Patterns of vegetation along contrasting elevation gradients in Oaxaca and Veracruz, Mexico
Silvia H. Salas-Morales a, Guadalupe Williams-Linera b, *
a Sociedad para el Estudio de los Recursos Bióticos de Oaxaca, A.C., Camino Nacional 80, 71246 San Sebastián Tutla, Oaxaca, Mexico
b Instituto de Ecología, A.C., Carretera Antigua a Coatepec 351, El Haya, 91073 Xalapa, Veracruz, Mexico
*Corresponding author: guadalupe.williams@inecol.mx (G. Williams-Linera)
Abstract
Elevation gradients have been widely documented, but few studies have compared patterns of variation between contrasting transects. Our objective was to compare vegetation structure and tree species composition of forest communities on 2 extended gradients located along the Pacific coast (Oaxaca, 0-3,600 m), and the Gulf of Mexico coast (Veracruz, 70-4,000 m), Mexico. We established 21 one-ha plots on each gradient. A total of 4,229 trees were measured and identified. Results showed that with increased elevation, basal area decreased unimodally in Oaxaca, and increased monotonically in Veracruz, whereas taxa richness decreased non-linearly in both gradients. Oaxaca was warmer and drier than Veracruz, however, richness was higher in Oaxaca (260 species) than in Veracruz (210 species). A multinomial classification model identified 58 species as Oaxaca specialist and 41 as Veracruz specialists, but only 12 species were generalist in both gradients. Canonical correspondence analyses for species, genus, and family consistently separated dry forests related to temperature and potential evapotranspiration from high elevation conifer forests. Mid-elevation montane forest differed between gradients. We conclude that climate is differentially important in vegetation structure and taxa distribution, but geographical location and disturbance history should be discussed for each gradient.
Keywords: Disturbance history; Multinomial classification model; Oaxaca; Precipitation; Species richness; Temperature; Vegetation structure; Veracruz
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. Este es un artículo Open Access bajo la licencia CC BY-NC-ND
Patrones de vegetación en gradientes altitudinales contrastantes en Oaxaca y Veracruz, México
Resumen
Los gradientes altitudinales han sido ampliamente documentados, pero pocos estudios han comparado patrones de variación entre transectos contrastantes. El objetivo fue comparar la estructura de la vegetación y diversidad de especies de árboles en 2 gradientes extensos ubicados en las costas del Pacífico (Oaxaca, 0-3,600 m) y golfo de México (Veracruz, 70-4,000 m). Se establecieron 21 parcelas de 1 ha en cada gradiente y se midieron e identificaron un total de 4,229 árboles. Al aumentar la elevación, el área basal disminuyó unimodalmente en Oaxaca y aumentó monotónicamente en Veracruz, mientras que la riqueza disminuyó en ambos gradientes. Oaxaca es más cálido y seco que Veracruz, sin embargo, la riqueza es mayor en Oaxaca (260 especies) que en Veracruz (210 especies). El modelo de clasificación multinomial reveló 58 especies como especialistas de Oaxaca y 41 como especialistas de Veracruz, pero solo 12 generalistas para ambos gradientes. Los análisis de correspondencia canónica separaron consistentemente selvas secas relacionadas con temperatura y evapotranspiración potencial de bosques de coníferas, pero los bosques mesófilos difieren entre gradientes. En conclusión, el clima es diferencialmente importante en la estructura vegetal y distribución de taxones, pero debe discutirse la ubicación geográfica y la historia de perturbación de los gradientes.
Palabras clave: Historia de perturbación; Modelo de clasificación multinomial; Oaxaca; Precipitación; Riqueza de especies; Temperatura; Estructura de la vegetación; Veracruz
© 2019 Universidad Nacional Autónoma de México, Instituto de Biología. This is an open access article under the CC BY-NC-ND license
Introduction
One of the fundamental challenges of ecology is to determine which factors influence the distribution of organisms on Earth (Sanders & Rahbek, 2012). Gradients are the most commonly utilized tool for analyzing the response of the biota to environmental change, and elevation gradients are well-studied systems, since there are diverse life zones along these gradients, with particular and diverse collections of organisms, where different types of vegetation can be observed. Due to strong climatic variation over short distances, biotic zones and vegetation types are both compressed into a small area (Liao et al., 2014).
Biodiversity along elevation gradients shows variation in patterns depending on the group under study and the geographic location of the gradient itself (Grytnes & Beaman, 2006). Among the diverse patterns of elevation variation, the unimodal pattern is the most common (Rahbek, 1995) although there are many diverse responses of the biota to environmental changes over elevation gradients. For example, in a study on Mt. Rinjani, Lombok, Indonesia, it was determined that the alpha diversity of understory, low-canopy and canopy plants decreases with increasing elevation, while that of the creeping plants shows a unimodal pattern (Dossa et al., 2013). Along a 2,700 m elevation transect in Costa Rica, the maximum diversity of woody species was found at 400-600 m elevation (Clark et al., 2015) whereas in Nepal, maximum diversity of tropical genera was found below the midpoint of the elevation gradient, and the diversity of temperate genera presented a unimodal pattern (Li & Feng, 2015).
The range of patterns of elevation variation challenges to propose a general model; the variation has been attributed to the use of different sampling methods (Nogués-Bravo et al., 2008), analysis of incomplete elevation gradients (Grytnes & Vetaas, 2002) and the effect of scale (Rahbek, 2005). To obtain a general explanation of the underlying causes of the patterns of elevation variation, it is advisable to adopt similar sampling methods, standardize both the area sampled and the monitoring of environmental data and include complete elevation gradients (Lomolino, 2001). Studies based on only part of a gradient face an important limitation and, their results can only apply to that part of the gradient (Grytnes & Vetaas, 2002). Frequently, the lowest and highest parts of mountains have been severely disturbed by human activities (Nogués-Bravo et al., 2008), such that the native vegetation has largely been altered and replaced by other land uses (Arévalo et al., 2010; Da et al., 2009; González-Abraham et al., 2015; Piperno, 2006).
Diverse studies have documented patterns of elevation variation of species richness, diversity and environmental factors (e.g., Salas-Morales & Meave, 2012; Sanders & Rahbek, 2012; Toledo-Garibaldi & Williams-Linera, 2014). Several hypotheses have been proposed to explain which factors underlie the elevation variation of organisms. The hypotheses include area, biogeographic interpretations, climate, environmental heterogeneity, geological and climatic history, geometric restrictions, productivity, and soil characteristics (Colwell & Lees, 2000; Hawkins et al., 2003; Kitayama & Aiba, 2002; Latham & Ricklefs, 1993; Li & Feng, 2015; Rowe, 2009; Sanders, 2002; Wang et al., 2009). More recently, elevation gradients are central to study plant and animal responses in the face of global climate change since some species could potentially migrate upslope, but others will go extinct under most projections of global temperature increases (Clark et al., 2015; Colwell et al., 2008; Feeley et al., 2013).
Few studies have compared patterns of change over several elevation transects. Sanders (2002) analyzed ant species richness along elevation transects in 3 states in the USA: Colorado, Nevada and Utah. Grytnes (2003) compared 7 transects in Norway in order to determine patterns of elevation richness variation in vascular plants. Rowe (2009) studied patterns of richness of non-flying mammals over 4 elevation gradients located close together in North America. In northeast China, Wang et al. (2009) analyzed regional patterns of forest plant species on 6 elevation gradients, and Kessler et al. (2011) determined patterns of elevation variation in ferns over 20 gradients located at diverse sites around the world. To the best of our knowledge, there is no study yet comparing extended gradients facing 2 different oceans.
Mexico is a land of mountains flanked by the Pacific and Atlantic Oceans on the western and eastern sides, respectively, and thus offers a great opportunity to compare gradients in the Neotropics. The objective of this study was to contrast the variation in vegetation structure and characteristics of the arboreal component of 2 extended and environmentally distinct elevation gradients. We hypothesized that if precipitation, air temperature and potential evapotranspiration (PET) vary over elevation gradients then differential patterns in vegetation structure and tree species composition would relate to different climatic variables. Alternative explanations would be related to mountain range location and disturbance history.
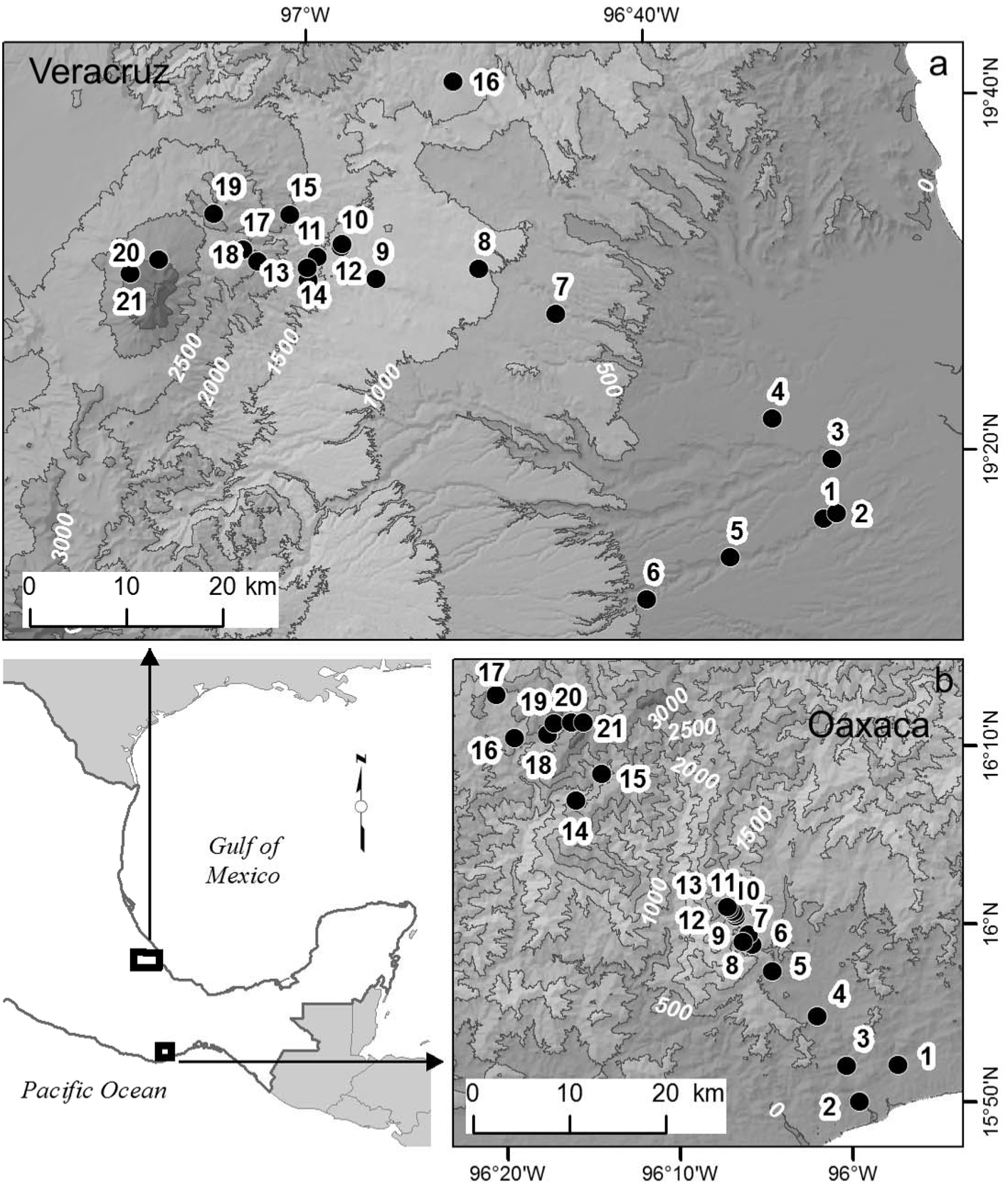
Materials and methods
We studied a Pacific coast elevation gradient in the state of Oaxaca, and a Gulf of Mexico coast gradient in the state of Veracruz, both in Mexico (Fig. 1). In both gradients, selected sites were distributed along the entire elevation gradients, had relatively little disturbance, and field work was conducted during the same years, 2010 and 2011. The Oaxaca elevation gradient is located on the southern slope of the Sierra Madre del Sur and vegetation is well conserved; 21 sites were located from 70 to 3,600 m elevation at the summit. The climate is sub-humid with a marked rainy season in the summer months. Mean annual temperature decreases from 27 °C at the lowest to 9 °C at the highest sites, total annual precipitation varies from 437 mm at the lowest site to 1,632 mm at mid-elevations (Salas-Morales & Meave, 2012). The Veracruz elevation gradient is located in the central part of the state; 21 sites were located from 97 m to 4,000 m elevation at the tree line on the Cofre de Perote Volcano. The vegetation along the gradient has historically been disturbed, but well-preserved sites are scattered throughout the landscape. Mean annual temperature decreases from 25 °C at the lowest to 8 °C at the highest sites, total annual precipitation ranges from 932 mm at lower elevations, to ca. 2,000 mm at mid-elevations (Toledo-Garibaldi & Williams-Linera, 2014). Hereafter, the Oaxaca and the Veracruz elevation gradients will be referred as Oaxaca and Veracruz, respectively.
Meteorological stations are scarce along the elevation gradients; however, we used the few that are available to corroborate data obtained from WorldClim (Hijmans et al., 2005) for each study site. We analyzed 8 variables extracted from WorldClim at a 1-km spatial resolution (mean temperature of the warmest and coldest quarter, annual mean temperature, precipitation of the warmest and coldest quarter, precipitation of the wettest and driest quarter and annual precipitation). In addition, we estimated PET as an indicator of dryness where the annual PET exceeds annual precipitation (Harris et al., 2013).
At each site, a 0.1 ha plot was established. In each plot, we counted the number of individuals and identified the species of all trees ≥ 5 cm diameter at 1.3 m in height (dbh). Vouchers were deposited at the SERO herbarium of the Sociedad para el Estudio de los Recursos Bióticos de Oaxaca, and the XAL herbarium of the Instituto de Ecología, A.C.
To identify groups of taxa that are specialists in each gradient, we used a classification model (CLAM; Chazdon et al., 2011). This is a multinomial statistical method that uses the relative abundance of taxa to classify specialists and generalists (Chazdon et al., 2011). We used a K-level of 0.5 for the simple-majority rule or liberal threshold, with a P-level of 0.005 as has been suggested when the objective is whole community analysis (Chao & Lin, 2011; Chazdon et al., 2011). We excluded morphospecies from the classification analysis; for analysis of the species and genera, we also excluded individuals identified to family level.
For each site, we calculated basal area (m2/ha), density (individuals/ha), species, genus, and family richness, and the Shannon diversity index (H´). Differences in climate variables between the gradients were analyzed using analyses of variance. To determine patterns of distribution, vegetation structure, taxa richness, diversity and climatic data, we fitted each variable to linear and polynomial models using generalized linear models. The best model was selected with the Akaike Information Criterion for small sample size, AICc (Burnham & Anderson, 2002). For the number of taxa (counts), we used a Poisson distribution and log link function. Data were analyzed using R project software version 3.4.2 (R Core Team, 2017).
Canonical correspondence analysis (CCA) was used to examine the relationship between plant taxa and climate variables along environmental gradients. The species, genus and family matrices consisted of the number of individuals of each taxa recorded in each of the 42 sites. The environmental data matrix included elevation and 8 climatic variables. Monte Carlo permutation tests were performed to determine whether the observed patterns differed from a random relationship. The forward selection procedure was used to determine the statistical significance of each environmental variable. Analyses were performed with CANOCO software version 4.5 (ter Braak & Šmilauer, 2002).
Results
Mean annual temperature decreased linearly with increasing elevation in both Oaxaca and Veracruz (Fig. 2a, b). However, in Oaxaca, the mean temperature values in both the warmest and coldest quarters were higher than those in Veracruz. The temperature difference between the coldest and the warmest quarters was smaller along the Oaxaca gradient (1.1 to 2.7 °C) than in Veracruz (3.2 to 5.7 °C; F7, 34 = 207.10, p < 0.0001). Mean rainfall presented a unimodal relationship with elevation (Fig. 2c, d). A slight peak was observed between 500 and 1,200 m asl in Oaxaca and between 1,500 and 1,800 m asl in Veracruz. Climatic differences between the gradients were clear for PET below 2,000 m asl in elevation (Fig. 2e, f). These values indicated that Oaxaca is drier than Veracruz.
A total of 4,229 individuals were recorded belonging to 435 species, 212 genera, 85 families and 19 morphospecies on the 2 gradients. Along the Oaxaca gradient, 1,678 individuals were measured and 260 species, 146 genera and 66 families were identified (Appendix). Along the Veracruz gradient, 2,551 trees were measured and 210 species, 124 genera and 63 families were identified (Appendix). The families represented by the highest number of individuals and species were Leguminosae (53 species), Fagaceae (22 species), Euphorbiaceae (21 species), Rubiaceae (21 species), Malvaceae (19 species), Burseraceae (11 species) and Pinaceae (11 species). The genera with the highest number of species were Quercus (21 species), Bursera (10 species) and Pinus (10 species) (Appendix).
Classification of 388 species into groups of gradient specialization by CLAM indicated that, from 31 shared species, only 12 presented a relatively similar abundance in both gradients for classification as generalist (Appendix). The CLAM identified 58 species as Oaxaca specialists, while 41 were identified as Veracruz specialists (Appendix). Classification of 212 genera showed that, from 58 shared genera, 19 were generalist; 43 genera were Oaxaca specialists (e.g., Amphyterygium, Arbutus, Jacquinia, Phenax, Poeppigia) whereas 24 genera were Veracruz specialists (e.g., Fagus, Hedyosmum, Liquidambar, Savia, Turpinia). The classification of 85 families indicated that 16 families were generalists; 22 were Oaxaca specialists and 14 were Veracruz specialists (Table 1).
Basal area showed a unimodal pattern in Oaxaca and a monotonic pattern in Veracruz (Fig. 3a; Table 2). Density of trees showed inverse linear patterns on the studied gradients; in Oaxaca, density decreased with increasing elevation, while in Veracruz density increased with elevation (Fig. 3b; Table 2).
Overall, species, genus and family richness and Shannon diversity index tended to decrease with increasing elevation along Oaxaca and Veracruz (Fig. 3c-f; Table 2). Richness and diversity were higher in Oaxaca than in Veracruz; however, above 1,800-2,000 m elevation, these parameters were similar on both gradients (Fig. 3c-f).
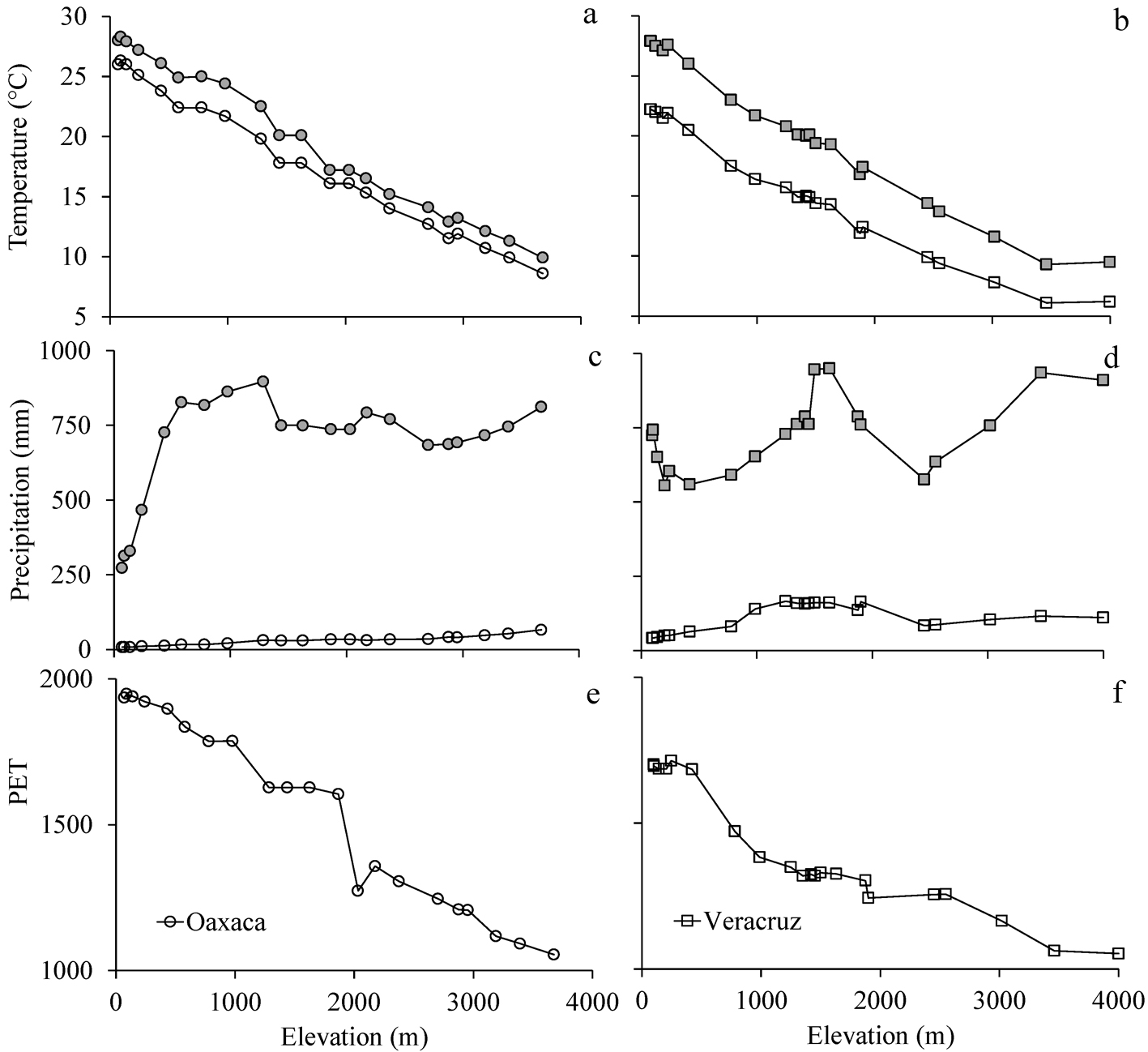
Table 1
Families in the elevational gradients of Oaxaca and Veracruz, Mexico, classified as generalists to both gradients, Oaxaca specialists and Veracruz specialists according to the CLAM analysis. Values are number of individuals recorded along each gradient. Superscripts indicate biogeographical distribution of each family: 1, tropical; 2, temperate; 3, cosmopolitan.
|
Generalist |
Oaxaca Specialist |
Veracruz Specialist |
||||||
|
Family |
Oax |
Ver |
Family |
Oax |
Ver |
Family |
Oax |
Ver |
|
Actinidiaceae3 |
12 |
28 |
Anacardiaceae3 |
62 |
21 |
Betulaceae2 |
26 |
127 |
|
Burseraceae1 |
61 |
111 |
Annonaceae1 |
20 |
6 |
Celastraceae1 |
1 |
11 |
|
Caricaceae1 |
5 |
15 |
Apocynaceae1 |
31 |
13 |
Chloranthaceae1 |
0 |
40 |
|
Lauraceae1 |
11 |
30 |
Araliaceae1 |
24 |
15 |
Clethraceae3 |
13 |
60 |
|
Malvaceae1 |
101 |
84 |
Bignoniaceae1 |
30 |
20 |
Convolvulaceae3 |
0 |
15 |
|
Moraceae1 |
9 |
16 |
Boraginaceae3 |
14 |
3 |
Fagaceae2 |
48 |
392 |
|
Myrsinaceae1 |
5 |
10 |
Clusiaceae1 |
14 |
0 |
Hamamelidaceae2 |
0 |
84 |
|
Myrtaceae3 |
37 |
51 |
Combretaceae1 |
14 |
0 |
Melastomataceae1 |
0 |
19 |
|
Nyctaginaceae1 |
9 |
2 |
Ericaceae3 |
48 |
8 |
Pinaceae2 |
267 |
632 |
|
Polygonaceae3 |
8 |
12 |
Euphorbiaceae3 |
124 |
101 |
Sapindaceae1 |
1 |
16 |
|
Rhamnaceae3 |
2 |
11 |
Hernandiaceae1 |
17 |
4 |
Staphyleaceae2 |
0 |
75 |
|
Rosaceae2 |
10 |
15 |
Julianiaceae1 |
14 |
0 |
Styracaceae2 |
0 |
27 |
|
Rubiaceae1 |
66 |
70 |
Leguminosae3 |
207 |
134 |
Symplocaceae1 |
0 |
11 |
|
Rutaceae1 |
4 |
13 |
Meliaceae1 |
33 |
6 |
Theaceae1 |
0 |
26 |
|
Ulmaceae3 |
9 |
7 |
Myricaceae3 |
16 |
0 |
|||
|
Verbenaceae1 |
8 |
15 |
Oleaceae3 |
17 |
0 |
|||
|
Proteaceae1 |
9 |
0 |
||||||
|
Salicaceae3 |
33 |
6 |
||||||
|
Sapotaceae1 |
18 |
0 |
||||||
|
Simaroubaceae1 |
12 |
1 |
||||||
|
Theophrastaceae1 |
13 |
0 |
||||||
|
|
|
|
Urticaceae1 |
37 |
0 |
|
|
|
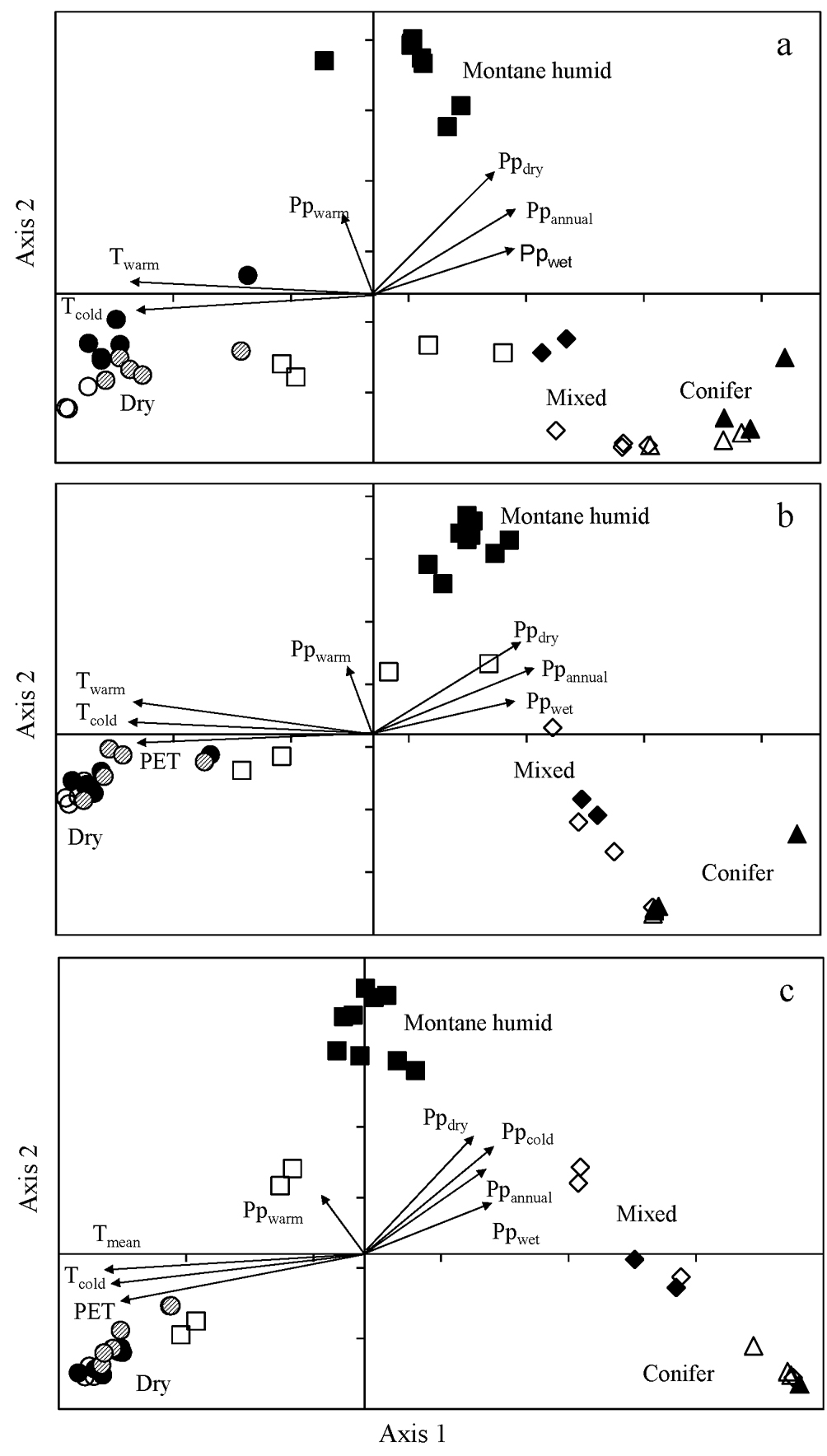
Ordination by CCA of the 42 sites for species, genus and family abundance was significant for the first axis (Monte Carlo test, F = 1.74, 2.52, 5.29, respectively, p = 0.002) and all canonical axes (Monte Carlo test, F = 1.56, 1.95, 2.66, respectively, p = 0.002), showing that the observed patterns differed from a random relationship. For species, the first 2 axes accounted for 12.9 and 12.4% of the cumulative variance, respectively (Fig. 4a). For genus, axis 1 and axis 2 accounted for 18.6% and 16.3%, respectively (Fig. 4b). For family, axis 1 and axis 2 described 30.3% and 24.6% of the cumulative variance, respectively (Fig. 4c). For species, genus and family, the first axis may be interpreted by temperature gradients whereas the second axis was related to precipitation and PET (Fig. 4). The retained significant variables in each CCA are shown in table 3.
In general, the CCA for species, genus and family, consistently separated 3 groups of sites (Fig. 4). The biplots indicated that, on axis 1, pine-oak and coniferous forests in Oaxaca and Veracruz had positive scores while dry forest sites had negative scores. On axis 2, all montane cloud forests of Veracruz had positive scores; however, montane forest sites on Oaxaca gradient had negative scores for species and were separated from the Veracruz group.
Discussion
Differences in climate along the gradients of both, Oaxaca and Veracruz, are related to their geographic location and the meteorological phenomena affecting them (Espinosa et al., 2008). On Veracruz gradient, the frequent presence of mist is attributed to the dominant warm marine current of the Gulf of Mexico, whereas the Oaxaca gradient on the Pacific side is influenced by dry wind and cold marine currents (Espinosa et al., 2008). Moreover, from November to March, cold northerly winds across the Gulf of Mexico, contribute to the lower temperatures reported for the Veracruz gradient, and bring rains and fog during the relative dry season (Holwerda et al., 2010). PET plays an important role in determining community types since same amount of rainfall manifests different in warm than in cold environments. PET values clearly indicated that Oaxaca is drier than Veracruz, but only below 2,000 m elevation.
The CCA results evidenced the relationship between vegetation and climate on these 2 gradients. Several authors have emphasized the importance of climate, not only in large-scale patterns, but also at the local level (Francis & Currie, 2003; Hawkins et al., 2003). The groups of forest types were differentially related to precipitation or temperature variables (Toledo-Garibaldi & Williams-Linera, 2014). On the Oaxaca gradient, temperature was the most important environmental factor (Salas-Morales et al., 2015). In Oaxaca, groups of sites were related to low and high temperatures, separating tropical from temperate vegetation (Salas-Morales & Meave, 2012). Temperature is a variable that is highly associated with altitude and with elevation patterns in floristic and vegetation variation (Grubb, 1977; Sang, 2009). On the Veracruz gradient, 3 groups of sites were distinguished: lowland dry forests and highland temperate forests related to high and low temperatures, respectively, and montane cloud forests related to humidity. These forests are found in particular sites on the mountains of Mexico, in an elevation belt where there is a frequent influence of fog (Holwerda et al., 2010).
Forests on the Oaxaca gradient display lower basal area and density of trees than on Veracruz. Differences become complicated because along the whole gradients, BA and density tend to increase in Veracruz whereas in Oaxaca tend to decrease with elevation. Differences are greater in vegetation structure in the temperate forests of higher elevations, and may be related to humidity, since during the warmest, coldest, and driest quarters, the Veracruz gradient receives twice the precipitation in the highest-altitude sites than Oaxaca. Different patterns of vegetation structure along gradients have been observed in a number of studies, and generality is not expected when comparing tropical elevation transects (Clark et al., 2015). For example, on Mount Kinabalu in Borneo, stem density increased with elevation, and basal area in non-ultrabasic soils increased monotonically, while in the ultrabasic soils presented a unimodal pattern (Aiba & Kitayama, 1999). However, on an elevation gradient on the Barba Volcano in Costa Rica, there were 2 peaks in tree density, at 400 m and 2,800 m elevation, while the basal area varied little along the gradient and was the highest in the 2,800 m plot (Clark et al., 2015).
Variation in elevation patterns is not limited to vegetation structure. Elevation gradients display variation in richness, diversity and taxa composition. For both gradients, richness and diversity decrease with increasing elevation; however, the patterns differ from each other. On the Oaxaca gradient above 1,800 – 2,000 m occurs a rapid decreasing trend in species, genera and family richness and diversity related to the low tolerance of some tropical taxa to relatively cold temperatures (Salas-Morales et al., 2015). While in Oaxaca the elevation pattern seems to be related to a critical elevation, the richness pattern in Veracruz decreased smoothly related to the mixture of temperate and tropical taxa and more humid conditions (Challenger & Soberón, 2008). Likewise, in Eastern Asia forests with tropical and temperate genera are found in similar elevation gradients (Li & Feng, 2015; Liao et al., 2014). Li and Feng (2015) reported that tropical genera are found below and temperate genera above mid-point in Nepal.
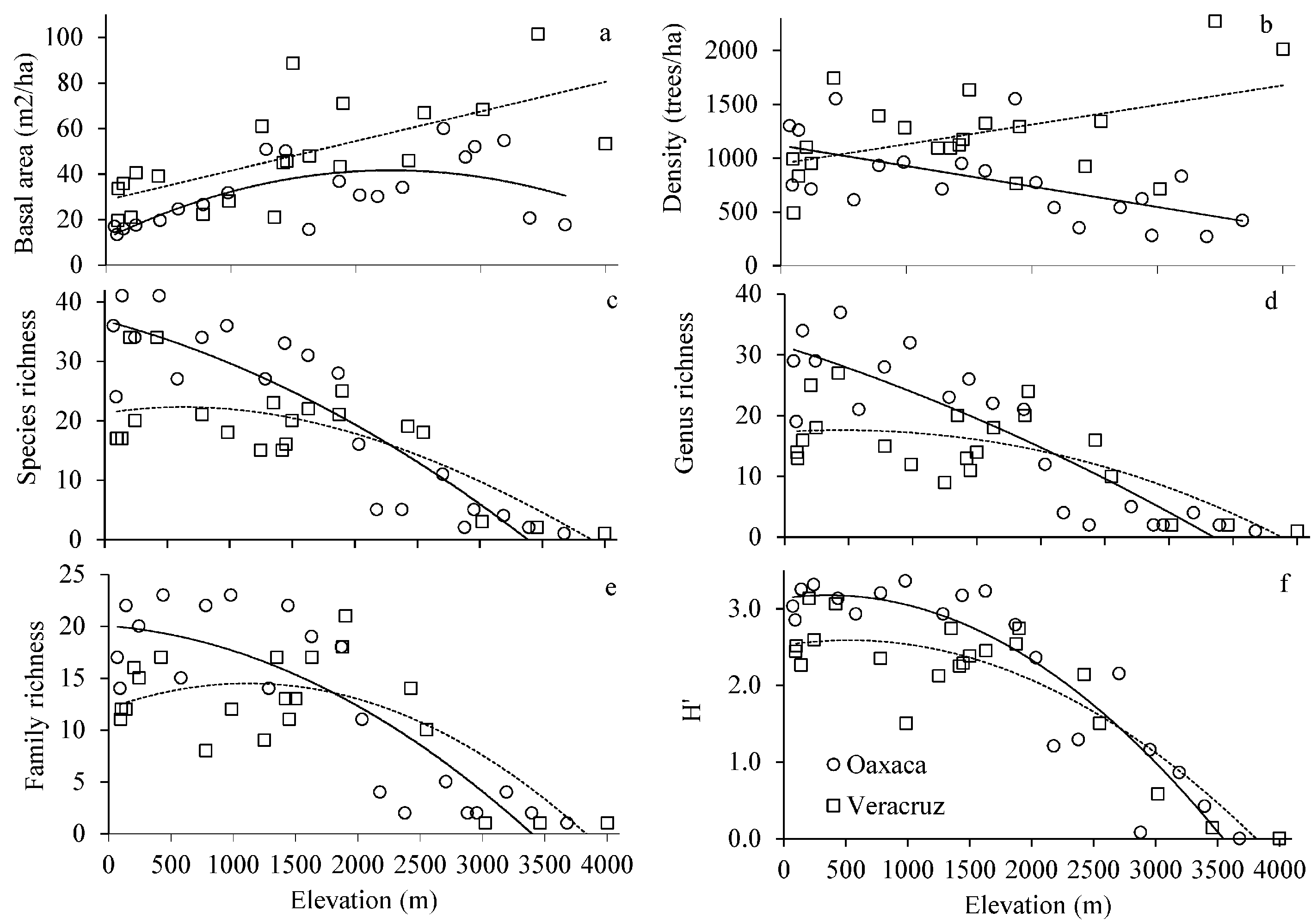
Table 2
Model fitting of species, genus and family richness, Shannon’s diversity index, basal area and density in relation to elevation in Oaxaca and Veracruz, Mexico. Results shown are residual deviance, deviance explained (%), Χ2 and P. AICc is corrected Akaike information criterion and Δi is the AICc difference between the AICc of the best model and that of the model i. Note that models having ΔAICc within 2 of the best model have substantial support and receive consideration (Burnham & Anderson, 2002). Boldface indicates the best model. Model 1 is linear; Model 2 is quadratic, Model 3 is cubic. *p is < 0.05, ** is < 0.01, *** is < 0.001.
|
Oaxaca |
Veracruz |
|||||||||
|
Model |
Residual deviance |
Percentage deviance explained |
Χ2 |
AICc |
Δi |
Residual deviance |
Percentage deviance explained |
Χ2 |
AICc |
Δi |
|
Species richness |
||||||||||
|
1 |
74.9 |
69.84 |
173.45*** |
173.2 |
41.1 |
61.61 |
38.76 |
38.99*** |
161.1 |
25.4 |
|
2 |
31.12 |
87.47 |
217.24*** |
132.1 |
0 |
36.57 |
63.65 |
64.03*** |
138.8 |
3.1 |
|
3 |
31.07 |
87.49 |
217.28*** |
135.2 |
3.1 |
30.32 |
69.86 |
70.27*** |
135.7 |
0 |
|
Genus richness |
||||||||||
|
1 |
63.91 |
71.56 |
160.81*** |
156.7 |
33.2 |
55.72 |
36 |
31.34*** |
150.4 |
19.2 |
|
2 |
27.99 |
87.54 |
196.73*** |
123.5 |
0 |
37 |
57.5 |
50.06*** |
134.4 |
3.2 |
|
3 |
26.43 |
88.24 |
198.28*** |
125.1 |
1.6 |
30.67 |
64.77 |
56.40*** |
131.2 |
0 |
|
Family richness |
||||||||||
|
1 |
50.89 |
62.92 |
86.36*** |
139.6 |
26.4 |
53.02 |
24.17 |
16.90*** |
143.5 |
27.8 |
|
2 |
21.75 |
84.15 |
115.50*** |
113.2 |
0 |
28.08 |
59.84 |
41.84*** |
121.3 |
5.6 |
|
3 |
20.62 |
84.98 |
116.63*** |
115.2 |
2 |
19.4 |
72.25 |
50.52*** |
115.7 |
0 |
|
H’ |
|
|
|
|
|
|
|
|
|
|
|
1 |
6.44 |
76.38 |
30.31*** |
42.2 |
7.3 |
5.45 |
64 |
21.45*** |
38.7 |
7.78 |
|
2 |
3.93 |
85.59 |
40.69*** |
34.9 |
0 |
3.25 |
78.53 |
32.32*** |
30.9 |
0 |
|
3 |
3.41 |
87.5 |
43.69*** |
35.4 |
0.5 |
3.25 |
78.53 |
32.32*** |
34.4 |
3.5 |
|
Basal area |
||||||||||
|
1 |
3413.5 |
24.66 |
5.95* |
173.9 |
3 |
5508.1 |
44.19 |
12.25** |
183 |
0 |
|
2 |
2549.2 |
43.74 |
12.08** |
170.9 |
0 |
5348.6 |
45.81 |
12.86** |
186.4 |
3.4 |
|
3 |
2375.1 |
47.58 |
13.56** |
172.9 |
2 |
4965.2 |
49.69 |
14.43** |
188.4 |
5.4 |
|
Density |
||||||||||
|
1 |
1762528 |
36.61 |
9.57** |
305.1 |
0 |
2805447 |
23.33 |
5.58* |
314.9 |
0.6 |
|
2 |
1735128 |
37.59 |
9.90** |
307.9 |
2.8 |
2655046 |
27.44 |
6.74* |
316.8 |
2.5 |
|
3 |
1728317 |
37.84 |
9.98* |
311.3 |
6.2 |
1997907 |
45.4 |
12.71** |
314.3 |
0 |
Oaxaca was more diverse (260 species) than Veracruz (210 species), which was contrary to expected given that the Veracruz gradient is more humid. In the Neotropics, plant species richness is strongly correlated with total annual precipitation (Gentry, 1988), but the most diverse dry forest are not the wettest ones, but rather the western Mexico dry forests (Gentry, 1995). Thus, the dry forest is more diverse in the Pacific coast than in the Gulf of Mexico, contributing greatly to the high diversity of Oaxaca.
The family with the highest number of individuals for both, Oaxaca and Veracruz, was Pinaceae. In elevations above 2,200 and 2,450 m on the Oaxaca and Veracruz gradients, respectively, the forests are dominated by the genus Pinus, which shares dominance with Quercus in these elevation belts. This result is consistent throughout the mountains of Mexico where forests are dominated by pine-oak and pine forests, and although Mexico has more than 150 species of oaks and more than 40 species of pines (Gernandt & Pérez-de la Rosa, 2014; Valencia, 2004), in each particular site they were represented by a few species (Challenger & Soberón, 2008). Gentry (1988) indicated that Neotropical plant communities are together in nonrandom ways. In Oaxaca and Veracruz, families classified by CLAM as generalists (e.g., Lauraceae, Rubiaceae) were the families that contributed most to species richness in the Neotropics according to Gentry (1988). Oaxaca specialist families identified by CLAM were mainly of tropical affinity (e.g., Euphorbiaceae, Leguminosae), whereas in Veracruz, several of the indicator families were of temperate affinity (e.g., Betulaceae, Fagaceae, Pinaceae; Table 1).
It is likely that difference in temperature and precipitation was not the only factor to affect forest development and composition. Other variables, such as soil characteristics and land use history or legacy play a role, and they are alternative explanations as has been shown in previous studies (Aiba & Kitayama, 1999; Arévalo et al., 2010; Da et al., 2009; Kitayama & Aiba, 2002; Piperno, 2006). Fragmentation and worldwide forest disappearance are mostly due to a long history of human activities (Da et al., 2009; González-Abraham et al., 2015; Piperno, 2006). Veracruz has a long history of land use before and after the arrival of the Spaniards, and the center of Veracruz was intensively used and deforested, since this gradient is located along a major route to Mexico City (González-Abraham et al., 2015). In contrast, up until 50 years ago, the Oaxaca gradient lacked paved roads and the human impact on the vegetation is therefore more recent and confined to lower altitudes (Salas-Morales & Meave, 2012).
Our results support the hypothesis that climate is one of the main underlying factors related to differential patterns in vegetation structure and taxa distribution along elevation gradients. However, climate influence depends on other local factors such as mountain range location, physiography, slope, and disturbance. The results strongly indicate differential influence of climate, since humidity is apparently an important environmental factor for the vegetation of the Gulf of Mexico, while temperature is the determining factor on the Pacific coast. The Oaxaca gradient displayed higher taxa richness than Veracruz gradient, particularly in the lower elevations. In both gradients richness decreases with increased elevation, but in Veracruz there is a smooth transition from tropical to temperate vegetation whereas in Oaxaca richness at mid-elevation shows an abrupt decreased related to temperature.
Acknowledgements
We thank the associate editor and two anonymous reviewers who kindly made suggestions to this manuscript, and to Rosario Landgrave for preparing the map in figure 1. This project was funded by the Mexican Council of Science and Technology through a postdoctoral scholarship to SHSM and a grant ref. CB-2014-01-238831 to GWL.
Table 3
Results of the forward selection procedure to choose climate explanatory variables for CCA analyses of species, genera and family along the elevation gradients of Oaxaca and Veracruz, Mexico. Boldface indicates significant p values.
|
|
Species |
Genus |
Family |
||||||
|
Variable |
λA |
F |
p |
λA |
F |
p |
λA |
F |
p |
|
Elevation |
0.65 |
1.41 |
0.058 |
0.33 |
1.37 |
0.128 |
0.06 |
0.63 |
0.900 |
|
Annual mean temperature |
0.46 |
1.02 |
0.434 |
0.26 |
1.1 |
0.326 |
0.85 |
6.75 |
0.002 |
|
Temperature of warmest quarter |
0.97 |
1.95 |
0.002 |
0.92 |
3.27 |
0.002 |
0.12 |
1.12 |
0.292 |
|
Temperature of coldest quarter |
0.79 |
1.66 |
0.002 |
0.34 |
1.38 |
0.044 |
0.15 |
1.55 |
0.040 |
|
Annual precipitation |
0.75 |
1.58 |
0.002 |
0.52 |
1.98 |
0.002 |
0.27 |
2.42 |
0.002 |
|
Precipitation of wettest quarter |
0.63 |
1.39 |
0.016 |
0.46 |
1.8 |
0.006 |
0.24 |
2.21 |
0.002 |
|
Precipitation of driest quarter |
0.93 |
1.89 |
0.002 |
0.72 |
2.65 |
0.002 |
0.55 |
4.74 |
0.002 |
|
Precipitation of warmest quarter |
0.69 |
1.47 |
0.010 |
0.44 |
1.72 |
0.008 |
0.19 |
1.83 |
0.008 |
|
Precipitation of coldest quarter |
0.51 |
1.14 |
0.288 |
0.35 |
1.43 |
0.102 |
0.17 |
1.68 |
0.048 |
|
Potential evapotranspiration |
0.62 |
1.36 |
0.064 |
0.39 |
1.59 |
0.048 |
0.19 |
1.87 |
0.028 |
Appendix. Tree species and family recorded along the elevation gradients of Oaxaca (OAX) and Veracruz (VER), Mexico. The numbers are species abundance in 21 sites in each gradient. Classification is based on CLAM analysis as OAX specialist, VER specialist, generalist or too rare to classify. Bold type indicates the specialist species in each elevation gradient. Individuals identified to family level and morphospecies were excluded. When more than 1 species was not identified within a genus, they were numbered.
|
Continued |
|||
|
Species |
OAX |
VER |
Classification |
|
ACTINIDIACEAE |
|||
|
Saurauia leucocarpa Schltdl. |
0 |
22 |
VER specialist |
|
Saurauia pedunculata Hook. |
0 |
6 |
Too rare to classify |
|
Saurauia pringlei Rose |
12 |
0 |
OAX specialist |
|
ADOXACEAE |
|||
|
Sambucus nigra L. |
0 |
3 |
Too rare to classify |
|
Viburnum tiliifolium (Oerst.) Hemsl. |
0 |
2 |
Too rare to classify |
|
ALTINGIACEAE |
|||
|
Liquidambar styraciflua L. |
0 |
84 |
VER specialist |
|
ANACARDIACEAE |
|||
|
Amphipterygium adstringens (Schltdl.) Standl. |
14 |
0 |
OAX specialist |
|
Astronium graveolens Jacq. |
13 |
0 |
OAX specialist |
|
Comocladia engleriana Loes. |
45 |
16 |
OAX specialist |
|
Spondias mombin L. |
0 |
2 |
Too rare to classify |
|
Spondias purpurea L. |
4 |
0 |
Too rare to classify |
|
Spondias sp. |
0 |
2 |
Too rare to classify |
|
Tapirira mexicana Marchand |
0 |
1 |
Too rare to classify |
|
ANNONACEAE |
|||
|
Annona cherimola Mill. |
0 |
4 |
Too rare to classify |
|
Annona squamosa L. |
2 |
0 |
Too rare to classify |
|
Annona sp. |
6 |
0 |
Too rare to classify |
|
Sapranthus microcarpus (Donn. Sm.) R.E. Fr. |
0 |
1 |
Too rare to classify |
|
APOCYNACEAE |
|||
|
Tonduzia longifolia (A. DC.) Markgr. |
9 |
0 |
OAX specialist |
|
Plumeria rubra L. |
8 |
1 |
OAX specialist |
|
Stemmadenia obovata K. Schum. |
2 |
11 |
Generalist |
|
Tabernaemontana litoralis Kunth |
2 |
0 |
Too rare to classify |
|
Thevetia ovata (Cav.) A. DC. |
10 |
0 |
OAX specialist |
|
Thevetia sp. |
0 |
1 |
Too rare to classify |
|
AQUIFOLIACEAE |
|||
|
Ilex discolor Hemsl. |
0 |
9 |
Too rare to classify |
|
Ilex sp. |
0 |
1 |
Too rare to classify |
|
ARALIACEAE |
|||
|
Dendropanax arboreus (L.) Decne. & Planch. |
1 |
0 |
Too rare to classify |
|
Dendropanax sp. |
0 |
2 |
Too rare to classify |
|
Oreopanax xalapensis (Kunth) Decne. & Planch. |
15 |
13 |
Generalist |
|
ARECACEAE |
|||
|
Acrocomia aculeata (Jacq.) Lodd. ex Mart. |
3 |
0 |
Too rare to classify |
|
ASPARAGACEAE |
|||
|
Nolina longifolia (Karw. ex Schult. f.) Hemsl. |
3 |
0 |
Too rare to classify |
|
Yucca elephantipes Regel |
0 |
2 |
Too rare to classify |
|
ASTERACEAE |
|||
|
Critonia sp. |
1 |
0 |
Too rare to classify |
|
Koanophyllon pittieri (Klatt) R.M. King & H. Rob. |
0 |
3 |
Too rare to classify |
|
Verbesina olivacea Klatt |
0 |
1 |
Too rare to classify |
|
Verbesina sp. |
0 |
1 |
Too rare to classify |
|
Vernonia sp. |
3 |
0 |
Too rare to classify |
|
BETULACEAE |
|||
|
Alnus acuminata Kunth |
0 |
1 |
Too rare to classify |
|
Alnus jorullensis Kunth |
26 |
0 |
OAX specialist |
|
Carpinus tropicalis (Donn. Sm.) Lundell |
0 |
126 |
VER specialist |
|
BIGNONIACEAE |
|||
|
Parmentiera aculeata (Kunth) Seem. |
1 |
0 |
Too rare to classify |
|
Handroanthus chrysanthus (Jacq.) S.O. Grose |
6 |
20 |
Generalist |
|
Handroanthus impetiginosus (Mart. ex DC.) Mattos |
19 |
0 |
OAX specialist |
|
Tecoma stans (L.) Juss. ex Kunth |
4 |
0 |
Too rare to classify |
|
BIXACEAE |
|||
|
Cochlospermum vitifolium (Willd.) Spreng. |
4 |
6 |
Too rare to classify |
|
BORAGINACEAE |
|||
|
Bourreria aff. purpusii Brandegee |
4 |
0 |
Too rare to classify |
|
Cordia alliodora (Ruiz & Pav.) Oken |
7 |
3 |
Generalist |
|
Cordia tinifolia Willd. ex Roem. & Schult. |
3 |
0 |
Too rare to classify |
|
BRUNELLIACEAE |
|||
|
Brunellia mexicana Standl. |
0 |
1 |
Too rare to classify |
|
BUDDLEJACEAE |
|||
|
Buddleja sp. |
1 |
0 |
Too rare to classify |
|
BURSERACEAE |
|||
|
Bursera aff. cinerea Engl. |
1 |
0 |
Too rare to classify |
|
Bursera aff. grandifolia (Schltdl.) Engl. |
8 |
0 |
OAX specialist |
|
Bursera aff. simaruba (L.) Sarg. |
3 |
0 |
Too rare to classify |
|
Bursera cinerea Engl. |
0 |
28 |
VER specialist |
|
Bursera excelsa (Kunth) Engl. |
8 |
0 |
OAX specialist |
|
Bursera fagaroides (Kunth) Engl. |
0 |
14 |
VER specialist |
|
Bursera graveolens (Kunth) Triana & Planch. |
6 |
10 |
Generalist |
|
Bursera heteresthes Bullock |
7 |
0 |
Too rare to classify |
|
Bursera simaruba (L.) Sarg. |
27 |
58 |
Generalist |
|
Bursera sp. |
1 |
0 |
Too rare to classify |
|
Protium copal (Schltdl. & Cham.) Engl. |
0 |
1 |
Too rare to classify |
|
CANNABACEAE |
|||
|
Celtis caudata Planch. |
1 |
5 |
Too rare to classify |
|
Trema micrantha (L.) Blume |
0 |
2 |
Too rare to classify |
|
CAPPARACEAE |
|||
|
Quadrella incana (Kunth) Iltis & Cornejo |
1 |
1 |
Too rare to classify |
|
Quadrella indica (L.) Iltis & Cornejo |
2 |
0 |
Too rare to classify |
|
CARICACEAE |
|||
|
Jacaratia mexicana A. DC. |
5 |
15 |
Generalist |
|
CELASTRACEAE |
|||
|
Euonymus mexicanus Benth. |
0 |
1 |
Too rare to classify |
|
CHLORANTHACEAE |
|||
|
Hedyosmum mexicanum C. Cordem. |
0 |
40 |
VER specialist |
|
CLETHRACEAE |
|||
|
Clethra lanata M. Martens & Galeotti |
13 |
0 |
OAX specialist |
|
Clethra macrophylla M. Martens & Galeotti |
0 |
60 |
VER specialist |
|
CLUSIACEAE |
|||
|
Clusia salvinii Donn. Sm. |
14 |
0 |
OAX specialist |
|
COMBRETACEAE |
|||
|
Bucida macrostachya Standl. |
14 |
0 |
OAX specialist |
|
CONVOLVULACEAE |
|||
|
Ipomoea wolcottiana Rose |
0 |
15 |
VER specialist |
|
CORNACEAE |
|||
|
Cornus excelsa Kunth |
0 |
2 |
Too rare to classify |
|
CUNONIACEAE |
|||
|
Weinmannia pinnata L. |
0 |
6 |
Too rare to classify |
|
CUPRESSACEAE |
|||
|
Cupressus lusitanica Mill. |
0 |
5 |
Too rare to classify |
|
DIPENTODONTACEAE |
|||
|
Perrottetia ovata Hemsl. |
0 |
1 |
Too rare to classify |
|
Wimmeria sp. |
1 |
0 |
Too rare to classify |
|
Zinowiewia sp. 1 |
0 |
2 |
Too rare to classify |
|
Zinowiewia sp. 2 |
0 |
7 |
Too rare to classify |
|
EBENACEAE |
|||
|
Diospyros salicifolia Humb. & Bonpl. ex Willd. |
1 |
0 |
Too rare to classify |
|
ERICACEAE |
|||
|
Arbutus sp. |
1 |
0 |
Too rare to classify |
|
Arbutus xalapensis Kunth |
47 |
0 |
OAX specialist |
|
Gaultheria acuminata Schltdl. & Cham. |
0 |
1 |
Too rare to classify |
|
Vaccinium leucanthum Schltdl. |
0 |
8 |
Too rare to classify |
|
ERYTHROXYLACEAE |
|||
|
Erythroxylum havanense Jacq. |
3 |
0 |
Too rare to classify |
|
Erythroxylum pallidum Rose |
1 |
0 |
Too rare to classify |
|
EUPHORBIACEAE |
|||
|
Acalypha adenostachya Müll. Arg. |
0 |
7 |
Too rare to classify |
|
Alchornea latifolia Sw. |
0 |
7 |
Too rare to classify |
|
Bernardia mexicana (Hook. & Arn.) Müll. Arg. |
0 |
4 |
Too rare to classify |
|
Cnidoscolus spinosus Lundell |
0 |
16 |
VER specialist |
|
Cnidoscolus tubulosus (Müll. Arg.) I.M. Johnst. |
37 |
0 |
OAX specialist |
|
Cnidoscolus multilobus (Pax) I.M. Johnst. |
0 |
3 |
Too rare to classify |
|
Cnidoscolus sp. |
0 |
2 |
Too rare to classify |
|
Croton cortesianus Kunth |
0 |
4 |
Too rare to classify |
|
Croton draco Schltdl. & Cham. |
2 |
0 |
Too rare to classify |
|
Croton reflexifolius Kunth |
0 |
15 |
VER specialist |
|
Croton septemnervius McVaugh |
41 |
0 |
OAX specialist |
|
Croton sp. |
1 |
0 |
Too rare to classify |
|
Drypetes sp. |
6 |
0 |
Too rare to classify |
|
Euphorbia calcarata (Schltdl.) V.W. Steinm. |
1 |
3 |
Too rare to classify |
|
Euphorbia pulcherrima Willd. ex Klotzsch |
2 |
0 |
Too rare to classify |
|
Euphorbia schlechtendalii Boiss. |
0 |
5 |
Too rare to classify |
|
Gymnanthes longipes Müll. Arg. |
0 |
4 |
Too rare to classify |
|
Gymnanthes sp. |
0 |
1 |
Too rare to classify |
|
Jatropha malacophylla Standl. |
12 |
0 |
OAX specialist |
|
Jatropha sympetala S.F. Blake & Standl. |
2 |
0 |
Too rare to classify |
|
Sapium glandulosum (L.) Morong |
16 |
0 |
OAX specialist |
|
Sebastiania pavonia (Müll. Arg.) Müll. Arg. |
4 |
0 |
Too rare to classify |
|
FAGACEAE |
|||
|
Fagus grandifolia Ehrh. |
0 |
52 |
VER specialist |
|
Quercus acherdophylla Trel. |
0 |
1 |
Too rare to classify |
|
Quercus acutifolia Née |
0 |
4 |
Too rare to classify |
|
Quercus candicans Née |
8 |
0 |
OAX specialist |
|
Quercus corrugata Hook. |
0 |
15 |
VER specialist |
|
Quercus cortesii Liebm. |
0 |
17 |
VER specialist |
|
Quercus crassifolia Bonpl. |
0 |
5 |
Too rare to classify |
|
Quercus delgadoana S. Valencia, Nixon & L.M. Kelly |
0 |
43 |
VER specialist |
|
Quercus germana Schltdl. & Cham. |
0 |
29 |
VER specialist |
|
Quercus glabrescens Benth. |
0 |
12 |
VER specialist |
|
Quercus lancifolia Schltdl. & Cham. |
0 |
61 |
VER specialist |
|
Quercus laurina Bonpl. |
11 |
0 |
OAX specialist |
|
Quercus peduncularis Née |
2 |
0 |
Too rare to classify |
|
Quercus pinnativenulosa C.H. Mull. |
0 |
2 |
Too rare to classify |
|
Quercus rugosa Née |
14 |
0 |
OAX specialist |
|
Quercus sapotifolia Liebm. |
0 |
84 |
VER specialist |
|
Quercus sartorii Liebm. |
0 |
17 |
VER specialist |
|
Quercus xalapensis Bonpl. |
0 |
48 |
VER specialist |
|
Quercus sp. 1 |
8 |
0 |
OAX specialist |
|
Quercus sp. 2 |
0 |
2 |
Too rare to classify |
|
Quercus sp. 3 |
4 |
0 |
Too rare to classify |
|
Quercus sp. 4 |
1 |
0 |
Too rare to classify |
|
HERNANDIACEAE |
|||
|
Gyrocarpus americanus Jacq. |
0 |
4 |
Too rare to classify |
|
Gyrocarpus mocinnoi Espejo |
17 |
0 |
OAX specialist |
|
LAMIACEAE |
|||
|
Vitex hemsleyi Briq. |
8 |
0 |
OAX specialist |
|
LAURACEAE |
|||
|
Beilschmiedia mexicana (Mez) Kosterm. |
0 |
3 |
Too rare to classify |
|
Cinnamomun effusum (Meisn.) Kosterm. |
0 |
12 |
VER specialist |
|
Cinnamomum triplinerve (Ruiz & Pav.) Kosterm. |
1 |
0 |
Too rare to classify |
|
Licaria misantlae (Brandegee) Kosterm. |
0 |
5 |
Too rare to classify |
|
Litsea glaucescens Kunth |
2 |
1 |
Too rare to classify |
|
Nectandra salicifolia (Kunth) Nees |
4 |
1 |
Too rare to classify |
|
Ocotea effusa (Meisn.) Hemsl. |
1 |
0 |
Too rare to classify |
|
Ocotea psychotrioides Kunth |
0 |
5 |
Too rare to classify |
|
Persea americana Mill. |
3 |
3 |
Too rare to classify |
|
LEGUMINOSAE |
|||
|
Acaciella angustissima (Mill.) Britton & Rose |
1 |
0 |
Too rare to classify |
|
Apoplanesia paniculata C. Presl |
1 |
0 |
Too rare to classify |
|
Bauhinia sp. |
0 |
10 |
Too rare to classify |
|
Caesalpinia eriostachys Benth. |
11 |
0 |
OAX specialist |
|
Caesalpinia sp. |
0 |
1 |
Too rare to classify |
|
Calliandra houstoniana (Mill.) Standl. |
1 |
0 |
Too rare to classify |
|
Calliandra rubescens (M. Martens & Galeotti) Standl. |
0 |
1 |
Too rare to classify |
|
Chloroleucon mangense (Jacq.) Britton & Rose |
1 |
0 |
Too rare to classify |
|
Cojoba arborea (L.) Britton & Rose |
5 |
1 |
Too rare to classify |
|
Coulteria platyloba S. Watson |
1 |
0 |
Too rare to classify |
|
Coulteria velutina (Britton & Rose) Standl. |
4 |
0 |
Too rare to classify |
|
Dalbergia granadillo Pittier |
5 |
0 |
Too rare to classify |
|
Diphysa carthagenensis Jacq. |
0 |
9 |
Too rare to classify |
|
Erythrina lanata Rose |
5 |
0 |
Too rare to classify |
|
Erythrina sp. |
0 |
1 |
Too rare to classify |
|
Gliricidia sepium (Jacq.) Kunth ex Walp. |
9 |
12 |
Generalist |
|
Inga oerstediana Benth. ex Seem. |
3 |
0 |
Too rare to classify |
|
Inga paterno Harms |
5 |
0 |
Too rare to classify |
|
Inga punctata Willd. |
16 |
0 |
OAX specialist |
|
Leucaena lanceolata S. Watson |
6 |
20 |
Generalist |
|
Leucaena leucocephala (Lam.) de Wit |
0 |
3 |
Too rare to classify |
|
Lonchocarpus aff. magallanesii M. Sousa |
9 |
0 |
OAX specialist |
|
Lonchocarpus constrictus Pittier |
13 |
0 |
OAX specialist |
|
Lonchocarpus emarginatus Pittier |
8 |
0 |
OAX specialist |
|
Lonchocarpus lanceolatus Benth. |
6 |
0 |
Too rare to classify |
|
Lonchocarpus molinae Standl. & L.O. Williams |
4 |
0 |
Too rare to classify |
|
Lonchocarpus sp. |
2 |
0 |
Too rare to classify |
|
Lysiloma acapulcense (Kunth) Benth. |
0 |
22 |
VER specialist |
|
Lysiloma auritum (Schltdl.) Benth. |
0 |
4 |
Too rare to classify |
|
Lysiloma divaricatum (Jacq.) J.F. Macbr. |
0 |
6 |
Too rare to classify |
|
Lysiloma microphyllum Benth. |
27 |
0 |
OAX specialist |
|
Machaerium biovulatum Micheli |
2 |
0 |
Too rare to classify |
|
Myrospermum frutescens Jacq. |
7 |
0 |
Too rare to classify |
|
Piptadenia obliqua (Pers.) J.F. Macbr. |
3 |
0 |
Too rare to classify |
|
Piscidia piscipula (L.) Sarg. |
0 |
16 |
VER specialist |
|
Poeppigia procera C. Presl |
17 |
0 |
OAX specialist |
|
Pterocarpus rohrii Vahl |
16 |
0 |
OAX specialist |
|
Pterocarpus sp. |
1 |
0 |
Too rare to classify |
|
Senegalia polyphylla DC. |
6 |
0 |
Too rare to classify |
|
Senna atomaria (L.) H.S. Irwin & Barneby |
0 |
6 |
Too rare to classify |
|
Senna pendula (Humb. & Bonpl. ex Willd.) H.S. Irwin &Barneby |
0 |
1 |
Too rare to classify |
|
Senna pallida (Vahl) H.S. Irwin & Barneby |
0 |
1 |
Too rare to classify |
|
Senna skinneri (Benth.) H.S. Irwin & Barneby |
1 |
0 |
Too rare to classify |
|
Senna sp. 1 |
0 |
2 |
Too rare to classify |
|
Senna sp. 2 |
0 |
5 |
Too rare to classify |
|
Styphnolobium conzattii (Standl.) M. Sousa & Rudd |
3 |
0 |
Too rare to classify |
|
Tara cacalaco (Bonpl.) Molinari & Sánchez Och. |
0 |
3 |
Too rare to classify |
|
Vachellia collinsii Saff. |
1 |
0 |
Too rare to classify |
|
Vachellia cornigera (L.) Willd. |
0 |
1 |
Too rare to classify |
|
Vachellia farnesiana (L.) Willd. |
0 |
2 |
Too rare to classify |
|
Vachellia hindsii Benth. |
5 |
0 |
Too rare to classify |
|
Vachellia pennatula (Schltdl. & Cham.) Benth. |
0 |
7 |
Too rare to classify |
|
Zapoteca sp. |
2 |
0 |
Too rare to classify |
|
LYTHRACEAE |
|||
|
Ginoria nudiflora (Hemsl.) Koehne |
2 |
0 |
Too rare to classify |
|
MAGNOLIACEAE |
|||
|
Magnolia schiedeana Schltdl. |
0 |
2 |
Too rare to classify |
|
MALPIGHIACEAE |
|||
|
Bunchosia aff. gracilis Nied. |
3 |
0 |
Too rare to classify |
|
Malpighia glabra L. |
0 |
1 |
Too rare to classify |
|
MALVACEAE |
|||
|
Bernoullia flammea Oliv. |
6 |
0 |
Too rare to classify |
|
Ceiba aesculifolia (Kunth) Britten & Baker f. |
3 |
9 |
Generalist |
|
Gossypium aridum (Rose & Standl.) Skovst. |
1 |
0 |
Too rare to classify |
|
Guazuma ulmifolia Lam. |
10 |
6 |
Generalist |
|
Hampea mexicana Fryxell |
2 |
0 |
Too rare to classify |
|
Heliocarpus americanus L. |
0 |
3 |
Too rare to classify |
|
Heliocarpus donnellsmithii Rose |
0 |
43 |
VER specialist |
|
Heliocarpus terebinthinaceus (DC.) Hochr. |
8 |
0 |
OAX specialist |
|
Heliocarpus sp. 1 |
9 |
0 |
OAX specialist |
|
Heliocarpus sp. 2 |
11 |
0 |
OAX specialist |
|
Heliocarpus sp. 3 |
6 |
0 |
Too rare to classify |
|
Hibiscus purpusii Brandegee |
2 |
0 |
Too rare to classify |
|
Luehea candida (DC.) Mart. |
8 |
22 |
Generalist |
|
Luehea speciosa Willd. |
0 |
1 |
Too rare to classify |
|
Malvaviscus arboreus Cav. |
2 |
0 |
Too rare to classify |
|
Melochia oaxacana Dorr & L.C. Barnett |
3 |
0 |
Too rare to classify |
|
Pseudobombax ellipticum (Kunth) Dugand |
16 |
0 |
OAX specialist |
|
Robinsonella speciosa Fryxell |
1 |
0 |
Too rare to classify |
|
Tilia americana L. |
13 |
0 |
OAX specialist |
|
MELASTOMATACEAE |
|||
|
Conostegia arborea Steud. |
0 |
2 |
Too rare to classify |
|
Miconia glaberrima (Schltdl.) Naudin |
0 |
13 |
VER specialist |
|
Miconia mexicana (Bonpl.) Naudin |
0 |
3 |
Too rare to classify |
|
Miconia oligotricha (DC.) Naudin |
0 |
1 |
Too rare to classify |
|
MELIACEAE |
|||
|
Cedrela oaxacensis C. DC. & Rose |
2 |
0 |
Too rare to classify |
|
Cedrela salvadorensis Standl. |
2 |
0 |
Too rare to classify |
|
Guarea sp. 1 |
1 |
0 |
Too rare to classify |
|
Guarea sp. 2 |
1 |
0 |
Too rare to classify |
|
Swietenia humilis Zucc. |
8 |
0 |
OAX specialist |
|
Trichilia havanensis Jacq. |
17 |
0 |
OAX specialist |
|
Trichilia hirta L. |
2 |
0 |
Too rare to classify |
|
Trichilia trifolia L. |
0 |
6 |
Too rare to classify |
|
MENISPERMACEAE |
|||
|
Hyperbaena jalcomulcensis E. Pérez & Cast.-Campos |
0 |
1 |
Too rare to classify |
|
Hyperbaena mexicana Miers |
1 |
0 |
Too rare to classify |
|
Hyperbaena sp. |
1 |
0 |
Too rare to classify |
|
MONIMIACEAE |
|||
|
Mollinedia viridiflora Tul. |
0 |
2 |
Too rare to classify |
|
Siparuna andina (Tul.) A. DC. |
2 |
0 |
Too rare to classify |
|
MORACEAE |
|||
|
Brosimum alicastrum Sw. |
1 |
14 |
VER specialist |
|
Ficus citrifolia Mill. |
1 |
0 |
Too rare to classify |
|
Ficus pertusa L. f. |
1 |
0 |
Too rare to classify |
|
Ficus sp. |
0 |
1 |
Too rare to classify |
|
Maclura tinctoria (L.) D. Don ex Steud. |
3 |
1 |
Too rare to classify |
|
Trophis mexicana (Liebm.) Bureau |
3 |
0 |
Too rare to classify |
|
MYRICACEAE |
|||
|
Myrica lindeniana C. DC. |
16 |
0 |
OAX specialist |
|
MYRSINACEAE |
|||
|
Ardisia compressa Kunth |
2 |
0 |
Too rare to classify |
|
Ardisia revoluta Kunth |
1 |
0 |
Too rare to classify |
|
Ardisia sp. |
2 |
0 |
Too rare to classify |
|
MYRTACEAE |
|||
|
Calyptranthes schiedeana O. Berg |
0 |
26 |
VER specialist |
|
Eugenia liebmannii Standl. |
0 |
18 |
VER specialist |
|
Eugenia mexicana Steud. |
0 |
5 |
Too rare to classify |
|
Eugenia xalapensis (Kunth) DC. |
0 |
2 |
Too rare to classify |
|
Eugenia sp. 1 |
6 |
0 |
Too rare to classify |
|
Eugenia sp. 2 |
31 |
0 |
OAX specialist |
|
NYCTAGINACEAE |
|||
|
Neea tenuis Standl. |
0 |
1 |
Too rare to classify |
|
Neea sp. |
2 |
0 |
Too rare to classify |
|
Pisonia sp. |
0 |
1 |
Too rare to classify |
|
Torrubia macrocarpa Miranda |
7 |
0 |
Too rare to classify |
|
OLEACEAE |
|||
|
Fraxinus uhdei (Wenz.) Lingelsh. |
17 |
0 |
OAX specialist |
|
ONAGRACEAE |
|||
|
Hauya elegans DC. |
2 |
0 |
Too rare to classify |
|
OPILIACEAE |
|||
|
Agonandra obtusifolia Standl. |
2 |
0 |
Too rare to classify |
|
Agonandra racemosa (DC.) Standl. |
3 |
0 |
Too rare to classify |
|
PAPAVERACEAE |
|||
|
Bocconia frutescens L. |
3 |
0 |
Too rare to classify |
|
PENTAPHYLACACEAE |
|||
|
Cleyera integrifolia (Benth.) Choisy |
0 |
14 |
VER specialist |
|
Ternstroemia sylvatica Schltdl. & Cham. |
0 |
12 |
VER specialist |
|
PHYLLANTHACEAE |
|||
|
Phyllanthus sp. |
0 |
3 |
Too rare to classify |
|
Savia sessiliflora (Sw.) Willd. |
0 |
29 |
VER specialist |
|
PICRAMNIACEAE |
|||
|
Alvaradoa amorphoides Liebm. |
12 |
0 |
OAX specialist |
|
Picramnia mexicana Brandegee |
0 |
1 |
Too rare to classify |
|
PINACEAE |
|||
|
Abies religiosa (Kunth) Schltdl. & Cham. |
0 |
222 |
VER specialist |
|
Pinus ayacahuite C. Ehrenb. ex Schltdl. |
8 |
40 |
VER specialist |
|
Pinus douglasiana Martínez |
1 |
0 |
Too rare to classify |
|
Pinus hartwegii Lindl. |
112 |
265 |
VER specialist |
|
Pinus herrerae Martínez |
11 |
0 |
OAX specialist |
|
Pinus maximinoi H.E. Moore |
23 |
0 |
OAX specialist |
|
Pinus montezumae Lamb. |
8 |
0 |
OAX specialist |
|
Pinus patula Schltdl. & Cham. |
0 |
100 |
VER specialist |
|
Pinus pseudostrobus Brongn. |
101 |
5 |
OAX specialist |
|
Pinus sp. 1 |
1 |
0 |
Too rare to classify |
|
Pinus sp. 2 |
2 |
0 |
Too rare to classify |
|
PIPERACEAE |
|||
|
Piper umbricola C. DC. |
1 |
0 |
Too rare to classify |
|
PODOCARPACEAE |
|||
|
Podocarpus matudae Lundell |
0 |
5 |
Too rare to classify |
|
POLYGONACEAE |
|||
|
Coccoloba liebmannii Lindau |
1 |
0 |
Too rare to classify |
|
Coccoloba schiedeana Lindau |
4 |
0 |
Too rare to classify |
|
Coccoloba sp. |
0 |
7 |
Too rare to classify |
|
Podopterus mexicanus Bonpl. |
0 |
1 |
Too rare to classify |
|
Ruprechtia fusca Fernald |
3 |
0 |
Too rare to classify |
|
Ruprechtia pallida Standl. |
0 |
1 |
Too rare to classify |
|
Ruprechtia sp. |
0 |
3 |
Too rare to classify |
|
PRIMULACEAE |
|||
|
Bonellia nervosa (C. Presl) B. Ståhl & Källersjö |
13 |
0 |
OAX specialist |
|
Myrsine coriacea (Sw.) R. Br. ex Roem. & Schult. |
0 |
10 |
Too rare to classify |
|
PROTEACEAE |
|||
|
Roupala montana Aubl. |
9 |
0 |
OAX specialist |
|
RESEDACEAE |
|||
|
Forchhammeria pallida Liebm. |
3 |
0 |
Too rare to classify |
|
RHAMNACEAE |
|||
|
Colubrina triflora Brongn. ex G. Don |
1 |
6 |
Too rare to classify |
|
Rhamnus capreifolia Schltdl. |
0 |
3 |
Too rare to classify |
|
Rhamnus longistyla C.B. Wolf |
0 |
1 |
Too rare to classify |
|
Rhamnus mcvaughii L.A. Johnst. & M.C. Johnst. |
0 |
1 |
Too rare to classify |
|
Rhamnus sp. |
1 |
0 |
Too rare to classify |
|
ROSACEAE |
|||
|
Cercocarpus macrophyllus C.K. Schneid. |
2 |
0 |
Too rare to classify |
|
Prunus brachybotrya Zucc. |
6 |
3 |
Too rare to classify |
|
Prunus rhamnoides Koehne |
0 |
7 |
Too rare to classify |
|
Prunus samydoides Schltdl. |
0 |
3 |
Too rare to classify |
|
Prunus tetradenia Koehne |
2 |
0 |
Too rare to classify |
|
Prunus sp. 1 |
0 |
1 |
Too rare to classify |
|
Prunus sp. 2 |
0 |
1 |
Too rare to classify |
|
RUBIACEAE |
|||
|
Arachnothryx capitellata (Hemsl.) Borhidi |
0 |
34 |
VER specialist |
|
Calycophyllum candidissimum (Vahl) DC. |
4 |
0 |
Too rare to classify |
|
Chiococca pachyphylla Wernham |
3 |
8 |
Too rare to classify |
|
Chiococca sp. |
1 |
0 |
Too rare to classify |
|
Chomelia crassifolia Borhidi |
1 |
0 |
Too rare to classify |
|
Deppea grandiflora Schltdl. |
0 |
2 |
Too rare to classify |
|
Deppea sp. |
1 |
0 |
Too rare to classify |
|
Exostema caribaeum (Jacq.) Schult. |
2 |
0 |
Too rare to classify |
|
Guettarda sp. |
3 |
0 |
Too rare to classify |
|
Hamelia patens Jacq. |
10 |
0 |
OAX specialist |
|
Hintonia latiflora (DC.) Bullock |
2 |
0 |
Too rare to classify |
|
Palicourea padifolia (Humb. & Bonpl. ex Schult.) C.M. Taylor & Lorence |
0 |
4 |
Too rare to classify |
|
Psychotria galeottiana (M. Martens) C.M. Taylor & Lorence |
0 |
1 |
Too rare to classify |
|
Randia aculeata L. |
0 |
7 |
Too rare to classify |
|
Randia armata (Sw.) DC. |
3 |
0 |
Too rare to classify |
|
Randia monantha Benth. |
0 |
14 |
VER specialist |
|
Randia nelsonii Greenm. |
3 |
0 |
Too rare to classify |
|
Randia oaxacana Standl. |
1 |
0 |
Too rare to classify |
|
Randia tetracantha (Cav.) DC. |
3 |
0 |
Too rare to classify |
|
Rogiera langlassei (Standl.) Borhidi |
24 |
0 |
OAX specialist |
|
Solenandra mexicana (A. Gray) Borhidi |
5 |
0 |
Too rare to classify |
|
RUTACEAE |
|||
|
Esenbeckia berlandieri Baill. |
4 |
0 |
Too rare to classify |
|
Ptelea sp. |
0 |
1 |
Too rare to classify |
|
Zanthoxylum melanostictum Schltdl. & Cham. |
0 |
10 |
Too rare to classify |
|
Zanthoxylum sp. |
0 |
2 |
Too rare to classify |
|
SABIACEAE |
|||
|
Meliosma alba (Schltdl.) Walp. |
0 |
1 |
Too rare to classify |
|
Meliosma dentata (Liebm.) Urb. |
0 |
3 |
Too rare to classify |
|
SALICACEAE |
|||
|
Bartholomaea sessiliflora (Standl.) Standl. & Steyerm. |
3 |
0 |
Too rare to classify |
|
Casearia nitida Jacq. |
7 |
1 |
Too rare to classify |
|
Casearia obovata Schltdl. |
1 |
0 |
Too rare to classify |
|
Casearia sylvestris Sw. |
0 |
5 |
Too rare to classify |
|
Casearia tremula (Griseb.) Griseb. ex C. Wright |
8 |
0 |
OAX specialist |
|
Casearia sp. 1 |
1 |
0 |
Too rare to classify |
|
Casearia sp. 2 |
3 |
0 |
Too rare to classify |
|
Prockia crucis P. Browne ex L. |
1 |
0 |
Too rare to classify |
|
Samyda mexicana Rose |
1 |
0 |
Too rare to classify |
|
Xylosma sp. 1 |
1 |
0 |
Too rare to classify |
|
Xylosma sp. 2 |
1 |
0 |
Too rare to classify |
|
Xylosma sp. 3 |
4 |
0 |
Too rare to classify |
|
Xylosma sp. 4 |
1 |
0 |
Too rare to classify |
|
Xylosma sp. 5 |
1 |
0 |
Too rare to classify |
|
SAPINDACEAE |
|||
|
Matayba oppositifolia (A. Rich.) Britton |
0 |
1 |
Too rare to classify |
|
Sapindus saponaria L. |
0 |
1 |
Too rare to classify |
|
Thouinidium decandrum (Bonpl.) Radlk. |
0 |
14 |
VER specialist |
|
Thouinia villosa DC. |
1 |
0 |
Too rare to classify |
|
SAPOTACEAE |
|||
|
Chrysophyllum mexicanum Brandegee ex Standl. |
10 |
0 |
OAX specialist |
|
Sideroxylon capiri (A. DC.) Pittier |
6 |
0 |
Too rare to classify |
|
Sideroxylon salicifolium (L.) Lam. |
1 |
0 |
Too rare to classify |
|
Sideroxylon sp. |
1 |
0 |
Too rare to classify |
|
SOLANACEAE |
|||
|
Cestrum sp. |
2 |
0 |
Too rare to classify |
|
Solanum nigricans M. Martens & Galeotti |
0 |
2 |
Too rare to classify |
|
STAPHYLEACEAE |
|||
|
Turpinia insignis (Kunth) Tul. |
0 |
75 |
VER specialist |
|
STYRACACEAE |
|||
|
Styrax glabrescens Benth. |
0 |
27 |
VER specialist |
|
SYMPLOCACEAE |
|||
|
Symplocos limoncillo Bonpl. |
0 |
11 |
VER specialist |
|
TAXACEAE |
|||
|
Taxus globosa Schltdl. |
0 |
5 |
Too rare to classify |
|
THYMELAEACEAE |
|||
|
Daphnopsis sp. |
2 |
0 |
Too rare to classify |
|
ULMACEAE |
|||
|
Aphananthe monoica (Hemsl.) J.-F. Leroy |
8 |
0 |
OAX specialist |
|
URTICACEAE |
|||
|
Cecropia obtusifolia Bertol. |
2 |
0 |
Too rare to classify |
|
Gyrotaenia microcarpa (Wedd.) Fawc. & Rendle |
10 |
0 |
OAX specialist |
|
Phenax mexicanus Wedd. |
17 |
0 |
OAX specialist |
|
Urera pacifica V.W. Steinm. |
10 |
0 |
OAX specialist |
|
VERBENACEAE |
|||
|
Citharexylum berlandieri B.L. Rob. |
0 |
2 |
Too rare to classify |
|
Citharexylum caudatum L. |
0 |
4 |
Too rare to classify |
|
Citharexylum ligustrinum Van Houtte |
0 |
3 |
Too rare to classify |
|
Citharexylum mocinnoi D. Don |
0 |
6 |
Too rare to classify |
|
WINTERACEAE |
|||
|
Drimys granadensis L. f. |
0 |
2 |
Too rare to classify |
References
Aiba, S., & Kitayama, K. (1999). Structure, composition and species diversity in an altitude-substrate matrix of rain forest tree communities on Mount Kinabalu, Borneo. Plant Ecology, 140, 139–157. https://doi.org/10.1023/A:1009710618040
Arévalo, J. R., Otto. R., Escudero, C., Fernández-Lugo, S., Arteaga, M., Delgado, J. D. et al. (2010). Do anthropogenic corridors homogenize plant communities at a local scale? A case studied in Tenerife (Canary Islands). Plant Ecology, 209, 23–35. https://doi.org/10.1007/s11258-009-9716-y
Burnham, K. P., & Anderson, D. R. (2002). Model selection and multimodel inference: a practical information-theoretic approach. New York: Springer-Verlag.
Challenger, A., & Soberón, J. (2008). Los ecosistemas terrestres. In Capital Natural de México, Vol. I. Conocimiento actual de la biodiversidad (pp. 87–108). México: Conabio.
Chao, A., & Lin, S. Y. (2011). Program CLAM (Classification Method). Program and User’s Guide. Retrieved on August 18, 2015 from http://purl.oclc.org/clam
Chazdon, R. L., Chao, A., Colwell, R. K., Lin, S. Y., Norden, N., Letcher, S. G. et al. (2011). A novel statistical method for classifying habitat generalists and specialists. Ecology, 92, 1332–1343. https://doi.org/10.1890/10-1345.1
Clark, D. B., Hurtado, J., & Saatchi, S. S. (2015). Tropical rain forest structure, tree growth and dynamics along a 2700-m elevational transect in Costa Rica. Plos One, 10, e0122905. https://doi.org/10.1371/journal.pone.0122905
Colwell, R. K., Brehm, G., Cardelús, C. L., Gilman, A. C., & Longino, J. T. (2008). Global warming, elevational range shifts, and lowland biotic attrition in the wet tropics. Science, 322, 258–261. https://doi.org/10.1126/science.1162547
Colwell, R. K., & Lees, D. C. (2000). The mid-domain effect: geometric constraints on the geography of species richness. Trends in Ecology and Evolution, 15, 70–76. https://doi.org/10.1016/S0169-5347(99)01767-X
Da, L. J., Kang, M. M., Song, K., Shang, K. K., Yang, Y. C., Xia, A. M. et al. (2009). Altitudinal zonation of human-disturbed vegetation on Mt. Tianmu, eastern China. Ecological Research, 24, 1287–1299. https://doi.org/10.1007/s11284-009-0613-6
Dossa, G. G. O., Paudel, E., Fujinuma, J., Yu, H., Chutipong, W., Zhang, Y. et al. (2013). Factors determining forest diversity and biomass on a tropical volcano, Mt. Rinjani, Lombok, Indonesia. Plos One, 8, e67720. https://doi.org/10.1371/journal.pone.0067720
Espinosa, D. O., Ocegueda, S. C., Aguilar, C., Flores, O., Llorente-Bousquets, J., & Vázquez, B. (2008). El conocimiento biogeográfico de las especies y su regionalización natural. In Capital Natural de México, Vol. I. Conocimiento actual de la biodiversidad (pp. 33–65). México: Conabio.
Feeley, K. J., Hurtado, J., Saatchi, S., Silman, M. R., & Clark, D. B. (2013). Compositional shifts in Costa Rican forests due to climate-driven species migrations. Global Change Biology, 19, 3472–3480. https://doi.org/10.1111/gcb.12300
Francis, A. P., & Currie, D. J. (2003). A globally consistent richness-climate relationship for angiosperms. The American Naturalist, 161, 523–536. https://doi.org/10.1086/368223
Gentry, A. H. (1988). Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden, 75, 1–34. https://doi.org/10.2307/2399464
Gentry, A. H. (1995). Diversity and floristic composition of neotropical dry forests. In S. H. Bullock, H. A. Mooney, & E. Medina (Eds.), Seasonally dry tropical forests. (pp. 146–194). Cambridge University Press. https://doi.org/10.1017/CBO9780511753398.007
Gernandt, D. S., & Pérez-de la Rosa, J. A. (2014). Biodiversidad de Pinophyta (coníferas) en México. Revista Mexicana de Biodiversidad (Supl.) 85, S126–S133. https://doi.org/10.7550/rmb.32195
González-Abraham, C., Ezcurra, E., Garcillán, P. P., Ortega-Rubio, A., Kolb, M., & Bezaury Creel, J. E. (2015). The human footprint in Mexico: physical geography and historical legacies. Plos One, 10, e0121203. https://doi.org/10.1371/journal.pone.0121203
Grubb, P. J. (1977). Control of forest growth and distribution on wet tropical mountains: with special reference to mineral nutrition. Annual Review of Ecology and Systematics, 8, 83–107. https://doi.org/10.1146/annurev.es.08.110177.000503
Grytnes, J. A. (2003). Species-richness patterns of vascular plants along seven altitudinal transects in Norway. Ecography, 26, 291–300. https://doi.org/10.1034/j.1600-0587.2003.03358.x
Grytnes, J. A., & Beaman, J. H. (2006). Elevational species richness patterns for vascular plants on Mount Kinabalu, Borneo. Journal of Biogeography, 33, 1838–1849. https://doi.org/10.1111/j.1365-2699.2006.01554.x
Grytnes, J. A., & Vetaas, O. R. (2002). Species richness and altitude: a comparison between null models and interpolated plant species richness along the Himalayan altitudinal gradient, Nepal. The American Naturalist, 159, 294–304. https://doi.org/10.1086/338542
Harris, I., Jones, P. D., Osborn, T. J., & Lister, D. H. (2013). Updated high-resolution grids of monthly climatic observations – the CRU TS3.10 Dataset. International Journal of Climatology, 34, 623–642. https://doi.org/10.1002/joc.3711
Hawkins, B. A., Field, R., Cornell, H. V., Currie, D. J., Guégan, J.-F., Kaufman, D. M. et al. (2003). Energy, water, and broad-scale geographic patterns of species richness. Ecology, 84, 3105–3117. https://doi.org/10.1890/03-8006
Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25, 1965–1978. https://doi.org/10.1002/joc.1276
Holwerda, F., Bruijnzeel, L. A., Muñoz-Villers, L. E., Equihua, M., & Asbjornsen, H. (2010). Rainfall and cloud water interception in mature and secondary lower montane cloud forests of central Veracruz, Mexico. Journal of Hydrology, 384, 84–96. https://doi.org/10.1016/j.jhydrol.2010.01.012
Kessler, M., Kluge, J., Hemp, A., & Ohlemüller, R. (2011). A global comparative analysis of elevational species richness patterns of ferns. Global Ecology and Biogeography, 20, 868–880. https://doi.org/10.1111/j.1466-8238.2011.00653.x
Kitayama, K., & Aiba, S. (2002). Ecosystem structure and productivity of tropical rain forests along altitudinal gradients with contrasting soil phosphorus pools on Mount Kinabalu, Borneo. Journal of Ecology, 90, 37–51. https://doi.org/10.1046/j.0022-0477.2001.00634.x
Latham, R. E., & Ricklefs, R. E. (1993). Global patterns of tree species richness in moist forests: energy-diversity does not account for variation in species richness. Oikos, 67, 325–333. https://doi.org/10.2307/3545479
Li, M., & Feng, J. (2015). Biogeographical interpretation of elevational patterns of genus diversity of seed plants in Nepal. Plos One, 10, e0140992. https://doi.org/10.1371/journal.pone.0140992
Liao, C. C., Liu, M., Su, M. H., & Wang, J. C. (2014). Compression and overlap of unique vegetation system of subtropical mountain resembling tropical and temperate forests along elevation. Journal of Forestry Research, 19, 215–225. https://doi.org/10.1007/s10310-013-0409-y
Lomolino, M. V. (2001). Elevation gradients of species-density: historical and prospective views. Global Ecology and Biogeography, 10, 3–13. https://doi.org/10.1046/j.1466-822x.2001.00229.x
Nogués-Bravo, D., Araújo, M. B., Romdal T., & Rahbek, C. (2008). Scale effects and human impact on the elevational species richness gradients. Nature, 453, 216–220. https://doi.org/10.1038/nature06812
Piperno, D. R. (2006). Quaternary environmental history and agricultural impact on vegetation in Central America. Annals of the Missouri Botanical Garden, 93, 274–296. https://doi.org/10.3417/0026-6493(2006)93[274:QEHAAI]2.0.CO;2
R Core Team (2017). R: a language and environment for statistical computing. R Foundation for Statistical Computing. Retrieved on November 13, 2017 from: http://www.Rproject.org
Rahbek, C. (1995). The elevation gradient of species richness: a uniform pattern? Ecography, 18, 200–205. https://doi.org/10.1111/j.1600-0587.1995.tb00341.x
Rahbek, C. (2005). The role of spatial scale and the perception of large-scale species-richness patterns. Ecology Letters, 8, 224–239. https://doi.org/10.1111/j.1461-0248.2004.00701.x
Rowe, R. J. (2009). Environmental and geometric drivers of small mammal diversity along elevational gradients in Utah. Ecography, 32, 411–422. https://doi.org/10.1111/j.1600-0587.2008.05538.x
Salas-Morales, S. H., & Meave, J. A. (2012). Elevational patterns in the vascular flora of a highly diverse region in southern Mexico. Plant Ecology, 213, 1209–1220. https://doi.org/10.1007/s11258-012-0077-6
Salas-Morales, S. H., Meave, J. A., & Trejo, I. (2015). The relationship of meteorological patterns with changes in floristic richness along a large elevational gradient in a seasonally dry region of southern Mexico. International Journal of Biometeorology, 59, 1861–1874. https://doi.org/10.1007/s00484-015-0993-y
Sanders, N. J. (2002). Elevational gradients in ant species richness: area, geometry, and Rapoport’s rule. Ecography, 25, 25–32. https://doi.org/10.1034/j.1600-0587.2002.250104.x
Sanders, N. J., & Rahbek, C. (2012). The patterns and causes of elevational diversity gradients. Ecography, 35, 1–3. https://doi.org/10.1111/j.1600-0587.2011.07338.x
Sang, W. (2009). Plant diversity patterns and their relationship with soil and climatic factors along an altitudinal gradient in the middle Tianshan Mountain area, Xinjiang, China. Ecological Research, 24, 303–314. https://doi.org/10.1007/s11284-008-0507-z
ter Braak, C. J. F., & Šmilauer, P. (2002). CANOCO Reference manual and CanoDraw for Windows User’s Guide: software for canonical community ordination (version 4.5). Ithaca, New York: Microcomputer Power.
Toledo-Garibaldi, M., & Williams-Linera, G. (2014). Tree diversity patterns in successive vegetation types along an elevation gradient in the mountains of eastern Mexico. Ecological Research, 29, 1097–1104. https://doi.org/10.1007/s11284-014-1196-4
Valencia, S. (2004). Diversidad del género Quercus (Fagaceae) en México. Boletín de la Sociedad Botánica de México, 75, 33–53. https://doi.org/10.17129/botsci.1692
Wang, X., Fang, J., Sanders, N. J., White, P. S. & Tang, Z. (2009). Relative importance of climate vs local factors in shaping the regional patterns of forest plant richness across northeast China. Ecography, 32, 133–142. https://doi.org/10.1111/j.16000587.2008.05507.x



