Jannete Medina-Estrada a, Daniela Remolina-Figueroa b, Patricia Ramírez-Bastida c, Leopoldo D. Vázquez-Reyes c, *
a Universidad Nacional Autónoma de México, Facultad de Ciencias, Museo de Zoología, Apartado postal 70-399, 04510 Ciudad de México, Mexico
b Universidad Nacional Autónoma de México, Posgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México, Edificio D, 1er piso, Unidad de Posgrado, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, Mexico
c Universidad Nacional Autónoma de México, Facultad de Estudios Superiores Iztacala, Avenida de los Barrios # 1, Los Reyes Ixtacala, 54090 Tlalnepantla de Baz, Estado de México, Mexico
*Corresponding author: leopoldo.vazquez@unam.mx (L.D. Vázquez-Reyes)
Received: 24 November 2020; accepted: 9 April 2021
Abstract
Nesting resources for cavity-adopter birds commonly have spatial aggregation patterns within tropical dry forests. Spatial aggregation occurs because large trees, carrying large cavities, are restricted within small semideciduous forest areas. In contrast, deciduous forests occupy most of the coverage with smaller trees and cavities. Consequently, semideciduous forest loss could imperil cavity-adopter birds with large bodies. To test this hypothesis, we performed an intensive search in a tropical dry forest in Central-Mexico. We surveyed 5 transects —0.2 ha— in both deciduous and semideciduous forest, totalizing a survey of 2 ha. There were no differences in resource density between deciduous (4 ± 6.51 cavities/ha) and semideciduous forest (11 ± 6.51 cavities/ha). However, semideciduous forest cavities had wider entrances and were in larger trees. Besides, 90% of nesting resources for birds with bodies > 6 cm were restricted within the semideciduous forest, including Megascops seductus, an endemic owl, and Ara militaris, a threatened macaw. Bird-excavated cavities were associated with deciduous forest and Pachycereus weberi cacti. In contrast, decay cavities were associated with semideciduous forest and Enterolobium cyclocarpum trees. Our results suggest that the conservation of large-bodied cavity-adopter birds within dry forest depends on semideciduous forest coverage.
Keywords: Neotropics; Nesting resources; Secondary cavity nesters; Tree cavities
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Disponibilidad de recursos de anidación para aves adoptadoras de cavidades en un bosque tropical seco del centro de México
Resumen
Los recursos para aves adoptadoras de cavidades, por lo común, están agregados espacialmente dentro del bosque tropical seco. Esto ocurre porque los árboles grandes, con cavidades de mayor tamaño, están restringidos en áreas pequeñas de bosque subcaducifolio. En contraste, el bosque caducifolio, con árboles y cavidades menores, ocupa mayores extensiones. Como consecuencia, la pérdida de bosque subcaducifolio podría amenazar a las aves adoptadoras de cavidades. Para evaluar esta hipótesis, muestreamos por búsqueda intensiva un bosque tropical seco del centro de México, considerando 5 transectos de 0.2 ha en cada tipo de bosque, totalizando 2 ha muestreadas. No hubo diferencias entre bosque caducifolio (4 ± 6.51 cavidades/ha) y subcaducifolio (11 ± 6.51 cavidades/ha). Sin embargo, las cavidades del bosque subcaducifolio tuvieron entradas más anchas y estaban en árboles más grandes. El 90% de los recursos para aves con cuerpos > 6 cm, como Megascops seductus y Ara militaris, estuvieron en el bosque subcaducifolio. Las cavidades excavadas se asociaron con el bosque caducifolio y el cactus Pachycereus weberi, mientras que las cavidades formadas por decaimiento se asociaron con el bosque subcaducifolio y árboles de Enterolobium cyclocarpum. Nuestros resultados indican que la conservación de aves adoptadoras de cavidades depende de la cobertura de bosque subcaducifolio.
Palabras clave: Neotrópico; Recursos de anidamiento; Anidadores secundarios de cavidad; Cavidades de árboles
© 2022 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Introduction
Secondary cavity nesters constitute up to 21% of bird species inhabiting Neotropical forests (Cockle et al., 2011; Monterrubio-Rico & Escalante-Pliego, 2006). Secondary cavity nesters need holes in trees to nest but cannot excavate them themselves, so they depend on the available cavities (Newton, 1994). However, not all existing cavities within forest habitat are suitable for nesting. Nesting sites are selected according to their characteristics related to predator exclusion and provision of an appropriate microhabitat for brood development (Enkerlin-Hoeflich, 1995; Newton, 1994; Saunders et al., 1982). If cavities with appropriate depth, entrance size, and height above the ground are scarce, their availability could be a limiting factor for bird populations (Newton, 1994). Consequently, secondary cavity nesters are more vulnerable to habitat loss and nesting resources than other birds in tropical forests (Monterrubio-Rico & Escalante-Pliego, 2006; van der Hoek et al., 2017).
In tropical forests, wood decay, including processes like breaking of branches, insect pests, and fungal infections, form most of the cavities used for nesting (Cockle et al., 2011; Gibbs et al., 1993). In the tropics, the same environmental conditions that favor cavity formation, such as high temperature and humidity, also reduce the useful life of cavities because of the rapid decay of wood. Accelerated decay processes could therefore make nesting resources abundant, but available only for a short time (Cockle et al., 2011; Cornelius et al., 2008). Another source of cavities is ecosystem engineering by excavator bird species, i.e. woodpeckers (Şekercioğlu et al., 2016). However, within tropical forests, the abundance and richness of woodpeckers is low compared to the richness of secondary cavity nesters. Therefore, woodpeckers are apparently less important for cavity formation than wood decay processes (Cockle et al., 2011; Cornelius et al., 2008; Sandoval & Barrantes, 2009).
The Mexican tropical dry forest is an ecosystem with high spatial heterogeneity in vegetation structure and composition because of its complex hilly orography. The vegetation coverage of the tropical dry forest is dominated by deciduous forests, covering most of the hilly slopes, while semideciduous forests are restricted to stream beds and narrow valleys between hills, where higher humidity allows the growth of larger trees (Balvanera et al., 2002; Bezaury-Creel, 2010; Holdridge, 1967). Notably, habitat heterogeneity can drive spatial aggregation patterns of nesting resources. For example, on the Pacific coast of Mexico, trees within the tropical semideciduous forests, such as Astronium graveolens, Brosimum alicastrum, and mostly Piranhea mexicana monodominant forests, account for up to 80% of all nesting cavities within tropical dry forests, despite being only 14% of the available habitat (Vázquez & Renton, 2015). This aggregation pattern implies a serious conservation issue: loss of semideciduous forest equals the loss of the largest trees, the same ones carrying both most of the larger cavities and available nesting resources. Therefore, deforestation of the semideciduous forest a severe threat to the conservation of secondary cavity nester birds (Cockle et al., 2011; Salinas-Melgoza et al., 2009).
The eastern section of the Balsas basin, the Alto Balsas, is a global Important Bird Area (IBA) because of its endemic richness (Birdlife International, 2020b). Tropical lowlands within the Alto Balsas are habitat to a considerable richness of cavity-nesting species. Of at least 150 bird species that inhabit the region (Vázquez-Reyes et al., 2018), 22 use cavities to nest, and 11 are obligate cavity-adopters. The families Strigidae and Psittacidae include species that may be restricted to cavities in the semideciduous forest because their large body size means that they only fit in large cavities, located in large trees (Monterrubio-Rico & Escalante-Pliego, 2006). For example, the Balsas Screech-Owl, Megascops seductus, is a threatened endemic owl, and the Military Macaw, Ara militaris, is considered vulnerable at a global scale (Berlanga et al., 2010; Birdlife International, 2020a). Unfortunately, habitat loss of tropical dry forests due to human activities show rates of 1.4% annually (Sánchez-Azofeifa & Portillo-Quintero, 2011; Trejo & Dirzo, 2000). Consequently, the biodiversity in the Alto Balsas forests is threatened (Vázquez-Reyes et al., 2017). The semideciduous forest suffers direct impacts of habitat loss because it is converted to agricultural activities such as crops and orchards (Sánchez-Colón et al., 2009). Furthermore, tree species typical of this habitat, like Enterolobium cyclocarpum, are used for timber (Pennington & Sarukhán, 2005). Thus, the threat to the small area of semideciduous forest bearing nesting resources for obligate cavity-adopter birds, particularly for large-bodied birds, is a severe issue (Salinas-Melgoza et al., 2009; Vázquez & Renton, 2015).
In this study, we aimed to assess the availability of nesting resources for secondary cavity nester birds within a tropical dry forest matrix in the Alto Balsas. We hypothesized that the availability of cavities useful as nesting resources for birds would be higher in semideciduous forest than in deciduous forest, particularly for secondary cavity nesters of larger body sizes. To test this hypothesis, we compared the density and characteristics of tree cavities, considering both the deciduous and semideciduous forest within the study area. This study highlights the ecological importance of the semideciduous forest areas within the Alto Balsas in providing the essential resource of nesting sites for tropical dry forest birds in Central Mexico (Renton et al., 2018; Salinas-Melgoza et al., 2009).
Materials and methods
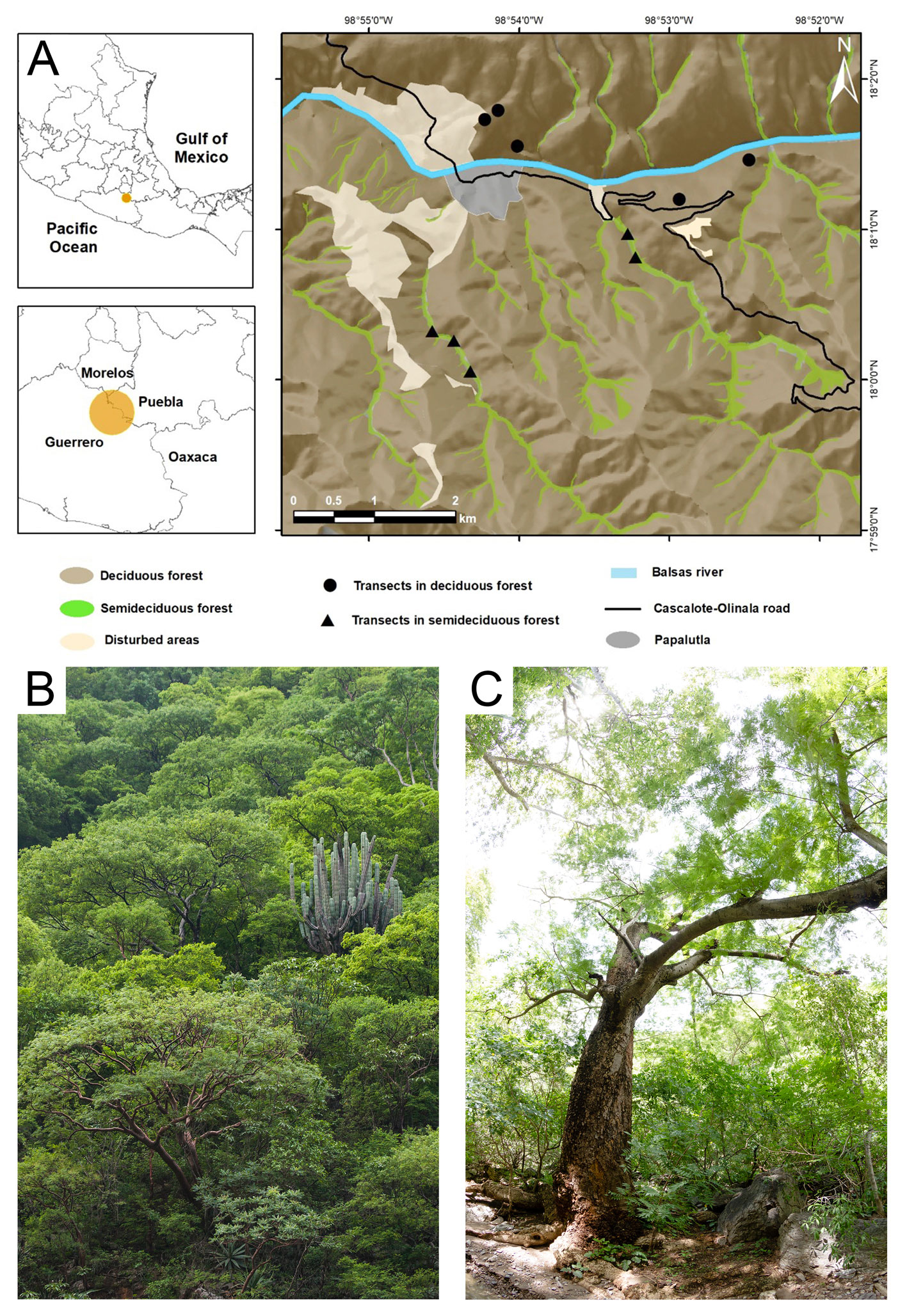
The study area is located in the Balsas River Basin in northeast Guerrero, Mexico, at central coordinates of 18°01’ N, 98° 53’ W and with an elevational gradient from 650 to 1,950 m (Fig. 1). The climate is warm and semiarid, with a mean temperature of 26 ºC, a summer rainy season (Aw according to the Köppen classification) and annual precipitation of 780 mm (Meza & López-García, 1997). The focus of our study is the tropical lowlands, between 650 and 1,400 m. Here, dominant vegetation types are tropical deciduous forest on the hillsides and tropical semideciduous forest in valleys and along stream beds. Above 1,400 m, vegetation is oak forests, dominated by species with a Nearctic floristic affinity (Martínez et al., 1997; Rzedowski, 2006). Deciduous forests have a noteworthy diversity of Bursera and Lysiloma tree genera, as well as floristic elements such as Cyrtocarpa procera, Amphipterigium adstringens, and Ceiba aesculifolia, and columnar cacti, such as Pachycereus weberi. The tree stratum of the semideciduous forest includes Enterolobium cyclocarpum, Ficus periolaris, and Lonchocarpus spp. trees (Martínez et al., 1997).
To estimate cavity density within the tropical dry forest, we considered both deciduous and semideciduous forest areas. We sampled in forest areas where the original vegetation cover remained unchanged and human activity was limited to occasional transit of people (Vázquez-Reyes et al., 2017). We defined the location of the sampling areas according to the floristic composition and spatial distribution of vegetation. We used Google Earth satellite images to identify deciduous and semideciduous forest areas, considering that semideciduous vegetation foliage remains green even during the dry season. Using these satellite images, we estimated that 91.1% (3,926 ha) of the study area is covered by deciduous forest, while 8.9% (382 ha) is semideciduous forest. At least 3 observers, including JME, DRM, and LDVR, sampled 5 transects —100 × 20 m— within each habitat type. Thus, our sample area included 1 ha per habitat type, totalizing a survey of 2 ha of tropical dry forest. The distance between neighboring transects was always > 100 m (Fig. 1). The transects’ locations and dimensions were defined using a measuring tape, flagging tape, and GPS equipment.
To assess the cavity availability, all trees within the transect areas were intensively searched for cavities by JME and LDVR using 10 × 40 binoculars. We defined cavities as hollows in trees that had walls and a floor, an entrance > 3 cm wide and with an internal depth > 12 cm. For each cavity, we recorded the height above the ground, depth, entrance width, and the diameter of the supporting trunk or branch. In the case of cavities with more than 1 entrance, we recorded the data for the largest opening, since this size determines whether an organism (either the occupant or a predator) can access the cavity (Vázquez & Renton, 2015).
Cavities were categorized according to their origin as excavated by birds or from tree decay. Excavated cavities are easily recognized due to their round, almost symmetrical shape, and smooth borders, while non-excavated cavities have irregular shapes (Aitken & Martin, 2007). Cavities less than 4 m above the ground were accessed using a ladder and measured with a measuring tape. For cavities more 4 m above the ground, we measured the entrance and the diameter of the support with a 15 m extendable measuring pole. A nylon polymer ruler graduated in cm was attached to the end of the pole. To measure cavity depth, a reel of nylon string with a round weight tied to the end was mounted on the polymer ruler and the weight was smoothly lowered into the cavities to avoid injuring any potential occupants; the displacement distance was the depth of the cavity. We did not found any living organisms occupying cavities during our survey. We recorded the number of cavities, the diameter at breast height (DBH), and the height of each tree with at least 1 cavity.
We considered cavities with an entrance width of 3.4 to 14.7 cm to be potentially appropriate nesting resources for birds. These measurements correspond to the body width range of the secondary cavity nester assemblage within our study area (Table 1). Surveys to define the list of regional bird biodiversity and also potential cavity-adopter birds was performed by LDVR between 2007 and 2017 (Vázquez-Reyes et al., 2018). This criterion also takes into account that the cavity entrance size is constrained by the size of occupants on one hand and the exclusion of potential predators and lower predation risk on the other (Enkerlin-Hoeflich, 1995; Newton, 1994; Saunders et al., 1982).
We used specimens from scientific collections (Museo de Zoología de la Facultad de Ciencias, and Colección Nacional de Aves, both at the National Autonomous University of Mexico, UNAM) to determine the body width of cavity-adopter birds in our study area. We use a graduated ruler to measure the body width (bird back-width at the scapula, in cm) for each considered specimen. Only the specimens whose taxidermy represents the live specimen’s body shape properly were considered. Five specimens of each species, except for Ara militaris and Megascops seductus, for which only 4 specimens were available. Specimens from localities as close as possible to the study area were measured to reduce biases in estimating the birds’ sizes at our study site due to geographic variation in body size.
Table 1
Cavity-nesting birds within deciduous and semideciduous forests of the Alto Balsas. Taxonomical criteria correspond to International Ornithological Committee (Gill et al., 2020). ? Obligate adopter (bird that only nest inside cavities, however, are not capable to excavate them). ‡ Facultative adopter (bird that could nest within cavities but also in other substrates). § Excavator (bird that excavate their own nesting cavities). ¶ Bird species whose body width is larger than the mean entrance width of deciduous forest cavities.
|
Order |
Family |
Species |
Body width (cm ± standard deviation) |
|
Anseriformes |
Anatidae |
Dendrocygna autumnalis ‡ |
9.58 ± 0.58 ¶ |
|
Strigiformes |
Tytonidae |
Tyto furcata ‡ |
10.76 ± 0.77 ¶ |
|
Strigidae |
Megascops seductus † |
7.73 ± 0.30 ¶ |
|
|
Bubo virginianus ‡ |
14.78 ± 1.04 ¶ |
||
|
Strix virgata † |
9.84 ± 0.36 ¶ |
||
|
Glaucidium palmarum † |
5 ± 0.35 |
||
|
Glaucidium brasilianum † |
4.5 ± 0.19 |
||
|
Micrathene whitneyi † |
4.58 ± 0.31 |
||
|
Trogoniformes |
Trogonidae |
Trogon elegans † |
5.7 ± 0.14 |
|
Falconiformes |
Falconidae |
Caracara cheriway ‡ |
12.3 ± 0.52 ¶ |
|
Herpetotheres cachinnans ‡ |
11.5 ± 0.37 ¶ |
||
|
Psittaciformes |
Psittacidae |
Ara militaris † |
12.28 ± 0.76 ¶ |
|
Piciformes |
Picidae |
Dryobates scalaris § |
3.44 ± 0.36 |
|
|
Melanerpes chrysogenys § |
4.94 ± 0.42 |
|
|
|
|
Melanerpes hypopolius § |
4.7 ± 0.23 |
|
Campephilus guatemalensis § |
6.7 ± 0.14 |
||
|
Passeriformes |
Tyrannidae |
Pyrocephalus rubinus † |
3.42 ± 0.52 |
|
Myiarchus tuberculifer † |
3.68 ± 0.20 |
||
|
Myiarchus nuttingi † |
4 ± 0.27 |
||
|
Myiarchus tyrannulus ? |
4.4 ± 0.41 |
||
|
Corvidae |
Corvus corax ‡ |
12.6 ± 0.45 ¶ |
|
|
Hirundinidae |
Tachycineta thalassina † |
3.84 ± 0.43 |
Shapiro-Wilk normality test were performed, which showed that neither the cavity density data nor their characteristics had a normal distribution. Therefore, all comparisons between deciduous and semideciduous forests were computed using non-parametric Mann-Whitney U tests. To compute the statistical comparisons of cavity density between forest types, we made separate comparisons of the total cavities, cavities suitable for birds, excavated cavities, and wood-decay formed cavities. These comparisons take account of the net number of recorded cavities per transect (Table 2). However, we also want to show a density measurement that allows comparisons with other secondary cavity-nesting birds’ studies (Cockle et al., 2008; de la Parra et al., 2015; Vázquez & Renton, 2015). Hence, we show the cavity/transect values scaled to cavities/ha values. The compared cavity characteristics were height from the ground, entrance width, depth, and supporting trunk or branch diameter. The diameter at breast height (DBH) and the trees’ total height where cavities were found were also compared. We computed Chi-square contingency tables to assess whether the origin of the cavities and the availability of suitable cavities were associated with habitat type. We performed the data analysis using the Past3 statistical package (Hammer et al., 2001).
Results
A total of 51 cavities within the sampled transects in the Alto Balsas tropical dry forest were recorded: 20 in deciduous forest and 31 in semideciduous forest. The overall mean cavity density, expressed as a density measure ± standard deviation, was 25.5 ± 16.06 cavities/ha. The mean density in the deciduous forest was 20 ± 16.95 cavities/ha and in the semideciduous forest 31 ± 14.74 cavities/ha. The difference in total cavities per transect between habitat types was not significant (Table 2). Dividing cavities by their origin, the deciduous forest had a mean density of 13 ± 13.5 wood-decay cavities/ha and a mean density of 7 ± 15.65 excavated cavities/ha. The semideciduous forest had a mean density of wood-decay cavities of 30 ± 16.2 cavities/ha and of excavated cavities of 1 ± 2.23 cavities/ha. This difference between habitat types was not significant (Table 2). When considering only cavities that were suitable as a nesting resource for secondary cavity nesters, the deciduous forest had a mean density of 4 ± 6.51 cavities/ha, while in the semideciduous forest, the mean density was 11 ± 6.51 cavities/ha. There were no significant differences in suitable cavities density between habitat types (Table 2).
Table 2
Number of cavities recorded by habitat type within the tropical dry forest of Alto Balsas. Mean values ± standard deviation values are shown. Because our survey found only one cavity for the excavated and the wood-decay categories, computing the statistical tests was not possible.
|
Cavities |
Deciduous forest |
Semideciduous forest |
Statistical test |
|
All |
4 ± 3.39 |
6.2 ± 2.94 |
H1,10 = 0.69, p = 0.4 |
|
Excavated |
1.4 ± 3.13 |
0.2 ± 0.44 |
H1,10 = 0.01, p = 0.88 |
|
Wood-decay |
2.6 ± 2.7 |
6 ± 3.24 |
H1,10 = 2.13, p = 0.14 |
|
Bird-suitable |
0.8 ± 1.3 |
2.2 ± 1.3 |
H1,10 = 2.79, p = 0.08 |
|
Excavated |
0 |
0.2 ± 0 |
N. A. |
|
Wood-decay |
0.2 ± 0 |
0 |
N. A. |
Comparisons of cavity characteristics by habitat type are expressed as the characteristic measure in the appropriate unit ± standard deviation. Considering all of the cavities in both forest types (n = 51), the mean height above the ground was 4.12 ± 3.48 m, entrance width was 11.39 ± 7.51 cm, depth was 27.57 ± 28.23 cm, and support diameter was 36.13 ± 33.97 cm. The mean DBH of trees with cavities was 65.37 ± 52.95 cm, and their mean height was 10.15 ± 5.13 m. There were no significant differences between habitat types either for cavities’ height, depth, or the DBH of the tree where the cavity was placed. However, the semideciduous forests’ cavities had wider entrances, larger diameter supports, and were located in taller trees than cavities in the deciduous forest. This pattern occurs for all cavities but also the bird-suitable cavities subset (Table 3). Woodpeckers formed the 87.5% of the cavities recorded in the deciduous forest, compared to 30.3% in the semideciduous forest. According to the contingency tables, there was an association between cavity origin and habitat type (c2 = 9.28, p = 0.0023).
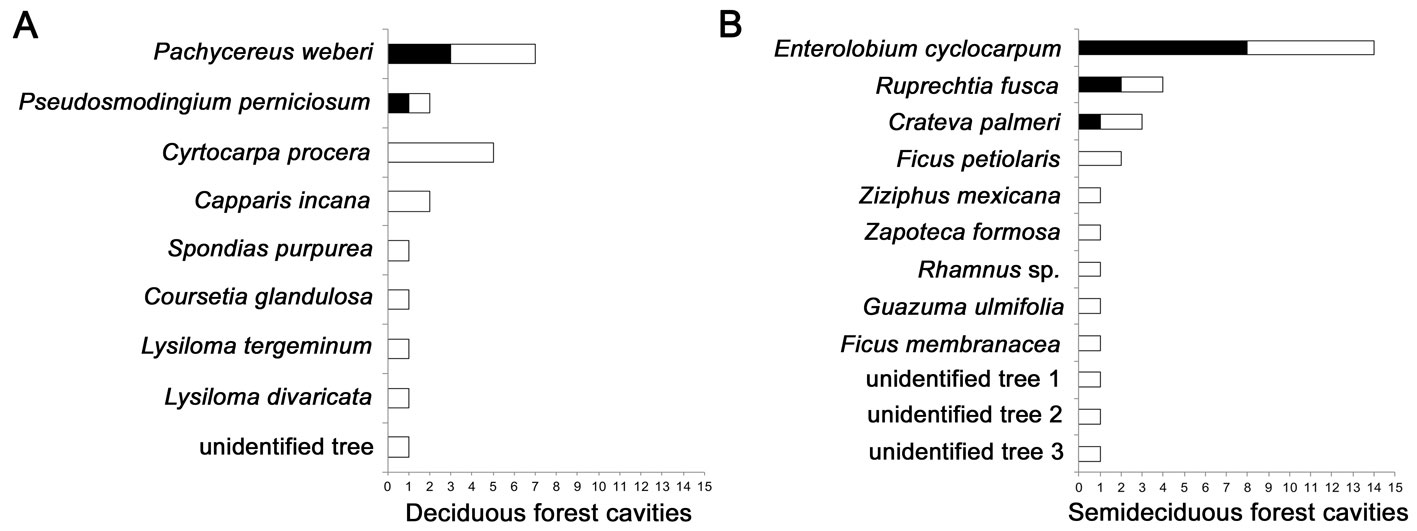
A total of 17 different plant species with cavities were identified within the survey, plus 4 plant species that could not be identified (Fig. 2). Within the deciduous forest 8 different species had cavities, but 70% of all of the cavities recorded occurred in just 3 species: Pachycereus weberi (7 cavities), Cyrtocarpa procera (5 cavities); and Pseudosmondigium perniciosum (2 cavities). Besides, all of the bird-suitable cavities occurred in P. weberi (3 cavities, 75%) and P. perniciosum (1 cavity, 25%). Within the semideciduous forest, we identified 9 tree species with cavities, plus 3 unidentified species. Again, 3 species contained ~ 70% of the total cavities in this forest type: Enterolobium cyclocarpum (14 cavities), Ruprechtia fusca (4 cavities), and Crateva palmeri (3 cavities); and these same 3 species housed all of the cavities suitable for birds (E. cyclocarpum: 8 cavities, 72.72%; R. fusca: 2 cavities, 18.18%; C. palmeri: 1 cavity, 9.09%).
The mean body width of the obligate cavity-adopter birds of the tropical dry forest was 5.8 ± 2.61 cm, with a range of 3.4 to 13.4 cm. We find that birds with body sizes close to 8 cm, such as Megascops seductus, coincide only marginally with the upper quartile of cavities’ width-entrance within the deciduous forest. Indeed, larger birds such as Strix virgata and Ara militaris, actually exceed the width of any available cavity size within this habitat. However, large bird body sizes fall within the range of cavities available in the sub-deciduous forest. Figure 3 shows the correspondence between cavity nesters’ body size and the entrance width distribution of bird-suitable cavities available in each habitat type.
Discussion
The Alto Balsas’ tropical dry forest has a high density of nesting resources for birds (25.5 total cavities/ha and 7.5 suitable cavities/ha) compared to other Neotropical forests. For example, studies in South American cloud forests have found values between 12.8 and 16.8 total cavities/ha; and between 3.9 and 4.5 suitable cavities/ha (Cockle et al., 2008, 2010; Politi et al., 2010). However, the density of nesting resources in the Alto Balsas is low compared to the dry tropics of the Mexican Pacific, where density nesting resources is 40 cavities/ha in semideciduous forest, and 64.8 cavities/ha in Piranhea mexicana forest (Vázquez & Renton, 2015). In comparison, the semideciduous forest of the Alto Balsas had a density of 11 suitable cavities/ha.
A possible explanation for the difference in cavity density could be the prevailing ecological conditions in Central Mexico. Although tropical dry forests cover both the Pacific slope and the Balsas depression, the coastal humidity in Western Mexico drives higher rainfall and humidity (Rzedowski, 2006). High humidity favor wood-decay processes and could increase the abundance of tree cavities (Cockle et al., 2011; Vázquez & Renton et al., 2015). In contrast, the lowlands across the Balsas river basin have lower rainfall and humidity because of the rain shadow effect of the mountains shaping the basin (Rzedowski, 2006). Two associated factors can explain the lower abundance of cavities. First, the wood-decay processes could be slower due to the lower environmental humidity (Gibbs et al., 1993; Cockle et al., 2011). At the same time, lower humidity could lead to slower plant growth, reducing the abundance of large trees with an appropriate size to bear the large cavities that are useful as nesting resources for large birds (Meza & López-García, 1997).
No differences in cavity density between the deciduous and the semideciduous forest were found. In contrast, in western Mexico, semideciduous forests have up to 3.5 times higher cavity density than deciduous forests (Vázquez & Renton, 2015). However, according to our hypothesis, the resource density for the large obligate cavity-adopter species within the Alto Balsas is highly restricted within the semideciduous forest. Birds with body width between 6 and 8 cm may only access the 25% of available nesting resources within the deciduous forest. In contrast, around 90% of suitable cavities in the semideciduous forest are useful for the large birds. Thus, nesting resources for the large obligate cavity-adopter species are restricted within the 8.9% of the area of the tropical dry forest in the Alto Balsas.
The differences in the cavity measurements suggest that different bird species may use different forest matrix elements as breeding habitat. Suitable cavities for small body size birds are available in both types of forest. Therefore, small secondary cavity-nesters such as the Colima Pygmy-Owl (Glaucidium palmarum), the Ferruginous Pygmy-Owl (G. brasilianum), and Nutting’s Flycatcher (Myiarchus nuttingi) could potentially find resources in both types of habitat. Conversely, birds that require larger cavities only find resources within the semideciduous forest, where the entrance widths and support diameters are significantly larger than in the deciduous forest (de la Parra et al., 2015; Saunders et al., 1982). This includes the Balsas Screech-Owl, the Mottled Owl (Strix virgata), and potentially, the Military Macaw. Until now, Military Macaw nests only have been recorded on rocky cliffs within the study area (Jiménez-Arcos et al., 2012). However, some of the largest cavities recorded on the E. cyclocarpum trees of the semideciduous forest could fulfill the characteristics selected as nest sites by macaws in other regions (de la Parra et al., 2015). When available tree cavities have suitable characteristics, the Military Macaw could nest in cliffs or trees facultatively (Rivera-Ortíz et al., 2016).
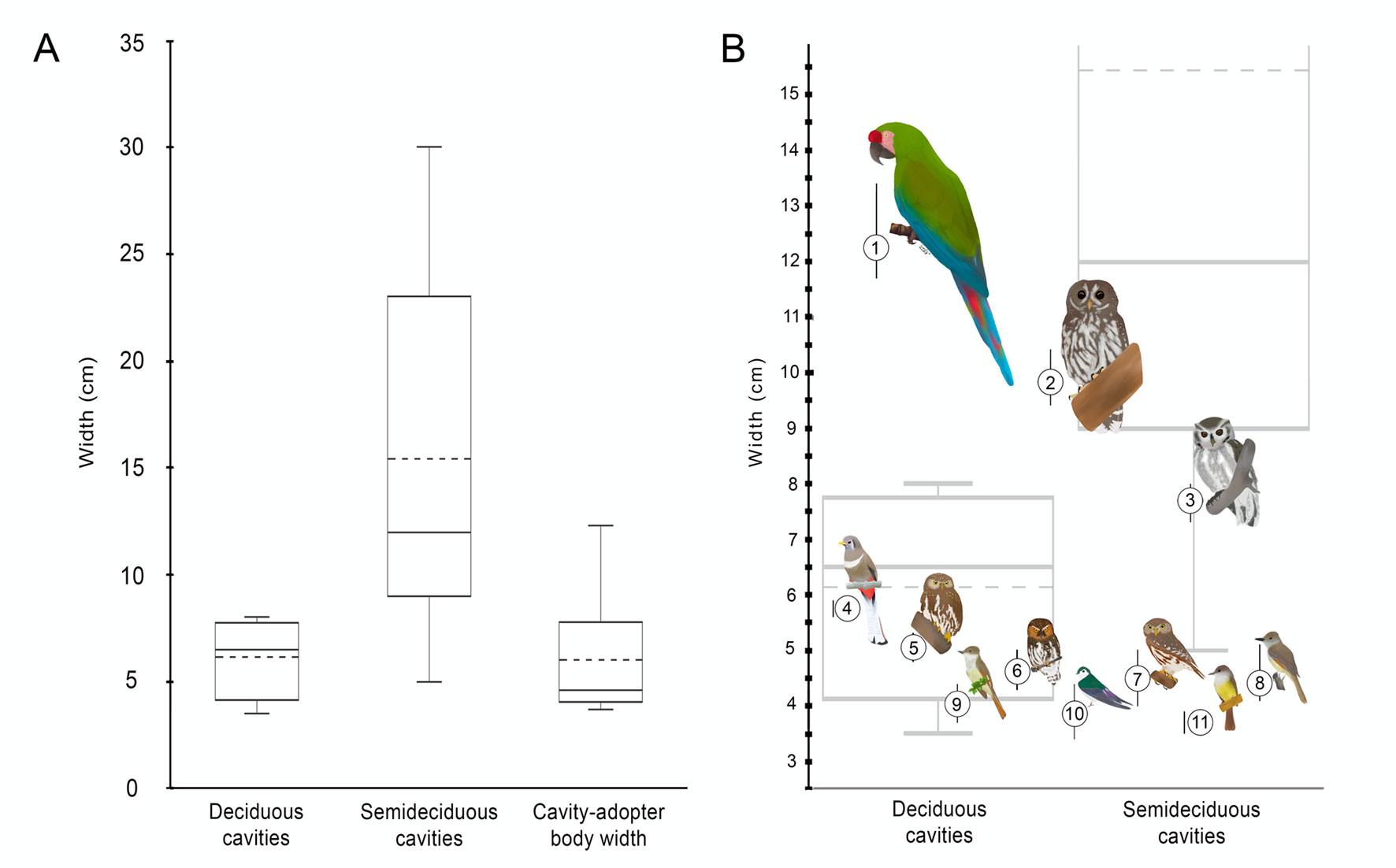
Table 3
Characteristics of the cavities recorded within the tropical dry forest of Alto Balsas. Mean values ± standard deviation are shown. * = Significant differences (p < 0.05).
|
All cavities |
Deciduous forest |
Semideciduous forest |
Statistical test |
|
Height to ground (m) |
3.6 ± 2.8 |
4.42 ± 3.83 |
U = 0.37, p = 0.70 |
|
*Entrance width (cm) |
8 ± 7.75 |
13.5 ± 6.5 |
U = 3.79, p < 0.001 |
|
Depth (cm) |
26.3 ± 28.7 |
28.3 ± 28.3 |
U = 0.87, p = 0.38 |
|
*Support diameter (cm) |
25.35 ± 32.8 |
43.09 ± 33.3 |
U = 3.7, p < 0.001 |
|
Tree DBH (cm) |
44.6 ± 35.7 |
78.7 ± 58.2 |
U = 1.83, p = 0.06 |
|
*Tree total height (m) |
6.8 ± 1.76 |
12.3 ± 5.43 |
U = 3.05, p = 0.001 |
|
Bird-suitable cavities |
Deciduous forest |
Semideciduous forest |
Statistical test |
|
Height to ground (m) |
6.51 ± 1.65 |
6.6 ± 2.7 |
U = 0.52, p = 0.6 |
|
*entrance width (cm) |
6.12 ± 1.93 |
15.45 ± 8.33 |
U = 2.42, p = 0.01 |
|
Depth (cm) |
33.5 ± 27.3 |
24.9 ± 10.04 |
U = 0.13, p = 0.89 |
|
*Support diameter (cm) |
16 ± 6.48 |
44.27 ± 26.66 |
U = 2.41, p = 0.01 |
|
Tree DBH (cm) |
55.7 ± 20.5 |
105.4 ± 59.3 |
U = 1.12, p = 0.26 |
|
**Tree total height (m) |
7.87 ± 0.58 |
15.09 ± 4.61 |
U = 1.85, p = 0.05 |
Beyond the density or their spatial distribution of cavities, territorial behavior and defense of the nest area by birds, could also restrict the actual availability of nesting resources. In western Mexico, the Lilac-crowned Parrot (Amazona finschi) actively chases other birds from the nesting area, which restricts the use suitable cavities close to existing nests (Salinas-Melgoza et al., 2009). Because the Strix and Megascops owls, as well Ara macaws are intensely territorial around their nesting areas, the actual availability of nesting resources will likely be reduced due to competition (Belthoff & Ritchison, 1990; Enriquez & Cheng, 2008; Gehlbach & Stoleson, 2010; Gerhardt et al., 1994; Renton & Brightsmith, 2009).
Seemingly, the local environmental conditions that define changes in the floristic composition at the local scale (Balvanera et al., 2002; Meza & López-García, 1997; Trejo & Dirzo, 2002) also could influence the spatial distribution patterns and availability of nesting resources for birds (Cockle et al., 2008, 2011). Thus, our results show that the deciduous and semideciduous forests play complementary roles in providing nesting resources for cavity-adopting birds of the tropical dry forest within the Alto Balsas, depending on the ecological conditions and plant species composition within vegetation types.
Eighty-seven percent of the total excavated cavities were in the deciduous forest, and excavator bird activity on the large columnar cactus Pachycereus weberi accounted for 75% of the nesting resources within this habitat type. The entrance width (7 ± 1cm) and depth (20 ± 5cm) of these cavities suggest that were formed by excavator birds like the Golden-cheeked Woodpecker (Melanerpes chrysogenys) the most common woodpecker in the tropical lowlands of Alto Balsas, or perhaps by the Grey-breasted Woodpecker (M. hypopolius). These woodpeckers prefer to use soft substrates to excavate their nests because they require less energy expenditure (Schepps et al., 1999). Therefore, it is plausible that the low density of the plant tissue could favor the selective use of columnar cacti as a nesting substrate by woodpeckers. Notably, columnar cacti are common nesting substrates for woodpeckers in many arid and semi-arid zones of Mexico (Arizmendi & Espinosa de los Monteros, 1996; Hendricks et al., 1990; Leonard, 2000; Zwartjes & Nordell, 1998). Hence, excavator birds within the Alto Balsas’ deciduous forests seem to play an essential role in the provision of nesting resources while they excavate their nests and forage (Cockle et al., 2011; Şekercioğlu et al., 2016).
On the other hand, cavities formed by wood-decay processes were associated with the semideciduous forest, and 72.3% of suitable cavities were in Enterolobium cyclocarpum trees. This species grows in habitats with high humidity, where wood-decay favors cavity formation. Additionally, by exceeding a height of 15 m and 1.5 m DBH, E. cyclocarpum trees had the largest cavities in our survey. However, due to accelerated wood-decay, high humidity also reduces the useful lifespan and density of cavities as nesting resources (Cockle et al., 2011; Sandoval & Barrantes, 2009). Notably, we found only 1 suitable cavity formed by wood-decay within the deciduous forest, and only 1 cavity formed by excavator birds within the semideciduous forest. It is possible that the extreme conditions of heat and insolation in deciduous forest habitat slows down the process of cavity formation by wood-decay. In contrast, high humidity and temperature within semideciduous forests could accelerate wood-decay, reducing the useful lifespan of recently excavated cavities (Sandoval & Barrantes, 2009).
The higher availability of cavities with wider entrances within the semideciduous forest involves a spatial aggregation of nesting resources and a serious conservation concern. This forest grows in areas traditionally preferred by local people for agriculture because of their flat terrain, humidity, and fertile soil. Consequently, semideciduous forest cover is lost more rapidly than the deciduous forest (Sánchez-Colón et al., 2009). Furthermore, large E. cyclocarpum individuals are exploited locally as a timber resource (Pineda-Herrera et al., 2012), and trees with appropriate characteristics to bear nesting sites are selectively harvested. Indeed, between 2015 and 2017, we documented the loss of at least 3 individuals of E. cyclocarpum with height < 15 m and DBH < 1.5 m. Forest cover loss and selective logging are serious threats that imperil secondary cavity nester populations in the Alto Balsas. For example, Megascops seductus is a threatened, endemic, obligate cavity adopter bird (Berlanga et al., 2010; Egan, 2020), whose nesting resources are restricted within semideciduous forest areas.
Unfortunately, the loss of semideciduous forest, because it is converted to agricultural activities, is a widespread phenomenon throughout the distributional area of the tropical dry forest through Mexico and even the Mesoamerican region (Sánchez-Azofeifa & Portillo-Quintero, 2011; Sánchez-Colón et al., 2009). Furthermore, the loss of large trees from the sub-deciduous forest to be used as timber (Pennington & Sarukhán, 2005) is a critical issue. The loss of these trees could seriously affect large cavity adopter birds. Besides, the distribution of the dry forest of central and western Mexico, where between 11% and 29% of bird diversity requires cavities to nest (Monterrubio-Rico & Escalante-Pliego, 2006), corresponds to some of the areas with higher bird endemism in Mexico (Navarro-Sigüenza et al., 2014). Besides, the Alto Balsas region has received, at best, marginal governmental support for biodiversity conservation (Bezaury-Creel, 2010; Vázquez-Reyes et al., 2018), even though the area meets the criteria to be considered a protected area in Mexico and is an IBA (BirdLife International, 2020b). Stop, and eventually reverse, the loss of semideciduous forest cover is urgent to conserve the large cavity-adopters bird diversity. Those actions should consider the tree species that provide nesting resources, especially for large cavity-adopter bird species.
Acknowledgements
Fieldwork was funded by the Conanp PROCER/DRCEN/06/2016 project (LDV-R, Naturam Sequi AC) and by Biosphera Picture A.C. We are grateful to the local authorities and inhabitants of the Papalutla community and the members of S.C. ANP Cerro Tecaballo for the facilities provided to perform the study. Juan Esteban, Josué Esteban, Raúl Caballero and Jezreel B. Rivadeneyra provided helpful support during fieldwork. We thank Adolfo G. Navarro-Sigüenza (Museo de Zoología, Facultad de Ciencias, UNAM) and Patricia Escalante-Pliego (CNA, Instituto de Biología, UNAM) for the facilities provided to review collection specimens. Amira Ruiz-Rodríguez performed the GIS work for Figure 1. Montserrat Serra Rojas de la Barrera illustrated the birds in Figure 2. Lynna Kiere provided valuable help for the English writing. Héctor Godínez and Leopoldo Vázquez S. provided logistical support for the development of the cavity measurement tools. Adolfo Navarro, Alejandro Gordillo, Horacio Paz, Víctor Jiménez, and Francisco Rivera provided valuable comments that improved the manuscript.
References
Aitken, K. E. H., & Martin, K. (2007). The importance of excavators in hole-nesting communities: availability and use of natural tree holes in old mixed forest in western Canada. Journal of Ornithology, 148, 425–434. https://doi.org/10.1007/s10336-007-0166-9
Arizmendi, M. C., & Espinosa de los Monteros, A. (1996). Avifauna de los bosques de cactaceas columnares del Valle de Tehuacan, Puebla. Acta Zoológica Mexicana, 67, 25–46.
Balvanera, P., Lott, E., Segura, G., Siebe, C., & Islas, A. (2002). Patterns of b-diversity in a Mexican tropical dry forest. Journal of Vegetation Science, 13, 145–158. https://doi.org/10.1111/j.1654-1103.2002.tb02034.x
Belthoff, J. R., & Ritchison, G. (1990). Nest-site selection by Eastern Screech-Owls in central Kentucky. The Condor, 92, 982–990. https://doi.org/10.2307/1368734
Berlanga, H., Kennedy, J. A., Rich, T. D., Arizmendi, M. C., Beardmore, C. J., Blancher, P. J. et al. (2010). Saving our shared birds: partners in flight tri-national vision for landbird conservation. Ithaca, NY: Cornell Lab. of Ornithology.
Bezaury-Creel, J. E. (2010). Las selvas secas del Pacífico mexicano en el contexto mundial. In G. Ceballos, L. Martínez, A. García, E. Espinoza, J. E. Bezaury-Creel, & R. Dirzo (Eds.), Diversidad, amenazas y áreas prioritarias para la conservación de las selvas secas del Pacífico de México (pp. 21–40). México D.F.: Comisión Nacional para el Conocimiento y Uso de la Biodiversidad/ Fondo de Cultura Económica.
BirdLife International (2020a). Ara militaris. The IUCN Red List of Threatened Species 2016: e.T22685548A93079238. Available: https://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22685548A93079238.en
BirdLife International (2020b). Important Bird areas factsheet: Papalutla, Sierra de Tecaballo. Available: http://datazone.birdlife.org/site/factsheet/papalutla-sierra-de-tecaballo-iba-mexico
Cockle, K. L., Martin, K., & Drever, M. C. (2010). Supply of nest holes limits nest density of cavity-nesting birds in primary and logged subtropical Atlantic forests. Biological Conservation, 143, 2851–2857. http://doi:10.1016/j.biocon.2010.08.002
Cockle, K. L., Martin, K., & Wesolowski, T. (2011). Woodpeckers, decay, and the future of cavity-nesting vertebrate communities worldwide. Frontiers in Ecology and the Environment, 9, 377–382. https://doi.org/10.1890/110013
Cockle, K., Martin, K., & Wiebe, K. (2008). Availability of cavities for nesting birds in the Atlantic forest, Argentina. Ornitologia Neotropical, 19, 269–278.
Cornelius, C., Cockle, K., Politi, N., Berkunsky, I., Sandoval, L., Ojeda, V. et al. (2008). Cavity nesting birds in neotropical forests: cavities as a potentially limiting resource. Ornitologia Neotropical, 19, 253–268.
de la Parra-Martínez, S. M., Renton, K., Salinas-Melgoza, A., & Muñoz-Lacy, L. G. (2015). Tree-cavity availability and selection by a large-bodied secondary cavity-nester: the Military Macaw. Journal of Ornithology, 156, 489–498. https://doi.org/10.1007/s10336-014-1150-9
Egan, S. (2020). Balsas Screech-Owl (Megascops seductus), version 1.0. In T. S. Schulenberg (Ed.). Birds of the World. Cornell Lab of Ornithology. https://doi.org/10.2173/bow.basowl.01
Enkerlin-Hoeflich, E. C. (1995). Comparative ecology and reproductive biology of three species of Amazona parrots in northeastern Mexico (PhD. Thesis). Texas A&M University, College Station, USA.
Enríquez, P. L., & Cheng, K. M. (2008). Natural History of the Threatened Bearded Screech-Owl (Megascops barbarus) in Chiapas, Mexico. Journal of Raptor Research, 42, 180–187. https://doi.org/10.3356/JRR-07-30.1
Gehlbach, F. R., & Stoleson, S. H. (2010). Western Screech-Owl (Megascops kennicottii). In J. L. Cartron (Ed.), Raptors of New Mexico (pp. 511–523). Albuquerque: University of New Mexico Press.
Gerhardt, R. P., Bonilla-González, N., McAnnis-Gerhardt, D., & Flatten, C. J. (1994). Breeding biology and home range of two ciccaba owls. The Wilson Bulletin, 106, 629–639.
Gibbs, J. P., Hunter, M. L. Jr., & Melvin, S. M. (1993). Snag availability and communities of cavity nesting birds in tropical versus temperate forests. Biotropica, 25, 236–241. https://doi:10.2307/2389188
Gill, F., Donsker, D., & Rasmussen, P. (Eds). (2020). Master list. IOC World Bird List (v10.2). Available: http://www.worldbirdnames.org/
Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4, 4A.
Hendricks, P., McAuliffe, J. R., & Valiente-Banuet, A. (1990). On communal roosting and associated winter social behavior of Gray-breasted Woodpeckers. The Condor, 92, 254–255. https://doi.org/10.2307/1368413
Holdridge, L. R. (1967). Life zone Ecology. San José, Costa Rica: Tropical Science Center.
Jiménez-Arcos, V. H., Santacruz-Padilla, S. A., Escalona-López, A., Arizmendi-Arriaga, M. C., & Vázquez-López, L. D. (2012). Ampliación de la distribución y presencia de una colonia reproductiva de la guacamaya verde (Ara militaris) en el alto Balsas de Guerrero, México. Revista Mexicana de Biodiversidad, 83, 864–867. https://doi.org/10.7550/rmb.27460
Leonard, D. L. Jr. (2000). Breeding and life history observations of the Gray-breasted Woodpecker (Melanerpes hypopolius). Ornitologia Neotropical, 11, 341–348.
Martínez, G. M., Valencia, A. S., & Calónico, S. J. (1997). Flora de Papalutla, Guerrero y de sus alrededores. Anales del Instituto de Biología, Universidad Nacional Autónoma de México, Serie Botánica, 68, 107–133.
Meza, L., & López-García, J. (1997). Vegetación y mesoclima de Guerrero. Estudios Florísticos en Guerrero. No. 1. México D.F.: Facultad de Ciencias, UNAM.
Monterrubio-Rico, T. C., & Escalante-Pliego, P. (2006). Richness, distribution and conservation status of cavity nesting birds in Mexico. Biological Conservation, 128, 67–78. https://doi.org/10.1016/j.biocon.2005.09.017
Navarro-Sigüenza, A. G., Rebón-Gallardo, M. F., Gordillo-Martínez, A., Peterson A. T., Berlanga-García, H., & Sánchez-González, L. A. (2014). Biodiversidad de aves en México. Revista Mexicana de Biodiversidad, 85 (Supl.), S476–S495. https://doi.org/10.7550/rmb.41882
Newton, I. (1994). The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biological Conservation, 70, 265–276. https://doi.org/10.1016/0006-3207(94)90172-4
Pennington, T. D., & Sarukhán, J. (2005). Árboles tropicales de México. Manual de identificación de las principales especies, 3th Ed. Ciudad de México: UNAM/ Fondo de Cultura Económica.
Pineda-Herrera, E., Pérez-Olvera, C. P., Dávalos-Sotelo, R., & Valdez-Hernández, J. I. (2012). Características tecnológicas de la madera de dos especies de Costa Grande, Guerrero, México. Madera y Bosques, 18, 53–71. https://doi.org/10.21829/myb.2012.183358
Politi, N., Hunter, M., & Rivera, L. (2010). Availability of cavities for avian cavity nesters in selectively logged subtropical montane forest of the Andes. Forest Ecology and Management, 260, 893–906. https://doi.org/10.1016/j.foreco.2010.06.009
Renton, K., & Brightsmith, D. (2009). Cavity use and reproductive success of nesting macaws in lowland forest of southeast Peru. Journal of Field Ornithology, 80, 1–8. https://doi.org/10.1111/j.1557-9263.2009.00198.x
Renton, K., Salinas-Melgoza, A., Rueda-Hernández, R., & Vázquez-Reyes, L. D. (2018). Differential resilience to extreme climate events of tree phenology and cavity resources in tropical dry forest: Cascading effects on a threatened species. Forest Ecology and Management, 426, 164–175. https://doi.org/10.1016/j.foreco.2017.10.012
Rivera-Ortíz, F. A., Oyama, K., Villar-Rodríguez, C. L., Contreras-González, A. M., & Arizmendi, M. C. (2016). The use of tree cavities and cliffs by the Military Macaw (Ara militaris) in Salazares Nayarit, Mexico. Revista Mexicana de Biodiversidad, 87, 540–544. https://doi.org/10.1016/j.rmb.2016.02.002
Rzedowski, J. (2006). Vegetación de México. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Available: https://www.biodiversidad.gob.mx/publicaciones/librosDig/pdf/VegetacionMx_Cont.pdf
Salinas-Melgoza, A., Salinas-Melgoza, V., & Renton, K. (2009). Factors influencing nest spacing of a secondary cavity nesting parrot: Habitat heterogeneity and proximity of conespecifics. The Condor, 111, 305–313. https://doi.org/10.1525/cond.2009.090017
Sánchez-Azofeifa, G. A., & Portillo-Quintero, C. (2011). Extent and drivers of change of neotropical seasonally dry tropical forests. In R. Dirzo, H. S. Young, H. A. Mooney, & G. Ceballos (Eds.), Seasonally dry tropical forests: ecology and conservation (pp. 45–58). Washington, DC: Island Press.
Sánchez-Colón, S., Flores-Martínez, A., Cruz-Leyva, I. A., & Velázquez, A. (2009). Estado y transformación de los ecosistemas terrestres por causas humanas. In J. Sarukhán (Coord.), Capital natural de México, vol. II. Estado de conservación y tendencias de cambio (pp. 75–129). México D.F.: Conabio.
Sandoval, L., & Barrantes, G. (2009). Relationship between species richness of excavator birds and cavity adopters in seven tropical forests in Costa Rica. The Wilson Journal of Ornithology, 121, 75 – 81. https://doi.org/10.1676/07-165.1
Saunders, D. A., Smith, G. T., & Rowley, I. (1982). The availability and dimensions of tree hollows that provide nest sites for cockatoos (Psittaciformes) in Western Australia. Australian Wildlife Research, 9, 541–556. https://doi.org/10.1071/WR9820541
Schepps, J., Lohr, S., & Martin, T. (1999). Does tree hardness influence nest-site selection by primary cavity nesters? The Auk, 116, 658–665. https://doi.org/10.2307/4089327
Şekercioğlu, C. H., Wenny, D. G., & Whelan, C.J. (Eds.). (2016). Why birds matter. Avian ecological function and ecosystem services. Chicago: Chicago University Press.
Trejo, I., & Dirzo, R. (2000). Deforestation of seasonally dry tropical forest: a national and local analysis in Mexico. Biological Conservation, 94, 133–142. https://doi.org/10.1016/S0006-3207(99)00188-3
Trejo, I., & Dirzo, R. (2002). Floristic diversity of Mexican seasonally dry tropical forests. Biodiversity and Conservation, 11, 2063–2084. https://doi.org/10.1023/A:1020876316013
van der Hoek, Y., Gaona, G., & Martin, K. (2017). The diversity, distribution and conservation status of the tree-cavity nesting birds of the world. Diversity and Distributions, 23, 1120 – 1131. https://doi.org/10.1111/ddi.12601
Vázquez, L. D., & Renton, K. (2015). High density of tree-cavities and snags in Tropical dry forest of western Mexico raises questions for a latitudinal gradient. Plos One, 10, e0116745. https://doi.org/10.1371/journal.pone.0116745
Vázquez-Reyes, L. D., Arizmendi, M. C., Godínez-Álvarez, H. O., & Navarro-Sigüenza, A. G. (2017). Directional effects of biotic homogenization of bird communities in Mexican seasonal forests. The Condor, 119, 275–288. https://doi.org/10.1650/CONDOR-16-116.1
Vázquez-Reyes, L. D., Jiménez-Arcos, V. H., Santa Cruz-Padilla, S. A., García-Aguilera, R., Aguirre-Romero, A., Arizmendi, M. C. et al. (2018). Aves del Alto Balsas de Guerrero: diversidad e Identidad de una región prioritaria para la conservación. Revista Mexicana de Biodiversidad, 89, 873–897. https://doi.org/10.22201/ib.20078706e.2018.3.2314
Zwartjes, P. W., & Nordell, S. E. (1998). Patterns of cavity-entrance orientation by Gilded Flickers (Colaptes chrysoides) in Cardón Cactus. The Auk, 115, 119–126. https://doi.org/10.2307/4089117




