On the Mexican ricinuleids: a new species of the genus Pseudocellus (Arachnida: Ricinulei: Ricinoididae) from the cloud forest of Chiapas, Mexico
Alejandro Valdez-Mondragón a, c, *, Mayra R. Cortez-Roldán b, d,
Emmanuel F. Campuzano-Granados e
a Conacyt Research Fellow, Laboratorio de Aracnología, Laboratorio Regional de Biodiversidad y Cultivo de Tejidos Vegetales, Instituto de Biología, Universidad Nacional Autónoma de México, sede Tlaxcala, Ex-Fábrica San Manuel, San Miguel Contla, 90640 Santa Cruz Tlaxcala, Tlaxcala, Mexico
b Laboratorio Regional de Biodiversidad y Cultivo de Tejidos Vegetales, Instituto de Biología, Universidad Nacional Autónoma de México, sede Tlaxcala, Ex-Fábrica San Manuel, San Miguel Contla, 90640 Santa Cruz Tlaxcala, Tlaxcala, Mexico
c Colección Nacional de Arácnidos, Departamento de Zoología, Instituto de Biología, Universidad Nacional Autónoma de México, 04510 México City, Mexico
d Posgrado en Ciencias Biológicas, Centro Tlaxcala de Biología de la Conducta, Universidad Autónoma de Tlaxcala, Carretera Federal Tlaxcala-Puebla, Km. 1.5, 90062 Tlaxcala, Tlaxcala, Mexico
e El Colegio de la Frontera Sur, Unidad Tapachula, carretera Antiguo Aeropuerto Km. 2.5, Centro, 30700 Tapachula de Córdova y Ordoñez, Chiapas, Mexico
*Corresponding author: lat_mactans@yahoo.com.mx (A. Valdez-Mondragón)
Received: 28 September 2019; accepted: 13 January 2020
http://zoobank.org/urn:lsid:zoobank.org:pub:920B9FFA-3E68-4C4D-BFF3-19DBA3FC9EBF
Abstract
A new species of epigean ricinuleid of the genus Pseudocellus Platnick, 1980 is described from a cloud forest of the state of Chiapas, Mexico. The species is described based on adult males and females: Pseudocellus franckei sp. nov. This is the sixth species described from Chiapas, holding the highest number of ricinuleids species for the country. The total number of described species of Pseudocellus from Mexico increases to 19, having the highest number of Ricinulei worldwide.
Keywords: Ricinuleids; Taxonomy; New species; Epigean species
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Sobre los ricinúlidos mexicanos: una especie nueva del género Pseudocellus (Arachnida: Ricinulei: Ricinoididae) de bosque de niebla de Chiapas, Mexico
Resumen
Una especie nueva de ricinúlido epigeo del género Pseudocellus Platnick, 1980 es descrita de un bosque de niebla del estado de Chiapas, México. La especie es descrita basada en machos y hembras adultos: Pseudocellus franckei sp. nov. Esta es la sexta especie descrita de Chiapas, manteniendo el más alto número de especies de ricinúlidos del país. El número total de especies descritas de Pseudocellus de México se incrementa a 19, teniendo el mayor número de Ricinulei en todo el mundo.
Palabras clave: Ricinúlidos; Taxonomía; Especie nueva; Especie epigea
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Ricinulei currently comprises 90 species worldwide (including the new species herein described) and 22 fossil species (Harvey, 2003; Selden, 1992; Valdez-Mondragón et al., 2018; Wunderlich, 2017). Traditionally, the order is composed by the suborders Palaeoricinulei Selden, 1992 and Neoricinulei Selden, 1992, each including extinct and living taxa respectively (Harvey, 2003; Selden, 1992). Wunderlich (2017) recognized 2 new monogeneric families of extinct ricinuleids, both described into the Primoricinulei: Hirsutisomidae Wunderlich, 2017 and Monooculricinuleidae Wunderlich, 2017.
The superfamily Ricinoidoidea Ewing, 1929 comprises 3 genera: Cryptocellus Westwood, 1874, Pseudocellus Platnick, 1980 and Ricinoides Ewing, 1929; with 42, 35 and 11 described species respectively (Armas, 2017; Armas & Agreda, 2016; Botero-Trujillo & Flórez, 2017; Botero-Trujillo & Valdez-Mondragón, 2016; Harvey, 2003; Valdez-Mondragón et al., 2018). Only Cryptocellus and Pseudocellus are distributed in the New World, whereas Ricinoides is restricted to western and central Africa countries (Naskrecki, 2008; Penney et al., 2009; Tuxen, 1974). Pseudocellus is primarily distributed in North and Central America with some species described from the Caribbean islands, whereas Cryptocellus is predominantly South American (Armas, 2017; Armas & Agreda, 2016; Botero-Trujillo & Flórez, 2017; Botero-Trujillo & Pérez 2008, 2009; Botero-Trujillo & Valdez-Mondragón, 2016; Harvey, 2003; Pinto-da Rocha & Andrade, 2012; Teruel & Armas, 2008; Tourinho & Azevedo, 2007; Tourinho & Saturnino, 2010; Tourinho et al., 2010, 2014; Valdez-Mondragón & Francke 2011, 2013; Valdez-Mondragón et al., 2018).
In the last 8 years, 6 epigean and 2 troglobitic species have been described from Mexico (Valdez-Mondragón & Francke 2011, 2013; Valdez-Mondragón et al., 2018). Recently, the first 2 sympatric epigean species were described from North America by Valdez-Mondragón et al. (2018) from Veracruz, Mexico; describing for the very first time for any ricinuleids, pores with an unknown function on the membrane below the female spermathecae. Ricinuleids of the genus Pseudocellus from Mexico are typically found in the soil of lowland tropical rainforests, into the leaf litter, underlying layers, under rocks as well as under rotten logs (Harvey, 2003; Platnick, 1980; Valdez-Mondragón et al., 2018). Several species, all belonging to Pseudocellus are found frequently inside caves, including true troglobites with distinct troglomorphisms. Mexico is the country with the largest number of known troglobitic ricinuleids, so far, with 8 described species: Pseudocellus bolivari (Gertsch, 1971), P. boneti (Bolívar & Pieltain, 1942), P. monjarazi Valdez-Mondragón & Francke, 2013, P. osorioi (Bolívar & Pieltain, 1946), P. oztotl Valdez-Mondragón & Francke, 2011, P. platnicki Valdez-Mondragón & Francke, 2011, P. reddelli (Gertsch, 1971), and P. sbordonii (Brignoli, 1974), holding the first place in number of known ricinuleid species worldwide, with 19 out of 35 valid species of Pseudocellus (not including the new species described herein).
In this contribution, we describe, based on adult males and females, a new species of Pseudocellus from a cloud forest in Chiapas, southern Mexico.
Materials and methods
All the material is deposited at Colección de Arácnidos del Sureste de México (ECOTAAR), Colegio de la Frontera Sur (ECOSUR), Unidad Tapachula, Chiapas, México (Curator: Dr. Guillermo Ibarra Núñez), and Colección Nacional de Arácnidos (CNAN), Instituto de Biología, Universidad Nacional Autónoma de México (IBUNAM), Ciudad de México (Curator: Dr. Oscar F. Francke Ballvé). The specimens were collected under Scientific Collector Permit: SGPA/DGVS/02823/15 issued to Guillermo Ibarra Núñez. Specimens were examined in a Zeiss DiscoveryV8 stereomicroscope. Photographs were obtained with a Zeiss Axiocam 506 digital camera attached to a Zeiss AXIO ZoomV16 stereomicroscope. Photography was conducted with specimens and structures submerged in commercial-use gel alcohol (to hold them in the appropriate position), and the preparation completely covered with 80% ethanol. Spermathecae were stained using a drop of chlorazol (1%) for a few seconds followed by wash with 80% ethanol. Images were edited in Adobe Photoshop CS6. Distribution map was produced using QGIS v3.4. Measurements, in millimeters, were obtained using the methodology outlined by Cooke and Shadab (1973). Terminology used for referring to leg segments follows Gertsch (1971), whereas that used for the copulatory structures follows Pittard and Mitchell (1972) and Valdez-Mondragón et al. (2018). The length/diameter (l/d) ratio of femur II of males was calculated in prolateral view. The following abbreviations are used for some copulatory structures following Valdez-Mondragón et al. (2018): ac, accessory piece of the male copulatory apparatus; Lc, lamina cyathiformis; MT, metatarsus of leg III; mP, metatarsal process; st, spermatheca; tP, tarsal process.
Description
Order Ricinulei Thorell, 1876
Family Ricinoididae Ewing, 1929
Genus Pseudocellus Platnick, 1980
Type species: Pseudocellus dorotheae (Gertsch & Mulaik, 1939).
Pseudocellus franckei sp. nov. Valdez-Mondragón & Cortez-Roldán
Figs. 1-37
http://zoobank.org/urn:lsid:zoobank.org:act:A5351AC7-
70F8-49EB-AE47-58D83A05AABC
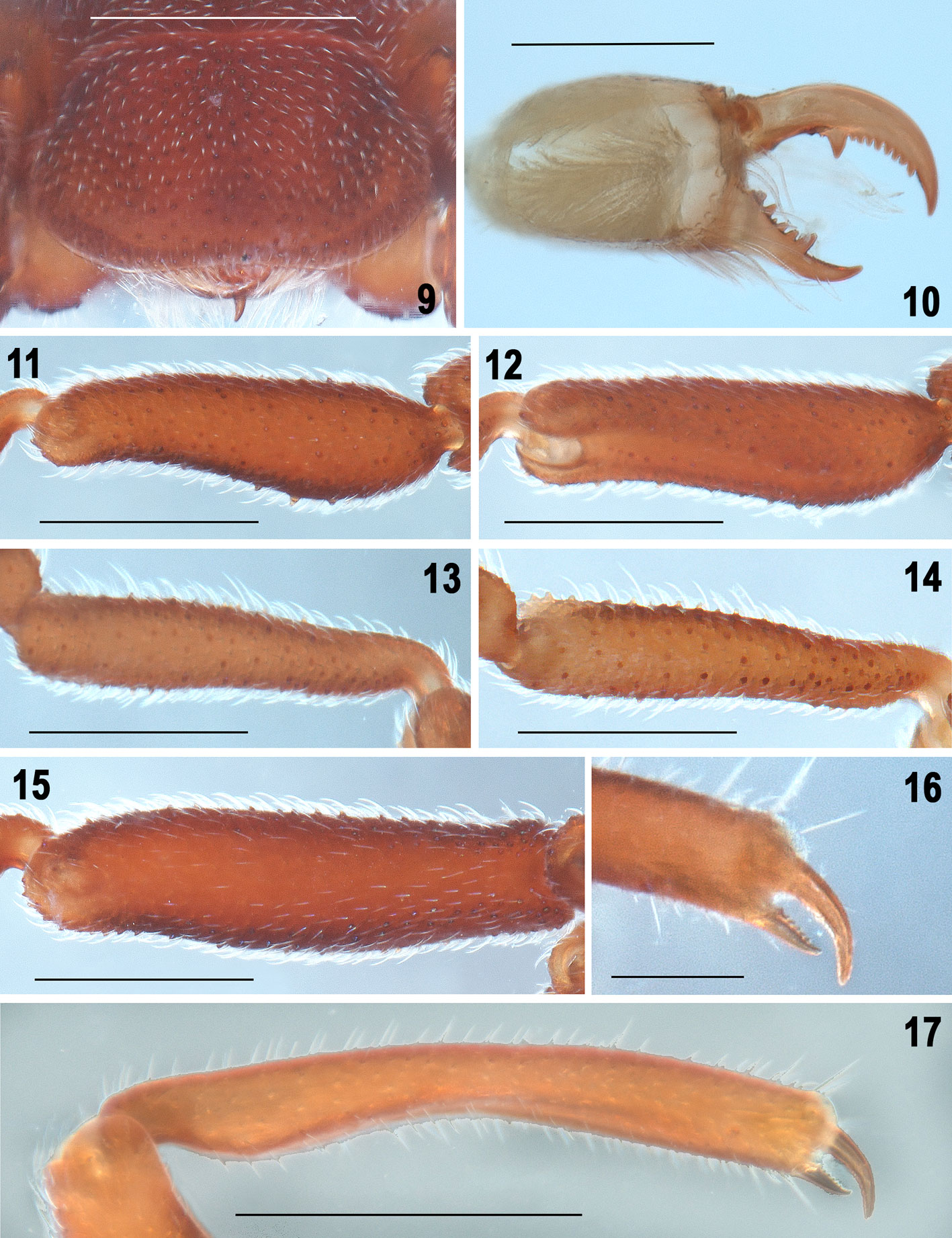
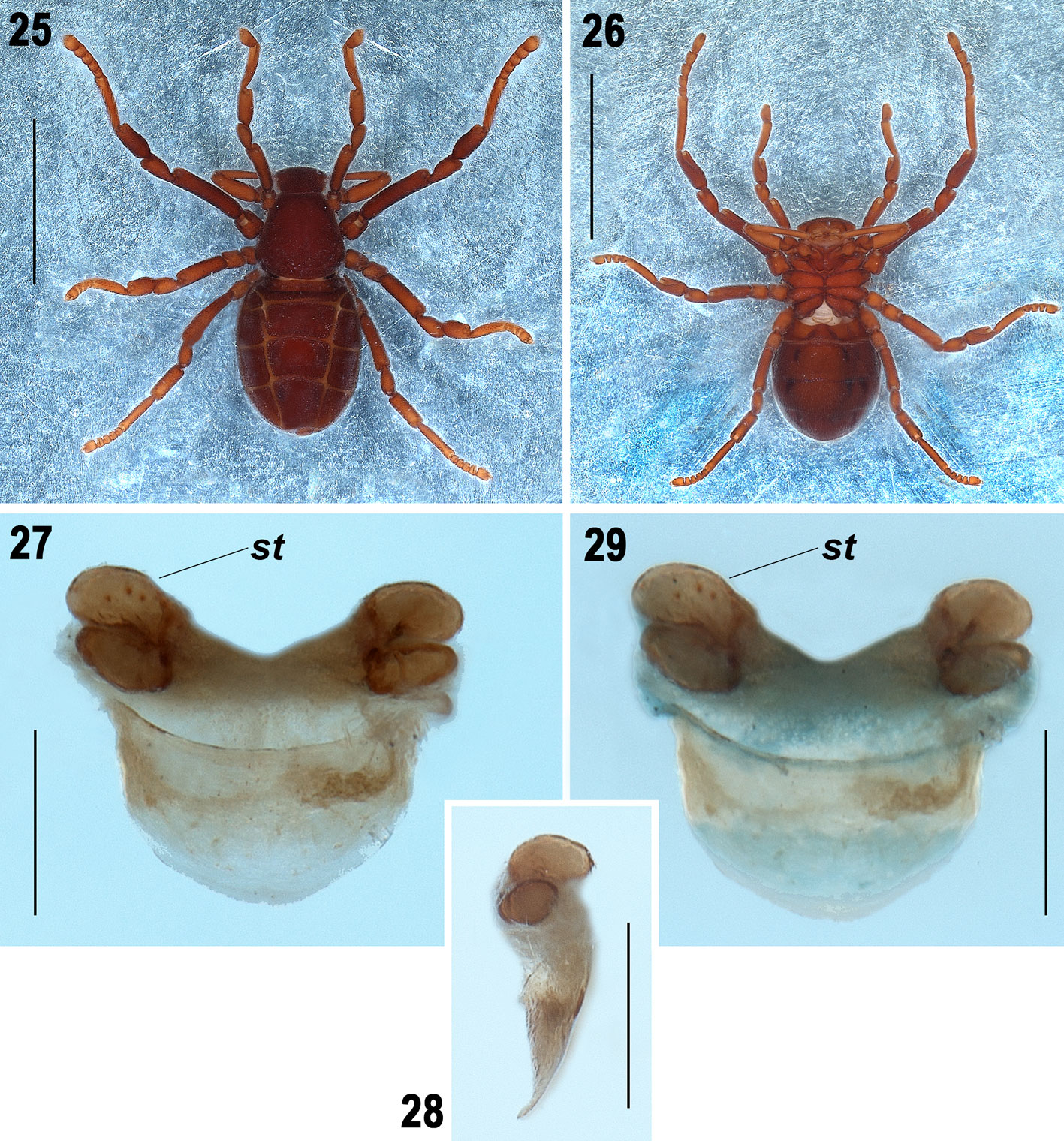
Diagnosis. Males can be distinguished by the following combination of features: 1) femora thin, without apophyses, only some inconspicuous sharp-tipped granules (Fig. 15); 2) tibia II simple, slightly curved dorsally, curved ventrally, without longitudinal rows of curved spines or apophyses, only some inconspicuous sharp-tipped granules (Figs. 11, 12); 3) tarsal process (tP) of copulatory apparatus of leg III thin and J-shaped in prolateral view (Figs. 19, 21, 23), wide and slightly sigmoidal in dorsal view (Fig. 22), apically with small and inconspicuous tips (Figs. 22-24); 4) accessory piece (ac) sigmoid, thin and single, not bifurcated distally (Fig. 21), and 5) tarsal process (tP) with a longitudinal prolateral carina (Fig. 21). Females can be distinguished by the spermathecae with 2 oval lobules on each side, one slightly longer than the other (Figs. 27-29).
Description. Male holotype (ECOTAAR-Ri00013). Measurements (in mm). Total length (carapace + opisthosoma including pygidium) 3.50. Carapace 1.34 long, 1.38 wide (widest part). Cucullus 0.61 long, 0.95 wide. Opisthosoma 1.06 long (not including pygidium), 1.86 wide (widest part). Femur II length/diameter (l/d): 3.11. Legs tarsal formula (leg I to IV): 1-5-4-5. Leg lengths; I: coxa 0.63/ trochanter 1 0.33/ trochanter 2 -/ femur 0.82/ patella 0.33/ tibia 0.58/ metatarsus 0.63/ tarsus 0.46/ total 3.78; II: 0.76/ 0.44/ -/ 1.25/ 0.51/ 0.93/ 1/ 1.26/ 6.15; III: 0.70/ 0.36/ 0.41/ 1/ 0.47/ 0.63/ 1.14/ 0.47/ 5.18; IV: 0.55/ 0.38/ 0.34/ 1.03/ 0.36/ 0.66/ 0.68/ 0.84/ 4.84. Leg length formula: 4123. Coloration. Cucullus, carapace and sternal region reddish, coxae paler reddish basally (Figs. 1–4). Pedipalps and leg II, darker reddish than leg I, III and IV; metatarsi and tarsi of legs paler reddish than other segments (Figs. 1, 2). Opisthosoma reddish dorsally, darker ventrally (Figs. 5, 6). Carapace (Fig. 3). As long as wide, trapezoidal, widest at posterior margin near coxae III. Tegument covered with abundant, fine translucent setae and rounded granules. Anterior margin straight, lateral margins not parallel, narrowing anteriorly; posterior margin curved. Translucent areas completely absent. Carapace with 6 depressions: median longitudinal depression, ¾ the length of carapace; 2 small circular depressions at level of coxae II; 1 long and curved depression on each side toward coxae III and IV, close to posterior margin. Cucullus (Fig. 9). Wider than long, widest distally; anterior margin straight, lateral margins rounded on anterior corners, were widest. Tegument covered with abundant translucent setae and granules like those on carapace; granules become slightly larger and more conspicuous distally; 1 depression on basal part and 1 depression each side; cuticular pits absent. Distal margin with medium size translucent setae. Chelicera (Fig. 10). Fixed finger with 6 teeth, distalmost slightly larger than others, which are subequal in size. Movable finger with 9 teeth: basalmost considerably longest, the rest with about the same size. Sternal region (Figs. 2, 4). Coxae covered with abundant translucent setae and granules similar to those on carapace. Coxa I rhomboidal, II trapezoidal, III and IV conical. Coxa II considerably larger than others; coxa IV smallest. Coxae I not meeting tritosternum; coxa II meeting tritosternum along 1/3 of its length. Coxae II anterior margins slightly curved, posterior margins perpendicular to median axis of prosoma; coxae III anterior margins slightly curved, their posterior margins forming an obtuse angle (> 90°) with each other; coxae IV oblique, their posterior margins forming an angle of 90° with each other. Opisthosoma (Figs. 5-7). Longer than wide, widest at level of anterior part of tergite XII. Tegument covered with abundant setae and granules like those on carapace; cuticular pits absent. Median plates of tergites XI-XIII with paired longitudinal and slightly curved depressions, those of tergite XI being the smallest. Tergite X widest and shortest, trapezoidal-shaped, curved anteriorly. Median tergite XI trapezoidal, wider than long; XII wider than long, curved posteriorly; median tergite XIII slightly longer than wide, curved anteriorly, with posterior corners straight and parallel. Lateral tergites in oblique position; X smallest, XII and XIII largest. Lateral tergites X and XIII triangular, lateral tergites XI and XII square. Sternites XI-XIII with paired and slightly curved depressions. Sternites XI and XII darker medially. Pygidium segments without notch (Fig. 8). Pedipalps (Figs. 4, 16, 17). Coxa without cuticular pits, with fine translucent setae and rounded granules on basal half. Trochanter 1 rounded, with sparse fine translucent setae and few granules; trochanter 2 conical, without setae or granules. Femur without granules, slightly curved, wider basally, with deep prolateral concavity in distal half close to the tibial joint; tegument with few and small translucent setae. Tibia curved, with a curved concavity medially in prolateral part, with numerous thin translucent setae which are longer on distal half of segment. Movable claw longer than fixed claw. Legs. Without cuticular pits but with translucent setae and rounded granules on all segments (Figs. 1, 11-15). Leg II longest (Figs. 1, 2). Femur II slightly wider and longer than the others (Fig. 1), femur and patella II ventrally with some inconspicuous sharp-tipped granules (Fig. 15). Tibia II ventrally curved, without longitudinal rows of curved spines (Figs. 11, 12). Metatarsus II with scattered sharp-tipped granules, not forming rows, dorsal ones longer than ventral (Figs. 13, 14). Tarsomeres of leg II with sharp-tipped granules on distal margins. Femur I ventrally with few sharp-tipped granules. Femora III and IV without sharp-tipped granules, only few and scattered rounded granules. Patellae I with granules, patellae III and IV with few granules. Tibiae I, III and IV with granules. All metatarsi dorsally with V-shaped invaginations distally; metatarsus I, III, and IV ventrally without granules, metatarsus IV without granules ventrally, only few slightly sharp-tipped granules dorso-distally. Tarsomere of leg I with few granules, tarsomeres of legs II with few slightly sharp-tipped granules dorso-distally, tarsomeres of leg III without granules, and tarsomeres of leg IV only with few and scattered rounded granules dorsally. Leg III and copulatory apparatus. Metatarsus semi-conical, with long translucent setae (Figs. 18, 19); metatarsal process (mP) almost straight dorsally and curved ventrally (Figs. 18, 19). Lamina cyathiformis (Lc) of tarsomere 2 conical, with a small notch basally in retrolateral view (Fig. 18), with long translucent setae throughout. All tarsomeres ventrally with long curved-tip setae. Tarsomere 4 with 2 curved claws (Figs. 18-20).
Female paratype (ECOTAAR-Ri00015). Measurements. Total length (without pygidium) 3.68. Carapace 1.35 long, 1.42 wide. Cucullus 0.63 long, 0.98 wide. Opisthosoma (without pygidium) 2.30 long, 1.96 wide. Femur II length/diameter (l/d): 4.27. Legs tarsal formula (leg I to IV): 1-5-4-5. Leg lengths; I: coxa 0.55/ trochanter 1 0.34/ trochanter 2 -/ femur 0.87/ patella 0.42/ tibia 0.66/ metatarsus 0.79/ tarsus 0.55/ total 4.18; II: 0.81/ 0.38/ -/ 1.34/ 0.63/ 1.03 / 0.69/ 0.38/ 5.26; III: 0.66/ 0.38/ 0.42/ 0.95/ 0.55/ 0.69/ 0.79/ 0.41/ 4.85; IV: 0.62/ 0.44/ 0.39/ 1.03/ 0.38/ 0.73/ 0.50/ 0.26/ 4.35. Leg length formula: 4123. Differs from male as follows: Body coloration reddish darker than the male (Figs. 25, 26). Carapace in posterior part with more marked edges than the male (Fig. 25). Tibia of the pedipalp almost straight, without curved concavity in prolateral part as the male. Opisthosoma wider than the male (Fig. 25). Median tergite XIII of opisthosoma with posterior corners slightly curved, not parallel as those in male (Fig. 25). Sternites XI-XIII of the opisthosoma with paired and slightly curved depressions more marked than male (Figs. 2, 26).
Larva (ECOTAAR-Ri00012) (Figs. 30, 31). Appendages and body coloration pale orange, femora darker than the other segments. Carapace and cucullus dark orange. Tibia of the pedipalp dark orange in distal half. Carapace wider than long, trapezoidal, with rounded corners in posterior part, with numerous rounded granules throughout. Cucullus wider than long, oval shaped, distal margin with long translucent setae, with numerous rounded granules along. Sternal region, legs and opisthosoma covered with abundant, fine translucent setae and rounded granules, except pedipalps where granules are absent. Opisthosoma wider than long, widest at the level of tergite XI. Tergite X thin, wider than long. Tergites XI-XII rectangular, considerably wider than long. Tergite XIII trapezoidal, wider than long. Tergites without depressions. Lateral tergite longer than wide, X smallest, lateral tergites XI-XIII larger. Lateral tergites XI-XII rectangular, XIII triangular. All tergites widely separated from each other. Sternites X-XIII clearly visible and separated from each other. Sternites XI-XIII dark medially. Pygidium segments without notch. Measurements: Total length (without pygidium) 1.55. Carapace 0.65 long, 0.77 wide (widest part). Cucullus 0.31 long, 0.48 wide. Opisthosoma (without pygidium) 0.84 long, 0.98 wide. Legs tarsal formula (leg I to III): 1-2-2.
Protonymph (ECOTAAR-Ri00012) (Figs. 32, 33). Appendages and body coloration orange, paler than the larva, femora, patellae and tibiae darker than the other segments. Carapace and cucullus dark orange, darker than the larva. Tibia of the pedipalp dark orange in distal half. Carapace as in the larva, wider than long, trapezoidal, with rounded corners in posterior part, with numerous rounded granules throughout. Cucullus as the larva, wider than long, oval shaped, distal margin with long translucent setae, with numerous rounded granules throughout. Sternal region, legs and opisthosoma covered with abundant, fine translucent setae and rounded granules as the larva, except pedipalps where granules are absent. Opisthosoma wider than long, widest at the level between tergite XI and XII. Tergite X thin, wider than long. Tergites XI-XII as in the larva, rectangular, considerably wider than long. Tergite XIII trapezoidal, wider than long. Tergites XI-XIII with paired inconspicuous depressions. Lateral tergite longer than wide, X smallest, lateral tergites XI-XIII largest. Lateral tergites XI-XII rectangular, XIII triangular. All tergites closer to each other than in the larva. Sternites X-XIII clearly visible and less separated from each other than the larva. Sternites XI-XIII dark medially as the larva. Pygidium segments without notch. Measurements: total length (without pygidium) 2.00. Carapace 0.82 long, 0.90 wide (widest part). Cucullus 0.36 long, 0.54 wide. Opisthosoma (without pygidium) 1.12 long, 1.22 wide. Legs tarsal formula (leg I to IV): 1-4-3-2.
Deutonymph (ECOTAAR-Ri00016) (Figs. 34, 35). Appendages and body coloration orange, same coloration than in the protonymph. Femora, patellae and tibiae darker than the other segments as in the protonymph. Carapace and cucullus dark orange, darker than the protonymph. Tibia of the pedipalp dark orange in distal half as the protonymph. Carapace slightly wider than long, trapezoidal, with rounded corners in posterior part as the protonymph, with numerous rounded granules throughout. Cucullus wider than long, somewhat hexagonal shaped, distal margin with long translucent setae, with numerous rounded granules along. Sternal region, legs and opisthosoma covered with abundant, fine translucent setae and rounded granules as in the protonymph, except pedipalps where granules are absent. Opisthosoma as longer as wide, widest at the level between tergite XI and XII as in the protonymph. Tergite X thin, wider than long. Tergites XI‒XII as the protonymph, rectangular, considerably wider than long. Tergite XIII trapezoidal, wider than long. Tergites XI-XIII with paired depressions, more visible than in the protonymph. Lateral tergites longer than wide, X smallest, lateral tergites XI-XIII larger. Lateral tergites XI-XII rectangular, XIII triangular as in the protonymph. All tergites separated from each other as in the protonymph. Sternites X-XIII clearly visible and separated from each other as in the protonymph. Sternites XI-XIII dark medially as the protonymph. Pygidium segments without notch. Measurements: total length (without pygidium) 2.40. Carapace 0.95 long, 1.00 wide (widest part). Cucullus 0.42 long, 0.58 wide. Opisthosoma (without pygidium) 1.42 long, 1.42 wide. Legs tarsal formula (leg I to IV): 1-5-4-4.
Tritonymph (ECOTAAR-Ri00016) (Figs. 36, 37). Appendages and body coloration darker orange than the deutonymph. Femora, patellae and tibiae darker than the other segments as the deutonymph. Carapace and cucullus dark orange, darker than the deutonymph. Tibia of the pedipalp darker orange in distal half than the deutonymph. Carapace wider than long, trapezoidal, with rounded corners in posterior part as the deutonymph, with numerous rounded granules throughout. Cucullus wider than long, oval shaped, distal margin with long translucent setae, with numerous rounded granules throughout. Sternal region, legs and opisthosoma covered with abundant, fine translucent setae and rounded granules as the deutonymph, except pedipalps where granules are absent. Opisthosoma longer than wide, widest at the level between tergite XI and XII as in the deutonymph. Tergite X thin, wider than long. Tergites XI-XII as in the deutonymph, rectangular, considerably wider than long. Tergite XIII trapezoidal, wider than long. Tergites XI-XIII with paired depressions as in the deutonymph. Lateral tergites longer than wide, X smallest, lateral tergites XI-XIII larger. Lateral tergites XI-XII rectangular, XIII triangular as the deutonymph. All tergites separated from each other as in the deutonymph. Sternites X-XIII clearly visible and closer to each other than in the deutonymph. Sternites XI-XIII dark orange. Pygidium segments without notch. Measurements: total length (without pygidium) 3.00. Carapace 1.12 long, 1.20 wide (widest part). Cucullus 0.52 long, 0.80 wide. Opisthosoma (without pygidium) 1.85 long, 1.67 wide. Legs tarsal formula (leg I to IV): 1-5-4-5.
Taxonomic summary
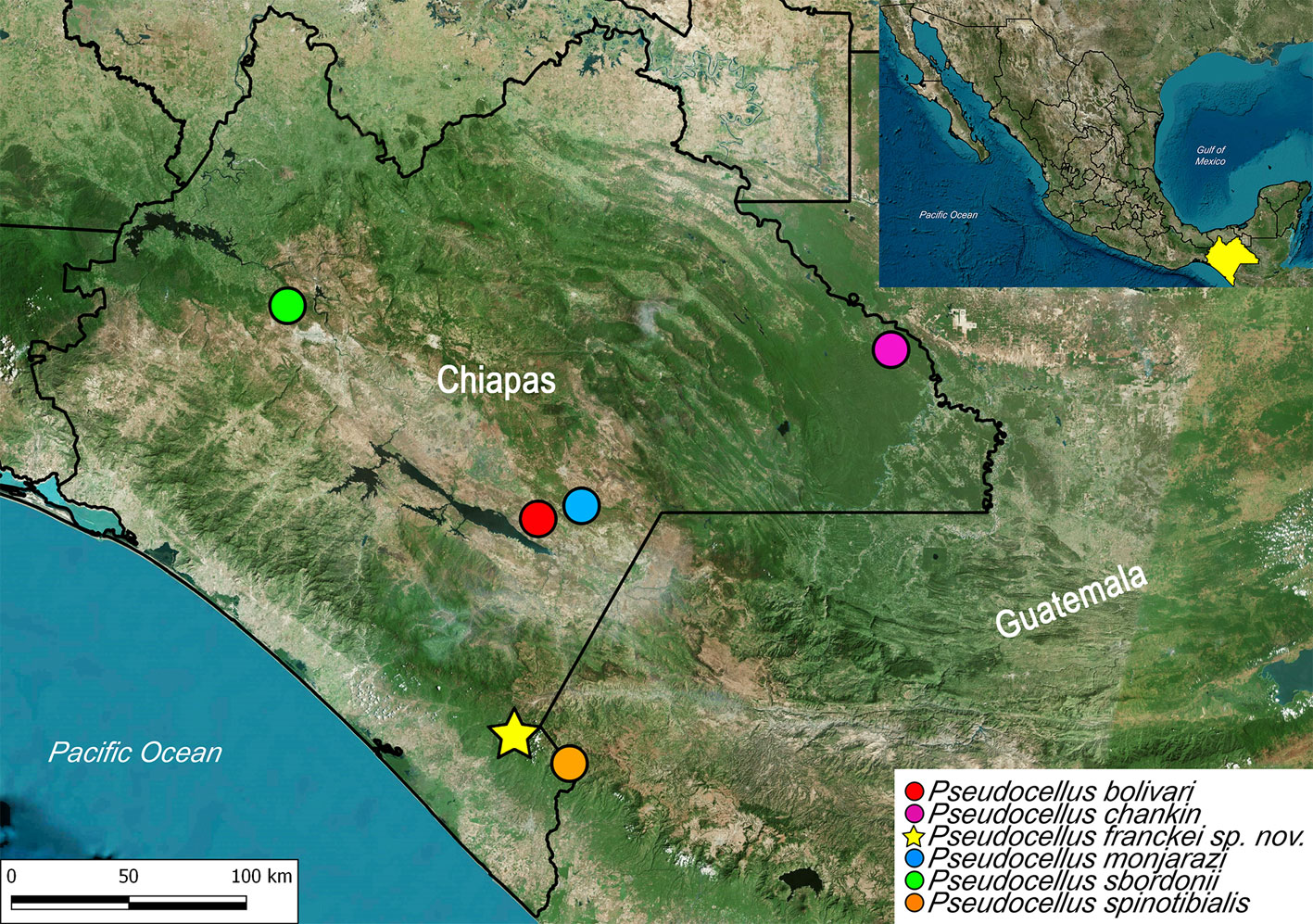
Type material: Mexico: Chiapas: male holotype (ECOTAAR-Ri00013) from Cerro Boquerón (N 15.22989°, W -92.30589°, 2,388 m asl), Ejido Boquerón, Municipality Motozintla, 12-VI-2015, E. Campuzano, L. Gallegos, H. Montaño, S. Moreno Cols. Paratypes: Mexico: Chiapas: female (ECOTAAR-Ri00015) from same locality as holotype, 14-16-VII-2015, L. Gallegos, H. Montaño, S. Moreno, G. Angulo, D. Blé Cols. 1 male, 4 females (CNAN-T01363) from same locality as holotype, 14-16-VII-2015, L. Gallegos, H. Montaño, S. Moreno, G. Angulo, D. Blé Cols. 2 protonymphs, 2 deutonymphs, 1 tritonymph (ECOTAAR-Ri00014), same data as holotype. One female, 1 larva, 3 protonymphs, 2 deutonymphs, 2 tritonymphs (ECOTAAR-Ri00012) from same locality as holotype, 1-V-2015, E. Campuzano, L. Gallegos, S. Moreno, E. Chamé, G. Sánchez Cols. One male, 3 females, 3 protonymphs, 3 deutonymphs, 2 tritonymphs (ECOTAAR-Ri00016) from same locality as holotype, 27-III-2015, E. Campuzano, L. Gallegos, H. Montaño, S. Moreno Cols.
Etymology: this species is dedicated to the renowned arachnologist Dr. Oscar F. Francke Ballvé in recognition of his contribution to the knowledge of the ricinuleids and other arachnids from Mexico.
Natural history: the specimens were collected in a cloud forest between 2,388 and 2,422 m asl, using a Berlese funnel for leaf litter samples and pitfall tramps.
Distribution: known only from the type locality in Chiapas, Mexico (Fig. 38).


Remarks
Pseudocellus franckei sp. nov. is morphologically similar to Pseudocellus quetzalcoatl Valdez-Mondragón, Francke & Botero-Trujillo, 2018 from Parque Ecológico Jaguaroundi, Municipality Coatzacoalcos, Veracruz, Mexico. These species resemble each other in overall size, body shape and proportions of femur II of the males. However, several morphological differences distinguish both species. The carapace of the male of P. franckei sp. nov. is almost trapezoidal (Fig. 3), whereas that of P. quetzalcoatl is more square-shaped (Valdez- Mondragón et al., 2018: fig. 3). The opisthosoma of the male of P. franckei sp. nov. is wide and oval (Figs. 1, 2, 5, 6), whereas that of P. quetzalcoatl is longer and thinner. Males of the new species have the femur of leg II thinner than P. quetzalcoatl, with a proportion of 4.0 and 3.2 times longer than wide respectively (Fig. 15). Males of P. franckei sp. nov. and P. quetzalcoatl have similar shape of tibia of leg II, however the new species lacks the 2 ventral rows of small conical spines that P. quetzalcoatl has, the new species only has some inconspicuous sharp-tipped granules (Figs. 11, 12; Valdez-Mondragón et al., 2018: figs. 11-12). The tarsal process (tP) of male leg III of P. franckei sp. nov. is longer than in P. quetzalcoatl (Figs. 21, 23; Valdez-Mondragón et al., 2018: fig. 19). The metatarsal process (mP) of males of P. quetzalcoatl is slightly sigmoidal (Valdez-Mondragón et al., 2018: figs. 18-19), whereas in the new species it is almost straight dorsally and curved ventrally (Figs. 18, 19). The tarsal process (tP) of leg III of the male in P. franckei sp. nov. is thin and J-shaped, with small and inconspicuous tips apically and with prolateral carina along (Figs. 21-24), whereas in P. quetzalcoatl is wide, canoe-shaped, with 3 rounded and conspicuous tips or lobes apically and dorsal carina on distal half (Valdez-Mondragón et al., 2018: figs. 21-26). The spermathecae of the female of P. franckei sp. nov. has 2 oval lobules on each side, one slightly longer than the other (Figs. 27-29), whereas in P. quetzalcoatl the spermathecae are slightly oval apically and with 2 long lobules on each side: 1 large and curved in a upside-down J-shape, and other smaller and straight (Valdez-Mondragón et al., 2018: figs. 33-35).



Acknowledgments
First author wishes to thank the program “Cátedras Conacyt”, Consejo Nacional de Ciencia y Tecnología (Conacyt), Mexico for the scientific support for project No. 59: “Laboratorio Regional de Biodiversidad y Cultivo de Tejidos Vegetales (LBCTV) del Instituto de Biología (IBUNAM), Tlaxcala”. To Secretaría de Fomento Agropecuario del Estado de Tlaxcala (SEFOA) and the Government of the state of Tlaxcala for the facilities and support to this research. To the reviewers for their comments and suggestions that improved our manuscript. To the project SEP-Conacyt No. 179476. EFCG thanks to Conacyt for scholarship (No- 355707) during the PhD of Biological Sciences and the members of the Colección de Arácnidos del Sureste de México (CASEM) for their field work support. First author especially thanks Dr. Guillermo Ibarra Núñez, from El Colegio de la Frontera Sur, Unidad Tapachula, Chiapas, Mexico, for the loan of the biological material for the description.
References
Armas, L. F. (2017). Cuatro especies nuevas de Pseudocellus de Cuba (Arachnida: Ricinulei). Revista Ibérica de Aracnología, 30, 87–99.
Armas, L.F., & Agreda, E.O. (2016). Una nueva especie de Pseudocellus (Ricinulei: Ricinoididae) del suroeste de Guatemala. Revista Ibérica de Aracnología, 28, 79–83.
Botero-Trujillo, R., & Flórez, D. E. (2017). Two new ricinuleid species from Ecuador and Colombia belonging to the peckorum species-group of Cryptocellus Westwood (Arachnida, Ricinulei). Zootaxa, 4286, 483–498. https://doi.org/10.11646/zootaxa.4286.4.2
Botero-Trujillo, R., & Pérez, G. A. (2008). A new species of Cryptocellus (Arachnida, Ricinulei) from northwestern Colombia. Journal of Arachnology, 36, 468–471. https://doi.org/10.1636/ch07-95sc.1
Botero-Trujillo, R., & Pérez, G. A. (2009). A new species of Cryptocellus (Arachnida, Ricinulei) from the Kófan Territory in southwestern Colombia. Zootaxa, 2050, 56–64. https://doi.org/10.11646/zootaxa.2050.1.3
Botero-Trujillo, R., & Valdez-Mondragón, A. (2016). A remarkable new species of the magnus species-group of Cryptocellus (Arachnida, Ricinulei) from Ecuador, with observations on the taxonomy of the New World genera. Zootaxa, 4107, 321–337. https://doi.org/10.11646/zootaxa.4107.3.2
Cooke, J. A. L., & Shadab, M. U. (1973) New and little known ricinuleids of the genus Cryptocellus (Arachnida, Ricinulei). American Museum Novitates, 2530, 1–25.
Gertsch, W. J. (1971). Three new Ricinuleids from Mexican caves (Arachnida, Ricinulei). Bulletin of the Association for Mexican Cave Studies, 4, 127–135.
Harvey, M. S. (2003). Catalogue of the smaller Arachnid orders of the World. CSIRO Publishing, Collinwood, Victoria, Australia. https://doi.org/10.1071/9780643090071
Naskrecki, P. (2008). A new ricinuleid of the genus Ricinoides Ewing (Arachnida, Ricinulei) from Ghana. Zootaxa, 1698, 57–64. https://doi.org/10.11646/zootaxa.1698.1.4
Penney, D., Marusik, Y., Wheater, C. P., & Langan, A. M. (2009). First Gambian Ricinulei (Arachnida: Ricinoididae): northernmost African record for the order. Zootaxa, 2021, 66–68. https://doi.org/10.11646/zootaxa.2021.1.5
Pinto-da Rocha, R., & Andrade, R. (2012). A new Cryptocellus (Arachnida: Ricinulei) from Eastern Amazonia. Zoologia, 29, 474–478. https://doi.org/10.1590/s1984-46702012000500012
Pittard, K., & Mitchell, R. (1972). Comparative morphology of the life stages of Cryptocellus pelaezi (Arachnida, Ricinulei). Graduate Studies, Texas Tech University, 1, 1–77.
Platnick, N. I. (1980). On the phylogeny of Ricinulei. In J. Gruber (Ed.), Verhandlungen des 8 (pp. 349–353). Internationalen Arachnologen-Kongress. Wien: H. Egermann.
Selden, P. A. (1992). Revision of the fossil ricinuleids. Transactions of the Royal Society of Edinburgh: Earth Sciences, 83, 595–634. https://doi.org/10.1017/s0263593300003333
Teruel, R., & Armas, L. F. (2008). Nuevo Pseudocellus Platnick 1980 de Cuba oriental y nuevos registros de Pseudocellus paradoxus (Cooke 1972) (Ricinulei: Ricinoididae). Boletín de la Sociedad Entomológica Aragonesa, 43, 29–33.
Tourinho, A. L., & Azevedo, C. S. (2007). A new Amazonian Cryptocellus Westwood (Arachnida, Ricinulei). Zootaxa, 1540, 55–60. https://doi.org/10.11646/zootaxa.1540.1.2
Tourinho, A. L., Lo Man-Hung, N. F., & Bonaldo, A. B. (2010). A new species of Ricinulei of the genus Cryptocellus Westwood (Arachnida) from northern Brazil. Zootaxa, 2684, 63–68. https://doi.org/10.11646/zootaxa.2684.1.7
Tourinho, A. L., Lo Man-Hung, N. F., & Salvatierra, L. (2014). A new Amazonian species of Cryptocellus (Arachnida, Ricinulei), with descriptions of its integumental structures and all free-living life stages. Zootaxa, 3814, 81–95. https://doi.org/10.11646/zootaxa.3814.1.4
Tourinho, A. L., & Saturnino, R. (2010). On the Cryptocellus peckorum and Cryptocellus adisi groups, and description of a new species of Cryptocellus from Brazil (Arachnida: Ricinulei). Journal of Arachnology, 38, 425–432. https://doi.org/10.1636/ha09-108.1
Tuxen, S. L. (1974). The African genus Ricinoides (Arachnida, Ricinulei). Journal of Arachnology, 1, 85–106.
Valdez-Mondragón, A., & Francke, O. F. (2011). Four new species of the genus Pseudocellus (Arachnida: Ricinulei: Ricinoididae) from Mexico. Journal of Arachnology, 39, 365–377. https://doi.org/10.1636/ha11-02.1
Valdez-Mondragón, A., & Francke, O. F. (2013). Two new species of ricinuleids of the genus Pseudocellus (Arachnida: Ricinulei: Ricinoididae) from southern Mexico. Zootaxa, 3635, 545–556. https://doi.org/10.11646/zootaxa.3635.5.4
Valdez-Mondragón, A., Francke, O. F., & Botero-Trujillo, R. (2018). New morphological data for the order Ricinulei with the description of two new species of Pseudocellus (Arachnida: Ricinulei: Ricinoididae) from Mexico. Journal of Arachnology, 46, 114–132. https://doi.org/10.1636/joa-s-17-054r1.1
Wunderlich, J. (2017). New extinct taxa of the arachnid order Ricinulei, based on new fossils preserved in Mid Cretaceous Burmese amber. In J. Wunderlich (Ed.), Beiträge zur Araneologie 10: Spinnen des erdmittelalters (pp. 48–71). Germany: Joerg Wunderlich, Hirschberg.



