploration of the sub-tidal zone is needed to investigate the diversity of Lobophora species in the region.
Two other interesting questions are: why do L. variegata and L. dispersa not occur in the same localities even when both occur in the intertidal zone, and why is there no molecular confirmation of L. variegata in the southern and great part of the northern Gulf of Mexico? Although this could be the effect of limited sampling, it could be alternatively related to the water temperature of each marine region delimiting the distribution of both species and a biogeographic factor. In this regard, the existence of a transition zone between the Gulf of Mexico and the Caribbean biotic components on the Yucatán peninsula has been suggested, which is attributed to the influence of seaweed diversity and endemicity of each region (Vilchis et al., 2018).
In conclusion, we have expanded the knowledge of this genus by reporting the occurrence of L. dispersa in Veracruz, updating the distribution of L. variegata along the coast of Quintana Roo, and pointing out some insights into the genetic diversity of Lobophora in the region. However, there is a clear need for a broader and more efficient sampling effort to represent more accurately the genetic diversity occurring throughout the Gulf of Mexico and the Caribbean Sea. It is well known that further sampling in poorly explored areas of species with wide distributions can result in better-supported conclusions (Dijoux et al., 2014; Lee et al., 2013; Zuccarello et al., 2006). As Lobophora is reported in Tamaulipas and Campeche (as L. variegata; Dreckmann, 1998; García-García et al., 2021) the occurrence of other species, as well as some co-existing in the same localities, could have been overlooked.
Acknowledgements
We thank Uri O. García Vázquez from the Molecular Systematics Laboratory at FES Zaragoza, and the Molecular Systematics Laboratory at Instituto de Biología (UNAM) for facilitating the molecular work. We thank the “Carrera de Biología” from FES Zaragoza for providing transport and facilities, Arturo Ubaldo-Fuentes for his help taking slide photographs, and we also thank the reviewers who assisted in improving this manuscript. This work was supported by “Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica” (PAPIIT) funding IA204921 (Universidad Nacional Autónoma de México). LHA thanks the “Posgrado en Ciencias Biológicas, UNAM” and received fellowship 779816 from Conahcyt.
References
Ardehed, A., Johansson, D., Schagerström, E., Kautsky, L., Johannesson K., & Pereyra R. T. (2015). Complex spatial clonal structure in the macroalgae Fucus radicans with both sexual and asexual recruitment. Ecology and Evolution, 5, 4233–4245. https://doi.org/10.1002/ece3.1629
Camacho, O., Fernández-García, C., Vieira, C., Gurgel, C. F. D., Norris, J. N., Freshwater, D. W. et al. (2019). The systematics of Lobophora (Dictyotales, Phaeophyceae) in the western Atlantic and eastern Pacific oceans: eight new species. Journal of Phycology, 55, 611–624. https://doi.org/10.1111/jpy.12850
Clement, M., Posada, D., & Crandall, K. A. (2000). TCS: a computer program to estimate gene genealogies. Molecular Ecology, 9, 1657–1659. https://doi.org/10.1046/j.1365-294x.2000.01020.x
Couceiro, L., Maneiro, I., Ruiz J. M., & Barreiro R. (2011). Multiscale genetic structure of an endangered seaweed Ahnfeltiopsis pusilla (Rhodophyta): implications for its conservation. Journal of Phycology, 47, 259–268. https://doi.org/10.1111/j.1529-8817.2011.00959.x
Díaz-Martínez, S., Zuccarello, G. C., Chávez, G. A. S., Pedroche, F. F., & Avila-Ortiz A. G. (2016). Species of Padina (Dictyotales, Phaeophyceae) in tropical Mexican waters based on molecular-assisted taxonomy. Phycologia, 55, 673–687. https://doi.org/10.2216/16-15.1
Dijoux, L., Viard, F., & Payri, C. (2014). The more we search, the more we find: discovery of a new lineage and a new species complex in the genus Asparagopsis. Plos One, 9, 1–13. https://doi.org/10.1371/journal.pone.0103826
Doyle, J. J., & Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin, 19, 11–15.
Dreckmann, K. M. (1998). Clasificación y nomenclatura de las macroalgas marinas bentónicas del Atlántico mexicano. Mexico D.F.: UAM, Iztapalapa/ Conabio.
Dreckmann, K. M., & Sentíes, A. (2013). Las arribazones de algas marinas en el Caribe mexicano, evento biológico natural o basura en las playas. Biodiversitas, 107, 7–11.
Dreckmann, K. M., Núñez-Resendiz, M. L., & Sentíes, A. (2018). Gracilaria microcarpa sp. nov. (Gracilariaceae, Rhodophyta) from the southwestern Gulf of Mexico. Botanica Marina, 61, 115–125. https://doi.org/10.1515/bot-2017-0068
García-García, A. M. E., Cabrera-Becerril, E., Núñez-Reséndiz, M. L., Dreckmann, K. M., & Sentíes, A. (2021). Actualización taxonómica de las algas pardas (Phaeophyceae, Ochrophyta) marinas bentónicas del Atlántico mexicano. Acta Botanica Mexicana, 128, e1968. https://doi.org/10.21829/abm128.2021.1968
Godínez-Ortega, J. L., Cabrera, L. I., García-Sandoval, R., Wynne, M. J., Olivares-Rubio, H. F., Ramírez-García, P. et al. (2018). Morphological and molecular characterization of Lobophora declerckii and L. variegata (Dictyotales, Ochrophyta) on the Atlantic coast of Mexico. Phytotaxa, 382, 57–73. https://doi.org/10.11646/phytotaxa.382.1.2
Guiry, M. D., & Guiry, G. M. (2022). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. Recovered on July 1, 2022: http://www.algaebase.org
Hernández, O. E., Dreckmann, K. M., Nuñez-Resendiz, M. L., & Sentíes, A. (2021). Patrones de distribución de la familia Solieriaceae (Gigartinales, Rhodophyta) en México. Acta Botanica Mexicana, 128, e1994. https://doi.org/10.21829/abm128.2021.1994
Huelsenbeck, J. P., & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17, 745–775.
Kamiya, M., & West, J. A. (2014). Cryptic diversity in the euryhaline red alga Caloglossa ogasawaraensis (Delesseriaceae, Ceramiales). Phycologia, 53, 374–382. https://doi.org/10.2216/13-242.1
Krueger-Hadfield, S. A., Collén, J., Daguin-Thiébaut, C., & Valero, M. (2011). Genetic population structure and mating system in Chondrus crispus (Rhodophyta). Journal of Phycology, 47, 440–450. https://doi.org/10.1111/j.1529-8817.2011.00995.x
Krueger-Hadfield, S. A., Roze, D., Mauger, S., & Valero, M. (2013). Intergametophytic selfing and microgeographic genetic structure shape populations of the intertidal red seaweed Chondrus crispus. Molecular Ecology, 22, 3242–3260. https://doi.org/10.1111/mec.12191
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549. https://doi.org/10.1093/molbev/msy096
Lanfear, R., Calcott, B., Ho, S. Y. W., & Guindon, S. (2012). PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Molecular Biology and Evolution, 29, 1695–1701. https://doi.org/10.1093/molbev/mss020
Lee, K. M., Boo, S. M., Kain, J. M., & Sherwood, A. R. (2013). Cryptic diversity and biogeography of the widespread brown alga Colpomenia sinuosa (Ectocarpales, Phaeophyceae). Botanica Marina, 56, 15–25. https://doi.org/10.1515/bot2012-0211
Leigh, J. W., & Bryant, D. (2015). POPART: full-feature software for haplotype network construction. Methods
in Ecology and Evolution, 6, 1110–1116. https://doi.org/10.1111/2041-210X.12410
Li, J. J., Hu, Z. M., Liu, R. Y., Zhang, J., Liu, S. L., & Duan, D. L. (2016). Phylogeographic surveys and apomictic genetic connectivity in the North Atlantic red seaweed Mastocarpus stellatus. Molecular Phylogenetics and Evolution, 94, 463–472. https://doi.org/10.1016/j.ympev.2015.10.029
Muangmai, N., Preuss, M., & Zuccarello, G. C. (2015). Comparative physiological studies on the growth of cryptic species of Bostrychia intricata (Rhodomelaceae, Rhodophyta) in various salinity and temperature conditions. Phycological Research, 63, 300–306. https://doi.org/10.1111/pre.12101
Nauer, F., Gurgel, C. F., Ayres-Ostrock, L. M., Plastino, E. M., & Oliveira, M. C. (2019). Phylogeography of the Hypnea musciformis species complex (Gigartinales, Rhodophyta) with the recognition of cryptic species in the western Atlantic Ocean. Journal of Phycology, 55, 676–687. https://doi.org/10.1111/jpy.12848
Nguyen, L. T., Schmidt, H. A., Von Haeseler, A., & Minh, B. Q. (2015). IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Phylogenetics and Evolution, 32, 268–274. https://doi.org/10.1093/molbev/msu300
Núñez-Resendiz, M. L., Dreckmann, K. M., Sentíes, A., & León-Tejera, H. P. (2019). Meristotheca spinella Núñez-Resendiz, Dreckmann & Sentíes, sp. nov. (Solieriaceae, Rhodophyta) a new cylindrical species from the southwestern Gulf of Mexico. Cryptogamie, Algologie, 40, 63–72. https://doi.org/10.5252/cryptogamie-algologie2019v40a6
Núñez-Resendiz, M. L., Zuccarello, G. C., Dreckmann, K. M., & Sentíes, A. (2017). Phylogeography of Hydropuntia cornea/Hydropuntia usneoides complex (Gracilariales, Rhodophyta) in the Yucatán Peninsula. Phycologia, 56, 14–20. https://doi.org/10.2216/16-46.1
Ortega, M., Godínez-Ortega, J., & Garduño, G. (2001). Catálogo de algas bénticas de las costas mexicanas del golfo de México y mar Caribe. Cuadernos. Mexico D.F.: UNAM/ Conabio.
Pindell, J. L., & Kennan, L. (2009). Tectonic evolution of the Gulf of Mexico, Caribbean and northern South America in the mantle reference frame: an update. Geological Society London, Special Publications, 328, 1–55. https://doi.org/10.1144/SP328.1
Puk, L. D., Vieira, C., Roff, G., De Clerck, O., & Mumby, P. J. (2020). Cryptic diversity in the macroalgal genus Lobophora (Dictyotales) reveals environmental drivers of algal assemblages. Marine Biology, 167, 188. https://doi.org/10.1007/s00227-020-03802-x
Rambaut, A., Drummond, A. J., Xie, D., Baele, G., & Suchard, M. A. (2018). Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67, 901–904. https://doi.org/10.1093/sysbio/syy032
Schultz, N. E., Lane, C. E., Le Gall, L., Gey, D., Bigney, A. R., De Reviers, B. et al. (2015). A barcode analysis of the genus Lobophora (Dictyotales, Phaeophyceae) in the western Atlantic Ocean with four novel species and the epitypification of L. variegata (J.V. Lamouroux) E.C. Oliveira. European Journal of Phycology, 50, 481–500. https://doi.org/10.1080/09670262.2015.1078500
Silberfeld, T., Bittner, L., Fernández-García, C., Cruaud, C., Rousseau, F., de Reviers, B. et al. (2013). Species diversity, phylogeny and large scale biogeographic patterns of the genus Padina (Phaeophyceae, Dictyotales). Journal of Phycology, 49, 130–142. https://doi.org/10.1111/jpy.12027
Thiers, B. (2022). Index Herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. Recovered on August 2, 2022: http://sweetgum.nybg.org/science/ih/
Torres-Conde, E. G., Zúñiga-Delgado, J. G., Reyes-Pérez, D. L., & Suárez, A. M. (2021). New records of the genus Lobophora (Dictyotales: Phaeophyceae) for the marine flora of Cuba and their distribution in the Greater Caribbean Sea. Revista Mexicana de Biodiversidad, 92, e923538. https://doi.org/10.22201/IB.20078706E.2021.92.3538
Vieira, C. (2020). Lobophora-coral interactions and phase shifts: summary of current knowledge and future directions. Aquatic Ecology, 54, 1–20. https://doi.org/10.1007/s10452-019-09723-2
Vieira, C., Aharonov, A., Paz, G., Engelen, A. H., Tsiamis, K., Einav, R. et al. (2019). Diversity and origin of the genus Lobophora in the Mediterranean Sea including the description of two new species. Phycologia, 58, 163–168. https://doi.org/10.1080/00318884.2018.1534923
Vieira, C., Camacho, O., Wynne, M. J., Mattio, L., Anderson, R. J., Bolton, J. J. et al. (2016). Shedding new light on old algae: matching names and sequences in the brown algal genus Lobophora (Dictyotales, Phaeophyceae). Taxon, 65, 689–707. https://doi.org/10.12705/654.1
Vieira, C., D’hondt, S., De Clerck, O., & Payri, C. E. (2014). Toward an inordinate fondness for stars, beetles and Lobophora? Species diversity of the genus Lobophora (Dictyotales, Phaeophyceae) in New Caledonia. Journal of Phycology, 50, 1101–1119. https://doi.org/10.1111/jpy.12243
Vieira, C., De Clerckm O., Millet, L., & Payri, C. E. (2019). Description of ten new Lobophora species from the Bismarck Sea (Papua New Guinea). Phycological Research, 67, 228–238. https://doi.org/10.1111/pre.12372
Vieira, C., Henriques, F., D’hondt, S., Neto, A., Almada, C. H., Kaufmann, M. et al. (2020). Lobophora (Dictyotales)
species richness, ecology and biogeography across the north-Eastern Atlantic archipelagos and description of two new species. Journal of Phycology, 56, 346–357. https://doi.org/10.1111/jpy.12956
Vieira, C., Morrow, K., D’Hondt, S., Camacho, O., Engelen, A. H., Payri, C. E. et al. (2020). Diversity, ecology, biogeography, and evolution of the prevalent brown algal genus Lobophora in the Greater Caribbean Sea, including the description of five new species. Journal of Phycology, 56, 592–607. https://doi.org/10.1111/jpy.12986
Vieira, C., Rasoamanendrika, F. A., Zubia, M., Bolton, J. J., Anderson, R. J., Engelen, A. H. et al. (2021). Lobophora (Dictyotales, Phaeophyceae) from the western Indian Ocean: diversity and biogeography. South African Journal of Botany, 142, 230–246. https://doi.org/10.1016/j.sajb.2021.06.015
Vieira, C., Steen, F., D’hondt, S., Bafort, Q., Tyberghein, L., Fernandez-García, C. et al. (2021). Global biogeography
and diversification of a group of brown seaweeds (Phaeophyceae) driven by clade-specific evolutionary processes. Journal of Biogeography, 48, 703–715. https://doi.org/10.1111/jbi.14047
Vilchis, M. I., Dreckmann, K. M., García-Trejo, E. A., Hernández, O. E., & Sentíes, A. (2018). Patrones de distribución de las grandes macroalgas en el golfo de México y el Caribe mexicano: una contribución a la biología de la conservación. Revista Mexicana de Biodiversidad, 89, 183–192. https://doi.org/10.22201/ib.20078706e.2018.1.2226
Yoon, H. S., Hackett, J. D., & Bhattacharya, D. (2002). A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proceedings of the National Academy of Sciences of the United States of America, 99, 11724–11729. https://doi.org/10.1073/pnas.172234799
Zuccarello, G. C., Buchanan, J., & West, J. A. (2006). Increased sampling for inferring phylogeographic patterns in Bostrychia radicans/B. moritziana (Rhodomelaceae, Rhodophyta) in the eastern USA. Journal of Phycology, 42, 1349–1352.
https://doi.org/10.1111/j.1529-8817.2006.00292.x