Mariano C. Michat a, b, *, Miguel Archangelsky c, Yves Alarie d
a Laboratorio de Entomología, Departamento de Biodiversidad y Biología Experimental, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Av. Int. Guiraldes 2160, 1428 Buenos Aires, Argentina
b Instituto de Biodiversidad y Biología Experimental y Aplicada, Universidad de Buenos Aires, Consejo Nacional de Investigaciones Científicas y Técnicas, Av. Int. Guiraldes 2160, 1428 Buenos Aires, Argentina
c Laboratorio de Investigaciones en Ecología y Sistemática Animal, Centro de Investigaciones Esquel de Montaña y Estepa Patagónica (Consejo Nacional de Investigaciones Científicas y Técnicas – Universidad Nacional de la Patagonia San Juan Bosco), Roca 780, 9200 Esquel, Chubut, Argentina
d Department of Biology, Laurentian University, Ramsey Lake Road, Sudbury, Ontario, Canada
*Corresponding author: marianoide@gmail.com (M.C. Michat)
Received: 29 May 2020; accepted: 2 July 2020
Abstract
Larvae of 2 Neotropical species of Haliplus Latreille, 1802 (H. indistinctus Zimmermann, 1928 and H. subseriatus Zimmermann, 1921) are described and illustrated including detailed morphometric and chaetotaxic analyses of the cephalic capsule, head appendages, and legs. Except for the legs, this is the first treatment of larval primary chaetotaxy for the family Haliplidae. The larvae studied herein have 10 abdominal segments, with segment X forking into 2 caudal projections, 1 pretarsal claw, a clasping device on prothoracic legs, and numerous short tracheal gills on the body, characteristics typically found in haliplid larvae. Regarding primary chaetotaxy, they are characterized by the absence of several setae and pores which are commonly present among other families of Hydradephaga, namely seta FR6 on the frontoclypeus, seta PA16 on the parietal, setae AN2 and AN3 and pore ANg on the antenna, setae MX13 and MX14 on the maxilla, and seta CO6 on the coxa. Mandibular seta MN2 is also strongly developed as compared to other hydradephagans. The larvae of the 2 described species can be separated by comparing the distance from the base of segment X to the point at which it forks into caudal projections.
Keywords: Crawling water beetles; Haliplus indistinctus; H. subseriatus; Larva; Sensilla
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Morfología y quetotaxia de larvas neotropicales de Haliplus (Coleoptera: Haliplidae)
Resumen
Se describen e ilustran las larvas de 2 especies neotropicales de Haliplus Latreille, 1802 (H. indistinctus Zimmermann, 1928 y H. subseriatus Zimmermann, 1921), incluyendo análisis detallados de la morfometría y quetotaxia de la cápsula cefálica, apéndices cefálicos y patas. Excepto por las patas, este es el primer tratamiento de la quetotaxia primaria larval para la familia Haliplidae. Las larvas estudiadas presentan 10 segmentos abdominales, con el segmento X biburcado en 2 proyecciones caudales, 1 única uña pretarsal, 1 dispositivo de agarre en las patas protorácicas, y numerosas branquias traqueales cortas en el cuerpo, todas características típicamente encontradas en larvas de halíplidos. Con respecto a la quetotaxia primaria, están caracterizadas por la ausencia de varias setas y poros que están comúnmente presentes en otras familias de Hydradephaga, como la seta FR6 en el frontoclípeo, la seta PA16 en el parietal, las setas AN2 y AN3 y el poro ANg en la antena, las setas MX13 y MX14 en la maxila, y la seta CO6 en la coxa. Además, la seta mandibular MN2 está fuertemente desarrollada en comparación con la de otros hidradéfagos. Las larvas de las 2 especies descritas pueden separarse comparando la distancia desde la base del segmento X hasta el punto en el cual éste se bifurca en las proyecciones caudales.
Palabras clave: Escarabajos acuáticos gateadores; Haliplus indistinctus; H. subseriatus; Larva; Sensilios
© 2020 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
Haliplidae, commonly known as crawling water beetles due to the particular swimming habits of adults, are a small yet very characteristic family of aquatic beetles of the suborder Adephaga. They are represented in all major biogeographic regions by about 238 small to medium-sized species (adult body length 1.5-5.0 mm) which are more commonly encountered in stagnant or slowly running, permanent to semi-permanent or temporary waters (Vondel, 2005, 2016). Most species prefer water bodies with growing filamentous algae or characeans where both adults and larvae feed upon (Matheson, 1912). The larvae are unable to swim but crawl slowly between algal filaments and are rarely recognized during field work due to their perfect camouflage with the substrate (Vondel, 1997, 2016). The family is composed of 5 genera, with the largest genus Haliplus Latreille, 1802 having a world-wide distribution (Vondel, 2016). In the Neotropical region, Haliplus counts with about 50 species, the adults of which have been revised by Vondel and Spangler (2008), and there are more local treatments by Vidal-Sarmiento and Grosso (1970, 1971), Archangelsky and Michat (2014) for Argentina, and Moroni (1980) for Chile.
Larval morphology of Haliplidae, in general, and of Haliplus in particular, is still poorly known. The first descriptions or treatments of larvae of this genus were presented by Schiødte (1864), Matheson (1912), Hickman (1931), Jaboulet (1960), and Bertrand (1972 and references therein). More recently, Beutel (1986) studied the skeleton and musculature of the larval head of 1 European species, and Vondel (1986, 1996, 2004, 2011a, 2011b, 2012) described larvae of several species mainly from the Netherlands, Australia, Lebanon, and the USA. With respect to the Neotropical region, only 2 descriptions (restricted to the third instar) are found in the literature, Moroni (1989) treating H. valdiviensis Moroni, 1980, and Vondel (2001) describing H. subseriatus Zimmermann, 1921. All these studies have provided a useful and detailed account of the larval morphology of this genus. With regards to the primary chaetotaxy, however, studies are still in a more preliminary stage, with a few (Nilsson, 1988; Vondel, 2011a, 2012) having considered this character system, and restricted to the legs.
In recent years, detailed studies of the primary chaetotaxy of aquatic beetle larvae have been developed, in combination with more traditional morphological treatments. The utility of exploring the character set provided by chaetotaxy relies in that the presence/absence and variations in position, size, and shape of sensilla have proven to provide a large number of characters useful to distinguish taxa at different taxonomic levels, and to study the phylogenetic relationships amongst these taxa (Alarie, Michat et al., 2011; Michat, Alarie et al., 2017). In this context, a system of nomenclature for the primary sensilla (i.e., setae and so called pores) present in first instar larvae was developed for several hydradephagan families, namely Aspidytidae (Alarie & Bilton, 2005), Hygrobiidae (Alarie et al., 2004), Dytiscidae (Alarie & Michat, 2014), Gyrinidae (Michat, Gustafson et al., 2017), Meruidae (Alarie, Short et al., 2011b), and Noteridae (Urcola et al., 2019).
Fieldwork performed several years ago in 2 distinct areas of Argentina, namely Patagonia in the south and Buenos Aires City in the central-east, allowed us to collect several interesting larvae belonging to 2 different species of Haliplus: H. indistinctus Zimmermann, 1928 and H. subseriatus. These findings prompted this study in which we present, for the first time, detailed descriptions of the morphology and chaetotaxy of these 2 species (the third instar of H. subseriatus is redescribed after Vondel [2001]). Apart from the more traditional morphological study, we include an in-depth treatment of the chaetotaxy in the context of modern works on hydradephagan larvae, i.e. including detailed descriptions and illustrations of sensilla of the cephalic capsule, head appendages, and legs. We also discuss chaetotaxic features in a broader context including comparisons with other families of Hydradephaga.
Material and methods
Descriptions are based on larvae collected in association with adults of the 2 species. Identification of the larvae is definite as these were the only species collected as adults in the respective localities (see below for details). Larvae were cleared in lactic acid, dissected, and mounted on glass slides in polyvinyl-lacto-glycerol. Microscopic examination at magnifications up to 1,000× and drawings were made using an Olympus CX31 (Olympus Corporation, Tokyo, Japan) compound microscope equipped with a camera lucida. Drawings were scanned and digitally inked using a Genius PenSketch tablet (KYE Corporation, Taipei, Taiwan).
The following measurements were taken, with abbreviations shown in parentheses: head length (HL, total head length measured medially); head width (HW, measured at the widest point of head); length of frontoclypeus (FRL, measured medially from anterior to posterior margin); occipital foramen width (OCW, maximum width measured along dorsal margin); length of mandible (MNL, measured from laterobasal angle to apex); width of mandible (MNW, maximum width measured at base); length of galea (GA); length of antenna (A), maxillary (MP) and labial (LP) palpi were derived by adding the lengths of the individual segments; each segment is denoted by the corresponding letter(s) followed by a number (e.g., A1, first antennomere). Length of leg, including the longest claw (CL), was derived by adding the lengths of the individual segments; each leg is denoted by the letter L followed by a number (e.g., L1, prothoracic leg); the leg was considered as being composed of 6 segments (Lawrence, 1991); length of undivided (proximal) portion of abdominal segment X (SXA, measured from base to fork); length of caudal projections of abdominal segment X (SXB, measured from fork to apex). These measurements were used to calculate several ratios that characterize body shape.
For chaetotaxy analysis, primary setae and pores were distinguished in the cephalic capsule, head appendages and legs. Sensilla were coded by 2 capital letters, in most cases corresponding to the first 2 letters of the name of the structure on which they are located, and a number (setae) or a lower-case letter (pores). The following abbreviations were used: AN, antenna; CO, coxa; FE, femur; FR, frontoclypeus; LA, labium; MN, mandible; MX, maxilla; PA, parietal; PT, pretarsus; TA, tarsus; TI, tibia; TR, trochanter. Setae and pores present in the first-instar larvae of the species studied were labeled by comparison with previous papers dealing with primary chaetotaxy of other Adephagan families such as Carabidae (Bousquet & Goulet, 1984), Gyrinidae (Michat, Gustafson et al., 2017), Aspidytidae (Alarie & Bilton, 2005), Hygrobiidae (Alarie et al., 2004), Noteridae (Urcola et al., 2019), Meruidae (Alarie, Short et al., 2011), and Dytiscidae (Alarie & Michat, 2014). Primary chaetotaxy of the legs was also compared with previous papers dealing with this subject in other haliplid species (Nilsson, 1988; Vondel, 2011a). Homologies were recognized using the criterion of similarity of position (Wiley, 1981). Setae located at the apices of the maxillary and labial palpi were extremely difficult to distinguish due to their position and small size. Accordingly, they are not well represented in the drawings.
Description
Genus Haliplus Latreille, 1802
Figs. 1-5
Larvae of both studied species are similar in all aspects described in this section. Differences among them are discussed after the general description.
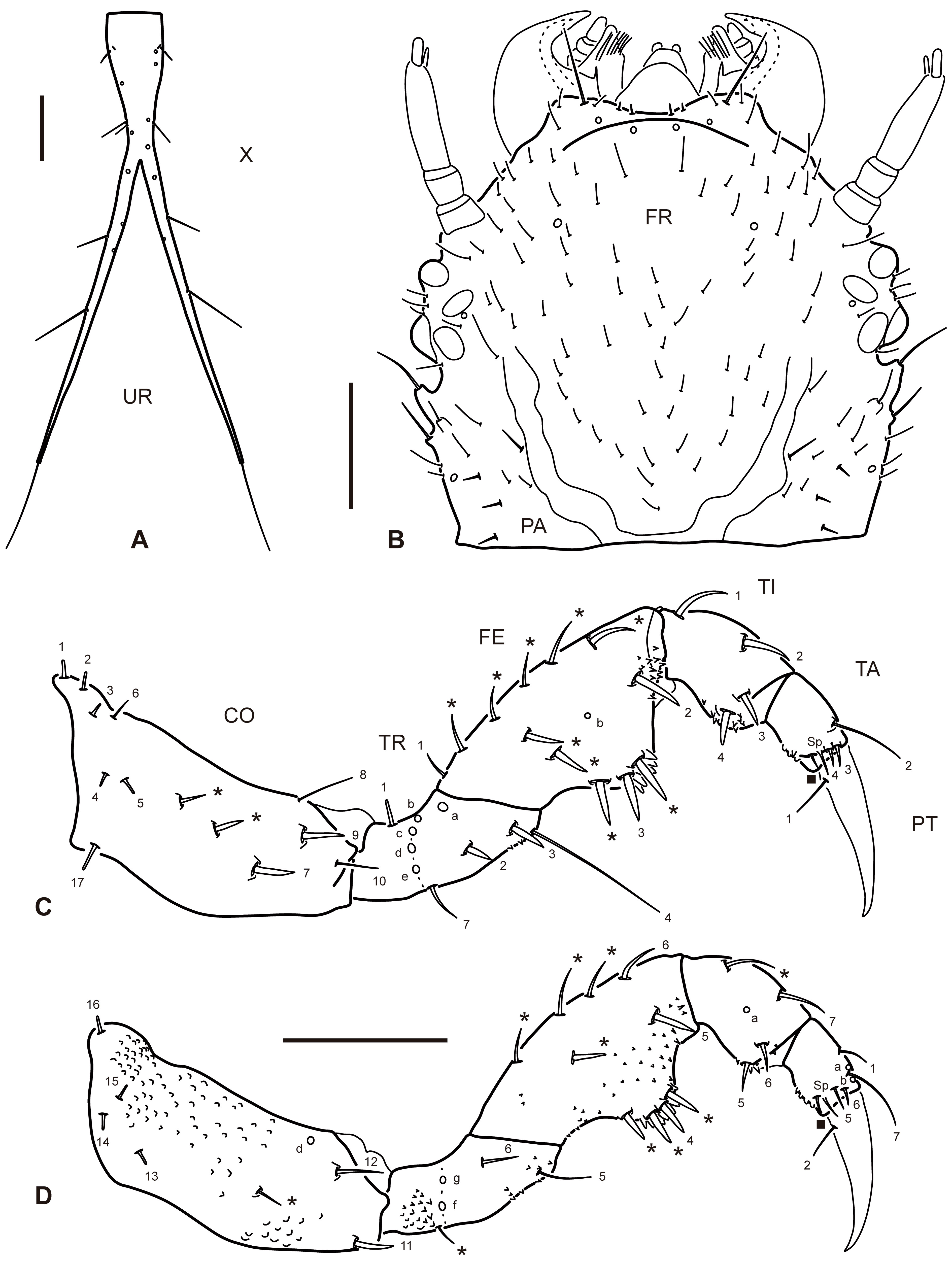
Instar I. Color. Testaceous, with sclerotized parts in general slightly darker than membranous parts, lacking distinct color pattern; mouth parts sometimes somewhat darkened, likely depending on the level of sclerotization of the specimen. Body (Fig. 1A). Slender, subcylindrical, narrowing towards abdominal apex. Measurements and ratios that characterize body shape are shown in Table 1. Head (Fig. 1A, B). Cephalic capsule subovate in dorsal view, somewhat broader than long, widest at level of stemmata; neck constriction and occipital suture absent; ecdysial sutures broad, well visible except on anterior portion, coronal suture absent; frontoclypeus large, extending from anterior to posterior margin of cephalic capsule, anterior margin rounded medially, anterolateral lobes well developed, well projected beyond anterior margin; egg bursters present laterally at about mid length; parietal with occipital foramen broad, not indented ventrally; ocularium rounded, composed of 6 stemmata, 4 visible dorsally and 2 ventrally; few setiferous tubercles present dorsolaterally behind stemmata; tentorial pits visible ventrally on each side of midline at about mid length. Antenna (Fig. 2A-B) considerably shorter than head width, composed of 4 antennomeres; A1 and A2 shortest, sub-equal in length, A3 longest, much longer than the others, A4 somewhat longer than A1 and A2, very slender, bearing a minute dorsodistal spinula; A3′ prominent, reaching about half of length of A4. Mandible (Fig. 2C) short, robust, very broad basally, distal half progressively narrowed to sharp apex projected inwards; mesal margin with a denticle bearing some robust spinulae (interpreted as the retinaculum); mandibular channel present, with proximal opening on dorsoproximal region and distal opening at apex. Maxilla (Fig. 2D, E) very short, robust; cardo well developed, subovate; stipes broad, subcylindrical, bearing a tight group of elongate spinulae near base of galea (interpreted as the lacinia); galea short, broad, only distal galeomere evident; palpifer completely fused to stipes and to MP1, not recognizable except for presence of seta MX10; palpus very short, composed of 3 palpomeres, MP1 not well differentiated from stipes/palpifer (more evident in dorsal view), MP2 very short, MP3 longest, about twice longer than MP2. Labium (Fig. 3A, B) very small; postmentum fused to ventral wall of cephalic capsule; prementum about as long as broad, dorsal margin prominent, somewhat projected forward, anterolateral margins bearing few short spinulae; palpus very short, composed of 2 palpomeres, LP2 about twice longer than LP1. Thorax (Fig. 1A). Terga convex, pronotum somewhat shorter than meso- and metanotum combined, meso- and metanotum subequal; dorsal sclerites transverse, lacking anterior transverse carina, with small spinulae on the surface and few setiferous tubercles (lateral ones longer than central ones); sagittal line visible on the 3 tergites; ventral sclerites not visible, sterna with 1 small setiferous tubercle on each side of midline. Legs (Fig. 4A-C) composed of 6 segments; L3 longest, L2 slightly shorter than L3, L1 considerably shorter than L3; CO robust, elongate, TR weakly divided into 2 parts by an incipient annulus, FE, TI and TA slender, subcylindrical, PT with a single long, slender, slightly curved claw; proFE and to a lesser extent proTI with a ventral bulbous extension bearing both spinulae and setae (clasping apparatus); TA with 1 elongate spinula on both anterodistal and posterodistal surfaces; surface of leg articles (except claw) covered in part with small spinulae. Abdomen (Fig. 1A). 10-segmented; segments I-IX subequal in shape but progressively narrowing to apex, with both a dorsal (more visible) and a ventral (hardly visible) sclerite; dorsal sclerites I-IX transverse, lacking anterior transverse carina, with small spinulae on surface and few setiferous tubercles; ventral sclerites I-IX very indistinct, with small spinulae on surface and few setiferous tubercles; segment X slender, completely sclerotized, bearing small spinulae, with basoventral anal region and continued posteriorly in a long postanal prolongation which forks into 2 caudal 1-segmented projections (urogomphi?); fork occurs proximally on segment X, therefore caudal projections are longer than undivided proximal portion.
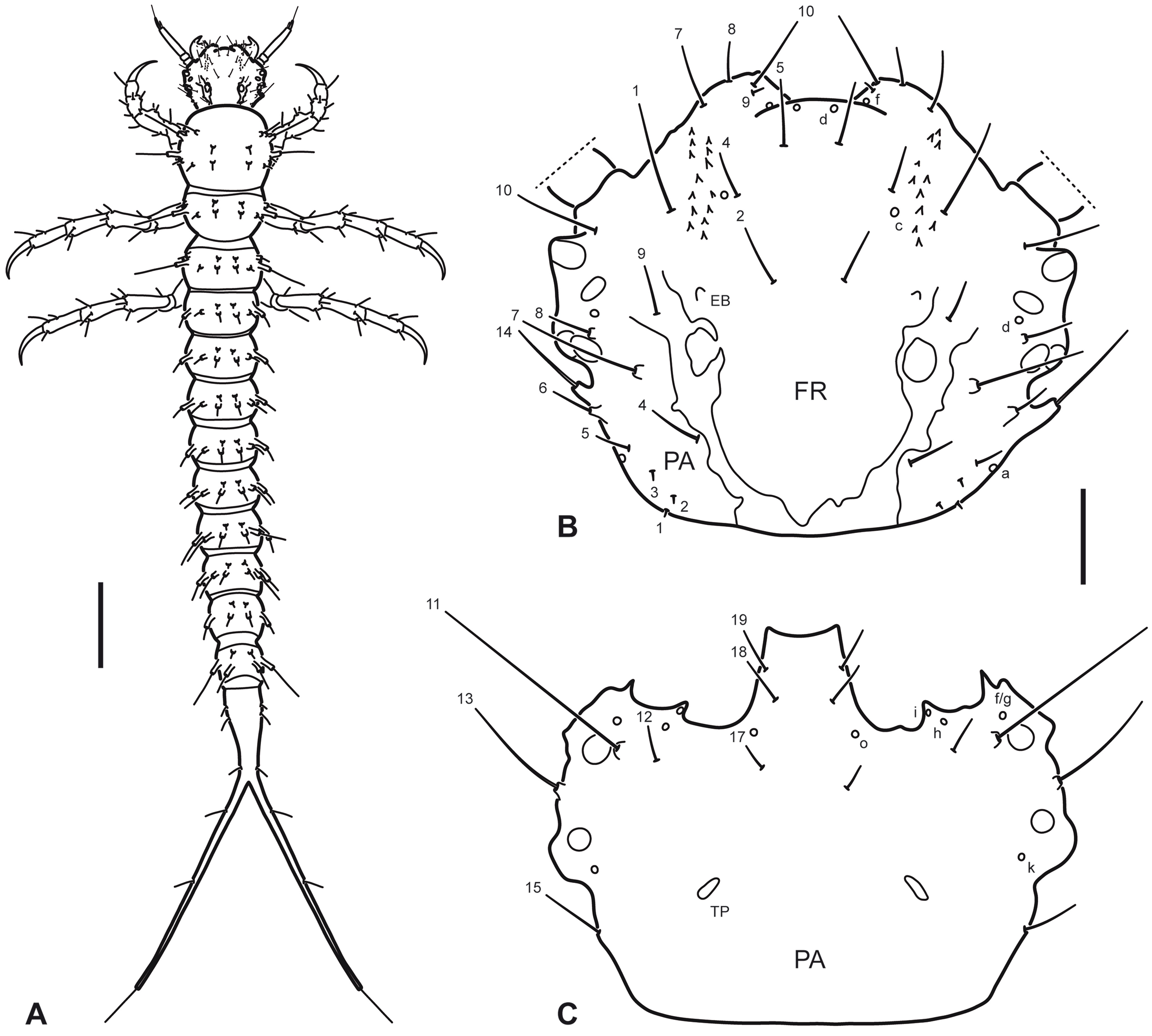
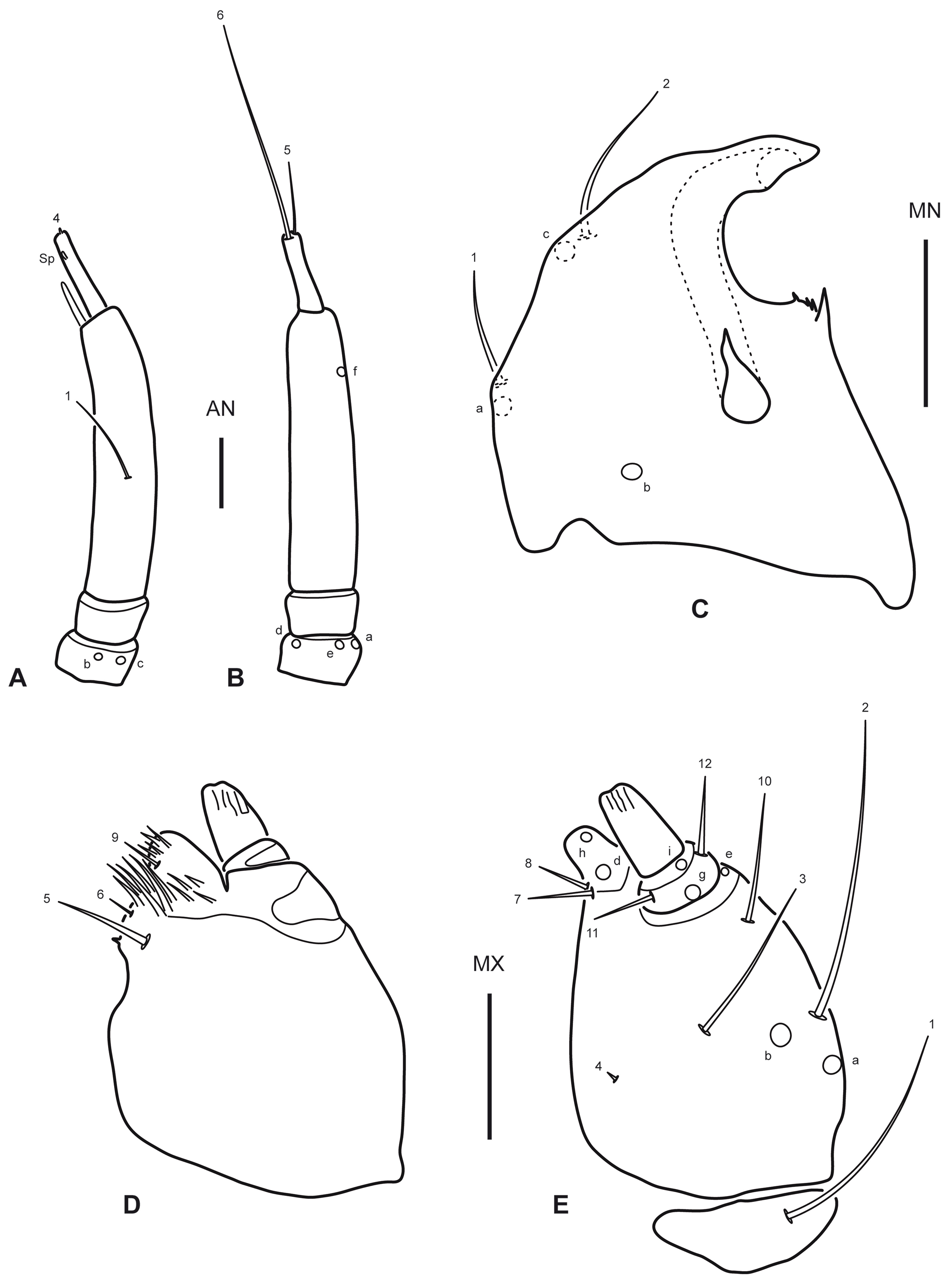
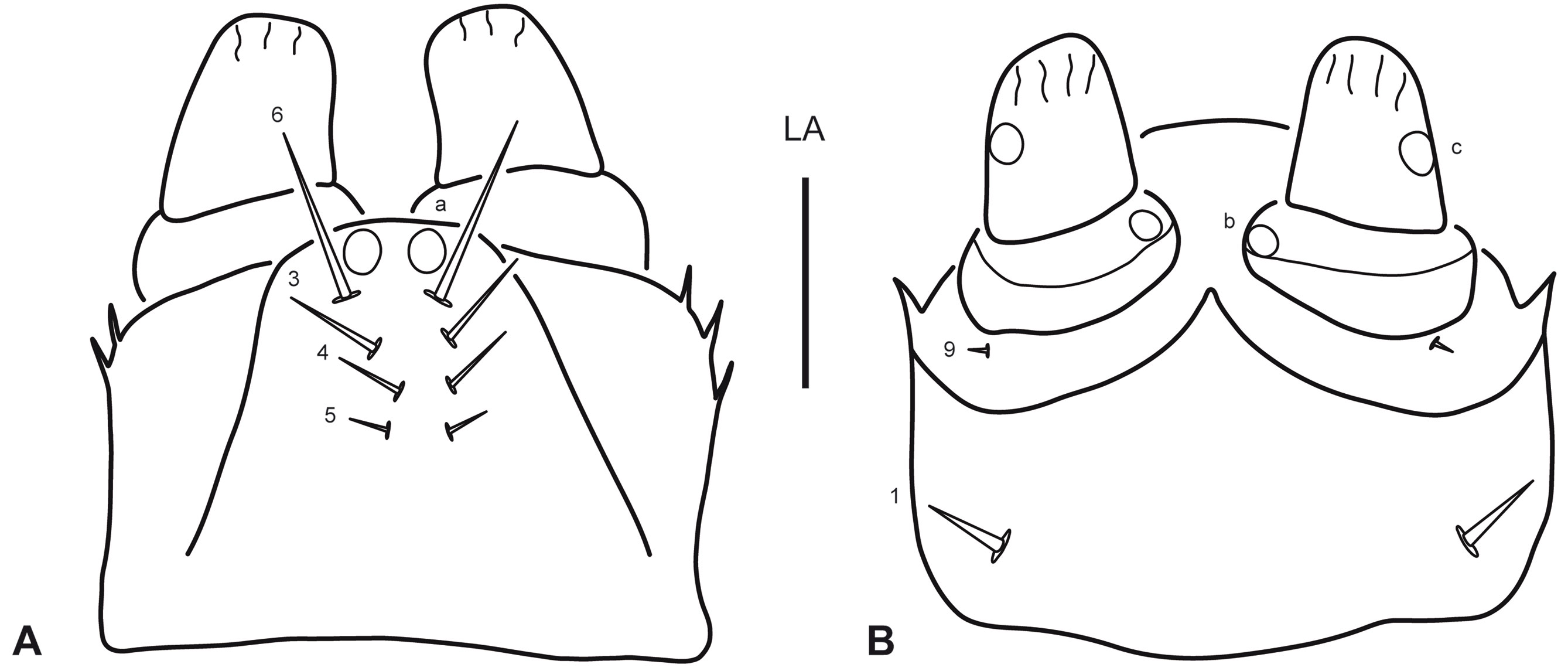
Table 1
Measurements and ratios for the larvae of Haliplus indistinctus Zimmermann, 1928 and Haliplus subseriatus Zimmermann, 1921.
|
Haliplus indistinctus |
Haliplus subseriatus |
||||
|
Measure |
Instar I (n = 3) |
Instar II (n = 2) |
Instar I (n = 1)* |
Instar II (n = 2) |
Instar III (n = 3) |
|
HL (mm) |
0.22-0.24 |
0.32 |
0.27 |
0.36-0.38 |
0.50-0.52 |
|
HW (mm) |
0.27-0.29 |
0.39 |
0.39 |
0.42 |
0.59-0.61 |
|
FRL (mm) |
0.22-0.24 |
0.32 |
0.27 |
0.36-0.38 |
0.50-0.52 |
|
OCW (mm) |
0.17-0.18 |
0.28 |
0.29 |
0.28 |
0.50-0.52 |
|
HL/HW |
0.80-0.84 |
0.82 |
0.71 |
0.86-0.90 |
0.86 |
|
HW/OCW |
1.56-1.61 |
1.39 |
1.34 |
1.47-1.50 |
1.16-1.19 |
|
A/HW |
0.69-0.72 |
0.57-0.58 |
0.52 |
0.53-0.56 |
0.40-0.43 |
|
A1/A3 |
0.14-0.16 |
0.17-0.18 |
0.18 |
1.19-1.21 |
0.24-0.28 |
|
A2/A3 |
0.13-0.16 |
0.17-0.18 |
0.18 |
0.20-0.23 |
0.26-0.31 |
|
A4/A3 |
0.25-0.34 |
0.24-0.25 |
0.27-0.29 |
0.27-0.32 |
0.25-0.26 |
|
A3’/A4 |
0.50-0.61 |
0.61-0.67 |
0.53-0.54 |
0.61-0.70 |
0.90-1.03 |
|
MNL/MNW |
1.00-1.12 |
1.14-1.25 |
1.37 |
1.21-1.24 |
1.44-1.50 |
|
MNL/HL |
0.40-0.41 |
0.44 |
0.46 |
0.39-0.42 |
0.39 |
|
A/MP |
4.58-5.09 |
4.74-5.29 |
4.67-4.74 |
4.09-4.64 |
3.46-3.73 |
|
GA/MP1 |
1.00-1.40 |
1.14-1.23 |
1.50 |
1.38-1.57 |
1.60-1.89 |
|
MP3/MP1 |
1.25-1.60 |
1.14-1.15 |
1.25-1.33 |
1.19-1.29 |
1.10-1.26 |
|
MP3/MP2 |
1.88-2.29 |
1.88-2.14 |
1.88-2.00 |
1.64-1.90 |
1.41-1.71 |
|
MP/LP |
2.75-3.00 |
2.92-3.17 |
2.50-2.57 |
2.47-2.65 |
2.38-2.71 |
|
LP2/LP1 |
2.00 |
2.00 |
1.80 |
2.00-2.40 |
1.78-2.00 |
|
L3 (mm) |
0.90-0.97 |
1.35-1.38 |
1.00 |
1.35-1.40 |
1.80-2.03 |
|
L3/L1 |
1.55-1.62 |
1.76-1.85 |
1.46-1.47 |
1.66-1.67 |
1.69-1.80 |
|
L3/L2 |
1.03-1.06 |
1.05-1.07 |
1.04 |
1.07-1.09 |
1.08-1.13 |
|
L3/HW |
3.30-3.42 |
3.56 |
2.60 |
3.24-3.27 |
3.31-3.40 |
|
L3 (CO/FE) |
0.85-0.89 |
0.89-0.90 |
0.96 |
0.88-0.90 |
0.89-0.96 |
|
L3 (TI/FE) |
0.87-0.91 |
0.86-0.89 |
0.97 |
0.95-0.96 |
0.85-0.89 |
|
L3 (TA/FE) |
0.72-0.77 |
0.66-0.68 |
0.83 |
0.74-0.78 |
0.60-0.72 |
|
L3 (CL/TA) |
1.53-1.79 |
1.34-1.47 |
1.53 |
1.27-1.33 |
1.16-1.28 |
|
SXA (mm) |
0.32-0.36 |
1.11-1.17 |
– |
1.17-1.32 |
2.68-3.03 |
|
SXB (mm) |
0.70-0.83 |
1.11-1.31 |
– |
0.81-0.83 |
0.99-1.33 |
|
SXA/HW |
1.18-1.30 |
2.87 |
– |
2.81-3.17 |
4.63-5.15 |
|
SXA/SXB |
0.41-0.45 |
0.82-1.04 |
– |
1.36-1.45 |
2.73 |
*: badly preserved specimen, measures may not be confident.
Instar II. As for instar I except as follows. Body. Measurements and ratios that characterize body shape shown in Table 1. Head. Egg bursters absent. Thorax. Ventral sclerites present, difficult to see; setiferous tubercles on thoracic segments more numerous. Abdomen. Dorsal and ventral sclerites more developed; setiferous tubercles on abdominal segments more numerous, most so on segment X; segment X more elongate, with fork occurring more distally, therefore caudal projections vary from subequal to somewhat shorter than undivided proximal portion.
Instar III. As for instar II except as follows. Color. Somewhat darker in general. Body. Measurements and ratios that characterize body shape shown in Table 1. Head (Fig. 5B). A1, A2 and A4 subequal in length; A3′ subequal in length to A4. Thorax. Ventral sclerites present, well visible; setiferous tubercles on thoracic segments much more numerous; spiracles present lateroventrally on meso- and metathorax, those of metathorax weakly developed, unfunctional. Abdomen. Dorsal and ventral sclerites more developed; setiferous tubercles on abdominal segments much more numerous both dorsally and ventrally; on segments I-IX tubercles are grouped in short posterolateral extensions; segments VIII-IX with 2 short posterodorsal extensions (those of segment VIII less evident); segments I-VII with 4 indistinct posterodorsal extensions; segment X more elongate, with fork occurring more distally, therefore caudal projections are considerably shorter than undivided proximal portion; spiracles present lateroventrally on segments I-VIII, those of segment VIII weakly developed, unfunctional.
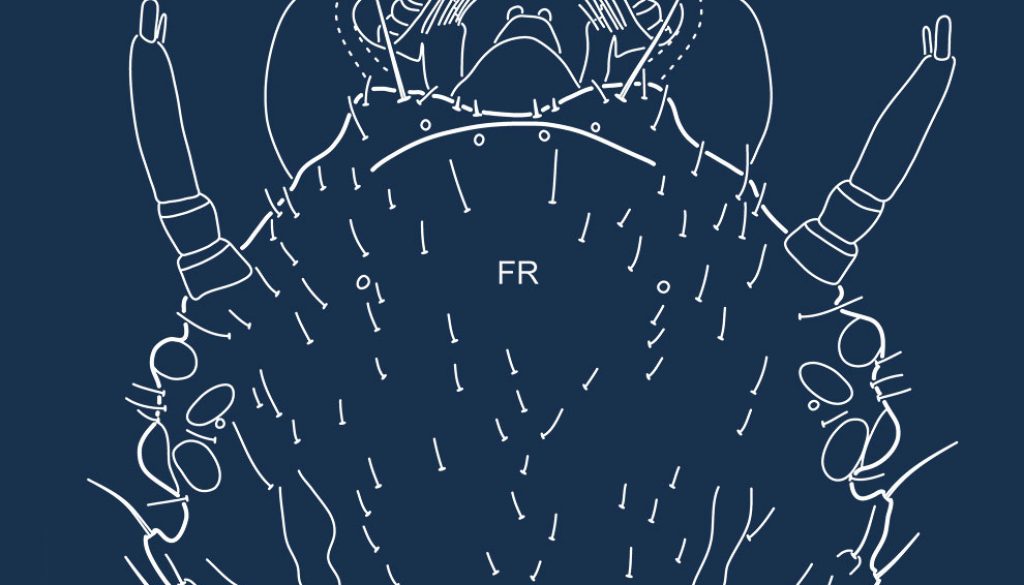
Primary chaetotaxy (instar I). Head (Fig. 1B, C). Frontoclypeus with 3 setae (FR1, FR2 and FR4) and 1 pore (FRc) anteriorly to egg bursters, 1 seta (FR5) and 1 pore (FRd) near anterior margin, and 4 setae (FR7, FR8, FR9, FR10) and 1 pore (FRf) on anterolateral lobes; dorsal surface of parietal with 5 setae (PA1, PA2, PA3, PA4, PA5) and 1 pore (PAa) on basal region, 5 setae (PA6, PA7, PA8, PA9, PA14) and 1 pore (PAd) on medial region, and 1 seta (PA10) on distal region; ventral surface of parietal with 2 setae (PA13, PA15) and 1 pore (PAk) on lateral margin, 2 setae (PA11, PA12) and 3 pores (PAf /g, PAh, PAi) on anterolateral region, and 3 setae (PA17, PA18, PA19) and 1 pore (PAo) on anteromedial region. Antenna (Fig. 2A-B). Antennomere 1 with 2 pores (ANb, ANc) on dorsal surface and 3 pores (ANa, ANd, ANe) on ventral surface; antennomere 3 with 1 seta (AN1) on dorsal surface at about mid length and 1 pore (ANf) distally on ventral surface; antennomere 4 with 3 apical setae (AN4, AN5, AN6). Mandible (Fig. 2C). With 1 pore (MNb) on dorsobasal region and 2 setae (MN1, MN2) and 2 pores (MNa, MNc) on external margin. Maxilla (Fig. 2D-E). Cardo with 1 seta (MX1); stipes with 2 setae (MX5, MX6) on dorsal surface near base of galea, and 3 setae (MX2, MX3, MX4) and 2 pores (MXa, MXb) on ventral surface; galea with 1 seta (MX9) on dorsal surface, and 2 setae (MX7, MX8) and 2 pores (MXd, MXh) on ventral surface; palpifer with 1 seta (MX10) on ventral surface; palpomere 1 with 1 pore (MXe) on ventral surface; palpomere 2 with 2 setae (MX11, MX12) and 2 pores (MXg, MXi) on ventral surface; palpomere 3 with an unidentified structure (possibly a placoid sensillum) on external margin. Labium (Fig. 3A, B). Prementum with 4 setae (LA3, LA4, LA5, LA6) and 1 pore (LAa) on dorsal surface, and 1 seta (LA1) on ventral surface; palpomere 1 with 1 seta (LA9) and 1 pore (LAb) on ventral surface; palpomere 2 with 1 pore (LAc) on ventral surface. Legs (Fig. 4A-C). Anterior surface of coxa with 7 setae (CO1, CO2, CO3, CO4, CO5, CO17, CO18) on proximal portion and 4 setae (CO7, CO8, CO9, CO10) on distal portion; seta CO10 more weakly developed and inserted somewhat more dorsally on prothoracic leg; posterior surface of coxa with 4 setae (CO13, CO14, CO15, CO16) on proximal portion, and 2 setae (CO11, CO12) and 1 pore (COd) on distal portion; anterior surface of trochanter with 2 setae (TR1, TR7) and 4 pores (TRb, TRc, TRd, TRe) on proximal portion, and 3 setae (TR2, TR3, TR4) and 1 pore (TRa) on distal portion; posterior surface of trochanter with 2 pores (TRf, TRg) on proximal portion and 2 setae (TR5, TR6) on distal portion; anterior surface of femur with 1 seta (FE1) and 1 pore (FEb) on proximal portion and 2 setae (FE2, FE3) on distal portion; posterior surface of femur with 3 setae (FE4, FE5, FE6) on distal portion; on prothoracic leg, setae FE3 and FE4 are located more basally, on the ventral bulbous extension; anterior surface of tibia with 1 seta (TI1) on proximal portion and 3 setae (TI2, TI3, TI4) on distal portion); posterior surface of tibia with 1 pore (TIa) on proximal portion and 3 setae (TI5, TI6, TI7) on distal portion; on prothoracic leg, setae TI4 and TI5 are located on the ventral bulbous extension; anterior surface of tarsus with 4 setae (TA2, TA3, TA4 and 1 additional seta) on distal portion; posterior surface of tarsus with 5 setae (TA1, TA5, TA6, TA7 and 1 additional seta) and 2 pores (TAa, TAb) on distal portion; pretarsus with 2 setae (PT1, PT2) on basoventral region.
Secondary chaetotaxy. Instar II. Cephalic capsule with several secondary setae, almost exclusively restricted to the dorsal surface; mandible with 1 secondary seta on basoexternal margin; stipes with 1 secondary seta on basoexternal margin; thoracic segments with several setae mainly on dorsal surface and associated to the tubercles; secondary setation on legs detailed in Table 2; abdominal segments I-IX with several setae restricted to dorsal surface and associated to the tubercles; basal (undivided) part of segment X with numerous setae associated to the tubercles.
Instar III. Cephalic capsule with numerous secondary setae, almost exclusively restricted to the dorsal surface (Fig. 5B); mandible with 2 secondary setae on basoexternal margin; stipes with 3-4 secondary setae on basoexternal margin; thoracic segments with a larger number of setae mainly on dorsal surface and associated to the tubercles; secondary setation on legs detailed in figure 5C, D and Table 2; abdominal segments I-IX with a larger number of setae both on dorsal and ventral surfaces, associated to the tubercles.
Taxonomic summary
Haliplus indistinctus: Argentina, 5 instar I, 2 instar II; Buenos Aires city, Plaza República del Ecuador; 14 June 2001; Michat leg.; temporary pond. Haliplus subseriatus: Argentina, 1 instar I, 4 instar II, 3 instar III; Santa Cruz province, road 5 at km 118; 28 Jan. 2001; Archangelsky leg.; pond. The material is deposited in the Entomology Collection of the Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Buenos Aires, Argentina.

Remarks
Although we collected several larvae, we were unable to get third instars of H. indistinctus, and the only first instar of H. subseriatus we could observe was in rather bad condition, and consequently morphometric measurements may not be totally accurate for several structures (primary chaetotaxy, however, could be confidently studied, but no differences among species were found). Therefore, only the second instar was used for the comparison. Second instars of both species share very similar morphology and chaetotaxy. The only reliable difference we found to separate them is the distance from the base at which abdominal segment X forks. Indeed in H. indistinctus the fork seems to occur at about mid length of the segment, and therefore caudal projections are subequal in length to undivided proximal portion (ratio SXA/SXB = 0.82-1.04). In H. subseriatus, on the other hand, the fork occurs more distally on segment X, and therefore caudal projections are shorter than the undivided proximal portion (ratio SXA/SXB = 1.36-1.45) (Table 1).
Table 2
Number and position of secondary setae on the legs of larvae of Haliplus indistinctus Zimmermann, 1928 and Haliplus subseriatus Zimmermann, 1921. Numbers between slash marks refer to pro-, meso- and metathoracic leg, respectively. A = Anterior, D = dorsal, P = posterior, Pr = proximal, V = ventral, Total = total number of secondary setae on the segment (excluding primary setae).
|
Haliplus indistinctus |
Haliplus subseriatus |
|||
|
Segment |
Position |
Instar II (n = 2) |
Instar II (n = 3) |
Instar III (n = 3) |
|
Coxa |
A |
0-1 / 0 / 0 |
0 / 0 / 0 |
0-2 / 2-4 / 2-6 |
|
P |
0 / 0 / 0 |
0 / 0 / 0 |
0-2 / 0-3 / 0-2 |
|
|
V |
0 / 0 / 0 |
0 / 0 / 0 |
0 / 0-1 / 0-1 |
|
|
Total |
0-1 / 0 / 0 |
0 / 0 / 0 |
0-4 / 2-8 / 2-9 |
|
|
Trochanter |
Pr |
0 / 0 / 0 |
0 / 0 / 0 |
0-1 / 1-2 / 0-3 |
|
Total |
0 / 0 / 0 |
0 / 0 / 0 |
0-1 / 1-2 / 0-3 |
|
|
Femur |
A |
2 / 1-2 / 1-2 |
1-2 / 1 / 1-2 |
1-2 / 1-2 / 1-3 |
|
AD |
3-4 / 4-5 / 3-5 |
4-6 / 4-6 / 4-7 |
4-5 / 5-8 / 5-6 |
|
|
AV |
1-2 / 2-3 / 2-4 |
2 / 2-3 / 1-2 |
2 / 2-3 / 2-3 |
|
|
P |
1 / 1-2 / 1-3 |
1 / 1-2 / 1 |
0-2 / 1-3 / 1-2 |
|
|
PD |
1-2 / 1-2 / 1 |
1 / 1 / 0-2 |
3-5 / 1-4 / 3-5 |
|
|
PV |
2 / 0 / 0 |
2 / 0-1 / 0 |
3 / 0-1 / 0-1 |
|
|
Total |
11-13 / 10-13 / 8-13 |
11-14 / 10-12 / 8-13 |
14-17 / 12-18 / 13-18 |
|
|
Tibia |
AD |
0 / 1 / 1-2 |
0 / 1 / 1 |
0 / 1 / 1-2 |
|
PD |
1-2 / 2-3 / 1-2 |
1 / 1-2 / 1-2 |
1 / 2 / 1-3 |
|
|
Total |
1-2 / 3-4 / 2-4 |
1 / 2-3 / 2-3 |
1 / 3 / 2-4 |
Discussion
Larvae of Haliplidae are highly characteristic among Hydradephaga, showing several unique or distinctive features, the most commonly known being the presence of 10 abdominal segments (9 in Peltodytes Régimbart, 1878), a single tarsal claw, and numerous variously shaped tracheal gills mainly on the thoracic and abdominal segments. Many larvae of this family are also well known for bearing a clasping device on prothoracic legs, formed by ventral extensions of the profemur and sometimes also of the protibia. Another distinctive feature is the abdominal segment X, which in many species forks into 2 caudal projections of variable length (Vondel, 2016). Our study covers only 2 Neotropical Haliplus species, and is therefore far from being complete. However, it is the most comprehensive study of larval chaetotaxy presented so far, and we find worth discussing several characters (mainly chaetotaxic) in a broader context, comparing them with those found in other families of aquatic Adephaga.
The head of the larvae studied herein lacks a true coronal suture, which contradicts Strand and Spangler (1994) and Vondel (1997, 2016) who postulated that a short coronal suture is present in Haliplidae. This is based on the observation that in our larvae the frontoclypeal region extends posteriorly up (or almost so) to the posterior margin of the cephalic capsule, which makes the recognition of the coronal suture impossible. This gives the frontoclypeal region a U-shaped appearance, more similar to that in Noteridae (Urcola et al., 2019) and Meruidae (Alarie, Short et al., 2011), than to the typical Y-shaped configuration found in most hydradephagan larvae. Regarding primary chaetotaxy, the head is characterized by the absence of several setae and pores which are commonly present among other hydradephagan families. Indeed, larvae of Haliplus are unique in the absence of setae FR6 on the frontoclypeus and PA16 on the parietal. They also characteristically lack seta FR3 and pores FRa and FRb on the frontoclypeus, and pores PAb, PAc, PAj, PAm and PAp on the parietal. These sensilla are only sporadically absent in other hydradephagan families, such as FR3 in Aspidytidae, FRa in Dytiscidae, and FRb, PAb, PAc, PAj, PAm and PAp in Gyrinidae and few Dytiscidae.
The head appendages of Haliplus larvae also exhibit several interesting features worthy of mention. The antennomere 3 lacks a ventroapical spinula which is commonly present within Hydradephaga. Outside Haliplidae, absence of this structure is only observed in restricted groups within Dytiscidae (Michat, Alarie et al., 2017). The antenna also lacks the primary setae AN2 and AN3 and the primary pores ANg and ANi. If we exclude pore ANi, which is lacking within the dytiscid subfamily Hydroporinae, all these sensilla are consistently present within Hydradephaga. Another unique chaetotaxic characteristic of Haliplus larvae is the strong development of the mandibular primary seta MN2. This sensillum has either the appearance of a pore or minute seta in almost all hydradephagans, or at most of a short hair-like seta such as in Gyrinidae and in the dytiscid subfamily Hydrovatinae. The maxillary palpus is characteristically noticeably short, to the point that the palpifer is completely fused to the stipes and is only recognizable by the presence of seta MX10. Moreover, the palpomere 1 is also partially fused to the stipes. Such abbreviation of the palpus is not observed in other hydradephagan larvae. In addition to its typical morphology, the maxilla is also highly characteristic in that setae MX13 and MX14 and pores MXf and MXj are lacking. Except for pores MXf and MXj, which are absent in members of the dytiscid tribes Laccophilini and Vatellini respectively, all these sensilla are consistently present amongst Hydradephaga. Finally, in addition to be extremely short, the labial palpus characterizes by the absence of the primary seta LA11, which summed to the absence of the premental primary seta LA2 represent very characteristic features within Hydradephaga (seta LA2 is sporadically absent in some hydroporine groups).
Leg primary chaetotaxy of Haliplus larvae was first studied by Nilsson (1988), with subsequent treatments by Vondel (2011a, 2012). The only difference with the larva of H. lineolatus Mannerheim, 1844 studied by Nilsson (1988) is that our larvae lack coxal primary seta CO6. Absence of this seta is not observed in any other hydradephagan larva and is therefore a highly distinctive feature of the larvae studied here. The coxa also lacks primary pore COa, and the tarsus lacks primary pores TAc, TAd, TAe and TAf. These 2 features are also unique within Hydradephaga, except that pore COa is absent in the dytiscid tribe Pachydrini, and pores TAc, TAd, TAe and TAf are absent in the dytiscid tribe Aciliini.
Acknowledgments
This project was supported by Agencia Nacional de Promoción Científica y Tecnológica (grant PICT-2017-1177) and Universidad de Buenos Aires (grant UBACyT-20020150100170BA).
References
Alarie, Y., Beutel, R. G., & Watts, C. H. S. (2004). Larval morphology of three species of Hygrobiidae (Coleoptera: Adephaga: Dytiscoidea) with phylogenetic considerations. European Journal of Entomology, 101, 293–311. https://doi.org/10.14411/eje.2004.039
Alarie, Y., & Bilton, D. T. (2005). Larval morphology of Aspidytidae (Coleoptera: Adephaga) and its phylogenetic implications. Annals of the Entomological Society of America, 98, 417–430. https://doi.org/10.1603/0013-8746(2005)098[0417:LMOACA]2.0.CO;2
Alarie, Y., & Michat, M. C. (2014). Bridging ecology and systematics: 25 years of study of larval morphology of world Dytiscidae (Coleoptera). In D. A. Yee (Ed.), Ecology, systematics, and the natural history of predaceous diving beetles (Coleoptera: Dytiscidae) (pp. 17–47). New York: Springer. https://doi.org/10.1007/978-94-017-9109-0_2
Alarie, Y., Michat, M. C., & Miller, K. B. (2011). Notation of primary setae and pores on larvae of Dytiscinae (Coleoptera: Dytiscidae), with phylogenetic considerations. Zootaxa, 3087, 1–55. https://doi.org/10.11646/zootaxa.3087.1.1
Alarie, Y., Short, A. E. Z., García, M., & Joly, L. (2011). Larval morphology of Meruidae (Coleoptera: Adephaga) and its phylogenetic implications. Annals of the Entomological Society of America, 104, 25–36. https://doi.org/10.1603/AN10054
Archangelsky, M., & Michat, M. C. (2014). Coleoptera, Haliplidae. In S. Roig-Juñent, L. E. Claps & J. J. Morrone (Eds.), Biodiversidad de artrópodos argentinos, Vol. 3 (pp. 467–473). San Miguel de Tucumán: Editorial INSUE/ Universidad Nacional de Tucumán.
Bertrand, H. (1972). Larves et nymphes des coléoptères aquatiques du globe. Paris: F. Paillart. https://doi.org/10.1002/iroh.19730580416
Beutel, R. G. (1986). Skelet und Muskulatur des Kopfes der Larve von Haliplus lineatocollis Mrsh. (Coleoptera: Haliplidae). Stuttgarter Beiträge zur Naturkunde, Ser. A, 390, 1–15.
Bousquet, Y., & Goulet, H. (1984). Notation of primary setae and pores on larvae of Carabidae (Coleoptera: Adephaga). Canadian Journal of Zoology, 62, 573–588. https://doi.org/10.1139/z84-085
Hickman, J. R. (1931). Contribution to the biology of the Haliplidae (Coleoptera). Annals of the Entomological Society of America, 24, 129–142.
Jaboulet, M. C. (1960). Contribution à l’étude des larves d’Haliplides. Travaux du Laboratoire de Zoologie et de la Station Aquicole Grimaldi de la Faculté des Sciences de Dijon, 31, 1–15.
Lawrence, J. F. (1991). Order Coleoptera. In F. W. Stehr (Ed.), Immature insects, Vol.2 (pp. 144–658). Dubuque: Kendall/ Hunt Publishing Company.
Matheson, R. (1912). The Haliplidæ of North America, north of Mexico. Journal of the New York Entomological Society, 20, 156–193.
Michat, M. C., Alarie, Y., & Miller, K. B. (2017). Higher-level phylogeny of diving beetles (Coleoptera: Dytiscidae) based on larval characters. Systematic Entomology, 42, 734–767. https://doi.org/10.1111/syen.12243
Michat, M. C., Gustafson, G. T., & Bergsten, J. (2017). Larval description and chaetotaxic analysis of Dineutus sinuosipennis Laporte, 1840, with a key for the identification of larvae of the tribe Dineutini (Coleoptera, Gyrinidae). Zookeys, 718, 95–114. https://doi.org/10.3897/zookeys.718.20726
Moroni, J. C. (1980). Aporte al conocimiento de los Haliplidae de Chile. I. (Coleoptera: Dytiscoidea). Revista Chilena de Entomología, 10, 29–33.
Moroni, J. C. (1989). Aporte al conocimiento de los Haliplidae de Chile. II. Descripción del tercer instar larval de Haliplus valdiviensis Moroni, 1980 (Coleoptera: Dytiscoidea). Revista Chilena de Entomología, 17, 89–93.
Nilsson, A. N. (1988). A review of primary setae and pores on legs of larval Dytiscidae (Coleoptera). Canadian Journal of Zoology, 66, 2283–2294. https://doi.org/10.1139/z88-339
Schiødte, J. C. (1864). De Metamorphosi Eleutheratorum observationes: Bidrag til Insekternes Udviklingshistorie. Naturhistorisk Tidsskrift, 3 raekke 3, 131–224.
Strand, R. M., & Spangler, P. J. (1994). The natural history, distribution, and larval description of Brychius hungerfordi Spangler (Coleoptera: Haliplidae). Proceedings of the Entomological Society of Washington, 96, 208–213.
Urcola, J. I., Alarie, Y., Benetti, C. J., Rodriguez, G., & Michat, M. C. (2019). Larval morphology and analysis of primary chaetotaxy in the genus Suphis Aubé, 1836 (Coleoptera: Noteridae). Zootaxa, 4619, 121–138. https://doi.org/10.11646/zootaxa.4619.1.5
Vidal-Sarmiento, J., & Grosso, L. E. (1970). Notas sobre haliplidos argentinos. I. (Coleoptera). Revista de la Sociedad Entomológica Argentina, 32, 63–67.
Vidal-Sarmiento, J., & Grosso, L. E. (1971). Notas sobre haliplidos argentinos (Coleoptera). II. Revisión de las especies argentinas. Revista de la Sociedad Entomológica Argentina, 33, 147–157.
Vondel, B. J. van (1986). Description of the second and third-instar larvae of Haliplus laminatus (Schaller) with notes on the subgeneric status (Coleoptera: Haliplidae). Entomologische Berichten, 46, 128–132.
Vondel, B. J. van (1996). Description of the second and third instar larva of Haliplus varius with notes on the subgeneric status (Coleoptera: Haliplidae). Entomologische Berichten, 56, 9–11.
Vondel, B. J. van (1997). Insecta: Coleoptera: Haliplidae. SuBwasserfauna von Mitteleuropa, 20, 1–95.
Vondel, B. J. van (2001). Description of the third instar larva of Haliplus subseriatus (Coleoptera: Haliplidae). Entomologische Berichten, 61, 14–16.
Vondel, B. J. van (2004). First description of larvae of Haliplus-species from Australia (Coleoptera: Haliplidae). Tijdschrift voor Entomologie, 147, 57–61. https://doi.org/10.1163/22119434-900000140
Vondel, B. J. van (2005). Haliplidae. In A. N. Nilsson & B. J. van Vondel (Eds.), World Catalogue of Insects. Amphizoidae, Aspidytidae, Haliplidae, Noteridae and Paelobiidae (Coleoptera, Adephaga) (pp. 20–86). Stenstrup: Apollo Books.
Vondel, B. J. van (2011a). Description of Haliplus larvae from Lebanon (Coleoptera: Haliplidae). Koleopterologische Rundschau, 81, 41–54.
Vondel, B. J. van (2011b). Description of the third instar larva of Haliplus variomaculatus Brigham & Sanderson with notes on larvae of Nearctic Haliplidae (Coleoptera). Tijdschrift voor Entomologie, 154, 127–133. https://doi.org/10.1163/22119434-900000310
Vondel, B. J. van (2012). Description of larvae of four Haliplus species from Australia (Coleoptera: Haliplidae). Tijdschrift voor Entomologie, 155, 193–208. https://doi.org/10.1163/22119434-00002012
Vondel, B. J. van (2016). Haliplidae. In R. G. Beutel, & R. A. B. Leschen (Eds.), Handbook of Zoology. Arthropoda: Insecta. Coleoptera, Beetles. Volume 1: morphology and systematics, 2nd edition (pp. 87–96). Berlin: Walter de Gruyter. https://doi.org/10.1515/9783110373929
Vondel, B. J. van, & Spangler, P. J. (2008). Revision of the Haliplidae of the Neotropical Region including Mexico (Coleoptera: Haliplidae). Koleopterologische Rundschau, 78, 69–194.
Wiley, E. O. (1981). Phylogenetics. The theory and practice of phylogenetic systematics. New York: John Wiley & Sons.